- 1Division of Pediatrics, University Hospitals Cleveland Medical Center, Cleveland, OH, United States
- 2Division of Infectious Diseases, Rainbow Babies and Children’s Hospital, Cleveland, OH, United States
- 3School of Medicine and Department of Population and Quantitative Health Sciences, Case Western Reserve University, Cleveland, OH, United States
- 4School of Health and Rehabilitation Sciences, Ohio State University School of Health and Rehabilitation Sciences, Columbus, OH, United States
- 5Joint Clinical Research Centre, Kampala, Uganda
- 6Makerere University, Kampala, Uganda
Introduction: Perinatally acquired HIV infection (PHIV) occurs during a critical window of immune development. We investigated changes in systemic inflammation and immune activation in adolescents with PHIV and those without HIV (HIV-) in Uganda.
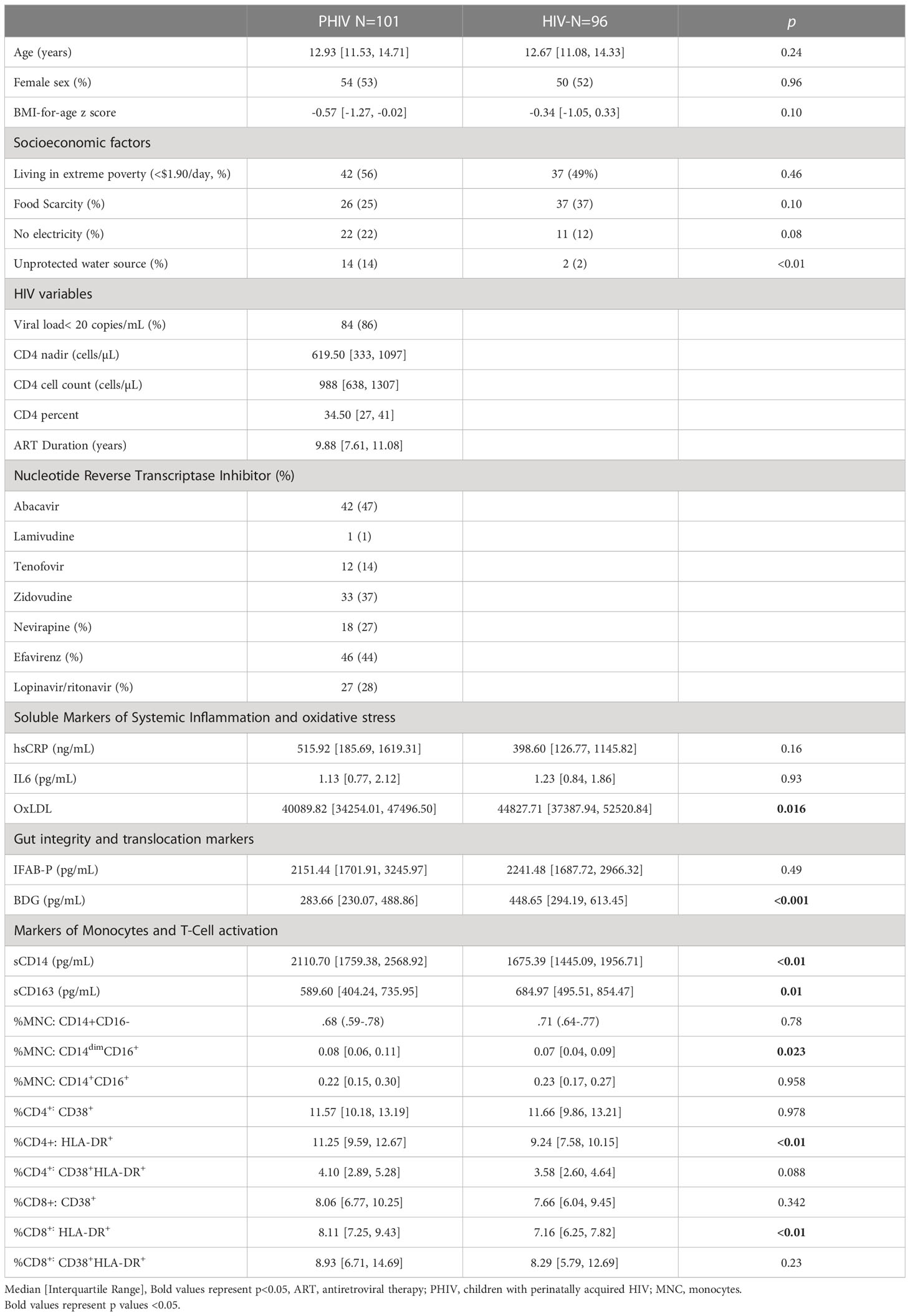
Methods: A prospective observational cohort study was performed in 2017-2021 in Uganda. All participants were between 10-18 years of age and without active co-infections. PHIVs were on ART with HIV-1 RNA level ≤400 copies/mL. We measured plasma and cellular markers of monocyte activation, T-cell activation (expression of CD38 and HLA-DR on CD4+ and CD8+), oxidized LDL, markers of gut integrity and fungal translocation. Groups were compared using Wilcoxon rank sum tests. Changes from baseline were examined with 97.5% confidence intervals on relative fold change. P values were adjusted for false discovery rate.
Results: We enrolled 101 PHIV and 96 HIV-; among these, 89 PHIV and 79 HIV- also had measurements at 96 weeks. At baseline, median (Q1, Q3) age was 13 yrs (11,15), and 52% were females. In PHIV, median CD4+ cell counts were 988 cells/µL (638, 1308), ART duration was 10 yrs (8, 11), and 85% had viral load <50 copies/mL throughout the study, 53% of participants had a regimen switch between visits, 85% of whom switched to 3TC, TDF and DTG. Over 96 weeks, while hsCRP decreased by 40% in PHIV (p=0.12), I-FABP and BDG both increased by 19 and 38% respectively (p=0.08 and ≤0.01) and did not change in HIV- (p≥0.33). At baseline, PHIVs had higher monocyte activation (sCD14) (p=0.01) and elevated frequencies of non-classical monocytes (p<0.01) compared to HIV- which remained stable over time in PHIV but increased by 34% and 80% respectively in HIV-. At both time points, PHIVs had higher T cell activation (p ≤ 0.03: CD4+/CD8+ T cells expressing HLA-DR and CD38). Only in PHIV, at both timepoints, oxidized LDL was inversely associated with activated T cells(p<0.01). Switching to dolutegravir at week 96 was significantly associated an elevated level of sCD163 (β=0.4, 95% CI=0.14,0.57, p<0.01), without changes in other markers.
Conclusion: Ugandan PHIV with viral suppression have some improvement in markers of inflammation over time, however T-cell activation remains elevated. Gut integrity and translocation worsened only in PHIV over time. A deeper understanding of the mechanisms causing immune activation in ART treated African PHIV is crucial.
Introduction
Adolescents are a growing population of interest in HIV and our understanding of ongoing immune dysregulation in this population is lacking. Wide access to antiretroviral therapy (ART) has transformed HIV from a fatal condition into a chronic disease. Despite the high prevalence of HIV in children living in sub-Saharan Africa, there are limited data on the activation status of immune cells in perinatally acquired HIV (PHIV) in the setting of ART. Perinatal HIV transmission refers to HIV transmission from mother to child during either pregnancy, labor and delivery, or breastfeeding and accounts for the majority of childhood HIV infections. With the universal recommendation for ART treatment, understanding the side effects of ART exposure and the immunologic perturbations that persist despite viral suppression in PHIV is paramount to reducing long-term complications as children age into early adulthood. There is a need for further studies in African PHIV and for exercising caution when extrapolating data from PHIV in the global North to PHIV in the global South.
We have previously assessed the consequences of PHIV and its treatment with ART in a cohort of adolescents in Uganda (1–5) and have reported significant differences in the immune profiles of HIV- and PHIV children. In this study, we assessed changes over 96 weeks of sustained ART in soluble and cellular markers of monocyte activation and T-cell activation, plasma markers of systemic inflammation, oxidized lipids, and gut integrity in youth with PHIV, in comparison to age and sex matched Ugandan youth without HIV (HIV-). The primary objectives of this study were to 1) assess changes in inflammatory biomarkers over 96 weeks by study arm; and 2) to determine the association between gut integrity/fungal translocation, oxidized lipids and T-cell and monocyte activation over time 3) and lastly explore in this pediatric cohort, followed since 2017, immune changes pre- and post- integrase strand transfer inhibitors (INSTIs).
Methods
Study design
This is a prospective observational cohort of PHIV and HIV- children prospectively enrolled at the Joint Clinical Research Center (JCRC) in Kampala, Uganda between 2017 and 2021. The study was approved by the Research Ethics Committee in Uganda, the Ugandan National Council of Science and Technology as well as the IRB of the University Hospitals Cleveland Medical Center, Cleveland, Ohio. Caregivers gave written informed consent. All participants were 10-18 years of age and they provided written informed assent as per research guidelines in Uganda. Timing of HIV acquisition in PHIV participants could not be ascertained but started in utero, at birth or shortly thereafter. PHIV participants were on ART for at least 2 years with a stable regimen for at least the last 6 months with HIV-1 RNA < 400 copies/mL. Evidence of self-reported or documented diarrhea or acute infection (malaria, tuberculosis, helminthiasis, pneumonia, meningitis) in the last 3 months, as well as moderate or severe malnutrition were exclusionary. Adolescents with pregnancy or intent to become pregnant were excluded. All participants lived in Kampala and surrounding areas (urban or peri-urban).
Study evaluations
Participants were seen at study entry (baseline) and at week 96 during which a Ugandan pediatrician (RN) performed a physical exam and measured body mass index (BM)I and sexual maturity rating or Tanner staging. Blood was drawn after at least an 8-hour fast. Blood was processed and plasma, serum, and PBMCs were cryopreserved for shipment to University Hospitals Cleveland Medical Center, Cleveland, Ohio. All assays were performed in batches and without prior thaw.
Cellular markers of monocyte and T-cell activation
Monocytes and T-cells were phenotyped by flow cytometry as previously described by Dr. Funderburg (6). CD4+ and CD8+ T-cell activation was measured by expression of CD38 and HLA-DR. Monocyte subsets were determined by the relative expression of CD14 and CD16.
Inflammation, soluble immune activation and gut markers
We selected intestinal biomarkers based on prior data in PHIV in Uganda as well as in adults living with HIV suggesting a potential role in cardiovascular disease (CVD) and inflammation (1). Beta D glucan (BDG, Mybiosource Inc. CA), is a polysaccharide cell wall component of most fungal species. Intestinal fatty acid binding protein (I-FABP, R &D Systems, Minneapolis, Minnesota, USA) is considered a marker of enterocyte inflammation or damage (7, 8). Soluble CD14 (sCD14, R &D Systems, Minneapolis, Minnesota) is a marker of monocyte activation, and is associated with mortality and progression of atherosclerosis[26]. We have shown that oxidized lipids (oxLDL) upregulates monocyte activation in HIV (9), making oxLDL a potentially important mediator on the causal pathway of monocyte activation.
sCD163, hsCRP, IL6 and ox LDL were measured by ELISA (R &D Systems, Minneapolis, Minnesota, USA, ALPCO, Salem, New Hampshire, USA and Mercodia, Uppsala, Sweden). The intra-assay variability ranged between 4-8% and inter-assay variability was less than 10% for all markers. All assays were performed at Dr. Funderburg’s laboratory at Ohio State University, Columbus, OH. Laboratory personnel were blinded to group assignments.
Statistical analysis
All variables were compared between groups using Wilcoxon rank sum tests or Fisher’s exact tests, as appropriate. Biomarkers were analyzed baseline and at week 96. Biomarker changes over time were calculated as the mean difference between week 96 values and baseline values on the log10 scale and back transformed to represent mean fold-change from baseline. Evidence for change over time are presented with 97.5% confidence intervals (CI), where 1 indicates no change. Shifts in the distribution of changes from baseline were evaluated using Wilcoxon rank sum tests and described as relative fold-change with corresponding p-values. FDR adjusted p-values using the Benjamini-Hochberg method to control for false discovery rates are also provided. The relationship between inflammatory markers and predictor variables of interest were assessed using Spearman correlation analyses. We fitted generalized estimating equations (GEE) with unstructured correlations on the inflammatory biomarkers over 96 weeks to estimate the effect of medication switch and dolutegravir. The GEE models included terms for observation time, medication switch, and an interaction term medication switch x time. Models were fit for each biomarker separately. We then estimated the effect of switching to dolutegravir with GEE models by including terms for observation time, switch to dolutegravir and an interaction terms dolutegravir switch x time. All statistical analyses were performed using R 4.2.1 and STATA 17.0 BE
Results
Participant characteristics
Baseline characteristics are highlighted in Table 1. Of the 197 participants recruited at baseline (101 PHIV, 96 HIV-), 168 (89 PHIV, 79 HIV-) had measurements at 96 weeks. Reasons for drop out before 96 weeks included loss to follow-up (n=10), one pregnancy, fear/inability to come to the clinic during the first wave of the COVID-19 pandemic (n=10) and relocation (n=9). Median age at enrollment was 13 years and 53% of participants were female. All participants, regardless of HIV status, were exposed to high levels of socioeconomic adversity. PHIV were more likely to have lack of access to clean water and electricity. Over the study period, 30% of participants went through puberty and reached Tanner stage 4-5.
At baseline, 86% PHIV had viral load < 50 copies/mL and remained undetectable at week 96. At baseline 72% were on a non-nucleotide reverse transcriptase inhibitor (NNRTI) containing regimen, 28% were on lopinavir/ritonavir (LPV/r) and 2 participants were on dolutegravir; 71% had a past history of thymidine analogue exposure (AZT or D4T). During the 96 week study period, 53% of participants had drug substitutions, 85% of whom switched to lamivudine, tenofovir and dolutegravir (3TC, TDF and DTG) for regimen optimization based on the Ugandan Ministry of Health HIV Guidelines (10).
Median ART duration at baseline was 10 years, with 8 participants initiating ART under the age of 1 and the rest between the ages of 2 and 10. All but 3 PHIV were on cotrimoxazole prophylaxis throughout the study and none of the participants were receiving antifungal or tuberculosis medications.
Changes in markers of systemic inflammation, gut integrity and oxidative lipids
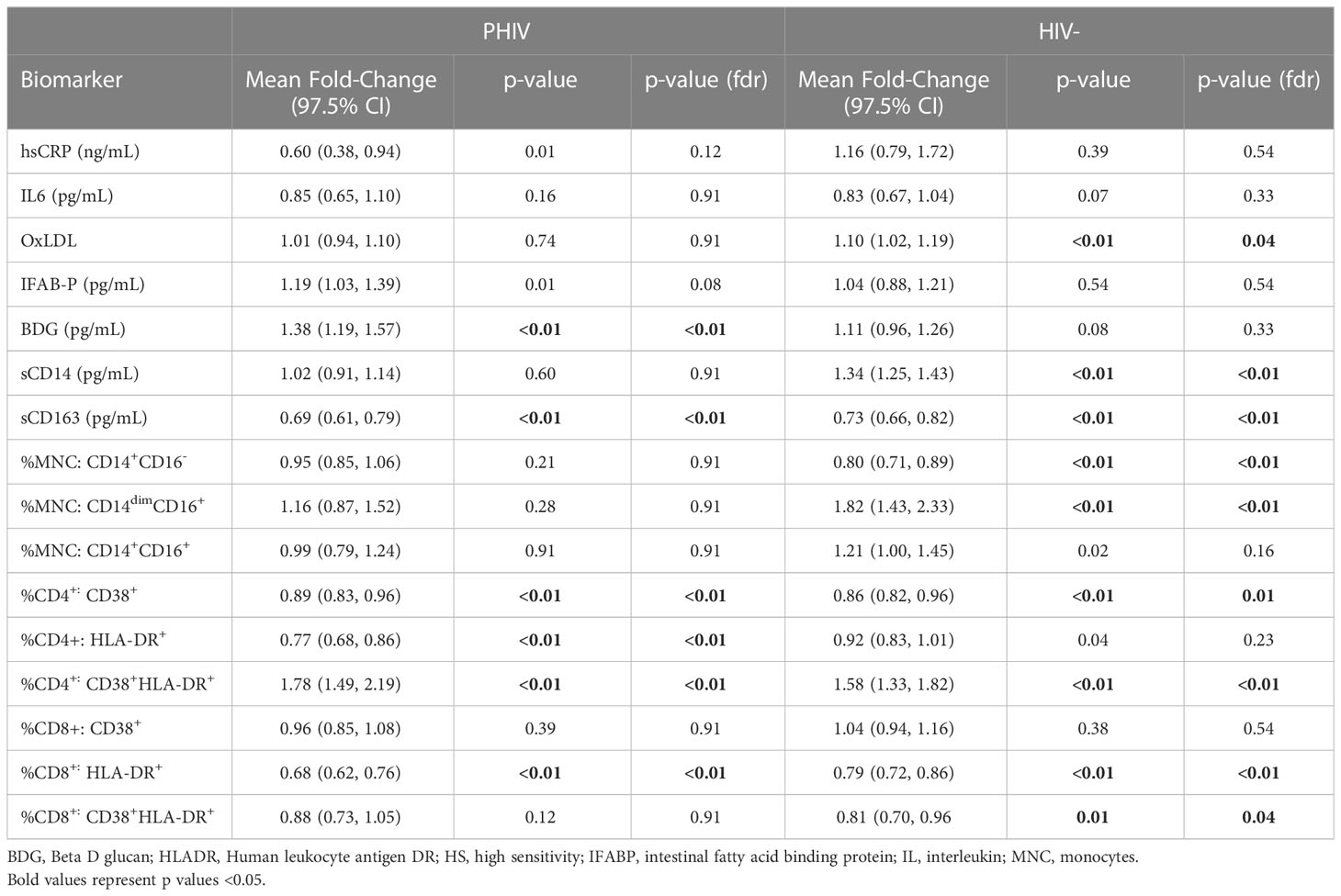
Mean fold change in biomarkers from baseline over time by study group are presented in Table 2; Figures 1, 2. At week 96, hsCRP levels in PHIV decreased by 40% and remained stable in HIV-. At both time points, markers of systemic inflammation hsCRP and IL6 were not different between the arms (p≥0.19).
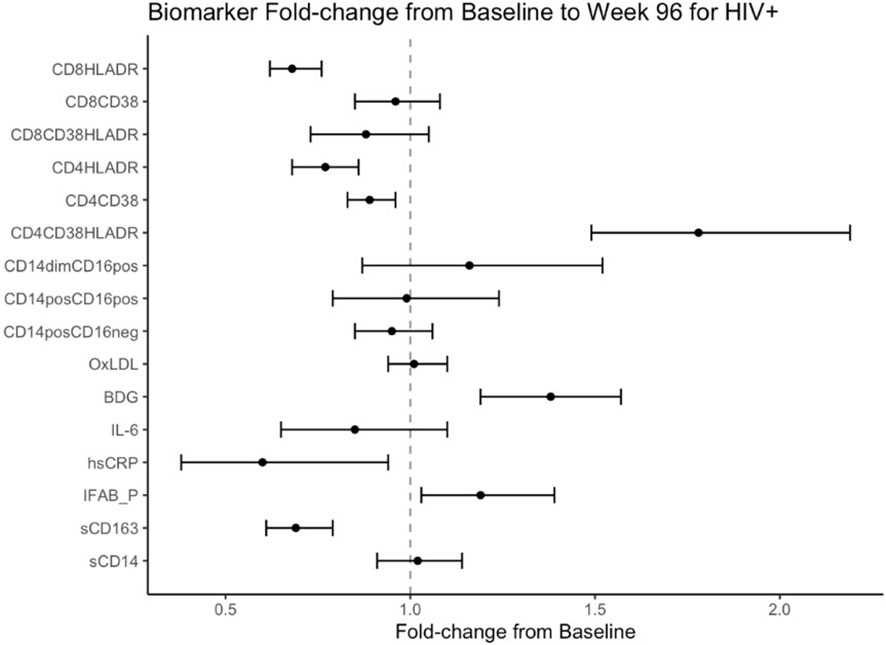
Figure 1 Fold Change from baseline over 96 weeks. Point estimates and error bars reflect mean and 95% confidence intervals.
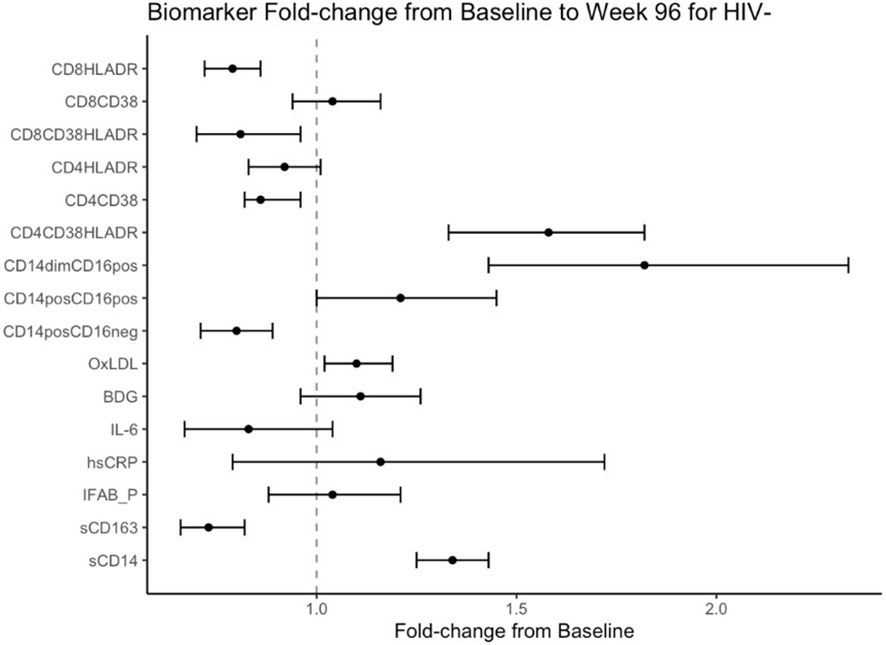
Figure 2 Fold Change from baseline over 96 weeks in HIV- participants. Point estimates and error bars reflect mean and 95% confidence intervals.
In contrast and despite the decreases in systemic inflammation in PHIV, I-FABP and BDG increased at week 96 and were 19% and 38% higher than baseline levels respectively; levels did not change significantly in HIV-. Formal comparisons of changes in I-FABP did not hold after FDR adjustment, however the consistency in magnitude and direction is suggestive of increased gut integrity damage over time in PHIV.
Oxidized LDL was significantly worse in HIV- at both times points (p ≤ 0.01) compared to PHIV and increased 10% in HIV- over 96 weeks but remained stable in PHIV.
Changes in markers of monocytes and T-cell activation
At baseline, PHIVs had higher monocyte activation; higher sCD14 (p<0.01) and a higher proportion of non-classical CD14dimCD16+ monocytes (p<0.02) compared to these indices in HIV-. At week 96, sCD14 and proportion of all monocytes remained relatively stable in PHIV, however sCD14 and the proportion of CD14dimCD16+ monocytes increased by 34% and 80% respectively in HIV-.
sCD163 was significantly higher in HIV- at baseline and week 96 (p ≤ 0.01) and decreased in both arms at week 96, by 69% and 73% in PHIV and HIV- respectively.
At both time points, PHIVs had higher T cell activation (p ≤ 0.03: CD4+ and CD8+ T cells expressing HLA-DR) compared to HIV- children. Over 96 weeks, CD4+ T cell expressing HLA-DR and CD38 worsened in both groups by 78% in PHIV and 58% in HIV- (p<0.01 for both). CD8+T cell expressing both HLA-DR and CD38 decreased by 19% in HIV- (p=0.01).
Role of sex and sexual maturity
Biomarkers were not different by sex among all participants at baseline or at week 96 (p≥0.09). Among PHIV participants, only hsCRP was higher in males at baseline (p=0.003), but all other biomarkers were similar between sexes at baseline and week 96 in PHIV and HIV- participants (p≥0.09).
Among all participants, higher sexual maturity rating or tanner stage correlated with higher hsCRP (r=0.16, p=0.05) and lower fungal translocation (r=-0.17, p=0.02).
Correlations
At baseline, markers of systemic inflammation (hsCRP), and fungal translocation (BDG), correlated with monocyte and T- cell activation in PHIV (r=0.24 - 0.33, p<0.03). In HIV-, however, only markers of systemic inflammation (hsCRP and IL6) positively correlated with CD8 activated T cells (r= 0.24-0.28; p<0.03). Only in PHIV, at both time points, oxidized LDL was inversely associated with monocyte and activated T cells (r ranges between -0.44 and -0.15, p<0.01).
Role of ART and HIV factors
At baseline, there was no correlation between ART duration, PI or NNRTI use and plasma, cellular markers of monocyte activation or T-cell activation (p≥0.32). Lower nadir CD4+ cell count correlated with proportions of traditional (CD14+CD16-) and non-classical monocytes (r=0.27-0.28), as well as CD4 and CD8+ T-cells expressing HLA-DR and CD38 (r= 0.24-0.28; p ≤ 0.03 for all).
We examined the effects of switching ART regimen during the study period, in separate models for each marker. The only significant interaction between medication switch and study duration was for sCD163. The results of a combination of coefficients, medication switch plus interaction term, showed that there was a significant increase at week 96 for participants who switched ART during the study (β=0.29, 95% CI: 0.07, 0.50, p= 0.01);. At week 96, sCD163 was lower for those who continued the same medication and those who switched medications had higher sCD163.
We then looked specifically at the effects of switching to dolutegravir (Table 3) Switching to dolutegravir at week 96 was significantly associated elevated levels of sCD163 (β=0.4, 95% CI:0.14,0.57, p<0.01) and oxidized LDL (β=0.08, 95%CI: 0.009, 0.16, p=0.03) and had a marginal effect in reducing sCD14 levels and IL6.
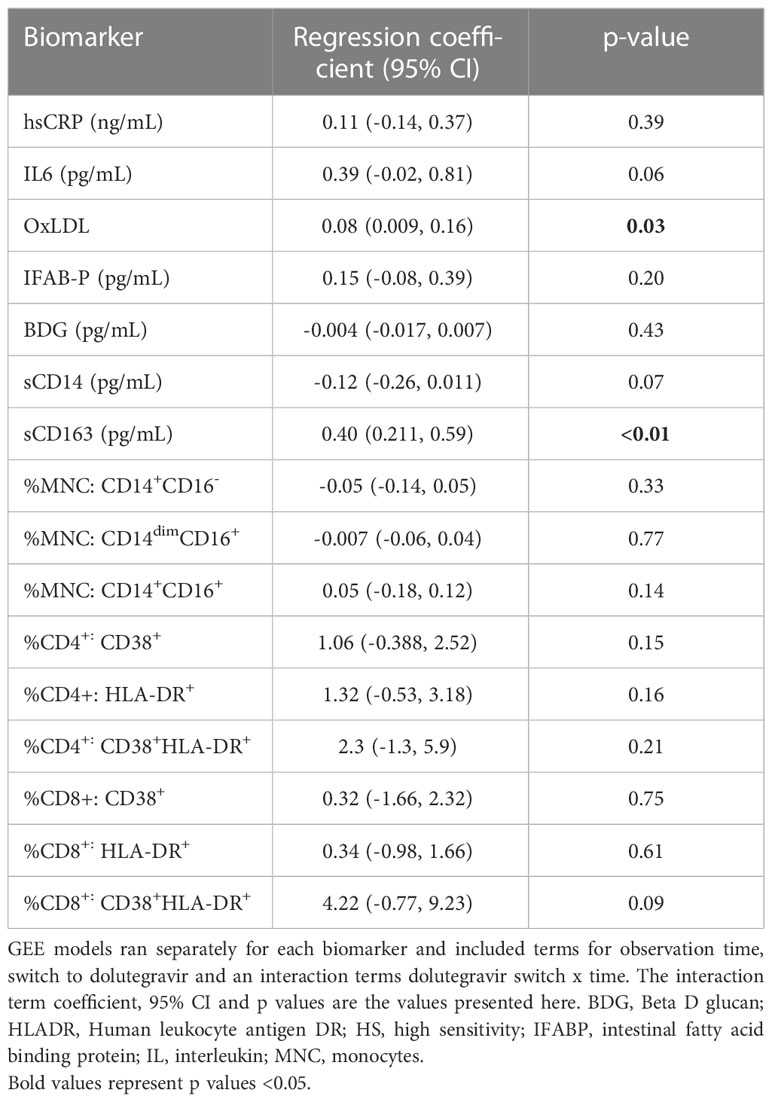
Table 3 Analysis of association between dolutegravir switch during study and changes in inflammatory biomarkers.
Discussion
Our data highlight that children with PHIV display ongoing CD4 T cell immune activation and evidence of ongoing gut structural damage despite long term viral suppression on ART. Although systemic inflammation improved in PHIV, levels of I-FABP, a marker of enterocyte inflammation, and BDG, a marker of fungal translocation, increased over time.
PHIV is lifelong and started in utero, at birth or shortly thereafter. People with PHIV have endured HIV and ART throughout their immunological development. Non-perinatal HIV in adults is typically acquired after immunological development is complete. Many children and youth with PHIV started ART with more toxic combination regimens (e.g., thymidine analogues), whereas more recently infected adults or adolescents have access to less toxic ART (e.g., integrase strand transfer inhibitors). Repeat challenges to the developing immune system, including HIV and ART, may lead to an immune disturbances and trajectory not seen in adults with HIV. There is a dearth of research assessing immune dysfunction in perinatal HIV on ART as they age towards adulthood.
Similarly to adults with HIV, we found that some indices of immune activation and systemic inflammation improved over time in PHIV with ART (11), yet, levels may not reach those of populations without HIV (12, 13). To our knowledge, this is the first comprehensive and longitudinal study investigating changes in inflammatory biomarkers and T-cells and monocyte activation in PHIV on ART in sub-Saharan Africa and in age and sex matched children without HIV.
Most participants in our cohort initiated ART early, after 2 years of age, however, this is likely too late to eliminate residual inflammation during ART. Evidence from the RV254/RV304 in a cohort of adults with HIV suggest that ART initiation during HIV seroconversion may mitigate or eliminate persistent inflammation (14). Findings in adults with HIV suggest that ongoing viral replication is likely not responsible for elevated levels of inflammation and immune activation once plasma viremia is consistently suppressed by ART and the strongest associations found for markers of HIV persistence on ART were pre-ART levels of plasma, and cell associated HIV-1 DNA (15). These findings suggest that strategies to reduce inflammation and immune activation in HIV need to focus on 1) reversing the damage induced by HIV prior to ART initiation and/or 2) on the timing between seroconversion and ART initiation. Perinatal HIV infection presents a unique opportunity to decrease the reservoir and limit ongoing inflammation through early ART (16) and studies within the International Maternal, Pediatric, Adolescent AIDS Clinical Trials (IMPAACT) network are currently examining the long-term clinical, immunologic and virologic profiles of children who received early combination ART (ART initiated within 12 weeks of birth). In a cohort of 440 infants who initiated pre-emptive ART within 48 hours, 34 of whom were diagnosed with in utero HIV infection, and continued ART with virologic suppression for 2 years, the authors found that 89% tested HIV-1 antibody negative and 70% had non-detectable cell-associated HIV-1 DNA through age 2 years (17). These findings suggest that infants with very early ART initiation may achieve restricted HIV-1 reservoirs.
Persistent immune activation plays a key role in HIV pathogenesis and co-morbidities (18–20) and may be a result of ongoing inflammation triggered by gut dysfunction and microbial translocation (21). Our findings support this hypothesis as well as earlier observations made by our group in adults living with HIV that 1) intestinal integrity markers like I-FABP do not improve overtime despite successful ART and that 2) fungal translocation as measured by BDG is associated with inflammation and immune activation (22). BDG is known to be highly immunogenic (23, 24). Similar to adults with HIV, we demonstrated a correlation between serum levels of BDG and markers of monocyte and T cell activation that was not seen in uninfected controls. This suggests that fungal translocation from an inflamed gut mucosa may contribute to the ongoing inflammation and immune activation seen in PHIV despite viral suppression on ART.
We have previously found that oxLDL is a main driver of systemic inflammation and monocyte activation in adults (9, 25), therefore we assessed the correlation between oxLDL and measured biomarkers. We found that lower oxidized LDL was associated with a weak but consistent increase in T cell and monocyte activation throughout the study. Oxidized LDL is an oxidized form of LDL cholesterol that may cause endothelial and smooth muscle cell dysfunction, can modulate innate and adaptive immunity or be taken up by macrophages to form atherosclerotic plaques (26). OxLDL is associated with coronary heart disease in adults (27, 28). In the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE), the largest primary CVD prevention trial in HIV, higher levels of oxLDL was associated with male sex, residence in high-income regions, white race and higher BMI (29). These findings highlight how there might be differences in systemic immune pathways in the global North and South. However, our findings are contrary to what has been found in adults living with HIV. We hypothesize that this may be due to the fact that Ugandan PHIV in our study had normal BMI and lower LDL cholesterol (5), which may explain why oxidized LDL may not appear to be in the pathway of immune activation in younger populations.
Among the uninfected participants, we found that sCD163 levels were higher than levels in PHIV and that significant markers of oxidative stress (oxidized LDL), monocyte activation (sCD14), and the proportions of patrolling monocytes and activated CD4+ T cells all increased over 96 weeks. Our findings highlight non-HIV sources of inflammation that are likely endemic to Sub-Saharan Africa. Exposure to multiple co-occurring adversities likely influence inflammation in this setting and may include exposure to social adversity, such as economic hardship, food insecurity, chronic stress (30–34) and exposure to environmental adversity, such as ambient air pollution – which has been increasing in SSA over the past decade (29) – has also been linked to inflammation (35). Although acute co-infections were exclusionary in this study, both groups in this study were exposed to high levels of economic insecurity, and PHIV were more likely to lack access to clean water and electricity. Several factors may limit the natural progression of inflammation in PHIV and buffer the adverse effects of socioeconomic adversity in addition to early and successful ART, and may include access to routine health care and the anti-inflammatory properties of co-trimoxazole (36).
There are limited data on assessing immune dysfunction in PHIV on ART as children age and go through puberty. In this study, 30% of participants underwent puberty during the study period. This is relevant as the function of the immune system peaks during puberty (37). We have previously reported a lack of sex related differences in immune markers in a younger Ugandan cohort (38) (Kamari paper) and hypothesized that our findings were likely secondary to the prepubertal stage of the participants. Similarly, in this study, we found a lack of sex differences both at baseline and week 96, regardless of HIV status. However, women living with HIV have been reported to have higher systemic immune activation and decreased gut integrity markers compared to men (39–43). We hypothesize that our findings could be secondary to a combination of factors including differences in microbiomes, early acquisition of HIV during immune development may lead to similar immune setpoints regardless of sex differences, and or sex-specific genetic and epigenetic regulation may not become relevant until after puberty and could explain the relationship between tanner staging and higher hsCRP.
Our study is the first to look at the effect of switching to dolutegravir on markers of inflammation in PHIV youth in this setting. Participants in our study were switched to 3TC, TDF and DTG during the study period following regimen optimization based on the Ugandan Ministry of Health HIV Guidelines (10). We found that most markers did not change when switching to DTG and that only sCD14 and IL6 slightly improved while sCD163 and oxidized LDL worsened. Interpretation of our results must be done with caution as our analysis was done in a non-randomized population with different ART backbones. These findings, however, are consistent with data from adult studies in virally suppressed participants on ART switching to a DTG-based regimen. In a randomized open-label Phase IIIb study in adults virally suppressed on ART randomized to switch to ABC, DTG and 3TC or continue on their current ART regimen (either 2 NRTIs plus either a PI or an NNRTI), participants who switched to a DTG based regimen had significant decrease in sCD14 and I-FABP and a small but not significant increase in sCD163 (44). In a retrospective case-cross over study where virologically suppressed patients were switched from 3TC and a PI to 3TC and DTG, similarly to our findings, sCD14 decreased, while other markers remained stable (45). Further studies are needed to assess whether DTG will have a differential impact on inflammatory biomarkers or any evidence on downstream clinical outcomes in this young population especially as DTG is now used as the preferred ART for children and adolescents worldwide.
Our study includes well-characterized cohorts of adolescents with and without HIV that are age and gender matched and are from the same country and urban area for comparison. In addition, our study is strengthened by the longitudinal design and the long-standing viral suppression in PHIV. The biomarkers selected for this analysis are likely part of an interrelated group and may consist of multiple steps and feedback loops, we therefore corrected for multiple comparisons. There are a few limitations to our study including the lack of assessment of the composition of the gastrointestinal microbiome and mycobiome, or of bacterial translocation. Although we excluded participants with active infections, we did not assess for potential helminthiasis or perform nutritional assessments, all of which could affect the microbiome. In addition, a little over half of the participants had an ART regimen switch during the study period.
In conclusion, our study showed that children who acquired HIV perinatally have evidence of ongoing T cell activation, damage to the gut integrity and fungal translocation despite ART and viral suppression over 2 years. Further research is warranted in this population to investigate the links between intestinal barrier function, intestinal microbiota composition, and immune activation, and the implications of life long exposure to the consequences of decreased gut-barrier function on potential comorbidities in HIV-infected children. In addition, our findings highlight the need for continued research on newer ART regimens and early ART initiation in PHIV to decrease inflammation and prevent long-term comorbidities.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, on a case by case basis.
Ethics statement
The studies involving human participants were reviewed and approved by University Hospitals Medical Center IRB and Joint Clinical Research Center IRB. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
SD-F and GM conceptualized and designed the study. Data collection was performed by CG, RN, and SD-F. Data analysis and interpretation was performed by AS, MS and SD-F. Immune assays were performed by KA and NF. The article was drafted by SD-F and revisions and final approval by all co-authors. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health [K23HD088295 to SD-F] and by the National Institute of Allergy and Infectious Diseases [U01AI168630 to SD-F and NF]. This publication was made possible through funding support of University Hospitals Cleveland Medical Center Clinical Research Center (UHCRC) and the Clinical and Translational Science Collaborative of Cleveland, UL1TR002548 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of UHCRC or the NIH.
Acknowledgments
The authors would like to thank the patients and their parents who participated in this research.
Conflict of interest
NF has received funding from Gilead. Grace McComsey served as a scientific consultant for Gilead, ViiV, Merck, Theratechnologies and Janssen, and has received grant support from Astellas, Tetraphase, Roche, Redhill, Pfizer, Redhill, Cognivue, Genentech.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dirajlal-Fargo S, Albar Z, Bowman E, Labbato D, Sattar A, Karungi C, et al. Increased monocyte and T-cell activation in treated HIV+ Ugandan children: Associations with gut alteration and HIV factors. AIDS (London England) (2020) 34(7):1009–18. doi: 10.1097/QAD.0000000000002505
2. Dirajlal-Fargo S, Albar Z, Sattar A, Kulkarni M, Bowman E, Funderburg N, et al. Relationship between economic insecurity, inflammation, monocyte activation and intestinal integrity in children living with HIV in Uganda. AIDS Care (2020) 32(11):1451–6. doi: 10.1080/09540121.2020.1776822
3. Dirajlal-Fargo S, Shan L, Sattar A, Bowman E, Gabriel J, Kulkarni M, et al. Insulin resistance and intestinal integrity in children with and without HIV infection in Uganda. HIV Med (2020) 21(2):119–27. doi: 10.1111/hiv.12808
4. Dirajlal-Fargo S, Shan L, Sattar A, Kulkarni M, Bowman E, Funderburg N, et al. Micronutrients, metabolic complications, and inflammation in Ugandan children with HIV. J Pediatr Gastroenterol Nutr (2020) 70(5):e100–e5. doi: 10.1097/MPG.0000000000002630
5. Dirajlal-Fargo S, Albar Z, Bowman E, Labbato D, Sattar A, Karungi C, et al. Subclinical vascular disease in children with human immunodeficiency virus in Uganda is associated with intestinal barrier dysfunction. Clin Infect Dis (2020) 71(12):3025–32. doi: 10.1093/cid/ciz1141
6. Funderburg NT, Jiang Y, Debanne SM, Storer N, Labbato D, Clagett B, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis (2014) 58(4):588–95. doi: 10.1093/cid/cit748
7. Pelsers MM, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, Hermens WT, et al. Intestinal-type and liver-type fatty acid-binding protein in the intestine. tissue distribution and clinical utility. Clin Biochem (2003) 36(7):529–35. doi: 10.1016/s0009-9120(03)00096-1
8. El Kamari V, Moser C, Hileman CO, Currier JS, Brown TT, Johnston L, et al. Lower pretreatment gut integrity is independently associated with fat gain on antiretroviral therapy. Clin Infect Diseases. (2018) 68(8):1394–401. doi: 10.1093/cid/ciy716
9. Zidar DA, Juchnowski S, Ferrari B, Clagett B, Pilch-Cooper HA, Rose S, et al. Oxidized LDL levels are increased in HIV infection and may drive monocyte activation. J acquired Immune deficiency syndromes (1999). (2015) 69(2):154–60. doi: 10.1097/QAI.0000000000000566
10. Health UMo. Consolidated guidelines for the prevention and treatment of HIV and AIDS in Uganda. (Republic of Uganda: Ministry of Health) (2020).
11. Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol (2013) 119:51–83. doi: 10.1016/B978-0-12-407707-2.00002-3
12. Lederman MM, Calabrese L, Funderburg NT, Clagett B, Medvik K, Bonilla H, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect diseases. (2011) 204(8):1217–26. doi: 10.1093/infdis/jir507
13. Jong E, Louw S, van Gorp EC, Meijers JC, ten Cate H, Jacobson BF. The effect of initiating combined antiretroviral therapy on endothelial cell activation and coagulation markers in south African HIV-infected individuals. Thromb haemostasis. (2010) 104(6):1228–34. doi: 10.1160/TH10-04-0233
14. Sereti I, Krebs SJ, Phanuphak N, Fletcher JL, Slike B, Pinyakorn S, et al. Persistent, albeit reduced, chronic inflammation in persons starting antiretroviral therapy in acute HIV infection. Clin Infect Dis (2017) 64(2):124–31. doi: 10.1093/cid/ciw683
15. Gandhi RT, McMahon DK, Bosch RJ, Lalama CM, Cyktor JC, Macatangay BJ, et al. Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PloS pathogens. (2017) 13(4):e1006285. doi: 10.1371/journal.ppat.1006285
16. Rainwater-Lovett K, Luzuriaga K, Persaud D. Very early combination antiretroviral therapy in infants: Prospects for cure. Curr Opin HIV AIDS. (2015) 10(1):4–11. doi: 10.1097/COH.0000000000000127
17. Deborah Persaud EGC, Nelson BS, Tierney C, Cotton MF, Coletti A, Costello D, et al. TWO-YEAR VIROLOGIC OUTCOMES OF VERY EARLY ART FOR INFANTS IN THE IMPAACT P1115 STUDY [CROI Abstract 31] (2022). In Special Issue: Abstracts From the 2022 Conference on Retroviruses and Opportunistic Infections. Top Antiv Med (2022) 30(1s):11.
18. Peterson TE, Baker JV. Assessing inflammation and its role in comorbidities among persons living with HIV. Curr Opin Infect Diseases. (2019) 32(1):8–15. doi: 10.1097/QCO.0000000000000510
19. Dirajlal-Fargo S, Funderburg N. HIV And cardiovascular disease: the role of inflammation. Curr Opin HIV AIDS. (2022) 17(5):286–92. doi: 10.1097/COH.0000000000000755
20. Nasi M, De Biasi S, Gibellini L, Bianchini E, Pecorini S, Bacca V, et al. Ageing and inflammation in patients with HIV infection. Clin Exp Immunol (2016) 187(1):44–52. doi: 10.1111/cei.12814
21. Roider JM, Muenchhoff M, Goulder PJR. Immune activation and paediatric HIV-1 disease outcome. Curr Opin HIV AIDS. (2016) 11(2):146–55. doi: 10.1097/COH.0000000000000231
22. Weiner LD, Retuerto M, Hager CL, El Kamari V, Shan L, Sattar A, et al. Fungal translocation is associated with immune activation and systemic inflammation in treated HIV. AIDS Res Hum Retroviruses (2019) 35(5):461–72. doi: 10.1089/aid.2018.0252
23. Hoenigl M, Pérez-Santiago J, Nakazawa M, de Oliveira MF, Zhang Y, Finkelman MA, et al. (1→3)-β-d-Glucan: A biomarker for microbial translocation in individuals with acute or early HIV infection? Front Immunol (2016) 7. doi: 10.3389/fimmu.2016.00404
24. Morris A, Hillenbrand M, Finkelman M, George MP, Singh V, Kessinger C, et al. Serum (1–>3)-beta-D-glucan levels in HIV-infected individuals are associated with immunosuppression, inflammation, and cardiopulmonary function. J acquired Immune deficiency syndromes (1999). (2012) 61(4):462–8. doi: 10.1097/QAI.0b013e318271799b
25. Hileman CO, Turner R, Funderburg NT, Semba RD, McComsey GA. Changes in oxidized lipids drive the improvement in monocyte activation and vascular disease after statin therapy in HIV. AIDS (London England) (2016) 30(1):65–73. doi: 10.1097/QAD.0000000000000885
26. Rhoads JP, Major AS. How oxidized low-density lipoprotein activates inflammatory responses. Crit Rev Immunol (2018) 38(4):333–42. doi: 10.1615/CritRevImmunol.2018026483
27. Koenig W, Karakas M, Zierer A, Herder C, Baumert J, Meisinger C, et al. Oxidized LDL and the risk of coronary heart disease: Results from the MONICA/KORA augsburg study. Clin Chem (2011) 57(8):1196–200. doi: 10.1373/clinchem.2011.165134
28. Nou E, Lu MT, Looby SE, Fitch KV, Kim EA, Lee H, et al. Serum oxidized low-density lipoprotein decreases in response to statin therapy and relates independently to reductions in coronary plaque in patients with HIV. AIDS (London England) (2016) 30(4):583–90. doi: 10.1097/QAD.0000000000000946
29. Looby SE, Kantor A, Burdo TH, Currier JS, Fichtenbaum CJ, Overton ET, et al. Factors associated with systemic immune activation indices in a global primary cardiovascular disease prevention cohort of people with human immunodeficiency virus on antiretroviral therapy. Clin Infect Dis (2022) 75(8):1324–33. doi: 10.1093/cid/ciac166
30. Milaniak I, Jaffee SR. Childhood socioeconomic status and inflammation: A systematic review and meta-analysis. Brain behavior immunity. (2019) 78:161–76. doi: 10.1016/j.bbi.2019.01.018
31. Prendergast A, Kelly P. Enteropathies in the developing world: Neglected effects on global health. Am J Trop Med hygiene. (2012) 86(5):756–63. doi: 10.4269/ajtmh.2012.11-0743
32. Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect diseases. (2011) 203(10):1474–83. doi: 10.1093/infdis/jir060
33. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet (London England) (2013) 382(9903):1525–33. doi: 10.1016/S0140-6736(13)61809-7
34. Calderon-Garciduenas L, Villarreal-Calderon R, Valencia-Salazar G, Henriquez-Roldan C, Gutierrez-Castrellon P, Torres-Jardon R, et al. Systemic inflammation, endothelial dysfunction, and activation in clinically healthy children exposed to air pollutants. Inhalation toxicol (2008) 20(5):499–506. doi: 10.1080/08958370701864797
35. Gencer B, Mach F. Air pollution triggers inflammation and cardiovascular events: Now is the time to act. Eur Heart J (2021) 42(7):773–5. doi: 10.1093/eurheartj/ehaa1020
36. Bourke CD, Gough EK, Pimundu G, Shonhai A, Berejena C, Terry L, et al. Cotrimoxazole reduces systemic inflammation in HIV infection by altering the gut microbiome and immune activation. Sci Trans Med (2019) 11(486). doi: 10.1126/scitranslmed.aav0537
37. Wood CL, Lane LC, Cheetham T. Puberty: Normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab (2019) 33(3):101265. doi: 10.1016/j.beem.2019.03.001
38. Dirajlal-Fargo S, El-Kamari V, Weiner L, Shan L, Sattar A, Kulkarni M, et al. Altered intestinal permeability and fungal translocation in Ugandan children with human immunodeficiency virus. Clin Infect Dis (2020) 70(11):2413–22. doi: 10.1093/cid/ciz561
39. Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect diseases. (2013) 208(11):1737–46. doi: 10.1093/infdis/jit508
40. Ticona E, Bull ME, Soria J, Tapia K, Legard J, Styrchak SM, et al. Biomarkers of inflammation in HIV-infected Peruvian men and women before and during suppressive antiretroviral therapy. AIDS (London England). (2015) 29(13):1617–22. doi: 10.1097/QAD.0000000000000758
41. Mathad JS, Gupte N, Balagopal A, Asmuth D, Hakim J, Santos B, et al. Sex-related differences in inflammatory and immune activation markers before and after combined antiretroviral therapy initiation. J acquired Immune deficiency syndromes (1999). (2016) 73(2):123–9. doi: 10.1097/QAI.0000000000001095
42. Griesbeck M, Scully E, Altfeld M. Sex and gender differences in HIV-1 infection. Clin Sci (London England: 1979). (2016) 130(16):1435–51. doi: 10.1042/CS20160112
43. Dirajlal-Fargo S, Mussi-Pinhata MM, Weinberg A, Yu Q, Cohen R, Harris DR, et al. HIV-Exposed uninfected infants have increased inflammation and monocyte activation. AIDS (London England) (2019) 33(5):845–53. doi: 10.1097/QAD.0000000000002128
44. Lake J, Currier JS, Koteff J, Brennan C, Granier C, Shaefer M, et al. Cardiovascular biomarkers after switch to ABC/DTG/3TC: The STRIIVING study [CROI abstract 660]. In Special Issue: Abstract From the 2016 Conferenc eon REtroviruses and Opportunistic Infectious. Top Antiv Med. (2016) 24(1):59–81.
45. Lombardi F, Belmonti S, Borghetti A, Ciccullo A, Baldin G, Cauda R, et al. Reduced soluble CD14 levels after switching from a dual regimen with lamivudine plus boosted protease inhibitors to lamivudine plus dolutegravir in virologically suppressed HIV-infected patients. HIV Res Clin Practice. (2019) 20(3):92–8. doi: 10.1080/25787489.2019.1653512
Keywords: inflammation, immune activation, gut integrity, microbial translocation, children, HIV, sub-Saharan Africa
Citation: Dirajlal-Fargo S, Strah M, Ailstock K, Sattar A, Karungi C, Nazzinda R, Kityo C, Musiime V, Funderburg N and McComsey GA (2023) Persistent immune activation and altered gut integrity over time in a longitudinal study of Ugandan youth with perinatally acquired HIV. Front. Immunol. 14:1165964. doi: 10.3389/fimmu.2023.1165964
Received: 14 February 2023; Accepted: 14 March 2023;
Published: 28 March 2023.
Edited by:
Hongshuo Song, University of Maryland, United StatesReviewed by:
Anthony Jaworowski, RMIT University, AustraliaSouheil-Antoine Younes, Emory University, United States
Copyright © 2023 Dirajlal-Fargo, Strah, Ailstock, Sattar, Karungi, Nazzinda, Kityo, Musiime, Funderburg and McComsey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sahera Dirajlal-Fargo, U2FoZXJhLmRpcmFqbGFsLWZhcmdvQHVoaG9zcGl0YWxzLm9yZw==
†These authors share senior authorship
 Sahera Dirajlal-Fargo
Sahera Dirajlal-Fargo Monika Strah3
Monika Strah3 Nicholas Funderburg
Nicholas Funderburg