- Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Multiple sclerosis (MS) is a chronic autoimmune disorder of the central nervous system (CNS) that predominantly affects young adults. However, current disease-modifying therapies demonstrate limited efficacy in addressing progressive disease subtypes, underscoring the urgent need for novel therapeutic strategies. Here, we systematically review the neuroimmune interactions underlying the pathogenesis of MS, with a focus on three key aspects: the immune niche, immune cell types, and cell-based therapies. We first discuss the evolution of brain-immune concepts, from early notions of immune privilege to modern understandings of brain-border immune niches (meninges, choroid plexus, and perivascular spaces). These compartments serve as critical interfaces where peripheral immune cells interact with CNS-resident immune cells. We then analyze the roles of specific immune cell subsets (e.g., T/B cells, myeloid cells and microglia) in disease progression, highlighting their functional heterogeneity across different MS subtypes. Furthermore, we highlight emerging MS immunotherapies-including chimeric antigen receptor (CAR) T regimens, mesenchymal stem cell interventions, microbiome modulation, and nanodelivery systems, which strategically target mechanistic nodes spanning neuroimmune niche regulation, inflammatory cascade blockade, and CNS neurorestorative capacities.
1 Introduction
Multiple sclerosis (MS) is a widespread chronic inflammatory disorder of the central nervous system (CNS), driven by immune-mediated processes and serving as the leading cause of acquired neurological disability among individuals aged 18 to 45 (1). Globally, approximately 2.5 million people globally are impacted by MS, with around 350,000 cases reported in the United States (2, 3). Most patients experience progressive neurological decline 10 to 15 years after onset. MS manifests in diverse clinical manifestations, encompassing clinically isolated syndrome (CIS), relapsing-remitting MS (RRMS), primary progressive MS (PPMS), and secondary progressive MS (SPMS). Notably, RRMS, the most common subtype, affects approximately 80% of patients, predominantly young women (4). Experimental autoimmune encephalomyelitis (EAE) model is widely regarded as the standard for investigating RRMS. Despite ongoing research, the exact cause and development of MS continue to be unclear, with contributions from both genetic predisposition and environmental influences suspected (5). The disease is marked by extensive demyelination, axonal degeneration, and astrogliosis in CNS white matter, leading to progressive neurological impairment (6).
The immune microenvironment within the central nervous system constitutes a sophisticated and dynamically regulated network. Brain border immune niches are emerging as pivotal anatomical compartments for investigating neuroimmune regulatory mechanisms. These particular niches function as entry points facilitating the migration of immune cells from the peripheral regions into the CNS, facilitating intricate crosstalk between immunocytes, cytokines, and membrane-bound receptors that orchestrates inflammatory modulation and tissue repair. Conventional multiple sclerosis research has historically underappreciated the mechanistic connection between brain border immune niches and disease pathogenesis. Emerging evidence demonstrates structural compromise of the blood-brain barrier (BBB), blood-cerebrospinal fluid barrier (BCSFB), and meningeal layers in both MS and EAE. These observations collectively indicate that dynamic remodeling of brain border immune niches is mechanistically intertwined with MS pathophysiology.
Activated antigen-presenting cells (APCs) and autoreactive T cells produce proinflammatory cytokines such as IFN-γ, TNF-α, IL-17, and IL-23, which promote cell-mediated immune reactions within the CNS. Conversely, Th2 cytokines (e.g., IL-4, IL-5, IL-10, TGF-β) exhibit protective effects. Dysfunctional regulatory T lymphocytes (Tregs), marked by Foxp3 expression, have played a role in the development of MS (7). Within the immunopathogenesis of neuroinflammatory conditions, IL-23 critically orchestrates the lineage commitment of lymphocytes toward pro-inflammatory effector profiles. This cytokine-mediated polarization is pathologically amplified in demyelinating autoimmune pathologies, with particular mechanistic relevance observed in CD4+ lymphocyte subsets demonstrating robust IL-17 synthesis capacity - a cellular population designated as Th17 lymphocytes (8). Recent evidence underscores the role of various immune cell subsets in the pathogenesis of MS, including peripherally derived and infiltrating T follicular helper (Tfh) cells (9), dendritic cells(DC) (10), NK cells, B cells (11, 12), and CNS-resident glial subsets (microglia (13), oligodendrocytes, astrocytes), which collectively orchestrate neuroinflammatory cascades. Methodological breakthroughs in high-resolution sequencing platforms, such as single-cell RNA sequencing (scRNA-seq) and single-nucleus RNA sequencing (snRNA-seq), have enabled multidimensional characterization of immune pathway crosstalk, cytokine-cell interplay, and proteome-wide interaction networks in MS, revolutionizing our understanding of spatiotemporal heterogeneity within disease-driving immune compartments. This review synthesizes contemporary advances in the pathogenesis of MS, alongside novel therapeutic targeting strategies, conceptualized through the framework of brain border immune niche.
2 Interactions between the immune system and the brain
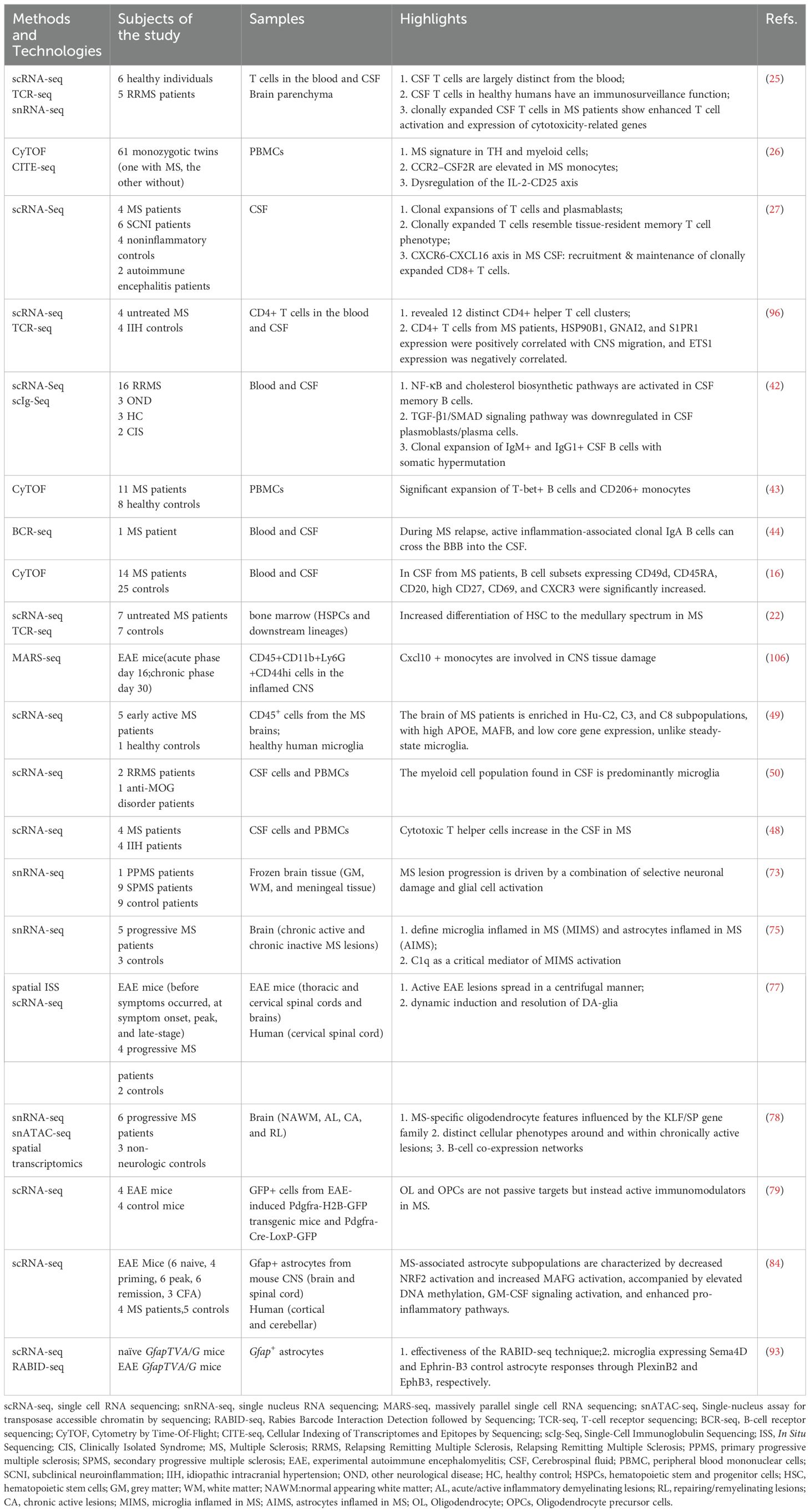
Breakthroughs in high-throughput methodologies, such as flow cytometry and transcriptomic analysis, have dramatically expanded the multiplex capacity for concurrent measurement of immune parameters. This capability enables high-resolution quantitative profiling of neuroimmune responses within the CNS of individuals with MS. Leveraging these approaches, scientists have constructed analytical frameworks to investigate crosstalk between immune pathways, dynamic cytokine-cell interplay, and proteome-wide mapping of cellular interactions, advancing systematic characterization of neuroimmune networks in the human CNS. Consequently, high-throughput sequencing technology is increasingly utilized to elucidate pathogenic mechanisms in MS research (Table 1).
2.1 Early perspectives and “immune privilege”
Conventionally, the brain has been considered an organ possessing “immune privilege”, where immune cell infiltration was believed to occur only during CNS inflammation and disease. Peripheral immune cell infiltration-induced focal inflammation holds a pivotal role in the neuropathological processes and development of MS. Nevertheless, recent research has unveiled a novel perspective, revealing that immune responses extend beyond their traditional role and actively participate in CNS maintenance and repair (14, 15). Discoveries have indicated that immune cells influence neurogenesis and cognition (16, 17).
2.2 Modern perspectives: immune niches in the brain
The anatomical boundaries of the brain include the BBB, BCSFB, and meninges (18). In this context, the BBB is considered the primary interface separating the periphery from the brain, with other structures serving the additional role of protective barriers that facilitate immune cell surveillance and defense functions. The notion of immune niches within the brain pertains to its border areas that support the presence and function of various immune cells, both innate and adaptive, thereby orchestrating brain function and repair (14, 19). These specialized immune niches include key areas like the choroid plexus (CP), perivascular spaces, and the meninges. Within these niches, peripheral immune cells infiltrate the brain parenchyma, inducing inflammation and cytokine release that modulate neuronal and glial cell activity and function, and orchestrate cross-system interactions (nervous, hematopoietic, lymphatic, and endocrine systems) to mediate disease pathogenesis (Figure 1). The meninges, divided into the inner pia mater, middle arachnoid membrane, and outer dura mater, each have distinct immune functions. In this regard, the dura mater is recognized as the fundamental immune ecological niche capable of sensing and presenting antigens, as well as releasing substantial quantities of cytokines (15). The subarachnoid lymphoid meninges (SLYM) within the subarachnoid space acts as a filtration mesh for material exchange, preventing the passage of CSF solutes exceeding 3 kDa in size (20). The choroid plexus, which houses a substantial population of immune cells, detects and responds to peripheral immune signals. The choroid plexus constitutes the BCSFB, serving as a pivotal location for immune cell infiltration and functioning as an essential brain barrier and immune hub (21). Moreover, perivascular spaces, including small penetrating blood vessels supplying blood to the brain and adjacent interstitial compartments, enable immune surveillance and response tailored to the brain’s immune requirements. In addition, the interconnected immune microenvironment encompassing immune niches also includes the skull bone marrow, offering a swift access route for immune cells (22), alongside the cervical lymph nodes (CLNs) responsible for draining the brain, which collect metabolic waste via the meningeal lymphatic vasculature (23). The existence and functional significance of these immune niches underscores the intricate interplay between the brain and the immune system, surpassing traditional views. Beyond their role in pathogen defense, these niches significantly impact neurogenesis, cognitive functions, and neural repair mechanisms.
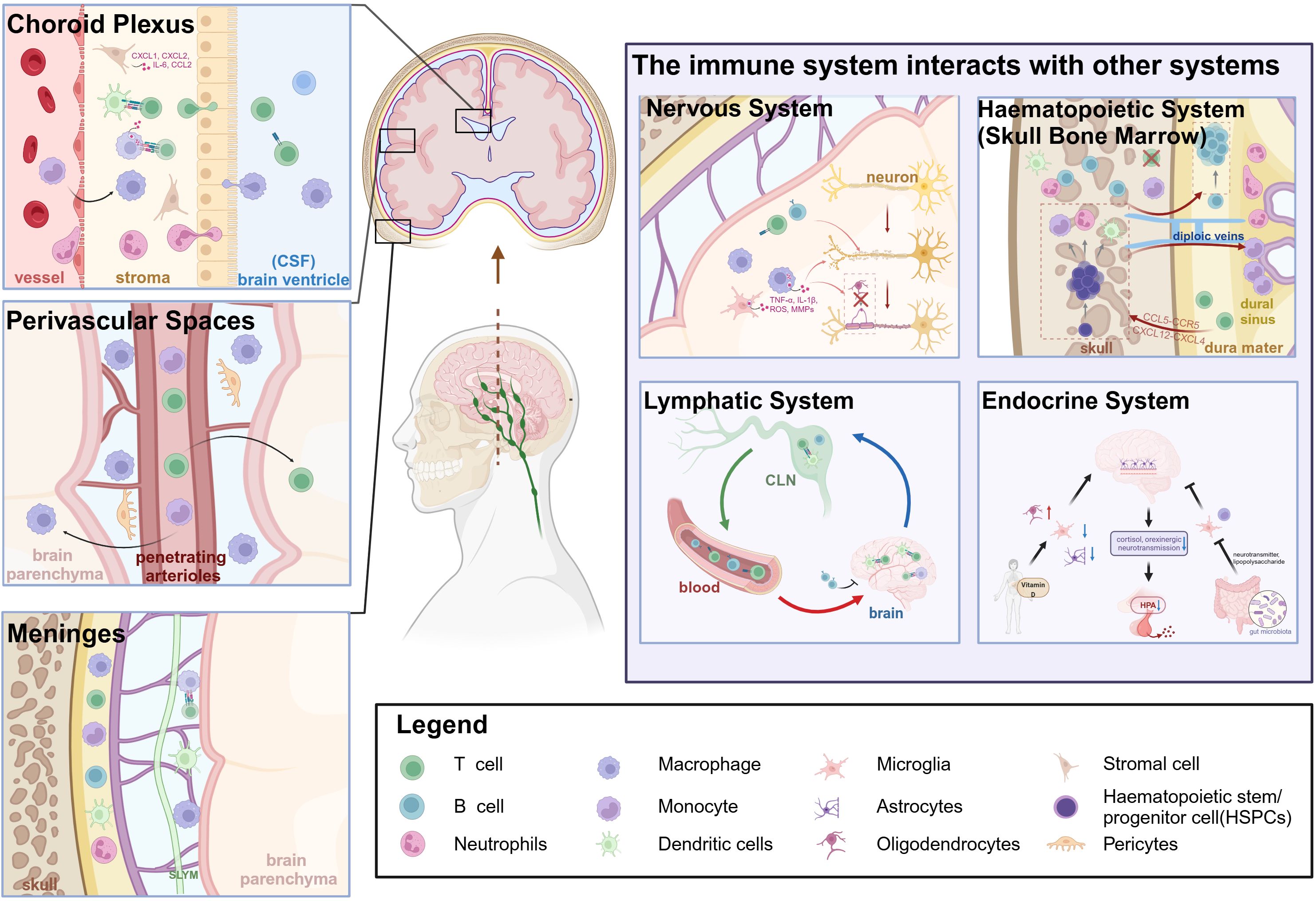
Figure 1. Overview of the pathogenesis of cerebral immune niches in Multiple Sclerosis and their interactions with other systems. Created in BioRender. Xiaodi, S. (2025) https://BioRender.com/a6k520m.
2.3 Peripheral immune cells
Immune cells located in the periphery, along with their secreted mediators, possess the ability to penetrate into the brain parenchyma via specialized immune niches at the brain’s borders. These cells and factors play a crucial role in initiating and sustaining the pathological mechanisms underlying MS (24).
2.3.1 T cells
The predominant presence of T cells within lesions is a crucial aspect of the pathogenesis of MS. Hafler’s team used a dual approach, integrating scRNA-seq with T-cell receptor sequencing (TCR-seq), to describe the enduring transcriptional signatures displayed by T-cells in the blood and CSF of healthy subjects (25). Their results showed that the T-cell populations in the CSF of healthy individuals comprised distinct clusters of memory CD4+ and CD8+ T cells and two major clusters: naive CD4+ T-cells and naive CD8+ T-cells in the blood. It is worth mentioning that the proliferation of these T cells was not extensively induced, and consequently did not trigger inflammatory cascades that are characteristically associated with MS, thereby underscoring their non-pathogenic nature within the context of healthy physiology. At the level of transcription, patients suffering from MS demonstrated heightened expression of genes involved in TCR recognition and the activation of T-cells, particularly within clonally expanded T-cell populations. Florian Ingelfinger et al. conducted an analysis on 61 sets of identical twins, where one twin was diagnosed with MS while the other, despite carrying the highest genetic predisposition, remained asymptomatic. The study found that the greatest difference in immune profiles between the twins was in cytokine receptors, which are the communication modes of immune cells (26). Furthermore, Beltrán et al. demonstrated the CSF of MS patients and subclinical neuroinflammation (SCNI) contained clonally expanded CD4+ T cells, CD8+ T cells, and B cells (27). In both MS and EAE, the migration of pathogenic CD4+ T cells into the CNS is the key event.
In the EAE model, we observed that the CD45+ immune cell subset, particularly CD4+ T cells, accumulates within the CP during the early stages of the disease progression and remains at high levels during the chronic phase, while the levels of these cells in the brain and spinal cord decrease (28). Chemokine receptor 6+(CCR6+) Th17 cells utilize the CP as an alternative pathway for accessing the CNS, thereby initiating EAE (29). Cytokines from Th17 cells, like IFN-γ and IL-17, stimulate the CP to release CCL20, facilitating the migration of pre-activated B cells and T cells into the CSF (30). The research conducted by Wu Zheng and colleagues revealed that knocking down CP-A2AR can inhibit the CCR6-CCL20 axis, thereby facilitating the reduction in the transit of Th17 cells across the CP into the brain parenchyma (21). Sabela discovered that in comparison to controls without neuroinflammation, patients with progressive MS exhibited a higher density of CD8+ T cells within the stroma of the choroid plexus. Furthermore, it was noted that MHCII+ antigen-presenting cells were frequently located in close vicinity to T cells, hinting at the CP involvement in immune surveillance within the CNS (31).
CD4+ T cells, also known as helper T (TH) cells, are crucial in orchestrating the immune response in MS (32). In the pathogenesis of MS, various subsets of Th cells exist, namely Th1, Th2, Th17, and Tregs, each fulfilling a unique role. Initially considered to be the primary causative cells of MS, Th1 cells secrete pro-inflammatory cytokines including IFN-γ and TNF-α (33, 34). Similarly, Th17 cells, distinguished by their secretion of IL-17, hold a significant role in MS progression (35). Elevated IL-17 levels in CSF and MS lesions correlate positively with disease severity. Tregs maintain immune tolerance and prevent autoimmunity, but in MS, their function and number are often impaired (36). It is now widely accepted that an imbalance between Treg cells and Teff cells within the CNS constitutes a novel pathophysiological mechanism in MS. Correction of this imbalance has been posited as a potentially efficacious therapeutic strategy. However, therapies targeting only T cells have proven ineffective in treating RRMS (37). Interestingly, within MS CNS lesions CD8+ cytotoxic T cells outnumber CD4+ helper T cells, highlighting their central role (38, 39).
2.3.2 B cells
T lymphocytes have historically been regarded as central to the initiation and driving the progression of MS (40). However, due to the successful application of B cell depletion therapy specifically targeting CD20, the importance of B cells in MS immunopathogenesis has received increasing attention (11, 41). In the CSF of patients with MS, strong clonal expansion has been detected in the plasma cell population, with 90% of clones showing expansion, though 20% also show expansion in SCNI (27). In parallel, Ramesh et al. observed a 13-fold elevation in the number of B cells in the CSF of RRMS patients compared to healthy controls, accompanied by a skewed distribution of IgG1+ B cells favoring the CSF over the blood, and a balanced presence of IgG1+ and IgM+ B cells in the CSF. Notably, IgM-bearing cells were prevalent in naive and unswitched memory subsets, whereas IgG1-expressing cells were primarily restricted to plasma blast/plasma cells, switched memory B cells, and double-negative subsets (42). In the peripheral blood of early-stage MS patients there was an increase in B-cell subsets expressing CXCR3 (T-bet) and classic CD206+ monocyte subsets. Conversely, patients with aggressive MS exhibited an enrichment of B-cell subsets expressing CXCR3 (T-bet) (43). Furthermore, the presence of IgA-producing B cells (CD19+) within the inflamed CNS highlights the involvement of IgA-secreting cells in the pathogenesis of MS (44). Utilizing mass cytometry on both blood and CSF samples, Johansson and colleagues identified a novel subpopulation of small B-cells that is associated with MS. These cells exhibited a unique phenotype, including CD49d, CD69, CD27, CXCR3, and HLA-DR expression, which partially overlapped with memory B cells but did not fully match established B cell subsets, sharing similarities with plasma cells due to CD27 and CD20 expression, yet remaining distinct (16).
B cells located within the immune niches at the brain’s border primarily originate from two sources: the dura mater and the cranial bone marrow. The dura-associated lymphoid tissue (DALT) harbors germinal center B cells capable of differentiating into plasma cells upon antigenic challenge. These cells are adept at recognizing local antigens and can swiftly mount a humoral immune response (45). The cranial bone marrow functions as a direct reservoir for immune cells of the nervous system, with minute micro-osseous channels within the cranial marrow cavity linking to the trabecular veins of the meninges (46). B cells originating from the cranial bone marrow traverse microvascular channels within the cranial endothelium to migrate towards the dura mater, providing a continuous supply route of B cells to the CNS (47).
MS progression is influenced by the multifaceted functions of B-cells, acting as precursors to antibody-generating plasma cells. During inflammatory phases, these plasma cells release substances that have the capacity to cause demyelination in the CNS. Additionally, mature and memory B cells fulfill the function of APCs, releasing cytokines and chemokines. Notably, the involvement of pathogenic B cells may vary among different MS patient subgroups. Collectively, these results underscore the intricate nature and crucial importance of B-cells in MS, emphasizing the imperative for ongoing research into their functions and potential therapeutic applications.
2.3.3 Macrophages and monocytes
Ingelfinger et al. discovered a monocyte subset in MS-affected twins characterized by high expression of CCR2 and granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor-specific subunit CD116, accompanied by low CD14 expression and no CD16 expression (26). Shafflick et al. identified a monocyte subset termed “Mono2,” predominantly derived from cerebrospinal fluid. These Mono2 cells exhibit unique gene characteristics, including classical markers such as CD9, CD163, EGR1, and BTG2, alongside non-canonical markers like C1QA, C1QB, MAF, and CSF1R (also known as CD115). Furthermore, they express markers associated with vascular macrophages (LYVE1), microglia (including TREM2, TMEM119, and GPR34), as well as microglia resident at the CNS boundaries (STAB1 and CH25H) (48). The gene characteristics of Mono2 are similar to those of steady-state microglia described in previous studies (49, 50). Circulating monocytes can be classified into classical (phagocytic) monocytes, non-classical monocytes, and monocytes that display an intermediate transcriptional profile. Notably, both non-classical monocytes and those with an intermediate transcriptional profile characterized by high CD16 expression, possess patrol functions. Upon stimulation, they rapidly produce pro-inflammatory cytokines (51). In multiple sclerosis, increased circulating frequencies or frequencies in cerebrospinal fluid have been reported for these monocytes (52). Additionally, they produce heightened production of pro-inflammatory cytokines, notably including IL-6 and IL-12.
When monocytes enter tissues, they differentiate into tissue-infiltrating macrophages. Macrophages derived from the same monocyte lineage can display diverse functional profiles. M1 macrophages, characterized by their destructive nature, exhibit high levels of costimulatory molecules, cytokines that promote inflammation, and reactive oxygen species (ROS). These macrophages drive the differentiation of pro-inflammatory T cells, specifically Th1 and Th17 subsets. Macrophages that are alternatively activated, referred to as M2 macrophages, exhibit increased secretion of IL-10. These macrophages promote Th2 cell differentiation, display enhanced phagocytic abilities, and exhibit regulatory and homeostatic properties (51). In physiological states, the macrophages present within the CNS encompass parenchymal microglia and macrophages associated with the borders, referred to as border-associated macrophages (BAMs), together playing a crucial role in sustaining the homeostasis of the CNS (53). BAMs are composed of diverse macrophage subsets, such as choroid plexus macrophages, meningeal macrophages, and perivascular macrophages (54), with heterogeneous origins. For instance, some macrophages in the leptomeninges originate from progenitors in the yolk sac and fetal liver, enabling them to self-renew (55). In contrast, a subset of monocytes in the dura mater originates directly from adjacent skull bone marrow (56). Additionally, infiltrating monocyte-derived macrophages (MDMs) from the bone marrow hematopoietic system serve as another significant source of brain macrophages, such as those in the choroid plexus. Blood-derived macrophages are considered key contributors to the initiation of immunopathology in CNS diseases (53). A study has demonstrated that blocking the insulin-like growth factor-1 (IGF-1) signaling mechanism in BAMs results in a significant reduction of central nervous system inflammation in EAE mice (57). This finding validates the feasibility of targeting brain macrophages as a therapeutic strategy.
2.3.4 Neutrophils
During inflammation, neutrophils enhance the permeability of the BBB, infiltrate leaky regions of the BBB and blood-spinal cord barrier (BSCB), and subsequently enter the CNS through compromised choroid plexus and leptomeninges, thereby exerting pathogenic effects (58). The choroid plexus epithelium synthesizes CXCL1 and CXCL2 chemokines, while the increased expression of adhesion molecules on its blood vessels of the choroid plexus facilitates the infiltration of blood-derived leukocytes into the brain parenchyma (31, 59). Neutrophils adhere via β2 integrins, specifically LFA-1 and Mac-1 (60), subsequently extravasate, and migrate into brain endothelium and other neural tissues. Additionally, they can obstruct blood flow by stalling in cerebral micro vessels, inducing ischemic phenomena (61). Neutrophils also release inflammatory mediators, such as ROS and cytotoxic granules, thereby exacerbating tissue injury (62). In murine models of cerebral ischemia, neutrophils release proteases and neutrophil extracellular traps (NETs), increasing BBB permeability and promoting inflammation and tissue injury (63). Despite the pathophysiological cascades through which neutrophils induce blood-spinal cord barrier dysfunction in multiple sclerosis remaining enigmatic, the decoding of their cellular choreography may establish novel therapeutic avenues targeting the neurovascular unit.
2.3.5 Dendritic cells
DCs are highly efficient APCs that are essential to both innate and adaptive immune systems, capable of promoting or suppressing myelin antigen-specific immune responses (64). In CNS, DCs are localized within the choroid plexus, the meninges, as well as CSF. Their primary physiological role is to capture antigens that enter the CSF and conveying them to regional lymph nodes to activate antigen-specific responses (20). In EAE and MS, DCs exhibit both proinflammatory and immunosuppressive properties, which vary depending on the disease stage, course, and DC subtype. DCs are categorized into two primary subsets: conventional DCs (cDCs) and plasmacytoid DCs (pDCs) (65). Research indicates that DCs are major producers of IL-23, a cytokine with a pathogenic role in EAE and MS (66). An elevation in cDCs has been observed to exacerbate inflammation, whereas pDCs have been shown to foster the growth of Tregs, exerting an anti-inflammatory influence in EAE. A reduction in pDCs is associated with increased CNS inflammation and worsened clinical symptoms in EAE due to a reduced immune response involving Th1 and Th17 cells (67). Furthermore, both cDC and pDC numbers are significantly higher in the CSF of MS patients, in comparison to those with non-inflammatory conditions (68, 69).
David Schafflick and his colleagues employed scRNA-seq to study the cell atlas of CSF and blood, revealing the presence of previously unidentified myeloid dendritic cells (mDCs) in CSF. Their findings highlighted the increase in cellular heterogeneity in CSF and alterations in the transcriptional blueprint of blood cells in MS patients (48). Since mDCs are crucial for Treg differentiation, these results support earlier studies suggesting impaired Treg development in MS, potentially due to a deficiency of mDCs (70). In 2019, Böttcher et al. analyzed peripheral blood mononuclear cells from individuals at the onset of MS and found a marked decrease in CD141+ CD68low mDCs compared to healthy individuals (71). In MS, the transport, accumulation, and migration of DCs to the CNS are disrupted, contributing to disease pathology (72).
2.4 Resident immune cells in the CNS
2.4.1 Microglia
Microglia are tissue-resident macrophages in the CNS that originate from the embryonic yolk sac. The heterogeneity of microglia has attracted significant attention, yet methods based on morphology, location, and surface markers cannot differentiate all subgroups. To tackle this issue, a study employed scRNA-seq to examine microglia (70). Researchers isolated cortical microglia from brains without CNS lesions and compared them to brains from early active MS cases. They identified four microglial clusters in the healthy control group, similar to clusters observed in previous mouse studies. Integrating scRNA-seq data from both MS and healthy brains, the study revealed seven microglial clusters: three exclusives to healthy brains, representing steady-state microglia; one group with cells from both MS and healthy brains, likely in a pre-activated state; and three clusters have been identified as being enriched or exclusive to MS brains. Of these, the unique ones exhibit reduced expression of core genes but heightened expression of APOE and MAFB. This diversity, influenced by spatial localization and the microenvironment, indicates that microglia play specific roles in MS pathology. Another study used single-nucleus RNA sequencing technology to analyze brain cells from MS lesions (73). Microglia from MS brains show activated phagocytic and amoeboid markers, confirmed in vitro. A separate study employing multimodal imaging techniques, which, while not isolating individual cells, analyzed cell morphology and location, distinguished blood-derived and tissue-resident microglia, differing in markers and morphology based on lesion type (74). This study highlighted the upregulation of C1q components in microglia bordering chronic active lesions, correlating with the presence of complement-related risk variants in patients with active lesions. Additionally, Martina Absinta et al. used scRNA-seq to investigate active lesion regions as well as healthy white matter. They identified two cell clusters specific to the edge of lesions: microglia active in MS inflammation (MIMS) and astrocytes (AIMS) (75). Beth Stevens and her team further identified nine microglial subgroups with distinct transcription patterns, each exhibiting unique gene expression profiles (76). This extensive analysis highlights the intricate roles played by MS and their significant contribution to the disease’s pathophysiology.
2.4.2 Oligodendrocytes
While oligodendrocytes were previously considered solely as victims of immune cell attacks, Kukanja P’s study showed that oligodendrocytes are active not only in the peripheral zones of lesions but also throughout the spinal cord and brain (77). This raises the question of whether they are suppressed or potentially driving the disease in MS. Elkjaer ML et al.’s single-cell multi-omics study demonstrated that the KLF/SP gene family exerts a substantial influence on oligodendrocyte genetics in progressive MS, potentially through the mechanism of autocrine iron uptake signaling (78). Furthermore, these oligodendrocytes displayed inflammatory characteristics and were present both at the periphery and within chronically active lesions. Similarly, Falcão AM et al. discovered that oligodendrocytes and their progenitor cells in a murine model of MS possess immune cell properties, suggesting they are not only targets of immune attacks but also actively interact with immune cells, which contributes to disease regulation and progression (79). Additionally, oligodendrocyte precursor cells play a role in removing damaged myelin. Genes associated with MS susceptibility are active in both oligodendrocytes and their progenitor cells. These discoveries underscore the promising potential of oligodendrocytes as therapeutic targets, offering novel pathways for the development of future MS treatments and emphasizing their critical role in the disease.
2.4.3 Astrocytes
Astrocytes are a crucial and highly heterogeneous component of the CNS (80). Astrocytes play a pivotal role in the development of lesions, enabling the infiltration of peripheral immune cells into the CNS (81, 82). By utilizing spatial transcriptomics and in situ hybridization methods, reactive astrocytes induced by inflammation are localized to distinct brain areas (83). Additionally, Francisco J. Quintana analyzed cells from the CNS of animals in an EAE model using Drop-seq technology, discovering multiple transcriptionally distinct subgroups of astrocytes. Among these, the Cluster 4 subgroup showed the greatest expansion during EAE induction. Further analysis revealed that within the Cluster4 subgroup, the expression of Nfe2l2, which encodes the transcription factor NRF2, was low. Conversely, the Cluster5 subgroup exhibited upregulated MAFG expression, which, in coordination with MAT2α, augments DNA methylation, also suppress transcription processes related to antioxidant and anti-inflammatory responses (84). These findings underscore the complex and dynamic roles of astrocytes in the pathology of MS and highlight potential as targets for therapeutic strategies.
3 Immune regulatory networks
The immune system comprises a sophisticated network consisting of a vast array of cells, receptors, and secreted factors. To be effective, immune responses require the seamless integration and orchestration of these diverse elements. To predict immune interaction outcomes and manage immune responses precisely, it is essential to study immune functions and dysfunctions at the pathway level rather than focusing on individual components. Understanding these underlying interaction networks is important for the development of precision therapies.
3.1 The disease-relevant immune cell crosstalk between the brain immune niche and the CNS
Interactions between resident immune-related cells and peripherally infiltrated immune cells can be observed in the brain. The expression of MHC molecules on APCs is indispensable for the recognition of antigens by TCRs. Antigen-presenting compartments in the CNS are constituted by both circulating mononuclear phagocytes of hematopoietic origin and tissue-embedded microglia, which are specialized myeloid sentinels endowed with CNS-specific maintenance functions. The co-stimulatory molecules they provide can effectively facilitate T-cell activation or suppression. For example, IL-12 and IL-6 play a crucial role in inducing the differentiation of Th1 and Th17 cells towards a pro-inflammatory state. Conversely, IL-10 fosters the development of Th2 cells, which exhibit an anti-inflammatory phenotype (85). Conversely, differentiated T-cells can also modulate the phenotypic conversion of APCs.
Follicular Th cells residing in lymphoid tissues secrete high levels of IL-21, which stimulates B-cell growth and differentiation into plasma cells, and potentiates follicular B-cell and Th17 responses, thereby exacerbating neuroinflammation (86, 87). Effector molecules, including granzyme B and perforin, are highly expressed by CD8+ T cells and have the capability to directly trigger the death of oligodendrocytes and neurons (88). Cytokines including TNF-α, lymphotoxin-α, and IL-6, which are secreted by B cells, can augment the proliferation of Th1 and Th17 cells, while anti-CD20 therapy targeting B cells can suppress this pathological reaction (89). B cells, especially memory B cells, also secrete GM-CSF, inducing the activation of pro-inflammatory myeloid cells (90). The upregulation of the CD22 protein, a B-cell receptor functioning as an inhibitory modulator of phagocytosis in microglia of aged individuals, may be correlated with decreased cognitive function in aged mice (90).
It has been demonstrated that by regulating microglial activation and the behavior of other non-neuronal cells, adaptive immune cells can exert indirect influences on synaptic processes and neuronal functioning. For instance, the presence of CD4+ T cells is essential for the progression and maturation of microglia (91). B cells infiltrate the brain during early mouse development and facilitate the proliferation of oligodendrocyte precursor cells via immunoglobulin M-Fcα/μR signal transduction, thus promoting myelin formation (92). Specifically, after demyelinating injury occurs in mice, myeloid cells with proinflammatory properties demonstrate a more aggressive role in myelin repair. MyD88-dependent signaling triggers these proinflammatory microglia to secrete TNFα, thereby promoting the generation of oligodendrocytes capable of new myelin formation (85).Francisco J. Quintana validated a novel single-cell sequencing technology called RABID-seq, which uncovered communication between microglia and astrocytes involving the EphB3-Ephrin-B3 and Sema4D-PlexinB2 signaling pathways (93). In chronic MS and its animal models, NK cells accumulate in the subventricular zone, particularly near neural stem cells that generate IL-15 and maintain NK cell function (94).
3.2 Immune cells cross the BBB
The BBB serves as a vital shield, safeguarding the central nervous system from external deleterious substances. It is comprised mainly consists of endothelial cells, pericytes with their basement membrane, astrocytes, and perivascular macrophages (95). In the context of MS, the interplay between immune cells and endothelial cells, along with the BBB, serves as a pivotal factor in the advancement of the disease. Studies have shown that immune cells, such as T and B cells, have the ability to cross the BBB, penetrate into the CNS, and trigger an inflammatory response through specific molecular pathways. These intricate mechanisms encompass the upregulation of cell adhesion molecules, the secretion of chemokines, and modifications in BBB permeability. Kendirli A et al. tracked the migration of CD4+ T cells from blood to CSF using scRNA-seq and TCR-seq techniques. They demonstrated that the expression of the migration-promoting genes HSP90B1, GNAI2, and S1PR1 was positively correlated with their ability to migrate to the CNS, whereas the negative regulator ETS1 exhibited a negative correlation with the migratory ability of CD4+ T cells from MS patients (96). IFN-γ and TLR9-activated T-bet-high IgG1 B cells may potentiate their recruitment via CXCR3 and amplify local immune responses within the CNS of MS patients (97). In comparison to healthy controls, brain microvascular endothelial cells sourced from individuals with MS exhibited compromised junctional integrity, reduced barrier functions, and decreased efflux pump activity. Furthermore, these cells demonstrated an inflammatory profile, characterized by elevated levels of adhesion molecule expression and heightened interaction with immune cells. Activation of Wnt/β-catenin signaling in endothelial progenitor cells isolated from MS was found to enhance barrier characteristics and attenuate the inflammatory response. Post-mortem brain tissue analysis of patients with MS revealed a reduction or disruption of proteins integral to tight and adhesion junctions, such as occludin, claudin-5, and vascular endothelial cadherin. Notably, extravascular leakage of serum components like IgG and fibrinogen was observed in the damaged BBB. Additionally, the crucial efflux pump P-glycoprotein (P-gp) was impaired within the lesions of MS patients. During MS, the endothelial cells of the BBB underwent an immunophenotypic shift, with the upregulation of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and atypical chemokine receptor 1 (ACKR1), thereby facilitating increased immune cell infiltration into the CNS (98).
Within the choroid plexus stroma, stromal cells release pro-inflammatory mediators, including IL-6, CCL2, CXCL1, and CXCL2 (99), in response to IL-1β produced by infiltrating activated APCs. Stimulated by cytokines such as IFN-γ and IL-17, the CP epithelium upregulates specialized trafficking molecules and secretes the chemokine ligand CCL20, thereby facilitating B and T cell migration into the cerebrospinal fluid (30).
Conversely, strategies aimed at inhibiting lymphocyte entry into the CNS, either by adhering to or sequestering lymphocytes in primary lymphoid organs, have proven effective in treating both MS and EAE. Adhesion blockade can be achieved with natalizumab, while lymphocyte sequestration can be induced using sphingosine-1-phosphate (S1P) receptor modulators such as siponimod and ozanimod.
3.3 The immune system interacts with other systems
The pathogenesis of MS transcends classical immune dysregulation, involving coordinated multiorgan crosstalk among the neurovascular unit, hypothalamic-pituitary-adrenal axis, and bone marrow-derived myeloid effector circuits.
The CNS constitutes the principal target of immunopathological assault in MS, with cardinal pathomechanisms centered on demyelination, axonal degeneration, and neurodegenerative processes. It has been shown that migratory Th cells from patients with MS express cytokines that target CNS homing molecules, thereby potentially fostering the proliferation of pathogenic CD4+ effector memory T cells within the inflamed CNS. TRM cells have been implicated in increased myeloid differentiation in MS (26). TRM cells are also recognized for their role in surveying the brain parenchyma in the absence of inflammation. It is postulated that CD8+ T-cell clones exhibiting a TRM phenotype, which are expanded, play a pivotal role in CNS injury. These cells constitute the primary lymphocyte population that persists and is shared between the CNS and CSF (27). Moreover, we identified a correlation between the expansion of clonal B-cells and gadolinium enhancement observed on MRI, hinting that the proliferation of clonal B-cells in the CSF becomes more pronounced during active demyelination and disruption of the BBB. This discovery extends a recent research that associated a heightened IgG index with elevated levels of neurofilament light chains in CSF, which serves as an indicator of axonal damage (100). The elevated somatic hypermutation (SHM) observed in IgM+ cells within CSF and the presence of a greater proportion of basic residues in the heavy chain complementarity-determining region 3 (H-CDR3) align with the exposure to neoantigens in the CSF. Furthermore, IgG1+ B cells exhibit longer H-CDR3 sequences compared to their counterparts in the blood, providing additional evidence of neoantigen exposure within the CNS (42).
Hypothalamic-pituitary-adrenal axis dysfunction is frequently observed in MS, which researchers suggest may be associated with orexinergic neurotransmission disturbances and abnormal cortisol secretion (101). Vitamin D plays a crucial role in regulating the immune system in MS by exerting anti-inflammatory and potential neuroprotective effects. It aids in myelin regeneration through oligodendrocyte precursor cells (OPCs), inhibits the activation of reactive astrocytes and M1 microglia to counteract neurodegenerative and oxidative stress processes, fosters the upregulation of neuroprotective factors, as well as regulates blood-brain barrier permeability (102). Alterations in the gut microbiota impact the susceptibility of mice in the EAE mice to neuroinflammatory demyelinating diseases. The gut-brain axis constitutes a sophisticated communication network encompassing the gut microbiota and the immune, nervous, and endocrine systems, whereby through neuroendocrine, neurotransmitter, and neuroimmune signaling mechanisms, the gut microbiota exerts an influence on the CNS (103), and can generate immunogenic endotoxins, such as lipopolysaccharide (LPS) (104), inducing autoimmune and neuroinflammatory responses within the brain. In MS, immune cells like microglia and meningeal NK cells become activated by antigens originating from the host or gut microbiota, leading to their migration into the CNS. This migration influences the formation and function of astrocytes (105), thereby promoting neuroinflammatory processes.
Through integrated single-cell transcriptomics and clonal lineage tracing, Liu et al. revealed pathological expansion of myeloid compartments in both MS patients and EAE models. Mechanistically, CNS-autoreactive T cells were shown to undergo CXCL12-CXCR4-mediated bone marrow homing, where they activate the CCL5-CCR5 chemotactic circuit to drive pathogenic myeloid expansion. Molecular profiling reveals dysregulated transcriptional networks in MS hematopoietic stem/progenitor cells (HSPCs), marked by enhanced granulocyte-monocyte progenitor (GMP) programs (AZU1+/MPO+/S100A8+) and hyperactivated monocyte-dendritic precursors (MDPs) with CST3hi/IFITM3hi/CTSShi signatures, along with upregulated myeloid-lineage transcription factors (CEBPZ↑, RARA↑, IRF8↑) that orchestrate pathological differentiation trajectories (22). Mildner et al. further distinguished eight monocyte subsets and three DC subsets in the acute and chronic phases of MS, highlighting the pathogenic Cxcl10+ and Saa3+ monocyte subsets originating from early myeloid progenitors (106). Collectively, these findings delineate a cell-autonomous bias of hematopoietic stem cells towards accelerated myeloid commitment in MS pathogenesis. The aberrant myelopoiesis drives excessive production of inflammatory neutrophils and monocytic effectors that breach the blood-brain barrier, creating self-reinforcing neuroinflammation through feedforward amplification of demyelinating cascades. Through the analysis of twin pairs, F. Ingelfinger and colleagues discovered that twins with MS exhibited a shift in bone marrow compartments, characterized by a transition from nonclassical monocytes to inflammatory classical monocytes, accompanied by a reduction in the type 1 interferon gene signature. These monocyte subpopulations demonstrated heightened expression of CCR2 and GM-CSF receptors, indicating their heightened sensitivity to inflammatory stimuli (26).
3.4 Immunological microenvironment comparison among different types of MS
MS is characterized by a dynamic disease continuum and its course must be viewed from a developmental perspective. In the developmental trajectory of MS, CIS represents the initial stage, suggesting that some patients are at risk of progressing to RRMS and that over time the majority of patients with RRMS will progress to SPMS. The presence of focal plaques is a central pathological characteristic that is common to all types of MS, which manifest pathologically as demyelination in the peripheral area of small post-capillary veins. However, these subtypes exhibit significant differences in their immunological microenvironments. Firstly, RRMS is characterized by intermittent disease attacks and remissions. The immunological microenvironment in RRMS shows periodic exacerbation and alleviation of inflammatory responses. scRNA-seq of lesion tissues in RRMS patients reveals numerous active inflammatory T cells, especially Th1 and Th17 cells, as well as abnormally activated B cells that produce autoantibodies. During remission periods, Tregs are enhanced, helping to suppress inflammation (107). Secondly, SPMS presents as a gradually worsening disability with relatively stable inflammatory reactions. In SPMS patients, single-cell sequencing shows fewer increases in immune cells but a significant decrease in neuroprotective Tregs. This reduction leads to ongoing inflammation and neuronal damage. The imbalance in the immunoregulatory network results in the loss of neuroprotective mechanisms, accelerating disease progression (42). Thirdly, in PPMS, the disease presents with an earlier onset of progressive disability, with no obvious intermittent inflammatory attacks. Lesion tissues in PPMS patients, analyzed using single-cell sequencing, reveal persistently activated inflammatory T cells, especially Th17 cells, and an abnormal increase in macrophages. Additionally, the function of Tregs is inhibited, leading to sustained autoimmune responses without effective control (108). In summary, RRMS is dominated by periodic inflammatory fluctuations and enhanced Treg roles. In contrast, SPMS and PPMS show sustained activation of inflammatory cells and an imbalance in the inflammatory regulatory network, contributing to progressive disease deterioration. Understanding these differences in the immunological microenvironments provides a theoretical basis for tailored treatment and intervention strategies targeting specific immune cell subtypes in different types of MS.
3.5 Immunological microenvironment comparison between MS and other autoimmune systemic diseases
MS is a complex disorder involving neurons, immune cells, and glial cells, marked by clinical and pathological heterogeneity. The MS microenvironment comprises various signals that regulate remyelination and myelin sheath disruption. Traditional bulk genomic and transcriptomic analyses have provided significant insights into disease remission and activity but may obscure critical signals from specific cell populations. Understanding these signals is crucial for improving MS treatment responses and advancing stem cell and immune cell therapies (42). Compared to other autoimmune systemic diseases, the immunological microenvironment of MS displays unique features. This group of cells primarily consists of T cells, B cells and macrophages, all of which play a role in the inflammatory process. In RRMS patients, single-cell sequencing has revealed numerous active inflammatory T cells, particularly Th1 and Th17 cells, along with abnormally activated B cells that produce autoantibodies (107). In other autoimmune diseases, like systemic lupus erythematosus (SLE) (100) and rheumatoid arthritis (RA)[82], the types of inflammatory cells involved may vary, with T cells, B cells, and macrophages playing more prominent roles. Additionally, the specificity of autoantibodies to target organs differs. In MS, B cells produce autoantibodies against self-antigens, affecting nervous system tissues. In contrast, SLE and RA involve autoantibodies targeting different tissue organs (109). Additionally, MS exhibits abnormal cytokine expression patterns, including pro-inflammatory cytokines like IL-17 and IFN-γ, as well as anti-inflammatory cytokines such as IL-10 and TGF-β. In other autoimmune diseases, the patterns and characteristics of cytokine expression may differ. In MS, the dysfunction of immunoregulatory cells, such as Treg cells, gives rise to a disruption in immune tolerance, ultimately causing ineffective control of autoimmune attacks. While immunoregulatory cells also play essential roles in other autoimmune diseases, their mechanisms of action may differ. In conclusion, significant differences exist in the immunological microenvironment between MS and other autoimmune systemic diseases, contributing to distinct pathological features and clinical manifestations. Understanding these differences is crucial for elucidating the pathogenesis and pathological processes of various autoimmune diseases, providing new perspectives and strategies for personalized treatment and intervention. However, additional research is necessary to fully understand the intricate mechanisms underlying these differences.
4 Targeted therapeutic strategies for immunomodulation
Inflammation plays a pivotal role in the early stages of MS, which is known as the “golden window” for treatment. During this phase, widespread neurological damage has not yet accumulated, and the body’s reparative mechanisms are still active, making Disease Modifying Therapies (DMTs) most effective. However, as the disease progresses, the capacity for repair diminishes, leading to irreversible disability. The clinical manifestations and treatment responses of MS vary extensively, thus presenting challenges for accurate diagnosis and effective therapy. Therefore, personalized molecular targeted therapy is urgently needed.
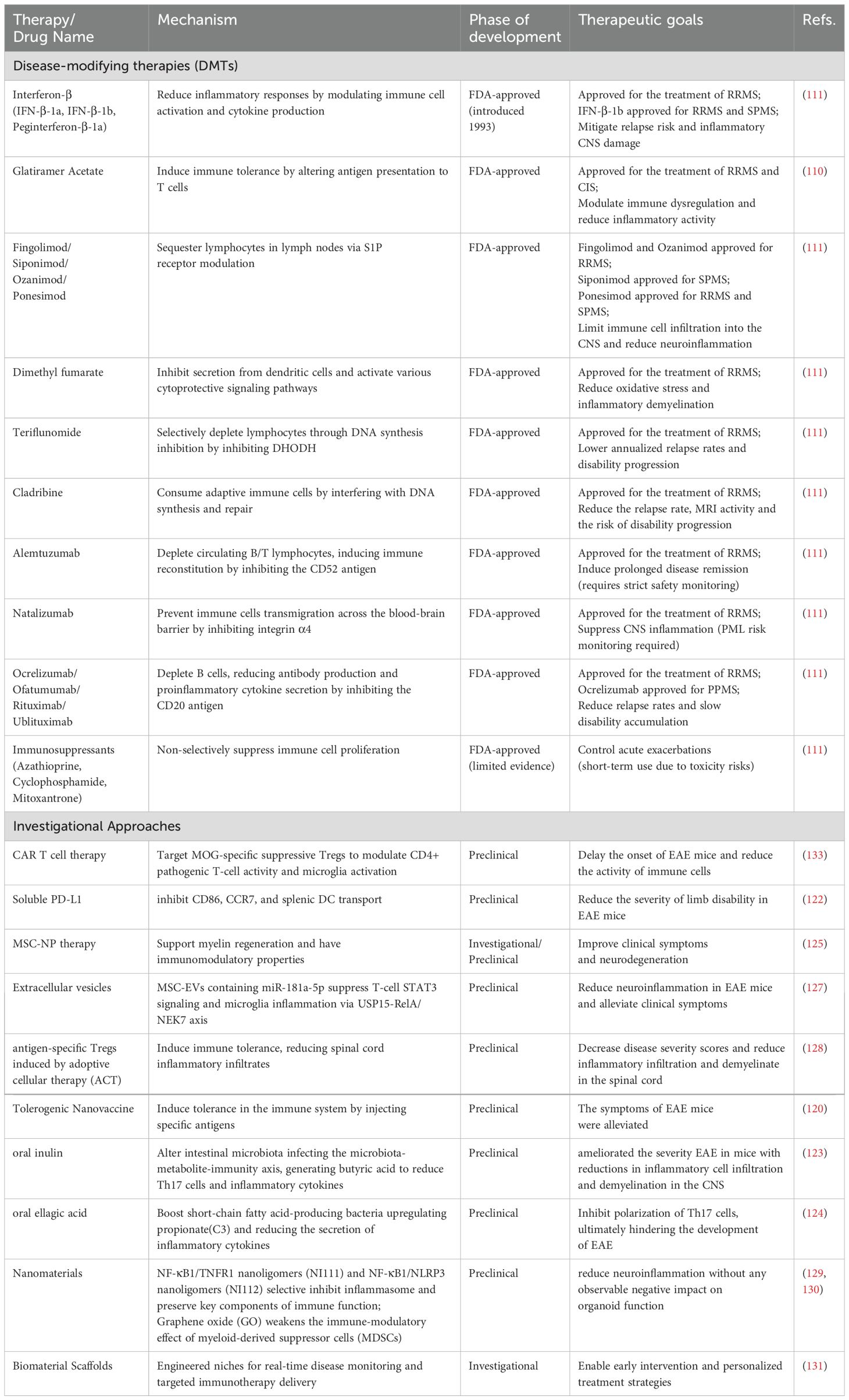
Since the introduction of IFN-β for the treatment of MS in 1993, a growing array of immunomodulatory medications has been shown to be effective and has gained widespread utilization. These drugs help reduce inflammatory brain damage and decrease the likelihood of clinical relapse through various mechanisms. Key therapeutic drugs include IFN β-1a, β-1b, and pegylated interferon β-1a, which reduce inflammatory responses; glatiramer acetate, which affects antigen presentation to T cells (110). Fingolimod, siponimod, ozanimod, and ponesimod, which regulate immune cell movement by affecting S1P receptors; dimethyl fumarate, which inhibits secretion from dendritic cells; and DNA synthesis inhibitors such as cladribine and teriflunomide (111). Additionally, some immunosuppressive agents, including azathioprine, cyclophosphamide, glucocorticoids, IVIG, and mitoxantrone, are used based on limited evidence.
Compared to traditional treatments, monoclonal antibodies can more precisely target specific immune pathways or cells, reducing damage to healthy tissues and demonstrating better efficacy and safety in individual patients. For example, CD52 inhibitors such as alemtuzumab modulate the immune system by inhibiting the CD52 antigen, reducing inflammatory responses. Natalizumab prevents immune cells from entering the CNS by inhibiting integrin α4, thereby reducing inflammation. CD20 inhibitors, including ocrelizumab, ofatumumab, rituximab, and ublituximab, modulate the immune system by inhibiting the CD20 antigen, thus reducing inflammation. An overview of therapeutic targets for DMTs in MS is shown in Figure 2.
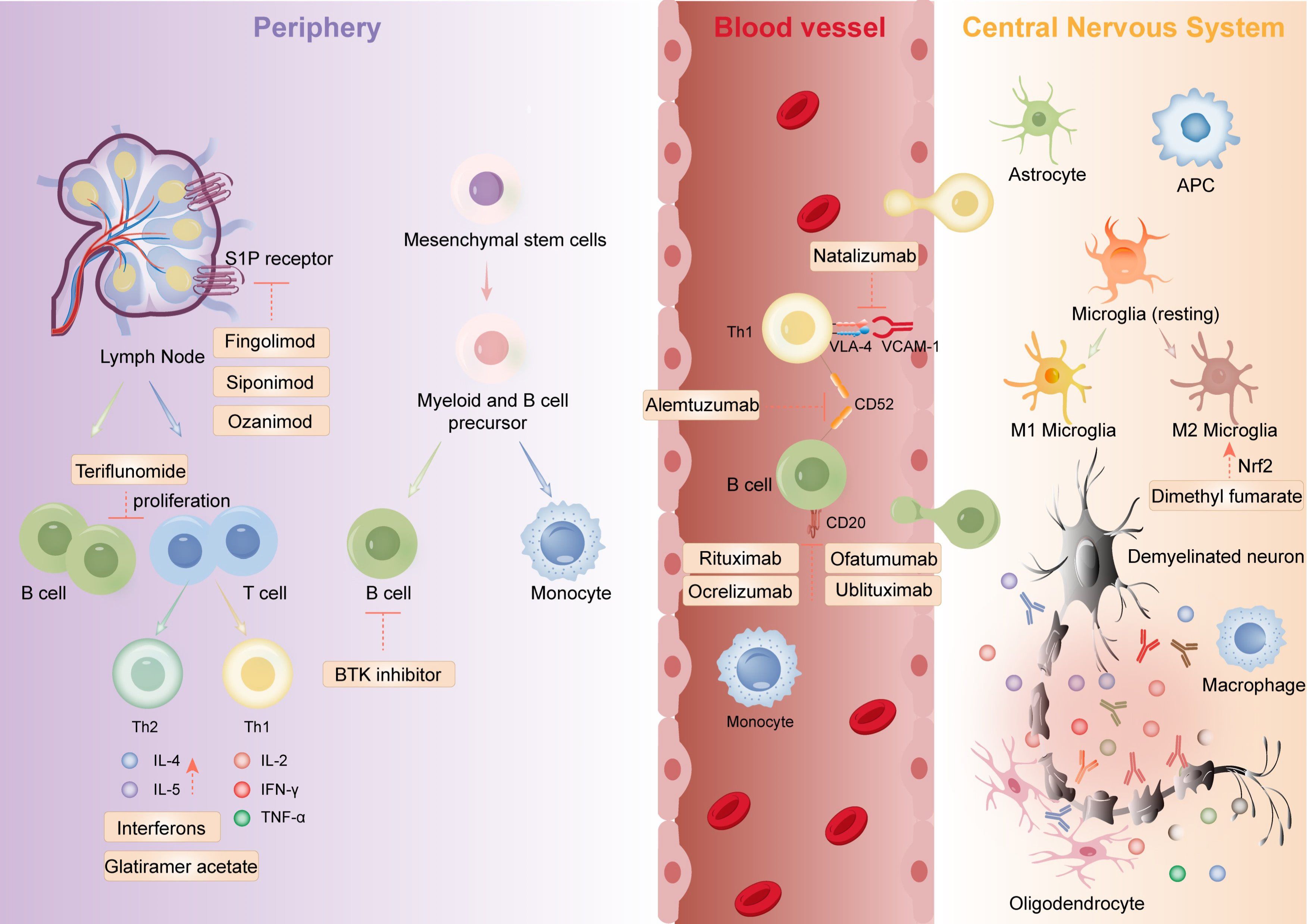
Figure 2. The mechanistic framework of marketed immune-targeting therapeutics for multiple sclerosis encompasses: orchestrating lymphocyte polarization, suppressing pathogenic Th17/Treg imbalance, enforcing CNS infiltration blockade via endothelial adhesion modulation, and stimulating oligodendrocyte-mediated remyelination. Figure created with Adobe Illustrator.
Advancements in single-cell sequencing technology have led to more precise targeted therapies. β-interferon confirmed immunomodulatory drugs’ efficacy in MS. Immunosuppressants like azathioprine and glucocorticosteroids have also been used. Since then, DMTs has shifted to targeted agents like glatiramer acetate, cladribine, and teriflunomide. Monoclonal antibodies, with their precision in targeting immune pathways, have emerged as key therapies, offering tailored efficacy and safety.
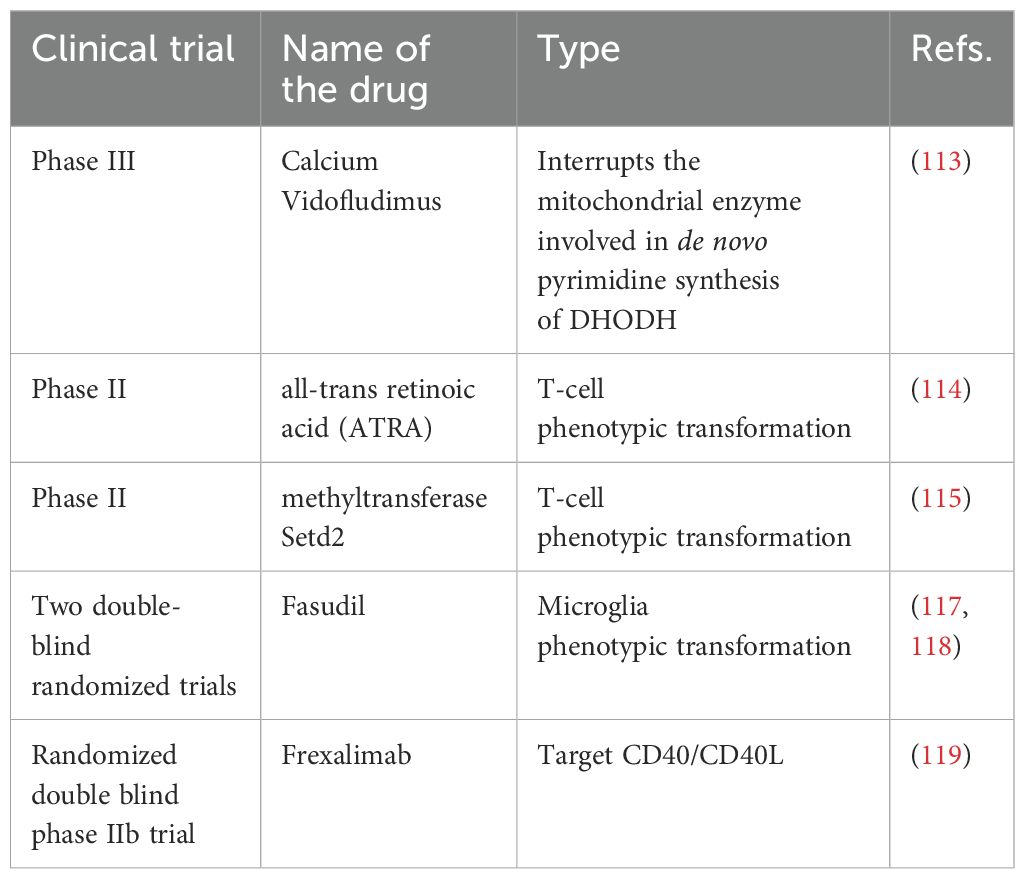
Furthermore, several preclinical and clinical trial drugs are being developed for MS treatment, showing considerable efficacy in experimental animal models (Table 2). For instance, in EAE models, oral administration of adrenocorticotropic hormone (ACTH) reduces IL-17 and IFN-γ in the CNS (112). By blocking the Dihydroorotate Dehydrogenase (DHODH) enzyme, Calcium vidofludimus (IMU-838; Immunic AG, Germany) inhibits the intracellular metabolic processes of activated T and B cells (113). Repeated immunization with all-trans retinoic acid (ATRA) and CAF16 liposomal adjuvant in an EAE model resulted in T-cell phenotypic transformation (114). Methyltransferase Setd2, an epigenetic regulator, inhibits the development of Th17 cells while promoting iTreg cell polarization through phospholipid remodeling (115). B-cell activation factor(BAFF) shifts B-cell pools towards a regulated phenotype, affecting B-cell function (116). Fasudil inhibits microglia-mediated neuroinflammation (117, 118). Frexalimab blocks the co-stimulatory CD40/CD40L cell pathway (119). Immune Cell Tolerance Therapy vaccines induce tolerance in the immune system by injecting specific antigens, suppressing autoimmune responses, and reducing disease symptoms (120). There are several factors to consider when choosing and combining medicines, including the patient’s medical condition, the severity of the disease, and the patient’s tolerance to medicines. The comprehensive use of different drug categories can effectively control MS symptoms and progression.
There are some preclinical drugs that have shown effectiveness in animal models of MS disease. The use of suppressor Tregs to induce durable tolerogenic autologous chimeric antigen receptor (CAR) T cell therapy targeting myelin oligodendrocyte glycoprotein (MOG), effectively modulates CD4+ pathogenic cell activity and diminishes microglia activation, thereby significantly reducing inflammation in the CNS of EAE mice (121). Programmed cell death 1 (PD-1) interacts with programmed cell death ligand 1(PD-L1) to maintain immune tolerance. Soluble PD-L1 (sPD-L1) alleviates clinical symptoms of MOG-induced EAE by inhibiting CD86, CCR7, and splenic DC transport (122). The microbiota-metabolite-immunity axis also has the potential to influence EAE disease progression. Ning and Li,Xinyan observed that oral inulin alters intestinal microbiota, reducing Th17 cells and inflammatory cytokines (123). Additionally, Bing and Han, Lin found that oral ellagic acid(EA) boosts short-chain fatty acid-producing bacteria(e.g., Alloprevotella), upregulating propionate(C3) and reducing the secretion of inflammatory cytokines by pathogenic Th17 cells (124). Autologous mesenchymal stem cell (MSC) therapy addresses both immune and neurodegenerative mechanisms in MS. Furthermore, MSC-derived neural progenitor cells (MSC-NPs) support myelin regeneration and have immunomodulatory properties (125). Extracellular vesicles (EVs) derived from MSCs, containing miR-181a-5p, have been shown to decrease STAT3 expression in T-cell regulation. These EVs also inhibit microglia inflammation and cellular death through the USP15-mediated RelA/NEK7 axis (126, 127). Furthermore, antigen-specific Tregs induced by adoptive cellular therapy (ACT) in treated mice resulted in a decrease in inflammatory infiltration and demyelination within the spinal cord (128). Nanomaterials are also used in MS therapy. The combination of nanoligomers (NF-κB1 + TNFR1, known as NI111, and NF-κB1 + NLRP3, known as NI112) reduce neuroinflammation without adverse effects on organoid function (129). Graphene oxide (GO) is biocompatible, but its immunomodulatory effect weakens when myeloid-derived suppressor cells (MDSCs) are exposed to rougher rGO (90), where cellular size correlates with apoptotic activation (130). Morris and Rad, among others, have devised a biomaterial scaffold intended to replace diseased tissue and establish engineered immunological niches for monitoring disease progression. This enables the recognition of pathological signals at the onset of disease and the prompt application of targeted immunotherapy (131, 132). Table 3 presents a comparative analysis of promising investigational therapeutic strategies and clinically validated DMTs in MS management.
5 Conclusion and future perspectives
The CNS was traditionally viewed as immune-privileged, with mechanisms in place to limit immune responses and prevent potential neurological damage. However, emerging research has revealed that the CNS and immune system are interconnected more intricately and dynamically. Beyond the resident immune cells of the CNS, specifically microglia, which are responsible for local immunosurveillance and regulation, there is a broader cerebral immune network involving multiple peripheral immune components. This network includes the meninges, the skull’s bone marrow, the choroid plexus, and the lymphatic drainage system, all of which contribute to neuroprotection, antigen presentation, and immune regulation. However, In MS, this immune microenvironment is disrupted by intense inflammation and myelin damage, resulting in either acute or chronic neurological harm. Peripherally derived T cells, B cells, and macrophages cross the BBB and enter the CNS, releasing numerous inflammatory factors and creating localized inflammatory foci.
Advancements in technologies like single-cell sequencing and spatial transcriptomics now allow for a more detailed mapping of immune cells within the CNS, revealing their dynamic changes and functional characteristics. These developments are crucial for understanding the intricate immune microenvironment of the CNS and the immune system and are paving the way for novel treatment approaches for neurological diseases.
Author contributions
XS: Writing – original draft, Writing – review & editing, Conceptualization, Investigation. FZ: Writing – original draft, Writing – review & editing. LW: Writing – original draft, Writing – review & editing. GL: Visualization, Writing – original draft. SY: Visualization, Writing – original draft. DZ: Visualization, Writing – original draft. BC: Visualization, Writing – original draft. BH: Writing – review & editing. YZ: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the grants from National Key Research and Development Program of China (grant 2024YFC3044800 to BH), Young Elite Scientists Sponsorship Program by CAST (No.2022QNRC001), the National Natural Science Foundation of China (No. 82371341 to YZ, No. 82330040 and 82090044 to BH, No.81820108010 to BH), Noncommunicable Chronic Diseases-National Science and Technology Major Project (grant 2024ZD0527901 to BH), Hubei Province Key R&D Program (No. 2022BCA008 to BH). Core medical technology project of union hospital (No. 2024JBGS2008 to BH).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Reich DS, Lucchinetti CF, and Calabresi PA. Multiple sclerosis. New Engl J Med. (2018) 378:169–80. doi: 10.1056/nejmra1401483
2. Sejvar J. H1 Connect Recommendation of GBD 2015 Neurological Disorders Collaborator Group. Lancet Neurol. 16(11:877-897)]. In H1 Connect. (2018). doi: 10.3410/f.731253574.793550809
3. Wallin MT, Culpepper WJ, Campbell JD, Nelson LM, Langer-Gould A, Marrie RA, et al. The prevalence of MS in the United States. Neurology. (2019) 92:e1029–40. doi: 10.1212/wnl.0000000000007035
4. Noseworthy JH, Lucchinetti C, Rodriguez M, and Weinshenker BG. Multiple sclerosis. New Engl J Med. (2000) 343:938–52. doi: 10.1056/nejm200009283431307
5. Sawcer S, Franklin RJM, and Ban M. Multiple sclerosis genetics. Lancet Neurology. (2014) 13:700–9. doi: 10.1016/s1474-4422(14)70041-9
6. Lassmann H. Multiple sclerosis pathology: evolution of pathogenetic concepts. Brain Pathology. (2005) 15:217–22. doi: 10.1111/j.1750-3639.2005.tb00523.x
7. Venken K, Hellings N, Liblau R, and Stinissen P. Disturbed regulatory T cell homeostasis in multiple sclerosis. Trends Mol Med. (2010) 16:58–68. doi: 10.1016/j.molmed.2009.12.003
8. van Langelaar J, van der Vuurst de Vries RM, Janssen M, Wierenga-Wolf AF, Spilt IM, Siepman TA, et al. T helper 17.1 cells associate with multiple sclerosis disease activity: perspectives for early intervention. Brain. (2018) 141:1334–49. doi: 10.1093/brain/awy069
9. Holm Hansen R, Talbot J, Højsgaard Chow H, Bredahl Hansen M, Buhelt S, Herich S, et al. Increased intrathecal activity of follicular helper T cells in patients with relapsing-remitting multiple sclerosis. Neurol Neuroimmunology Neuroinflammation. (2022) 9:e200009. doi: 10.1212/nxi.0000000000200009
10. Comabella M, Montalban X, Münz C, and Lünemann JD. Targeting dendritic cells to treat multiple sclerosis. Nat Rev Neurology. (2010) 6:499–507. doi: 10.1038/nrneurol.2010.112
11. Cencioni MT, Mattoscio M, Magliozzi R, Bar-Or A, and Muraro PA. B cells in multiple sclerosis — from targeted depletion to immune reconstitution therapies. Nat Rev Neurology. (2021) 17:399–414. doi: 10.1038/s41582-021-00498-5
12. Jelcic I, Al Nimer F, Wang J, Lentsch V, Planas R, Jelcic I, et al. Memory B cells activate brain-homing, autoreactive CD4(+) T cells in multiple sclerosis. Cell. (2018) 175:85–100 e123. doi: 10.1016/j.cell.2018.08.011
13. Voet S, Prinz M, and van Loo G. Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol Med. (2019) 25:112–23. doi: 10.1016/j.molmed.2018.11.005
14. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. (2015) 523:337–41. doi: 10.1038/nature14432
15. Castellani G, Croese T, Peralta Ramos JM, and Schwartz M. Transforming the understanding of brain immunity. Science. (2023) 380:eabo7649. doi: 10.1126/science.abo7649
16. Johansson D, Rauld CL, Roux J, Regairaz C, Galli E, Callegari I, et al. Mass cytometry of CSF identifies an MS-associated B-cell population. Neurol Neuroimmunology Neuroinflammation. (2021) 8:e943. doi: 10.1212/nxi.0000000000000943
17. Minhas PS, Latif-Hernandez A, McReynolds MR, Durairaj AS, Wang Q, Rubin A, et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature. (2021) 590:122–8. doi: 10.1038/s41586-020-03160-0
18. Prinz M, Masuda T, Wheeler M A, and Quintana F J. Microglia and central nervous system-associated macrophages-from origin to disease modulation. Annu Rev Immunol. (2021) 39:251–77. doi: 10.1146/annurev-immunol-093019-110159
19. Rustenhoven J, Drieu A, Mamuladze T, de Lima KA, Dykstra T, Wall M, et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell. (2021) 184:1000–1016.e1027. doi: 10.1016/j.cell.2020.12.040
20. Møllgård K, Beinlich FRM, Kusk P, Miyakoshi LM, Delle C, Plá V, et al. A mesothelium divides the subarachnoid space into functional compartments. Science. (2023) 379:84–8. doi: 10.1126/science.adc8810
21. Zheng W, Feng Y, Zeng Z, Ye M, Wang M, Liu X, et al. Choroid plexus-selective inactivation of adenosine A2A receptors protects against T cell infiltration and experimental autoimmune encephalomyelitis. J Neuroinflammation. (2022) 19:52. doi: 10.1186/s12974-022-02415-z
22. Shi K, Li H, Chang T, He W, Kong Y, Qi C, et al. Bone marrow hematopoiesis drives multiple sclerosis progression. Cell. (2022) 185:2234–47.e2217. doi: 10.1016/j.cell.2022.05.020
23. Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. (2015) 212:991–9. doi: 10.1084/jem.20142290
24. Tan LY, Cunliffe G, Hogan MP, Yeo XY, Oh C, Jin B, et al. Emergence of the brain-border immune niches and their contribution to the development of neurodegenerative diseases. Front Immunol. (2024) 15:1380063. doi: 10.3389/fimmu.2024.1380063
25. Pappalardo JL, Zhang L, Pecsok MK, Perlman K, Zografou C, Raddassi K, et al. Transcriptomic and clonal characterization of T cells in the human central nervous system. Sci Immunol. (2020) 5:1–14. doi: 10.1126/sciimmunol.abb8786
26. Ingelfinger F, Gerdes LA, Kavaka V, Krishnarajah S, Friebel E, Galli E, et al. Twin study reveals non-heritable immune perturbations in multiple sclerosis. Nature. (2022) 603:152–8. doi: 10.1038/s41586-022-04419-4
27. Beltrán E, Gerdes LA, Hansen J, Flierl-Hecht A, Krebs S, Blum H, et al. Early adaptive immune activation detected in monozygotic twins with prodromal multiple sclerosis. J Clin Invest. (2019) 129:4758–68. doi: 10.1172/jci128475
28. Lazarevic I, Soldati S, Mapunda JA, Rudolph H, Rosito M, de Oliveira AC, et al. The choroid plexus acts as an immune cell reservoir and brain entry site in experimental autoimmune encephalomyelitis (vol 20, 39, 2023). Fluids Barriers Cns. (2023) 20:39. doi: 10.1186/s12987-023-00457-w
29. Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. (2009) 10:514–23. doi: 10.1038/ni.1716
30. Haas J, Rudolph H, Costa L, Faller S, Libicher S, Wuerthwein C, et al. The choroid plexus is permissive for a preactivated antigen-experienced memory B-cell subset in multiple sclerosis. Front Immunol. (2021) 11:618544. doi: 10.3389/fimmu.2020.618544
31. Rodriguez-Lorenzo S, Konings J, van der Pol S, Kamermans A, Amor S, Van Horssen J, et al. Inflammation of the choroid plexus in progressive multiple sclerosis: accumulation of granulocytes and T cells (vol 8, 9, 2020). Acta Neuropathologica Commun. (2020) 8:9. doi: 10.1186/s40478-020-00899-5
32. Ruterbusch M, Pruner KB, Shehata L, and Pepper M. In vivo CD4(+) T cell differentiation and function: revisiting the th1/th2 paradigm. Annu Rev Immunol. (2020) 38:705–25. doi: 10.1146/annurev-immunol-103019-085803
33. Ando DG, Clayton J, Kono D, Urban JL, and Sercarz EE. Encephalitogenic T cells in the B10.PL model of experimental allergic encephalomyelitis (EAE) are of the Th-1 lymphokine subtype. Cell Immunol. (1989) 124:132–43. doi: 10.1016/0008-8749(89)90117-2
34. Panitch HS, Hirsch RL, Haley AS, and Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. (1987) 1:893–5. doi: 10.1016/s0140-6736(87)92863-7
35. Park E, Barclay WE, Barrera A, Liao TC, Salzler HR, Reddy TE, et al. Integrin alpha3 promotes T(H)17 cell polarization and extravasation during autoimmune neuroinflammation. Sci Immunol. (2023) 8:eadg7597. doi: 10.1126/sciimmunol.adg7597
36. Ferraro D, De Biasi S, Simone AM, Orlandi R, Nasi M, Vitetta F, et al. Modulation of tregs and iNKT by fingolimod in multiple sclerosis patients. Cells. (2021) 10:3324. doi: 10.3390/cells10123324
37. van Oosten BW, Lai M, Hodgkinson S, Barkhof F, Miller DH, Moseley IF, et al. Treatment of multiple sclerosis with the monoclonal anti-CD4 antibody cM-T412: Results of a randomized, double-blind, placebo-controlled MR-monitored phase II trial. Neurology. (1997) 49:351–7. doi: 10.1212/wnl.49.2.351
38. Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, et al. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. (2000) 192:393–404. doi: 10.1084/jem.192.3.393
39. MaChado-Santos J, Saji E, Tröscher AR, Paunovic M, Liblau R, Gabriely G, et al. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain. (2018) 141:2066–82. doi: 10.1093/brain/awy151
40. Sospedra M and Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. (2005) 23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707
41. Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. (2008) 358:676–88. doi: 10.1056/NEJMoa0706383
42. Ramesh A, Schubert RD, Greenfield AL, Dandekar R, Loudermilk R, Sabatino JJ Jr., et al. A pathogenic and clonally expanded B cell transcriptome in active multiple sclerosis. Proc Natl Acad Sci U S A. (2020) 117:22932–43. doi: 10.1073/pnas.2008523117
43. Couloume L, Ferrant J, Le Gallou S, Mandon M, Jean R, Bescher N, et al. Mass cytometry identifies expansion of T-bet(+) B cells and CD206(+) monocytes in early multiple sclerosis. Front Immunol. (2021) 12:653577. doi: 10.3389/fimmu.2021.653577
44. Probstel AK, Zhou X, Baumann R, Wischnewski S, Kutza M, Rojas OL, et al. Gut microbiota-specific IgA(+) B cells traffic to the CNS in active multiple sclerosis. Sci Immunol. (2020) 5:eabc7191. doi: 10.1126/sciimmunol.abc7191
45. Zanluqui NG, Fitzpatrick Z, Rosenblum JS, Tuong ZK, Chandrashekhar V, Negro-Demontel ML, et al. Venous plexus-associated lymphoid hubs support meningeal humoral immunity. J Immunol. (2023) 210:612–9. doi: 10.4049/jimmunol.210.Supp.76.23
46. Herisson F, Frodermann V, Courties G, Rohde D, Sun Y, Vandoorne K, et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat Neurosci. (2018) 21:1209–17. doi: 10.1038/s41593-018-0213-2
47. Brioschi S, Wang W-L, Peng V, Wang M, Shchukina I, Greenberg ZJ, et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science. (2021) 373:eabf9277. doi: 10.1126/science.abf9277
48. Schafflick D, Xu CA, Hartlehnert M, Cole M, Schulte-Mecklenbeck A, Lautwein T, et al. Integrated single cell analysis of blood and cerebrospinal fluid leukocytes in multiple sclerosis. Nat Commun. (2020) 11:247. doi: 10.1038/s41467-019-14118-w
49. Masuda T, Sankowski R, Staszewski O, Böttcher C, Amann L, Sagar, et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. (2019) 566:388–92. doi: 10.1038/s41586-019-0924-x
50. Esaulova E, Cantoni C, Shchukina I, Zaitsev K, Bucelli RC, Wu GF, et al. Single-cell RNA-seq analysis of human CSF microglia and myeloid cells in neuroinflammation. Neurol Neuroimmunology Neuroinflammation. (2020) 7:e732. doi: 10.1212/nxi.0000000000000732
51. Waschbisch A, Schroeder S, Schraudner D, Sammet L, Weksler B, Melms A, et al. Pivotal role for CD16+ monocytes in immune surveillance of the central nervous system. J Immunol. (2016) 196:1558–67. doi: 10.4049/jimmunol.1501960
52. Moore CS, Rao VTS, Durafourt BA, Bedell BJ, Ludwin SK, Bar-Or A, et al. miR-155 as a multiple sclerosis-relevant regulator of myeloid cell polarization. Ann Neurology. (2013) 74:709–20. doi: 10.1002/ana.23967
53. Sun R and Jiang H. Border-associated macrophages in the central nervous system. J Neuroinflammation. (2024) 21:67. doi: 10.1186/s12974-024-03059-x
54. Kierdorf K, Masuda T, Jordão MJC, and Prinz M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat Rev Neurosci. (2019) 20:547–62. doi: 10.1038/s41583-019-0201-x
55. Goldmann T, Wieghofer P, Jordão MJ, Prutek F, Hagemeyer N, Frenzel K, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. (2016) 17:797–805. doi: 10.1038/ni.3423
56. Cugurra A, Mamuladze T, Rustenhoven J, Dykstra T, Beroshvili G, Greenberg ZJ, et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science. (2021) 373:eabf7844. doi: 10.1126/science.abf7844
57. Ivan DC, Berve KC, Walthert S, Monaco G, Borst K, Bouillet E, et al. Insulin-like growth factor-1 receptor controls the function of CNS-resident macrophages and their contribution to neuroinflammation. Acta Neuropathol Commun. (2023) 11:35. doi: 10.1186/s40478-023-01535-8
58. Santos-Lima B, Pietronigro EC, Terrabuio E, Zenaro E, and Constantin G. The role of neutrophils in the dysfunction of central nervous system barriers. Front Aging Neurosci. (2022) 14:965169. doi: 10.3389/fnagi.2022.965169
59. Szmydynger-Chodobska J, Strazielle N, Gandy JR, Keefe TH, Zink BJ, Ghersi-Egea JF, et al. Posttraumatic invasion of monocytes across the blood-cerebrospinal fluid barrier. J Cereb Blood Flow Metab. (2012) 32:93–104. doi: 10.1038/jcbfm.2011.111
60. Gorina R, Lyck R, Vestweber D, and Engelhardt B. β2 integrin-mediated crawling on endothelial ICAM-1 and ICAM-2 is a prerequisite for transcellular neutrophil diapedesis across the inflamed blood-brain barrier. J Immunol. (2014) 192:324–37. doi: 10.4049/jimmunol.1300858
61. El Amki M, Glück C, Binder N, Middleham W, Wyss MT, Weiss T, et al. Neutrophils obstructing brain capillaries are a major cause of no-reflow in ischemic stroke. Cell Rep. (2020) 33:108260. doi: 10.1016/j.celrep.2020.108260
62. DiStasi MR and Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol. (2009) 30:547–56. doi: 10.1016/j.it.2009.07.012
63. Kang L, Yu H, Yang X, Zhu Y, Bai X, Wang R, et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun. (2020) 11:2488. doi: 10.1038/s41467-020-16191-y
64. Sie C and Korn T. Dendritic cells in central nervous system autoimmunity. Semin Immunopathol. (2017) 39:99–111. doi: 10.1007/s00281-016-0608-7
65. Macri C, Pang ES, Patton T, and O’Keeffe M. Dendritic cell subsets. Semin Cell Dev Biol. (2018) 84:11–21. doi: 10.1016/j.semcdb.2017.12.009
66. Hiltensperger M and Korn T. The interleukin (IL)-23/T helper (Th)17 axis in experimental autoimmune encephalomyelitis and multiple sclerosis. Cold Spring Harb Perspect Med. (2018) 8:a029637. doi: 10.1101/cshperspect.a029637
67. Isaksson M, Ardesjö B, Rönnblom L, Kämpe O, Lassmann H, Eloranta ML, et al. Plasmacytoid DC promote priming of autoimmune Th17 cells and EAE. Eur J Immunol. (2009) 39:2925–35. doi: 10.1002/eji.200839179
68. Pashenkov M, Huang Y-M, Kostulas V, Haglund M, Söderström M, and Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain. (2001) 124:480–92. doi: 10.1093/brain/124.3.480
69. Longhini ALF, von Glehn F, Brandão CO, de Paula RFO, Pradella F, Moraes AS, et al. Plasmacytoid dendritic cells are increased in cerebrospinal fluid of untreated patients during multiple sclerosis relapse. J Neuroinflammation. (2011) 8:2. doi: 10.1186/1742-2094-8-2
70. Haas J, Schwarz A, Korporal-Kuhnke M, Jarius S, and Wildemann B. Myeloid dendritic cells exhibit defects in activation and function in patients with multiple sclerosis. J Neuroimmunology. (2016) 301:53–60. doi: 10.1016/j.jneuroim.2016.10.007
71. Noster R, Riedel R, Mashreghi M-F, Radbruch H, Harms L, Haftmann C, et al. IL-17 and GM-CSF expression are antagonistically regulated by human T helper cells. Sci Trans Med. (2014) 6:241ra280. doi: 10.1126/scitranslmed.3008706
72. De Laere M, Berneman ZN, and Cools N. To the brain and back: migratory paths of dendritic cells in multiple sclerosis. J Neuropathology Exp Neurology. (2018) 77:178–92. doi: 10.1093/jnen/nlx114
73. Schirmer L, Velmeshev D, Holmqvist S, Kaufmann M, Werneburg S, Jung D, et al. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature. (2019) 573:75–82. doi: 10.1038/s41586-019-1404-z
74. Lawson LJ, Perry VH, Dri P, and Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. (1990) 39:151–70. doi: 10.1016/0306-4522(90)90229-w
75. Absinta M, Maric D, Gharagozloo M, Garton T, Smith MD, Jin J, et al. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature. (2021) 597:709–14. doi: 10.1038/s41586-021-03892-7
76. Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity. (2019) 50:253–271 e256. doi: 10.1016/j.immuni.2018.11.004
77. Kukanja P, Langseth CM, Rubio Rodríguez-Kirby LA, Agirre E, Zheng C, Raman A, et al. Cellular architecture of evolving neuroinflammatory lesions and multiple sclerosis pathology. Cell. (2024) 187:1990–2009.e1919. doi: 10.1016/j.cell.2024.02.030
78. Elkjaer ML, Hartebrodt A, Oubounyt M, Weber A, Vitved L, Reynolds R, et al. Single-cell multi-omics map of cell type–specific mechanistic drivers of multiple sclerosis lesions. Neurol Neuroimmunology Neuroinflammation. (2024) 11:e200213. doi: 10.1212/nxi.0000000000200213
79. Falcão AM, van Bruggen D, Marques S, Meijer M, Jäkel S, Agirre E, et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat Med. (2018) 24:1837–44. doi: 10.1038/s41591-018-0236-y
80. Lee H-G, Wheeler MA, and Quintana FJ. Function and therapeutic value of astrocytes in neurological diseases. Nat Rev Drug Discovery. (2022) 21:339–58. doi: 10.1038/s41573-022-00390-x
81. Brosnan CF and Raine CS. The astrocyte in multiple sclerosis revisited. Glia. (2013) 61:453–65. doi: 10.1002/glia.22443
82. Farina C, Aloisi F, and Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. (2007) 28:138–45. doi: 10.1016/j.it.2007.01.005
83. Hasel P, Rose IVL, Sadick JS, Kim RD, and Liddelow SA. Neuroinflammatory astrocyte subtypes in the mouse brain. Nat Neurosci. (2021) 24:1475–87. doi: 10.1038/s41593-021-00905-6
84. Wheeler MA, Clark IC, Tjon EC, Li Z, Zandee SEJ, Couturier CP, et al. MAFG-driven astrocytes promote CNS inflammation. Nature. (2020) 578:593–9. doi: 10.1038/s41586-020-1999-0
85. Bar-Or A and Li R. Cellular immunology of relapsing multiple sclerosis: interactions, checks, and balances. Lancet Neurol. (2021) 20:470–83. doi: 10.1016/s1474-4422(21)00063-6
86. Vinuesa CG, Linterman MA, Yu D, and MacLennan IC. Follicular helper T cells. Annu Rev Immunol. (2016) 34:335–68. doi: 10.1146/annurev-immunol-041015-055605
87. Guo J, Zhao C, Wu F, Tao L, Zhang C, Zhao D, et al. T follicular helper-like cells are involved in the pathogenesis of experimental autoimmune encephalomyelitis. Front Immunol. (2018) 9:944. doi: 10.3389/fimmu.2018.00944
88. Saxena A, Bauer J, Scheikl T, Zappulla J, Audebert M, Desbois S, et al. Cutting edge: Multiple sclerosis-like lesions induced by effector CD8 T cells recognizing a sequestered antigen on oligodendrocytes. J Immunol. (2008) 181:1617–21. doi: 10.4049/jimmunol.181.3.1617
89. Bar-Or A, Fawaz L, Fan B, Darlington PJ, Rieger A, Ghorayeb C, et al. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol. (2010) 67:452–61. doi: 10.1002/ana.21939
90. Li R, Rezk A, Miyazaki Y, Hilgenberg E, Touil H, Shen P, et al. Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med. (2015) 7:310ra166. doi: 10.1126/scitranslmed.aab4176
91. Pasciuto E, Burton OT, Roca CP, Lagou V, Rajan WD, Theys T, et al. Microglia require CD4 T cells to complete the fetal-to-adult transition. Cell. (2020) 182:625–640.e624. doi: 10.1016/j.cell.2020.06.026
92. Tanabe S and Yamashita T. B-1a lymphocytes promote oligodendrogenesis during brain development. Nat Neurosci. (2018) 21:506–16. doi: 10.1038/s41593-018-0106-4
93. Clark IC, Gutiérrez-Vázquez C, Wheeler MA, Li Z, Rothhammer V, Linnerbauer M, et al. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science. (2021) 372:eabf1230. doi: 10.1126/science.abf1230
94. Liu Q, Sanai N, Jin W-N, La Cava A, Van Kaer L, and Shi F-D. Neural stem cells sustain natural killer cells that dictate recovery from brain inflammation. Nat Neurosci. (2016) 19:243–52. doi: 10.1038/nn.4211
95. Ortiz GG, Pacheco-Moisés FP, Macías-Islas M, Flores-Alvarado LJ, Mireles-Ramírez MA, González-Renovato ED, et al. Role of the blood-brain barrier in multiple sclerosis. Arch Med Res. (2014) 45:687–97. doi: 10.1016/j.arcmed.2014.11.013
96. Kendirli A, de la Rosa C, Lämmle KF, Eglseer K, Bauer IJ, Kavaka V, et al. A genome-wide in vivo CRISPR screen identifies essential regulators of T cell migration to the CNS in a multiple sclerosis model. Nat Neurosci. (2023) 26:1713–25. doi: 10.1038/s41593-023-01432-2
97. van Langelaar J, Rijvers L, Janssen M, Wierenga-Wolf AF, Melief MJ, Siepman TA, et al. Induction of brain-infiltrating T-bet-expressing B cells in multiple sclerosis. Ann Neurol. (2019) 86:264–78. doi: 10.1002/ana.25508
98. Nishihara H, Perriot S, Gastfriend BD, Steinfort M, Cibien C, Soldati S, et al. Intrinsic blood-brain barrier dysfunction contributes to multiple sclerosis pathogenesis. Brain. (2022) 145:4334–48. doi: 10.1093/brain/awac019
99. Shimada A and Hasegawa-Ishii S. Increased cytokine expression in the choroid plexus stroma and epithelium in response to endotoxin-induced systemic inflammation in mice. Toxicol Rep. (2021) 8:520–8. doi: 10.1016/j.toxrep.2021.03.002
100. Fortuna G and Brennan MT. Systemic lupus erythematosus: epidemiology, pathophysiology, manifestations, and management. Dent Clin North Am. (2013) 57:631–55. doi: 10.1016/j.cden.2013.06.003
101. Burfeind KG, Yadav V, and Marks DL. Hypothalamic dysfunction and multiple sclerosis: implications for fatigue and weight dysregulation. Curr Neurol Neurosci Rep. (2016) 16:98. doi: 10.1007/s11910-016-0700-3
102. Sangha A, Quon M, Pfeffer G, and Orton SM. The role of vitamin D in neuroprotection in multiple sclerosis: an update. Nutrients. (2023) 15:2978. doi: 10.3390/nu15132978
103. Zhang W, Wang Y, Zhu M, Liu K, and Zhang HL. Gut flora in multiple sclerosis: implications for pathogenesis and treatment. Neural Regener Res. (2024) 19:1480–8. doi: 10.4103/1673-5374.387974
104. Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, et al. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol. (2009) 183:6041–50. doi: 10.4049/jimmunol.0900747
105. Johann L and Waisman A. The astrocyte LAMP lights a T cell TRAIL of death. Neuron. (2021) 109:1423–5. doi: 10.1016/j.neuron.2021.04.009
106. Giladi A, Wagner LK, Li H, Dorr D, Medaglia C, Paul F, et al. Cxcl10(+) monocytes define a pathogenic subset in the central nervous system during autoimmune neuroinflammation. Nat Immunol. (2020) 21:525–34. doi: 10.1038/s41590-020-0661-1
107. Straeten F, Zhu J, Borsch AL, Zhang B, Li K, Lu IN, et al. Integrated single-cell transcriptomics of cerebrospinal fluid cells in treatment-naive multiple sclerosis. J Neuroinflammation. (2022) 19:306. doi: 10.1186/s12974-022-02667-9
108. Amezcua L. Progressive multiple sclerosis. CONTINUUM: Lifelong Learn Neurology. (2022) 28:1083–103. doi: 10.1212/con.0000000000001157
109. Möckel T, Basta F, Weinmann-Menke J, and Schwarting A. B cell activating factor (BAFF): Structure, functions, autoimmunity and clinical implications in Systemic Lupus Erythematosus (SLE). Autoimmun Rev. (2021) 20:102736. doi: 10.1016/j.autrev.2020.102736
110. Arnon R and Aharoni R. Mechanism of action of glatiramer acetate in multiple sclerosis and its potential for the development of new applications. Proc Natl Acad Sci. (2004) 101:14593–8. doi: 10.1073/pnas.0404887101
111. Konen FF, Möhn N, Witte T, Schefzyk M, Wiestler M, Lovric S, et al. Treatment of autoimmunity: The impact of disease-modifying therapies in multiple sclerosis and comorbid autoimmune disorders. Autoimmun Rev. (2023) 22:103312. doi: 10.1016/j.autrev.2023.103312
112. Dittel LJ, Dittel BN, and Brod SA. Ingested (oral) adrenocorticotropic hormone (ACTH) inhibits interleukin-17 in the central nervous system after adoptive transfer of T helper (Th)1/Th17 T cells in the mouse model of multiple sclerosis, experimental autoimmune encephalomyelitis. J Neurological Sci. (2024) 456:122779. doi: 10.1016/j.jns.2023.122779
113. Muehler A, Peelen E, Kohlhof H, Gröppel M, and Vitt D. Vidofludimus calcium, a next generation DHODH inhibitor for the Treatment of relapsing-remitting multiple sclerosis. Multiple Sclerosis Related Disord. (2020) 43:102129. doi: 10.1016/j.msard.2020.102129
114. Wørzner K, Zimmermann J, Buhl R, Desoi A, Christensen D, Dietrich J, et al. Repeated immunization with ATRA-containing liposomal adjuvant transdifferentiates Th17 cells to a Tr1-like phenotype. J Autoimmunity. (2024) 144:103174. doi: 10.1016/j.jaut.2024.103174
115. Chen Y, Chen K, Zhu H, Qin H, Liu J, and Cao X. Methyltransferase Setd2 prevents T cell–mediated autoimmune diseases via phospholipid remodeling. Proc Natl Acad Sci. (2024) 121:e2314561121. doi: 10.1073/pnas.2314561121
116. Wang AA, Luessi F, Neziraj T, Pössnecker E, Zuo M, Engel S, et al. B cell depletion with anti-CD20 promotes neuroprotection in a BAFF-dependent manner in mice and humans. Sci Trans Med. (2024) 16:eadi0295. doi: 10.1126/scitranslmed.adi0295
117. Liu C, Li Y, Yu J, Feng L, Hou S, Liu Y, et al. Targeting the shift from M1 to M2 macrophages in experimental autoimmune encephalomyelitis mice treated with fasudil. PloS One. (2013) 8:e54841. doi: 10.1371/journal.pone.0054841
118. Liu C, Guo S, Liu R, Guo M, Wang Q, Chai Z, et al. Fasudil-modified macrophages reduce inflammation and regulate the immune response in experimental autoimmune encephalomyelitis. Neural Regeneration Res. (2023) 19:671–9. doi: 10.4103/1673-5374.379050
119. Vermersch P, Granziera C, Mao-Draayer Y, Cutter G, Kalbus O, Staikov I, et al. Inhibition of CD40L with frexalimab in multiple sclerosis. New Engl J Med. (2024) 390:589–600. doi: 10.1056/nejmoa2309439
120. Park J, Le QV, Wu Y, Lee J, and Oh YK. Tolerogenic nanovaccine for prevention and treatment of autoimmune encephalomyelitis. Advanced Materials. (2022) 35:e2202670. doi: 10.1002/adma.202202670
121. Jihane F, Marion D, Xavier M, Damien T, Yue C, Sandrine R, et al. MOG-specific CAR Tregs: a novel approach to treat multiple sclerosis. J Neuroinflammation. (2024) 21:268. doi: 10.1186/s12974-024-03262-w
122. Mi Y, Dong J, Liu C, Zhang Q, Zheng C, Wu H, et al. Amelioration of experimental autoimmune encephalomyelitis by exogenous soluble PD-L1 is associated with restraining dendritic cell maturation and CCR7-mediated migration. Int Immunopharmacology. (2024) 143:113398. doi: 10.1016/j.intimp.2024.113398
123. Li N, Han X, Ruan M, Huang F, Yang L, Xu T, et al. Prebiotic inulin controls Th17 cells mediated central nervous system autoimmunity through modulating the gut microbiota and short chain fatty acids. Gut Microbes. (2024) 16:2402547. doi: 10.1080/19490976.2024.2402547
124. Han B, Shi L, Bao M-Y, Yu F-L, Zhang Y, Lu X-Y, et al. Dietary ellagic acid therapy for CNS autoimmunity: Targeting on Alloprevotella rava and propionate metabolism. Microbiome. (2024) 12:114. doi: 10.1186/s40168-024-01819-8
125. Ghareghani M, Arneaud A, and Rivest S. The evolution of mesenchymal stem cell-derived neural progenitor therapy for Multiple Sclerosis: from concept to clinic. Front Cell Neurosci. (2024) 18:1428652. doi: 10.3389/fncel.2024.1428652
126. Park S-M, Oh Y-H, Lim G-H, Yun G-H, Kim K-B, An J-H, et al. Deferoxamine preconditioning of canine stem cell derived extracellular vesicles alleviates inflammation in an EAE mouse model through STAT3 regulation. Sci Rep. (2024) 14:19273. doi: 10.1038/s41598-024-68853-2
127. Shi Z, Sun H, Tian X, Song X, Fan J, Sun S, et al. Extracellular vesicles containing miR-181a-5p as a novel therapy for experimental autoimmune encephalomyelitis-induced demyelination. Int Immunopharmacology. (2024) 135:112326. doi: 10.1016/j.intimp.2024.112326
128. Yang T-T, Liu P-J, Sun Q-Y, Wang Z-Y, Yuan G-B, Fan Z-X, et al. CD4+CD25+ regulatory T cells ex vivo generated from autologous naïve CD4+ T cells suppress EAE progression. Sci Rep. (2024) 14:112326. doi: 10.1038/s41598-024-56739-2
129. Sharma S, Risen S, Gilberto VS, Boland S, Chatterjee A, Moreno JA, et al. Targeted-neuroinflammation mitigation using inflammasome-inhibiting nanoligomers is therapeutic in an experimental autoimmune encephalomyelitis mouse model. ACS Chem Neurosci. (2024) 15:1596–608. doi: 10.1021/acschemneuro.4c00024
130. Camacho-Toledano C, Machin-Diaz I, Lebron-Galan R, Gonzalez-Mayorga A, Palomares FJ, Serrano MC, et al. Graphene oxide films as a novel tool for the modulation of myeloid-derived suppressor cell activity in the context of multiple sclerosis. Nanoscale. (2024) 16:7515–31. doi: 10.1039/d3nr05351b
131. Rad LM, Hughes KR, Wheeler SN, Decker JT, Orbach SM, Galvan A, et al. Engineered immunological niche directs therapeutic development in models of progressive multiple sclerosis. Proc Natl Acad Sci U S A. (2025) 122:e2409852122. doi: 10.1073/pnas.2409852122
132. Morris AH, Hughes KR, Oakes RS, Cai MM, Miller SD, Irani DN, et al. Engineered immunological niches to monitor disease activity and treatment efficacy in relapsing multiple sclerosis. Nat Commun. (2020) 11:3871. doi: 10.1038/s41467-020-17629-z
Keywords: multiple sclerosis, autoimmune, neuroimmune, cerebral immune niches, targeted therapy
Citation: Sun X, Zhang F, Wang L, Lee G, Yang S, Zhou D, Chang B, Hu B and Zhou Y (2025) Immunological microenvironment and targeted therapeutics in multiple sclerosis: new insights in crosstalk between immune niches and CNS. Front. Immunol. 16:1604987. doi: 10.3389/fimmu.2025.1604987
Received: 02 April 2025; Accepted: 21 July 2025;
Published: 01 August 2025.
Edited by:
Barbara Rossi, University of Verona, ItalyReviewed by:
Yuji Tomizawa, Juntendo University, JapanAdil Maarouf, Assistance Publique Hôpitaux de Marseille, France
Copyright © 2025 Sun, Zhang, Wang, Lee, Yang, Zhou, Chang, Hu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Hu, aHVib0BodXN0LmVkdS5jbg==; Yifan Zhou, eWlmYW4wMTgwQGh1c3QuZWR1LmNu
†These authors have contributed equally to this work
 Xiaodi Sun
Xiaodi Sun Feng Zhang†
Feng Zhang† Bo Hu
Bo Hu Yifan Zhou
Yifan Zhou