- 1Department of Microbiology, Virology and Microbial Toxins, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
- 2Microbial Toxins Physiology Group (MTPG), Universal Scientific Education and Research Network (USERN), Rasht, Iran
- 3Member of Research Committee, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
Introduction: Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which appeared in 2019, has been classified as critical and non-critical according to clinical signs and symptoms. Critical patients require mechanical ventilation and intensive care unit (ICU) admission, whereas non-critical patients require neither mechanical ventilation nor ICU admission. Several factors have been recently identified as effective factors, including blood cell count, enzymes, blood markers, and underlying diseases. By comparing blood markers, comorbidities, co-infections, and their relationship with mortality, we sought to determine differences between critical and non-critical groups.
Method: We used Scopus, PubMed, and Web of Science databases for our systematic search. Inclusion criteria include any report describing the clinical course of COVID-19 patients and showing the association of the COVID-19 clinical courses with blood cells, blood markers, and bacterial co-infection changes. Twenty-one publications were eligible for full-text examination between 2019 to 2021.
Result: The standard difference in WBC, lymphocyte, and platelet between the two clinical groups was 0.538, -0.670, and -0.421, respectively. Also, the standard difference between the two clinical groups of CRP, ALT, and AST was 0.482, 0.402, and 0.463, respectively. The odds ratios for hypertension and diabetes were significantly different between the two groups. The prevalence of co-infection also in the critical group is higher.
Conclusion: In conclusion, our data suggest that critical patients suffer from a suppressed immune system, and the inflammation level, the risk of organ damage, and co-infections are significantly high in the critical group and suggests the use of bacteriostatic instead of bactericides to treat co-infections.
Introduction
The SARS Coronavirus 2 (SARS-CoV-2) originated in China and spread to most countries worldwide in 2019. Generally, more than 200 million confirmed cases and more than 4 million deaths have been reported. SARS-CoV-2 is more infectious than SARS-CoV (1–3). The new Coronavirus is classified into critical and non-critical cases based on symptoms. Description of critical patients require mechanical ventilation and intensive care unit (ICU) admission, and Non-critical patients don’t require mechanical ventilation and ICU admission (3, 4). Accordingly, comparing critical and non-critical groups can describe the difference between the presence and absence of co-infection. After three years of the first appearance of COVID-19, researchers examined essential factors for evaluating COVID-19 disease.
As a first step, the blood cell count was evaluated. Some papers suggest that the number of white blood cells (WBCs), lymphocytes, and platelets may vary as a result of COVID-19, including critical, mild, moderate, and severe cases (5, 6). The difference between these cells may show the infection and inflammation in critical and non-critical groups (7, 8). Another factor associated with COVID-19 may be enzymes and proteins. Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), and C reactive protein (CRP) levels were inconsistent between critical and non-critical groups. The level of these markers can determine the prognosis of the two groups (9, 10).
Underlying diseases such as high blood pressure, diabetes, cardiovascular disease, and dyslipidemia can play a crucial role in the clinical course of COVID-19 patients. Critical and non-critical patients exhibit varying levels of comorbidities; these have a different impact on morbidity (11). Moreover, there is evidence to suggest that the mortality rate can be affected by comorbidities (12, 13).
Co-infection is an essential factor in morbidity and mortality. Globally, the prevalence of bacterial co-infection in COVID-19 patients is unknown and different micro-organisms lead to co-infection (14). Most of the organisms differ in their distribution in different organs, such as the respiratory, blood, and urinary tracts (15). As a result, the reaction of critical and non-critical groups to co-infections will be considerably variable. Bacterial co-infection plays a vital role in the clinical course of the COVID-19 disease, which can be treated with various antibiotics.
The mortality rate of COVID-19 disease can be affected by factors such as blood cell count, blood markers, comorbidities, and co-infections (4). Therefore, the mortality rate can differ between critical and non-critical groups. In this study, blood markers, comorbidities, co-infections, and their relationship to mortality rates were compared between critical and non-critical patients.
Method
Search strategy
We reported this systematic review meta-analysis using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The studies were identified using the following PICOS principle: Patients = Patients with COVID-19, Intervention = dividing patients into critical and non-critical based on ICU admission and mechanical ventilation, Control, Outcome = Comparison of immunological and clinical factors between critical and non-critical groups, Study design = case-control, prospective or retrospective studies (16). We used Scopus, PubMed, and Web of Science databases for our systematic search. The search terms used in the database were included (“COVID-19” OR “SARS COV-2” OR “Coronavirus infection”) AND (“critical” OR “Non-sever”) AND (“non-critical”) AND (“Co-infection” OR “Secondary infection” OR “bacterial infection”). We searched English publications and stored and checked articles using Endnote software as a citation manager. All selected articles were published in the 2019 to Jan 2022 date range. We reviewed the search results’ titles, abstracts, and full text for screening and study selection based on the inclusion criteria. Inclusion criteria include any original study that evaluated differences in 1) blood cells and blood markers, 2) bacterial co-infection, 3) Comorbidities, and 4) mortality Rate between critical and non-critical COVID-19 patients. Viral co-infection, case reports, reviews, and duplicate studies are generally excluded from this systematic review.
Quality assessment and data extraction
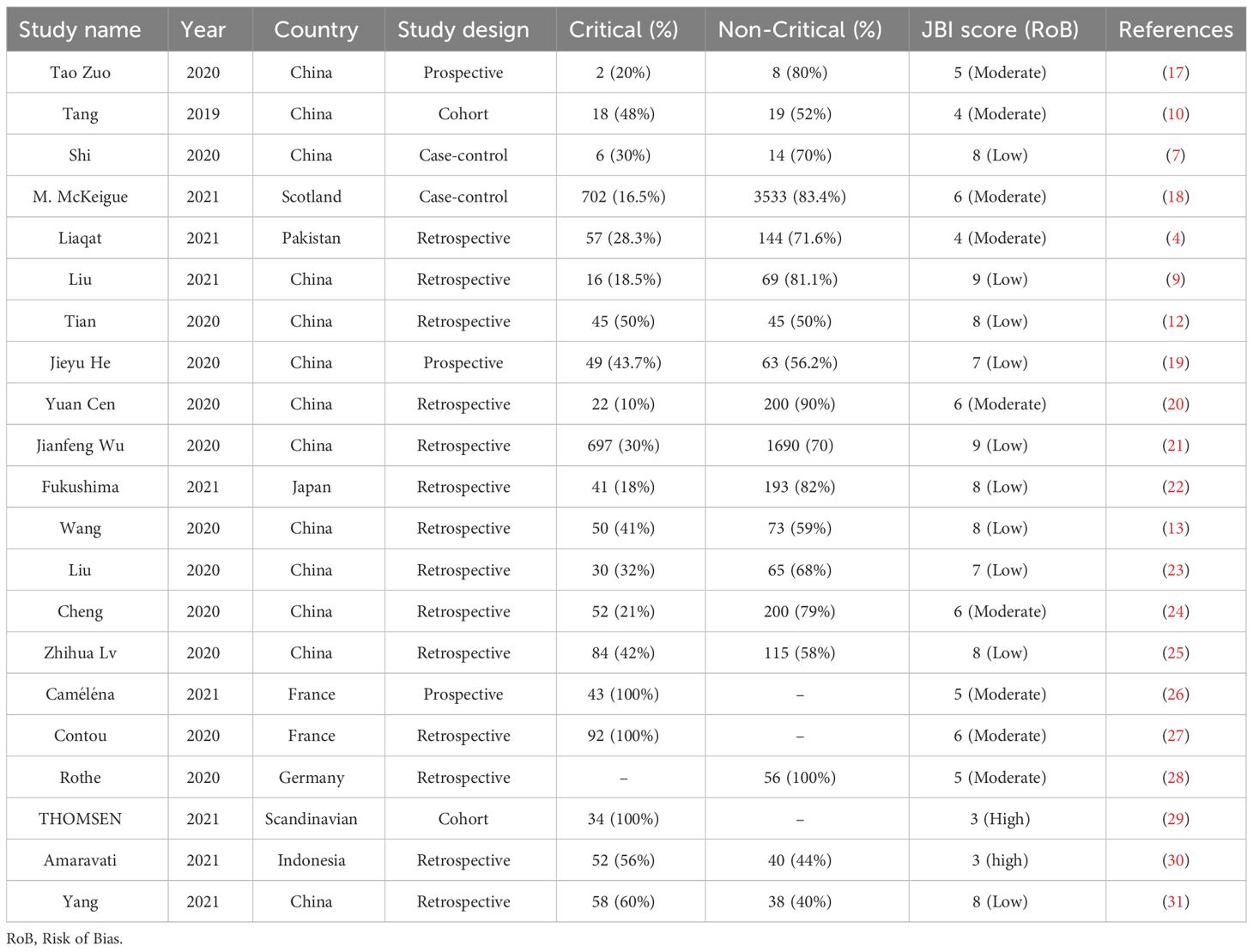
Rayyan platform was used for screening and data extraction of included studies. Using the nine-point Joanna Briggs Institute critical appraisal checklist for studies, two researchers conducted the quality assessment (A. B and M. H) and disagreements were resolved by consensus (H. S). The included studies met more than half of the quality assessment parameters. Based on Table 1, we (A. B. and M. H.) extracted the publication year, country, the number of patients, the clinical course (ICU admission or mechanical ventilation), and physiological data from the studies. The prevalence of bacteria and co-infections were determined using nasal and pharyngeal swabs, blood serum, and urine analysis samples for the respiratory, bloodstream, and urinary systems, respectively in selected studies. As a part of our investigation, the following information was obtained: publication year, study design and research question, number of articles, number of each type of study, language, and country of study, device used, patient characteristics, and statistical methodology.
Data analysis
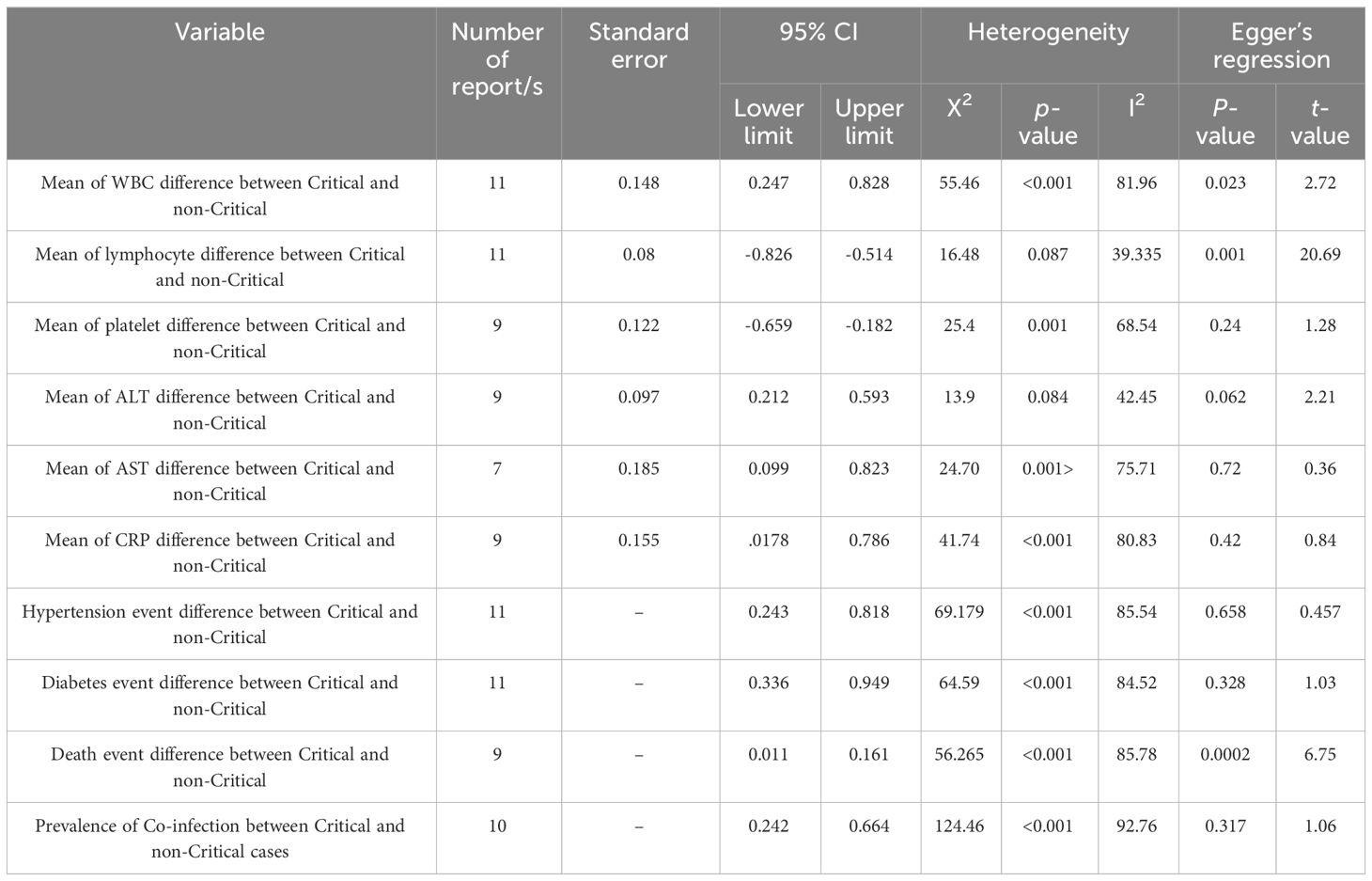
The statistical analysis and construction of graphs were performed with a comprehensive meta-analysis (CMA) version 3 (Biostat Inc., Englewood, NJ) with a random effect model plotted on forest plots since this model is more reasonable in the presence of heterogeneity than the fixed model. The pooled standard difference in mean with 95% CI gave the summary estimate. To test heterogeneity, we used the I-squared (I2). Visual bias was assessed using a funnel plot, and Egger’s regression test confirmed it (p < 0.05 was considered a statistically significant publication bias).
Result
Search outcome and study characteristics
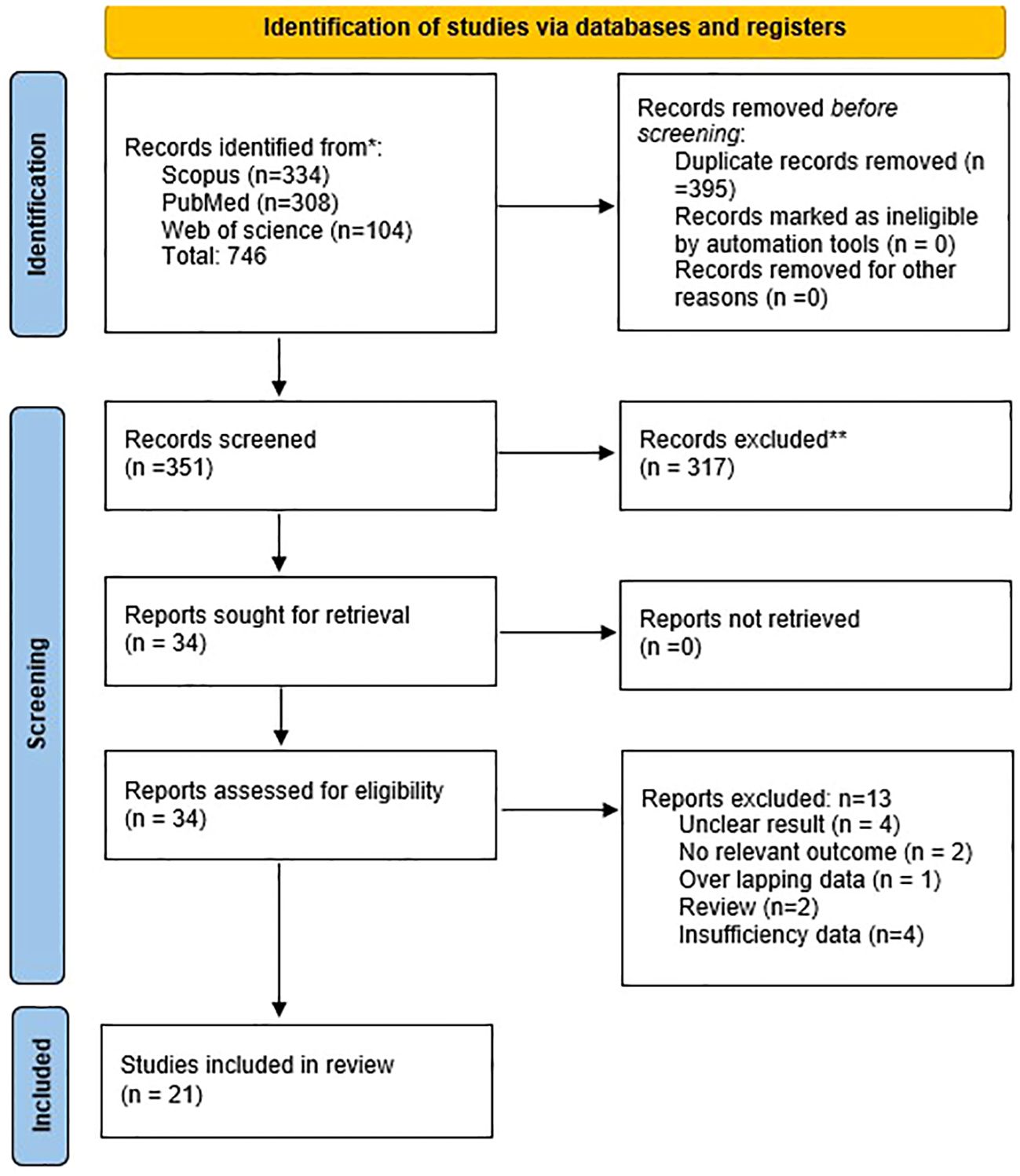
Considering the objectives of this study, we identified 746 publications in Scopus, PubMed, and Web of Science databases. After removing duplicate studies and screening based on inclusion and exclusion, 21 publications were eligible for full-text examination (Figure 1). Studies have been conducted in the following countries: China (13), France (2), and one study from each of the following: Germany, Scotland, Pakistan, Japan, Scandinavian, and Indonesia. Among the studies, there were 14 retrospective studies, three prospective studies, two case-control studies, and two cohort studies.
Comparison of blood cell count between critical and non-critical groups
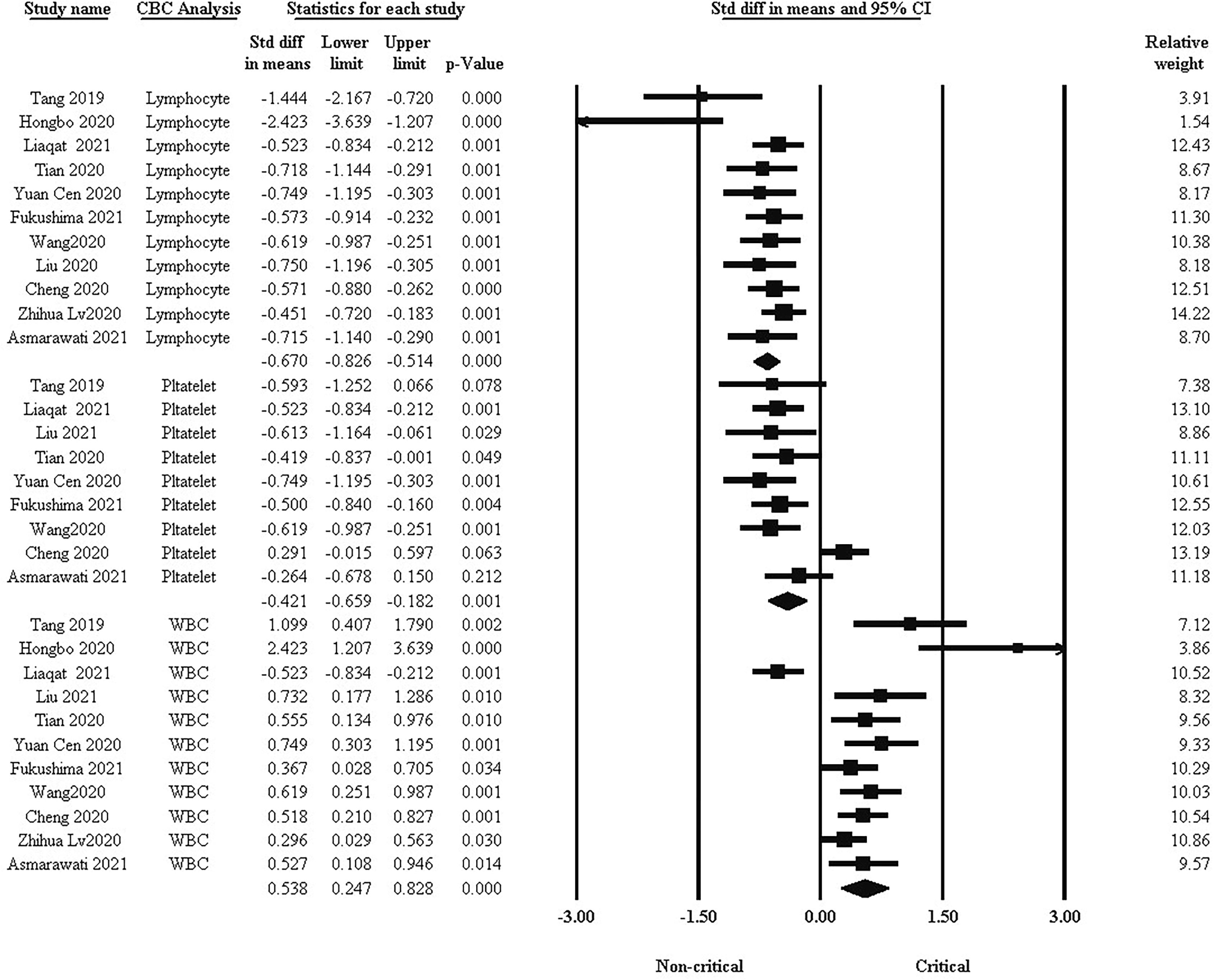
One of the basic factors in the clinical course of COVID-19 is blood cells. We analyzed WBC, lymphocyte, and platelet differences between the two groups. As shown in Figure 2, the standard difference in means indicated that lymphocytes and platelets were significantly higher in non-critical patients than in critical patients, while WBCs were higher in critical patients (std: -0.670, -0.421, and 0.538, respectively, 95% CI, P <0.001).
Comparison biomarkers level between critical and non-critical groups
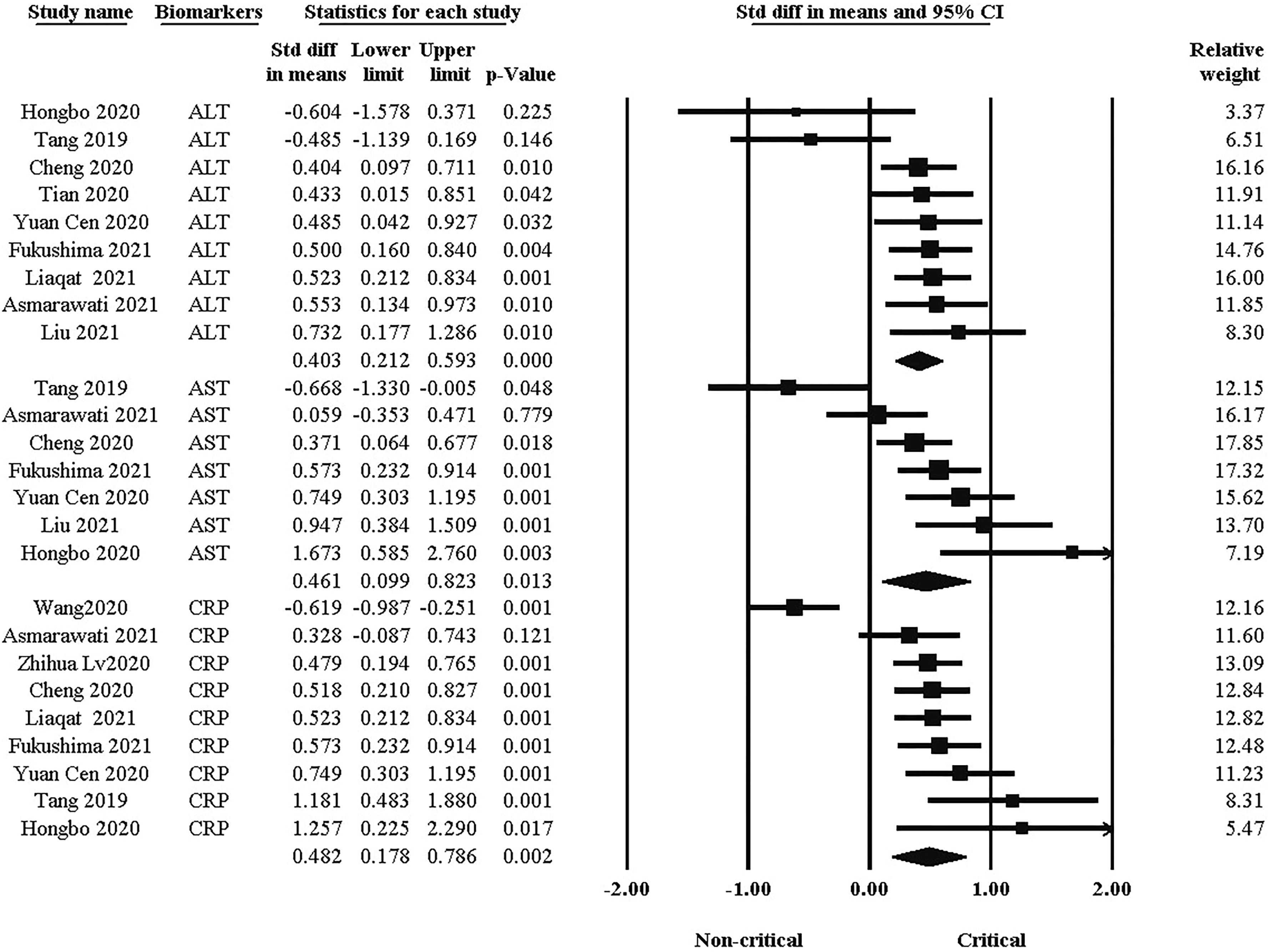
Biomarkers and enzymes, such as CRP, ALT, and AST are other factors in the clinical course of COVID-19. As shown in Figure 3, there was a significant difference in blood markers between the two groups. the standard difference of the mean for ALT was 0.403 (95% CI: 0.212, 0.593. P <0.001), while for AST it was 0.461 (95% CI: 0.099, 0.823. P = 0.013). In addition, the standard difference of the mean for CRP was 0.482 (95% CI: 0.178, 0.786. P = 0.002). The critical group had significantly higher levels of each of these factors than the non-critical group.
Comparison of mortality and comorbidities between critical and non-critical groups
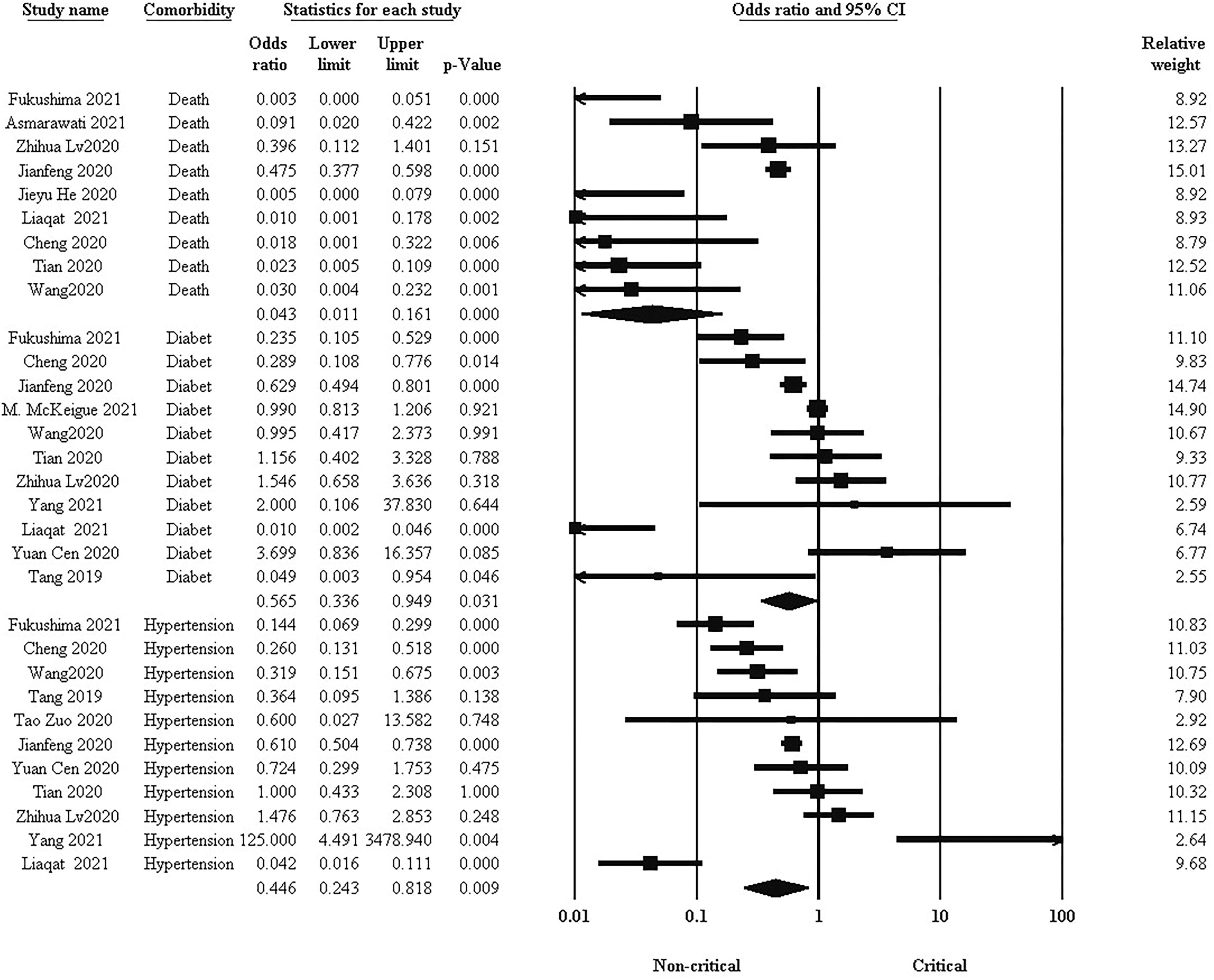
Comorbidities such as hypertension and diabetes are important factors related to mortality and complications of COVID-19 patients. Pooled results in Figure 4 showed that there was a significantly higher prevalence of hypertension, diabetes, and subsequent mortality rate in the critical group ((OR: 0.446, 95% CI: 0.243, 0.818. P = 0.009), (OR: 0.565, 95% CI: 0.336, 0.949. P = 0.031), and (OR: 0.043, 95% CI: 0.011, 0.161. P < 0.001) respectively).
Comparison of bacterial co-infection between critical and non-critical groups
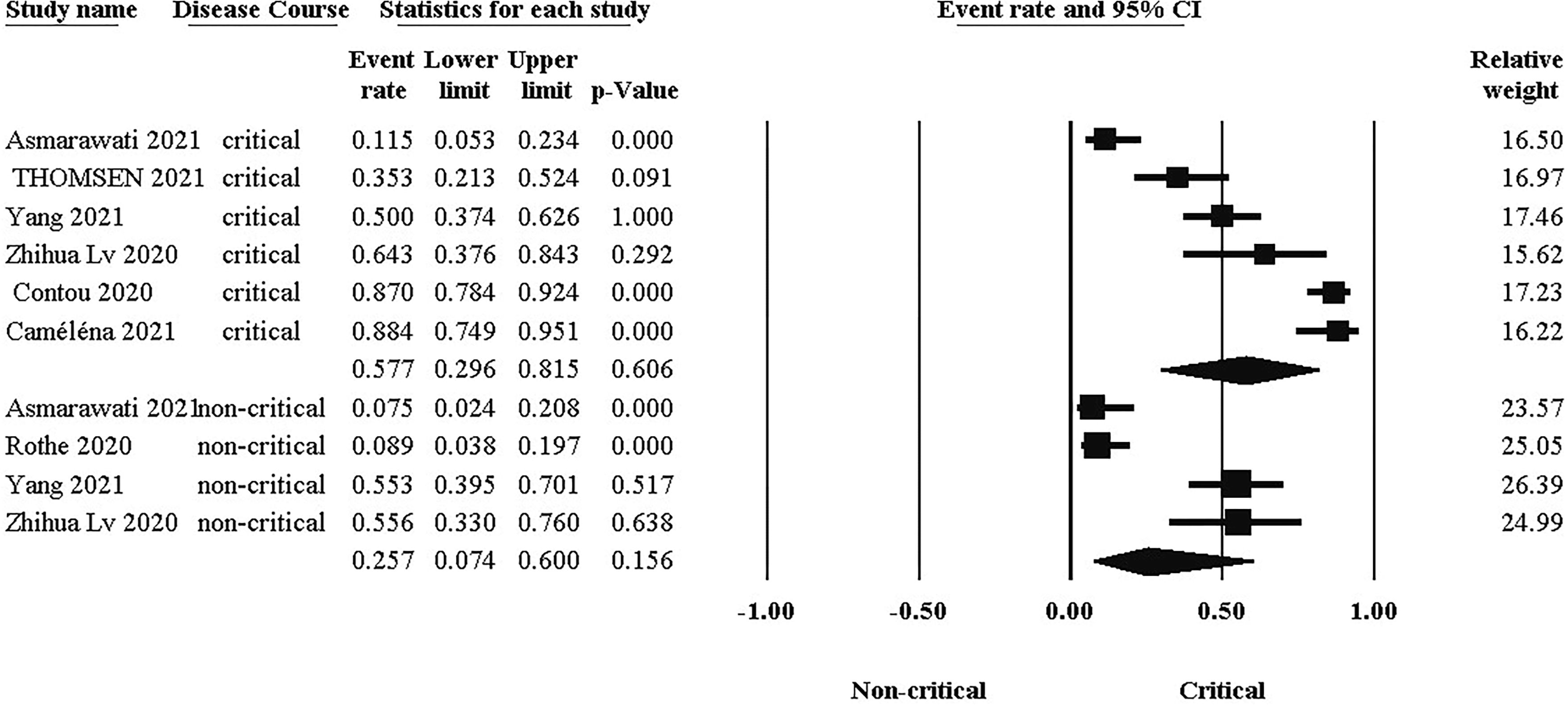
The probability of bacterial co-infection differs significantly between groups, as illustrated in Figure 5. As a result, the difference in co-infection prevalence between critical (Event rate:57.7%, 95% CI: 0.296, 0.816) and non-critical (Event rate:25.7%, 95% CI: 0.074, 0.60) groups was 32%.
Publication bias
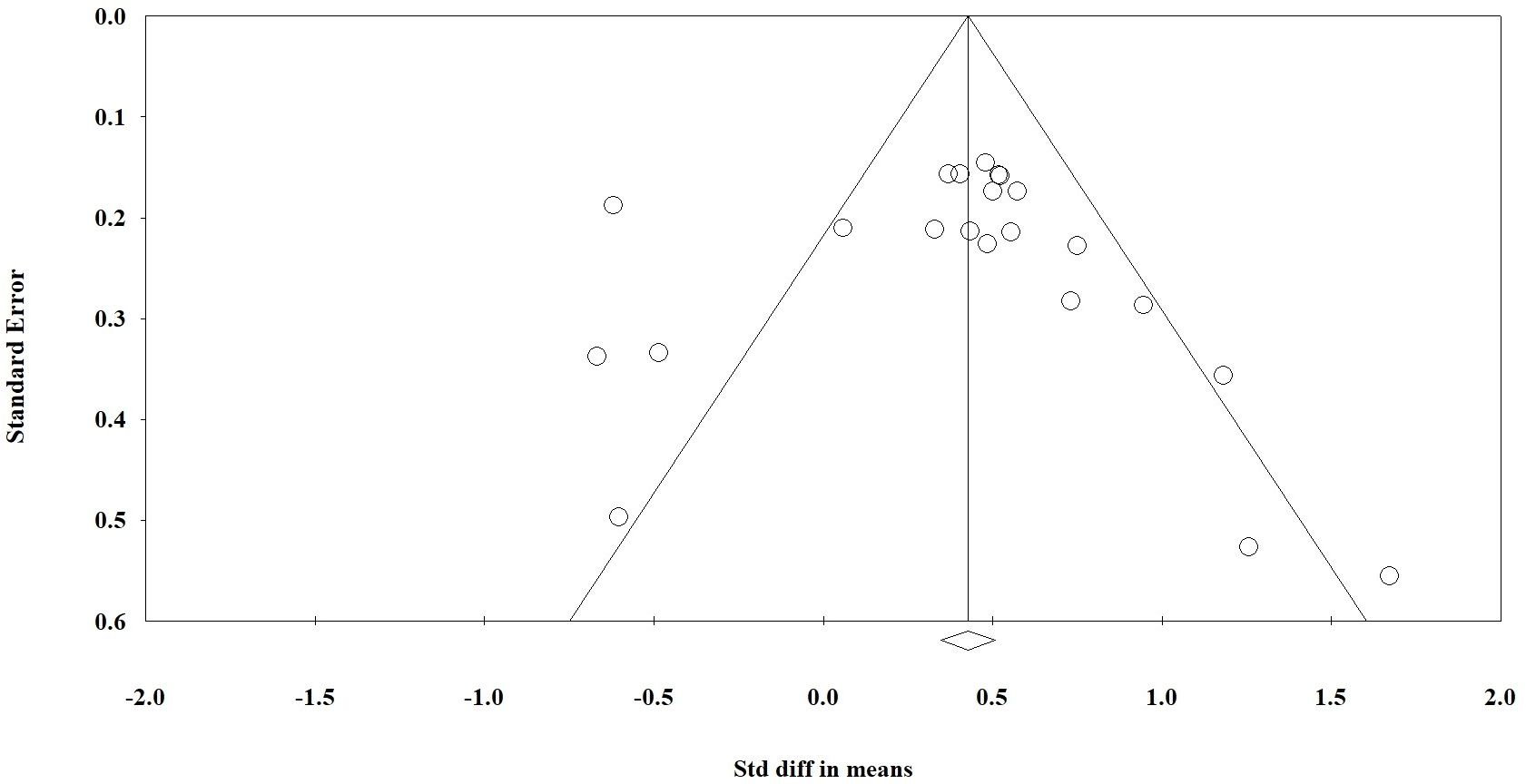
A funnel plot was used for visual evaluation (Figure 6, 5S) and Egger’s test was used to determine bias (Table 2). Egger’s test indicated publication bias for three of the ten variables. According to Egger’s test, we found significant bias in WBC and lymphocyte mean and mortality rate differences between the two groups. Using Table 2, it appears that variables with a P value < 0.05 are heterogeneous in terms of heterogeneity analysis. Although heterogeneous data does not necessarily indicate bias, the Egger test must be significant (P< 0.05). We have also attached the results of one removed study plots as Supplementary Figures 1–3.
Discussion
The COVID-19 pandemic has reached a global scale, and medical systems in many countries are experiencing severe problems as a result (32, 33). The COVID-19 pandemic in 2019 has caused significant hospitalizations and deaths. According to the clinical course on COVID-19, we can classify patients with COVID-19 into critical and non-critical groups (4). The results showed that there are several differences between critical and non-critical groups. In this study, we examined the blood cell count, blood markers, and the comorbidities difference between critical and non-critical groups.
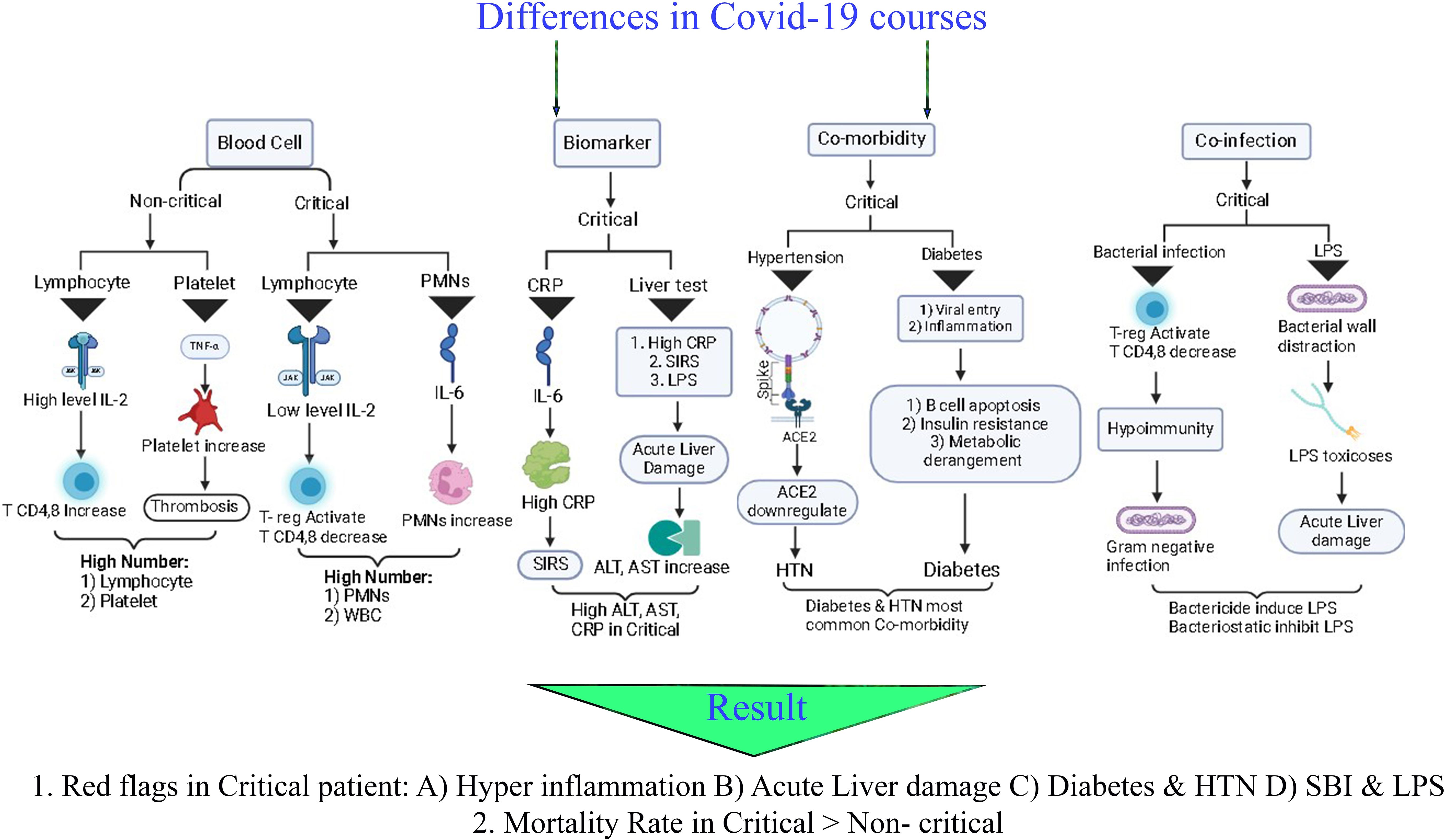
Due to the correlation between the immune system function and the clinical course of COVID-19, we compared the blood cell count between groups (34). The production of cytokines is crucial for the growth and specialization of immune cells. In COVID-19 pneumonia patients, certain inflammatory cytokines like IL-6 and IL-10 were found to be elevated in critical cases (35). However, IL-2 levels were increased in non-critical patients but decreased in critical ones. When present in low concentrations, IL-2 can prevent CD4+ T and CD8+ T-cell activation by maintaining T regulatory cell activity and survival (36). As a result, this could lead to a significant drop in CD8+ T-cells and lymphocytes in COVID-19 critical patients (37). Furthermore, critical patients had significantly lower T-cell, B-cell, and NK cell counts compared to controls (38). A gradual decrease in peripheral blood lymphocytes is a common early indicator of adult patients with non-critical and critical illnesses (39). IL-6 can stimulate T cell differentiation, and its increased levels are associated with producing acute-phase proteins like CRP and inflammatory cytokines. It is also possible that increased WBC in critical patients with low lymphocytes may be caused by an increase in PMNs, which can be indicated by an increase in CRP levels.
This study analysis indicated the higher platelet count in non-critical patients. Platelets and other related indicators play a crucial role in inflammation and prothrombotic responses during numerous viral infections (19). Apart from their traditional function in hemostasis and thrombosis, platelets also contribute significantly to the immune and inflammatory processes. Research suggests that platelets express surface receptors that enable them to bind and allow entry to various viruses. Furthermore, the rise in platelets and neutrophils could be due to anti-apoptotic cytokines and stimulation by specific pro-inflammatory cytokines (40). In addition to the immune system, enzymes and inflammation markers play an essential role in the course of COVID-19 disease (9). As a result of this study, ALT, AST, and CRP levels are significantly higher in the critical group than in the non-critical group. During acute inflammatory responses to COVID-19, there is usually a rapid and significant increase in serum CRP levels. Elevated CRP fluctuation during hospitalization has been identified as the primary cause of ICU admission with a poor prognosis (41). Analysis revealed that critical patients have higher CRP levels, indicating a more significant inflammatory response than non-critical patients (13). Although CRP is a sensitive indicator of disease activity and an independent risk factor for various diseases, studies have shown that CRP fluctuation is a better indicator of inflammation severity for guiding treatment in sepsis, systemic inflammatory response syndrome (SIRS), and community-acquired pneumonia (9, 42).
An elevated CRP level in critical patients may hint to SIRS and multi organ damage. An elevated level of ALT and AST in critically ill patients may indicate liver damage and a change in bacterial co-infection in COVID-19 disease, both of which are associated with mortality (10). Lipopolysaccharides (LPS) are always considered a major contributor to liver damage (43). In critical patients with elevated liver enzymes that are indicative of acute liver damage, LPS may be one of the contributing factors. Our analysis of critical patients reveals a high prevalence of bacterial co-infection and LPS is predominantly present in bacterial cell walls. LPS is generally released from bacterial walls during bacterial proliferation or destruction (44). Therefore, it is possible that the overused broad-spectrum antibiotics in COVID-19 patients may suddenly destroy gram-negative bacteria and induce liver damage with a large amount of LPS toxin (45). Bactericide antibiotics may cause bacteria to release LPS, so bacteriostatic are recommended instead. The bacteriostatic inhibits the proliferation of bacteria, but does not kill them, therefore the level of LPS remains low until the body can recover from COVID-19. Once COVID-19 has been eliminated, bactericide antibiotics can be used. ALT, AST, and CRP levels are associated with ICU admission risk based on the results of this study and according to the definition of critical patients.
In univariable analysis, hypertension, diabetes, cardiovascular disease, and cancer were associated with critical illnesses (24). In this study, a statistical meta-analysis revealed that comorbidities, such as hypertension and diabetes, are more prevalent in critical groups than in non-critical groups. However, some previous studies state that comorbidities are common in non-critical groups, contrary to recent studies and our meta-analysis (46). A common element of COVID-19 patients with hypertension and diabetes is the use of angiotensin-converting enzyme inhibitors (ACEI). A membrane receptor known as ACE2 is responsible for binding SARS-CoV-2 to cells and promoting its entry into the respiratory tract. The downregulation of ACE2 by SARS-Cov-2 spike protein binding reduces the protective effects of ACE2 during acute inflammation (47). ACE inhibitors may induce the ACE2 expression, the cellular receptor for SARS-Cov-2, and can aggravate the disease course (48). It has been identified that SARS-CoV-2 is able to invade cells via this previously established cell receptor which is facilitating the invasion of SARS-CoV-2 cells (49). The higher incidence of diabetes in critically ill patients can be attributed to three well-defined mechanisms (50): 1) The direct entry of viruses through various receptors in β-cells can directly cause β-cell dysfunction and apoptosis or trigger β-cell autoimmunity. Alternatively, viruses can enter pancreatic cells that express viral receptors, leading to structural and functional changes, local inflammation, and the creation of a pro-diabetic environment. This can disrupt the integrity of nearby non-infected β-cells in a paracrine manner, potentially leading to loss or dysfunction of these cells (51). 2) Targeting putative viral receptor-expressing cells in metabolic organs like the liver and adipose tissue can induce insulin resistance and result in the loss of disease tolerance mechanisms (52). 3) Induction of systemic inflammation and accumulation of prediabetic metabolites can lead to metabolic derangement and maladaptive functions (53).
Critical patients with COVID-19 pneumonia exhibit a state of immune deficiency and hypo immunity. These factors can further worsen the situation by causing severe infection and leading to fatal outcomes (54). The prevalence of bacterial co-infection in COVID-19 patients can also be another difference between critical and non-critical patients. Our meta-analysis showed bacterial co-infection is more common in critical than non-critical patients. Bronchoalveolar lavage (BAL) and sputum are usually collected in the first week of ICU admission. The majority of COVID-19 patients with bacterial co-infection previously received antibiotics. Overall, our results revealed that the frequency of bacterial co-infection is higher in critical patients following ICU admission than in non-critical patients.
Therefore, the risk of inflammation, organ damage, and previous disease is significantly higher in the critical group. According to the comparison of co-infection rates, critical patients are more likely to have co-infections than non-critical patients. Also, the critical group had a higher death rate than the non-critical group (Graphical abstract).
In conclusion, our findings suggested that critical patients have a suppressed immune system and that inflammation, organ damage, and co-infections are significantly higher. Due to these factors, critical groups have a worsened course of the disease and a high mortality rate, so these patients require rapid diagnosis and careful management. Additionally, bactericide antibiotics may cause liver failure in critical patients due to the risk of liver damage. Therefore, we suggest that this relationship be fully evaluated in future studies.
Limitations
Incomplete and vague definitions of some articles about critical and non-critical phases.
More than three-quarters of the studies we included were from China describing patients at the start of the pandemic.
Most patients with COVID-19 patients do not require hospitalization but patients in the studies included in this review were predominantly hospitalized.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
MH-C: Project administration, Resources, Supervision, Writing – review & editing. HE-S: Investigation, Methodology, Software, Validation, Writing – review & editing. AB: Conceptualization, Methodology, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1341168/full#supplementary-material
References
1. Cevik M, Bamford C, Ho A. COVID-19 pandemic—a focused review for clinicians. Clin Microbiol Infection. (2020) 26:842–7. doi: 10.1016/j.cmi.2020.04.023
2. Saberi A, Ghayeghran A, Hatamian H, Hosseini-Nejad M, Bakhshayesh Eghbali B. COVID-19-associated myelitis, para/post infectious or infectious myelitis: A case report from the North of Iran. Caspian J Neurological Sci. (2020) 6:132–8. doi: 10.32598/CJNS
3. Baghaei A, Alipour N, Abbaszadeh P, Bakhshi A, Fallah A, Zeraatpishe M, et al. Identifying and combating the Covid-19 pandemic using Artificial Intelligence. Tobacco Regul Sci (TRS). (2022), 313–30. doi: 10.18001/TRS.8.2.20
4. Liaqat A, Ali-Khan RS, Asad M, Rafique Z. Evaluation of myocardial injury patterns and ST changes among critical and non-critical patients with coronavirus-19 disease. Sci Rep. (2021) 11:1–8. doi: 10.1038/s41598-021-84467-4
5. Tian R, Wu W, Wang C, Pang H, Zhang Z, Xu H, et al. Clinical characteristics and survival analysis in critical and non-critical patients with COVID-19 in Wuhan, China: a single-center retrospective case control study. Sci Rep. (2020) 10:17524. doi: 10.1038/s41598-020-74465-3
6. Xu K, Cai H, Shen Y. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. J Zhejiang Univ (Med Sci). (2022) 2:1–12. doi: 10.24205/03276716.2020.4015
7. Shi H, Wang W, Yin J, Ouyang Y, Pang L, Feng Y, et al. The inhibition of IL-2/IL-2R gives rise to CD8+ T cell and lymphocyte decrease through JAK1-STAT5 in critical patients with COVID-19 pneumonia. Cell Death Dis. (2020) 11:1–8. doi: 10.1038/s41419-020-2636-4
8. Read KA, Powell MD, McDonald PW, Oestreich KJ. IL-2, IL-7, and IL-15: multistage regulators of CD4+ T helper cell differentiation. Exp Hematol. (2016) 44:799–808. doi: 10.1016/j.exphem.2016.06.003
9. Liu Z, Wu D, Han X, Jiang W, Qiu L, Tang R, et al. Different characteristics of critical COVID-19 and thinking of treatment strategies in non-elderly and elderly severe adult patients. Int Immunopharmacol. (2021) 92:107343. doi: 10.1016/j.intimp.2020.107343
10. Tang L, Gu S, Gong Y, Li B, Lu H, Li Q, et al. Clinical significance of the correlation between changes in the major intestinal bacteria species and COVID-19 severity. Engineering. (2020) 6:1178–84. doi: 10.1016/j.eng.2020.05.013
11. Choi GJ, Kim HM, Kang H. The potential role of dyslipidemia in COVID-19 severity: An umbrella review of systematic reviews. J Lipid Atheroscl. (2020) 9:435. doi: 10.12997/jla.2020.9.3.435
12. Tian R, Wu W, Wang C, Pang H, Zhang Z, Xu H, et al. Clinical characteristics and survival analysis in critical and non-critical patients with COVID-19 in Wuhan, China: a single-center retrospective case control study. Sci Rep. (2020) 10:1–8. doi: 10.1038/s41598-020-74465-3
13. Wang W, Zhao Z, Liu X, Liu G, Xie D, Xu Z, et al. Clinical features and potential risk factors for discerning the critical cases and predicting the outcome of patients with COVID-19. J Clin Lab Anal. (2020) 34:e23547. doi: 10.1002/jcla.23547
14. Huttner B, Catho G, Pano-Pardo J, Pulcini C, Schouten J. COVID-19: don’t neglect antimicrobial stewardship principles! Clin Microbiol Infection. (2020) 26:808. doi: 10.1016/j.cmi.2020.04.024
15. Zhang H, Zhang Y, Wu J, Li Y, Zhou X, Li X, et al. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerging Microbes infections. (2020) 9:1958–64. doi: 10.1080/22221751.2020.1812437
16. Rahim Khorasani M, Rostami S, Bakhshi A, Sheikhi R. Global evaluation of the antibacterial activity of Ceftolozane/Tazobactam against ESBLs-producing Escherichia coli and Klebsiella pneumoniae: a systematic review and meta-analysis. Ther Adv Infect Dis. (2023) 10:20499361231212074. doi: 10.1177/20499361231212074
17. Zuo T, Liu Q, Zhang F, Lui GC-Y, Tso EY, Yeoh YK, et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. (2021) 70:276–84. doi: 10.1136/gutjnl-2020-322294
18. McKeigue PM, Kennedy S, Weir A, Bishop J, McGurnaghan SJ, McAllister D, et al. Relation of severe COVID-19 to polypharmacy and prescribing of psychotropic drugs: the REACT-SCOT case-control study. BMC Med. (2021) 19:1–11. doi: 10.1186/s12916-021-01907-8
19. He J, Wei Y, Chen J, Chen F, Gao W, Lu X. Dynamic trajectory of platelet-related indicators and survival of severe COVID-19 patients. Crit Care. (2020) 24:1–4. doi: 10.1186/s13054-020-03339-x
20. Cen Y, Chen X, Shen Y, Zhang XH, Lei Y, Xu C, et al. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019-a multi-centre observational study. Clin Microbiol Infect. (2020) 26:1242–7. doi: 10.1016/j.cmi.2020.05.041
21. Wu J, Huang J, Zhu G, Wang Q, Lv Q, Huang Y, et al. Elevation of blood glucose level predicts worse outcomes in hospitalized patients with COVID-19: a retrospective cohort study. BMJ Open Diabetes Res Care. (2020) 8:1–7. doi: 10.1136/bmjdrc-2020-001476
22. Fukushima K, Yamada Y, Fujiwara S, Tanaka M, Kobayashi T, Yajima K, et al. Development of a risk prediction score to identify high-risk groups for the critical coronavirus disease 2019 (COVID-19) in Japan. Jpn J Infect Dis. (2021) 74:344–51. doi: 10.7883/yoken.JJID.2020.789
23. Liu D, Lan L, Luo D, Zhao B, Wei G, He Y, et al. Lymphocyte subsets with the lowest decline at baseline and the slow lowest rise during recovery in COVID-19 critical illness patients with diabetes mellitus. Diabetes Res Clin Pract. (2020) 167:108341. doi: 10.1016/j.diabres.2020.108341
24. Cheng S, Wu D, Li J, Zou Y, Wan Y, Shen L, et al. Risk factors for the critical illness in SARS-CoV-2 infection: a multicenter retrospective cohort study. Respir Res. (2020) 21:1–12. doi: 10.1186/s12931-020-01492-z
25. Lv Z, Cheng S, Le J, Huang J, Feng L, Zhang B, et al. Clinical characteristics and co-infections of 354 hospitalized patients with COVID-19 in Wuhan, China: a retrospective cohort study. Microbes Infect. (2020) 22:195–9. doi: 10.1016/j.micinf.2020.05.007
26. Caméléna F, Moy AC, Dudoignon E, Poncin T, Deniau B, Guillemet L, et al. Performance of a multiplex polymerase chain reaction panel for identifying bacterial pathogens causing pneumonia in critically ill patients with COVID-19. Diagn Microbiol Infect Dis. (2021) 99:115183. doi: 10.1016/j.diagmicrobio.2020.115183
27. Contou D, Claudinon A, Pajot O, Micaëlo M, Longuet Flandre P, Dubert M, et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intensive Care. (2020) 10:119. doi: 10.1186/s13613-020-00736-x
28. Rothe K, Feihl S, Schneider J, Wallnöfer F, Wurst M, Lukas M, et al. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: a retrospective cohort study in light of antibiotic stewardship. Eur J Clin Microbiol Infect Dis. (2021) 40:859–69. doi: 10.1007/s10096-020-04063-8
29. Thomsen K, Pedersen HP, Iversen S, Wiese L, Fuursted K, Nielsen HV, et al. Extensive microbiological respiratory tract specimen characterization in critically ill COVID-19 patients. Apmis. (2021) 129:431–7. doi: 10.1111/apm.13143
30. Asmarawati TP, Rosyid AN, Suryantoro SD, Mahdi BA, Windradi C, Wulaningrum PA, et al. The clinical impact of bacterial co-infection among moderate, severe and critically ill COVID-19 patients in the second referral hospital in Surabaya. F1000Res. (2021) 10:113. doi: 10.12688/f1000research
31. Yang S, Hua M, Liu X, Du C, Pu L, Xiang P, et al. Bacterial and fungal co-infections among COVID-19 patients in intensive care unit. Microbes Infect. (2021) 23:104806. doi: 10.1016/j.micinf.2021.104806
32. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
33. Bakhshi A, Eslami N, Norouzi N, Letafatkar N, Amini-Salehi E, Hassanipour S. The association between various viral infections and multiple sclerosis: An umbrella review on systematic review and meta-analysis. Rev Med Virol. (2024) 34:e2494. doi: 10.1002/rmv.2494
34. Palladino M. Complete blood count alterations in COVID-19 patients: A narrative review. Biochem Med (Zagreb). (2021) 31:030501. doi: 10.11613/issn.1846-7482
35. Azaiz MB, Jemaa AB, Sellami W, Romdhani C, Ouslati R, Gharsallah H, et al. Deciphering the balance of IL-6/IL-10 cytokines in severe to critical COVID-19 patients. Immunobiology. (2022) 227:152236. doi: 10.1016/j.imbio.2022.152236
36. Ghanbari Naeini L, Abbasi L, Karimi F, Kokabian P, Abdi Abyaneh F, Naderi D. The important role of interleukin-2 in COVID-19. J Immunol Res. (2023) 2023. doi: 10.1155/2023/7097329
37. Shi H, Wang W, Yin J, Ouyang Y, Pang L, Feng Y, et al. The inhibition of IL-2/IL-2R gives rise to CD8+ T cell and lymphocyte decrease through JAK1-STAT5 in critical patients with COVID-19 pneumonia. Cell Death Dis. (2020) 11:429. doi: 10.1038/s41419-020-2636-4
38. Li X, Cheng W, Zhang J, Li D, Wang F, Cui N. Early alteration of peripheral blood lymphocyte subsets as a risk factor for delirium in critically ill patients after cardiac surgery: A prospective observational study. Front Aging Neurosci. (2022) 14:950188. doi: 10.3389/fnagi.2022.950188
39. Fouladseresht H, Ghamar Talepoor A, Eskandari N, Ghezelbash B, Nejadghaderi SA, Kolahi A-A, et al. Potential immune indicators for predicting the prognosis of COVID-19 and trauma: similarities and disparities. Front Immunol. (2022) 12:785946. doi: 10.3389/fimmu.2021.785946
40. Scherlinger M, Richez C, Tsokos GC, Boilard E, Blanco P. The role of platelets in immune-mediated inflammatory diseases. Nat Rev Immunol. (2023) 23:495–510. doi: 10.1038/s41577-023-00834-4
41. Nazemi P, SeyedAlinaghi S, Azarnoush A, Mabadi A, Khaneshan AS, Salehi M. Serum C-reactive protein greater than 75 mg/dL as an early available laboratory predictor of severe COVID-19: A systematic review. Immunity Inflammation Dis. (2023) 11:e1130. doi: 10.1002/iid3.1130
42. Mouliou DS. C-reactive protein: pathophysiology, diagnosis, false test results and a novel diagnostic algorithm for clinicians. Diseases. (2023) 11:132. doi: 10.3390/diseases11040132
43. Gandhi CR. Pro- and anti-fibrogenic functions of gram-negative bacterial lipopolysaccharide in the liver. Front Med (Lausanne). (2020) 7:130. doi: 10.3389/fmed.2020.00130
44. Matsuura M. Structural modifications of bacterial lipopolysaccharide that facilitate gram-negative bacteria evasion of host innate immunity. Front Immunol. (2013) 4:48636. doi: 10.3389/fimmu.2013.00109
45. Heinbockel L, Weindl G, Martinez-de-Tejada G, Correa W, Sanchez-Gomez S, Bárcena-Varela S, et al. Inhibition of lipopolysaccharide- and lipoprotein-induced inflammation by antitoxin peptide Pep19-2.5. Front Immunol. (2018) 9:1704. doi: 10.3389/fimmu.2018.01704
46. Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) 8:475–81. doi: 10.1016/S2213-2600(20)30079-5
47. Gallo G, Calvez V, Savoia C. Hypertension and COVID-19: current evidence and perspectives. High Blood Pressure Cardiovasc Prev. (2022) 29:115–23. doi: 10.1007/s40292-022-00506-9
48. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. (2020) 8:e21. doi: 10.1016/S2213-2600(20)30116-8
49. Naserghandi A, Saffarpour R, Allameh SF. Exploring the causes of mild COVID-19 involvement in pediatric patients. New Microbes New Infections. (2020) 37:100741. doi: 10.1016/j.nmni.2020.100741
50. Lotfy MA, Shama AA. Intensive insulin therapy improves the survival probability of non-diabetic COVID-19 patients presenting with acute hyperglycemia. Egyptian J Anaesthesia. (2022) 38:211–9. doi: 10.1080/11101849.2022.2060636
51. Geravandi S, Mahmoudi-Aznaveh A, Azizi Z, Maedler K, Ardestani A. SARS-CoV-2 and pancreas: a potential pathological interaction? Trends Endocrinol Metab. (2021) 32:842–5. doi: 10.1016/j.tem.2021.07.004
52. D’Orazio MAGN. COVID-19 and obesity: overlapping of two pandemics. (2021). 6:579–85. doi: 10.1159/000518386
53. Lima-Martínez MM, Boada CC, Madera-Silva MD, Marín W, Contreras M. COVID-19 and diabetes: A bidirectional relationship. In: Clínica e Investigación En Arteriosclerosis (English Edition) (Spain: Elsevier). (2021). 151–7.
Keywords: COVID-19, critical, co-infection, mortality, sever
Citation: Hedayati-Ch M, Ebrahim-Saraie HS and Bakhshi A (2024) Clinical and immunological comparison of COVID-19 disease between critical and non-critical courses: a systematic review and meta-analysis. Front. Immunol. 15:1341168. doi: 10.3389/fimmu.2024.1341168
Received: 19 November 2023; Accepted: 28 March 2024;
Published: 16 April 2024.
Edited by:
Athanasia Mouzaki, University of Patras, GreeceReviewed by:
Stelvio Tonello, University of Eastern Piedmont, ItalyMaria Lagadinou, University of Patras, Greece
Copyright © 2024 Hedayati-Ch, Ebrahim-Saraie and Bakhshi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arash Bakhshi, QS5iYWtoc2hpLmIxM0BnbWFpbC5jb20=
†ORCID: Arash Bakhshi, orcid.org/0000-0002-0642-4651
 Mojtaba Hedayati-Ch1,2
Mojtaba Hedayati-Ch1,2 Arash Bakhshi
Arash Bakhshi