- 1Departments of Medical Oncology, The First People’s Hospital of Xiaoshan District, Hangzhou, Zhejiang, China
- 2Department of Pathology, The First People’s Hospital of Xiaoshan District, Hangzhou, Zhejiang, China
- 3Outpatient infusion Room, Chengxiang Health Service Center, Xiaoshan District, Hangzhou, China
- 4Department of Anorectal Surgery, The First People’s Hospital of Xiaoshan District, Hangzhou, Zhejiang, China
This report describes a 56-year-old female diagnosed with lymphoepithelioma-like carcinoma with carcinosarcoma of the endometrium (LELCCSE). She experienced vaginal bleeding and lower abdominal discomfort without any obvious cause following six years of menopause. She sought treatment at our hospital in Hangzhou, China. An ultrasound examination revealed a hypoechoic mass within the uterus. Subsequently, a biopsy confirmed it as lymphoepithelioma-like carcinoma (LELC) with carcinosarcoma.The patient underwent a total hysterectomy, bilateral salpingo-oophorectomy, and associated lymphadenectomy. The postoperative pathology suggested LELC complicated with carcinosarcoma and an IIC grade by the 2023 FIGO staging system. She received adjuvant chemotherapy and radiotherapy without any molecular testing due to her financial constraints. A gene which encodes the catalytic subunit of DNA polymerase epsilon(POLE) mutation was found by the retrospective molecular testing; however, no recurrence or metastasis occurred after 12 follow-up months.
1 Introduction
Lymphoepithelial carcinoma is an undifferentiated carcinoma that was initially identified in the nasopharynx. Associated with the Epstein-Barr virus (EBV), it is characterized by undifferentiated epithelial tumor cell clusters and significant lymphocytic as well as plasma cell infiltration (1–3). Cancers with similar morphological features and occurring outside the nasopharynx are called “lymphoepithelioma-like carcinoma” (LELC). Although they have been reported in several anatomical sites (4–6), their occurrences in the endometrium are rare (7–13). The main clinical symptom of endometrial LELC is vaginal bleeding post-menopause, similar to the symptoms of other common gynecological conditions. The diagnosis of endometrial LELC relies on pathological assessment, immunohistochemistry, and molecular pathology analysis. Moreover, immunohistochemical markers like Epithelial Membrane Antigen(EMA) and Cytokeratin(CK) show positive expressions, while molecular Small RNA encoded by Epstein-Barr virus(EBER) displays a negative result. Additionally, endometrial carcinosarcoma is complex and highly malignant (14–16). Because of variable biological behaviors and prognostic characteristics, its existence with endometrial LELC makes both diagnosis and treatment difficult. Recent molecular pathology advancements have promoted the relevance of gene mutation analysis for classifying and assessing the prognosis of endometrial cancer. Notably, the POLE gene’s mutation status is significantly associated with a better prognosis for patients (17, 18). We have described a 56-year-old female patient diagnosed with POLE-mutated Lymphoepithelioma-like Carcinoma with Carcinosarcoma of the Endometrium (LELCCSE). This rare case highlights the necessity of molecular pathology and offers new perspectives for timely and appropriate diagnosis as well as treatment in clinical practice.
2 Case report
A 56-year-old female who had been postmenopausal for six years reported a single episode of minimal bright red vaginal bleeding and mild lower abdominal pain six months ago. However, she did not seek immediate medical attention and was admitted to our hospital in Hangzhou, China, 11 days ago. A physical examination indicated a normal general condition. Moreover, the gynecological assessment showed no abnormalities in the vulva, vagina, or cervix. The uterus was in a normal position, firm, without any palpable masses, and also showed moderate mobility without any tenderness. No significant masses were observed in the adnexal regions.
An ultrasound revealed that the uterus was in a normal position. The uterine cavity was completely compressed by a hypoechoic mass with clear borders, measuring approximately 2.4 x 2.3 x 1.4 on the left uterine body’s posterior wall (Figure 1). Its endometrial thickness (single layer) was 0.7 cm. Heterogeneous echogenicity and blood flow signals were also observed. In the left ovary, a cystic dark area, measuring approximately 3.0 x 2.7 x 2.4 cm, contained clear internal fluid. The right ovary was faintly visible, smaller, and displayed a relatively solid echogenicity. A pelvic Magnetic Resonance Imaging (MRI) scan with enhancement was performed, and revealed a soft tissue mass measuring approximately 3.0 x 2.0 cm within the uterine cavity. The serum tumor markers were: alpha-fetoprotein at 1.39 ng/mL (normal range: 0.89-8.78 ng/mL), carcinoembryonic antigen at 2.58 ng/mL (normal range: 0.00-5.00 ng/mL), and carbohydrate antigen 125 at 12.70 U/mL (normal range: 0.00-35.00 U/mL).
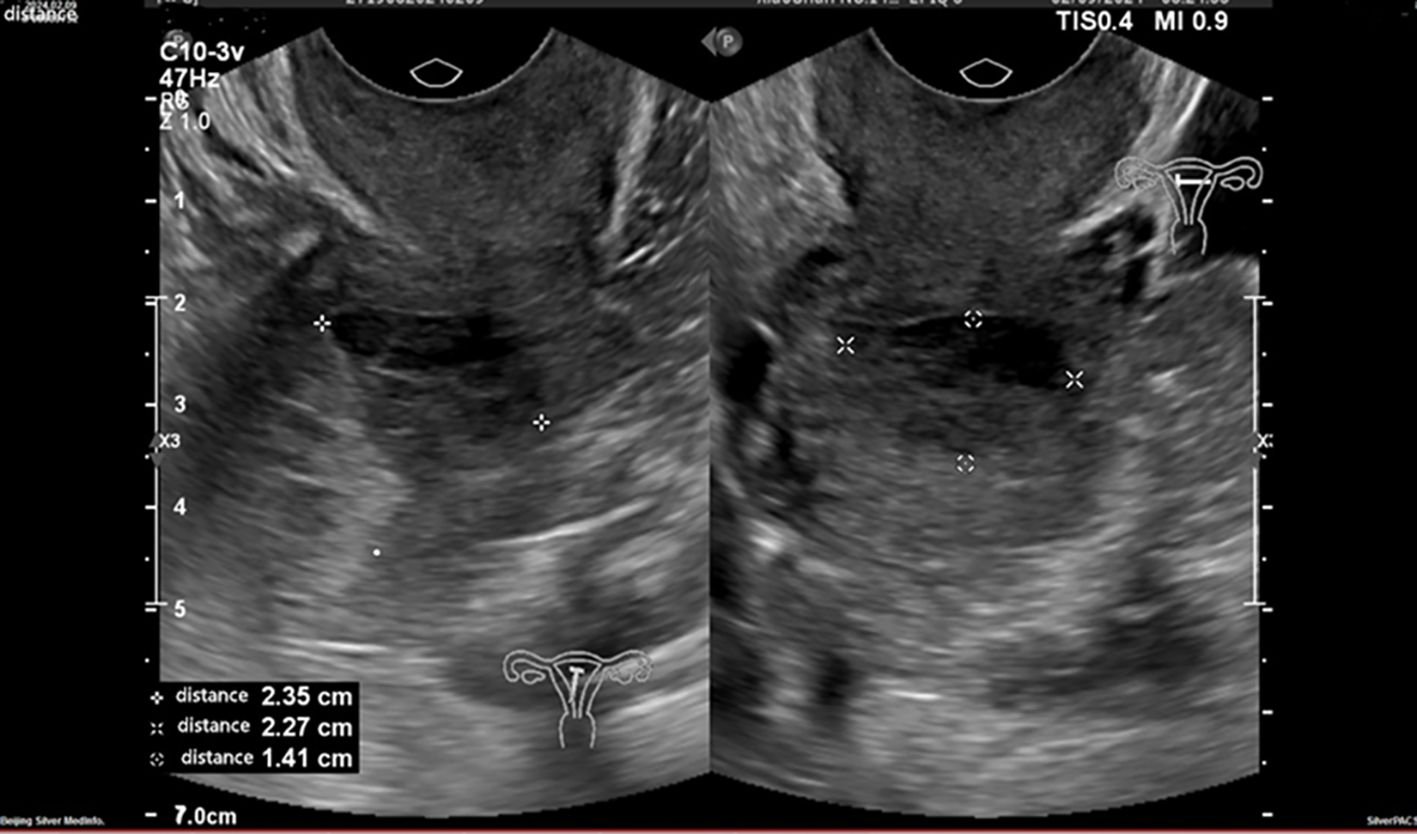
Figure 1. An ultrasound examination reveals a hypoechoic mass in the left posterior wall of the uterus.
Married at 21 years, the patient had four pregnancies: four full-term, none preterm, two abortions, and four living children. Her endometrial biopsy showed a fragmented dark red tissue, measuring 3x2x1 cm. Microscopic examination revealed the tumor comprised nests of undifferentiated carcinoma cells with prominent and vacuolated nuclei, a small carcinosarcoma component, and extensive lymphocytic and plasma cell infiltration (Figure 2a).
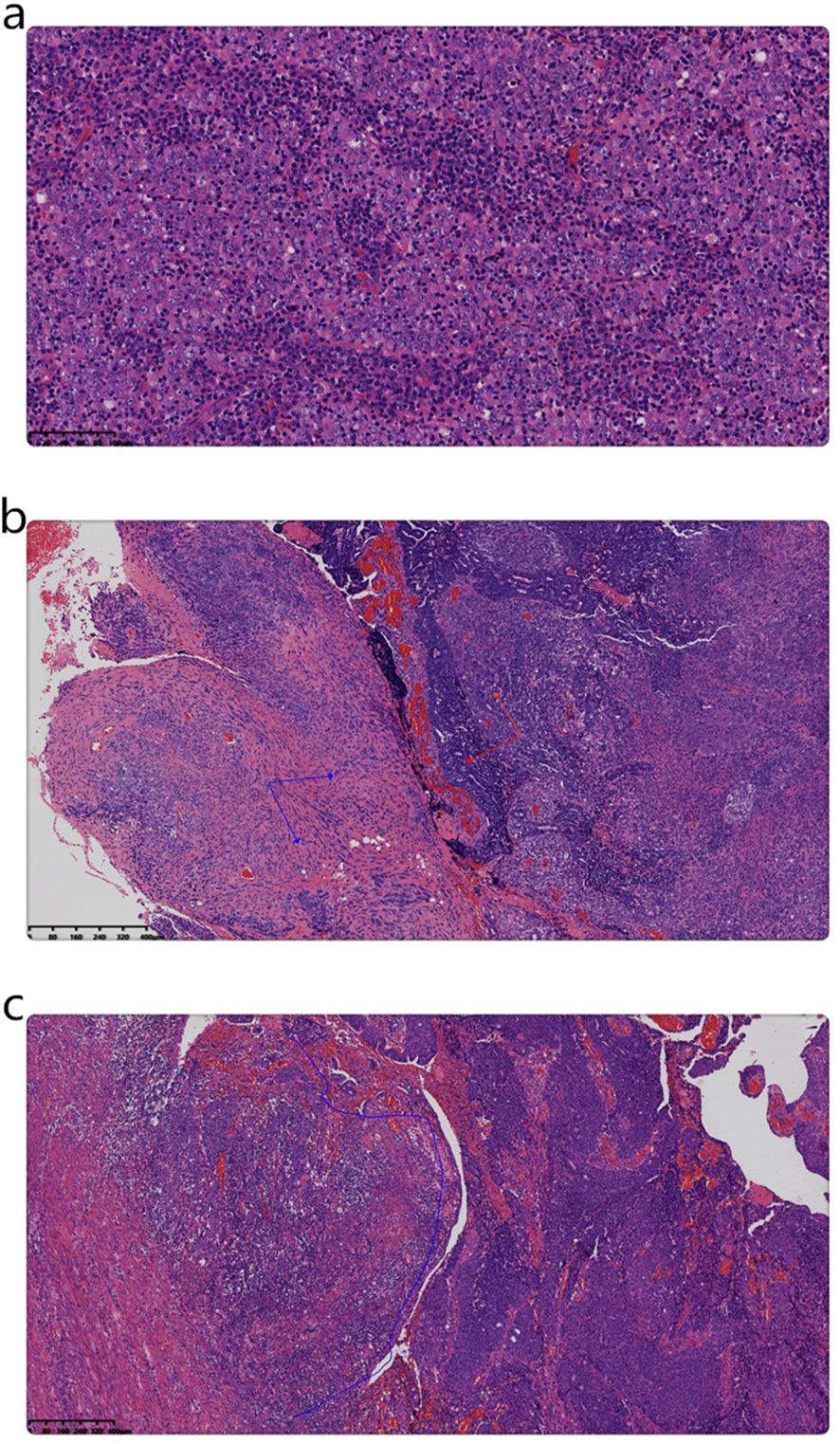
Figure 2. (a) Microscopic examination of the biopsy indicates that the tumor was composed of nests of undifferentiated carcinoma cells, including syncytial cells and vacuolated nuclei, along with abundant lymphocytic and plasma cell infiltration. (Magnification, x200; the scale bar measured 100µm; H&E). (b) Microscopic examination of the surgical specimen shows that the tumor comprised poorly differentiated endometrioid carcinoma (red arrow) and intermediate-grade sarcoma (blue arrow) components. (Magnification, x50; the scale bar measured 400µm; H&E). (c) At the base of the mass, poorly differentiated endometrioid carcinoma (right-side blue line area) was observed adjacent to a smaller LELC component (left area of the blue line). (Magnification, x50; the scale bar measured 400µm; H&E).
Subsequently, the patient underwent a laparoscopic transabdominal total hysterectomy, bilateral ovariectomy, salpingectomy, pelvic lymph node dissection, and para-aortic lymph node resection. The gross examination revealed an intact uterine specimen. The uterine body volume measured 6x5x3 cm. Although the endometrium was thin, the muscle wall thickness ranged from 1-2 cm. A polypoid mass measuring 3 x 2 x 2 cm was found at the uterine cavity’s base. The cut section was gray with a medium texture and displayed superficial muscle infiltration. The cervix measured 3x2.5x2 cm and exhibited a smooth surface.
The gynecologic specimen’s microscopic examination revealed that the tumor comprised a mixture of poorly differentiated endometrioid carcinoma and intermediate-grade spindle cell sarcoma. Additionally, a small area of LELC was observed adjacent to the tumor base (Figures 2b, c). It infiltrated the superficial myometrium (infiltration 0.5/total thickness 1.5cm) without any evidence of vascular invasion.
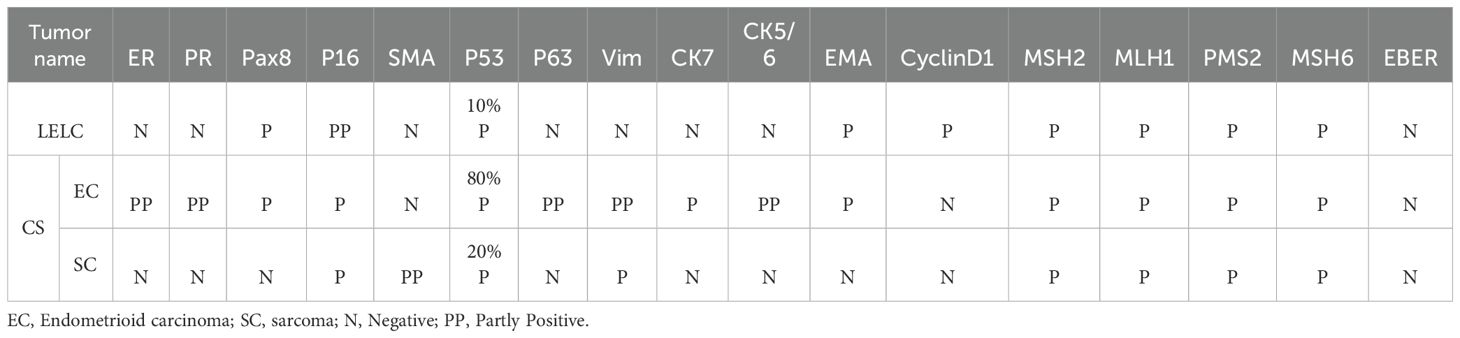
Table 1 displays the results of immunohistochemical and molecular pathology assessments. The LELC expressed both EMA and Pax8 (Figures 3a, b). Conversely, a part of the sarcoma expressed smooth muscle actin (SMA) also (Figure 3c). However, in situ hybridization evaluation was negative for EBER.
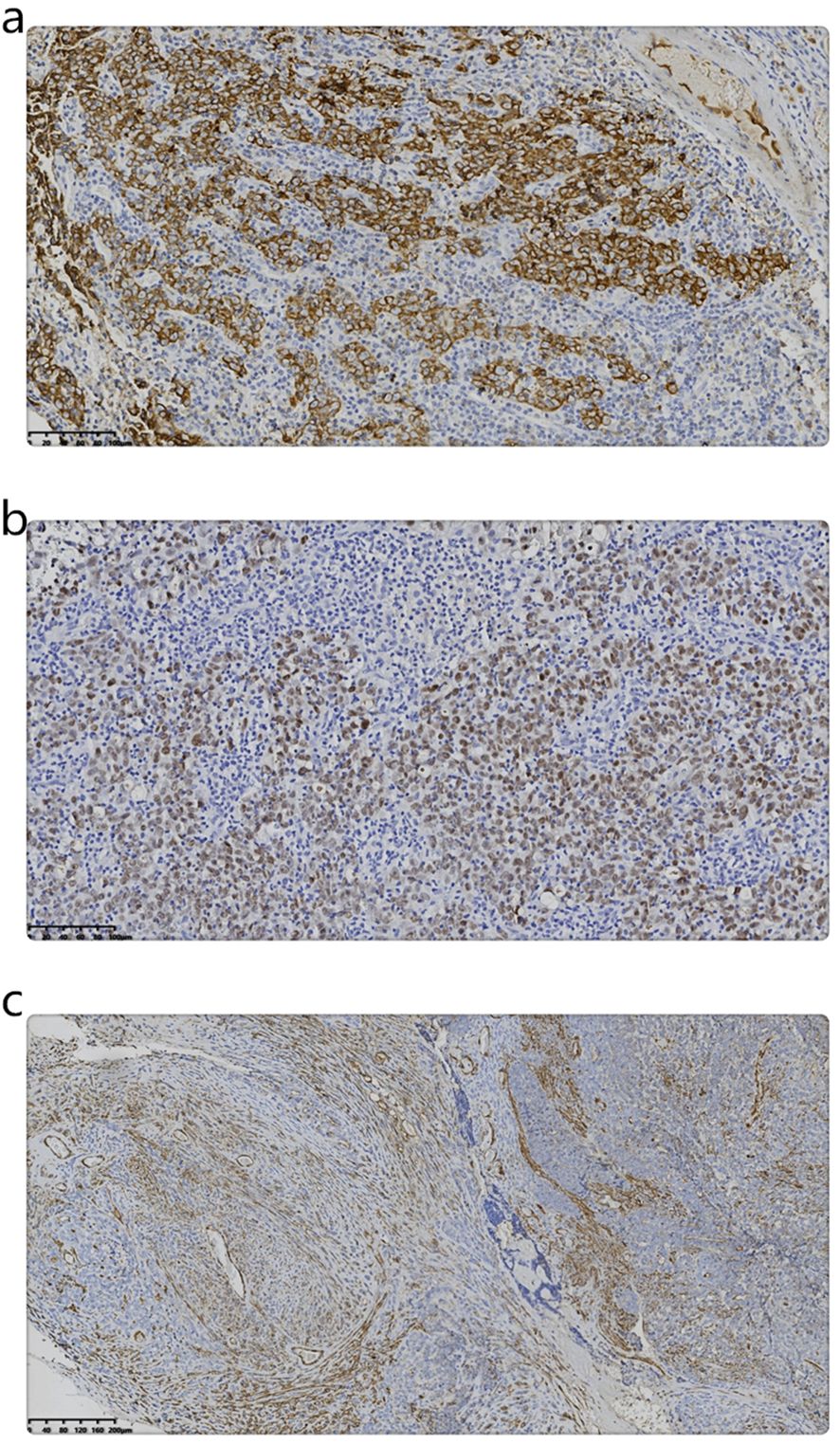
Figure 3. (a) Immunohistochemistry shows the expression of EMA in LELC. (Magnification, IHC x200; the scale bar measured 100 µm). (b) Immunohistochemistry shows the expression of Pax8 in LELC. (Magnification, IHC x200; the scale bar measured 100 µm). (c) Immunohistochemistry shows the expression of SMA in sarcoma. (Magnification, IHC x100; the scale bar measured 200 µm).
The pathological diagnosis was lymphoepithelioma-like Carcinoma with Carcinosarcoma of the Endometrium (LELCCSE). Infiltrating the superficial myometrium(<1/2), it did not involve the cervix. Bilateral ovaries and fallopian tubes exhibited serous cystadenofibromas and salpingitis, respectively. Bilateral pelvic lymph nodes showed negative results in ten nodes, and para-aortic lymph nodes were not detected. Thus, it was graded as IIC by the 2023 FIGO staging system.
Treatment: After gynecologic surgery, the patient went to another hospital as she declined molecular testing. She underwent four intravenous carboplatin and paclitaxel chemotherapy cycles, four uterine afterloading treatments, and two additional intravenous carboplatin and paclitaxel chemotherapy cycles. Due to myelosuppression, the carboplatin dose was adjusted between 550-680 mg/dose, and the paclitaxel dosage was maintained at 260 mg/dose.
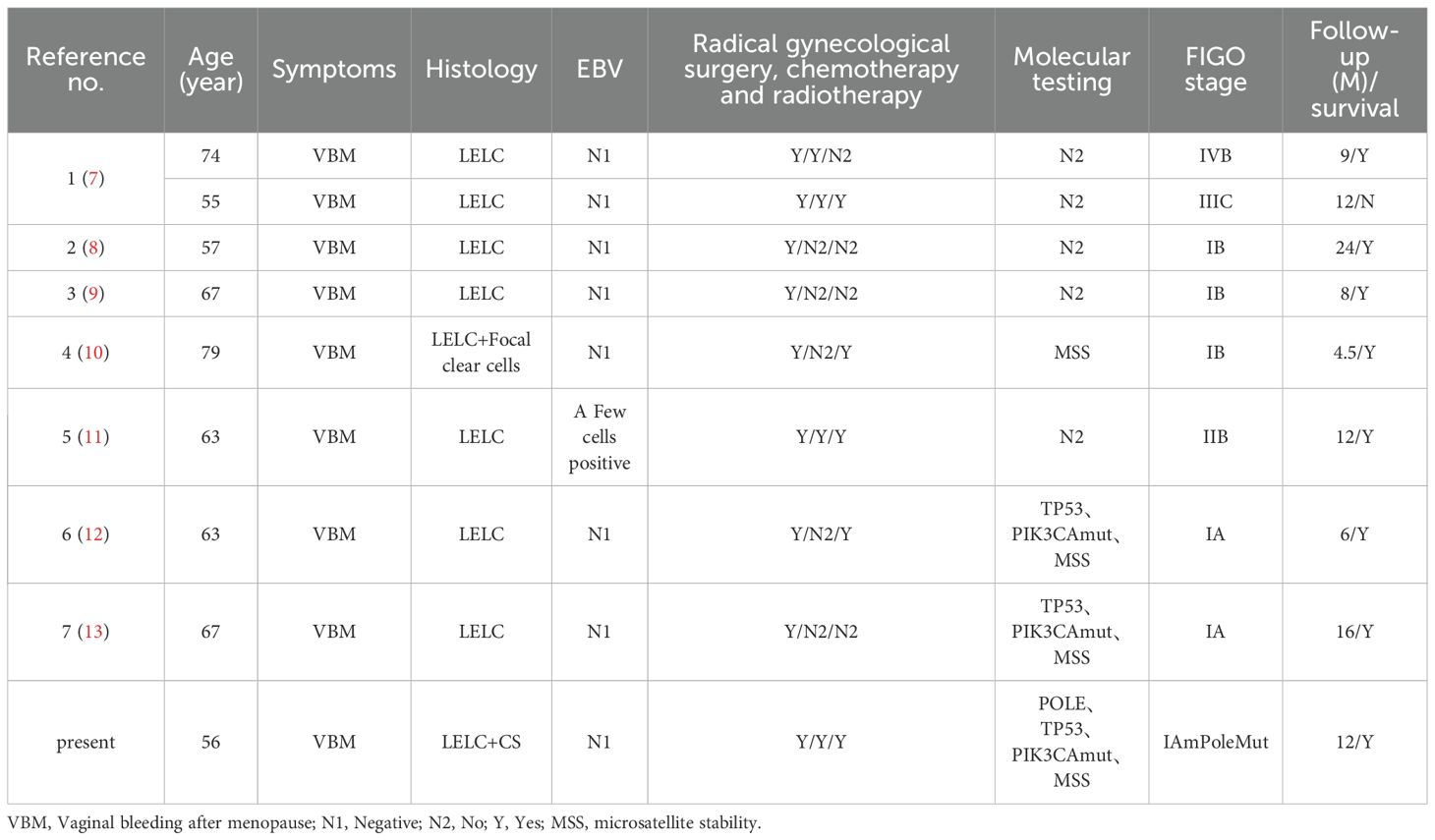
Retrospective molecular testing: A complementary, independent endometrial cancer-related gene sequencing identified LELC and carcinosarcoma tissues (Jinan Kingmed Medical Laboratory Center Co. Ltd). Using the GRCh37/hg19 reference genome, the sequencing was performed using next-generation sequencing (NGS) on the Illumina platform (NextSeq 550/NovaSeq 6000). The samples primarily included single-nucleotide variants (SNVs), small fragment deletions and insertions (INDELs), and copy number variations (CNVs). These findings support the molecular classification and prognostic evaluation of endometrial cancer, as well as predict responses to targeted therapies and immunotherapy.
Our SNV/INDEL results revealed POLE mutations (c.1376C>T p.S459F) occurring at frequencies of 18.53% and 42.8% in LELC and carcinosarcoma (Figure 4), respectively. These mutations were classified as Level I, thereby indicating a favorable prognosis and a good response to immunotherapy. Since TP53 mutations (c.637C>T p.R213*) were found at frequencies of 7.86% and 42.1%, they were also classified as Level I. Moreover, TP53 mutations (c.844C>T p.R282W) were identified at frequencies of 4.84% and 41.2%, and classified as Level I. Microsatellite instability was absent.
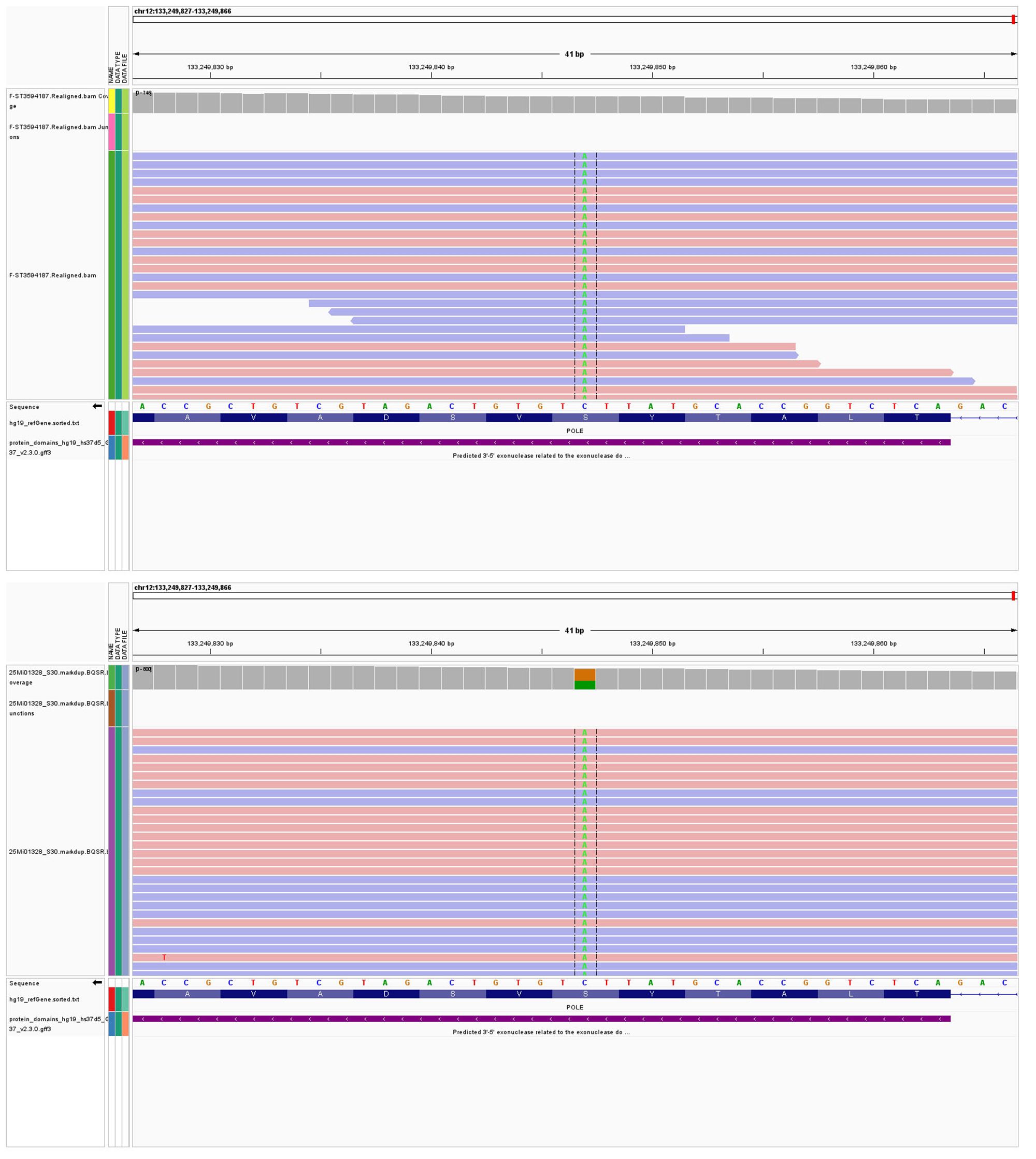
Figure 4. (A) POLE mutations (c.1376C>T p.S459F) occurring at frequencies of 18.53% in LELC. (B) POLE mutations (c.1376C>T p.S459F) occurring at frequencies of 42.8% in carcinosarcoma.
Finally, the pathological results were updated. Endometrial lymphoepithelioma-like carcinoma with carcinosarcoma was classified as POLE mut and IAm-POLE mut by the WHO molecular classification and 2003 FIGO staging system, respectively.
Follow-up: There was no recurrence or metastasis post-12 follow-up months.
3 Discussion
We have presented a rare case of LELCCSE to highlight its important morphological, immunohistochemical, and molecular pathology characteristics. Endometrial lymphoepithelioma-like carcinoma is a rare instance, and only nine cases have been reported together, including this case to date (7–13). Although the primary clinical presentation was postmenopausal bleeding, eight cases were EBV-negative, and only one case showed EBV positivity in a few tumor cells (11). A total of four tumors, including this case, were assessed as microsatellite stable (10, 12, 13) (Table 2). The diagnosis depends on pathological examination, and its clinical symptoms are similar to those of other gynecological diseases. Therefore, this case might provide valuable insights for related studies. Molecular testing in this case also revealed a Pole mutation. The pathogenesis of endometrial lymphoepithelioma-like carcinoma is not clearly related to EBV, but TP53 gene mutation and PIK3CA gene mutation have been found (12, 13), which are speculated to be related to these two gene mutations. We found a Pole mutation and added a new reference.
The molecular classification of endometrial cancer significantly affects a patient’s prognosis, especially in POLE mutation cases, which are typically associated with more favorable outcomes (17, 18). The “ultra-mutated” nature of POLE-mutant endometrial cancer displays a high mutational burden and improves responses to immunotherapy (19). Additionally, TP53 mutations are frequently correlated with tumor aggressiveness, highlighting the importance of the interaction between these genetic mutations in patient prognosis. According to the WHO molecular classification for endometrial cancer, the cases with concurrent POLE and TP53 mutations should be classified as POLE mutant type (14, 20). In the present case, the identified POLE mutation (p.S459F) and TP53 mutations (p.R213* and p.R282W) offered valuable insights into the tumor’s biological behavior and helped categorize this patient as a POLE mutant type. Additionally, the IIC stage was changed to IAm-POLE mut in the 2023 FIGO staging system.
Furthermore, the pathological examination revealed that endometrial LELC is morphologically similar to undifferentiated carcinoma of the nasopharynx (7–13). Microscopically, there were clusters of undifferentiated epithelial tumor cells and prominent infiltration of lymphocytes and plasma cells, with vacuolated nuclei and prominent nucleoli. Uterine carcinosarcomas comprise both epithelial and mesenchymal components, showcasing variable morphologies. Epithelial carcinomas might present as endometrioid, serous, or clear cell carcinomas (14, 15). The sarcomatous component might include homologous subtypes, like leiomyosarcoma, endometrial stromal sarcoma, fibrosarcoma, and undifferentiated sarcoma, or heterologous subtypes, including rhabdomyosarcoma, chondrosarcoma, osteosarcoma, and liposarcoma (16). Although LELC shows immunohistochemical CK and EMA expressions, in situ hybridization often results in negative EBER findings. Thus, our pathological, immunohistochemical, and molecular findings support the diagnosis of LELCCSE. However, the diversity of tumor components might affect the patient’s treatment options and prognosis.
Endometrial carcinosarcoma is highly malignant, and its treatment includes surgery, radiotherapy, as well as chemotherapy (14, 16). However, endometrial cancer patients with POLE mutations get better clinical results after appropriate treatment, which can guide clinical practice (21). With the coexistence of POLE and TP53 mutations, the POLE mutation might still dominate its biological behavior. Bogani G. et al. reported that 20 endometrial cancer patients (64.5%) with both POLE and TP53 mutations displayed a good prognosis (22). However, this patient refused molecular testing due to monetary reasons and sought chemotherapy and radiotherapy in other hospitals, according to the diagnosis of high-grade endometrial cancer. The 12-month follow-up showed no recurrence or metastasis. POLE mutations were identified by retrospective and complementary molecular testing. Due to the limited number of LELCCSE patients with POLE mutations, more cases are required to improve treatment options and prognosis.
This report had a few limitations. Since our patient did not undergo timely molecular testing due to financial reasons, she underwent radiotherapy and chemotherapy for the diagnosis of high-grade endometrial cancer. This might be categorized as overtreatment for early-stage POLE mutation patients. Due to a mixed carcinosarcoma component, the molecular detection post-tissue segmentation could not be performed. Thus, we were unable to determine whether LELC and ESC ensued from the same clonal origin.
Conclusion
Future research should analyze more similar cases to elucidate the clinical characteristics, prognostic factors, and treatment responses associated with POLE mutations in endometrial cancers. Additionally, molecular pathology advancements might lead to novel targeted therapies and prognostic strategies, thereby enabling personalized treatment approaches. In summary, this case has enhanced the understanding of endometrial cancer research and can serve as an important reference for future clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Xiaoshan District First People’s Hospital (Hangzhou, China). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JX: Writing – original draft, Conceptualization. QZ: Data curation, Project administration, Conceptualization, Writing – review & editing. BH: Writing – original draft, Investigation, Writing – review & editing. YZ: Formal analysis, Methodology, Validation, Investigation, Writing – review & editing. JS: Writing – review & editing, Writing – original draft, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Topal O and Erinanc H. Coexistence of lymphoepithelial carcinoma of the paroti-d gland and submandibular gland pleomorphic adenoma. J Craniofac Surg. (2017) 28:e453–4. doi: 10.1097/SCS.0000000000003662
2. Aurilio G, Ricci V, De Vita F, Fasano M, Fazio N, Orditura M, et al. A possible connective tissue primary lymphoepithelioma-like carcinoma (LELC). Ecancermedicalscience. (2010) 4:197. doi: 10.3332/ecancer.2010.197
3. Jakobiec FA, Stagner AM, and Rubin PAD. Lymphoepithelial carcinoma of the nasolacrimal duct: clinical, radiologic, and immunopathologic features. Ophthalmic Plast Reconstr Surg. (2017) 33:S18–21. doi: 10.1097/IOP.0000000000000478
4. Trihia H, Siatra H, Gklisty H, Diamantopoulos P, Arapantoni-Dadiotis P, and Kalogerakos K. Lymphoepithelioma-like carcinoma of the breast: cytological and histological features and review of the literature. Acta Cytol. (2012) 56:85–91. doi: 10.1159/000330677
5. Lopez-Beltran A, Paner G, Blanca A, Montironi R, Tsuzuki T, Nagashima Y, et al. Lymphoepithelioma-like carcinoma of the upper urinary tract. Virchows Arch. (2017) 470:703–9. doi: 10.1007/s00428-017-2117-z
6. Weng W, Sheng W, and Wang L. Human papillomavirus-associated lymphoepithelioma-like carcinoma of the anal canal: A case report and literature revi-ew. Front Med (Lausanne). (2021) 8:766960. doi: 10.3389/fmed.2021.766960
7. Vargas MP and Merino MJ. Lymphoepitheliomalike carcinoma: an unusual variant of endometrial cancer. A report of two cases. Int J Gynecol Pathol. (1998) 17:272–6. doi: 10.1097/00004347-199807000-00013
8. Rahimi S, Lena A, and Vittori G. Endometrial lymphoepitheliomalike carcinoma: absence of Epstein-Barr virus genomes. Int J Gynecol Cancer. (2007) 17:532–5. doi: 10.1111/j.1525-1438.2007.00793.x
9. Ambrosio MR, Rocca BJ, Mourmouras V, Onorati O, Mastrogiulio Mg, Di Mari N, et al. Lymphoepithelioma-like carcinoma of the endometrium. Pathologica. (2010) 102:57–61.
10. Jones H, Gold MA, Giannico G, Troutman A, Vnencak-Jones CL, Schultenover SJ, et al. Lymphoepithelioma-like carcinoma of the endometrium: immunophenotypic characterization of a rare tumor with microsatellite instability testing. Int J Gynecol Pathol. (2014) 33:64–73. doi: 10.1097/PGP.0b013e318276310b
11. Makannavar JH, KishanPrasad HL, and Shetty JK. Lymphoepithelioma-like carc-inoma of endometrium; A rare case report. Indian J Surg Oncol. (2015) 6:130–4. doi: 10.1007/s13193-015-0405-0
12. Němejcová K, Hájková N, Tichá I, Němejcová K, Hájková N, Tichá I, et al. Lymphoepithelioma-like carcinoma of the endometrium: Case report of a rare tumour with comprehensive immunohistochemical and molecular analysis. Pol J Pathol. (2018) 69:87–92. doi: 10.5114/pjp.2018.75342
13. Bienfait L, D’Haene N, Catteau X, and Noël JC. PIK3CA and p53 mutations by next generation sequencing in lymphoepithelioma-like carcinoma of the endometrium. Case Rep Pathol. (2018), 5894589. doi: 10.1155/2018/5894589
14. WHO Classification of Tumours Editorial Board. WHO classification of tumours. In: Female genital tumours, 5th ed. IARC Press, Lyon (2020).
15. Bogani G, Ray-Coquard I, Concin N, Ngoi NYL, Morice P, Caruso G, et al. Endometrial carcinosarcoma. Int J Gynecol. Cancer. (2023) 33:147–74. doi: 10.1136/ijgc-2022-004073
16. Pezzicoli G, Moscaritolo F, Silvestris E, Silvestris F, Cormio G, Porta C, et al. Uterine carcinosarcoma: An overview. Crit Rev Oncol Hematol. (2021) 163:103369. doi: 10.1016/j.critrevonc.2021.103369
17. Carlson J and McCluggage WG. Reclassifying endometrial carcinomas with a combined morphological and molecular approach. Curr Opin Oncol. (2019) 31:411–9. doi: 10.1097/CCO.0000000000000560
18. Du NN, Liu Y, Ren CX, Wang YX, Du J, Yang J, et al. Clinical application of TCGA molecular classification in endometrial endometrioid carcinoma. Zhonghua Bing Li Xue Za Zhi. (2019) 48:596–603. doi: 10.3760/cma.j.issn.0529-5807.2019.08.003
19. Ying J, Yang L, Yin JC, Xia G, Xing M, Chen X, et al. Additive effects of variants of unknown significance in replication repair-associated DNA polymerase genes on mutational burden and prognosis across diverse cancers. J Immunother Cancer. (2021) 9:1–13. doi: 10.1136/jitc-2021-002336
20. Tabata J, Takenaka M, and Okamoto A. Molecular typing guiding treatment and prognosis of endometrial cancer. Gynecology Obstetrics Clin Med. (2023) 3:7–17. doi: 10.1016/j.gocm.2023.01.011
21. Yu S, Sun Z, Zong L, Yan J, Yu M, Chen J, et al. Clinicopathological and molecular characteriz-ation of high-grade endometrial carcinoma with POLE mutation: a single centerstudy. J Gynecol Oncol. (2022) 33:e38. doi: 10.3802/jgo.2022.33.e38
Keywords: carcinosarcoma, clinicopathology, endometrium, lymphoepithelioma-like carcinoma, molecular pathology
Citation: Xu J, Huang B, Zhu Y, Shi J and Zhou Q (2025) Lymphoepithelioma-like carcinoma with carcinosarcoma of the endometrium: a case report and literature review. Front. Immunol. 16:1636575. doi: 10.3389/fimmu.2025.1636575
Received: 28 May 2025; Accepted: 30 June 2025;
Published: 21 July 2025.
Edited by:
Tullio Golia D’Augè, Sapienza University of Rome, ItalyReviewed by:
Dmitry Aleksandrovich Zinovkin, Gomel State Medical University, BelarusTeodor Florin Georgescu, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2025 Xu, Huang, Zhu, Shi and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Huang, aDc0MDkyNUBzaW5hLmNvbQ==; Jianping Shi, c2pwMDEyMzYzQDE2My5jb20=; Qiang Zhou, NDcwMzY1MzI5QHFxLmNvbQ==
 Jialing Xu1
Jialing Xu1 Bin Huang
Bin Huang Jianping Shi
Jianping Shi