- Department of Ophthalmology, Section of Immunobiology, LMU University Hospital, LMU Munich, Munich, Germany
Although TNF-α-blockade is an approved treatment for several autoimmune diseases there are cases of paradoxical induction of autoimmunity appearing under anti-TNF therapy, even of diseases that are usually treated with TNF-blockers. The case of a patient with hidradenitis suppurativa (HS) from the department of dermatology was referred to our eye hospital due to blurred vision and subsequently diagnosed Vogt-Koyanagi-Harada-like disease (VKHLD) as an adverse effect of her long-term adalimumab therapy. A novel antibody targeting IL-17, bimekizumab, was introduced as a therapy for her skin disease with concomitant improvement of her ocular symptoms. In contrast to formerly tested anti-IL-17-antibodies with moderate therapeutic effect on uveitis this novel antibody targets both, IL-17A and -F and is thus expected to be more effective. This case inspired us to further analyze the paradoxical immune mechanisms of TNF-α blockade that might induce autoimmunity and the pathomechanisms of HS and VKHD, revealing an interplay of TNF-α and IL-17 signaling pathways. This led us to a hypothesis explaining the paradox of inducing autoimmunity by TNF-blockade in context with IL-17-mediated autoimmune diseases and an explanation why VKHD should be treated with anti-IL-17A/F rather than with anti-TNF-α.
Introduction
TNF-α blockade is a successful treatment for several autoimmune diseases. However, in rare cases a paradoxical induction of an additional autoimmune disease occurs. According to the literature, the frequency of anti-TNF-induced autoimmunity ranges from 0.03% (demyelinating CNS events) to 10.7% (psoriasis) (1). With her informed consent, we report the case of a 33-year-old woman, who suffered from hidradenitis suppurativa (HS), an inflammatory skin disease, and then developed Vogt-Koyanagi-Harada-like disease (VKHLD) affecting her eyes and brain under adalimumab therapy. Uveitis has been described as a rare adverse event of anti-TNF-α therapy, and in a retrospective study four patients were described with a co-occurrence of HS and uveitis, two with anterior uveitis, one with iridocyclitis and one with panuveitis. These patients developed uveitis between one and twelve years before HS was diagnosed, notably, none of them were on anti-TNF-α therapy. Thus far, no cases of HS and VKHLD have been reported in the literature (2, 3). Our patient had experienced sudden onset of blurred vision in the left eye, which was diagnosed as acute posterior uveitis, and persistent headaches. Adalimumab was discontinued, and she was hospitalized for treatment with systemic corticosteroids, cyclosporine and mycophenolate mofetil. Following treatment, the patient gradually improved and achieved remission of her VKHLD during a 12-week follow-up period. For the treatment of her HS she was later switched to bimekizumab, a novel anti-IL-17A/F antibody. Here we discuss common pathomechanisms of HS and Vogt-Koyanagi-Harada disease (VKHD) and immune mechanisms of TNF-alpha blockade that might lead to autoimmunity. Additionally, we discuss the action of the new therapeutic antibody, which targets IL-17A and IL-17F, and the consequences of blocking IL-17F with respect to the gut microbiome and autoimmune diseases.
Hidradenitis suppurativa (HS) is an inflammatory, chronic skin disease characterized by recurrent abscesses, with a key role of tumor necrosis factor-alpha (TNF-α) in the pathogenesis (4). TNF-α inhibitors are used to treat various inflammatory and autoimmune diseases, including HS (5), but in rare cases, adalimumab has been associated with paradoxical inflammatory adverse events (PAEs), such as posterior uveitis (2). Uveitis comprises any type of intraocular inflammation and may occur as an isolated disease or be accompanied by other inflammatory disease. In addition to uveitis, TNF-alpha inhibition can also induce other autoimmune diseases such as demyelination, sarcoidosis, vasculitis, vitiligo, alopecia areata, psoriasis and thyroiditis (6–8) as well as VKH syndrome-like disease (VKHLD). VKHD is a rare inflammatory disorder that affects multiple organs, including the eyes, ears, meninges, and skin. The target of the immune response are melanocyte-specific proteins (7, 9, 10). VKHD typically begins with a prodromal phase characterized by symptoms such as headache and neck stiffness, followed by an acute uveitis phase with inflammation of the choroid, optic nerve, and retina. As the disease progresses and becomes chronic it often causes sequelae such as scarring and pigment changes in the affected parts of the retina and choroid and depigmentation of skin and hair (10).
Case report
A 33-year-old Asian woman with a history of hidradenitis suppurativa (HS) and ongoing adalimumab therapy for two years presented with sudden blurred vision in her left eye, along with severe headache and fever. She used to feel very stressed because of her hidradenitis, which was only slightly improved by adalimumab treatment. When she started adalimumab, her chest-X-ray was normal for Tb, but she received 2 x 300 mg rifampicin as a prophylactic anti-Tb treatment. One month before deterioration of vision the patient had a severe cold but was tested negative for COVID. At presentation best corrected visual acuity (BCVA) was normal in the right eye (logMAR 0.0) and count fingers (logMAR 1.9) in the left. Intraocular pressure was 19 and 21 mm Hg in the right and left eye, respectively, and slit-lamp examination of the anterior segment of both eyes was unremarkable. Her family history was negative for HS and VKH disease.
Diagnostic assessment
Funduscopic examination of the right eye was normal (Figure 1A) and the left eye revealed macular thickening indicated by yellowish discoloration and increased fundus autofluorescence (Figure 1B). Optical coherence tomography (OCT) showed exudative retinal detachment, detachment of the retinal pigment epithelial and bacillary layer detachment (Figure 2).
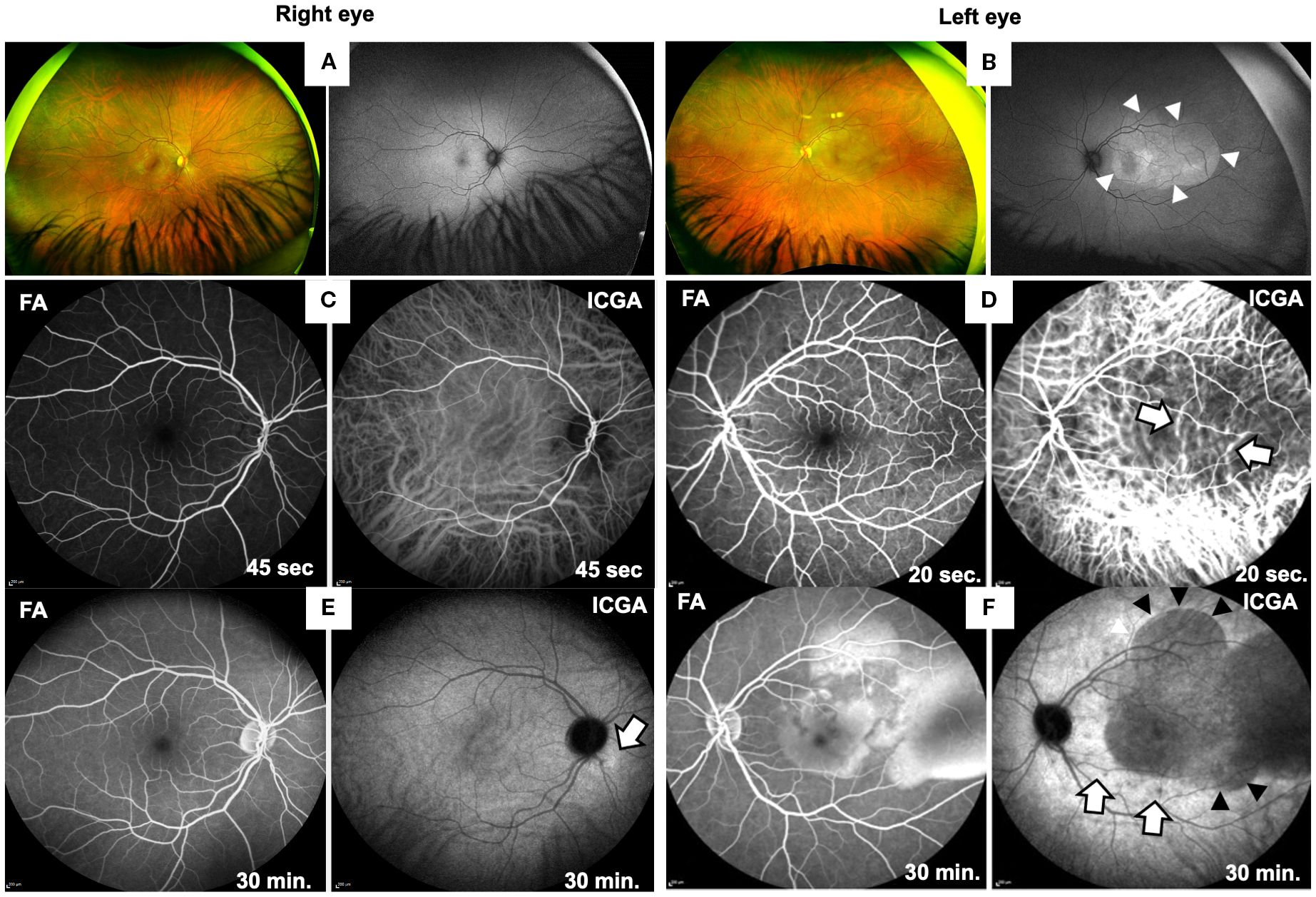
Figure 1. Ocular findings at first presentation of the patient. Fundus widefield photographs of right (A) and left eye (B). The fundus and fundus autofluorescence (FAF) imaging of the right eye (A) appeared normal, while wide-field photography of the left eye (B) reveals irregular areas of yellowish fundus discoloration in the posterior pole, caused by retinal edema and subretinal fluid accumulation. FAF imaging demonstrates an oval-shaped area of hyperautofluorescence (arrowheads), indicating RPE activation. FA and ICGA images of both eyes from early (C, D; after 45 or 20 sec) and late phases (E, F; after 30 min) are shown. Right eye (C, E) shows normal early phases (45 sec) of FA and ICGA (C) and in late phase at 30 min normal FA but a faint hyperfluorescence spot in the late phase of ICGA (E; arrow). Left eye (D, F) shows normal FA in the early phase (D, 20 sec), while ICGA reveals choroidal vasculitis (20 sec, white arrows). At 30 min (F) FA shows exudative retinal detachment caused by subretinal fluid accumulation and ICGA confirms subretinal fluid (black arrowheads) and shows additional hypofluorescent spots (white arrows), suggestive of choroidal granulomas.
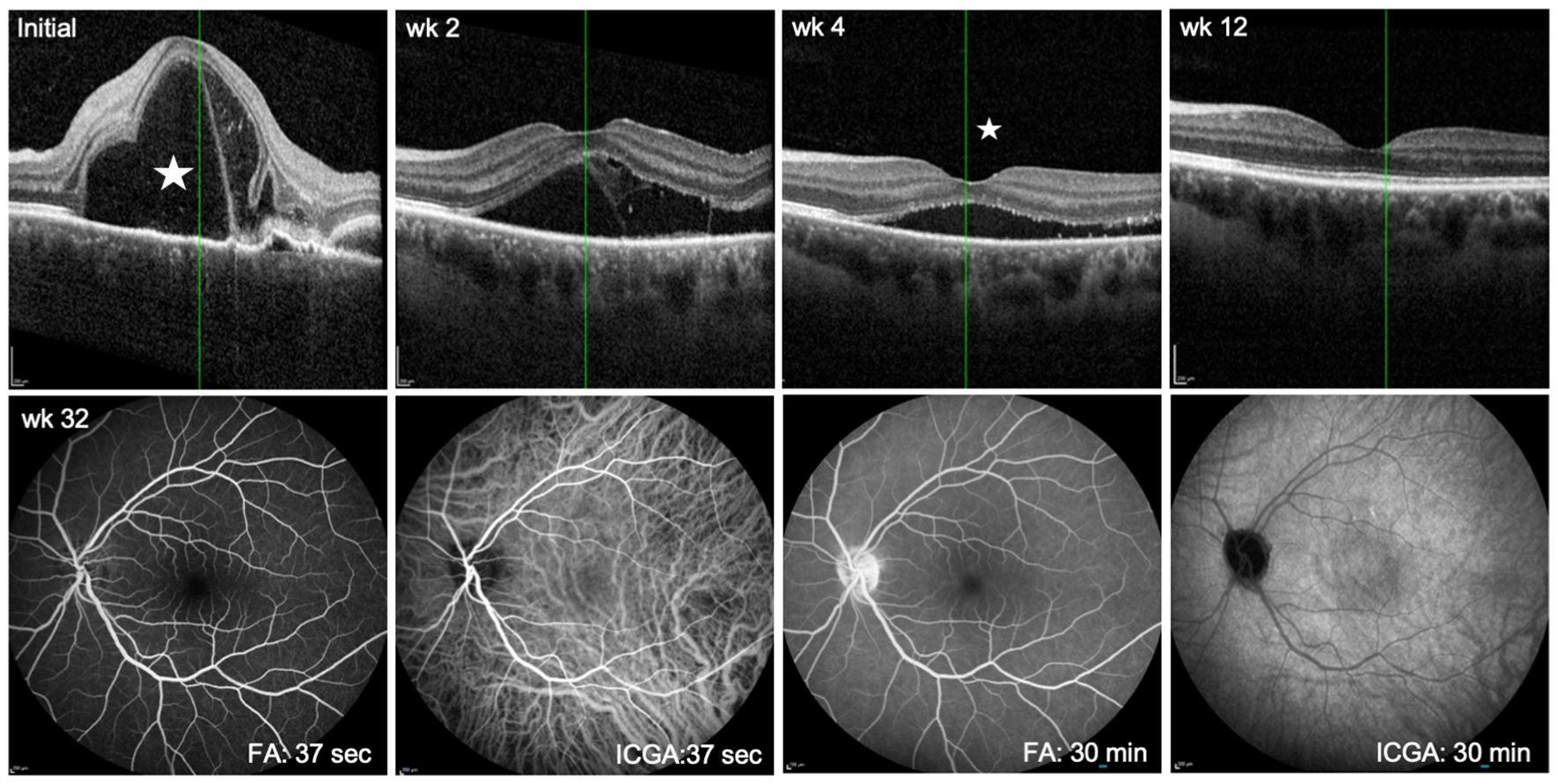
Figure 2. Follow up showing immunosuppressive treatment effect in the predominantly affected left eye. Upper panel: OCT scans at first presentation (Initial) and at follow up 2, 4, and 12 weeks after initiation of treatment. Asterisk at initial presentation indicates prominent subretinal fluid accumulation and right to it bacillary layer detachment. At 4 weeks, the asterisk reveals only a small amount of submacular exudation. By week 12, the exudation is completely resolved. Lower panel: FA/ICGA at week 32 of treatment shows resolution of choroidal vasculitis (ICGA at early phase, 37 sec and late phase, 30 min) and complete resorption of subretinal fluid at 30 min of FA.
In the left eye early phase of indocyanine green angiography (ICGA) showed fuzzy hyperfluorescent choroidal vessels (Figure 1D, white arrows, right panel) and in the late phase hypofluorescent, dark dots indicating choroidal stromal granulomas (Figure 1F, white arrows) and extensive hypofluorescent areas corresponding to regions of exudative retinal detachment (Figure 1F, right panel, black arrowheads). On late-phase fluorescein angiography (FA) the left eye showed areas of hyperfluorescence (Figure 1F, left panel), consistent with leakage and pooling in areas of serous retinal detachment. The right eye appeared normal on FA, but ICGA showed few choroidal infiltrates (Figure 1E, right panel, white arrow).
Additional laboratory tests for angiotensin-converting enzyme (ACE) and soluble interleukin-2 receptor (sIL-2R) levels were within normal limits. Serologic tests for toxoplasmosis, lues, and Lyme disease were negative. Cerebrospinal fluid analysis revealed mild, unspecific intrathecal immunoglobulin production and oligoclonal bands, as previously reported for acute VKH syndrome (11).
The HLA-type of the patient was negative for all of the VKHD-associated HLA molecules described in the literature (12). Nevertheless, based on the clinical findings the patient was diagnosed with acute VKH-like (VKHL) syndrome in both eyes, with her left eye more severely affected.
Therapeutic intervention
As the patient had no further underlying disease and had been treated with the TNF-alpha inhibitor adalimumab for 2 years, a diagnosis of paradoxical adalimumab-induced disease was made.
Adalimumab therapy was discontinued, and the treatment regimen included high-dose intravenous prednisolone (500 mg daily) for 5 days, and oral immunosuppressive therapy with cyclosporine (2 x 100 mg) as well as 2 x 1000 mg mycophenolate mofetil. After 5 days of i.v. steroids, she was switched to oral methyprednisolone 80 mg and tapered off within 4 weeks. Six weeks later, due to intolerance of the medication CyA and MMF were reduced to daily doses of 150 mg and 1500 mg, respectively.
Follow up and outcome
Twelve weeks after initiation of treatment there was complete resolution of both intraretinal and subretinal fluid in the left eye (see OCT in Figure 2). In addition, FLA/ICGA angiography at week 32 had normalized (Figure 2). Visual acuity improved to logMAR -0.1 in the left eye and to logMAR -0.1 in the right eye. Although the patient no longer complained of headaches or blurred vision anymore, she was still concerned about the smoldering activity of HS.
Then, after 27 weeks of treatment with cyclosporine and mycophenolate mofetil a flare-up of the patient’s HS occurred. She was then started on a therapy with the IL-17A and IL-17F-inhibitor bimekizumab (320 mg every 2 weeks), which resulted in a full control of the skin disease within 6 weeks. After 6 weeks of combined therapy VKHLD and HS remained stable, allowing the cessation of cyclosporine and another 6 weeks later MMF was stopped without any signs of a relapse of the VKH-like disease and stable visual acuity of logMAR -0.1 in both eyes. Since beginning the treatment with bimekizumab five months ago, the VKH-like disease with associated uveitis as well as the HS have remained in remission even after reducing the dose of bimekizumab to 320 mg every 4 weeks. During her last visit, the patient reported feeling relieved and showed no more clinical symptoms of her HA or VKHLD.
Discussion
Most autoimmune diseases are driven by Th1 cells secreting IFN-γ, and/or Th17 cells characterized by IL-17 secretion. Both cell types also produce the pleiotropic cytokine TNF-α, which is the major mediator of destructive inflammation. Therefore, targeting TNF-α is thought to simultaneously downregulate both T cell types and the inflammatory responses of TNF-α-producing innate cells.
Adalimumab is a widely used biologic anti-TNF-α-agent for moderate to severe hidradenitis suppurativa and many other autoimmune diseases, including rheumatoid arthritis, ankylosing spondylitis, Crohn’s disease, and uveitis of the posterior segment. It works by blocking the binding of TNF-α to its receptors (4, 5). TNF-α exists in two forms, soluble and membrane-bound, both of which signal through TNFR1 and TNFR2 receptors.
The soluble form of TNF-α plays a central role in systemic inflammation by driving cytokine production, recruiting immune cells, and activating endothelial signaling pathways. The membrane-bound TNF-α can be cleaved off to generate soluble TNF-α but also serves as a receptor to promote cell-to-cell communication and regulation of the immune response. Adalimumab binds primarily to soluble TNF-α, preventing it from binding to TNF receptors, and even can scavenge already receptor-bound TNF-α (13, 14).
There are two receptors for TNF-α. TNFR1 is ubiquitously expressed at low levels on most cell types, whereas TNFR2 is inducible on endothelial and immune cells, such as monocytes, macrophages, natural killer cells, and T and B lymphocytes (13). While membrane-bound TNF-α activates both, TNFR1 and TNFR2, soluble TNF-α only activates TNFR1 (15, 16). The two TNF receptors trigger different signal transduction cascades and therefore have different effector functions. TNFR1-activation is pro-inflammatory and induces apoptosis or even necroptosis via its intracellular death domain. In contrast, TNFR2 binding leads to cell survival, tissue regeneration and activation by interaction with the cytoplasmic TNF-receptor associated factor (TRAF2), finally leading to antiapoptotic stimulation of various kinases (17).
When TNF-α binds TNFR2, IL-17A production is inhibited, because TNFR2-binding activates the anti-inflammatory TNF-α-induced protein 3 (TNFAIP3/A20). A20, the gene product of TNFAIP3, blocks TNF-α-induced NF-kB signaling via degradation of the receptor interacting protein kinase 1 (RIP1), resulting in decreased IL-17A production. However, blocking the interaction of TNF-α with TNFR2 inhibits TNFAIP3/A20 and thus promotes IL-17 production (18). Since many mutations resulting in TNFAIP3 dysfunction have been described that cause severe inflammatory syndromes and diseases (reviewed in (19)), many of the patients not responding to anti-TNF-α therapy may have a mutation in their TNFAIP3 gene. Anti-TNF-α-mediated prevention of TNFR2-binding disrupts anti-inflammatory processes mediated by nuclear factor-kappa B (NF-κB), which results in the production of other pro-inflammatory cytokines such as IL-6, IL-1, IL-2, granulocyte-macrophage colony-stimulating factor (GM-CSF), and interferon-γ, upregulating inflammatory responses via TNFR1 activation. On the other hand, TNF-α also induces negative feedback regulators, such as IL-10 and corticosteroids, thus playing a key role in maintaining the balance between pro- and anti-inflammatory responses (20, 21). Inhibiting TNF-α can disrupt this balance, potentially enhancing the activity of pro-inflammatory mediators such as IL-6 or IL-17 and type I interferons via TNFR1 while downregulating anti-inflammatory cytokines via TNFR2. This may lead to a dysregulation and promotion of autoreactive T cells resulting in pro-inflammatory paradoxical adverse events (PAEs) (5, 6, 8, 13, 22). The “severe cold” the patient reported before onset of her VKH-like symptoms might have contributed to the disbalance by upregulating type-1-IFNs and other inflammatory cytokines to fight her infection.
Both, hidradenitis suppurativa and VKHD are driven by IL-17A and IL-17F (23–29). Th17 cells co-produce IL-17 and TNF-α, which act synergistically on melanocytes by inducing IL-6, IL-8, IL-1b, and CXCL1 and inhibit the pigment production of melanocytes in the skin. This results in vitiligo, one of the most common signs seen in VKHD, which, however, was not observed in our patient (30–32). In addition to the melanocytes of skin and choroid also the meninges in the brain and pigmented cells of the inner ear are affected by VKHD, causing decreased vision, headaches, tinnitus and hearing loss. Melanocyte-specific proteins of the tyrosinase family are thought to be the targeted autoantigens in VKHD, as they are recognized by T cells from VKH patients and can induce VKH disease-like symptoms in rats (10).
Due to the co-expression of IL-17 and TNF-α in Th17 cells, anti-TNF-α is expected to inhibit these cells and thus to downregulate also IL-17 production. Therefore, TNF-α blockers have been used to treat HS as well as VKHD and many other autoimmune diseases. However, under anti-TNF-α therapy some patients show increased disease activity or even the onset of new autoimmune diseases, such as our patient, who developed VKHLD. Patients with rheumatoid arthritis who developed paradoxical psoriasis under TNF-α inhibitor therapy showed an increase in Th17 cells and IL-17A production. Interestingly, in patients with inflammatory bowel disease and anti-TNF-α therapy the increase in Th17 and IL-17A levels indicates a better outcome, while the opposite is observed in MS patients, who worsen as IL-17 increases under TNF-α blockade (33–35).
As blocking TNF-α can lead to increased IL-17 production, which is counterproductive in several IL-17-driven autoimmune diseases (such as HS and VHK or VKHLD as described here), disease exacerbations or new autoimmune diseases during TNF-α blocker therapy should be carefully monitored. Anti-TNF-α should probably be replaced by anti-IL-17 in diseases where IL-17 is known to be the disease-driving cytokine. A new therapeutic antibody, bimekizumab, which blocks both IL-17A and IL-17F, is approved for the treatment of severe psoriasis and currently being tested for its effect on HS. IL-17A and IL-17F are very similar in structure and function and the differences between both are not yet clear. Former therapeutic antibodies that have only targeted IL-17A were not efficient in treating uveitis, since blocking IL-17A might have been compensated by IL-17F. Interestingly, the inhibition of IL-17F rather than IL-17A, has been shown to induce regulatory T cells. This suggests that the therapeutic efficiency of bimekizumab may extend beyond merely blocking a proinflammatory cytokine. This has been demonstrated in an experimental colitis model and may also apply to other autoimmune diseases (36). In mice with experimental colitis an increase in Prevotella species within the gut microbiome was observed, which correlated with the severity of the disease. A dominance of the “pathogenic” commensal bacterium Prevotella was also found in patients with inflammatory bowel disease and rheumatoid arthritis. Neutralizing IL-17F, but not IL-17A, decreased Prevotella in the gut. This allowed the expansion of “protective” commensals and ameliorated colitis (37). Interestingly, Prevotella spp. are also abundantly found in the skin lesion of patients with HS and are suspected to outcompete other skin commensals, as it was found in the guts of colitis mice (38). This may explain the therapeutic effect of bimekizumab due to its neutralization of IL-17F. Additionally, Prevotella dominance was also was found in the gut microbiome of VKH patients (39), suggesting a similar mechanism by which IL-17F neutralization ameliorates the disease. However, since we have no data of our patient regarding gut microbiota and/or Prevotella prevalence in the skin lesions, the aforementioned pathomechanism remains speculative. Nevertheless, the therapeutic success we have seen in our patient supports this thesis.
Our patient was switched to treatment with this antibody and her VKHLD was also controlled by bimekizumab. Therefore, targeting both IL-17A and -F might be considered as a novel therapy for VKHD and VKHLD, especially when TNF-α blockade is ineffective or induces paradoxical effects.
Although adalimumab plays a crucial role in the treatment of autoimmune diseases by specifically inhibiting TNF-α and is significantly improving outcomes for many patients, its use is not without risks. Infections or neoplasms due to immunosuppression, as well as rare but serious paradoxical reactions such as uveitis can occur. Since other autoimmune diseases are usually induced earlier under TNF-alpha blocking therapy, we speculate that the Patient’s flu-like infection could have initiated an unspecific stimulation and dysregulation of her immune system. This may have resulted in a bystander activation of a latent but so far controlled ocular autoimmunity, or antigenic mimicry of pathogen and ocular autoantigen epitopes, or both (40).
In cases where TNF-α inhibition leads to paradoxical inflammation, switching to an alternative approach, such as anti-IL-17A/F therapy, may be beneficial. However, further clinical studies are needed to prove that bimekizumab is an effective alternative to TNF-α blockade for VKH and VKHLD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by Ethics committee of the Medical Faculty of the Ludwigs-Maximilians-University Munich. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this Case Report.
Author contributions
MG: Data curation, Investigation, Writing – original draft, Writing – review & editing. ST: Data curation, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing. GW: Conceptualization, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. De Stefano L, Pallavicini FB, Mauric E, Piccin V, Vismara EM, Montecucco C, et al. Tumor necrosis factor-A Inhibitor-related autoimmune disorders. Autoimmun Rev. (2023) 22:103332. doi: 10.1016/j.autrev.2023.103332
2. Kowalski T and Mack HG. Ocular complications of tumour necrosis factor alpha inhibitors. Clin Exp Optom. (2020) 103:148–54. doi: 10.1111/cxo.12904
3. Saygın D, Syed AU, Lowder CY, Srivastava S, Maya JJ, and Hajj-Ali RA. Characteristics of inflammatory eye disease associated with hidradenitis suppurativa. Eur J Rheumatol. (2018) 5:165–8. doi: 10.5152/eurjrheum.2018.17163
4. Pandey A. Essentials of hidradenitis suppurativa: A comprehensive review of diagnostic and treatment perspectives. Ann Med Surg (Lond). (2024) 86:5304–13. doi: 10.1097/MS9.0000000000002345
5. Mitoma H, Horiuchi T, Tsukamoto H, and Ueda N. Molecular mechanisms of action of anti-tnf-alpha agents - comparison among therapeutic tnf-alpha antagonists. Cytokine. (2018) 101:56–63. doi: 10.1016/j.cyto.2016.08.014
6. Iwahashi C, Ono H, Haruta M, Minami T, Mashimo H, Shimojo H, et al. New onset or exacerbation of uveitis with infliximab: paradoxical effects? BMJ Open Ophthalmol. (2019) 4:e000250. doi: 10.1136/bmjophth-2018-000250
7. Toussirot E and Aubin F. Paradoxical reactions under tnf-alpha blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. (2016) 2:e000239. doi: 10.1136/rmdopen-2015-000239
8. Zheng Q, Zhu Y, Cheng H, and Zhu K. Uveitis occurring in a patient with psoriasis during adalimumab therapy: A case report. Indian J Dermatol. (2022) 67:207. doi: 10.4103/ijd.ijd_366_21
9. Maguire AD, Bethea JR, and Kerr BJ. Tnfalpha in ms and its animal models: implications for chronic pain in the disease. Front Neurol. (2021) 12:780876. doi: 10.3389/fneur.2021.780876
10. Lavezzo MM, Sakata VM, Morita C, Rodriguez EE, Abdallah SF, da Silva FT, et al. Vogt-koyanagi-harada disease: review of a rare autoimmune disease targeting antigens of melanocytes. Orphanet J Rare Dis. (2016) 11:29. doi: 10.1186/s13023-016-0412-4
11. Algahtani H, Shirah B, Algahtani R, Alkahtani A, and Alwadie S. Vogt koyanagi harada syndrome mimicking multiple sclerosis: A case report and review of the literature. Mult Scler Relat Disord. (2017) 12:44–8. doi: 10.1016/j.msard.2017.01.003
12. Ng JYW, Luk FOJ, Lai TYY, and Pang C-P. Influence of molecular genetics in vogt-koyanagi-harada disease. J Ophthalmic Inflammation Infect. (2014) 4:20. doi: 10.1186/s12348-014-0020-1
13. Tracey D, Klareskog L, Sasso EH, Salfeld JG, and Tak PP. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol Ther. (2008) 117:244–79. doi: 10.1016/j.pharmthera.2007.10.001
14. van Loo G and Bertrand MJM. Death by tnf: A road to inflammation. Nat Rev Immunol. (2023) 23:289–303. doi: 10.1038/s41577-022-00792-3
15. MacEwan DJ. Tnf ligands and receptors–a matter of life and death. Br J Pharmacol. (2002) 135:855–75. doi: 10.1038/sj.bjp.0704549
16. Wajant H and Siegmund D. TNFR1 and TNFR2 in the control of the life and death balance of macrophages. Front Cell Dev Biol. (2019) 7:91. doi: 10.3389/fcell.2019.00091
17. Dostert C, Grusdat M, Letellier E, and Brenner D. The TNF family of ligands and receptors: communication modules in the immune system and beyond. Physiol Rev. (2019) 99:115–60. doi: 10.1152/physrev.00045.2017
18. Urbano PCM, Aguirre-Gamboa R, Ashikov A, van Heeswijk B, Krippner-Heidenreich A, Tijssen H, et al. TNF-alpha-induced protein 3 TNF-alpha, TNFAIP3 acts as a master switch in tnf-alpha blockade-driven IL-17A expression. J Allergy Clin Immunol. (2018) 142:517–29. doi: 10.1016/j.jaci.2017.11.024
19. Bagyinszky E and An SSA. Genetic mutations associated with TNFAIP3 (A20) haploinsufficiency and their impact on inflammatory diseases. Int J Mol Sci. (2024) 25(15). doi: 10.3390/ijms25158275
20. Huynh L, Kusnadi A, Park SH, Murata K, Park-Min K-H, and Ivashkiv LB. Opposing regulation of the late phase TNF response by MTORC1-IL-10 signaling and hypoxia in human macrophages. Sci Rep. (2016) 6:31959. doi: 10.1038/srep31959
21. Kruckow KL, Murray E, Shayhidin E, Rosenberg AF, Bowdish DME, and Orihuela CJ. Chronic tnf exposure induces glucocorticoid-like immunosuppression in the alveolar macrophages of aged mice that enhances their susceptibility to pneumonia. Aging Cell. (2024) 23:e14133. doi: 10.1111/acel.14133
22. Raffeiner B, Ometto F, Bernardi L, Botsios C, and Punzi L. Inefficacy or paradoxical effect? Uveitis in ankylosing spondylitis treated with etanercept. Case Rep Med. (2014) 2014:471319. doi: 10.1155/2014/471319
23. Kimball AB, Loesche C, Prens EP, Bechara FG, Weisman J, Rozenberg I, et al. IL-17A is a pertinent therapeutic target for moderate-to-severe hidradenitis suppurativa: combined results from a pre-clinical and phase ii proof-of-concept study. Exp Dermatol. (2022) 31:1522–32. doi: 10.1111/exd.14619
24. Malvaso D, Calabrese L, Chiricozzi A, Antonelli F, Coscarella G, Rubegni P, et al. IL-17 inhibition: A valid therapeutic strategy in the management of hidradenitis suppurativa. Pharmaceutics. (2023) 15(10). doi: 10.3390/pharmaceutics15102450
25. Rastrick J, Edwards H, Ferecskó AS, Le Friec G, Manghera A, Page M, et al. The roles of IL-17A and IL-17F in hidradenitis suppurativa pathogenesis: evidence from human in vitro preclinical experiments and clinical samples. Br J Dermatol. (2024) 192:660–71. doi: 10.1093/bjd/ljae442
26. Shu Q, Yang P, Hou S, Li F, Chen Y, Du L, et al. Interleukin-17 gene polymorphism is associated with vogt-koyanagi-harada syndrome but not with behcet’s disease in a chinese han population. Hum Immunol. (2010) 71:988–91. doi: 10.1016/j.humimm.2010.06.020
27. Li F, Yang P, Liu X, Wang C, Hou S, and Kijlstra A. Upregulation of interleukin 21 and promotion of interleukin 17 production in chronic or recurrent vogt-koyanagi-harada disease. Arch Ophthalmol. (2010) 128:1449–54. doi: 10.1001/archophthalmol.2010.265
28. Chi W, Yang P, Li B, Wu C, Jin H, Zhu X, et al. IL-23 promotes cd4+ T cells to produce IL-17 in vogt-koyanagi-harada disease. J Allergy Clin Immunol. (2007) 119:1218–24. doi: 10.1016/j.jaci.2007.01.010
29. Chang R, Yi S, Tan X, Huang Y, Wang Q, Su G, et al. Microrna-20a-5p suppresses IL-17 production by targeting osm and ccl1 in patients with vogt-koyanagi-harada disease. Br J Ophthalmol. (2018) 102:282–90. doi: 10.1136/bjophthalmol-2017-311079
30. Kotobuki Y, Tanemura A, Yang L, Itoi S, Wataya-Kaneda M, Murota H, et al. Dysregulation of melanocyte function by th17-related cytokines: significance of th17 cell infiltration in autoimmune vitiligo vulgaris. Pigment Cell Melanoma Res. (2012) 25:219–30. doi: 10.1111/j.1755-148X.2011.00945.x
31. Toosi S, Orlow SJ, and Manga P. Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J Invest Dermatol. (2012) 132:2601–9. doi: 10.1038/jid.2012.181
32. Wang CQF, Akalu YT, Suarez-Farinas M, Gonzalez J, Mitsui H, Lowes MA, et al. IL-17 and tnf synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. (2013) 133:2741–52. doi: 10.1038/jid.2013.237
33. Chen DY, Chen YM, Chen HH, Hsieh CW, Lin CC, and Lan JL. Increasing levels of circulating TH17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-tnf-A Therapy. Arthritis Res Ther. (2011) 13:R126. doi: 10.1186/ar3431
34. Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. (2015) 43:727–38. doi: 10.1016/j.immuni.2015.09.003
35. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Tnf neutralization in ms: results of a randomized, placebo-controlled multicenter study. The lenercept multiple sclerosis study group and the university of british columbia ms/mri analysis group. Neurology. (1999) 53:457–65. doi: 10.1212/WNL.53.3.457
36. Tang C, Kakuta S, Shimizu K, Kadoki M, Kamiya T, Shimazu T, et al. Suppression of IL-17F, but Not of IL-17A, Provides Protection against Colitis by Inducing Treg Cells through Modification of the Intestinal Microbiota. Nat Immunol. (2018) 19:755–65. doi: 10.1038/s41590-018-0134-y
37. Jiraskova Zakostelska Z, Reiss Z, Tlaskalova-Hogenova H, and Rob F. Paradoxical reactions to anti-tnfα and anti-IL-17 treatment in psoriasis patients: are skin and/or gut microbiota involved? Dermatol Ther. (2023) 13:911–33. doi: 10.1007/s13555-023-00904-4
38. Świerczewska Z, Lewandowski M, Surowiecka A, and Barańska-Rybak W. Microbiome in hidradenitis suppurativa-what we know and where we are heading. Int J Mol Sci. (2022) 23(19). doi: 10.3390/ijms231911280
39. Ye Z, Wu C, Zhang N, Du L, Cao Q, Huang X, et al. Altered gut microbiome composition in patients with vogt-koyanagi-harada disease. Gut Microbes. (2020) 11:539–55. doi: 10.1080/19490976.2019.1700754
Keywords: hidratenitis suppurativa, Vogt-Koyanagi-Harada-like disease, TNF-α blockade, TNF-receptors, IL-17A, IL-17F, regulatory T cells, microbiome & dysregulation
Citation: Gehrke M, Thurau S and Wildner G (2025) Case Report: Treating one autoimmune disease induces another: the paradox of TNF-alpha inhibitor therapies. Front. Immunol. 16:1640455. doi: 10.3389/fimmu.2025.1640455
Received: 03 June 2025; Accepted: 22 September 2025;
Published: 10 October 2025.
Edited by:
Vlad Pădureanu, University of Medicine and Pharmacy of Craiova, RomaniaReviewed by:
Joseph Larkin, University of Florida, United StatesYoichi Nakayama, Kyoto University, Japan
Copyright © 2025 Gehrke, Thurau and Wildner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephan Thurau, c3RlcGhhbi50aHVyYXVAbWVkLnVuaS1tdWVuY2hlbi5kZQ==; Gerhild Wildner, Z2VyaGlsZC53aWxkbmVyQG1lZC51bmktbXVlbmNoZW4uZGU=
 Miranda Gehrke
Miranda Gehrke Stephan Thurau*
Stephan Thurau* Gerhild Wildner
Gerhild Wildner