- Department of Medicine, Section of Immunology, University of Verona, Verona, Italy
Pattern recognition receptors are primitive sensors that arouse a preconfigured immune response to broad stimuli, including nonself pathogen-associated and autologous damage-associated molecular pattern molecules. These receptors are mainly expressed by innate myeloid cells, including granulocytes, monocytes, macrophages, and dendritic cells. Recent investigations have revealed new insights into these receptors as key players not only in triggering inflammation processes against pathogen invasion but also in mediating immune suppression in specific pathological states, including cancer. Myeloid-derived suppressor cells are preferentially expanded in many pathological conditions. This heterogeneous cell population includes immunosuppressive myeloid cells that are thought to be associated with poor prognosis and impaired response to immune therapies in various cancers. Identification of pattern recognition receptors and their ligands increases the understanding of immune-activating and immune-suppressive myeloid cell functions and sheds light on myeloid-derived suppressor cell differences from cognate granulocytes and monocytes in healthy conditions. This review summarizes the different expression, ligand recognition, signaling pathways, and cancer relations and identifies Toll-like receptors as potential new targets on myeloid-derived suppressor cells in cancer, which might help us to decipher the instruction codes for reverting suppressive myeloid cells toward an antitumor phenotype.
Introduction
Myeloid-derived suppressor cells (MDSCs) have recently emerged as key players in regulating host immune responses in many pathologies. Although this heterogeneous population of myeloid cells was identified in many tumor histotypes, its relevance in orchestrating innate and adaptive immune responses has also been ascribed in infectious diseases, such as sepsis (1) and COVID-19 (2, 3), as well as in pregnancy (4, 5). MDSCs include two main subsets of myeloid cells—monocytic (M-MDSC) and polymorphonuclear (PMN-MDSC). Although phenotypically these immune regulators resemble normal monocyte and polymorphonuclear counterparts, MDSCs are endowed with peculiar molecular, metabolic, and immune suppressive features toward natural killer (NK), T and B lymphocyte activation and proliferation (6). In mice, M-MDSCs and PMN-MDSCs cannot be distinguished from inflammatory monocytes and granulocytes/polymorphonuclear leukocytes (PMNs) according to surface markers (M-MDSC: CD11b+ Ly6Chi cells; PMN-MDSC: CD11b+ Ly6Cint Ly6G+cells) (7). In humans, three main MDSC subsets have been identified: M-MDSCs and PMN-MDSCs are defined as CD11b+ CD14+ CD15- CD33+ HLA-DR- and CD11b+ CD14- CD15+ (or CD66b+) CD33+-expressing cells, respectively. Recently a third MDSC population, named early-stage MDSCs (eMDSCs), which is negative for CD14, CD15, and HLA-DR markers and expresses CD33, was identified and represented a small cell subset of myeloid precursors (7). These days, PMN-MDSCs can be separated from neutrophils in human peripheral blood samples by employing gradient separation or novel disclosed markers such as lectin-type oxidized LDL receptor 1 (LOX-1), whereas M-MDSCs are characterized by the low expression of HLA-DR, which is instead highly present in monocytes (7).
Despite these common features, several studies have shown that specific immunological contexts define the direction of myelopoietic routes toward the generation of PMNs, monocytes, or their immunosuppressive counterparts. Indeed myeloid cells patrol immune cells, promptly reaching the infection site to fight pathogen spreading by many mechanisms, including phagocytosis, killing, neutralization, inflammation, priming, and support of adaptive immune responses. A pathogen infection results in the fast mobilization of both PMNs and monocytes toward the inflammation site in an attempt to defeat pathogen dissemination. Classical myeloid cell activation occurs through sensing a broad spectrum of danger-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) by their pattern recognition receptors (PRRs). PRRs are composed of several parts and include ligand recognition domains, intermediate domains, and effector domains. After recognizing and binding to their respective ligands, they recruit adaptor molecules and initiate downstream signaling pathways such as cell activation and transcription of inflammatory genes (8). PAMPs can be expressed by pathogens or invasive microbes, whereas DAMPs are stress signals released or exposed on the membrane of damaged cells and include components that are normally found intracellularly, such as adenosine triphosphate, calreticulin, annexin A1, high mobility group box 1 (HMGB1), heat shock (HSP), and S100 proteins (9). Dr. William Coley, acknowledged as the forefather of cancer immune therapy, provided in 1891 the first evidence that bacterial immune toxins could reactivate the host immune system to fight tumor progression in inoperable cancer patients, without knowing either the mechanism of action of those PAMPs or the target receptors and immune cells (10). Recently, we demonstrated that hyperthermic-mediated secondary apoptosis of tumor cells induces the release of HMGB1, which, in turn, supports the activation of dendritic cells and sustains the activation of a tumor-specific adaptive immune response that restricts prostate cancer outgrowth (11). Thus, DAMPs can protect the host by supporting immunogenic cell death, thus alerting the immune system to recognize tumor cell components. However, chronic DAMP stimulation in sterile inflammatory conditions, sustained directly (actively secreted molecules) or indirectly (because of cell death) by tumors, shows opposite effects by supporting the recruitment and immunosuppressive functions of MDSCs and TAMs, contributing to cancer cell survival, local invasion, and metastatic dissemination in many neoplasias (12). Injured or stressed cancer cells release redundant DAMPs that potently work on specific PRRs on MDSCs (13). Moreover, the linkage between specific PRRs and cancer-associated microorganisms is well documented, including Helicobacter pylori (14) and Epstein–Barr virus (15) in gastric cancer, hepatitis B virus and hepatitis C virus in hepatocellular carcinoma, human papillomavirus (16) in cervical cancer, and dysbiotic gut microorganisms (for example, Bacteroides fragilis) (17) in pancreatic cancer and colorectal carcinoma (CRC) (18). The immune contexture, timing, and concentration of DAMPs and PAMPs can altogether orchestrate different effects on the host immune system, participating in defining the fate of cancer therapy. Chronic exposure to inflammatory stimuli usually associated with unresolved infections and chronic inflammation, cancer included, sustains a pathological deviation of host myelopoiesis with increased dysfunctional monocytes and PMNs. MDSC expansion and acquisition of immune suppressive abilities take place through sequential events that occur in different microenvironments. In the first phase, chronic stimulation by granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), and granulocyte macrophage colony-stimulating factor (GM-CSF) sustains MDSC generation and expansion in the bone marrow. Those cells are then mobilized in the periphery and can acquire more pronounced immune suppressive features if exposed to a specific microenvironment, such as in the tumor core, by vascular endothelial growth factor, interleukins, or a hypoxic state (5). Activation of signal transducer and activator of transcription 3 (STAT3), CCAAT/enhancer binding protein β (C/EBPβ) (19), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (20) signaling is crucially involved in both stages. Although many cytokines can promote pathological hematopoiesis deviation, recent studies underlined that MDSC generation and suppression can be recapitulated in vitro by the combination of GM-CSF + IL6 and GM-CSF + G-CSF in mouse and human bone marrow progenitors, respectively (19). Cancer sustains this chronic inflammation to corrupt host hematopoiesis toward the generation and recruitment of immune regulatory cells, including MDSCs. These cells can support tumor progression and metastatic spreading by hijacking cancer immune surveillance by restricting T cell entrance into the tumor and by establishing a hostile tumor microenvironment (TME) impairing the fitness of cytotoxic effectors (21). Moreover, MDSCs can directly support cancer progression by promoting epithelial–mesenchymal transition and tumor stemness and establishing a microenvironment suitable for seeding metastatic tumor cells, named the premetastatic niche (22–24). Many studies have associated MDSC expansion with worse patient clinical outcomes in solid tumors, including skin (25), pancreatic (26), brain (27), prostate (28), lung (29), and breast cancers (30, 31), and have uncovered MDSC enumeration as a prognostic biomarker of response to immune therapies (32, 33). Thus, deciphering MDSC ontogeny and selective targeting strategies remain as cornerstones for overcoming the current limitations of cancer immunotherapies.
It has become apparent that, in many cancer types, PRRs play a central role in modulating a vast array of tumor-inhibiting and tumor-promoting cellular responses both in immune cells within the tumor microenvironment and directly in cancer cells. MDSCs share with physiological myeloid cells the expression of many Toll-like receptors (TLRs), including TLR2 and TLR7-8, in both mice and humans (34, 35). Thus, TLR agonists/antagonists were recently employed in both preclinical and translational research to repolarize myeloid cells (including MDSCs) and support the activation and efficacy of adaptive antitumor immunity. Whether PRRs are differentially expressed in MDSCs, monocytes, and PMNs and whether their triggering activates different signaling pathways are still open questions. In this review, we discuss the expression of PRRs on MDSCs, how PRRs contribute to MDSC development and function, the signaling pathways included, and the targeting approaches, with the final aim of unveiling the promise of potential agonists to target myeloid immune regulatory functions and to restore host cancer immune surveillance.
PRR expression on MDSCs
In the 1990s, the hypothesis of PAMPs and their specific receptors was proposed by Janeway, which was of epoch-making significance and changed research on innate immunity (36). TLRs are one of the earliest PRRs discovered in the innate immune system, and TLR4 can induce the activation of NF-κB and the expression of the costimulatory molecules CD80 and CD86, giving indispensable second signals for T lymphocyte activation (37, 38). After the discovery of TLR4, many PRRs and their corresponding ligands were discovered. PRRs can be classified into five groups based on protein domain homology: TLRs, nucleotide oligomerization domain (NOD)-like receptors (NLRs), retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs), C-type lectin receptors (CLRs), and absent in melanoma-2 (AIM2)-like receptors (ALRs) (7, 39). With the advances in studying PRRs’ structure, function, and distribution, the role of PRRs in arranging MDSC function in different types of cancer has become clearer, as summarized in Figure 1.
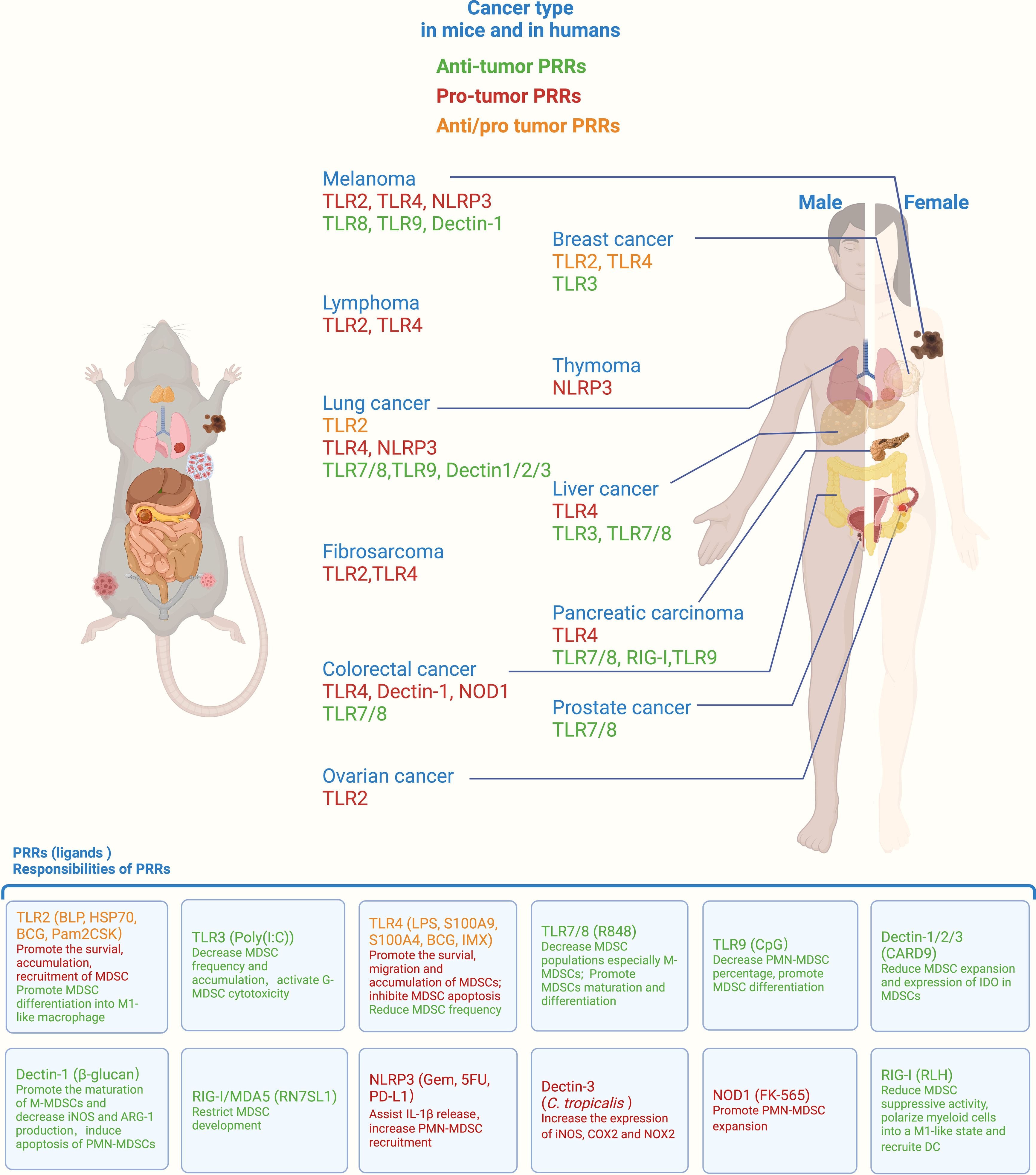
Figure 1 Roles of PRRs in orchestrating myeloid-derived suppressor cell (MDSC) function across different cancers. PRRs represent the primary MDSC receptors for integrating signals from pathogens or damaged cells. By recognizing different ligands, PRRs orchestrate MDSC immunosuppressive function, survival, migration, accumulation, differentiation, and soluble molecule release, thus exerting protumor or antitumor effects in mice and humans. PRRs, pattern recognition receptors; TLR, Toll-like receptor; NLRP3, NLR family pyrin domain containing 3; NOD1, nucleotide oligomerization domain; RIG-I, retinoic acid-inducible gene-I; BLP, bacterial lipoprotein; HSP70, heat shock protein 70; BCG, Bacille–Calmette–Guerín; LPS, lipopolysaccharide; S100A9, S100 calcium-binding protein A9; CARD9, caspase activation and recruitment domain 9; Gem, gemcitabine; 5FU, 5-fluorouracil; PD-L1, programmed death-ligand 1; RLH, RIG-I-like helicases. All listed PRRs were demonstrated to regulate MDSC function in mice, whereas only the PRRs indicated with lines were proven to modulate MDSC function in human cancers. The role of ALRs is not shown above due to the lack of relevant reports.
Toll-like receptors
TLRs are the first family of PRRs to be discovered and defined. In total, 13 types of TLRs (TLR1–10 in humans and TLR1–9 and TLR 11–13 in mice) have been identified. All TLRs are type I transmembrane glycoproteins and share a similar domain organization: an extracellular N-terminal ectodomain contains leucine-rich repeats (LRRs, responsible for recognition of DAMPs and PAMPs), a single transmembrane region, and an intracellular cytosolic Toll/IL-1R (TIR) region that is responsible for signal transduction (8, 40). TLRs localize in different positions of the cells. TLR1, 2, 4, 5, 6, and 10 are exposed on the cell surface, usually in the form of heterodimers or homodimers, and recognize pathogen membrane components, such as lipids, peptidoglycans, lipoproteins, or DAMPs, such as HSP. TLR3, TLR7, TLR8, and TLR9 are expressed in the form of homodimers on endosomal membranes and recognize intracellular RNA or DNA from microorganisms or damaged host cells. In monocytes and neutrophils, the activation of TLRs results in an inflammatory response by producing and secreting a variety of proinflammatory and antiviral factors (41). The consequence of different TLR activation in MDSCs in cancer is controversial and possibly context dependent. In general, cell surface TLR activation contributes more to MDSC suppressive function and a pro-tumor effect, yet endosomal TLR activation mainly decreases the ability of MDSCs and exerts an antitumor effect (42, 43). Cell surface TLRs, including TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10, can actively capture extracellular information, including tumor cell-released DAMPs or apoptotic cancer cell fragments, such as HMGB1, HSP, and S100, as well as extracellular vesicles containing changed cell components. The corresponding signal transduction will amplify the effect of the immune-suppressive response by producing a variety of proteins, including arginase 1 (ARG1), inducible nitric oxide synthase (iNOS, also named NOS2), indoleamine 2,3-dioxygenase 1 (IDO1), prostaglandin E2 (PGE2), programmed death-ligand 1 (PD-L1), CD40, tumor necrosis factor (TNF), IL-1β, IL-6, cyclooxygenase-2 (COX2), etc. Thus, creating an immunosuppressive TME with less antigen presentation hampers T cell or NK cell activation and proliferation and reduces cytotoxicity (44). Intracellular TLRs, including TLR3, TLR7, TLR8, and TLR9, are capable of detecting intracellular nucleic acids and inducing the production of type I IFN (IFN1), which plays a role in antitumor immunity by diverse mechanisms, including reducing ARG1 and NOS2 production and improving the survival of cytotoxic lymphocytes.
TLR2 usually forms heterodimers with TLR1 or TLR6 to recognize ligands, including bacterial lipoteichoic acid, triacyl lipopeptide, arabinomannan, peptidoglycan, pore protein, and fungal zymosan. In MDSCs, the recognition pattern is changed. TLR1/2 exhibit duality in regulating MDSC behavior during tumor progression. Indeed TLR2 activation with bacterial lipoproteins in MDSCs supports cancer growth by promoting MDSC survival, accumulation, and recruitment to the tumor microenvironment in mouse lymphoma, melanoma, lung cancer, fibrosarcoma, and CRC models and is verified to be correlated with worse prognosis in human CRC tissues (45–47). However, an opposite correlation was found by other groups who showed that TLR1/2 expression and activation were associated with better prognosis in lung cancer patients, which was further confirmed in mouse lung cancer and breast cancer models by promoting MDSC differentiation into M1 macrophages (48, 49). In tuberculosis, which is associated with a higher lung cancer risk (50), Mycobacterium bovis Bacille–Calmette–Guerín (BCG) infection induces TLR2 and TLR4 expression in mouse MDSCs, further upregulating CD40, PD-L1, and CD69 expression in both G-MDSCs and M-MDSCs as well as iNOS expression, leading to increased nitric oxide (NO) production required for the suppression of T cell proliferation in BCG-infected mice (51). However, hematopoietic stem cells exposed to BCG showed stronger anti-tuberculosis immune protection when engrafted in a new host by producing “trained” monocytes/macrophages (52). These findings challenge the current vision of adaptive immunity as the only immune system components endowed with enhanced functional capacity. Several recent studies have demonstrated further evidence of memory-like or stimulus-imprinted innate immune education through PAMP-PRR recognition in PMNs (53, 54). In CRC patients, increased circulating MDSCs were found in peripheral blood as well as accumulated in tumor tissues, and this process was driven by S100 calcium-binding protein A9 (S100A9) via TLR4-mediated NF-κB signaling pathways in MDSCs (55). C-X-C motif chemokine ligand 10 (CXCL10) plays an important role in hepatic disease. Recently, it was reported that CXCL10 contributes to hepatocellular apoptosis through TLR4 on MDSCs instead of its common receptor—CXC motif receptor 3—in patients with hepatocellular carcinoma (HCC), thus leading to cancer recurrence. Knockout or inhibition of CXCL10/TLR4 significantly reduced the M-MDSC levels and constrained tumor growth in a mouse recurrent tumor model (56). S100A4 can also bind to TLR4 on MDSCs in fibrosarcoma-, melanoma-, and lung cancer-bearing mouse models (57). The soluble protein involved in pancreatic ductal adenocarcinoma (PDAC) tumorigenesis—named pancreatic adenocarcinoma upregulated factor—binds to TLR4 on MDSCs and triggers the production of ARG1, NO, and reactive oxygen species (ROS) in pancreatic tumor-bearing mice (58). Soluble calreticulin has also been demonstrated to be involved in the migration and survival process of tumor-derived MDSCs via interaction with TLR4 (59). Notably, TLR4 activation in MDSCs [activated by BCG (60) and Immunomax (61)] reduced the MDSC frequency and thereby suppressed rat bladder cancer progression or mouse breast cancer metastasis, respectively.
Nevertheless, LPS-activated TLR4 has been proven to expand MDSCs in vivo. Recently, it has aroused interest again on its long-term functional “training” on myeloid cells in the pathogenesis of chronic inflammatory diseases (62, 63), which goes along with extensive long-lasting epigenetic memory in hematopoietic stem cells, provoking drastic changes in the landscape of substream myeloid progenitor cells (64). An increased transcriptional response of open myeloid enhancers to secondary stimulation was observed to help defend against secondary infection from gram-negative bacteria.
Endosome-localized TLR3, TLR7/8, and TLR9 are typical nucleotide sensors. TLR3 and TLR9 agonists reduce MDSCs in an EG7 lymphoma mouse model (65). TLR3 activates G-MDSC cytotoxicity and inhibits tumor growth through the production of ROS/reactive nitrogen species (RNS) (66).
TLR7/8 and TLR9 agonists have been successfully used in cancer treatment. In CRC mouse models, applying the TLR9 ligand CpG decreased the PMN-MDSC percentage, prevented MDSC suppressive functions on T cell proliferation, and promoted their differentiation into mature myeloid cells with enhanced expression of the macrophage differentiation marker F4/80, the DC maturation markers MHCII and CD11c, and the costimulatory molecule CD80 (67). CpG also decreased the percentage of MDSCs in the spleen and inhibited their infiltration into mouse B16 melanoma tumors (68). The TLR7/8 agonist resiquimod (R848) decreases both intratumoral and circulating MDSC populations, especially M-MDSCs, with up to fivefold decrease in the tumor. R848 promoted MDSC maturation and differentiation with upregulation of the surface molecules CD11c, F4/80, MHC-I, and MHC-II (69). Similarly, TLR7 agonists reduced MDSCs by 50% and nearly doubled the M1/M2 ratio to shift the TME toward a more inflammatory state, thus enhancing the efficacy of CAR-T therapy in 4T1 breast cancer models (70).
NOD-like receptors
Unlike TLRs localized on the cell membrane, NLRs are intracellular PRRs consisting of three binding domains: central NOD (nucleotide-binding and oligomerization domain), N-terminal effector domain, and C-terminal LRRs. According to the different N-terminal domains, NLRs are divided into four subgroups: the acidic transactivation domain, the baculoviral inhibitory repeat-like domain, the caspase activation and recruitment domain (CARD; NLRC), and the pyrin domain (NLRP) (71). Sensing of danger signals by NLRs leads to their oligomerization into large macromolecular scaffolds and the rapid deployment of effector signaling cascades to restore homeostasis (72). NLR activation in MDSCs is closely related to colitis-associated cancer. NOD1 (a main member of the NLRC) activation by FK-565 arouses systemic inflammation, with expanded PMN-MDSCs, followed by the later recruitment of M-MDSCs into the peritoneal compartment, and increases the expression of ARG1 to exert immunosuppressive function, thus driving carcinogenesis toward CRC (73). Some recent studies have also confirmed the key role of NLRP3 in MDSC promotion of tumor progression. Some chemotherapeutic agents, gemcitabine (Gem) and 5-fluorouracil, were also proven to induce the expression of IL-1β in an NLRP3-dependent manner in MDSCs, thus curtailing anticancer immunity (74). NLRP3 inhibition diminished PMN-MDSC recruitment in response to anti-PD-1 Ab therapy in a mouse melanoma model (75, 76), and NLRP3 inflammasome blockade reduced the frequencies of MDSCs in the tumor tissues, spleen, and peripheral blood in lymphoma and decreased the expression of immunosuppressive genes (Pdcd1l1, Arg1, Il10, and Tgfb1) in PMN-MDSCs isolated from tumor-bearing mice (77, 78). In addition, NLRP3-deficient MDSCs (isolated from knockout tumor-bearing mice) are less efficient in reaching the tumor site (79).
RIG-I-like receptors
Retinoic acid-inducible gene 1 executes its antiviral activity by recognizing viral RNA and releasing interferon-beta and other proinflammatory cytokines (8). RIG-I-like receptors are intracellular PRRs. RLRs include several molecules, such as retinoic acid-inducible gene I, melanoma differentiation-associated protein 5 (MDA5), and LGP2 (80). Mimicking viral infections with immune-activating RNA species is a potential strategy for tumor immunotherapy because of the shared fundamental features of immune responses against viruses and tumors. RIG-I-like helicase triggering induces an IFN-driven immune response and reprograms the TME of pancreatic cancer by reducing MDSC suppressive activity, polarizing myeloid cells into an M1-like state and recruiting DCs (81). Since the low rate of tumor-infiltrating immune cells, the development of lymphocyte exhaustion, and antigen insufficiency are common mechanisms that limit chimeric antigen receptor (CAR)-T cell therapeutic effectiveness (82), CAR-T cells engineered to deliver RIG-I/MDA5 agonists were able to restrict MDSC development by reducing their release of anti-inflammatory cytokines (e.g., TGF-β) and fostered DC subsets with costimulatory features, thus enhancing CAR-T cell efficacy in B16 melanoma-bearing mice, even when heterogeneous CAR antigen tumors lack adequate neoantigens (83).
C-type lectin receptors
CLRs are a class of transmembrane phagocytic PRRs. They play a role in both the innate immune system and acquired immunity. It recognizes and binds to PAMPs and places pathogens in cytoplasmic vesicles for direct digestion and elimination to control the infection (84). CLRs include three members: Dectin-1, Dectin-2, and Dectin-3 (8). A study indicated that LOX-1 (member of the Dectin-1 subgroup) was a specific marker for human PMN-MDSCs since LOX-1 could not be detected in the neutrophils of healthy donors, whereas LOX-1+ neutrophils (5–15% in cancer patients and 15–50% in tumor tissues) had potent immune suppressive activity and other biochemical characteristics of PMN-MDSCs (85). C. tropicalis facilitates the immunosuppressive function of MDSCs by increasing the expression of iNOS, COX2, and NOX2 and the production of NO and ROS via C-type lectin receptor Dectin-3-dependent pathways. NO produced by MDSCs enhanced aerobic glycolysis. Furthermore, C. tropicalis upregulates the expression of HIF-1α target genes encoding the glycolytic enzymes GLUT1, HK2, PKM2, LDHA, and PDK1 in aerobic glycolysis, which drives colorectal cancer (86). The CLR downstream signaling molecule adapter protein CARD9, which is highly expressed in myeloid cells, was proven to reduce MDSC expansion and the expression of indoleamine 2,3-dioxygenase in MDSCs to slow down the lung cancer progression (87).
Fungal-derived polysaccharide β-glucan (a major ligand of Dectin-1) induces the differentiation of M-MDSCs (monocytic MDSCs) into a more mature population with a CD11c+ F4/80+ phenotype and drastically decreases iNOS and ARG-1 production via the dectin-1 pathway in vitro, thereby leading to delayed tumor progression in a mouse Lewis lung cancer model (88). Another study also demonstrated that β-glucan induced subsequent apoptosis in PMN-MDSCs while converting M-MDSCs to potent antigen-presenting cells, which could promote antigen-specific CD4 and CD8 T cell responses (89). Furthermore, recent reports have shown a novel and profound effect of β-glucan in promoting a sustained and enhanced response of myeloid cells to secondary infectious or inflammatory challenges as a representation of trained innate immunity (90, 91). Nevertheless, β-glucan training teaches hematopoietic progenitors toward better control of melanoma and lung cancer progression. Indeed β-glucan stimulation caused transcriptomic and epigenetic rewiring of granulopoiesis toward an antitumor phenotype. This imprinting was carried for a long term in adoptively transferred neutrophils from β-glucan-trained mice to naive recipients and suppressed tumor growth in a ROS-dependent manner, giving us new insight into antitumor immunity regulation through the induction of trained immunity (92).
Signaling pathways in PRRs of MDSCs
Despite the large number of PRRs discovered as well as their ligands, there are only a few types of signal transduction pathways that are shared by them, and all pathways are cross-talking. As we gained deeper insight into the role of MDSCs in cancer, these pathways were found to be involved in MDSC polarization and immunosuppressive function (Figure 2).
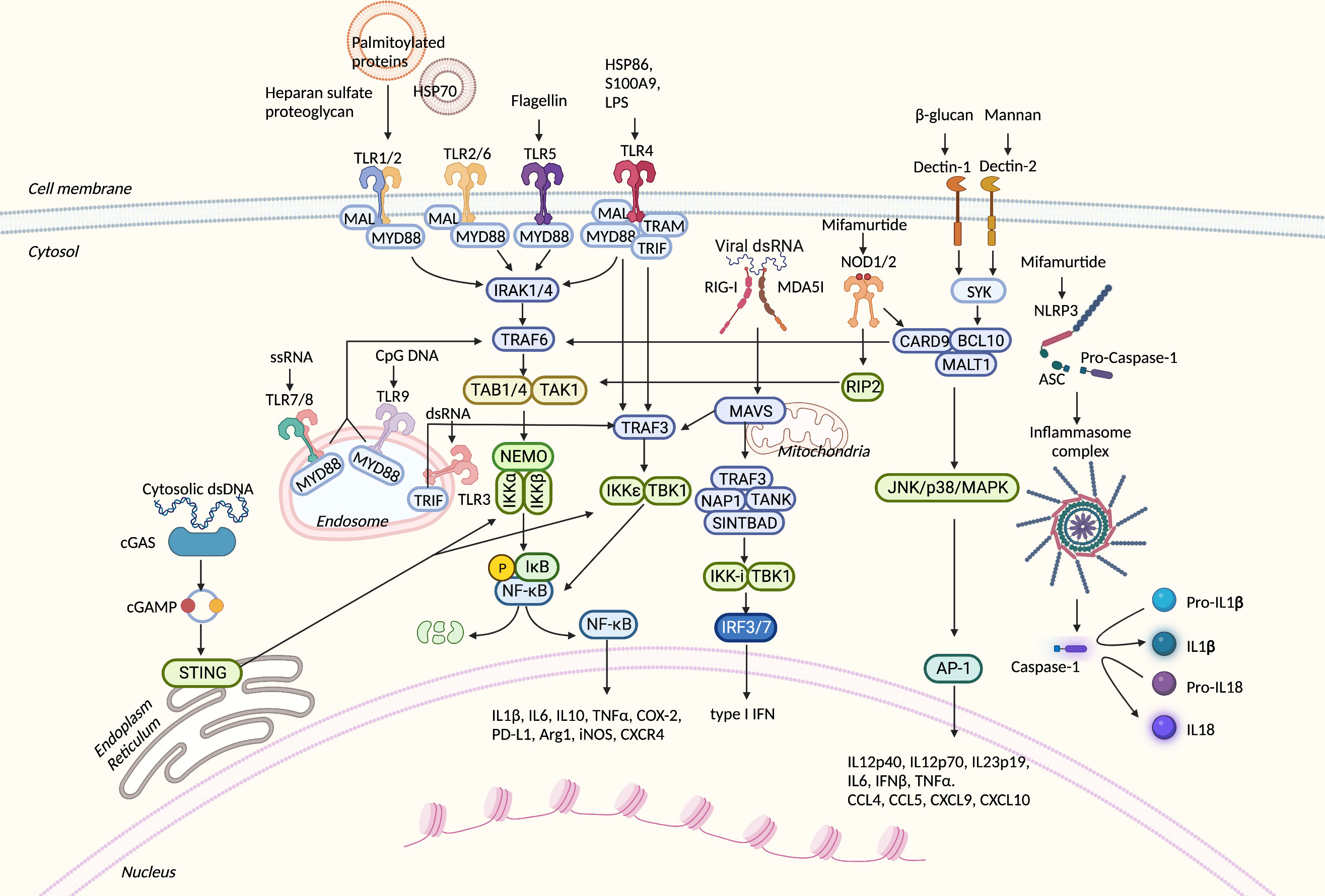
Figure 2 Overview of PRR-related signaling pathways in myeloid-derived suppressor cells (MDSCs). PRRs can sense numerous danger signals released by pathogens and damaged normal and neoplastic cells. After binding to proper ligands, PRRs recruit adaptor proteins and trigger downstream signal transduction. These processes result in the translocation of transcription factors into the nucleus, expression of inflammatory-related genes, or direct cytokine activation from their inactive forms. PRRs, pattern recognition receptors; TLR, Toll-like receptor; HSP70, heat shock protein 70; LPS, lipopolysaccharide; S100A9, S100 calcium-binding protein A9; MyD88, myeloid differentiation primary TAK-binding proteins 1; IKK, IκB kinase; NEMO, NF-κB essential modulator; Mal, MyD8response 88; RIP2, serine–threonine protein 2; SYK, spleen tyrosine kinase; IRAK4, IL-1R-related kinase 4; TRAF6, TNF receptor-associated factor 6; TAK1, TGF-β-activated kinase 1; TAB1, 8-adapter-like; TRIF, TIR domain-containing adaptor protein-inducing interferon β; TRAM, TRIF-related adaptor molecule; NOD, nucleotide oligomerization domain; cGAS, cyclic GMP–AMP synthase; cGAMP, 2′3′ cyclic GMP–AMP; STING, stimulator of interferon genes; TBK1, TANK-binding kinase 1; RIG-I, retinoic acid-inducible gene-I; MDA5, melanoma differentiation-associated gene 5; MAVS, mitochondrial antiviral signaling protein; TANK, TRAF family member-associated NFKB activator; SINTBAD, similar to NAP1 TBK1 adaptor; IRF3, interferon regulatory factor 3; CARD9, caspase activation and recruitment domain 9; BCL10, B-cell lymphoma/leukemia 10; MALT1, mucosa-associated lymphoid tissue lymphoma translocation protein 1; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; AP-1, activator protein 1; NLRP3, NLR family pyrin domain containing 3; ASC, apoptosis-associated speck-like protein containing CARD; Arg1, Arginase1; iNOS, inducible nitric oxide synthase; COX2, cyclooxygenase-2; TNF, tumor necrosis factor; PD-L1, programmed death-ligand 1; CXCR4, C-X-C chemokine receptor type 4; dsRNA, double-strand RNA; ssRNA, single-strand RNA; IFN-β, interferon-β; TNF-α, tumor necrosis factor-α; CXCL9, C-X-C motif chemokine ligand 9; CCL4, C-C motif chemokine ligand 4.
NF-κB signaling
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) plays a key role in the process of cellular inflammation and immune response. NF-κB is a heterodimer composed of two molecules, p50 and p65, and it is usually inactive by binding to the inhibitory protein IκB under normal conditions. Toll-like receptors are considered universally conserved activators of NF-κB signaling in MDSCs (93).
After TLRs bind to corresponding PAMPs and DAMPs, the TIR domains conduct signals by myeloid differentiation primary response 88 (MyD88)-dependent and MyD88-independent pathways. MyD88 binds to the TIR domain of TLRs, recruits IL-1R-related kinase 4 (IRAK4) and activates IRAK1 and IRAK2. Ubiquitin ligase TNF receptor-associated factor 6 (TRAF6) is recruited to form a complex with TGF-β-activated kinase 1 (TAK1) and two TAK-binding proteins (TAB1 and TAB4). TRAF6 is then degraded. The TAK1–TAB1–TAB4 complex then phosphorylates the IκB kinase (IKK) complex, causing its own degradation by ubiquitination. Afterward, NF-κB is released and translocated into the nucleus, thereby regulating the transcription of inflammatory genes, including TNF, IL-6, IL-1, and other chemokines (8). Recently, it was reported that murine fibrosarcoma-derived PGE2 induced the accumulation of p50 in the nucleus, which led to strengthened binding of STAT1 to selected IFNγ-dependent genes in M-MDSCs, resulting in higher NOS2 expression. This process modulated M-MDSCs into an NO-mediated immunosuppression phenotype and reduced TNFα expression that can support tumors in return (94). Furthermore, the cellular FADD-like IL-1β-converting enzyme-inhibitory protein expression in M-MDSCs of PDAC patients was reported to be increased significantly, which played a major role in chemotherapy resistance, including commonly applied 5-fluorouracil (5-FU) and Gem, in part through the activation of the canonical NF-κB signaling pathway (20).
The immunosuppression function directed by NF-κB signaling has been well described in anti-inflammatory processes after long-term TLR activation. Sustained activation of TLRs causes persistent production of proinflammatory cytokines, such as TNF or IL-6, leading to tissue damage (95, 96). Similarly, PRR activation in MDSCs also plays an important role in the cancer process. Tumor cells can generate abnormal surface components that activate PRRs on MDSCs. Melanoma cells change the glycocalyx structure on their heparan sulfate proteoglycan, which can bind to TLR2 on MDSCs and facilitate the recruitment of MDSCs via the TLR2/MyD88/IL‐6/STAT3 pathway, leading to the inhibition of NK cell recruitment and cytotoxicity and ultimately tumor progression and metastasis (97). Melanoma can also release heat shock protein 86, acting as a DAMP, which is recognized by TLR4 on MDSCs. Both adaptors (MyD88 and TRIF) accumulate to activate NF-κB, with a pronounced expression of IL1β, IL6, IL10, TNFα, COX-2, and PD-L1, leading to a strong immunosuppressive capacity toward T lymphocytes (98). S100A9 was found in the CRC microenvironment to activate the MDSC immune suppressive program through TLR4-dependent NF-κB signaling pathways, resulting in upregulated Arg1, iNOS, and IL-10 production (55). Tumors exploit extracellular vesicles (EVs) to deliver long-distance information that can regulate the host immune system. Renal cancer-derived exosomes encapsulate HSP70 to recognize TLR2 and promote MDSC expansion and production of iNOS, ARG1, and ROS through the MyD88/TRAF6/P38/AP-1 pathway (99). Exosomes derived from prostate cancer cells can upregulate CXC motif receptor 4 (CXCR4) via the TLR2/NF-κB signaling pathway, eventually promoting the migration of MDSCs into the tumor microenvironment in a CXCR4–CXCL12 axis-dependent manner (100). Conventional monocytes readily take up palmitoylated proteins from acute myeloid leukemia-derived EVs, activating the TLR2/NF-κB signaling pathway, and subsequently undergo MDSC differentiation, expressing a typical HLA-DRlow phenotype, releasing IDO-1, and gaining T cell suppression ability (101). Nevertheless, some TLRs can also be activated to abstain from MDSC immunosuppressive functions. In contrast to glucans, nCKAP-2, a branched arabinan-type polysaccharide purified from Curcuma kwangsiensis, was discovered to exert an anti-MDSC effect by inducing the apoptosis of MDSCs through the TLR4-mediated NF-κB signaling pathway (102). Mifamurtide, a prodrug that can release free muramyl dipeptide and initiate NOD2-NF-κB signaling in patients with cancer, has been successfully used to treat osteosarcoma with markedly reduced MDSC accumulation (103).
MAPK signaling
Mitogen-activated protein kinase (MAPK) signaling is an evolutionarily conserved pathway linking extracellular signals to fundamental cellular processes such as growth, proliferation, differentiation, migration, and apoptosis. In the MyD88-dependent pathway of TLRs, IRAK-1 is activated and interacts with TRAF6 and then activates the IKK complex. In this step, IRAK-1 can also cause the activation of MAPKs, including three subfamilies: c-Jun N-terminal kinase (JNK), p38, and MAPK. When NLRs are activated because they sense bacterial components, downstream CARD9 can be recruited, thereby activating p38, JNK, and finally the MAPK pathway to promote the release of proinflammatory factors.
MAPK is reported to modulate MDSC plasticity. LPS could convert the potentially suppressive Gr1+CD115+ monocytes toward an inflammation stimulatory monocyte via the p38/MAPK signaling pathway, reducing its ability to convert conventional CD4+ T cells into CD4+CD25+Foxp3+ Tregs (104). The ERK/MAPK pathway inhibition was related to suppressed tumor growth and promoted MDSC apoptosis (105). Immune checkpoint protein V-domain immunoglobulin suppressor of T cell activation dampened the TLR-mediated activation of both MAPK/AP-1 and IKK/NF-κB signaling cascades and restored T cell IFN-γ production in mice bearing melanoma and increased the cytokines IL12p40, IL12p70, IL23p19, IL6, IFNβ, and TNFα as well as the chemokines CCL4, CCL5, CXCL9, and CXCL10 inside the tumor tissue (106). Nevertheless, the complement system can sense DAMPs and apoptotic cells and trigger a sterile inflammatory response. Although complement activation on the tumor endothelium can reverse the endothelial barrier and fuel T cell entrance into the tumor microenvironment (107), C5a orchestrates MDSC infiltration and the immune suppression program in tumors by regulating the production of ROS and RNS (108). Accordingly, C3 can be produced by hepatic carcinoma cells to activate MDSCs to produce IL10 through p38/MAPK signaling (109).
TBK1–IRF-3 signaling
The major adaptor protein in the MyD88-independent pathway is TRIF, which mainly induces type I IFN expression. After binding to its cognate receptor, TRIF and TRAF3 activate signaling, leading to the recruitment of IKKϵ/TANK-binding kinase 1 (TBK1), which can phosphorylate IRF3 and activate type I IFN gene transcription. This cascade of events regulates innate anti-viral immune responses. RLRs such as RIG-I and MDA5 can detect viral RNAs. Then, MDA5 and RIG-I interact with their common adaptor mitochondrial antiviral signaling protein to dimerize and bind to TRAF3. In turn, TRAF3 recruits the adaptor proteins TANK, NAP1, and SINTBAD, which induce the phosphorylation of IRF-3 (8). IFN plays a central role in antiviral immunity and was recently considered to directly affect MDSC function (110). Importantly, IFN-I signaling in PMN-MDSCs was a prerequisite for their immunosuppressive effects, and the downregulation of IFN-I signaling in PMN-MDSCs led to the activation of the PI3K-Akt/mTOR pathway, which led PMN-MDSCs to obtain their immunosuppressive traits in peripheral blood (111).
Inflammasome signaling
Inflammasomes are cytosolic macromolecular complexes assembled by multiple PRRs in the cytoplasm. There are five types of inflammasomes, including the NLRP1 inflammasome, NLRP3 inflammasome, NLRC4 inflammasome, IPAF inflammasome, and AIM2 inflammasome. Inflammasomes are usually composed of apoptosis-associated speck-like protein containing a CARD (ASC), caspase protease, and a protein of the NLR family (e.g., NLRP3) or HIN-200 family protein (e.g., AIM2). Taking NLR inflammasome formation as an example, the dimerization of NLRs under the action of intracellular PAMPs or DAMPs makes pyrin domains (PYDs) polymerize and then activate the ASC complex, which activates the effector complex composed of CARD and caspase-1. Finally, NLRs (LRR + NACHT + PYD), ASC (PYD + CARD), and the effector complex (CARD + caspase-1) together constitute the inflammasome complex. After formation, inflammasomes can activate caspase-1. Activated caspase-1 splices proIL-1β/proIL-18 into the corresponding mature cytokines. NLRP3 is the most studied inflammasome sensor driving IL-1β-mediated conditions from sterile inflammation to rare hereditary syndromes (76).
MDSCs exhibit an increased activation of NLRP3 during tumor development. Ablation of the NLRP3/pro-IL-1β inflammasome rewires MDSC function, promotes melanoma (112) and breast cancer (113) regression, and causes cisplatin resistance (114). NLRP3 inhibition and anti-PD-1 treatment significantly increased the antitumor efficacy of monotherapy by limiting MDSC-mediated T cell suppression and melanoma tumor progression (76). Gemcitabine and 5-fluorouracil were reported to be able to activate the NLRP3 inflammasome in MDSCs, leading to the production of IL-1β, and IL-1β induced the secretion of IL-17 by CD4+ T cells, which blunted the chemotherapy efficacy (74).
However, diverse functions of the NLRP3 inflammasome in MDSCs have also been reported. The expression of the scavenger receptor macrophage receptor with collagenous structure (MARCO) on MDSCs correlates with poor prognosis and a “cold tumor” phenotype in PDAC patients. Anti-human MARCO (anti-hMARCO) antibody activated the NLRP3 inflammasome, resulting in IL-18 production, and could help T cells and NK cells produce IFNγ (115). Nevertheless, melanoma expresses high NLRP3-derived IL-1β expression, which specifically induces pSTAT3 and amplifies IL-6 secretion through the IL-1β/IL-6/STAT3 axis in vivo, leading to a further expansion of MDSCs (77). A previous preclinical report suggested that the limitation of 5-FU anticancer efficacy is due to IL-1β secretion by MDSCs, 5-FU-mediated NLRP3 activation, and subsequent caspase-1 activity in MDSCs driving the expression of IL-1β. The application of docosahexaenoic acid improved 5-FU efficacy by inhibiting IL-1β secretion and caspase-1 activity in an MDSC cell line (MSC-2) (116). Mifamurtide, except from binding to NOD2, also activates NLRP3 and promotes the activation of IL-1β in macrophages and monocytes. Application of mifamurtide significantly improves event-free survival and overall survival in osteosarcoma patients (117).
The STING pathway
The detection of cytosolic DNA is contributed, in a large part, by the cyclic GMP–AMP synthase (cGAS)–stimulator of interferon genes (STING) pathway, which has emerged as a critical mechanism for coupling the sensing of cellular perturbation to the powerful innate immune responses. In this pathway, after sensing dsDNA, cGAS allosterically activates its catalytic activity and leads to the production of 2′3′ cyclic GMP–AMP (cGAMP), a potent agonist of the endoplasmic reticulum (ER) membrane protein STING. Then, STING translocates from the ER to the Golgi, where STING can bind to TBK1 and then recruit and phosphorylate IRF3 and subsequently activate the production of type I IFNs.
The protumor vs. antitumor roles of STING have been argued. In B16 melanoma tumor-bearing mice, an intravenous administration of the STING agonist cGAMP decreased the number of GR1+ and especially Ly6G+ PMN-MDSCs in the spleen and tumor while suppressing the production of ROS and NO from MDSCs and abolishing their suppressive function (118). The application of cyclic diguanylate (c-di-GMP), a ligand for STING, significantly increased the production of IL-12 by MDSCs, reversing its suppressive function, in correlation with an improved vaccination response against metastatic breast cancer (119). The intrinsic activation of the unfolded protein response mediator PKR-like ER kinase (PERK) in MDSCs suppressed the STING pathway in cancer by decreasing the STING-driven production of type I IFN (120). Moreover, independent of IFN production, STING overexpression in MDSCs upregulated the suppressor of cytokine signaling 1 (SOCS1), a potent inhibitor of Janus kinase (JAK) 1/2 in the JAK/STAT signaling pathway (121), preventing STAT3 phosphorylation (122, 123) and inhibiting the production and release of IL-6 and GM-CSF, two known drivers of MDSC expansion in nasopharyngeal carcinoma (7).
TLRs as potential targets for MDSCs in cancer
PRRs widely exist in tumors and are not only related to the growth and proliferation of tumors themselves but are also closely related to the formation of the tumor microenvironment (124). Therefore, how to utilize and target these receptors has aroused extensive interest from researchers. Among PRRs, TLRs are the most widely studied and modulated. Tumor cells release DAMPs actively or when they die, such as HMGB1, HSP, and S100 (44). MDSCs capture these molecules to respond to these proteins through the TLR pathway to increase activity, expand proliferation, or increase penetration. A variety of drugs targeting TLRs have been developed and even used in the treatment of clinical tumor patients or combined with immune checkpoint inhibitors (ICIs). The major treatment strategies targeting PRRs are to use ligand analogs to activate them, antagonists to inhibit their activation, or antibodies and small molecules to inhibit the related signaling pathways (125). Today, most targeted drugs are mostly in the preclinical development stage. Since this future therapeutic approach will be developed not only to directly target tumor cells but also to modify the immune composition of the TME, targeting different pro-tumor immune cells via TLRs has also attracted attention, whether combined with ICIs (Table 1) or with other therapies (Table 2). Unfortunately, only a few clinical trials nowadays aim to evaluate the therapeutic impact of TLR targeting in reprogramming MDSC cell functionality and activity (Tables 1, 2).
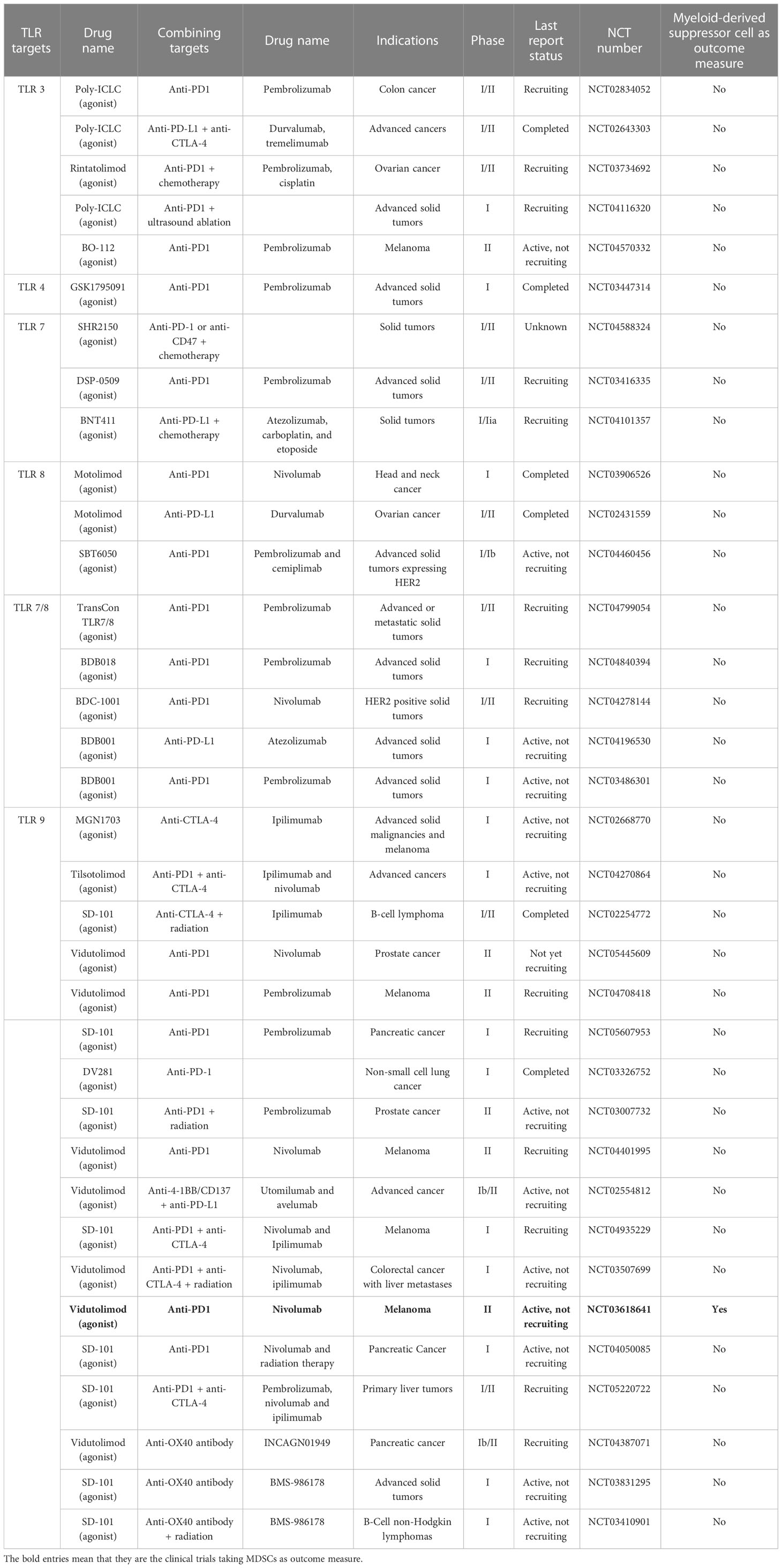
Table 1 Summary of clinical trials targeting Toll-like receptor (TLRs) in combination with immune checkpoint inhibitors.
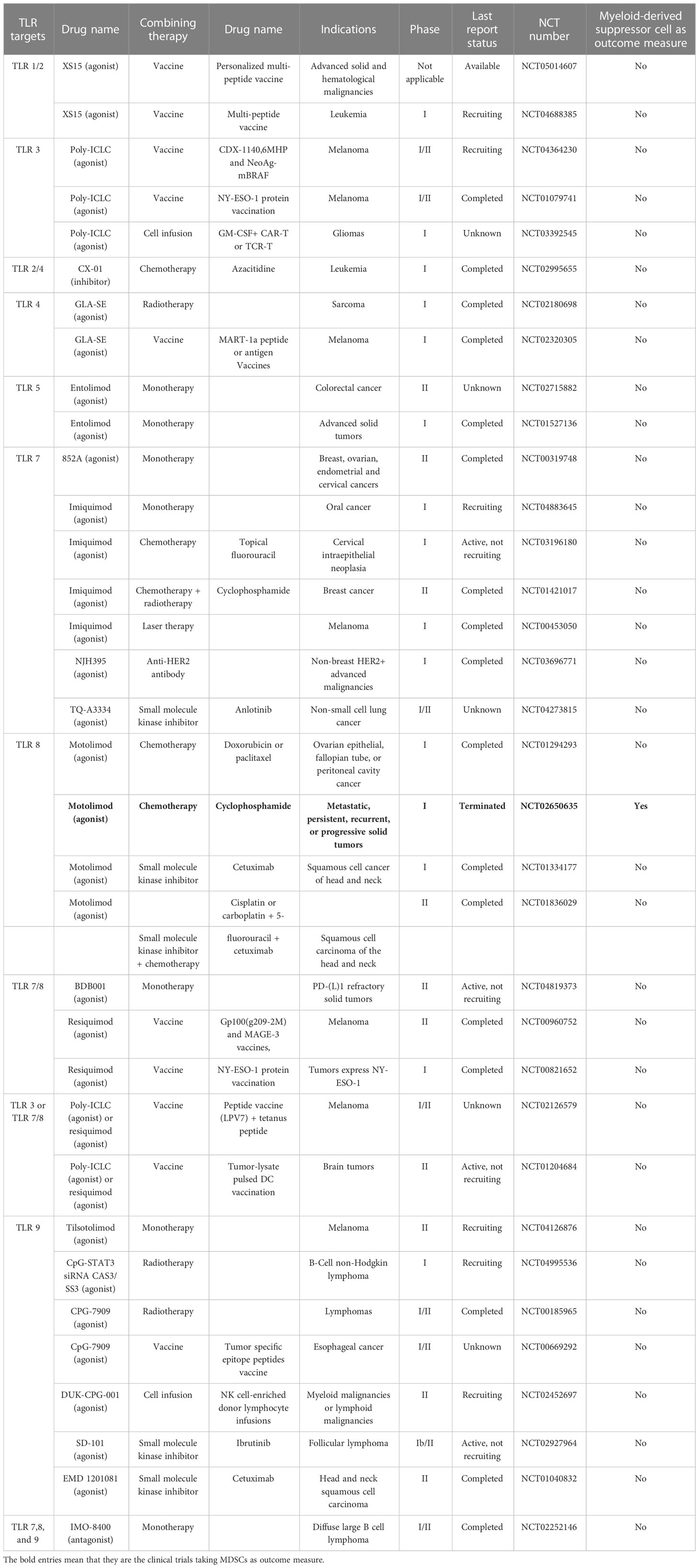
Table 2 Summary of clinical trials targeting Toll-like receptor (TLRs) as monotherapy or in combination with other treatments.
TLR1/2
Studies on TLR1/2 agonists in MDSCs are controversial. Pam3CSK4, an agonist of TLR1/2, can promote the differentiation of M-MDSCs in the blood of patients with colon, prostate, pancreas, or liver cancer into M2-type macrophages with highly immunosuppressive functions (34). In a melanoma study, Pam3CSK4 upregulated the expression of PD-L1 on immature myeloid cells (IMCs) (98). Both studies suggest that the activation of TLR1/2 on MDSCs promotes the formation of an immunosuppressive microenvironment. However, in another mouse model of HCC, Pam3CSK4 helps M-MDSCs polarize toward mature macrophages and DCs and exert antitumor functions to prevent tumor progression (126). In lung cancer, Deng et al. found that applying agonists of TLR1 and TLR2 using bacterial lipoprotein not only inhibited tumor growth but also reduced the number of M-MDSCs in mouse models (48). A more interesting study on colon cancer found that an important DAMP molecule released by dying tumor cells, translational control tumor protein (TCTP), activates TLR2 on M-MDSCs and promotes the secretion of CXCL1/2, recruiting PMN-MDSCs into the TME. They abolished TCTP using 55F3, a monoclonal antibody, and found reduced CXCL1/2 concentrations as well as reduced PMN-MDSC infiltration (46).
TLR3
Double-stranded RNA (dsRNA), released by viruses or damaged cells, is the common ligand for TLR3. The TLR3 agonist poly(I:C)/PIC, an artificial dsRNA analog that has significant potential to enhance the immune system, has been widely used in cancer therapy (127, 128). One recent study on lymphoma using irreversible electroporation combined with anti-PD1 and TLR3/9 agonists indicated a sound treatment response by reversing the immunosuppressive TME, including the reduction in MDSCs (65). PIC combined with low-dose cisplatin showed better drug tolerance in treating oral squamous cell carcinoma and enhanced antitumor effects (129). Reovirus has been actively studied as an oncolytic virus. Its antitumor immune effect is also considered to be closely related to TLR. When reovirus was internalized by MDSCs, the expression of TLR3 on MDSCs was significantly upregulated, accompanied by a decrease in immunosuppressive ability (130).
TLR4
HMGB1, as a DAMP released by cancer cells, can efficiently activate TLR4. Blocking HMGB1 led to remodeling of the TME, including a marked reduction in MDSCs. Although blocking HMGB1 alone did not improve survival, it enhanced the response to anti-PD1 therapy (131). Another study showed that, in lung cancer, tumor-dependent complement 5a increases the expression of the HMGB1 receptors TLR4 and RAGE (advanced glycation end products) on PMN-MDSCs, promoting their neutrophil extracellular trap (NET) generation. NETs encapsulate and shelter circulating tumor cells from attack by immune cells to support tumor metastasis in lung cancer. When the release of HMGB1 was inhibited using glycyrrhizin, NET formation was significantly reduced (132). The coculture of melanoma-derived extracellular vesicles with IMCs transformed them into immunosuppressive MDSCs with upregulated PD-L1 expression in vitro. However, when melanoma-derived EVs were added to TLR4-knockout IMCs, the upregulation of PD-L1 was blocked (98).
TLR5
Flagellin, a structural protein of the flagellum of bacteria, is the only known TLR5 ligand. An ongoing phase I study (NCT01527136) has shown that flagellin is well tolerated and relatively safe. Engated T cells with flagellin-secreting ability reshaped the TME by delivering the agonist locally at the tumor site and demonstrated good antitumor effects with reduced infiltration of MDSCs (133).
TLR7/8
TLR7/8 are the other hot targets among TLRs. R848, a classical TLR7/8 agonist, showed a profound influence on MDSCs. MDSCs isolated from the peripheral blood of breast cancer patients and stimulated with R848 showed a significantly reduced viability, while antigen-presenting cells such as dendritic cells and macrophages increased significantly (134). R848 injection in colon tumors induced a significant reduction in MDSCs in mice (69). Moonkyu Lee et al. also cultured MDSCs with R848 conditioned medium in vitro and confirmed their reduced expansion (135). R848 can even improve the drug resistance of oxaliplatin during the treatment of colon cancer, which promotes the differentiation of MDSCs into M1 macrophages, modifying the immunosuppressive TME and enhancing the antitumor effect of oxaliplatin (136). Other TLR7/8 agonist-related clinical trials are underway, such as the combination of cyclophosphamide and the TLR8 agonist VTX-2337 in the treatment of solid tumors (NCT02650635). A phase Ib clinical study combining neoadjuvant cetuximab and the TLR8 agonist R848 in head and neck cancer showed hampered MDSC suppressive signals in an NF-kB-mediated manner (137).
TLR9
The role of TLR9 in MDSCs is also controversial and may be relevant to cancer types. Synthetic unmethylated CpG oligodeoxynucleotide (ODN), a commonly utilized TLR9 receptor agonist, did not achieve the expected results in clinical trials when used alone; however, great results characterized by an increase in the antitumor T cell-mediated immune response have been achieved in a plethora of clinical trials in which CpG-ODN has been synergistically codelivered with other therapeutic approaches (40, 138, 139). Studies have shown that CpG can effectively block the inhibitory activity of MDSCs (67). In liver cancer, local application of the TLR agonist ODN2395 in tumors induced the differentiation of MDSCs into mature macrophages (140). When using CpG-siRNA conjugates that specifically silence STAT3 in TLR9-positive myeloid cells in mouse prostate cancer cells, the downstream STAT3 pathway activation blockage decreased the expression and activity of Arg-1, thus weakening the immunosuppression ability of MDSCs (141). In pancreatic cancer, CpG application leads to the recruitment of regulatory T cells and the proliferation of MDSCs by binding to TLR9 (124). Furthermore, treatment with ODN2395 and SD101 induced MDSC apoptosis and increased the M1/M2 macrophage ratio in an NFκB-dependent manner (140).
There are many PRRs worth investigating, such as the NLRP family, RLRs, and CLRs. However, even for the TLR family, what role it plays in MDSCs, when it needs to be stimulated, or when it needs to be blocked is still an open question. Regarding the widespread use of TLR-related drugs and their strong impact on the tumor immune microenvironment, in-depth related research is worthwhile.
Conclusions and perspectives
The wide range of PRRs affords a potentially unprecedented opportunity to arm the cancer immunotherapy arsenal. Their ability not only to link innate and adaptive immunity but also to shape numerous aspects of the immune response in the tumor microenvironment, such as the modulation of cancer cells themselves, makes PRR-targeting drugs continuously growing. Most studies on the PRR family have used TLR agonists in clinical trials. Combining PRR agonists with conventional cancer therapies showed enhanced antitumor efficacy (Figure 3). Motolimod in combination with chemotherapy produced clinical benefits in ovarian cancer and HPV-positive head and neck squamous cell carcinoma patients (142, 143) (Table 2). Moreover, TLR9 agonists combined with targeted therapy, such as the small molecule kinase inhibitor cetuximab, showed good tolerability, although there was no incremental clinical efficacy in SCCHN patients (144), suggesting that more studies are required to achieve final conclusions. Ionizing radiation can activate the cGAS/STING pathway to trigger tumor antigen uptake and cross-presentation, inducing antitumor T cell immunity, and several clinical trials are currently testing the efficacy of radiation therapy in combination with TLR agonists (145) (Table 2). Similarly, tumor vaccination together with the TLR4 agonist GLA (glucopyranosyl) proved to have good tolerability in melanoma patients, and the clinical results will soon be disclosed (146). Other combinations (IFN-γ, Poly I:C plus STING agonists) are still in the preclinical testing stage and have shown promising results (147).
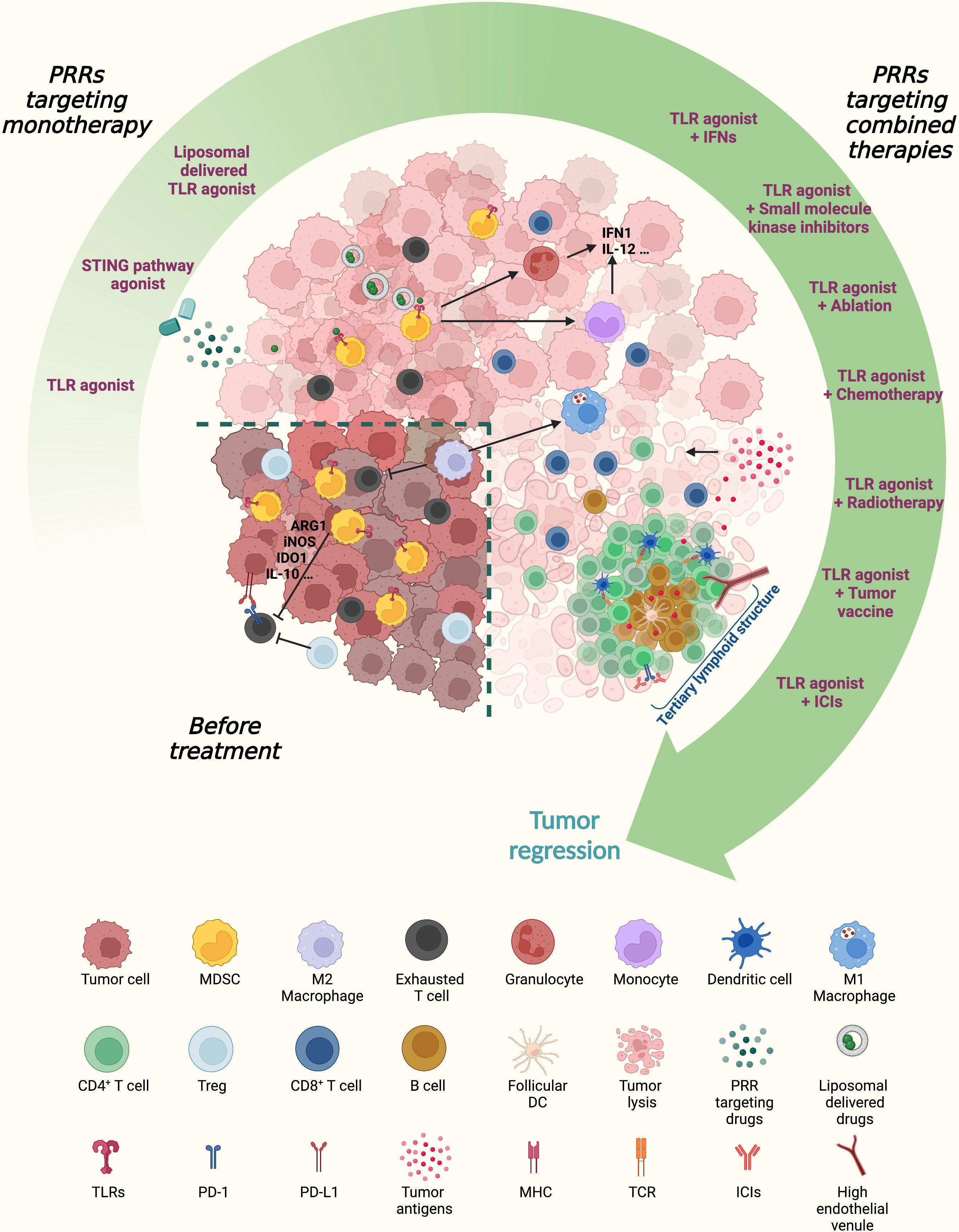
Figure 3 Next-generation PRR-based therapeutic approaches for cancer patients. PRR-targeting agonists, especially when combined with anti-inflammatory and/or immunostimulatory agents, can switch an immunosuppressive tumor microenvironment (TME) hostile for T cell trafficking and fitness toward an immunogenic milieu. PRR agonists promote MDSC maturation into inflammatory monocytes and granulocytes and increase the M1/M2 macrophage ratio. Combining PRR-targeting therapies with classic immunotherapy might support the establishment of TLSs containing a CD3+ T cell zone with mature dendritic cells (DCs) and a germinal center with B cells and follicular DCs. The coordinated actions of tumor antigen presentation through DCs and cytotoxic effector T cells and B cells enable the in situ priming and sustainment of antitumor adaptive immunity. MDSCs, myeloid-derived suppressor cells; DCs, dendritic cells; Treg, regulatory T cell; PRRs, pattern recognition receptors; TLR, Toll-like receptor; STING, stimulator of interferon genes; IFN, interferon; ICIs, immune checkpoint inhibitors; Arg1, arginase 1; iNOS, inducible nitric oxide synthase; IDO1, indoleamine 2,3-dioxygenase 1; PD1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; TLS, tertiary lymphoid structure; MHC, major histocompatibility complex; TCR, T cell receptor.
At present, the greatest enthusiasm is for the association of TLR agonists with immune checkpoint-targeted inhibitors (such as anti-CTLA-4 or anti-PD-L1 antibodies), as what emerged from the ongoing study evaluating the efficacy of the intratumoral TLR9 agonist tilsotolimod (IMO-2125) in combination with ICIs such as ipilimumab and nivolumab in different solid tumors (ILLUMINATE-206). TLR9 agonists in combination with nivolumab have already shown prolonged disease control in advanced NSCLC patients (NCT03326752). The TLR4 agonist GSK1795091 plus immunotherapy [GSK3174998 (anti‐OX40 monoclonal antibody), GSK3359609 (anti‐ICOS monoclonal antibody), or pembrolizumab] showed good safety performance in patients with solid tumors (148) (Table 2 and Figure 3).
Moreover, the continuous understanding of the immune regulation mechanism of PRRs along with the burgeoning of nanotechnology has improved targeting and therapeutic effects in fighting tumor immune escape (Figure 3). A tumor-targeting liposomal formulation (PCL8-U75) that arouses cytotoxic effects, particularly depleting MDSCs and tumor-associated macrophages in the TME, has been investigated in combination with immune-activating therapies such as the TLR7 agonist R848, which showed a potent antitumoral effect (149). Remarkably, the growing development of proper nanosized materials that can protect biological ligands from degradation may represent the future golden age in cancer therapy to deliver PRR agonists directly into cancer cells, harnessing not only the role of PRRs in immune regulation but also their direct effect on tumors.
Notably, it has been reported that TLR9 agonists together with anti-PD-1 therapy can induce the neogenesis of tertiary lymphoid structures (TLSs) in mouse NSCLC tumors, which are composed of a well-organized lymphoid framework including B cells, DCs, and T cells in close contact adjacent within the TME (150). TLSs function as tumor-adjacent lymphoid sites for the priming and maintenance of adaptive humoral and cell-mediated responses against tumor antigens (151). TLS establishment in the TME correlates with long-term survival and a better response to immunotherapy in multiple human solid cancers (152, 153). TLR agonists in combination with ICIs can altogether sculpt the TME toward an antitumor milieu, thus enhancing ICI efficacy (Figure 3).
Furthermore, new technologies such as transcriptomic profiling, which have recently emerged in the field of precision medicine, can provide opportunities to gain insight into the complexity of the TME, where PRR plays a crucial role. Recently, an accessible multiomics platform that is able to classify and visualize the whole tumor composition, integrating transcriptomic and genomic data, has been developed. Their transcriptomic analysis of more than 10,000 cancer patients portrayed four distinct TME subtypes conserved across 20 different cancers, which correlate with patient response to immunotherapy in multiple cancers (154). More efforts should be put into unpuzzling the PRR complexity in immune cells to unveil the arrangement of how immune cells choose to open or close their “gates” of environmental recognition in response to stimuli in pathological contexts.
Collectively, MDSC PRRs have a key role in the TME-modulating process, and growing evidence on their regulatory proinflammatory and antitumor mechanisms combined with the development of novel analytic strategies, such as spatial transcriptomic analysis and precise refinement of the timing and method of administration of drug therapies based on PRRs, fuels promising anticancer therapeutic applications.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the PRIN programs of the Italian Ministry of University and Research (MUR, PI: SU; CUP: B38D19000140006, PI: FDS; CUP: B39J22001200001), Associazione Italiana per la Ricerca sul Cancro (AIRC, PI: SU; Project: 21509), Fondazione Cariverona (PI: SU; Enact project), and PNRR programs of the Italian MUR (Project “National Center for Gene Therapy and Drugs Based on RNA Technology”, application code CN00000041, Mission 4, Component 2 Investment 1.4, funded from the European Union - NextGenerationEU, MUR Directorial Decree No. 1035 of 17 June 2022, CUP B33C22000630001; PI: SU).
Acknowledgments
We thank Prof. Vincenzo Bronte and all components of the Immunology section at the University of Verona (Bronte lab; #Immunologia1) for providing feedback on this manuscript. We would like to thank Biorender for providing a platform for creative artwork.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers (2016) 2:16045. doi: 10.1038/nrdp.2016.45
2. Bost P, De Sanctis F, Cane S, Ugel S, Donadello K, Castellucci M, et al. Deciphering the state of immune silence in fatal COVID-19 patients. Nat Commun (2021) 12(1):1428. doi: 10.1038/s41467-021-21702-6
3. Schulte-Schrepping J, Reusch N, Paclik D, Bassler K, Schlickeiser S, Zhang B, et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell (2020) 182(6):1419–40.e23. doi: 10.1016/j.cell.2020.08.001
4. Pawelec G, Verschoor CP, Ostrand-Rosenberg S. Myeloid-derived suppressor cells: Not only in tumor immunity. Front Immunol (2019) 10:1099. doi: 10.3389/fimmu.2019.01099
5. Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol (2018) 19(2):108–19. doi: 10.1038/s41590-017-0022-x
6. Hofer F, Di Sario G, Musiu C, Sartoris S, De Sanctis F, Ugel S. A complex metabolic network confers immunosuppressive functions to myeloid-derived suppressor cells (MDSCs) within the tumour microenvironment. Cells (2021) 10(10):1–34. doi: 10.3390/cells10102700
7. Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun (2016) 7:12150. doi: 10.1038/ncomms12150
8. Li D, Wu M. Pattern recognition receptors in health and diseases. Signal Transduct Target Ther (2021) 6(1):291. doi: 10.1038/s41392-021-00687-0
9. Hoden B, DeRubeis D, Martinez-Moczygemba M, Ramos KS, Zhang D. Understanding the role of toll-like receptors in lung cancer immunity and immunotherapy. Front Immunol (2022) 13:1033483. doi: 10.3389/fimmu.2022.1033483
10. Coley WB. II. contribution to the knowledge of sarcoma. Ann Surg (1891) 14(3):199–220. doi: 10.1097/00000658-189112000-00015
11. De Sanctis F, Sandri S, Martini M, Mazzocco M, Fiore A, Trovato R, et al. Hyperthermic treatment at 56 degrees c induces tumour-specific immune protection in a mouse model of prostate cancer in both prophylactic and therapeutic immunization regimens. Vaccine (2018) 36(25):3708–16. doi: 10.1016/j.vaccine.2018.05.010
12. Hernandez C, Huebener P, Schwabe RF. Damage-associated molecular patterns in cancer: a double-edged sword. Oncogene (2016) 35(46):5931–41. doi: 10.1038/onc.2016.104
13. Man SM, Jenkins BJ. Context-dependent functions of pattern recognition receptors in cancer. Nat Rev Cancer (2022) 22(7):397–413. doi: 10.1038/s41568-022-00462-5
14. Dooyema SDR, Noto JM, Wroblewski LE, Piazuelo MB, Krishna U, Suarez G, et al. Helicobacter pylori actively suppresses innate immune nucleic acid receptors. Gut Microbes (2022) 14(1):2105102. doi: 10.1080/19490976.2022.2105102
15. Shehab M, Sherri N, Hussein H, Salloum N, Rahal EA. Endosomal toll-like receptors mediate enhancement of interleukin-17A production triggered by Epstein-Barr virus DNA in mice. J Virol (2019) 93(20):1–12. doi: 10.1128/JVI.00987-19
16. Hasan UA, Zannetti C, Parroche P, Goutagny N, Malfroy M, Roblot G, et al. The human papillomavirus type 16 E7 oncoprotein induces a transcriptional repressor complex on the toll-like receptor 9 promoter. J Exp Med (2013) 210(7):1369–87. doi: 10.1084/jem.20122394
17. Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, et al. The toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science (2011) 332(6032):974–7. doi: 10.1126/science.1206095
18. Fan L, Xu C, Ge Q, Lin Y, Wong CC, Qi Y, et al. A. muciniphila suppresses colorectal tumorigenesis by inducing TLR2/NLRP3-mediated M1-like TAMs. Cancer Immunol Res (2021) 9(10):1111–24. doi: 10.1158/2326-6066.CIR-20-1019
19. Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity (2010) 32(6):790–802. doi: 10.1016/j.immuni.2010.05.010
20. Fiore A, Ugel S, De Sanctis F, Sandri S, Fracasso G, Trovato R, et al. Induction of immunosuppressive functions and NF-kappaB by FLIP in monocytes. Nat Commun (2018) 9(1):5193. doi: 10.1038/s41467-018-07654-4
21. De Sanctis F, Lamolinara A, Boschi F, Musiu C, Caligola S, Trovato R, et al. Interrupting the nitrosative stress fuels tumor-specific cytotoxic T lymphocytes in pancreatic cancer. J Immunother Cancer (2022) 10(1):1–17. doi: 10.1136/jitc-2021-003549
22. Trovato R, Cane S, Petrova V, Sartoris S, Ugel S, De Sanctis F. The engagement between MDSCs and metastases: Partners in crime. Front Oncol (2020) 10:165. doi: 10.3389/fonc.2020.00165
23. Ugel S, De Sanctis F, Mandruzzato S, Bronte V. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest (2015) 125(9):3365–76. doi: 10.1172/JCI80006
24. De Sanctis F, Bronte V, Ugel S. Tumor-induced myeloid-derived suppressor cells. Microbiol Spectr (2016) 4(3):1–22. doi: 10.1128/microbiolspec.MCHD-0016-2015
25. Sade-Feldman M, Kanterman J, Klieger Y, Ish-Shalom E, Olga M, Saragovi A, et al. Clinical significance of circulating CD33+CD11b+HLA-DR- myeloid cells in patients with stage IV melanoma treated with ipilimumab. Clin Cancer Res (2016) 22(23):5661–72. doi: 10.1158/1078-0432.CCR-15-3104
26. Trovato R, Fiore A, Sartori S, Cane S, Giugno R, Cascione L, et al. Immunosuppression by monocytic myeloid-derived suppressor cells in patients with pancreatic ductal carcinoma is orchestrated by STAT3. J Immunother Cancer (2019) 7(1):255. doi: 10.1186/s40425-019-0734-6
27. Abdelfattah N, Kumar P, Wang C, Leu JS, Flynn WF, Gao R, et al. Single-cell analysis of human glioma and immune cells identifies S100A4 as an immunotherapy target. Nat Commun (2022) 13(1):767. doi: 10.1038/s41467-022-28372-y
28. Idorn M, Kollgaard T, Kongsted P, Sengelov L, Thor Straten P. Correlation between frequencies of blood monocytic myeloid-derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration-resistant metastatic prostate cancer. Cancer Immunol Immunother (2014) 63(11):1177–87. doi: 10.1007/s00262-014-1591-2
29. Huang A, Zhang B, Wang B, Zhang F, Fan KX, Guo YJ. Increased CD14(+)HLA-DR (-/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol Immunother (2013) 62(9):1439–51. doi: 10.1007/s00262-013-1450-6
30. Palazon-Carrion N, Jimenez-Cortegana C, Sanchez-Leon ML, Henao-Carrasco F, Nogales-Fernandez E, Chiesa M, et al. Circulating immune biomarkers in peripheral blood correlate with clinical outcomes in advanced breast cancer. Sci Rep (2021) 11(1):14426. doi: 10.1038/s41598-021-93838-w
31. Grover A, Sanseviero E, Timosenko E, Gabrilovich DI. Myeloid-derived suppressor cells: A propitious road to clinic. Cancer Discovery (2021) 11(11):2693–706. doi: 10.1158/2159-8290.CD-21-0764
32. Martens A, Wistuba-Hamprecht K, Geukes Foppen M, Yuan J, Postow MA, Wong P, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res (2016) 22(12):2908–18. doi: 10.1158/1078-0432.CCR-15-2412
33. Koh J, Kim Y, Lee KY, Hur JY, Kim MS, Kim B, et al. MDSC subtypes and CD39 expression on CD8(+) T cells predict the efficacy of anti-PD-1 immunotherapy in patients with advanced NSCLC. Eur J Immunol (2020) 50(11):1810–9. doi: 10.1002/eji.202048534
34. Wang J, Shirota Y, Bayik D, Shirota H, Tross D, Gulley JL, et al. Effect of TLR agonists on the differentiation and function of human monocytic myeloid-derived suppressor cells. J Immunol (2015) 194(9):4215–21. doi: 10.4049/jimmunol.1402004
35. Dang Y, Rutnam ZJ, Dietsch G, Lu H, Yang Y, Hershberg R, et al. TLR8 ligation induces apoptosis of monocytic myeloid-derived suppressor cells. J Leukoc Biol (2018) 103(1):157–64. doi: 10.1002/JLB.5AB0217-070R
36. Medzhitov R, Janeway CA Jr. Innate immunity: impact on the adaptive immune response. Curr Opin Immunol (1997) 9(1):4–9. doi: 10.1016/s0952-7915(97)80152-5
37. Fang H, Ang B, Xu X, Huang X, Wu Y, Sun Y, et al. TLR4 is essential for dendritic cell activation and anti-tumor T-cell response enhancement by DAMPs released from chemically stressed cancer cells. Cell Mol Immunol (2014) 11(2):150–9. doi: 10.1038/cmi.2013.59
38. Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol (2004) 4(7):499–511. doi: 10.1038/nri1391
39. Miller CL, Sagiv-Barfi I, Neuhofer P, Czerwinski DK, Artandi SE, Bertozzi CR, et al. Systemic delivery of a targeted synthetic immunostimulant transforms the immune landscape for effective tumor regression. Cell Chem Biol (2022) 29(3):451–62.e8. doi: 10.1016/j.chembiol.2021.10.012
40. Fitzgerald KA, Kagan JC. Toll-like receptors and the control of immunity. Cell (2020) 180(6):1044–66. doi: 10.1016/j.cell.2020.02.041
41. Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med (2013) 45(12):e66. doi: 10.1038/emm.2013.97
42. Zhou H, Jiang M, Yuan H, Ni W, Tai G. Dual roles of myeloid-derived suppressor cells induced by toll-like receptor signaling in cancer. Oncol Lett (2021) 21(2):149. doi: 10.3892/ol.2020.12410
43. Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity (2010) 32(3):305–15. doi: 10.1016/j.immuni.2010.03.012
44. Jang GY, Lee JW, Kim YS, Lee SE, Han HD, Hong KJ, et al. Interactions between tumor-derived proteins and toll-like receptors. Exp Mol Med (2020) 52(12):1926–35. doi: 10.1038/s12276-020-00540-4
45. Shime H, Maruyama A, Yoshida S, Takeda Y, Matsumoto M, Seya T. Toll-like receptor 2 ligand and interferon-gamma suppress anti-tumor T cell responses by enhancing the immunosuppressive activity of monocytic myeloid-derived suppressor cells. Oncoimmunology (2017) 7(1):e1373231. doi: 10.1080/2162402X.2017.1373231
46. Hangai S, Kawamura T, Kimura Y, Chang CY, Hibino S, Yamamoto D, et al. Orchestration of myeloid-derived suppressor cells in the tumor microenvironment by ubiquitous cellular protein TCTP released by tumor cells. Nat Immunol (2021) 22(8):947–57. doi: 10.1038/s41590-021-00967-5
47. Maruyama A, Shime H, Takeda Y, Azuma M, Matsumoto M, Seya T. Pam2 lipopeptides systemically increase myeloid-derived suppressor cells through TLR2 signaling. Biochem Biophys Res Commun (2015) 457(3):445–50. doi: 10.1016/j.bbrc.2015.01.011
48. Deng Y, Yang J, Qian J, Liu R, Huang E, Wang Y, et al. TLR1/TLR2 signaling blocks the suppression of monocytic myeloid-derived suppressor cell by promoting its differentiation into M1-type macrophage. Mol Immunol (2019) 112:266–73. doi: 10.1016/j.molimm.2019.06.006
49. Liu Y, Zhang L, Zhu X, Wang Y, Liu W, Gong W. Polysaccharide agaricus blazei murill stimulates myeloid derived suppressor cell differentiation from M2 to M1 type, which mediates inhibition of tumour immune-evasion via the toll-like receptor 2 pathway. Immunology (2015) 146(3):379–91. doi: 10.1111/imm.12508
50. Yu YH, Liao CC, Hsu WH, Chen HJ, Liao WC, Muo CH, et al. Increased lung cancer risk among patients with pulmonary tuberculosis: a population cohort study. J Thorac Oncol (2011) 6(1):32–7. doi: 10.1097/JTO.0b013e3181fb4fcc
51. John V, Kotze LA, Ribechini E, Walzl G, Du Plessis N, Lutz MB. Caveolin-1 controls vesicular TLR2 expression, p38 signaling and T cell suppression in BCG infected murine monocytic myeloid-derived suppressor cells. Front Immunol (2019) 10:2826. doi: 10.3389/fimmu.2019.02826
52. Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonca LE, Pacis A, et al. BCG Educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell (2018) 172(1-2):176–90.e19. doi: 10.1016/j.cell.2017.12.031
53. Moorlag S, Rodriguez-Rosales YA, Gillard J, Fanucchi S, Theunissen K, Novakovic B, et al. BCG Vaccination induces long-term functional reprogramming of human neutrophils. Cell Rep (2020) 33(7):108387. doi: 10.1016/j.celrep.2020.108387
54. Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, et al. Bacille calmette-guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U.S.A. (2012) 109(43):17537–42. doi: 10.1073/pnas.1202870109
55. Huang M, Wu R, Chen L, Peng Q, Li S, Zhang Y, et al. S100A9 regulates MDSCs-mediated immune suppression via the RAGE and TLR4 signaling pathways in colorectal carcinoma. Front Immunol (2019) 10:2243. doi: 10.3389/fimmu.2019.02243
56. Liu H, Ling CC, Yeung WHO, Pang L, Liu J, Zhou J, et al. Monocytic MDSC mobilization promotes tumor recurrence after liver transplantation via CXCL10/TLR4/MMP14 signaling. Cell Death Dis (2021) 12(5):489. doi: 10.1038/s41419-021-03788-4
57. Li Q, Dai C, Xue R, Wang P, Chen L, Han Y, et al. S100A4 protects myeloid-derived suppressor cells from intrinsic apoptosis via TLR4-ERK1/2 signaling. Front Immunol (2018) 9:388. doi: 10.3389/fimmu.2018.00388
58. Song J, Lee J, Kim J, Jo S, Kim YJ, Baek JE, et al. Pancreatic adenocarcinoma up-regulated factor (PAUF) enhances the accumulation and functional activity of myeloid-derived suppressor cells (MDSCs) in pancreatic cancer. Oncotarget (2016) 7(32):51840–53. doi: 10.18632/oncotarget.10123
59. He XY, Gong FY, Chen Y, Zhou Z, Gong Z, Gao XM. Calreticulin fragment 39-272 promotes B16 melanoma malignancy through myeloid-derived suppressor cells In vivo. Front Immunol (2017) 8:1306. doi: 10.3389/fimmu.2017.01306
60. Wang Y, Liu J, Yang X, Liu Y, Liu Y, Li Y, et al. Bacillus calmette-guerin and anti-PD-L1 combination therapy boosts immune response against bladder cancer. Onco Targets Ther (2018) 11:2891–9. doi: 10.2147/OTT.S165840
61. Ghochikyan A, Pichugin A, Bagaev A, Davtyan A, Hovakimyan A, Tukhvatulin A, et al. Targeting TLR-4 with a novel pharmaceutical grade plant derived agonist, Immunomax(R), as a therapeutic strategy for metastatic breast cancer. J Transl Med (2014) 12:322. doi: 10.1186/s12967-014-0322-y
62. Zhou XY, Gao R, Hu J, Gao DP, Liao YL, Yang JJ. Trained innate immunity by repeated low-dose lipopolysaccharide injections displays long-term neuroprotective effects. Mediators Inflammation (2020) 2020:8191079. doi: 10.1155/2020/8191079
63. De Zuani M, Dal Secco C, Tonon S, Arzese A, Pucillo CEM, Frossi B. LPS guides distinct patterns of training and tolerance in mast cells. Front Immunol (2022) 13:835348. doi: 10.3389/fimmu.2022.835348
64. Jentho E, Ruiz-Moreno C, Novakovic B, Kourtzelis I, Megchelenbrink WL, Martins R, et al. Trained innate immunity, long-lasting epigenetic modulation, and skewed myelopoiesis by heme. Proc Natl Acad Sci U.S.A. (2021) 118(42):1–10. doi: 10.1073/pnas.2102698118
65. Babikr F, Wan J, Xu A, Wu Z, Ahmed S, Freywald A, et al. Distinct roles but cooperative effect of TLR3/9 agonists and PD-1 blockade in converting the immunotolerant microenvironment of irreversible electroporation-ablated tumors. Cell Mol Immunol (2021) 18(12):2632–47. doi: 10.1038/s41423-021-00796-4
66. Shime H, Matsumoto M, Seya T. Double-stranded RNA promotes CTL-independent tumor cytolysis mediated by CD11b(+)Ly6G(+) intratumor myeloid cells through the TICAM-1 signaling pathway. Cell Death Differ (2017) 24(3):385–96. doi: 10.1038/cdd.2016.131
67. Zoglmeier C, Bauer H, Noerenberg D, Wedekind G, Bittner P, Sandholzer N, et al. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin Cancer Res (2011) 17(7):1765–75. doi: 10.1158/1078-0432.CCR-10-2672
68. Xu X, Jin Z, Liu Y, Gong H, Sun Q, Zhang W, et al. Carbohydrate-based adjuvants activate tumor-specific Th1 and CD8(+) T-cell responses and reduce the immunosuppressive activity of MDSCs. Cancer Lett (2019) 440-441:94–105. doi: 10.1016/j.canlet.2018.10.013
69. Spinetti T, Spagnuolo L, Mottas I, Secondini C, Treinies M, Ruegg C, et al. TLR7-based cancer immunotherapy decreases intratumoral myeloid-derived suppressor cells and blocks their immunosuppressive function. Oncoimmunology (2016) 5(11):e1230578. doi: 10.1080/2162402X.2016.1230578
70. Luo W, Napoleon JV, Zhang F, Lee YG, Wang B, Putt KS, et al. Repolarization of tumor-infiltrating myeloid cells for augmentation of CAR T cell therapies. Front Immunol (2022) 13:816761. doi: 10.3389/fimmu.2022.816761
71. Kim YK, Shin JS, Nahm MH. NOD-like receptors in infection, immunity, and diseases. Yonsei Med J (2016) 57(1):5–14. doi: 10.3349/ymj.2016.57.1.5
72. Zhong Y, Kinio A, Saleh M. Functions of NOD-like receptors in human diseases. Front Immunol (2013) 4:333. doi: 10.3389/fimmu.2013.00333
73. Maisonneuve C, Tsang DKL, Foerster EG, Robert LM, Mukherjee T, Prescott D, et al. Nod1 promotes colorectal carcinogenesis by regulating the immunosuppressive functions of tumor-infiltrating myeloid cells. Cell Rep (2021) 34(4):108677. doi: 10.1016/j.celrep.2020.108677
74. Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, et al. Chemotherapy-triggered cathepsin b release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med (2013) 19(1):57–64. doi: 10.1038/nm.2999
75. Theivanthiran B, Evans KS, DeVito NC, Plebanek M, Sturdivant M, Wachsmuth LP, et al. A tumor-intrinsic PD-L1/NLRP3 inflammasome signaling pathway drives resistance to anti-PD-1 immunotherapy. J Clin Invest (2020) 130(5):2570–86. doi: 10.1172/JCI133055
76. Tengesdal IW, Menon DR, Osborne DG, Neff CP, Powers NE, Gamboni F, et al. Targeting tumor-derived NLRP3 reduces melanoma progression by limiting MDSCs expansion. Proc Natl Acad Sci U.S.A. (2021) 118(10):1–11. doi: 10.1073/pnas.2000915118
77. Tengesdal IW, Dinarello A, Powers NE, Burchill MA, Joosten LAB, Marchetti C, et al. Tumor NLRP3-derived IL-1beta drives the IL-6/STAT3 axis resulting in sustained MDSC-mediated immunosuppression. Front Immunol (2021) 12:661323. doi: 10.3389/fimmu.2021.661323
78. Lu F, Zhao Y, Pang Y, Ji M, Sun Y, Wang H, et al. NLRP3 inflammasome upregulates PD-L1 expression and contributes to immune suppression in lymphoma. Cancer Lett (2021) 497:178–89. doi: 10.1016/j.canlet.2020.10.024
79. van Deventer HW, Burgents JE, Wu QP, Woodford RM, Brickey WJ, Allen IC, et al. The inflammasome component NLRP3 impairs antitumor vaccine by enhancing the accumulation of tumor-associated myeloid-derived suppressor cells. Cancer Res (2010) 70(24):10161–9. doi: 10.1158/0008-5472.CAN-10-1921
80. Barral PM, Sarkar D, Su ZZ, Barber GN, DeSalle R, Racaniello VR, et al. Functions of the cytoplasmic RNA sensors RIG-I and MDA-5: key regulators of innate immunity. Pharmacol Ther (2009) 124(2):219–34. doi: 10.1016/j.pharmthera.2009.06.012
81. Metzger P, Kirchleitner SV, Kluge M, Koenig LM, Horth C, Rambuscheck CA, et al. Immunostimulatory RNA leads to functional reprogramming of myeloid-derived suppressor cells in pancreatic cancer. J Immunother Cancer (2019) 7(1):288. doi: 10.1186/s40425-019-0778-7
82. Flugel CL, Majzner RG, Krenciute G, Dotti G, Riddell SR, Wagner DL, et al. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat Rev Clin Oncol (2022) 20(1):49–62. doi: 10.1038/s41571-022-00704-3
83. Johnson LR, Lee DY, Eacret JS, Ye D, June CH, Minn AJ. The immunostimulatory RNA RN7SL1 enables CAR-T cells to enhance autonomous and endogenous immune function. Cell (2021) 184(19):4981–95.e14. doi: 10.1016/j.cell.2021.08.004
84. Freeman SA, Grinstein S. Phagocytosis: Receptors, signal integration, and the cytoskeleton. Immunol Rev (2014) 262(1):193–215. doi: 10.1111/imr.12212
85. Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol (2016) 1(2):1–32. doi: 10.1126/sciimmunol.aaf8943
86. Zhang Z, Zheng Y, Chen Y, Yin Y, Chen Y, Chen Q, et al. Gut fungi enhances immunosuppressive function of myeloid-derived suppressor cells by activating PKM2-dependent glycolysis to promote colorectal tumorigenesis. Exp Hematol Oncol (2022) 11(1):88. doi: 10.1186/s40164-022-00334-6
87. Qu J, Liu L, Xu Q, Ren J, Xu Z, Dou H, et al. CARD9 prevents lung cancer development by suppressing the expansion of myeloid-derived suppressor cells and IDO production. Int J Cancer (2019) 145(8):2225–37. doi: 10.1002/ijc.32355
88. Tian J, Ma J, Ma K, Guo H, Baidoo SE, Zhang Y, et al. Beta-glucan enhances antitumor immune responses by regulating differentiation and function of monocytic myeloid-derived suppressor cells. Eur J Immunol (2013) 43(5):1220–30. doi: 10.1002/eji.201242841
89. Albeituni SH, Ding C, Liu M, Hu X, Luo F, Kloecker G, et al. Yeast-derived particulate beta-glucan treatment subverts the suppression of myeloid-derived suppressor cells (MDSC) by inducing polymorphonuclear MDSC apoptosis and monocytic MDSC differentiation to APC in cancer. J Immunol (2016) 196(5):2167–80. doi: 10.4049/jimmunol.1501853
90. Bekkering S, Arts RJW, Novakovic B, Kourtzelis I, van der Heijden C, Li Y, et al. Metabolic induction of trained immunity through the mevalonate pathway. Cell (2018) 172(1-2):135–46.e9. doi: 10.1016/j.cell.2017.11.025
91. Chavakis T, Mitroulis I, Hajishengallis G. Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation. Nat Immunol (2019) 20(7):802–11. doi: 10.1038/s41590-019-0402-5
92. Kalafati L, Kourtzelis I, Schulte-Schrepping J, Li X, Hatzioannou A, Grinenko T, et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell (2020) 183(3):771–85.e12. doi: 10.1016/j.cell.2020.09.058
93. Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-kappaB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther (2020) 5(1):209. doi: 10.1038/s41392-020-00312-6
94. Porta C, Consonni FM, Morlacchi S, Sangaletti S, Bleve A, Totaro MG, et al. Tumor-derived prostaglandin E2 promotes p50 NF-kappaB-Dependent differentiation of monocytic MDSCs. Cancer Res (2020) 80(13):2874–88. doi: 10.1158/0008-5472.CAN-19-2843
95. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol (2010) 11(5):373–84. doi: 10.1038/ni.1863
96. Bhattarai S, Li Q, Ding J, Liang F, Gusev E, Lapohos O, et al. TLR4 is a regulator of trained immunity in a murine model of duchenne muscular dystrophy. Nat Commun (2022) 13(1):879. doi: 10.1038/s41467-022-28531-1
97. Cherfils-Vicini J, Iltis C, Cervera L, Pisano S, Croce O, Sadouni N, et al. Cancer cells induce immune escape via glycocalyx changes controlled by the telomeric protein TRF2. EMBO J (2019) 38(11):1–20. doi: 10.15252/embj.2018100012
98. Fleming V, Hu X, Weller C, Weber R, Groth C, Riester Z, et al. Melanoma extracellular vesicles generate immunosuppressive myeloid cells by upregulating PD-L1 via TLR4 signaling. Cancer Res (2019) 79(18):4715–28. doi: 10.1158/0008-5472.CAN-19-0053
99. Gao Y, Xu H, Li N, Wang H, Ma L, Chen S, et al. Renal cancer-derived exosomes induce tumor immune tolerance by MDSCs-mediated antigen-specific immunosuppression. Cell Commun Signal (2020) 18(1):106. doi: 10.1186/s12964-020-00611-z
100. Li N, Wang Y, Xu H, Wang H, Gao Y, Zhang Y. Exosomes derived from RM-1 cells promote the recruitment of MDSCs into tumor microenvironment by upregulating CXCR4 via TLR2/NF-kappaB pathway. J Oncol (2021) 2021:5584406. doi: 10.1155/2021/5584406
101. Tohumeken S, Baur R, Bottcher M, Stoll A, Loschinski R, Panagiotidis K, et al. Palmitoylated proteins on AML-derived extracellular vesicles promote myeloid-derived suppressor cell differentiation via TLR2/Akt/mTOR signaling. Cancer Res (2020) 80(17):3663–76. doi: 10.1158/0008-5472.CAN-20-0024
102. Jiang S, Ma J, Li Y, Lu B, Du J, Xu J, et al. A polysaccharide from native curcuma kwangsiensis and its mechanism of reversing MDSC-induced suppressive function. Carbohydr Polym (2022) 297:120020. doi: 10.1016/j.carbpol.2022.120020
103. Wen X, Zheng P, Ma Y, Ou Y, Huang W, Li S, et al. Salutaxel, a conjugate of docetaxel and a muramyl dipeptide (MDP) analogue, acts as multifunctional prodrug that inhibits tumor growth and metastasis. J Med Chem (2018) 61(4):1519–40. doi: 10.1021/acs.jmedchem.7b01407
104. Yang Y, Zhang R, Xia F, Zou T, Huang A, Xiong S, et al. LPS converts gr-1(+)CD115(+) myeloid-derived suppressor cells from M2 to M1 via P38 MAPK. Exp Cell Res (2013) 319(12):1774–83. doi: 10.1016/j.yexcr.2013.05.007
105. Yu J, Li H, Zhang Z, Lin W, Wei X, Shao B. Targeting the MDSCs of tumors In situ with inhibitors of the MAPK signaling pathway to promote tumor regression. Front Oncol (2021) 11:647312. doi: 10.3389/fonc.2021.647312
106. Xu W, Dong J, Zheng Y, Zhou J, Yuan Y, Ta HM, et al. Immune-checkpoint protein VISTA regulates antitumor immunity by controlling myeloid cell-mediated inflammation and immunosuppression. Cancer Immunol Res (2019) 7(9):1497–510. doi: 10.1158/2326-6066.CIR-18-0489
107. Facciabene A, De Sanctis F, Pierini S, Reis ES, Balint K, Facciponte J, et al. Local endothelial complement activation reverses endothelial quiescence, enabling t-cell homing, and tumor control during t-cell immunotherapy. Oncoimmunology (2017) 6(9):e1326442. doi: 10.1080/2162402X.2017.1326442
108. Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, et al. Modulation of the antitumor immune response by complement. Nat Immunol (2008) 9(11):1225–35. doi: 10.1038/ni.1655
109. Wang N, Tan HY, Lu Y, Chan YT, Wang D, Guo W, et al. PIWIL1 governs the crosstalk of cancer cell metabolism and immunosuppressive microenvironment in hepatocellular carcinoma. Signal Transduct Target Ther (2021) 6(1):86. doi: 10.1038/s41392-021-00485-8
110. Yu R, Zhu B, Chen D. Type I interferon-mediated tumor immunity and its role in immunotherapy. Cell Mol Life Sci (2022) 79(3):191. doi: 10.1007/s00018-022-04219-z
111. Sun Y, Han X, Shang C, Wang Y, Xu B, Jiang S, et al. The downregulation of type I IFN signaling in G-MDSCs under tumor conditions promotes their development towards an immunosuppressive phenotype. Cell Death Dis (2022) 13(1):36. doi: 10.1038/s41419-021-04487-w
112. Papafragkos I, Grigoriou M, Boon L, Kloetgen A, Hatzioannou A, Verginis P. Ablation of NLRP3 inflammasome rewires MDSC function and promotes tumor regression. Front Immunol (2022) 13:889075. doi: 10.3389/fimmu.2022.889075
113. Tengesdal IW, Li S, Powers NE, May M, Neff CP, Joosten LAB, et al. Activation of host-NLRP3 inflammasome in myeloid cells dictates response to anti-PD-1 therapy in metastatic breast cancers. Pharm (Basel) (2022) 15(5):1–13. doi: 10.3390/ph15050574
114. Liang M, Liu Y, Zhang Z, Yang H, Dai N, Zhang N, et al. Fusobacterium nucleatum induces MDSCs enrichment via activation the NLRP3 inflammosome in ESCC cells, leading to cisplatin resistance. Ann Med (2022) 54(1):989–1003. doi: 10.1080/07853890.2022.2061045
115. Sarhan D, Eisinger S, He F, Bergsland M, Pelicano C, Driescher C, et al. Targeting myeloid suppressive cells revives cytotoxic anti-tumor responses in pancreatic cancer. iScience (2022) 25(11):105317. doi: 10.1016/j.isci.2022.105317
116. Dumont A, de Rosny C, Kieu TL, Perrey S, Berger H, Fluckiger A, et al. Docosahexaenoic acid inhibits both NLRP3 inflammasome assembly and JNK-mediated mature IL-1beta secretion in 5-fluorouracil-treated MDSC: implication in cancer treatment. Cell Death Dis (2019) 10(7):485. doi: 10.1038/s41419-019-1723-x
117. Fujiwara T, Healey J, Ogura K, Yoshida A, Kondo H, Hata T, et al. Role of tumor-associated macrophages in sarcomas. Cancers (Basel) (2021) 13(5):1–17. doi: 10.3390/cancers13051086
118. Cheng H, Xu Q, Lu X, Yuan H, Li T, Zhang Y, et al. Activation of STING by cGAMP regulates MDSCs to suppress tumor metastasis via reversing epithelial-mesenchymal transition. Front Oncol (2020) 10:896. doi: 10.3389/fonc.2020.00896
119. Chandra D, Quispe-Tintaya W, Jahangir A, Asafu-Adjei D, Ramos I, Sintim HO, et al. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol Res (2014) 2(9):901–10. doi: 10.1158/2326-6066.CIR-13-0123
120. Mohamed E, Sierra RA, Trillo-Tinoco J, Cao Y, Innamarato P, Payne KK, et al. The unfolded protein response mediator PERK governs myeloid cell-driven immunosuppression in tumors through inhibition of STING signaling. Immunity (2020) 52(4):668–82.e7. doi: 10.1016/j.immuni.2020.03.004
121. Liau NPD, Laktyushin A, Lucet IS, Murphy JM, Yao S, Whitlock E, et al. The molecular basis of JAK/STAT inhibition by SOCS1. Nat Commun (2018) 9(1):1558. doi: 10.1038/s41467-018-04013-1
122. Zhang CX, Ye SB, Ni JJ, Cai TT, Liu YN, Huang DJ, et al. STING signaling remodels the tumor microenvironment by antagonizing myeloid-derived suppressor cell expansion. Cell Death Differ (2019) 26(11):2314–28. doi: 10.1038/s41418-019-0302-0
123. Zhang CX, Huang DJ, Baloche V, Zhang L, Xu JX, Li BW, et al. Galectin-9 promotes a suppressive microenvironment in human cancer by enhancing STING degradation. Oncogenesis (2020) 9(7):65. doi: 10.1038/s41389-020-00248-0
124. Zambirinis CP, Levie E, Nguy S, Avanzi A, Barilla R, Xu Y, et al. TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J Exp Med (2015) 212(12):2077–94. doi: 10.1084/jem.20142162
125. Owen AM, Fults JB, Patil NK, Hernandez A, Bohannon JK. TLR agonists as mediators of trained immunity: Mechanistic insight and immunotherapeutic potential to combat infection. Front Immunol (2020) 11:622614. doi: 10.3389/fimmu.2020.622614
126. Li S, Li F, Xu L, Liu X, Zhu X, Gao W, et al. TLR2 agonist promotes myeloid-derived suppressor cell polarization via Runx1 in hepatocellular carcinoma. Int Immunopharmacol (2022) 111:109168. doi: 10.1016/j.intimp.2022.109168
127. De Waele J, Marcq E, Van Audenaerde JR, Van Loenhout J, Deben C, Zwaenepoel K, et al. Poly(I:C) primes primary human glioblastoma cells for an immune response invigorated by PD-L1 blockade. Oncoimmunology (2018) 7(3):e1407899. doi: 10.1080/2162402X.2017.1407899
128. Kyi C, Roudko V, Sabado R, Saenger Y, Loging W, Mandeli J, et al. Therapeutic immune modulation against solid cancers with intratumoral poly-ICLC: A pilot trial. Clin Cancer Res (2018) 24(20):4937–48. doi: 10.1158/1078-0432.CCR-17-1866
129. Ding L, Ren J, Zhang D, Li Y, Huang X, Ji J, et al. The TLR3 agonist inhibit drug efflux and sequentially consolidates low-dose cisplatin-based chemoimmunotherapy while reducing side effects. Mol Cancer Ther (2017) 16(6):1068–79. doi: 10.1158/1535-7163.MCT-16-0454
130. Katayama Y, Tachibana M, Kurisu N, Oya Y, Terasawa Y, Goda H, et al. Oncolytic reovirus inhibits immunosuppressive activity of myeloid-derived suppressor cells in a TLR3-dependent manner. J Immunol (2018) 200(8):2987–99. doi: 10.4049/jimmunol.1700435
131. Hubert P, Roncarati P, Demoulin S, Pilard C, Ancion M, Reynders C, et al. Extracellular HMGB1 blockade inhibits tumor growth through profoundly remodeling immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. J Immunother Cancer (2021) 9(3):1–18. doi: 10.1136/jitc-2020-001966
132. Ortiz-Espinosa S, Morales X, Senent Y, Alignani D, Tavira B, Macaya I, et al. Complement C5a induces the formation of neutrophil extracellular traps by myeloid-derived suppressor cells to promote metastasis. Cancer Lett (2022) 529:70–84. doi: 10.1016/j.canlet.2021.12.027
133. Geng D, Kaczanowska S, Tsai A, Younger K, Ochoa A, Rapoport AP, et al. TLR5 ligand-secreting T cells reshape the tumor microenvironment and enhance antitumor activity. Cancer Res (2015) 75(10):1959–71. doi: 10.1158/0008-5472.CAN-14-2467
134. Safarzadeh E, Mohammadi A, Mansoori B, Duijf PHG, Hashemzadeh S, Khaze V, et al. STAT3 silencing and TLR7/8 pathway activation repolarize and suppress myeloid-derived suppressor cells from breast cancer patients. Front Immunol (2020) 11:613215. doi: 10.3389/fimmu.2020.613215
135. Lee M, Park CS, Lee YR, Im SA, Song S, Lee CK. Resiquimod, a TLR7/8 agonist, promotes differentiation of myeloid-derived suppressor cells into macrophages and dendritic cells. Arch Pharm Res (2014) 37(9):1234–40. doi: 10.1007/s12272-014-0379-4
136. Liu Z, Xie Y, Xiong Y, Liu S, Qiu C, Zhu Z, et al. TLR 7/8 agonist reverses oxaliplatin resistance in colorectal cancer via directing the myeloid-derived suppressor cells to tumoricidal M1-macrophages. Cancer Lett (2020) 469:173–85. doi: 10.1016/j.canlet.2019.10.020
137. Shayan G, Kansy BA, Gibson SP, Srivastava RM, Bryan JK, Bauman JE, et al. Phase ib study of immune biomarker modulation with neoadjuvant cetuximab and TLR8 stimulation in head and neck cancer to overcome suppressive myeloid signals. Clin Cancer Res (2018) 24(1):62–72. doi: 10.1158/1078-0432.CCR-17-0357
138. Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol (2010) 28(28):4324–32. doi: 10.1200/JCO.2010.28.9793
139. Goldinger SM, Dummer R, Baumgaertner P, Mihic-Probst D, Schwarz K, Hammann-Haenni A, et al. Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8(+) T-cell responses in melanoma patients. Eur J Immunol (2012) 42(11):3049–61. doi: 10.1002/eji.201142361
140. Ghosh CC, Heatherton KR, Connell KPO, Alexander IS, Greer DA, LaPorte J, et al. Regional infusion of a class c TLR9 agonist enhances liver tumor microenvironment reprogramming and MDSC reduction to improve responsiveness to systemic checkpoint inhibition. Cancer Gene Ther (2022) 29(12):1854–65. doi: 10.1038/s41417-022-00484-z
141. Hossain DM, Pal SK, Moreira D, Duttagupta P, Zhang Q, Won H, et al. TLR9-targeted STAT3 silencing abrogates immunosuppressive activity of myeloid-derived suppressor cells from prostate cancer patients. Clin Cancer Res (2015) 21(16):3771–82. doi: 10.1158/1078-0432.CCR-14-3145
142. Monk BJ, Facciabene A, Brady WE, Aghajanian CA, Fracasso PM, Walker JL, et al. Integrative development of a TLR8 agonist for ovarian cancer chemoimmunotherapy. Clin Cancer Res (2017) 23(8):1955–66. doi: 10.1158/1078-0432.CCR-16-1453
143. Ferris RL, Saba NF, Gitlitz BJ, Haddad R, Sukari A, Neupane P, et al. Effect of adding motolimod to standard combination chemotherapy and cetuximab treatment of patients with squamous cell carcinoma of the head and neck: The Active8 randomized clinical trial. JAMA Oncol (2018) 4(11):1583–8. doi: 10.1001/jamaoncol.2018.1888
144. Ruzsa A, Sen M, Evans M, Lee LW, Hideghety K, Rottey S, et al. Phase 2, open-label, 1:1 randomized controlled trial exploring the efficacy of EMD 1201081 in combination with cetuximab in second-line cetuximab-naive patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN). Invest New Drugs (2014) 32(6):1278–84. doi: 10.1007/s10637-014-0117-2
145. Walshaw RC, Honeychurch J, Choudhury A, Illidge TM. Toll-like receptor agonists and radiation therapy combinations: An untapped opportunity to induce anticancer immunity and improve tumor control. Int J Radiat Oncol Biol Phys (2020) 108(1):27–37. doi: 10.1016/j.ijrobp.2020.04.020
146. Grewal EP, Erskine CL, Nevala WK, Allred JB, Strand CA, Kottschade LA, et al. Peptide vaccine with glucopyranosyl lipid a-stable oil-in-water emulsion for patients with resected melanoma. Immunotherapy (2020) 12(13):983–95. doi: 10.2217/imt-2020-0085
147. He M, Soni B, Schwalie PC, Husser T, Waltzinger C, De Silva D, et al. Combinations of toll-like receptor 8 agonist TL8-506 activate human tumor-derived dendritic cells. J Immunother Cancer (2022) 10(6):1–16. doi: 10.1136/jitc-2021-004268
148. Steeghs N, Hansen AR, Hanna GJ, Garralda E, Park H, Strauss J, et al. Manufacturing-dependent change in biological activity of the TLR4 agonist GSK1795091 and implications for lipid a analog development. Clin Transl Sci (2022) 15(11):2625–39. doi: 10.1111/cts.13387
149. Ringgaard L, Melander F, Eliasen R, Henriksen JR, Jolck RI, Engel TB, et al. Tumor repolarization by an advanced liposomal drug delivery system provides a potent new approach for chemo-immunotherapy. Sci Adv (2020) 6(36):1–11. doi: 10.1126/sciadv.aba5628
150. Gallotta M, Assi H, Degagne E, Kannan SK, Coffman RL, Guiducci C. Inhaled TLR9 agonist renders lung tumors permissive to PD-1 blockade by promoting optimal CD4(+) and CD8(+) T-cell interplay. Cancer Res (2018) 78(17):4943–56. doi: 10.1158/0008-5472.CAN-18-0729
151. Johansson-Percival A, Ganss R. Therapeutic induction of tertiary lymphoid structures in cancer through stromal remodeling. Front Immunol (2021) 12:674375. doi: 10.3389/fimmu.2021.674375
152. Fridman WH, Meylan M, Petitprez F, Sun CM, Italiano A, Sautes-Fridman C. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat Rev Clin Oncol (2022) 19(7):441–57. doi: 10.1038/s41571-022-00619-z
153. Kang W, Feng Z, Luo J, He Z, Liu J, Wu J, et al. Tertiary lymphoid structures in cancer: The double-edged sword role in antitumor immunity and potential therapeutic induction strategies. Front Immunol (2021) 12:689270. doi: 10.3389/fimmu.2021.689270
Keywords: PRR, MDSC, TLRs, NLRs, cancer, immune therapy
Citation: Wang T, Hu Y, Dusi S, Qi F, Sartoris S, Ugel S and De Sanctis F (2023) "Open Sesame" to the complexity of pattern recognition receptors of myeloid-derived suppressor cells in cancer. Front. Immunol. 14:1130060. doi: 10.3389/fimmu.2023.1130060
Received: 22 December 2022; Accepted: 30 January 2023;
Published: 22 February 2023.
Edited by:
Sergej Tomić, Institute for the Application of Nuclear Energy (INEP), SerbiaReviewed by:
Иван Јовановић, University of Kragujevac, SerbiaSasa R. Vasilijic, Stanford University, United States
Copyright © 2023 Wang, Hu, Dusi, Qi, Sartoris, Ugel and De Sanctis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefano Ugel, c3RlZmFuby51Z2VsQHVuaXZyLml0
 Tian Wang
Tian Wang Yushu Hu
Yushu Hu Silvia Dusi
Silvia Dusi Stefano Ugel
Stefano Ugel Francesco De Sanctis
Francesco De Sanctis