- 1Clinical Research Center, The First People’s Hospital of Xiaoshan District, Xiaoshan Affiliated Hospital of Wenzhou Medical University, Hangzhou, China
- 2The Office of Drug Clinical Trial Institution, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
Introduction: The global increase in the elderly population has heightened the need to address nutritional risks in this vulnerable group. However, the relationship between overall dietary antioxidant intake and nutritional risk in the elderly remains unclear. This study aimed to investigate this association using the composite dietary antioxidant index (CDAI) and the geriatric nutritional risk index (GNRI).
Methods: We analyzed data from the National Health and Nutrition Examination Survey (2010–2018), focusing on 4,208 participants aged ≥65 years. CDAI was calculated based on the intake of vitamins A, C, E, selenium, zinc, and carotenoids, while GNRI was derived from serum albumin and body weight. Multivariate regression models were employed to assess associations between CDAI, individual dietary antioxidants, and GNRI. Smooth curve fitting and two-piecewise linear regression were further performed to identify the non-linear relationships and determine the corresponding inflection points.
Results: A statistically significant positive correlation was observed between the CDAI and GNRI, indicating that increased dietary antioxidant intake is linked to reduced nutritional risk. Vitamin C, selenium, zinc, and carotenoids were strongly associated with higher GNRI scores, with vitamin C and zinc showing the most robust effects. Subgroup analyses further revealed that men, diabetic individuals, and those without cancer exhibited greater improvements in nutritional risk with higher CDAI levels. Threshold effect analysis identified an optimal range for CDAI, beyond which the nutritional benefits diminished.
Conclusions: Our findings highlight the critical role of dietary antioxidants, especially vitamin C and zinc, in mitigating nutritional risk among the elderly. These results support the importance of balanced dietary intake of antioxidants to optimize nutritional health in aging populations.
Introduction
The global demographic shift towards an aging population presents significant health challenges, particularly in the realm of nutrition (1). Elderly individuals are at increased risk of nutritional deficiencies caused by reduced appetite, impaired nutrient absorption, and chronic diseases, which in turn exacerbate age-related conditions (2, 3). Consequently, addressing nutritional risks in the elderly has become a critical public health priority.
Recent studies have underscored the heterogeneous nature of the elderly population, classifying individuals into subgroups such as healthy, pre-frail, and frail, each exhibiting distinct nutritional requirements and metabolic profiles (4, 5). Among these subgroups, frail elderly individuals demonstrate heightened susceptibility to oxidative stress and chronic inflammation, factors that exacerbate nutritional decline (4, 5). Oxidative stress arises from an imbalance between reactive oxygen species and endogenous antioxidant defenses, a process that not only accelerates aging but also contributes to the pathogenesis of age-related diseases (6, 7). Dietary antioxidants have been demonstrated to mitigate oxidative stress, thereby potentially reducing the risk of chronic diseases in elderly populations (8). Given their protective effects, dietary antioxidants may serve as a vital component in strategies aimed at improving nutritional status and overall health in aging populations.
The geriatric nutritional risk index (GNRI) is a widely used tool for assessing nutritional risk in the elderly, incorporating parameters such as serum albumin levels and body weight (9). While GNRI has proven useful in predicting morbidity and mortality in older adults (10, 11), it has limitations, including its reliance on static measures that may not fully capture the dynamic nature of nutritional status. There is a growing need for more robust indicators that can provide a holistic assessment of nutritional risk, particularly in relation to dietary factors.
The composite dietary antioxidant index (CDAI) is a novel measure that aggregates the intake of multiple dietary antioxidants, providing a comprehensive assessment of antioxidant exposure (12). By considering the combined effects of various antioxidants, CDAI offers a more nuanced understanding of their role in mitigating nutritional risk. This study leverages CDAI to explore the relationship between dietary antioxidants and nutritional status in the elderly, addressing a critical gap in the existing literature.
Methods
Study design and population
This research analyzes data obtained from the National Health and Nutrition Examination Survey (NHANES), which is a nationally representative study administered by the National Center for Health Statistics (NCHS) to evaluate the health and nutritional status of the U.S. population. Our analysis specifically examines NHANES data collected between 2010 and 2018, providing a comprehensive and up-to-date dataset. All protocols from NHANES were approved by the NCHS Research Ethics Review Board, and informed consent was secured in writing from each participant.
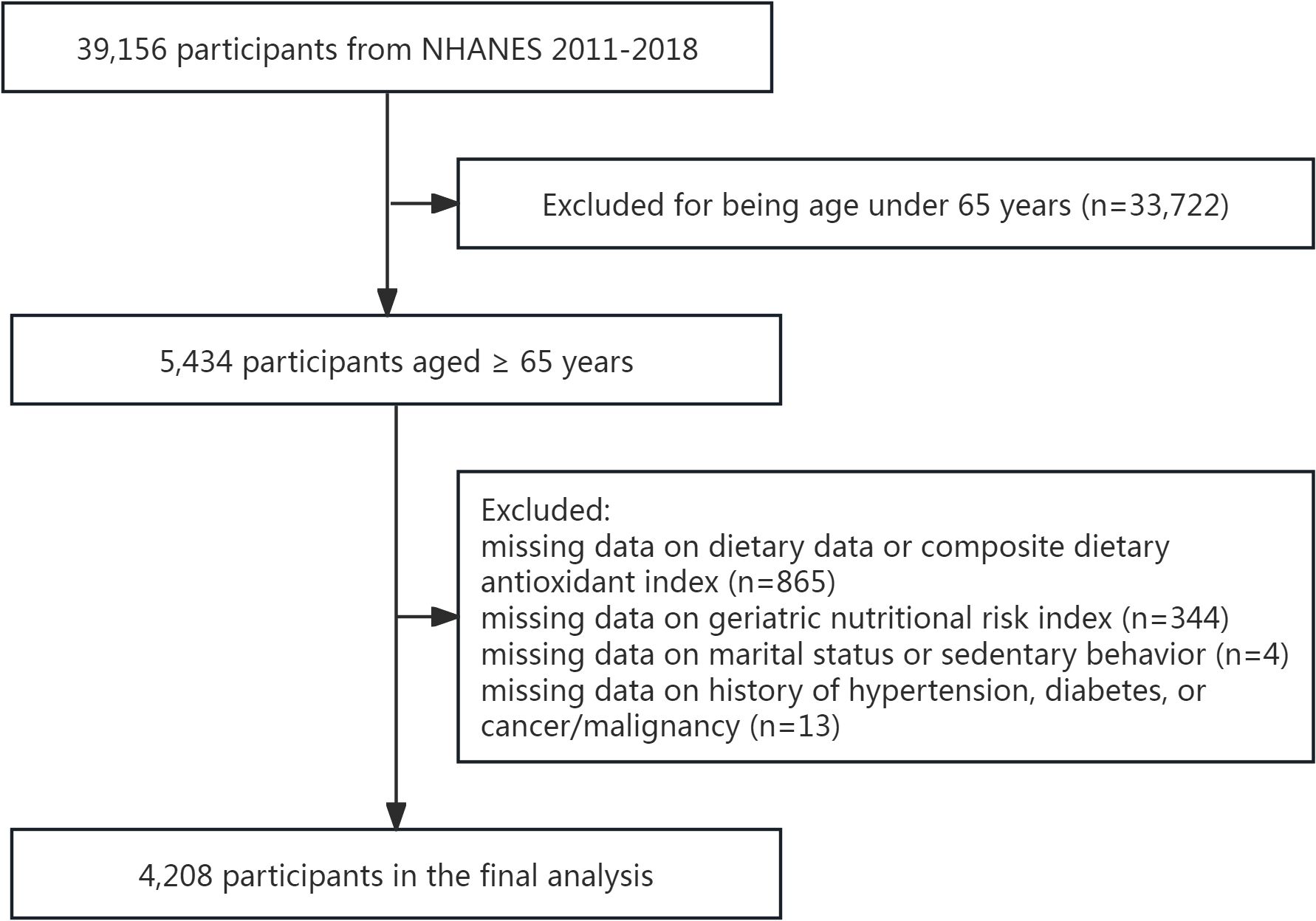
Initially, 5,434 participants aged ≥65 years were included. After applying exclusion criteria—removing individuals with incomplete data for calculating CDAI (n=865) or GNRI (n=344), missing marital status (n=3), undefined sedentary behavior (n=1), or undocumented histories of hypertension, diabetes, or cancer (n=13)—the final analysis included 4,208 elderly subjects (Figure 1).
CDAI measurement
The nutritional assessment in NHANES utilized a 24-hour dietary recall method, conducted at mobile examination centers over two non-consecutive days, with the first interview followed by a telephone interview conducted 3 to 10 days later. To reduce bias and improve accuracy, the nutrient intake for each individual was calculated as the average of the two days, compensating for any missing data from one day by using values from the other.
The CDAI was computed using a modified methodology adapted from Wright et al. (13), which incorporates six dietary antioxidants: vitamins A, C, and E, selenium, zinc, and carotenoids. The carotenoid component comprised α-carotene, lycopene, β-carotene, β-cryptoxanthin, and lutein with zeaxanthin. In alignment with prior research (14, 15), the CDAI composite score was calculated by summing the standardized values of individual micronutrients, obtained through mean-centering and scaling by their respective standard deviations. The calculation adheres to the formula: CDAI=∑ (each intake - mean value)/SD (n = 6).
GNRI measurement
The GNRI was computed using the formula: GNRI = [1.489 × serum albumin (g/L)] + [41.7 × (body weight (kg)/ideal body weight (kg))], where ideal body weight is defined as 22 times the square of the height in meters. If the ratio of actual weight to ideal weight surpasses 1, it is normalized to 1.
Covariates
Selected covariates were based on previous literature and clinical experience, including: (1). demographic data: age, sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American, other race/ethnicity), education level (less than high school, high school, more than high school), marital status (married/living with partner, widowed/divorced/separated, never married), and poverty income ratio (categorized as low [<1.3], medium [1.3–3.5], or high [>3.5]); (2). examination data: body mass index; (3). questionnaire data: sedentary behavior (defined as the absence of moderate or vigorous physical activity) and history of hypertension, diabetes, and cancer//malignancy. Demographic data were collected through in-home interviews, examination data were measured at mobile examination centers, and questionnaire data were obtained via self-reported conditions.
Statistical analyses
The baseline characteristics of the subjects were categorized according to the quartiles of the CDAI. Continuous variables are reported as mean ± standard deviation, whereas categorical variables are expressed as percentages. To assess differences between groups, we utilized χ² tests for categorical data, one-way ANOVA for normally distributed continuous data, and Kruskal-Wallis H tests for data with skewed distributions.
To examine the associations between CDAI, individual dietary antioxidants, and GNRI, we implemented a tiered multivariate linear regression analysis following the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines (16). Three sequential models were constructed: Model 1 (crude): unadjusted analysis to assess raw associations; Model 2 (partially adjusted): controlled for core demographic confounders (age, sex, race/ethnicity); and Model 3 (fully adjusted): further adjusted for socioeconomic, lifestyle, and clinical covariates. Subgroup analyses assessed effect modification by stratifying key covariates, with multiplicative interaction terms tested for statistical significance. To address non-linearity, we applied: generalized additive models with smoothing splines, which flexibly capture non-parametric trends without presupposing functional forms; and two-piecewise linear regression to objectively identify inflection points where the relationship shifted. This method was favored over polynomial regression due to its clinical utility in defining actionable thresholds.
Statistical analyses were carried out using R software (version 3.4.3) and EmpowerStats (X&Y Solutions, Inc., Boston, MA). A two-sided P value of less than 0.05 was regarded as statistically significant.
Results
Study population characteristics
The characteristics of the study population categorized by quartiles of CDAI are detailed in Table 1. A total of 4,208 participants were divided into four quartiles, revealing significant disparities in demographic and health-related factors among the groups. The percentage of men increased from 36.6% in Q1 to 61.9% in Q4, while the proportion of women decreased correspondingly. Individuals with education beyond high school increased from 36.3% in Q1 to 65.1% in Q4. The frequency of sedentary behavior decreased from 70.5% in Q1 to 50.8% in Q4. The prevalence of hypertension, diabetes, and cancer showed an inverse relationship with CDAI quartiles. Additionally, dietary assessments indicated a significant rise in both energy and antioxidants intake, while GNRI demonstrated a slight increase across the quartiles.
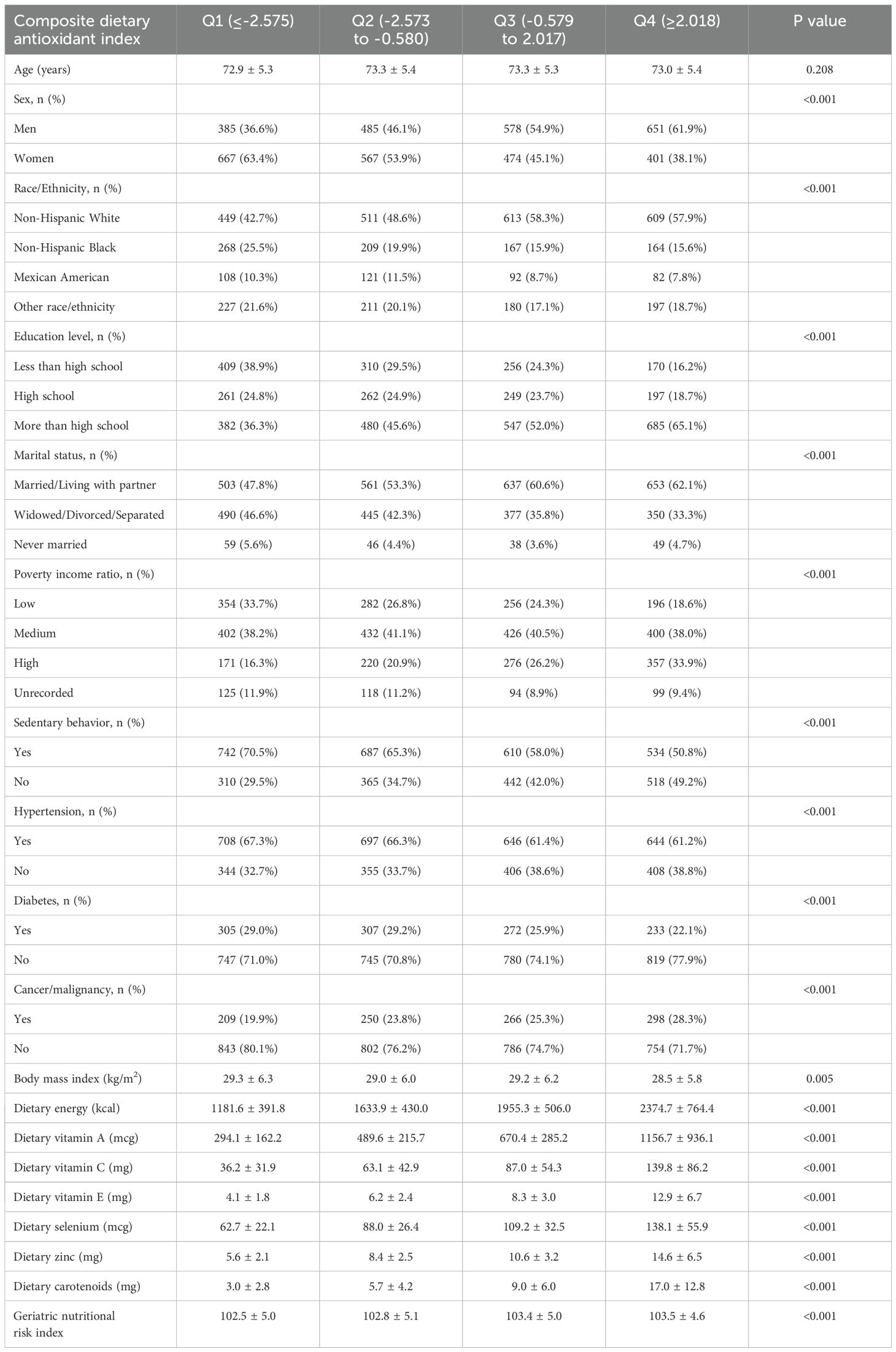
Table 1. Characteristics of study population based on composite dietary antioxidant index quartiles.
Associations between antioxidants and GNRI
Table 2 presents the associations between the CDAI, individual dietary antioxidants, and the GNRI. The CDAI exhibited a significant positive correlation with the GNRI across all models (Model 3: β=0.121, 95% CI: 0.072, 0.171). Dietary intake of vitamin C, selenium, zinc, and carotenoids was positively associated with the GNRI, particularly for vitamin C (Model 3: β=0.005, 95% CI: 0.003, 0.007) and zinc (Model 3: β=0.076, 95% CI: 0.039, 0.113). These relationships, along with potential non-linear trends, are illustrated in Figures 2, 3.

Table 2. Association between composite dietary antioxidant index, six dietary antioxidants and geriatric nutritional risk index.
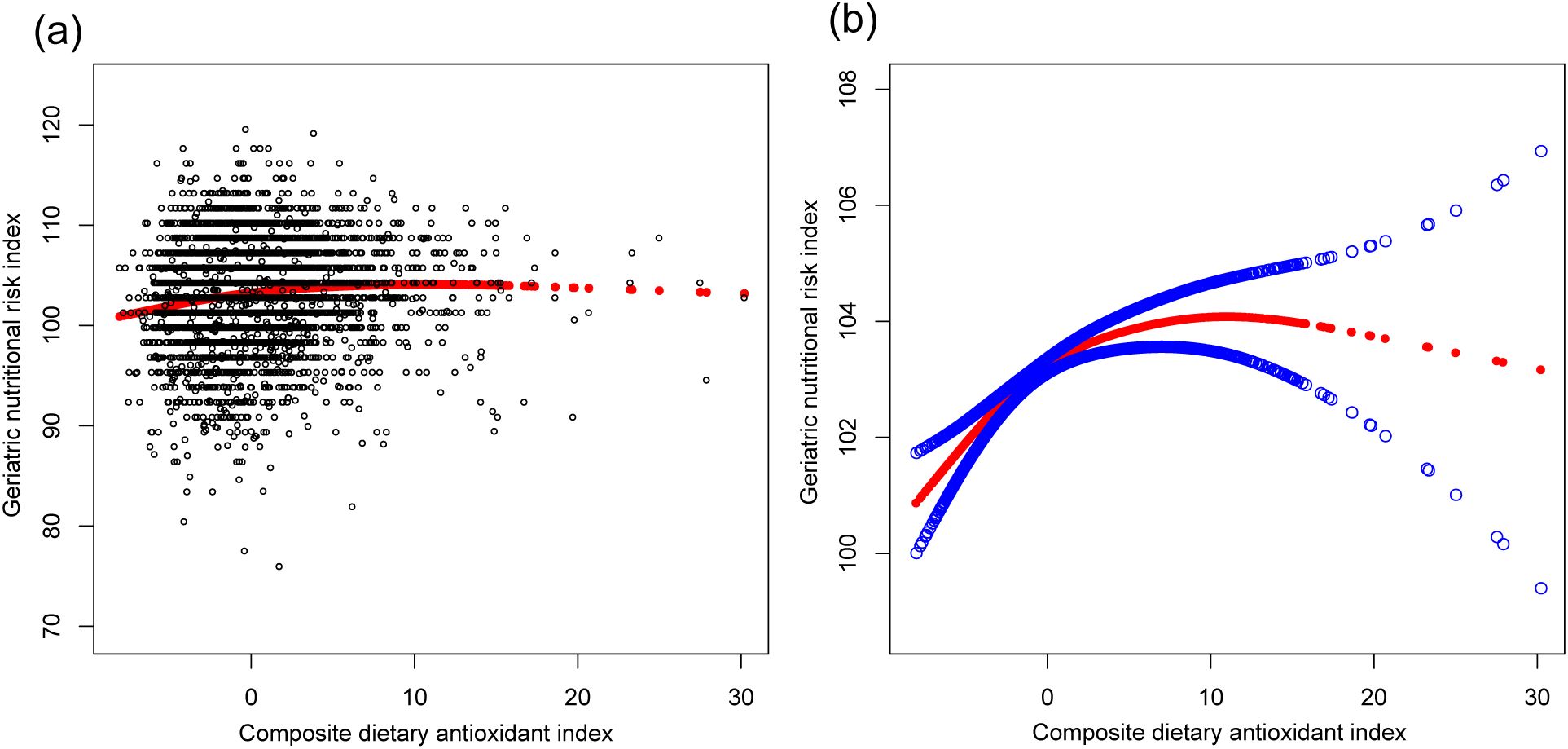
Figure 2. The association between composite dietary antioxidant index and geriatric nutritional risk index. (a) Each black point represents a sample. (b) Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. Age, sex, race, education level, marital status, poverty income ratio, sedentary behavior, body mass index, dietary energy, history of hypertension, diabetes, and cancer were adjusted.
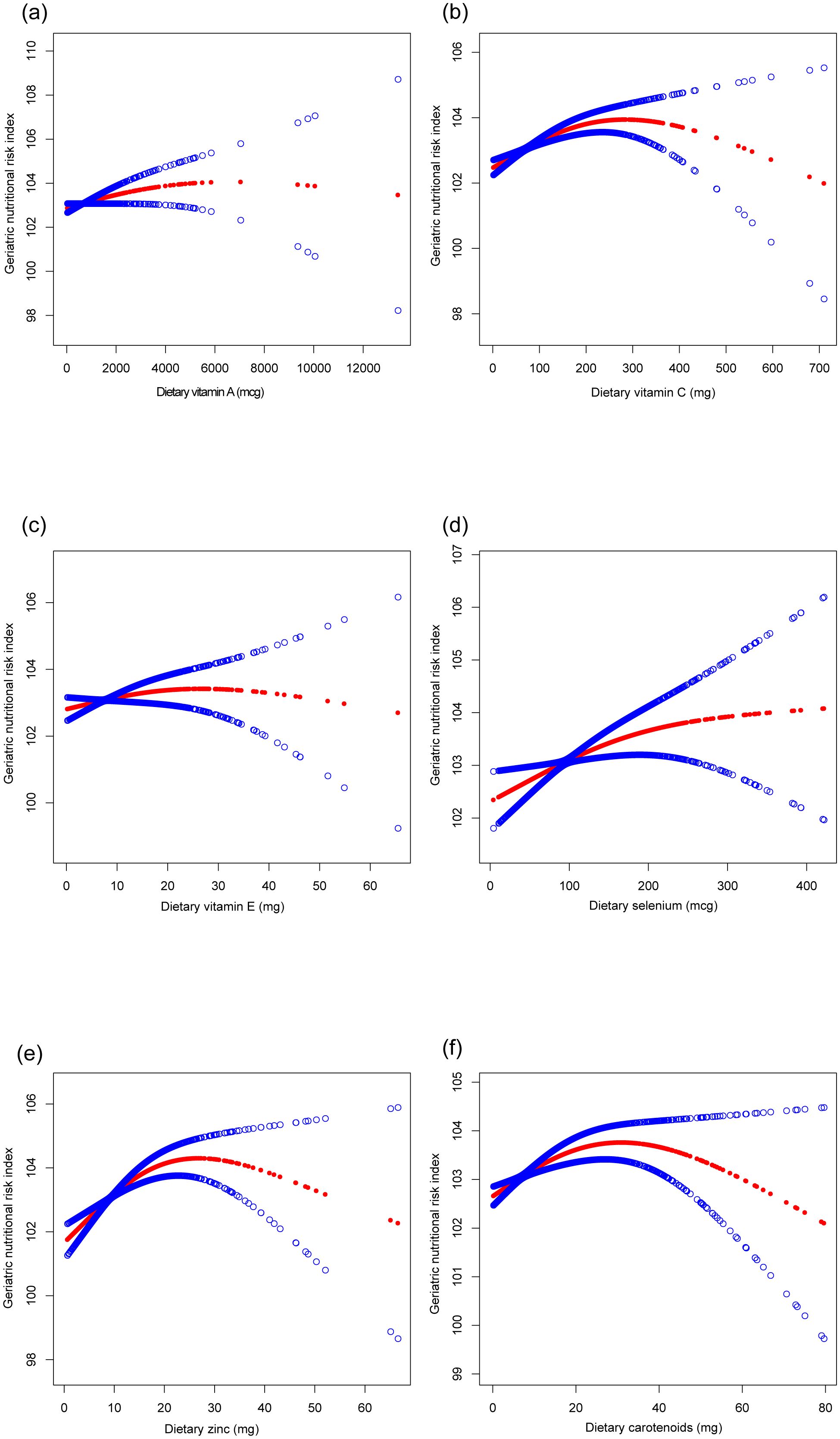
Figure 3. The association between six dietary antioxidants intake and geriatric nutritional risk index. (a) Dietary vitamin A; (b) Dietary vitamin C; (c) Dietary vitamin E; (d) Dietary selenium; (e) Dietary zinc; (f) Dietary carotenoids. Age, sex, race, education level, marital status, poverty income ratio, sedentary behavior, body mass index, dietary energy, history of hypertension, diabetes, and cancer were adjusted.
Subgroup analysis
Figure 4 illustrates the subgroup analysis concerning the CDAI and the GNRI. The β coefficient for men was higher (0.137, 95% CI: 0.070, 0.204) compared to women (0.104, 95% CI: 0.029, 0.178). Participants with diabetes had a β coefficient of 0.152 (95% CI: 0.044, 0.261), greater than that of non-diabetic individuals (β=0.106, 95% CI: 0.051, 0.161). Participants without cancer presented a significantly elevated β coefficient (0.152, 95% CI: 0.096, 0.209) compared to those with cancer (β=0.040, 95% CI: -0.063, 0.143). Potential non-linear relationships stratified by sex, history of hypertension, diabetes, and cancer are further confirmed in Figure 5.
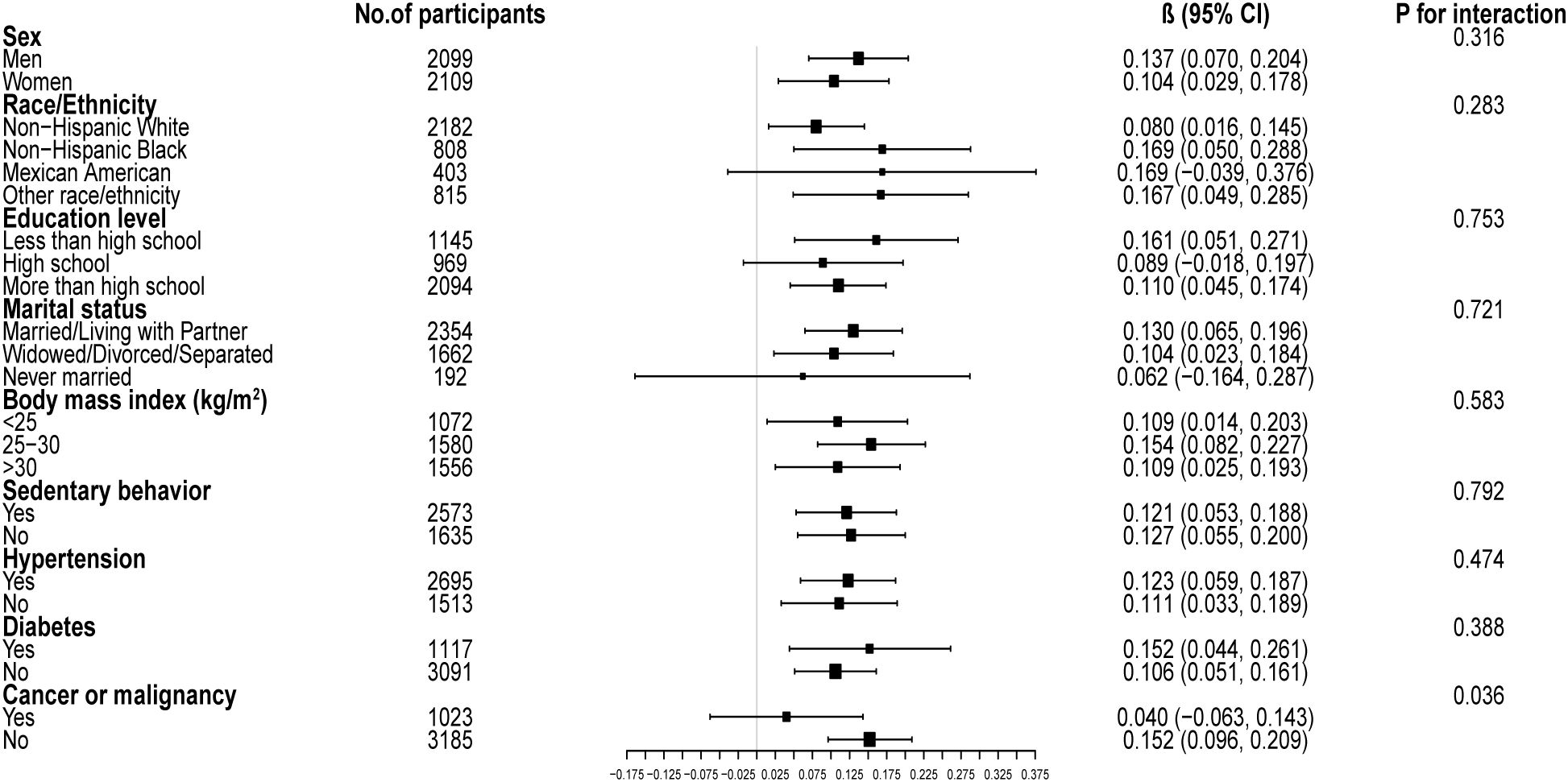
Figure 4. Subgroup analysis of the associations between composite dietary antioxidant index and geriatric nutritional risk index. Age, sex, race, education level, marital status, poverty income ratio, sedentary behavior, body mass index, dietary energy, history of hypertension, diabetes, and cancer were adjusted. In the subgroup analysis, the model is not adjusted for the stratification variable itself.
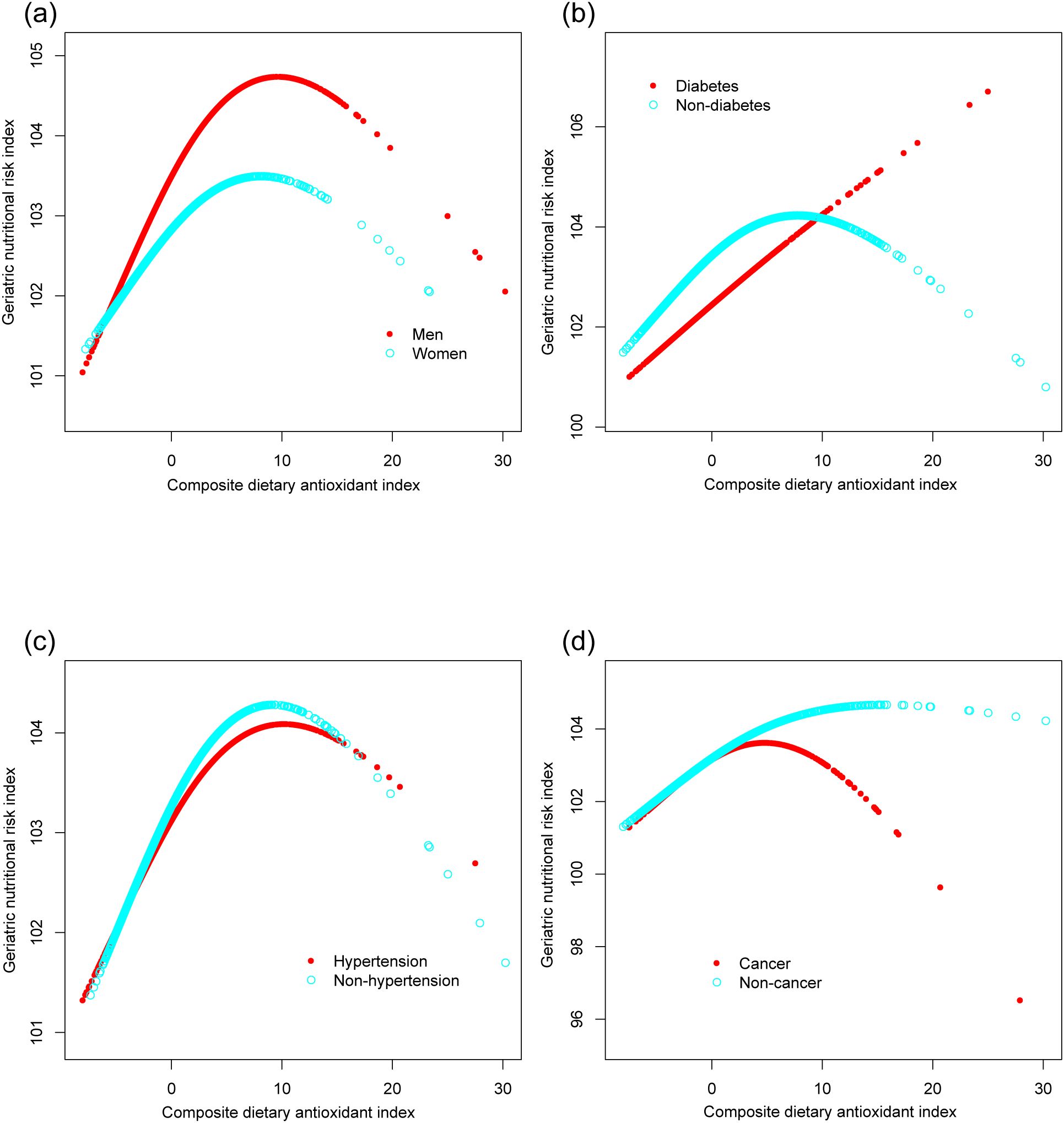
Figure 5. The associations between composite dietary antioxidant index and geriatric nutritional risk index, stratified by sex (a), history of diabetes (b), hypertension (c), and cancer (d). Age, sex, race, education level, marital status, poverty income ratio, sedentary behavior, body mass index, dietary energy, history of hypertension, diabetes, and cancer were adjusted. In the subgroup analysis, the model is not adjusted for the stratification variable itself.
Threshold effect analysis
Analysis of the threshold effect of CDAI on GNRI revealed distinct patterns across various subgroups (Table 3). For men, the inflection point was determined at 10, indicating a significant positive association below this threshold (β=0.201, 95% CI: 0.122, 0.279) and a non-significant negative association above it (β=-0.177, 95% CI: -0.394, 0.040). Women exhibited a similar trend, with an inflection point at 8.5. Participants without diabetes showed a significant positive association when the CDAI was below 8.5 (β=0.167, 95% CI: 0.102, 0.232), whereas those with cancer displayed a notable inflection point at 5, with significant associations observed both below and above this threshold.
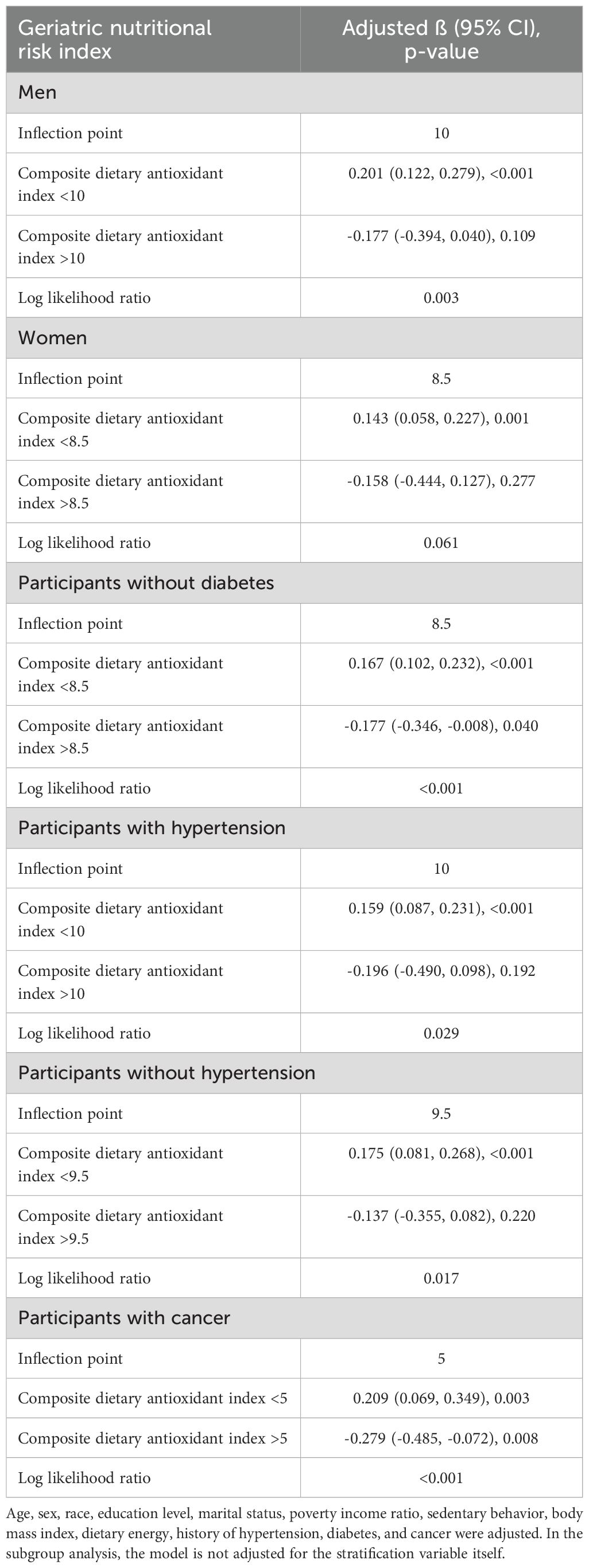
Table 3. Threshold effect analysis of composite dietary antioxidant index on geriatric nutritional risk index using two-piecewise linear regression model.
Discussion
Our study demonstrates a significant positive association between CDAI and GNRI, indicating that higher dietary antioxidant intake is linked to reduced nutritional risk in elderly individuals. Specifically, vitamin C, selenium, zinc, and carotenoids showed strong positive associations with GNRI, with vitamin C and zinc exhibiting the most pronounced effects. These findings highlight the critical role of dietary antioxidants in mitigating nutritional risk in aging populations.
The CDAI-GNRI correlation demonstrates that dietary antioxidants mitigate age-related nutritional decline. This effect may occur through dual mechanisms of reducing oxidative stress and inflammation, which are key drivers of nutritional decline that impair cellular function and exacerbate muscle wasting, ultimately helping preserve nutritional status in elderly populations (17–19). The robust associations observed for vitamin C and zinc may be attributed to their essential roles in immune function and cellular repair. Vitamin C plays a crucial role in cellular antioxidant defense by scavenging free radicals in the aqueous phase, thereby preventing oxidative damage to proteins, lipids, and carbohydrates. Additionally, it contributes to the regeneration of vitamin E, which is vital for maintaining the integrity of the cellular antioxidant network (20, 21). Zinc, on the other hand, is crucial for decreased oxidative stress biomarkers and decreased inflammatory cytokines in the elderly, and its deficiency is closely linked to malnutrition in the elderly (22, 23). The differential effects of antioxidants may also reflect variations in bioavailability and synergistic interactions. For instance, carotenoids, being fat-soluble, are better absorbed in the presence of dietary fats, whereas vitamin C, being water-soluble, is more readily excreted (24, 25).
Our findings are consistent with prior studies highlighting the beneficial effects of dietary antioxidants on health outcomes in the elderly, a population particularly vulnerable to nutrition-related risks. Evidence highlights their protective effects against cardiovascular diseases, with studies showing that high antioxidants intake reduces myocardial infarction risk (26). Antioxidants also support cognitive health, with plant foods rich in antioxidants linked to significant beneficial effects on cognitive functions risk (27, 28). Additionally, antioxidants counteract osteoporosis by mitigating oxidative damage, potentially accelerating fracture healing (29). Furthermore, they contribute to cancer prevention by neutralizing oxidative stress, a key pathogenic factor in carcinogenesis, which is particularly relevant for the elderly due to cumulative lifetime exposure (30). Our threshold analysis identified distinct optimal ranges for antioxidant intake, with CDAI values below 10.0 in men and 8.5 in women demonstrating maximal nutritional benefits. These gender-specific inflection points may reflect fundamental differences in body composition, oxidative stress response thresholds, and nutrient utilization efficiency. These findings suggest that geriatric nutritional interventions should incorporate sex-specific antioxidant recommendations to optimize therapeutic outcomes.
This study advances current knowledge by introducing CDAI as a novel tool for assessing dietary antioxidant intake and its relationship with nutritional risk. The observed inverted U-shaped relationship between antioxidant intake and GNRI may be attributed to nutrient saturation and the pro-oxidant effects of high doses of certain antioxidants. The concept of nutrient saturation indicates that antioxidant benefits peak at specific intake levels, beyond which additional consumption yields no further advantages and may pose potential risks (31, 32). High doses of certain antioxidants, such as vitamin C, can exhibit pro-oxidant activity, increasing oxidative stress and potentially damaging cellular components, including DNA and proteins (33, 34). Our findings support the important role of dietary antioxidants in reducing nutritional risk among elderly populations. Specifically, vitamin C-rich foods such as citrus fruits, berries, kiwi and bell peppers (33), zinc-rich foods including legumes, seafood, lean meats and fortified cereals (35), and balanced dietary patterns like the Mediterranean diet (36) may collectively help mitigate age-related nutritional deficiencies. These findings underscore the need for public health initiatives to develop tailored dietary guidelines for the elderly, prioritizing a varied intake of antioxidant-rich whole foods to optimize nutritional risk reduction. Importantly, given the observed threshold effects and potential pro-oxidant risks associated with high-dose supplementation, excessive isolated antioxidant intake should be discouraged. To facilitate implementation, clinicians and policymakers could incorporate CDAI-based screening into routine geriatric nutritional assessments, enabling early identification of individuals with suboptimal antioxidant intake and guiding personalized dietary interventions.
To the best of our knowledge, this study represents the first investigation into the relationship between CDAI and GNRI within a nationally representative sample of elderly individuals. However, several limitations should be acknowledged. First, the NHANES database predominantly utilizes a cross-sectional design, which inherently restricts the ability to infer causality. While our findings demonstrate a significant association between antioxidants intake and nutritional risk, they do not establish whether increased antioxidant consumption directly reduces nutritional risk. The observed association between higher antioxidant intake and reduced nutritional risk may be influenced by reverse causality, wherein individuals with better baseline nutritional status are more likely to consume antioxidant-rich foods. To elucidate the causal relationship and temporal dynamics, future longitudinal cohort studies or randomized controlled trials are warranted. Second, dietary data in NHANES are collected through 24-hour dietary recalls, a method susceptible to recall bias. This limitation is particularly relevant for elderly participants, who may experience memory decline or cognitive impairments, potentially leading to inaccuracies in reporting dietary intake. Underreporting or overreporting of specific antioxidants may introduce measurement bias into the CDAI calculation, thereby either attenuating or inflating the observed associations. Third, although the study adjusted for a range of covariates, residual confounding may persist due to unmeasured variables such as genetic predispositions, environmental exposures, cognitive assessment or the severity of chronic diseases, which could influence the observed associations. For instance, cognitive impairment, a prevalent condition among the elderly yet unmeasured in our analysis, may systematically influence dietary recall accuracy, introducing potential confounding. Furthermore, the severity of underlying chronic conditions could act as an effect modifier in the antioxidant-GNRI relationship. These limitations underscore the necessity for future studies incorporating multidimensional clinical evaluations to validate and refine these observational associations.
Conclusion
In summary, this study underscores the critical role of dietary antioxidants in reducing nutritional risk among elderly individuals. The robust positive association between CDAI and GNRI, particularly driven by vitamin C and zinc, highlights the potential of these nutrients to enhance nutritional health in aging populations. The observed inverted U-shaped relationship between antioxidants intake and GNRI suggests that while moderate consumption offers significant protective benefits, excessive intake may result in diminishing returns or adverse effects. These findings advocate for a balanced dietary approach, emphasizing diverse food intake to achieve optimal antioxidant levels and improve nutritional outcomes, particularly in elderly populations at higher risk of deficiencies.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Ethics statement
The ethics review board of the National Center for Health Statistics approved all NHANES protocols. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZZ: Writing – original draft. YH: Writing – original draft. HC: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bojang KP and Manchana V. Nutrition and healthy aging: A review. Curr Nutr Rep. (2023) 12:369–75. doi: 10.1007/s13668-023-00473-0
2. Volkert D, Delzenne N, Demirkan K, Schneider S, Abbasoglu O, Bahat G, et al. Nutrition for the older adult - Current concepts. Report from an ESPEN symposium. Clin Nutr. (2024) 43:1815–24. doi: 10.1016/j.clnu.2024.06.020
3. Kaur D, Rasane P, Singh J, Kaur S, Kumar V, Mahato DK, et al. Nutritional interventions for elderly and considerations for the development of geriatric foods. Curr Aging Sci. (2019) 12:15–27. doi: 10.2174/1874609812666190521110548
4. Dzięgielewska-Gęsiak S and Muc-Wierzgoń M. Inflammation and oxidative stress in frailty and metabolic syndromes-two sides of the same coin. Metabolites. (2023) 13(4):475. doi: 10.3390/metabo13040475
5. Li W, Wu Z, Liao X, Geng D, Yang J, Dai M, et al. Nutritional management interventions and multi-dimensional outcomes in frail and pre-frail older adults: A systematic review and meta-analysis. Arch Gerontol Geriatr. (2024) 125:105480. doi: 10.1016/j.archger.2024.105480
6. Yang J, Luo J, Tian X, Zhao Y, Li Y, and Wu X. Progress in understanding oxidative stress, aging, and aging-related diseases. Antioxid (Basel). (2024) 13(4):394. doi: 10.3390/antiox13040394
7. Ashraf MV, Khan S, Misri S, Gaira KS, Rawat S, Rawat B, et al. High-altitude medicinal plants as promising source of phytochemical antioxidants to combat lifestyle-associated oxidative stress-induced disorders. Pharm (Basel). (2024) 17(8):975. doi: 10.3390/ph17080975
8. Beetch M, Harandi-Zadeh S, Shen K, Lubecka K, Kitts DD, O'Hagan HM, et al. Dietary antioxidants remodel DNA methylation patterns in chronic disease. Br J Pharmacol. (2020) 177:1382–408. doi: 10.1111/bph.v177.6
9. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. (2005) 82:777–83. doi: 10.1093/ajcn/82.4.777
10. Feng M, Liu Y, Li Q, Yang X, Wei F, Cheng H, Lyu J, et al. Association between geriatric nutritional risk index and adverse outcomes in critical ill patients with chronic obstructive pulmonary disease: a cohort study of 2824 older adults. BMC Pulm Med. (2024) 24:634. doi: 10.1186/s12890-024-03454-3
11. Serrato P, Craft S, Sayeed S, Hengartner AC, Belkasim S, Sadeghzadeh S, et al. Impact of preoperative nutritional status on morbidity and mortality in elderly patients undergoing subdural hematoma evacuation: the role of the Geriatric Nutritional Risk Index. J Neurosurg. (2024) 142(4):1–11. doi: 10.3171/2024.7.JNS24875
12. Bao H, Deng F, and Lei S. The composite dietary antioxidant index is associated with common non-communicable diseases: the mediation and joint effects of inflammatory indices. Biol Res Nurs. (2025) 3:10998004251320591. doi: 10.1177/10998004251320591
13. Wright ME, Mayne ST, Stolzenberg-Solomon RZ, Li Z, Pietinen P, Taylor PR, et al. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol. (2004) 160:68–76. doi: 10.1093/aje/kwh173
14. Jin X, Tong W, Sun L, Lu S, Sun P, Li H, and Liu Y. Association of composite dietary antioxidant index with high risk of prostate cancer in middle-aged and elderly men: insights from NHANES. Front Immunol. (2025) 16:1530174. doi: 10.3389/fimmu.2025.1530174
15. Wang Z, Tang F, Zhao B, Yan H, Shao X, and Yang Q. Composite dietary antioxidant index and abdominal aortic calcification: a national cross-sectional study. Nutr J. (2024) 23:130. doi: 10.1186/s12937-024-01029-w
16. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, and Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
17. Tan BL, Norhaizan ME, Liew WP, and Sulaiman Rahman H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front Pharmacol. (2018) 9:1162. doi: 10.3389/fphar.2018.01162
18. Deledda A, Annunziata G, Tenore GC, Palmas V, Manzin A, Velluzzi F, et al. Diet-derived antioxidants and their role in inflammation, obesity and gut microbiota modulation. Antioxid (Basel). (2021) 10(5):708 . doi: 10.3390/antiox10050708
19. Terao J. Revisiting carotenoids as dietary antioxidants for human health and disease prevention. Food Funct. (2023) 14:7799–824. doi: 10.1039/D3FO02330C
20. Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. (2003) 22:18–35. doi: 10.1080/07315724.2003.10719272
21. Duarte TL and Lunec J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res. (2005) 39:671–86. doi: 10.1080/10715760500104025
22. Gammoh NZ and Rink L. Zinc in infection and inflammation. Nutrients. (2017) 9(6):624. doi: 10.20944/preprints201705.0176.v1
23. Prasad AS. Zinc is an antioxidant and anti-inflammatory agent: its role in human health. Front Nutr. (2014) 1:14. doi: 10.3389/fnut.2014.00014
24. Bas TG. Bioactivity and bioavailability of carotenoids applied in human health: technological advances and innovation. Int J Mol Sci. (2024) 25(14):7603. doi: 10.3390/ijms25147603
25. Villagran M, Ferreira J, Martorell M, and Mardones L. The role of vitamin C in cancer prevention and therapy: A literature review. Antioxid (Basel). (2021) 10(12):1894. doi: 10.3390/antiox10121894
26. Klipstein-Grobusch K, Geleijnse JM, den Breeijen JH, Boeing H, Hofman A, Grobbee DE, et al. Dietary antioxidants and risk of myocardial infarction in the elderly: the Rotterdam Study. Am J Clin Nutr. (1999) 69:261–6. doi: 10.1093/ajcn/69.2.261
27. Baroni L, Sarni AR, and Zuliani C. Plant foods rich in antioxidants and human cognition: A systematic review. Antioxid (Basel). (2021) 10(5):714. doi: 10.3390/antiox10050714
28. Devore EE, Grodstein F, van Rooij FJ, Hofman A, Stampfer MJ, Witteman JC, et al. Dietary antioxidants and long-term risk of dementia. Arch Neurol. (2010) 67:819–25. doi: 10.1001/archneurol.2010.144
29. Sheweita SA and Khoshhal KI. Calcium metabolism and oxidative stress in bone fractures: role of antioxidants. Curr Drug Metab. (2007) 8:519–25. doi: 10.2174/138920007780866852
30. Khan N, Afaq F, and Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. (2008) 10:475–510. doi: 10.1089/ars.2007.1740
31. Halliwell B. Establishing the significance and optimal intake of dietary antioxidants: the biomarker concept. Nutr Rev. (1999) 57:104–13. doi: 10.1111/j.1753-4887.1999.tb06933.x
32. Jarrett SG, Cuenco J, and Boulton M. Dietary antioxidants provide differential subcellular protection in epithelial cells. Redox Rep. (2006) 11:144–52. doi: 10.1179/135100006X116646
33. Doseděl M, Jirkovský E, Macáková K, Krčmová LK, Javorská L, Pourová J, et al. Vitamin C-sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients. (2021) 13(2):615. doi: 10.3390/nu13020615
34. Banihani SA and Alawneh RF. Human semen samples with high antioxidant reservoir may exhibit lower post-cryopreservation recovery of sperm motility. Biomolecules. (2019) 9(3):111. doi: 10.3390/biom9030111
35. Mohamad NS, Tan LL, Ali NIM, Mazlan NF, Sage EE, Hassan NI, et al. Zinc status in public health: exploring emerging research trends through bibliometric analysis of the historical context from 1978 to 2022. Environ Sci pollut Res Int. (2023) 30:28422–45. doi: 10.1007/s11356-023-25257-5
Keywords: dietary intake, nutritional risk, aged, antioxidant, nutrition survey
Citation: Zhu Z, Han Y and Chen H (2025) Dietary antioxidants and nutritional risk in the elderly: insights from the composite dietary antioxidant index and geriatric nutritional risk index. Front. Immunol. 16:1596663. doi: 10.3389/fimmu.2025.1596663
Received: 20 March 2025; Accepted: 26 May 2025;
Published: 10 June 2025.
Edited by:
Reinaldo B. Oria, Federal University of Ceara, BrazilReviewed by:
Sylwia Dziegielewska-Gesiak, Medical University of Silesia, PolandVeronique Sante-Lhoutellier, INRAE Clermont-Auvergne-Rhône-Alpes, France
Copyright © 2025 Zhu, Han and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huafang Chen, Y2hlbmh1YWZhbmdAd211LmVkdS5jbg==
†These authors have contributed equally to this work
 Zhongxin Zhu
Zhongxin Zhu Yueyuan Han1†
Yueyuan Han1† Huafang Chen
Huafang Chen