- Division of Hematology and Oncology, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI, United States
Allogeneic Hematopoietic stem cell transplantation (HSCT) offers a potential cure for patients with hematologic malignancies. Unfortunately, graft-versus-host disease (GVHD) remains a major obstacle to the greater success of this treatment. Despite intensive research efforts over the past several decades, GVHD is still a major cause of morbidity and mortality in patients receiving allogeneic HSCT. The genetic disparity between donor and recipient is the primary factor that dictates the extent of alloimmune response and the severity of acute GVHD (aGVHD). However, some nongenetic factors are also actively involved in GVHD pathogenesis. Thus, identifying host factors that can be readily modified to reduce GVHD risk is of important clinical significance. We are particularly interested in the potential role of nutrition, as a nongenetic factor, in the etiology and management of aGVHD. In this article, we summarize recent findings regarding how different routes of nutritional support and various dietary factors affect aGVHD. Since diet is one of the most important factors that shape gut microbiota, we also provide evidence for a potential link between certain nutrients and gut microbiota in recipients of allogeneic HSCT. We propose a shifting role of nutrition from support to therapy in GVHD by targeting gut microbiota.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative treatment for patients with hematologic malignancies including leukemia, lymphoma, and multiple myeloma (1). Although the pretransplant conditioning regimen, consisting of chemotherapy with or without radiation, can kill some malignant cells directly, the curative potential of allogeneic HSCT largely depends on a graft-versus-leukemia (GVL) effect that is mediated by mature donor T cells in the allograft (2). Unfortunately, donor T cells can also play a detrimental role and afflict patients with graft-versus-host-disease (GVHD) (3, 4). Acute GVHD (aGVHD) occurs because donor T cells recognize the genetically incompatible host as foreign and mount a strong alloimmune response, resulting in multiple-organ damage of the transplant recipient. Severe aGVHD can be fatal, and it remains a major obstacle to the greater success of allogeneic HSCT (5–7).
The genetic disparity between donor and recipient is the primary factor that dictates the extent of alloimmune response and the severity of aGVHD. In clinical settings, some established GVHD risk factors also include sex-mismatched transplants, older recipient age, more intense conditioning regimens, etc. (8). These observations suggest that some nongenetic factors are actively involved in GVHD pathogenesis. Unfortunately, these factors cannot be changed within clinics to reduce GVHD risk. Therefore, identifying host factors that can be readily modified to reduce GVHD risk is of important clinical significance. We are particularly interested in the potential role of nutrition, as a nongenetic factor, in the etiology and management of aGVHD. The dietary patterns and nutritional status of allogeneic HSCT recipients vary substantially, but how these factors contribute to aGVHD risk has not been well explored. Also, diet and nutrients can be easily modified with the hope of gaining therapeutic benefits. However, the potential value of using nutritional interventions to reduce the complications of allogeneic HSCT has largely been overlooked. In this article, we summarize recent findings regarding how different routes of nutritional support and certain dietary factors affect aGVHD. We also discuss a potential link between certain nutrients and gut microbiota during aGVHD. We propose that dietary and nutritional intervention may be useful as a safe, easily applicable, and inexpensive monotherapy or adjunct therapy for aGVHD.
Routes of nutritional support and GVHD
Nutritional status is a significant variable among patients receiving allogeneic HSCT. Prior to transplantation, patients have dietary habits and nutritional statuses that are substantially different from each other. After transplantation, they often develop macronutrient and micronutrient deficiencies secondary to changes in dietary intakes, severe mucositis, gastrointestinal GVHD, therapeutic toxicity, etc. (9, 10). Malnutrition, low levels of serum albumin, and severe weight loss have been associated with poor prognosis in patients after HSCT (11–14). These patients often cannot tolerate adequate oral intake and therefore must rely on parenteral nutrition (PN) and/or enteral nutrition (EN). However, prolonged dependence on PN can cause metabolic problems including fluid and electrolyte imbalances, hyperglycemia, and hyperlipidemia. These changes in host metabolism may have negative impacts on GVHD. For example, severe hyperglycemia post-transplant has been shown to be associated with increased risk of developing aGVHD in two clinical studies (15, 16). These observations may be partially explained by increased inflammatory cytokines associated with hyperglycemia.
Diet management practices vary across transplant centers. Despite higher costs and associated complications, PN often remains a standard route and sometimes sole option for many HSCT patients. Some studies have examined whether routes of nutritional administration affect GVHD outcomes. Three large, retrospective studies have compared clinical outcomes between EN and PN in HSCT patients. In general, these three studies observed lower non-relapse mortality and fewer incidences of severe aGVHD in patients that received EN compared to patients who received PN. One study observed this trend in pediatric patients (17), while the other two observed a similar trend in adult patients (18, 19). In addition to PN and EN, Beckerson et al. also included a group that received inadequate oral nutrition, and these patients had similar outcomes to those that received PN. It is worth mentioning that published evidence favoring EN is of retrospective nature. Also, more critically ill patients (e.g., early sepsis, severe aGVHD) tend to require PN more often. An interesting question that should be addressed in future studies is whether EN is still better if PN includes intestinal villus protection as is used in intensive care units.
Several smaller trials also indicate a beneficial trend towards EN rather than PN for better nutritional management for HSCT patients, despite limited patient number and mixed results (20–22). In these studies, patients who received EN showed improvement in a variety of parameters. Notably, Guieze et al. saw a lower rate of transfer to intensive care, and Seguy et al. observed significantly lower incidence of grade III/IV acute GVHD and decreased early 100-day mortality rate due to infection.
Given that EN is shown to be more beneficial than PN for GVHD outcomes, oral nutrition may also be beneficial for this patient group when it can be tolerated. One study applied a stepwise upgrade diet protocol to children suffering from gastrointestinal aGVHD. This dietary management appeared to contribute to a faster improvement of GI GVHD in these patients (23). A different group implemented an evidence-based nutrition support pathway for HSCT patients. As a result, these patients showed improved oral intake and relied less on PN than those patients who were not involved in a nutrition support pathway (24). Another study found that out of 228 HSCT patients, 144 were able to eat daily during their initial hospital stay, and 28 of those 144 patients did not need additional PN. The 84 patients on PN who were unable to tolerate any oral intake were divided into groups based on how many days they went with no oral intake. These researchers found a correlation between days without oral intake and grade III/IV aGVHD. Patients who went more than 9 days without oral intake had the poorest prognosis (25). Thus, oral nutrition may have important prognostic impacts in patients after allogeneic HSCT. If PN is necessary, as it often is for patients with severe aGVHD, reintroduction of oral intake as soon as it can be tolerated, even in addition to PN, may better support these patients. Of course, this should be initiated carefully and gradually with consideration of the patient’s overall condition and gut function. Improving food taste and smell may help increase oral intake. Further studies and clinical trials should attempt to optimize the protocol of transitioning patients from PN dependency to sole oral feeding cautiously and effectively.
Overall, there seems to be a beneficial trend toward EN in HSCT patients. Therefore, if total parenteral nutrition (TPN) is initiated, early reintroduction of oral intake is likely to improve clinical outcomes. The observation that GVHD was reduced due to better oral intake in patients receiving home care compared with hospital care further supports this premise (26). Atrophy of GI mucosa, weakness of gut barrier function, and lack of gut bacterial fermentation associated with sole PN support may partially explain poor patient outcomes. A comprehensive evaluation of individual patients’ nutritional status will be the foundation for optimal dietary management for this patient group (27).
Nutritional factors and GVHD
Amino acids
Tryptophan is an essential amino acid found in high-protein foods. It is a precursor to serotonin and melatonin and is metabolized through the kynurenine, serotonin, and indole pathways (28). The breakdown of tryptophan along the kynurenine axis is mediated by the rate-limiting enzyme indoleamine 2,3-dioxygenase (IDO). Tryptophan depletion or the accumulation of its cytotoxic metabolites can cause T-cell anergy and apoptosis, facilitating the induction of immune tolerance (29). In preclinical studies, Jasperson and colleagues found that IDO expression was increased in HSCT recipient intestinal tissues. IDO deficiency in recipient mice accelerated GVHD lethality with significantly increased colonic T cell infiltration and inflammation, demonstrating a protective role of IDO induction during intestinal GVHD (30). Further, the administration of exogenous tryptophan metabolites led to reduced GVHD (31). Dysregulated tryptophan metabolism in the brain was also evident in mouse models of GVHD (32). Human studies confirmed the involvement of IDO in regulating intestinal GVHD (33). In addition, tryptophan catabolism was induced in patients after allogeneic HSCT, and kynurenine levels were associated with GVHD severity (34). Tryptophan can also be utilized by gut bacteria (28). Bacterial catabolism of tryptophan produces indoles that improve intestinal barrier function and gut health. Importantly, recent studies have shown indole and its derivatives can improve gut barrier integrity and protect against GVHD in mouse models (35).
Glutamine is a non-essential amino acid but serves as a preferable nutrient for enterocytes, hepatocytes, lymphocytes, and macrophages. Glutamine deficiency can lead to increased gut permeability and higher secretion of inflammatory cytokines (36). Interestingly, it has been reported that oral glutamine supplementation improves intestinal barrier function and reduces GVHD-associated tissue damage in animal models (37). The idea of supplementing glutamine to HSCT patients has been proposed and investigated (38). One meta-analysis included 17 randomized controlled trials of HSCT patients with supplemental glutamine (39). Most of these studies were small and varied in terms of recipient age, transplant type (autologous or allogeneic), route of administration (oral or intravenous), and administration schedule. Overall, there was no effect of oral or intravenous glutamine on transplant-related mortality at day 100. Oral glutamine might reduce mucositis in HSCT recipients. Intravenous glutamine might suppress infection but increase the risk of relapse according to two small studies (39). However, these observations should be interpreted cautiously since they were mostly based on a few small trials. Large, well-designed trials may provide insight into whether glutamine can improve GVHD.
Dietary fat
Allogeneic HSCT is associated with significant changes in host lipid metabolism (40). These changes can result in dyslipidemia which is often observed in transplant recipients (40). There is evidence that obesity is associated with worse clinical outcomes in patients receiving allogeneic HSCT (41, 42). A trend toward increased risk of grade II-IV aGVHD with increased body mass index (BMI) has been reported (43). Recent studies demonstrated that obesity causes changes in host gut microbiota and augments GVHD after allogeneic HSCT (44, 45).
Statins are lipid-lowering drugs frequently used to treat high serum cholesterol, but their potential immunomodulatory effect has been studied in the context of aGVHD (46–48). In one retrospective study, pravastin was used for endothelial protection and reduced non-relapse mortality after HSCT, though not due to a lower incidence of aGVHD. However, patients who did suffer from aGVHD benefited more from statin treatment than patients without aGVHD (49). A murine study found that atorvastatin reduced aGVHD lethality without affecting GVL effect when both donor and recipient were dosed with the drug. This result was attributed to the drug’s effect on the polarization of donor T cells and the function of host antigen presenting cells (APCs) (50). Another group observed similar results when testing the effects of simvastatin on human APCs in vitro (51). These results also indicate the involvement of lipids in GVHD pathogenesis. In addition to the immunomodulatory effects, statins lower lipid and cholesterol levels. Fatty acid metabolism is implicated in the clonal expansion and function of donor alloreactive T-cells (52–54). Use of statins may help reduce the negative effect that a high-fat and high-cholesterol diet potentially has on HSCT patients. Lipidomic studies using patient blood samples have shown that pretransplant lipid signature has the potential to be used as a biomarker of GVHD (55).
Essential fatty acids (EFAs) are polyunsaturated fatty acids (PUFAs) that cannot be synthesized by the body and must be obtained from diet (56). There are two classes of EFAs: omega-6 and omega-3. Each plays a different role in regulating inflammation. omega-6 PUFAs promote inflammation by producing proinflammatory cytokines while omega-3 PUFAs are generally considered to have anti-inflammatory properties. The potential effects of EFAs on GVHD have been explored in a B6→Balb/c mouse model of allogeneic HSCT with a chemotherapy-based conditioning regimen. Interestingly, omega-3-rich fish oil supplementation worsens GVHD by reducing the reconstitution of regulatory T cells (57). The precise role of dietary fatty acids in GVHD warrants further investigation.
Vitamins
Vitamin D is a steroid hormone known to exert an anti-inflammatory function and promote immune tolerance (58, 59). The active form of vitamin D is 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] (60). Vitamin D interacts with vitamin D receptors (VDRs) expressed in various immune and nonimmune cells. Given the broad and critical role of vitamin D in the immune system, many studies have investigated the relationship between vitamin D status and clinical outcomes in HSCT patients (61–64).
Lower serum vitamin D levels were reported before and/or after HSCT in nearly all studies, despite different thresholds to define vitamin D insufficiency/deficiency (65–75). Some studies found significant associations between vitamin D insufficiency/deficiency with decreased survival (65, 70, 72, 74) or higher incidence of chronic GVHD (69, 72), while other studies observed no significant associations. Interestingly, one study reported a more frequent incidence of acute GVHD among patients with sufficient vitamin D levels (70). The inconsistency between studies may be attributed to different patient populations, various cut-off levels for vitamin D deficiency, different HSCT conditioning regimens, and lack of information on VDR expression levels etc. A prospective multi-center phase I/II clinical trial observed that vitamin D supplementation was associated with a significantly lower incidence of chronic GVHD at one year after transplantation (76). Further studies showed that patient VDR polymorphism contributes to the outcomes of vitamin D supplementation (77). Overall, vitamin D insufficiency/deficiency is quite common in patients undergoing HSCT. Whether vitamin D levels are associated with GVHD risk and whether vitamin D supplementation improves transplant-associated complications such as aGVHD remain inconclusive. Defining the best serum marker for vitamin D levels, standardizing cut-off levels of vitamin D deficiency, and identifying which group of patients are most likely to benefit from vitamin D supplementation will advance research in this area.
Vitamin A is another fat-soluble vitamin that has been implicated in GVHD pathogenesis (78, 79). Retinoic acid (RA), the active metabolite of vitamin A, has a broad range of effects on the immune system. RA binds to retinoic acid receptors (RARs), and either an immunosuppressive or a proinflammatory response is observed depending on the context of RA/RAR signaling. Whether vitamin A levels affect aGVHD risk remains unclear. Some clinical studies reported negative impacts of lower serum vitamin A levels on GVHD severity in pediatric patients, suggesting potential benefits of supplementing vitamin A to these patients (80, 81). However, it has also been reported that pre-transplant serum vitamin A levels do not appear to affect the development of acute GVHD in adult patients undergoing allogeneic HSCT (82). Importantly, a population of RA-responsive pathogenic donor CD8+ T cells has been identified in patients with gastrointestinal GVHD (83).
Preclinical studies examining the relationship between RA signaling and GVHD also reported mixed results (84–88). Yang et al. observed a protective effect of RA against GVHD in a B6 → B6D2F1 mouse model of aGVHD (84). By contrast, our group and others showed that enhancing RA/RAR signaling by exogenous RA exacerbated the development of gastrointestinal GVHD (85–88). Furthermore, our recent studies showed that enhancing RA signaling may negatively impact gut barrier integrity during GVHD (89). Given the pleiotropic effects of RA on immune and nonimmune cells as well as its highly context-dependent effects, it is not surprising that different and sometimes contradictory results are found in the literature. Overall, both preclinical and clinical evidence indicates an important role of RA/RAR signaling in the development of gastrointestinal GVHD. Serum vitamin A levels may provide some useful information, but the expression of RARs or RA levels in tissue sites such as the gut seem more valuable to define their relation to GVHD outcomes. Such information may be obtained from tissue biopsy specimens obtained when diagnosing and staging gastrointestinal GVHD. Further studies are needed to determine how to modify RA signaling or use vitamin A advantageously for GVHD patients.
Choline
Some dietary and nutritional factors have the potential to worsen GVHD. A recent study showed that a choline-rich diet accelerated GVHD-associated mortality in mice (90). Choline is an essential nutrient for humans and is required in many biological processes including the formation of cell membranes and the synthesis of neurotransmitters (91). Choline can also be utilized by intestinal bacteria, producing the toxic metabolite Trimethylamine N-oxide (TMAO) which can cause vascular inflammation (92). Wu et al. found that choline-derived or exogenously supplied TMAO aggravated experimental GVHD. Mechanistic studies revealed that TMAO enhanced M1 macrophage polarization via NLRP3 inflammasome activation. A high-choline diet significantly increased serum TMAO levels and resulted in more severe GVHD target organ damage (90). This study highlighted the detrimental effects of some dietary components on GVHD that are often unrecognized by clinicians.
Gut microbiota and GVHD
Our gut microbial communities contain up to 1000 bacterial species and have immense impact on human metabolism, nutrition, and immune function (93). Disruption of gut microbiota has been linked to many diseases including cancer, obesity, diabetes, malnutrition, and inflammatory bowel disease (94–98). Perturbation of gut microbiota is commonly observed in recipients of allogeneic HSCT (99–103). Changes in the diversity and composition of gut microbiota can be due to several factors including conditioning-induced gut damage, changes in diet, prophylactic and/or therapeutic use of antibiotics, and inflammation caused by GVHD (101, 103–105). Patients with steroid-refractory aGVHD have a poor prognosis and this detrimental complication is associated with endothelial dysfunction. Early sepsis is an endothelial complication that can already be predicated prior to transplantation (106–109), which often results in significantly higher antibiotic exposure of these patients. It is thus conceivable that endothelial dysfunction can influence the gut microbiome by higher incidence of sepsis.
In the gut microbiota of allogenic HSCT patients, there is often a loss of microbial diversity, dominance of a single bacterial species, and the expansion of opportunistic pathogens (110–113). Studies found that patients with more diverse gut microbiota had better survival rates and lower non-relapse mortality (111, 112). The increased abundance of certain bacterial species, especially Blautia, was associated with reduced GVHD mortality (110). Antibiotics with broad-spectrum effects appear to correlate with higher GVHD-related mortality (114). Mechanistically, antibiotics such as carbapenem can expand mucus-degrading bacteria that impair gut barrier function and aggravate aGVHD (115). Thus, the antibiotics used in this patient group should be carefully chosen. It is clear from these studies that certain bacteria are beneficial within the GI tract of HSCT patients while others could be harmful. Higher diversity of gut microbiota and enrichment of beneficial bacteria are crucial for better GVHD outcomes.
Given this well-demonstrated trend, strategies that aim at preserving diversity and potentially enhancing the richness of beneficial gut microbiota have been proposed and investigated. One approach is the introduction of live microbes through fecal microbiota transplant (FMT). Pilot trials have shown that successful FMT from patients’ spouses, relatives, or even random, healthy donors led to partial or complete resolution of gastrointestinal GVHD (116, 117). Subsequent reports provided further evidence that FMT improved gut microbiota diversity and composition in GVHD patients (118, 119). Despite these positive results, the potential risk of bacteremia induced by FMT needs to be further addressed before this approach can be used more widely in this patient group (120). Probiotics may also be used to introduce live microbes. The effects of probiotics have been tested in animal models of HSCT. Oral administration of Lactobacillus rhamnosus GG led to improved post-transplant overall survival and reduced experimental GVHD (121). A more recent study demonstrated that a single strain of Bacteroides fragilis can improve gut barrier integrity and mitigates GVHD in mice (122). In human studies, the use of probiotics appeared to be safe in HSCT patients, but whether they improve transplant outcomes remains to be determined (123, 124).
Nutrients that are considered prebiotics and postbiotics have also been investigated. Prebiotics stimulate the growth of beneficial bacteria in the gut. Unlike probiotics, these are nutrients found in food and supplements and do not introduce live microbes. Some commercially available prebiotics include inulin, galacto-oligosaccharides (GOS), and fructo-oligosaccharides. Fructo-oligosaccharides are well-tolerated when given to patients receiving allogeneic HSCT and can modify gut microbiota composition and phenotypes of some immune cells (125). Yoshifuji et al. showed that the combination of resistant starch and other dietary prebiotics effectively reduced mucosal damage after transplantation, preserved microbiota diversity, and mitigated GVHD (126). Patients with higher baseline gut microbiota diversity appeared to benefit most from prebiotic supplementation, likely because fermentation of prebiotics requires a relatively intact gut microbiota. Holmes et al. recently found that mice supplemented with GOS had improved post-transplant survival in a well-established murine GVHD model, but only when control and experimental mice were also treated with antibiotics. This suggests that prebiotics may mitigate injury to the gut microbiome caused by antibiotics (127).
Given the potential infection risk associated with probiotics in immunocompromised HSCT patients and the requirement of a healthy gut microbial community to digest prebiotics, postbiotics seem to be a safer and more effective strategy (103, 128). Postbiotics are metabolites of beneficial gut bacteria. The most promising and well-studied postbiotic is butyrate, a short-chain fatty acid that can promote gut health. Indeed, it has been demonstrated that butyrate can mitigate experimental gastrointestinal GVHD (129).
Interplay between nutritional factors and gut microbiota during GVHD
Diet is one major factor that shapes gut microbiota in humans and experimental animals (130). Dietary macronutrients and micronutrients can influence the diversity and composition of gut microbiota. It has been well established that a high-fat, high-animal protein, and low-fiber dietary pattern contributes to the development of many metabolic and inflammatory diseases such as obesity, cancer, and cardiovascular diseases (131). Gut dysbiosis is often observed in these patients and is linked to their eating habits.
Accumulating evidence supports the active interplay between nutrients and gut microbiota during GVHD. We have already discussed the interaction of tryptophan, choline, and prebiotics with gut microbiota during GVHD. There is also evidence that tyrosine, a nonessential amino acid, impacts gut microbiota. In a mouse model of allogeneic HSCT, tyrosine was found to be reduced in the gut of GVHD mice (132). This was accompanied by a dramatic reduction of tyrosine metabolites as revealed by metabolomic analyses. Recipient mice fed a tyrosine-supplemented diet showed prolonged survival and decreased tissue damage in the early stages of GVHD. Tyrosine supplementation led to changes in the composition of gut microbiota and host metabolism, demonstrating a crosstalk between this amino acid and gut microbiota during GVHD (132).
There is also ample evidence that vitamins, particularly fat-soluble vitamins A and D, can actively participate in regulating gut microbiota (133–136). In the setting of allogeneic HSCT, we recently showed that vitamin A can modulate gut microbiota during GVHD (89). Vitamin A-supplemented mice had a reduced gut microbiota diversity. The abundance of opportunistic pathogens such as Escherichia-Shigella was increased in these mice, but the abundance of Bacteroides and Lactobacillus, two beneficial bacteria, were significantly decreased. In our model, vitamin A supplementation is associated with a higher degree of gut dysbiosis and inferior transplant outcomes.
Lactose, a nutrient commonly found in dairy products, has been shown to be critically involved in GVHD development (137). Lactose is required for the expansion of Enterococcus which can intensify gut inflammation. A lactose-free diet reduced the abundance of Enterococcus and mitigated GVHD severity in mice. Fecal domination by Enterococcus early after transplantation predicts a reduced overall survival and more severe GVHD. This study provides direct evidence of a causal relationship linking a common nutrient, gut microbiota, and the severity of intestinal and systemic alloimmune responses. Many transplant centers now provide a lactose-free diet to patients with intestinal GVHD (138).
Modification of gut microbiota is likely to be an important mechanism by which nutritional factors affect GVHD development, as being primarily discussed in this article. However, certain nutrients and/or their metabolites can also directly act on immune or nonimmune cells that are critically involved in the pathogenesis of aGVHD. For example, vitamins A and D can have pleiotropic effects on donor T cells and host APCs/DCs to modulate GVHD risk as reviewed previously (62).
Discussion and future directions
Despite the limitations that GVHD exhibits on the success of allogeneic HSCT, there are currently few effective options for prevention and treatment of the disease. Accumulating evidence points to nutrition as an important and modifiable nongenetic factor that may influence GVHD risk. A nutritional approach may be beneficial as dietary changes and nutritional supplements can be quickly and easily implemented by physicians into patient care plans.
As outlined in the above sections, enteral nutrition provides nutritional support superior to parenteral nutrition for patients with GVHD. Accumulating evidence also indicates that certain nutrients confer protective effects while other nutrients can worsen GVHD. Tryptophan and tyrosine supplementation appear to lead to better GVHD outcomes in animal models, but more human studies are needed to confirm these results. Nutrients that are considered essential for the general population could sometimes be detrimental for recipients of allogeneic HSCT. For example, choline accelerated GVHD mortality in one murine study. Vitamin A appears to have mixed results in animal studies. Due to its known influence on the immune system, vitamin A deserves to be studied further in the context of aGVHD. Pre-transplant nutritional status should be carefully considered and assessed for any potential indicators of GVHD risk.
From a therapeutic standpoint, we propose that nutritional intervention may be an attractive option for patients with GVHD. It is encouraging that various dietary intervention trials are ongoing (Table 1). There is convincing evidence indicating that gut microbiota diversity is directly related to the severity of gastrointestinal GVHD. Thus, interventions that can preserve intact diversity and restore injured gut microbiota should be investigated. Fecal microbiota transplant and probiotics are two options that deserve further exploration. There is also a clear interplay between the status of gut microbiota and dietary habits. This is true for all populations but is especially crucial in the context of allogeneic HSCT. The combination of fecal microbiota transplant and dietary intervention should be considered in clinical trials. Prebiotics and certain nutrients may also be used to restore microbiota diversity and composition to reduce GVHD. Unlike drug-based therapeutics that are expensive and require lengthy approval processes, nutrition-based approaches are expected to be inexpensive and easily applicable in the clinic with good safety profile. However, it is important to keep in mind that the translation of findings in animal models into clinical settings isn’t always straightforward and cannot be taken for granted. Also, severe aGVHD is a catabolic catastrophe that is unlikely to be managed by pure nutritional intervention. Instead, nutritional intervention can be easily incorporated into existing GVHD prevention or treatment protocols to improve patient outcomes. By adding beneficial nutritional and/or dietary factors, the use of drugs with significant side effects, such as steroids, may be reduced.
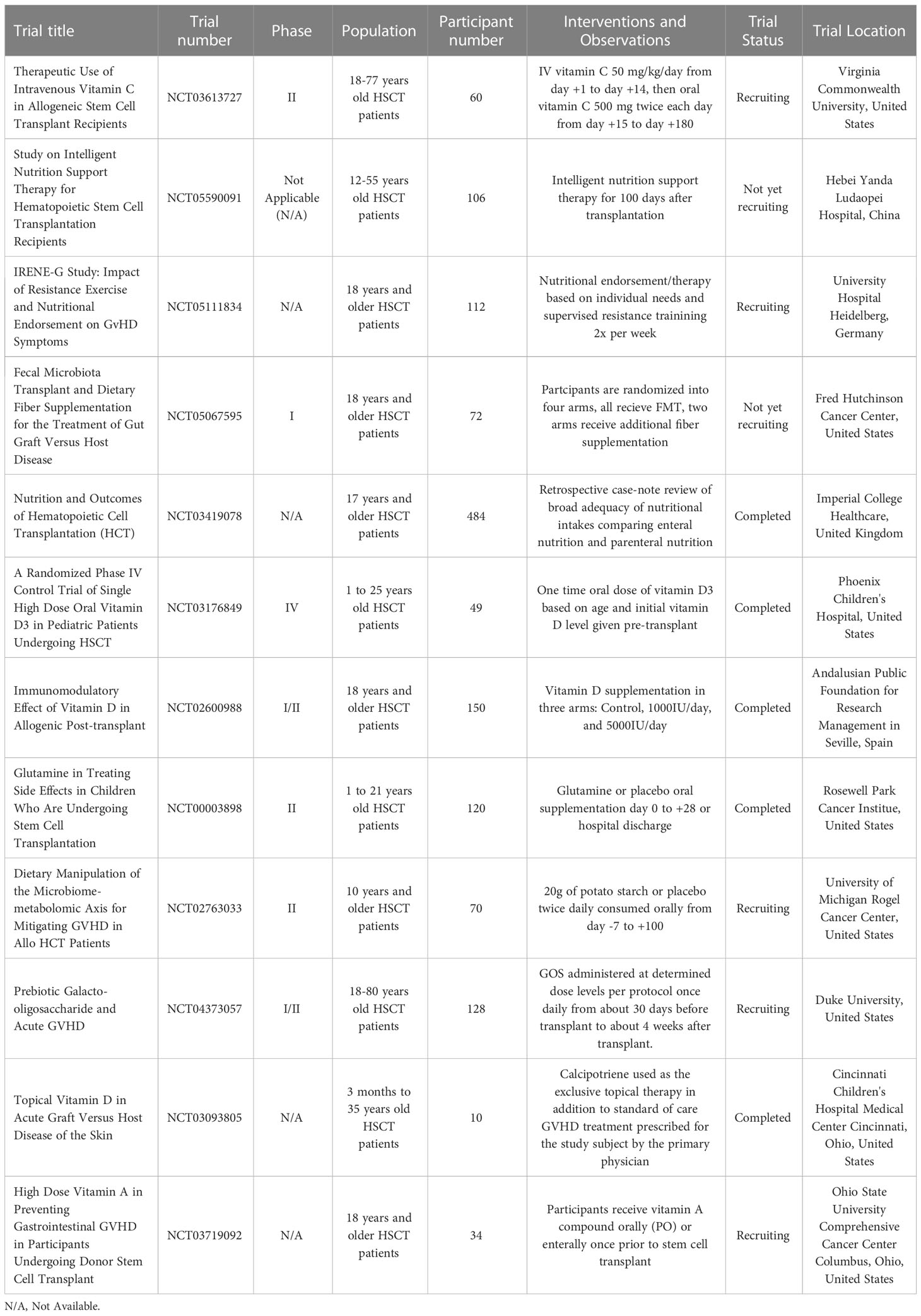
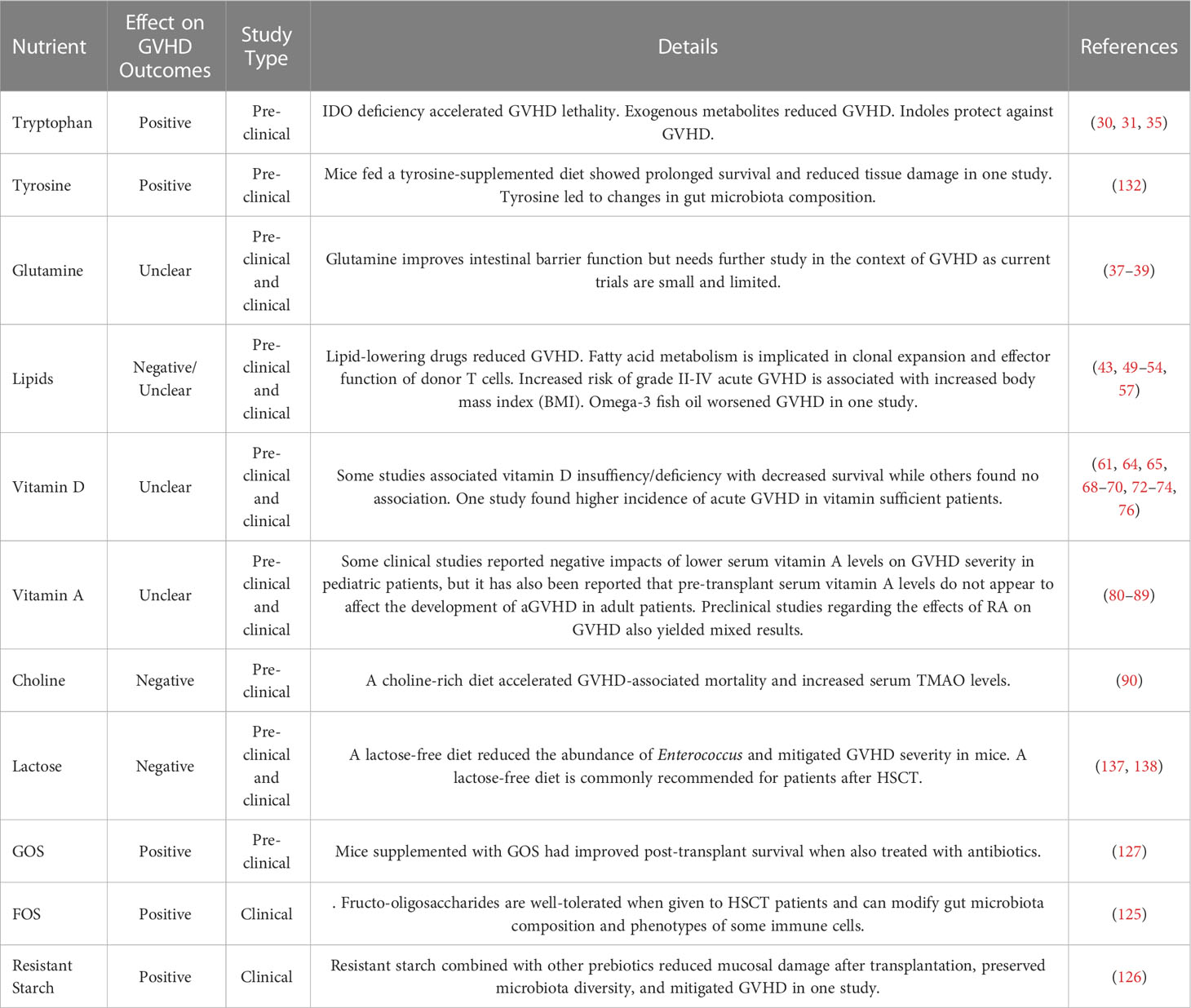
Table 1 Some human clinical trials of dietary and nutritional interventions for managing GVHD in HSCT patients.
In summary, nutritional management should be an integral part of medical care for patients undergoing allogeneic HSCT. Carefully designed and well-formulated nutritional support will not only improve the overall wellbeing of these patients but help mitigate transplant complications in this medically fragile patient group. In this regard, the role of nutrition could be shifting from support to therapy. There are numerous vitamins, minerals, amino acids, and other nutritional factors that impact human health and have immunomodulatory effects, many of which have not been explored in the specific context of allogeneic HSCT and GVHD. The current evidence proves that this is an area worth exploring further. There could be one or several nutritional components that are severely affecting GVHD patients, both positively and negatively (Table 2, Figure 1). Identifying these elements will help improve evidence-based guidelines for nutrition before and after transplantation. More well-designed clinical trials will help to determine potential links between nutrition and aGVHD in humans. Nutritional assessment as a standard before HSCT can also reveal how pretransplant nutritional status affects aGVHD pathogenesis. Gut microbiota is likely to serve as an important nexus linking nutrition and aGVHD outcomes during nutritional intervention. Further, in an era of precision medicine, tailored dietary intervention based on the unique nutritional status and microbial composition of individual patients is expected to achieve optimal results. We believe that a better understanding of the complex interplay between nutritional factors, gut microbiota, and mucosal immune responses will shed new light on aGVHD pathogenesis and management. We propose the use of dietary and nutritional intervention as a safe, easily applicable, and inexpensive monotherapy or adjunct therapy for patients with aGVHD by targeting gut microbiota.
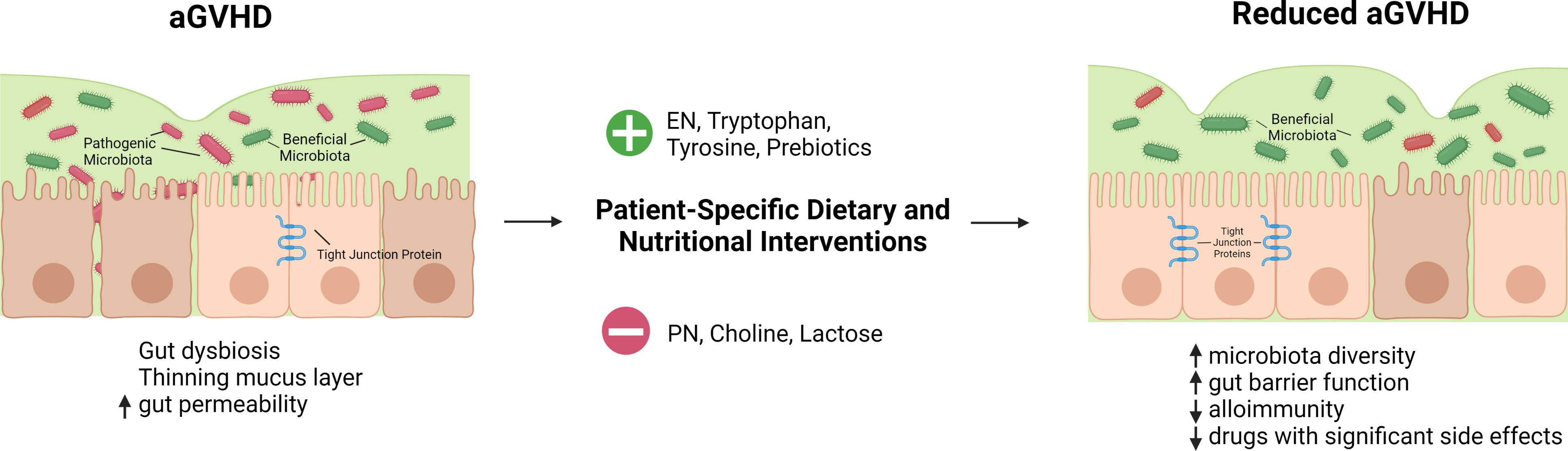
Figure 1 Hypothetical summary of nutritional and dietary interventions that may help reduce aGVHD. HSCT conditioning regimen, reduced oral intake, and transplant-associated inflammation lead to a thinning mucus layer, gut dysbiosis, and increased gut permeability. Gut dysbiosis is characterized by a reduction in the diversity of gut microbiota and short chain fatty acid-producing beneficial microbes as well as an expansion of pathogenic bacteria. Dietary and nutritional interventions tailored to patients' specific needs can improve these outcomes. Enteral nutrition is favorable over parenteral nutrition for patients after allogeneic HSCT. Tryptophan, and tyrosine supplementation as well as the use of prebiotics may have a beneficial effect while choline and lactose may have a negative impact. Nutritional interventions may correct gut dysbiosis, restore gut barrier function, and mitigate alloimmune response with the net outcome of reduced GVHD.  -supply
-supply  -avoid. Created with BioRender.com.
-avoid. Created with BioRender.com.
Author contributions
RL wrote and edited the manuscript. PP wrote the manuscript. L-SW discussed the work. XC conceived the concept and topic, supervised the work, wrote, and edited the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
This research was supported in part by National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases grants RO1 AI125334 and R21 AI144424.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
APCs, antigen presenting cells; BMI, body mass index; EFA, essential fatty acid; EN, enteral nutrition; FMT, fecal microbiota transplant; GOS, galacto-oligosaccharides; GVHD, graft-versus-host disease; GVL, graft-versus-leukemia HSCT, hematopoietic stem cell transplantation; IDO, indoleamine 2,3-dioxygenase; PN, parenteral nutrition; PUFA, polyunsaturated fatty acid RA, retinoic acid; RAR, retinoic acid receptor; TMAO, trimethylamine N-oxide; TPN, total parenteral nutrition; VDR, vitamin D receptor.
References
1. Gratwohl A, Pasquini MC, Aljurf M, Atsuta Y, Baldomero H, Foeken L, et al. One million haemopoietic stem-cell transplants: a retrospective observational study. Lancet Haematol (2015) 2:e91–100. doi: 10.1016/S2352-3026(15)00028-9
2. Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood (1990) 75:555–62. doi: 10.1182/blood.V75.3.555.555
4. Ferrara JL, Smith CM, Sheets J, Reddy P, Serody JS. Altered homeostatic regulation of innate and adaptive immunity in lower gastrointestinal tract GVHD pathogenesis. J Clin Invest (2017) 127:2441–51. doi: 10.1172/JCI90592
5. Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol (2007) 25:139–70. doi: 10.1146/annurev.immunol.25.022106.141606
6. Choi SW, Reddy P. Current and emerging strategies for the prevention of graft-versus-host disease. Nat Rev Clin Oncol (2014) 11:536–47. doi: 10.1038/nrclinonc.2014.102
7. Zeiser R, Blazar BR. Acute graft-versus-Host disease - biologic process, prevention, and therapy. N Engl J Med (2017) 377:2167–79. doi: 10.1056/NEJMra1609337
8. Hill GR, Betts BC, Tkachev V, Kean LS, Blazar BR. Current concepts and advances in graft-Versus-Host disease immunology. Annu Rev Immunol (2021) 39:19–49. doi: 10.1146/annurev-immunol-102119-073227
9. van der Meij BS, de Graaf P, Wierdsma NJ, Langius J a. E, Janssen JJWM, van Leeuwen P a. M, et al. Nutritional support in patients with GVHD of the digestive tract: state of the art. Bone Marrow Transplant (2013) 48:474–82. doi: 10.1038/bmt.2012.124
10. Bassim CW, Fassil H, Dobbin M, Steinberg SM, Baird K, Cole K, et al. Malnutrition in patients with chronic GVHD. Bone Marrow Transplant (2014) 49:1300–6. doi: 10.1038/bmt.2014.145
11. Ayuk F, Bussmann L, Zabelina T, Veit R, Alchalby H, Wolschke C, et al. Serum albumin level predicts survival of patients with gastrointestinal acute graft-versus-host disease after allogeneic stem cell transplantation. Ann Hematol (2014) 93:855–61. doi: 10.1007/s00277-013-1957-0
12. Ferreira EE, Guerra DC, Baluz K, de Resende Furtado W, da Silva Bouzas LF. Nutritional status of patients submitted to transplantation of allogeneic hematopoietic stem cells: a retrospective study. Rev Bras Hematol E Hemoter (2014) 36:414–9. doi: 10.1016/j.bjhh.2014.07.014
13. Fuji S, Einsele H, Savani BN, Kapp M. Systematic nutritional support in allogeneic hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant (2015) 21:1707–13. doi: 10.1016/j.bbmt.2015.07.003
14. Baumgartner A, Bargetzi M, Bargetzi A, Zueger N, Medinger M, Passweg J, et al. Nutritional support practices in hematopoietic stem cell transplantation centers: a nationwide comparison. Nutr Burbank Los Angel Cty Calif (2017) 35:43–50. doi: 10.1016/j.nut.2016.10.007
15. Fuji S, Kim S-W, Mori S, Fukuda T, Kamiya S, Yamasaki S, et al. Hyperglycemia during the neutropenic period is associated with a poor outcome in patients undergoing myeloablative allogeneic hematopoietic stem cell transplantation. Transplantation (2007) 84:814–20. doi: 10.1097/01.tp.0000296482.50994.1c
16. Gebremedhin E, Behrendt CE, Nakamura R, Parker P, Salehian B. Severe hyperglycemia immediately after allogeneic hematopoietic stem-cell transplantation is predictive of acute graft-versus-host disease. Inflammation (2013) 36:177–85. doi: 10.1007/s10753-012-9533-7
17. Gonzales F, Bruno B, Alarcón Fuentes M, De Berranger E, Guimber D, Behal H, et al. Better early outcome with enteral rather than parenteral nutrition in children undergoing MAC allo-SCT. Clin Nutr Edinb Scotl (2018) 37:2113–21. doi: 10.1016/j.clnu.2017.10.005
18. Seguy D, Duhamel A, Rejeb MB, Gomez E, Buhl ND, Bruno B, et al. Better outcome of patients undergoing enteral tube feeding after myeloablative conditioning for allogeneic stem cell transplantation. Transplantation (2012) 94:287–94. doi: 10.1097/TP.0b013e3182558f60
19. Beckerson J, Szydlo RM, Hickson M, Mactier CE, Innes AJ, Gabriel IH, et al. Impact of route and adequacy of nutritional intake on outcomes of allogeneic haematopoietic cell transplantation for haematologic malignancies. Clin Nutr Edinb Scotl (2019) 38:738–44. doi: 10.1016/j.clnu.2018.03.008
20. Seguy D, Berthon C, Micol J-B, Darré S, Dalle J-H, Neuville S, et al. Enteral feeding and early outcomes of patients undergoing allogeneic stem cell transplantation following myeloablative conditioning. Transplantation (2006) 82:835–9. doi: 10.1097/01.tp.0000229419.73428.ff
21. Imataki O, Nakatani S, Hasegawa T, Kondo M, Ichihashi K, Araki M, et al. Nutritional support for patients suffering from intestinal graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Am J Hematol (2006) 81:747–52. doi: 10.1002/ajh.20700
22. Guièze R, Lemal R, Cabrespine A, Hermet E, Tournilhac O, Combal C, et al. Enteral versus parenteral nutritional support in allogeneic haematopoietic stem-cell transplantation. Clin Nutr Edinb Scotl (2014) 33:533–8. doi: 10.1016/j.clnu.2013.07.012
23. Koç N, Gündüz M, Azık MF, Tavil B, Gürlek-Gökçebay D, Özaydın E, et al. Stepwise diet management in pediatric gastrointestinal graft versus host disease. Turk J Pediatr (2016) 58:145–51. doi: 10.24953/turkjped.2016.02.004
24. Andersen S, Brown T, Kennedy G, Banks M. Implementation of an evidenced based nutrition support pathway for haematopoietic progenitor cell transplant patients. Clin Nutr Edinb Scotl (2015) 34:536–40. doi: 10.1016/j.clnu.2014.06.006
25. Mattsson J, Westin S, Edlund S, Remberger M. Poor oral nutrition after allogeneic stem cell transplantation correlates significantly with severe graft-versus-host disease. Bone Marrow Transplant (2006) 38:629–33. doi: 10.1038/sj.bmt.1705493
26. Svahn B-M, Remberger M, Heijbel M, Martell E, Wikström M, Eriksson B, et al. Case-control comparison of at-home and hospital care for allogeneic hematopoietic stem-cell transplantation: the role of oral nutrition. Transplantation (2008) 85:1000–7. doi: 10.1097/TP.0b013e31816a3267
27. Fuji S, Cheng J, Yakushijin K, Wanitpongpun C. Nutritional support in allogeneic hematopoietic stem cell transplantation Asian perspective. Blood Cell Ther (2022) 5:54–60. doi: 10.31547/bct-2021-024
28. Modoux M, Rolhion N, Mani S, Sokol H. Tryptophan metabolism as a pharmacological target. Trends Pharmacol Sci (2021) 42:60–73. doi: 10.1016/j.tips.2020.11.006
29. Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol (2004) 4:762–74. doi: 10.1038/nri1457
30. Jasperson LK, Bucher C, Panoskaltsis-Mortari A, Taylor PA, Mellor AL, Munn DH, et al. Indoleamine 2,3-dioxygenase is a critical regulator of acute graft-versus-host disease lethality. Blood (2008) 111:3257–65. doi: 10.1182/blood-2007-06-096081
31. Jasperson LK, Bucher C, Panoskaltsis-Mortari A, Mellor AL, Munn DH, Blazar BR. Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood (2009) 114:5062–70. doi: 10.1182/blood-2009-06-227587
32. Belle L, Zhou V, Stuhr KL, Beatka M, Siebers EM, Knight JM, et al. Host interleukin 6 production regulates inflammation but not tryptophan metabolism in the brain during murine GVHD. JCI Insight (2017) 2:93726. doi: 10.1172/jci.insight.93726
33. Ratajczak P, Janin A, Peffault de Larour R, Koch L, Roche B, Munn D, et al. IDO in human gut graft-versus-host disease. Biol Blood Marrow Transplant (2012) 18:150–5. doi: 10.1016/j.bbmt.2011.08.002
34. Landfried K, Zhu W, Waldhier MC, Schulz U, Ammer J, Holler B, et al. Tryptophan catabolism is associated with acute GVHD after human allogeneic stem cell transplantation and indicates activation of indoleamine 2,3-dioxygenase. Blood (2011) 118:6971–4. doi: 10.1182/blood-2011-06-357814
35. Swimm A, Giver CR, DeFilipp Z, Rangaraju S, Sharma A, Ulezko Antonova A, et al. Indoles derived from intestinal microbiota act via type I interferon signaling to limit graft-versus-host disease. Blood (2018) 132:2506–19. doi: 10.1182/blood-2018-03-838193
36. Wischmeyer PE. Glutamine: role in gut protection in critical illness. Curr Opin Clin Nutr Metab Care (2006) 9:607–12. doi: 10.1097/01.mco.0000241672.09676.03
37. Noth R, Häsler R, Stüber E, Ellrichmann M, Schäfer H, Geismann C, et al. Oral glutamine supplementation improves intestinal permeability dysfunction in a murine acute graft-vs.-host disease model. Am J Physiol Gastrointest Liver Physiol (2013) 304:G646–654. doi: 10.1152/ajpgi.00246.2012
38. Crowther M. Hot topics in parenteral nutrition. a review of the use of glutamine supplementation in the nutritional support of patients undergoing bone-marrow transplantation and traditional cancer therapy. Proc Nutr Soc (2009) 68:269–73. doi: 10.1017/S0029665109001384
39. Crowther M, Avenell A, Culligan DJ. Systematic review and meta-analyses of studies of glutamine supplementation in haematopoietic stem cell transplantation. Bone Marrow Transplant (2009) 44:413–25. doi: 10.1038/bmt.2009.41
40. Kagoya Y, Seo S, Nannya Y, Kurokawa M. Hyperlipidemia after allogeneic stem cell transplantation: prevalence, risk factors, and impact on prognosis. Clin Transplant (2012) 26:E168–175. doi: 10.1111/j.1399-0012.2012.01628.x
41. Weiss BM, Vogl DT, Berger NA, Stadtmauer EA, Lazarus HM. Trimming the fat: obesity and hematopoietic cell transplantation. Bone Marrow Transplant (2013) 48:1152–60. doi: 10.1038/bmt.2012.201
42. Nakao M, Chihara D, Niimi A, Ueda R, Tanaka H, Morishima Y, et al. Impact of being overweight on outcomes of hematopoietic SCT: a meta-analysis. Bone Marrow Transplant (2014) 49:66–72. doi: 10.1038/bmt.2013.128
43. Fuji S, Kim S-W, Yoshimura K, Akiyama H, Okamoto S, Sao H, et al. Japan Marrow donor program. possible association between obesity and posttransplantation complications including infectious diseases and acute graft-versus-host disease. Biol Blood Marrow Transplant (2009) 15:73–82. doi: 10.1016/j.bbmt.2008.10.029
44. Khuat LT, Le CT, Pai C-CS, Shields-Cutler RR, Holtan SG, Rashidi A, et al. Obesity induces gut microbiota alterations and augments acute graft-versus-host disease after allogeneic stem cell transplantation. Sci Transl Med (2020) 12:eaay7713. doi: 10.1126/scitranslmed.aay7713
45. Khuat LT, Vick LV, Choi E, Dunai C, Merleev AA, Maverakis E, et al. Mechanisms by which obesity promotes acute graft-Versus-Host disease in mice. Front Immunol (2021) 12:752484. doi: 10.3389/fimmu.2021.752484
46. Hamadani M, Gibson LF, Remick SC, Wen S, Petros W, Tse W, et al. Sibling donor and recipient immune modulation with atorvastatin for the prophylaxis of acute graft-versus-host disease. J Clin Oncol (2013) 31:4416–23. doi: 10.1200/JCO.2013.50.8747
47. Rotta M, Storer BE, Storb RF, Martin PJ, Heimfeld S, Peffer A, et al. Donor statin treatment protects against severe acute graft-versus-host disease after related allogeneic hematopoietic cell transplantation. Blood (2010) 115:1288–95. doi: 10.1182/blood-2009-08-240358
48. Efebera YA, Geyer S, Andritsos L, Vasu S, Jaglowski S, Bingman A, et al. Atorvastatin for the prophylaxis of acute graft-versus-Host disease in patients undergoing HLA-matched related donor allogeneic hematopoietic stem cell transplantation (allo-HCT). Biol Blood Marrow Transplant (2016) 22:71–9. doi: 10.1016/j.bbmt.2015.07.034
49. Pabst C, Schreck N, Benner A, Hegenbart U, Schönland S, Radujkovic A, et al. Statin-based endothelial prophylaxis and outcome after allogeneic stem cell transplantation. Eur J Clin Invest (2023) 53:e13883. doi: 10.1111/eci.13883
50. Zeiser R, Youssef S, Baker J, Kambham N, Steinman L, Negrin RS. Preemptive HMG-CoA reductase inhibition provides graft-versus-host disease protection by Th-2 polarization while sparing graft-versus-leukemia activity. Blood (2007) 110:4588–98. doi: 10.1182/blood-2007-08-106005
51. Shimabukuro-Vornhagen A, Liebig T, von Bergwelt-Baildon M. Statins inhibit human APC function: implications for the treatment of GVHD. Blood (2008) 112:1544–5. doi: 10.1182/blood-2008-04-149609
52. Byersdorfer CA, Tkachev V, Opipari AW, Goodell S, Swanson J, Sandquist S, et al. Effector T cells require fatty acid metabolism during murine graft-versus-host disease. Blood (2013) 122:3230–7. doi: 10.1182/blood-2013-04-495515
53. Raha S, Raud B, Oberdörfer L, Castro CN, Schreder A, Freitag J, et al. Disruption of de novo fatty acid synthesis via acetyl-CoA carboxylase 1 inhibition prevents acute graft-versus-host disease. Eur J Immunol (2016) 46:2233–8. doi: 10.1002/eji.201546152
54. Zou Y, Chen BJ. T Cell metabolism in graft-versus-host disease. Blood Sci Baltim Md (2020) 2:16–21. doi: 10.1097/BS9.0000000000000035
55. Contaifer D, Roberts CH, Kumar NG, Natarajan R, Fisher BJ, Leslie K, et al. A preliminary investigation towards the risk stratification of allogeneic stem cell recipients with respect to the potential for development of GVHD via their pre-transplant plasma lipid and metabolic signature. Cancers (2019) 11:1051. doi: 10.3390/cancers11081051
56. Kominsky DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol Baltim Md 1950 (2010) 184:4062–8. doi: 10.4049/jimmunol.0903002
57. Al Hashmi S, Sadeghi B, Hassan Z, Abedi-Valugerdi M, Lindskog M, Hassan M. Omega-3 from fish oil augments GVHD through the enhancement of chemotherapy conditioning regimen and selective FoxP3 depletion. Bone Marrow Transplant (2013) 48:843–8. doi: 10.1038/bmt.2012.227
58. Hewison M. Vitamin D. And immune function: an overview. Proc Nutr Soc (2012) 71:50–61. doi: 10.1017/S0029665111001650
59. Cantorna MT, Snyder L, Lin Y-D, Yang L. Vitamin d and 1,25(OH)2D regulation of T cells. Nutrients (2015) 7:3011–21. doi: 10.3390/nu7043011
60. Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ. Vitamin d: metabolism. Endocrinol Metab Clin North Am (2010) 39:243–53. doi: 10.1016/j.ecl.2010.02.002
61. Benrashid M, Moyers K, Mohty M, Savani BN. Vitamin d deficiency, autoimmunity, and graft-versus-host-disease risk: implication for preventive therapy. Exp Hematol (2012) 40:263–7. doi: 10.1016/j.exphem.2012.01.006
62. Chen X, Mayne CG. The role of micronutrients in graft-VS.-Host disease: immunomodulatory effects of vitamins a and d. Front Immunol (2018) 9:2853. doi: 10.3389/fimmu.2018.02853
63. Flamann C, Peter K, Kreutz M, Bruns H. Regulation of the immune balance during allogeneic hematopoietic stem cell transplantation by vitamin d. Front Immunol (2019) 10:2586. doi: 10.3389/fimmu.2019.02586
64. Soto JR, Anthias C, Madrigal A, Snowden JA. Insights into the role of vitamin d as a biomarker in stem cell transplantation. Front Immunol (2020) 11:966. doi: 10.3389/fimmu.2020.00966
65. Wallace G, Jodele S, Howell J, Myers KC, Teusink A, Zhao X, et al. Vitamin d deficiency and survival in children after hematopoietic stem cell transplant. Biol Blood Marrow Transplant (2015) 21:1627–31. doi: 10.1016/j.bbmt.2015.06.009
66. Sproat L, Bolwell B, Rybicki L, Dean R, Sobecks R, Pohlman B, et al. Vitamin d level after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant (2011) 17:1079–83. doi: 10.1016/j.bbmt.2010.12.704
67. Robien K, Strayer LG, Majhail N, Lazovich D, Baker KS, Smith AR, et al. Vitamin d status among long-term survivors of hematopoietic cell transplantation. Bone Marrow Transplant (2011) 46:1472–9. doi: 10.1038/bmt.2010.326
68. Urbain P, Ihorst G, Biesalski H-K, Bertz H. Course of serum 25-hydroxyvitamin D(3) status and its influencing factors in adults undergoing allogeneic hematopoietic cell transplantation. Ann Hematol (2012) 91:759–66. doi: 10.1007/s00277-011-1365-2
69. Glotzbecker B, Ho VT, Aldridge J, Kim HT, Horowitz G, Ritz J, et al. Low levels of 25-hydroxyvitamin d before allogeneic hematopoietic SCT correlate with the development of chronic GVHD. Bone Marrow Transplant (2013) 48:593–7. doi: 10.1038/bmt.2012.177
70. Hansson MEA, Norlin A-C, Omazic B, Wikström A-C, Bergman P, Winiarski J, et al. Vitamin d levels affect outcome in pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transplant (2014) 20:1537–43. doi: 10.1016/j.bbmt.2014.05.030
71. Campos DJ, Boguszewski CL, Funke VAM, Bonfim CMS, Kulak CAM, Pasquini R, et al. Bone mineral density, vitamin d, and nutritional status of children submitted to hematopoietic stem cell transplantation. Nutr Burbank Los Angel Cty Calif (2014) 30:654–9. doi: 10.1016/j.nut.2013.10.014
72. von Bahr L, Blennow O, Alm J, Björklund A, Malmberg K-J, Mougiakakos D, et al. Increased incidence of chronic GvHD and CMV disease in patients with vitamin d deficiency before allogeneic stem cell transplantation. Bone Marrow Transplant (2015) 50:1217–23. doi: 10.1038/bmt.2015.123
73. Myers KC, Howell JC, Wallace G, Dandoy C, El-Bietar J, Lane A, et al. Poor growth, thyroid dysfunction and vitamin d deficiency remain prevalent despite reduced intensity chemotherapy for hematopoietic stem cell transplantation in children and young adults. Bone Marrow Transplant (2016) 51:980–4. doi: 10.1038/bmt.2016.39
74. Beebe K, Magee K, McNulty A, Stahlecker J, Salzberg D, Miller H, et al. Vitamin d deficiency and outcomes in pediatric hematopoietic stem cell transplantation. Pediatr Blood Cancer (2018) 65. doi: 10.1002/pbc.26817
75. Wallace G, Jodele S, Myers KC, Dandoy CE, El-Bietar J, Nelson A, et al. Vitamin d deficiency in pediatric hematopoietic stem cell transplantation patients despite both standard and aggressive supplementation. Biol Blood Marrow Transplant (2016) 22:1271–4. doi: 10.1016/j.bbmt.2016.03.026
76. Caballero-Velázquez T, Montero I, Sánchez-Guijo F, Parody R, Saldaña R, Valcarcel D, et al. Immunomodulatory Effect of Vitamin D after Allogeneic Stem Cell Transplantation: Results of a Prospective Multicenter Clinical Trial. Clin Cancer Res Off J Am Assoc Cancer Res (2016) 22:5673–81. doi: 10.1158/1078-0432.CCR-16-0238
77. Carrillo-Cruz E, García-Lozano JR, Márquez-Malaver FJ, Sánchez-Guijo FM, Montero Cuadrado I, Ferra Coll I C, et al. Vitamin D Modifies the Incidence of Graft-versus-Host Disease after Allogeneic Stem Cell Transplantation Depending on the Vitamin D Receptor (VDR) Polymorphisms. Clin Cancer Res Off J Am Assoc Cancer Res (2019) 25:4616–4623. doi: 10.1158/1078-0432.CCR-18-3875
78. Larange A, Cheroutre H. Retinoic acid and retinoic acid receptors as pleiotropic modulators of the immune system. Annu Rev Immunol (2016) 34:369–94. doi: 10.1146/annurev-immunol-041015-055427
79. Zheng J, Taylor B, Chen X. Role of vitamin a in modulating graft-versus-Host disease. J Immunol Res Ther (2018) 3:124–8.
80. Lounder DT, Khandelwal P, Dandoy CE, Jodele S, Grimley MS, Wallace G, et al. Lower levels of vitamin a are associated with increased gastrointestinal graft-versus-host disease in children. Blood (2017) 129:2801–7. doi: 10.1182/blood-2017-02-765826
81. Carpenter PA. Vitamin a to reduce gut leak and GVHD? Blood (2017) 129:2715–7. doi: 10.1182/blood-2017-03-773226
82. Gjærde LK, Andersen NS, Friis LS, Kornblit B, Petersen SL, Schjødt I, et al. Pretransplantation vitamin a plasma levels and risk of acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant (2020) 55:1457–9. doi: 10.1038/s41409-019-0760-5
83. Ball JA, Clear A, Aries J, Charrot S, Besley C, Mee M, et al. Retinoic acid-responsive CD8 effector T cells are selectively increased in IL-23-rich tissue in gastrointestinal GVHD. Blood (2021) 137:702–17. doi: 10.1182/blood.2020005170
84. Yang H, Gu J, Zhu Q, Lu H, Wang K, Ni X, et al. Protection of acute GVHD by all-trans retinoic acid through suppression of T cell expansion and induction of regulatory T cells through IL-2 signaling. Int Immunopharmacol (2015) 28:911–6. doi: 10.1016/j.intimp.2015.03.042
85. Koenecke C, Prinz I, Bubke A, Schreder A, Lee C-W, Pabst O, et al. Shift of graft-versus-host-disease target organ tropism by dietary vitamin a. PloS One (2012) 7:e38252. doi: 10.1371/journal.pone.0038252
86. Chen X, Dodge J, Komorowski R, Drobyski WR. A critical role for the retinoic acid signaling pathway in the pathophysiology of gastrointestinal graft-versus-host disease. Blood (2013) 121:3970–80. doi: 10.1182/blood-2012-08-445130
87. Aoyama K, Saha A, Tolar J, Riddle MJ, Veenstra RG, Taylor PA, et al. Inhibiting retinoic acid signaling ameliorates graft-versus-host disease by modifying T-cell differentiation and intestinal migration. Blood (2013) 122:2125–34. doi: 10.1182/blood-2012-11-470252
88. Wang D, Yu Y, Haarberg K, Fu J, Kaosaard K, Nagaraj S, et al. Dynamic change and impact of myeloid-derived suppressor cells in allogeneic bone marrow transplantation in mice. Biol Blood Marrow Transplant (2013) 19:692–702. doi: 10.1016/j.bbmt.2013.01.008
89. Pan P, Atkinson SN, Taylor B, Zhu H, Zhou D, Flejsierowicz P, et al. Retinoic acid signaling modulates recipient gut barrier integrity and microbiota after allogeneic hematopoietic stem cell transplantation in mice. Front Immunol (2021) 12:749002. doi: 10.3389/fimmu.2021.749002
90. Wu K, Yuan Y, Yu H, Dai X, Wang S, Sun Z, et al. The gut microbial metabolite trimethylamine n-oxide aggravates GVHD by inducing M1 macrophage polarization in mice. Blood (2020) 136:501–15. doi: 10.1182/blood.2019003990
91. Zeisel SH, da Costa K-A. Choline: an essential nutrient for public health. Nutr Rev (2009) 67:615–23. doi: 10.1111/j.1753-4887.2009.00246.x
92. Liu Y, Dai M. Trimethylamine n-oxide generated by the gut microbiota is associated with vascular inflammation: new insights into atherosclerosis. Mediators Inflammation (2020) 2020:4634172. doi: 10.1155/2020/4634172
93. Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Ther Adv Gastroenterol (2013) 6:295–308. doi: 10.1177/1756283X13482996
94. Song M, Chan AT, Sun J. Influence of the gut microbiome, diet, and environment on risk of colorectal cancer. Gastroenterology (2020) 158:322–40. doi: 10.1053/j.gastro.2019.06.048
95. Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U.S.A. (2009) 106:2365–70. doi: 10.1073/pnas.0812600106
96. Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature (2012) 490:55–60. doi: 10.1038/nature11450
97. Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature (2011) 474:327–36. doi: 10.1038/nature10213
98. Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol (2017) 14:573–84. doi: 10.1038/nrgastro.2017.88
99. Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med (2012) 209:903–11. doi: 10.1084/jem.20112408
100. Docampo MD, Auletta JJ, Jenq RR. Emerging influence of the intestinal microbiota during allogeneic hematopoietic cell transplantation: control the gut and the body will follow. Biol Blood Marrow Transplant (2015) 21:1360–6. doi: 10.1016/j.bbmt.2015.02.016
101. Peled JU, Hanash AM, Jenq RR. Role of the intestinal mucosa in acute gastrointestinal GVHD. Blood (2016) 128:2395–402. doi: 10.1182/blood-2016-06-716738
102. Staffas A, Burgos da Silva M, van den Brink MRM. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood (2017) 129:927–33. doi: 10.1182/blood-2016-09-691394
103. Shono Y, van den Brink MRM. Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat Rev Cancer (2018) 18:283–95. doi: 10.1038/nrc.2018.10
104. Rafei H, Jenq RR. Microbiome-intestine cross talk during acute graft-versus-host disease. Blood (2020) 136:401–9. doi: 10.1182/blood.2019000950
105. Schwabkey ZI, Jenq RR. Microbiome anomalies in allogeneic hematopoietic cell transplantation. Annu Rev Med (2020) 71:137–48. doi: 10.1146/annurev-med-052918-122440
106. Korell F, Schreck N, Müller-Tidow C, Dreger P, Luft T. Taskforce allogeneic stem cell transplantation, university hospital heidelberg. pre-transplant EASIX and sepsis after allogeneic stem cell transplantation. Intensive Care Med (2022) 48:753–5. doi: 10.1007/s00134-022-06676-3
107. Luft T, Dietrich S, Falk C, Conzelmann M, Hess M, Benner A, et al. Steroid-refractory GVHD: T-cell attack within a vulnerable endothelial system. Blood (2011) 118:1685–92. doi: 10.1182/blood-2011-02-334821
108. Wall SA, Zhao Q, Yearsley M, Blower L, Agyeman A, Ranganathan P, et al. Complement-mediated thrombotic microangiopathy as a link between endothelial damage and steroid-refractory GVHD. Blood Adv (2018) 2:2619–28. doi: 10.1182/bloodadvances.2018020321
109. Zeisbrich M, Becker N, Benner A, Radujkovic A, Schmitt K, Beimler J, et al. Transplant-associated thrombotic microangiopathy is an endothelial complication associated with refractoriness of acute GvHD. Bone Marrow Transplant (2017) 52:1399–405. doi: 10.1038/bmt.2017.119
110. Jenq RR, Taur Y, Devlin SM, Ponce DM, Goldberg JD, Ahr KF, et al. Intestinal blautia is associated with reduced death from graft-versus-Host disease. Biol Blood Marrow Transplant (2015) 21:1373–83. doi: 10.1016/j.bbmt.2015.04.016
111. Holler E, Butzhammer P, Schmid K, Hundsrucker C, Koestler J, Peter K, et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant (2014) 20:640–5. doi: 10.1016/j.bbmt.2014.01.030
112. Taur Y, Jenq RR, Perales M-A, Littmann ER, Morjaria S, Ling L, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood (2014) 124:1174–82. doi: 10.1182/blood-2014-02-554725
113. Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis (2012) 55:905–14. doi: 10.1093/cid/cis580
114. Shono Y, Docampo MD, Peled JU, Perobelli SM, Velardi E, Tsai JJ, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med (2016) 8:339ra71. doi: 10.1126/scitranslmed.aaf2311
115. Hayase E, Hayase T, Jamal MA, Miyama T, Chang C-C, Ortega MR, et al. Mucus-degrading bacteroides link carbapenems to aggravated graft-versus-host disease. Cell (2022) 185:3705–19.e14. doi: 10.1016/j.cell.2022.09.007
116. Kakihana K, Fujioka Y, Suda W, Najima Y, Kuwata G, Sasajima S, et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood (2016) 128:2083–8. doi: 10.1182/blood-2016-05-717652
117. Spindelboeck W, Schulz E, Uhl B, Kashofer K, Aigelsreiter A, Zinke-Cerwenka W, et al. Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft-versus-host-disease. Haematologica (2017) 102:e210–3. doi: 10.3324/haematol.2016.154351
118. Kaito S, Toya T, Yoshifuji K, Kurosawa S, Inamoto K, Takeshita K, et al. Fecal microbiota transplantation with frozen capsules for a patient with refractory acute gut graft-versus-host disease. Blood Adv (2018) 2:3097–101. doi: 10.1182/bloodadvances.2018024968
119. Zhao Y, Li X, Zhou Y, Gao J, Jiao Y, Zhu B, et al. Safety and efficacy of fecal microbiota transplantation for grade IV steroid refractory GI-GvHD patients: interim results from FMT2017002 trial. Front Immunol (2021) 12:678476. doi: 10.3389/fimmu.2021.678476
120. DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, et al. Drug-resistant e. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med (2019) 381:2043–50. doi: 10.1056/NEJMoa1910437
121. Gerbitz A, Schultz M, Wilke A, Linde H-J, Schölmerich J, Andreesen R, et al. Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood (2004) 103:4365–7. doi: 10.1182/blood-2003-11-3769
122. Sofi MH, Wu Y, Ticer T, Schutt S, Bastian D, Choi H-J, et al. A single strain of bacteroides fragilis protects gut integrity and reduces GVHD. JCI Insight (2021) 6. doi: 10.1172/jci.insight.136841
123. L Ej, B M, C L, S E, P A, B Dm, et al. The safety and feasibility of probiotics in children and adolescents undergoing hematopoietic cell transplantation. Bone Marrow Transplant (2016) 51. doi: 10.1038/bmt.2015.275
124. Gorshein E, Wei C, Ambrosy S, Budney S, Vivas J, Shenkerman A, et al. Lactobacillus rhamnosus GG probiotic enteric regimen does not appreciably alter the gut microbiome or provide protection against GVHD after allogeneic hematopoietic stem cell transplantation. Clin Transplant (2017) 31. doi: 10.1111/ctr.12947
125. Andermann TM, Fouladi F, Tamburini FB, Sahaf B, Tkachenko E, Greene C, et al. A fructo-oligosaccharide prebiotic is well tolerated in adults undergoing allogeneic hematopoietic stem cell transplantation: a phase I dose-escalation trial. Transplant Cell Ther (2021) S2666-6367(21):01074–5. doi: 10.1016/j.jtct.2021.07.009
126. Yoshifuji K, Inamoto K, Kiridoshi Y, Takeshita K, Sasajima S, Shiraishi Y, et al. Prebiotics protect against acute graft-versus-host disease and preserve the gut microbiota in stem cell transplantation. Blood Adv (2020) 4:4607–17. doi: 10.1182/bloodadvances.2020002604
127. Holmes ZC, Tang H, Liu C, Bush A, Neubert BC, Jiao Y, et al. Prebiotic galactooligosaccharides interact with mouse gut microbiota to attenuate acute graft-versus-host disease. Blood (2022) 140:2300–4. doi: 10.1182/blood.2021015178
128. Andermann TM, Rezvani A, Bhatt AS. Microbiota manipulation with prebiotics and probiotics in patients undergoing stem cell transplantation. Curr Hematol Malig Rep (2016) 11:19–28. doi: 10.1007/s11899-016-0302-9
129. Mathewson ND, Jenq R, Mathew AV, Koenigsknecht M, Hanash A, Toubai T, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol (2016) 17:505–13. doi: 10.1038/ni.3400
130. Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature (2018) 555:210–5. doi: 10.1038/nature25973
131. Christ A, Lauterbach M, Latz E. Western Diet and the immune system: an inflammatory connection. Immunity (2019) 51:794–811. doi: 10.1016/j.immuni.2019.09.020
132. Li X, Lin Y, Li X, Xu X, Zhao Y, Xu L, et al. Tyrosine supplement ameliorates murine aGVHD by modulation of gut microbiome and metabolome. EBioMedicine (2020) 61:103048. doi: 10.1016/j.ebiom.2020.103048
133. Singh P, Rawat A, Alwakeel M, Sharif E, Al Khodor S. The potential role of vitamin d supplementation as a gut microbiota modifier in healthy individuals. Sci Rep (2020) 10:21641. doi: 10.1038/s41598-020-77806-4
134. Naderpoor N, Mousa A, Fernanda Gomez Arango L, Barrett HL, Dekker Nitert M, de Courten B. Effect of vitamin d supplementation on faecal microbiota: a randomised clinical trial. Nutrients (2019) 11:E2888. doi: 10.3390/nu11122888
135. Cantorna MT, McDaniel K, Bora S, Chen J, James J. Vitamin d, immune regulation, the microbiota, and inflammatory bowel disease. Exp Biol Med Maywood NJ (2014) 239:1524–30. doi: 10.1177/1535370214523890
136. Cantorna MT, Snyder L, Arora J. Vitamin a and vitamin d regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit Rev Biochem Mol Biol (2019) 54:184–92. doi: 10.1080/10409238.2019.1611734
137. Stein-Thoeringer CK, Nichols KB, Lazrak A, Docampo MD, Slingerland AE, Slingerland JB, et al. Lactose drives enterococcus expansion to promote graft-versus-host disease. Science (2019) 366:1143–9. doi: 10.1126/science.aax3760
138. Toenges R, Greinix H, Lawitschka A, Halter J, Baumgartner A, Simon A, et al. Current practice in nutrition after allogeneic hematopoietic stem cell transplantation – results from a survey among hematopoietic stem cell transplant centers. Clin Nutr (2021) 40:1571–7. doi: 10.1016/j.clnu.2021.02.030
Keywords: graft-versus-host disease, nutrition, nutritional intervention, gut microbiota, intestinal barrier
Citation: Limpert R, Pan P, Wang L-S and Chen X (2023) From support to therapy: rethinking the role of nutrition in acute graft-versus-host disease. Front. Immunol. 14:1192084. doi: 10.3389/fimmu.2023.1192084
Received: 22 March 2023; Accepted: 19 May 2023;
Published: 08 June 2023.
Edited by:
Daniel Peltier, University of Michigan, United StatesReviewed by:
Tomomi Toubai, Yamagata University, JapanThomas Luft, Heidelberg University Hospital, Germany
Copyright © 2023 Limpert, Pan, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Chen, eGNoZW5AbWN3LmVkdQ==
†These authors share first authorship
 Rachel Limpert
Rachel Limpert Pan Pan
Pan Pan Li-Shu Wang
Li-Shu Wang Xiao Chen
Xiao Chen