- 1Department of Hematology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
- 2Department of Hematology, The Affiliated Hospital of Shandong University of Traditional Chinese Medical, Jinan, Shandong, China
- 3Department of Hematology, The Affiliated Taian City Centeral Hospital of Qingdao University, Taian, Shandong, China
- 4Department of Hematology, Shandong Provincial Hospital, Shandong University, Jinan, Shandong, China
- 5Branch of National Clinical Research Center for Hematologic Diseases, Jinan, Shandong, China
- 6National Clinical Research Center for Hematologic Diseases, the First Affiliated Hospital of Soochow University, Suzhou, China
Background: Eltrombopag has demonstrated efficacy in treating low platelet (PLT) levels, but it remains unclear whether eltrombopag can promote PLT engraftment after hematopoietic stem cell transplantation (HSCT).
Methods: Forty-one HSCT patients received eltrombopag 50 mg/d from +1 day until PLT >50 × 109/L or 1 month after HSCT. Fifty-one patients in the same period received thrombopoietin (TPO) to promote PLT graft after HSCT and served as a control group.
Results: A total of 51 patients who applied TPO during the same period were treated as a control. In the eltrombopag group, the median time to white blood cells (WBC) graft was 12 days (range, 10-17 days) and the PLT graft was 15 days (range, 10-30 days), whereas for the patients in the TPO group, the median time to WBC and PLT graft was 12 days (range, 9-23 days) and 15.5 days (range, 9-41 days), respectively. In the first month after HSCT, the median WBC count in the eltrombopag group was 4.41 × 109/L (range, 0.87-40.01 × 109/L) and the median PLT was 89x109/L (range, 30-401 × 109/L); the median WBC and PLT \counts in the TPO group were 4.65 × 109/L (range, 0.99-23.63 × 109/L) and 86 × 109/L (range, 5-512 × 109/L), respectively. Patients in the TPO or eltrombopag group did not experience serious side effects after drug administration, and the difference in side effects on liver and kidney function between the two groups was not statistically significant.
Conclusion: Eltrombopag is safe and similarly promotes platelet engraftment to thrombopoietin after allogeneic HSCT.
1 Introduction
Hematopoietic stem cell transplantation (HSCT) is an effective curative measure for many hematologic malignancies and some nonmalignant diseases. Neutrophil and platelet (PLT) implantation after HSCT is essential for optimal results (1). Prolonged isolated thrombocytopenia (PT) is a very common complication for all patients with HSCT. PT patients are usually faced with uniformly poor outcomes (2, 3). Thus, how to reduce the incidence of PT and successfully manage thrombocytopenia after HSCT remains a major challenge.
Thrombopoietin (TPO) is a cytokine, and previous studies have shown that TPO is the main physiological cytokine that stimulates platelet production (4, 5). A prospective randomized controlled study has shown that recombinant human TPO (rhTPO) promotes platelet engraftment in patients after HSCT (6). The safety of rhTPO has been demonstrated in many previous trials (6, 7).
Eltrombopag is a small molecule non-peptide oral formulation that is an agonist of the thrombopoietin receptor, and it can increase PLT counts in patients with thrombocytopenia. Although initially used to improve thrombocytopenia in chronic immune thrombocytopenia (ITP), it was later found to be effective in other causes of thrombocytopenia (8). Since thrombopoietin receptors are expressed on both megakaryocytes and hematopoietic stem cells, hematopoietic stem cell can be stimulated by eltrombopag (9–11). More recently, eltrombopag has been used for the treatment of thrombocytopenia and graft failure after HSCT (12).
The effects of eltrombopag on poor graft function of HSCT patients have been studied and were shown to be effective (13, 14). However, it remains unclear whether eltrombopag can promote PLT engraftment after HSCT.
Herein, we conducted a retrospective, single-arm clinical trial to evaluate the effect of eltrombopag on platelet engraftment in patients after transplantation and the safety of clinical use of eltrombopag.
2 Materials and methods
2.1 Study design
We enrolled a total of 94 patients diagnosed with hematological malignancies who underwent HSCT from three hospitals from 21 January 2019 to 10 November 2021. The three hospitals both used TPO or eltrombopag after transplantation as standard treatment. These patients were divided into two groups. Patients were administered 50 mg (qd) from +1 day until PLT >50 × 109/L or 1 month after HSCT were classified as the eltrombopag group, and patients who used TPO only during the same period were divided into the TPO Group. We verified ANC and PLT counts daily for post-transplant patients. Additionally, we monitored kidney and liver function every three days. We compared the implantation of white blood cells (WBC) and PLT of the two groups after HSCT. We also analyzed the side effects of both drugs on patients’ liver function, kidney injury, and outcomes. This study was approved by the institutional ethics board of Shandong Provincial Hospital Affiliated to Shandong First Medical University, the Affiliated Hospital of Shandong University of Traditional Chinese Medicine and Taian City Central Hospital. This study was performed in accordance with the Helsinki Declaration of 1975 and was approved by the institutional ethics board of Shandong Provincial Hospital (Jinan, China), the Affiliated Hospital of Shandong University of Traditional Chinese Medicine (Jinan, China), the Affiliated Taian City Center Hospital of Qingdao University (Taian, China). Informed consent.
2.2 Conditioning regimen and prophylaxis of GVHD
All patients received myeloablative conditioning regimens including busulfan + cyclophosphamide (busulfan 0.8 mg/kg in 9 doses, cyclophosphamide 50 g/kg/day, days -3, -2), busulfan + fludarabine (busulfan 0.8 mg/kg in 9 doses, fludarabine 30mg/kg/day, from day -6 to day-2),or total body irradiation + cyclophosphamide (total body irradiation 3Gy/day, from day -6 to day-4, cyclophosphamide 50 g/kg/day, days -3, -2)-based regimens. To prevent GVHD, cyclosporine A, mycophenolate, and short-term methotrexate were administered in all patients. Patients who underwent matched HSCT from sibling donors did not receive ATG, and the other patients received ATG (2.5 mg/kg/day, from day -5 to day -2).
2.3 Stem cell mobilization and infusion
Granulocyte colony-stimulating factor (5-10 μg/kg/day) was used to mobilize hematopoietic stem cells into the peripheral blood. Peripheral blood stem cells were collected on the fifth day after mobilization. The ideal infused mononuclear cells were more than 5 × 108/kg, and CD34+ cells should were more than 2 × 106/kg.
2.4 Indicators and definitions
WBC engraftment after HSCT was defined as neutrophil granulocyte count greater than 0.5 × 109/L for three consecutive days. PLT engraftment was defined as a PLT count exceeding 20 × 109/L for three consecutive days without transfusion support. We also compared the time achieved for the WBC count>0.2 after HSCT and the number of WBC and PLT at 1 and 3 months after HSCT between the two groups. Liver function was assessed by analyzing the levels of glutamic oxaloacetic transaminase (AST), glutamic-pyruvic transaminase(ALT), alkaline phosphatase (ALP) and total bilirubin (TBIL). Kidney injury was assessed by measuring urinary protein levels (Upro), blood urea nitrogen (BUN), and creatinine (CREA). The grade of liver and kidney injury was determined according to the World Health Organization (WHO) classification system. Parallelly, we collected the number of MKCs in the bone marrow of patients one month after HSCT. Acute GVHD (aGVHD) was accessed according to the Glucksberg criteria (15) and cGvHD was graded based on the revised Seattle criteria (16). Overall survival (OS) was defined as the time between HSCT and death. Progression-free survival (PFS) was defined as the time between HSCT and disease recurrence or death.
2.5 Statistical analysis
The T test was used to compare continuous variables and the chi-square test was used to compare categorical variables. The Kaplan-Meier method and Cox proportional hazard model were used to estimate leukocyte and PLT engraftment, OS, PFS, and GVHD. SPSS v.25.0 was used for data analysis. P<0.05 based on the 2-sided hypothesis tests were considered statistically significant.
3 Results
3.1 Patients’ characteristics
This study involved 94 consecutive patients. A total of 43 patients received eltrombopag after HSCT, among whom 2 individuals experienced severe nausea and vomiting, making oral medication intolerable. Subsequently, they switched to treatment with thrombopoietin (TPO). As these two patients underwent cross-over treatment with two different medications, they were not included in a specific treatment group for analysis. A total of 41 patients finally enrolled in the eltrombopag group. A total of 51 patients used TPO and were enrolled in the TPO group. The patient characteristics are summarized in Table 1. There was a significant difference in follow-up time between the eltrombopag group and the TPO group. There were no significant differences in sex, recipient’s sex, patient’s age, pre-HSCT CR, donor-recipient HLA and other indexes between the two groups (Table 1).
3.2 Engraftment
All patients achieved engraftment, and none displayed primary graft rejection. The median time to recovery from WBC was 12 days (range, 10-17 days) in the eltrombopag group and 12 days (range, 9-23 days) in the TPO group (P = 0.174, HR = 1.344, 95%CI: 0.877-2.059) (Figure 1A). Two patients had poor engraftment of PLT in the TPO group. The median time to PLT engraftment was 15 days (range, 10-30 days) in the eltrombopag and 15.5 days (range, 9-41 days) in the TPO group (P = 0.299, HR = 1.249, 95%CI: 0.821-1.901) (Figure 1B).
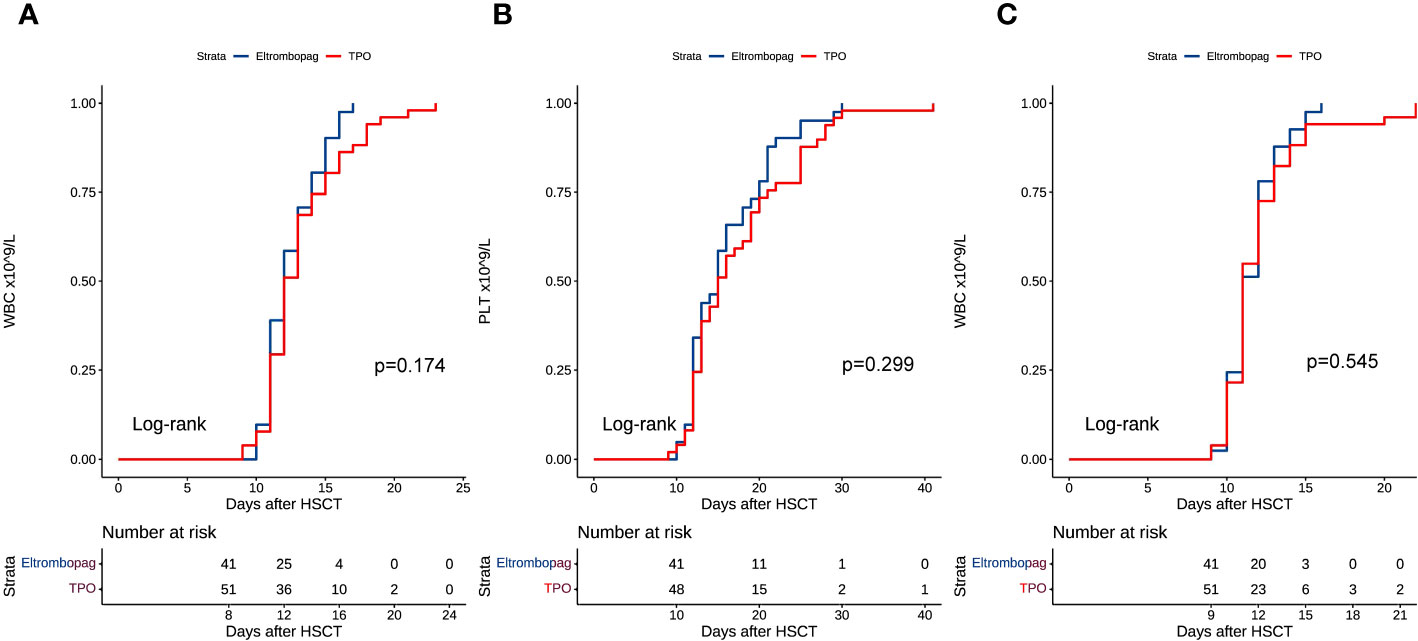
Figure 1 (A) WBC engraftment after HSCT. (B) PLT engraftment after HSCT. (C) Time course of WBC counts >0.2 × 109/L after HSCT.
We compared the time to WBC counts >0.2 × 109/L in both groups. In the eltrombopag and TPO groups, the median time to WBC >0.2 × 109/L was 11 days (range, 9-16 days) and 11 days (range, 9-22 days), respectively (eltrombopag vs TPO: P = 0.545, HR = 1.138, 95%CI: 0.749-1.729) (Figure 1C). We collected the = WBC and PLT counts at 1 and 3 months after HSCT. At the first month after HSCT, the median WBC count was 4.41 × 109/L (range, 0.87-40.01 × 109/L) in the eltrombopag group and 4.65 × 109/L (range, 0.99-23.63 × 109/L) in the TPO group, respectively (P = 0.720, HR = 1.079, 95%CI: 0.711-1.637), and 3.96 × 109/L (range, 2.19-21.69 × 109/L) and 4.21 × 109/L (range, 1.59-10.41 × 109/L) at the third month after HSCT, respectively (P = 0.371, HR = 0.819, 95%CI: 0.529-1.268). While for PLT it was 89 × 109/L (range, 30-401 × 109/L) and 86 × 109/L (range, 5-512 × 109/L) in the TPO and eltrombopag group at the 1st month, respectively (eltrombopag vs. TPO: P = 0.198, HR = 0.761, 95%CI: 0.503-1153). The median PLT count was 91 × 109/L (range, 3-299 × 109/L) and 90 × 109/L (range, 9-261 × 109/L) in the two groups at the third month, respectively (Eltrombopag vs TPO: P = 0.625, HR = 0.897, 95%CI: 0.581-1.387).
One month after HSCT, all patients in the eltrombopag group had PLT >25 × 109/L, while in the TPO group 89% of the patients had PLT >25. Overall, 89% of the patients in the eltrombopag group had PLT >25 × 109/L, while in the TPO group, 89% patients had PLT >25 × 109/L. Approximately, 89% of patients in the eltrombopag group had PLT >50 × 109/L, while 58% patients in the TPO group had PLT >50 × 109/L. In the eltrombopag group, the PLT counts of 51% patients were over 100 × 109/L, and in the TPO group 42% patients had counts greater than 100. Three months after HSCT, in the eltrombopag group, 90% patients had PLT >25 × 109/L, compared with 89% patients in the TPO group, whereas 77% of the patients in the eltrombopag group had PLT >50 × 109/L, and 71% of patients in the TPO group had PLT >50 × 109/L. There were 52% patients in the eltrombopag group with PLT >100 × 109/L, whereas 49% patients of the TPO group.
The MKCs in the bone marrow were assayed 1 month after HSCT. No MKCs were found in the bone marrow of 3 patients in the Eltrombopag group and 3 patients in the TPO group. The median level of MKC was 44 (range, 2-345) and 58 (range, 2-788) in the eltrombopag and TPO group, respectively (P = 0.425).
3.3 Graft-versus-host disease
Sixteen patients developed grade II-IV aGVHD in the eltrombopag group, while 17 developed grade II-IV aGVHD in the TPO group. At 100 days after HSCT, no significant differences were observed in cumulative aGVHD in the eltrombopag (39%, 95% CI: 23%-54%) vs TPO groups (33.3%, 95% CI: 19%-46%) (P = 0.734, HR = 1.125, 95%CI: 0.569-2.228) (Figure 2A). One (2%, 95% CI: 1%-7%) patient developed grade II-IV cGVHD in the eltrombopag group and 9 (17%, 95% CI: 6%-28%) developed grade II-IV cGVHD in the TPO group. The number of patients developing cGVHD in the Eltrombopag and TPO group was not significantly different (P = 0.188, HR = 0.246, 95%CI: 0.030-1.988) (Figure 2B).
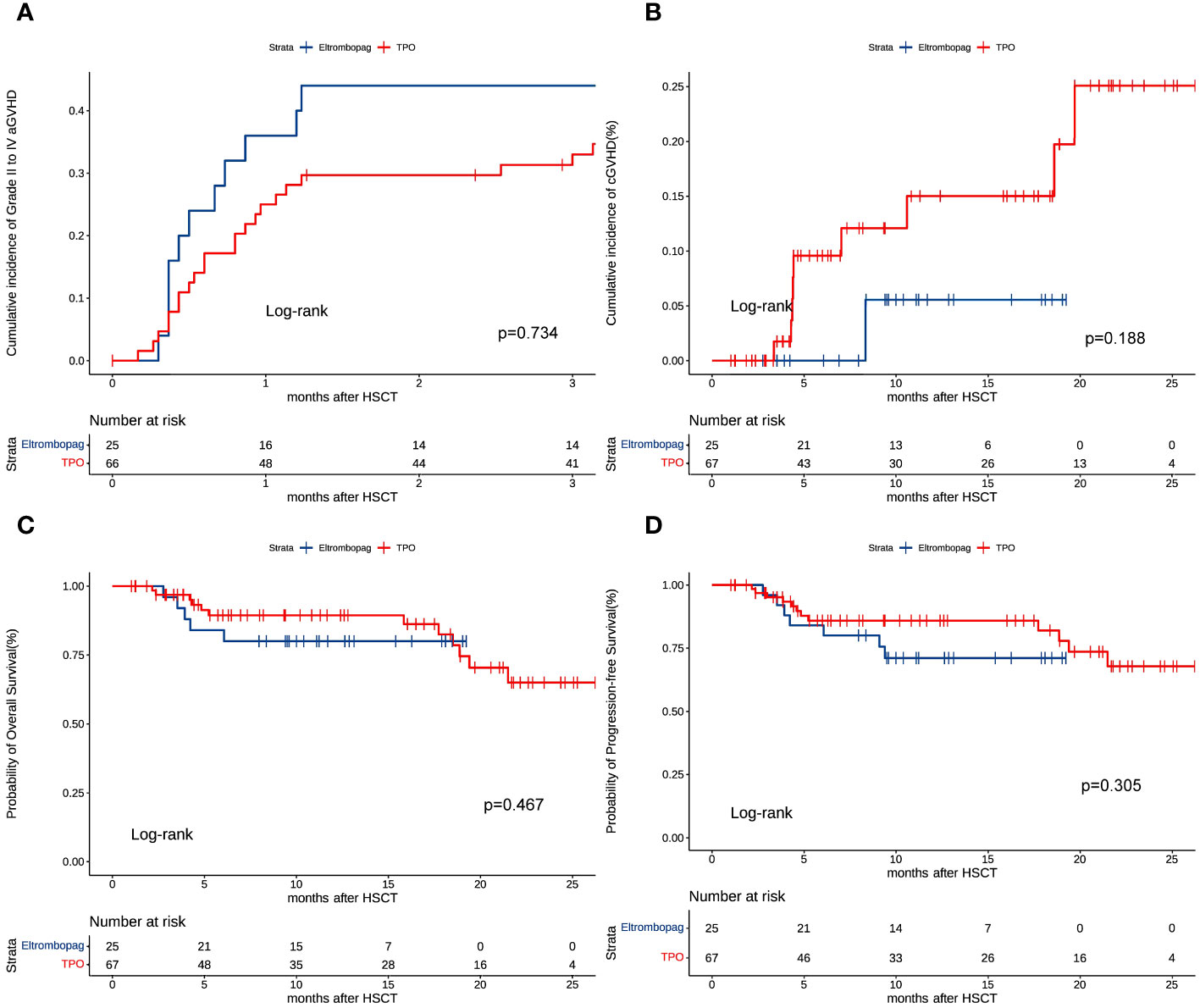
Figure 2 (A) Cumulative incidence of grade II to IV aGVHD. (B) Cumulative incidence of grade II to IV cGVHD. (C) Overall survival (OS). (D) Progressive-free survival (PFS).
3.4 Survival
Survival analysis revealed no statistical difference (eltrombopag vs. TPO: P = 0.467, HR = 1.490, 95%CI: 0.509-4.364) in the 2-year OS in the eltrombopag (85%, 95% CI 74%-96%) vs TPO groups (78%, 95% CI 66%-90%) (Figure 2C). The two-year PFS was similar in the eltrombopag group and in the TPO group (80%, 95CI: 67%-93% vs 78%, 95CI: 66%-90%, P = 0.305, HR = 1.664, 95%CI: 0.629-4.403, Figure 2D). Six (17%) and 11 (24%) patients died in the Eltrombopag and TPO group, respectively (P = 0.394).
We also compared the adverse effects on the liver and kidney in the eltrombopag and TPO groups after HSCT. We evaluated liver and kidney function throughout the treatment period after transplantation. Based on TBIL levels, 37 patients in the eltrombopag group were assessed as having grade 0 liver injury, 4 patients had grade I liver injury. Forty-two patients were evaluated as having grade 0 liver injury and 7 patients had grade I liver injury in the TPO group, respectively (P = 0.514). According to ALP levels, all patients in the eltrombopag group had a grade 0 liver injury, 44 patients had a grade 0 liver injury, and 5 patients had a grade I liver injury in the TPO group, respectively (P = 0.060). Based on BUN levels, there were 39 patients with grade 0 kidney injury and 2 patients with grade I injury in the eltrombopag group, whereas 45 patients with grade 0 kidney injury and 4 patients with grade I in the TPO group TPO (P = 0.685). Based on CREA levels, all patients had grade 0 kidney injury in the eltrombopag group, 48 of the patients had grade 0 kidney injury, and I patient had grade 1 in the TPO group (P > 0.999). Depending on Upro levels, 24 of the patients had grade 0 kidney injury and 17 patients had grade I in the eltrombopag group. Twenty-four patients had grade 0 kidney injury and 25 patients had grade I in the TPO group TPO (P = 0.365).
4 Discussion
HSCT is the only curable treatment for many hematological malignant diseases and some non-malignant diseases, but according to the literature, approximately 5%-44% of patients will experience thrombocytopenia after HSCT (17–19). Long-term thrombocytopenia after transplantation is also a risk factor for a poor prognosis (20). Currently, there is no standard treatment for thrombocytopenia after transplantation. Gamma globulin, androgen, and recombinant human thrombocytopenia (rhTPO) are commonly used in clinical treatment of thrombocytopenia after transplantation. Eltrombopag is an oral small molecule agonist for the thrombopoietin receptor. As early as 2008, eltrombopag was approved by the US FDA for the treatment of primary immune thrombocytopenia (ITP) (21), and effective rates were reported to be 59% to 85% (22). Eltrombopag has been shown to have promising results in patients with ITP and refractory severe aplastic anemia (rSAA), with almost 80% of patients with ITP and 40% of rSAA showing platelet recovery after treatment (23–25). In addition, eltrombopag has recently been used for the treatment of thrombocytopenia after HSCT and showed high effectiveness (13, 26–30). However, it is not clear whether Eltrombopag can promote platelet implantation after HSCT.
TPO is a key regulator of thrombogenesis, which promotes proliferation and multiploidy of megakaryocytes by stimulating stem cells to differentiate into megakaryocytes, thus promoting thrombogenesis (5, 31, 32). Sun et al. (33) treated 24 patients with chronic isolated thrombocytopenia (PT) after HSCT with rhTPO, and the study showed that 45.8% of the patients responded to rhTPO treatment and eventually achieved platelet implantation. Delayed platelet implantation (DPE) is also a common complication after allo-HSCT, Kanda (34) showed that 5-37% of patients who received allo-HSCT developed DPE (19), while Wang et al. (35) demonstrated that rhTPOT could promote platelet transplantation after HSC. IFN-γ, a key pro-inflammatory cytokine, was reported to be involved in the destruction of HSPC by inhibiting TPO signaling, while eltrombopag can bypass this inhibition in vitro by activating c-MPL signaling (36, 37). Since the thrombopoietin receptor is expressed in both megakaryocytes and hematopoietic stem cells, eltrombopag can promote the formation and maturation of megakaryocytes to release platelets and promote stem cell generation (38, 39). It has also been reported that not only PLT, but also hemoglobin and WBCs increased significantly with eltrombopag treatment after allogeneic HSCT (25, 40, 41). These studies suggest that eltrombopag may play a better role in promoting hematopoietic stem cell graft.
In our study, there were 2 cases of poor platelet implantation after transplantation among patients with TPO. Except for these 2 cases of poor platelet implantation, the remaining 51 patients in the TPO group and the 41 patients in the Eltrombopag group were successfully implanted with white blood cells and platelets after transplantation. The median leucocyte and platelet grafting time after transplantation were the same in both groups. Furthermore, we found that the patients with the highest leukocyte or platelet implantation after transplantation were both in the TPO group. We also analyzed the number of WBCs and PLTs in both groups at 1 and 3 months after transplantation. WBCs were higher in the TPO group at 1 and 3 months after transplantation, whereas PLT counts were higher in the eltrombopag group at 1 month after transplantation. The number of MKC in the bone marrow 1 month after transplantation was similar between the two groups and there are no statistical differences. From our study, it can be seen that eltrombopag can promote PLT implantation after HSCT, which is the same as confirms findings from a previous study that found that eltrombopag could be used to treat graft dysfunction after transplantation (42, 43).
Early treatment with TPO and Eltrombopag after HSCT had tolerable side effects and high safety (44–47). Han et al. (6) conducted a study including 120 patients with HSCT and found that there was no difference in adverse events involving liver function, kidney function, coagulation function and GVHD between rhTPO group and the control group, and the probability of OS was similar. Another study involved 38 patients who received eltrombopag for thrombocytopenia after HSCT and found that all patients were well tolerated. Twenty-three patients developed aGVHD, but all of these patients recovered without discontinuing eltrombopag. Other serious adverse reactions such as severe liver injury and thrombosis were not observed (48). In our study, only two patients who used TPO developed III-IV aGVHD, none of the patients in the two groups had grade III-IV aGVHD, the liver injury and kidney injury were mild, and none of the patients had other serious adverse reactions. Additionally, patients treated with eltrombopag and TPO had similar OS and PFS. Our study showed the tolerance and safety of TPO and eltrombopag was in accordance with other previously published data (7, 49).
It should be noted that due to the retrospective nature of this study, we cannot determine to what extent platelet recovery after transplantation is affected by TPO or Eltrombopag. Furthermore, this study is limited by the small sample size of patients, which can cause data bias. More prospective randomized controlled large-sample clinical studies are needed to confirm the exact efficacy of early application of Eltrombopag after transplantation.
However, this study has confirmed that eltrombopag can be used to promote platelet implantation in patients early after transplantation, its efficacy is not inferior to that of TPO in promoting platelet implantation, and its side effects can be tolerated and are safe.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the institutional ethic board of Shandong Provincial Hospital Affiliated to Shandong First Medical University, the Affiliated Hospital of Shandong University of TCM and Taian City Central Hospital and followed the Helsinki Declaration of 1975. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YL: Data curation, Software, Writing – original draft. FK: Supervision, Writing – review & editing. GB: Supervision, Writing – review & editing. YJ: Data curation, Methodology, Writing – review & editing. WZ: Software, Writing – review & editing. XS: Methodology, Writing – review & editing. XHS: Methodology, Writing – review & editing. YL: Resources, Writing – review & editing. MD: Resources, Writing – review & editing. DY: Software, Writing – review & editing. XW: Supervision, Writing – review & editing. XF: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Technology Projects of Jinan (No. 202019044); 2021 Shandong Medical Association Clinical Research Fund—Qilu Special Project; and Young scholars research of the Chinese medical association hematology—Sansheng Pharmaceutical Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gupta AK, Srinivasan P, Das G, Meena JP, Tanwar P, Seth R. Eltrombopag post autologous hematopoietic stem cell transplant - an emerging indication in younger pediatric patients. Am J Blood Res. (2021) 11:168–71.
2. Tang FF, Sun YQ, Mo XD, Lv M, Chen YH, Wang Y, et al. Incidence, risk factors, and outcomes of primary prolonged isolated thrombocytopenia after haploidentical hematopoietic stem cell transplant. Biol Blood Marrow Transplant. (2020) 26:1452–8. doi: 10.1016/j.bbmt.2020.03.024
3. Zhang XH, Wang QM, Chen H, Chen YH, Han W, Wang FR, et al. Clinical characteristics and risk factors of Intracranial hemorrhage in patients following allogeneic hematopoietic stem cell transplantation. Ann Hematol. (2016) 95:1637–43. doi: 10.1007/s00277-016-2767-y
4. Qian S, Fu F, Li W, Chen Q, de Sauvage FJ. Primary role of the liver in thrombopoietin production shown by tissue-specific knockout. Blood. (1998) 92:2189–91. doi: 10.1182/blood.V92.6.2189
5. Alexander WS, Roberts AW, Nicola NA, Li R, Metcalf D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood. (1996) 87:2162–70. doi: 10.1182/blood.V87.6.2162.bloodjournal8762162
6. Han TT, Xu LP, Liu DH, Liu KY, Wang FR, Wang Y, et al. Recombinant human thrombopoietin promotes platelet engraftment after haploidentical hematopoietic stem cell transplantation: a prospective randomized controlled trial. Ann Hematol. (2015) 94:117–28. doi: 10.1007/s00277-014-2158-1
7. Liu DH, Huang XJ, Liu KY, Xu LP, Chen YH, Wang Y, et al. Safety of recombinant human thrombopoietin in adults after related donor haploidentical haematopoietic stem cell transplantation: a pilot study. Clin Drug Investig. (2011) 31:135–41. doi: 10.1007/BF03256939
8. Boyers D, Jia X, Crowther M, Jenkinson D, Fraser C, Mowatt G. Eltrombopag for the treatment of chronic idiopathic (immune) thrombocytopenic purpura (ITP). Health Technol Assess. (2011) 15 Suppl 1:23–32. doi: 10.3310/hta15Suppl1/03
9. Ecsedi M, Lengline E, Knol-Bout C, Bosman P, Eikema DJ, Afanasyev B, et al. Use of eltrombopag in aplastic anemia in Europe. Ann Hematol. (2019) 98:1341–50. doi: 10.1007/s00277-019-03652-8
10. Fattizzo B, Levati G, Cassin R, Barcellini W. Eltrombopag in immune thrombocytopenia, aplastic anemia, and myelodysplastic syndrome: from megakaryopoiesis to immunomodulation. Drugs. (2019) 79:1305–19. doi: 10.1007/s40265-019-01159-0
11. Konishi A, Nakaya A, Fujita S, Satake A, Nakanishi T, Azuma Y, et al. Evaluation of eltrombopag in patients with aplastic anemia in real-world experience. Leuk Res Rep. (2019) 11:11–3. doi: 10.1016/j.lrr.2019.03.002
12. Bento L, Bastida JM, Garcia-Cadenas I, Garcia-Torres E, Rivera D, Bosch-Vilaseca A, et al. Thrombopoietin receptor agonists for severe thrombocytopenia after allogeneic stem cell transplantation: experience of the spanish group of hematopoietic stem cell transplant. Biol Blood Marrow Transplant. (2019) 25:1825–31. doi: 10.1016/j.bbmt.2019.05.023
13. Marotta S, Marano L, Ricci P, Cacace F, Frieri C, Simeone L, et al. Eltrombopag for post-transplant cytopenias due to poor graft function. Bone Marrow Transplant. (2019) 54:1346–53. doi: 10.1038/s41409-019-0442-3
14. Aydin S, Dellacasa C, Manetta S, Giaccone L, Godio L, Iovino G, et al. Rescue treatment with eltrombopag in refractory cytopenias after allogeneic stem cell transplantation. Ther Adv Hematol. (2020) 11:2040620720961910. doi: 10.1177/2040620720961910
15. Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. (1995) 15:825–8.
16. Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transpl. (2003) 9:215–33. doi: 10.1053/bbmt.2003.50026
17. Ramírez P, Brunstein CG, Miller B, Defor T, Weisdorf D. Delayed platelet recovery after allogeneic transplantation: a predictor of increased treatment-related mortality and poorer survival. Bone Marrow Transplant. (2011) 46:981–6. doi: 10.1038/bmt.2010.218
18. Bruno B, Gooley T, Sullivan KM, Davis C, Bensinger WI, Storb R, et al. Secondary failure of platelet recovery after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2001) 7:154–62. doi: 10.1053/bbmt.2001.v7.pm11302549
19. Yamazaki R, Kuwana M, Mori T, Okazaki Y, Kawakami Y, Ikeda Y, et al. Prolonged thrombocytopenia after allogeneic hematopoietic stem cell transplantation: associations with impaired platelet production and increased platelet turnover. Bone Marrow Transplant. (2006) 38:377–84. doi: 10.1038/sj.bmt.1705444
20. Bolwell B, Pohlman B, Sobecks R, Andresen S, Brown S, Rybicki L, et al. Prognostic importance of the platelet count 100 days post allogeneic bone marrow transplant. Bone Marrow Transplant. (2004) 33:419–23. doi: 10.1038/sj.bmt.1704330
21. Garnock-Jones KP, Keam SJ. Eltrombopag. Drugs. (2009) 69:567–76. doi: 10.2165/00003495-200969050-00005
22. Saleh MN, Bussel JB, Cheng G, Meyer O, Bailey CK, Arning M, et al. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Blood. (2013) 121:537–45. doi: 10.1182/blood-2012-04-425512
23. Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, Aivado M, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. (2011) 377:393–402. doi: 10.1016/S0140-6736(10)60959-2
24. Wong RSM, Saleh MN, Khelif A, Salama A, Portella MSO, Burgess P, et al. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. (2017) 130:2527–36. Blood, 2018. 131(6): p. 709.
25. Desmond R, Townsley DM, Dumitriu B, Olnes MJ, Scheinberg P, Bevans M, et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. (2014) 123:1818–25. doi: 10.1182/blood-2013-10-534743
26. Raut SS, Shah SA, Sharanangat VV, Shah KM, Patel KA, Anand AS, et al. Safety and efficacy of eltrombopag in post-hematopoietic stem cell transplantation (HSCT) thrombocytopenia. Indian J Hematol Blood Transfus. (2015) 31:413–5. doi: 10.1007/s12288-014-0491-0
27. Master S, Dwary A, Mansour R, Mills GM, Koshy N. Use of eltrombopag in improving poor graft function after allogeneic hematopoietic stem cell transplantation. Case Rep Oncol. (2018) 11:191–5. doi: 10.1159/000487229
28. Tanaka T, Inamoto Y, Yamashita T, Fuji S, Okinaka K, Kurosawa S, et al. Eltrombopag for treatment of thrombocytopenia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. (2016) 22:919–24. doi: 10.1016/j.bbmt.2016.01.018
29. Yuan C, Boyd AM, Nelson J, Patel RD, Varela JC, Goldstein SC, et al. Eltrombopag for treating thrombocytopenia after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. (2019) 25:1320–4. doi: 10.1016/j.bbmt.2019.01.027
30. Masetti R, Vendemini F, Quarello P, Girardi K, Prete A, Fagioli F, et al. Eltrombopag for thrombocytopenia following allogeneic hematopoietic stem cell transplantation in children. Pediatr Blood Cancer. (2020) 67:e28208. doi: 10.1002/pbc.28208
31. de Sauvage FJ, Villeval JL, Shivdasani RA. Regulation of megakaryocytopoiesis and platelet production: lessons from animal models. J Lab Clin Med. (1998) 131:496–501. doi: 10.1016/S0022-2143(98)90057-9
32. Kaushansky K, Lok S, Holly RD, Broudy VC, Lin N, Bailey MC, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. (1994) 369:568–71. doi: 10.1038/369568a0
33. Sun YQ, Kong Y, Zhang XH, Wang Y, Shi MM, Song Y, et al. A novel recombinant human thrombopoietin for treating prolonged isolated thrombocytopenia after allogeneic stem cell transplantation. Platelets. (2019) 30:994–1000. doi: 10.1080/09537104.2018.1557613
34. Kanda Y, Chiba S, Hirai H, Sakamaki H, Iseki T, Kodera Y, et al. Allogeneic hematopoietic stem cell transplantation from family members other than HLA-identical siblings over the last decade (1991-2000). Blood. (2003) 102:1541–7. doi: 10.1182/blood-2003-02-0430
35. Wang H, Huang M, Zhao Y, Qi JQ, Chen C, Tang YQ, et al. Recombinant human thrombopoietin promotes platelet engraftment and improves prognosis of patients with myelodysplastic syndromes and aplastic anemia after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2017) 23:1678–84. doi: 10.1016/j.bbmt.2017.06.010
36. Alvarado LJ, Huntsman HD, Cheng H, Townsley DM, Winkler T, Feng X, et al. Eltrombopag maintains human hematopoietic stem and progenitor cells under inflammatory conditions mediated by IFN-γ. Blood. (2019) 133:2043–55. doi: 10.1182/blood-2018-11-884486
37. Kao YR, Chen J, Narayanagari SR, Todorova TI, Aivalioti MM, Ferreira M, et al. Thrombopoietin receptor-independent stimulation of hematopoietic stem cells by eltrombopag. Sci Transl Med. (2018) 10(458). doi: 10.1126/scitranslmed.aas9563
38. Kuter DJ. Thrombopoietin and thrombopoietin mimetics in the treatment of thrombocytopenia. Annu Rev Med. (2009) 60:193–206. doi: 10.1146/annurev.med.60.042307.181154
39. Gill H, Wong RSM, Kwong YL. From chronic immune thrombocytopenia to severe aplastic anemia: recent insights into the evolution of eltrombopag. Ther Adv Hematol. (2017) 8:159–74. doi: 10.1177/2040620717693573
40. Olnes MJ, Scheinberg P, Calvo KR, Desmond R, Tang Y, Dumitriu B, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. (2012) 367:11–9. doi: 10.1056/NEJMoa1200931
41. Yamazaki H, Ohta K, Iida H, Imada K, Obara N, Tokumine Y, et al. Hematologic recovery induced by eltrombopag in Japanese patients with aplastic anemia refractory or intolerant to immunosuppressive therapy. Int J Hematol. (2019) 110:187–96. doi: 10.1007/s12185-019-02683-1
42. Dyba J, Tinmouth A, Bredeson C, Matthews J, Allan DS. Eltrombopag after allogeneic haematopoietic cell transplantation in a case of poor graft function and systematic review of the literature. Transfus Med. (2016) 26:202–7. doi: 10.1111/tme.12300
43. Li S, Wu R, Wang B, Fu L, Zhu G, Zhou X, et al. Eltrombopag for delayed platelet recovery and secondary thrombocytopenia following allogeneic stem cell transplantation in children. J Pediatr Hematol Oncol. (2019) 41:38–41. doi: 10.1097/MPH.0000000000001263
44. Tang B, Huang L, Liu H, Cheng S, Song K, Zhang X, et al. Recombinant human thrombopoietin promotes platelet engraftment after umbilical cord blood transplantation. Blood Adv. (2020) 4:3829–39. doi: 10.1182/bloodadvances.2020002257
45. Tang G, Wang XM, Meng JX, Luan CL, Chen JF, Wu YQ, et al. [Efficacy of recombinant human thrombopoietin and recombinant human interleukin 11 for treatment of chemotherapy indu-ced thrombocytopenia in acute myeloid leukaemia patients]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. (2018) 26:234–8.
46. Yaman Y, Elli M, Şahin Ş, Özdilli K, Bilgen H, Bayram N, et al. Eltrombopag for treatment of thrombocytopenia after allogeneic hematopoietic cell transplantation in children: Single-centre experience. Pediatr Transplant. (2021) 25:e13962. doi: 10.1111/petr.13962
47. Mahat U, Rotz SJ, Hanna R. Use of thrombopoietin receptor agonists in prolonged thrombocytopenia after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2020) 26:e65–73. doi: 10.1016/j.bbmt.2019.12.003
48. Fu H, Zhang X, Han T, Mo X, Wang Y, Chen H, et al. Eltrombopag is an effective and safe therapy for refractory thrombocytopenia after haploidentical hematopoietic stem cell transplantation. Bone Marrow Transplant. (2019) 54:1310–8. doi: 10.1038/s41409-019-0435-2
Keywords: eltrombopag, thrombopoietin, safety, prognosis, allogeneic hematopoietic stem cell transplantation, platelet engraftment
Citation: Li Y, Kong F, Bai G, Jiang Y, Zhang W, Sun X, Sui X, Li Y, Ding M, Yuan D, Wang X and Fang X (2024) Eltrombopag can promote platelet implantation after allogeneic hematopoietic stem cell transplantation as safely and similarly to thrombopoietin. Front. Immunol. 15:1340908. doi: 10.3389/fimmu.2024.1340908
Received: 19 November 2023; Accepted: 25 March 2024;
Published: 08 April 2024.
Edited by:
Ryotaro Nakamura, City of Hope National Medical Center, United StatesReviewed by:
Erlie Jiang, Chinese Academy of Medical Sciences & Peking Union Medical College, ChinaJan Styczynski, Nicolaus Copernicus University in Toruń, Poland
Copyright © 2024 Li, Kong, Bai, Jiang, Zhang, Sun, Sui, Li, Ding, Yuan, Wang and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaosheng Fang, ZnhzaF8xMDEwQDEyNi5jb20=; Xin Wang, eGludzAwN0AxMjYuY29t
†These authors have contributed equally to this work
 Yahan Li
Yahan Li Fansheng Kong2†
Fansheng Kong2† Wenlu Zhang
Wenlu Zhang Xin Wang
Xin Wang Xiaosheng Fang
Xiaosheng Fang