- 1Department of Oncology, Affiliated Hospital of Shandong Second Medical University, School of Clinical Medicine, Shandong Second Medical University, Weifang, Shandong, China
- 2Jinming Yu Academician Workstation of Oncology, Shandong Second Medical University, Shandong, China
- 3Department of Infectious Diseases, Affiliated Hospital of Shandong Second Medical University, School of Clinical Medicine, Shandong Second Medical University, Weifang, Shandong, China
- 4Medical Center, University of Chicago, Cancer Center, Medical Center, Chicago, IL, United States
Objective: To evaluate the prognostic significance of the Naples Prognostic Score (NPS) in patients with locally advanced non-small cell lung cancer (NSCLC) after neoadjuvant chemotherapy and surgery.
Methods: A retrospective review was done of 126 patients with locally advanced NSCLC who were surgically treated Affiliated Hospital of Weifang Medical University. from September 2012 to April 2019. According to the neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), albumin, and total cholesterol before neoadjuvant chemotherapy, NPS was divided into separate groups: group 0, group 1, and group 2. Kaplan-Meier method was used to analyze survival curves for the NPS. Univariate and multivariate Cox analysis of overall survival (OS) and progression-free survival (PFS) was then conducted.
Results: This study included 60 male and 66 female patients, with the median age being 59 (59.94 ± 11.77). Based on the NPS system, the three groups were divided: Group 0, 41(32.5%) patients; Group 1, 55(43.7%) patients; and Group 2, 30(23.8%) patients. Smoking status (P=0.032) and KPS score (P=0.018) were significantly different among the three NPS groups, but it had no statistical relevance in regards to gender (P=0.849), age (P=0.474), clinical stage (P=0.101), pathology (P=0.819), tumor location (P=0.304), degree of differentiation (P=0.889), surgical method (P=0.436), chemotherapy (P=0.718), postoperative complications (P=0.177) or CEA level (P=0.447). Univariate Analysis showed that clinical stage (P=0.004), KPS score (P=0.003), surgery approach (P=0.042) and NPS (Group 2 vs. Group 0, P< 0.001; Group 1 vs. Group 0, P=0.005) were predictors of OS in patients with locally advanced NSCLC, and that clinical stage (P=0.005), KPS score (P=0.002), and NPS (Group 2 vs. Group 0, P< 0.001; Group 1 vs. group 0, P=0.001) were significantly associated with PFS. Based on the positive results of univariate analysis, we performed multivariate analysis. Multivariate Cox Regression showed that the NPS was a significant independent predictor of worse OS (Group 2 vs. Group 0, P=0.006; Group 1 vs. group 0, P=0.017) and PFS (group 2 vs. group 0, P=0.006; Group 1 vs group 0, P=0.011).
Conclusion: As a clinically accessible blood indicator, NPS has vital value in predicting the prognosis of resected locally advanced NSCLC patients receiving neoadjuvant chemotherapy and surgery.
Introduction
Lung cancer is the second most diagnosed cancer in both men and women but the most common the leading cause in cancer related deaths (1). Lung cancer brings high economic burden for governments and families (2, 3). Most patients with early-stage NSCLC fall outside of the optimal treatment window the treatment due to lack of clinical manifestations (1). Stage III lung cancer, also known as locally advanced lung cancer, is a type of disease with very strong clinical heterogeneity (4). Patients with locally advanced NSCLC have a poor prognosis, with a 5-year survival rate of only 15%-40% (5). Therefore, there is an urgent need to stratify patients with locally advanced NSCLC according to individual heterogeneity, to explore effective and reliable prognostic indicators. In current clinical practice, researchers have confirmed that inflammation-related hematological biomarkers can be used as markers for predicting patient prognosis, including C-reactive protein (CRP), mean platelet volume/platelet count ratio (MPV/PC ratio), platelet-to-lymphocyte ratio (PLR), lymphocyte-white blood cell ratio(LWR), lymphocyte to CRP ratio (LCR) and CRP to albumin ratio (CAR) etc (6–13). Additionally, inflammatory markers such as Neutrophil-to-Lymphocyte Ratio (NLR) and lymphocyte-to-monocyte ratio (LMR), and other prognostic factors that represent or reflect the nutritional or immune status of patients have also been confirmed by various studies to be key factors in predicting the survival rate of osteosarcoma (14, 15). Besides, prognostic nutritional index (PNI) like albumin, cholesterol, controlled nutritional status (CONUT) score, systemic immune inflammation index (SII) also shows prognostic value (16–20). Therefore, a multidimensional prognostic evaluation system containing multiple markers or factors together may have better predictive efficacy than a single prognostic factor.
The Naples prognostic score (NPS) is a comprehensive prognostic scoring system, calculated according to serum albumin and total cholesterol concentrations, LMR and NLR (21, 22). As an immune and nutritional evaluation method, it has been used to predict the prognosis of various solid tumors including gastrointestinal (GI) cancers, non-small-cell-lung cancer, gallbladder cancer (23–26). Moreover, it also predicts other diseases development, such as adult asthma (21), heart failure mortality (27), pulmonary arterial hypertension (28). Up to now, the significance of the NPS prognostic score in the prognostic value of surgically resected locally advanced NSCLC patients has not been widely studied.
Herein, the purpose of this study is to evaluate the correlation between the NPS prognostic score and the clinicopathological characteristics as well as the long-term prognosis of patients with locally advanced NSCLC. Furthermore, the NPS prognostic score was compared with previously developed scoring systems and the classic TNM staging system to evaluate whether the Naples prognostic score has a predictive value for the prognosis of patients with locally advanced NSCLC who underwent surgery after neoadjuvant chemotherapy.
Data and methods
Clinical data
A total of 126 patients with locally advanced NSCLC who were eligible for surgery at the Affiliated Hospital of Weifang Medical College between September 2012 and April 2019 were selected. Inclusion criteria: (1) aged over 18 years; (2) no anti-tumor treatment before admission; (3) patients diagnosed with NSCLC by histological pathology. (4) Karnofsky (KPS) functional status score within 80–100 points; (5) complete peripheral blood test collected 1 week prior to treatment initiation, including neutrophils, monocytes, lymphocytes, albumin, cholesterol, tumor markers, etc. (6) no other major medical morbidities, and were otherwise deemed ideal candidates for chemotherapy and surgery. (7) patients and their families agreed to chemotherapy and surgery and signed informed consent for chemotherapy and surgery. Exclusion criteria: (1) patients with other malignant tumors. (2) patients previously treated with nonsteroidal anti-inflammatory drugs or antibiotics; (3) patients with active or chronic infectious diseases or inflammatory conditions such as blood diseases, liver diseases, and immune system diseases. This retrospective study was approved by the ethics committee of affiliated hospital of Weifang Medical University (NO. wyfy-2024-ky-490).
Data collection
The following information of all patients collected from the electronic medical record system of the Affiliated Hospital of Weifang Medical College was included: age, sex, smoking status, KPS score, pathological type, tumor location, degree of differentiation, clinical stage, surgical method, chemotherapy regimen, postoperative complications, CEA level, overall survival (OS), and progression-free survival (PFS). In addition, serum albumin and total cholesterol levels as well as lymphocyte, neutrophil, and monocyte counts were collected 1 week prior to the initiation of neoadjuvant chemotherapy.
NPS prognostic score
The NPS prognostic score is calculated based on plasma albumin and cholesterol levels, NLR, and LMR. The neutrophil count divided by the lymphocyte count equals the NLR, and the lymphocyte count divided by the monocyte count equals the LMR. Based on previous reports, an albumin concentration <4 mg/dL is scored as 1 point, and ≥4 mg/dL is scored as 0 point. A total cholesterol level ≤180 mg/dL is scored as 1, and a total cholesterol level >180 mg/dL is scored as 0. An NLR ≥2.96 is scored as 1 point, and an NLR <2.96 is scored as 0 point. An LMR ≤4.44 is scored as 1, and an LMR >4.44 is scored as 0. The NPS prognostic score is the sum of the plasma albumin and cholesterol levels, NLR, and LMR scores (6). According to the NPS prognostic score, patients were divided into three groups: Group 0, patients with a prognostic score of 0; Group 1, patients with a prognostic score of 1 or 2; Group 2, patients with a prognostic score of 3 or 4. The systemic inflammation score (SIS) is defined as follows: 2 points for serum albumin concentration <4 mg/dL and LMR ≤ 4.44; 0 points for serum albumin concentration ≥4 mg/dL and LMR>4.44; 1 point for serum albumin concentration <4 mg/dL or LMR ≤ 4.44.
Treatment methods
According to the Clinical Diagnosis and Treatment Guidelines for Lung Cancer of the Oncology Branch of the Chinese Medical Association (2021 edition), senior oncology experts discussed and analyzed the treatment plans for all chemotherapy patients. Specific chemotherapy plans include: pemetrexed combined with platinum, paclitaxel combined with platinum, gemcitabine combined with platinum, and pemetrexed or paclitaxel combined with platinum chemotherapy, respectively. The specific dosage of the drug needs to be determined in combination with the patient’s body tolerance and tumor condition to determine the dosage and time of chemotherapy drugs. The conclusion of chemotherapy is then followed by a two-week resting period. If there are no surgical contraindications, then a patient is recommended to undergo surgical treatment. If the patient does have surgical contraindications, then it is recommended that the resting period be extended, and surgery can be initiated if there are any indications. est time should be appropriately extended, and surgical treatment should be performed after there are indications. The specific surgical method depends on each individual patient’s tumor characteristics. The surgical methods include thoracoscopic surgery and thoracotomy. For the choice of the two surgical methods, at least 3 thoracic surgeons will make a comprehensive consideration to ensure the safety of the operation and reduce patient trauma.
Follow-up
All patients are followed up regularly after the initiation of treatment. According to the follow-up system regulations, patients are mainly contacted through outpatient examinations or telephone calls. The follow-up interval is every 3 months for the first 3 years and every 6 months for the next 6 years. Routine physical examinations, laboratory tests, chest and abdominal CT, cranial MRI and other imaging examinations are performed. OS is defined as the time from the first treatment to death (event) or the last follow-up (review), and PFS is defined as the time from the start of treatment to disease progression (including metastasis, recurrence or death).
Statistical analysis
IBM SPSS Statistics 20.0 (SPSS, Inc., Chicago, IL) and Graphpad (version 8.0) were used for all statistical analyses. The receiver operating characteristic (ROC) curve analysis method was used to determine the predictive accuracy of NPS and its component parameters. The Kaplan-Meier method and Log-rank test were used to compare the differences in survival between NPS groups. Univariate and multivariate Cox proportional hazards regression analysis was used to determine prognostic factors. The hazard ratio (HR) and its 95% confidence interval (95%CI) were also calculated.
Results
Clinical characteristics of patients
A total of 126 patients with locally advanced NSCLC were included in this study. All patients underwent surgical treatment after neoadjuvant chemotherapy, and no data were missing during follow-up (Figure 1). The average age of these patients at the time of treatment was 59 years (59.94 ± 11.77 years). 98 (77.8%) patients had a KPS score of 100 points, and 28 (22.2%) had a KPS score of 80–90 points. 66 (46.8%) patients had a history of smoking. There were 54 (42.9%) patients with squamous cell carcinoma and 72 (57.1%) patients with adenocarcinoma; according to the TNM staging system, there were 82 (65.1%) patients in stage IIIA and 44 (34.9%) patients in stage IIIB. All patients received neoadjuvant chemotherapy, including 59 (46.8%) patients who received paclitaxel combined with platinum, 30 (23.8%) patients who received pemetrexed combined with platinum, and 37 (29.4%) patients who received gemcitabine combined with platinum. The baseline characteristics and results of the patients are summarized in Table 1.
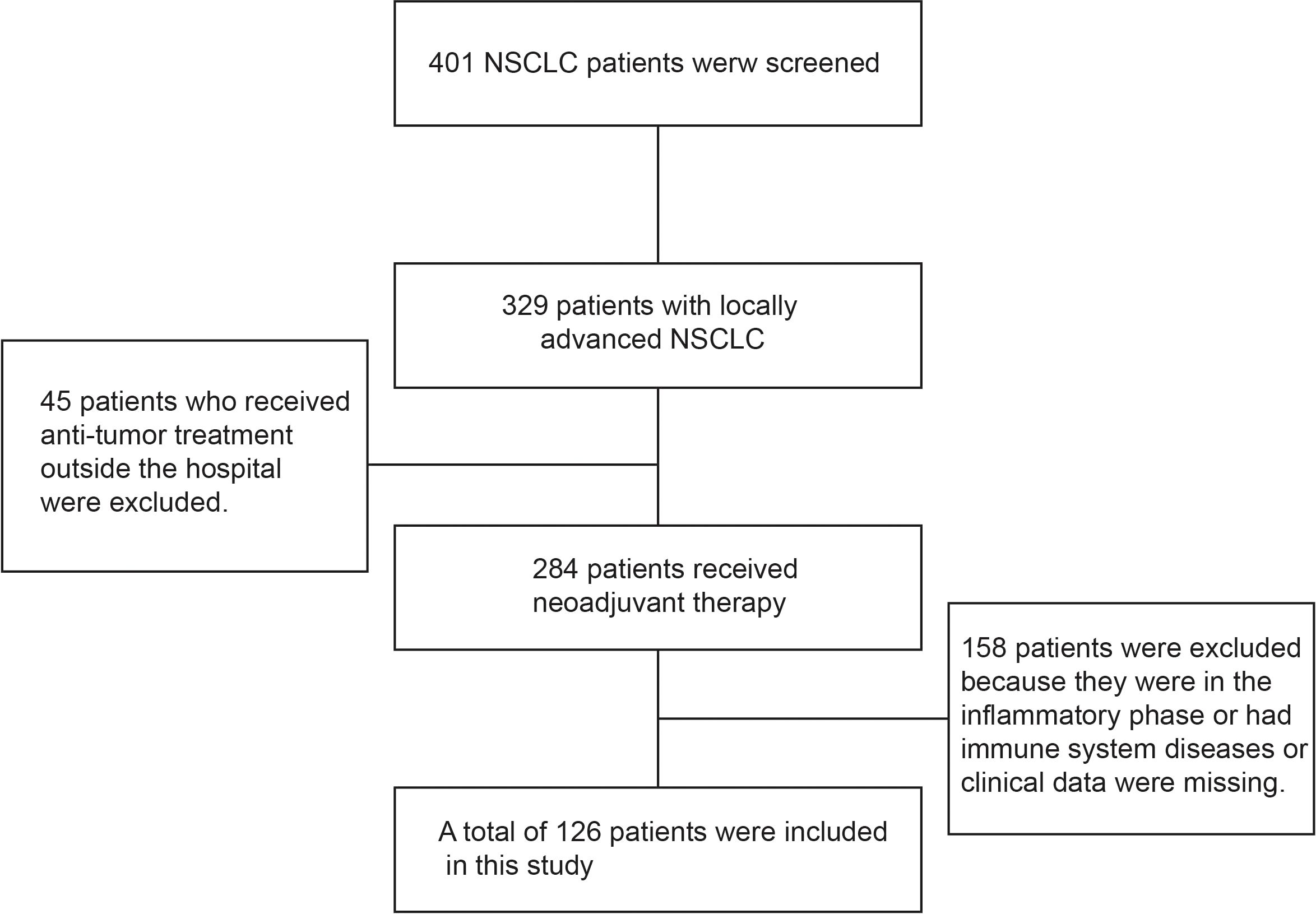
Figure 1. Demonstrated case screening process. Totally, 401 patients with NSCLC were screened, including 329 patients with locally advanced disease, and preliminarily excluded 45 patients who had undergone relevant anti-tumor therapy in other hospitals. A further 284 patients received neoadjuvant therapy, and 158 patients with inflammatory phase or immune system diseases or lack of clinical data were excluded, for a total of 126 patients were included in this study.

Table 1. Relationship between NPS prognostic score and clinicopathological characteristics in patients with locally advanced NSCLC.
Relationship between NPS prognostic score and clinicopathological characteristics
According to the calculation of NPS prognostic scoring standards, 41 (32.5%) patients with a score of 0 points were included in the NPS group 0, 55 (43.7%) patients with a score of 1–2 points were included in the NPS group 1, and the remaining 30 (23.8%) patients with scores of 3 to 4 were included in NPS group 2. The NPS prognostic score is significantly correlated with some clinicopathological features. The results revealed statistically significant differences in smoking status (P=0.032) and KPS score (P=0.018) in the three groups. However, gender (P=0.849), age (P=0.474), and clinical stage (P= 0.101), pathological type (P=0.819), tumor location (P=0.304), degree of differentiation (P=0.889), surgical method (P=0.436), chemotherapy regimen (P=0.718), postoperative complications (P= 0.177) and CEA level (P=0.447) had no statistical significance among the three groups of NPS prognostic score.
Laboratory indicator analysis
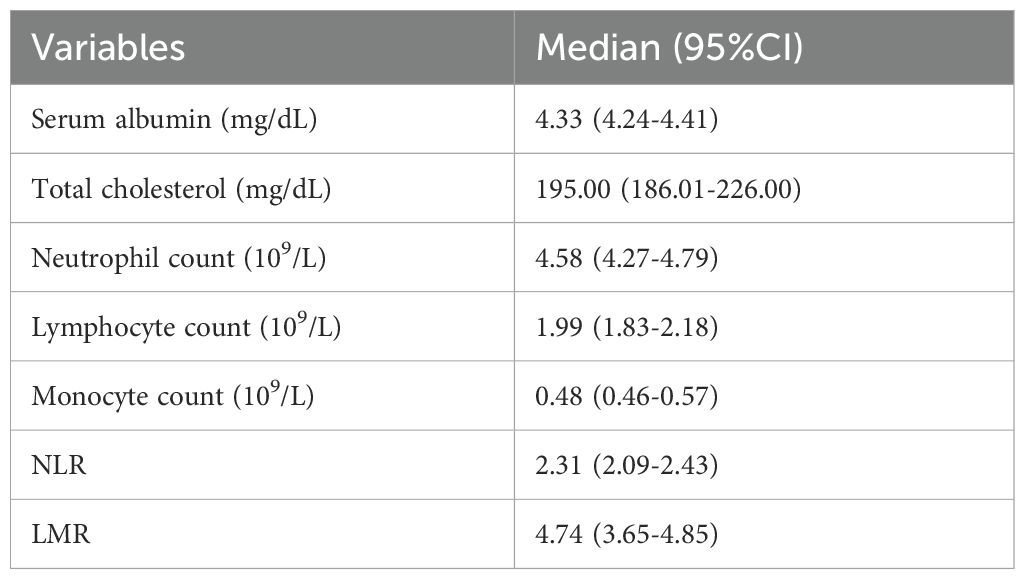
The median values of neutrophil count, lymphocyte count, monocyte count, NLR, LMR, albumin and total cholesterol were 4.58 (95%CI: 4.27-4.79) and 1.99 (95%CI: 1.83-2.18) respectively.), 0.48 (95%CI: 0.46-0.57), 2.31 (95%CI: 2.09-2.43), 4.74 (95%CI: 3.65-4.85), 4.33 (95%CI: 4.24-4.41) mg/dL and 195.00 (95% CI: 186.01-226.00) mg/dL (Table 2).
Survival difference analysis
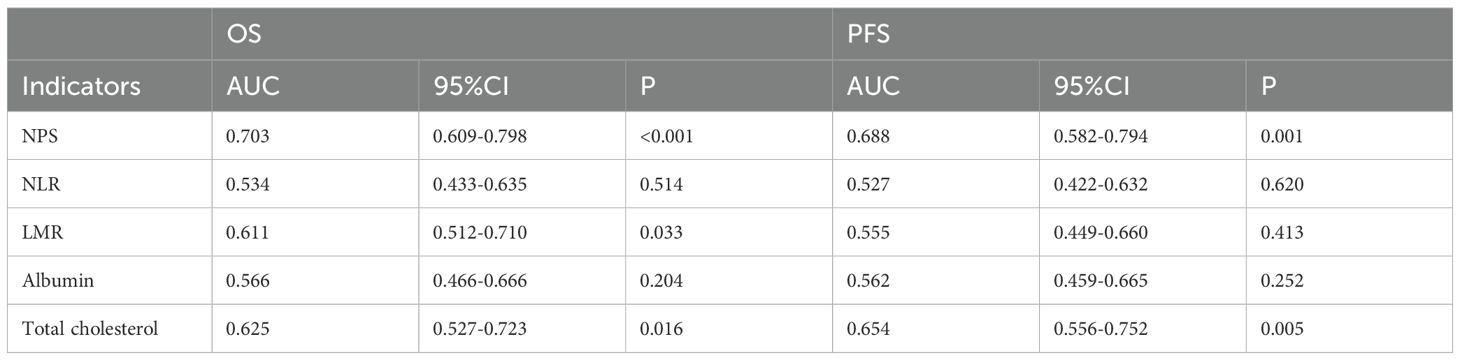
The predictive performance of NPS, NLR, LMR, albumin, and total cholesterol was evaluated by ROC curve analysis. Table 3 shows the area under the curve (AUC), 95% confidence interval (95%CI), and P value of NPS, NLR, LMR, albumin, and total cholesterol. The results showed that the NPS prognostic score had a higher predictive value for OS (AUC=0.703) and PFS (AUC=0.688). NLR (P=0.534, P=0.527), LMR (P=0.611, P=0.555), albumin (P=0.566, P=0.562), and total cholesterol (P=0.625, P=0.654) had lower predictive values for OS and PFS. The ROC curve can clearly show the predictive ability of the NPS prognostic score (Figure 2). The OS and PFS survival curves of NPS prognostic scores (Figure 3). For all enrolled patients, patients in NPS 0 group had better OS compared with patients in NPS 2 and NPS 1 groups (group 2 vs group 0, P < 0.001, group 1 vs group 0, P = 0.005); in terms of PFS, NPS 0 group had better PFS than NPS 2 and NPS 1 groups (group 2 vs group 0, P < 0.001, group 1 vs group 0, P = 0.001).
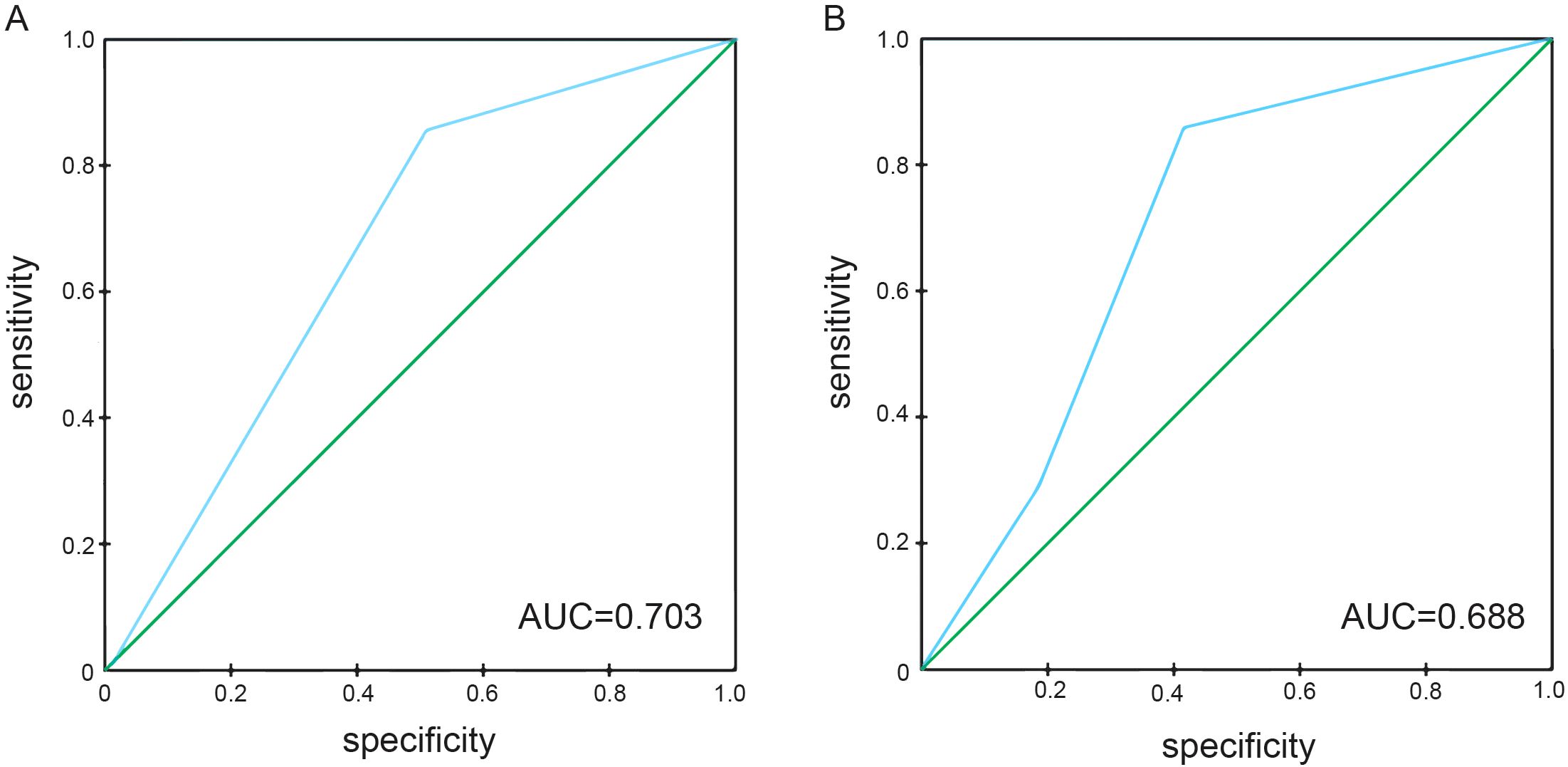
Figure 2. ROC curves of NPS for predicting survival prognosis in patients with locally advanced NSCLC. (A) The ROC curve of NPS score predicting OS in patients with locally advanced NSCLC, area under the curve AUC=0.703. (B) The ROC curve of NPS score predicted PFS in patients with locally advanced NSCLC, and the area under the curve AUC=0.688.
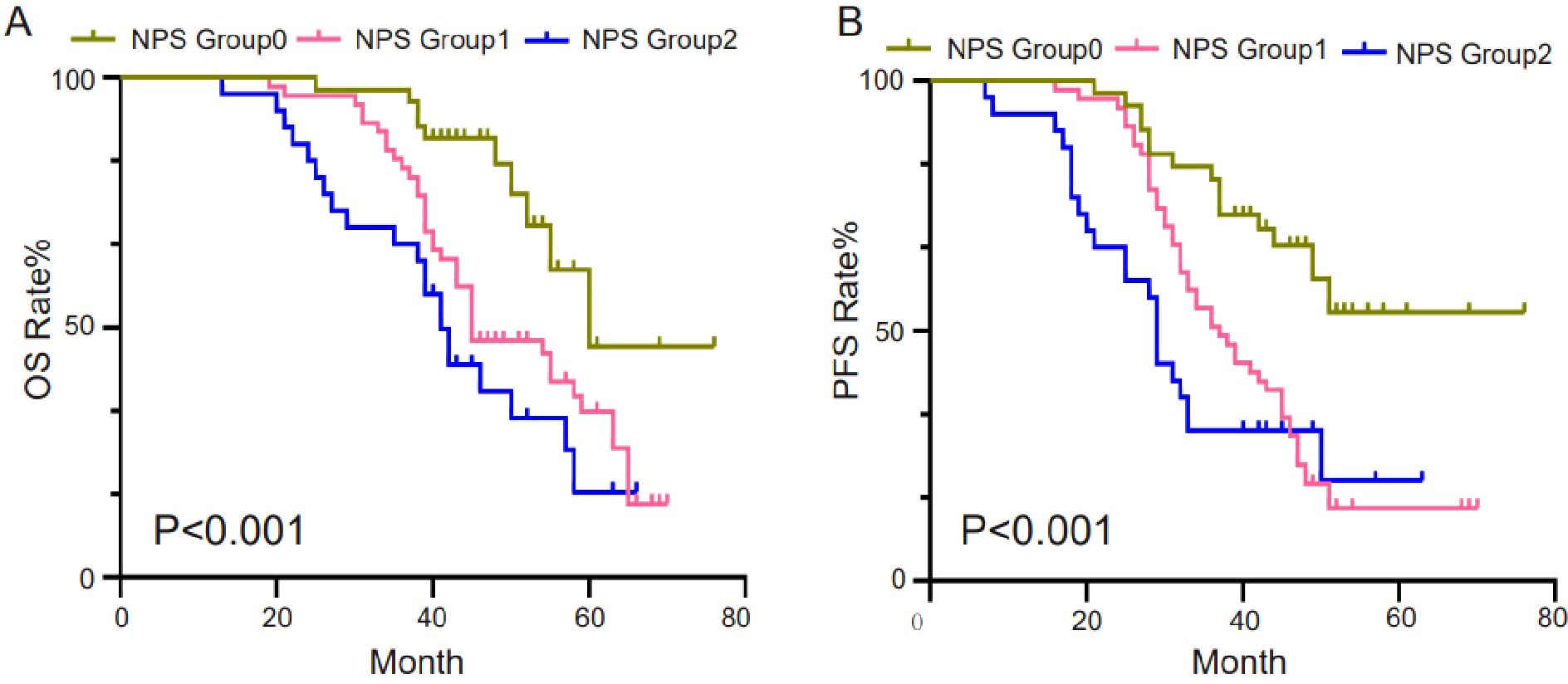
Figure 3. Survival curves of survival prognosis of patients involved in different groups. (A) For all enrolled patients, patients in the NPS 0 group had better OS compared to patients in the NPS 2, NPS 1 groups (2 groups vs 0 groups, P<0.001, 1 group vs 0 groups, P=0.005). (B) In terms of PFS, NPS 0 had better PFS than NPS 2 and NPS 1 (2 vs 0, P<0.001, 1 vs 0, P=0.001).
Single factor and multi-factor analysis
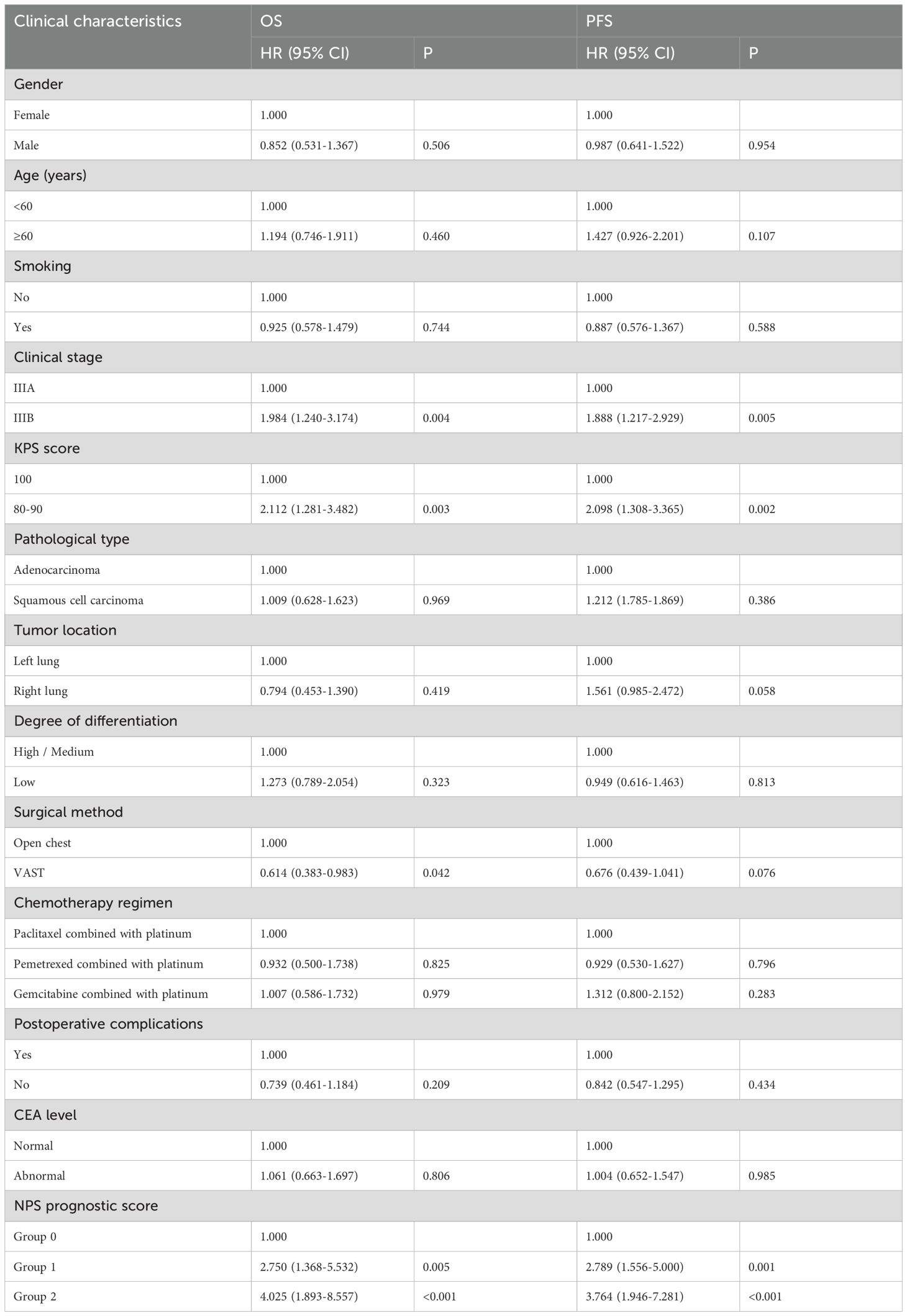
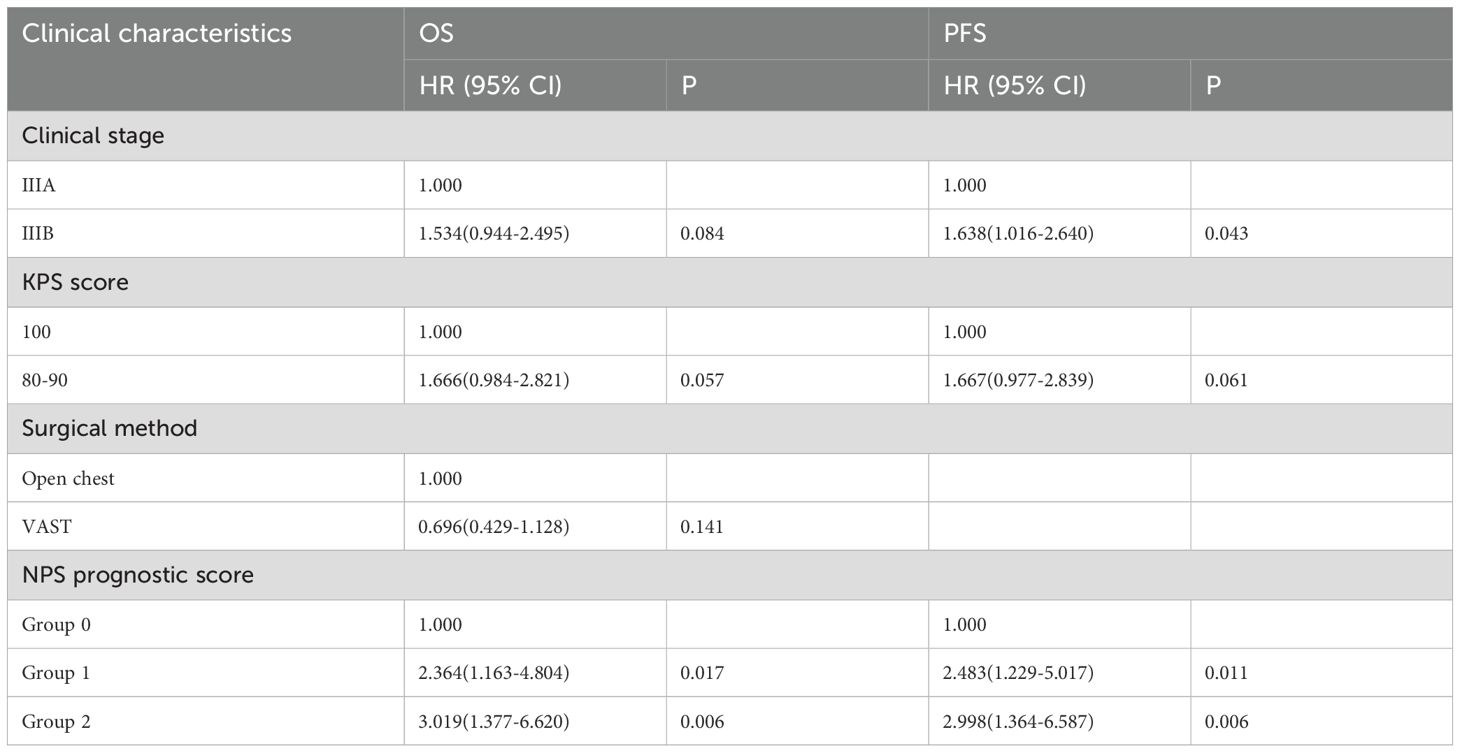
To explore the relationship between various clinicopathological factors and OS and PFS in patients with locally advanced NSCLC, univariate analysis was performed for each factor (Table 4). Univariate analysis showed clinical stage (P=0.004), KPS score (P=0.003), surgical method (P=0.042) and NPS prognostic score (group 2 vs group 0, P<0.001; group 1 vs group 0, P= There is a significant correlation between 0.005) and OS. However, gender (P=0.506), age (P=0.460), smoking status (P=0.744), pathological type (P=0.969), tumor location (P=0.419), and degree of differentiation (P=0.323) were not found and CEA level (P=0.806) were related to OS. Clinical stage (P=0.005), KPS score (P=0.002) and NPS prognostic score (group 2 vs group 0, P<0.001; group 1 vs group 0, P=0.001) were related to PFS. Indicators that were meaningful in the univariate analysis of OS (NPS prognostic score, clinical stage, KPS score, and surgical approach) were included in the multivariate analysis. Multivariate analysis results revealed that NPS prognostic score was an independent prognostic factor for OS (group 2 vs group 0, P=0.006; group 1 vs group 0, P=0.017); however, clinical stage (P=0.084), KPS score (P=0.057) and surgical method (P=0.141) were not statistically significant. Based on the results of PFS single-factor analysis, clinical stage, KPS score (P=0.057) and NPS prognostic score were included in multi-factor analysis, and the results showed that NPS prognostic score (group 2 vs group 0, P=0.006; group 1 vs 0 group, P=0.011) is an independent prognostic predictor of PFS in patients with locally advanced NSCLC (Table 5).
Discussion
Inflammation related factors play important roles in the occurrence and development of tumors (29). Systemic inflammation is an essential component of the tumor microenvironment (30–32). For instance, acute inflammatory response products promote tumor cell proliferation, metastasis and invasion through various pathways. Inflammatory mediators like Interleukin-6 (IL-6) and Tumor Necrosis Factor-alpha (TNF-alpha) activate STAT3 and NF-κB signaling pathways to promote cancer cell proliferation (33, 34). Besides, inflammatory signals can induce epithelial-to-mesenchymal transition (EMT) (35), angiogenesis (36) and extracellular matrix (ECM) remodeling (37) for cancer cell metastasis and invasion. Besides, inflammatory factors also promote tumor angiogenesis by upregulating the expression of vascular endothelial growth factor (38–42). In current clinical practice, researchers have confirmed that inflammation-related hematological biomarkers can be used as markers for predicting patient prognosis, including C-reactive protein (CRP), NLR, LMR, mean platelet volume to platelet ratio (MPV/PCT), etc (6–8). In addition to inflammatory markers such as NLR and LMR, other prognostic factors that represent or reflect the nutritional or immune status of patients have also been confirmed by various studies to be key factors in predicting the survival rate of other malignancies, such as osteosarcoma, including prognostic nutritional index (PNI), albumin, cholesterol, controlled nutritional status (CONUT) score, systemic immune inflammation index (SII), etc (16–20). Therefore, multi-dimensional prognostic evaluation systems may have better predictive power than a single prognostic factor. Recently, the NPS prognostic score, which is calculated from serum albumin, total cholesterol concentration, LMR, and NLR, has attracted attention. The NPS prognostic score is a comprehensive prognostic scoring system that includes the currently used inflammatory and nutritional prognostic markers. However, the significance of the NPS prognostic score in the prognostic value of locally advanced NSCLC patients who underwent surgical resection has not been extensively studied. During the preparation of the manuscript for this study, Zou et al. also reported the value of the NPS prognostic score in the survival prognosis of patients with locally advanced non-small cell lung cancer who underwent resection after neoadjuvant therapy (43).
In this research, we evaluated the correlation between the NPS prognostic score and the clinicopathological characteristics as well as long-term prognosis of patients with locally advanced NSCLC. In addition, the NPS prognostic score was compared with previously developed scoring systems and the classic TNM staging system to evaluate its performance. Our results are consistent with previous studies. Our study revealed that the NPS prognostic score combines inflammation, nutrition and immune parameters, and is better than a single indicator in predicting the survival of patients with locally advanced NSCLC who received surgery after neoadjuvant chemotherapy, comparing to other prognostic indicators related to immunity and nutrition.
Although our study has achieved certain results, this study still has the inevitable limitation of sample size. To more clearly predict the prognosis of lung cancer or even other types of cancer, a larger sample size is needed in the future. First, this study is a retrospective study with a small sample from a single center. Secondly, among the components of NPS, including NLR, LMR, albumin and total cholesterol, the cutoff values of the above indicators are determined by previous literature, and this article does not set its own cutoff values based on our study. Secondly, all patients with locally advanced NSCLC included in this study received neoadjuvant chemotherapy and surgical treatment, but some patients received chemoradiotherapy and some patients received chemotherapy alone after surgery. There are differences in treatment methods, which may affect the prediction of survival by NPS to a certain extent. The patients selected for this study had strict inclusion criteria, but laboratory indicators such as serum albumin, total cholesterol, neutrophils, lymphocytes, and monocytes are easily affected by various factors and cannot be accurately controlled.
In summary, the NPS prognostic score is a simple and reliable preoperative prognostic scoring system that can independently predict the OS and PFS of patients with locally advanced NSCLC who undergo surgical treatment. The NPS prognostic score reflects the importance of biomarkers related to inflammation and nutrition. By reflecting the inflammatory and nutritional status of tumor patients, it can accurately stratify patients into risk groups, play a positive role in adjusting treatment strategies, and enable patients to obtain survival benefits.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Affiliated Hospital of Shandong Second Medical University, school of Clinical Medicine, Shandong Second Medical University, Weifang 261053, Shandong, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YZ: Writing – original draft, Formal analysis, Investigation, Software. CT: Writing – original draft, Conceptualization. FL: Data curation, Writing – review & editing. YW: Investigation, Writing – original draft. LQ: Supervision, Writing – review & editing. JL: Writing – review & editing. MY: Data curation, Writing – original draft. SL: Data curation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. In this work, Yanfei Zhang is supported by Shandong Provincial Health Commission(M-2022054). Jingjing Li is supported by the National Natural Science Foundation of China (Grant No. 82104289), Shandong Provincial Health Commission(M-2022053), Science and Technology Innovation Plan from Weifang Medical University (041004), Yuandu Scholar Grant of Weifang City to LJJ, Weifang Science and Technology Bureau Plan Project (2021YX081), Science and technology project jointly established by the Science and Technology Department of the State Administration of Traditional Chinese Medicine (GZY-KJS-SD-2023-079), Shandong Provincial Medical Association Young Talent Promotion Project (2023_GJ_0039). Science and Technology Innovation Plan from Weifang Medical University (041011). The formation mechanism and clinical significance of PD-L1-containing exosomes in the early metastasis of breast cancer (2023FYM005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Wagle NS, and Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. Islami F, Marlow EC, Zhao J, Wiese D, Asare S, Bandi P, et al. Person-years of life lost and lost earnings from cigarette smoking-attributable cancer deaths, United States, 2019. Int J Cancer. (2022) 151:2095–106. doi: 10.1002/ijc.v151.12
3. Yabroff KR, Mariotto A, Tangka F, Zhao J, Islami F, Sung H, et al. Annual report to the nation on the status of cancer, part 2: patient economic burden associated with cancer care. J Natl Cancer Inst. (2021) 113:1670–82. doi: 10.1093/jnci/djab192
4. Petrella F, Rizzo S, Attili I, Passaro A, Zilli T, Martucci F, et al. Stage III non-small-cell lung cancer: an overview of treatment options. Curr Oncol. (2023) 30:3160–75. doi: 10.3390/curroncol30030239
5. Miyamoto Y, Hiyoshi Y, Daitoku N, Okadome K, Sakamoto Y, Yamashita K, et al. Naples prognostic score is a useful prognostic marker in patients with metastatic colorectal cancer. Diseases of the Colon & Rectum. (2019) 62:1485–93. doi: 10.1097/DCR.0000000000001484
6. Gregory AD and McGarry Houghton A. Tumor-associated neutrophils: new targets for cancer therapy. Cancer research. (2011) 71:2411–6. doi: 10.1158/0008-5472.CAN-10-2583
7. Uryvaev A, Passhak M, Hershkovits D, Sabo E, and Bar-Sela G. The role of tumor-infiltrating lymphocytes (TILs) as a predictive biomarker of response to anti-PD1 therapy in patients with metastatic non-small cell lung cancer or metastatic melanoma. Medical oncology. (2018) 35:1–9. doi: 10.1007/s12032-018-1080-0
8. Tan KW, Chong SZ, Wong FH, Evrard M, Tan SML, Keeble J, et al. Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-A bioavailability and secreting VEGF-D. The Journal of the American Society of Hematology. (2013) 122:3666–77. doi: 10.1182/blood-2012-11-466532
9. Cui LJ, Yu FH, Cheng ZX, Su F, Chen YY, and Tan HY. Prognostic value of inflammation-related biomarkers in patients with gastroenteropancreatic neuroendocrine neoplasms: A systematic review and meta-analysis. J Neuroendocrinol. (2024) 36:e13381. doi: 10.1111/jne.13381
10. Padhy AK, Gopinath PG, Basu AK, and Reddy KG. Scintigraphic findings in a case of hydatid cyst of the liver communicating with the biliary system. Clin Nucl Med. (1986) 11:281. doi: 10.1097/00003072-198604000-00017
11. Deng Q, Long Q, Liu Y, Yang Z, Du Y, and Chen X. Prognostic value of preoperative peripheral blood mean platelet volume/platelet count ratio (MPV/PC) in patients with resectable cervical cancer. BMC Cancer. (2021) 21:1282. doi: 10.1186/s12885-021-09016-8
12. Shen X, Xiang M, Tang J, Xiong G, Zhang K, Xia T, et al. Evaluation of peripheral blood inflammation indexes as prognostic markers for colorectal cancer metastasis. Sci Rep. (2024) 14:20489. doi: 10.1038/s41598-024-68150-y
13. Ruan GT, Xie HL, Yuan KT, Lin SQ, Zhang HY, Liu CA, et al. Prognostic value of systemic inflammation and for patients with colorectal cancer cachexia. J Cachexia Sarcopenia Muscle. (2023) 14:2813–23. doi: 10.1002/jcsm.13358
14. Nost TH, Alcala K, Urbarova I, Byrne KS, Guida F, Sandanger TM, et al. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur J Epidemiol. (2021) 36:841–8. doi: 10.1007/s10654-021-00752-6
15. Mosca M, Nigro MC, Pagani R, De Giglio A, and Di Federico A. Neutrophil-to-lymphocyte ratio (NLR) in NSCLC, gastrointestinal, and other solid tumors: immunotherapy and beyond. Biomolecules. (2023) 13. doi: 10.3390/biom13121803
16. Jansen CS, Prokhnevska N, Master VA, Sanda MG, Carlisle JW, Bilen MA, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. (2019) 576:465–70. doi: 10.1038/s41586-019-1836-5
17. Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer research. (2009) 69:5383–91. doi: 10.1158/0008-5472.CAN-08-3845
18. Wang S, Sun J, Chen K, Ma P, Lei Q, Xing S, et al. Perspectives of tumor-infiltrating lymphocyte treatment in solid tumors. BMC medicine. (2021) 19:140. doi: 10.1186/s12916-021-02006-4
19. Boenisch O, Lopez M, Elyaman W, Magee C, Ahmad U, and Najafian N. Ex vivo expansion of human Tregs by rabbit ATG is dependent on intact STAT3-signaling in CD4+ T cells and requires the presence of monocytes. American Journal of Transplantation. (2012) 12:856–66. doi: 10.1111/j.1600-6143.2011.03978.x
20. Dou A and Fang J. Heterogeneous myeloid cells in tumors. Cancers. (2021) 13:3772. doi: 10.3390/cancers13153772
21. Zhu N, Lin S, Yu H, Liu F, Huang W, and Cao C. Naples prognostic score as a novel prognostic prediction indicator in adult asthma patients: A population-based study. World Allergy Organ J. (2023) 16:100825. doi: 10.1016/j.waojou.2023.100825
22. Galizia G, Lieto E, Auricchio A, Cardella F, Mabilia A, Podzemny V, et al. Naples prognostic score, based on nutritional and inflammatory status, is an independent predictor of long-term outcome in patients undergoing surgery for colorectal cancer. Dis Colon Rectum. (2017) 60:1273–84. doi: 10.1097/DCR.0000000000000961
23. Lieto E, Auricchio A, Tirino G, Pompella L, Panarese I, Del Sorbo G, et al. Naples prognostic score predicts tumor regression grade in resectable gastric cancer treated with preoperative chemotherapy. Cancers. (2021) 13:4676. doi: 10.3390/cancers13184676
24. Li J, Yang W, Yuan Y, Zuo M, Li T, Wang Z, et al. Preoperative Naples prognostic score is a reliable prognostic indicator for newly diagnosed glioblastoma patients. Frontiers in Oncology. (2022) 12:775430. doi: 10.3389/fonc.2022.775430
25. Chen F, Xie C, Ren K, and Xu X. Prognostic value of the naples prognostic score in patients with gastrointestinal cancers: A meta-analysis. Nutr Cancer. (2023) 75:1520–30. doi: 10.1080/01635581.2023.2212426
26. Yang J, Lv L, Zhao F, Mei X, Zhou H, and Yu F. The value of the preoperative Naples prognostic score in predicting prognosis in gallbladder cancer surgery patients. World J Surg Oncol. (2023) 21:303. doi: 10.1186/s12957-023-03198-0
27. Kilic O, Suygun H, Mustu M, Karadeniz FO, Ozer SF, Senol H, et al. Is the Naples prognostic score useful for predicting heart failure mortality. Kardiologiia. (2023) 63:61–5. doi: 10.18087/cardio.2023.3.n2328
28. Arugaslan E, Kalayci S, and Tufekcioglu O. Naples prognostic score and clinical outcomes in pulmonary arterial hypertension patients. Sisli Etfal Hastan Tip Bul. (2023) 57:374–9. doi: 10.14744/SEMB.2023.82783
29. Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. (2021) 6:263. doi: 10.1038/s41392-021-00658-5
30. Tokunaga R, Sakamoto Y, Nakagawa S, OhuchiM , Izumi D, Kosumi K, et al. CONUT: a novel independent predictive score for colorectal cancer patients undergoing potentially curative resection. International journal of colorectal disease (2017) 32:99–106. doi: 10.1007/s00384-016-2668-5
31. Lai C-C, You J-F, Yeh C-Y, Chen J-S, Tang R, Wang J-Y, et al. Low preoperative serum albumin in colon cancer: a risk factor for poor outcome. International journal of colorectal disease. (2011) 26:473–81. doi: 10.1007/s00384-010-1113-4
32. Li Z, Xu Z, Huang Y, Zhao R, Cui Y, Zhou , et al. Prognostic values of preoperative platelet-to-lymphocyte ratio, albumin and hemoglobin in patients with non-metastatic colon cancer. (2019), 3265–74. doi: 10.2147/CMAR.S191432
33. Zhou Q, Qi F, Zhou C, Ji J, Jiang J, Wang C, et al. VPS35 promotes gastric cancer progression through integrin/FAK/SRC signalling-mediated IL-6/STAT3 pathway activation in a YAP-dependent manner. Oncogene. (2024) 43:106–22. doi: 10.1038/s41388-023-02885-2
34. Cruceriu D, Baldasici O, Balacescu O, and Berindan-Neagoe I. The dual role of tumor necrosis factor-alpha (TNF-alpha) in breast cancer: molecular insights and therapeutic approaches. Cell Oncol (Dordr). (2020) 43:1–18. doi: 10.1007/s13402-019-00489-1
35. Masuda T, Fukuda A, Yamakawa G, Omatsu M, Namikawa M, Sono M, et al. Pancreatic RECK inactivation promotes cancer formation, epithelial-mesenchymal transition, and metastasis. J Clin Invest. (2023) 133. doi: 10.1172/JCI161847
36. Zhang M, Zhou K, Wang Z, Liu T, Stevens LE, Lynce F, et al. A subpopulation of luminal progenitors secretes pleiotrophin to promote angiogenesis and metastasis in inflammatory breast cancer. Cancer Res. (2024) 84:1781–98. doi: 10.1158/0008-5472.CAN-23-2640
37. He L, Kang Q, Chan KI, Zhang Y, Zhong Z, and Tan W. The immunomodulatory role of matrix metalloproteinases in colitis-associated cancer. Front Immunol. (2022) 13:1093990. doi: 10.3389/fimmu.2022.1093990
38. Morhij R, Mahendra A, Jane M, and McMillan DC. The modified Glasgow prognostic score in patients undergoing surgery for bone and soft tissue sarcoma. Reconstructive & Aesthetic Surgery. (2017) 70:618–24. doi: 10.1016/j.bjps.2017.01.016
39. Hardt J, Pilz L, Magdeburg J, Kienle P, Post S, and Magdeburg R. Preoperative hypoalbuminemia is an independent risk factor for increased high-grade morbidity after elective rectal cancer resection. International journal of colorectal disease. (2017) 32:1439–46. doi: 10.1007/s00384-017-2884-7
40. Yvan-Charvet L, Wang N, and Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arteriosclerosis, thrombosis, and vascular biology. (2010) 30:139–43. doi: 10.1161/ATVBAHA.108.179283
41. Bonacina F, Pirillo A, Catapano AL, and Norata GD. Cholesterol membrane content has a ubiquitous evolutionary function in immune cell activation: the role of HDL. Current opinion in lipidology. (2019) 30:462–9. doi: 10.1097/MOL.0000000000000642
42. Kopecka J, Godel M, and Riganti C. Cholesterol metabolism: At the cross road between cancer cells and immune environment. Biochemistry & Cell Biology (2020) 129:105876. doi: 10.1016/j.biocel.2020.105876
Keywords: Naples prognostic score, NSCLC, neoadjuvant chemotherapy, NPS, LMR
Citation: Zhang Y, Tang C, Yang M, Li S, Li F, Wang Y, Qi L and Li J (2025) The predictive value of Naples prognostic score for patients with locally advanced non-small cell lung cancer undergoing surgery after neoadjuvant chemotherapy. Front. Immunol. 16:1578896. doi: 10.3389/fimmu.2025.1578896
Received: 18 February 2025; Accepted: 30 April 2025;
Published: 22 May 2025.
Edited by:
Vinay Kumar, The Pennsylvania State University, United StatesReviewed by:
Sabyasachi Mohanty, University of Nebraska-Lincoln, United StatesManish Shukla, Defence Institute of Physiology and Allied Sciences (DRDO), India
Copyright © 2025 Zhang, Tang, Yang, Li, Li, Wang, Qi and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Qi, cWlsaTExMDRAaG90bWFpbC5jb20=; Jingjing Li, amluZ2ppbmdsaS5tZC5waGRAZ21haWwuY29t
 Yanfei Zhang1,2
Yanfei Zhang1,2 Jingjing Li
Jingjing Li