- 1Department of Preventive Medicine, School of Public Health and Management, Wenzhou Medical University, Wenzhou, Zhejiang, China
- 2State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, China-Singapore Belt and Road Joint Laboratory on Infection Research and Drug Development, National Medical Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
- 3Yuhang Institute for Collaborative Innovation and Translational Research in Life Sciences and Technology, Hangzhou, Zhejiang, China
- 4Department of Intensive Care Unit, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
- 5Department of Anesthesiology, Affiliated Hospital of Nantong University, Nantong, Jiangsu, China
- 6Lishui Key Laboratory of Brain Health and Severe Brain Disorders, Department of Rehabilitation, Lishui Second People’s Hospital, Lishui, Zhejiang, China
Depression, a highly prevalent and relapsing mental disorder, exacts profound personal and socioeconomic tolls globally, warranting urgent scientific and clinical attention. Emerging evidence from both preclinical models and human clinical investigations has established the microbiota-gut-brain axis (MGBA) as a critical determinant in depression pathogenesis. This intricate bidirectional network integrates gut microbiota with central nervous system function, influencing mental health through mechanisms previously underrecognized. This review systematically synthesizes gut microbiota alterations associated with depression and their impacts on neuroendocrine, neuroimmune, and metabolic pathways. Advanced therapeutic strategies targeting the MGBA are discussed, including probiotics, fecal microbiota transplantation, and artificial intelligence-enabled microbiome interventions for depression management. While challenges in standardization, mechanistic understanding, efficacy and safety remain, MGBA-centered approaches offer a promising shift toward microbiota-based diagnostics and personalized treatments for depression.
Introduction
Depression, particularly major depressive disorder (MDD), is a debilitating mental health condition marked by persistent low mood, loss of interest in activities, cognitive impairments, and suicidal tendencies, significantly impacting patients’ social functioning and quality of life (1–3). In 2008, the World Health Organization (WHO) identified MDD as the third leading cause of global disease burden (4). The prevalence of MDD continues to rise, with approximately 280 million individuals worldwide affected each year, including 700,000 deaths by suicide (5). Notably, the COVID-19 pandemic further exacerbated this issue, with increased social isolation, economic stress, and health-related anxieties contributing to a significant rise in depression rates, placing additional strain on both societal and familial structures (6, 7). Despite the widespread use of first-line antidepressants such as selective serotonin reuptake inhibitors (e.g., fluoxetine, sertraline) and serotonin-norepinephrine reuptake inhibitors (e.g., venlafaxine, duloxetine), these medications often show delayed efficacy (typically 4–6 weeks) and may cause adverse side effects including sexual dysfunction, weight gain, and gastrointestinal disturbances. Evidence indicates that over one-third of patients have a poor or minimal response to these treatments (8), highlighting the incomplete understanding of depression’s underlying pathophysiology and the urgent need for alternative therapeutic targets.
The therapeutic challenge in treating MDD has sparked growing interest in the microbiota-gut-brain axis (MGBA), a potential mechanism for both understanding the disorder and exploring new interventions. The MGBA concept evolved over decades of research, from early discoveries of gut-brain hormonal interactions to the current view of the gut microbiome as a key regulator of neuropsychiatric health (9, 10). The human gut microbiota, made up of trillions of microorganisms (11), acts as a “second brain,” influencing the central nervous system (CNS) through multiple pathway (12). Dysbiosis of gut microbiota refers to significant changes in the quantity and function of gut microbiota, which can significantly affect host physiology through MGBA, contributing to disorders like Parkinson’s disease, autism, bipolar disorder, and schizophrenia (13–15). In MDD, both clinical and preclinical studies show microbial composition changes during depressive states (16–18). Fecal microbiota transplantation (FMT) from depressed individuals can induce depression-like behaviors in animals (19–22), while certain probiotics have shown promise in alleviating depressive symptoms (23–26). This growing body of evidence emphasizes the potential of microbiota-based interventions in managing MDD.
As research into the gut microbiota’s role in depression expands, new potential targets and mechanisms are emerging. Current studies have identified three key pathways through which gut microbiota influence depression via the MGBA: immune regulation (e.g., cytokine release) (27–29), endocrine modulation (e.g., hypothalamic-pituitary-adrenal (HPA) axis activity) (30, 31), and neural signaling (e.g., vagus nerve communication and neurotransmitter regulation) (32, 33). This evolving MGBA framework highlights the diagnostic and therapeutic potential of gut microbiota in managing depression, positioning microbiota-targeted interventions as a promising avenue for antidepressant development. This review explores the contributions of MGBA to MDD pathogenesis, focusing on key regulatory nodes in the immuno-neuro-endocrine network, and also discusses innovative treatment strategies that utilize gut microbiota modulation, showcasing their potential for next-generation depression therapies.
Gut dysbiosis and depression
Gut microbiota has emerged as a crucial factor in the development and progression of depression, with increasing evidence supporting its influence on mental health through various mechanisms. Research in both preclinical and clinical studies has shown that gut microbiota can modulate brain function and behavior through MGBA. In preclinical studies, the gut microbiota has been found to affect neurotransmitter production, inflammation, and stress response systems, all of which are involved in depression. Animal models have demonstrated that altering the gut microbiota can lead to changes in mood and behavior, mimicking symptoms of depression or showing improvements with specific microbiota compositions. Clinical studies have further reinforced these findings, showing that individuals with depression often have an altered gut microbiome compared to healthy controls. Additionally, Moreover, interventions aimed at modifying the gut microbiota—such as probiotics, prebiotics, or FMT—have shown promising effects in alleviating depressive symptoms in some patients. These studies suggest that gut microbiota may play a significant role in depression and could offer new therapeutic avenues for managing the condition.
Preclinical studies
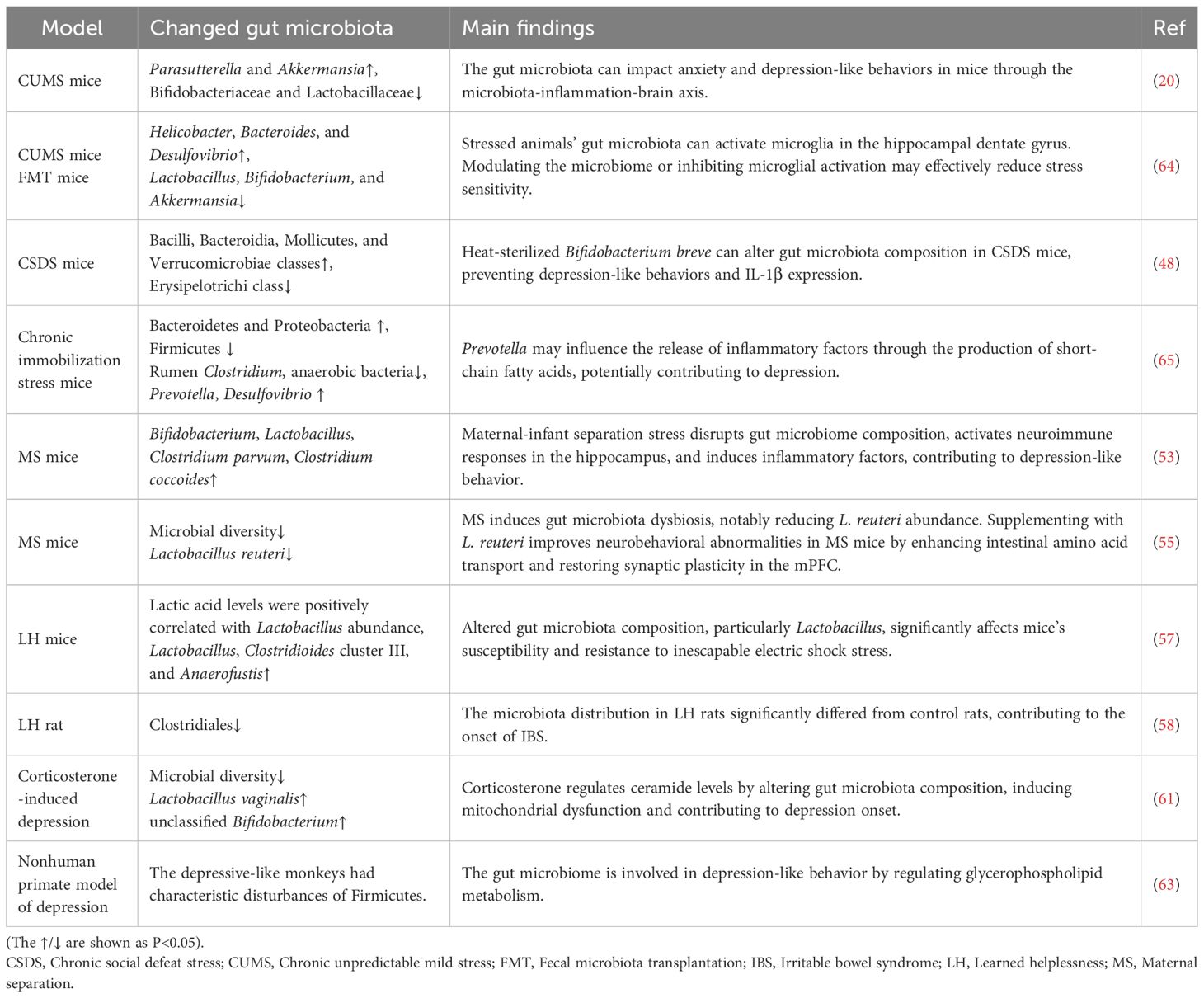
The pivotal role of gut microbiota in the pathophysiology of psychiatric disorders, such as MDD, has become increasingly recognized (34–37). However, the precise mechanisms through which the microbiota influences mental health remain unclear. As outlined in in Table 1, a key focus of current research involves investigating the dynamic interplay between changes in microbial communities and psychiatric phenotypes through animal models. This approach has become central in the field, seeking to elucidate the underlying processes connecting gut microbiota to mental health disorders.
A widely employed animal model in this research is the chronic unpredictable mild stress (CUMS) model, which induces stress-related behaviors by exposing animals to a series of unpredictable, mild stressors, mimicking the symptoms of depression (35, 38–41). In mice subjected to CUMS, significant alterations in the gut microbiota occur, including an increase in Proteobacteria and Verrucomicrobia, coupled with a decrease in beneficial bacteria such as Bifidobacteriaceae and Lactobacillaceae. Notably, FMT from CUMS mice to healthy recipients successfully replicates depressive phenotypes (20), establishing a direct link between gut dysbiosis and depression. Furthermore, these depressive-like behaviors can be reversed by probiotic interventions, which operate through three synergistic mechanisms: (1) enhancing serotoninergic neurotransmission by promoting 5-HT synthesis and TPH expression, (2) suppressing neuroinflammation via IDO inhibition, and (3) normalizing hyperactivity of the HPA axis (42). Similarly, microbial metabolites such as short-chain fatty acids (SCFAs), including butyrate and propionate, have proven effective in alleviating CUMS-induced behavioral deficits, reinforcing the therapeutic potential of microbiota-targeted strategies (43–45).
The chronic social defeat stress (CSDS) model is another frequently used animal model to study depression, specifically targeting psychological and social stressors that contribute to depressive behaviors. In this model, mice are subjected to repeated episodes of social subjugation by a dominant conspecific, inducing severe psychological stress that leads to persistent behavioral changes. These behaviors typically include social withdrawal and anhedonia, both defining features of depression (46). Recent research has highlighted the connection between CSDS-induced depression-like behaviors and alterations in the gut microbiota, offering valuable insights into the underlying mechanisms. Mice exposed to CSDS display significant gut microbiota dysbiosis, characterized by reduced alpha diversity and a notable decline in Lactobacillus abundance (47). Additionally, studies by Aika Kosuge et al. have identified shifts in the abundance of bacterial classes, including an increase in Bacilli, Bacteroidia, Mollicutes, and Verrucomicrobiae, which are linked to metabolic and immune-modulatory functions. These microbial changes may influence the stress response and contribute to depressive symptoms. Conversely, the reduction in Erysipelotrichi, a class associated with inflammation and metabolic disturbances, further underscores the role of gut microbiota in modulating depression-like behaviors (48). Therapeutic interventions targeting specific bacterial strains have demonstrated efficacy in alleviating depression-like behaviors in CSDS mice, suggesting that microbiota modulation could serve as a promising therapeutic avenue for depression treatment (49, 50).
The maternal separation (MS) model, which involves early deprivation of pup-mother interactions, induces core depressive phenotypes such as anhedonia, reduced exploratory behavior, and diminished interest during developmental maturation. This model is widely used to simulate the long-term impact of early-life stress on neurodevelopment and subsequent behavioral outcomes (51, 52). Emerging studies have shown that MS results in significant changes in gut microbiota composition in mice (53, 54), which appear to mediate the neuropsychiatric effects associated with early-life stress. Notably, oral administration of multi-strain probiotic formulations has demonstrated significant improvements in anxiety and depressive symptoms in MS-exposed mice, suggesting that gut microbiota dysbiosis plays a pivotal role in the pathophysiology of MS-induced neuropsychiatric symptoms. This could be mediated through gut-brain axis and neuroimmune signaling pathways (55, 56). The growing body of evidence emphasizes the profound impact of early-life stressors on both the gut microbiome and brain, opening new possibilities for therapeutic interventions aimed at modulating the gut microbiota to alleviate mood and anxiety disorders.
The learned helplessness (LH) model is a widely recognized animal model for studying depression. It consists of two phases: initially, animals are subjected to inescapable stress, followed by a re-exposure to escapable stress, during which they demonstrate a marked reduction in escape responses. Studies show that LH mice experience a significant decrease in gut microbial diversity, with a notable reduction in beneficial bacteria such as Lactobacillaceae (57) and Clostridiales incertae sedis (58). This disruption in gut microbiota is thought to contribute to the development of depression-like behaviors. Interestingly, dietary supplementation with prebiotics has been shown to increase Lactobacillus abundance, helping to alleviate these behaviors in LH mice (59). Additionally, compared to LH-resilient rats, LH-susceptible rats exhibit a notable increase in certain bacterial genera in the gut, including Asaccharobacter, Eisenbergiella, and Klebsiella (60). Alongside the LH model, other methods such as drug treatments and surgical interventions are also used to induce depression in rodents. For example, corticosterone (CORT) is commonly administered to mice to induce depression, which results in a decrease in microbial diversity and an increase in Lactobacillus vaginalis and unclassified Bifidobacterium species (61). A study by Jiang et al. underscores the importance of gut microbiota in reducing anxiety and depression-like behaviors in post-stroke mice (62).
In addition to rodents, non-human primates are also used to establish depression models due to their close similarities to humans in terms of physiology, cognitive abilities, neuroanatomy, social complexity, reproduction, and development. In female cynomolgus monkeys, which naturally display depression-like behaviors, characteristic dysbiosis of the Firmicutes phylum has been observed (63). When healthy male cynomolgus monkeys of varying ages are exposed to CUMS, significant differences in microbial composition and gut-brain metabolic characteristics emerge between adolescent and adult monkeys. Dysbiosis of Clostridium and Haemophilus is observed only in adolescent depressed monkeys, while it is absent in adults. These findings strongly suggest that gut microbial dysbiosis is not just a consequence of depression but also plays a critical role in its development. The connection between gut microbiota and mood disorders underscores the potential of microbiome-based therapeutic strategies aimed at modulating the gut-brain axis to alleviate depression-like behaviors. Overall, these cross-species studies emphasize the pivotal role of the gut microbiome in influencing neuropsychiatric outcomes, making it a promising target for future therapeutic approaches.
Clinical studies
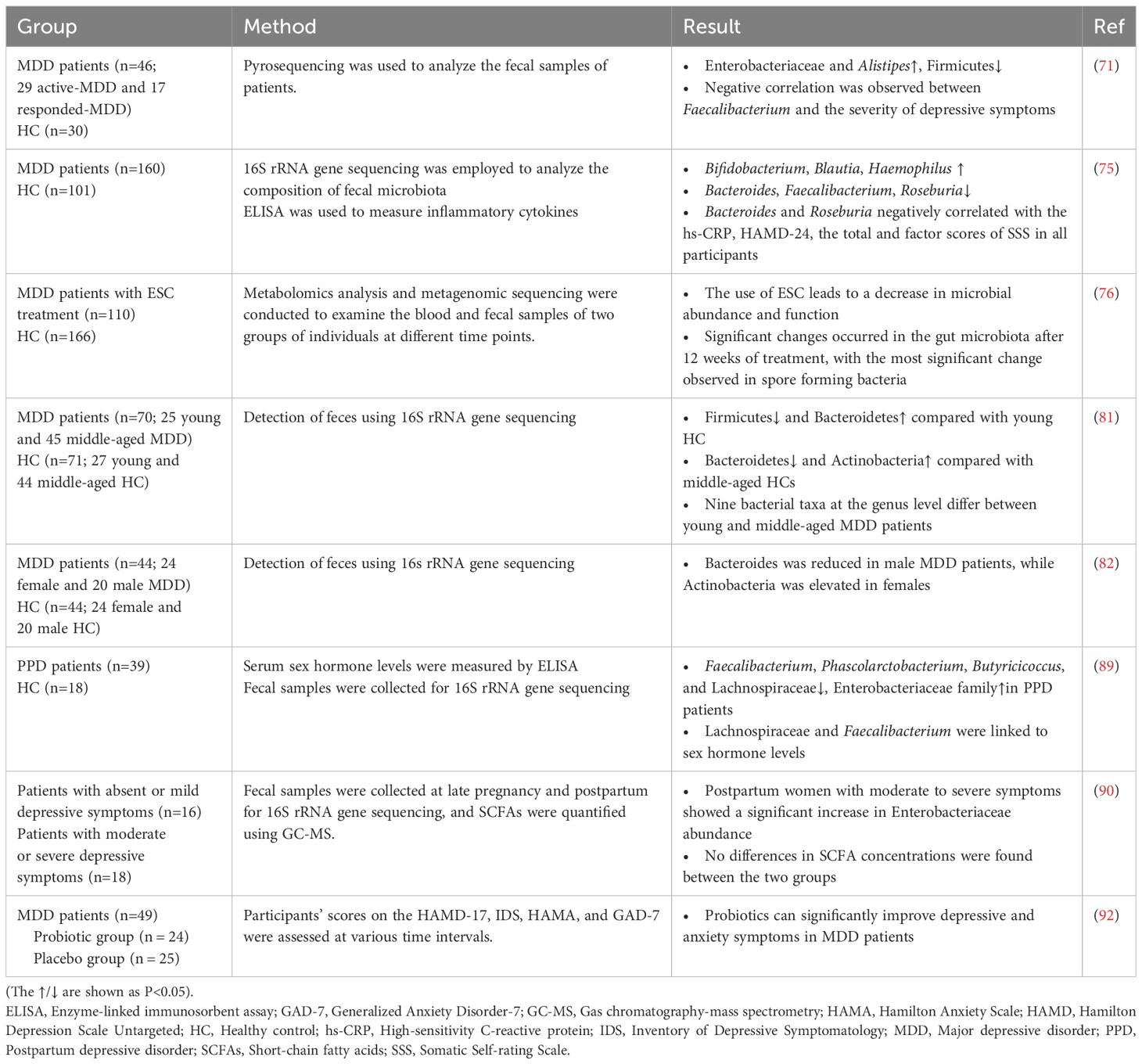
As shown in Table 2, numerous clinical studies have highlighted significant differences in the gut microbiota composition between individuals with MDD and healthy controls, emphasizing the critical role of the MGBA in the pathophysiology of depression (28, 66–69). In healthy individuals, the gut microbiome is predominantly composed of Firmicutes (79%), followed by Bacteroidetes (17%), Actinobacteria (3%), Proteobacteria (0.9%), and Verrucomicrobia (0.1%) (70). However, notable alterations in microbiota have been reported in MDD patients, with increased abundance of Enterobacteriaceae and Alistipes, alongside a reduction in Faecalibacterium levels, which correlate with the severity of depressive symptoms (71). Similarly, female MDD patients exhibit an enrichment of Bacteroidetes, Proteobacteria, and Fusobacteria, while healthy controls show higher levels of Firmicutes and Actinobacteria (72). Subsequent meta-analyses have confirmed these findings, showing consistently reduced levels of Coprococcus and Faecalibacterium in MDD patients (73). Furthermore, probiotic interventions have been shown to significantly alleviate depressive symptoms, supporting the therapeutic potential of microbiota modulation in treating depression (74). Importantly, changes in the gut microbiota of MDD patients are considered closely related to their somatic symptoms (75). Notably, antidepressant treatment can induce dynamic changes in the gut microbiota. In a longitudinal study by Wang et al., untargeted metabolomics and metagenomic sequencing were performed on blood and fecal samples collected from 110 MDD patients at three timepoints (baseline, week 2, and week 12) during escitalopram (ESC) treatment (76). The findings showed that while the gut microbial composition did not change significantly by week 2, notable alterations emerged by week 12, with the most pronounced changes observed in spore-forming bacteria.
In addition to shifts in microbial composition, the dynamic evolution of the gut microbiota throughout the lifespan further complicates the relationship between microbiota and depression (77–80). Emerging evidence suggests that MDD patients across various age groups may display distinct microbial profiles, highlighting the importance of age-specific approaches in microbiota-based therapies (81). Additionally, differences in gut microbiota composition have been observed between male and female MDD patients. Notably, the level of Bacteroides was significantly reduced only in male MDD patients, while the level of Actinobacteria was significantly elevated only in female MDD patients (82). Alterations in the gut microbiota have also been linked to specific physiological periods, such as pregnancy, which can further impact the onset and progression of depression (83–85). Postpartum depression (PPD), a common subtype of MDD affecting around 10%-15% of women (86), not only negatively affects maternal health and mother-infant bonding but also carries long-term implications for child development (87, 88). Recent studies have revealed significant differences in microbial diversity and composition between PPD patients and healthy postpartum women. For instance, Zhou et al. found partial differences in microbial diversity between PPD patients and controls, with certain bacterial taxa, such as Lachnospiraceae and Faecalibacterium, correlating with fluctuations in sex hormone levels (89). Additionally, an increased abundance of Enterobacteriaceae has been observed in postpartum women experiencing severe depressive symptoms (90).
More strikingly, several clinical trials have validated the effectiveness of probiotic supplementation in alleviating depressive symptoms by modulating the gut microbiota (29, 91). One randomized controlled trial (RCT) reported significant reductions in depression scale scores following probiotic supplementation (92). Additionally, numerous studies have confirmed that specific probiotic strains can significantly improve maternal mood, further supporting the therapeutic benefits of microbiota-based interventions (93). Furthermore, studies have shown that supplementation with the right probiotics can effectively enhance maternal mood (93). Meanwhile, probiotics have been found to improve verbal episodic memory and increase serum levels of brain-derived neurotrophic factor (BDNF) in MDD patients (25). In parallel, microbiota-based biomarkers are increasingly being utilized to differentiate between depressed individuals and healthy controls. Recent advancements in genomic sequencing, such as single-nucleotide-resolved amplicon sequence variants (ASVs) of human gut microbiomes, have allowed the identification of depression phenotypes in healthy cohorts, emphasizing the value of gut microbiota profiling in clinical diagnostics (94). These findings not only highlight the clinical significance of microbiota dysbiosis as a potential biomarker for depression but also underscore the promising therapeutic potential of modulating the microbiota-gut-brain axis in psychiatric treatments.
MGBA in depression pathogenesis
The relationship between gut microbiota and depression has garnered increasing attention in recent years, as research efforts aim to unravel the complex mechanisms that link these two entities and identify potential therapeutic avenues. The gut-brain axis, a bidirectional communication network between the gastrointestinal tract and the central nervous system, is integral to maintaining physiological homeostasis. Gut microbiota plays a central role in this axis, influencing brain function through multiple signaling pathways. As such, the MGBA hypothesis has gained prominence as a potential framework for understanding the pathophysiology of depression. Current research suggests that gut microbiota influence the onset and progression of depressive disorders through three primary mechanisms: neural signaling, endocrine modulation, and immune regulation (Figure 1). These pathways contribute to altering brain function and mood regulation, providing new insights into the therapeutic potential of targeting the gut microbiota in treating depression.
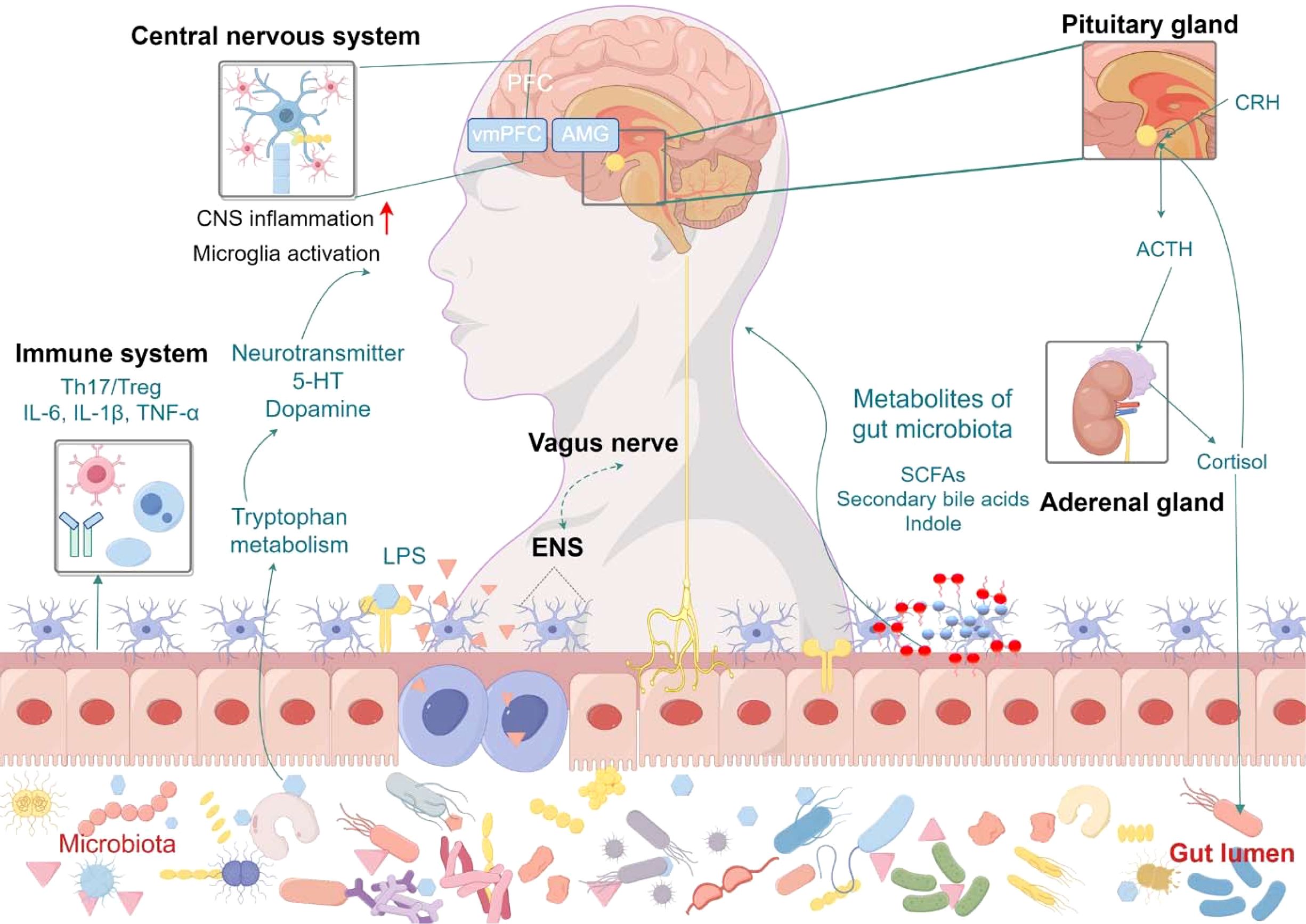
Figure 1. Mechanisms of gut microbiota in depression. The gut microbiota influences depression through three interconnected pathways: neural signaling, endocrine regulation, and immune modulation. Vagus nerve-mediated bidirectional communication allows gut-derived neurotransmitters (e.g., serotonin, GABA) to directly affect central nervous system activity, while activation of the hypothalamic-pituitary-adrenal (HPA) axis by microbial metabolites (e.g., short-chain fatty acids, bile acids) induces cortisol release, promoting neuroinflammation and synaptic dysfunction in brain regions like the hippocampus. Intestinal barrier dysfunction permits bacterial components and proinflammatory cytokines (e.g., IL-6, TNF-α) to translocate systemically, activating microglia and disrupting Th17/Treg cell balance in both gut and brain, thereby exacerbating depressive symptomatology. ACTH, Adrenocorticotropic Hormone; AMG, Amygdala; CNS, Central Nervous System; CRH, Corticotropin Releasing Hormone; ENS, Enteric Nervous System; 5-HT, 5-hydroxytryptamine; LPS, Lipopolysaccharide; SCFAs, Short-chain fatty acids; vmPFC, Ventromedial Prefrontal Cortex.
Neural signaling
In the MGBA, the neural signaling network plays a crucial role in facilitating rapid communication between the gut and the brain. This network integrates the enteric nervous system (ENS), the vagus nerve, and spinal nerves, each of which contributes to the regulation of gut-brain signaling pathways and the pathophysiology of neuropsychiatric disorders, including depression (95). The ENS, an intricate and expansive neural network embedded within the gastrointestinal tract, not only regulates the gut environment but also shares neurotransmitters with the CNS. Importantly, the ENS has been implicated in depression-related alterations, contributing to the development of mood disorders (96). In addition to its regulatory role in intestinal functions, the ENS modulates gut microbial composition, influences microbiota-derived metabolites, adjusts neurotransmitter levels, and participates in immune signaling (10, 97, 98). Moreover, the ENS is subject to modulation by the gut microbiota, with its development, function, and renewal being heavily influenced by microbial interactions (99). Pathological disruptions in ENS function can exacerbate the course of depression by disturbing key processes such as gut secretion, immune responses, and intestinal barrier integrity (100).
The vagus nerve is another critical component of the MGBA, especially in the context of depression. It represents one of the most direct pathways for microbial signals to reach the brain (101, 102). Notably, early work by Bravo et al. (103) demonstrated that oral administration of Lactobacillus rhamnosus alleviated stress-induced depressive-like behaviors in mice, with this effect being blocked following vagotomy, underscoring the vagus nerve’s central role in microbial-mediated signaling (103). Subsequent studies have provided further insights into the vagus nerve’s involvement in depression. Zhang’s group found that lipopolysaccharide (LPS)-induced depressive-like behaviors in mice were entirely abolished following subdiaphragmatic vagotomy (SDV) (104). This work revealed that LPS administration altered gut microbiota composition, with a notable increase in the abundance of Firmicutes and Bacteroidetes, changes that were not observed in SDV mice, suggesting the critical role of the vagus nerve in mediating microbial-induced alterations in the gut. Moreover, the same research demonstrated that continuous administration of Lactobacillus intestinalis and Lactobacillus reuteri for 14 days induced behavioral despair, accompanied by elevated plasma IL-6 levels and reduced prefrontal cortex synaptic protein expression, effects which were effectively blocked by SDV (105). In a RCT, Mörkl et al. (106) observed significant improvements in vagal nerve function following probiotic treatment in patients with MDD, which correlated with increased abundances of Christensenellales and Akkermansia muciniphila (106). Together, these studies emphasize the pivotal role of the vagus nerve in mediating the gut-brain communication in depression.
Neurotransmitters serve as fundamental mediators of neural communication, and their dysregulation is a key factor in the pathogenesis of depression. Most contemporary antidepressant therapies target the modulation of synaptic neurotransmitter concentrations (107). Among these, serotonin (5-HT) deficiency is strongly linked to mood disorders (108), with gut microbiota exerting a significant influence on 5-HT levels, and gut microbiota can influence emotional states by regulating 5-HT levels. Selective serotonin reuptake inhibitors (SSRIs), which act on this system, have become widely used for treating mood disorders (109, 110). Notably, approximately 95% of the body’s 5-HT is synthesized in the gut (111), highlighting the critical role of the intestinal microbiota in regulating emotional states by modulating 5-HT production. In seminal work by William et al., germ-free (GF) mice exhibited a 2.8-fold reduction in 5-HT levels compared to conventionally raised controls, suggesting the essential role of the microbiota in regulating serotonin levels (112). Moreover, Zhou et al. demonstrated that FMT from healthy adolescent volunteers significantly elevated 5-HT concentrations in the brain and colon of adolescent mice subjected to chronic restraint stress (CRS), indicating that the microbiota directly influences CNS neurotransmitter levels (113). Several studies have also demonstrated the antidepressant effects of probiotics such as Bifidobacterium (114, 115) and Lactobacillus (116) strains through the modulation of 5-HT levels, offering potential therapeutic avenues for mood disorders. Additionally, oral administration of 5-HT not only alleviated depression-like behaviors in mice but also reversed depression-induced alterations in SCFAs concentrations and brain-derived neurotrophic factor (BDNF) levels, while restoring gut microbiota balance (117).
Beyond serotonin, the gut microbiota also influences the synthesis of other neurotransmitters such as gamma-aminobutyric acid (GABA), dopamine, and acetylcholine, all of which are integral to emotional regulation. The genus Bacteroides, for instance, encodes glutamate decarboxylase (GAD), a key enzyme involved in GABA synthesis (118). Both Bifidobacterium and Lactobacillus strains have also been shown to synthesize GABA (119), with the administration of GABA-producing strains leading to depression-like behaviors in animal models (120). Furthermore, research suggests that chronic stress can reduce the abundance of urease-positive bacteria in the gut, leading to lower peripheral ammonia levels. This disruption in ammonia homeostasis decreases glutamine synthesis in astrocytes, which in turn promotes GABAergic dysfunction (121). Furthermore, the gut microbiota significantly influences dopamine metabolism, a neurotransmitter central to reward processing and mood regulation (122). For instance, Enterococcus faecalis has been shown to alleviate depressive symptoms in mice through dopaminergic pathways (123). In a randomized, double-blind, placebo-controlled clinical trial, treatment with Bifidobacterium breve BB05 reduced fecal levels of acetylcholine (ACh), epinephrine (Epi), and norepinephrine (NE), which was accompanied by improvements in both anxiety and depressive symptoms (124). Thus, the gut microbiota profoundly impacts the neural signaling pathways within MGBA, modulating neurotransmitter synthesis and influencing brain-gut communication, ultimately affecting the pathophysiology of depression.
Endocrine modulation
The HPA axis is a central neuroendocrine system (125) that plays a crucial role in regulating the body’s response to stress, and it is intimately linked with the MGBA through bidirectional interactions (126, 127). Dysregulation of the HPA axis is frequently observed in individuals with depression, characterized by elevated secretion of corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH), increased plasma cortisol concentrations, and a disrupted negative feedback mechanism (128). These alterations in HPA axis function are believed to contribute to gastrointestinal inflammation, compromised gut barrier integrity, and neuronal damage (129–132), ultimately resulting in shifts in the gut microbiome composition. Conversely, gut microbiota can exert significant influence on HPA axis function. Dysbiosis has been shown to facilitate the translocation of pro-inflammatory cytokines such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) across the blood-brain barrier, which in turn activates the HPA axis, intensifying the stress response (30). Notably, the GABA signaling pathway plays a critical role in the negative feedback regulation of the HPA axis. It modulates the axis’s responsiveness to stress, potentially alleviating HPA axis dysfunction (133). Numerous studies have demonstrated that the gut microbiota can influence GABA metabolism, further emphasizing its role in the regulation of HPA axis activity (118, 134). For instance, Li et al. reported that Bifidobacterium breve 207–1 modulates GABA and related hormones in the context of the gut-brain axis, suggesting a potential mechanistic link between microbiota and HPA axis regulation (135).
In addition to GABA signaling, the gut microbiota can also affect enteroendocrine cells via microbial metabolites like SCFAs and secondary bile acids. SCFAs, which are produced through bacterial fermentation of partially indigestible polysaccharides such as dietary fiber and resistant starch, are among the most crucial microbial metabolites (136). The primary SCFAs—acetate, propionate, and butyrate—constitute over 95% of total SCFA production, with other metabolites, such as lactic acid, present in smaller amounts (137). These SCFAs exert a variety of physiological effects within the gut, including regulation of redox balance, maintenance of intestinal pH homeostasis, promotion of hormone secretion, and involvement in epigenetic modifications (138–141). Recent research has spotlighted the significant role of SCFAs in neuropsychiatric disorders, particularly depression. Studies have demonstrated that chronic stress leads to a marked reduction in SCFA levels—such as acetate, propionate, and valerate—in animal models. Moreover, a positive correlation has been observed between the abundance of Allobaculum and acetic acid levels in these models (142). Similarly, in post-stroke depression models, decreased levels of butyrate, acetate, and valerate are linked with alterations in lipid metabolism (143). Notably, the depletion of butyrate, a metabolite known for its anti-inflammatory effects, has been directly associated with the manifestation of depressive symptoms (144). Additionally, research on rats exposed to blue light during sleep revealed increased lactic acid levels in the cerebrospinal fluid and lateral habenula, correlating with the onset of depressive behaviors. Importantly, inhibiting lactic acid production in these rats alleviated these depressive-like symptoms (145). SCFAs may contribute to the pathophysiology of depression through several mechanisms, including immunomodulation of Th17/Treg cells (146), activation of the TLR4/NF-κB inflammatory pathway (147), regulation of acetyl-CoA synthetase short-chain family member 2 (ACSS2) (148), and histone epigenetic regulation (149). Emerging evidence suggests that SCFAs play a crucial role in maintaining blood-brain barrier (BBB) integrity (150, 151), while BBB impairment has been implicated in depression pathogenesis (152, 153). These findings underscore the pivotal role of SCFAs in modulating the MGBA, suggesting that strategies aimed at modulating SCFA production or signaling could hold therapeutic potential for treating depression.
Furthermore, the gut microbiota significantly influences bile acid metabolism, with specific bacteria, such as Clostridium and Bacteroides, being capable of synthesizing secondary bile acids (96). Clinical and animal studies have revealed a compelling relationship between bile acids and depressive disorders. In a comparison of serum and fecal samples from individuals with MDD and healthy controls, researchers found significantly elevated levels of 2,3-deoxycholic acid in MDD patients, while taurolithocholic acid (TLCA), glycolithocholic acid (GLCA), and 3-sulfolithocholic acid were found at lower concentrations (154). Notably, a positive correlation was observed between the abundance of Verrucomicrobium and the levels of TLCA and GLCA, further illustrating the microbiota-bile acid axis in depression. Similarly, increased dissociation of conjugated bile acids and enhanced biosynthesis of secondary bile acids have been observed in CUMS mice, with an increased abundance of Ruminococacae promoting the biosynthesis of deoxycholic acid (DCA) (155). These findings collectively highlight the intricate role of bile acid metabolism in the pathophysiology of depression and suggest that modulation of the bile acid microbiota could represent a novel avenue for therapeutic intervention.
Gut microbiota plays a crucial role in the pathogenesis of depression through the metabolism of tryptophan into indole and its derivatives. Gut microbes, equipped with a variety of catalytic enzymes, transform tryptophan into several indole metabolites, including indole-3-propionic acid (IPA), indole-3-acetaldehyde (IAld), indole-3-acetic acid (IAA), and indole-3-lactic acid (ILA) (156). A growing body of evidence has linked these indole metabolites to depression-related behavioral changes. For example, Brydges et al. found that serum concentrations of indoxyl sulfate (IS), an indole metabolite, are positively correlated with the severity of depressive and anxiety symptoms (157). Similarly, a prospective observational study revealed significantly elevated urinary IS concentrations in women with recurrent depressive episodes (158). Other indole derivatives have also been implicated in depression’s pathophysiology. In one study, Qian et al. showed that hippocampal ILA levels were significantly reduced in a mouse model of depression. Supplementation with Bifidobacteria not only increased ILA concentrations but also alleviated neuroinflammation and improved depression-related phenotypes by activating the aryl hydrocarbon receptor (AhR) signaling pathway (159). Furthermore, other studies have shown that reduced IAld levels are associated with worsened depressive symptoms in obese patients (160). In animal models, indole-3-carboxaldehyde (I3C) was found to mediate depressive behaviors induced by chronic restraint stress. Both I3C supplementation and administration of Lactobacillus reuteri, an I3C-producing strain, were effective in ameliorating these behavioral deficits (161).
Immune regulation
Recent studies have increasingly highlighted the connection between immune system activation and the onset of depression, suggesting that inflammation plays a crucial role in its pathophysiology (159, 162–164). Key pro-inflammatory cytokines implicated in depression include IL-6, TNF-α, IL-1β, IL-10, IL-1ra, transforming growth factor-β, and C-reactive protein (CRP) (165, 166). The regulation of these cytokines occurs through a complex immune signaling network that integrates both innate and adaptive immune responses in the gut, brain, and systemic circulation, thereby facilitating the interaction between immune functions, gut microbiota, and depression (167–169).
One of the central mechanisms through which inflammation influences depression is altered intestinal permeability, commonly referred to as leaky gut syndrome (LGS) (170). LGS results from dysbiosis of the gut microbiota, epithelial damage, and compromised intestinal barrier function, which collectively contribute to neuroinflammation—a critical driver in the pathogenesis of depression (171). Furthermore, the compromised gut barrier allows for the translocation of LPS-producing Gram-negative bacteria, triggering immune responses that exacerbate depression (172). The role of LPS in MDD has been well-established (173), with LPS-induced inflammation models serving as valuable tools for investigating the mechanisms underlying MDD (174–176). Targeting the regulation of gut microbiota has emerged as a promising strategy for mitigating LPS-induced depressive symptoms. For example, Limosilactobacillus fermentum L18 has been shown to restore intestinal epithelial permeability by enhancing the expression of tight junction proteins such as occludin and E-cadherin, which can improve gut-barrier integrity (177). Similarly, Ramalho et al. demonstrated that administering Lactococcus lactis in LPS-induced depressive mice improved related behaviors by modulating oxidative stress and pro-inflammatory cytokine levels in the hippocampus (178).
The concept of the gut microbiota-immune-glia axis has also been introduced, offering a deeper understanding of the bidirectional communication between the gut microbiota and glial cells in the brain (169). Glial cells, including microglia (179, 180), astrocytes (181), oligodendrocytes (182), and ependymal cells (183), have been shown to play significant roles in the development of depression. The gut microbiota influences the activation and function of these glial cells through neural and chemical signaling pathways. In particular, microglia exhibit dynamic shifts between pro-inflammatory and anti-inflammatory states, and dysregulation of this process is thought to contribute to the neuroinflammation observed in depression (184, 185). For instance, rifaximin, a gut microbiota-targeted treatment, has been found to alleviate depressive behaviors in CUMS mice. This effect correlates with shifts in the gut microbiota, particularly the relative abundance of Ruminococcaceae and Lachnospiraceae, which subsequently influence brain microglia and peripheral cytokine levels (such as TNF-α, IL-1β, IL-10) (186). Similarly, minocycline has demonstrated antidepressant effects via its modulation of the gut microbiota-microglia regulatory pathway (187). Collectively, these findings suggest that targeting microglial activation through modulation of the gut-brain axis may represent a promising therapeutic strategy for depression (188, 189).
Immune cells, particularly macrophages, also play critical roles in the pathophysiology of depression. Macrophages are central to innate immune responses and activate adaptive immunity, including the differentiation of T lymphocytes into pro-inflammatory and anti-inflammatory subsets (190). Within the context of depression, the balance between Th17 and Treg cells—two distinct T cell subsets—has gained attention. Th17 and Treg cells, which are influenced by the gut microbiota (191, 192), have been implicated in regulating brain development, neuroinflammation, and the activation of glial cells such as microglia and astrocytes during periods of stress (184, 193, 194). Increasing evidence suggests that an imbalance between Th17 and Treg cells plays a role in depression (195–197). The dysregulated ratio of Th17 to Treg cells may contribute to the chronic inflammation observed in depressed individuals, and the restoration of this balance could have therapeutic benefits. Research by Westfall et al. revealed that metabolites from the gut microbiota can modulate the Th17/Treg ratio, alleviating stress-induced inflammatory responses and improving resilience against anxiety and depressive behaviors (146). Notably, this gut microbiota-mediated regulation of the Th17/Treg imbalance is also thought to influence the efficacy of antidepressant treatments, such as ketamine (198–200). Thus, modulating immune responses and restoring balance within the immune system and gut microbiota may provide valuable insights into novel approaches for treating depression.
Therapeutic potential of gut microbiota in depression treatment
MGBA axis plays a pivotal role in the regulation of neurological function and mental health, with growing evidence supporting its involvement in the pathophysiology and treatment of depression. Unlike the static nature of human genetics, the gut microbiota is a highly dynamic, evolving, and diverse ecosystem that is responsive to various external influences. This plasticity presents an opportunity for therapeutic intervention aimed at restoring balance within the microbiota to improve mental health outcomes. Recent research has highlighted the potential of modulating the gut microbiota through various approaches, such as dietary interventions, administration of probiotics, prebiotics, synbiotics, and postbiotics, and the use of FMT. These strategies can promote beneficial shifts in the gut microbiome, which, in turn, can positively influence the gut-brain signaling pathways involved in depression. By combining these microbiota-modulating strategies with traditional antidepressant therapies, there is significant promise in enhancing the efficacy of depression treatment through a more comprehensive approach that targets both the microbiome and the CNS. The emerging therapeutic role of the gut microbiota in depression underscores the importance of the gut-brain axis as a critical modulator of mental health and provides a promising avenue for the development of novel treatment strategies (Figure 2).
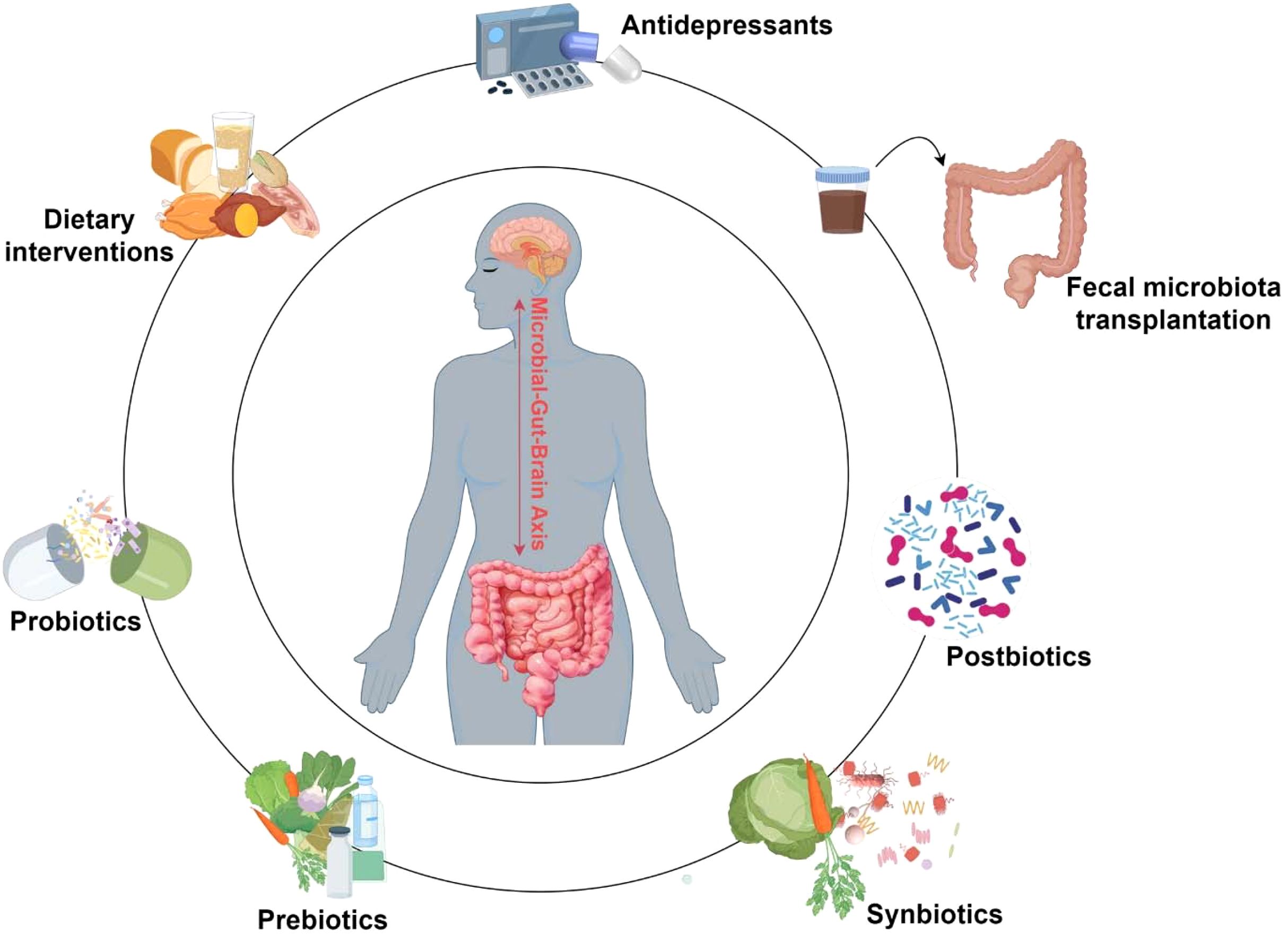
Figure 2. Applications of gut microbiota in depression treatment. This diagram summarizes emerging gut microbiota modulation strategies for depression treatment. Antidepressants, dietary interventions, and microbiota-based therapies (including probiotics, prebiotics, synbiotics, postbiotics, and fecal microbiota transplantation) can influence gut microbial composition and function, potentially alleviating depressive symptoms. These strategies—used individually or synergistically—have demonstrated substantial promise as adjunctive therapies for depression, offering novel pathways to enhance treatment efficacy.
Interaction between gut microbiota and antidepressants
Pharmacological intervention remains the cornerstone of treatment for depression, with oral antidepressants being the most prescribed modality. These pharmacological agents can have both direct and indirect effects on the gut microbiota, influencing its composition and function (201, 202). Antidepressants, such as selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), and selective serotonin and norepinephrine reuptake inhibitors (SNRIs), have been shown to alter microbial diversity and composition in various ways (203–207). For instance, SSRIs have been reported to increase the abundance of Bacteroides, while TCAs are associated with elevated levels of Clostridium in the gastrointestinal tract (208). Additionally, norepinephrine reuptake inhibitors, such as reboxetine (RBX), have been found to significantly reduce Lactobacillus populations and decrease the Firmicutes/Bacteroidetes ratio, both of which are key indicators of microbial dysbiosis (209). These alterations in gut microbiota may contribute to the gastrointestinal side effects often observed with antidepressant treatment, such as nausea, bloating, and altered bowel movements (210).
Conversely, the gut microbiota can influence the pharmacokinetics and pharmacodynamics of antidepressants. Gut bacteria may modulate the metabolism of these drugs through enzymatic mechanisms, which can impact both their therapeutic efficacy and toxicity (211, 212). For example, Klünemann et al. demonstrated that intestinal bacteria, such as Streptococcus salivarius and Escherichia coli IAI1, can enhance the bioaccumulation of the SNRI venlafaxine, potentially reducing its bioavailability and therapeutic effectiveness (213). This highlights a crucial interaction in which the microbiota could diminish the expected clinical response to antidepressants. Furthermore, treatment resistance in MDD has been linked to specific gut microbial profiles (214). Patients who exhibit resistance to standard antidepressant treatments often have elevated levels of Proteobacteria, Tenericutes, and Peptostreptococcaceae, while the presence of Thaumarchaeota, Yersinia, and Peptococcus may be indicative of treatment failure (215). In the context of novel therapeutic approaches, ketamine has emerged as a promising alternative due to its higher efficacy and lower side-effect profile. Recent studies suggest that the gut microbiota plays a role in potentiating ketamine’s antidepressant effects, with specific taxa, such as Actinobacteria and Coriobacteria, being identified as facilitators of its therapeutic action (216). Despite its potential, antidepressant treatment, especially in the context of MDD, often comes with a spectrum of adverse effects, including weight gain, gastrointestinal disturbances, hormonal imbalances, blurred vision, and an increased risk of cardiovascular events (210, 217–220). Probiotics, as an adjunctive treatment, have shown promise in alleviating some of these side effects, further emphasizing the potential of microbiota-targeted therapies in depression management (221).
It is important to note that the current body of research primarily focuses on single drug-microbe interactions. However, in clinical settings, many patients with MDD are prescribed a combination of medications, which may lead to more complex interactions with the gut microbiome. This polypharmacy approach could result in a more intricate regulatory network within the microbiota, potentially amplifying or mitigating the effects of individual drugs. Although the precise mechanisms underlying these interactions are still not fully understood, preliminary evidence supports the potential of microbiota-modulated antidepressant therapies. As our understanding of these complex interactions evolves, microbiome-based adjunctive treatments are expected to become an increasingly integral component of depression therapy.
Dietary interventions in gut microbiota-depression interactions
The composition and functionality of the gut microbiota are highly responsive to dietary influences, which, in turn, can significantly impact mental health outcomes, including depression (222). A growing body of evidence supports the notion that dietary interventions can play a crucial role in preventing and managing depression by modulating the gut microbiota. Research has consistently shown that healthy dietary patterns can enhance mental well-being, while poor dietary choices can exacerbate depressive symptoms (223). The Western diet, which is emblematic of modern eating habits, is typically characterized by high consumption of ultra-processed foods, red meat, refined sugars, and trans fats, with a concomitant deficiency in fruits, vegetables, and whole grains (224). This dietary pattern is associated with several detrimental effects on gut microbiota, including a reduction in microbial diversity, an increase in the abundance of pathogenic bacteria, and the promotion of systemic inflammation, all of which are linked to an elevated risk of depression (225, 226). Specifically, an imbalance in gut microbial populations caused by the Western diet may trigger neuroinflammatory pathways that contribute to mood disorders. In contrast, the Mediterranean diet has garnered significant attention for its protective effects against depression (227). This diet, rich in fruits, vegetables, whole grains, legumes, nuts, and olive oil, with moderate intake of fish and poultry, has been shown to reduce the incidence and severity of depressive symptoms (228). The Mediterranean diet promotes a favorable gut microbiota composition by decreasing the prevalence of pathogenic bacteria such as Escherichia coli and increasing the abundance of beneficial species, including Bifidobacterium. These microbial shifts lead to enhanced production of SCFAs, particularly butyrate, which has been identified as a key player in mediating the positive effects of this diet on gut and brain health (229). SCFAs have been shown to reduce systemic inflammation and neuroinflammation, thereby lowering the risk of depression (230, 231).
Additionally, diets from countries such as Norway and Japan, which are rich in omega-3 polyunsaturated fatty acids, whole grains, olive oil, soy products, and low-fat dairy, have been associated with a decreased risk of depression (232–234). The inclusion of ω-3 fatty acids, in particular, has been linked to beneficial changes in gut microbiota composition and a reduction in neuroinflammation, further supporting the idea that dietary patterns can modulate both gut health and mental well-being. The rising popularity of vegetarian diets has also spurred interest in their relationship with depression. Vegetarianism, which emphasizes plant-based foods such as vegetables, whole grains, legumes, nuts, seeds, and fruits (235), has been suggested in some studies to offer protective effects against depression (236). However, findings on the relationship between vegetarianism and depression are mixed, with some studies reporting a reduced risk of depressive symptoms, while others suggest no significant effects or even potential risks due to nutrient deficiencies (237, 238). These discrepancies underscore the need for more research to elucidate the mechanisms underlying the effects of vegetarian diets on mental health. The ketogenic diet, characterized by high fat, moderate protein, and very low carbohydrate intake, is another dietary intervention that has attracted attention for its potential antidepressant effects. Through its effects on neurotransmitter systems, particularly glutamate and GABA transmission, as well as its ability to modulate oxidative stress and inflammation, the ketogenic diet has shown promise in treating depression (239–241). However, the long-term sustainability of the ketogenic diet remains a concern due to its potential to cause nutritional imbalances, particularly with respect to carbohydrates and micronutrients, which could lead to metabolic disruptions if followed over extended periods.
Taken together, there is substantial evidence supporting the role of dietary patterns in shaping the gut microbiota and influencing mental health, particularly in relation to depression. While various diets, such as the Mediterranean, Norwegian, Japanese, and vegetarian diets, have demonstrated benefits in modulating depression risk, further studies are needed to clarify the underlying mechanisms and optimize dietary interventions as adjunctive treatments for mood disorders.
Microecologics in adjunctive depression therapy
Microecologics encompass viable bacteria, non-viable bacteria, and their metabolites, including probiotics, prebiotics, synbiotics, and postbiotics. These agents have garnered attention for their ability to modulate the gut microbiota, promote beneficial microbial growth, suppress pathogenic bacteria, and maintain intestinal homeostasis. Recent studies have highlighted the potential of microecologics in influencing gut-brain interactions to alleviate depressive symptoms, positioning them as a promising adjunctive therapeutic approach for depression.
Probiotics, a key group within microecologics, have gained considerable recognition for their therapeutic potential in treating various conditions, including depression. They exert their effects primarily through the MGBA, influencing brain function by reducing systemic inflammation, repairing the intestinal barrier, and mitigating neuroinflammation (29). Several studies have identified specific probiotic strains with antidepressant potential (67). For example, Lactobacillus and Bifidobacterium species are among the most used strains in clinical settings due to their documented efficacy in modulating mood disorders (114, 242–244). However, results remain inconsistent across studies, with some reporting no significant improvement in depressive symptoms following probiotic administration (245, 246). This variability is likely due to factors such as strain specificity, multi-strain synergism, and the overall dosage (247, 248). An umbrella meta-analysis revealed that probiotic efficacy in treating depression is contingent upon both the dosage and duration of the intervention. Significant alleviation of depressive symptoms was observed only when the probiotic dose exceeded 10×109 colony-forming units (CFUs) and the intervention lasted for more than 8 weeks (249). Additionally, certain microorganisms, particularly those difficult to culture, have demonstrated antidepressant effects. Akkermansia spp., for instance, has been shown to improve depressive behaviors in chronic stress models by restoring the balance of depression-related molecules such as corticosterone, dopamine, and BDNF (250). However, a gap remains between preclinical findings and clinical application, necessitating further research into the therapeutic potential of probiotics for depression.
Prebiotics, defined as selectively fermented compounds that confer health benefits, include substances such as fructooligosaccharides (FOS), galactooligosaccharides (GOS), inulin, and other soluble fibers (251). These compounds influence the gut microbiota indirectly by promoting the growth of beneficial bacteria. Evidence suggests that prebiotics may alleviate depressive symptoms by modulating the gut microbiota. For example, long-term administration of FOS and GOS has been shown to reduce depressive and anxious behaviors in animal models by enhancing acetate-producing bacteria (23). However, clinical evidence regarding the efficacy of prebiotics in depression is mixed. In a RCT involving 110 patients, a probiotic supplement significantly improved depressive symptoms compared to a placebo, while a prebiotic intervention had no substantial effect (252). This may be due to the indirect nature of prebiotics’ action, which works by modulating the gut microbiota rather than acting directly on the host. As a result, prebiotics are often used in conjunction with probiotics to optimize therapeutic outcomes. One study demonstrated that a complex probiotic formulation containing inulin was more effective than single supplements in improving psychological well-being and reducing inflammation in patients with depression (253).
The synergy between probiotics and prebiotics has led to the development of synbiotics, which combine both components to enhance therapeutic efficacy. Synbiotics offer potential benefits in treating depression by regulating gut microbiota composition and their metabolic products, reducing pro-inflammatory cytokines, and alleviating oxidative stress (254). A synbiotic formulation containing Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium species, and inulin has been shown to alleviate depressive symptoms in overweight or obese individuals (255). Similarly, a combination of probiotics (L. acidophilus T16, Bifidobacterium BIA-6, B. lactis BIA-27, B. longum BIA-4) and prebiotics improved depression severity and serum BDNF levels in patients undergoing hemodialysis (256). While synbiotics hold promise as a therapeutic intervention, their efficacy depends on identifying the optimal combinations of probiotics and prebiotics, highlighting the need for further research to refine formulations.
Postbiotics, comprising inactivated microbial cells and their bioactive metabolites, have emerged as another avenue for modulating gut-brain interactions. Postbiotics have been shown to alleviate depressive-like behaviors by influencing the gut-brain axis, offering a potential advantage over live probiotics in certain populations (257). For example, a postbiotic formulation derived from heat-inactivated Lactobacillus helveticus has been found to reduce anxiety and depressive-like symptoms in animal models while modulating dopamine and serotonin receptor expression in the brain (258). Given the potential risks associated with live bacterial administration, particularly in vulnerable populations such as critically ill patients, postbiotics may represent a safer and equally effective alternative for managing depression.
Collectively, microecologics, including probiotics, prebiotics, synbiotics, and postbiotics, represent a promising frontier in depression therapy. Although preclinical and early clinical studies show promise, further research is essential to fully understand their mechanisms of action and optimize their use for clinical applications in the treatment of depression.
FMT for depression treatment
FMT represents an innovative therapeutic approach aimed at restoring the gut microbiota by transferring functional microbiota from a healthy donor’s feces into the recipient’s gastrointestinal tract. This concept, though relatively modern in clinical application, has ancient roots dating back to 4th-century China, where “Huang Tang”, a fecal suspension, was utilized to treat conditions such as severe diarrhea and food poisoning (259). By the Ming Dynasty, this practice expanded to include a broader range of gastrointestinal disorders, including constipation and abdominal pain (260). The therapeutic potential of fecal matter was also recognized in 18th-century Europe, particularly by Metchnikoff, who noted substantial health improvements from incorporating fermented foods into his diet (261). Over time, FMT evolved primarily as a treatment for various intestinal disorders, especially infectious conditions (262, 263), and its scope has since broadened to encompass non-infectious diseases, such as Parkinson’s disease, melanoma, non-alcoholic fatty liver disease, and type 2 diabetes (264–267).
Recent studies have increasingly focused on the role of FMT in managing psychiatric conditions, particularly depression. Depression, often associated with dysbiosis or an imbalance in the gut microbiota, has been linked to altered gut-brain interactions. The therapeutic potential of FMT in depression is under investigation, with studies revealing a notable ability of fecal microbiota to influence depressive phenotypes (268). Notably, research has demonstrated that fecal microbiota from depressed individuals or animal models can induce depression related phenotypes in healthy recipients. For instance, in an animal model, Flinders Resistant Line (FRL) rats exhibited marked depressive-like behaviors following FMT from patients diagnosed with MDD. This transfer of depressive symptoms was accompanied by significant shifts in the microbiota, notably an increase in Ruminococcaceae and Lachnospira, and a decrease in Coprococcus, suggesting a direct link between specific microbial taxa and depression-related phenotypes (269). In a similar vein, FMT from rheumatoid arthritis patients has been shown to induce depressive-like behaviors in mice, elevating pro-inflammatory cytokines, such as IL-6 and TNF-α, thereby highlighting the role of gut microbiota in modulating systemic inflammation and immune responses, which may contribute to the development of depression (270). In contrast, several studies have demonstrated the potential of FMT to alleviate depression-related phenotypes. For instance, in an animal model subjected to CUMS, FMT led to significant improvements in depressive behaviors, alongside an increase in Firmicutes abundance and a reduction in intestinal and neuroinflammation (271). Clinical trials further support the therapeutic potential of FMT in depression, particularly in patients with comorbid irritable bowel syndrome, a condition frequently associated with depression. These studies revealed that FMT administration not only reduced depressive symptoms but also promoted a shift in gut microbiota composition, including the reduction of pathogenic bacteria such as Faecalibacterium, Eubacterium, and Escherichia coli, which are often implicated in gastrointestinal and mood disorders (272).
Despite the promising therapeutic effects, the clinical application of FMT has been accompanied by reports of side effects, including transient diarrhea, constipation, abdominal pain, and low-grade fever (273). Moreover, the efficacy of FMT is influenced by various factors, including fecal dosage, infusion frequency, delivery route, and donor-recipient compatibility (274). Recent advancements in personalized precision FMT have introduced the potential of artificial intelligence (AI)-driven donor-recipient matching, which can significantly optimize the treatment process by tailoring the procedure to the unique microbiota profiles of both the donor and recipient. AI-based algorithms can analyze large-scale microbiome data to predict the most compatible donor-recipient pairs, thereby enhancing the therapeutic outcomes and minimizing adverse effects. These variables underscore the need for careful optimization of FMT protocols to maximize therapeutic benefits while reducing the risk of side effects. As the understanding of gut microbiota’s role in depression continues to expand, further research is essential to refine FMT techniques, integrate AI-driven precision matching, explore long-term effects, and determine the optimal use of FMT in the treatment of depression. The incorporation of AI-based typing and personalized microbiota-based transplantation approaches holds promise for making FMT a more reliable and effective treatment for patients suffering from depression.
Conclusion & future perspectives
The gut microbiota has emerged as a central player in depression pathogenesis, with compelling evidence linking microbial dysbiosis to altered neuroimmune signaling, neurotransmitter metabolism, and HPA axis regulation through the MGBA. Recent advances in multi-omics technologies have identified specific microbial signatures (e.g., reduced Faecalibacterium and increased Enterobacteriaceae) and their neuroactive metabolites (e.g., diminished SCFAs, elevated LPS) as potential diagnostic biomarkers and therapeutic targets. While traditional antidepressants remain the cornerstone of treatment, emerging microbiome-based approaches, including probiotics, prebiotics, postbiotics, and FMT, have shown promise in alleviating depressive symptoms in both preclinical and clinical studies. Furthermore, innovative interventions such as next-generation probiotics engineered for targeted neuroactive compound delivery, phage-based microbial modulation, and AI-optimized FMT protocols, demonstrate transformative potential in the management of depression. Despite the promising advancements, several key challenges remain. First, the need to establish causal mechanisms using humanized gnotobiotic models and cutting-edge techniques like single-cell spatial metabolomics is crucial to unraveling the precise relationships between the microbiome and depression. Second, clinical translation is complicated by the heterogeneity of microbiome profiles, necessitating the development of standardized, multi-omics-defined microbial consortia for consistent therapeutic outcomes. Third, there is a pressing need to advance precision medicine approaches that integrate microbiome-host interactions with individual genetic, epigenetic, and lifestyle factors to ensure the most effective and personalized treatments. The integration of synthetic biology, including CRISPR-modified psychobiotics, nanotechnology (e.g., blood-brain barrier-penetrating microbial vesicles), and computational psychiatry is on the horizon, and could usher in a new era of microbiome-based depression therapies. Realizing the full potential of these approaches will demand interdisciplinary collaboration across fields like microbial genomics, neuroimmunology, and computational sciences. By bridging mechanistic insights with clinically actionable solutions, these innovations hold the potential to revolutionize the treatment of mental health disorders, offering a paradigm shift in the prevention and management of depression.
Author contributions
ZZ: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. YC: Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. XL: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. XX: Data curation, Formal analysis, Methodology, Writing – original draft. WD: Data curation, Formal analysis, Methodology, Writing – original draft. ZL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. JL: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. GC: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This present work was funded by the grants of the National S&T Major Project of China (2023YFC2308400), the Research Project of China National Health Development Research Center (WKZX2022JG0105), the Fundamental Research Funds for the Central Universities (2025ZFJH03), the Zhejiang Provincial Natural Science Foundation of China (LQ24H090005), Shandong Provincial Laboratory Project (SYS202202), the Taishan Scholar Foundation of Shandong Province (tsqn202103119), and the Foundation of China’s State Key Laboratory for Diagnosis and Treatment of Infectious Diseases (ZZ202316 and ZZ202319).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen M, Xie CR, Shi YZ, Tang TC, and Zheng H. Gut microbiota and major depressive disorder: A bidirectional Mendelian randomization. J Affect Disord. (2022) 316:187–93. doi: 10.1016/j.jad.2022.08.012
2. Wang Q, Zhao Y, Qin X, and Tian J. Deciphering relationship between depression and microbial molecules based on multi-omics: A case study of Chaigui Granules. Chin Herb Med. (2024) 16:612–21. doi: 10.1016/j.chmed.2023.12.003
3. Dobrek L and Głowacka K. Depression and its phytopharmacotherapy-A narrative review. Int J Mol Sci. (2023) 24:4772. doi: 10.3390/ijms24054772
4. Malhi GS and Mann JJ. Depression. Lancet. (2018) 392:2299–312. doi: 10.1016/s0140-6736(18)31948-2
5. Góralczyk-Bińkowska A, Szmajda-Krygier D, and Kozłowska E. The microbiota-gut-brain axis in psychiatric disorders. Int J Mol Sci. (2022) 23:11245. doi: 10.3390/ijms231911245
6. Gałecki P, Bliźniewska-Kowalska K, Maes M, and Su KP. Neuroimmunology and (Epi)Genetics in depressive disorder. J Pers Med. (2021) 11:670. doi: 10.3390/jpm11070670
7. Covid-19 mental disorders collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. (2021) 398:1700–12. doi: 10.1016/s0140-6736(21)02143-7
8. Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Focus (Am Psychiatr Publ). (2018) 16:420–9. doi: 10.1176/appi.focus.16407
9. Hsiao EY, Mcbride SW, Hsien S, Sharon G, Hyde ER, Mccue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. (2013) 155:1451–63. doi: 10.1016/j.cell.2013.11.024
10. Margolis KG, Cryan JF, and Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology. (2021) 160:1486–501. doi: 10.1053/j.gastro.2020.10.066
11. Douglas GM, Bielawski JP, and Langille MGI. Re-evaluating the relationship between missing heritability and the microbiome. Microbiome. (2020) 8:87. doi: 10.1186/s40168-020-00839-4
12. Ridaura V and Belkaid Y. Gut microbiota: the link to your second brain. Cell. (2015) 161:193–4. doi: 10.1016/j.cell.2015.03.033
13. Pellegrini C, Fornai M, D’antongiovanni V, Antonioli L, Bernardini N, and Derkinderen P. The intestinal barrier in disorders of the central nervous system. Lancet Gastroenterol Hepatol. (2023) 8:66–80. doi: 10.1016/s2468-1253(22)00241-2
14. Mcintyre RS, Subramaniapillai M, Shekotikhina M, Carmona NE, Lee Y, Mansur RB, et al. Characterizing the gut microbiota in adults with bipolar disorder: a pilot study. Nutr Neurosci. (2021) 24:173–80. doi: 10.1080/1028415x.2019.1612555
15. Checa-Ros A, Jeréz-Calero A, Molina-Carballo A, Campoy C, and Muñoz-Hoyos A. Current evidence on the role of the gut microbiome in ADHD pathophysiology and therapeutic implications. Nutrients. (2021) 13:249. doi: 10.3390/nu13010249
16. Zhu B, Gu Z, Hu H, Huang J, Zeng Z, Liang H, et al. Altered gut microbiota contributes to acute-respiratory-distress-syndrome-related depression through microglial neuroinflammation. Res (Wash D C). (2025) 8:636. doi: 10.34133/research.0636
17. Xu Q, Sun L, Chen Q, Jiao C, Wang Y, Li H, et al. Gut microbiota dysbiosis contributes to depression-like behaviors via hippocampal NLRP3-mediated neuroinflammation in a postpartum depression mouse model. Brain Behav Immun. (2024) 119:220–35. doi: 10.1016/j.bbi.2024.04.002
18. Tian X, Wang G, Teng F, Xue X, Pan J, Mao Q, et al. Zhi Zi Chi decoction (Gardeniae fructus and semen Sojae Praeparatum) attenuates anxious depression via modulating microbiota-gut-brain axis in corticosterone combined with chronic restraint stress-induced mice. CNS Neurosci Ther. (2024) 30:e14519. doi: 10.1111/cns.14519
19. Kelly JR, Borre Y, O'Brien C, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. (2016) 82:109–18. doi: 10.1016/j.jpsychires.2016.07.019
20. Li N, Wang Q, Wang Y, Sun A, Lin Y, Jin Y, et al. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress. (2019) 22:592–602. doi: 10.1080/10253890.2019.1617267
21. Liu P, Liu Z, Wang J, Wang J, Gao M, Zhang Y, et al. Immunoregulatory role of the gut microbiota in inflammatory depression. Nat Commun. (2024) 15:3003. doi: 10.1038/s41467-024-47273-w
22. Ritz NL, Brocka M, Butler MI, Cowan CSM, Barrera-Bugueño C, Turkington CJR, et al. Social anxiety disorder-associated gut microbiota increases social fear. Proc Natl Acad Sci U.S.A. (2024) 121:e2308706120. doi: 10.1073/pnas.2308706120
23. Paiva IHR, Maciel LM, Silva RSD, Mendonça IP, Souza JRB, and Peixoto CA. Prebiotics modulate the microbiota-gut-brain axis and ameliorate anxiety and depression-like behavior in HFD-fed mice. Food Res Int. (2024) 182:114153. doi: 10.1016/j.foodres.2024.114153
24. Ullah H, Di Minno A, Esposito C, El-Seedi HR, Khalifa SAM, Baldi A, et al. Efficacy of a food supplement based on S-adenosyl methionine and probiotic strains in subjects with subthreshold depression and mild-to-moderate depression: A monocentric, randomized, cross-over, double-blind, placebo-controlled clinical trial. BioMed Pharmacother. (2022) 156:113930. doi: 10.1016/j.biopha.2022.113930
25. Schneider E, Doll JPK, Schweinfurth N, Kettelhack C, Schaub AC, Yamanbaeva G, et al. Effect of short-term, high-dose probiotic supplementation on cognition, related brain functions and BDNF in patients with depression: a secondary analysis of a randomized controlled trial. J Psychiatry Neurosci. (2023) 48:E23–e33. doi: 10.1503/jpn.220117
26. Reininghaus EZ, Platzer M, Kohlhammer-Dohr A, Hamm C, Mörkl S, Bengesser SA, et al. PROVIT: supplementary probiotic treatment and vitamin B7 in depression-A randomized controlled trial. Nutrients. (2020) 12:3422. doi: 10.3390/nu12113422
27. Agirman G, Yu KB, and Hsiao EY. Signaling inflammation across the gut-brain axis. Science. (2021) 374:1087–92. doi: 10.1126/science.abi6087
28. Sah RK, Nandan A, Kv A, S P, S S, Jose A, et al. Decoding the role of the gut microbiome in gut-brain axis, stress-resilience, or stress-susceptibility: A review. Asian J Psychiatr. (2024) 91:103861. doi: 10.1016/j.ajp.2023.103861
29. Singh J, Vanlallawmzuali, Singh A, Biswal S, Zomuansangi R, Lalbiaktluangi C, et al. Microbiota-brain axis: Exploring the role of gut microbiota in psychiatric disorders - A comprehensive review. Asian J Psychiatr. (2024) 97:104068. doi: 10.1016/j.ajp.2024.104068
30. Chidambaram SB, Essa MM, Rathipriya AG, Bishir M, Ray B, Mahalakshmi AM, et al. Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: Tales of a vicious cycle. Pharmacol Ther. (2022) 231:107988. doi: 10.1016/j.pharmthera.2021.107988
31. Averina OV, Zorkina YA, Yunes RA, Kovtun AS, Ushakova VM, Morozova AY, et al. Bacterial metabolites of human gut microbiota correlating with depression. Int J Mol Sci. (2020) 21:9234. doi: 10.3390/ijms21239234
32. Schneider KM, Blank N, Alvarez Y, Thum K, Lundgren P, Litichevskiy L, et al. The enteric nervous system relays psychological stress to intestinal inflammation. Cell. (2023) 186:2823–2838.e2820. doi: 10.1016/j.cell.2023.05.001
33. Cheng L, Wu H, Cai X, Zhang Y, Yu S, Hou Y, et al. A Gpr35-tuned gut microbe-brain metabolic axis regulates depressive-like behavior. Cell Host Microbe. (2024) 32:227–243.e226. doi: 10.1016/j.chom.2023.12.009
34. Pearson-Leary J, Zhao C, Bittinger K, Eacret D, Luz S, Vigderman AS, et al. The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Mol Psychiatry. (2020) 25:1068–79. doi: 10.1038/s41380-019-0380-x
35. Rincel M, Aubert P, Chevalier J, Grohard PA, Basso L, Monchaux De Oliveira C, et al. Multi-hit early life adversity affects gut microbiota, brain and behavior in a sex-dependent manner. Brain Behav Immun. (2019) 80:179–92. doi: 10.1016/j.bbi.2019.03.006
36. Zhu R, Fang Y, Li H, Liu Y, Wei J, Zhang S, et al. Psychobiotic Lactobacillus plantarum JYLP-326 relieves anxiety, depression, and insomnia symptoms in test anxious college via modulating the gut microbiota and its metabolism. Front Immunol. (2023) 14:1158137. doi: 10.3389/fimmu.2023.1158137
37. Li D, Liu R, Wang M, Peng R, Fu S, Fu A, et al. 3β-Hydroxysteroid dehydrogenase expressed by gut microbes degrades testosterone and is linked to depression in males. Cell Host Microbe. (2022) 30:329–339.e325. doi: 10.1016/j.chom.2022.01.001
38. Zhang Y, Huang R, Cheng M, Wang L, Chao J, Li J, et al. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome. (2019) 7:116. doi: 10.1186/s40168-019-0733-3
39. Sun L, Zhang H, Cao Y, Wang C, Zhao C, Wang H, et al. Fluoxetine ameliorates dysbiosis in a depression model induced by chronic unpredicted mild stress in mice. Int J Med Sci. (2019) 16:1260–70. doi: 10.7150/ijms.37322
40. Hao W, Ma Q, Wang L, Yuan N, Gan H, He L, et al. Gut dysbiosis induces the development of depression-like behavior through abnormal synapse pruning in microglia-mediated by complement C3. Microbiome. (2024) 12:34. doi: 10.1186/s40168-024-01756-6
41. Li Y, Li J, Cheng R, Liu H, Zhao Y, Liu Y, et al. Alteration of the gut microbiome and correlated metabolism in a rat model of long-term depression. Front Cell Infect Microbiol. (2023) 13:1116277. doi: 10.3389/fcimb.2023.1116277
42. He H, Zhao Z, Xiao C, Li L, Liu YE, Fu J, et al. Gut microbiome promotes mice recovery from stress-induced depression by rescuing hippocampal neurogenesis. Neurobiol Dis. (2024) 191:106396. doi: 10.1016/j.nbd.2023.106396
43. Palepu MSK, Gajula SNR, K M, Sonti R, and Dandekar MP. SCFAs supplementation rescues anxiety- and depression-like phenotypes generated by fecal engraftment of treatment-resistant depression rats. ACS Chem Neurosci. (2024) 15:1010–25. doi: 10.1021/acschemneuro.3c00727
44. Liu J, Fang Y, Cui L, Wang Z, Luo Y, Gao C, et al. Butyrate emerges as a crucial effector of Zhi-Zi-Chi decoctions to ameliorate depression via multiple pathways of brain-gut axis. BioMed Pharmacother. (2022) 149:112861. doi: 10.1016/j.biopha.2022.112861
45. Xiao W, Li J, Gao X, Yang H, Su J, Weng R, et al. Involvement of the gut-brain axis in vascular depression via tryptophan metabolism: A benefit of short chain fatty acids. Exp Neurol. (2022) 358:114225. doi: 10.1016/j.expneurol.2022.114225
46. Wang W, Liu W, Duan D, Bai H, Wang Z, and Xing Y. Chronic social defeat stress mouse model: Current view on its behavioral deficits and modifications. Behav Neurosci. (2021) 135:326–35. doi: 10.1037/bne0000418
47. Zhu X, Sakamoto S, Ishii C, Smith MD, Ito K, Obayashi M, et al. Dectin-1 signaling on colonic γδ T cells promotes psychosocial stress responses. Nat Immunol. (2023) 24:625–36. doi: 10.1038/s41590-023-01447-8
48. Kosuge A, Kunisawa K, Arai S, Sugawara Y, Shinohara K, Iida T, et al. Heat-sterilized Bifidobacterium breve prevents depression-like behavior and interleukin-1β expression in mice exposed to chronic social defeat stress. Brain Behav Immun. (2021) 96:200–11. doi: 10.1016/j.bbi.2021.05.028
49. Tian T, Xu B, Qin Y, Fan L, Chen J, Zheng P, et al. Clostridium butyricum miyairi 588 has preventive effects on chronic social defeat stress-induced depressive-like behaviour and modulates microglial activation in mice. Biochem Biophys Res Commun. (2019) 516:430–6. doi: 10.1016/j.bbrc.2019.06.053
50. Yang C, Fujita Y, Ren Q, Ma M, Dong C, and Hashimoto K. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci Rep. (2017) 7:45942. doi: 10.1038/srep45942
51. Wang D, Levine JLS, Avila-Quintero V, Bloch M, and Kaffman A. Systematic review and meta-analysis: effects of maternal separation on anxiety-like behavior in rodents. Transl Psychiatry. (2020) 10:174. doi: 10.1038/s41398-020-0856-0
52. Nishi M. Effects of early-life stress on the brain and behaviors: implications of early maternal separation in rodents. Int J Mol Sci. (2020) 21:7212. doi: 10.3390/ijms21197212
53. Amini-Khoei H, Haghani-Samani E, Beigi M, Soltani A, Mobini GR, Balali-Dehkordi S, et al. On the role of corticosterone in behavioral disorders, microbiota composition alteration and neuroimmune response in adult male mice subjected to maternal separation stress. Int Immunopharmacol. (2019) 66:242–50. doi: 10.1016/j.intimp.2018.11.037
54. Enqi W, Jingzhu S, Lingpeng P, and Yaqin L. Comparison of the gut microbiota disturbance in rat models of irritable bowel syndrome induced by maternal separation and multiple early-life adversity. Front Cell Infect Microbiol. (2020) 10:581974. doi: 10.3389/fcimb.2020.581974
55. Zhu J, Zhong Z, Shi L, Huang L, Lin C, He Y, et al. Gut microbiota mediate early life stress-induced social dysfunction and anxiety-like behaviors by impairing amino acid transport at the gut. Gut Microbes. (2024) 16:2401939. doi: 10.1080/19490976.2024.2401939
56. De Santa F, Strimpakos G, Marchetti N, Gargari G, Torcinaro A, Arioli S, et al. Effect of a multi-strain probiotic mixture consumption on anxiety and depression symptoms induced in adult mice by postnatal maternal separation. Microbiome. (2024) 12:29. doi: 10.1186/s40168-024-01752-w
57. Zhang K, Fujita Y, Chang L, Qu Y, Pu Y, Wang S, et al. Abnormal composition of gut microbiota is associated with resilience versus susceptibility to inescapable electric stress. Transl Psychiatry. (2019) 9:231. doi: 10.1038/s41398-019-0571-x
58. Takajo T, Tomita K, Tsuchihashi H, Enomoto S, Tanichi M, Toda H, et al. Depression promotes the onset of irritable bowel syndrome through unique dysbiosis in rats. Gut Liver. (2019) 13:325–32. doi: 10.5009/gnl18296
59. Mika A, Day HE, Martinez A, Rumian NL, Greenwood BN, Chichlowski M, et al. Early life diets with prebiotics and bioactive milk fractions attenuate the impact of stress on learned helplessness behaviours and alter gene expression within neural circuits important for stress resistance. Eur J Neurosci. (2017) 45:342–57. doi: 10.1111/ejn.13444
60. Wang X, Eguchi A, Fujita Y, Wan X, Chang L, Yang Y, et al. Abnormal compositions of gut microbiota and metabolites are associated with susceptibility versus resilience in rats to inescapable electric stress. J Affect Disord. (2023) 331:369–79. doi: 10.1016/j.jad.2023.03.073
61. Wang G, Cao L, Li S, Zhang M, Li Y, Duan J, et al. Gut microbiota dysbiosis-mediated ceramides elevation contributes to corticosterone-induced depression by impairing mitochondrial function. NPJ Biofilms Microbiomes. (2024) 10:111. doi: 10.1038/s41522-024-00582-w
62. Jiang ST, Sun YH, Li Y, Wang MQ, Wang XY, and Dong YF. Gut microbiota is necessary for pair-housing to protect against post-stroke depression in mice. Exp Neurol. (2024) 378:114834. doi: 10.1016/j.expneurol.2024.114834
63. Zheng P, Wu J, Zhang H, Perry SW, Yin B, Tan X, et al. The gut microbiome modulates gut-brain axis glycerophospholipid metabolism in a region-specific manner in a nonhuman primate model of depression. Mol Psychiatry. (2021) 26:2380–92. doi: 10.1038/s41380-020-0744-2
64. He H, He H, Mo L, You Z, and Zhang J. Priming of microglia with dysfunctional gut microbiota impairs hippocampal neurogenesis and fosters stress vulnerability of mice. Brain Behav Immun. (2024) 115:280–94. doi: 10.1016/j.bbi.2023.10.031
65. Zhu HZ, Liang YD, Ma QY, Hao WZ, Li XJ, Wu MS, et al. Xiaoyaosan improves depressive-like behavior in rats with chronic immobilization stress through modulation of the gut microbiota. BioMed Pharmacother. (2019) 112:108621. doi: 10.1016/j.biopha.2019.108621
66. Singh R, Panganiban K, Au E, Ravikumar R, Pereira S, Prevot TD, et al. Human-fecal microbiota transplantation in relation to gut microbiome signatures in animal models for schizophrenia: A scoping review. Asian J Psychiatr. (2024) 102:104285. doi: 10.1016/j.ajp.2024.104285
67. Liu L, Wang H, Zhang H, Chen X, Zhang Y, Wu J, et al. Toward a deeper understanding of gut microbiome in depression: the promise of clinical applicability. Adv Sci (Weinh). (2022) 9:e2203707. doi: 10.1002/advs.202203707
68. Nikolova VL, Smith MRB, Hall LJ, Cleare AJ, Stone JM, and Young AH. Perturbations in gut microbiota composition in psychiatric disorders: A review and meta-analysis. JAMA Psychiatry. (2021) 78:1343–54. doi: 10.1001/jamapsychiatry.2021.2573
69. Ling Z, Cheng Y, Chen F, Yan X, Liu X, Shao L, et al. Changes in fecal microbiota composition and the cytokine expression profile in school-aged children with depression: A case-control study. Front Immunol. (2022) 13:964910. doi: 10.3389/fimmu.2022.964910
70. Mudimela S, Vishwanath NK, Pillai A, Morales R, Marrelli SP, Barichello T, et al. Clinical significance and potential role of trimethylamine N-oxide in neurological and neuropsychiatric disorders. Drug Discov Today. (2022) 27:103334. doi: 10.1016/j.drudis.2022.08.002
71. Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. (2015) 48:186–94. doi: 10.1016/j.bbi.2015.03.016
72. Chen YH, Xue F, Yu SF, Li XS, Liu L, Jia YY, et al. Gut microbiota dysbiosis in depressed women: The association of symptom severity and microbiota function. J Affect Disord. (2021) 282:391–400. doi: 10.1016/j.jad.2020.12.143
73. Sanada K, Nakajima S, Kurokawa S, Barceló-Soler A, Ikuse D, Hirata A, et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J Affect Disord. (2020) 266:1–13. doi: 10.1016/j.jad.2020.01.102
74. Zheng P, Yang J, Li Y, Wu J, Liang W, Yin B, et al. Gut microbial signatures can discriminate unipolar from bipolar depression. Adv Sci (Weinh). (2020) 7:1902862. doi: 10.1002/advs.201902862
75. Liu P, Jing L, Guo F, Xu Y, Cheng J, Liu S, et al. Characteristics of gut microbiota and its correlation with hs-CRP and somatic symptoms in first-episode treatment-naive major depressive disorder. J Affect Disord. (2024) 356:664–71. doi: 10.1016/j.jad.2024.04.011
76. Wang Y, Zhou J, Ye J, Sun Z, He Y, Zhao Y, et al. Multi-omics reveal microbial determinants impacting the treatment outcome of antidepressants in major depressive disorder. Microbiome. (2023) 11:195. doi: 10.1186/s40168-023-01635-6
77. Zhao S, Lieberman TD, Poyet M, Kauffman KM, Gibbons SM, Groussin M, et al. Adaptive evolution within gut microbiomes of healthy people. Cell Host Microbe. (2019) 25:656–667.e658. doi: 10.1016/j.chom.2019.03.007
78. Elhenawy W, Tsai CN, and Coombes BK. Host-specific adaptive diversification of crohn’s disease-associated adherent-invasive Escherichia coli. Cell Host Microbe. (2019) 25:301–312.e305. doi: 10.1016/j.chom.2018.12.010
79. Barroso-Batista J, Pedro MF, Sales-Dias J, Pinto CJG, Thompson JA, Pereira H, et al. Specific eco-evolutionary contexts in the mouse gut reveal Escherichia coli metabolic versatility. Curr Biol. (2020) 30:1049–1062.e1047. doi: 10.1016/j.cub.2020.01.050
80. Yilmaz B, Mooser C, Keller I, Li H, Zimmermann J, Bosshard L, et al. Long-term evolution and short-term adaptation of microbiota strains and sub-strains in mice. Cell Host Microbe. (2021) 29:650–663.e659. doi: 10.1016/j.chom.2021.02.001
81. Chen JJ, He S, Fang L, Wang B, Bai SJ, Xie J, et al. Age-specific differential changes on gut microbiota composition in patients with major depressive disorder. Aging (Albany NY). (2020) 12:2764–76. doi: 10.18632/aging.102775
82. Chen JJ, Zheng P, Liu YY, Zhong XG, Wang HY, Guo YJ, et al. Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatr Dis Treat. (2018) 14:647–55. doi: 10.2147/ndt.S159322
83. Smid MC, Ricks NM, Panzer A, Mccoy AN, Azcarate-Peril MA, Keku TO, et al. Maternal gut microbiome biodiversity in pregnancy. Am J Perinatol. (2018) 35:24–30. doi: 10.1055/s-0037-1604412
84. Fasano A, Chassaing B, Haller D, Flores Ventura E, Carmen-Collado M, Pastor N, et al. Microbiota during pregnancy and early life: role in maternal-neonatal outcomes based on human evidence. Gut Microbes. (2024) 16:2392009. doi: 10.1080/19490976.2024.2392009
85. Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. (2012) 150:470–80. doi: 10.1016/j.cell.2012.07.008
86. Arifunhera J and Mirunalini R. An update on the pharmacotherapy of postpartum depression. Int J Gynaecol Obstet. (2025) 168:933–43. doi: 10.1002/ijgo.15980
87. Fan Q, Long Q, De Silva V, Gunarathna N, Jayathilaka U, Dabrera T, et al. Prevalence and risk factors for postpartum depression in Sri Lanka: A population-based study. Asian J Psychiatr. (2020) 47:101855. doi: 10.1016/j.ajp.2019.101855
88. Tambelli R, Tosto S, and Favieri F. Psychiatric risk factors for postpartum depression: A systematic review. Behav Sci (Basel). (2025) 15:173. doi: 10.3390/bs15020173
89. Zhou Y, Chen C, Yu H, and Yang Z. Fecal microbiota changes in patients with postpartum depressive disorder. Front Cell Infect Microbiol. (2020) 10:567268. doi: 10.3389/fcimb.2020.567268
90. Mota AS, Sparvoli LG, Vanzele P, Naspolini NF, Tobaruela EC, Yoshizaki CT, et al. Longitudinal gut microbiota composition during perinatal period in women with different intensities of depressive symptoms. Braz J Psychiatry. (2024). doi: 10.47626/1516-4446-2024-3721
91. Kim CS, Cha L, Sim M, Jung S, Chun WY, Baik HW, et al. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: A randomized, double-blind, placebo-controlled, multicenter trial. J Gerontol A Biol Sci Med Sci. (2021) 76:32–40. doi: 10.1093/gerona/glaa090
92. Nikolova VL, Cleare AJ, Young AH, and Stone JM. Acceptability, tolerability, and estimates of putative treatment effects of probiotics as adjunctive treatment in patients with depression: A randomized clinical trial. JAMA Psychiatry. (2023) 80:842–7. doi: 10.1001/jamapsychiatry.2023.1817
93. Vicariotto F, Malfa P, Torricelli M, Lungaro L, Caio G, and De Leo V. Beneficial Effects of Limosilactobacillus reuteri PBS072 and Bifidobacterium breve BB077 on Mood Imbalance, Self-Confidence, and Breastfeeding in Women during the First Trimester Postpartum. Nutrients. (2023) 15:3513. doi: 10.3390/nu15163513
94. Stevens BR, Roesch L, Thiago P, Russell JT, Pepine CJ, Holbert RC, et al. Depression phenotype identified by using single nucleotide exact amplicon sequence variants of the human gut microbiome. Mol Psychiatry. (2021) 26:4277–87. doi: 10.1038/s41380-020-0652-5
95. Chang L, Wei Y, and Hashimoto K. Brain-gut-microbiota axis in depression: A historical overview and future directions. Brain Res Bull. (2022) 182:44–56. doi: 10.1016/j.brainresbull.2022.02.004
96. Reyes-Martínez S, Segura-Real L, Gómez-García AP, Tesoro-Cruz E, Constantino-Jonapa LA, Amedei A, et al. Neuroinflammation, microbiota-gut-brain axis, and depression: the vicious circle. J Integr Neurosci. (2023) 22:65. doi: 10.31083/j.jin2203065
97. Ganz J. Revealing the complexity of the gut’s brain. Nat Neurosci. (2021) 24:1–2. doi: 10.1038/s41593-020-00769-2
98. Niesler B, Kuerten S, Demir IE, and Schäfer KH. Disorders of the enteric nervous system - a holistic view. Nat Rev Gastroenterol Hepatol. (2021) 18:393–410. doi: 10.1038/s41575-020-00385-2
99. Dicks LMT. Gut bacteria and neurotransmitters. Microorganisms. (2022) 10:1838. doi: 10.3390/microorganisms10091838
100. Fried S, Wemelle E, Cani PD, and Knauf C. Interactions between the microbiota and enteric nervous system during gut-brain disorders. Neuropharmacology. (2021) 197:108721. doi: 10.1016/j.neuropharm.2021.108721
101. Morais LH, Schreiber HLT, and Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. (2021) 19:241–55. doi: 10.1038/s41579-020-00460-0
102. Pu Y, Tan Y, Qu Y, Chang L, Wang S, Wei Y, et al. A role of the subdiaphragmatic vagus nerve in depression-like phenotypes in mice after fecal microbiota transplantation from Chrna7 knock-out mice with depression-like phenotypes. Brain Behav Immun. (2021) 94:318–26. doi: 10.1016/j.bbi.2020.12.032
103. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U.S.A. (2011) 108:16050–5. doi: 10.1073/pnas.1102999108
104. Zhang J, Ma L, Chang L, Pu Y, Qu Y, and Hashimoto K. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl Psychiatry. (2020) 10:186. doi: 10.1038/s41398-020-00878-3
105. Wang S, Ishima T, Zhang J, Qu Y, Chang L, Pu Y, et al. Ingestion of Lactobacillus intestinalis and Lactobacillus reuteri causes depression- and anhedonia-like phenotypes in antibiotic-treated mice via the vagus nerve. J Neuroinflamm. (2020) 17:241. doi: 10.1186/s12974-020-01916-z
106. Mörkl S, Narrath M, Schlotmann D, Sallmutter MT, Putz J, Lang J, et al. Multi-species probiotic supplement enhances vagal nerve function - results of a randomized controlled trial in patients with depression and healthy controls. Gut Microbes. (2025) 17:2492377. doi: 10.1080/19490976.2025.2492377
107. Jacobsen JP, Medvedev IO, and Caron MG. The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philos Trans R Soc Lond B Biol Sci. (2012) 367:2444–59. doi: 10.1098/rstb.2012.0109
108. Bertollo AG, Mingoti MED, and Ignácio ZM. Neurobiological mechanisms in the kynurenine pathway and major depressive disorder. Rev Neurosci. (2025) 36:169–87. doi: 10.1515/revneuro-2024-0065
109. Strawn JR, Mills JA, Schroeder H, Mossman SA, Varney ST, Ramsey LB, et al. Escitalopram in adolescents with generalized anxiety disorder: A double-blind, randomized, placebo-controlled study. J Clin Psychiatry. (2020) 81:20m13396. doi: 10.4088/JCP.20m13396
110. Kato M, Baba H, Takekita Y, Naito M, Koshikawa Y, Bandou H, et al. Usefulness of mirtazapine and SSRIs in late-life depression: post hoc analysis of the GUNDAM study. Eur J Clin Pharmacol. (2023) 79:1515–24. doi: 10.1007/s00228-023-03563-8
111. Madabushi JS, Khurana P, Gupta N, and Gupta M. Gut biome and mental health: do probiotics work? Cureus. (2023) 15:e40293. doi: 10.7759/cureus.40293
112. Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U.S.A. (2009) 106:3698–703. doi: 10.1073/pnas.0812874106
113. Zhou M, Fan Y, Xu L, Yu Z, Wang S, Xu H, et al. Microbiome and tryptophan metabolomics analysis in adolescent depression: roles of the gut microbiota in the regulation of tryptophan-derived neurotransmitters and behaviors in human and mice. Microbiome. (2023) 11:145. doi: 10.1186/s40168-023-01589-9
114. Tian P, Chen Y, Zhu H, Wang L, Qian X, Zou R, et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav Immun. (2022) 100:233–41. doi: 10.1016/j.bbi.2021.11.023
115. Tian P, Wang G, Zhao J, Zhang H, and Chen W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J Nutr Biochem. (2019) 66:43–51. doi: 10.1016/j.jnutbio.2019.01.007
116. Sarkawi M, Raja Ali RA, Abdul Wahab N, Abdul Rathi ND, and Mokhtar NM. A randomized, double-blinded, placebo-controlled clinical trial on Lactobacillus-containing cultured milk drink as adjuvant therapy for depression in irritable bowel syndrome. Sci Rep. (2024) 14:9478. doi: 10.1038/s41598-024-60029-2
117. Wu L, Ran L, Wu Y, Liang M, Zeng J, Ke F, et al. Oral administration of 5-hydroxytryptophan restores gut microbiota dysbiosis in a mouse model of depression. Front Microbiol. (2022) 13:864571. doi: 10.3389/fmicb.2022.864571
118. Otaru N, Ye K, Mujezinovic D, Berchtold L, Constancias F, Cornejo FA, et al. GABA production by human intestinal bacteroides spp.: prevalence, regulation, and role in acid stress tolerance. Front Microbiol. (2021) 12:656895. doi: 10.3389/fmicb.2021.656895
119. Aggarwal S, Ahuja V, and Paul J. Dysregulation of GABAergic signalling contributes in the pathogenesis of diarrhea-predominant irritable bowel syndrome. J Neurogastroenterol Motil. (2018) 24:422–30. doi: 10.5056/jnm17100
120. Gomez-Nguyen A, Basson AR, Dark-Fleury L, Hsu K, Osme A, Menghini P, et al. Parabacteroides distasonis induces depressive-like behavior in a mouse model of Crohn’s disease. Brain Behav Immun. (2021) 98:245–50. doi: 10.1016/j.bbi.2021.08.218
121. Wang P, Wu PF, Wang HJ, Liao F, Wang F, and Chen JG. Gut microbiome-derived ammonia modulates stress vulnerability in the host. Nat Metab. (2023) 5:1986–2001. doi: 10.1038/s42255-023-00909-5
122. Luqman A, Nega M, Nguyen MT, Ebner P, and Götz F. SadA-expressing staphylococci in the human gut show increased cell adherence and internalization. Cell Rep. (2018) 22:535–45. doi: 10.1016/j.celrep.2017.12.058
123. Villageliú D and Lyte M. Dopamine production in Enterococcus faecium: A microbial endocrinology-based mechanism for the selection of probiotics based on neurochemical-producing potential. PloS One. (2018) 13:e0207038. doi: 10.1371/journal.pone.0207038
124. Wang Y, Wang Y, Ding K, Liu Y, Liu D, Chen W, et al. Effectiveness of psychobiotic bifidobacterium breve BB05 in managing psychosomatic diarrhea in college students by regulating gut microbiota: A randomized, double-blind, placebo-controlled trial. Nutrients. (2024) 16:1989. doi: 10.3390/nu16131989
125. Fu X, Wang Y, Zhao F, Cui R, Xie W, Liu Q, et al. Shared biological mechanisms of depression and obesity: focus on adipokines and lipokines. Aging (Albany NY). (2023) 15:5917–50. doi: 10.18632/aging.204847
126. Cryan JF and O’mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. (2011) 23:187–92. doi: 10.1111/j.1365-2982.2010.01664.x
127. Frankiensztajn LM, Elliott E, and Koren O. The microbiota and the hypothalamus-pituitary-adrenocortical (HPA) axis, implications for anxiety and stress disorders. Curr Opin Neurobiol. (2020) 62:76–82. doi: 10.1016/j.conb.2019.12.003
128. Zhao S, Zhu L, and Yang J. Association between depression and macrovascular disease: a mini review. Front Psychiatry. (2023) 14:1215173. doi: 10.3389/fpsyt.2023.1215173
129. Łaniewski P and Herbst-Kralovetz MM. Connecting microbiome and menopause for healthy ageing. Nat Microbiol. (2022) 7:354–8. doi: 10.1038/s41564-022-01071-6
130. Couch CE, Neal WT, Herron CL, Kent ML, Schreck CB, and Peterson JT. Gut microbiome composition associates with corticosteroid treatment, morbidity, and senescence in Chinook salmon (Oncorhynchus tshawytscha). Sci Rep. (2023) 13:2567. doi: 10.1038/s41598-023-29663-0
131. Doney E, Cadoret A, Dion-Albert L, Lebel M, and Menard C. Inflammation-driven brain and gut barrier dysfunction in stress and mood disorders. Eur J Neurosci. (2022) 55:2851–94. doi: 10.1111/ejn.15239
132. Sonali S, Ray B, Ahmed Tousif H, Rathipriya AG, Sunanda T, Mahalakshmi AM, et al. Mechanistic insights into the link between gut dysbiosis and major depression: an extensive review. Cells. (2022) 11:1362. doi: 10.3390/cells11081362
133. Almeida FB, Pinna G, and Barros HMT. The role of HPA axis and allopregnanolone on the neurobiology of major depressive disorders and PTSD. Int J Mol Sci. (2021) 22:5495. doi: 10.3390/ijms22115495
134. Mutoh N, Moriya M, Xu C, Kato K, Arai S, Iwabuchi N, et al. Bifidobacterium breve M-16V regulates the autonomic nervous system via the intestinal environment: A double-blind, placebo-controlled study. Behav Brain Res. (2024) 460:114820. doi: 10.1016/j.bbr.2023.114820
135. Li J, Li Y, Zhao J, Li L, Wang Y, Chen F, et al. Effects of Bifidobacterium breve 207–1 on regulating lifestyle behaviors and mental wellness in healthy adults based on the microbiome-gut-brain axis: a randomized, double-blind, placebo-controlled trial. Eur J Nutr. (2024) 63:2567–85. doi: 10.1007/s00394-024-03447-2
136. Mann ER, Lam YK, and Uhlig HH. Short-chain fatty acids: linking diet, the microbiome and immunity. Nat Rev Immunol. (2024) 24:577–95. doi: 10.1038/s41577-024-01014-8
137. O’riordan KJ, Collins MK, Moloney GM, Knox EG, Aburto MR, Fülling C, et al. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol Cell Endocrinol. (2022) 546:111572. doi: 10.1016/j.mce.2022.111572
138. Hoffman JD, Yanckello LM, Chlipala G, Hammond TC, Mcculloch SD, Parikh I, et al. Dietary inulin alters the gut microbiome, enhances systemic metabolism and reduces neuroinflammation in an APOE4 mouse model. PloS One. (2019) 14:e0221828. doi: 10.1371/journal.pone.0221828
139. Van Hoek MJ and Merks RM. Redox balance is key to explaining full vs. partial switching to low-yield metabolism. BMC Syst Biol. (2012) 6:22. doi: 10.1186/1752-0509-6-22
140. Donohoe DR, Holley D, Collins LB, Montgomery SA, Whitmore AC, Hillhouse A, et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. (2014) 4:1387–97. doi: 10.1158/2159-8290.Cd-14-0501
141. Modina SC, Aidos L, Rossi R, Pocar P, Corino C, and Di Giancamillo A. Stages of gut development as a useful tool to prevent gut alterations in piglets. Anim (Basel). (2021) 11:1412. doi: 10.3390/ani11051412
142. Wu M, Tian T, Mao Q, Zou T, Zhou CJ, Xie J, et al. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl Psychiatry. (2020) 10:350. doi: 10.1038/s41398-020-01038-3
143. Jiang W, Chen J, Gong L, Liu F, Zhao H, Yan Z, et al. Microbiota-derived short-chain fatty acids may participate in post-stroke depression by regulating host’s lipid metabolism. J Psychiatr Res. (2023) 161:426–34. doi: 10.1016/j.jpsychires.2023.03.032
144. Liu RT, Rowan-Nash AD, Sheehan AE, Walsh RFL, Sanzari CM, Korry BJ, et al. Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain Behav Immun. (2020) 88:308–24. doi: 10.1016/j.bbi.2020.03.026
145. Li Y, Zou X, Ma Y, Cheng J, Yu X, Shao W, et al. Lactic acid contributes to the emergence of depression-like behaviors triggered by blue light exposure during sleep. Ecotoxicol Environ Saf. (2025) 289:117643. doi: 10.1016/j.ecoenv.2024.117643
146. Westfall S, Caracci F, Zhao D, Wu QL, Frolinger T, Simon J, et al. Microbiota metabolites modulate the T helper 17 to regulatory T cell (Th17/Treg) imbalance promoting resilience to stress-induced anxiety- and depressive-like behaviors. Brain Behav Immun. (2021) 91:350–68. doi: 10.1016/j.bbi.2020.10.013
147. Sun Y, Yan T, Gong G, Li Y, Zhang J, Wu B, et al. Antidepressant-like effects of Schisandrin on lipopolysaccharide-induced mice: Gut microbiota, short chain fatty acid and TLR4/NF-κB signaling pathway. Int Immunopharmacol. (2020) 89:107029. doi: 10.1016/j.intimp.2020.107029
148. Chen N, Xu X, Guo Y, Zhao M, Li Y, Zhou T, et al. Brain short-chain fatty acids induce ACSS2 to ameliorate depressive-like behavior via PPARγ-TPH2 axis. Res (Wash D C). (2024) 7:400. doi: 10.34133/research.0400
149. Behrens LMP, Gasparotto J, Rampelotto PH, Escalona M, Da Silva LDS, Carazza-Kessler FG, et al. Sodium propionate oral supplementation ameliorates depressive-like behavior through gut microbiome and histone 3 epigenetic regulation. J Nutr Biochem. (2024) 130:109660. doi: 10.1016/j.jnutbio.2024.109660
150. Chenghan M, Wanxin L, Bangcheng Z, Yao H, Qinxi L, Ting Z, et al. Short-chain fatty acids mediate gut microbiota-brain communication and protect the blood-brain barrier integrity. Ann N Y Acad Sci. (2025) 1545:116–31. doi: 10.1111/nyas.15299
151. Fock E and Parnova R. Mechanisms of blood-brain barrier protection by microbiota-derived short-chain fatty acids. Cells. (2023) 12:657. doi: 10.3390/cells12040657
152. Varghese SM, Patel S, Nandan A, Jose A, Ghosh S, Sah RK, et al. Unraveling the role of the blood-brain barrier in the pathophysiology of depression: recent advances and future perspectives. Mol Neurobiol. (2024) 61:10398–447. doi: 10.1007/s12035-024-04205-5
153. Shi W, Zhang S, Yao K, Meng Q, Lu Y, Ren Y, et al. Breakdown of the blood-brain barrier in depressed mice induced by chronic unpredictable mild stress. J Psychiatr Res. (2024) 180:138–46. doi: 10.1016/j.jpsychires.2024.10.004
154. Sun N, Zhang J, Wang J, Liu Z, Wang X, Kang P, et al. Abnormal gut microbiota and bile acids in patients with first-episode major depressive disorder and correlation analysis. Psychiatry Clin Neurosci. (2022) 76:321–8. doi: 10.1111/pcn.13368
155. Qu Y, Su C, Zhao Q, Shi A, Zhao F, Tang L, et al. Gut microbiota-mediated elevated production of secondary bile acids in chronic unpredictable mild stress. Front Pharmacol. (2022) 13:837543. doi: 10.3389/fphar.2022.837543
156. Roager HM and Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. (2018) 9:3294. doi: 10.1038/s41467-018-05470-4
157. Brydges CR, Fiehn O, Mayberg HS, Schreiber H, Dehkordi SM, Bhattacharyya S, et al. Indoxyl sulfate, a gut microbiome-derived uremic toxin, is associated with psychic anxiety and its functional magnetic resonance imaging-based neurologic signature. Sci Rep. (2021) 11:21011. doi: 10.1038/s41598-021-99845-1
158. Philippe C, Szabo De Edelenyi F, Naudon L, Druesne-Pecollo N, Hercberg S, Kesse-Guyot E, et al. Relation between mood and the host-microbiome co-metabolite 3-indoxylsulfate: results from the observational prospective NutriNet-santé Study. Microorganisms. (2021) 9:716. doi: 10.3390/microorganisms9040716
159. Qian X, Li Q, Zhu H, Chen Y, Lin G, Zhang H, et al. Bifidobacteria with indole-3-lactic acid-producing capacity exhibit psychobiotic potential via reducing neuroinflammation. Cell Rep Med. (2024) 5:101798. doi: 10.1016/j.xcrm.2024.101798
160. Delgado I, Cussotto S, Anesi A, Dexpert S, Aubert A, Aouizerate B, et al. Association between the indole pathway of tryptophan metabolism and subclinical depressive symptoms in obesity: a preliminary study. Int J Obes (Lond). (2022) 46:885–8. doi: 10.1038/s41366-021-01049-0
161. Chen C, Xiao Q, Wen Z, Gong F, Zhan H, Liu J, et al. Gut microbiome-derived indole-3-carboxaldehyde regulates stress vulnerability in chronic restraint stress by activating aryl hydrocarbon receptors. Pharmacol Res. (2025) 213:107654. doi: 10.1016/j.phrs.2025.107654
162. Katamanin OM, Tan IJ, Barry J, and Jafferany M. Role of inflammation and cytokine dysregulation in depression in patients with inflammatory skin conditions. Am J Clin Dermatol. (2025) 26:35–43. doi: 10.1007/s40257-024-00905-9
163. Li L and Dai F. Comparison of the associations between Life’s Essential 8 and Life’s Simple 7 with depression, as well as the mediating role of oxidative stress factors and inflammation: NHANES 2005-2018. J Affect Disord. (2024) 351:31–9. doi: 10.1016/j.jad.2024.01.200
164. Hu C, Ge M, Liu Y, Tan W, Zhang Y, Zou M, et al. From inflammation to depression: key biomarkers for IBD-related major depressive disorder. J Transl Med. (2024) 22:997. doi: 10.1186/s12967-024-05758-8
165. Petralia MC, Mazzon E, Fagone P, Basile MS, Lenzo V, Quattropani MC, et al. The cytokine network in the pathogenesis of major depressive disorder. Close to translation? Autoimmun Rev. (2020) 19:102504. doi: 10.1016/j.autrev.2020.102504
166. Orsolini L, Pompili S, Tempia Valenta S, Salvi V, and Volpe U. C-reactive protein as a biomarker for major depressive disorder? Int J Mol Sci. (2022) 23:1616. doi: 10.3390/ijms23031616
167. Cryan JF, O’riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. (2019) 99:1877–2013. doi: 10.1152/physrev.00018.2018
168. Fung TC. The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol Dis. (2020) 136:104714. doi: 10.1016/j.nbd.2019.104714
169. Rudzki L and Maes M. The microbiota-gut-immune-glia (MGIG) axis in major depression. Mol Neurobiol. (2020) 57:4269–95. doi: 10.1007/s12035-020-01961-y
170. Kinashi Y and Hase K. Partners in leaky gut syndrome: intestinal dysbiosis and autoimmunity. Front Immunol. (2021) 12:673708. doi: 10.3389/fimmu.2021.673708
171. Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. (2019) 68:1516–26. doi: 10.1136/gutjnl-2019-318427
172. Horn J, Mayer DE, Chen S, and Mayer EA. Role of diet and its effects on the gut microbiome in the pathophysiology of mental disorders. Transl Psychiatry. (2022) 12:164. doi: 10.1038/s41398-022-01922-0
173. Van Eeden WA, Van Hemert AM, Carlier IVE, Penninx B, Lamers F, Fried EI, et al. Basal and LPS-stimulated inflammatory markers and the course of individual symptoms of depression. Transl Psychiatry. (2020) 10:235. doi: 10.1038/s41398-020-00920-4
174. Yu X, Yao H, Zhang X, Liu L, Liu S, and Dong Y. Comparison of LPS and MS-induced depressive mouse model: behavior, inflammation and biochemical changes. BMC Psychiatry. (2022) 22:590. doi: 10.1186/s12888-022-04233-2
175. Zheng XX, Zhang CF, Li LQ, Ye JR, Ren SY, Zhang Z, et al. Improvement of astrocytic gap junction involves the anti-depressive effect of celecoxib through inhibition of NF-κB. Brain Res Bull. (2024) 207:110871. doi: 10.1016/j.brainresbull.2024.110871
176. Wang J, Yang Y, Liu J, Qiu J, Zhang D, Ou M, et al. Loss of sodium leak channel (NALCN) in the ventral dentate gyrus impairs neuronal activity of the glutamatergic neurons for inflammation-induced depression in male mice. Brain Behav Immun. (2023) 110:13–29. doi: 10.1016/j.bbi.2023.02.013
177. Kaur S, Sharma P, Mayer MJ, Neuert S, Narbad A, and Kaur S. Beneficial effects of GABA-producing potential probiotic Limosilactobacillus fermentum L18 of human origin on intestinal permeability and human gut microbiota. Microb Cell Fact. (2023) 22:256. doi: 10.1186/s12934-023-02264-2
178. Ramalho JB, Spiazzi CC, Bicca DF, Rodrigues JF, Sehn CP, Da Silva WP, et al. Beneficial effects of Lactococcus lactis subsp. cremoris LL95 treatment in an LPS-induced depression-like model in mice. Behav Brain Res. (2022) 426:113847. doi: 10.1016/j.bbr.2022.113847
179. Wang H, He Y, Sun Z, Ren S, Liu M, Wang G, et al. Microglia in depression: an overview of microglia in the pathogenesis and treatment of depression. J Neuroinflamm. (2022) 19:132. doi: 10.1186/s12974-022-02492-0
180. Fan C, Li Y, Lan T, Wang W, Long Y, and Yu SY. Microglia secrete miR-146a-5p-containing exosomes to regulate neurogenesis in depression. Mol Ther. (2022) 30:1300–14. doi: 10.1016/j.ymthe.2021.11.006
181. Li S, Sun Y, Song M, Song Y, Fang Y, Zhang Q, et al. NLRP3/caspase-1/GSDMD-mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression. JCI Insight. (2021) 6:e146852. doi: 10.1172/jci.insight.146852
182. Zhou B, Zhu Z, Ransom BR, and Tong X. Oligodendrocyte lineage cells and depression. Mol Psychiatry. (2021) 26:103–17. doi: 10.1038/s41380-020-00930-0
183. Seo JS, Mantas I, Svenningsson P, and Greengard P. Ependymal cells-CSF flow regulates stress-induced depression. Mol Psychiatry. (2021) 26:7308–15. doi: 10.1038/s41380-021-01202-1
184. Carlessi AS, Borba LA, Zugno AI, Quevedo J, and Réus GZ. Gut microbiota-brain axis in depression: The role of neuroinflammation. Eur J Neurosci. (2021) 53:222–35. doi: 10.1111/ejn.14631
185. Fung TC, Olson CA, and Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. (2017) 20:145–55. doi: 10.1038/nn.4476
186. Li H, Xiang Y, Zhu Z, Wang W, Jiang Z, Zhao M, et al. Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat. J Neuroinflamm. (2021) 18:254. doi: 10.1186/s12974-021-02303-y
187. Schmidtner AK, Slattery DA, Gläsner J, Hiergeist A, Gryksa K, Malik VA, et al. Minocycline alters behavior, microglia and the gut microbiome in a trait-anxiety-dependent manner. Transl Psychiatry. (2019) 9:223. doi: 10.1038/s41398-019-0556-9
188. Abdel-Haq R, Schlachetzki JCM, Glass CK, and Mazmanian SK. Microbiome-microglia connections via the gut-brain axis. J Exp Med. (2019) 216:41–59. doi: 10.1084/jem.20180794
189. Zhang L, Zhang J, and You Z. Switching of the microglial activation phenotype is a possible treatment for depression disorder. Front Cell Neurosci. (2018) 12:306. doi: 10.3389/fncel.2018.00306
190. Huang C, Zhang F, Li P, and Song C. Low-dose IL-2 attenuated depression-like behaviors and pathological changes through restoring the balances between IL-6 and TGF-β and between th17 and treg in a chronic stress-induced mouse model of depression. Int J Mol Sci. (2022) 23:13856. doi: 10.3390/ijms232213856
191. Brockmann L, Tran A, Huang Y, Edwards M, Ronda C, Wang HH, et al. Intestinal microbiota-specific Th17 cells possess regulatory properties and suppress effector T cells via c-MAF and IL-10. Immunity. (2023) 56:2719–2735.e2717. doi: 10.1016/j.immuni.2023.11.003
192. Kedmi R, Najar TA, Mesa KR, Grayson A, Kroehling L, Hao Y, et al. A RORγt(+) cell instructs gut microbiota-specific T(reg) cell differentiation. Nature. (2022) 610:737–43. doi: 10.1038/s41586-022-05089-y
193. Bauer ME and Teixeira AL. Neuroinflammation in mood disorders: role of regulatory immune cells. Neuroimmunomodulation. (2021) 28:99–107. doi: 10.1159/000515594
194. Pandiyan P, Bhaskaran N, Zou M, Schneider E, Jayaraman S, and Huehn J. Microbiome dependent regulation of T(regs) and Th17 cells in mucosa. Front Immunol. (2019) 10:426. doi: 10.3389/fimmu.2019.00426
195. Ghosh R, Mitra P, Kumar P, Goyal T, and Sharma P. T helper cells in depression: central role of Th17 cells. Crit Rev Clin Lab Sci. (2022) 59:19–39. doi: 10.1080/10408363.2021.1965535
196. Kim J, Suh YH, and Chang KA. Interleukin-17 induced by cumulative mild stress promoted depression-like behaviors in young adult mice. Mol Brain. (2021) 14:11. doi: 10.1186/s13041-020-00726-x
197. Schiweck C, Valles-Colomer M, Arolt V, Müller N, Raes J, Wijkhuijs A, et al. Depression and suicidality: A link to premature T helper cell aging and increased Th17 cells. Brain Behav Immun. (2020) 87:603–9. doi: 10.1016/j.bbi.2020.02.005
198. Ambrée O, Ruland C, Zwanzger P, Klotz L, Baune BT, Arolt V, et al. Social defeat modulates T helper cell percentages in stress susceptible and resilient mice. Int J Mol Sci. (2019) 20:3512. doi: 10.3390/ijms20143512
199. Westfall S, Caracci F, Estill M, Frolinger T, Shen L, and Pasinetti GM. Chronic stress-induced depression and anxiety priming modulated by gut-brain-axis immunity. Front Immunol. (2021) 12:670500. doi: 10.3389/fimmu.2021.670500
200. Cui M, Dai W, Kong J, and Chen H. Th17 cells in depression: are they crucial for the antidepressant effect of ketamine? Front Pharmacol. (2021) 12:649144. doi: 10.3389/fphar.2021.649144
201. Ait Chait Y, Mottawea W, Tompkins TA, and Hammami R. Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Sci Rep. (2020) 10:17878. doi: 10.1038/s41598-020-74934-9
202. Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. (2018) 555:623–8. doi: 10.1038/nature25979
203. Diviccaro S, Giatti S, Cioffi L, Falvo E, Piazza R, Caruso D, et al. Paroxetine effects in adult male rat colon: Focus on gut steroidogenesis and microbiota. Psychoneuroendocrinology. (2022) 143:105828. doi: 10.1016/j.psyneuen.2022.105828
204. Zhang W, Qu W, Wang H, and Yan H. Antidepressants fluoxetine and amitriptyline induce alterations in intestinal microbiota and gut microbiome function in rats exposed to chronic unpredictable mild stress. Transl Psychiatry. (2021) 11:131. doi: 10.1038/s41398-021-01254-5
205. Deng Y, Zhou M, Wang J, Yao J, Yu J, Liu W, et al. Involvement of the microbiota-gut-brain axis in chronic restraint stress: disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes. (2021) 13:1–16. doi: 10.1080/19490976.2020.1869501
206. Shen Y, Yang X, Li G, Gao J, and Liang Y. The change of gut microbiota in MDD patients under SSRIs treatment. Sci Rep. (2021) 11:14918. doi: 10.1038/s41598-021-94481-1
207. Duan J, Huang Y, Tan X, Chai T, Wu J, Zhang H, et al. Characterization of gut microbiome in mice model of depression with divergent response to escitalopram treatment. Transl Psychiatry. (2021) 11:303. doi: 10.1038/s41398-021-01428-1
208. Vich Vila A, Collij V, Sanna S, Sinha T, Imhann F, Bourgonje AR, et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun. (2020) 11:362. doi: 10.1038/s41467-019-14177-z
209. Aydin S, Ozkul C, Yucel NT, and Karaca H. Gut microbiome alteration after reboxetine administration in type-1 diabetic rats. Microorganisms. (2021) 9:1948. doi: 10.3390/microorganisms9091948
210. Oliva V, Lippi M, Paci R, Del Fabro L, Delvecchio G, Brambilla P, et al. Gastrointestinal side effects associated with antidepressant treatments in patients with major depressive disorder: A systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 109:110266. doi: 10.1016/j.pnpbp.2021.110266
211. Cussotto S, Clarke G, Dinan TG, and Cryan JF. Psychotropic drugs and the microbiome. Mod Trends Psychiatry. (2021) 32:113–33. doi: 10.1159/000510423
212. Weersma RK, Zhernakova A, and Fu J. Interaction between drugs and the gut microbiome. Gut. (2020) 69:1510–9. doi: 10.1136/gutjnl-2019-320204
213. Klünemann M, Andrejev S, Blasche S, Mateus A, Phapale P, Devendran S, et al. Bioaccumulation of therapeutic drugs by human gut bacteria. Nature. (2021) 597:533–8. doi: 10.1038/s41586-021-03891-8
214. Fontana A, Manchia M, Panebianco C, Paribello P, Arzedi C, Cossu E, et al. Exploring the role of gut microbiota in major depressive disorder and in treatment resistance to antidepressants. Biomedicines. (2020) 8:311. doi: 10.3390/biomedicines8090311
215. Nigam K, King FT, and Espi Forcen F. Ketamine for refractory depression: Save the best for last? J Psychopharmacol. (2025) 39:5–7. doi: 10.1177/02698811241282646
216. Huang N, Hua D, Zhan G, Li S, Zhu B, Jiang R, et al. Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression. Pharmacol Biochem Behav. (2019) 176:93–100. doi: 10.1016/j.pbb.2018.12.001
217. Pavlidi P, Kokras N, and Dalla C. Antidepressants’ effects on testosterone and estrogens: What do we know? Eur J Pharmacol. (2021) 899:173998. doi: 10.1016/j.ejphar.2021.173998
218. Zhou Q, Li X, Yang D, Xiong C, and Xiong Z. A comprehensive review and meta-analysis of neurological side effects related to second-generation antidepressants in individuals with major depressive disorder. Behav Brain Res. (2023) 447:114431. doi: 10.1016/j.bbr.2023.114431
219. Gill H, Gill B, El-Halabi S, Chen-Li D, Lipsitz O, Rosenblat JD, et al. Antidepressant medications and weight change: A narrative review. Obes (Silver Spring). (2020) 28:2064–72. doi: 10.1002/oby.22969
220. Cao H, Baranova A, Zhao Q, and Zhang F. Bidirectional associations between mental disorders, antidepressants and cardiovascular disease. BMJ Ment Health. (2024) 27:e300975. doi: 10.1136/bmjment-2023-300975
221. Johnson D, Thurairajasingam S, Letchumanan V, Chan KG, and Lee LH. Exploring the role and potential of probiotics in the field of mental health: major depressive disorder. Nutrients. (2021) 13:1728. doi: 10.3390/nu13051728
222. Ross FC, Mayer DE, Gupta A, Gill CIR, Del Rio D, Cryan JF, et al. Existing and future strategies to manipulate the gut microbiota with diet as a potential adjuvant treatment for psychiatric disorders. Biol Psychiatry. (2024) 95:348–60. doi: 10.1016/j.biopsych.2023.10.018
223. Wu PY, Chen KM, and Belcastro F. Dietary patterns and depression risk in older adults: systematic review and meta-analysis. Nutr Rev. (2021) 79:976–87. doi: 10.1093/nutrit/nuaa118
224. Clemente-Suárez VJ, Beltrán-Velasco AI, Redondo-Flórez L, Martín-Rodríguez A, and Tornero-Aguilera JF. Global impacts of western diet and its effects on metabolism and health: A narrative review. Nutrients. (2023) 15:2749. doi: 10.3390/nu15122749
225. Dinan TG, Stanton C, Long-Smith C, Kennedy P, Cryan JF, Cowan CSM, et al. Feeding melancholic microbes: MyNewGut recommendations on diet and mood. Clin Nutr. (2019) 38:1995–2001. doi: 10.1016/j.clnu.2018.11.010
226. Noble EE, Hsu TM, and Kanoski SE. Gut to brain dysbiosis: mechanisms linking western diet consumption, the microbiome, and cognitive impairment. Front Behav Neurosci. (2017) 11:9. doi: 10.3389/fnbeh.2017.00009
227. Zielińska M, Łuszczki E, Michońska I, and Dereń K. The mediterranean diet and the western diet in adolescent depression-current reports. Nutrients. (2022) 14:4390. doi: 10.3390/nu14204390
228. Ventriglio A, Sancassiani F, Contu MP, Latorre M, Di Salvatore M, Fornaro M, et al. Mediterranean diet and its benefits on health and mental health: A literature review. Clin Pract Epidemiol Ment Health. (2020) 16:156–64. doi: 10.2174/1745017902016010156
229. Krznarić Ž, Vranešić Bender D, and Meštrović T. The Mediterranean diet and its association with selected gut bacteria. Curr Opin Clin Nutr Metab Care. (2019) 22:401–6. doi: 10.1097/mco.0000000000000587
230. Seethaler B, Nguyen NK, Basrai M, Kiechle M, Walter J, Delzenne NM, et al. Short-chain fatty acids are key mediators of the favorable effects of the Mediterranean diet on intestinal barrier integrity: data from the randomized controlled LIBRE trial. Am J Clin Nutr. (2022) 116:928–42. doi: 10.1093/ajcn/nqac175
231. Merra G, Noce A, Marrone G, Cintoni M, Tarsitano MG, Capacci A, et al. Influence of Mediterranean diet on human gut microbiota. Nutrients. (2020) 13:7. doi: 10.3390/nu13010007
232. Deacon G, Kettle C, Hayes D, Dennis C, and Tucci J. Omega 3 polyunsaturated fatty acids and the treatment of depression. Crit Rev Food Sci Nutr. (2017) 57:212–23. doi: 10.1080/10408398.2013.876959
233. Lassale C, Batty GD, Baghdadli A, Jacka F, Sánchez-Villegas A, Kivimäki M, et al. Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol Psychiatry. (2019) 24:965–86. doi: 10.1038/s41380-018-0237-8
234. Nishi D, Su KP, Usuda K, Chang JP, Hamazaki K, Ishima T, et al. Plasma estradiol levels and antidepressant effects of omega-3 fatty acids in pregnant women. Brain Behav Immun. (2020) 85:29–34. doi: 10.1016/j.bbi.2019.02.014
235. Alcorta A, Porta A, Tárrega A, Alvarez MD, and Vaquero MP. Foods for plant-based diets: challenges and innovations. Foods. (2021) 10:293. doi: 10.3390/foods10020293
236. Shen YC, Chang CE, Lin MN, and Lin CL. Vegetarian diet is associated with lower risk of depression in Taiwan. Nutrients. (2021) 13:1059. doi: 10.3390/nu13041059
237. Iguacel I, Huybrechts I, Moreno LA, and Michels N. Vegetarianism and veganism compared with mental health and cognitive outcomes: a systematic review and meta-analysis. Nutr Rev. (2021) 79:361–81. doi: 10.1093/nutrit/nuaa030
238. Ocklenburg S and Borawski J. Vegetarian diet and depression scores: A meta-analysis. J Affect Disord. (2021) 294:813–5. doi: 10.1016/j.jad.2021.07.098
239. Brietzke E, Mansur RB, Subramaniapillai M, Balanzá-Martínez V, Vinberg M, González-Pinto A, et al. Ketogenic diet as a metabolic therapy for mood disorders: Evidence and developments. Neurosci Biobehav Rev. (2018) 94:11–6. doi: 10.1016/j.neubiorev.2018.07.020
240. Guan YF, Huang GB, Xu MD, Gao F, Lin S, Huang J, et al. Anti-depression effects of ketogenic diet are mediated via the restoration of microglial activation and neuronal excitability in the lateral habenula. Brain Behav Immun. (2020) 88:748–62. doi: 10.1016/j.bbi.2020.05.032
241. Danan A, Westman EC, Saslow LR, and Ede G. The ketogenic diet for refractory mental illness: A retrospective analysis of 31 inpatients. Front Psychiatry. (2022) 13:951376. doi: 10.3389/fpsyt.2022.951376
242. Rudzki L, Ostrowska L, Pawlak D, Małus A, Pawlak K, Waszkiewicz N, et al. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. (2019) 100:213–22. doi: 10.1016/j.psyneuen.2018.10.010
243. Zhang X, Chen S, Zhang M, Ren F, Ren Y, Li Y, et al. Effects of fermented milk containing lacticaseibacillus paracasei strain shirota on constipation in patients with depression: A randomized, double-blind, placebo-controlled trial. Nutrients. (2021) 13:2238. doi: 10.3390/nu13072238
244. Ibejekwe AN, Dashen MM, Ngene AC, and Egbere OJ. Probiotic potential and modulatory effects of mixed culture of lactic acid bacteria on mice infected with Escherichia coli O157:H7. Adv Gut Microbiome Res. (2025) 2025:7392790. doi: 10.1155/agm3/7392790
245. Dinan TG, Butler MI, and Cryan JF. Psychobiotics: evolution of novel antidepressants. Mod Trends Psychiatry. (2021) 32:134–43. doi: 10.1159/000510424
246. Vaghef-Mehrabany E, Maleki V, Behrooz M, Ranjbar F, and Ebrahimi-Mameghani M. Can psychobiotics “mood” ify gut? An update systematic review of randomized controlled trials in healthy and clinical subjects, on anti-depressant effects of probiotics, prebiotics, and synbiotics. Clin Nutr. (2020) 39:1395–410. doi: 10.1016/j.clnu.2019.06.004
247. Bambury A, Sandhu K, Cryan JF, and Dinan TG. Finding the needle in the haystack: systematic identification of psychobiotics. Br J Pharmacol. (2018) 175:4430–8. doi: 10.1111/bph.14127
248. Chapman CM, Gibson GR, and Rowland I. In vitro evaluation of single- and multi-strain probiotics: Inter-species inhibition between probiotic strains, and inhibition of pathogens. Anaerobe. (2012) 18:405–13. doi: 10.1016/j.anaerobe.2012.05.004
249. Musazadeh V, Zarezadeh M, Faghfouri AH, Keramati M, Jamilian P, Jamilian P, et al. Probiotics as an effective therapeutic approach in alleviating depression symptoms: an umbrella meta-analysis. Crit Rev Food Sci Nutr. (2023) 63:8292–300. doi: 10.1080/10408398.2022.2051164
250. Ding Y, Bu F, Chen T, Shi G, Yuan X, Feng Z, et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl Microbiol Biotechnol. (2021) 105:8411–26. doi: 10.1007/s00253-021-11622-2
251. He Q, Si C, Sun Z, Chen Y, and Zhang X. The intervention of prebiotics on depression via the gut-brain axis. Molecules. (2022) 27:3671. doi: 10.3390/molecules27123671
252. Kazemi A, Noorbala AA, Azam K, Eskandari MH, and Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin Nutr. (2019) 38:522–8. doi: 10.1016/j.clnu.2018.04.010
253. Moludi J, Khedmatgozar H, Nachvak SM, Abdollahzad H, Moradinazar M, and Sadeghpour Tabaei A. The effects of co-administration of probiotics and prebiotics on chronic inflammation, and depression symptoms in patients with coronary artery diseases: a randomized clinical trial. Nutr Neurosci. (2022) 25:1659–68. doi: 10.1080/1028415x.2021.1889451
254. Freijy TM, Cribb L, Oliver G, Metri NJ, Opie RS, Jacka FN, et al. Effects of a high-prebiotic diet versus probiotic supplements versus synbiotics on adult mental health: The “Gut Feelings” randomised controlled trial. Front Neurosci. (2022) 16:1097278. doi: 10.3389/fnins.2022.1097278
255. Hadi A, Sepandi M, Marx W, Moradi S, and Parastouei K. Clinical and psychological responses to synbiotic supplementation in obese or overweight adults: A randomized clinical trial. Complement Ther Med. (2019) 47:102216. doi: 10.1016/j.ctim.2019.102216
256. Haghighat N, Rajabi S, and Mohammadshahi M. Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: a randomized, double-blinded, clinical trial. Nutr Neurosci. (2021) 24:490–9. doi: 10.1080/1028415x.2019.1646975
257. Wu Y, Wang Y, Hu A, Shu X, Huang W, Liu J, et al. Lactobacillus plantarum-derived postbiotics prevent Salmonella-induced neurological dysfunctions by modulating gut-brain axis in mice. Front Nutr. (2022) 9:946096. doi: 10.3389/fnut.2022.946096
258. Maehata H, Kobayashi Y, Mitsuyama E, Kawase T, Kuhara T, Xiao JZ, et al. Heat-killed Lactobacillus helveticus strain MCC1848 confers resilience to anxiety or depression-like symptoms caused by subchronic social defeat stress in mice. Biosci Biotechnol Biochem. (2019) 83:1239–47. doi: 10.1080/09168451.2019.1591263
259. Zhang F, Luo W, Shi Y, Fan Z, and Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. (2012) 107:1755; author reply p.1755–1756. doi: 10.1038/ajg.2012.251
260. D T and Venkatesh MP. Fecal microbiota transplantation: History, procedure and regulatory considerations. Presse Med. (2023) 52:104204. doi: 10.1016/j.lpm.2023.104204
261. De Groot PF, Frissen MN, De Clercq NC, and Nieuwdorp M. Fecal microbiota transplantation in metabolic syndrome: History, present and future. Gut Microbes. (2017) 8:253–67. doi: 10.1080/19490976.2017.1293224
262. Borody TJ, Paramsothy S, and Agrawal G. Fecal microbiota transplantation: indications, methods, evidence, and future directions. Curr Gastroenterol Rep. (2013) 15:337. doi: 10.1007/s11894-013-0337-1
263. Zhang W, Zou G, Li B, Du X, Sun Z, Sun Y, et al. Fecal microbiota transplantation (FMT) alleviates experimental colitis in mice by gut microbiota regulation. J Microbiol Biotechnol. (2020) 30:1132–41. doi: 10.4014/jmb.2002.02044
264. Vendrik KE, Chernova VO, Kuijper EJ, Terveer EM, Van Hilten JJ, and Contarino MF. Safety and feasibility of faecal microbiota transplantation for patients with Parkinson’s disease: a protocol for a self-controlled interventional donor-FMT pilot study. BMJ Open. (2023) 13:e071766. doi: 10.1136/bmjopen-2023-071766
265. Davar D, Dzutsev AK, Mcculloch JA, Rodrigues RR, Chauvin JM, Morrison RM, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. (2021) 371:595–602. doi: 10.1126/science.abf3363
266. Xue L, Deng Z, Luo W, He X, and Chen Y. Effect of fecal microbiota transplantation on non-alcoholic fatty liver disease: A randomized clinical trial. Front Cell Infect Microbiol. (2022) 12:759306. doi: 10.3389/fcimb.2022.759306
267. Wu Z, Zhang B, Chen F, Xia R, Zhu D, Chen B, et al. Fecal microbiota transplantation reverses insulin resistance in type 2 diabetes: A randomized, controlled, prospective study. Front Cell Infect Microbiol. (2022) 12:1089991. doi: 10.3389/fcimb.2022.1089991
268. Koh A and Bäckhed F. From association to causality: the role of the gut microbiota and its functional products on host metabolism. Mol Cell. (2020) 78:584–96. doi: 10.1016/j.molcel.2020.03.005
269. Knudsen JK, Michaelsen TY, Bundgaard-Nielsen C, Nielsen RE, Hjerrild S, Leutscher P, et al. Faecal microbiota transplantation from patients with depression or healthy individuals into rats modulates mood-related behaviour. Sci Rep. (2021) 11:21869. doi: 10.1038/s41598-021-01248-9
270. Pu Y, Zhang Q, Tang Z, Lu C, Wu L, Zhong Y, et al. Fecal microbiota transplantation from patients with rheumatoid arthritis causes depression-like behaviors in mice through abnormal T cells activation. Transl Psychiatry. (2022) 12:223. doi: 10.1038/s41398-022-01993-z
271. Rao J, Xie R, Lin L, Jiang J, Du L, Zeng X, et al. Fecal microbiota transplantation ameliorates gut microbiota imbalance and intestinal barrier damage in rats with stress-induced depressive-like behavior. Eur J Neurosci. (2021) 53:3598–611. doi: 10.1111/ejn.15192
272. Lin H, Guo Q, Wen Z, Tan S, Chen J, Lin L, et al. The multiple effects of fecal microbiota transplantation on diarrhea-predominant irritable bowel syndrome (IBS-D) patients with anxiety and depression behaviors. Microb Cell Fact. (2021) 20:233. doi: 10.1186/s12934-021-01720-1
273. Dailey FE, Turse EP, Daglilar E, and Tahan V. The dirty aspects of fecal microbiota transplantation: a review of its adverse effects and complications. Curr Opin Pharmacol. (2019) 49:29–33. doi: 10.1016/j.coph.2019.04.008
Keywords: depression, gut microbiota, microbiota-gut-brain axis, neuroinflammation, therapeutic interventions
Citation: Zhu Z, Cheng Y, Liu X, Xu X, Ding W, Ling Z, Liu J and Cai G (2025) The microbiota-gut-brain axis in depression: unraveling the relationships and therapeutic opportunities. Front. Immunol. 16:1644160. doi: 10.3389/fimmu.2025.1644160
Received: 10 June 2025; Accepted: 09 September 2025;
Published: 25 September 2025.
Edited by:
Tommy Regen, Johannes Gutenberg-University Mainz, GermanyReviewed by:
Eduardo Duarte-Silva, University of Texas Health Science Center at Houston, United StatesAmeer Luqman, Chongqing University, China
Copyright © 2025 Zhu, Cheng, Liu, Xu, Ding, Ling, Liu and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zongxin Ling, bGluZ3pvbmd4aW5Aemp1LmVkdS5jbg==; Jiaming Liu, d3pqaWFtaW5nX2xpdUAxNjMuY29t; Guangyong Cai, Z3Vhbmd5b25nX2NhaUAxNjMuY29t
†These authors have contributed equally to this work
‡ORCID: Zongxin Ling, orcid.org/0000-0001-9662-099X
 Zhangcheng Zhu1†
Zhangcheng Zhu1† Xia Liu
Xia Liu Zongxin Ling
Zongxin Ling Jiaming Liu
Jiaming Liu