- 1Department of Hematology and Oncology, Shenzhen University General Hospital, International Cancer Center, Hematology Institution, Haoshi Cell Therapy Institute of Shenzhen University, Shenzhen University Medical School, Shenzhen University, Shenzhen, China
- 2Guangdong Key Laboratory for Biomedical Measurements and Ultrasound Imaging, National-Regional Key Technology Engineering Laboratory for Medical Ultrasound, School of Biomedical Engineering, Shenzhen University Medical School, Shenzhen, China
- 3R&D Department, Shenzhen Haoshi Biotechnology Co., Ltd, Shenzhen, China
Background: T (8; 21) acute myeloid leukemia (AML) is a special type of acute leukemia, and exhibits a heterogeneous prognosis, with a long-term relapse rate of about 40%. Once t(8; 21) AML patients experience relapse, they have an extremely poor prognosis, with a 5-year overall survival rate of less than 15%. Therefore, it is crucial to develop effective strategies to improve the prognosis of relapsed/refractory (R/R) t(8; 21) AML. CD19 is a specific B-cell surface marker, but it is aberrantly expressed in 50-80 % of t(8; 21) AML patients. CAR-T cells targeting aberrant cell-surface antigens could induce the depletion of tumor cells without the destruction of hematopoiesis. Therefore, CD19 might be a promising target for CAR-T cell therapy in R/R t(8; 21) AML with aberrant CD19 expression. The present study is aimed to explore the efficacy and safety of CD19 CAR-T cell therapy in R/R t(8;21) AML with aberrant CD19 expression.
Methods: In the present study, 3 R/R t(8;21) AML patients with aberrant CD19 expression were enrolled. After lymphodepleting chemotherapy, 3 patients received autologous CAR-T cell infusion at a dose of 1.0 × 10^6 cells/kg, 2.0 × 10^6 cells/kg, and 2.0 × 10^6 cells/kg, respectively.
Results: They all achieved CD19 negativity approximately half a month after CD19 CAR-T cell infusion. These indicate CD19 CAR-T cell therapy is effective in R/R t(8;21) AML with aberrant CD19 expression. However, patient 1 and patient 2 rapidly relapsed within 3 months after CD19 CAR-T cell therapy. Subsequently, patient 1 received allogeneic hematopoietic stem cell transplantation (allo-HSCT). Fortunately, patient 1 achieved mCR 2 months after allo-HSCT.
Conclusion: Considering the short-term remission of CD19 CAR-T cell therapy in R/R t(8;21) AML, allo-HSCT might be performed as soon as possible to consolidate the efficacy of CAR-T cell therapy and reduce the risk of relapse.
1 Introduction
Acute myeloid leukemia (AML) is the most common form of acute leukemia in adults. It has a poor prognosis, with a dismal 5-year survival rate of only 24%. T (8; 21) AML is a special type of acute leukemia with RUNX1::RUNX1T1 fusion, which presents with favorable karyotype and accounts for 5% ~ 15% of AML (1). However, the prognosis of t (8; 21) AML is heterogeneous, and approximately 40% of t (8; 21) AML patients experienced relapse (2). Once t (8; 21) AML patients experience relapse, the prognosis of them is extremely poor, and the survival rate is usually less than 15%. It is well known that allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only potentially curative treatment option for relapsed/refractory (R/R) AML. However, approximately 40% of AML patients still experience relapse after allo-HSCT (3). Considering that the effective therapeutic options for R/R AML patients are currently limited, it is crucial to explore novel and effective therapeutic strategies to improve the prognosis of R/R AML.
Chimeric antigen receptor (CAR)-T cell therapy is a major breakthrough in cancer treatment, and it has achieved unprecedented responses in R/R B-cell malignancies in recent years, such as B-cell acute lymphoblastic leukemia (B-ALL), B-cell non-Hodgkin lymphoma (B-NHL), and multiple myeloma (MM) (4–6). However, due to antigen heterogeneity and the lack of specific target antigens, CAR-T cell therapy for R/R AML remains a major challenge. In particular, due to the overexpression of target antigens on hematopoietic stem cells (HSCs), such as CD123 and CD33, CAR-T cells targeting these co-expressed antigens on leukemic blast cells and HSCs may not only deplete malignant cells but also induce the clearance of bone marrow cells, resulting in severe myelosuppression (7, 8). This off-tumor on-target toxicity may be fatal and further limit the application of CAR-T cell therapy in R/R AML. In addition, CAR-T cell therapy targeting these co-expressed surface antigens are mostly at the preclinical stage.
At present, a number of studies have demonstrated that approximately 50-80% of t(8;21) AML patients present with aberrant expression of B-cell-specific surface marker CD19 on leukemic blast cells (9–13). Targeting aberrant CD19 expression could result in the depletion of these CD19-positive tumor cells without the destruction of hematopoiesis (14). Therefore, CD19 may also serve as a potential immuno-therapeutic target for AML with aberrant CD19 expression, such as t(8;21) AML and mixed-phenotype acute leukemia (14, 15). It is well-known that CD19 CAR-T cell therapy has achieved satisfactory efficacy in B-cell malignancies, including B-ALL and B-NHL. However, there is very little data on the application of CD19 CAR-T cell therapy in CD19-positive R/R AML (10). Considering the frequent expression of CD19 in t(8;21) AML patients, the present study is aimed to investigate the efficacy and safety of CD19 CAR-T cell therapy in R/R t(8;21) AML with aberrant CD19 expression.
2 Methods
2.1 Study approval and clinical protocols
Three R/R t(8;21) AML patients with CD19 aberrant expression were enrolled in the clinical trial of CD19 CAR-T cell therapy, which was approved by the Institutional Review Board at Shenzhen University General Hospital. Written informed consent was obtained from all the participants. Before CAR-T cell infusion, all patients received lymphodepleting chemotherapy with FC regimen (fludarabine 50 mg/m2 day -5 to day -3, cyclophosphamide 400 mg/m2 day -5 to day -3). Day 0 referred to the day on which CAR-T cells were infused.
2.2 CAR-T cell manufacturing
Peripheral blood mononuclear cells (PBMCs) were collected by apheresis and further isolated and purified by Ficoll density gradient centrifugation. Then T cells were isolated and activated by CD3/28 Dynabeads. After activation for 2 days, the lentiviral vector encoding CD19 CAR was applied for the T cells transduction. The CAR structure consists of a humanized anti- CD19 single-chain variable fragments (scfv), a CD8α hinge region, a 4-1BB co-stimulatory domain, and a CD3ζ signaling domain, as well as a truncated human epidermal growth factor receptor (tEGFR). The CAR-T cells were further expanded in X-vivo medium containing 10 ng/mL IL-7, 5 ng/mL IL-15, and 30 ng/mL IL-21 till the cell quantity reached the dose requirement.
2.3 Flow cytometry
To detect CD19-positive leukemic blasts, 8 mL bone marrow samples were collected in EDTA tubes at different time points. After red blood cell lysis, the remaining cells from each tube were stained with anti-human antibodies, including CD45-KO (Beckman Coulter, B36294), CD34-APC (BD biosciences, 652837), cMPO-FITC (BD biosciences,340580), CD11B-FITC (Beckman Coulter, IM0530), HLA-DR APC (BD Bioscience, 662909), CD64-PE (BD biosciences, 652830), CD14-APC (BD biosciences, 6657505), CD117-PE (BD Bioscience, 664936), CD33-PE-Cy5.5 (Beckman Coulter, B36289), CD13-PE-Cy7 (BD Bioscience, 662910), CD38-BV421(BD biosciences, 562444), CD19-PE-Cy7 (Beckman Coulter, IM3628), CD16-ECD (Beckman Coulter, B49216), and CD56-ECD (Beckman Coulter, B49214) for flow cytometric analysis. To detected the expansion of CAR-T cells which incorporate a truncated EGFR element, the following antibodies were used: EGFR-APC (Biolegend, 52906) and CD3-APC/Cyanine7 (Biolegend, 300426).
2.4 ELISA
Serum levels of IL-6, IL-8, and IL-10 were detected by commercial ELISA kits (R&D Systems, Minneapolis, USA) and the protocols were adopted according to manufacturer’s instructions.
3 Results
3.1 Patient characteristics
Three R/R t(8;21) AML patients were enrolled, and patient 3 had c-KIT mutation. The characteristics of the enrolled patients are summarized in Table 1. All the patients had been heavily pretreated, and the detailed information is available below.
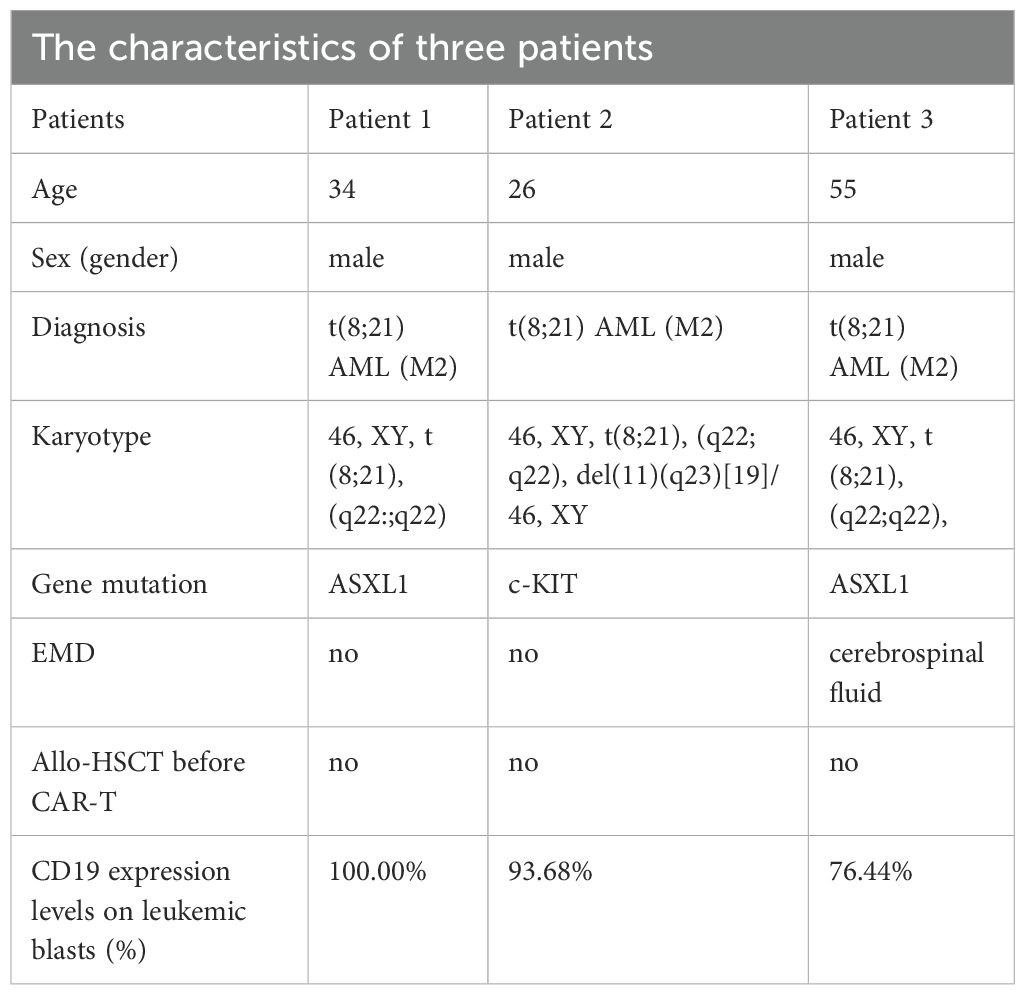
Patient 1 is a 34-year-old man diagnosed with t(8;21) AML by cytogenetic and fluorescence-in-situ hybridization (FISH) studies in February 2023. Bone marrow smear and flow cytometry (FCM) showed more than 80.0% of leukemic blasts. His karyotype was 46, XY, t(8;21) (q22; q22). RUNX1::RUNX1T1 rearrangement and ASXL1 mutation were detected. The patient received standard “3 + 7” regimen with idarubicin and cytarabine (IA) as induction therapy, and then received three cycles of high dose cytarabine as consolidation therapy. After induction and consolidation therapy, he achieved molecular complete remission (mCR) with RUNX1::RUNX1T1 gene copies and ASXL1 mutation undetectable. Due to pulmonary infections, the patient didn’t receive allo-HSCT. In February 2024, FCM showed 55.33% blasts in peripheral blood, which indicated AML relapse. Then, the patient received two cycles of CDHAA regimen (Chidamide 30 mg/d d1 and d3; Decitabine 20 mg/d d1-5; Harringtonine 2mg/d d3-7; Cytarabine 100mg/d d3-7; Aclarubicin 20mg/d d3-7), and the patient achieved hematological complete remission (hCR) with 0.56% of RUNX1::RUNX1T1 fusion gene detectable. Due to minimal residual disease (MRD) positivity after multi-line of chemotherapy and the high levels of CD19 expression on leukemic blast cells (Figure 1A), the patient was enrolled in the clinical trial of CD19 CAR-T cell therapy to prevent hematological relapse. After lymphodepleting chemotherapy, he was treated with a single infusion of autologous CD19 CAR-T cells at a dose of 1.0 × 10^6 cells/kg on August 5, 2024.
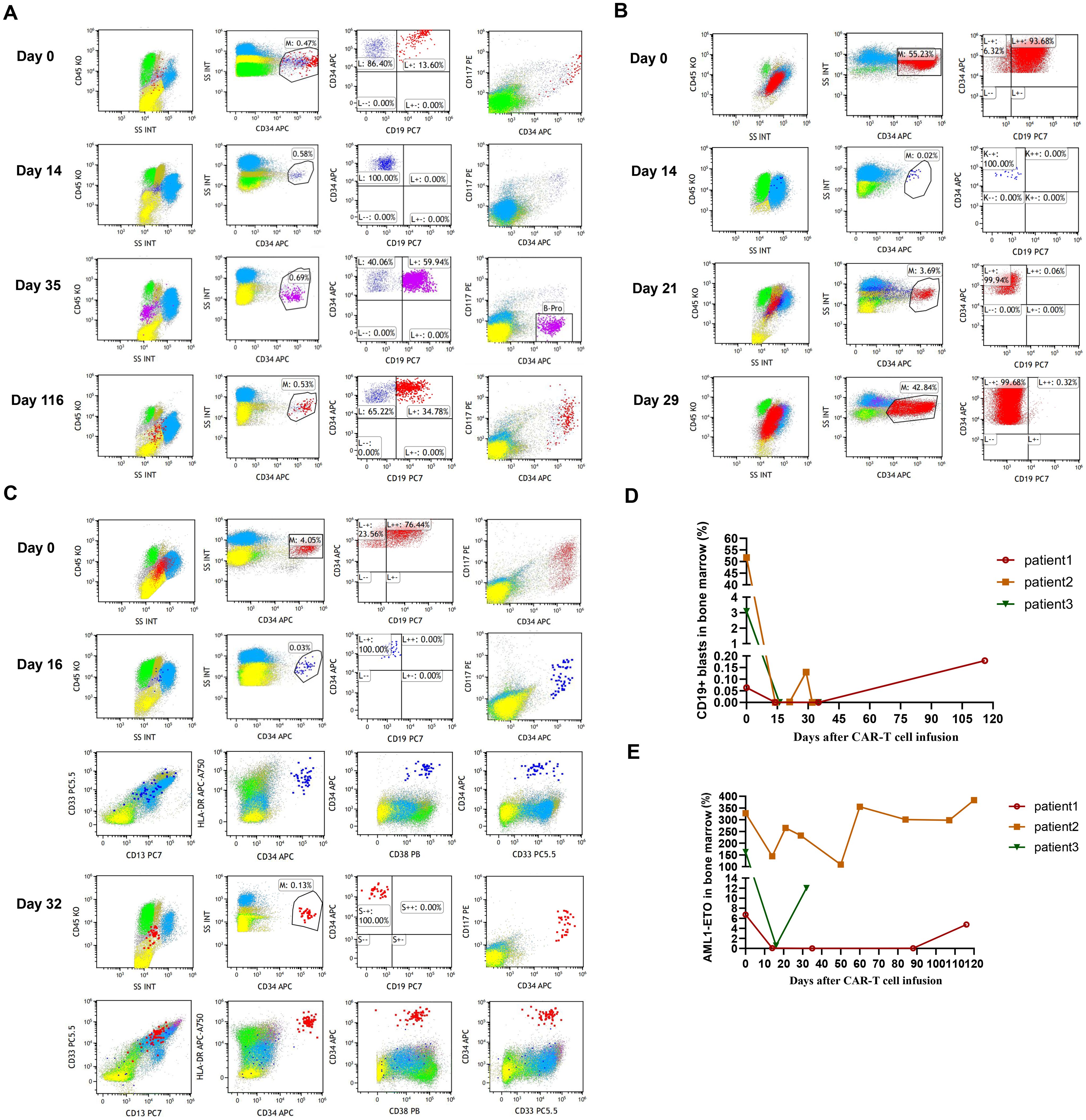
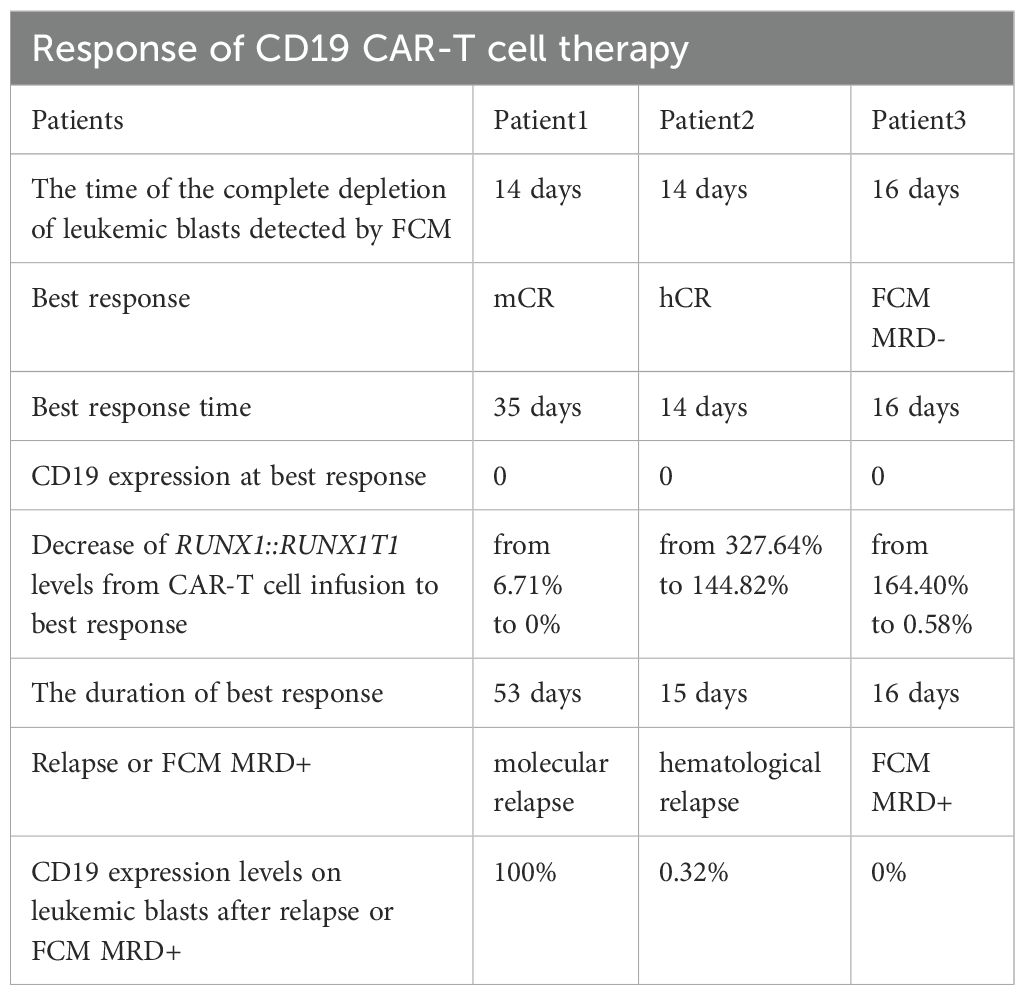
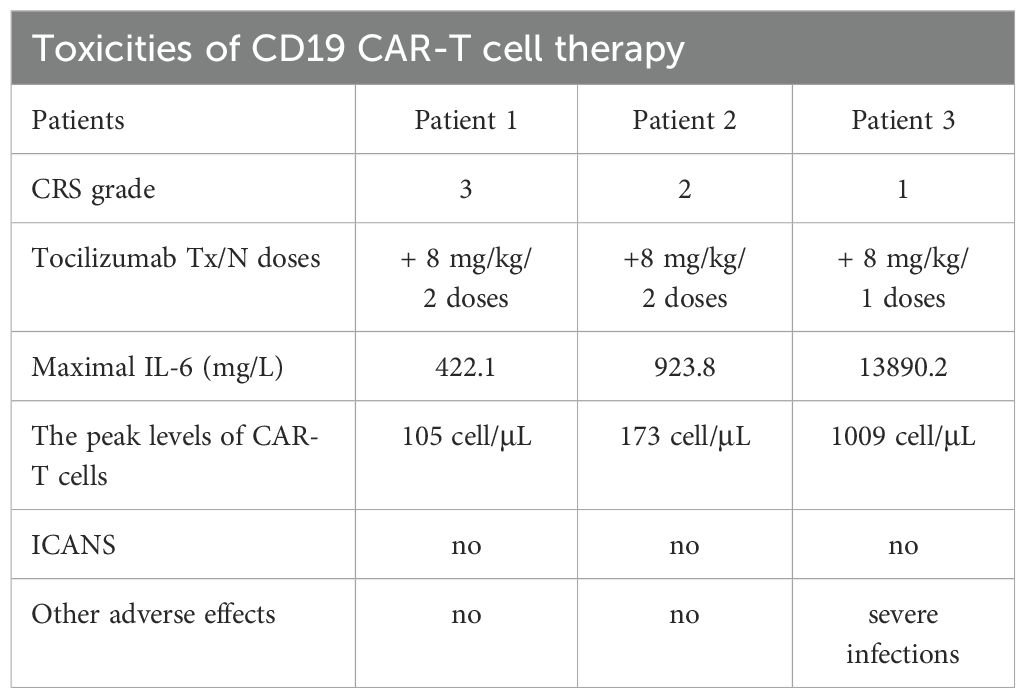
Figure 1. (A) The percentage of leukemic blasts in bone marrow after CAR-T cell infusion in patient 1. Red dots represent leukemic blasts and dark blue dots represent normal primitive hematopoietic cells, which are also applicable to patient 2 and patient 3. Purple dots represent B-progenitor cells. (B) The percentage of leukemic blasts in bone marrow after CAR-T cell infusion in patient 2. (C) The percentage of leukemic blasts in bone marrow after CAR-T cell infusion in patient 3. On day 16, no leukemic blasts were detected, as shown by the normal development and differentiation of primitive hematopoietic cells. On day 32, 0.13% of leukemic blasts were detected by FCM in bone marrow, which abnormally expressed several markers, such as CD38 dim, bright CD33, bright CD13, and bright HLA-DR. (D) The changes of CD19+ leukemic blasts in bone marrow after CAR-T cell infusion in 3 patients. (E) The transcript levels of RUNX1::RUNX1T1 in bone marrow after CAR-T cell infusion in 3 patients.
Patient 2 is a 26-year-old man diagnosed with DLBCL in January 2022, and underwent 5 cycles of R-CHOP regimen as well as radiotherapy. Unfortunately, the patient failed to achieve remission and diagnosed with therapy-related t(8;21) AML with c-KIT mutation by cytogenetic and FISH studies in August 2024. His karyotype was 46, XY, t(8;21) (q22;q22), del(11) (q23). For the treatment of t(8;21) AML, the patient received IA regimen and CDHAA regimen. However, the patient failed to achieve hCR and experienced disease progression. Due to 55.23% of leukemic blasts and high levels of aberrant CD19 expression in bone marrow (Figure 1B), the patient was enrolled in the clinical trial of CD19 CAR-T cell therapy to deplete leukemic blasts. After lymphodepleting chemotherapy, he was infused with a single infusion of autologous CD19 CAR-T cells at a dose of 2.0 × 10^6 cells/kg on October 21, 2024.
Patient 3 is a 55-year-old man diagnosed with t(8;21) AML confirmed by cytogenetic and FISH studies in August 2017, and then received standard “3 + 7” IA regimen. After 2 cycles of IA regimen and 1 cycle of intermediate-dose cytarabine for consolidation therapy, the patient achieved mCR on December 2017. Due to the lack of suitable donors, the patient didn’t receive allo-HSCT. Unfortunately, 3.38% of RUNX1::RUNX1T1 fusion gene was detected in December 2020, which suggested molecular relapse. Then, the patient underwent multi-line of chemotherapy, including cytarabine combined with etoposide, 4 cycles of DCAG (DAC 15 mg d1-5, Acla 20 mg d4-7, Ara-C 20 mg d1-7, G-CSF 300 μg d1-7), and 2 cycles of CDHAA regimen. After a variety of chemotherapy regimens, the patient still didn’t achieve molecular remission. Unfortunately, leukemic blasts were detectable in the cerebrospinal fluid, which indicated central nervous system leukemia (CNS-L). After intrathecal injection of methotrexate, cytarabine, and dexamethasone, CNS-L was controlled. Given MRD positivity and 76.44% of CD19 expression on leukemic blasts (Figure 1C), the patient was enrolled in the clinical trial of CD19 CAR-T cell therapy to prevent hematological and CNS relapse. After lymphodepleting chemotherapy, he was treated with a single infusion of autologous CD19 CAR-T cells at a dose of 2.0 × 10^6 cells/kg on October 21, 2024.
3.2 The kinetics of CD19 CAR-T cell expansion in R/R t(8;21) AML patients and objective responses
The efficacy of CD19 CAR-T cell therapy in R/R t(8;21) AML with CD19 aberrant expression was evaluated by bone marrow aspiration within half a month to 3 months.
After lymphodepleting chemotherapy, patient 1 received autologous CD19 CAR-T cell infusion at a dose of 1.0 × 10^6 cells/kg on August 5, 2024. CAR-T cell expansion reached peak levels of 105 cells/μL in peripheral blood on day 9 as detected by FCM (Figure 2B). Surprisingly, leukemic blasts were completely depleted 14 days after CD19 CAR-T cell infusion (Figures 1A, D), and the levels of RUNX1::RUNX1T1 fusion gene were decreased from 6.71% to 0.06% (Figure 1E). The patient achieved mCR with RUNX1::RUNX1T1 fusion gene undetectable 35 days after CD19 CAR-T infusion (Figure 1E). Meanwhile, CD19 CAR-T cells were virtually undetectable 35 days after CD19 CAR-T infusion and B-progenitor cells were detected in bone marrow (Figures 1A, 2B), which indicated CAR-T cell exhaustion. Unfortunately, 88 days after CD19 CAR-T cell therapy, 0.05% of RUNX1::RUNX1T1 fusion gene was detected in bone marrow, which suggested molecular relapse in patient 1 (Figure 1E). Subsequently, CD19-positive leukemic blasts reemerged 116 days after CAR-T cell infusion (Figures 1A, D). Considering MRD positivity, the patient underwent matched sibling donor allo-HSCT on December 16, 2024. The total dose of CD34+ cells was 6.07 × 10^6 cells/kg. The patient achieved neutrophil and platelet engraftment 13 and 14 days after the infusion of allogeneic HSCs, respectively. On January 10, 2025, bone marrow chimerism testing by STR-PCR showed 99.85% donor chimerism. On January 23, 2025, 2.92% of leukemic blasts were detected in bone marrow by FCM, and the transcript levels of RUNX1::RUNX1T1 fusion increased to 32.92%. Considering disease progression, cyclosporin A was discontinued. Subsequently, the patient developed grade III skin acute graft-versus-host disease (aGVHD) and grade II gut aGVHD, and presented with generalized rash and gastrointestinal symptoms, including abdominal pain, diarrhea, nausea, and vomiting. Fortunately, aGVHD was controlled by the combination treatment of cyclosporin A, mycophenolate mofetil, ruxolitinib, and methylprednisolone. On February 12, 2025, leukemic blasts and RUNX1::RUNX1T1 fusion gene were not detected in bone marrow, and bone marrow chimerism testing by STR-PCR showed 100% donor chimerism. We speculate that leukemic blasts were depleted by graft versus leukemia effects. So far, patient 1 remained in mCR.
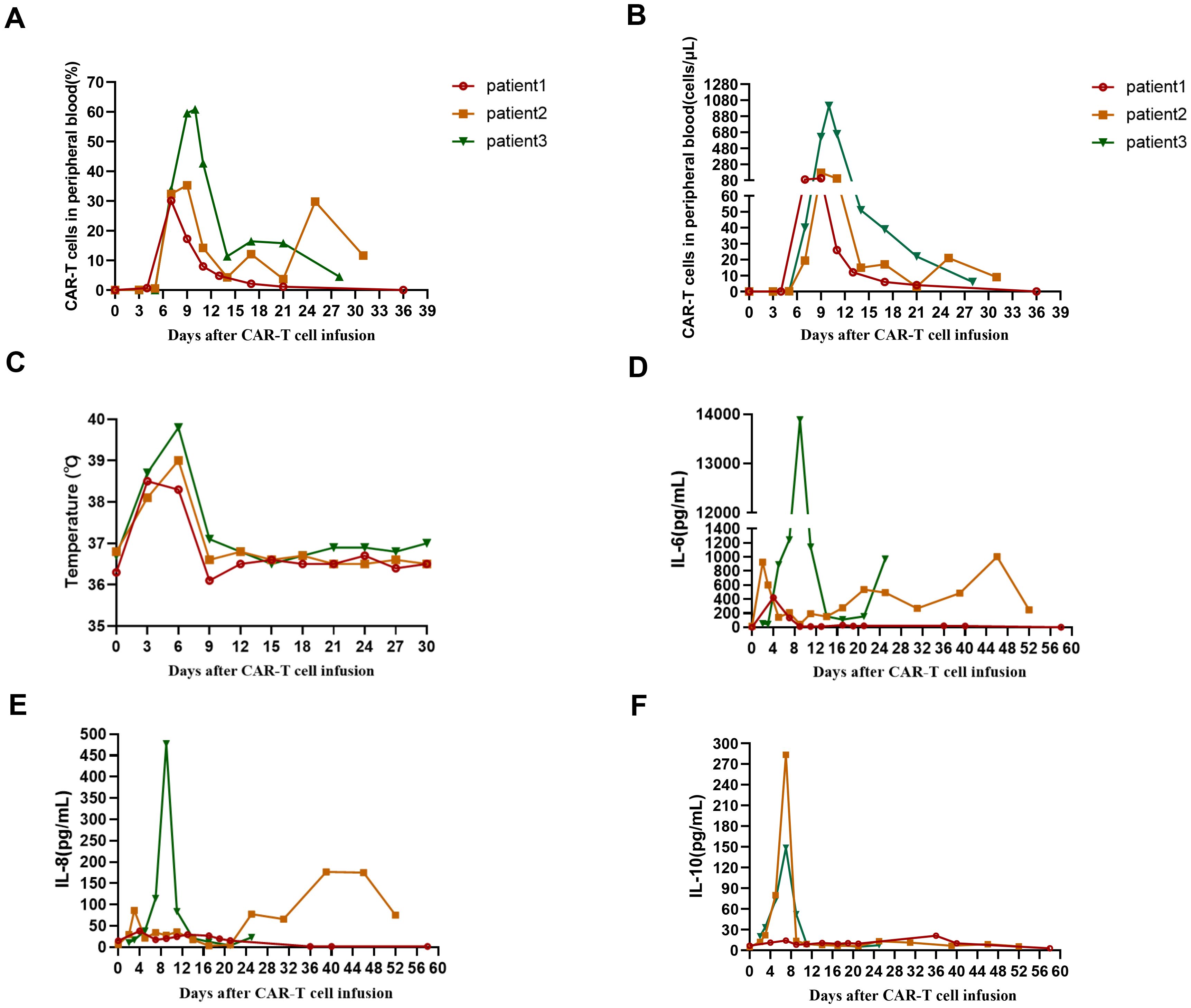
Figure 2. (A) The percentage of CD19 CAR-T cells in peripheral blood from 3 patients after CAR-T cell infusion. (B) The absolute number of CAR-T cells in peripheral blood from 3 patients after CAR-T cell infusion. (C) The changes of temperature in 3 patients after CAR-T cell infusion. (D) The levels of IL-6 in 3 patients after CAR-T cell infusion. (E) The levels of IL-8 in 3 patients after CAR-T cell infusion. (F) The levels of IL-10 in 3 patients after CAR-T cell infusion.
After lymphodepleting chemotherapy, patient 2 received autologous CD19 CAR-T cell infusion at a dose of 2.0 × 10^6 cells/kg on October 21, 2024. CAR-T cell expansion reached peak levels of 173 cells/μL in peripheral blood on day 9 as detected by FCM (Figure 2B). Surprisingly, leukemic blasts were completely depleted 14 days after CD19 CAR-T cell infusion, including CD19-positive tumor cells (Figures 1B, D), and the levels of RUNX1::RUNX1T1 fusion gene decreased from 327.64% to 144.82% (Table 2, Figure 1E). These suggested that patient 2 achieved hCR14 days after CD19 CAR-T cell therapy. Unfortunately, 3.69% of leukemic blasts were detected in bone marrow by FCM 21 days after CD19 CAR-T cell therapy (Figure 1B), and the levels of leukemic blasts remarkably increased to 42.84% on 29 day (Figure 1B), which indicated the patient rapidly experienced hematological relapse.
After lymphodepleting chemotherapy, patient 3 was also treated with a single infusion of autologous CD19 CAR-T cells at a dose of 2.0 × 10^6 cells/kg on October 21, 2024. CAR-T cell expansion reached peak levels of 1009 cells/μL in peripheral blood on day 10 (Figure 2B). Sixteen days after CD19 CAR-T cell infusion, flow cytometric analysis showed that CD19-positive leukemic blasts were completely depleted (Figures 1C, D), and the copy numbers of RUNX1::RUNX1T1 fusion gene decreased from 187233 copies/μg to 1908 copies/μg, and the relative values of RUNX1::RUNX1T1 fusion gene also decreased from 164.40% to 0.58% (Table 2, Figure 1E). However, 32 days after CD19 CAR-T cell therapy, 0.13% of leukemic blasts were detected in bone marrow by FCM and showed CD19-negative expression (Figure 1C), and the copy number of RUNX1::RUNX1T1 fusion gene in bone marrow was 19024 copies/μg, and the relative values of RUNX1::RUNX1T1 fusion gene was 12.03% (Figure 1E). Ultimately, he died of septic shock 49 days after CD19 CAR-T cell infusion.
3.3 Adverse effects of CD19 CAR-T cell therapy in 3 R/R t(8;21) AML patients
Cytokine release syndrome (CRS) occurred in all 3 patients. Patient 1 experienced grade 3 CRS (Table 3), mainly manifested as fever and hypotension, which was controlled by intravenous rehydration, tocilizumab, norepinephrine, and dopamine. Patient 2 and patient 3 experienced grade 2 and grade 1 CRS, respectively (Table 3). Especially, patient 3 presented with persistent high fever for several days, and the peak levels of IL-6 reached 13890.20 pg/mL on day 9 (Figure 2D, Table 3). To alleviate the clinical symptoms of CRS, patient 2 and patient 3 were also treated with tocilizumab. Due to agranulocytosis mediated by long-term chemotherapy as well as CAR-T cell therapy, all 3 patients received prophylactic anti-infective treatment, including acyclovir, voriconazole, and imipenem, and patient 3 received granulocyte colony-stimulating factor. Unfortunately, patient 3 still suffered from multiple severe infections within 49 days after CD19 CAR-T cell infusion, mainly manifested as bacterial infections, including Klebsiella pneumoniae and Pseudomonas aeruginosa. Eventually, patient 3 died of septic shock 49 days after CD19 CAR-T cell infusion, despite aggressive anti-infective treatment, such as a combination of meropenem and tigecycline. Immune effector cell-associated neurotoxicity syndrome (ICANS) was not observed in all 3 patients. Furthermore, due to thrombocytopenia and severe anemia, patient 2 and patient 3 received multiple platelet and leukocyte-depleted red blood cell transfusions within 1 month after CAR-T cell infusion.
4 Discussion
R/R AML has a poor prognosis with a 5-year survival rate of about 24%. Up to now, the therapeutic options for R/R AML are limited. Allo-HSCT is the only curative treatment option, but approximately 40% of AML patients still relapse after allo-HSCT. Therefore, it is crucial to explore effective therapeutic strategies to improve the prognosis of R/R AML. In recent years, CAR-T cell therapy has revolutionized the outcomes of R/R B-cell malignancies and shown great promise in the treatment of R/R AML. However, there are various difficulties for CAR-T therapy in R/R AML, including the lack of specific target antigens and off-target toxicities. In particular, due to the co-expression of pan-myeloid markers on leukemic blast cells and hematopoietic cells, such as CD33, CD123, CLL-1, CD70, and CD44v6, CAR-T cells targeting these antigens may result in a higher risk of the destruction of hematopoiesis (7, 16, 17). The prolonged myeloablation after CAR-T cell therapy in AML is fatal, which is mainly manifested as neutropenic infections and bleeding (16). Thereby, identification of novel antigens which are expressed on leukemic blasts but not normal hematopoietic cells may help to improve the safety of CAR-T cell therapy in R/R AML.
Aberrant antigen expression is a hallmark of AML. Aberrantly expressed antigens are absent from normal hematopoietic stem and progenitor cells, so targeting these surface antigens may result in the depletion of tumor cells without myelotoxicity. It has been demonstrated that more than 50% of t(8;21) AML patients present with aberrant CD19 expression on leukemic blasts (13), which indicates that CD19 is a promising target for CAR-T cell therapy in CD19-positive t(8;21) AML patients. However, there is very little data on the application of CD19 CAR-T cell therapy in R/R t(8;21) AML. Therefore, the present study investigated the efficacy and safety of CD19 CAR-T cell therapy in R/R t(8;21) AML with aberrant CD19 expression. In the present study, flow cytometric analysis showed leukemic blasts were rapidly depleted in all three patients within 16 days after CAR-T cell infusion (Table 2, Figures 1A–C). Patient 1 achieved mCR with RUNX1::RUNX1T1 fusion gene undetectable 35 days after CD19 CAR-T cell therapy (Table 2, Figure 1E), and patient 2 achieved hCR 14 days after CD19 CAR-T cell infusion (Table 2, Figures 1B, E). Flow cytometric analysis showed leukemic blasts were completely depleted 16 days after CD19 CAR-T cell infusion in patient 3 (Table 2, Figure 1C), and the levels of RUNX1::RUNX1T1 fusion gene rapidly decreased from 164.40% to 0.58% (Table 2, Figure 1E). These demonstrated that CD19 CAR-T cell therapy was effective in R/R t(8;21) AML patients with aberrant CD19 expression, which might further expand the application of CD19 CAR-T cell therapy, beyond B-cell malignancies. However, patient 1 and patient 2 rapidly relapsed after CD19 CAR-T cell therapy in the present study, especially patient 2. Patient 1 experienced molecular relapse 88 days after CD19 CAR-T cell therapy, with MRD positivity and CD19-positive expression detected by FCM 116 days after CAR-T cell infusion. These may be partially attributed to CD19 CAR-T cell exhaustion (Figures 2A, B), which was consistent with the regeneration of B-progenitor cells detected by FCM 35 days after CAR-T cell infusion (Figure 1A). Patient 2 experienced hematological relapse 29 days after CAR-T cell infusion, and the vast majority of leukemic blasts were CD19-negative (Figure 1B). Similarly, 0.13% of leukemic blasts were detected in bone marrow by FCM 32 days after CAR-T cell infusion in patient 3, manifested by CD19 negativity, and the transcript levels of RUNX1::RUNX1T1 in bone marrow increased to 12.03%. Unfortunately, patient 3 died of septic shock 49 days after CD19 CAR-T cell infusion, which may be associated with the impaired immune function mediated by previous long-term chemotherapy and CD19 CAR-T cell therapy (18).
It is well known that there are several leading causes contributing to tumor recurrence after CAR-T cell therapy, including antigen escape and CAR-T cell exhaustion (19–21), which were also observed in our study. On the one hand, the potent selective pressure of CD19 CAR-T cell therapy may trigger the mutation or loss of the target antigen CD19, also known as CD19 antigen escape (19, 20, 22). In addition, CD19 CAR-T cells may not eradicate all tumor cells due to the pre-existing CD19-negative leukemic subclones. These CD19-negative leukemic subclones may also become predominant subsets under the potent selective pressure of CD19 CAR-T cell therapy (23), as seen in patient 2 and patient 3, which might eventually result in CD19-negative relapse. CD19-negative relapse after CD19 CAR-T cell therapy in R/R t(8;21) AML was also observed in the previous study (10). On the other hand, CD19 CAR-T cell exhaustion could result in CD19-positive relapse, as seen in patient 1. Regardless, CD19 CAR-T cells rapidly reduced tumor burden in R/R t(8;21) AML patients with aberrant CD19 expression in our study. Therefore, CD19 CAR-T cell therapy may be serve as a promising bridging therapy prior to allo-HSCT in CD19-positive R/R t(8;21) AML patients. Furthermore, considering that the duration of remission induced by CD19 CAR-T cell therapy is short in the present study, allo-HSCT should be performed as soon as possible to eradicate the residual tumor cells, including CD19-negative subclones, and then reduce the risk of relapse and achieve long-term tumor remission in R/R t(8;21) AML (10, 22).
In addition, patient 2 is a CD19-positive R/R t(8;21) AML patient with c-KIT D816V mutation, which is recognized to an independent adverse prognostic factor in t(8; 21) AML and could significantly increase the risk of tumor recurrence (24). Several studies have confirmed that CAR-T cell therapy is able to overcome the unfavorable prognosis in high-risk lymphoma and multiple myeloma, such as double hit or double expressor lymphomas, and multiple myeloma with extramedullary disease or high-risk cytogenetics (25–27). In the present study, it seems that CAR-T cell therapy is unlikely to overcome the poor prognosis of c-KIT mutation in R/R t(8;21) AML. At present, it has been demonstrated that avapritinib could specifically target c-KIT mutation and induce rapid and deep remission in t(8; 21) AML (28–30). Unfortunately, avapritinib was ineffective in patient 2. Subsequently, he will receive allo-HSCT as salvage therapy.
5 Conclusion
In conclusion, CD19 CAR-T cell therapy for R/R t(8;21) AML with CD19 aberrant expression is effective and safe, which broadens the application of CD19 CAR-T cell therapy in hematological malignancies, beyond B-cell malignancies. It provides a new strategy for the treatment of R/R t(8;21) AML. Although CD19 CAR- T cell therapy could reduce CD19-positive tumor burden in R/R t(8;21) AML patients with CD19 aberrant expression, tumor relapses occur rapidly due to CAR-T cell exhaustion or antigen escape. Therefore, allo-HSCT should be performed as soon as possible to consolidate the efficacy of CD19 CAR-T cell therapy and eradicate the residual tumor cells. CD19 CAR-T cell therapy combined with allo-HSCT may be an alternative strategy to overcome the heterogeneity of R/R t(8; 21) AML with CD19 aberrant expression. In addition, due to the limited number of patients in the present study, large-scale clinical trials are required to confirm the efficacy of CD19 CAR T- cell therapy as well as the combination of CD19 CAR T- cell therapy and allo-HSCT in CD19-positive R/R t(8;21) AML.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the institutional review boards of Shenzhen University General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XZ: Formal Analysis, Writing – original draft, Validation, Conceptualization, Funding acquisition, Methodology. LXW: Writing – original draft, Resources, Project administration, Methodology, Investigation. JQQ: Resources, Project administration, Methodology, Writing – original draft. SW: Supervision, Visualization, Investigation, Writing – original draft, Validation. LJW: Writing – original draft, Visualization, Investigation, Validation. LL: Methodology, Formal Analysis, Investigation, Writing – original draft, Visualization. JDQ: Resources, Formal Analysis, Data curation, Writing – original draft, Investigation. ZC: Visualization, Methodology, Investigation, Writing – original draft, Supervision. WH: Investigation, Project administration, Visualization, Writing – original draft, Methodology. YZ: Formal Analysis, Writing – original draft, Visualization, Data curation, Investigation. HP: Data curation, Investigation, Visualization, Project administration, Writing – original draft. JM: Validation, Resources, Methodology, Investigation, Writing – original draft. HW: Project administration, Investigation, Writing – original draft, Resources. CY: Visualization, Writing – original draft, Project administration, Investigation. YL: Validation, Writing – original draft, Formal Analysis, Resources, Investigation. LY: Methodology, Funding acquisition, Conceptualization, Writing – original draft, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (82030076, 82470229, 82405133), Shenzhen Clinical Research Center for hematologic disease (LCYSSQ20220823091401002), Shenzhen Science and Technology Program(JCYJ20241202124225031), Program for Youzuzhikeyan of Shenzhen University, Sanming Project of Medicine in Shenzhen (SZSM202111004), Shenzhen Key Laboratory Foundation (ZDSYS20200811143757022), and Medicine Plus Program of Shenzhen University(000003011601).
Acknowledgments
We thank all the staff for the clinical support and technical support from the Department of Hematology and Oncology, Shenzhen University General Hospital, and Shenzhen University-Haoshi Cell Therapy Institute.
Conflict of interest
Authors CY and YL were employed by the company Shenzhen Haoshi Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. (2010) 116:354–65. doi: 10.1182/blood-2009-11-254441
2. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. (2017) 129:424–47. doi: 10.1182/blood-2016-08-733196
3. Tsirigotis P, Byrne M, Schmid C, Baron F, Ciceri F, Esteve J, et al. Relapse of AML after hematopoietic stem cell transplantation: methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transplant. (2016) 51:1431–8. doi: 10.1038/bmt.2016.167
4. Bock TJ, Colonne CK, Fiorenza S, and Turtle CJ. Outcome correlates of approved CD19-targeted CAR T cells for large B cell lymphoma. Nat Rev Clin Oncol. (2025) 22:241–61. doi: 10.1038/s41571-025-00992-5
5. Zhao WH, Wang BY, Chen LJ, Fu WJ, Xu J, Liu J, et al. Four-year follow-up of LCAR-B38M in relapsed or refractory multiple myeloma: A phase 1, single-arm, open-label, multicenter study in China (LEGEND-2). J Hematol Oncol. (2022) 15:86. doi: 10.1186/s13045-022-01301-8
6. Jacobson CA, Chavez JC, Sehgal AR, William BM, Munoz J, Salles G, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. (2022) 23:91–103. doi: 10.1016/S1470-2045(21)00591-X
7. Baroni ML, Sanchez Martinez D, Gutierrez Aguera F, Roca Ho H, Castella M, Zanetti SR, et al. 41BB-based and CD28-based CD123-redirected T-cells ablate human normal hematopoiesis. In Vivo. J Immunother Cancer. (2020) 8:e000845. doi: 10.1136/jitc-2020-000845
8. O’Hear C, Heiber JF, Schubert I, Fey G, and Geiger TL. Anti-CD33 chimeric antigen receptor targeting of acute myeloid leukemia. Haematologica. (2015) 100:336–44. doi: 10.3324/haematol.2014.112748
9. Ball ED, Davis RB, Griffin JD, Mayer RJ, Davey FR, Arthur DC, et al. Prognostic value of lymphocyte surface markers in acute myeloid leukemia. Blood. (1991) 77:2242–50. doi: 10.1182/blood.V77.10.2242.2242
10. Liu S, Yin Z, Yu X, Zhao Y, Pan J, and Song Y. CD19-specific CAR-T cell therapy for relapsed/refractory non-B-cell acute leukaemia with CD19 antigen expression. Eur J Cancer. (2021) 153:1–4. doi: 10.1016/j.ejca.2021.04.042
11. Walter K, Cockerill PN, Barlow R, Clarke D, Hoogenkamp M, Follows GA, et al. Aberrant expression of CD19 in AML with t(8;21) involves a poised chromatin structure and PAX5. Oncogene. (2010) 29:2927–37. doi: 10.1038/onc.2010.56
12. Shang L, Chen X, Liu Y, Cai X, Shi Y, Shi L, et al. The immunophenotypic characteristics and flow cytometric scoring system of acute myeloid leukemia with t(8;21) (q22;q22);. Int J Lab Hematol. (2019) 41:23–31. doi: 10.1111/ijlh.12916
13. Kita K, Nakase K, Miwa H, Masuya M, Nishii K, Morita N, et al. Phenotypical characteristics of acute myelocytic leukemia associated with the t(8;21) (q22;q22) chromosomal abnormality: frequent expression of immature B-cell antigen CD19 together with stem cell antigen CD34. Blood. (1992) 80:470–7. doi: 10.1182/blood.V80.2.470.470
14. Ma G, Wang Y, Ahmed T, Zaslav AL, Hogan L, Avila C, et al. Anti-CD19 chimeric antigen receptor targeting of CD19 + acute myeloid leukemia. Leuk Res Rep. (2018) 9:42–4. doi: 10.1016/j.lrr.2018.03.002
15. Danylesko I, Shem-Tov N, Yerushalmi R, Jacoby E, Toren A, Shouval R, et al. Point of care CD19 chimeric antigen receptor (CAR) T-cells for relapsed/refractory acute myeloid leukemia (AML) with aberrant CD19 antigen expression. Curr Res Transl Med. (2024) 72:103471. doi: 10.1016/j.retram.2024.103471
16. Xu J, Zhang H, Zhao Y, Zhang X, Guo S, Shi X, et al. Infectious complications distribution following CLL1 CAR-T cell therapy for acute myeloid leukemiass. Cancer Immunol Immunother. (2025) 74:149. doi: 10.1007/s00262-025-03998-1
17. Restelli C, Ruella M, Paruzzo L, Tarella C, Pelicci PG, and Colombo E. Recent advances in immune-based therapies for acute myeloid leukemia. Blood Cancer Discov. (2024) 5:234–48. doi: 10.1158/2643-3230.BCD-23-0202
18. Park JH, Romero FA, Taur Y, Sadelain M, Brentjens RJ, Hohl TM, et al. Cytokine release syndrome grade as a predictive marker for infections in patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with chimeric antigen receptor T cells. Clin Infect Dis. (2018) 67:533–40. doi: 10.1093/cid/ciy152
19. Orlando EJ, Han X, Tribouley C, Wood PA, Leary RJ, Riester M, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med. (2018) 24:1504–6. doi: 10.1038/s41591-018-0146-z
20. Jacoby E, Nguyen SM, Fountaine TJ, Welp K, Gryder B, Qin H, et al. CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat Commun. (2016) 7:12320. doi: 10.1038/ncomms12320
21. Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. (2015) 21:581–90. doi: 10.1038/nm.3838
22. Ruella M and Maus MV. Catch me if you can: Leukemia Escape after CD19-Directed T Cell Immunotherapies. Comput Struct Biotechnol J. (2016) 14:357–62. doi: 10.1016/j.csbj.2016.09.003
23. Francis J, Dharmadhikari AV, Sait SN, Deeb G, Wallace PK, Thompson JE, et al. CD19 expression in acute leukemia is not restricted to the cytogenetically aberrant populations. Leuk Lymphoma. (2013) 54:1517–20. doi: 10.3109/10428194.2012.754096
24. Paschka P, Marcucci G, Ruppert AS, Mrózek K, Chen H, Kittles RA, et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B Study. J Clin Oncol. (2006) 24:3904–11. doi: 10.1200/JCO.2006.06.9500
25. Qiang W, Lu J, Jia Y, Liu J, Liu J, He H, et al. B-cell maturation antigen/CD19 dual-targeting immunotherapy in newly diagnosed multiple myeloma. JAMA Oncol. (2024) 10:1259–63. doi: 10.1001/jamaoncol.2024.2172
26. Neelapu SS, Dickinson M, Munoz J, Ulrickson ML, Thieblemont C, Oluwole OO, et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat Med. (2022) 28:735–42. doi: 10.1038/s41591-022-01731-4
27. Karmali R, Shouse G, Torka P, Moyo TK, Romancik J, Barta SK, et al. Double hit & double expressor lymphomas: a multicenter analysis of survival outcomes with CD19-directed CAR T-cell therapy. Blood Cancer J. (2025) 15:43. doi: 10.1038/s41408-025-01250-8
28. Wang Q, Hu Y, Gao L, Zhang S, Lu J, Li B, et al. Pediatric acute myeloid leukemia with t(8;21) and KIT mutation treatment with avapritinib post-stem cell transplantation: a report of four cases. Ann Hematol. (2024) 103:3795–800. doi: 10.1007/s00277-024-05810-z
29. Xue S, Huang W, Liu F, Zhang Y, Hao Q, Cui B, et al. Rapid response to avapritinib of acute myeloid leukemia with t(8;21) and KIT mutation relapse post allo-HSCT. Leuk Lymphoma. (2022) 63:2247–50. doi: 10.1080/10428194.2022.2064994
30. Kong J, Zheng FM, Wang ZD, Zhang YY, Cheng YF, Fu HX, et al. Avapritinib is effective for treatment of minimal residual disease in acute myeloid leukemia with t (8;21) and kit mutation failing to immunotherapy after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. (2023) 58:777–83. doi: 10.1038/s41409-023-01973-x
Keywords: t(8; 21) acute myeloid leukemia, aberrant CD19 expression, CD19 CAR-T cell therapy, hematological remission, molecular remission
Citation: Zhang X, Wang L, Qiao J, Wang S, Wang L, Liu L, Qin J, Chen Z, Huang W, Zheng Y, Peng H, Mei J, Wang H, Yu C, Li Y and Yu L (2025) Anti-CD19 CAR-T cell therapy in relapsed/refractory t(8;21) acute myeloid leukemia with aberrant CD19 expression. Front. Immunol. 16:1617589. doi: 10.3389/fimmu.2025.1617589
Received: 24 April 2025; Accepted: 30 June 2025;
Published: 21 July 2025.
Edited by:
Liang Huang, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Jian Zhou, Henan Provincial Cancer Hospital, ChinaJohn Marra, Università Cattolica del Sacro Cuore, Italy
Copyright © 2025 Zhang, Wang, Qiao, Wang, Wang, Liu, Qin, Chen, Huang, Zheng, Peng, Mei, Wang, Yu, Li and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Yu, eXVsaUBzenUuZWR1LmNu; Yisheng Li, eXNsaUBoYW9zaGliaW8uY29t
 Xiaomin Zhang
Xiaomin Zhang Lixin Wang
Lixin Wang Jingqiao Qiao1
Jingqiao Qiao1 Lian Liu
Lian Liu Jiading Qin
Jiading Qin Wenfa Huang
Wenfa Huang Chuan Yu
Chuan Yu Yisheng Li
Yisheng Li