- 1Department of Dermatology and Venereology, University of Tartu, Dermatology Clinic, Tartu University Hospital, Tartu, Estonia
- 2Department of Dermatology and Venereology, Faculty of Medicine, Riga Stradins University, Riga, Latvia
- 3Department of Skin and Venereal Diseases, Lithuanian University of Health Sciences (LSMU), Hospital of LSMU Kauno Klinikos, European Reference Network for Rare and Complex Diseases of the Skin (ERN-Skin) Member, Kaunas, Lithuania
- 4Dermatology Department, State Research Center of Dermatovenereology and Cosmetology, Moscow, Russia
- 5Dermatology Department, Markusovszky University Teaching Hospital, Szombathely, Hungary
- 6Ambulatory for Specialized Medical Care, Skin and Venereal Diseases, Sofia, Bulgaria
- 7AbbVie SRL Romania, Bucharest, Romania
- 8AbbVie Deutschland GmbH & Co. KG, Ludwigshafen, Germany
- 9AbbVie Portugal, Amadora, Portugal
- 10IInd Department of Dermatology, Colentina Clinical Hospital, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania
Psoriasis is a common, life-long skin disease with a significant negative health and societal impact. Data on rates of disease control and treatment strategies are lacking in Central and Eastern European countries. We aimed to describe the real-world disease severity, control, and treatment strategies for psoriasis in patients from Central and Eastern European countries. CRYSTAL (EUPAS36459) was a cross-sectional, retrospective study in adults (18–75 years) from Bulgaria, Estonia, Hungary, Latvia, Lithuania, Romania, and Russia. We enrolled patients with moderate-to-severe psoriasis receiving continuous systemic treatment for ≥24 weeks. We used the Psoriasis Area and Severity Index (PASI) to describe disease severity and the Dermatology Life Quality Index (DLQI) to assess quality of life (QoL) and collected other outcomes [psoriasis work productivity and activity impairment (WPAI-PSO), patient satisfaction] at enrollment. Analyses were descriptive. A total of 690 patients were included in the analyses. Median disease duration was 11.8 years. Current treatment was monotherapy for most patients (95.8%) with either biological (BIO group; 88.4%) or conventional (NON-BIO group; 7.4%) agents. Mean (± standard deviation) absolute PASI scores were 3.5 ± 5.7, 3.1 ± 5.3, and 6.6 ± 7.4 in the overall population, the BIO group, and the NON-BIO group, respectively. Among patients treated with monotherapy, absolute PASI scores ≤1, ≤3, and ≤5 were observed for 44.1%, 72.0%, and 82.6% of BIO patients and 21.6%, 33.3%, and 49.0% of NON-BIO patients. Mean DLQI total score was 3.3 ± 5.1; higher scores were noted for higher absolute PASI. The most impacted WPAI-PSO domain was presenteeism; for all domains, impact increased with increased absolute PASI. A total of 91.8% of BIO patients and 74.5% of NON-BIO patients were satisfied with the current treatment. We observed a better disease control in BIO than NON-BIO patients. However, around half of BIO patients did not reach clear skin status and reported an impact on QoL. An improvement in treatment strategies is still needed in Central and Eastern European countries to optimize outcomes of moderate-to-severe psoriasis.
1 Introduction
Psoriasis is a chronic, immune-mediated inflammatory skin disease affecting 2%–4% of the general population in Western countries (1, 2) and generating a high burden in terms of patients’ quality of life (QoL), comorbidity, and social costs (3–5). The Global Burden of Disease group has estimated more than 64.6 million psoriasis cases globally in 2017 (6) and 4,622,594 incident cases in 2019 (7). In Europe, country-specific prevalence estimates for psoriasis as diagnosed by physicians/dermatologists ranged from 0.51% to 2.36%, and an overall lower prevalence was reported in Central and Eastern European than in Western European countries (8). However, more recent reports indicate a higher burden of disease in Central and Eastern Europe: in Latvia, the estimated annual incidence between 2015 and 2020 was 2.1–2.2 cases per 1,000 person-years (9), and, in Romania, a prevalence of 4% was estimated between November 2018 and February 2019 (10).
The severity of psoriasis depends to a significant degree on the extension of lesions, which may range from a few scattered red, scaly plaques to involvement of almost the entire body surface, impacting severely the individual’s QoL. Several factors such as disease severity, gender, age, anatomical sites of lesion, comorbidity(ies), psychological distress and burden, and time needed for treatment have been associated with a reduced health-related QoL (HRQoL) (11). Most psoriasis patients also experience negative impact on work, emotions, and relationships (12).
Treatment effectiveness and convenience have been proposed as measures of patients’ satisfaction with therapy under real-world conditions (13), and treatment regimens are frequently adjusted in order to maximize effectiveness.
The growing variety of treatments have increased expectations to achieve a complete/almost complete resolution of disease symptoms, as observed in clinical trials (14). International guidelines (15–17) recently incorporated the Psoriasis Area and Severity Index (PASI) absolute scores (e.g., PASI ≤2) as treatment targets (in addition to relative scores), as they reflect the efficacy of a treatment regardless of disease severity at baseline. Moreover, absolute PASI is known to better correlate with the Dermatology Life Quality Index (DLQI) than relative PASI.
Biological therapy has shown good effectiveness in real-world settings (18) and greater efficacy and improvement in the patients’ HRQoL compared to conventional agents (19, 20). However, the use of biologics in psoriasis patients is not uniformly implemented in Central and Eastern Europe (21) and recent data on the type of treatment used in real-life settings are lacking (21, 22). The main objective of this study was to describe psoriasis severity, by absolute PASI scores, for patients from Central and Eastern European countries with moderate-to-severe psoriasis under systemic treatment in the clinical setting. We also aimed to describe treatment patterns, HRQoL, work and activity impairment, and treatment satisfaction in these patients.
2 Material and methods
2.1 Study design and participants
We conducted an epidemiological, multi-country, multicenter, cross-sectional, retrospective study between September 2020 and February 2021, in 29 hospital centers/clinics/practices (public or private) specialized in dermatology from seven Central and Eastern European countries: Bulgaria, Estonia, Hungary, Latvia, Lithuania, Romania, and Russia. The study involved a single visit where eligibility was assessed, informed consent was obtained, and study data were collected. All treatments were administered according to routine clinical practice. Study approvals were obtained from National and/or local Ethics Committees in all participating countries. The study was designed and conducted in accordance with the Declaration of Helsinki, the Good Pharmacoepidemiology Practices guidelines of the International Society for Pharmacoepidemiology, as well as local regulations.
This study included patients meeting all selection criteria who accepted to participate. Eligible patients were aged 18–75 years with confirmed diagnosis of moderate-to-severe chronic plaque–type psoriasis and treated with any approved systemic treatment for psoriasis (mono- or combination therapy) continuously for at least 24 weeks, who had absolute PASI assessed at the start of their current systemic treatment (including during a window between 30 days prior and 7 days after) and were expected to have absolute PASI assessment at enrollment (i.e., the study visit). Patients receiving treatment with any investigational intervention or who had received treatment within 1 month or 5 half-lives of the agent were not eligible.
The study is registered in The European Union electronic Register of Post-Authorization Studies (EUPAS36459).
2.2 Data collection
The data collected at the study baseline visit included medical history and baseline demographic and behavioral characteristics, including smoking habits. Disease severity by absolute PASI [calculated as described in Text S1 (23)], comorbidities, treatment for psoriasis, and patient-reported outcomes in terms of HRQoL [DLQI (24)], work productivity and activity impairment [WPAI (25)], and patient satisfaction with treatment were collected. Disease characteristics at psoriasis diagnosis, clinically relevant medical history (including psoriatic arthritis), past treatments for psoriasis, and information about the current treatment from its initiation until enrollment (i.e., date of initiation, starting dosage, and dosage intensifications) were also recorded. All data were entered into a password-protected, web-based electronic data capture system by the physician.
Patients completed the DLQI, EQ-5D-5L [including the EuroQol-visual analog scale (EQ-VAS)], and WPAI-PSO questionnaires (paper forms), and scores were calculated as described in Supplementary Text S1.
2.3 Statistical analysis
Sample size calculation was based on the primary endpoint. A sample size of 630 patients was estimated to produce a two-sided 95% confidence interval with a distance from the mean to limits equal to 0.078 for an estimated standard deviation (SD) of 1.0.
Statistical analyses were mainly descriptive and were performed on the full analysis set, including all eligible patients with available data. In addition, where indicated by the study objectives, analyses were also performed in the study subpopulations by current systemic treatment option and by absolute PASI score at the study visit, as applicable. Exploratory statistical tests were used only in the context of examining the correlation between HRQoL/DLQI/EQ-VAS and the absolute PASI score at the study visit, and the potential association of factors of interest with primary and secondary outcomes.
Continuous variables were examined with the Shapiro–Wilk test for normality. The correlation between continuous variables was evaluated by use of the Spearman’s ρ correlation coefficient. The effect of factors of interest on the primary outcome variable (absolute PASI score at the study visit) was assessed by linear regression models. The potential influence of confounding factors on the associations was examined through multivariable linear regression analysis. The following variables were entered in the initial step of the stepwise procedure based on minimization of the Akaike’s information criterion:
• For absolute PASI score at study visit: absolute PASI at the start of current treatment (or most recent assessment), comorbid psoriatic arthritis and/or spondylitis and/or enthesitis and/or dactylitis at start of current treatment, current systemic treatment with biological agents, disease duration at the start of current treatment, duration of current systemic treatment, gender, number of previous treatment courses with biological agents, physician-reported disease severity at the start of current treatment, positive family history of psoriasis, nail psoriasis at the start of current treatment, comorbidities diagnosed prior to the start of current treatment, and prior use of biological agent(s) before the start of current treatment. Gender was not identified as a confounder and thus was included in the initial step of the stepwise procedure. Patients’ age at the start of current treatment was identified as a confounder of the association between absolute PASI score at study visit and disease duration (years) at the start of current treatment (continuous); therefore, it was excluded from the stepwise process and was added in the final model.
• For PASI ≤1 achievement at study visit: absolute PASI at the start of current treatment (or most recent assessment), comorbid psoriatic arthritis and/or spondylitis and/or enthesitis and/or dactylitis at the start of current treatment, current systemic treatment with biologics, disease duration at the start of current treatment, duration of current systemic treatment, gender, number of previous treatment courses with biologic agents, patients’ age at the start of current treatment, physician-reported disease severity at the start of current treatment, positive family history of psoriasis, nail psoriasis at the start of current treatment, comorbidities diagnosed prior to the start of current treatment, and prior use of biologic agent(s) before the start of current treatment.
Variables that were examined in both continuous and categorical forms by univariable regression models were included in the multivariable model in the form corresponding to a lower p-value in the univariable analysis. Independent variables with a missing data rate exceeding 10% were not included in the multivariable analysis. All statistical tests were two-sided and were performed at a 0.05 significance level.
Analyses were performed using the SAS statistical software package.
3 Results
3.1 Patients, disease, and treatment characteristics
Of 694 enrolled patients, 690 were included in the full analysis set. For four patients, inclusion criteria were not met (“age between 18 and 75 years old” for two patients and “continuous systemic treatment for psoriasis for at least 24 weeks” for two patients).
The median age at enrollment was 49.7 [interquartile range (IQR), 39.4–60.2] years, and most patients were men (64.9%; 448). Nearly half (46.5%; 321) of patients had at least one comorbidity (Table 1), and 89 (13.0%) of 682 patients with available data had active psoriatic arthritis.
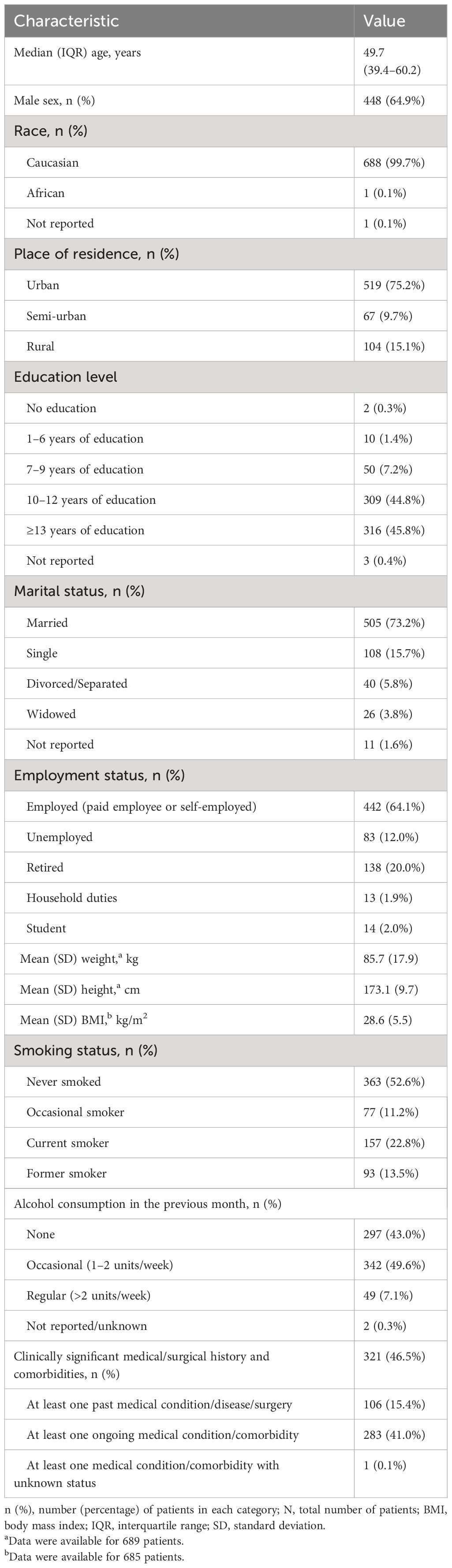
Table 1 Patient sociodemographic, anthropometric, lifestyle, and clinical characteristics at study visit (full analysis set, N = 690).
Median disease duration was 11.8 (IQR, 5.8–21.8) years (Table 2). At the current systemic treatment initiation, 95.2% (657) of patients had received at least one prior treatment, which had been discontinued at current systemic treatment initiation, with 552 (80.0%) and 625 (90.6%) patients having received systemic and non-systemic treatment, respectively. For the previous systemic treatment, the type of treatment received was conventional agents [73.9% (68.7% methotrexate)], phototherapy (62.0%), biological agents {27.1% [20% tumor necrosis factor (TNF) inhibitors, 6.7% interleukin-17 (IL-17) inhibitors, and 3.3% IL-12/23 inhibitors]}. The current systemic treatment had been received over a median period of 27.7 months. Most patients (95.8%) were receiving monotherapy with either biological (88.4%) or conventional (7.4%) agents, whereas 4.2% of patients were under a combination treatment (Table 2). During the current systemic treatment, 47.0% (324) of patients had received at least one concomitant topical treatment, which was ongoing at enrollment in the study for 29.4% (203) of patients (with keratolytic agents such as salicylic acid for 21.7% and corticosteroids for 14.6% of patients). Among patients receiving biological monotherapy, treatment was intensified (i.e., dose increased or time between doses decreased) in 3.1% (19) of patients (mainly due to insufficient response).
3.2 PASI scores
The mean (SD) absolute PASI score was 3.5 (5.7) (Table 2). The mean absolute PASI was 3.1 (5.3) and 6.6 (7.4) for biological agents and non-biological agents. The proportions of patients with absolute PASI scores ≤1, ≤3, and ≤5 were 42.3%, 69.1%, and 80.0%, respectively. An absolute PASI score <1 was achieved by 44.1% and 21.6% of patients for biologics (monotherapy) and non-biologics, respectively. Absolute PASI scores ≤1, ≤3, and ≤5 were observed for 44.1%, 72.0%, and 82.6% of patients receiving monotherapy with biological agents and 21.6%, 33.3%, and 49.0% of patients receiving monotherapy with conventional agents (Figure 1).
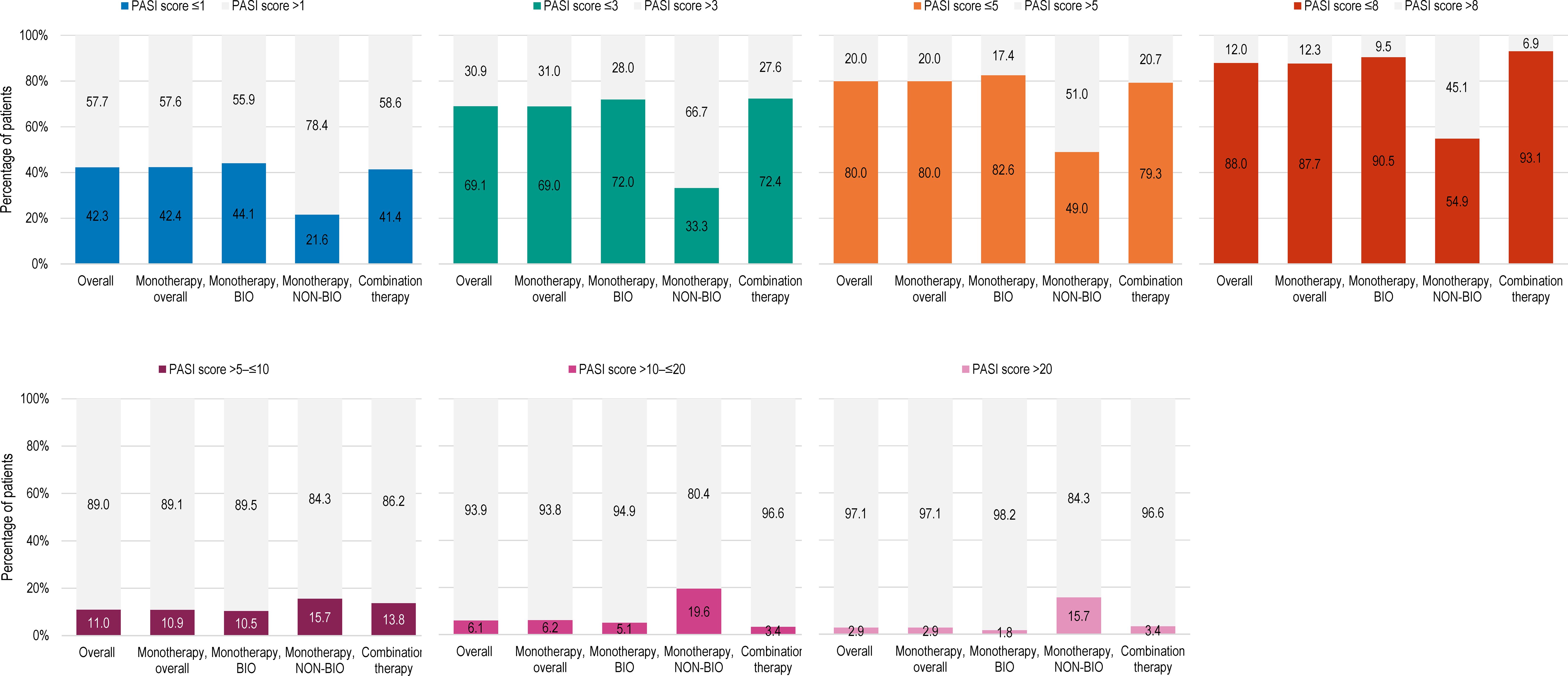
Figure 1 Distribution of patients by PASI score at study visit, in the overall population, and by current systemic treatment received (full analysis set). BIO, patients treated with biological agents; NON-BIO, patients treated with non-biological (conventional) agents; PASI, Psoriasis Area and Severity Index.
In multivariate analyses, lower absolute PASI scores at the start of current systemic treatment, current systemic treatment with biologics, higher disease duration at the start of current treatment, higher duration of current systemic treatment, and lower patient age at the start of current treatment were associated with decreased absolute PASI scores at the study visit. Lower patient age at the start of treatment, disease duration ≥10 years, current systemic treatment with biologics, absence of nail psoriasis, moderate psoriasis at the start of the current treatment, and female gender were associated with increased odds of reaching an absolute PASI score of ≤1 (Table 3).

Table 3 Results of multivariable linear regression analysis with factors of interest for absolute PASI score at study visit and for absolute PASI ≤1 achievement at study visit (N = 679).
3.3 Quality of life
The mean (SD) DLQI total score in the overall population was 3.3 (5.1) (Table 4). Impairments in HRQoL, as shown by DLQI scores >5 were observed in 20.0% (138) of patients (Figure 2A). DLQI scores increased with higher PASI scores; among the patients with an absolute PASI scores >1 and >3, 30.7% (122/398) and 45.5% (97/213), respectively, had DLQI scores >5 (Figure 2B).
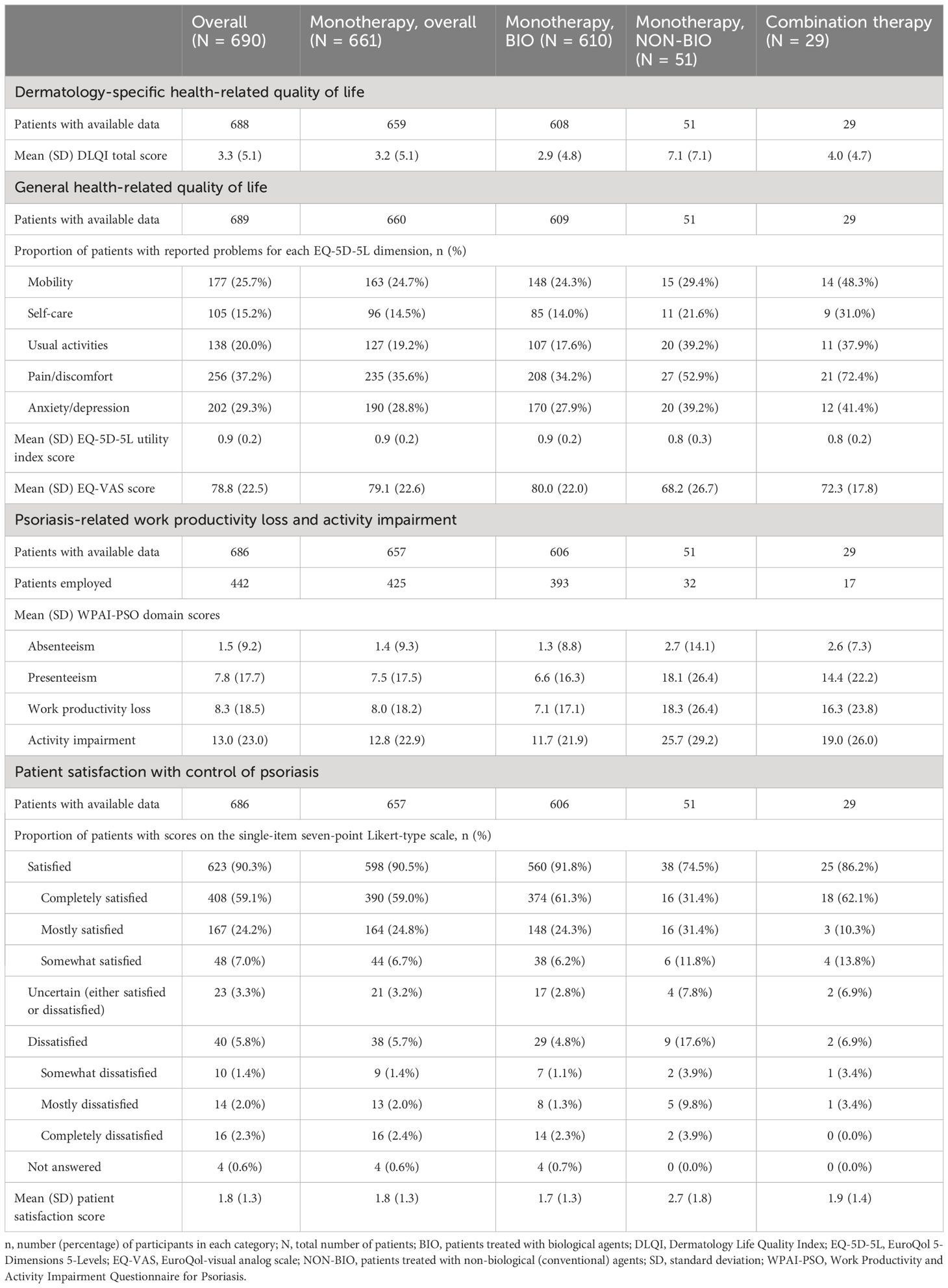
Table 4 Patient-reported outcomes at study visit, in the overall population, and by current systemic treatment received (full analysis set).
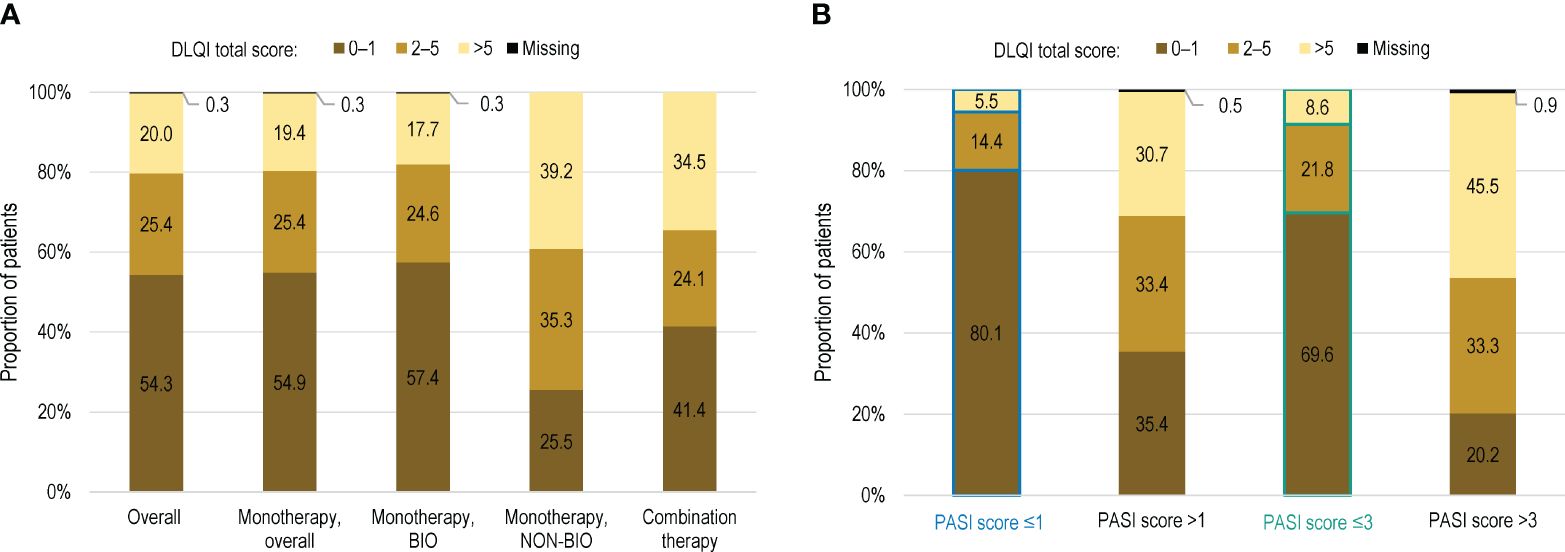
Figure 2 Distribution of patients by DLQI score in (A) the overall population, by current systemic treatment received; and (B) in the overall population with PASI score >1 and >3 (full analysis set). BIO, patients treated with biological agents; DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area and Severity Index; NON-BIO, patients treated with non-biological (conventional) agents.
In the overall population, the correlation between the DLQI total score and the absolute PASI score was positive (Spearman ρ = 0.591, p < 0.001) (Table 5).
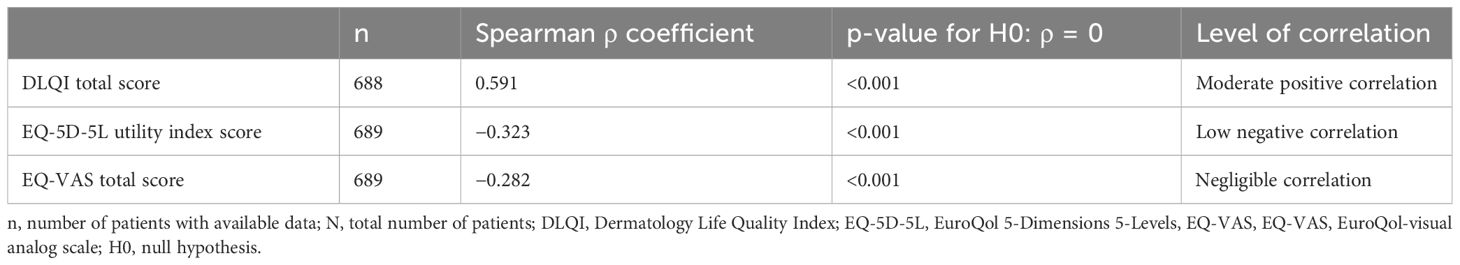
Table 5 Correlation of dermatology-specific and generic health-related quality of life with absolute PASI score as assessed by the Spearman’s rho correlation coefficient (full analysis set, N = 690).
The mean (SD) EQ-5D-5L utility score in the overall population was 0.9 (0.2). The most commonly reported negatively affected dimension was pain/discomfort, followed by anxiety/depression. The mean EQ-VAS score was 78.8 (22.5) (Table 4).
3.4 Work productivity and activity impairment
Overall, 686 (99.4%) patients completed the WPAI-PSO questionnaire, of whom 442 (64.4%) were employed (Table 4). The domains with the highest impact of disease severity were presenteeism, followed by work productivity loss and activity impairment. For all domains, a higher impact was observed at higher PASI scores (Figure 3, Supplementary Table S1).
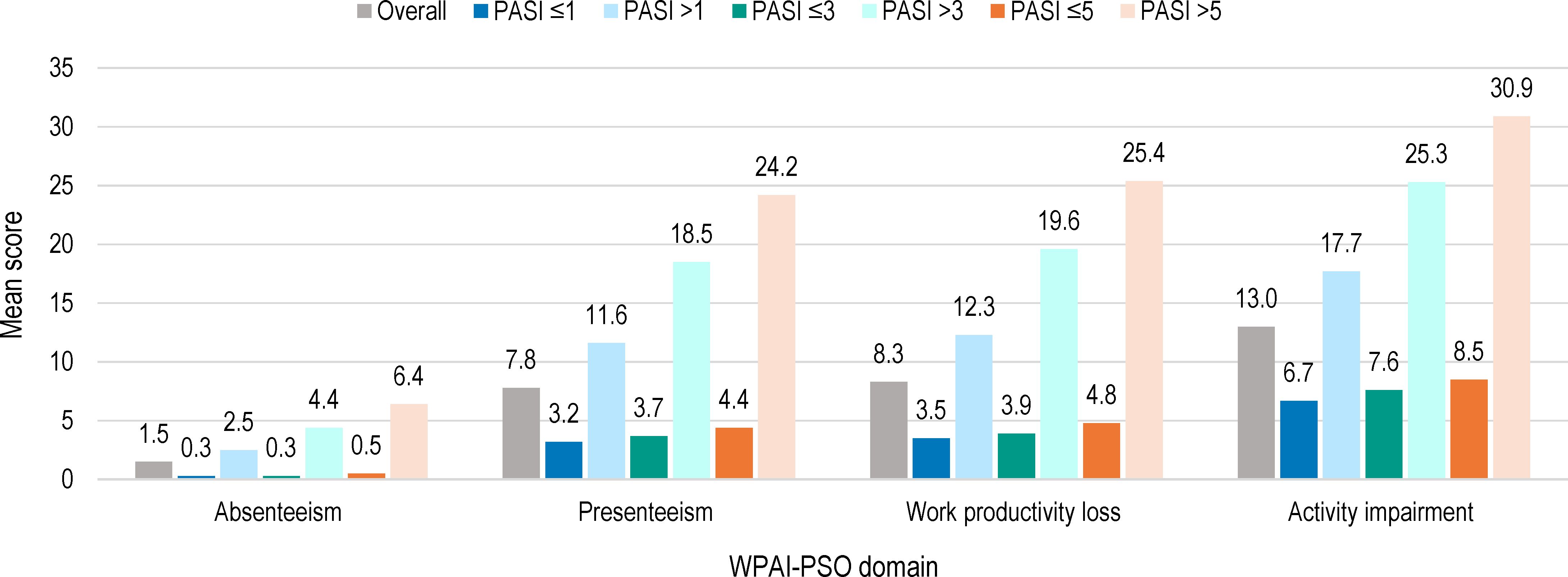
Figure 3 WPAI-PSO domain scores by absolute PASI scores at study visit (full analysis set). WPAI-PSO, Work Productivity and Activity Impairment Questionnaire for Psoriasis; PASI, Psoriasis Area and Severity Index. Note: Of the 690 patients in the full analysis set, 442 were employed.
3.5 Patient satisfaction
Patient satisfaction questionnaires were completed by 99.4% of patients. In the overall population, most patients (90.3%) were satisfied with the overall control of the disease achieved with their current systemic treatment. The percentage of patients who were satisfied varied by systemic treatment: 91.8%, 74.5%, and 86.2% in patients receiving monotherapy with biological agents, monotherapy with conventional agents and combination therapy, respectively (Table 4).
4 Discussion
This large retrospective study is the first to provide extensive evidence on the control and treatment patterns of psoriasis in a large population of patients with moderate-to-severe disease, managed with systemic therapy in routine clinical settings across seven Central and Eastern European countries.
The median age at initial diagnosis was 32.9 years, most patients (64.9%) were men, and almost half (46.5%) had comorbidities. The characteristics of patients in our study compared well to those previously reported in another study conducted in 2008 in 913 patients from Central and Eastern Europe, considered as typical of a population of psoriasis patients (26).
We found that, after a median duration of 2.3 years of systemic treatment, mainly comprising biological monotherapy (in 88.4% of patients), more than 40% of participants with initial moderate-to-severe disease had absolute PASI scores ≤1, about two-thirds had scores ≤3, and 20% had not achieved scores ≤5. Absolute PASI scores can be an additional predictor for clinical response, which is more readily available in routine clinical settings than score improvements (27). Absolute PASI scores have been proposed as a measure of treatment goals, with values ≤2 or ≤3 indicating success and >5 indicating the need for a change in treatment, regardless of the baseline values (28). In our study, a higher proportion of patients with absolute PASI scores ≤3 (72.0% versus 33.3%) and a lower proportion with absolute scores >5 (17.4% versus 51.0%) were observed among the group treated with systemic biological therapy than those under conventional therapy. This finding is an indicator for better efficacy for systemic treatment using biological agents compared with conventional ones, in line with previous observations (29). In Europe, the recently updated EuroGuiDerm guidelines recommend initiation of systemic treatment for moderate-to-severe cases of psoriasis, using conventional agents as first-line and biological agents in the case of inadequate response, contraindications, or lack of tolerance to the conventional systemic treatment. However, initiation with biologics is recommended as a first-line treatment for severe psoriasis, when lack of success is anticipated with use of conventional agents (16). In contrast, French and British guidelines do not include biologics as first-line therapy, only as an option in case of lack of response to at least two conventional systemic therapies or in case of intolerance/contraindications (15, 17). The use of biologics can vary from one country to another and is impacted not only by national practices but also by criteria for coverage or access to specific agents. In contrast with previous reports from Central and Eastern European countries (21), our study indicated a wide use of biological therapy among patients with moderate-to-severe psoriasis, in line with current European recommendations (16).
Almost half of patients in our study had a DLQI score >1 and 20% of patients had a score >5. We observed a higher impact of the disease on HRQoL in patients treated with conventional agents than those treated with biological therapy. However, even among the latter, an impact on the QoL was reported by >40% of patients. In addition, a DLQI score >2 was still noted in around 20% of patients with absolute PASI score <1. This moderate impact of psoriasis is expected in the study population, comprising patients treated for at least 24 weeks, but still confirms the negative effect of the disease on the patient’s QoL. In a recent survey conducted in adult patients from 20 countries worldwide, 54% reported a very large to extremely large impact of psoriasis on their HRQoL (12), as indicated by DLQI scores ≥10. In a previous study conducted in Russia, psoriasis was shown to have a negative impact on patients’ HRQoL (with a reported mean DLQI score of 7.1) and work productivity (dropping by 33.2%), which increased further with disease severity (30). In a survey conducted in Romania, 35.7% of patients indicated that the disease affected their relationship with family and friends and 46.1% reported reduced social comfort in public places (31). We also established a moderate positive correlation between the DLQI and disease severity as indicated by PASI, in line with previous findings (32). Nevertheless, the effect of psoriasis on the QoL and the patient’s perception on achieving a therapeutic goal should be considered in a broader context, beyond a single point in time. Thus, the concept of cumulative life course impairment was previously proposed to express the ongoing, accumulated impact of psoriasis and its associated stigmatization and physical and psychological comorbidities over a patient’s life course (33), potentially preventing psoriasis patients from realizing their full life potential (34).
In our study, WPAI-PSO scores were low in the overall population, driven by the group of patients under biological systemic treatment, for which a positive impact on work productivity has been established (35–37). The patients’ satisfaction with the current systemic treatment was also higher in patients receiving biological agents compared to those taking conventional agents, with 91.8% versus 74.5% of responders being satisfied with their evolution, even among patients on biological therapy with PASI scores >5.
While our study helps building recent real-world evidence across Central and Eastern European countries about psoriasis control in patients with moderate-to-severe disease, the cross-sectional and retrospective design is associated with inherent limitations. We attempted to minimize patient selection bias by enrolling all consecutive patients reporting to study sites for visits and patient recall bias by using patient-reported outcomes that employ short or no recall period. The study was conducted during the COVID-19 pandemic; thus, the patients’ mental health and perceived QoL may have been adversely impacted, hindering direct comparisons with other studies. In addition, the results may have been influenced by differences between countries on multiple levels. National criteria for disease severity, reimbursement rules, and treatment guidelines differ from country to country (21), and this may have impacted the generalizability of our results. The use of the reimbursed treatment may have limited timely achievement of an adequate PASI score. For instance, in Latvia, biologic therapy is fully reimbursed only if initiated with adalimumab and the clinical effect must be observed for ≥16 weeks (38), thus potentially limiting the possibility of reaching the target PASI score faster by switching to a more effective biologic therapy.
5 Conclusion
A large proportion of patients with moderate-to-severe psoriasis are treated with bio-logical systemic therapy in Central and Eastern Europe and show low absolute PASI scores after at least 24 weeks of continuous treatment and an overall good satisfaction with their evolution. However, around half of patients with biological treatment did not reach clear skin status and reported an impact of the disease on the QoL, indicating that improvement in treatment strategies is still needed in Central and Eastern European countries to optimize the outcomes of patients with moderate-to-severe psoriasis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Bulgaria – Bulgarian Drug Agency approval number 28037/10.07.2020 (no requirement for explicit EC approval in observational studies at the time of study start), Estonia - Research Ethics Committee of the National Institute for Health Development (approval no. 430/27-Aug-2020), Ethics Committee of Medical Research Council of Medical Research Council (“ETT TUKEB”, approval no. IV/3394-7/2020/EKU/03-Jun-2020), Latvia - The Rīga Stradiņš University Research Ethics Committee (approval no. 280520-2L/28-May-2020), Lithuania – Lithuanian Bioethics Committee (approval no. L20-4/1 and L20-4/2/07-Jul-2020), Romania - National Bioethics Committee of Medicines and Medical Devices Romania (approval no. 9SNI/09-Jul-2020) and Russia - Independent Interdisciplinary Committee on Ethical Review of Clinical Studies (approval following the protocol meeting minutes no. 12/03-Jul-2020). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LR: Investigation, Validation, Writing – original draft, Writing – review & editing. IH: Investigation, Validation, Writing – original draft, Writing – review & editing. SV: Investigation, Validation, Writing – original draft, Writing – review & editing. AK: Investigation, Validation, Writing – original draft, Writing – review & editing. ET: Investigation, Validation, Writing – original draft, Writing – review & editing. IB: Investigation, Validation, Writing – original draft, Writing – review & editing. DM: Conceptualization, Data curation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. SR: Conceptualization, Data curation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. TA: Conceptualization, Data curation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. MC: Investigation, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was sponsored by AbbVie.
Acknowledgments
Medical writing support was provided by Petronela M. Petrar (PhD) and Ana Maria Iordan (MD and MSc) of MedInteractiv (Bucharest, Romania) and funded by AbbVie in accordance with Good Publication Practice (GPP3) guidelines. Statistical analyses were provided by Qualitis SA (part of Optimapharm Group) and were funded by AbbVie.
Conflict of interest
LR reports honoraria for lectures and support for attending meetings from AbbVie. IH reports grants or contracts from Abbvie and Galderma; consulting fees from AbbVie and Janssen; honoraria for lectures and presentations at educational events from AbbVie, Janssen, and Novartis; and support for attending meetings and/or travel from AbbVie and Janssen. SV reports Investigator agreement and payment from AbbVie. AK reports honoraria for lectures and/or Investigator agreements from AbbVie, Eli Lilly, Pfizer, Regeneron, Janssen, and Leo Pharma. IB reports personal and institution payments from AbbVie. DM, SR, and TA are full-time employees of Abbvie and may hold AbbVie stock and/or stock options. MC reports consulting fees from AbbVie, Novartis Pharma, Eli Lilly, and Pfizer; honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Abbvie, Novartis Pharma, Eli Lilly, Pfizer, Janssen, UCB, Sun Pharma, Genesis Pharma, and Amring; payment for expert testimony from UCB and Amring; and support for attending meetings and/or travel from Terapia and for participation on Data Safety Monitoring or Advisory Board from UCB, Amring, and Novartis Pharma.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from AbbVie. The funder contributed to the design, participated in the collection, analysis, and interpretation of data, and in writing, reviewing, and approval of the final version.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1410540/full#supplementary-material
References
1. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. (2013) 133:377–85. doi: 10.1038/jid.2012.339
2. Richard MA, Paul C, Nijsten T, Gisondi P, Salavastru C, Taieb C, et al. Prevalence of most common skin diseases in Europe: a population-based study. J Eur Acad Dermatol Venereol. (2022) 36:1088–96. doi: 10.1111/jdv.18050
3. Feldman SR, Burudpakdee C, Gala S, Nanavaty M, Mallya UG. The economic burden of psoriasis: a systematic literature review. Expert Rev Pharmacoecon Outcomes Res. (2014) 14:685–705. doi: 10.1586/14737167.2014.933671
4. Feldman SR, Zhao Y, Shi L, Tran MH. Economic and comorbidity burden among patients with moderate-to-severe psoriasis. J Manag Care Spec Pharm. (2015) 21:874–88. doi: 10.18553/jmcp.2015.21.10.874
5. Hawro T, Zalewska A, Hawro M, Kaszuba A, Królikowska M, Maurer M. Impact of psoriasis severity on family income and quality of life. J Eur Acad Dermatol Venereol. (2015) 29:438–43. doi: 10.1111/jdv.12572
6. GBD. 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/s0140–6736(18)32279–7
7. Damiani G, Bragazzi NL, Karimkhani Aksut C, Wu D, Alicandro G, McGonagle D, et al. The global, regional, and national burden of psoriasis: results and insights from the Global Burden of Disease 2019 Study. Front Med (Lausanne). (2021) 8:743180. doi: 10.3389/fmed.2021.743180
8. Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. (2020) 369:m1590. doi: 10.1136/bmj.m1590
9. Bondare-Ansberga V, Hartmane I, Mikažāns I, Ivdra I. (2021). Psoriasis in Latvia from year 2015 till 2020, epidemiology, systemic therapy tendencies, in: RSU Research week 2021: Knowledge for Use in Practice, Rīga, Latvia, 24–26 March 2021.
10. Nicolescu AC, Bucur Ș, Giurcăneanu C, Gheucă-Solovăstru L, Constantin T, Furtunescu F, et al. Prevalence and characteristics of psoriasis in Romania-first study in overall population. J Pers Med. (2021) 11:523. doi: 10.3390/jpm11060523
11. World Health Organization. Global report on psoriasis (2016). Available online at: https://apps.who.int/iris/handle/10665/204417.
12. Armstrong A, Bohannan B, Mburu S, Alarcon I, Kasparek T, Toumi J, et al. Impact of psoriatic disease on quality of life: interim results of a global survey. Dermatol Ther (Heidelb). (2022) 12(4):1055–64. doi: 10.1007/s13555–022-00695–0
13. Duffin KC, Yeung H, Takeshita J, Krueger GG, Robertson AD, Troxel AB, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. (2014) 170:672–80. doi: 10.1111/bjd.12745
14. Blair HA. Risankizumab: A review in moderate to severe plaque psoriasis. Drugs. (2020) 80:1235–45. doi: 10.1007/s40265–020-01357–1
15. Amatore F, Villani AP, Tauber M, Viguier M, Guillot B. French guidelines on the use of systemic treatments for moderate-to-severe psoriasis in adults. J Eur Acad Dermatol Venereol. (2019) 33:464–83. doi: 10.1111/jdv.15340
16. Nast A, Smith C, Spuls PI, Avila Valle G, Bata-Csörgö Z, Boonen H, et al. EuroGuiDerm Guideline on the systemic treatment of psoriasis vulgaris - Part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol. (2020) 34:2461–98. doi: 10.1111/jdv.16915
17. Smith CH, Jabbar-Lopez ZK, Yiu ZZ, Bale T, Burden AD, Coates LC, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. (2017) 177:628–36. doi: 10.1111/bjd.15665
18. Colombo D, Bianchi L, Fabbrocini G, Corrao S, Offidani A, Stingeni L, et al. Real-world evidence of biologic treatments in moderate-severe psoriasis in Italy: Results of the CANOVA (EffeCtiveness of biologic treAtmeNts for plaque psOriasis in Italy: an obserVAtional longitudinal study of real-life clinical practice) study. Dermatol Ther. (2022) 35:e15166. doi: 10.1111/dth.15166
19. Kerdel F, Zaiac M. An evolution in switching therapy for psoriasis patients who fail to meet treatment goals. Dermatol Ther. (2015) 28:390–403. doi: 10.1111/dth.12267
20. Norlin JM, Steen Carlsson K, Persson U, Schmitt-Egenolf M. Switch to biological agent in psoriasis significantly improved clinical and patient-reported outcomes in real-world practice. Dermatology. (2012) 225:326–32. doi: 10.1159/000345715
21. Rencz F, Kemény L, Gajdácsi JZ, Owczarek W, Arenberger P, Tiplica GS, et al. Use of biologics for psoriasis in Central and Eastern European countries. J Eur Acad Dermatol Venereol. (2015) 29:2222–30. doi: 10.1111/jdv.13222
22. Eissing L, Rustenbach SJ, Krensel M, Zander N, Spehr C, Radtke MA, et al. Psoriasis registries worldwide: systematic overview on registry publications. J Eur Acad Dermatol Venereol. (2016) 30:1100–6. doi: 10.1111/jdv.13634
23. Armstrong AW, Parsi K, Schupp CW, Mease PJ, Duffin KC. Standardizing training for psoriasis measures: effectiveness of an online training video on Psoriasis Area and Severity Index assessment by physician and patient raters. JAMA Dermatol. (2013) 149:577–82. doi: 10.1001/jamadermatol.2013.1083
24. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. (1994) 19:210–6. doi: 10.1111/j.1365-2230.1994.tb01167.x
25. Reilly Associates. Work Productivity and Activity Impairment Questionnaire (WPAI) Scoring (2002). Available online at: http://www.reillyassociates.net/WPAI_Scoring.html.
26. Palota T, Szepietowski JC, Pec J, Arenberger P, Giurcaneanu C, Gyulai R, et al. A survey of disease severity, quality of life, and treatment patterns of biologically naive patients with psoriasis in central and eastern Europe. Acta Dermatovenerol Croat. (2010) 18:151–61.
27. Zweegers J, Roosenboom B, van de Kerkhof PC, van den Reek JM, Otero ME, Atalay S, et al. Frequency and predictors of a high clinical response in patients with psoriasis on biological therapy in daily practice: results from the prospective, multicenter BioCAPTURE cohort. Br J Dermatol. (2017) 176:786–93. doi: 10.1111/bjd.14888
28. Belinchón Romero I, Dauden E, Ferrándiz Foraster C, González-Cantero Á, Carrascosa Carrillo JM. Therapeutic goals and treatment response evaluation in moderate to severe psoriasis: an experts opinion document. Ann Med. (2021) 53:1727–36. doi: 10.1080/07853890.2021.1986637
29. Sbidian E, Chaimani A, Garcia-Doval I, Doney L, Dressler C, Hua C, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. (2021) 4:Cd011535. doi: 10.1002/14651858.CD011535.pub4
30. Kubanov AA, Bakulev AL, Fitileva TV, Novoderezhkina E, Gilloteau I, Tian H, et al. Disease burden and treatment patterns of psoriasis in Russia: a real-world patient and dermatologist survey. Dermatol Ther (Heidelb). (2018) 8:581–92. doi: 10.1007/s13555–018-0262–1
31. Boca AN, Ilies RF, Vesa S, Pop R, Tataru AD, Buzoianu AD. The first nation-wide study revealing epidemiologic data and life quality aspects of psoriasis in Romania. Exp Ther Med. (2019) 18:900–4. doi: 10.3892/etm.2019.7652
32. Reich K, Griffiths CE. The relationship between quality of life and skin clearance in moderate-to-severe psoriasis: lessons learnt from clinical trials with infliximab. Arch Dermatol Res. (2008) 300:537–44. doi: 10.1007/s00403–008-0885–7
33. Kimball AB, Gieler U, Linder D, Sampogna F, Warren RB, Augustin M. Psoriasis: is the impairment to a patient's life cumulative? J Eur Acad Dermatol Venereol. (2010) 24:989–1004. doi: 10.1111/j.1468-3083.2010.03705.x
34. Warren RB, Kleyn CE, Gulliver WP. Cumulative life course impairment in psoriasis: patient perception of disease-related impairment throughout the life course. Br J Dermatol. (2011) 164 Suppl 1:1–14. doi: 10.1111/bjd.2011.164.issue-s1
35. Armstrong AW, Lynde CW, McBride SR, Ståhle M, Edson-Heredia E, Zhu B, et al. Effect of ixekizumab treatment on work productivity for patients with moderate-to-severe plaque psoriasis: analysis of results from 3 randomized phase 3 clinical trials. JAMA Dermatol. (2016) 152:661–9. doi: 10.1001/jamadermatol.2016.0269
36. Kimball AB, Yu AP, Signorovitch J, Xie J, Tsaneva M, Gupta SR, et al. The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol. (2012) 66:e67–76. doi: 10.1016/j.jaad.2010.10.020
37. Vender R, Lynde C, Ho V, Chau D, Poulin-Costello M. Work productivity and healthcare resource utilization outcomes for patients on etanercept for moderate-to-severe plaque psoriasis: results from a 1-year, multicentre, open-label, single-arm study in a clinical setting. Appl Health Econ Health Policy. (2012) 10:343–53. doi: 10.1007/bf03261868
Keywords: real-world, severity of illness index, patient-reported outcomes, psoriasis, systemic therapy
Citation: Raam L, Hartmane I, Valiukevičienė S, Karamova AE, Telegdy E, Botev I, Marina D, Rubant S, Albuquerque T and Constantin MM (2024) Disease severity, treatment patterns, and quality of life in patients with moderate-to-severe psoriasis routinely managed with systemic treatment: results of the CRYSTAL observational study in Central and Eastern European countries. Front. Immunol. 15:1410540. doi: 10.3389/fimmu.2024.1410540
Received: 01 April 2024; Accepted: 06 May 2024;
Published: 23 May 2024.
Edited by:
Mihaela Adriana Ilie, Länssjukhuset i Kalmar, SwedenReviewed by:
Costantino Ricci, University of Bologna, ItalySanda Popescu, Wrightington, Wigan and Leigh NHS Foundation Trust, United Kingdom
Copyright © 2024 Raam, Hartmane, Valiukevičienė, Karamova, Telegdy, Botev, Marina, Rubant, Albuquerque and Constantin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Magdalena Constantin, ZHJtYWdkYWRpbnVAeWFob28uY29t
 Liisi Raam1
Liisi Raam1 Skaidra Valiukevičienė
Skaidra Valiukevičienė Arfenya E. Karamova
Arfenya E. Karamova Diana Marina
Diana Marina