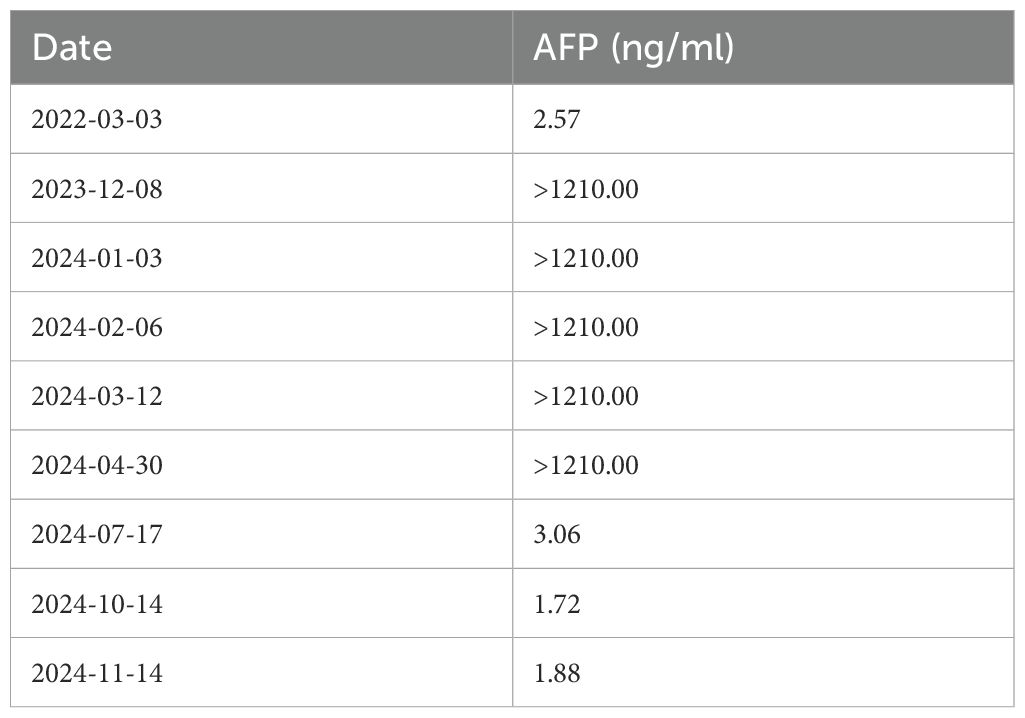
- 1Gastric Cancer Center/Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Department of Integrated Traditional and Western Medicine, West China Hospital, Sichuan University, Chengdu, China
Background: Hepatoid adenocarcinoma of the stomach (HAS) is a rare but highly aggressive subtype of gastric cancer (GC) associated with an unfavorable prognosis, particularly in advanced or metastatic stages. While the standard first-line treatment for advanced GC involves immune checkpoint inhibitors (ICIs) combined with chemotherapy, HAS often shows a poor therapeutic response to this regimen. The hypoxia in the tumor microenvironment is considered a key factor limiting ICI efficacy, and combining hyperbaric oxygen therapy (HBOT) with immunotherapy may offer a synergistic sensitizing effect.
Methods: We report a case of advanced HAS with peritoneal metastasis who received standard first-line immunochemotherapy (CAPOX plus sintilimab). After four cycles, the patient achieved only stable disease (SD) per RECIST 1.1 criteria. Consequently, HBOT was introduced as a sensitizing agent after the fifth cycle, and the patient subsequently completed the sixth cycle. This report was prepared using the CARE reporting guideline and checklist (Supplement A).
Results: Following the addition of HBOT, the patient’s tumor markers normalized. Subsequent imaging and endoscopic evaluations revealed a complete resolution of all lesions, meeting the criteria for a clinical complete response (cCR) under RECIST 1.1.
Conclusions: This case suggests that adding HBOT may enhance the efficacy of immunotherapy and overcome resistance to ICIs in advanced HAS. These promising findings warrant further investigation through prospective clinical studies to confirm this observation.
Introduction
Hepatoid adenocarcinoma of the stomach (HAS), a rare subtype of gastric cancer (GC), accounts for only 0.3%-15% of all GC cases (1). Histologically, HAS resembles hepatocellular carcinoma, characterized by abundant eosinophilic cytoplasm, centrally located nuclei, and frequent elevation of serum alpha-fetoprotein (AFP). Clinically, HAS exhibits a high propensity for vascular invasion and distant metastasis, particularly to the liver and peritoneum, a prognosis that is even worse than that of conventional gastric adenocarcinomas (2).
The peritoneum is one of the most common metastatic sites in GC, occurring in approximately 4%–14% of patients (3). Treatment of gastric cancer with peritoneal metastasis (GCPM) remains challenging and primarily relies on systemic therapy. While the combination of chemotherapy and immune checkpoint inhibitors (ICIs) has improved survival outcomes in advanced GC, achieving a complete clinical response (cCR) in unresectable cases with peritoneal dissemination is exceedingly rare. Moreover, primary or acquired resistance to ICIs is common, significantly limiting their therapeutic efficacy. Recent preclinical studies have highlighted the potential of hyperbaric oxygen therapy (HBOT) to favorably modulate the tumor microenvironment (TME), alleviate tumor hypoxia, and enhance the efficacy of ICIs (4). Therefore, combining HBOT with standard chemotherapy and ICIs offers a promising strategy for immunosensitization or overcoming ICI resistance.
This report describes the case of a 62-year-old male patient with advanced stage IVB (rT2N2M1) HAS with peritoneal metastasis. Initial systemic therapy with CAPOX chemotherapy combined with sintilimab yielded limited efficacy. Remarkably, after the incorporation of HBOT into his regimen, the patient achieved a cCR. To our knowledge, this is the first reported case of advanced HAS with peritoneal metastasis achieving cCR with this combination, highlighting the potential of HBOT as a novel immunosensitizing therapeutic strategy in advanced GC.
Case description
A timeline summarizing the key clinical events, from initial diagnosis to the last follow-up, is presented in Figure 1A.
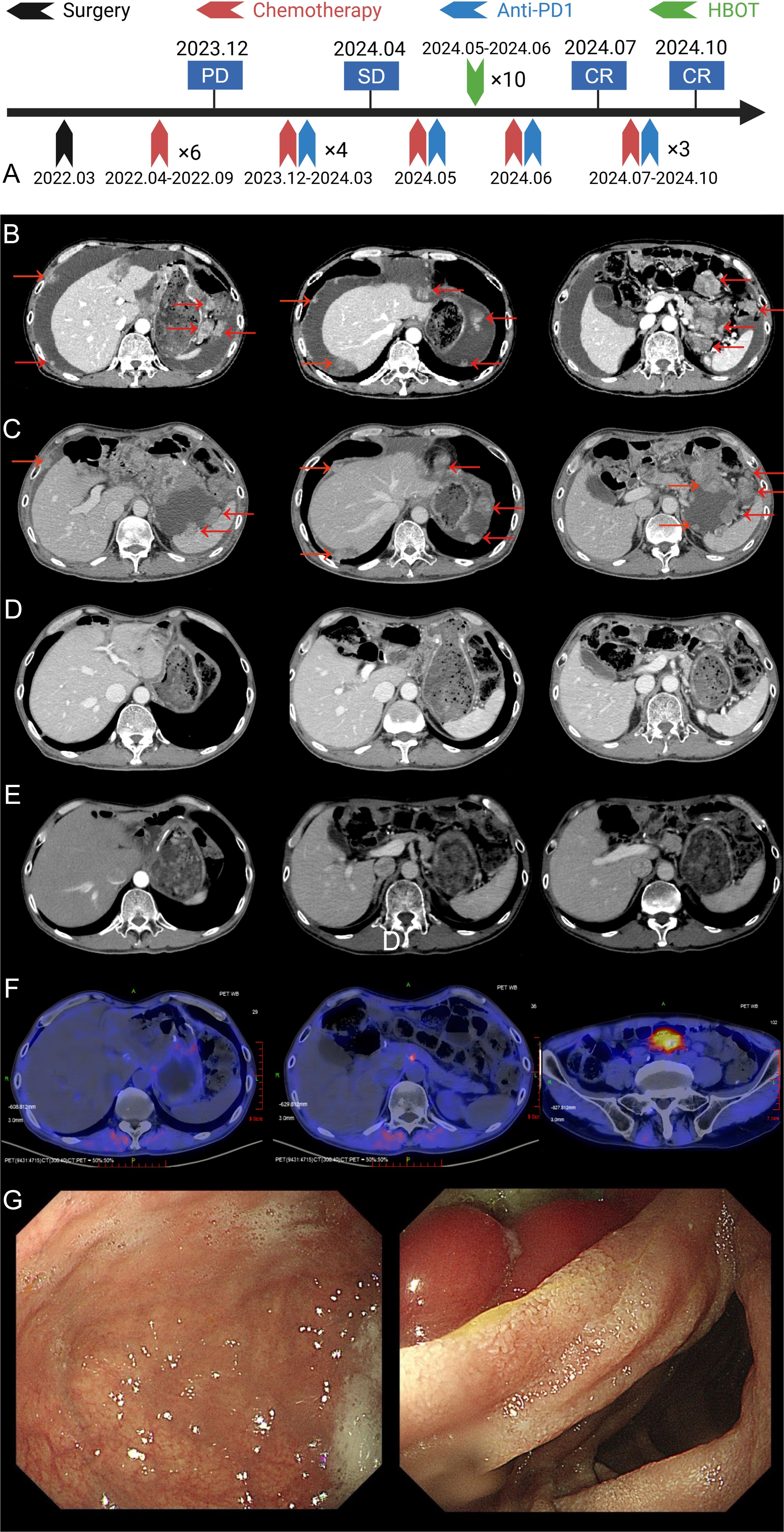
Figure 1. Imaging and treatment timeline of the patient. (A) The overall treatment timeline of the patient, including surgery, chemotherapy, anti-PD-1 therapy, and HBOT. (B) Abdominal enhanced CT at the time of recurrence showing tumor recurrence in the liver and surrounding tissues (tumor marked by red arrows). (C) Abdominal enhanced CT after 4 cycles of chemotherapy combined with ICIs treatment, showing partial tumor response (tumor marked by red arrows). (D) Abdominal enhanced CT after HBOT and an additional cycle of chemotherapy combined with ICIs, showing further tumor regression (tumor marked by red arrows). (E) Abdominal enhanced CT after 7–9 cycles of chemotherapy combined with ICIs, showing near-complete tumor resolution (tumor marked by red arrows). (F) FAPI PET/CT performed on January 3, 2025, revealing no evidence of tumor recurrence throughout the body. (G) Upper gastrointestinal endoscopy findings after 7–9 cycles of treatment. The gastric mucosa appeared smooth, and no active lesions were observed.
Patient background and initial diagnosis
In March 2022, a 62-year-old male with no history of smoking, alcohol use, or other chronic diseases presented with epigastric pain. Psychosocially, the patient was a retired civil servant, married with strong family support, and had no history of significant psychological distress. His family history was notable for gastric cancer in his father, and subsequent genetic testing revealed a pathogenic RAD51D germline mutation alongside a TP53 somatic mutation of uncertain significance. Physical examination identified left upper quadrant tenderness, though laboratory results, including tumor markers and organ function tests, were within normal limits. An initial computed tomography (CT) scan demonstrated thickening of the gastric wall and enlarged perigastric lymph nodes (Figure 2A). Gastroscopy revealed ulcers at the gastric angle, and biopsy confirmed moderately to poorly differentiated adenocarcinoma. Based on these findings, the patient was clinically staged as cT2N1M0 (Stage IIA) according to the AJCC 8th Edition Cancer Staging Manual.
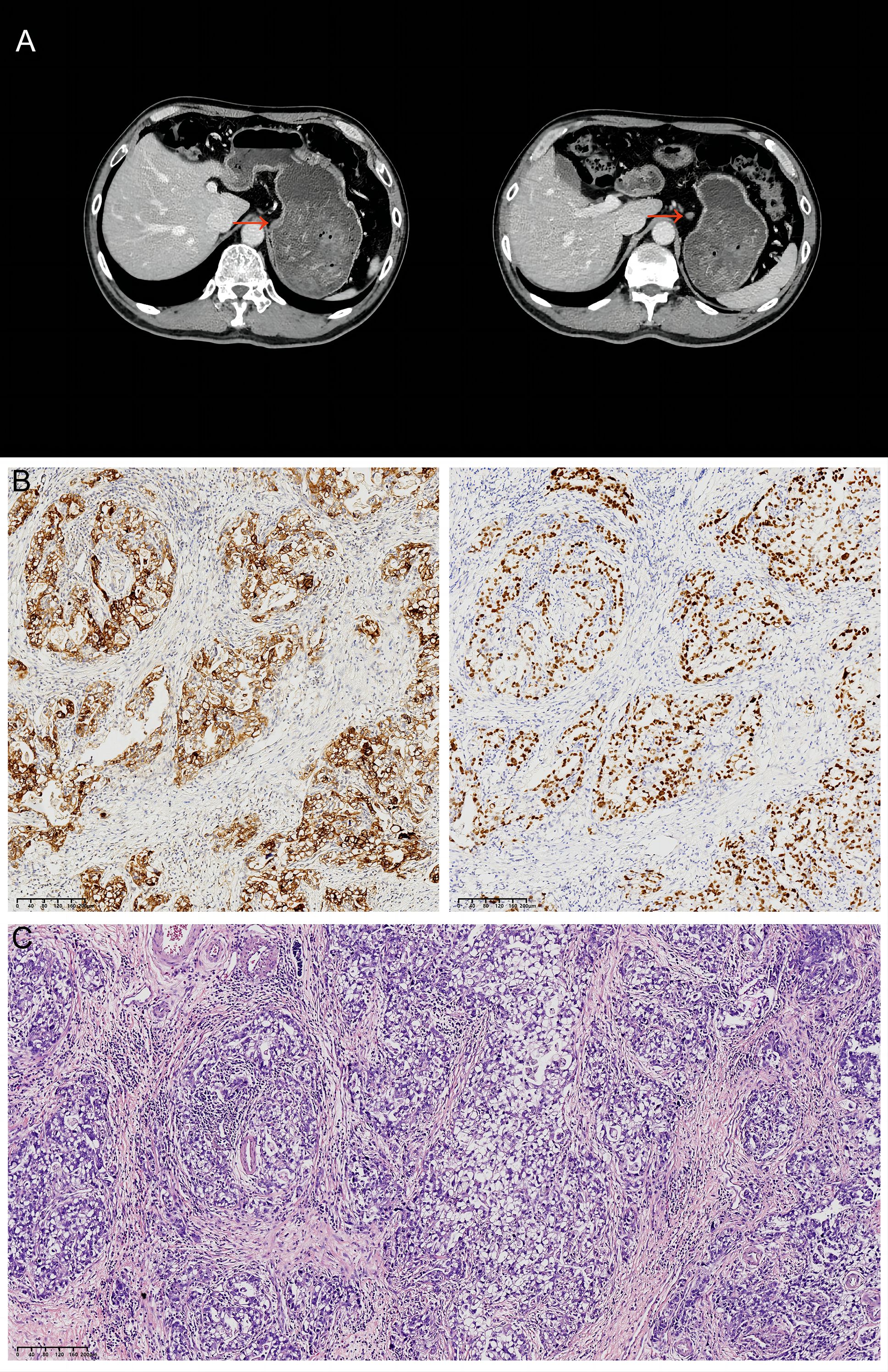
Figure 2. Initial CT findings and subsequent histopathology. (A) Abdominal CT from March 2022 showing thickening of the gastric lesser curvature (red arrows) and enlargement of the adjacent lymph nodes. (B) GPC3 (left) and SALL4 (right) immunohistochemical staining of the resected specimen. Original magnification:×100; scale bar = 100 μm. (C) Hematoxylin and eosin (H&E) staining of the resected specimen. Original magnification:×100; scale bar = 100 μm.
Surgical treatment and pathological findings
On March 16, 2022, the patient underwent laparoscopic radical distal gastrectomy with D2 lymphadenectomy, achieving a complete (R0) resection. Postoperative pathological examination of the resected specimen revealed a 4.8 × 4.0 cm, Borrmann type II tumor with a rough surface, which had focally infiltrated the muscularis propria. Histologically, the tumor was classified as an intestinal-type adenocarcinoma, that was predominantly tubular and graded as G2-G3 (moderately to poorly differentiated). Metastatic carcinoma was identified in three lymph nodes, one each from stations 3a, 7, and 11P.
Immunohistochemistry (IHC) analysis yielded the following results: HER2 (1+), CLDN18.2 (0), PD-L1 (CPS=2), and proficient mismatch repair (pMMR). PD-1 was positive in a minority of lymphocytes, while CDX2 and CK20 were positive, and Desmin was positive in smooth muscle. Epstein-Barr virus-encoded small RNA (EBER) in situ hybridization was negative. Additional IHC markers revealed positivity for SALL4 and GPC3, and negativity for CD34, AFP, and SOX10. These findings led to a final diagnosis of adenocarcinoma with features of both enteroblastic differentiation and hepatoid adenocarcinoma (Figures 2B, C). Based on the AJCC 8th Edition TNM staging system, the pathological stage was confirmed as pT2N2M0, Stage IIA.
Postoperative adjuvant chemotherapy and recurrence
Following surgery, the patient completed six cycles of adjuvant SOX chemotherapy and entered a period of surveillance. However, in December 2023, approximately one year after his initial surgery, he presented with abdominal distension. Laboratory tests revealed markedly elevated levels of serum AFP (>1210 ng/ml) and CA724 (16.10 U/ml). An abdominal CT scan subsequently confirmed the presence of peritoneal metastasis (Figure 1B). To establish a definitive diagnosis of recurrence, a therapeutic and diagnostic paracentesis was performed on December 8, 2023, which yielded approximately 700 mL of hemorrhagic ascitic fluid. Cytological analysis of this fluid confirmed recurrent adenocarcinoma. Consequently, the patient’s diagnosis was updated to recurrent hepatoid adenocarcinoma of the gastric angle with peritoneal metastasis, and he was restaged as rT2N2M1 (Stage IV) according to the AJCC staging system.
Systemic therapy
Following the diagnosis of recurrence, the patient received a single dose of intraperitoneal paclitaxel (60 mg) on December 9, 2023. Subsequently, from December 10, 2023, to March 12, 2024, he was treated with four cycles of first-line systemic immunochemotherapy. This regimen, administered every three weeks, consisted of CAPOX (capecitabine: 1.5 g orally, twice daily on days 1–14; oxaliplatin: 200 mg by intravenous infusion on day 1) combined with sintilimab (200 mg by intravenous infusion on day 1). An initial treatment response evaluation was performed in March 2024. An enhanced abdominal CT scan (Figure 1C) revealed stable disease (SD) according to the RECIST 1.1 criteria.
Effective combination therapy
The decision to add HBOT was prompted by the limited clinical benefit observed after the initial four cycles: imaging evaluation revealed stable disease (SD), and the patient’s abdominal distension had not significantly improved. To overcome potential resistance and prevent disease progression, and because the evaluation coincided with the scheduled start of the fifth cycle, the clinical team decided to introduce HBOT as a sensitizing strategy immediately after administering the next planned cycle of immunochemotherapy to maintain treatment continuity. Accordingly, on April 30, 2024, the patient received the 5th cycle of CAPOX and sintilimab with all drug doses maintained as in previous cycles. This was immediately followed by 10 sessions of HBOT (1-hour oxygen inhalation at 2 atmospheres absolute [ATA] per session). After completing the sixth cycle of the combined therapy on June 22, 2024, a follow-up evaluation in July 2024 showed a remarkable response. An enhanced abdominal CT scan revealed a complete resolution of the recurrent lesion at the gastric anastomosis and the peritoneal metastases (Figure 1D). Concurrently, tumor markers normalized, with AFP decreasing to 3.06 ng/mL (normal range: 0–7 ng/mL) and CA724 to 2.81 U/mL (normal range: 0–6.9 U/mL). The changes in AFP during treatment are illustrated in the Table 1. Based on RECIST 1.1 criteria, the patient had achieved complete response (CR).
The patient then proceeded to maintenance therapy with capecitabine and sintilimab. The maintenance regimen included the following: capecitabine: 1.5 g orally, twice daily, from day 1 to day 14; sintilimab: 200 mg by intravenous infusion, day 1. Each cycle was administered every three weeks. Follow-up imaging, including an enhanced chest and abdominal CT scan on October 15, 2024, confirmed a sustained cCR (Figure 1E). A fibroblast activation protein inhibitor (FAPI) PET/CT scan on January 3, 2025, showed a focal area of high tracer uptake in the peritoneum without a corresponding mass on CT, a finding suggestive of post-treatment inflammation rather than tumor recurrence (Figure 1F). Finally, a painless endoscopy on November 18, 2024, revealed no abnormalities in the remnant stomach (Figure 1G), further corroborating the complete response.
Patient perspective
The patient reported no increase in adverse effects after the addition of hyperbaric oxygen therapy and demonstrated good treatment compliance throughout the course.
Follow-up
The patient is currently undergoing follow-up, including hematological tests, imaging evaluations, and quality of life assessments. Compliance with the treatment plan is excellent, and the patient maintains a good quality of life, with no signs of recurrence or significant adverse effects observed. As of the last follow-up on March 29, 2025, the patient remained progression-free. The progression-free survival (PFS) since the confirmation of recurrence on December 8, 2023, has been ongoing for over 15 months.
Discussion
The management of HAS, a rare but highly aggressive subtype of GC, presents a significant clinical challenge due to its poor prognosis and limited response to standard therapies. Characterized by histological features resembling hepatocellular carcinoma, HAS is frequently associated with elevated serum AFP levels (2, 5). Despite its rarity, the high global burden of GC, one of the top five most common malignancies worldwide, means that a substantial number of patients are still diagnosed with HAS each year (6). Currently, the therapeutic strategies for HAS do not differ from those for conventional gastric adenocarcinoma. For patients with unresectable advanced or metastatic HAS, systemic therapy remains the primary treatment modality. Pivotal Phase III trials, such as KEYNOTE-859 (7), CheckMate-649 (8), and ORIENT-16 (9), have established the combination of chemotherapy and a PD-1 inhibitor as the standard of care for first-line treatment of HER2-negative advanced GC (with mPFS of 6.9–7.7 months), a recommendation endorsed by major clinical guidelines including NCCN, CSCO, and ESMO. Indeed, subsequent meta-analyses have confirmed that this combination provides a significant survival benefit compared to chemotherapy alone (10). However, HAS is associated with a poorer prognosis than conventional GC, which is attributed to its higher propensity for distant metastasis and limited responsiveness to immunotherapy (11). Consequently, developing strategies to overcome this resistance and sensitize HAS to immunotherapy represents a critical clinical challenge.
The present case offers evidence for the potential of HBOT. The patient, diagnosed with advanced HAS and peritoneal metastasis, initially showed a suboptimal response to four cycles of standard immunochemotherapy (CAPOX plus sintilimab), achieving only SD. However, a dramatic clinical turnaround was observed following the introduction of HBOT after the fifth cycle. Subsequent evaluations revealed a cCR, evidenced by the normalization of tumor markers and the disappearance of all lesions on imaging and endoscopic examinations. Notably, the patient’s progression-free survival has already exceeded 15 months. This remarkable improvement strongly suggests that HBOT acted as a potent immunosensitizing agent, overcoming the initial resistance and unlocking the therapeutic potential of the existing immunotherapy regimen.
The poor responsiveness of HAS to immunotherapy is largely attributed to its unique TME. HAS is typically characterized as an immunologically “cold” tumor, featuring diminished infiltration of CD8+ T cells, an abundance of regulatory T cells (Tregs) and M2-type macrophages, low PD-L1 expression, and a low tumor mutational burden (TMB). The majority of HAS cases are also microsatellite stable (MSS), all of which are hallmarks of poor responsiveness to immunotherapy (12). A salient feature of HAS is the elevation of serum AFP (11), a biomarker strongly associated with poor prognosis in GC. Elevated AFP not only indicates a poor outcome but also actively promotes tumor progression by enhancing proliferation, invasion, and migration (13). Furthermore, a distinctive feature of HAS is its extensive and abnormal tumor vasculature, which has been linked to the overexpression of vascular endothelial growth factor C (VEGF-C) and angiopoietin-like proteins (ANGPTLs) (14, 15). AFP itself can exacerbate this by upregulating VEGF expression and increasing microvessel density (14). However, this dysregulated angiogenesis results in dysfunctional vessels that are irregular, tortuous, and poorly branched. The consequent inefficient blood perfusion leads to extensive hypoxic regions within the tumor, creating a profoundly hypoxic and immunosuppressive TME (16). This tumor-induced hypoxia is a pivotal factor undermining the efficacy of ICIs. It triggers a cascade of immunosuppressive events, primarily through the activation of the hypoxia-inducible factor-1α (HIF-1α) signaling pathway. Activation of HIF-1α promotes the recruitment of inhibitory cells, including myeloid-derived suppressor cells (MDSCs), Tregs, and M2-type tumor-associated macrophages. It also upregulates PD-L1 expression on both cancer and dendritic cells, which directly inhibits cytotoxic T lymphocyte function and reinforces the immunosuppressive landscape (17–21). Additionally, hypoxic conditions are known to promote cancer stem cell maintenance, thereby increasing tumor invasiveness and therapeutic resistance (22, 23). Collectively, these features likely explain the limited efficacy of the initial immunochemotherapy regimen observed in our patient.
HBOT may enhance the efficacy of ICIs through multiple synergistic mechanisms. Foremost, HBOT directly counteracts the hypoxic tumor microenvironment. By increasing oxygen tension, it downregulates the expression of HIF-1α, thereby mitigating the recruitment of immunosuppressive cells such as Tregs and M2-macrophages (24–26). Concurrently, the hyperoxic state promotes the normalization of tumor vasculature, partly by upregulating the expression of platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31), which facilitates increased infiltration of immune cells into the tumor site (27). Beyond remodeling the TME, HBOT has been shown to directly augment the effector functions of immune cells. It can enhance the cytotoxic activity of both effector T cells and natural killer (NK) cells, boosting their antitumor capabilities (27). Furthermore, emerging evidence suggests that HBOT can amplify the efficacy of PD-1 blockade by activating the cGAS-STING signaling pathway, a key innate immune sensing mechanism (27). Another proposed mechanism involves the degradation of the extracellular matrix (ECM) by HBOT, which may improve the physical delivery and penetration of large-molecule therapeutics like anti-PD-1/PD-L1 monoclonal antibodies into the TME (28). Collectively, these multifaceted mechanisms provide a strong theoretical basis for the role of HBOT as an effective immunosensitizing strategy in cancer therapy.
Indeed, the “abnormal vasculature-hypoxia-immunosuppression axis” is not exclusive to HAS but is a common pathological feature of gastric adenocarcinoma, particularly in advanced stages (16, 29). This hypoxia-driven, immunosuppressive microenvironment is thought to be a key mechanism underlying the limited efficacy of ICIs in a significant proportion of GC patients. Consequently, this highlights the therapeutic potential of HBOT in the broader population of patients with advanced or metastatic gastric adenocarcinoma. Upon reviewing the existing clinical literature, we found that studies on HBOT in cancer treatment are limited, with most focusing on its role as an adjunct to radiotherapy or chemotherapy, primarily aimed at improving quality of life, and demonstrating good tolerability in most cases. To further investigate the immunosensitizing mechanisms of HBOT and to validate the efficacy of this combination therapy, our team has initiated a Phase Ib/II clinical trial. This study will evaluate CAPOX chemotherapy combined with the PD-1 inhibitor sintilimab and HBOT as a first-line treatment for advanced or metastatic gastric or gastroesophageal junction cancer (GC/GEJC) (NCT06742411).
To our knowledge, this is the first reported case of a patient with advanced, HER2-negative HAS and peritoneal metastasis achieving a cCR through the combination of HBOT with chemotherapy and immunotherapy. This outcome highlights the potential of HBOT as an effective immunosensitizing strategy in GC. The primary strengths of this report lie in its novelty, as well as the favorable safety profile and low cost of HBOT, which would facilitate its widespread adoption if proven effective in large-scale clinical trials. However, this case report has several limitations. Firstly, as a single case, it lacks a control group for comparison, and the influence of the patient’s specific, albeit of uncertain significance, genetic mutations on the observed efficacy of HBOT and ICIs remains unknown. Secondly, because no residual tumor was found during the follow-up endoscopy, we were unable to perform a comparative analysis of the TME before and after HBOT to elucidate the underlying mechanisms. Furthermore, predictive biomarkers to identify patients who would most benefit from the HBOT-immunochemotherapy combination are currently lacking. Although imaging techniques to assess tumor hypoxia in GC exist, their accuracy and correlation with treatment outcomes need to be validated in larger-scale clinical trials. Therefore, despite the promising results of this case, further prospective studies are essential to confirm these findings, explore the mechanisms of action, and define the optimal patient population for this novel therapeutic approach.
Conclusion
This case report details the successful treatment of a patient with advanced HAS who achieved a remarkable clinical CR. The addition of HBOT to a standard immunochemotherapy regimen appeared to overcome initial treatment resistance. These findings suggest that HBOT may serve as an effective immunosensitizing strategy, enhancing the efficacy of ICIs in the treatment of GC. Future clinical studies are warranted to further verify the efficacy and safety of HBOT combined with immunotherapy, explore its mechanisms of action, and provide new insights for the comprehensive treatment of advanced GC.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of West China Hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. JW: Investigation, Writing – review & editing. PZ: Writing – review & editing. MC: Investigation, Software, Writing – review & editing. MX: Writing – review & editing. LZ: Investigation, Project administration, Software, Writing – review & editing. ML: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Project administration, Software, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhu M, Chen E, Yu S, Xu C, Yu Y, Cao X, et al. Genomic profiling and the impact of MUC19 mutation in hepatoid adenocarcinoma of the stomach. Cancer Commun (Lond). (2022) 42:1032–5. doi: 10.1002/cac2.12336
2. Xia R, Zhou Y, Wang Y, Yuan J, and Ma X. Hepatoid adenocarcinoma of the stomach: current perspectives and new developments. Front Oncol. (2021) 11:633916. doi: 10.3389/fonc.2021.633916
3. Sirody J, Kaji AH, Hari DM, and Chen KT. Patterns of gastric cancer metastasis in the United States. Am J Surg. (2022) 224:445–8. doi: 10.1016/j.amjsurg.2022.01.024
4. Liu X, Ye N, Liu S, Guan J, Deng Q, Zhang Z, et al. Hyperbaric oxygen boosts PD-1 antibody delivery and T cell infiltration for augmented immune responses against solid tumors. Adv Sci (Weinh). (2021) 8:e2100233. doi: 10.1002/advs.202100233
5. Li M, Mei YX, Wen JH, Jiao YR, Pan QR, Kong XX, et al. Hepatoid adenocarcinoma-Clinicopathological features and molecular characteristics. Cancer Lett. (2023) 559:216104. doi: 10.1016/j.canlet.2023.216104
6. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
7. Rha SY, Oh D-Y, Yañez P, Bai Y, Ryu M-H, Lee J, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. (2023) 24:1181–95. doi: 10.1016/S1470-2045(23)00515-6
8. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
9. Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, et al. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: the ORIENT-16 randomized clinical trial. Jama. (2023) 330:2064–74. doi: 10.1001/jama.2023.19918
10. Pu W, Li S, Zhang J, Huang J, Li J, Jiang Y, et al. The efficacy and safety of PD-1/PD-L1 inhibitors in combination with chemotherapy as a first-line treatment for unresectable, locally advanced, HER2-negative gastric or gastroesophageal junction cancer: a meta-analysis of randomized controlled trials. Front Immunol. (2025) 16:1566939. doi: 10.3389/fimmu.2025.1566939
11. Jiang J, Ding Y, Lu J, Chen Y, Chen Y, Zhao W, et al. Integrative analysis reveals a clinicogenomic landscape associated with liver metastasis and poor prognosis in hepatoid adenocarcinoma of the stomach. Int J Biol Sci. (2022) 18:5554. doi: 10.7150/ijbs.71449
12. Taniguchi Y, Kiyozawa D, Kohashi K, Kawatoko S, Yamamoto T, Torisu T, et al. Volume of hepatoid component and intratumor M2 macrophages predict prognosis in patients with hepatoid adenocarcinoma of the stomach. Gastric Cancer. (2025) 28:41–50. doi: 10.1007/s10120-024-01562-x
13. Zhan Z, Chen B, Yu J, Zheng J, Zeng Y, Sun M, et al. Elevated serum alpha-fetoprotein is a significant prognostic factor for patients with gastric cancer: Results based on a large-scale retrospective study. Front Oncol. (2022) 12:901061. doi: 10.3389/fonc.2022.901061
14. Kamei S, Kono K, Amemiya H, Takahashi A, Sugai H, Ichihara F, et al. Evaluation of VEGF and VEGF-C expression in gastric cancer cells producing α-fetoprotein. J Gastroenterol. (2003) 38:540–7. doi: 10.1007/s00535-002-1099-y
15. Chen E, Tang C, Peng K, Cheng X, Wei Y, and Liu T. ANGPTL6-mediated angiogenesis promotes alpha fetoprotein-producing gastric cancer progression. Pathol-Res Pract. (2019) 215:152454. doi: 10.1016/j.prp.2019.152454
16. Chen Z, Han F, Du Y, Shi H, and Zhou W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduction Targeted Ther. (2023) 8:70. doi: 10.1038/s41392-023-01332-8
17. Greijer A and van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. (2004) 57:1009–14. doi: 10.1136/jcp.2003.015032
18. Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. (2003) 3:721–32. doi: 10.1038/nrc1187
19. Luo F, Lu F-T, Cao J-X, Ma W-J, Xia Z-F, Zhan J-H, et al. HIF-1α inhibition promotes the efficacy of immune checkpoint blockade in the treatment of non-small cell lung cancer. Cancer Lett. (2022) 531:39–56. doi: 10.1016/j.canlet.2022.01.027
20. Ding X-C, Wang L-L, Zhang X-D, Xu J-L, Li P-F, Liang H, et al. The relationship between expression of PD-L1 and HIF-1α in glioma cells under hypoxia. J Hematol Oncol. (2021) 14:92. doi: 10.1186/s13045-021-01102-5
21. Shurin MR and Umansky V. Cross-talk between HIF and PD-1/PD-L1 pathways in carcinogenesis and therapy. J Clin Invest. (2022) 132:e159473. doi: 10.1172/JCI159473
22. Bar EE, Lin A, Mahairaki V, Matsui W, and Eberhart CG. Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am J Pathol. (2010) 177:1491–502. doi: 10.2353/ajpath.2010.091021
23. Li P, Zhou C, Xu L, and Xiao H. Hypoxia enhances stemness of cancer stem cells in glioblastoma: an in vitro study. Int J Med Sci. (2013) 10:399. doi: 10.7150/ijms.5407
24. Zhang L, Ke J, Min S, Wu N, Liu F, Qu Z, et al. Hyperbaric oxygen therapy represses the Warburg effect and epithelial–mesenchymal transition in hypoxic NSCLC cells via the HIF-1α/PFKP axis. Front Oncol. (2021) 11:691762. doi: 10.3389/fonc.2021.691762
25. Wang P, Gong S, Pan J, Wang J, Zou D, Xiong S, et al. Hyperbaric oxygen promotes not only glioblastoma proliferation but also chemosensitization by inhibiting HIF1α/HIF2α-Sox2. Cell Death Discov. (2021) 7:103. doi: 10.1038/s41420-021-00486-0
26. Wu X, Zhu Y, Huang W, Li J, Zhang B, Li Z, et al. Hyperbaric oxygen potentiates doxil antitumor efficacy by promoting tumor penetration and sensitizing cancer cells. Adv Sci. (2018) 5:1700859. doi: 10.1002/advs.201700859
27. Chen S-Y, Tsuneyama K, Yen M-H, Lee J-T, Chen J-L, and Huang S-M. Hyperbaric oxygen suppressed tumor progression through the improvement of tumor hypoxia and induction of tumor apoptosis in A549-cell-transferred lung cancer. Sci Rep. (2021) 11:12033. doi: 10.1038/s41598-021-91454-2
28. Li K, Gong Y, Qiu D, Tang H, Zhang J, Yuan Z, et al. Hyperbaric oxygen facilitates teniposide-induced cGAS-STING activation to enhance the antitumor efficacy of PD-1 antibody in HCC. J ImmunoTher Cancer. (2022) 10:e004006. doi: 10.1136/jitc-2021-004006
Keywords: gastric cancer, hepatoid adenocarcinoma of the stomach, hyperbaric oxygen therapy, immune checkpoint inhibitors, immunosensitization, clinical complete response
Citation: Li W, Wei J, Zhang P, Cheng M, Xu M, Zhu L and Liu M (2025) Hyperbaric oxygen therapy as an immunosensitizing strategy in advanced gastric hepatoid adenocarcinoma: a case report. Front. Immunol. 16:1625273. doi: 10.3389/fimmu.2025.1625273
Received: 08 May 2025; Accepted: 11 June 2025;
Published: 27 June 2025.
Edited by:
Stavros P. Papadakos, Laiko General Hospital of Athens, GreeceReviewed by:
Spyridon Zouridis, Saint Louis University, United StatesEleni Myrto Maria Trifylli, National and Kapodistrian University of Athens, Greece
Copyright © 2025 Li, Wei, Zhang, Cheng, Xu, Zhu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Zhu, MTM4ODAyNjI4NjBAMTYzLmNvbQ==; Ming Liu, bGl1bWluZzYyOUB3Y2hzY3UuY24=
 Wenke Li
Wenke Li Jing Wei
Jing Wei Pengfei Zhang
Pengfei Zhang Mo Cheng
Mo Cheng Menghui Xu1
Menghui Xu1 Ming Liu
Ming Liu