- 1Department of Oncology, The Second Xiangya Hospital, Central South University, Changsha, China
- 2Xiangya School of Medicine, Central South University, Changsha, China
- 3Hunan Cancer Mega-Data Intelligent Application and Engineering Research Centre, Changsha, China
- 4Hunan Key Laboratory of Tumor Models and Individualized Medicine, The Second Xiangya Hospital, Central South University, Changsha, China
- 5Hunan Key Laboratory of Early Diagnosis and Precision Therapy in Lung Cancer, The Second Xiangya Hospital, Central South University, Changsha, China
Activation of the cGAS-STING pathway by cytoplasmic DNA induces the production of Type-1 interferons. Recent advances in research suggest that the cGAS-STING pathway is involved in different parts of the cancer-immunity cycle (CIC) to promote or suppress antitumor immune responses. Combination therapy of STING agonists has made certain progress in preclinical as well as clinical trials, but the selection of combination therapy regimens remains a challenge. In this review, we summarize the role of the cGAS-STING in all aspects of CIC, and focus on the combination immunotherapy strategies of STING agonists and current unsolved challenges.
1 Introduction
Cyclic GMP-AMP (cGAMP) synthase (cGAS) has been identified as a cytoplasmic double-stranded DNA sensor that plays a key role in Type-1 interferon and inflammatory responses via a Stimulator of Interferon Genes (STING)-dependent signaling pathway (1). This pathway has been demonstrated to have a regulatory role in metabolic endocrine diseases (2–5), viral infections (6, 7), autoimmune diseases (8, 9), and neurological disorders (10, 11). In recent years, there is increasing evidence that the cGAS-STING pathway is closely related to the occurrence, development and regression of cancer. The cGAS-STING pathway regulates various aspects of the Cancer-Immunity Cycle (CIC), including tumor antigen release (12), antigen presentation (13), the priming and activation of T cells (14), the trafficking and infiltration of T cells into tumor tissues (15), and the recognition and killing of tumor cells by T cells (16). The cGAS-STING pathway plays an anti-tumor or pro-tumor role.
In this review, we summarize the role of the cGAS-STING in all aspects of CIC, and focus on the combination immunotherapy strategies of STING agonists and current unsolved challenges.
2 Overview of the cGAS–STING signaling
cGAS is a cytosolic DNA receptor activated by double-stranded DNA (dsDNA) in a sequence-independent but length-dependent manner (1, 17). cGAS catalyzes the conversion of GTP and ATP to 2’3’-Cyclic GMP-AMP (2’3’-cGAMP) (18, 19), which binds to STING and promotes its translocation from the endoplasmic reticulum (ER) to Golgi (20, 21). STING recruits and activates TANK binding kinase-1 (TBK1), which in turn promotes the translocation of interferon regulatory factor 3 (IRF3) into the nucleus where it promotes the production of Type-1 interferon and the transcription of interferon-stimulated genes (ISGs) (22, 23). STING also binds and stimulates IκB kinase (IKK), which mediates the activation of canonical and non-canonical NF-kB pathways (24). After signal transduction is terminated, STING is transferred to endolysosomes for degradation (22).
3 The cGAS-STING pathway regulates the cancer-immunity cycle
Mounting evidence has demonstrated that the cGAS-STING pathway plays an important regulatory role in all stages of the cancer-immunity cycle, either activating or suppressing anti-tumor immune responses, depending on the strength and timing of the activation of the cGAS-STING pathway and the type and state of the tumors (14, 25–27).
3.1 The cGAS-STING pathway increases tumor antigen release by promoting apoptosis
During normal mitosis, cGAS has a higher affinity for nucleosomes compared to dsDNA, thus preventing cGAS dimerization and activation (1). However, when Taxane drugs interfere with mitosis leading to mitotic arrest, the accumulation of phosphorylated IRF3 which is induced by the cGAS inhibits the expression of the anti-apoptotic protein BCL-xL, triggering apoptosis via mitochondrial outer membrane permeabilization (MOMP) (12). In addition, Type-1 interferon and TNFα produced by the cGAS-STING activation can stimulate the expression of the pro-apoptotic molecule, NOXA, in neighboring cells via paracrine secretion. This induces apoptotic priming, meaning the cancer cells undergo MOMP propensity (28, 29). Analysis of The Cancer Genome Atlas (TCGA) datasets showed that lung and ovarian cancer patients with high cGAS expression were more sensitive to paclitaxel treatment (12). The 2 ‘ 3 ‘ -cGAMP analogue, c-di-AMP, activates the STING pathway to induce apoptosis in estrogen receptor-negative breast cancer cells, resulting in the release of tumor antigens (TAs) and propagation of the cancer-immunity cycle (30).
3.2 The cGAS-STING pathway facilitates the processing and presentation of tumor antigens
Dendritic cells (DCs) are considered to be the main antigen-presenting cells (APCs) responsible for the priming of anti-tumor T cells. Type-1 IFN production promotes DC maturation, upregulates the expression of molecules such as MHCI, MHCII, CD40, CD80, CD86 (13) on the DCs surface (31), and enhances DC migration to tumor draining lymph nodes (TDLNs) migration (32). Although T cell activation occurs mainly in TDLNs, STING signaling has been reported to induce the formation of intra-tumor tertiary lymphoid structures (TLS) in a mouse model of melanoma (33), where DCs may activate T cells, thereby skipping the need for migration to TDLNs (34). In addition, it has been reported that in the tumor microenvironment (TME), cancer cells transfer cGAMP into tumor-associated DCs via gap junctions, leading to the activation of pathways downstream of the cGAS-STING (31, 35).
3.3 The cGAS-STING pathway has a dichotomous effect on the priming and activation of T cells
Although it is well known that the cGAS-STING pathway plays a key role in the regulation of T cell priming and activation, the strength and timing of the activation of this signaling pathway may have opposing effects (14).
Moderate activation of the cGAS-STING pathway upregulates the expression of the TA-MHC I complex on the cell surface of DCs, which is recognized by TCRs, leading to the activation of CD8+ cytotoxic T cells (CTLs) (31).. Moreover, by increasing the expression of the transcription factor TCF1, the cGAS-STING pathway-mediated Type-1 interferon increases the activity of stem-like CD8+ T cells (36), which are capable of self-renewal, persistence, and differentiation potential (37–39). It has been reported that the cGAS agonist Manganese (40), low-dose STING agonists ADU-S100 (S100) (14), Vadimezan (DMXAA) (41), and STINGV155M (a constitutively activating mutation of STING) (42) all have the ability to enhance the activity of CTLs thereby producing durable antitumor immunity. Consistent with these findings, STING-deficiency reduces CD8+ T cell activity in mice (43).
However, high doses of ADU-S100 lead to substantial T cell death and impaired antitumor immunity (14). This may be attributed to the activation of the non-type I IFN domain of STING that disrupts calcium homeostasis, thereby stimulating T cells to be highly responsive to TCR signaling-induced endoplasmic reticulum stress, leading to T cell death (26, 27).
3.4 Activation of the cGAS-STING pathway promotes the trafficking and infiltration of T cells
CTLs need to leave TDLNs and enter the tumor tissue via blood vessels in order to recognize and kill cancer cells (44). The cGAS-STING pathway-induced Type-1 interferon response drives the expression of multiple chemokines such as CXCL9, CXCL10, and CCL5, that act as chemical gradients to direct CTLs into the tumor tissue (45–47). IFN I signaling also increases the expression of E selectin, VCAM-1, and ICAM-1 in endothelial cells, enhances vascular permeability, and facilitates immune cell extravasation, thus enhancing the antitumor effect (15).
The tumor vasculature is disorganized and immature, with loose connections and low pericyte coverage. In addition, this vascular system does not provide a continuous blood supply to the tumor tissue, thus increasing the distant metastasis of tumor cells and decreasing the tropism of CTLs to TME (48–51). The cGAS-STING pathway-induced activation of Type-1 interferon upregulates the vascular normalization genes such as Cdh5, Angpt1, Pdgfrb, Mcam, and Col4a. These genes induce the normalization of tumor vasculature with increased pericyte coverage and more intact basement membrane, facilitating infiltration of CTLs into tumor tissue (33, 52, 53). Consistent with these findings, STING deficiency reduces the expression of these genes (53). However, vascular endothelial growth factor (VEGF)/VEGFR2 can negatively regulate Type-1 interferon signaling through ubiquitin-mediated IFNAR degradation, leading to the inhibition of Type-1 interferon action in VEGF-rich tumor tissues (53). Combining STING agonists with VEGFR2 blockers not only attenuates the negative effects of VEGF, but also synergistically promotes tumor vascular normalization (53).
3.5 The cGAS-STING pathway has a dichotomous effect on the recognition and killing of cancer cells by T cells
Activation of the cGAS-STING pathway not only induces CTLs-mediated cancer cell death by upregulating MHC-I expression on the surface of cancer cells (54), but also activates NK cells to kill tumor cells, especially those with reduced or absent MHC-I expression (55–58). In addition, in the tumor microenvironment (TME), tumor derived cGAMP can be transferred from tumor cells to immune cells to trigger the STING pathway in immune cells and activate the antitumor response of NK cells (59). The death of cancer cells induces the release of tumor antigens, leading to the initiation of a new round of CIC.
The programmed cell death protein 1 (PD-1) expressed by T cells binds to the ligand PD-L1 on the surface of tumor cells, which inhibits the clearance of tumor cells by effector T cells (60–63). Activation of the cGAS-STING pathway has been demonstrated to increase the expression of PD-L1 on the surface of tumor cells and thus attenuate the activity of CTLs, which has been confirmed in models of liver cancer (64), melanoma (65), non-small cell lung cancer (NSCLC) (16) and small cell lung cancer (SCLC) (46, 66). The antitumor effects of STING agonists were enhanced when combined with PD-L1 or PD-1 blockers (40, 67, 68).
It has been found that activation of the cGAS-STING pathway may induce the formation of immunosuppressive TMEs and negatively regulate the killing effect of CTLs (69, 70). (IDO1) is an enzyme that catalyzes tryptophan into kynurenine, which inhibits the proliferation of T cells and promotes the differentiation of Tregs and the infiltration of myeloid-derived suppressor cells (MDSCs) (71, 72). Activation of the cGAS-STING pathway increases IDO1 expression (73), which has been validated in colorectal cancer (74, 75). Analysis of the TCGA dataset revealed that infiltration of Tregs and MDSCs positively correlated with STING expression in pancreatic cancer, bladder urothelial carcinoma, breast cancer, liver cancer, prostate adenocarcinoma, and thyroid cancer (76). Interestingly, Eslam Mohamed et al. (77) proposed that PERK-deficient MDSCs lead to activation of their own STING signaling, reprogramming immunosuppressed MDSCs into myeloid cells that activate CD8+ T cell-mediated anticancer immunity.
In addition, DNA damage-mediated activation of the cGAS-independent non-canonical STING signaling primarily activates NF-kB and promotes IL-6 production, which is associated with pro-tumor response (78–80). 2’3’ -cGAMP transferred from tumor cells to astrocytes activates NF-κB signaling, thereby promoting brain metastasis and chemoresistance (81). Since TBK1 and STING inhibitors do not block non-canonical STING, NF-kB inhibitors may be an option to reduce the pro-tumorigenic response (79).
4 The mechanism underlying the inhibition of the cGAS-STING pathway in tumor
An increasing number of investigations have indicated that the activity of the cGAS-STING pathway is inhibited in several tumors due to the regulation of multiple mechanisms. Mutant p53 inhibits the activation of the cGAS-STING-TBK1-IRF3 pathway and promotes tumor progression by interacting with and inhibiting TBK1 activity (82). Mutant NF2 is induced by activated IRF3 to form cellular condensates, which inhibit TBK1 activity, particularly in human vestibular nerve sheath tumors (83). As a hydrolase of cGAMP, ecto-nucleotide pyrophosphatases 1 (ENPP1) impedes the antitumor immune response by blocking cGAMP transfer from tumor cells to immune cells to trigger the STING pathway (84). Hypoxia, a feature of solid cancers, upregulates RNASEH2A via HIF2α, which may limit activation of the cGAS-STING signaling by reducing nuclear DNA release. Hypoxia is associated with poor prognosis of hepatocellular carcinoma (85). In a mouse model of ovarian cancer, the SETDB1-TRIM28 complex inhibited the formation of micronuclei in the cytoplasm, thereby inhibiting the activity of the cGAS-STING pathway and suppressing anti-tumor immunity (86). TIM-3 may inhibit the activation of the cGAS-STING pathway by suppressing the uptake of extracellular DNA by DCs, which has been demonstrated in breast cancer models (87).
Thus, blocking the mechanism underlying the inhibition of the cGAS-STING pathway may be an option for the treatment of tumors with suppressed activity of the cGAS-STING, though the existing intervention methods remain immature. In contrast, using agonists to activate the cGAS-STING signaling pathway, thereby antagonizing the inhibitory signals of this pathway and reversing the immunosuppressive state, may be a more feasible approach, which is expected to break the resistance bottleneck of these tumor immunotherapies.
5 Immune combination therapy of the cGAS-STING
As previously mentioned, the regulation of tumor immunity by the cGAS-STING pathway is dichotomous; therefore, STING agonists applied alone may carry the side effect of immunosuppression. However, combined STING agonists with other suitable antitumor therapies can mechanistically synergize, as demonstrated in clinical and preclinical models (Figure 1).
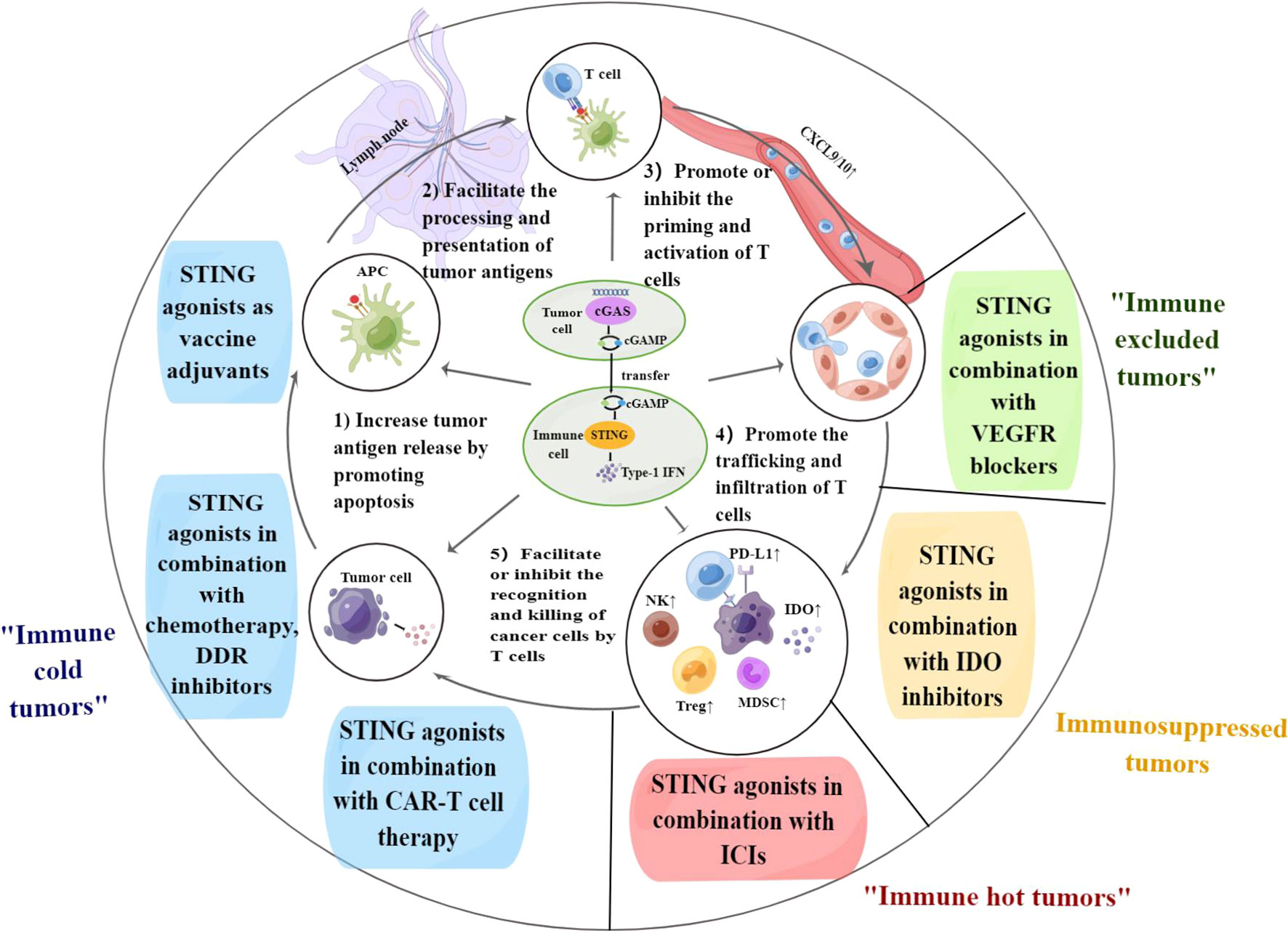
Figure 1 The cGAS-STING pathway regulates each step of the cancer-immunity cycle. Combination therapy of STING agonists can target different steps of the cancer-immunity cycle and contribute to solving immunotherapy challenges in the corresponding immune types of tumors. APC, antigen-presenting cell; PD-L1, programmed death-ligand 1; DDR, DNA damage response; VEGF, vascular endothelial growth factor; IDO, indoleamine 2,3-dioxygenase; ICIs, immune checkpoint inhibitors; CAR-T cell, chimeric antigen receptor-T cell. By Figdraw.
5.1 Combination therapy to promote tumor antigen release and presentation
Due to the low mutational burden and low expression of antigen-presentation markers, “immune cold tumors” lack infiltration of CTLs both inside and at the margins of the tumor, which respond poorly to immune checkpoint inhibitors (ICIs) and are often associated with poor prognosis (88–90). Therefore, such combination therapies are essential to overcome the immune deficiency and convert cold tumors into hot tumors.
5.1.1 STING agonists in combination with chemotherapy
STING agonists in combination with chemotherapy have shown promising efficacy in preclinical trials. The combination of cisplatin and cGAMP showed effective CXCR3-dependent antitumor effects in a mouse model of head and neck squamous cell carcinoma (HNSCC) (91). However, several clinical trials of STING agonists in combination with chemotherapy have been completed without achieving expected efficacy. The poor performance of the STING agonist ASA404 in clinical trials may be due to the fact that ASA404 selectively binds to mice, but not to human STING. Therefore, STING agonists with higher affinity for humans need to be rationally designed to enhance antitumor efficacy.
5.1.2 STING agonists in combination with DNA damage response inhibitors
Homologous recombination repair (HRR)-deficient tumors result in a higher tumor mutational load, including KEAP1-mutated non-small cell lung cancer (NSCLC) (92), BRCA1/2-deficient tumors (93, 94), microsatellite instability (MSI) colorectal cancer (CRC) (95), and small cell lung cancer (SCLC) (66) characterized by widespread deletion of two key regulators of the cell cycle checkpoint pathway, TP53 and RB1. Such tumors exhibit sensitivity to DDR inhibitors, and persistent high levels of DNA damage in their cells contribute to activation of the cGAS-STING pathway. It was revealed that combination therapy of DDR inhibitors (including PARP inhibitor olaparib and CHK1 inhibitor prexasertib) and STING agonists demonstrated beneficial therapeutic effects in such tumors, superior to both drugs monotherapy (66, 92–95). Thus, combination therapy with DDR inhibitors and STING agonists is expected to be a promising treatment for HRR-deficient tumors.
5.1.3 STING agonists as vaccine adjuvants
Recently, several studies have demonstrated that STING agonists can serve as adjuvants for tumor vaccines and exert beneficial effects in antitumor therapy. Matteo Rossi et al. (96) discovered that the combination of STING agonists with therapeutic protein vaccines significantly reduced the rate of tumor growth and improved the efficacy of therapeutic vaccination, which was demonstrated in a variety of mouse tumor models. CDGSF, a novel STING agonist that induces a “hot” tumor microenvironment to inhibit melanoma progression, has been shown to induce a robust adaptive immune response as an adjuvant to SARS-CoV-2 stinger protein and has great potential to be an adjuvant for cancer vaccines (97).
5.2 STING agonists combined with VEGFR blockers to promote the trafficking and infiltration of T cells
The combination of STING agonists and VEGFR blockers collaboratively drives the infiltration of CTLs into the tumor core, which is essential for “immune excluded tumors”. In immune excluded tumors, CTLs aggregate at the tumor border but cannot invade the tumor interior, possibly due to the lack of T-cell chemokines or abnormal tumor vascular formation barriers (69). Anlotinib, a tyrosine kinase inhibitor (TKI), inhibits tumor angiogenesis by blocking multiple targets such as VEGFR, PDGFR, and FGFR. A recent study revealed that the antitumor effects of anlotinib were also associated with activation of the cGAS-STING pathway, which was confirmed in a mouse model of gastric cancer (98). Another study confirmed that triple immunotherapy with STING agonists, anti-VEGFR2 antibodies, and anti-PD-1 or anti-CTLA-4 antibodies was more potent and durable in mouse models of lung and colon cancer, extending survival in mice resistant to ICIs or anti-angiogenic therapy (53).
5.3 Combination therapy to facilitate the recognition and killing of tumor cells by T cells
5.3.1 STING agonists in combination with chimeric antigen receptor -T cell therapy
CAR-T cell therapy is one of the promising anti-cancer therapies that has achieved excellent efficacy in treating hematologic tumors (99), but has a lower success rate in treating patients with solid tumors, which may be due to insufficient infiltration of CAR T cells into tumor tissue, immunosuppression TME-induced functional suppression, and CAR T cell exhaustion (100, 101).
In situ mouse mammary tumor model, administration of STING agonists DMXAA or cGAMP at sites distant from the tumor significantly enhanced the efficacy of Th/Tc17 CAR T cells, which may be related to the upregulation of chemokines CXCL9 and CXCL10 by STING agonists to promote the infiltration of CAR T cells into the tumor tissue. Furthermore, sustained tumor regression was only achieved in combination with anti-PD-1 monoclonal antibodies, possibly due to anti-PD-1 antibodies reversing CAR T-cell exhaustion (102). Feng Ji et al. also confirmed that PARPi can activate the cGAS-STING pathway to enhance the efficacy of CD70 CAR-T cells on renal cancer (103).
5.3.2 STING agonists in combination with immune checkpoint inhibitors
“Hot tumors” already contain large numbers of infiltrating T cells that were once activated but are depleted or malfunctioning due to the expression of a range of immunosuppressive receptors, including CTLA4 and PD-1 (69). As mentioned previously, activation of the cGAS-STING pathway promotes the infiltration of CTLs into tumor tissue and upregulates the expression of PD-L1 on the surface of cancer cells. While the therapeutic efficacy of immune checkpoint inhibitors (ICIs) correlates with the baseline infiltration level of CTLs in tumor tissue. Therefore, the combination of STING agonists and ICIs for the treatment of immune hot tumors may synergize.
The combination of STING agonists and ICIs is currently achieving some efficacy in clinical trials. A multicenter Phase 2 clinical trial demonstrated a complete response of 16.7% and a partial response of 83.3% (NCT03937141) when ADU-S100 (a STING agonist) and pembrolizumab were used together in the treatment of recurrent or metastatic head and neck cancer. An open-label phase 1 clinical trial for patients with advanced metastatic solid tumors showed that Mn2+, which can activate cGAS in combination with anti-PD-1 antibodies, has promising efficacy, with an objective response rate of 45.5% and a disease control rate of 90.9% (NCT03991559) (40). In preclinical model of HPV + oral cancer, intratumoral injection of STING agonist combined with systemic treatment with anti-PD-1 antibodies and anti-CTLA-4 antibodies resulted in sustained tumor regression in 71% of mice, significantly higher than the efficacy of PD-1blocker alone (104). In mouse melanoma models with B16F10 and BRAF mutations, the combination use of LP-cGAMP and anti-PD-L1 antibody achieved stronger and more durable efficacy than LP-cGAMP or anti-PD-L1 alone (105).
5.3.3 STING agonists in combination with IDO inhibitors
In immunosuppressed tumors, immune infiltration is present in the tumor lesion, but the degree of infiltration is not high (69). As previously mentioned, while activation of the cGAS-STING pathway promotes immune infiltration, it also upregulates the expression of the immunosuppressive factor IDO. Therefore, combining STING agonists with IDO inhibitors may be a promising option to reverse immunosuppression and promote immunosuppressed tumors to become hot tumors, thereby improving the efficacy of ICIs.
The combination of STING agonist and IDO inhibitor is currently in preclinical. In a mouse colorectal cancer model, the STING agonist diABZI in combination with the IDO inhibitor 1-MT significantly inhibited tumor growth, promoting the recruitment of CTLs and inhibiting the infiltration of MDSCs (75).
6 Conclusion and perspectives
The cGAS-STING pathway mediates various aspects of the cancer immune cycle (CIC) to enhance or attenuate anti-tumor immune responses. Combination therapy of STING agonists can target different steps of the cancer-immunity cycle and contribute to solving immunotherapy challenges in the corresponding tumor immune-phenotype. In addition, the activity of the cGAS-STING pathway is inhibited in several tumors due to negative regulation by multiple mechanisms such as TIM-3, ENPP1.
However, the following challenges need to be solved for STING agonists to be clinically applied on a large scale. First, for specific patients, whether STING agonists are immunopromoting or immunosuppressive is unclear and may be related to their tumor type and immune microenvironmental characteristics, which need to be further explored. Second, STING agonists with higher affinity for humans need to be rationally designed to enhance antitumor efficacy. Third, more potential STING agonist combination therapy strategies need to be explored, such as STING agonist in combination with TIM-3 inhibitor, ENPP1 inhibitors.
In summary, we believe that the cGAS-STING pathway manipulation will have a promising future in tumor immunotherapy.
Author contributions
ZT wrote the manuscript. ZT, YZ, YP, JL and FW revised the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by: 1) Hunan Provincial Science Fund for Excellent Young Scholars (Grant No. 2021JJ20088); 2) Changsha Municipal Science and Technology Bureau (Grant No. kq1907077).
Acknowledgments
The authors sincerely thank Dr. Feng Liu providing language help and valuable discussion points. The authors sincerely thank the multidisciplinary team (MDT) of thoracic oncology, the Second Xiangya Hospital, Central South University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bai J, Liu F. Nuclear cGAS: sequestration and beyond. Protein Cell (2021) 13(2):90–101. doi: 10.1007/s13238-021-00869-0
2. Bai J, Liu F. cGAS−STING signaling and function in metabolism and kidney diseases. J Mol Cell Biol (2021) 13:728–38. doi: 10.1093/jmcb/mjab066
3. Bai J, Liu F. The cGAS-cGAMP-STING pathway: A molecular link between immunity and metabolism. Diabetes (2019) 68:1099–108. doi: 10.2337/dbi18-0052
4. Bai J, Cervantes C, He S, He J, Plasko GR, Wen J, et al. Mitochondrial stress-activated cGAS-STING pathway inhibits thermogenic program and contributes to overnutrition-induced obesity in mice. Commun Biol (2020) 3:257. doi: 10.1038/s42003-020-0986-1
5. Bai J, Cervantes C, Liu J, He S, Zhou H, Zhang B, et al. DsbA-l prevents obesity-induced inflammation and insulin resistance by suppressing the mtDNA release-activated cGAS-cGAMP-STING pathway. Proc Natl Acad Sci U.S.A. (2017) 114(46):12196–201. doi: 10.1073/pnas.1708744114
6. Xu G, Liu C, Zhou S, Li Q, Feng Y, Sun P, et al. Viral tegument proteins restrict cGAS-DNA phase separation to mediate immune evasion. Mol Cell (2021) 81:2823–2837.e2829. doi: 10.1016/j.molcel.2021.05.002
7. Jin M, Shiwaku H, Tanaka H, Obita T, Ohuchi S, Yoshioka Y, et al. Tau activates microglia via the PQBP1-cGAS-STING pathway to promote brain inflammation. Nat Commun (2021) 12:6565. doi: 10.1038/s41467-021-26851-2
8. Chen R, Du J, Zhu H, Ling Q. The role of cGAS-STING signalling in liver diseases. JHEP Rep Innovation Hepatol (2021) 3:100324. doi: 10.1016/j.jhepr.2021.100324
9. Motwani M, McGowan J, Antonovitch J, Gao KM, Jiang Z, Sharma S, et al. cGAS-STING pathway does not promote autoimmunity in murine models of SLE. Front Immunol (2021) 12:605930. doi: 10.3389/fimmu.2021.605930
10. Chen K, Lai C, Su Y, Bao WD, Yang LN, Xu PP, et al. cGAS-STING-mediated IFN-I response in host defense and neuroinflammatory diseases. Curr Neuropharmacol (2022) 20:362–71. doi: 10.2174/1570159X19666210924110144
11. Jiang GL, Yang XL, Zhou HJ, Long J, Liu B, Zhang LM, et al. cGAS knockdown promotes microglial M2 polarization to alleviate neuroinflammation by inhibiting cGAS-STING signaling pathway in cerebral ischemic stroke. Brain Res Bull (2021) 171:183–95. doi: 10.1016/j.brainresbull.2021.03.010
12. Zierhut C, Yamaguchi N, Paredes M, Luo JD, Carroll T, Funabiki H. The cytoplasmic DNA sensor cGAS promotes mitotic cell death. Cell (2019) 178:302–315.e323. doi: 10.1016/j.cell.2019.05.035
13. Wang Y, Luo J, Alu A, Han X, Wei Y, Wei X. cGAS-STING pathway in cancer biotherapy. Mol Cancer (2020) 19(1):136. doi: 10.1186/s12943-020-01247-w
14. Sivick KE, Desbien AL, Glickman LH, Reiner GL, Corrales L, Surh NH, et al. Magnitude of therapeutic STING activation determines CD8(+) T cell-mediated anti-tumor immunity. Cell Rep (2018) 25:3074–3085.e3075. doi: 10.1016/j.celrep.2018.11.047
15. Campisi M, Sundararaman SK, Shelton SE, Knelson EH, Mahadevan NR, Yoshida R, et al. Tumor-derived cGAMP regulates activation of the vasculature, frontiers in immunology. Font Immunol (2020) 11. doi: 10.3389/fimmu.2020.02090
16. Chabanon RM, Muirhead G, Krastev DB, Adam J, Morel D, Garrido M, et al. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J Clin Invest (2019) 129:1211–28. doi: 10.1172/JCI123319
17. Hopfner KP, Hornung V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nature reviews Molecular cell biology (2020) 21(9):501–21. doi: 10.1038/s41580-020-0244-x
18. Sceneay J, Goreczny GJ, Wilson K, Morrow S, DeCristo MJ, Ubellacker JM, et al. Interferon signaling is diminished with age and is associated with immune checkpoint blockade efficacy in triple-negative breast cancer. Cancer Discovery (2019) 9:1208–27. doi: 10.1158/2159-8290.CD-18-1454
19. Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol (2021) 21:548–69. doi: 10.1038/s41577-021-00524-z
20. Shang G, Zhang C, Chen ZJ, Bai X-c, Zhang X. Cryo-EM structures of STING reveal its mechanism of activation by cyclic GMP-AMP. Nature (2019) 567:389. doi: 10.1038/s41586-019-0998-5
21. Zhao B, Du F, Xu P, Shu C, Sankaran B, Bell SL, et al. A conserved PLPLRT/SD motif of STING mediates the recruitment and activation of TBK1. Nature (2019) 569:718. doi: 10.1038/s41586-019-1228-x
22. Kwon J, Bakhoum SF. The cytosolic DNA-sensing cGAS-STING pathway in cancer. Cancer Discovery (2020) 10:26–39. doi: 10.1158/2159-8290.CD-19-0761
23. Murthy AMV, Robinson N, Kumar S. Crosstalk between cGAS-STING signaling and cell death. Cell Death Differentiation (2020) 27:2989–3003. doi: 10.1038/s41418-020-00624-8
24. Bai J, Liu F. Nuclear cGAS: sequestration and beyond. Protein Cell (2022) 13:90–101. doi: 10.1007/s13238-021-00869-0
25. Zheng C, Song Q, Zhao H, Kong Y, Sun L, Liu X, et al. A nanoplatform to boost multi-phases of cancer-immunity-cycle for enhancing immunotherapy. Journal of controlled release : official journal of the Controlled Release Society (2021) 339:403–15.doi: 10.1016/j.jconrel.2021.10.011
26. Wu J, Dobbs N, Yang K, Yan N. Interferon-independent activities of mammalian STING mediate antiviral response and tumor immune evasion. Immunity (2020) 53:115–126.e115. doi: 10.1016/j.immuni.2020.06.009
27. Wu J, Chen YJ, Dobbs N, Sakai T, Liou J, Miner JJ, et al. STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J Exp Med (2019) 216:867–83. doi: 10.1084/jem.20182192
28. Lohard S, Bourgeois N, Maillet L, Gautier F, Fétiveau A, Lasla H, et al. STING-dependent paracriny shapes apoptotic priming of breast tumors in response to anti-mitotic treatment. Nat Commun (2020) 11:259. doi: 10.1038/s41467-019-13689-y
29. Lohard S, Juin PP, Barillé-Nion S. Mitotic stress-induced secretome primes cancer cells to apoptosis and maximizes paclitaxel response in breast tumors when combined with BCL-xL-targeting BH3 mimetics. Mol Cell Oncol (2020) 7:1735912. doi: 10.1080/23723556.2020.1735912
30. Vasiyani H, Shinde A, Roy M, Mane M, Singh K, Singh J, et al. The analog of cGAMP, c-di-AMP, activates STING mediated cell death pathway in estrogen-receptor negative breast cancer cells. Apoptosis Int J Programmed Cell Death (2021) 26:293–306. doi: 10.1007/s10495-021-01669-x
31. Storozynsky Q, Hitt MM. The impact of radiation-induced DNA damage on cGAS-STING-Mediated immune responses to cancer. Int J Mol Sci (2020) 21(22). doi: 10.3390/ijms21228877
32. Nagata M, Kosaka A, Yajima Y, Yasuda S, Ohara M, Ohara K, et al. A critical role of STING-triggered tumor-migrating neutrophils for anti-tumor effect of intratumoral cGAMP treatment. Cancer Immunol Immunother CII (2021) 70:2301–12. doi: 10.1007/s00262-021-02864-0
33. Chelvanambi M, Fecek RJ, Taylor JL, Storkus WJ. STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment. J Immunother Cancer (2021) 9(2). doi: 10.1136/jitc-2020-001906
34. Blobner J, Kilian M, Tan CL, Aslan K, Sanghvi K, Meyer J, et al. Comparative evaluation of T-cell receptors in experimental glioma-draining lymph nodes. Neuro-Oncol Adv (2021) 3:vdab147. doi: 10.1093/noajnl/vdab147
35. Schadt L, Sparano C, Schweiger NA, Silina K, Cecconi V, Lucchiari G, et al. Cancer-Cell-Intrinsic cGAS expression mediates tumor immunogenicity. Cell Rep (2019) 29:1236. doi: 10.1016/j.celrep.2019.09.065
36. Li W, Lu L, Lu J, Wang X, Yang C, Jin J, et al. cGAS-STING-mediated DNA sensing maintains CD8(+) T cell stemness and promotes antitumor T cell therapy. Sci Transl Med (2020) 12(549). doi: 10.1126/scitranslmed.aay9013
37. B.C. M, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol (2019) 20:326–36. doi: 10.1038/s41590-019-0312-6
38. Gautam S, Fioravanti J, Zhu W, Le Gall JB, Brohawn P, Lacey NE, et al. The transcription factor c-myb regulates CD8(+) T cell stemness and antitumor immunity. Nat Immunol (2019) 20:337–49. doi: 10.1038/s41590-018-0311-z
39. Jansen CS, Prokhnevska N, Master VA, Sanda MG, Carlisle JW, Bilen MA, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature (2019) 576:465–70. doi: 10.1038/s41586-019-1836-5
40. Lv M, Chen M, Zhang R, Zhang W, Wang C, Zhang Y, et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res (2020) 30:966–79. doi: 10.1038/s41422-020-00395-4
41. Li A, Yi M, Qin S, Song Y, Chu Q, Wu K. Activating cGAS-STING pathway for the optimal effect of cancer immunotherapy. J Hematol Oncol (2019) 12:35. doi: 10.1186/s13045-019-0721-x
42. Tse SW, McKinney K, Walker W, Nguyen M, Iacovelli J, Small C, et al. mRNA-encoded, constitutively active STING(V155M) is a potent genetic adjuvant of antigen-specific CD8(+) T cell response, molecular therapy. J Am Soc Gene Ther (2021) 29:2227–38. doi: 10.1016/j.ymthe.2021.03.002
43. Vieira RS, Nascimento MS, Noronha IH, Vasconcelos JRC, Benvenuti LA, Barber GN, et al. STING signaling drives production of innate cytokines, generation of CD8(+) T cells and enhanced protection against trypanosoma cruzi infection. Front Immunol (2021) 12:775346. doi: 10.3389/fimmu.2021.775346
44. Han BJ, Murphy JD, Qin S, Ye J, Uccello TP, Garrett-Larsen J, et al. Microspheres encapsulating immunotherapy agents target the tumor-draining lymph node in pancreatic ductal adenocarcinoma. Immunol Investigations (2020) 49:808–23. doi: 10.1080/08820139.2020.1765795
45. Gammelgaard KR, Sandfeld-Paulsen B, Godsk SH, Demuth C, Meldgaard P, Sorensen BS, et al. cGAS-STING pathway expression as a prognostic tool in NSCLC. Trans Lung Cancer Res (2021) 10:340–54. doi: 10.21037/tlcr-20-524
46. Sen T, Rodriguez BL, Chen L, Della Corte CM, Morikawa N, Fujimoto J, et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discovery (2019) 9:646–61. doi: 10.1158/2159-8290.CD-18-1020
47. Zheng J, Mo J, Zhu T, Zhuo W, Yi Y, Hu S, et al. Comprehensive elaboration of the cGAS-STING signaling axis in cancer development and immunotherapy. Mol Cancer (2020) 19:133. doi: 10.1186/s12943-020-01250-1
48. Yang T, Xiao H, Liu X, Wang Z, Zhang Q, Wei N, et al. Vascular normalization: A new window opened for cancer therapies. Front Oncol (2021) 11:719836. doi: 10.3389/fonc.2021.719836
49. Ayuso-Íñigo B, Méndez-García L, Pericacho M, Muñoz-Félix JM. The dual effect of the BMP9-ALK1 pathway in blood vessels: An opportunity for cancer therapy improvement? Cancers (2021) 13(21). doi: 10.3390/cancers13215412
50. Wang K, Chen Q, Liu N, Zhang J, Pan X. Recent advances in, and challenges of, anti-angiogenesis agents for tumor chemotherapy based on vascular normalization. Drug Discovery Today (2021) 26:2743–53. doi: 10.1016/j.drudis.2021.07.024
51. Baris AM, Fraile-Bethencourt E, Anand S. Nucleic acid sensing in the tumor vasculature. Cancers (2021) 13(17). doi: 10.3390/cancers13174452
52. Lee SJ, Yang H, Kim WR, Lee YS, Lee WS, Kong SJ, et al. STING activation normalizes the intraperitoneal vascular-immune microenvironment and suppresses peritoneal carcinomatosis of colon cancer. J Immunother Cancer (2021) 9(6). doi: 10.1136/jitc-2020-002195
53. Yang H, Lee WS, Kong SJ, Kim CG, Kim JH, Chang SK, et al. STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J Clin Invest (2019) 129:4350–64. doi: 10.1172/JCI125413
54. Grabosch S, Bulatovic M, Zeng F, Ma T, Zhang L, Ross M, et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene (2019) 38:2380–93. doi: 10.1038/s41388-018-0581-9
55. Nicolai CJ, Wolf N, Chang IC, Kirn G, Marcus A, Ndubaku CO, et al. NK cells mediate clearance of CD8(+) T cell-resistant tumors in response to STING agonists. Sci Immunol (2020) 5(45). doi: 10.1126/sciimmunol.aaz2738
56. Benci JL, Johnson LR, Choa R, Xu Y, Qiu J, Zhou Z, et al. Opposing functions of interferon coordinate adaptive and innate immune responses to cancer immune checkpoint blockade. Cell (2019) 178:933–948.e914. doi: 10.1016/j.cell.2019.07.019
57. Da Y, Liu Y, Hu Y, Liu W, Ma J, Lu N, et al. STING agonist cGAMP enhances anti-tumor activity of CAR-NK cells against pancreatic cancer. Oncoimmunology (2022) 11:2054105. doi: 10.1080/2162402X.2022.2054105
58. Yan X, Yao C, Fang C, Han M, Gong C, Hu D, et al. Rocaglamide promotes the infiltration and antitumor immunity of NK cells by activating cGAS-STING signaling in non-small cell lung cancer. Int J Biol Sci (2022) 18:585–98. doi: 10.7150/ijbs.65019
59. Marcus A, Mao AJ, Lensink-Vasan M, Wang L, Vance RE, Raulet DH. Tumor-derived cGAMP triggers a STING-mediated interferon response in non-tumor cells to activate the NK cell response. Immunity (2018) 49:754. doi: 10.1016/j.immuni.2018.09.016
60. Ghosh C, Luong G, Sun Y. A snapshot of the PD-1/PD-L1 pathway. J Cancer (2021) 12:2735–46. doi: 10.7150/jca.57334
61. Eckstein M, Cimadamore A, Hartmann A, Lopez-Beltran A, Cheng L, Scarpelli M, et al. PD-L1 assessment in urothelial carcinoma: a practical approach. Ann Trans Med (2019) 7:690. doi: 10.21037/atm.2019.10.24
62. Wu Y, Chen W, Xu ZP, Gu W. PD-L1 distribution and perspective for cancer immunotherapy-blockade. Knockdown Inhibition Front Immunol (2019) 10:2022. doi: 10.3389/fimmu.2019.02022
63. Rotman J, den Otter LAS, Bleeker MCG, Samuels SS, Heeren AM, Roemer MGM, et al. PD-L1 and PD-L2 expression in cervical cancer: Regulation and biomarker potential. Front Immunol (2020) 11:596825. doi: 10.3389/fimmu.2020.596825
64. Du SS, Chen GW, Yang P, Chen YX, Hu Y, Zhao QQ, et al. Radiation therapy promotes hepatocellular carcinoma immune cloaking via PD-L1 upregulation induced by cGAS-STING activation. Int J Radiat Oncol Biol Phys (2022) 112:1243–55. doi: 10.1016/j.ijrobp.2021.12.162
65. Jacquelot N, Yamazaki T, Roberti MP, Duong CPM, Andrews MC, Verlingue L, et al. Sustained type I interferon signaling as a mechanism of resistance to PD-1 blockade. Cell Res (2019) 29:846–61. doi: 10.1038/s41422-019-0224-x
66. Sen T. Identifying and targeting the Achilles heel of a recalcitrant cancer. Sci Trans Med (2021) 13(605). doi: 10.1126/scitranslmed.abj6946
67. Morel KL, Sheahan AV, Burkhart DL, Baca SC, Boufaied N, Liu Y, et al. EZH2 inhibition activates a dsRNA-STING-interferon stress axis that potentiates response to PD-1 checkpoint blockade in prostate cancer. Nat Cancer (2021) 2:444–56. doi: 10.1038/s43018-021-00185-w
68. Zhou L, Xu Q, Huang L, Jin J, Zuo X, Zhang Q, et al. Low-dose carboplatin reprograms tumor immune microenvironment through STING signaling pathway and synergizes with PD-1 inhibitors in lung cancer. Cancer Lett (2021) 500:163–71. doi: 10.1016/j.canlet.2020.11.049
69. Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discovery (2019) 18:197–218. doi: 10.1038/s41573-018-0007-y
70. Guo Y, Liu Y, Wu W, Ling D, Zhang Q, Zhao P, et al. Indoleamine 2,3-dioxygenase (Ido) inhibitors and their nanomedicines for cancer immunotherapy. Biomaterials (2021) 276:121018. doi: 10.1016/j.biomaterials.2021.121018
71. Schumacher NSG, Fernandes LGR, de Lima Zollner R. Aqueous extract of passiflora alata leaves modulates in vitro the indoleamine 2,3-dioxygenase (IDO) and CD86 expression in bone marrow-derived professional antigen-presenting cells polarizing NOD mice T cells to a treg profile. Cytokine (2022) 152:155832. doi: 10.1016/j.cyto.2022.155832
72. Lynch KT, Gradecki SE, Kwak M, Meneveau MO, Wages NA, Gru AA, et al. IDO1 expression in melanoma metastases is low and associated with improved overall survival. Am J Surg Pathol (2021) 45:787–95. doi: 10.1097/PAS.0000000000001622
73. Cheng AN, Cheng LC, Kuo CL, Lo YK, Chou HY, Chen CH, et al. Mitochondrial lon-induced mtDNA leakage contributes to PD-L1-mediated immunoescape via STING-IFN signaling and extracellular vesicles. J Immunother Cancer (2020) 8(2). doi: 10.1136/jitc-2020-001372
74. Chen B, Alvarado DM, Iticovici M, Kau NS, Park H, Parikh PJ, et al. Interferon-induced IDO1 mediates radiation resistance and is a therapeutic target in colorectal cancer. Cancer Immunol Res (2020) 8:451–64. doi: 10.1158/2326-6066.CIR-19-0282
75. Shi J, Liu C, Luo S, Cao T, Lin B, Zhou M, et al. STING agonist and IDO inhibitor combination therapy inhibits tumor progression in murine models of colorectal cancer. Cell Immunol (2021) 366:104384. doi: 10.1016/j.cellimm.2021.104384
76. An X, Zhu Y, Zheng T, Wang G, Li J, et al. An Analysis of the Expression and Association with Immune Cell Infiltration of the cGAS/STING Pathway in Pan-Cancer. Molecular therapy Nucleic acids (2019) 14:80–9. doi: 10.1016/j.omtn.2018.11.003
77. Mohamed E, Sierra RA, Trillo-Tinoco J, Cao Y, Innamarato P, Payne KK, et al. The unfolded protein response mediator PERK governs myeloid cell-driven immunosuppression in tumors through inhibition of STING signaling. Immun (2020) 52:668–682.e667. doi: 10.1016/j.immuni.2020.03.004
78. Dunphy G, Flannery SM, Almine JF, Connolly DJ, Paulus C, Jønsson KL, et al. Non-canonical activation of the DNA sensing adaptor STING by ATM and IFI16 mediates NF-κB signaling after nuclear DNA damage. Mol Cell (2018) 71:745–760.e745. doi: 10.1016/j.molcel.2018.07.034
79. Al-Asmari SS, Rajapakse A, Ullah TR, Pépin G, Croft LV, Gantier MP. Pharmacological targeting of STING-dependent IL-6 production in cancer cells. Front Cell Dev Biol (2021) 9:709618. doi: 10.3389/fcell.2021.709618
80. Bakhoum SF, Ngo B, Laughney AM, Cavallo J-A, Murphy CJ, Ly P, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature (2018) 553:467. doi: 10.1038/nature25432
81. Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer (vol 533. Nat (2017) 544:124–4. doi: 10.1038/nature21730
82. Ghosh M, Saha S, Bettke J, Nagar R, Parrales A, Iwakuma T, et al. Mutant p53 suppresses innate immune signaling to promote tumorigenesis. Cancer Cell (2021) 39:494–508.e495. doi: 10.1016/j.ccell.2021.01.003
83. Meng F, Yu Z, Zhang D, Chen S, Guan H, Zhou R, et al. Induced phase separation of mutant NF2 imprisons the cGAS-STING machinery to abrogate antitumor immunity. Mol Cell (2021) 81:4147–4164.e4147. doi: 10.1016/j.molcel.2021.07.040
84. Carozza JA, Brown JA, Böhnert V, Fernandez D, AlSaif Y, Mardjuki RE, et al. Structure-Aided Development of Small-Molecule Inhibitors of ENPP1, the Extracellular Phosphodiesterase of the Immunotransmitter cGAMP. Cell chemical biology (2020) 27:1347–58.e1345. doi: 10.1016/j.chembiol.2020.07.007
85. Zhao F, Liu A, Gong X, Chen H, Wei J, Chen B, et al. Hypoxia-induced RNASEH2A limits activation of cGAS-STING signaling in HCC and predicts poor prognosis. Tumori (2022) 108:63–76. doi: 10.1177/03008916211026019
86. Lin J, Guo D, Liu H, Zhou W, Wang C, Müller I, et al. The SETDB1-TRIM28 complex suppresses antitumor immunity. Cancer Immunol Res (2021) 9:1413–24. doi: 10.1158/2326-6066.CIR-21-0754
87. de Mingo Pulido Á, Hänggi K, Celias DP, Gardner A, Li J, Batista-Bittencourt B, et al. The inhibitory receptor TIM-3 limits activation of the cGAS-STING pathway in intra-tumoral dendritic cells by suppressing extracellular DNA uptake. Immunity (2021) 54:1154–1167.e1157. doi: 10.1016/j.immuni.2021.04.019
88. Gianni T, Leoni V, Sanapo M, Parenti F, Bressanin D, Barboni C, et al. Genotype of immunologically hot or cold tumors determines the antitumor immune response and efficacy by fully virulent retargeted oHSV. Viruses (2021) 13(9). doi: 10.3390/v13091747
89. Huang X, Tang T, Zhang G, Liang T. Identification of tumor antigens and immune subtypes of cholangiocarcinoma for mRNA vaccine development. Mol Cancer (2021) 20:50. doi: 10.1186/s12943-021-01342-6
90. McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nature reviews Cancer (2020) 20(4):203–17. doi: 10.1038/s41568-020-0246-1
91. Harabuchi S, Kosaka A, Yajima Y, Nagata M, Hayashi R, Kumai T, et al. Intratumoral STING activations overcome negative impact of cisplatin on antitumor immunity by inflaming tumor microenvironment in squamous cell carcinoma. Biochem Biophys Res Commun (2020) 522:408–14. doi: 10.1016/j.bbrc.2019.11.107
92. Marzio A, Kurz E, Sahni JM, Di Feo G, Puccini J, Jiang S, et al. EMSY inhibits homologous recombination repair and the interferon response, promoting lung cancer immune evasion. Cell (2022) 185:169–183.e119. doi: 10.1016/j.cell.2021.12.005
93. Ma H, Kang Z, Foo TK, Shen Z, Xia B. Disrupted BRCA1-PALB2 interaction induces tumor immunosuppression and T-lymphocyte infiltration in HCC through cGAS-STING pathway. Hepatol (Baltimore Md) (2022). doi: 10.1002/hep.32335
94. Groelly FJ, Porru M, Zimmer J, Benainous H, De Visser Y, Kosova AA, et al. Anti-tumoural activity of the G-quadruplex ligand pyridostatin against BRCA1/2-deficient tumours. EMBO Mol Med (2022) 14:e14501. doi: 10.15252/emmm.202114501
95. Kaneta A, Nakajima S, Okayama H, Matsumoto T, Saito K, Kikuchi T, et al. Role of the cGAS-STING pathway in regulating the tumor-immune microenvironment in dMMR/MSI colorectal cancer, cancer immunology, immunotherapy: CII. (2022) 71(11):2765–76. doi: 10.1007/s00262-022-03200-w
96. Rossi M, Carboni S, Di Berardino-Besson W, Riva E, Santiago-Raber ML, Belnoue E, et al. STING agonist combined to a protein-based cancer vaccine potentiates peripheral and intra-tumoral T cell immunity. Front Immunol (2021) 12:695056. doi: 10.3389/fimmu.2021.695056
97. Wu JJ, Zhao L, Han BB, Hu HG, Zhang BD, Li WH, et al. A novel STING agonist for cancer immunotherapy and a SARS-CoV-2 vaccine adjuvant. Chem Commun (Cambridge England) (2021) 57:504–7. doi: 10.1039/D0CC06959K
98. Yuan M, Guo XL, Chen JH, He Y, Liu ZQ, Zhang HP, et al. Anlotinib suppresses proliferation, migration, and immune escape of gastric cancer cells by activating the cGAS-STING/IFN-β pathway. Neoplasma (2022) 69(4):807–19. doi: 10.4149/neo_2022_211012N1441
99. Sauter CS, Senechal B, Rivière I, Ni A, Bernal Y, Wang X, et al. CD19 CAR T cells following autologous transplantation in poor-risk relapsed and refractory b-cell non-Hodgkin lymphoma. Blood (2019) 134:626–35. doi: 10.1182/blood.2018883421
100. Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. 'Off-the-shelf' allogeneic CAR T cells: development and challenges. Nat Rev Drug Discovery (2020) 19:185–99. doi: 10.1038/s41573-019-0051-2
101. Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J (2021) 11:69. doi: 10.1038/s41408-021-00459-7
102. Xu N, Palmer DC, Robeson AC, Shou P, Bommiasamy H, Laurie SJ, et al. STING agonist promotes CAR T cell trafficking and persistence in breast. The Journal of experimental medicine. (2021) 218(2):e20200844. doi: 10.1084/jem.20200844
103. Ji F, Zhang F, Zhang M, Long K, Xia M, Lu F, et al. Targeting the DNA damage response enhances CD70 CAR-T cell therapy for renal carcinoma by activating the cGAS-STING pathway. J Hematol Oncol (2021) 14:152. doi: 10.1186/s13045-021-01168-1
104. Dorta-Estremera S, Hegde VL, Slay RB, Sun R, Yanamandra AV, Nicholas C, et al. Targeting interferon signaling and CTLA-4 enhance the therapeutic efficacy of anti-PD-1 immunotherapy in preclinical model of HPV(+) oral cancer. J Immunother Cancer (2019) 7:252. doi: 10.1186/s40425-019-0728-4
Keywords: cGAS, STING, cancer-immunity cycle, immunotherapy, tumor
Citation: Tian Z, Zeng Y, Peng Y, Liu J and Wu F (2022) Cancer immunotherapy strategies that target the cGAS-STING pathway. Front. Immunol. 13:996663. doi: 10.3389/fimmu.2022.996663
Received: 19 July 2022; Accepted: 03 October 2022;
Published: 24 October 2022.
Edited by:
Samanthi Perera, Takeda Oncology, United StatesReviewed by:
Michael Paul Gantier, Hudson Institute of Medical Research, AustraliaJuli Bai, The University of Texas Health Science Center at San Antonio, United States
John Tanner Wilson, Vanderbilt University, United States
Copyright © 2022 Tian, Zeng, Peng, Liu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Wu, d3VmYW5nNDQ2MUBjc3UuZWR1LmNu
 Zhuoying Tian1,2
Zhuoying Tian1,2 Fang Wu
Fang Wu