- Department of Ophthalmology, Fourth People`s Hospital of Shenyang, Shenyang, Liaoning, China
Graves’ ophthalmopathy (GO), also known as thyroid eye disease (TED), is the most common extrathyroidal manifestation of Graves’ disease and a leading cause of visual morbidity. The disease primarily affects the orbital tissue and is characterized by inflammation, expansion of extraocular muscles, and remodeling of orbital fat, resulting in proptosis, diplopia, and even vision loss. Active GO poses significant therapeutic challenges and often requires prompt intervention to preserve visual function and improve quality of life. Over the past decade, considerable progress has been made in understanding the immunopathogenesis of GO, leading to the development of targeted pharmacological therapies that extend beyond traditional systemic corticosteroids. This review summarizes recent advances in the drug therapy of active GO, focusing on novel immunomodulators, biological agents such as monoclonal antibodies targeting CD20, IL-6 R, and insulin-like growth factor-1 receptor (IGF-1R), and evolving treatment strategies based on disease activity and severity. We also discuss current clinical practice guidelines, emerging therapeutic targets under investigation, and future perspectives in the individualized management of this vision-threatening autoimmune condition.
1 Introduction
Graves’ ophthalmopathy (GO), or thyroid-associated ophthalmopathy (TAO), is an autoimmune inflammatory disorder of the orbit that commonly coexists with Graves’ hyperthyroidism. It affects up to 20% of patients with Graves’ disease and significantly impacts functional vision, appearance, and psychosocial well-being(!!! INVALID CITATION!!! (1)). GO is marked by autoantibody-mediated activation of orbital fibroblasts, infiltration of immune cells, and overproduction of glycosaminoglycans, leading to tissue expansion, proptosis, and, in severe cases, compressive optic neuropathy (1, 2).
Based on the European Group on Graves’ Orbitopathy (EUGOGO) consensus, GO is categorized by disease activity (active vs. inactive) and severity (mild, moderate-to-severe, or sight-threatening) (3, 4). Active GO is particularly challenging due to persistent inflammation, progressive disfigurement, and the potential for irreversible visual impairment (5). Although systemic glucocorticoids have long been the mainstay of treatment, their efficacy is limited in some patients, and relapse is common after withdrawal. Additionally, high-dose steroids carry considerable systemic toxicity, prompting the search for safer and more effective therapies (6).
Recent years have witnessed a paradigm shift in GO treatment strategies, driven by deeper insights into disease pathophysiology and advances in immunotherapy. Biological agents targeting specific pathways, including anti-CD20 monoclonal antibodies (e.g., rituximab or obinutuzumab) (7), IL-6 receptor antagonists (e.g., tocilizumab) (8), and insulin-like growth factor-1 receptor (IGF-1R) inhibitors (e.g., teprotumumab) (9), have shown promising efficacy in clinical trials. Moreover, treatment algorithms are increasingly emphasizing early intervention during the active phase to prevent progression and optimize long-term outcomes.
This review provides a comprehensive update on recent pharmacologic advances in the management of active GO. It highlights the clinical efficacy, safety profiles, and mechanistic rationale of emerging therapies, discusses evolving treatment guidelines, and explores ongoing challenges and future directions in GO care.
2 Pathophysiology of graves’ ophthalmopathy
2.1 Autoantigens and immune activation
Two key autoantigens have been identified in GO pathogenesis: the thyroid-stimulating hormone receptor (TSHR) and the IGF-1R (10, 11). Orbital fibroblasts and preadipocytes express both TSHR and IGF-1R, forming a functional complex. Autoantibodies, particularly thyroid-stimulating immunoglobulins (TSIs), bind to TSHR on orbital fibroblasts, triggering proinflammatory signaling and adipogenic differentiation. Recent studies have shown that IGF-1R, when stimulated by its ligands or cross-talked with TSHR signaling, enhances fibroblast activation, hyaluronan synthesis, and proinflammatory cytokine secretion (12). This mechanism underlies the rationale for IGF-1R-targeted therapies, such as teprotumumab.
2.2 Orbital fibroblasts and adipogenesis
Orbital fibroblasts are the primary effector cells in GO. Upon activation by TSIs, IGF-1, or proinflammatory cytokines (e.g., IL-1β, TNF-α), these cells proliferate, secrete glycosaminoglycans like hyaluronic acid, and differentiate into either myofibroblasts or adipocytes, depending on their CD90 expression. CD90 (-) fibroblasts tend to undergo adipogenic differentiation, contributing to orbital fat expansion, while CD90 (+) fibroblasts are more prone to fibrosis (13, 14). This dual differentiation potential explains the heterogeneity in clinical presentations, ranging from proptosis-dominant to fibrotic restrictive forms of GO.
2.3 Cytokine network and inflammatory cascade
The GO orbit exhibits infiltration by T cells (especially Th1 in the early stage and Th2 in later phases), B cells, and macrophages. These immune cells release a host of cytokines, including IL-6, IL-17, IFN-γ, and TNF-α, which further amplify local inflammation and fibroblast activation (15). TGF-β plays a pivotal role in sustaining chronic inflammation and fibrosis, and has emerged as an actively pursued therapeutic target (16). The activation of the JAK-STAT signaling pathway downstream of cytokine receptors further drives the pathological changes in the orbital tissues (17).
2.4 Role of B cells and autoantibodies
B cells contribute to the pathogenesis of GO through both antibody-dependent and independent mechanisms (18). In addition to producing TSIs and anti-IGF-1R antibodies, B cells act as antigen-presenting cells and secrete cytokines that shape T cell responses. Clinical studies with anti-CD20 monoclonal antibodies such as rituximab have confirmed the pathogenic relevance of B cells in moderate-to-severe GO (19, 20).
2.5 Oxidative stress and hypoxia
Oxidative stress also plays a critical role in GO, particularly in enhancing orbital fibroblast responses. Hyperglycemia, hypoxia, and smoking all increase reactive oxygen species (ROS) production, which activates proinflammatory transcription factors such as NF-κB (21). These factors synergize with cytokine signaling to exacerbate tissue damage. Moreover, hypoxia-inducible factor 1-alpha (HIF-1α) has been implicated in the pathological angiogenesis and tissue remodeling observed in GO (22).
3 Traditional pharmacological therapies for moderate-to-severe active graves’ ophthalmopathy
Despite recent advances in targeted therapies, traditional pharmacologic treatments remain foundational in the management of active GO. These treatments primarily focus on immune suppression, inflammation control, and symptomatic relief during the active phase of the disease.
3.1 Glucocorticoids
3.1.1 Mechanism of action
Glucocorticoids exert potent anti-inflammatory and immunosuppressive effects by inhibiting the transcription of proinflammatory cytokines such as IL-1, IL-6, TNF-α, and IFN-γ, as well as reducing lymphocyte proliferation and migration. In GO, they dampen the autoimmune activation of orbital fibroblasts and reduce edema and inflammatory cell infiltration (23).
3.1.2 Administration routes and regimens
Intravenous methylprednisolone (IVMP) is currently considered the first-line therapy for moderate-to-severe active GO due to its superior efficacy and lower incidence of systemic side effects compared to oral prednisone (24, 25). The standard cumulative dose ranges from 4.5 to 7.5 g administered over 12 weeks, as supported by EUGOGO guidelines (26).
High-dose oral GCs are reserved for cases with contraindications to IV administration, although they are associated with a higher risk of adverse events such as osteoporosis, glucose intolerance, and weight gain (27).
3.1.3 Efficacy and limitations
IVMP achieves a positive response in improving clinical activity scores (CAS), proptosis, and diplopia, particularly when initiated during the early active phase (28). However, some patients are refractory to GCs, and recurrence is not uncommon. Moreover, cumulative doses above 8 g are associated with hepatotoxicity and cardiovascular risks, limiting long-term use (29).
3.2 Radiotherapy
Orbital radiotherapy (ORT) delivers localized low-dose radiation (20 Gy over 10 sessions) to suppress lymphocytic infiltration and fibroblast activity (30, 31). ORT is often used as an adjunct to GCs, especially in cases with diplopia and extraocular muscle involvement. Although its anti-inflammatory effects are modest, combination therapy with IVMP can yield additive benefits. However, its use is limited by delayed onset of action and concerns over radiation-induced retinopathy or optic neuropathy, especially in diabetic patients (32).
3.3 Immunosuppressive agents
3.3.1 Mycophenolate mofetil
MMF selectively inhibits inosine monophosphate dehydrogenase, thereby suppressing B and T cell proliferation. Recent meta-analysis suggests MMF, either alone or combined with GCs, is superior to GCs alone in improving CAS and reducing relapse (33, 34). MMF is well tolerated and represents a promising alternative or adjunct, particularly for steroid-resistant or relapsing cases.
3.3.2 Cyclosporine and azathioprine
The use of some traditional nonspecific immunosuppressants such as mycophenolate, cyclosporine and azathioprine appears useful in combination with steroid therapy to achieve stable results in the long term (35). Therefore, these agents have historically been used as adjuncts to GCs in refractory cases, though their efficacy is limited and toxicity profiles (nephrotoxicity, hepatotoxicity, bone marrow suppression) restrict widespread use (36).
3.4 Rituximab
Rituximab, an anti-CD20 monoclonal antibody, depletes B cells and reduces autoantibody production (37). Clinical trials on rituximab have yielded mixed results. While some studies demonstrated significant improvement in CAS and disease stabilization (18, 38), limited studies focusing on the steroid-resistant subpopulation of GO patients have shown inconsistencies in the response to Rituximab treatment (39–41). The discrepancy may be due to variation in disease stage and baseline activity. Nevertheless, rituximab remains a potential option in steroid-refractory and relapsing GO, particularly with high B cell activity.
3.5 Limitations of traditional therapies
Traditional therapies, especially GCs and radiotherapy, are largely non-specific and associated with significant systemic toxicity. They also fail to address the underlying molecular drivers of GO such as TSHR and IGF-1R signaling. Moreover, a substantial subset of patients are resistant or intolerant to these interventions, highlighting the need for novel, targeted approaches.
4 Emerging targeted therapies for active graves’ ophthalmopathy
With increasing insights into the molecular mechanisms of GO, a range of targeted biologics and small molecule inhibitors have emerged as promising alternatives or adjuncts to traditional immunosuppressive therapy. These therapies aim to interfere with key pathogenic pathways such as the IGF-1R, interleukin signaling, and the Janus kinase/signal transducer and activator of transcription (JAK-STAT) pathway.
4.1 Teprotumumab: IGF-1R inhibition
4.1.1 Mechanism of action
Teprotumumab is a fully human monoclonal antibody that inhibits IGF-1R, a receptor overexpressed in orbital fibroblasts of GO patients and functionally linked to the TSHR (42). Its blockade reduces fibroblast activation, hyaluronic acid production, and adipogenesis, key contributors to tissue expansion and inflammation in GO.
4.1.2 Clinical efficacy
Teprotumumab has demonstrated unprecedented efficacy in clinical trials (43, 44). The Open-Label Clinical Extension Study (OPTIC-X) evaluated the safety and efficacy of teprotumumab in GO patients who were nonresponsive or experienced disease flare after prior treatment in the OPTIC trial. Among 37 prior placebo recipients, 89.2% became proptosis responders with results comparable to those in the original OPTIC trial, despite a longer median disease duration (12.9 vs. 6.3 months). Response durability was high, with maintained improvements in proptosis, CAS, and diplopia at 48 weeks. Of the five initial nonresponders to teprotumumab, two responded upon re-treatment. Additionally, 62.5% of prior responders who flared showed benefit from re-treatment. No new safety concerns emerged, though mild hearing impairments were observed, reinforcing the need for continued postmarketing surveillance. These findings support the benefit of teprotumumab even in later disease stages and in cases requiring re-treatment (45).
4.1.3 Safety and limitations
The most common adverse events included muscle spasms, hyperglycemia, and hearing-related disorders (e.g., tinnitus, hearing loss), some of which may be irreversible. Teprotumumab’s high cost and limited availability currently restrict its global use, though its FDA approval marks a paradigm shift in GO treatment (46).
4.2 Tocilizumab: IL-6 receptor blockade
4.2.1 Mechanism of action
Tocilizumab is a humanized monoclonal antibody targeting the IL-6 receptor. IL-6 plays a central role in GO pathogenesis by promoting Th17 cell differentiation, B cell activation, and cytokine release. Inhibiting IL-6 signaling mitigates orbital inflammation and fibrosis (47, 48).
4.2.2 Clinical data
Recent systematic review analyzed 29 studies on tocilizumab for GO, mostly case reports and series, with only one randomized clinical trial (RCT) (49). Tocilizumab, primarily used in glucocorticoid-resistant or relapsing cases, showed effectiveness in reducing inflammation and proptosis, with a low relapse rate (8.2%) and no severe side effects reported (49). Although the only RCT found no statistically significant improvement at six months, a recent meta-analysis suggested that tocilizumab may be the most effective option for reducing proptosis (50). Further randomized trials comparing tocilizumab with other treatments are needed.
4.2.3 Adverse effects
Reported side effects include elevated liver enzymes, gastrointestinal discomfort, and risk of infections (51). Regular monitoring is essential, especially in long-term use. Its off-label use for GO, lack of large-scale randomized trials, and high cost limits its broader adoption.
4.3 Janus Kinase inhibitors
Recent study used a mouse model to investigate the role of transmembrane protein 2 (TMEM2) in GO. TMEM2 expression was significantly reduced in GO orbital tissues. Functional assays showed that TMEM2 suppresses inflammation, oxidative stress, and adipogenesis in orbital fibroblasts, while activating the JAK/STAT pathway. In vivo, TMEM2 overexpression alleviated inflammation, adipose tissue expansion, and fibrosis (17). These findings suggest TMEM2 as a key regulator in GO pathogenesis and a potential therapeutic target.
4.4 Other investigational agents
4.4.1 TNF-α inhibitors
Drugs such as etanercept and infliximab, targeting TNF-α, have been trialed in GO with limited and inconsistent results. Though conceptually attractive, their impact on orbital inflammation appears less robust, and risk of infections remains a concern (52).
4.4.2 IL-1 and IL-17 inhibitors
Agents targeting IL-1β (anakinra) and IL-17A (secukinumab) are under investigation due to their roles in Th1/Th17-mediated autoimmunity (53).
5 Comparison of traditional and emerging treatments: efficacy, safety, and clinical implications
As the therapeutic landscape for active Graves’ ophthalmopathy evolves, clinicians are increasingly faced with choices between traditional immunosuppressive therapies and emerging targeted agents. A comprehensive comparison of their efficacy, safety profiles, indications, and limitations is essential for personalized and evidence-based treatment planning.
5.1 Efficacy
5.1.1 Improvement in clinical activity score
Previous studies have reported that approximately 58–83% of patients receiving IVGC, compared to 51% among those treated with oral glucocorticoids (OGC) (54–56). Of which, in a RCT involving 70 patients with active moderate-to-severe GO, median CAS decreased from 5 to 2 in the IVGC group, while in the OGC group, it declined from 5 to 3 (54). Notably, 77% (27/35) of IVGC-treated patients showed a 3-point CAS reduction, compared to 51% (18/35) in the OGC group. Another RCT with 81 participants receiving IVGC reported reductions in mean CAS from 3.66 at baseline to 1.65 and 1.68 (in right and left eyes, respectively) after 36 weeks of treatment (57). A RCT involving 16 GO patients treated with IVGC reported that 75% experienced a ≥2-point reduction in CAS at 24 weeks, with 69% achieving CAS inactivation (<3) (56). Another RCT comparing IVGC plus atorvastatin versus IVGC alone showed that only 28% of the 39 patients receiving IVGC monotherapy achieved improvement in a composite endpoint (58). Additionally, in a larger RCT of 159 patients with active moderate-to-severe TED, three different cumulative IVGC doses (2.25 g, 4.98 g, and 7.47 g) were evaluated. At 12 weeks, 81–83% of those receiving the medium and high doses had CAS improvements of >2 points, compared to 58% in the low-dose group. Disease inactivation (CAS ≤2) was observed in 45–65% of patients, depending on the dose (55).
5.1.2 Proptosis reduction
One of the most significant limitations of traditional treatments is their limited effect on proptosis. Teprotumumab uniquely demonstrates clinically meaningful and statistically significant proptosis reduction (-3.0 mm for phase 2 study; -3.32 mm for phase 3 study), approaching the effects of orbital decompression surgery (59). Neither IVGCs nor cyclosporine can achieve such anatomical improvements.
5.1.3 Diplopia and visual Function
Diplopia often persists despite IVGC therapy. Tocilizumab and teprotumumab have shown improvements in extraocular muscle function and diplopia scores (60), suggesting that early intervention with targeted therapies may reduce the need for rehabilitative surgery.
5.2 Safety profile
5.2.1 Systemic glucocorticoids
Although effective, IVGCs are associated with considerable side effects, including hyperglycemia, weight gain, hypertension, osteoporosis, and hepatic dysfunction. These risks are particularly concerning in elderly patients and those with comorbidities. A previous retrospective cohort study compared the metabolic, immunological, and therapeutic effects of 4-week versus 12-week IVG therapy in 48 patients with active moderate-to-severe GO. The 12-week group showed significant increases in glucose and lipid levels, indicating a higher metabolic burden. Both groups had reduced bone metabolism markers and autoantibody levels, though thyroglobulin antibodies declined significantly only in the 4-week group. While both regimens improved clinical outcomes similarly, the 4-week group had greater ADC improvement and fewer metabolic side effects, supporting its use in patients with metabolic risk (61).
5.2.2 Traditional immunosuppressants
Agents such as azathioprine and cyclosporine carry risks of nephrotoxicity, hepatotoxicity, and increased susceptibility to infections (62). Long-term use often necessitates careful monitoring and dose adjustments.
5.2.3 Targeted therapies
Biologics, while generally better tolerated in the short term, have specific risks. Teprotumumab is associated with hearing abnormalities and glycemic disturbances; tocilizumab may cause elevated liver enzymes and neutropenia; JAK inhibitors have been linked to thrombosis and reactivation of latent infections. Thus, targeted therapies require individualized risk-benefit assessment and close monitoring.
5.3 Route of administration and treatment burden
Traditional treatments like IVGCs and immunosuppressants require either inpatient administration or frequent monitoring. Teprotumumab is administered via intravenous infusion every three weeks, which may be burdensome. Oral agents such as tofacitinib (a JAK inhibitor) offer greater convenience but remain investigational in GO.
5.4 Indications and patient selection
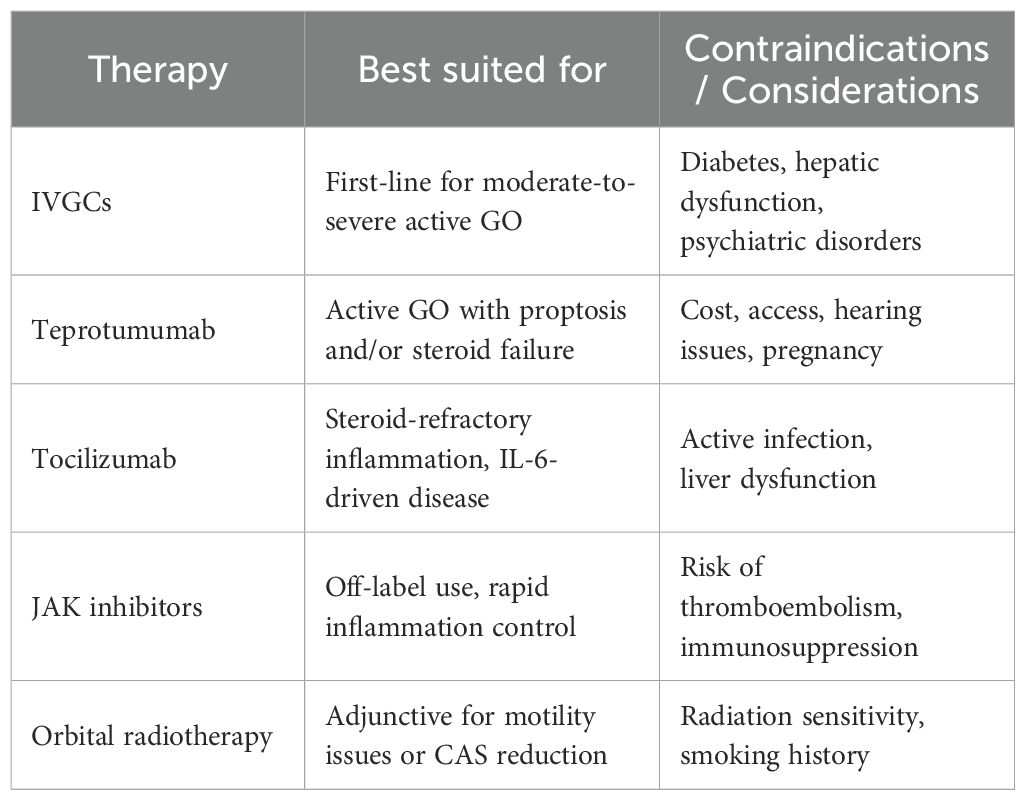
Therapies for active GO vary by severity and patient factors, with IV glucocorticoids as first-line and alternatives like teprotumumab, tocilizumab, JAK inhibitors, and radiotherapy used based on response and comorbidities (Table 1).
5.5 Long-term outcomes and surgery avoidance
Emerging data suggest that early initiation of biologics, particularly teprotumumab, may reduce the need for rehabilitative surgery by reversing or halting the anatomical and functional deterioration of orbital tissues. This represents a major advancement, as conventional approaches often require staged decompression, muscle correction, and eyelid surgery.
5.6 Cost-effectiveness and accessibility
The financial burden of biologics remains a significant barrier to widespread adoption. Teprotumumab is cost-prohibitive in many countries and not yet available outside the U.S. Conversely, IVGCs and immunosuppressants are inexpensive and widely accessible but less effective for key GO outcomes such as proptosis (63).
5.7 The limitations of drug therapy for GO
Pharmacological therapy remains the cornerstone of treatment for active moderate-to-severe GO, with systemic corticosteroids being the most widely used first-line option due to their potent anti-inflammatory effects. However, their benefits are often transient, with high relapse rates and significant adverse effects limiting long-term use. Immunosuppressive agents such as mycophenolate mofetil and cyclosporine have demonstrated varying degrees of efficacy, while newer biologics like teprotumumab have shown superior outcomes in randomized trials. Nevertheless, drug resistance, individual response variability, high costs, and limited accessibility, especially in low-resource settings, highlight the need for continued therapeutic innovation. Furthermore, current treatments often target inflammation but have limited impact on late-stage fibrotic changes, emphasizing the importance of early diagnosis and timely intervention.
6 Future directions and challenges
Despite notable advances in pharmacological therapies for active GO, significant challenges remain. GO is a complex autoimmune disorder characterized by dysregulated TSHR and IGF-1R signaling, elevated proinflammatory cytokines, and heterogeneous orbital fibroblast subsets, all of which offer therapeutic targets. Although biologics such as the IGF-1R inhibitor teprotumumab have shown promising efficacy, issues related to high cost, limited global availability, and variability in patient response underscore the unmet clinical need for novel and accessible therapeutic options. Emerging biologic strategies, such as TSHR antagonists, anti-cytokine agents (e.g., IL-1β, TNF-α inhibitors), and biosimilars of existing drugs—are under investigation, alongside innovative cell-based therapies including mesenchymal stem cells (MSCs) and exosome-based approaches.
To address the heterogeneity of GO, future management should prioritize individualized, mechanism-based treatments that balance efficacy, safety, and cost. Precision medicine approaches, incorporating biomarker-guided stratification, pharmacogenomics, and phenotype-specific therapy (targeting immuno-, fibrotic-, or adipogenic-dominant patterns), may offer more tailored and effective care. At the same time, the development of biosimilars, pricing reform, and wider guideline implementation are urgently needed to address global disparities in access to advanced treatments. Furthermore, long-term outcome monitoring, strategies for relapse prevention, and the evaluation of optimal combination regimens remain key areas of investigation. Multidisciplinary collaboration and the integration of digital health tools—including AI-assisted imaging analysis and mobile health platforms, will be instrumental in advancing personalized, cost-effective, and sustainable GO care.
Author contributions
LW: Software, Visualization, Writing – original draft, Writing – review & editing. LC: Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Du B, Wang Y, Yang M, and He W. Clinical features and clinical course of thyroid-associated ophthalmopathy: a case series of 3620 Chinese cases. Eye (Lond). (2021) 35:2294–301. doi: 10.1038/s41433-020-01246-7
2. Burch HB, Perros P, Bednarczuk T, Cooper DS, Dolman PJ, Leung AM, et al. Management of thyroid eye disease: a Consensus Statement by the American Thyroid Association and the European Thyroid Association. Eur Thyroid J. (2022) 11. doi: 10.1530/ETJ-22-0189
3. Perros P, Hegedus L, Bartalena L, Marcocci C, Kahaly GJ, Baldeschi L, et al. Graves’ orbitopathy as a rare disease in Europe: a European Group on Graves’ Orbitopathy (EUGOGO) position statement. Orphanet J Rare Dis. (2017) 12:72. doi: 10.1186/s13023-017-0625-1
4. Davies TF, Andersen S, Latif R, Nagayama Y, Barbesino G, Brito M, et al. Graves’ disease. Nat Rev Dis Primers. (2020) 6:52. doi: 10.1038/s41572-020-0184-y
5. Zhou M, Wu D, Cai L, Wang C, Su Y, Li Y, et al. Increased choroidal stromal area in patients with active Graves’ ophthalmopathy based on binarisation method of optical coherence tomographic images. BMJ Open Ophthalmol. (2024) 9. doi: 10.1136/bmjophth-2023-001443
6. Moghadam-Kia S and Werth VP. Prevention and treatment of systemic glucocorticoid side effects. Int J Dermatol. (2010) 49:239–48. doi: 10.1111/j.1365-4632.2009.04322.x
7. Manousou S, Holmberg M, Ekdahl E, Malmgren H, and Filipsson Nystrom H. Rituximab treatment as second-line therapy in glucocorticoid nonresponsive graves’ Orbitopathy: A nonrandomized, controlled, interventional study. Endocr Pract. (2025) 31:447–54. doi: 10.1016/j.eprac.2024.12.007
8. Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, Perez-Pampin E, Romo Lopez A, Rodriguez Alvarez FM, et al. Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant graves orbitopathy: A randomized clinical trial. Am J Ophthalmol. (2018) 195:181–90. doi: 10.1016/j.ajo.2018.07.038
9. Smith TJ, Cavida D, Hsu K, Kim S, Fu Q, Barbesino G, et al. Glycemic trends in patients with thyroid eye disease treated with teprotumumab in 3 clinical trials. Ophthalmology. (2024) 131:815–26. doi: 10.1016/j.ophtha.2024.01.023
10. Lee JY, Lee SB, Yang SW, and Paik JS. Linsitinib inhibits IGF-1-induced cell proliferation and hyaluronic acid secretion by suppressing PI3K/Akt and ERK pathway in orbital fibroblasts from patients with thyroid-associated ophthalmopathy. PloS One. (2024) 19:e0311093. doi: 10.1371/journal.pone.0311093
11. Latif R, Mezei M, and Davies TF. Mechanisms in thyroid eye disease: the TSH receptor interacts directly with the IGF-1 receptor. Endocrinology. (2025) 166. doi: 10.1210/endocr/bqaf009
12. Gulbins A, Horstmann M, Keitsch S, Soddemann M, Wilker B, Wilson GC, et al. Potential involvement of the bone marrow in experimental Graves’ disease and thyroid eye disease. Front Endocrinol (Lausanne). (2023) 14:1252727. doi: 10.3389/fendo.2023.1252727
13. Fang S, Huang Y, Zhong S, Li Y, Zhang Y, Li Y, et al. Regulation of orbital fibrosis and adipogenesis by pathogenic th17 cells in graves orbitopathy. J Clin Endocrinol Metab. (2017) 102:4273–83. doi: 10.1210/jc.2017-01349
14. Zeng F, Gao M, Liao S, Zhou Z, Luo G, and Zhou Y. Role and mechanism of CD90(+) fibroblasts in inflammatory diseases and Malignant tumors. Mol Med. (2023) 29:20. doi: 10.1186/s10020-023-00616-7
15. Zeitrag J, Benedicic M, Wolf J, Ammon T, Mayr V, Holthoff HP, et al. Inflammatory and tolerogenic dendritic cells and T lymphocytes in Graves’ thyroidal and orbital disease. Biochim Biophys Acta Mol Basis Dis. (2025) 1871:167747. doi: 10.1016/j.bbadis.2025.167747
16. Chang HH, Wu SB, and Tsai CC. A review of pathophysiology and therapeutic strategies targeting TGF-beta in graves’ Ophthalmopathy. Cells. (2024) 13. doi: 10.3390/cells13171493
17. Li H, Min J, Yang Y, Suo W, Wang W, Tian J, et al. TMEM2 inhibits the development of Graves’ orbitopathy through the JAK-STAT signaling pathway. J Biol Chem. (2024) 300:105607. doi: 10.1016/j.jbc.2023.105607
18. Salvi M, Vannucchi G, and Beck-Peccoz P. Potential utility of rituximab for Graves’ orbitopathy. J Clin Endocrinol Metab. (2013) 98:4291–9. doi: 10.1210/jc.2013-1804
19. Vannucchi G, Campi I, Covelli D, Curro N, Lazzaroni E, Palomba A, et al. Efficacy profile and safety of very low-dose rituximab in patients with graves’ Orbitopathy. Thyroid. (2021) 31:821–8. doi: 10.1089/thy.2020.0269
20. Wang Y, Hu H, Chen L, Zhang H, Yang T, Xu X, et al. Observation study of using a small dose of rituximab treatment for thyroid-associated ophthalmopathy in seven Chinese patients: One pilot study. Front Endocrinol (Lausanne). (2022) 13:1079852. doi: 10.3389/fendo.2022.1079852
21. Sha X, Ye H, Wang X, Xu Z, Sun A, Xiao W, et al. GSDMD mediated pyroptosis induced inflammation of Graves’ orbitopathy via the NF-kappaB/AIM2/Caspase-1 pathway. Exp Eye Res. (2024) 240:109812. doi: 10.1016/j.exer.2024.109812
22. Lee GE, Kim J, Lee JS, Ko J, Lee EJ, and Yoon JS. Role of proprotein convertase subtilisin/kexin type 9 in the pathogenesis of graves’ Orbitopathy in orbital fibroblasts. Front Endocrinol (Lausanne). (2020) 11:607144. doi: 10.3389/fendo.2020.607144
23. Xiang Q, Yang M, Luo W, Cao Y, Shuai S, Wei X, et al. Combined glucocorticoids and cyclophosphamide in the treatment of Graves’ ophthalmopathy: a systematic review and meta-analysis. BMC Endocr Disord. (2024) 24:12. doi: 10.1186/s12902-024-01545-0
24. Lee C, Lee JE, Kim K, and Woo KI. Effect of intravenous methylprednisolone on serum antibody levels in thyroid eye disease. Br J Ophthalmol. (2025) 109:516–23. doi: 10.1136/bjo-2024-325180
25. Wang L, Sun Y, Zhang M, He H, Wang J, Xu H, et al. Negative association between serum calcium and glucocorticoid-induced hypertension in thyroid-associated ophthalmopathy patients treated with methylprednisolone. Front Endocrinol (Lausanne). (2025) 16:1548953. doi: 10.3389/fendo.2025.1548953
26. Bartalena L, Kahaly GJ, Baldeschi L, Dayan CM, Eckstein A, Marcocci C, et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. (2021) 185:G43–67. doi: 10.1530/EJE-21-0479
27. Brennan-Speranza TC, Henneicke H, Gasparini SJ, Blankenstein KI, Heinevetter U, Cogger VC, et al. Osteoblasts mediate the adverse effects of glucocorticoids on fuel metabolism. J Clin Invest. (2012) 122:4172–89. doi: 10.1172/JCI63377
28. Moledina M, Damato EM, and Lee V. The changing landscape of thyroid eye disease: current clinical advances and future outlook. Eye (Lond). (2024) 38:1425–37. doi: 10.1038/s41433-024-02967-9
29. Kounatidis D, Vallianou NG, Kontos G, Kranidioti H, Papadopoulos N, Panagiotopoulos A, et al. Liver injury following intravenous methylprednisolone pulse therapy in multiple sclerosis: the experience from a single academic liver center. Biomolecules. (2025) 15. doi: 10.3390/biom15030437
30. San Miguel I, Arenas M, Carmona R, Rutllan J, Medina-Rivero F, and Lara P. Review of the treatment of Graves’ ophthalmopathy: The role of the new radiation techniques. Saudi J Ophthalmol. (2018) 32:139–45. doi: 10.1016/j.sjopt.2017.09.003
31. La Rocca M, Leonardi BF, Lo Greco MC, Marano G, Milazzotto R, Liardo RLE, et al. Orbital radiotherapy for graves’ Ophthalmopathy: single institutional experience of efficacy and safety. Diseases. (2025) 13. doi: 10.3390/diseases13020061
32. Kinaci-Tas B, Alderliesten T, Verbraak FD, and Rasch CRN. Radiation-induced retinopathy and optic neuropathy after radiation therapy for brain, head, and neck tumors: A systematic review. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15071999
33. Tu X, Dong Y, Zhang H, and Su Q. Corticosteroids for graves’ Ophthalmopathy: systematic review and meta-analysis. BioMed Res Int. (2018) 2018:4845894. doi: 10.1155/2018/4845894
34. Li LF, Xue JL, Guan L, Su FF, Wang H, and Zhang DF. Therapeutic outcomes of mycophenolate mofetil and glucocorticoid in thyroid-associated ophthalmopathy patients. Front Endocrinol (Lausanne). (2023) 14:1140196. doi: 10.3389/fendo.2023.1140196
35. Strianese D and Rossi F. Interruption of autoimmunity for thyroid eye disease: B-cell and T-cell strategy. Eye (Lond). (2019) 33:191–9. doi: 10.1038/s41433-018-0315-9
36. Quan LD, Thiele GM, Tian J, and Wang D. The development of novel therapies for rheumatoid arthritis. Expert Opin Ther Pat. (2008) 18:723–38. doi: 10.1517/13543776.18.7.723
37. Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. (1994) 83:435–45. doi: 10.1182/blood.V83.2.435.435
38. Stan MN, Garrity JA, Carranza Leon BG, Prabin T, Bradley EA, and Bahn RS. Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab. (2015) 100:432–41. doi: 10.1210/jc.2014-2572
39. Du Pasquier-Fediaevsky L, Andrei S, Berche M, Leenhardt L, Heron E, and Riviere S. Low-dose rituximab for active moderate to severe graves’ Orbitopathy resistant to conventional treatment. Ocul Immunol Inflammation. (2019) 27:844–50. doi: 10.1080/09273948.2018.1453078
40. Deltour JB, d’Assigny Flamen M, Ladsous M, Giovansili L, Cariou B, Caron P, et al. Efficacy of rituximab in patients with Graves’ orbitopathy: a retrospective multicenter nationwide study. Graefes Arch Clin Exp Ophthalmol. (2020) 258:2013–21. doi: 10.1007/s00417-020-04651-6
41. Bennedjai A, Bouheraoua N, Gatfosse M, Dupasquier-Fediaevsky L, Errera MH, Tazartes M, et al. Tocilizumab versus rituximab in patients with moderate to severe steroid-resistant graves’ Orbitopathy. Ocul Immunol Inflammation. (2022) 30:500–5. doi: 10.1080/09273948.2020.1808688
42. Huang W, Ou X, Lin S, Lin W, Chen G, Huang H, et al. Efficacy and safety of teprotumumab in thyroid eye disease: A systematic review and meta-analysis. Endocr Pract. (2025). doi: 10.1016/j.eprac.2025.01.012
43. Douglas RS, Kahaly GJ, Patel A, Sile S, Thompson EHZ, Perdok R, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. (2020) 382:341–52. doi: 10.1056/NEJMoa1910434
44. Kahaly GJ, Douglas RS, Holt RJ, Sile S, and Smith TJ. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diabetes Endocrinol. (2021) 9:360–72. doi: 10.1016/S2213-8587(21)00056-5
45. Douglas RS, Kahaly GJ, Ugradar S, Elflein H, Ponto KA, Fowler BT, et al. Teprotumumab efficacy, safety, and durability in longer-duration thyroid eye disease and re-treatment: OPTIC-X study. Ophthalmology. (2022) 129:438–49. doi: 10.1016/j.ophtha.2021.10.017
46. Huang J, Su A, Yang J, Zhuang W, and Li Z Sr. Postmarketing safety concerns of teprotumumab: A real-world pharmacovigilance assessment. J Clin Endocrinol Metab. (2024) 110:159–65. doi: 10.1210/clinem/dgae417
47. Boutzios G, Chatzi S, Goules AV, Mina A, Charonis GC, Vlachoyiannopoulos PG, et al. Tocilizumab improves clinical outcome in patients with active corticosteroid-resistant moderate-to-severe Graves’ orbitopathy: an observational study. Front Endocrinol (Lausanne). (2023) 14:1186105. doi: 10.3389/fendo.2023.1186105
48. Kulbay M, Tanya SM, Tuli N, Dahoud J, Dahoud A, Alsaleh F, et al. A comprehensive review of thyroid eye disease pathogenesis: from immune dysregulations to novel diagnostic and therapeutic approaches. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms252111628
49. Duarte AF, Xavier NF, Sales Sanz M, and Cruz AAV. Efficiency and safety of tocilizumab for the treatment of thyroid eye disease: A systematic review. Ophthalmic Plast Reconstr Surg. (2024) 40:367–73. doi: 10.1097/IOP.0000000000002573
50. Hu Y, Chen J, Lin K, and Yu X. Efficacy and Safety of intravenous monoclonal antibodies in patients with moderate-to-severe active Graves’ophthalmopathy: a systematic review and meta-analysis. Front Endocrinol (Lausanne). (2023) 14:1160936. doi: 10.3389/fendo.2023.1160936
51. Anger F, Wiegering A, Wagner J, Lock J, Baur J, Haug L, et al. Toxic drug-induced liver failure during therapy of rheumatoid arthritis with tocilizumab subcutaneously: a case report. Rheumatol (Oxford). (2017) 56:1628–9. doi: 10.1093/rheumatology/kex221
52. Nowak M, Nowak W, Marek B, Kos-Kudla B, Sieminska L, Londzin-Olesik M, et al. The position of monoclonal antibodies and small molecules in the treatment of thyroid orbitopathy. Endokrynol Pol. (2024) 75:604–16. doi: 10.5603/ep.98913
53. Khalilzadeh O, Anvari M, Esteghamati A, Mahmoudi M, Tahvildari M, Rashidi A, et al. Graves’ ophthalmopathy and gene polymorphisms in interleukin-1alpha, interleukin-1beta, interleukin-1 receptor and interleukin-1 receptor antagonist. Clin Exp Ophthalmol. (2009) 37:614–9. doi: 10.1111/j.1442-9071.2009.02093.x
54. Kahaly GJ, Pitz S, Hommel G, and Dittmar M. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab. (2005) 90:5234–40. doi: 10.1210/jc.2005-0148
55. Bartalena L, Krassas GE, Wiersinga W, Marcocci C, Salvi M, Daumerie C, et al. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab. (2012) 97:4454–63. doi: 10.1210/jc.2012-2389
56. Salvi M, Vannucchi G, Curro N, Campi I, Covelli D, Dazzi D, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab. (2015) 100:422–31. doi: 10.1210/jc.2014-3014
57. Kahaly GJ, Riedl M, Konig J, Pitz S, Ponto K, Diana T, et al. Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet Diabetes Endocrinol. (2018) 6:287–98. doi: 10.1016/S2213-8587(18)30020-2
58. Lanzolla G, Sabini E, Leo M, Menconi F, Rocchi R, Sframeli A, et al. Statins for Graves’ orbitopathy (STAGO): a phase 2, open-label, adaptive, single centre, randomised clinical trial. Lancet Diabetes Endocrinol. (2021) 9:733–42. doi: 10.1016/S2213-8587(21)00238-2
59. Winn BJ and Kersten RC. Teprotumumab: interpreting the clinical trials in the context of thyroid eye disease pathogenesis and current therapies. Ophthalmology. (2021) 128:1627–51. doi: 10.1016/j.ophtha.2021.04.024
60. Hiromatsu Y, Ishikawa E, Kozaki A, Takahashi Y, Tanabe M, Hayashi K, et al. A randomised, double-masked, placebo-controlled trial evaluating the efficacy and safety of teprotumumab for active thyroid eye disease in Japanese patients. Lancet Reg Health West Pac. (2025) 55:101464. doi: 10.1016/j.lanwpc.2025.101464
61. Chen X, Abudukerimu B, Li Q, Li Q, Qiao J, Lin D, et al. Influence of 4-week or 12-week glucocorticoid treatment on metabolic changes in patients with active moderate-to-severe thyroid-associated ophthalmopathy. Clin Transl Sci. (2021) 14:1734–46. doi: 10.1111/cts.12999
62. El-Awady SMM, El Afifi AM, Afifi R, Sabri NA, and Ahmed MA. Evaluation of the clinical outcomes of cyclosporine short infusion versus continuous infusion postallogenic stem cell transplantation. Eur J Drug Metab Pharmacokinet. (2025) 50:53–64. doi: 10.1007/s13318-024-00927-y
Keywords: graves’ ophthalmopathy, corticosteroids, immunosuppressants, teprotumumab, drug therapy
Citation: Wang L and Chen L (2025) Emerging therapeutic approaches in graves’ ophthalmopathy: an update on pharmacological interventions. Front. Immunol. 16:1647602. doi: 10.3389/fimmu.2025.1647602
Received: 16 June 2025; Accepted: 14 July 2025;
Published: 25 July 2025.
Edited by:
Vito Racanelli, University of Trento, ItalyReviewed by:
Rudolf Gesztelyi, University of Debrecen, HungaryChenjun Guo, Fourth Military Medical University, China
Copyright © 2025 Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linlin Chen, Y2xsbm5AaG90bWFpbC5jb20=
 Lin Wang
Lin Wang Linlin Chen*
Linlin Chen*