- 1Department of Pediatrics, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China
- 2Department of Pediatrics, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 3Department of Epidemiology and Health Statistics & Hunan Provincial Key Laboratory of Clinical Epidemiology, XiangYa School of Public Health, Central South University, Changsha, Hunan, China
- 4Department of Epidemiology and Health Statistics, Xiangya School of Public Health, Central South University, Changsha, Hunan, China
- 5Department of Rheumatology and Immunology, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 6Department of Nephrology and Rheumatology, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 7Department of Rheumatology and Immunology, Shenzhen Children’s Hospital, Shenzhen, China
- 8Department of Rheumatology and Immunology, Children’s Hospital of Nanjing Medical University, Nanjing, China
- 9Department of Immunology, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, China
Objective: This study evaluated the diagnostic accuracy of the 2012 SLICC and 2019 EULAR/ACR criteria in Chinese cSLE patients and aimed to develop an optimized classification schema based on the 2019 EULAR/ACR criteria, specifically tailored for cSLE.
Methods: Data from cSLE and control cases were extracted from the CAPRID database. Gold-standard diagnosis were established by consensus among 43 rheumatologists (≥80% agreement). From 1,390 consensus cases, a random selection of 1,045 cases (512 cSLE/533 non-cSLE) were allocated to derivation (n=522) and validation (n=523) cohorts. The 2012 SLICC and 2019 EULAR/ACR criteria were evaluated in the total cohort. Multiple optimization schemes were then developed through LASSO regression with expert consultation in the derivation cohort. All potential optimization schemes underwent validation in the validation cohort, from which the optimal scheme was selected and further evaluated in an ANA-positive subgroup.
Results: The 2012 SLICC criteria demonstrated sensitivity of 96.7% and specificity of 96.5%, while the 2019 EULAR/ACR criteria had sensitivity of 95.3% and specificity of 97.8%, with an optimal total score threshold of 10. When both the non-scarring alopecia and arthritis criteria were removed alongside the redefined urinary protein criterion, specificity significantly improved to 99.3% (P < 0.05), while sensitivity remained unaffected at 94.1% (P = 0.210). In the ANA-positive cohort, the optimized integrated scheme significantly improved specificity (97.7% vs. 86.4%, P = 0.012) while maintaining comparable sensitivity (96.2% vs. 97.8%, P = 0.138).
Conclusion: Both criteria performed well in Chinese cSLE patients. Optimizing the 2019 EULAR/ACR criteria by removing alopecia and arthritis criteria and modifying the urinary protein criterion enhanced specificity without compromising sensitivity.
Introduction
Systemic lupus erythematosus (SLE) is a complex autoimmune disorder characterized by immune dysregulation and chronic inflammation, with an overall mortality rate higher than that of the general population (1, 2). Childhood-onset systemic lupus erythematosus (cSLE), defined as SLE diagnosed before the age of 18, has an incidence rate of 3.97 cases per 100,000 person-years (95% CI: 3.93-4.01) in China (3). The most widely used classification criteria for both adult-onset SLE (aSLE) and cSLE are the 2012 Systemic Lupus International Collaborating Clinics (SLICC) criteria and the 2019 European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) criteria (4, 5). These criteria were primarily developed and validated in non-Asian aSLE populations. Subsequently, a dedicated study validated the applicability of the 2019 EULAR/ACR criteria in antinuclear antibody (ANA)-positive aSLE patients in China, demonstrating excellent classification performance (6). However, no specific classification criteria have been established for cSLE, as existing criteria are primarily derived from aSLE populations. Although multiple studies have evaluated the applicability of these classification criteria in cSLE, these results remain controversial (7–10).
It should be particularly noted that cSLE exhibits complex pathogenesis and demonstrates significant differences compared to aSLE, including distinct sex distribution patterns, more aggressive disease phenotypes, lower treatment response rates, and poorer prognosis (11, 12). A Turkish cohort study (13) revealed that cSLE patients have higher frequencies of renal, mucocutaneous, hematologic, and neuropsychiatric involvement, along with elevated positivity rates for anti-double-stranded DNA (anti-dsDNA) antibodies and anticardiolipin antibodies. These findings were further corroborated by a Canadian cohort study (14), which demonstrated significantly increased rates of neuropsychiatric manifestations and anticardiolipin antibody positivity in cSLE. Epidemiological data from both France and China (3, 15) consistently indicate that cSLE patients exhibit higher rates of renal and hematologic involvement, as well as greater disease severity. These differences not only underscore the importance of early diagnosis and treatment for cSLE, but also reveal the inherent limitations of applying adult-derived classification criteria to pediatric populations.
Therefore, this study aims to validate the applicability of the 2012 SLICC and 2019 EULAR/ACR classification criteria in Chinese cSLE and to explore potential optimizations to develop more appropriate classification criteria tailored to cSLE.
Patients and methods
Using a methodological workflow grounded in clinical epidemiology and expert consensus, the optimized cSLE classification criteria were developed in five phases (Figure 1): (1) data preparation, (2) gold standard establishment, (3) existing criteria validation, (4) optimization schemes derivation, and (5) validation of optimized schemes.
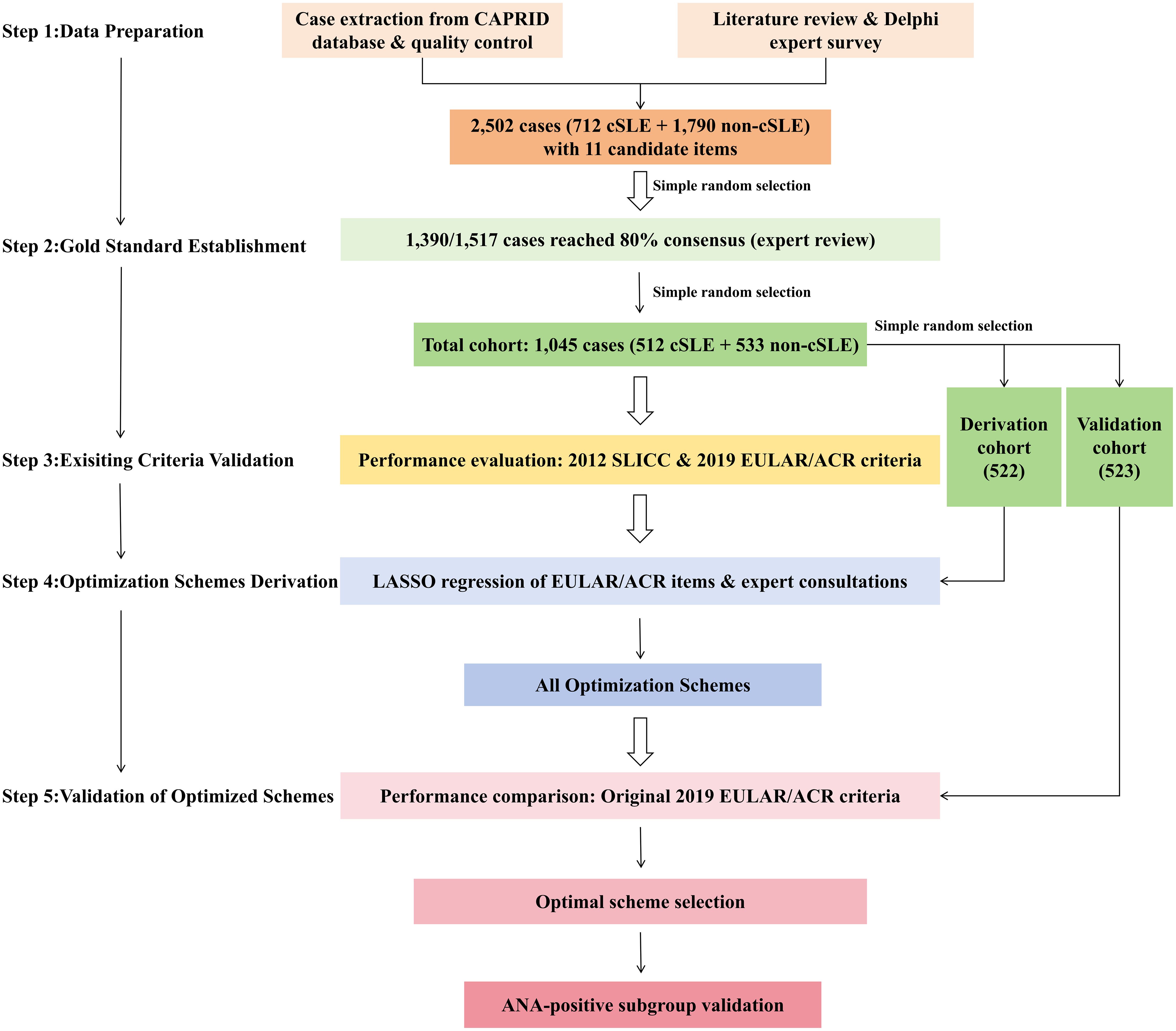
Figure 1. Methodological workflow for cSLE classification criteria optimization. cSLE, childhood-onset systemic lupus erythematosus; ANA, antinuclear antibody.
Stage 1: data preparation
Retrospective case data of pediatric rheumatic and immunological diseases were extracted from the Chinese Alliance of Pediatric Rheumatic and Immunologic Diseases (CAPRID) database, which comprises data from six Class-A tertiary hospitals. Cases included patients with an initial diagnosis of cSLE and those with other pediatric rheumatic diseases. Inclusion criteria were: age 5–18 years, confirmed diagnosis between 2017- 2023, and at least one year of follow-up. Exclusion criteria included neonatal lupus, monogenic lupus, overlap syndromes, infectious diseases, neoplastic diseases, and recurrent cases. After rigorous quality control procedures (including data completeness verification and outlier removal), we identified 2,502 eligible cases (712 cSLE/1,790 non-cSLE). From this pool, 1,517 cases (60.6%) were randomly selected for expert review using computer-generated randomization. Eleven supplementary candidate items were identified through systematic literature review and three-round Delphi surveys.
Stage 2: gold standard establishment
A panel of 43 rheumatologists with over ten years of clinical experience independently evaluated de-identified cases through a standardized electronic case report form (CRF). To eliminate institutional bias, reviewers were systematically excluded from evaluating cases originating from their own hospitals. For each case, experts selected one of three predefined classifications: (1) definite cSLE, (2) non-cSLE, or (3) indeterminate. A case was considered gold-standard confirmed when ≥80% of reviewers (≥4 of 5 raters) reached concordant classification. This rigorous process yielded 1,390 diagnostically validated cases (91.6% consensus rate). From these 1,390 confirmed cases, we randomly selected 1,045 cases (512 cSLE/533 non-cSLE) to form the total study cohort. This cohort was then randomly split into a derivation cohort (n=522) and a validation cohort (n=523) for subsequent analysis.
Stage 3: existing criteria validation
All analyses were performed using SPSS Statistics version 26.0 and R software version 4.1.1. Continuous variables were assessed for normality using Shapiro-Wilk tests. Continuous variables were assessed for normality using the Shapiro-Wilk test. Normally distributed data are presented as mean ± standard deviation (SD) and analyzed using independent t-tests (for two-group comparisons) or one-way analysis of variance (ANOVA) (for multi-group comparisons). Non-normally distributed data are expressed as median (interquartile range, IQR) and compared using Mann-Whitney U tests (two groups) or Kruskal-Wallis tests (multiple groups). Categorical variables are reported as counts (percentages) and analyzed using χ² tests or Fisher’s exact tests, as appropriate. All statistical tests were two-tailed with a significance level of α=0.05. The classification performance of the 2012 SLICC and 2019 EULAR/ACR criteria was evaluated in the total cohort. We calculated sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), Youden index, and accuracy, with 95% confidence intervals (CIs) estimated using Wilson’s method. Receiver operating characteristic (ROC) curve analysis was conducted for the 2019 EULAR/ACR criteria to determine the optimal score threshold.
Stage 4: optimization schemes derivation
In the derivation cohort (n = 522), we employed a dual approach combining statistical modeling and expert consensus to optimize the 2019 EULAR/ACR criteria. First, least absolute shrinkage and selection operator (LASSO) regression analysis was performed using R 4.1.1 software. The optimal penalty parameter (λ) was determined based on the minimum mean squared error (MSE) through 10-fold cross-validation, and variables with coefficients of zero were selected as optimizable items. The expert panel then discussed and identified potential optimization schemes.
Stage 5: validation of optimized schemes
All potential optimization schemes were compared with the original 2019 EULAR/ACR criteria in the validation cohort (n=523) using sensitivity, specificity, and ROC analyses. The optimal scheme was subsequently validated in an ANA-positive subgroup.
Results
Demographic and clinical characteristics of included study participants
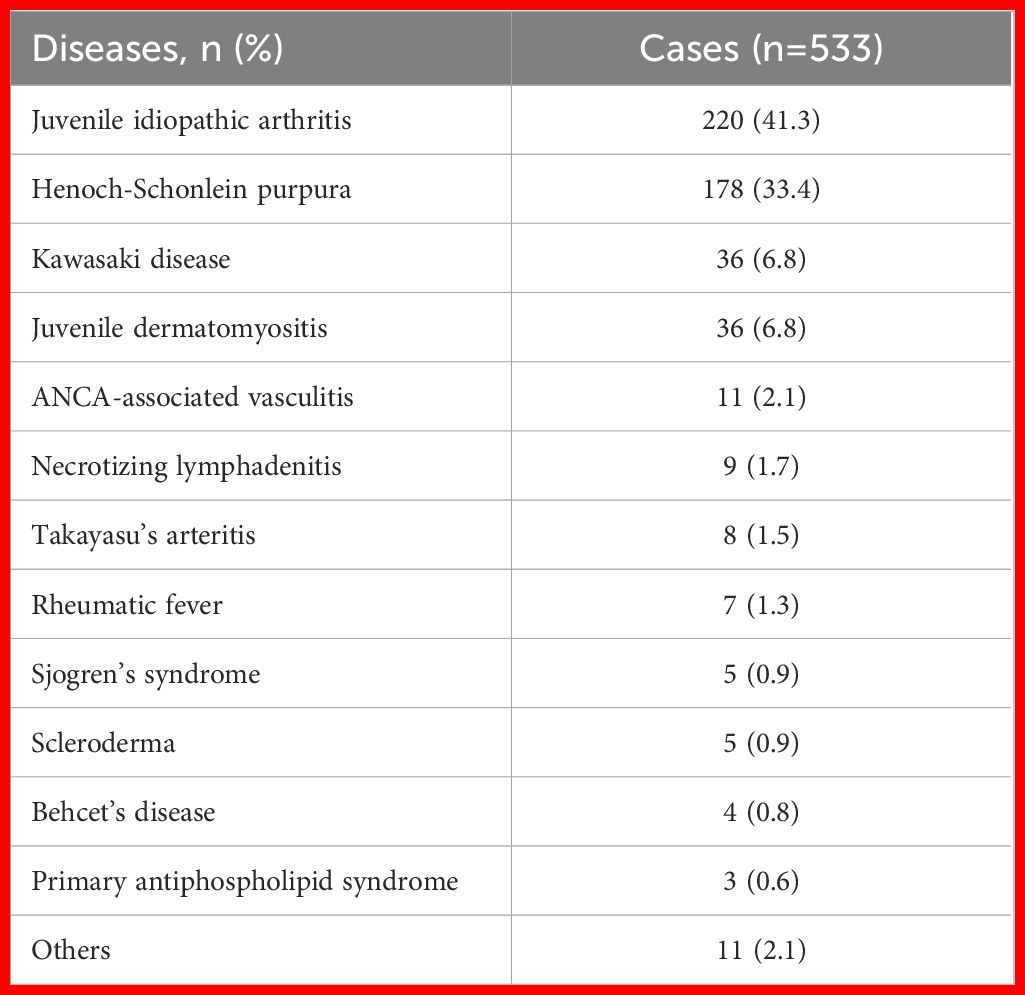
A total of 512 cSLE patients and 533 controls were included in this study. Table 1 summarizes the disease distribution in the control group, with juvenile idiopathic arthritis (JIA) accounting for 41.3% and Henoch-Schönlein purpura (HSP) for 33.4%, representing the primary differential diagnoses. The remaining 25.3% of cases included Kawasaki disease, juvenile dermatomyositis, ANCA-associated vasculitis, necrotizing lymphadenitis, Takayasu’s arteritis, rheumatic fever, and other miscellaneous disorders.
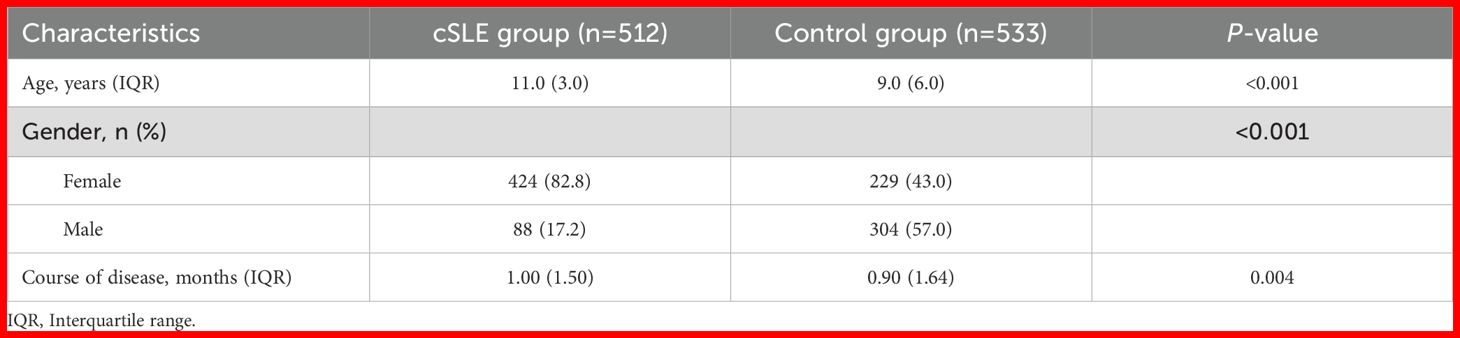
Demographic characteristics of the total cohort are presented in Table 2. The cSLE group was older (11.0 (3.0) vs 9.0 (6.0), P < 0.001) and had higher female predominance (82.8% vs 43.0%, P < 0.001) compared to controls. Additionally, disease duration at diagnosis was slightly longer in cSLE (1.00 vs 0.90, P = 0.004).
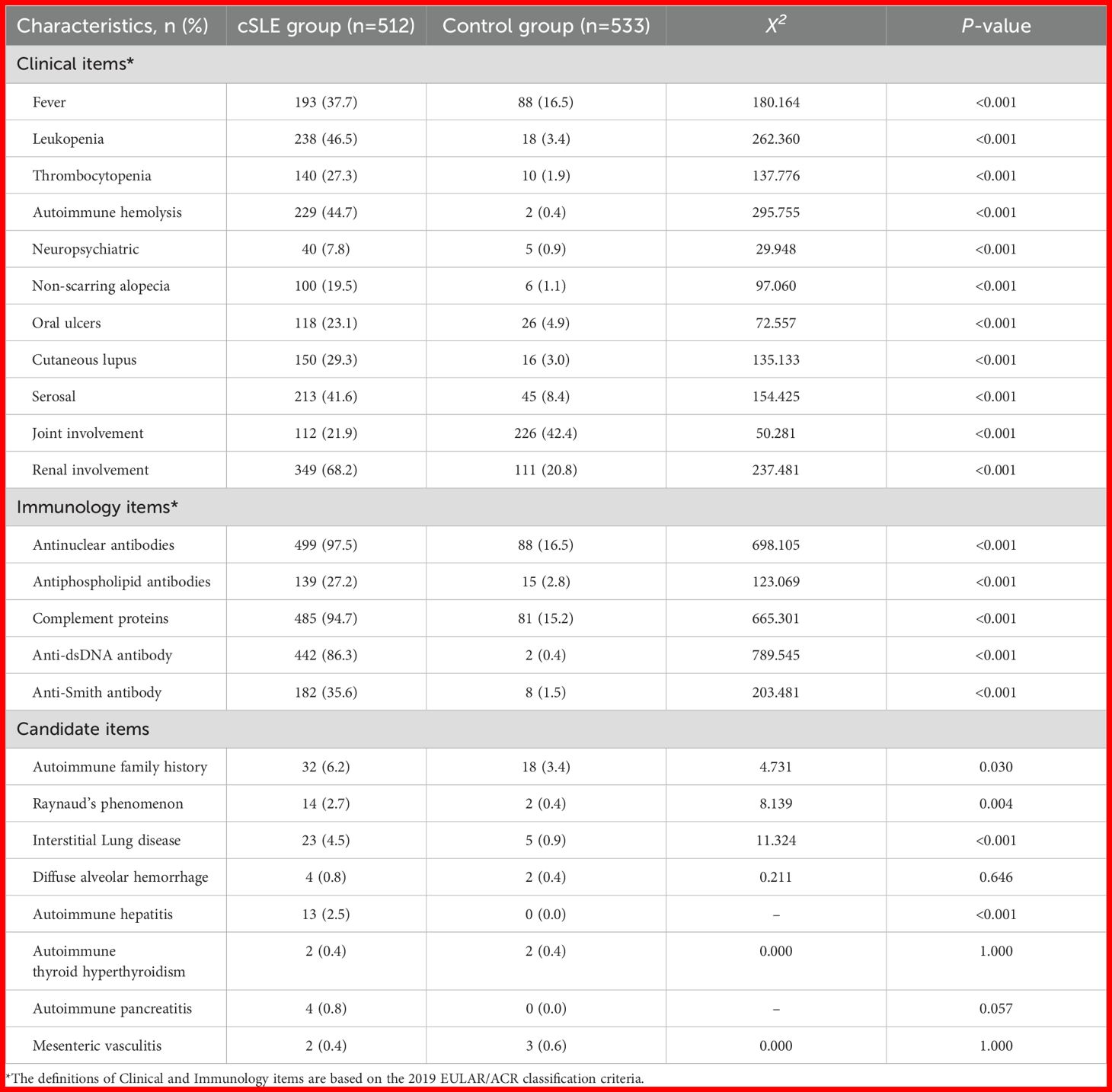
Table 3 compares clinical and immunological characteristics between groups. All cSLE classification criteria manifestations (e.g., fever, neuropsychiatric, joint involvement, renal involvement) and immunological characteristics (e.g., ANA, anti-dsDNA antibodies, complement levels) were significantly more frequent in cSLE (all P < 0.001). Among candidate items, cSLE showed higher positivity rates of autoimmune family history, Raynaud’s phenomenon, interstitial lung disease, and autoimmune hepatitis (P < 0.05), but no differences in diffuse alveolar hemorrhage, autoimmune hyperthyroidism, autoimmune pancreatitis, or mesenteric vasculitis (P > 0.05).
Validation of existing classification criteria
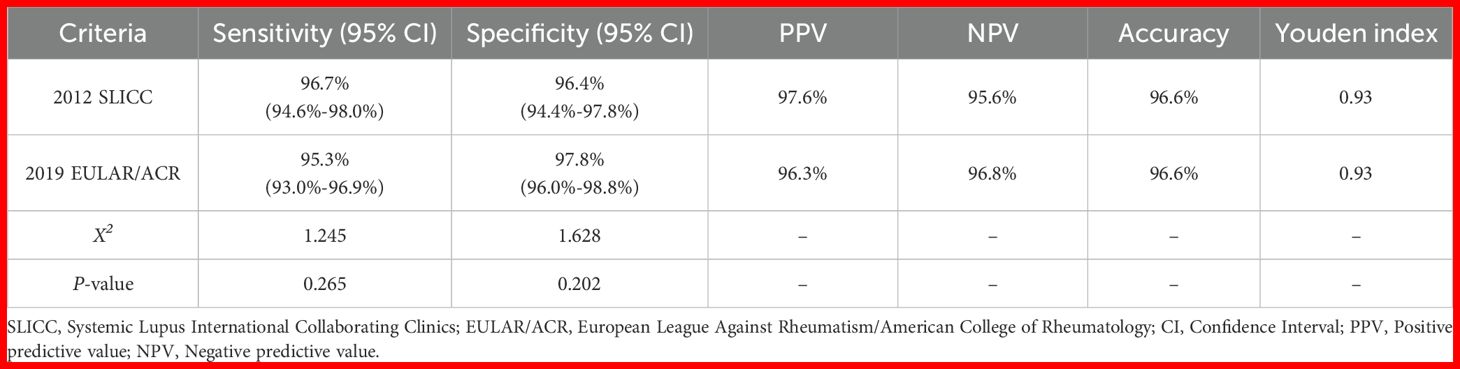
Table 4 evaluated the classification performance of the 2012 SLICC and 2019 EULAR/ACR criteria in the total cohort. The 2012 SLICC criteria showed 96.7% (95% CI: 94.6%-98.0%) sensitivity and 96.4% (95% CI: 94.4%-97.8%) specificity, while the 2019 EULAR/ACR criteria demonstrated 95.3% (95% CI: 93.0%-96.9%) sensitivity and 97.8% (95% CI: 96.0%-98.8%) specificity. Both criteria achieved 96.6% accuracy (Youden index=0.93). Figure 2A shows the ROC curve for the 2019 EULAR/ACR criteria score. The graph illustrates excellent diagnostic performance (AUC= 0.984, 95% CI 0.976-0.993), with a maximum Youden index of 0.946 corresponding to an optimal cutoff score of 10 (rounded from 9.5) for cSLE classification.
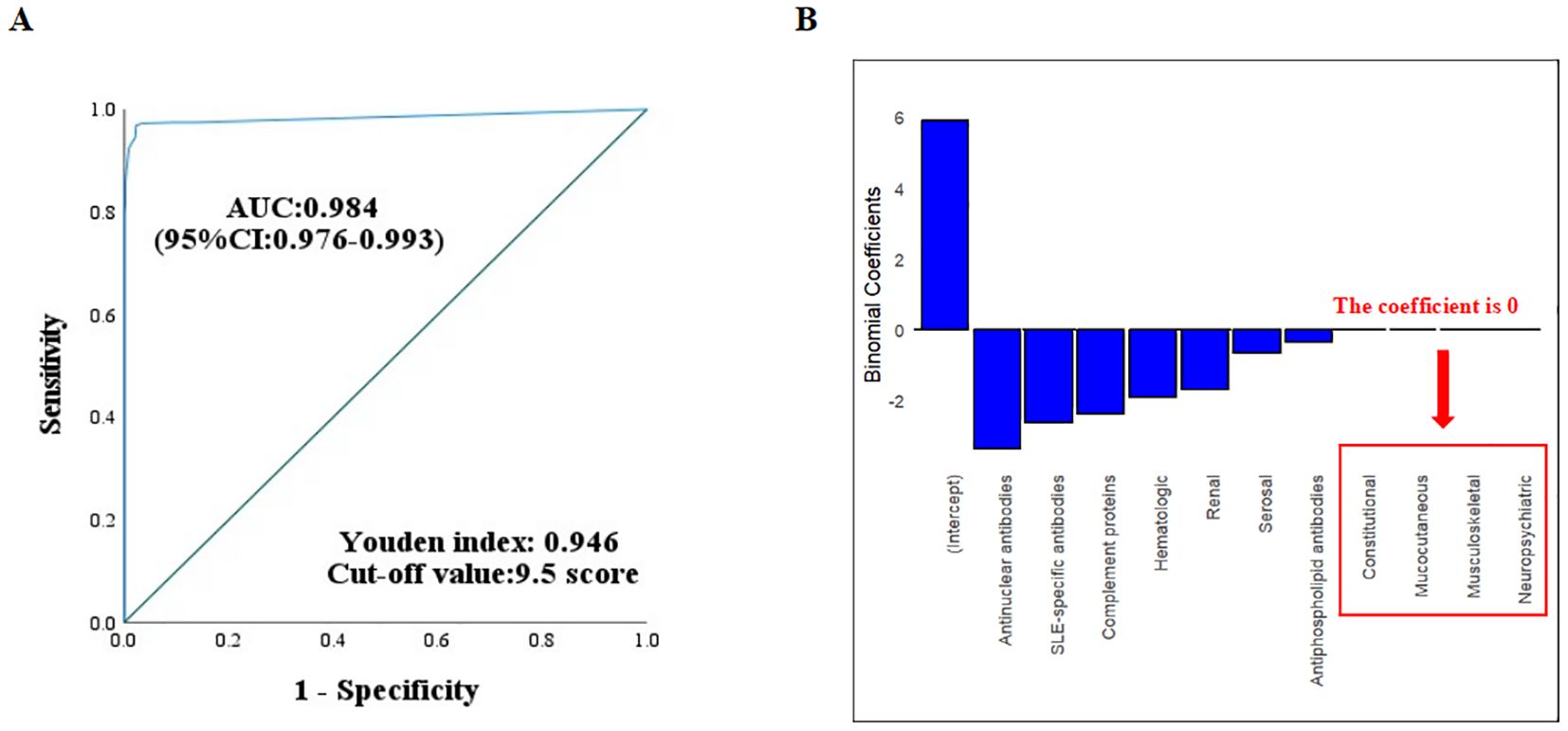
Figure 2. (A) ROC curve analysis of the 2019 EULAR/ACR criteria in the derivation cohort. The optimal cutoff value was determined by maximizing Youden’s index. (B) Regression coefficients for the 10 systemic domains in the 2019 EULAR/ACR criteria. Variables with 0 coefficients showed negligible weighting in the classification model.
Exploration of optimization in the derivation cohort using lasso regression and expert opinions
LASSO regression analysis of the ten 2019 EULAR/ACR-defined systemic domains in the derivation cohort showed zero coefficients for constitutional, mucocutaneous, neuropsychiatric, and musculoskeletal systems (Figure 2B). An expert panel reviewed the findings and reached a consensus. For non-scarring alopecia, musculoskeletal system involvement, and the assessment of urinary protein results, eliminations or definitional amendments were proposed individually or in combination, resulting in various optimization strategies. Among these, the criteria for urinary protein results were revised to include one of the following: 24-hour urinary protein > 150 mg, UPr/Cr > 0.2, or a positive qualitative urinary protein test. ROC analysis determined that the optimal total score cutoff for these optimization strategies was 9.5 points.
Evaluation of all optimization schemes in the validation cohort
In the validation cohort (n=523), all optimization schemes were systematically evaluated (Table 5). Individual exclusion of alopecia or arthritis criteria showed comparable sensitivities(95.3- 95.7% vs 96.5% original, P > 0.05) with modest specificity improvements (97.0-98.9% vs 95.9% original, P >0.05).The redefined proteinuria criteria maintained identical sensitivity (96.5%) and specificity (95.9%) compared to the original criteria (P = 1.000). However, combining the exclusion of both alopecia and arthritis criteria with the redefined proteinuria criteria significantly improved specificity to 99.3% (P < 0.05), while sensitivity remained comparable at 94.1% (P = 0.210).
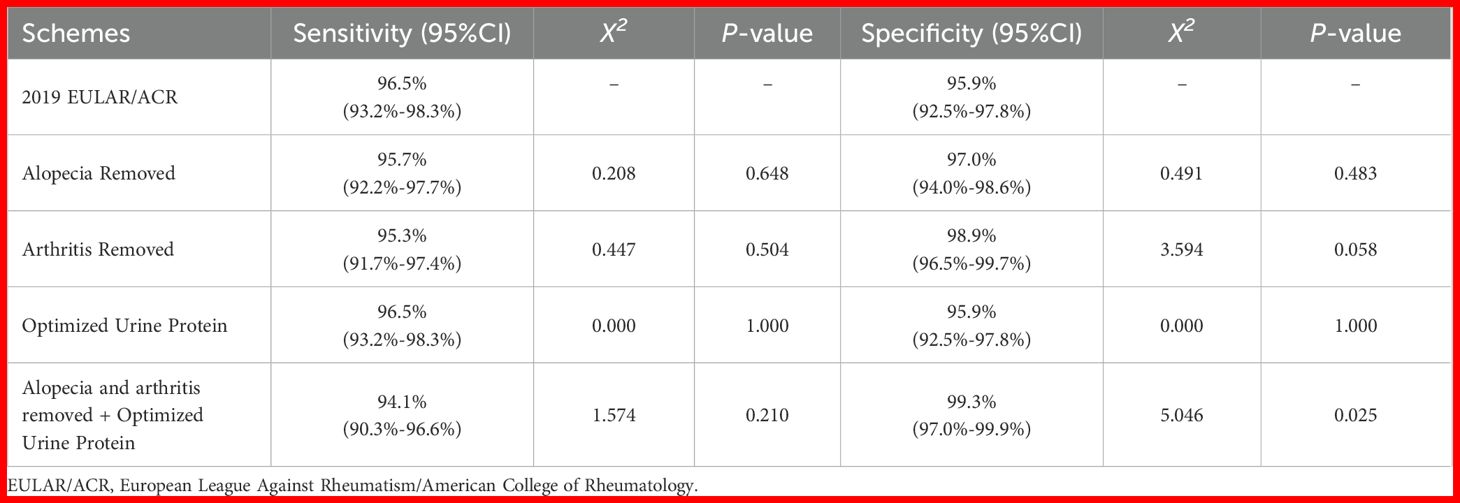
Table 5. Comparison of classification performance between optimized schemes and the 2019 EULAR/ACR criteria in the validation cohort.
Validation of the optimized integrated scheme in ANA-positive subgroup
To further evaluate the classification accuracy, we assessed the classification performance of the optimized integrated scheme in the ANA-positive subgroup, comprising 499 cSLE cases and 88 controls. The most frequent diagnoses among controls were JIA (48.9%) and juvenile dermatomyositis (21.6%). Comparative analysis showed that the optimized integrated scheme significantly improved specificity versus the 2019 EULAR/ACR criteria (97.7% vs 86.4%, P = 0.012) while maintaining comparable sensitivity (96.2% vs 97.8%, P = 0.138) (Table 6).
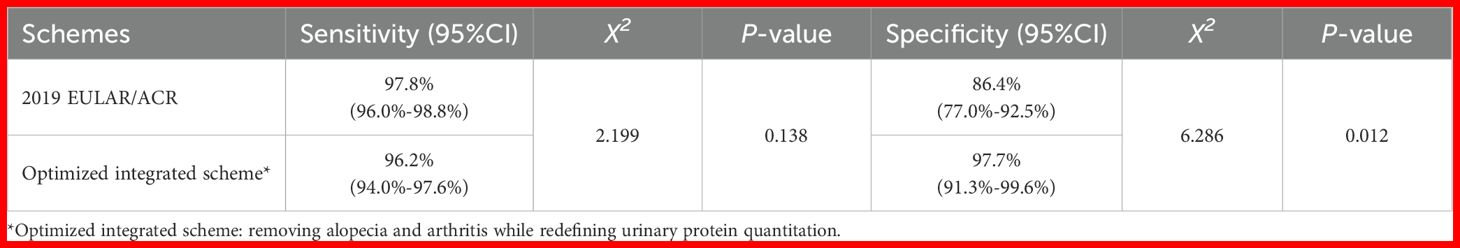
Table 6. Comparison of classification performance between the optimized integrated scheme and the 2019 EULAR/ACR Criteria in the ANA-positive cohort.
Discussion
This multicenter, large-sample retrospective study systematically evaluated the classification performance of the 2012 SLICC and 2019 EULAR/ACR criteria in a Chinese cSLE population. Through the integration of rigorous statistical analysis with expert consensus, we developed a revised classification scheme optimized for Chinese cSLE patients. The revised scheme significantly improved specificity while maintaining comparable sensitivity, with consistent performance in the ANA-positive subgroup. This provides clinically relevant improvements for cSLE classification in the Chinese population.
In the total cohort of this study, both the 2012 SLICC and 2019 EULAR/ACR criteria demonstrated comparable sensitivity (96.7% vs. 95.3%) and specificity (96.4% vs. 97.8%) at the time of diagnosis. Consistent with our findings, Ma et al. (16) also observed similar sensitivity and specificity between the two criteria in a retrospective cohort study of 156 cSLE cases. However, discrepancies exist among different studies. In a longitudinal study by Aslan et al. (17) involving 111 cSLE and 104 controls, the performance of three classification criteria was evaluated at diagnosis, 1-year follow-up, and final follow-up. The results showed that the 2019 EULAR/ACR criteria exhibited higher sensitivity at diagnosis and 1-year follow-up, and while its specificity was lower than that of the 1997 ACR criteria, it was superior to the 2012 SLICC criteria. A similar trend was observed in an Israeli multicenter pediatric cohort study (10), where the 2019 EULAR/ACR criteria demonstrated the highest sensitivity throughout the follow-up period, along with slightly better specificity than the 2012 SLICC criteria. However, an Omani multicenter study (9) found that although the 2019 EULAR/ACR criteria showed superior sensitivity at diagnosis, 1-year follow-up, and final follow-up, its specificity remained the lowest. Adjusting the threshold to 13 points improved specificity but at the cost of significantly reduced sensitivity.
Conversely, other studies indicated that the classification performance of the 2019 EULAR/ACR criteria was not superior to that of the 2012 SLICC criteria. A Turkish multicenter retrospective study (18) revealed that the 2012 SLICC criteria outperformed the 2019 EULAR/ACR criteria in both sensitivity and specificity. Additionally, a UK cohort study (19) demonstrated that the 2012 SLICC criteria had higher sensitivity than the 2019 EULAR/ACR criteria at both initial diagnosis and final follow-up, albeit with lower specificity. Similarly, a Brazilian single-center study (20) found that compared to the EULAR/ACR criteria (≥10 points), the SLICC criteria exhibited better specificity at initial diagnosis and 1-year follow-up, despite comparable sensitivity between the two. Raising the threshold to 13 points improved the specificity of the 2019 EULAR/ACR criteria but also led to a decline in sensitivity. While most existing studies have optimized classification criteria by adjusting thresholds, our cohort achieved ideal sensitivity and specificity at the 10-point threshold. Therefore, we focused on simplifying the classification criteria rather than modifying the score threshold, making this the first study to propose a simplified classification approach.
Despite good classification performance, the 2019 EULAR/ACR criteria have notable limitations, particularly in specificity. Requiring ANA positivity as an entry criterion limits its applicability to ANA-positive cases only, and specificity within this subgroup still needs improvement. Second, several methodological challenges emerged during the expert blind evaluation, including face-to-face discussions guided by extensive clinical experience and CRF data entry. Furthermore, direct application of criteria developed from adult populations to pediatric cohorts presents significant practical limitations. Key limitations include: (1) The rarity of typical non-scarring alopecia in pediatric populations, resulting in the absence of objective, standardized assessment criteria for alopecia in cSLE. Frequently, reported “alopecia” in medical records reflects patient or parental descriptions of “increased hair shedding” or “new-onset hair loss”, which often lack objective clinical confirmation as either alopecia areata or measurable hair density reduction upon physical examination. This introduces substantial subjectivity in assessing and documenting disease-related alopecia. Consequently, the expert panel reached consensus to consider excluding non-scarring alopecia from the optimized classification criteria.
Both the 2012 SLICC and 2019 EULAR/ACR classification criteria adopt consistent definitions for musculoskeletal involvement, mandating either: (1) synovitis involving ≥2 joints characterized by swelling or effusion; or (2) tenderness in ≥2 joints accompanied by morning stiffness persisting ≥30 minutes. Despite multiple studies (21–23) identifying musculoskeletal involvement as a common clinical feature in cSLE, only 21.9% of Chinese cSLE patients met the strict diagnostic criteria for joint involvement. Among cSLE patients classified as negative for joint involvement, common presentations included: (1) monoarticular involvement; (2) persistent arthralgia without objective swelling or radiographic evidence of effusion/synovitis; or (3) tenderness with limited range of motion but lacking the required morning stiffness duration. These clinical presentations may lead to false-negative classification of joint involvement in cSLE. To minimize selection bias, our study employed a control group representing the actual spectrum of pediatric rheumatic diseases treated across the six participating centers, rather than specifying particular disease controls. Notably, JIA was the predominant diagnosis in both the overall control group and ANA-positive subset, with 42.4% demonstrating musculoskeletal involvement-the sole criterion showing statistically significant intergroup differences, albeit lower in the SLE group. Thus, despite being a significant clinical manifestation in cSLE, musculoskeletal involvement showed zero contribution to disease classification in LASSO regression analysis. Consequently, musculoskeletal involvement was identified as a potential candidate for criteria optimization. Unsurprisingly, the proposal to exclude the arthritis criterion generated substantial discussion and controversy among the expert panel. Final analysis revealed that the optimized scheme, excluding joint involvement, maintained sensitivity while significantly improving specificity. However, we recognize that this approach may compromise sensitivity, particularly for early SLE identification.
The 2019 EULAR/ACR classification criteria define proteinuria as >500 mg/24-hour urine protein or an equivalent UPr/Cr ratio. However, pediatric rheumatology experts agree that this threshold is suboptimal for pediatric populations due to growth-related variations. Additionally, there are no established conversion factors between 24-hour urine protein and UPr/Cr for diverse pediatric ethnic groups. The optimal definition of proteinuria in children should incorporate body size adjustments, such as 24-hour urine protein/Kg or/m2, to enable better comparison across age groups in cSLE (24, 25). While UPr/Cr is a convenient alternative (26), complete 24-hour urine collections or UPr/Cr measurements are often unavailable in clinical practice, especially in control groups (e.g., JIA, JDM, KD) where renal involvement is uncommon. Given these challenges, we proposed a revised proteinuria definition incorporating: (1) >150 mg/24-hour urine protein; (2) UPr/Cr >0.2; or (3) positive qualitative proteinuria on routine urinalysis. This revised definition demonstrated comparable sensitivity and specificity to the original criteria. This finding aligns with the well-documented high prevalence of renal involvement and more severe organ damage in cSLE (27). We emphasize that these three methods are not fully interchangeable, and their appropriate use requires clinical judgment. The expert panel strongly affirms the clinical utility of both 24-hour urine protein quantification and UPr/Cr for evaluating renal involvement in cSLE. Future studies should establish validated, body size-adjusted cut-off values for 24-hour proteinuria and UPr/Cr specific to Chinese cSLE populations.
Despite LASSO regression analysis showing zero contribution from neuropsychiatric involvement to the classification criteria, pediatric rheumatologists argued that the reported 4.81% prevalence in Chinese cSLE patients substantially underestimates the true incidence. Globally, neuropsychiatric manifestations are reported in 13.5%-50.9% of cSLE cases (28). The reliability of children’s descriptions of neurological symptoms, such as headaches, personality changes, and memory impairment, varies significantly with age. Moreover, pediatric patients typically exhibit lower tolerance for invasive procedures like lumbar punctures compared to adults, which may contribute to underreporting of neuropsychiatric involvement. Data from a specialized center that routinely performs cerebrospinal fluid (CSF) analysis, electroencephalography (EEG), and brain positron emission tomography-computed tomography (PET-CT) in cSLE patients show frequent observation of elevated CSF pressure, non-epileptiform EEG abnormalities, and cerebral metabolic changes, even in asymptomatic patients. Notably, excluding neuropsychiatric criteria from our cohort did not improve specificity or sensitivity. Therefore, the expert panel decided to retain neuropsychiatric involvement in the refined classification criteria, based on this retrospective cohort study.
Furthermore, our analysis demonstrates that for ANA-positive cSLE patients, the most discriminative classification criteria include immunological markers and objectively quantifiable manifestations in the hematological system, kidneys, and serosal membranes. It is crucial to emphasize that classification criteria serve primarily to differentiate SLE from its mimickers, rather than comprehensively characterize the disease. Thus, our proposed optimization-removing non-scarring alopecia and arthritis while revising the proteinuria criterion-reinforces the essential classification framework for this population. Although derived from Chinese cSLE data, the optimized framework’s reliance on objective parameters (e.g., renal/hematologic metrics) and exclusion of subjective features (e.g., alopecia) may have transnational relevance. Given the biological consistency of pediatric SLE manifestations, this approach could inform global cSLE classification, though validation across ethnic populations remains imperative.
To ensure scientific rigor and objectivity, we implemented multiple measures, including data anonymization and independent expert blind review. Laboratory results were systematically extracted from a dedicated disease database using standardized codes to minimize data entry errors during CRF data entry. However, several inherent limitations remain. The primary limitation is the retrospective design, which relies on pre-existing medical records. Data completeness and accuracy are limited by the quality of source documentation, potentially affecting the precision of our results. Therefore, prospective studies are needed to validate these findings. Additionally, the small sample size of ANA-negative cSLE patients (n=14) limited our ability to conduct a detailed analysis of classification improvement in this subgroup. The proposed optimized scheme is specifically tailored for ANA-positive cSLE patients.
Conclusion
In this large Chinese cSLE cohort, both 2012 SLICC and 2019 EULAR/ACR classification criteria demonstrated robust performance. The 2019 EULAR/ACR criteria, with a total score threshold of 10, proved equally effective for cSLE classification. Furthermore, the removal of non-scarring alopecia and arthritis, along with the modification of the urinary protein criterion based on the 2019 EULAR/ACR classification criteria, significantly improved specificity without compromising sensitivity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of the second Xiangya Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
YZ: Formal analysis, Writing – original draft. TS: Project administration, Supervision, Validation, Writing – review & editing. SG: Data curation, Formal analysis, Investigation, Writing – review & editing. JXY: Methodology, Writing – review & editing. JS: Methodology, Writing – review & editing. XT: Data curation, Resources, Writing – review & editing. MW: Data curation, Resources, Writing – review & editing. JY: Data curation, Resources, Writing – review & editing. HY: Data curation, Resources, Writing – review & editing. HM: Data curation, Resources, Writing – review & editing. LS: Data curation, Resources, Writing – review & editing. YL: Data curation, Resources, Writing – review & editing. YC: Data curation, Resources, Writing – review & editing. XL: Data curation, Resources, Writing – review & editing. YW: Data curation, Resources, Writing – review & editing. QL: Data curation, Resources, Writing – review & editing. HS: Data curation, Project administration, Resources, Supervision, Writing – review & editing. XW: Data curation, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the National Key Research and Development Program of China: 2021YFC2702004 and National Natural Science Foundation of China Youth Project: 82101905.
Acknowledgments
This study gratefully acknowledges the participation of the following experts in the blind review process: Luo Chong, Zhang Yu, Tang Wenjing, Yang Xi, Wang Mo, Yang Haiping, Zhang Gaofu, Wan Junli, Wang Anshuo, Yang Qin, He Tingyan, Wang Linlin, Huang Yanyan, Xu Yongbin, Li Yan, Han Tongxin, Shu Zhou, Piao Yurong, Ma Le, Huang Na, Huang Hui, Guo Yihong, Qiu Lingzhi, Ma Huihui, Lu Meiping, Guo Li, Zheng Qi, Teng Liping, Zou Lixia, Ma Mingsheng, Jian Shan, Yu Zhongxun, and Zhou Yu. Special thanks also go to Li Huimin, Lu Xuan, Jiang Yufan, Zhang Huinan, Dong Yaohua, and Zheng Yajing for their assistance in data entry into the CRF.
Conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
ACR: American College of Rheumatology
ANA: Antinuclear antibody
anti-dsDNA: Anti-double-stranded DNA
ANOVA: One-way analysis of variance
aSLE: Adult-onset systemic lupus erythematosus
cSLE: Childhood-onset systemic lupus erythematosus
CIs: Confidence intervals
CRF: Case report form
CSF: Cerebrospinal fluid
EEG: Electroencephalography
EULAR: European League Against Rheumatism
HSP: Henoch-Schönlein purpura
IQR: Interquartile range
JIA: Juvenile idiopathic arthritis
LASSO: Least absolute shrinkage and selection operator
MSE: Minimum mean squared error
NPV: Negative predictive value
PET-CT: Positron emission tomography-computed tomography
PPV: Positive predictive value
ROC: Receiver operating characteristic
SD: Standard deviation
SLE: Systemic lupus erythematosus
SLICC: Systemic Lupus International Collaborating Clinics.
References
1. Arnaud L, Chasset F, and Martin T. Immunopathogenesis of systemic lupus erythematosus: An update. Autoimmun Rev. (2024) 23:103648. doi: 10.1016/j.autrev.2024.103648
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
2. Lee YH, Choi SJ, Ji JD, and Song GG. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. (2016) 25:727–34. doi: 10.1177/0961203315627202
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
3. Gao S, Yu Z, Ma X, Sun J, Ren A, Gao S, et al. Childhood-onset systemic lupus erythematosus in China, 2016-21: a nationwide study. Lancet Child Adolesc Health. (2024) 8:762–72. doi: 10.1016/S2352-4642(24)00172-X
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
4. Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. (2012) 64:2677–86. doi: 10.1002/art.34473
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
5. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European league against rheumatism/American college of rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. (2019) 71:1400–12. doi: 10.1002/art.40930
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
6. Chung YK, Ho LY, Lee C, To CH, and Mok CC. Validation of the 2019 EULAR/ACR classification criteria for systemic lupus erythematosus in ANA-positive Chinese patients. Ther Adv Musculoskelet Dis. (2022) 14:1759720X221100300. doi: 10.1177/1759720X221100300
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
7. Babgi E, Al Marri M, Al-Mayouf SM, Shehata R, Majeed M, Alsufyani K, et al. Comparison of systemic lupus international collaborating clinics 2012 classification criteria and European league against rheumatism/American college of rheumatology 2019 classification criteria for early detection of childhood onset systemic lupus erythematosus (multi-center study). Lupus. (2024) 33:629–37. doi: 10.1177/09612033241240830
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
8. Ohara A, Iwata N, Sugiura S, Abe N, Nakaseko H, and Kawabe S. Evaluation of the European League Against Rheumatism/American College of Rheumatology-2019 classification criteria in patients with childhood-onset systemic lupus erythematosus: a single-center retrospective study. Clin Rheumatol. (2022) 41:2483–9. doi: 10.1007/s10067-022-06138-7
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
9. Abdwani R, Al Masroori E, Abdullah E, Al Abrawi S, and Al-Zakwani I. Evaluating the performance of ACR, SLICC and EULAR/ACR classification criteria in childhood onset systemic lupus erythematosus. Pediatr Rheumatol Online J. (2021) 19:141. doi: 10.1186/s12969-021-00619-w
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
10. Levinsky Y, Broide M, Kagan S, Goldberg O, Scheuerman O, Tal R, et al. Performance of 2019 EULAR/ACR classification criteria for systemic lupus erythematosus in a paediatric population-a multicentre study. Rheumatol (Oxford). (2021) 60:5142–8. doi: 10.1093/rheumatology/keab140
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
11. Smith EMD, Lythgoe H, Midgley A, Beresford MW, and Hedrich CM. Juvenile-onset systemic lupus erythematosus: Update on clinical presentation, pathophysiology and treatment options. Clin Immunol. (2019) 209:108274. doi: 10.1016/j.clim.2019.108274
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
12. Avar-Aydın PÖ and Brunner HI. Revisiting childhood-onset systemic lupus erythematosus. Turk Arch Pediatr. (2024) 59:336–44. doi: 10.5152/TurkArchPediatr.2024.24097
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
13. Artim-Esen B, Şahin S, Çene E, Sahinkaya Y, Barut K, Adrovic A, et al. Comparison of disease characteristics, organ damage, and survival in patients with juvenile-onset and adult-onset systemic lupus erythematosus in a combined cohort from 2 tertiary centers in Turkey. J Rheumatol. (2017) 44:619–25. doi: 10.3899/jrheum.160340
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
14. Kim H, Levy DM, Silverman ED, Hitchon C, Bernatsky S, Pineau C, et al. A comparison between childhood and adult onset systemic lupus erythematosus adjusted for ethnicity from the 1000 Canadian Faces of Lupus Cohort. Rheumatol (Oxford). (2019) 25:kez006. doi: 10.1093/rheumatology/kez006
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
15. Kachaner A, Mageau A, Timsit JF, Rio J, Papo T, and Sacré K. SLE patients with childhood-onset: A nation-wide population-based study. Autoimmun Rev. (2025) 24:103802. doi: 10.1016/j.autrev.2025.103802
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
16. Ma M, Hui-Yuen JS, Cerise JE, Iqbal S, and Eberhard BA. Validation of the 2019 european league against rheumatism/american college of rheumatology criteria compared to the 1997 american college of rheumatology criteria and the 2012 systemic lupus international collaborating clinics criteria in pediatric systemic lupus erythematosus. Arthritis Care Res (Hoboken). (2020) 72:1597–601. doi: 10.1002/acr.24057
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
17. Aslan E, Sahin S, Bektas S, Akay N, Gul U, Kilic Konte E, et al. The performance of the 2019 EULAR/ACR classification criteria in childhood-onset systemic lupus erythematosus. Lupus. (2025) 34:511–8. doi: 10.1177/09612033251325321
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
18. Batu ED, Akca UK, Kısaarslan AP, Sag E, Demir F, Demir S, et al. The performances of the ACR 1997, SLICC 2012, and EULAR/ACR 2019 classification criteria in pediatric systemic lupus erythematosus. J Rheumatol. (2021) 48:907–14. doi: 10.3899/jrheum.200871
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
19. Smith EMD, Rasul S, Ciurtin C, Al-Abadi E, Armon K, Bailey K, et al. Limited sensitivity and specificity of the ACR/EULAR-2019 classification criteria for SLE in JSLE. Rheumatol (Oxford). (2021) 60:5271–81. doi: 10.1093/rheumatology/keab210
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
20. Rodrigues Fonseca A, Felix Rodrigues MC, Sztajnbok FR, Gerardin Poirot Land M, and Knupp Feitosa de Oliveira S. Comparison among ACR1997, SLICC and the new EULAR/ACR classification criteria in childhood-onset systemic lupus erythematosus. Adv Rheumatol. (2019) 59:20. doi: 10.1186/s42358-019-0062-z
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
21. Sukharomana M, Vonginyoo S, Piyaphanee N, and Charuvanij S. Musculoskeletal manifestations in childhood-onset systemic lupus erythematosus: an in-depth exploration. Ital J Pediatr. (2024) 50:149. doi: 10.1186/s13052-024-01725-7
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
22. Mahmoud K, Zayat A, and Vital EM. Musculoskeletal manifestations of systemic lupus erythmatosus. Curr Opin Rheumatol. (2017) 29:486–92. doi: 10.1097/BOR.0000000000000421
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
23. Ceccarelli F, Govoni M, Piga M, Cassone G, Cantatore FP, Olivieri G, et al. Arthritis in systemic lupus erythematosus: from 2022 international GISEA/OEG symposium. J Clin Med. (2022) 11:6016. doi: 10.3390/jcm11206016
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
24. Leung AK, Wong AH, and Barg SS. Proteinuria in children: evaluation and differential diagnosis. Am Fam Physician. (2017) 95:248–54.
25. Huang Y, Yang X, Zhang Y, Yue S, Mei X, Bi L, et al. Correlation of urine protein/creatinine ratios to 24-h urinary protein for quantitating proteinuria in children. Pediatr Nephrol. (2020) 35:463–8. doi: 10.1007/s00467-019-04405-5
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
26. Ambarsari CG, Utami DAP, Tandri CC, and Satari HI. Comparison of three spot proteinuria measurements for pediatric nephrotic syndrome: based on the International pediatric Nephrology Association 2022 Guidelines. Ren Fail. (2023) 45:2253324. doi: 10.1080/0886022X.2023.2253324
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
27. Groot N, de Graeff N, Marks SD, Brogan P, Avcin T, Bader-Meunier B, et al. European evidence-based recommendations for the diagnosis and treatment of childhood-onset lupus nephritis: the SHARE initiative. Ann Rheum Dis. (2017) 76:1965–73. doi: 10.1136/annrheumdis-2017-211898
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
28. Legge AC and Hanly JG. Recent advances in the diagnosis and management of neuropsychiatric lupus. Nat Rev Rheumatol. (2024) 20:712–28. doi: 10.1038/s41584-024-01163-z
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Keywords: childhood-onset systemic lupus erythematosus, classification criteria, SLICC-2012, EULAR/ACR-2019, criteria validation, criteria optimization
Citation: Zhang Y, Shen T, Gao S, Yan J, Shi J, Tang X, Wang M, Yang J, Yu H, Mao H, Shuai L, Li Y, Cao Y, Li X, Wang Y, Liu Q, Song H and Wu X (2025) Validation and optimization of classification criteria for childhood-onset systemic lupus erythematosus in a multi-center Chinese cohort. Front. Immunol. 16:1611349. doi: 10.3389/fimmu.2025.1611349
Received: 14 April 2025; Accepted: 30 June 2025;
Published: 17 July 2025.
Edited by:
Ozgur Kasapcopur, Istanbul University-Cerrahpasa, TürkiyeReviewed by:
Sezgin Sahin, Istanbul University-Cerrahpasa, TürkiyeKübra Öztürk, Istanbul Medeniyet University Göztepe Prof Dr Süleyman Yalçın City Hospital, Türkiye
Copyright © 2025 Zhang, Shen, Gao, Yan, Shi, Tang, Wang, Yang, Yu, Mao, Shuai, Li, Cao, Li, Wang, Liu, Song and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongmei Song, c29uZ2htMTAyMUAxMjYuY29t; Xiaochuan Wu, eGlhb2NodWFud3VAY3N1LmVkdS5jbg==
†These authors have contributed equally to this work
 Yudi Zhang1†
Yudi Zhang1† Tian Shen
Tian Shen Junxia Yan
Junxia Yan Xuemei Tang
Xuemei Tang Mo Wang
Mo Wang Jun Yang
Jun Yang Huawei Mao
Huawei Mao Lanjun Shuai
Lanjun Shuai Yongzhen Li
Yongzhen Li Ying Wang
Ying Wang Hongmei Song
Hongmei Song Xiaochuan Wu
Xiaochuan Wu