- 1Center for Excellence in Post-Harvest Technologies, North Carolina Agricultural and Technical State University, Kannapolis, NC, United States
- 2Department of Biology, North Carolina Agricultural and Technical State University, Greensboro, NC, United States
Introduction: MicroRNAs (miRs) play an essential role in adaptive and innate immune systems by regulating the development of immune cells. However, detailed studies of miRs’ role in food allergies are scarce compared to those of other allergic or non- allergic diseases. This systematic review aims to study miRs expression and its role in food allergies (FAs) and determine the signature miRs in FAs.
Method: Research articles published since 2015 were selected from various databases: Scopus, PubMed, ScienceDirect, and Web of Science. Randomized clinical trials, observational clinical studies, and in vivo studies were assessed via the Cochrane Risk of Bias 2 tool, the Newcastle-Ottawa scale, and SYRCLE method, respectively. The characteristics of the included studies, population characteristics, and experimental details were extracted, and the data were synthesized narratively.
Result: MiRs expression had been investigated in the context of cow milk allergy (CMA) and peanut allergy (PA) through both in vivo studies and clinical trials. Clinical trials included allergies to multiple combined foods, individual foods (such as milk, peanut, and what), and drugs and venom, while in vivo studies were conducted on milk, egg, and peanut allergies. MiR-146a, miR- 155, and miR-30a-5p were common miRs between in vivo studies and clinical trials. Moreover, few miRs were commonly studied between different types of food allergies. In clinical trials, miR-143-3p was studied in peanut allergy and non-celiac wheat sensitivity (NCWS), and miR-155 was studied in CMA and egg allergy in in vivo studies. Furthermore, the same miRs varied on their molecular target and effect depending on the type of food allergy.
Discussion: The study on signature miRs and their molecular target determination for the therapeutic purpose of food allergy is in its initial stage. For individual food allergies, miRs determination via next-generation sequencing (NGS), their validation via polymerase chain reaction (PCR), and target molecule determination via RNA interference (RNAi) should be the focus of future studies in order to determine reliable signature miRs of food allergy.
1 Introduction
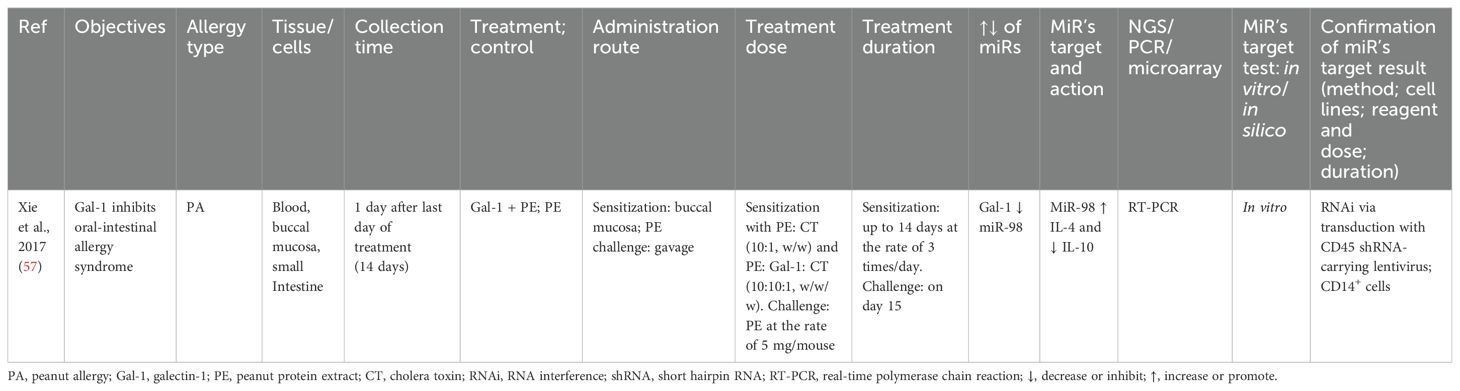
Approximately 2%–4% of adults and 4%–6% of children are affected by food allergies worldwide (1). Food allergies are adverse reactions of the body to food proteins. It can be divided into two categories: immunoglobulin E (IgE)-mediated and non-IgE-mediated (2, 3). The IgE-mediated food allergies occur in two stages: sensitization and effector phases (3, 4). In the sensitization phase, food proteins enter the body through the epithelial barrier, and they are captured by antigen-presenting cells (APCs) such as dendritic cells (DCs) or macrophages. These APCs engulf and break the allergens into peptides and are presented by major histocompatibility complex class II (MHC-II), which is recognized by naive CD4+ T cells in mesenteric lymph node (MLN) (2, 4–6). The naive T cell then convert into T helper 2 (Th2) cells in the presence of interleukin-4 (IL-4) and produce IL-4, IL-5, and IL-13. These cytokines, in combination with the interaction of CD40 of B cells and CD40-ligand of Th2 cells, activate and convert IgG-producing B cells into IgE-producing plasma cells (3–6). Moreover, according to a current review, T follicular helper 13 (Tfh13) cells, which produce IL-4 and IL-13, and type 2 innate lymphoid cells (ILC2), which produce IL-13, help to expand Tfh and B cells, which further induce IgE production from B cells (3). The plasma cell-produced IgE, binds to the α subunit of fragment crystallizable epsilon region R1 (FcεRI) receptors of mast cells or basophil cells. The re-exposure of the allergen (called effector phase) to IgE-FcεRI causes cross-linking of the two FcεRI-bound IgE, making an IgE-FcεRI–allergen complex, which degranulates mast cells or basophil cells. The degranulation leads to the production of various mediators such as histamines, β-hexosaminidase, prostaglandins, leukotrienes, platelet-activating factors (PAFs), pro-inflammatory cytokines, and chemokines and the development of allergic symptoms (2–5). In neutrophils and macrophages, the activation occurs due to IgG-FcγRIII–allergen or IgG-FcγRIV–allergen complex-releasing PAFs (3) (Figure 1).
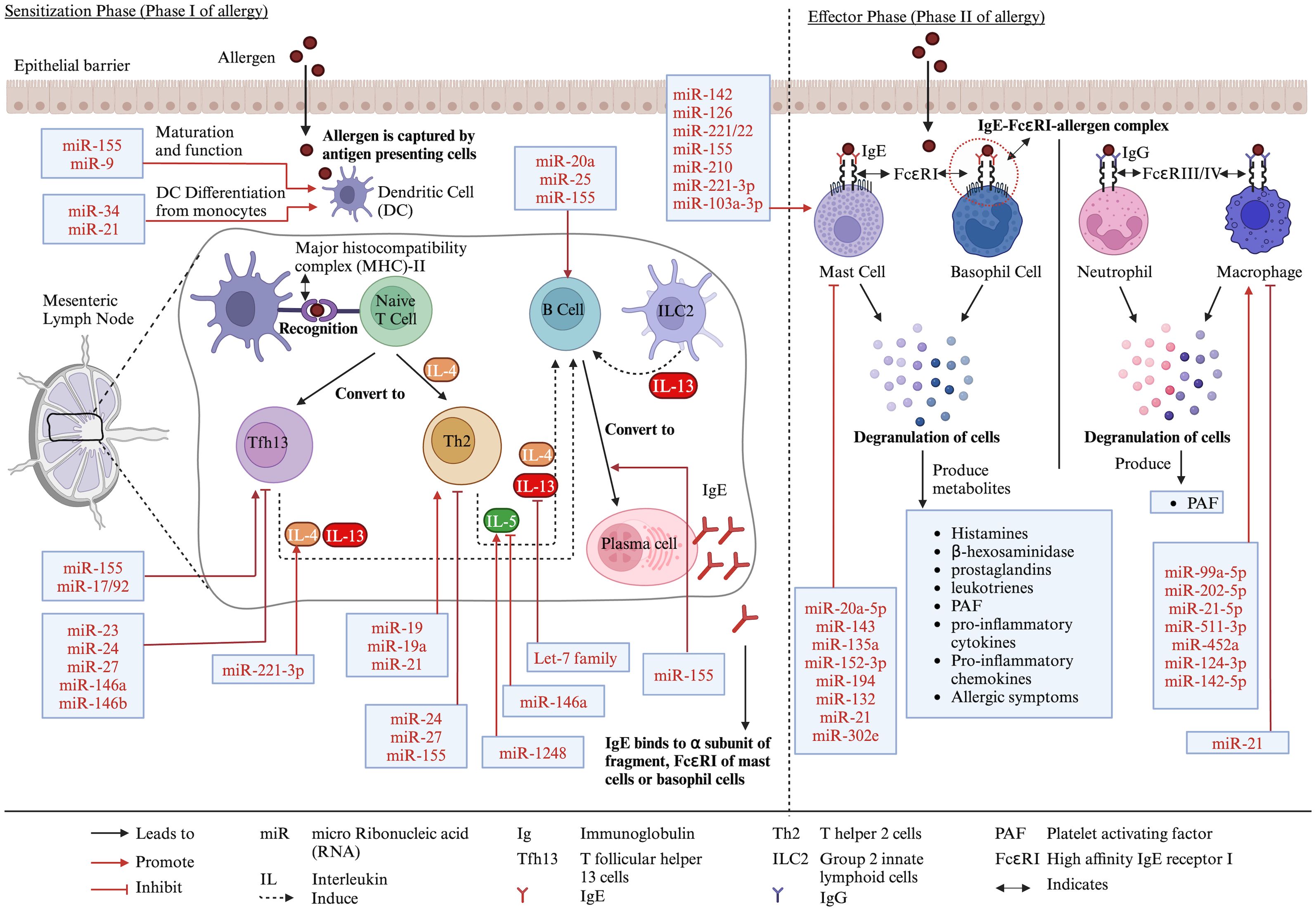
Figure 1. Different stages of food allergy development and role of miRs in the process. Created with BioRender.com.
MicroRNAs (miRs) are widely studied 19-22-nucleotide-long single-stranded non-coding RNAs that regulate gene expression post-transcriptionally (7). The miR-encoding genes are mainly located in the intron, and few are located in exons, intergenic regions, and 5′ and 3′ untranslated regions (UTRs). RNA polymerase-II transcribes miR-encoding genes, producing a single-stranded multiple hairpin structure protected by 5′-end caps and 3′-end poly-A tails, which are called primary miR transcripts (pri-miRs) (8–13). These pri-miRs are further processed by a class 2 RNase III endonuclease (Drosha) and its cofactor DiGeorge syndrome critical region 8 (DGCR8) in the nucleus into 65-nucleotide-long stem-loop-structured precursor miR (pre-miR). However, splicing of hairpin intron and modification with Lariat debranching (Ldbr) protein can also convert pri-miRs into pre-miRs (7, 10, 11, 13). Pre-miRs are exported from the nucleus to the cytoplasm by the ras-related nuclear protein (RanGTP)/exportin-5 complex. The exported pre-miRs are further processed (i.e., hairpin cut and convert pre-miR into -3p or -5p segments) by Dicer and transactivation response RNA-binding protein (TRBP) into 21 nucleotide long miR duplex with overhang, which are called double-stranded miRs (ds-miRs) intermediates. The ds-miRs unwind, and the guide strand of ds-miRs interacts with argonaute 2 (AGO2) protein, which results in functional miRs, while the passenger strand of the ds-miRs degrades. The functional miRs recruit components of the miR-induced silencing complex (miRISC), such as GW182, which interact with the AGO protein and bridge the AGO to other proteins involved in miRISC (e.g., 5′ de-capping deadenylase and exonuclease enzymes, which degrade target RNAs). Moreover, TRBP helps the binding of Dicer-miR with the RISC complex, which contains AGO2 and trinucleotide repeat-containing gene 6 protein (TNRC6) (7, 8, 10–12) (Figure 2).
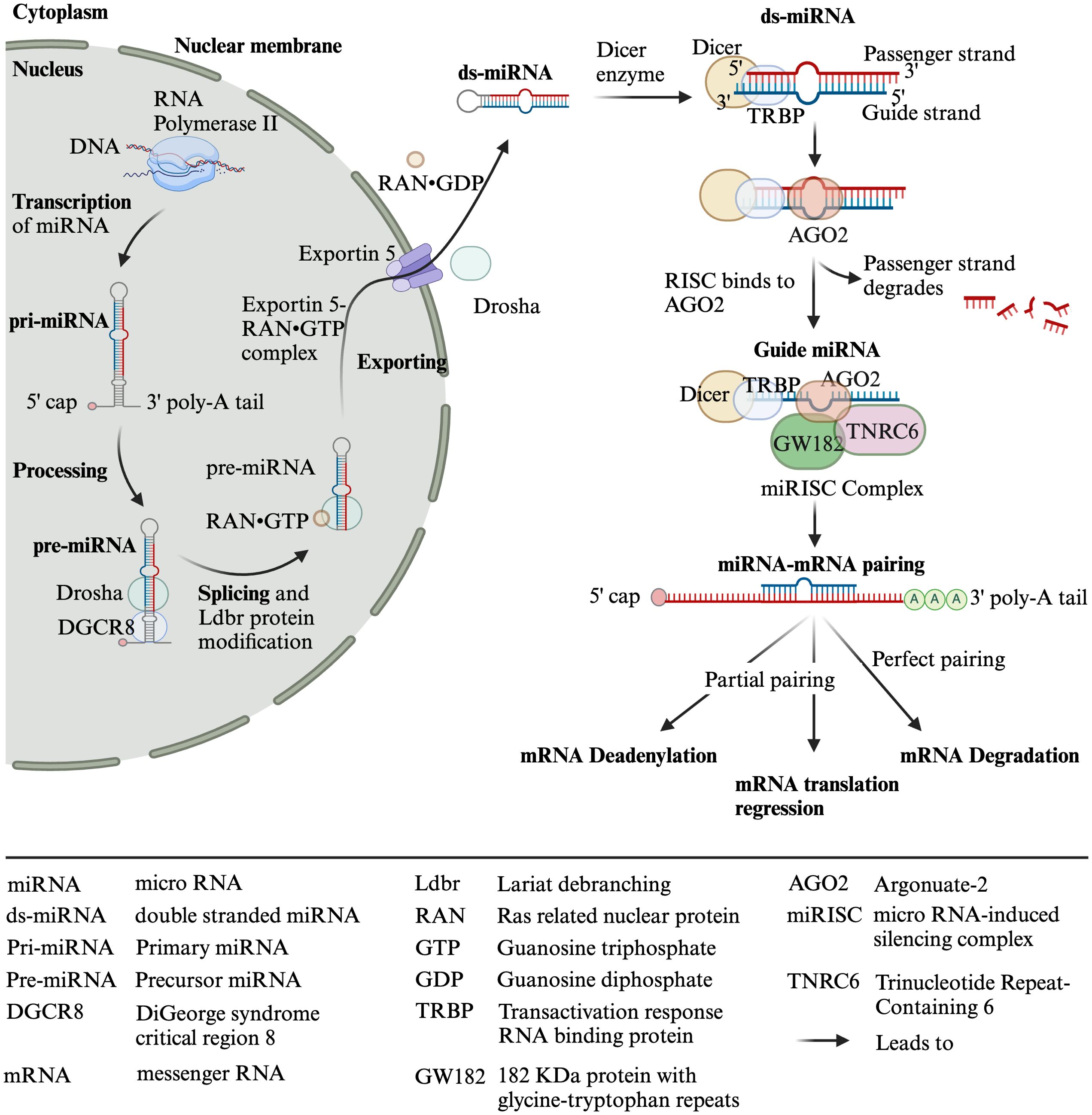
Figure 2. Biosynthesis of miRNAs. Created with BioRender.com.
Among four types of AGOs (AGO1–4), AGO2 is the most important for mammals, which has an active endonuclease pocket, binds to miRs, supports the DICER-dependent miR maturation process, and cleaves complementary RNA (10). MiRs bind on the 3′ or 5′ UTR or coding region of the target mRNAs, either degrading them (if completely paired with the target gene, which occurs occasionally in animals) or inhibiting their translation (if partially paired with the target) or promoting the translation of the target by binding to elements of the 5′ UTR (8–11, 13, 14) (Figure 2). Moreover, in the case of the complete pairing, AGO2 cleaves the target mRNA. However, in partial pairing, TNRC6 dominates the target mRNA’s deadenylation, degradation, or translation repression (7). In addition to direct binding to target genes, miRs also bind to RNA-binding proteins (RBPs), preventing them from binding and functioning as transcriptional activators or repressors (13).
MiRs are found in body fluids, reflecting the body’s physiological condition. Therefore, they have the potential to be used as signature biomarkers for various diseases (15). MiRs are essential in adaptive and innate immune systems, as they play important roles in the development, proliferation, activation, and inhibition of immune cells as well as cytokine production and their target genes, which are explained in detail somewhere else (9–11, 16–19). As an example, the role of miRs in immune cells related to allergy is described as follows. For T cells, miR-24, miR-27, and miR-155 reduced Th2 production, and miR-19, miR-19a, and miR-21 increased Th2 production (16, 19, 20). Moreover, miR-23, miR-24, miR-27, and miR-146a/b restricted Tfh cells, and miR-155 and miR-17/92 promoted Tfh cells (16). For DCs, miR-155 and miR-9 promoted DC maturation and function, and miR-34 and miR-21 promoted their differentiation from monocytes (16, 20). For B cells, miR-20a, miR-25, and miR-155 promoted B-cell responses (16). MiR-155 is also important for the differentiation of B cells into IgE-producing plasma cells (20). For macrophages, miR-99a-5p, miR-202-5p, miR-21-5p, miR-511-3p, miR-452a, miR-124-3p, and miR-142-5p promoted M2 macrophages, which are necessary for allergic reaction (17). However, miR-21 is also reported to negatively regulate macrophage activation (20). For mast cells, miR-142, miR-126, miR-221/22, miR-155, miR-210, miR-221-3p, and miR-103a-3p promote mast cell activation, leading to degranulation and cytokine release, while miR-20a-5p, miR-143, miR-135a, miR-152-3p, miR-194, miR-132, miR-21, and miR-302e decreased mast cell activation (21–23). For cytokines, miR-221-3p and miR-1248 upregulated IL-4 and IL-5, respectively, while miR-146a and let-7 family downregulated IL-5 and IL-13, respectively (19) (Figure 1).
It is noteworthy to mention that in addition to miRs, other epigenetic mechanisms such as histone modification and DNA methylation also affect allergies (24, 25). Moreover, these epigenetic mechanisms and miRs mutually regulate each other. For example, miRs can affect other epigenetic mechanisms by modulating enzymes important for histone modification and DNA methylation, such as DNA methyltransferases (DNMTs), histone deacetylases (HDACs), and polycomb-group genes. On the other hand, the epigenetic mechanisms could have transcriptional control on miRs expression (26–28). One of many reasons for miRs’ epigenetic regulation could be due to the fact that approximately 50% of miRs’ genes are located in CpG islands where DNA methylation occurs (27). Despite the interrelationship among epigenetics, miRs, and allergic disease/food allergy, that aspect is beyond the scope of this systematic review; thus, our current study only focuses on miRs expression in food allergy.
Moreover, the role of miRs in various types 2 immune diseases such as allergic rhinitis (29–31), atopic dermatitis (32–34), allergic asthma (7, 10), asthma (35–37), eosinophilic esophagitis (38), allergic contact dermatitis (39–41), and autoimmune diseases (11) have been studied and summarized elsewhere, and all agree that each miR has its distinct and critical role in type 2 immune system. However, compared to those of other diseases, studies on the effect of miRs on food allergy are scarce. Thus, this systematic review aims to study the signature miRs and their molecular targets in food allergies (FAs).
2 Materials and methods
2.1 Search strategy
Articles published from 2015 to June 14, 2024, in four databases were searched: ScienceDirect, Web of Science, Scopus, and PubMed. Two authors (T.S.R. and J.P.R.) used the following combinations of keywords (three to four sets) in each database to get relevant articles: in ScienceDirect, a) (“food hypersensitivity” OR “food allergy” OR “allergy”) AND (“MicroRNAs” OR “miR”) AND (“proanthocyanidin” OR “anthocyanidin” OR “procyanidin” OR “flavonoids”), b) (“food hypersensitivity” OR “food allergy” OR “allergy”) AND (“MicroRNA” OR “miR”) AND (“mast cell” OR “basophil” OR “RBL-2H3”), and c) (“MicroRNAs” OR “miR”) AND (“mast cell” OR “basophil” OR “RBL-2H3”) AND (“proanthocyanidin” OR “anthocyanidin” OR “ procyanidin” OR “flavonoids”). In Web of Science, a) (“food hypersensitivit*” OR “food allerg*” OR “allerg*”) AND (“MicroRNA*” OR “miR*”) AND (“proanthocyanidin*” OR “anthocyanidin*” OR “procyanidin*” OR “flavonoid*”), b) (“food hypersensitivit*” OR “food allerg*” OR “allerg*”) AND (“MicroRNA*” OR “miR*”) AND (“mast cell*” OR “basophil*” OR “RBL-2H3*”), and c) (“MicroRNA*” OR “miR*”) AND (“mast cell*” OR “basophil*” OR “RBL-2H3*”) AND (“proanthocyanidin*” OR “anthocyanidin*” OR “ procyanidin*” OR “flavonoid*”). In Scopus, a) (“food hypersensitivit*” OR “food allerg*” OR “allerg*”) AND (“MicroRNA*” OR “miR*”) AND (“proanthocyanidin*” OR “anthocyanidin*” OR “procyanidin*” OR “flavonoid*”), b) (“food hypersensitivit*” OR “food allerg*” OR “allerg*”) AND (“MicroRNA*” OR “miR*”) AND (“mast cell*” OR “basophil*” OR “RBL-2H3*”), and c) (“MicroRNA*” OR “miR*”) AND (“mast cell*” OR “basophil*” OR “RBL-2H3*”) AND (“proanthocyanidin*” OR “anthocyanidin*” OR “ procyanidin*” OR “flavonoid*”). In PubMed, a) (“Food Hypersensitivity”[Mesh]) AND “MicroRNAs”[Mesh], b) (“Proanthocyanidins”[Mesh]) AND “MicroRNAs”[Mesh], c) (“Mast Cells”[Mesh]) AND “MicroRNAs”[Mesh], and d) (“Anthocyanins”[Mesh]) AND “MicroRNAs”[Mesh]. The reference lists of related articles were also searched to include relevant articles. Duplicate articles and research articles not in English were removed, and a total of 642 articles were screened. After title and abstract screening, 111 articles were selected, out of which 10 articles were found to be related to miRs and food allergies. Seven articles from the selected articles’ reference list were also found; thus, in total, 17 articles were included in this study (Figure 3).
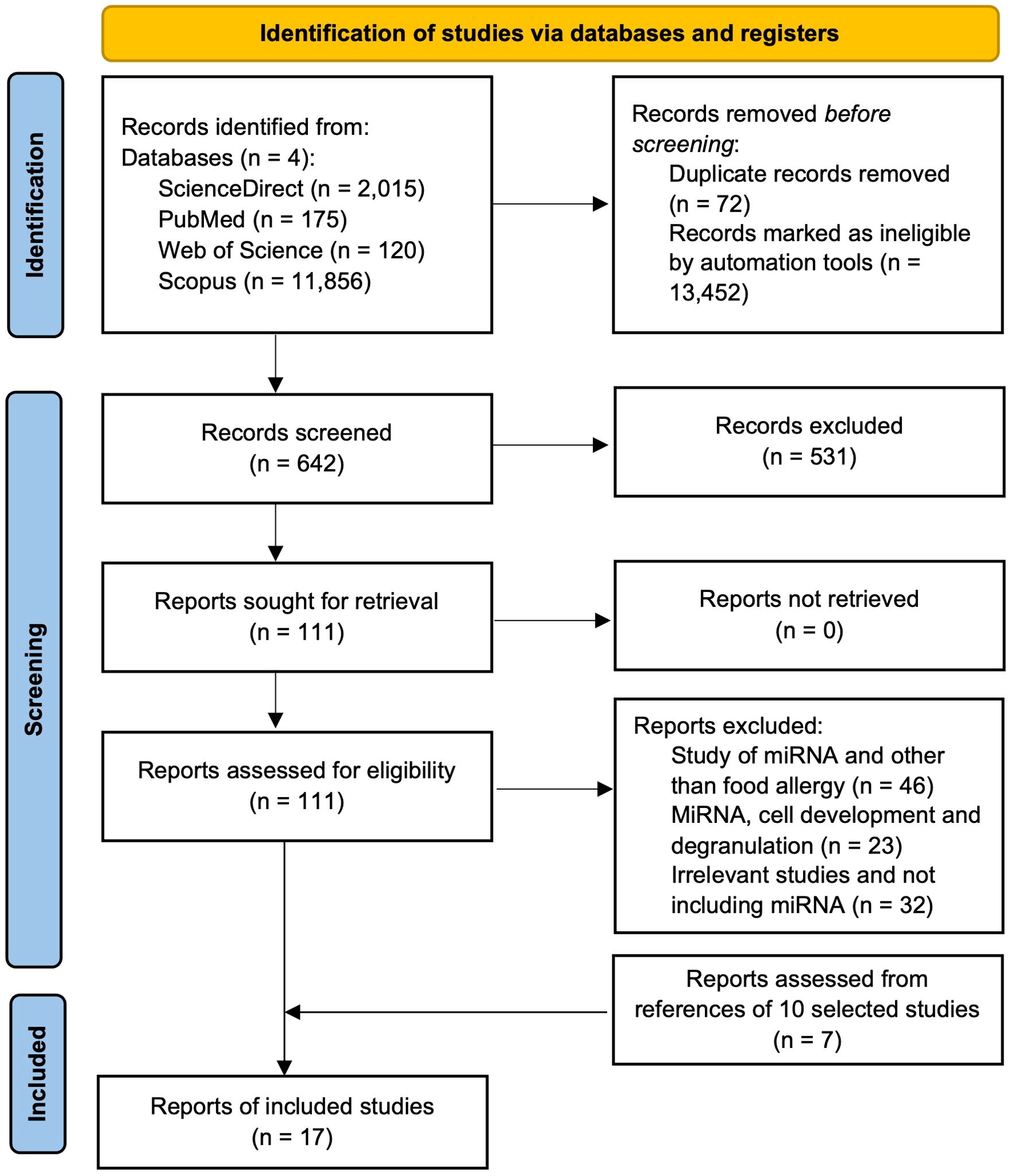
Figure 3. PRISMA flow diagram showing the steps of study selection process for the systematic review.
2.2 Inclusion and exclusion criteria
Various study designs were included, including randomized clinical trials (RCTs), observational clinical trials, and in vivo studies published in English. Initially, databases with the aforementioned keywords were searched; articles based on the publication dates (since 2015), types of articles (original research articles only), and language (English) were adjusted in the searched websites; the duplicate articles were removed using MS Excel, leading to 642 articles. Of the 642, articles related to miRs expression studies on allergic diseases other than food or food compounds, food allergy studies that did not include miRs expression, and articles that included miRs expression and their role in immunological cell growth and development were excluded from the title and abstract screening, leading to 111 articles. These articles were analyzed in detail (full article) again to filter out based on criteria used for the 642 articles, leading to 10 articles, and these 10 articles were further searched for references to find the remaining seven research articles, leading to a total of 17 articles included in this study. The automation tools/tabs from each database searched were used to adjust articles based on the aforementioned inclusion and exclusion criteria. Two authors (T.S.R and J.P.R.) screened the initially selected 642 articles separately. Articles were selected based on the aforementioned inclusion and exclusion criteria (Figure 3), and any disagreements were resolved by consulting with other authors (R.R.B and L.L.W).
2.3 Data extraction and analysis
We extracted the following information from included clinical studies: a) study characteristics: reference, country, funding, study setting, and study design; b) population characteristics: allergy type, age, sex, history of allergy, and number of participants; and c) experimental characteristics: objectives, tissue/sample type, sample collection time, treatment vs. control, clinical sign measured, change in miRs expression, method of miRs expression analysis, molecule or gene target of the miRs, and confirmation method of potential targets of miRs. In the in vivo studies, we collected similar information to that of clinical studies except for the study setting, study design, and history of allergies. Two authors (T.S.R. and J.P.R.) extracted that information separately, discussed it with the third author (R.R.B.), and agreed upon the results. Since we had diverse studies in terms of participants (animal and human), intervention, control, outcome, and study design, we synthesized the results in a narrative review. We synthesized the results based on the food allergy types in clinical trials or in the in vivo (mouse) model.
2.4 Risk of bias assessment
All the RCTs were evaluated using the Cochrane Risk of Bias 2 (ROB 2) tool (42). Observational clinical trials were assessed according to the Newcastle-Ottawa scale. All animal studies were evaluated using SYRCLE’s risk of bias tool for animal studies guide (43) by two authors (T.S.R. and R.R.B).
3 Results
We followed the PRISMA checklist guidelines to conduct the study. After we removed duplicates and ineligible articles by automation (i.e., only research articles in English and keywords in the title and abstract), 642 articles remained. After a title and abstract review of 642 articles, 111 articles were left. Out of 111, only 10 articles remained after the removal of unrelated articles. We obtained seven articles from reference searching of 10 articles, resulting in 17 total articles for this review (Figure 3).
3.1 Risk of bias
All in vivo studies did not mention randomization and blinding processes, and most of them were free of selective reporting. Most of the clinical studies were observational studies with moderate quality based on the Newcastle-Ottawa scales. One clinical study was a completely randomized controlled design with a high risk of bias according to the ROB 2 method (see Supplementary Material).
3.2 Study characteristics
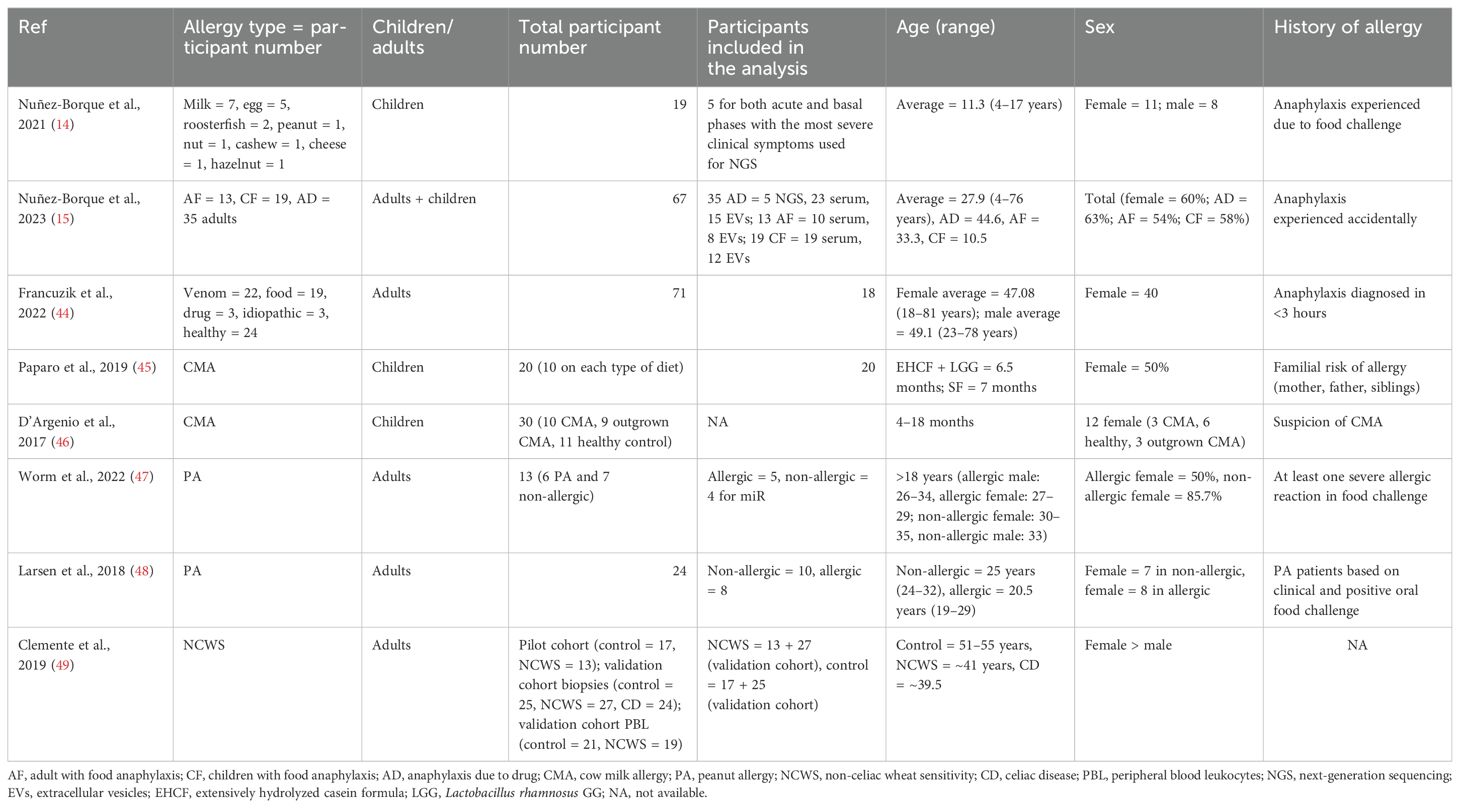
Out of eight clinical studies, three were on multiple food allergies, allergies to food and insect venom, or allergies to food and drugs. Both cow milk allergy (CMA) and peanut allergy had two studies each, and one study was related to non-celiac wheat sensitivity (NCWS) (Figure 4A). Five were cohort studies; one was an RCT, and two did not mention the study design. In a total of nine in vivo studies, five studies were on CMA (β-lactoglobulin), three studies were on egg [ovalbumin (OVA)] allergy, and one study was on peanut allergy. Clinical trials were primarily conducted in European countries: Italy, Spain, Germany, and Denmark (in the order of the highest to lowest number of studies) (Figure 4B). Of the included studies, 62.5% were government-funded, 25% were private company-funded, and 12.5% were funded by both private and government sectors (Figure 4C). All of the in vivo studies were conducted in China and funded by the government sector.
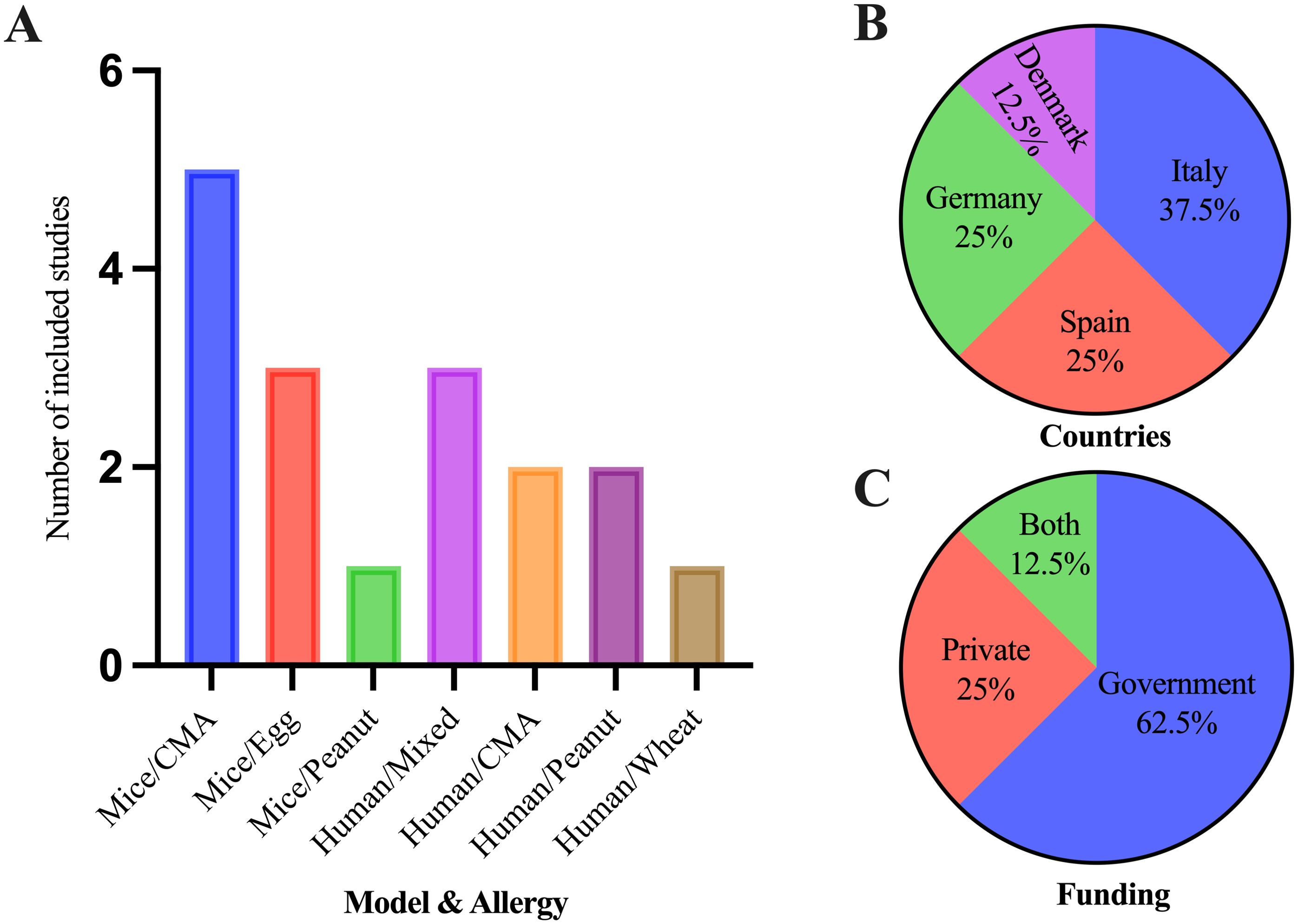
Figure 4. Characteristics of the included studies for the systematic review. (A) Number of studies included in both clinical and in vivo studies in different types of food allergies and miRs expression studies. (B) Countries where clinical trials of food allergies occurred. (C) Funding agencies of clinical trials for food allergies.
3.3 Population characteristics
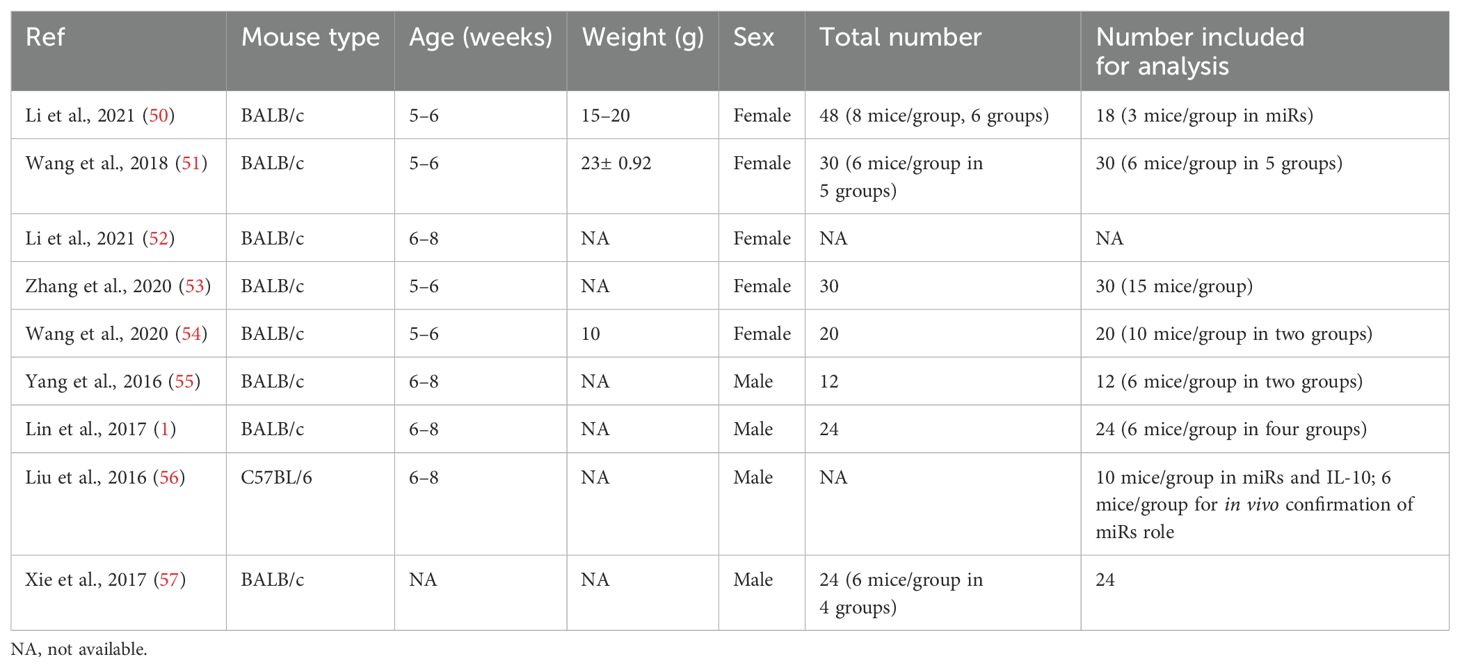
Most clinical trials included either adults or children, and only one study included both population categories. However, both sexes (male and female) were included in all studies. The included participants experienced allergy either due to food challenges or accidental anaphylaxis, and some were included based on familial risk for allergy. Seven out of eight total studies tested miRs expression based on allergic versus non-allergic participants. In contrast, one study included the effect of diet intervention on miRs expression (Table 1). In the in vivo model, eight studies used BALB/c mice, and one used C57BL/6 mice. Each study included either 5–8-week-old female or male mice. Half of the studies tested miRs expression in allergic and non-allergic mice, and the rest included the intervention of diet (human milk oligosaccharides), probiotics (Lactobacillus acidophilus), or anti-inflammatory protein (galectin-1) (Table 2).
3.4 Food allergy and miRs study in clinical trial
The clinical trials on food allergy included CMA, peanut allergy, NCWS, and mixed categories (food, drug, and venoms). The DNA was extracted from whole blood samples, serum, T cells, or peripheral blood-derived cells such as mononuclear cells, mast cells, or leukocytes (Tables 3–6). MiRs expression studies were conducted via next-generation sequencing (NGS) (five studies), microarray (two studies), or polymerase chain reaction (PCR) (one study). MiR-143-3p was commonly studied in peanut allergy and NCWS, and miR-191-5p and miR-3615 were commonly expressed in participants allergic to mixed food types (milk, fish, cheese, peanut, egg, cashew, and hazelnut) and milk (Tables 3–6).
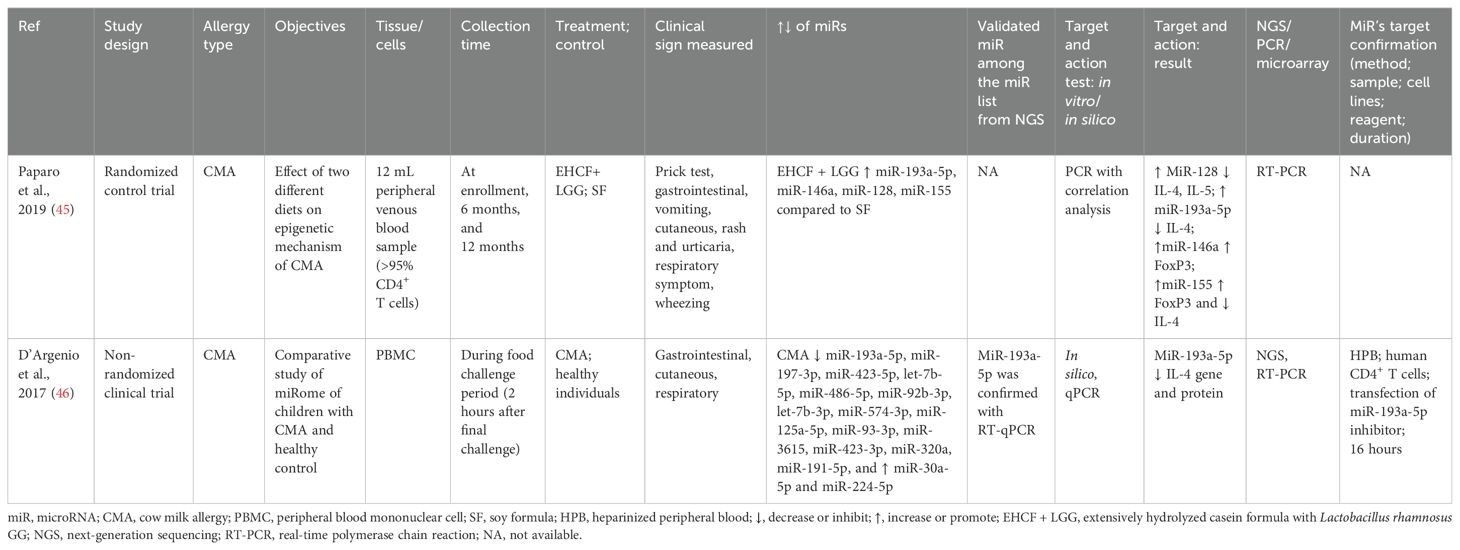
3.4.1 Cow milk allergy
Two studies were conducted on children aged 6-18 months. The first study was on the effect of diet (excessively hydrolyzed casein formula + Lactobacillus rhamnosus GG vs. soy formula) on CMA (45). In contrast, the second study compared the miRs expression in CMA children versus healthy children (46). Both studies analyzed peripheral blood samples generating CD4+ T cells and peripheral blood mononuclear cells (PBMCs). Both studies determined that CMA reduced miR-193a-5p, which was negatively correlated with IL-4, while extensively hydrolyzed casein formula (EHCF) + L. rhamnosus GG (LGG) increased this miR. The effect of miR-193-5p on targeting molecules was further tested in in vitro by RNA interference (RNAi) in human CD+ T cells (46). Furthermore, EHCF + LGG increased other miRs such as miR-128, which was negatively correlated to IL-4 and IL-5; miR-146a, which was positively correlated to FoxP3; and miR-155, which was positively correlated to FoxP3 and negatively correlated to IL-4 (45) (Table 3).
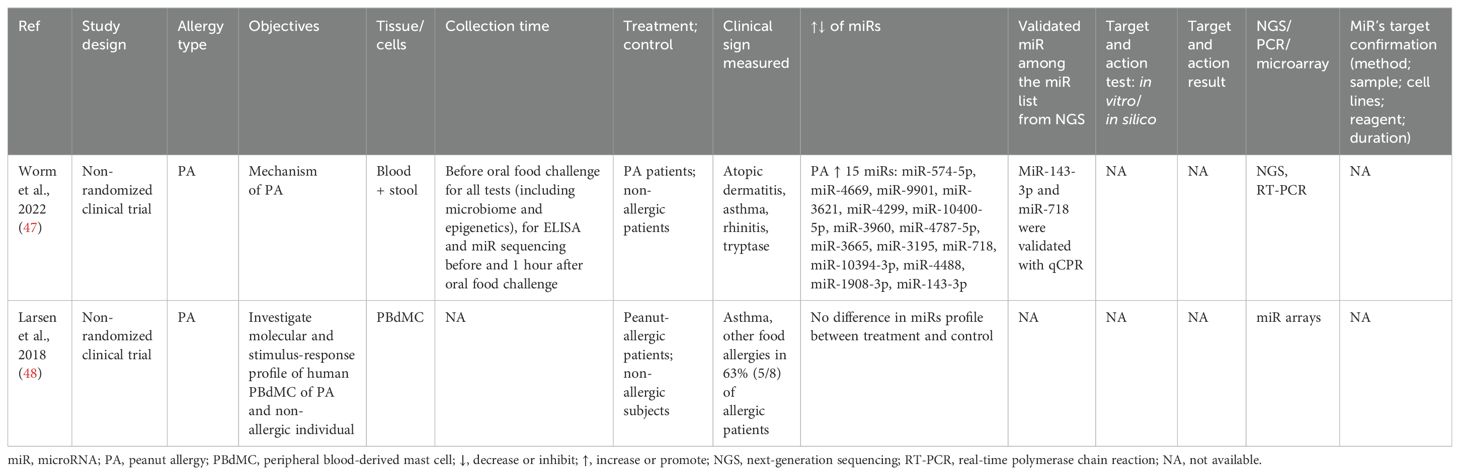
3.4.2 Peanut allergy
Two studies were on miRs expression in the peanut-allergic group versus the non-allergic group of 26–33-year-old adults. In one study, peanut allergy increased the expression of 15 miRs: miR-574-5p, miR-4669, miR-9901, miR-3621, miR-4299, miR-10400-5p, miR-3960, miR-4787-5p, miR-3665, miR-3195, miR-718, miR-10394-3p, miR-4488, miR-1908-3p, and miR-143-3p. However, only two were validated with qPCR: miR-143-3p and miR-718 (47). The second study found no differentially expressed miRs between allergic and non-allergic participants (48) (Table 4).
3.4.3 Non-celiac wheat sensitivity
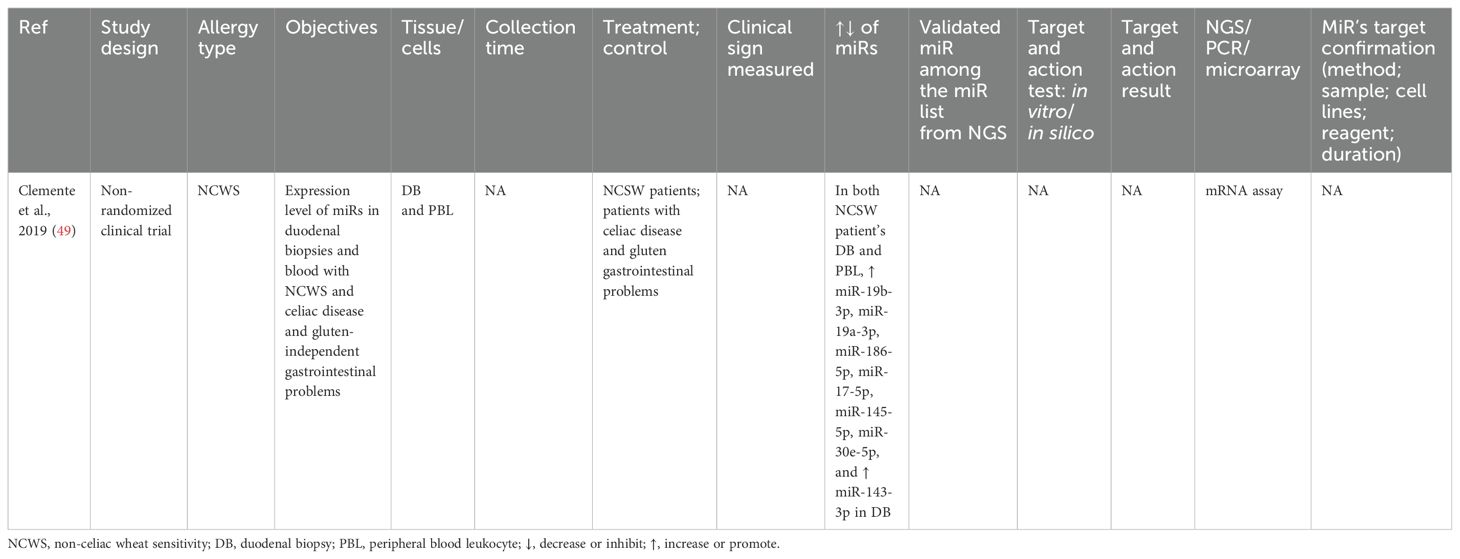
The miRs expression between NCWS and celiac disease and gluten-independent gastrointestinal problems in adults (40–55 years) was studied via miR array (49). NCWS increased miR-19b-3p, miR-19a-3p, miR-186-5p, miR-17-5p, miR-145-5p, and miR-30e-5p in blood and duodenal biopsies. The target molecules of miRs need to be determined (Table 5).
3.4.4 Food allergies, along with insect venom or drugs
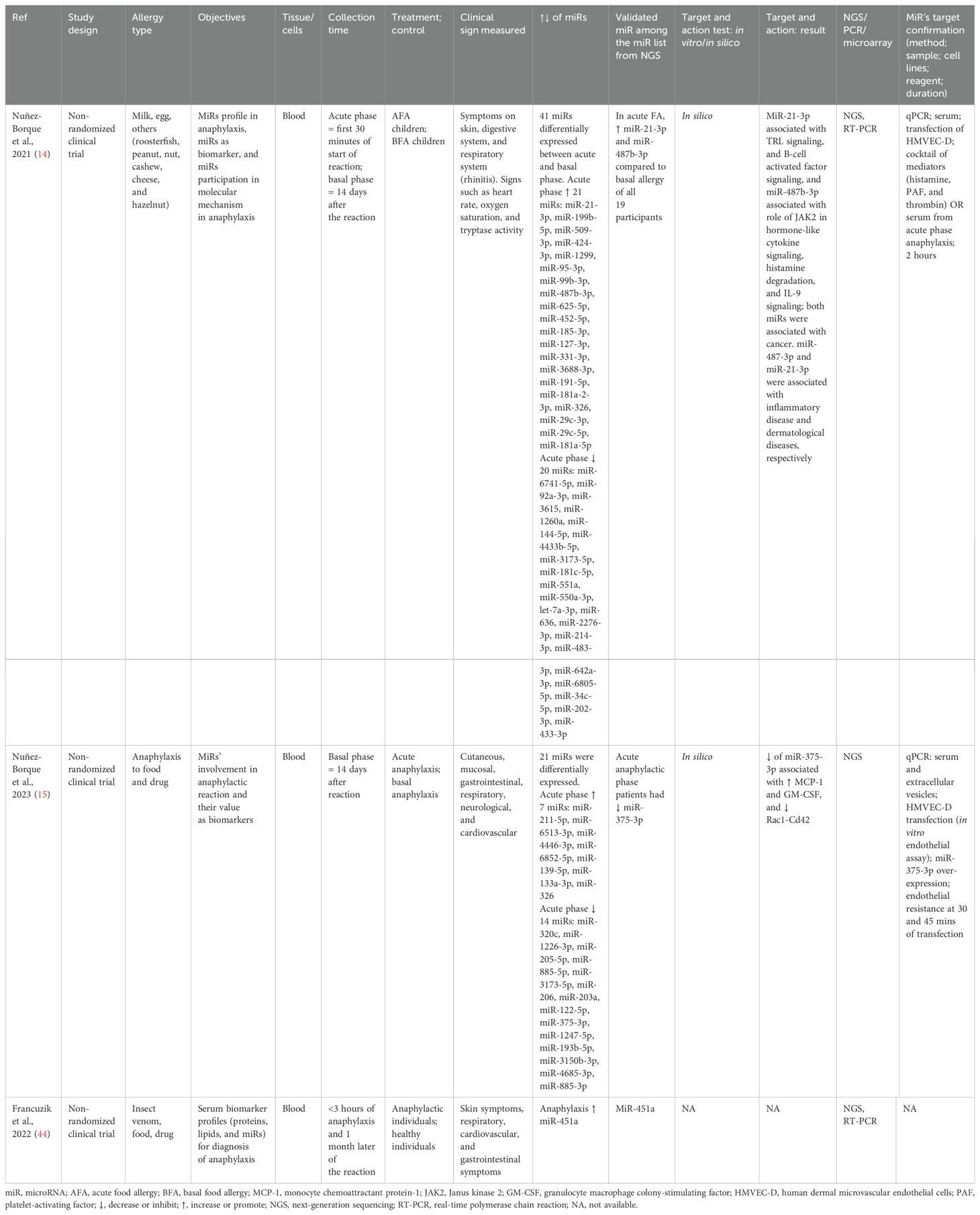
The first study included only food allergy in children (4–17 years) (14), the second study included food and drug allergy in adults (33–45 years) (15), and the third one included food, venom, and drug allergy in adults (18–81 years) (44). Blood samples were collected within 30 minutes to 3 hours of anaphylaxis for the acute phase and 14 days to 1 month later for the basal phase. Anaphylaxis due to food increased the expression of miR-21-3p and miR-487b-3p (14), anaphylaxis due to food and drug reduced the miR-375-3p expression (15), and anaphylaxis due to food, venom, and drugs increased the expression of miR-451a (44). These miRs were validated via qPCR after their differential expression was analyzed via NGS. MiR-21-3p was associated with Toll-like receptor (TLR) signaling and B-cell activation factor signaling, while miR-487b-3p was associated with JAK2’s role in cytokine signaling, histamine degranulation, and IL-9 signaling. Molecular targets of the validated miRs were conducted by in silico analysis and also via in vitro experiments either treating with a cocktail of serum of acute anaphylactic patients (14) or over-expressing miR-375-3p in human dermal microvascular endothelial cells (HMVEC-D) (15) (Table 6).
3.5 Food allergy and miRs study in in vivo model
The in vivo studies included CMA, egg allergy, and peanut allergy. The DNA was extracted from the colon, intestine, spleen, or specific types of cells such as B cells and macrophages. All studies used PCR for miRs expression analysis except one (which used NGS). All but one study used BALB/c mice aged 5–8 weeks, five studies used only female mice, and four used only male mice (Table 2). MiR-155 was found commonly in both milk (β-lactoglobulin) and egg (OVA) allergies (Tables 7, 8).
3.5.1 Cow milk (β-lactoglobulin) allergy
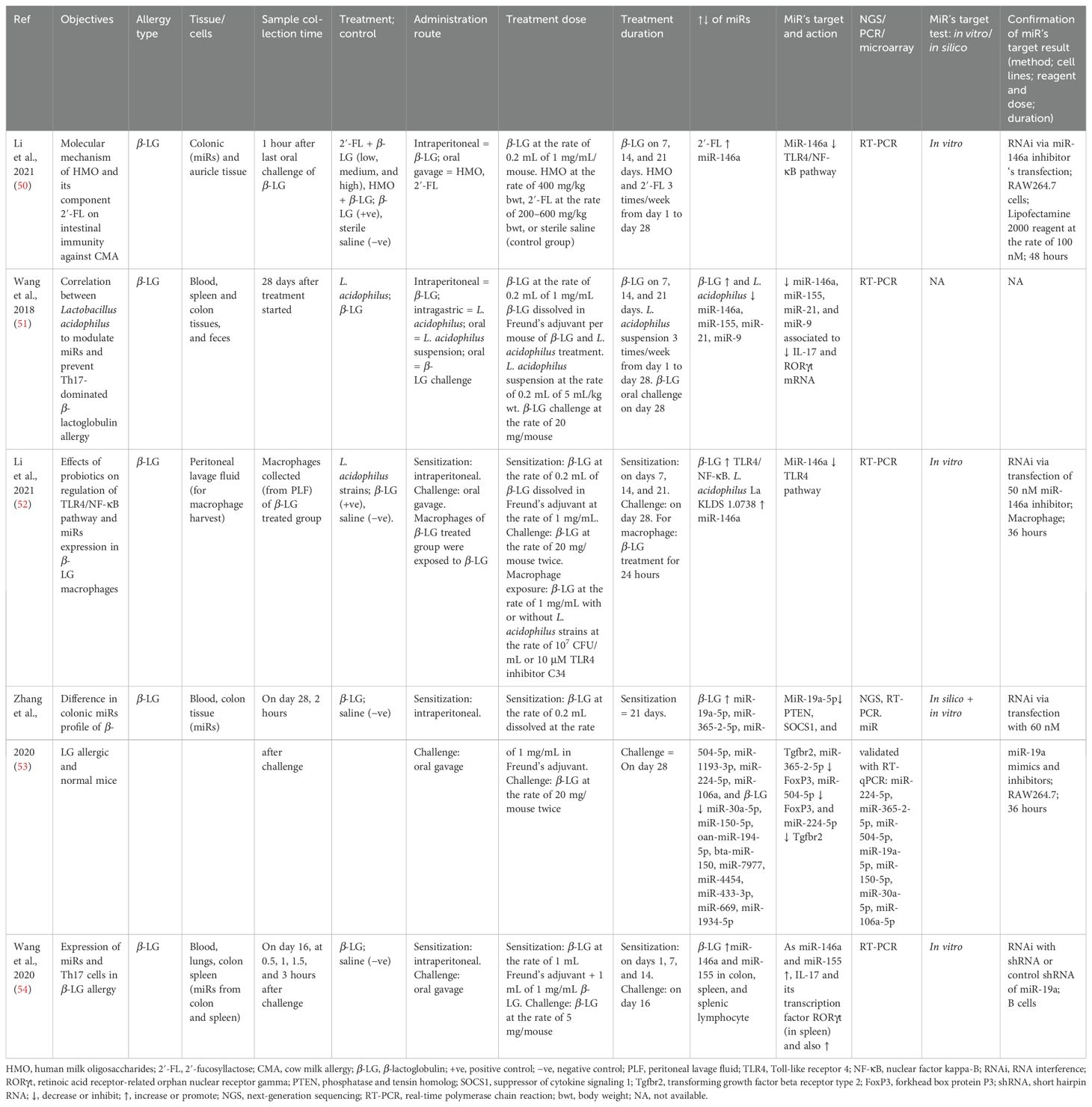
In BALB/c mice (female), the effect of human milk oligosaccharides (HMOs) and fucosyllactose (2′-FL) (50), L. acidophilus strains’ effect on CMA (51, 52), or difference on miRs between CMA and non-allergic mice were studied (53, 54). After sensitization for 21 days and challenging on the 28th day (except in one study) (54), the expression of selected miRs was assessed. MiR-146a (50–52, 54) and miR-155 (51, 54) were commonly expressed in most studies. MiR-146a had a negative association with TLR4/NF-κB signaling (52) and a positive association with IL-17 and RORγt (51, 54). Moreover, miR-9 was also positively associated with IL-17 and RORγt (54), and miR-19a was negatively associated with PTEN, Tgfbr4, and SOCS1. Both miR-365–2 and miR-504 were negatively associated with FoxP3, and miR-224 reduced Tgfbr2 (53). The targets of the miRs were validated via RNAi in B cells (54), macrophage (52), or RAW 264.7 (50, 53) (Table 7).
3.5.2 Egg (OVA) allergy
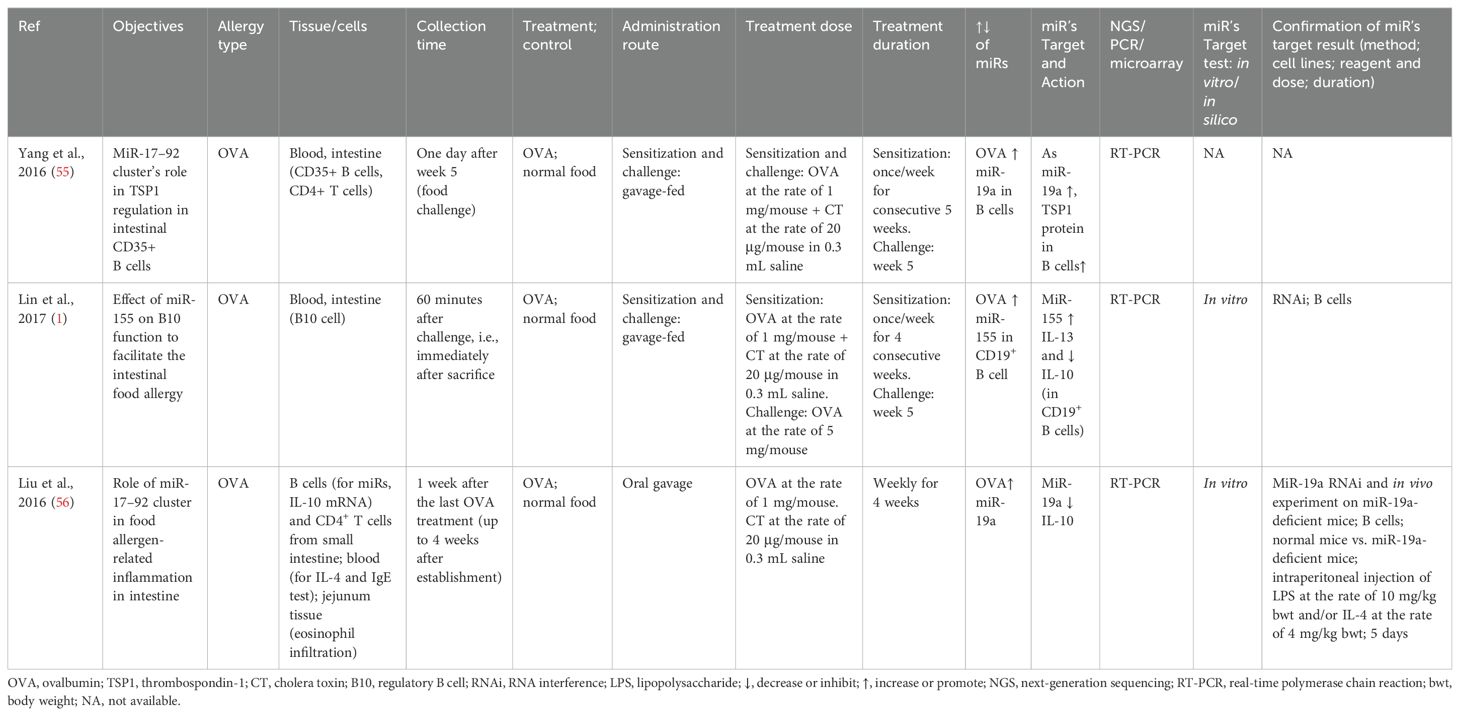
The miRs profile of allergic and non-allergic male BALB/c (1, 55) or C57BL/6 (56) mice were studied by sensitizing them with the oral treatment of OVA (1 mg/mouse) + cholera toxin (20 μg/mouse) for 4-5 weeks and challenged with OVA (1 or 5 mg/mouse). Allergies increased the expression of miR-19a and miR-155 in B cells. Both miRs reduced IL-10 (1, 56), miR-19a increased thrombospondin-1 (TSP1) (55), and miR-155 increased IL-13 (1). The target molecules of the miRs were validated via RNAi in B cells and in in vivo with miR-19a-deficient mice (Table 8).
3.5.3 Peanut allergy
The role of galectin-1 on intestinal allergy was studied in BALB/c male mice by sensitizing them until 14 days, three times/day, and challenged (5 mg/mouse) on the 15th day. Galectin-1 reduced miR-98, and miR-98 increased IL-4 and reduced IL-10. The target molecules of miRs was validated via RNAi of CD14+ cells isolated from lamina propria mononuclear cells (LPMCs) (57) (Table 9).
4 Discussion and future perspectives
The exclusion of miR studies related to allergies other than food allergies led to the determination of very few common miRs signatures among food allergies. However, those commonly studied miRs were also studied in other allergic as well as non-allergic diseases. We selected some of the miRs from this study for discussion based on the following criteria: a) miRs commonly expressed within or between clinical trials and in vivo studies, b) miRs whose target molecules were tested, c) miRs those were selectively used in the included studies based on results of previous studies, and d) miRs obtained from NGS and validated with PCR. In this study, the miRs commonly studied in both clinical trials and in vivo studies were miR-146a, miR-155, and miR-30a-5p (Figure 5). These miRs were also studied in other allergic diseases. For example, miR-146a, which was increased in CMA (50–52, 54), was reported to reduce allergic rhinitis (58–60), atopic dermatitis (61), and allergic asthma (62). MiR-155, which was increased in CMA (51, 54) and egg allergy (1), had a positive association with allergic rhinitis (63) and eosinophilic inflammation (64) and a negative association with allergic asthma (65). Moreover, miR-30a-5p, which was increased in CMA in a clinical trial (46) and decreased in CMA in an in vivo study (53), had a negative association with asthma (66) and a positive association with allergic rhinitis (67).
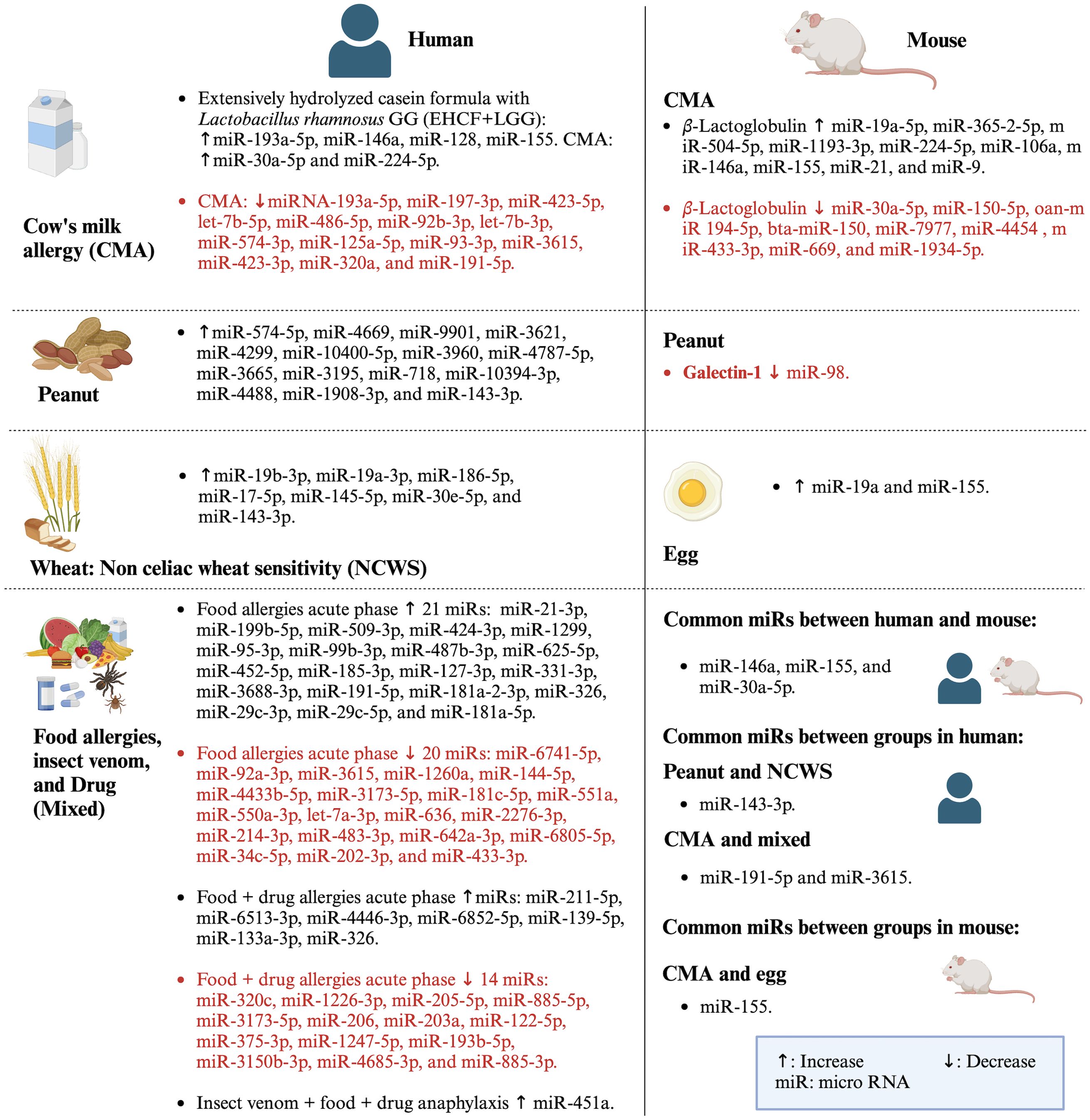
Figure 5. Summary of miRs studied in both clinical trials and in vivo studies along with common miRs expressed between and within the studies. Created with BioRender.com.
In addition to the aforementioned miRs, other miRs were also commonly studied. In clinical trials, miR-143-3p expression was increased in peanut (47) and wheat allergy (49). MiR-191-5p increased in both CMA and food allergy, and miR-3615 increased in CMA and decreased in food allergy. These miRs also related to other allergic and non-allergic diseases. For instance, miR-143-3p (68) and miR-191-5p (69) had negative and positive, respectively, regulation on allergic asthma. MiR-3615 was studied less in allergic diseases but was found to be positively correlated with other diseases such as hepatocellular carcinoma (70). Similarly, other miRs tested and validated with PCR in CMA, peanut allergy, and food allergy were found to be expressed in other diseases too. For example, miR-128 reduced in CMA (45) was positively associated with asthma (71), and miR-193a-5p, which had reduced (46) or enhanced (45) expression in CMA, also enhanced respiratory distress (72). MiR-21-3p increased in food allergy (14) also positively correlated with asthma (73). The miR-375-3p, which was reduced in food allergy (15), also negatively regulated atopic dermatitis (74). MiR-451a, which was increased due to allergy to food, venom, and drugs (44), reduced allergic asthma (75, 76). The miR-487b-3p was increased in food allergy (14) and reduced in allergic asthma (77, 78). The increased miR-718 in peanut allergy (47) was reported to inhibit autoimmune disease such as psoriasis (79).
In in vivo studies, miRs were expressed in various allergies. For instance, in CMA, miR-21, miR-9, miR-365-2-5p, miR-504-5p, miR-224-5p, and miR-106a were increased and miR-150-5p was decreased (51, 53); and miR-19a and miR-98 were increased in egg and peanut allergy, respectively (55, 57). These miRs were also studied in other allergic diseases. For example, miR-21 negatively regulated allergic rhinitis (80, 81) and positively regulated bronchial asthma (82) and eosinophilic esophagitis (83). MiR-9 was increased in neutrophilic asthma (84) and reduced in atopic dermatitis (85). MiR-365-2-5p promoted osteogenesis (86), and miR-504-5p suppressed glioblastomas (87). MiR-224-5p (88–90) and miR-106a (58–60) alleviated allergic rhinitis and atopic dermatitis (61). Moreover, miR-150-5p was found to increase allergic rhinitis (91) and rhinosinusitis (92). MiR-19a (93, 94) and miR-98 (95, 96) increased asthma and rhinosinusitis (97). These all reports and discussions indicate that the same miRs could have different effects in different physiological conditions/diseases, which may be because the same miRs can have different molecular targets for different diseases or physiological conditions. For instance, in the clinical trial of CMA, miR-146a had a positive association with FoxP3 (45), but in in vivo studies, it had a negative association with TLR4/NF-κB (50, 52) and a positive association with RORγt and IL-17 (51, 54). Moreover, miR-155, which positively regulated FoxP3 and IL-4 in clinical trials of CMA (45), also positively regulated RORγt, IL-17, and IL-13 and negatively regulated IL-10 in CMA and egg allergy (1, 51, 54). Thus, these evidences also imply that studying each miR for the specific context of food allergy is crucial before generalizing them as a common miR for food allergy.
There are both similarities and dissimilarities in the results of miRs expression studies included in this review and the findings of studies on the potential role of the miRs on immune cells or molecules involved in allergies. For example, miR-155, which was increased due to CMA in in vivo studies (51, 54), was reported to enhance the maturation and function of DCs, promote B-cell response, promote Tfh cells, and promote the conversion of B cells into plasma cells (Figure 1). Similarly, miR-9 and miR-19a were increased in CMA (51) and OVA allergies (55, 56), respectively, in in vivo studies, which were determined to increase the maturation and function of DCs and promote Th2 cells, respectively (Figure 1). Moreover, miR-21 was increased in CMA in in vivo studies (51) and was also reported to promote Th2 production and DC differentiation (Figure 1). Furthermore, let-7b-3p and let-7b-5p were reduced in CMA in clinical trials (46) and were mentioned to inhibit IL-13 production (Figure 1). However, miR-146a, which was increased in both in vivo studies (51, 54) and clinical trials (45), was found to inhibit proinflammatory cytokine and cells (IL-5 and Tfh13) (Figure 1). Moreover, miR-155 and miR-21 were also reported to reduce the Th2 production and activation of mast cells and macrophages, respectively (Figure 1). These dissimilarities in results may be due to differences in the studies parameters such as results from in vitro versus in vivo versus clinical trials. There is a need for further studies on the modulatory effect of differentially expressed miRs on immune cells and molecules involved in food allergies.
There are various limitations of this study. For example, most of the clinical studies included are observational studies; thus, the method used for their quality evaluation is a subjective approach based on the information provided in the articles and the supplementary materials. Moreover, some of the included clinical trials combined multiple food allergies or food allergies with drug or insect venom allergies, limiting the finding of these studies useful for specific food allergy-related signature miRs. Most animal studies were related to β-lactoglobulin, followed by OVA and peanut allergy. Furthermore, all of the mouse model studies were based on one country, i.e., China. Thus, more studies in different geographical areas targeting a particular type of food allergy are required to increase reliable miRs signatures and their molecular targets in food allergy. Moreover, in in vivo studies, one study used NGS for miRs profiling, and the rest of the studies used the previously studied miRs and quantified by PCR method. This may also led to a few common miRs shared between clinical trials and in vivo studies (Figure 5). Furthermore, more target molecules of differentially expressed miRs were studied in in vivo studies compared to those of clinical trials due to more available references on target molecules of the selected miRs. However, in clinical trials, half of the included studies did not focus on target molecules of differentially expressed miRs, again leaving more work to be conducted for molecular targets study of the miRs for them to be a reliable marker for a specific food allergy.
Because few studies were available on a particular food allergy, such as in peanut allergy and its related miRs expression, it is hard to generalize different signature miRs for a specific type of food allergy. Moreover, very few miRs are common among various kinds of food allergies within clinical trials or within in vivo studies. It indicates that more miR target-specific studies and studies specific to a particular food allergy are still required in clinical studies. In contrast, in in vivo studies, novel miRs may need to be explored via NGS. Additionally, the differentially expressed miRs via NGS are required to be validated via PCR, and those validated miRs need to be further studied for their target molecules with additional confirmation either in in vitro method via RNAi (e.g., transfection) or in in vivo method via target gene knockout/knockdown model.
In conclusion, very few common miRs were studied/expressed between or within the clinical trials and in vivo studies or within an individual study of food allergy. Moreover, the same miR can have different molecular targets and effects on specific diseases; thus, it is essential to study miRs and their targets in particular contexts of food allergy, such as peanut allergy, egg allergy, or β-lactoglobulin allergy, to determine their therapeutic target for each type of food allergy. Furthermore, future studies of food allergy should focus on the identification of novel miRs via NGS, their validation via PCR, and miR’s target molecules determination. This may increase the possibility for signature miRs identification for an individual food allergy type as well as for food allergy in general.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
TR: Conceptualization, Investigation, Methodology, Data curation, Writing – original draft, Writing – review & editing. RB: Investigation, Methodology, Writing – review & editing. JP: Investigation, Visualization, Writing – original draft. LW: Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the 1890 Capacity Building Grant Program from the United States Department of Agriculture’s National Institute of Food and Agriculture (Project award No. 2023-38821-39979).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1524392/full#supplementary-material
References
1. Lin R, Liu J, Lu H, Chen Y, Guan L, Liu Z, et al. Micro RNA-155 plays a critical role in the initiation of food allergen-related inflammation in the intestine. Oncotarget. (2017) 8:67497–505. doi: 10.18632/oncotarget.18723
2. Anvari S, Miller J, Yeh C, Davis CM. IgE-mediated food allergy. Clinic Rev Allerg Immunol. (2018) 57:244. doi: 10.1007/s12016-018-8710-3
3. Crespo JF, Cabanillas B. Recent advances in cellular and molecular mechanisms of IgE-mediated food allergy. Food Chem. (2023) 411:1–8. doi: 10.1016/j.foodchem.2023.135500
4. Rana TS, Bansode RR, Williams LL. Anti-allergic and anti-inflammatory signaling mechanisms of natural compounds/extracts in in vitro system of RBL-2H3 cell: A systematic review. Cells. (2024) 13:1–29. doi: 10.3390/cells13161389
5. Kumar S, Verma AK, Das M, Dwivedi PD. Molecular mechanisms of IgE mediated food allergy. Int Immunopharmacol. (2012) 13:432. doi: 10.1016/j.intimp.2012.05.018
6. Chinthrajah RS, Hernandez JD, Boyd SD, Galli SJ, Nadeau KC. Molecular and cellular mechanisms of food allergy and food tolerance. J Allergy Clin Immunol. (2016) 137:984. doi: 10.1016/j.jaci.2016.02.004
7. Weidner J, Bartel S, Kılıç A, Zissler UM, Renz H, Schwarze J, et al. Spotlight on microRNAs in allergy and asthma. Allergy. (2020) 76:1661. doi: 10.1111/all.14646
8. Bansode RR, Khatiwada JR, Losso JN, Williams LL. Targeting microRNA in cancer using plant-based proanthocyanidins. Diseases. (2016) 4:1–13. doi: 10.3390/diseases4020021
9. Johansson K, Weidner J, Rådinger M. MicroRNAs in type 2 immunity. Cancer Lett. (2018) 425:116. doi: 10.1016/j.canlet.2018.03.036
10. Guidi R, Wedeles CJ, Wilson MS. ncRNAs in type-2 immunity. ncRNA. (2020) 6:1–32. doi: 10.3390/ncrna6010010
11. Zhang L, Wu H, Zhao M, Chang C, Lu Q. Clinical significance of miRNAs in autoimmunity. J Autoimmun. (2020) 109:1–15. doi: 10.1016/j.jaut.2020.102438
12. O'Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol. (2018) 9:402. doi: 10.3389/fendo.2018.00402
13. Bian E, Zhao B, Huang C, Wang H, Meng X, Wu B, et al. New advances of DNA methylation in liver fibrosis, with special emphasis on the crosstalk between microRNAs and DNA methylation machinery. Cell Signalling. (2013) 25:1837. doi: 10.1016/j.cellsig.2013.05.017
14. Nuñez-borque E, Fernandez-bravo S, Rodriguez Del Rio P, Alwashali EM, Lopez-dominguez D, Gutierrez-blazquez MD, et al. Increased miR-21-3p and miR-487b-3p serum levels during anaphylactic reaction in food allergic children. Pediatr Allergy Immunol. (2021) 32:1296. doi: 10.1111/pai.13518
15. Nuñez-Borque E, Fernandez-Bravo S, Rodríguez Del Rio P, Palacio-García L, Di Giannatale A, Di Paolo V, et al. Novel mediator in anaphylaxis: decreased levels of miR-375-3p in serum and within extracellular vesicles of patients. Front Immunol. (2023) 14:1209874. doi: 10.3389/fimmu.2023.1209874
16. Cho S, Dong J, Lu L. Cell-intrinsic and -extrinsic roles of miRNAs in regulating T cell immunity. Immunological Rev. (2021) 304:126. doi: 10.1111/imr.13029
17. Ishibashi O, Muljo SA, Islam Z. Regulation of macrophage polarization in allergy by noncoding RNAs. ncRNA. (2023) 9:1–14. doi: 10.3390/ncrna9060075
18. Scalavino V, Liso M, Serino G. Role of microRNAs in the regulation of dendritic cell generation and function. Int J Mol Sci. (2020) 21:1–18. doi: 10.3390/ijms21041319
19. Specjalski K, Jassem E. MicroRNAs: potential biomarkers and targets of therapy in allergic diseases? Arch Immunol Ther Exp. (2019) 67:213. doi: 10.1007/s00005-019-00547-4
20. O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. (2010) 10:111. doi: 10.1038/nri2708
21. Shefler I, Salamon P, Mekori YA. MicroRNA involvement in allergic and non-allergic mast cell activation. IJMS. (2019) 20:1–12. doi: 10.3390/ijms20092145
22. Deng Q, Yao X, Fang S, Sun Y, Liu L, Li C, et al. Mast cell-mediated microRNA functioning in immune regulation and disease pathophysiology. Clin Exp Med. (2025) 25:1–12. doi: 10.1007/s10238-024-01554-2
23. Mayoral RJ, Deho L, Rusca N, Bartonicek N, Saini HK, Enright AJ, et al. MiR-221 influences effector functions and actin cytoskeleton in mast cells. PloS One. (2011) 6:1–13. doi: 10.1371/journal.pone.0026133
24. Cañas JA, Núñez R, Cruz-Amaya A, Gómez F, Torres MJ, Palomares F, et al. Epigenetics in food allergy and immunomodulation. Nutrients. (2021) 13:1–12. doi: 10.3390/nu13124345
25. Potaczek DP, Harb H, Michel S, Alhamwe BA, Renz H, Tost J. Epigenetics and allergy: from basic mechanisms to clinical applications. Epigenomics. (2017) 9:539. doi: 10.2217/epi-2016-0162
26. Chuang JC, Jones PA. Epigenetics and microRNAs. Pediatr Res. (2007) 61:24R. doi: 10.1203/pdr.0b013e3180457684
27. Piletič K, Kunej T. MicroRNA epigenetic signatures in human disease. Arch Toxicol. (2016) 90:2405. doi: 10.1007/s00204-016-1815-7
28. Sato F, Tsuchiya S, Meltzer SJ, Shimizu K. MicroRNAs and epigenetics. FEBS J. (2011) 278:1598. doi: 10.1111/j.1742-4658.2011.08089.x
29. Zhong Z, Huang X, Zhang S, Zheng S, Cheng X, Li R, et al. Blocking Notch signalling reverses miR-155-mediated inflammation in allergic rhinitis. Int Immunopharmacol. (2023) 116:1–9. doi: 10.1016/j.intimp.2023.109832
30. Li H, Wang Y, Han X. ESP-B4 promotes nasal epithelial cell-derived extracellular vesicles containing miR-146a-5p to modulate Smad3/GATA-3 thus relieving allergic rhinitis. Phytomedicine. (2022) 108:1–13. doi: 10.1016/j.phymed.2022.154516
31. Deng B, Zhao Y, Liu J. Downregulation of lncRNA CDKN2B-AS1 attenuates inflammatory response in mice with allergic rhinitis by regulating miR-98-5p/SOCS1 axis. Funct Integr Genomics. (2024) 24:1–13. doi: 10.1007/s10142-024-01318-x
32. Kim M, Lee S, Kim Y, Kwon Y, Park Y, Lee H, et al. Human adipose tissue-derived mesenchymal stem cells attenuate atopic dermatitis by regulating the expression of MIP-2, miR-122a-SOCS1 axis, and Th1/Th2 responses. Front Pharmacol. (2018) 9:1175. doi: 10.3389/fphar.2018.01175
33. Lee YS, Han S, Ham HJ, Park JH, Lee JS, Hwang DY, et al. IL-32γ suppressed atopic dermatitis through inhibition of miR-205 expression via inactivation of nuclear factor-kappa B. J Allergy Clin Immunol. (2020) 146:156. doi: 10.1016/j.jaci.2019.12.905
34. Yoon W, Kim E, Park Y, Kim S, Park Y, Yoo Y. Bacterially Delivered miRNA-Mediated Toll-like Receptor 8 Gene Silencing for Combined Therapy in a Murine Model of Atopic Dermatitis: Therapeutic Effect of miRTLR8 in AD. Microorganisms. (2021) 9:1–11. doi: 10.3390/microorganisms9081715
35. Fu D, Zhao H, Huang Y, Li J, Feng H, Li A, et al. Metformin regulates the effects of IR and IGF-1R methylation on mast cell activation and airway reactivity in diabetic rats with asthma through miR-152-3p/DNMT1 axis. Cell Biol Toxicol. (2022) 39:1851. doi: 10.1007/s10565-022-09787-1
36. Mohammed OA, Doghish AS, Alamri MMS, Alharthi MH, Alfaifi J, Adam MIE, et al. The role of matrix metalloproteinase-2 and miR-196a2 in bronchial asthma pathogenesis and diagnosis. Heliyon. (2024) 10:1–13. doi: 10.1016/j.heliyon.2024.e27694
37. Mohany KM, Gamal Y, Abdel Raheem YF. Heavy metal levels are positively associated with serum periostin and miRNA-125b levels, but inversely associated with miRNA-26a levels in pediatric asthma cases. A case-control study. J Trace Elements Med Biol. (2023) 82:1–10. doi: 10.1016/j.jtemb.2023.127364
38. Grueso-Navarro E, Navarro P, Laserna-Mendieta EJ, Lucendo AJ, Arias-González L. Blood-based biomarkers for eosinophilic esophagitis and concomitant atopic diseases: A look into the potential of extracellular vesicles. Int J Mol Sci. (2023) 24:1–24. doi: 10.3390/ijms24043669
39. Li W, Liu F, Wang J, Long M, Wang Z. MicroRNA-21-Mediated Inhibition of Mast Cell Degranulation Involved in the Protective Effect of Berberine on 2,4-Dinitrofluorobenzene-Induced Allergic Contact Dermatitis in Rats via p38 Pathway. Inflammation. (2017) 41:689. doi: 10.1007/s10753-017-0723-1
40. Wang Z, Yi T, Long M, Ding F, Ouyang L, Chen Z. Involvement of the negative feedback of IL-33 signaling in the anti-inflammatory effect of electro-acupuncture on allergic contact dermatitis via targeting microRNA-155 in mast cells. Inflammation. (2018) 41:859. doi: 10.1007/s10753-018-0740-8
41. Urgard E, Lorents A, Klaas M, Padari K, Viil J, Runnel T, et al. Pre-administration of PepFect6-microRNA-146a nanocomplexes inhibits inflammatory responses in keratinocytes and in a mouse model of irritant contact dermatitis. J Controlled Release. (2016) 235:195. doi: 10.1016/j.jconrel.2016.06.006
42. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:1–8. doi: 10.1136/bmj.l4898
43. Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Method. (2014) 14:43. doi: 10.1186/1471-2288-14-43
44. Francuzik W, Pažur K, Dalke M, Dölle-Bierke S, Babina M, Worm M. Serological profiling reveals hsa-miR-451a as a possible biomarker of anaphylaxis. JCI insight. (2022) 7:1–13. doi: 10.1172/jci.insight.156669
45. Paparo L, Nocerino R, Bruno C, Di Scala C, Cosenza L, Bedogni G, et al. Randomized controlled trial on the influence of dietary intervention on epigenetic mechanisms in children with cow’s milk allergy: the EPICMA study. Sci Rep. (2019) 9:1–10. doi: 10.1038/s41598-019-38738-w
46. D'Argenio V, Del Monaco V, Paparo L, De Palma FDE, Nocerino R, D'Alessio F, et al. Altered miR-193a-5p expression in children with cow's milk allergy. Allergy (Copenhagen). (2018) 73:379–86. doi: 10.1111/all.13299
47. Worm M, Alexiou A, Höfer V, Birkner T, Jeanrenaud ACSN, Fauchère F, et al. An interdisciplinary approach to characterize peanut-allergic patients—First data from the FOOD@ consortium. Clin Trans All. (2022) 12:1–13. doi: 10.1002/clt2.12197
48. Larsen LF, Juel-berg N, Hansen A, Hansen KS, Mills ENC, Van Ree R, et al. No difference in human mast cells derived from peanut allergic versus non-allergic subjects. Immun Inflam Dis. (2018) 6:416. doi: 10.1002/iid3.226
49. Clemente E, Efthymakis K, Carletti E, Capone V, Sperduti S, Bologna G, et al. An explorative study identifies miRNA signatures for the diagnosis of non-celiac wheat sensitivity. PloS One. (2019) 14:1–18. doi: 10.1371/journal.pone.0226478
50. Li A, Li Y, Zhang X, Zhang C, Li T, Zhang J, et al. The human milk oligosaccharide 2′-fucosyllactose attenuates β-lactoglobulin–induced food allergy through the miR-146a–mediated toll-like receptor 4/nuclear factor-κB signaling pathway. J Dairy Sci. (2021) 104:10473. doi: 10.3168/jds.2021-20257
51. Wang J, Li S, Li A, Zhang Q, Ni W, Li M, et al. Effect of Lactobacillus acidophilus KLDS 1.0738 on miRNA expression in in vitro and in vivo models of β-lactoglobulin allergy. Bioscience Biotechnology Biochem. (2018) 82:1955. doi: 10.1080/09168451.2018.1495551
52. Li A, Yang J, Zhang C, Chi H, Zhang C, Li T, et al. Lactobacillus acidophilus KLDS 1.0738 inhibits TLR4/NF-κB inflammatory pathway in β-lactoglobulin-induced macrophages via modulating miR-146a. J Food Biochem. (2021) 45:1–13. doi: 10.1111/jfbc.13662
53. Zhang Q, Ni W, Li Y, Zhang X, Hou J, Meng XC, et al. Analysis of altered miRNA profiling in the colon of a mouse model with β-lactoglobulin allergy. Allergologia Immunopathologia. (2020) 48:666. doi: 10.1016/j.aller.2020.05.007
54. Wang J, Zhang Y, Li H, Chen G, Zou Y, Rin K. Immune effects of miRNA and Th17 cells on β-Lg allergy in dietary milk based on mouse model. Saudi J Biol Sci. (2020) 27:3442. doi: 10.1016/j.sjbs.2020.08.028
55. Yang L, Li X, Qiu S, Zeng L, Li L, Feng B, et al. Micro RNA-19a suppresses thrombospondin-1 in CD35 + B cells in the intestine of mice with food allergy. Am J Trans Res. (2016) 8:5503–11.
56. Liu Z, Yang G, Geng X, Liu J, Mo L, Liu Z, et al. Micro RNA-17–92 cluster mediates interleukin-4-suppressed IL-10 expression in B cells. Am J Trans Res. (2016) 8:2317–24.
57. Xie R, Xu L, Yang L, Wang S, Liu Q, Liu Z, et al. Galectin-1 inhibits oral-intestinal allergy syndrome. Oncotarget. (2017) 8:13214–22. doi: 10.18632/oncotarget.14571
58. Wang J, Cui Z, Liu L, Zhang S, Zhang Y, Zhang Y, et al. MiR-146a mimic attenuates murine allergic rhinitis by downregulating TLR4/TRAF6/NF-κB pathway. Immunotherapy. (2019) 11:1095. doi: 10.2217/imt-2019-0047
59. Zhang Y, Yang Y, Guo J, Cui L, Yang L, Li Y, et al. miR-146a enhances regulatory T-cell differentiation and function in allergic rhinitis by targeting STAT5b. Allergy. (2021) 77:550. doi: 10.1111/all.15163
60. Luo X, Hong H, Tang J, Wu X, Lin Z, Ma R, et al. Increased expression of miR-146a in children with allergic rhinitis after allergen-specific immunotherapy. Allergy Asthma Immunol Res. (2016) 8:132–40. doi: 10.4168/aair.2016.8.2.132
61. Rebane A, Runnel T, Aab A, Maslovskaja J, Rückert B, Zimmermann M, et al. MicroRNA-146a alleviates chronic skin inflammation in atopic dermatitis through suppression of innate immune responses in keratinocytes. J Allergy Clin Immunol. (2014) 134:836. doi: 10.1016/j.jaci.2014.05.022
62. Laanesoo A, Urgard E, Periyasamy K, Laan M, Bochkov YA, Aab A, et al. Dual role of the miR-146 family in rhinovirus-induced airway inflammation and allergic asthma exacerbation. Clin Trans Med. (2021) 11:1–22. doi: 10.1002/ctm2.427
63. Hammad NM, Nabil F, Elbehedy EM, Sedeek R, Gouda MI, Arafa MA, et al. Role of microRNA-155 as a potential biomarker for allergic rhinitis in children. Can respiratory J. (2021) 2021:5554461–7. doi: 10.1155/2021/5554461
64. Malmhäll C, Alawieh S, Lu Y, Sjöstrand M, Bossios A, Eldh M, et al. MicroRNA-155 is essential for TH2-mediated allergen-induced eosinophilic inflammation in the lung. J Allergy Clin Immunol. (2013) 133:1429. doi: 10.1016/j.jaci.2013.11.008
65. Malmhäll C, Johansson K, Winkler C, Alawieh S, Ekerljung L, Rådinger M. Altered miR-155 expression in allergic asthmatic airways. Scand J Immunol. (2017) 85:300. doi: 10.1111/sji.12535
66. Li X, Wang B, Huang M, Wang X. miR-30a-3p participates in the development of asthma by targeting CCR3. Open Med. (2020) 15:483. doi: 10.1515/med-2020-0102
67. Zhao C, Wang W, Yao H, Wang X. SOCS3 is upregulated and targeted by miR30a-5p in allergic rhinitis. Int Arch Allergy Immunol. (2018) 175:209–19. doi: 10.1159/000486857
68. Gu J, Zhou D. Long non-coding RNA MEG3 knockdown represses airway smooth muscle cells proliferation and migration via sponging miR-143-3p/FGF9 in asthma. J Cardiothorac Surg. (2024) 19:1–10. doi: 10.1186/s13019-024-02798-5
69. Srinivasan A, Sundar IK. Recent updates on the role of extracellular vesicles in the pathogenesis of allergic asthma. Extracellular Vesicles Circulating Nucleic Acids. (2021) 2:127–47. doi: 10.20517/evcna.2021.03
70. Yuan X, Zhang Y, Yu Z. Expression and clinical significance of miR-3615 in hepatocellular carcinoma. J Int Med Res. (2021) 49:1–6. doi: 10.1177/0300060520981547
71. Farmanzadeh A, Qujeq D, Yousefi T. The interaction network of microRNAs with cytokines and signaling pathways in allergic asthma. MicroRNA. (2022) 11:104–17. doi: 10.2174/2211536611666220428134324
72. Parzibut G, Henket M, Moermans C, Struman I, Louis E, Malaise M, et al. A blood exosomal miRNA signature in acute respiratory distress syndrome. Front Mol Biosci. (2021) 8:640042. doi: 10.3389/fmolb.2021.640042
73. Xiao R, Noël A, Perveen Z, Penn AL. In utero exposure to second-hand smoke activates pro-asthmatic and oncogenic miRNAs in adult asthmatic mice. Environ Mol Mutagen. (2016) 57:190. doi: 10.1002/em.21998
74. Cheng S, Di Z, Hirman AR, Zheng H, Duo L, Zhai Q, et al. MiR-375-3p alleviates the severity of inflammation through targeting YAP1/LEKTI pathway in HaCaT cells. Bioscience Biotechnology Biochem. (2020) 84:2005. doi: 10.1080/09168451.2020.1783196
75. Wang T, Zhou Q, Shang Y. MiRNA-451a inhibits airway remodeling by targeting Cadherin 11 in an allergic asthma model of neonatal mice. Int Immunopharmacol. (2020) 83:1–9. doi: 10.1016/j.intimp.2020.106440
76. Wang T, Zhou Q, Shang Y. Downregulation of miRNA-451a Promotes the Differentiation of CD4+ T Cells towards Th2 Cells by Upregulating ETS1 in Childhood Asthma. J Innate Immun. (2025) 13:38. doi: 10.1159/000509714
77. Yamazumi Y, Sasaki O, Imamura M, Oda T, Ohno Y, Shiozaki-Sato Y, et al. The RNA binding protein mex-3B is required for IL-33 induction in the development of allergic airway inflammation. Cell Rep. (2016) 16:2456. doi: 10.1016/j.celrep.2016.07.062
78. Zhang X, Zhang X, Feng S, Wang X, Guo B, Liu J, et al. The specific microRNA profile and functional networks for children with allergic asthma. J Asthma Allergy. (2022) 15:1179. doi: 10.2147/jaa.s378547
79. Rani H, Saini N. MicroRNA-718 inhibits mitochondrial fusion and ameliorates IMQ-induced psoriasis inflammation mediated by PHB and STAT1. (2024). doi: 10.21203/rs.3.rs-4557621/v1
80. Chen RF, Huang HC, Ou CY, Hsu TY, Chuang H, Chang JC, et al. MicroRNA-21 expression in neonatal blood associated with antenatal immunoglobulin E production and development of allergic rhinitis. Clin Exp Allergy. (2010) 40:1482. doi: 10.1111/j.1365-2222.2010.03592.x
81. Song J, Wang T, Chen Y, Cen R. Long non-coding RNA growth arrest-specific 5 and its targets, microRNA-21 and microRNA-140, are potential biomarkers of allergic rhinitis. Clin Lab Anal. (2021) 35:1–8. doi: 10.1002/jcla.23938
82. Elkashef SMMAE, Ahmad SE, Soliman YMA, Mostafa MS. Role of microRNA-21 and microRNA-155 as biomarkers for bronchial asthma. Innate Immun. (2020) 27:61. doi: 10.1177/1753425920901563
83. Sawant DV, Yao W, Wright Z, Sawyers C, Tepper RS, Gupta SK, et al. Serum microRNA-21 as a biomarker for allergic inflammatory disease in children. MIRNA. (2015) 4:36. doi: 10.2174/2211536604666150220232507
84. Li JJ, Tay HL, Maltby S, Xiang Y, Eyers F, Hatchwell L, et al. MicroRNA-9 regulates steroid-resistant airway hyperresponsiveness by reducing protein phosphatase 2A activity. J Allergy Clin Immunol. (2015) 136:462. doi: 10.1016/j.jaci.2014.11.044
85. Kwon Y, Choi Y, Kim M, Jeong MS, Jung HS, Jeoung D. HDAC6 and CXCL13 mediate atopic dermatitis by regulating cellular interactions and expression levels of miR-9 and SIRT1. Front Pharmacol. (2021) 12:691279. doi: 10.3389/fphar.2021.691279
86. Hou C, Zhang Y, Lv Z, Luan Y, Li J, Meng C, et al. Macrophage exosomes modified by miR-365-2-5p promoted osteoblast osteogenic differentiation by targeting OLFML1. Regenerative Biomaterials. (2024) 11:1–14. doi: 10.1093/rb/rbae018
87. Seo J, Jin D, Choi C, Lee H. Integration of microRNA, mRNA, and protein expression data for the identification of cancer-related microRNAs. PloS One. (2017) 12:1–22. doi: 10.1371/journal.pone.0168412
88. Wu J, Wu L, Zhang L, Xu H, Wang M, Wang L, et al. Overexpression of miR-224-5p alleviates allergic rhinitis in mice via the TLR4/MyD88/NF-κB pathway. Exp Anim. (2021) 70:440–9. doi: 10.1538/expanim.20-0195
89. Li Y, An R, Wu M, He J, He X. miR-224-5p attenuates allergic responses in mice with allergic rhinitis by modulating the Th1/Th2 response. Analytical Cell Pathol. (2024) 2024:1–13. doi: 10.1155/2024/5531970
90. Zhuang Z, Zhou Y, Xu J, Pan L. Anti-asthmatic miR-224-5p inhibits the FHL1/MAPK pathway to repress airway smooth muscle cell proliferation in a murine model of asthma-like airway inflammation. Allergy Asthma Clin Immunol. (2022) 18:1–16. doi: 10.1186/s13223-022-00724-9
91. Fang S, Zhou Z, Sun Q, Liu X, Li C, Xie Y, et al. Plasma extracellular vesicles regulate the Functions of Th2 and ILC2 cells via miRNA-150-5p in patients with allergic rhinitis. Int Immunopharmacol. (2025) 144:1–10. doi: 10.1016/j.intimp.2024.113644
92. Ma Z, Shen Y, Zeng Q, Liu J, Yang L, Fu R, et al. MiR-150-5p regulates EGR2 to promote the development of chronic rhinosinusitis via the DC-Th axis. Int Immunopharmacol. (2017) 54:188. doi: 10.1016/j.intimp.2017.11.011
93. Jiang S, Piao X, Yu J, Feng Q. The “time-response” effect of Wenyang Pingchuan Formula on miR-19a in asthmatic mice experiment. Allergol Immunopathol. (2022) 50:129. doi: 10.15586/aei.v50i5.531
94. Haj-Salem I, Fakhfakh R, Bérubé J, Jacques E, Plante S, Simard MJ, et al. MicroRNA-19a enhances proliferation of bronchial epithelial cells by targetingTGFβR2gene in severe asthma. Allergy. (2014) 70:212. doi: 10.1111/all.12551
95. Chen L, Xu J, Chu X, Ju C. MicroRNA-98 interferes with thrombospondin 1 expression in peripheral B cells of patients with asthma. Bioscience Rep. (2017) 37:1–7. doi: 10.1042/bsr20170149
96. Midyat L, Gulen F, Karaca E, Ozkinay F, Tanac R, Demir E, et al. MicroRNA expression profiling in children with different asthma phenotypes. Pediatr Pulmonology. (2015) 51:582. doi: 10.1002/ppul.23331
97. Shin C, Byun J, Lee K, Kim B, Noh YK, Tran NL, et al. Exosomal miRNA-19a and miRNA-614 Induced by Air Pollutants Promote Proinflammatory M1 Macrophage Polarization via Regulation of RORα Expression in Human Respiratory Mucosal Microenvironment. J Immunol. (2025) 205:3179. doi: 10.4049/jimmunol.2000456
Keywords: food allergy, miRNA, RNAi, peanut allergy, CMA, BALB/c, NGS, ovalbumin
Citation: Rana TS, Bansode RR, Pakhrin Rana J and Williams LL (2025) MicroRNA expression and their molecular targets in food allergies: a systematic review. Front. Immunol. 16:1524392. doi: 10.3389/fimmu.2025.1524392
Received: 07 November 2024; Accepted: 07 April 2025;
Published: 12 May 2025.
Edited by:
Hongbing Chen, Nanchang University, ChinaReviewed by:
Vanesa Esteban, Health Research Institute Foundation Jimenez Diaz (IIS-FJD), SpainEmilio Nuñez-Borque, Hospital de la Princesa, Spain
Daniel P. Potaczek, University of Marburg, Germany
Copyright © 2025 Rana, Bansode, Pakhrin Rana and Williams. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leonard L. Williams, bGx3QG5jYXQuZWR1
 Tekan Singh Rana
Tekan Singh Rana Rishipal Rastrapal Bansode
Rishipal Rastrapal Bansode Jenny Pakhrin Rana
Jenny Pakhrin Rana Leonard L. Williams
Leonard L. Williams