- 1Guangzhou Chest Hospital, Guangzhou, China
- 2Guangdong Provincial Institute of Public Health, Guangdong Provincial Center for Disease Control and Prevention, Guangzhou, China
- 3Guangdong Provincial Center for Disease Control and Prevention, Guangzhou, China
- 4National Medical Products Administration (NMPA) Key Laboratory for Safety Evaluation of Cosmetics, Guangdong Provincial Key Laboratory of Tropical Disease Research, School of Public Health, Southern Medical University, Guangzhou, China
- 5Guangzhou Institute of Microbiology Group Co., Ltd., Guangzhou, China
- 6Xinjiang Uighur Autonomous Region Center for Disease Control and Prevention, Uighur, China
- 7Zhuhai Center for Disease Control and Prevention, Zhuhai, China
Atopic dermatitis (AD) is characterized by chronic and recurrent itching with a high burden of disability-adjusted life years (DALYs, a measure of overall disease burden). Traditional treatments mainly include corticosteroids, which have a good effect on controlling inflammation but adverse side effects. Recently, advancements in understanding the pathogenesis of AD have led to the emergence of a variety of novel therapeutic approaches, such as microbiome manipulation, offering renewed hope for more effective management of this condition. These strategies are particularly promising for mild-to-moderate AD, where dysbiosis and immune imbalance (e.g., Th2 skewing) are key drivers, though some approaches (e.g., fecal microbiota transplantation) are being explored for refractory cases. It has been shown that microbiome manipulation has the potential to improve disease states and regulates the balance of the inflammatory system in a variety of ways. Various approaches have been preclinically and clinically tested, including probiotics (and multiple co-applications), prebiotics, postbiotics, unmethylated CpG motifs, fecal microbiota transplantation, herbal fermentation technology with microorganisms and phage. In this review, we discuss these microbiome manipulation methods and emphasizes the potential of microbiome-based interventions to modulate Th1/Th2 balance with fewer side effects, ultimately leading to control of inflammation in AD. Further translational research in this field is needed to integrate when we apply this therapy and the capability for disease treatment and prevention.
1 Atopic dermatitis
Atopic dermatitis (AD) is a chronic, recurrent, inflammatory and pruritic dermatosis with complex pathophysiology, involving disruption of the epidermal barrier, microbial dysbiosis within affected lesions, and Th1/Th2-imbalanced immune responses to skin allergens (1). AD is a highly heterogeneous chronic inflammatory skin disease, and its clinical manifestations and etiology vary significantly among different age groups. In infancy, AD is mainly acute eczema; in childhood, AD is mainly flexor dermatitis; in adolescence and adulthood, AD presents as chronic lichenification; while in old age, AD shows a “reverse” distribution feature. AD in different age groups varies in terms of genetics, immunity, environment and psychological factors, but pruritus, skin barrier dysfunction and immune system abnormalities are the common pathological basis (2–4). AD has the highest DALY burden of all skin diseases and ranks 15th among all nonfatal diseases globally. The global burden of disease Study estimated that the general prevalence of AD was 15-20% among children and up to 10% among adults (5). Although most AD cases are mild-to-moderate (6), this condition is characterized by severe pruritus, which frequently results in skin injury, considerable sleep disruption, and a detrimental impact on the overall quality of life (7).
The exact mechanism underlying the complex progression of AD is not yet well understood. However, after a continuous exploration, current evidence indicates that the mechanism is related to genetic abnormalities, immunological dysfunction (mainly Th1/Th2 imbalance caused by excessive activation of Th2 immunity), and environmental factors as the prime drivers of AD exacerbation (8), as shown in Figure 1. Those factors contribute to skin barrier dysfunction and allow entry of external antigens and allergens, which activate immune responses across the skin surface.
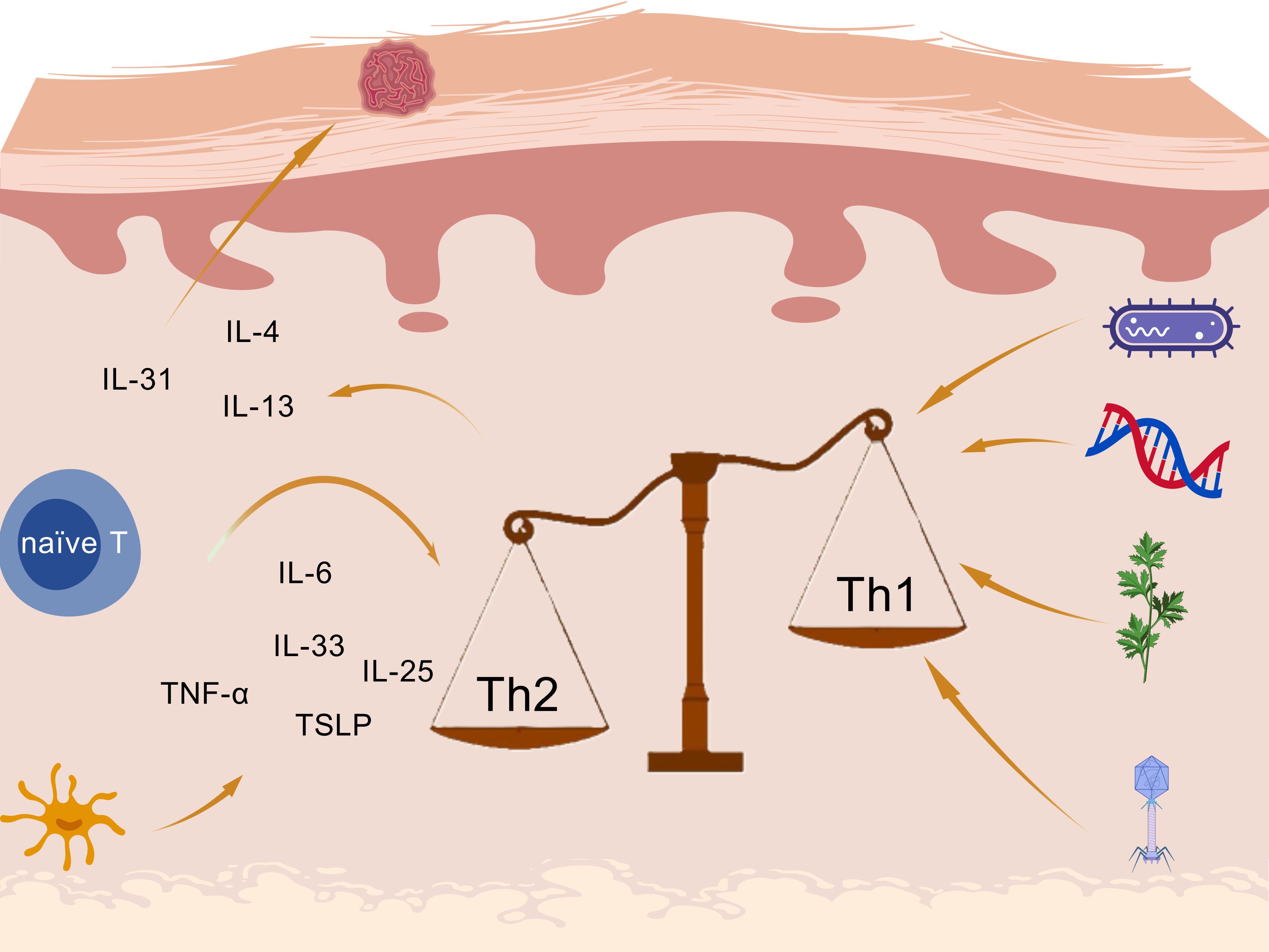
Figure 1. Inflammatory mechanisms in atopic dermatitis created with BioGDP.com (98).
Under allergen conditions, pro-inflammatory cytokines including interleukin (IL)-6, IL-25, IL-33, TNF-α, and thymic stromal lymphopoietin (TSLP) are produced by keratinocytes and Langerhans cells (LCs), leading to the activation of the Th2 immune axis through type 2 innate lymphoid cells (ILC2) and Th2 cells differentiate from naïve CD4+ T cells (9). Janus kinase (JAK) pathway is also activated, leading to a variety of cytokines and the enhance of Th2 cell differentiation (10, 11). The imbalanced and hyperactivated Th2-type immune responses induce the isotype class switch from IgM to IgE by mast cells, triggering a hypersensitivity reaction. Th2 cells secrete cytokines: IL-4 and IL-13 contribute to skin barrier defects, cutaneous infections, inflammation, thickening, and itching (12); IL-31 accounts for the stimulation of itch receptors, ultimately leading to pruritus in AD (13, 14).
Furthermore, increased Th2 polarization in AD not only disrupts the balance between Th1 and Th2 cells through excessive Th2 activation, but also contributes to pruritus. In response to itching, frequent scratching damages the skin barrier, increasing sensitivity to minor stimuli and facilitating the entry of external antigens and allergens such as dust. This, in turn, leads to further inflammation and itching, creating a vicious cycle.
2 Conventional and emerging therapeutics for atopic dermatitis
The management approaches for AD ought to be customized according to the unique attributes and advancement of the illness in each patient, with a primary focus on inflammation control. In most instances, corticosteroids and calcineurin inhibitors can be localized to the affected area and are widely used in the treatment of AD.
Topical corticosteroids (TCS) are widely recognized as first‐line anti‐inflammatory agents and are grouped into 7 classes, based on potency (15). This relies heavily on the physician’s experience and peer communication to adjust medications to balance efficacy and side effects (e.g. skin atrophy, purpura, telangiectasia, hypopigmentation, focal hypertrichosis, acneiform eruptions, and striae) (8). Topical calcineurin inhibitors (TCIs) exert their anti-inflammatory effects by inhibiting the activation and proliferation of calmodulin-dependent T cells (16), which do not cause skin atrophy but itching (17).
As for patients with chronic and severe AD, topical therapies are often insufficient for disease control. In such cases, systemic treatments, including oral and subcutaneous administration of medications (such as immunosuppressants and biologics), are required to manage symptoms and reduce disease severity (18, 19). Systemic corticosteroids are reserved for short-term use in acute flares due to risks like adrenal suppression and metabolic complications (20). Non-specific immunosuppressants like cyclosporine and methotrexate reduce inflammation by suppressing the immune response, widely employed in patients with refractory AD (21). Due to systemic administration, prolonged use of the above drugs can result significant adverse effects: cyclosporine is associated with nephrotoxicity (17); methotrexate is linked to hepatotoxicity (22). Therefore, careful monitoring is necessary during long-term treatment, and there is an urgent need for safer, long-term therapeutic options with fewer side effects.
Biologic therapies, particularly dupilumab, a monoclonal antibody targeting IL-4 and IL-13 signaling, have transformed treatment for moderate-to-severe AD (23, 24). Dupilumab is generally well tolerated and has shown sustained improvements in symptoms and quality of life (25). Additionally, JAK inhibitors (e.g., baricitinib, upadacitinib, abrocitinib) offer oral alternatives by interfering with cytokine signaling involved in inflammation (26–28). These agents are effective in moderate-to-severe AD but may carry risks such as infections, lipid elevations, and laboratory abnormalities, warranting individualized risk-benefit assessment (27, 29, 30).
Therefore, challenges remain — including drug-related adverse effects, long-term safety concerns, and high costs — underscoring the need for safer, more sustainable alternatives. In this context, microbiome-based therapies have gained attention for their potential to modulate immune responses and restore microbial balance with generally favorable tolerability.
Such approaches — including probiotics, prebiotics, postbiotics, phage therapy, and microbiota-derived compounds — are particularly promising for mild-to-moderate AD and may complement existing treatments or serve as preventive strategies, as shown in Figure 2. By addressing underlying dysbiosis, these interventions represent an innovative and evolving therapeutic avenue in AD management.
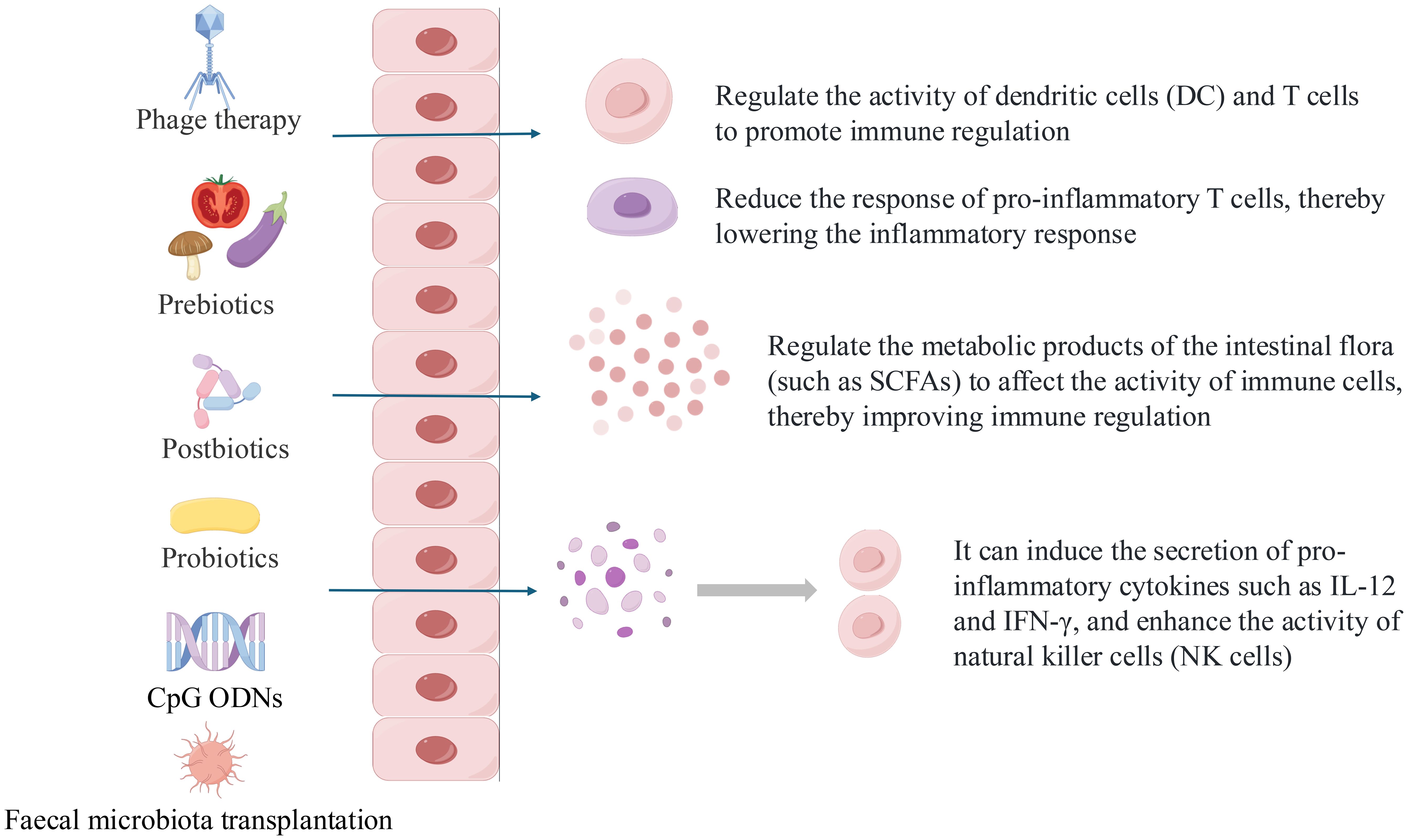
Figure 2. What are the possible mechanisms of microbiome operation methods and how do they affect humoral and cellular immunity.
3 Microbiome and AD
The human microbiome is composed of a collection of dynamic microbial communities (31). Accordingly, the balance of gut microbial environment has resulted in these communities playing a profound role in promoting human health and disruptions (mainly decreased bacterial diversity) may contribute to inflammation and susceptibility to allergy. Pretreatment with antibiotic combinations can eliminate pathogens, especially predominant pathogenic bacteria in dysbiosis and enhance the anti-inflammatory effects of probiotics by reducing pro-inflammatory cytokines and improving histological injury (32). Antibiotic-induced dysbiosis of intestinal flora enhances influenza virus damage to lung and intestinal mucosa and affects Th1/Th2 balance (33). Intervention studies have shown that probiotics improve intestinal dysbiosis in bronchial asthma (allergy), improve elevated serum IgE, IL-4, IL-5, IL-9, and IL-13 levels, and decrease Th1/Th2 ratio in asthmatic children (34). In the gut microbiota of AD patients, there is a lack of Bifidobacterium (except B. pseudocatenulatum), Enterococcus, Bacteroides, and Ruminococcus genus, while an overabundance of Staphylococcus aureus (S. aureus.), Clostridium spp. and Escherichia coli (35, 36).
The skin microbiome is a highly characterized ecosystem that varies significantly across different skin surfaces. Correspondingly, in the skin microbiota, there is an overabundance of S. aureus., S. epidermidis, and S. haemolyticus (37). In addition to overgrowth, S. aureus. strains in AD patients often exhibit high levels of antibiotic resistance. Studies have shown that patients with AD have significantly higher resistance rates to antibiotics such as penicillin, erythromycin, chloramphenicol, and tetracycline compared to the general population. For example, in a study involving 194 S. aureus strains, resistance to oxacillin was 1.4% in the AD group versus 0% in controls; erythromycin resistance was 45.1% in AD patients versus 23.4% in controls. These findings underscore the challenges in treating secondary infections in AD and highlight the importance of understanding microbial ecology and resistance patterns (38). Animal studies have demonstrated that changes in skin microbiota modulated immune cell dynamics, restoring the Th1/Th2 balance and leading to clinical improvement (39).
Recently, advancements in understanding the pathogenesis of AD have led to the emergence of a variety of novel therapeutic approaches, offering renewed hope for more effective management of this condition. Traditional therapies are associated with high side effects, as well as a lack of treatment and prevention for mild-to-moderate AD. In this review, we explore microbiome-based therapeutic strategies for AD and provide an updated perspective on chronic skin inflammation management. We focus on identifying treatment approaches that combine strong clinical efficacy with favorable safety profiles and high patient acceptability—attributes particularly important in chronic conditions like AD where long-term adherence is essential. The potential high acceptability of microbiome-targeted therapies stems from their non-invasive nature, avoidance of systemic immunosuppression, and alignment with growing patient preference for “natural” or physiology-modulating treatments over conventional pharmacotherapies.
4 Fecal microbiota transplantation
Fecal microbiota transplantation (FMT), a novel strategy for gut microbiota-induced disorders, allows long-lasting alteration of the recipient’s microbiome and temporary colonization to achieve the purpose of treatment. Briefly, intestinal probiotics are transplanted from healthy volunteers to receptors with gut microbiota disturbance, thus yielding the microecological balance in the rebuilt gut microbiota. The potential therapeutic effect of FMT was investigated by gut microbial ecology, immune system modulation, and fecal metabolite analysis using AD mice models.
After establishing AD mouse models, fecal microbiota transplantation (FMT) was found to reduce dermatitis scores compared to the negative control group (40, 41). Alpha diversity in the FMT group was initially lower than that of the donor group but increased following FMT treatment. Notably, the recovery of the gut microbial community showed significant variation starting from week 7 (40). In β-diversity analysis, there was no significant difference between the FMT and donor groups (41), whereas the negative control group showed a significant difference after just one week (42).
In further detail, at the phylum level, Firmicutes increased, while Bacteroidetes decreased in the AD model mice (41, 42), resulting increased the levels of SCFA (acetic, butyric, isobutyric, and propionic acid) levels (40). At the genus level, the abundance of Lactobacillus was increased in the FMT-treated groups compared with the negative control group (40, 42).
In the regulation of inflammatory factors, serum levels of IgE (40, 41) and IgG1 (41) were significantly decreased compared with the negative control group. The concentrations of Th2 cytokines (IL-4, IL-5, and IL-13), which are known to contribute to the development of AD, were significantly decreased compared with the negative control group after 7 weeks (40) and 2 weeks (41), and a study has reported that IL-4 and IL-13 were significantly decreased in 1 weeks (42). However, the concentrations of Th1 cytokines, such as IL-12, IFN-γ, and TNF-α, were significantly increased in the FMT group than in the negative control group (40).
FMT modulated Tregs through enhanced their surface PD-1 levels and Helioxs PD-1 Tregs levels and reduced cytokines levels secreted by Tregs (i.e., IL-10 and IL-1β) (41). Further mechanistic studies revealed that FMT treatment induced CD103 DCs and programmed cell death ligand 1 (PD-L1)/programmed cell death 1 (PD-1) expression in skin-draining lymph nodes and promoted Treg production to induce immune tolerance and suppress skin inflammation.
5 Oral supplements
Direct oral supplementation is another and more acceptable way to modulate the gut microbiota and microbial environment including probiotics, postbiotics, prebiotics and other products. The results of the randomized controlled trial showed a good effect, significantly reducing dermatitis scores and relieving itching. However, the pathway how achieves its effects of modulating inflammation remain a focus of research. The following explores the pathways of inflammation modulation and the development of new formulations.
5.1 Lactobacillus
In recent years, there has been more development and research on Lactobacillus and it has anti-allergic effects and modulating Th1/Th2 balance in AD models. A new train of the bacterium, Lactobacillus acidophilus (L. acidophilus) KBL409 reducing in serum IgE and regulating the mRNA levels of Th1 (Interferon-γ) and Th2 (IL-4, IL-5, IL-13, and IL-31) cytokines in skin tissues (43). Otherwise, Th17 (IL-17A) cytokines, anti-inflammatory cytokine IL-10 and the expression of Forkhead box protein P3 (Foxp3) were also increased.
A new strain, Lactocaseibacillus paracasei subsp. paracasei NTU 101 (NTU101), with similar effects including effectively alleviating skin symptoms and reducing the infiltration of inflammatory cells and the potential mechanism involves increasing the expression of Foxp3, with the same pathway about Foxp3 in the above (44). Further study verified NTU 101,and bacteria of the same genus Lactobacillus paracasei KBL382, influenced the proportion of CD4+CD25+Foxp3+ (Treg) cell to promote immune tolerance, and modulated the immune response by increasing the proportion of CD4+/IL-4+ (Th2) cells in the spleen and CD4+/IFN-γ+ (Th1) cells, achieving a rebalancing of the Th1/Th2 ratio (45, 46).
In the process of fractionating culture supernatants of Lactobacillus gasseri (L. gasseri), Chen selected a moonlighting protein, glycolytic glyceraldehyde 3-phosphate dehydrogenase (LGp40) and it can enhance skin barrier function and reduced infiltration of Langerhans cells in the dermis, and mitigated skewed Th2 and Th17 immune responses (47). Potential mechanisms include inhibiting allergen-induced keratinocyte apoptosis through the blockade of the caspa-se-3 cascade and reducing the NLR family pyrin domain containing 3 (NLRP3) in-flammasome in macrophages to regulate inflammation (48).
5.2 Lactiplantibacillus plantarum
Lactiplantibacillus plantarum (L. plantarum) is widely used in the pharmaceutical industries. Recent study found that the strains L. plantarum HD02 and MD159 demonstrated comparable preventive effects against allergies, reducing blood levels of mast cell protease-1 (MCPT-1) and total IgE (49). L. plantarum APsulloc 331261 downregulated Th2 expression, and upregulated Th1 expression in a colony-forming unit-dependent manner, showing its effect of relieving AD symptoms (50).
5.3 Pediococcus
By transforming dendritic cells into tolerogenic versions, Pediococcus (pentosaceus KF159) directly enhanced the production or secretion of IL10 through the induction of CD4+CD25+Foxp3(Treg) cells, which performed similarly to NTU101 (51) and Pediococcus acidilactici SRCM102024 dose-dependently improved the clinical symptoms similar to the positive-control (dexamethasone 3 mg/kg bw) and had fewer adverse effects (52).
5.4 Bifidobacteria
Past reviews have summarized the therapeutic effects of Bifidobacteria on atopic dermatitis: B. longum can reshape gut microbial composition and upregulated tryptophan metabolism of gut microbiota to increase I3C, which medicated AHR signaling pathway to improve clinical symptoms in AD combined metagenomic sequencing analysis and targeted tryptophan metabolic analysis (53). While considerable research has focused on exploring single bacterial species or consortia, the optimal strategies for microbiota-based therapeutics remain underexplored. Specifically, the comparative effectiveness of bacterial consortia versus individual species warrants further investigation. One of the innovative Strategies for Bifidobacterium is to combine with other Probiotics.
Two studies assessed the impact of bacterial consortium included L. plantarum, Bifidobacterium and Lacticaseibacillus casei, due to their synergistic effect on IL-10 production, an anti-inflammatory cytokine that plays a pivotal role in modeling the gut and skin microbiome: One found that the administration of bacterial consortium demonstrated enhanced therapeutic efficacy in experimental models(mice) of atopic dermatitis when compared to single strains. Bacterial consortium significantly restrained detrimental inflammatory immune responses, including reduced ear swelling, suppressed immune cell infiltration, and decreased serum immunoglobulin E(IgE) levels. These effects are likely attributed to the upregulation of intestinal regulatory CD103+CD11b+ dendritic cells and alterations in the microbiome communities (54); Another Randomized Controlled Trial (RCT) evaluated the therapeutic efficacy of a bacterial consortium in AD patients. The results demonstrated significant reductions in all clinical severity scores, including erythema, edema/papulation, excoriation, total inflammation score (TIS), and PRURISCORE, both at the group level and within individual patients. However, current evidence remains insufficient to determine whether these improvements result from synergistic effects among the bacterial strains, as no studies have specifically investigated this mechanistic aspect (55).
A new formulation of probiotics, which composed of one strain of Bifidobacterium and four strains of Lactobacillus, containing 5x1010 CFU active probiotics per gram, has been explored the efficacy in the treatment of AD and found that the formulation significantly alleviated skin inflammation of the MC903-induced AD in mice. Administration with the probiotics mixture induced increased production of regulatory T cells and regulatory dendritic cells (DCregs) in the mesenteric lymph nodes, thus modulating the immune response (56).
A new formulation of probiotics, YK4, including comprising Lactobacillus acidophilus CBT LA1, L. plantarum CBT LP3, Bifidobacterium breve CBT BR3, and B. lactis CBT BL3 and it regulates Th1/Th2 balance and suppresses inflammation through DCs, Tregs and Galectin-9 in DNCB-induced mice, indicating a potential role as an effective anti-inflammatory agent in AD patients (57).
5.5 Postbiotics
Defined by the International Scientific Association of Probiotics and Prebiotics (ISAPP) as preparations of inanimate microorganisms and/or their components that confer health benefits. Postbiotics include heat-killed bacteria, cell-free supernatants, and purified microbial components that maintain the beneficial properties of probiotics while ensuring safety and stability (58). As a high-temperature inactivated microbial preparation, postbiotics have the property of being easy to store and more acceptable compared to probiotics, but may only be able to exert part of the effect in relieving and treating inflammation.
A new postbiotic product has been reported and it can rebalance the population of Th1/Th2 cells in the spleen of HDM extraction-induced AD mice and was possibly related to modulate the proportion of IL-4+ CD4+ T cells and IFN-γ+ CD4+ T cells (59). Moreover, it has capability of decreasing serum IgE concentration to relieve the classic AD signs and the production process includes isolating two lactic acid bacteria strains (Lactococcus lactis subsp. cremoris MP01 and Lactobacillus paracasei subsp. paracasei MP02) from traditional fermented milk and being heat inactivated.
The recent review suggested that Lactobacillus postbiotics might be successfully used as adjuvant AD therapy in adults. Thus far, data do not indicate efficacy in pediatric patients. This age-dependent difference can be explained by three key factors observed in probiotic studies: different immunomodulatory mechanisms between the mature and developing immune systems, strain/dose response changes, and different stages of gut microbiota development (60–62). Standardizing nomenclatures and experimental procedures, as well as expanding the studies to more geographic locations and assessing comprehensively the effects on the gut microbiome would provide better perspectives of postbiotics as a therapeutic option for AD (63).
5.6 Prebiotics
Prebiotics are fermented ingredients that alter the composition and activity of the gut microflora, providing health benefits to the host. Xylooligosaccharide (XOS) is similar to fructo-oligosaccharides (FOS) and anti-inflammatory effects are achieved by promoting the reproduction of bifidobacteria. Laigaard et al. (64) addressed the effects of a prebiotic, XOS, on the gut microbiota and ear inflammation in an oxazolone-induced AD model. Feeding XOS alleviates oxazolone-induced hyperresponsiveness in AD mice. Serum IgE and ear tissue cytokine levels correlated significantly with the clinical scores. The prebiotic treatments (e.g. scGOS/lcFOS, inulin, and β- glucan) showed suppression of AD symptoms (65), Th2 cell differentiation, and AD-like skin lesions induced by DNCB and were immunomodulated via activation of galectin-9 and toll-like receptor 9 (TLR-9) (66).
6 CpG-ODNs
CpG oligodeoxynucleotides (CpG ODNs) are the artificial versions of unmethylated CpG motifs which were originally discovered in bacterial DNA, and demonstrated not only as potent immunoadjuvants but also as anticancer agents by triggering TLR9 activation (67). In humans, TLR9 is highly expressed in plasmacytoid DCs and B cells (68). CpG-ODN in high amount activates a TLR9-TRIF-non canonical NFκB-IRF3 cascade to mediate anti-inflammatory molecules such as IL-10, TGF-β and IDO (69). Consequently, such treatment at high dose combined with allergens could be an innovative strategy for the development of Tregs and B regulatory (Breg) cells during allergen-specific immunotherapy (AIT) protocol (70) such as high-dose CpG in combination with allergens without endotoxin toxicity to boost immune tolerance (71).
CpG-ODNs are strong Th1 response inducers while inhibiting Th2 responses, helping restore the balance of Th1/Th2. Studies have reported that CpG-ODNs prevent T helper 2 (Th2) allergic responses such as antigen-induced asthmatic responses, allergic rhinitis and AD (72) and trigger Th1 response via induction of IFN-α and-γ, as well as IL-12 to restore the balance of Th1/Th2. In addition, they can inhibit IgE production in B cells (73). Therefore CpG-ODNs are considered as adjuvant treatment for T helper 2 (Th2) allergic responses such as antigen-induced asthmatic responses, allergic rhinitis and AD (72).
Given that Bifidobacterium have specific immunostimulatory properties that influence the Th1/Th2 balance, these probiotics may have therapeutic potential for AD. A similar methylated CpG site capable to activate the in-vitro Treg cell differentiation was identified in the genomic DNA from the probiotic B. longum subsp. Infantis (74) and compared to Lactobacillus, Bifidobacterium have higher more CpG motifs (75).
Kim et al. (76) focused on the effects of three types of B. bifidum (probiotics, postbiotics, CpG ODN) and found that oral administration of the three types of B. bifidum alleviated the clinical symptoms of AD in the skin lesions by modulating the balance of Th1/Th2, Treg/Th1 and Treg/Th1+Th2.
Synthetic CpG-ODNs, which contain a partial or complete phosphorothioate (PS) backbone, exhibit immunotoxicity and hepatotoxicity, leading to conditions such as splenomegaly, liver necrosis, and hemorrhagic ascites (77). A low-toxic CpG-ODN with a phosphodiester backbone was synthesized and designated as 46O, and it suppressed IgE and IL-4 synthesis in mice with OVA-induced AD (78).
7 Herbal fermentation technology with microorganisms
Herbal fermentation technology with microorganisms refers to the process of culturing herbs with microorganisms to enhance their function and promote their absorption (79) and is expected to be the link between modern medicine and traditional Chinese medicine.
Compared with unfermented Portulaca oleracea L. (PO), PO fermented using 1% (v/v) Bacillus sp. DU-106 and 1% (v/v) Lactobacillus plantarum. improved the skin barrier in AD mice and limited the expression of inflammatory cytokines and exerts an anti-inflammatory effect by suppressing NF-κB signaling pathway and outperformed in reducing inflammation (80). Another study accessed postbiotics fermented from Smilaxchina L. leaves and Lactobacillus acidophilus (KCTC15475BP), indicating that the postbiotics significantly alleviated AD symptoms and suppressed the AD response by effectively regulating chemokines and cytokines through the reduction of NF-κB activity driven by inflammation (81). After probiotic fermentation, Morinda citrifolia significantly alleviated AD symptoms such as infiltration of inflammatory cells (e.g., mast cells and eosinophils), reduced the levels of IgE, TSLP and beneficially modulated the expressions of Th1, Th2(, Th17, and Th22)-mediated cytokines in lesioned skin and splenocytes (82).
8 Bacteriophages
Bacteriophages, also known as phages, are a type of virus that selectively target and infect bacteria (83). Compared with antibiotics, phages have stronger host specificity, which do not destroy the normal microflora of human body (84) and do have a profound impact on the bacteria that exist within and on humans.
Byrd et al. (85) analyzed microbial temporal dynamics from a cohort of pediatric AD patients sampled throughout the course of disease by Shotgun metagenomic sequence analysis and found that heterogeneous S. aureus. and Staphylococcus epidermidis are closely related to AD. Wang et al. (86) employed a random forest classifier to identify potential marker genes and highlighted the genetic diversity and functional implications of prophages in driving differentiation between strains from AD and healthy control group. Moreover, prophages in the healthy control group exhibited variously higher enrichment of differential functions and the AD group displayed a notable enrichment of virulence factors within their prophages, underscoring the important contribution of prophages to the pathogenesis of AD-associated strains.
Phage therapy has a theoretical basis to be an innovative solution for treating skin inflammation caused by certain specific strains of bacteria (87) and in recent years, lytic phages targeting specific bacterial groups (S. aureus.) are being developed and applied in humans.
Phage SAP71, which specifically lysed S. aureus, was able to significantly reduce inflammatory cell infiltration, and prevent the development of AD-like skin pathological changes in an AD model (88). Meanwhile, the bacteriophage-surfactant combination could increase the eradication of IgE-stimulated aggregation in vitro test, and significantly decolonized the pathogen with an efficacy double that of the phage-only treatment, and decreased the expression of pro-inflammatory cytokine genes (IL-1β, IL-12 and IFN-γ) for 5 days in vivo trial (89).
In summary, phage therapy has the potential to act as an alternative to antibiotics for S. aureus decolonization in patients with dermatitis and contribute to combat the antibiotic resistance.
9 Conclusion
This review has outlined the current landscape of microbiome-based therapies for AD, emphasizing their potential to modulate immune responses, restore microbial balance, and reduce inflammation. Approaches such as FMT, probiotics, prebiotics, postbiotics, phage therapy, and probiotic-fermented botanicals demonstrate promising results in reshaping the host microbiome and managing AD symptoms.
FMT has shown efficacy in modifying gut microbiota and is being explored in complex diseases such as autism and multiple sclerosis (90). However, its application in AD remains experimental and requires standardization in terms of microbial composition, dosage, formulation, and administration methods.
As research progresses, probiotics, prebiotics, postbiotics, and related products are increasingly applied in allergic diseases, including eczema. These interventions are generally considered well-tolerated based on existing studies, although comprehensive evaluations of adverse effects are still limited and further investigation is warranted.
Different reagents have different applications: Probiotics, is a fermented product that is expected to be used to modulate intestinal microbiota and maintain immune homeostasis in milk powder or other reagents. As the source of probiotic products, development of new strains remains a priority. It is also required to further explore the pairing of different flora and a more detailed mechanism. Although with a longer shelf life and lower biological activity than probiotics, postbiotics can be used for safe pharmaceutical and food-manufacturing applications. CpG ODNs, as potent immunoadjuvants, are being developed for different diseases, such as nanomedicines for tumor and nebulization therapy for asthma (91, 92). In the future, a potential therapeutic approach for AD could involve an ointment containing CpG ODNs, although this remains speculative and requires experimental validation.
Recent study has proved that probiotic-fermented herbs are able to alleviate AD symptoms and regulate Th1/Th2 balance in mice. PO has antioxidant, allergic and inflammatory effects. After fermentation, beneficial effects were observed along with elevated polyphenol and flavonoid content, indicating that it may work by increasing the beneficial phytocompounds. Through modern processing technology, we can not only enhance the beneficial effects of the original drugs (especially traditional Chinese medicine), but also help us to explore the mechanism.
Phage therapy is similar to antibiotic therapy in that it kills harmful flora (specifically S. aureus.) and reduces the inflammatory response, which also means that there is the problem in restoration of skin flora. In addition, the proteins and nucleic acids of the phage, as well as the excipients used in the administration of the drug, need to be taken into account to determine whether they are allergenic. Moreover, attention needs to be paid to whether the phage proteins, nucleic acids and pharmaceutical excipients can trigger allergies.
Based on the current research, the optimal treatment for mild-to-moderate AD includes TCS to rapidly control inflammation, combined with microbiome-based therapies to restore a healthy skin microecology, which may prove to be a useful therapeutic paradigm. In addition to TCS, calcineurin inhibitors play a crucial role in managing AD, especially in sensitive skin areas, and their use may complement microbial interventions aimed at restoring immune and microbial balance (93). The further goal is to use microbial therapies early and appropriately to reduce the use of hormonal medications and even to prevent the onset of AD. Beyond their application in atopic dermatitis, many of the microbial therapies discussed—such as probiotics, CpG-ODNs, and fecal microbiota transplantation—are being investigated for a broad spectrum of chronic inflammatory and immune-mediated diseases. For instance, FMT has shown promising results in conditions like ulcerative colitis, multiple sclerosis, and even autism spectrum disorders, highlighting the central role of the gut–immune axis (94–96). Similarly, CpG-ODNs are under development as immunotherapies for asthma and certain cancers due to their potent ability to shift Th2-skewed immunity toward a Th1-dominant response (40, 97). The success of these strategies in AD could pave the way for cross-condition translational therapies, especially in diseases that share common immunopathological features such as Th2 polarization or microbial dysbiosis. Future studies comparing microbial interventions across diseases may reveal shared microbial signatures and therapeutic targets, accelerating the design of more personalized and preventive treatments. Integrating microbiome-based strategies into a broader immunological framework could ultimately redefine how we treat not only skin disorders but systemic inflammatory diseases as well.
Author contributions
JM: Writing – original draft. YF: Writing – original draft. JH: Writing – original draft. SL: Writing – original draft. LZ: Writing – original draft. SC: Writing – original draft. ZL: Writing – review & editing. RM: Writing – review & editing. XY: Writing – review & editing. FZ: Writing – review & editing. GJ: Writing – review & editing. PL: Writing – review & editing. LC: Writing – review & editing. WW: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China, grant number 82073599 and 82373600, Special Support Program of Guangdong Province, grant number 2021TX06N107 and Nutrition Science Research Foundation of BYHEALTH, grant number TY202102002.
Acknowledgments
Figures were created using BioGDP.com. Accessed on 27 January 2025.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. David Boothe W, Tarbox JA, and Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. (2017) 1027:21–37. doi: 10.1007/978-3-319-64804-0_3
2. Maurelli M, Chiricozzi A, Peris K, Gisondi P, and Girolomoni G. Atopic dermatitis in the elderly population. Acta Derm Venereol. (2023) 103:adv13363. doi: 10.2340/actadv.v103.13363
3. Wang S, Zhu R, Gu C, Zou Y, Yin H, Xu J, et al. Distinct clinical features and serum cytokine pattern of elderly atopic dermatitis in China. J Eur Acad Dermatol Venereol. (2020) 34:2346–52. doi: 10.1111/jdv.16346
4. Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. (2015) 66 Suppl 1:8–16. doi: 10.1159/000370220
5. Laughter MR, Maymone MBC, Mashayekhi S, Arents BWM, Karimkhani C, Langan SM, et al. The global burden of atopic dermatitis: lessons from the global burden of disease study 1990-2017. Br J Dermatol. (2021) 184:304–9. doi: 10.1111/bjd.19580
6. Faye O, Flohr C, Kabashima K, Ma L, Paller AS, Rapelanoro FR, et al. Atopic dermatitis: A global health perspective. J Eur Acad Dermatol Venereol. (2024) 38:801–11. doi: 10.1111/jdv.19723
7. Lugović-Mihić L, Meštrović-Štefekov J, Potočnjak I, Cindrić T, Ilić I, Lovrić I, et al. Atopic dermatitis: disease features, therapeutic options, and a multidisciplinary approach. Life (Basel Switzerland). (2023) 13:1419. doi: 10.3390/life13061419
8. Weidinger S, Beck LA, Bieber T, Kabashima K, and Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. (2018) 4:1. doi: 10.1038/s41572-018-0001-z
9. Egawa G and Kabashima K. Multifactorial skin barrier deficiency and atopic dermatitis: essential topics to prevent the atopic march. J Allergy Clin Immunol. (2016) 138:350–8.e1. doi: 10.1016/j.jaci.2016.06.002
10. Bao L, Zhang H, and Chan LS. The involvement of the jak-stat signaling pathway in chronic inflammatory skin disease atopic dermatitis. Jakstat. (2013) 2:e24137. doi: 10.4161/jkst.24137
11. Rerknimitr P, Otsuka A, Nakashima C, and Kabashima K. The etiopathogenesis of atopic dermatitis: barrier disruption, immunological derangement, and pruritus. Inflammation Regener. (2017) 37:14. doi: 10.1186/s41232-017-0044-7
12. Bieber T. Interleukin-13: targeting an underestimated cytokine in atopic dermatitis. Allergy. (2020) 75:54–62. doi: 10.1111/all.13954
13. Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. Il-31: A new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. (2006) 117:411–7. doi: 10.1016/j.jaci.2005.10.033
14. Feld M, Garcia R, Buddenkotte J, Katayama S, Lewis K, Muirhead G, et al. The pruritus- and th2-associated cytokine il-31 promotes growth of sensory nerves. J Allergy Clin Immunol. (2016) 138:500–8.e24. doi: 10.1016/j.jaci.2016.02.020
15. Sidbury R, Alikhan A, Bercovitch L, Cohen DE, Darr JM, Drucker AM, et al. Guidelines of care for the management of atopic dermatitis in adults with topical therapies. J Am Acad Dermatol. (2023) 89:e1–e20. doi: 10.1016/j.jaad.2022.12.029
16. Nakahara T, Morimoto H, Murakami N, and Furue M. Mechanistic insights into topical tacrolimus for the treatment of atopic dermatitis. Pediatr Allergy Immunol. (2018) 29:233–8. doi: 10.1111/pai.12842
17. Eichenfield LF, Ahluwalia J, Waldman A, Borok J, Udkoff J, and Boguniewicz M. Current guidelines for the evaluation and management of atopic dermatitis: A comparison of the joint task force practice parameter and american academy of dermatology guidelines. J Allergy Clin Immunol. (2017) 139:S49–s57. doi: 10.1016/j.jaci.2017.01.009
18. Megna M, Napolitano M, Patruno C, Villani A, Balato A, Monfrecola G, et al. Systemic treatment of adult atopic dermatitis: A review. Dermatol Ther (Heidelb). (2017) 7:1–23. doi: 10.1007/s13555-016-0170-1
19. Stölzl D, Weidinger S, and Drerup K. A new era has begun: treatment of atopic dermatitis with biologics. Allergol Select. (2021) 5:265–73. doi: 10.5414/alx02259e
20. Yew YW, Alagappan U, Aw D, Chandran NS, Choo KJ, Chu R, et al. Updated consensus guidelines for management of moderate-to-severe atopic dermatitis in Singapore: integrating biologics, janus kinase inhibitors and conventional therapies. Ann Acad Med Singap. (2024) 53:670–82. doi: 10.47102/annals-acadmedsg.2024158
21. Goujon C, Viguier M, Staumont-Sallé D, Bernier C, Guillet G, Lahfa M, et al. Methotrexate versus cyclosporine in adults with moderate-to-severe atopic dermatitis: A phase iii randomized noninferiority trial. J Allergy Clin Immunol Pract. (2018) 6:562–9.e3. doi: 10.1016/j.jaip.2017.07.007
22. Roekevisch E, Schram ME, Leeflang MMG, Brouwer MWD, Gerbens LAA, Bos JD, et al. Methotrexate versus azathioprine in patients with atopic dermatitis: 2-year follow-up data. J Allergy Clin Immunol. (2018) 141:825–7.e10. doi: 10.1016/j.jaci.2017.09.033
23. Wollenberg A, Howell MD, Guttman-Yassky E, Silverberg JI, Kell C, Ranade K, et al. Treatment of atopic dermatitis with tralokinumab, an anti-Il-13 mab. J Allergy Clin Immunol. (2019) 143:135–41. doi: 10.1016/j.jaci.2018.05.029
24. Callewaert C, Nakatsuji T, Knight R, Kosciolek T, Vrbanac A, Kotol P, et al. Il-4rα Blockade by dupilumab decreases Staphylococcus aureus colonization and increases microbial diversity in atopic dermatitis. J Invest Dermatol. (2020) 140:191–202.e7. doi: 10.1016/j.jid.2019.05.024
25. Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. (2014) 371:130–9. doi: 10.1056/NEJMoa1314768
26. Guttman-Yassky E, Silverberg JI, Nemoto O, Forman SB, Wilke A, Prescilla R, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: A phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol. (2019) 80:913–21.e9. doi: 10.1016/j.jaad.2018.01.018
27. Nezamololama N, Fieldhouse K, Metzger K, and Gooderham M. Emerging systemic jak inhibitors in the treatment of atopic dermatitis: A review of abrocitinib, baricitinib, and upadacitinib. Drugs Context. (2020) 9. doi: 10.7573/dic.2020-8-5
28. Lugović-Mihić L, Meštrović-Štefekov J, Potočnjak I, Cindrić T, Ilić I, Lovrić I, et al. Atopic dermatitis: disease features, therapeutic options, and a multidisciplinary approach. Life (Basel). (2023) 13(6):1419. doi: 10.3390/life13061419
29. Napolitano M, Fabbrocini G, Ruggiero A, Marino V, Nocerino M, and Patruno C. The efficacy and safety of abrocitinib as a treatment option for atopic dermatitis: A short report of the clinical data. Drug Des Devel Ther. (2021) 15:1135–47. doi: 10.2147/dddt.S240866
30. Pereyra-Rodriguez JJ, Baldrich ES, Ruiz-Villaverde R, Torres EB, de la CDP, Nart IF, et al. Clinical approach to patients with moderate-to-severe atopic dermatitis: A Spanish Delphi consensus. Acta Derm Venereol. (2023) 103:adv12314. doi: 10.2340/actadv.v103.12314
31. Aggarwal N, Kitano S, Puah GRY, Kittelmann S, Hwang IY, and Chang MW. Microbiome and human health: current understanding, engineering, and enabling technologies. Chem Rev. (2023) 123:31–72. doi: 10.1021/acs.chemrev.2c00431
32. Xu J, Xu H, Guo X, Zhao H, Wang J, Li J, et al. Pretreatment with an antibiotics cocktail enhances the protective effect of probiotics by regulating Scfa metabolism and Th1/Th2/Th17 cell immune responses. BMC Microbiol. (2024) 24:91. doi: 10.1186/s12866-024-03251-2
33. Gao J, Chen H, Xu L, Li S, Yan H, Jiang L, et al. Effects of intestinal microorganisms on influenza-infected mice with antibiotic-induced intestinal dysbiosis, through the Tlr7 signaling pathway. Front Biosci (Landmark Ed). (2023) 28:43. doi: 10.31083/j.fbl2803043
34. Chen X, Yong SB, Yii CY, Feng B, Hsieh KS, and Li Q. Intestinal microbiota and probiotic intervention in children with bronchial asthma. Heliyon. (2024) 10:e34916. doi: 10.1016/j.heliyon.2024.e34916
35. Petersen EBM, Skov L, Thyssen JP, and Jensen P. Role of the gut microbiota in atopic dermatitis: A systematic review. Acta Derm Venereol. (2019) 99:5–11. doi: 10.2340/00015555-3008
36. Šuler Baglama Š and Trčko K. Skin and gut microbiota dysbiosis in autoimmune and inflammatory skin diseases. Acta Dermatovenerol Alp Pannonica Adriat. (2022) 31:105–9. doi: 10.15570/actaapa.2022.16
37. Khadka VD, Key FM, Romo-González C, Martínez-Gayosso A, Campos-Cabrera BL, Gerónimo-Gallegos A, et al. The skin microbiome of patients with atopic dermatitis normalizes gradually during treatment. Front Cell Infect Microbiol. (2021) 11:720674. doi: 10.3389/fcimb.2021.720674
38. Yeung C, Chow WC, Chan H, and Ho PL. Carriage of antibiotic-resistant Staphylococcus aureus in atopic dermatitis children attending paediatric outpatient clinics. Hong Kong J Dermatol Venereol. (2010) 18:125–31.
39. You Z, Zhang X, Huang S, Chen D, Zhu Y, Li G, et al. The influence of skin microbial ecology on γδ T cell immune pathways in allergic dermatitis models in mice. J Leukoc Biol. (2024) 117(5):qiae244. doi: 10.1093/jleuko/qiae244
40. Kim JH, Kim K, and Kim W. Gut microbiota restoration through fecal microbiota transplantation: A new atopic dermatitis therapy. Exp Mol Med. (2021) 53:907–16. doi: 10.1038/s12276-021-00627-6
41. Wang XZ, Huang JL, Zhang J, Li QH, Zhang PP, Wu C, et al. Fecal microbiota transplantation as a new way for ova-induced atopic dermatitis of juvenile mice. Int Immunopharmacol. (2024) 142:113183. doi: 10.1016/j.intimp.2024.113183
42. Jiang X, Liu Z, Ma Y, Miao L, Zhao K, Wang D, et al. Fecal microbiota transplantation affects the recovery of ad-skin lesions and enhances gut microbiota homeostasis. Int Immunopharmacol. (2023) 118:110005. doi: 10.1016/j.intimp.2023.110005
43. Kim WK, Jang YJ, Park S, Min SG, Kwon H, Jo MJ, et al. Lactobacillus acidophilus Kbl409 ameliorates atopic dermatitis in a mouse model. J Microbiol. (2024) 62:91–9. doi: 10.1007/s12275-024-00104-5
44. Liu CF, Shih TW, Lee CL, and Pan TM. The beneficial role of Lactobacillus paracasei subsp. Paracasei Ntu 101 in the prevention of atopic dermatitis. Curr Issues Mol Biol. (2024) 46:2236–50. doi: 10.3390/cimb46030143
45. Liu CF, Chao WY, Shih TW, Lee CL, and Pan TM. The enhancement of regulatory T cell maturation and Th1/Th2 balance through Foxp3 expression by Lactobacillus paracasei in an ovalbumin-induced allergic skin animal model. Curr Issues Mol Biol. (2024) 46:10714–30. doi: 10.3390/cimb46100636
46. Kim WK, Jang YJ, Han DH, Jeon K, Lee C, Han HS, et al. Lactobacillus paracasei Kbl382 administration attenuates atopic dermatitis by modulating immune response and gut microbiota. Gut Microbes. (2020) 12:1–14. doi: 10.1080/19490976.2020.1819156
47. Chen PC, Hsieh MH, Kuo WS, Wu LS, Kao HF, Liu LF, et al. Moonlighting glyceraldehyde-3-phosphate dehydrogenase (Gapdh) protein of lactobacillus gasseri attenuates allergic asthma via immunometabolic change in macrophages. J BioMed Sci. (2022) 29:75. doi: 10.1186/s12929-022-00861-8
48. Chen PC, Hsieh MH, Chen WL, Hsia YP, Kuo WS, Wu LS, et al. Moonlighting glyceraldehyde-3-phosphate dehydrogenase of lactobacillus gasseri inhibits keratinocyte apoptosis and skin inflammation in experimental atopic dermatitis. Asian Pac J Allergy Immunol. (2024). doi: 10.12932/ap-211123-1733
49. Kim AR, Jeon SG, Kim HR, Hong H, Yoon YW, Lee BM, et al. Preventive and therapeutic effects of lactiplantibacillus plantarum Hd02 and Md159 through mast cell degranulation inhibition in mouse models of atopic dermatitis. Nutrients. (2024) 16(17):3021. doi: 10.3390/nu16173021
50. Kim SY, Lee JO, Kim YJ, Jang YN, Lee JM, Park AY, et al. Effects of oral administration of lactiplantibacillus plantarum apsulloc 331261 (Gtb1(Tm)) isolated from green tea on atopic dermatitis (Ad)-like skin lesion mouse models. Evid Based Complement Alternat Med. (2022) 2022:4520433. doi: 10.1155/2022/4520433
51. Eom JE, Shin DU, Kim GD, Yoon JH, Shin HS, and Lee SY. Pediococcus pentosaceus kf159 alleviates house dust mite-induced atopic dermatitis by promoting Il10 production and regulatory T cell induction. Food Funct. (2024) 15:6975–87. doi: 10.1039/d4fo00933a
52. Jeong DY, Ryu MS, Yang HJ, Jeong SY, Zhang T, Yang HJ, et al. Pediococcus acidilactici intake decreases the clinical severity of atopic dermatitis along with increasing mucin production and improving the gut microbiome in nc/nga mice. BioMed Pharmacother. (2020) 129:110488. doi: 10.1016/j.biopha.2020.110488
53. Fang Z, Pan T, Li L, Wang H, Zhu J, Zhang H, et al. Bifidobacterium longum mediated tryptophan metabolism to improve atopic dermatitis via the gut-skin axis. Gut Microbes. (2022) 14:2044723. doi: 10.1080/19490976.2022.2044723
54. Lee C, Kim SW, Verma R, Noh J, Park JC, Park S, et al. Probiotic consortium confers synergistic anti-inflammatory effects in inflammatory disorders. Nutrients. (2024) 16(6):790. doi: 10.3390/nu16060790
55. Colombo D, Rigoni C, Cantù A, Carnevali A, Filippetti R, Franco T, et al. Probiotics and prebiotics orally assumed as disease modifiers for stable mild atopic dermatitis: an Italian real-life, multicenter, retrospective, observational study. Medicina (Kaunas). (2023) 59(12):2080. doi: 10.3390/medicina59122080
56. Xu B, Ling S, Xu X, Liu X, Wang A, Zhou Y, et al. A new formulation of probiotics attenuates calcipotriol-induced dermatitis by inducing regulatory dendritic cells. Front Immunol. (2021) 12:775018. doi: 10.3389/fimmu.2021.775018
57. Kim HW, Ju DB, Kye YC, Ju YJ, Kim CG, Lee IK, et al. Galectin-9 induced by dietary probiotic mixture regulates immune balance to reduce atopic dermatitis symptoms in mice. Front Immunol. (2019) 10:3063. doi: 10.3389/fimmu.2019.03063
58. Vinderola G, Sanders ME, Cunningham M, and Hill C. Frequently asked questions about the Isapp postbiotic definition. Front Microbiol. (2023) 14:1324565. doi: 10.3389/fmicb.2023.1324565
59. Chen HY, Chen YT, Li KY, Huang HW, Lin YC, and Chen MJ. A heat-killed probiotic mixture regulates immune T cells balance and Ige production in house dust mite extraction-induced atopic dermatitis mice. Microorganisms. (2022) 10(10):1881. doi: 10.3390/microorganisms10101881
60. Carucci L, Nocerino R, Paparo L, De Filippis F, Coppola S, Giglio V, et al. Therapeutic effects elicited by the probiotic lacticaseibacillus rhamnosus gg in children with atopic dermatitis. The results of the propad trial. Pediatr Allergy Immunol. (2022) 33:e13836. doi: 10.1111/pai.13836
61. Yoon W, Park SH, Lee JS, Byeon JH, Kim SH, Lim J, et al. Probiotic mixture reduces gut inflammation and microbial dysbiosis in children with atopic dermatitis. Australas J Dermatol. (2021) 62:e386–e92. doi: 10.1111/ajd.13644
62. Baquerizo Nole KL, Yim E, and Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. (2014) 71:814–21. doi: 10.1016/j.jaad.2014.04.050
63. Lima M and Paulino LC. Oral postbiotics as a therapeutic strategy for atopic dermatitis: A systematic review of randomized controlled trials. J Am Nutr Assoc. (2024) 43:139–46. doi: 10.1080/27697061.2023.2232021
64. Laigaard A, Krych L, Zachariassen LF, Ellegaard-Jensen L, Nielsen DS, Hansen AK, et al. Dietary prebiotics promote intestinal prevotella in association with a low-responding phenotype in a murine oxazolone-induced model of atopic dermatitis. Sci Rep. (2020) 10:21204. doi: 10.1038/s41598-020-78404-0
65. Kang M, Jung JH, Kim JY, Hong SH, and Her Y. Therapeutic and preventive effect of orally administered prebiotics on atopic dermatitis in a mouse model. Allergy Asthma Immunol Res. (2023) 15:303–15. doi: 10.4168/aair.2023.15.3.303
66. Kim JA, Kim SH, Kim IS, Yu DY, Kim GI, Moon YS, et al. Galectin-9 induced by dietary prebiotics regulates immunomodulation to reduce atopic dermatitis symptoms in 1-chloro-2,4-dinitrobenzene (Dncb)-treated nc/nga mice. J Microbiol Biotechnol. (2020) 30:1343–54. doi: 10.4014/jmb.2005.05017
67. Zhang Z, Kuo JC, Yao S, Zhang C, Khan H, and Lee RJ. Cpg oligodeoxynucleotides for anticancer monotherapy from preclinical stages to clinical trials. Pharmaceutics. (2021) 14(1):73. doi: 10.3390/pharmaceutics14010073
68. Jiang W, Lederman MM, Harding CV, Rodriguez B, Mohner RJ, and Sieg SF. Tlr9 stimulation drives naïve B cells to proliferate and to attain enhanced antigen presenting function. Eur J Immunol. (2007) 37:2205–13. doi: 10.1002/eji.200636984
69. Volpi C, Fallarino F, Pallotta MT, Bianchi R, Vacca C, Belladonna ML, et al. High doses of cpg oligodeoxynucleotides stimulate a tolerogenic Tlr9–Trif pathway. Nat Commun. (2013) 4:1852. doi: 10.1038/ncomms2874
70. Jacquet A. Nucleic acid vaccines and cpg oligodeoxynucleotides for allergen immunotherapy. Curr Opin Allergy Clin Immunol. (2021) 21:569–75. doi: 10.1097/aci.0000000000000772
71. Leonard C, Montamat G, Davril C, Domingues O, Hunewald O, Revets D, et al. Comprehensive mapping of immune tolerance yields a regulatory tnf receptor 2 signature in a murine model of successful fel D 1-specific immunotherapy using high-dose cpg adjuvant. Allergy. (2021) 76:2153–65. doi: 10.1111/all.14716
72. Serebrisky D, Teper AA, Huang CK, Lee SY, Zhang TF, Schofield BH, et al. Cpg oligodeoxynucleotides can reverse th2-associated allergic airway responses and alter the B7.1/B7.2 expression in a murine model of asthma. J Immunol. (2000) 165:5906–12. doi: 10.4049/jimmunol.165.10.5906
73. Kusunoki T, Sugai M, Gonda H, Nambu Y, Nagata-Nakajima N, Katakai T, et al. Cpg Inhibits Ige Class Switch Recombination through Suppression of Nf Kappa B Activity, but Not through Id2 or Bcl6. Biochem Biophys Res Commun. (2005) 328:499–506. doi: 10.1016/j.bbrc.2004.12.192
74. Li D, Cheng J, Zhu Z, Catalfamo M, Goerlitz D, Lawless OJ, et al. Treg-inducing capacity of genomic DNA of bifidobacterium longum subsp. Infantis Allergy Asthma Proc. (2020) 41:372–85. doi: 10.2500/aap.2020.41.200064
75. Ménard O, Gafa V, Kapel N, Rodriguez B, Butel MJ, and Waligora-Dupriet AJ. Characterization of immunostimulatory cpg-rich sequences from different bifidobacterium species. Appl Environ Microbiol. (2010) 76:2846–55. doi: 10.1128/aem.01714-09
76. Kim GI, Jeong HY, Kim IS, Lee SH, Kim SH, Moon YS, et al. Interconnection of the gut-skin axis in nc/nga mouse with atopic dermatitis: effects of the three types of bifidobacterium bifidum cbt-bf3 (Probiotics, postbiotics, and cytosine-phosphate-guanine oligodeoxynucleotide) on T cell differentiation and gut microbiota. Food Sci Anim Resour. (2024) 44:1417–39. doi: 10.5851/kosfa.2024.e100
77. Heikenwalder M, Polymenidou M, Junt T, Sigurdson C, Wagner H, Akira S, et al. Lymphoid follicle destruction and immunosuppression after repeated cpg oligodeoxynucleotide administration. Nat Med. (2004) 10:187–92. doi: 10.1038/nm987
78. Choi YJ, Lee KW, Kwon HJ, and Kim DS. Identification of immunostimulatory oligodeoxynucleotide from Escherichia coli genomic DNA. J Biochem Mol Biol. (2006) 39:788–93. doi: 10.5483/bmbrep.2006.39.6.788
79. Lee JH, Lee JH, and Jin JS. Fermentation of traditional medicine: present and future. Oriental Pharm Exp Med. (2012) 12:163–5. doi: 10.1007/s13596-012-0080-4
80. Zhao W, Zhang Y, Li W, Hu Q, Huang H, Xu X, et al. Probiotic-fermented portulaca oleracea L. Alleviated dnfb-induced atopic dermatitis by inhibiting the Nf-κb Signaling pathway. J Ethnopharmacol. (2023) 313:116613. doi: 10.1016/j.jep.2023.116613
81. Kim YK, Cho M, and Kang DJ. Anti-inflammatory response of new postbiotics in Tnf-α/Ifn-γ-induced atopic dermatitis-like hacat keratinocytes. Curr Issues Mol Biol. (2024) 46:6100–11. doi: 10.3390/cimb46060364
82. Kim SH, Seong GS, and Choung SY. Fermented morinda citrifolia (Noni) alleviates dncb-induced atopic dermatitis in nc/nga mice through modulating immune balance and skin barrier function. Nutrients. (2020) 12(1):249. doi: 10.3390/nu12010249
83. Kasman LM and Porter LD. Bacteriophages. In: Statpearls. StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC, Treasure Island (FL (2024).
84. Uyttebroek S, Chen B, Onsea J, Ruythooren F, Debaveye Y, Devolder D, et al. Safety and efficacy of phage therapy in difficult-to-treat infections: A systematic review. Lancet Infect Dis. (2022) 22:e208–e20. doi: 10.1016/s1473-3099(21)00612-5
85. Byrd AL, Deming C, Cassidy SKB, Harrison OJ, Ng WI, Conlan S, et al. Staphylococcus aureus and staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci Transl Med. (2017) 9(397):eaal4651. doi: 10.1126/scitranslmed.aal4651
86. Wang Z, Peng X, Hülpüsch C, Khan Mirzaei M, Reiger M, Traidl-Hoffmann C, et al. Distinct prophage gene profiles of staphylococcus aureus strains from atopic dermatitis patients and healthy individuals. Microbiol Spectr. (2024) 12:e0091524. doi: 10.1128/spectrum.00915-24
87. Gupta M, Anzelc M, Stetkevich S, and Burkhart C. Bacteriophages: an alternative to combat antibiotic resistance? J Drugs Dermatol. (2022) 21:1311–5. doi: 10.36849/jdd.6638
88. Geng H, Yang X, Zou C, Zhang W, Xiang J, Yang K, et al. Isolation of the novel phage sap71 and its potential use against staphylococcus aureus in an atopic dermatitis mouse model. Virus Genes. (2024) 60:737–46. doi: 10.1007/s11262-024-02106-2
89. Kim SG, Giri SS, Yun S, Kim HJ, Kim SW, Kang JW, et al. Synergistic phage-surfactant combination clears Ige-promoted staphylococcus aureus aggregation in vitro and enhances the effect in vivo. Int J Antimicrob Agents. (2020) 56:105997. doi: 10.1016/j.ijantimicag.2020.105997
90. Vendrik KEW, Ooijevaar RE, de Jong PRC, Laman JD, van Oosten BW, van Hilten JJ, et al. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol. (2020) 10:98. doi: 10.3389/fcimb.2020.00098
91. Tu ATT, Hoshi K, Ikebukuro K, Hanagata N, and Yamazaki T. Monomeric G-quadruplex-based cpg oligodeoxynucleotides as potent toll-like receptor 9 agonists. Biomacromolecules. (2020) 21:3644–57. doi: 10.1021/acs.biomac.0c00679
92. Hanagata N. Cpg oligodeoxynucleotide nanomedicines for the prophylaxis or treatment of cancers, infectious diseases, and allergies. Int J Nanomed. (2017) 12:515–31. doi: 10.2147/ijn.S114477
93. Ogonowska P, Szymczak K, Empel J, Urbaś M, Woźniak-Pawlikowska A, Barańska-Rybak W, et al. Staphylococcus aureus from atopic dermatitis patients: its genetic structure and susceptibility to phototreatment. Microbiol Spectr. (2023) 11:e0459822. doi: 10.1128/spectrum.04598-22
94. Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. (2015) 149:102–9.e6. doi: 10.1053/j.gastro.2015.04.001
95. Tankou SK, Regev K, Healy BC, Cox LM, Tjon E, Kivisakk P, et al. Investigation of probiotics in multiple sclerosis. Mult Scler. (2018) 24:58–63. doi: 10.1177/1352458517737390
96. Mashiah J, Karady T, Fliss-Isakov N, Sprecher E, Slodownik D, Artzi O, et al. Clinical efficacy of fecal microbial transplantation treatment in adults with moderate-to-severe atopic dermatitis. Immun Inflammation Dis. (2022) 10:e570. doi: 10.1002/iid3.570
97. Routy B, Lenehan JG, Miller WH Jr., Jamal R, Messaoudene M, Daisley BA, et al. Fecal microbiota transplantation plus anti-pd-1 immunotherapy in advanced melanoma: A phase I trial. Nat Med. (2023) 29:2121–32. doi: 10.1038/s41591-023-02453-x
Keywords: atopic dermatitis, microbial strategies, fecal microbiota transplantation, probiotics, postbiotics, prebiotics, CpG-ODNs, herbal fermentation technology
Citation: Ma J, Fang Y, Hu J, Li S, Zeng L, Chen S, Li Z, Meng R, Yang X, Zhang F, Ji G, Liao P, Chen L and Wu W (2025) Innovative microbial strategies in atopic dermatitis. Front. Immunol. 16:1605434. doi: 10.3389/fimmu.2025.1605434
Received: 03 April 2025; Accepted: 30 June 2025;
Published: 23 July 2025.
Edited by:
Zarqa Ali, Bispebjerg Hospital, DenmarkReviewed by:
Supriya Khanra, University of Glasgow, United KingdomSpela Suler Baglama, University Medical Centre Ljubljana, Slovenia
Copyright © 2025 Ma, Fang, Hu, Li, Zeng, Chen, Li, Meng, Yang, Zhang, Ji, Liao, Chen and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Chen, MTg5Mjg5Mjk3MjJAMTI2LmNvbQ==; Wei Wu, d3V3QGdkaXBoLm9yZy5jbg==
†These authors have contributed equally to this work
 Jingtai Ma
Jingtai Ma Yiting Fang
Yiting Fang Jinxing Hu
Jinxing Hu Shiqi Li2
Shiqi Li2 Lilian Zeng
Lilian Zeng Ruiling Meng
Ruiling Meng Xingfen Yang
Xingfen Yang Wei Wu
Wei Wu