- 1Department of Epidemiology, Noguchi Memorial Institute for Medical Research, College of Health Sciences, University of Ghana, Legon-Accra, Ghana
- 2Inovio Pharmaceuticals, Inc., Plymouth Meeting, PA, United States
- 3Department of Immunology, Noguchi Memorial Institute for Medical Research, College of Health Sciences, University of Ghana, Legon-Accra, Ghana
- 4Department of Global Public Health and Bioethics, Julius Center for Health Sciences and Primary Care, University Medical Center, Utrecht University, Utrecht, Netherlands
Background: Lassa fever (LF) is an acute viral hemorrhagic illness endemic to West Africa, with no licensed vaccines or targeted treatments available, highlighting a critical gap in global health preparedness. T cell-mediated immunity plays a central role in viral control and survival. Synthetic DNA vaccines offer a promising strategy to induce both humoral and cellular immunity against LF.
Methods: A Phase 1b, randomized, double-blind, placebo-controlled trial was conducted to assess the safety, tolerability, and immunogenicity of INO-4500, a DNA vaccine encoding the Lassa virus (Josiah strain) glycoprotein precursor (GPC). A total of 220 healthy adults were randomized to receive either 1 mg or 2 mg of INO-4500 (intervention), or placebo, administered intradermally (ID) followed by electroporation (EP) at Day 0 and Week 4. Safety was evaluated through Week 48. Primary immunogenicity endpoints included humoral and cellular immune responses at multiple timepoints post-vaccination.
Results: INO-4500 was well tolerated, with no Grade 3 or higher treatment-emergent adverse events (TEAEs) deemed to be related to the intervention; 88.6% of all TEAEs were Grade 1. No cases of attributable hearing loss were reported. INO-4500 groups demonstrated statistically significant increases in Lassa virus GPC-specific binding antibodies at Weeks 6 and 12 compared to placebo, with the 2 mg group eliciting the strongest responses. T cell responses remained elevated above baseline through Week 48 in both INO-4500 groups, indicating durable cellular immunity.
Conclusions: DNA vaccine INO-4500 was well tolerated and elicited durable humoral and cellular immune responses in healthy adults. These findings support further clinical development of INO-4500 as a potential preventive vaccine to reduce LF-associated morbidity and mortality in endemic regions.
Clinical Trial Registration: https://clinicaltrials.gov, identifier NCT04093076
Introduction
Lassa fever (LF), a devastating acute viral hemorrhagic zoonotic illness caused by the Lassa virus (LASV), poses a significant public health threat in West Africa (1, 2). An estimated 100,000 to 200,000 infections occur annually due to LASV infection in the region, resulting in approximately 5,000 deaths per year (3, 4). Symptoms range in severity, from mild disease to severe hemorrhagic fever leading to death (5). The disease’s severity is reflected in its case-fatality rate of 1-2% (6, 7), which rises to an estimated 15% among those hospitalized with severe symptoms (4, 7). Pregnant women are particularly impacted by LF, with case-fatality rates up to 30%, along with high rates of neonatal and fetal losses (8). One in five infections result in severe disease, impacting the liver, spleen and kidneys (7), and can include debilitating sequelae such as sensorineural hearing loss, polyserositis, vision distortion, vertigo, and back pain (5).
The absence of approved vaccines or therapeutics targeting LF underscores a critical gap in global health security (4, 5). This void, coupled with LF’s high incidence and severity, poses a significant threat to people living in endemic populations and travelers who visit LASV-endemic areas. The World Health Organization’s (WHO) designation of LASV as a priority pathogen requiring urgent research and development action highlights the need for effective interventions (9). Significant strides have been made in LF vaccine development, with multiple vaccine candidates progressing through clinical trials (4, 10–13). The Coalition for Epidemic Preparedness Innovations (CEPI) is further advancing these efforts by supporting the development of vaccines for priority pathogens such as LASV (9, 14).
Most studies to date, both in human and animal disease models like ours (15), provide evidence that T cell-mediated immunity is key to virus control and survival (15–19), although there is some evidence of non-neutralizing antibodies playing a protective role (5, 6, 20, 21). Potent memory CD4+ T cell responses targeting viral nucleoproteins and surface glycoproteins have been detected in healthy seropositive individuals living in LF endemic areas (19, 22), suggesting the activation of CD4+ T cells in mild and/or asymptomatic infections. Furthermore, in LASV infection, both CD4+ and CD8+ T cell-mediated immune responses are detectable in individuals who ultimately clear the virus, underscoring the essential role of these responses in effective viral clearance and recovery (18, 22, 23). While neutralizing antibodies alone are likely insufficient for protection (24, 25), non-neutralizing binding antibodies such as IgG may contribute to the overall immune defense, as evidenced by their production post-infection (5, 21). More research is needed to confirm these findings and further identify other factors that may contribute to protection against LF.
While the correlates of protection for LF are still being studied, it is currently believed that early virus-specific cellular responses constitute the predominant mode of protection, and are associated with survival, whereas delayed cellular responses are associated with fatal outcomes (5, 16, 18, 26, 27). This insight influences vaccine design strategy, as vaccines that predominantly induce cellular responses are more likely to provide protection against LASV (28).
Preclinical studies for one such vaccine candidate, INO-4500, has yielded encouraging results, demonstrating the presence of LASV-specific memory T cells in vaccinated non-human primates (NHPs) up to one year post-vaccination and conferring both short- and long-term protection against lethal LASV challenge (15). INO-4500 is a synthetic DNA vaccine encoding for the Lassa virus (Josiah strain) glycoprotein precursor (LASV GPC) and is injected intradermally (ID) and followed by electroporation (EP) to enhance cellular uptake. The vaccine targets the GPC protein of LASV, which plays a critical role in host entry, and represents the most conserved region in this genetically diverse virus (29, 30).
We present here the outcomes of a Phase 1b trial (LSV-002) conducted in Ghana, where we evaluated the safety, tolerability and immunogenicity of INO-4500 in healthy volunteers with no known prior exposure to LASV.
Materials and methods
Study design and population
LSV-002 was a Phase 1b, randomized, double-blinded within study group, placebo-controlled clinical trial to evaluate the safety, tolerability, and immunological profile of INO-4500 administered by ID injection followed by EP using the CELLECTRA® 2000 ID device (NCT04093076). INO-4500, the active investigational product used in this clinical trial, contains a DNA plasmid designed to express Lassa virus (Josiah strain) glycoprotein precursor. The study was performed in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice (GCP), and applicable regulatory requirements. The study was approved and conducted at the Clinical Trials Unit of the Noguchi Memorial Institute for Medical Research (NMIMR), University of Ghana, Legon.
Healthy adult volunteers, 18–50 years of age, were enrolled at a single location from within and around the University of Ghana community in Accra, Ghana. Written informed consent was obtained from all participants before any protocol procedures were performed. Inclusion criteria included the capability of following study procedures and practicing adequate contraception during the trial period. Exclusion criteria included: participants with a hearing level threshold greater than 30 dB for any frequency tested between 500 Hz – 8000 Hz; pregnant or lactating female participants.
Study procedures
Potential participants were consented and screened. Participants meeting eligibility criteria were randomized in a blinded fashion to one of four groups across the two dose regimens, either receiving active investigational product (INO-4500; 176 participants) or placebo (saline sodium citrate [SSC] buffer solution; 44 participants) (Figure 1). Blinding of INO-4500 and placebo was in place for the participants, sponsor, and all clinical site staff, except for the site pharmacy staff who were not involved in any post-vaccination assessments. Group A received a single 1 mg INO-4500 (0.1 ml of a 10 mg/ml solution) ID injection at Day 0 and Week 4 (± five days), totaling 2 mg, while Group B received two 1 mg INO-4500 (0.1 ml of a 10 mg/ml solution) ID injections on different limbs at the same timepoints, totaling 4 mg. Group C received a single ID injection of 0.1 ml SSC (placebo) at Day 0 and Week 4 (± five days), while Group D received two ID injections of 0.1 ml SSC (placebo) on different limbs at the same time points. All ID injections were followed by EP using the CELLECTRA™ 2000 ID device (Inovio Pharmaceuticals) delivering four pulses of 52 milliseconds and 0.2 amps. For all groups, the first dose of INO-4500 or placebo dose occurred at Day 0, within 60 days of screening.
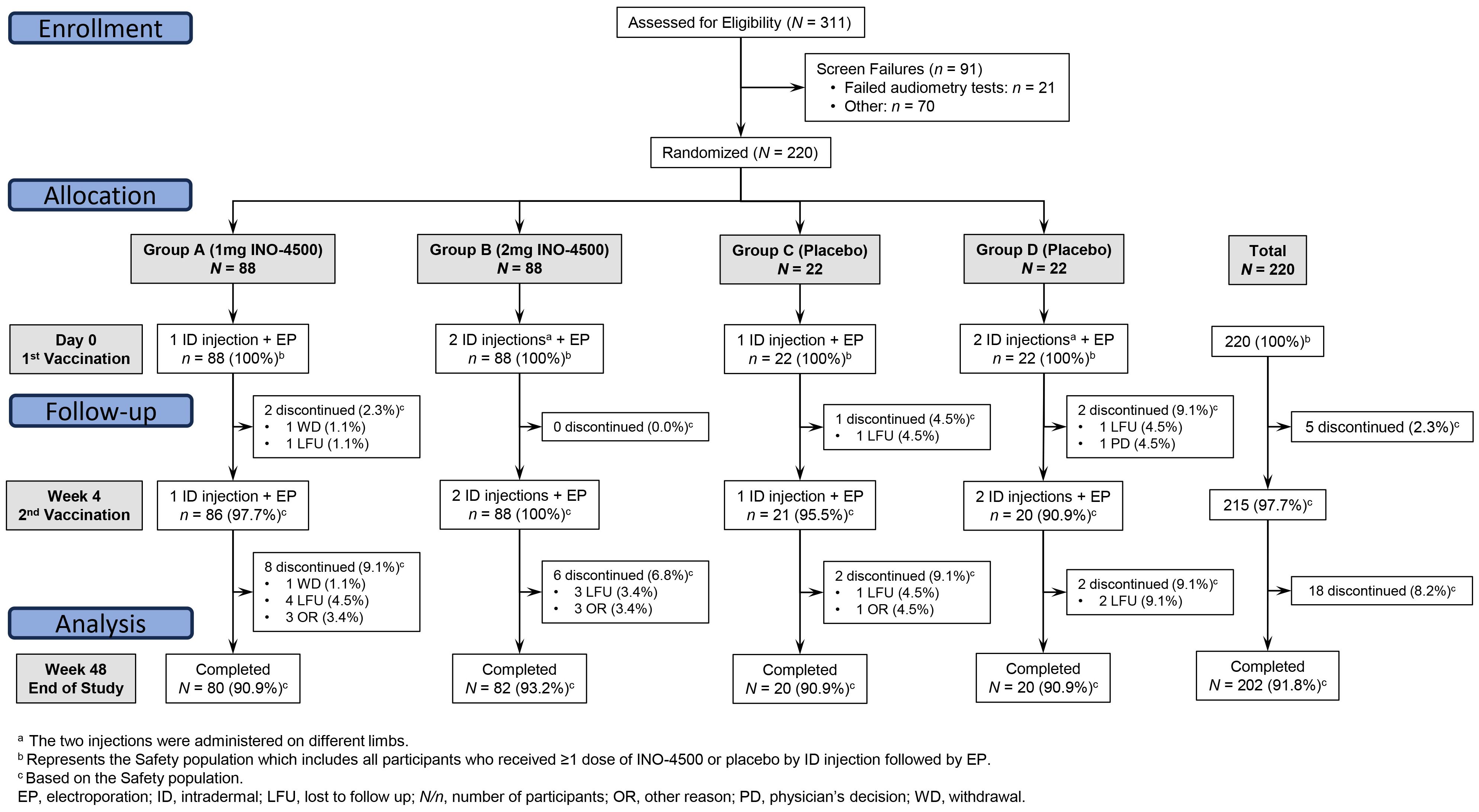
Figure 1. CONSORT diagram. LSV-002 was a Phase 1b, randomized, blinded, placebo-controlled trial enrolling participants at a ratio of 4:1 to receive a two-dose series of either INO-4500 or placebo. Eligibility was assessed for 311 subjects, and 220 participants were enrolled across four groups. Intervention arms were Group A with a single intradermal (ID) injection of 0.1 mL (1 mg) of INO-4500 on Day 0 and on Week 4; and Group B, with two ID injections (on separate limbs) of 0.1 mL (total dose of 2 mg) of INO-4500 on Day 0 and on Week 4. Placebo arms were Group C, with a single ID injection of 0.1 mL of saline sodium citrate (SSC) on Day 0 and on Week 4; and Group D, with two ID injections (on separate limbs) of 0.1 mL (total dose of 0.2 mL) of SSC on Day 0 and on Week 4. ID administration of INO-4500 or placebo was followed by electroporation (EP).
Objectives and endpoints
The primary objectives of this clinical trial were to evaluate the tolerability and safety, as well as humoral and cellular immunogenicity of INO-4500 administered by ID injection followed by EP in healthy adult participants. For the safety evaluation, the incidence, severity, and relationship of adverse events (AEs) to the study intervention were collected.
Safety evaluations
Participants were observed for immediate reactions to the study treatment for 30 minutes after each vaccination (Day 0 and Week 4) and asked to attend the study clinic on Days 2, 14 (Week 2), 28 (Week 4), 42 (Week 6), 84 (Week 12), 168 (Week 24) and 336 (Week 48) (end of study assessment). Physical examinations were performed at all visits except Week 12 and Week 24. Medical/clinical assessment, collection of whole blood and serum for immunology assessment, and safety laboratory analyses were performed at each visit except Day 2. Solicited local (injection site) and systemic AEs were collected via participant diary cards on the evening of the day of vaccinations (Day 0 and Week 4) and seven subsequent days following each vaccination. The injection site reactions were graded as mild, moderate or severe – Grades 1, 2 and 3 respectively – in accordance with the “Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials” (31).
Since hearing loss (HL) has been documented in LF survivors (32, 33), hearing assessments were conducted at screening, prior to second doses at Week 4 and at the final study visit at Week 48. Pure-tone audiometry was used to evaluate whether hearing was within normal-to-mild limits by evaluating hearing capabilities at certain frequencies: 500, 1000, 2000, 4000, 6000, and 8000 Hz. The hearing assessment included the hearing level (dB HL) at the measured frequencies. Degree of HL was defined as 0–15 dB: within normal limits; 16–25 dB: slight HL, 26–40 dB: mild HL; 41–55 dB: moderate HL; 56–70 dB: moderately severe HL; 71–90 dB: severe HL; 91+ dB: profound HL. Treatment-related attributable hearing loss measured as a change from baseline was reported as follows: AE, hearing loss of >30 dB in at least a single frequency and a change from one “Degree of Hearing Loss” category to another level, adverse event of special interest (AESI) as a hearing loss of >30 dB in three consecutive frequencies, and serious adverse event (SAE) as a hearing loss of ≥71 dB in at least one frequency (34).
AEs, including SAEs, treatment-emergent AEs (TEAEs), AESIs, as well as vital signs were evaluated throughout the study. The principal investigator (PI) assessed and graded clinical AEs or SAEs (based on discussions with participants) in accordance with the “Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials” (31). AESIs relevant to development of a LF vaccine that were monitored for during the study included, but were not limited to, sensorineural hearing loss, encephalitis, and thrombocytopenia. No significant safety findings were noted to cause a safety pause to be requested by the Data Safety Monitoring Board.
Immunogenicity assessments
Whole blood and serum were collected for immunogenicity assessments prior to the first dose (at screening and/or Day 0), and during the study. Determination of analysis of collected samples for immunological endpoints was determined on an ongoing basis throughout the study.
Humoral immune responses were evaluated using a standard binding enzyme-linked immunosorbent assay (ELISA) developed at Inovio to measure levels of total IgG antibodies specific for glycoprotein (GP) antigen in serum isolated from whole blood samples drawn on Day 0, Week 6, and Week 12. This GP antigen was from lineage IV (Recombinant LASV L-IV Prefusion Glycoprotein, Zalgen Labs), one of the three most prevalent lineages of LASV in Sierra Leone, Guinea, and Liberia (35). Post-vaccination end point IgG titers were reported as the dilution of sera at which the average optical density (OD) of triplicate wells was greater than the assay cutoff and average OD of the negative control, then converted to concentration (IU/mL) using the First WHO International Standard for anti-Lassa fever virus antibodies (NIBSC code: 20/202). The assay limit of detection (LOD) was 9.065 IU/mL. Seroconversion rate was defined as a two-fold rise over baseline (Day 0) values.
Cellular immune responses were assessed using an interferon-gamma (IFN-γ) Enzyme-Linked ImmunoSpot (ELISpot) assay performed on peripheral blood mononuclear cells (PBMCs) isolated from whole blood collected at Day 0, and Weeks 2, 4, 6, 12, 24 and 48. The number of antigen-specific IFN-γ secreting cells was determined in response to stimulation with two pools (GP1, GP2) of 15-mer peptides overlapping by 9 amino acids spanning the LASV GP precursor. Phorbol 12-myristate 13-acetate (PMA) and ionomycin (a calcium ionophore) were used in combination to stimulate cells as a positive control, while cells with no stimulant were used as negative controls for all assays. The assay LOD was 11 spot forming units (SFU)/106 PBMCs. ELISpot data were collected across two runs to support informal interim (Dataset 1) and long-term follow-up (Dataset 2) analyses. For the first analytical run (Dataset 1), samples collected at baseline (Day 0) and Weeks 6, 12, and 24 were analyzed. For the second analytical run (Dataset 2), samples collected at baseline and Weeks 2, 4, 24, and 48 were analyzed. Data were analyzed for Datasets 1 and 2 either individually or in combination, the latter using baseline subtraction. For each participant, a response threshold was calculated based on the mean SFU/106 PBMCs plus two standard deviations of triplicate at baseline (Day 0) plus assay limit of quantitation (20 SFU/106 PBMCs). Responder criterion was defined as any post-treatment value that exceeded the calculated response threshold.
Statistics
The safety analysis included all participants in the Safety population, who received at least one dose of INO-4500 or placebo administered by ID injection. TEAEs were defined for this trial as any AEs/SAEs that occurred on or after Day 0. All TEAEs and serious TEAEs were summarized by frequency and percentage. Laboratory response variables were descriptively summarized by time point and as changes from baseline. As an early clinical phase study, the safety evaluation focused on identifying safety signals or concerns of clinical relevance rather than potential statistical differences. Thus, no formal statistical testing was performed.
All primary immunogenicity analyses were conducted on participants in the modified intent-to-treat (mITT) population. Similar to the Safety population, the mITT population included all participants who received at least one dose of INO-4500 or placebo. Binding antibody levels were analyzed by dose regimen up to Week 12 using geometric mean titers with interquartile range or fold rise with associated 95% confidence intervals. Antigen-specific cellular immune responses above baseline were analyzed by dose regimen up to Week 48 as SFUs per 106 PBMCs with interquartile range or medians with 95% confidence intervals. Peak cellular response taken from any timepoint for each participant was used to calculate the mean peak SFU within each group. Percentage of participants with seroconversion (i.e., positive titer) or cellular response were analyzed within each study group. Statistical comparisons were performed within each study group between study weeks using the Wilcoxon signed-rank test and between groups at each study week using the Kruskal-Wallis test. The false discovery rate for multiple comparisons was controlled using the Benjamini-Hochberg adjustment and padj-values are reported. Statistical tests were performed using GraphPad Prism version 10.5.0.
Results
Participant demographics and baseline characteristics
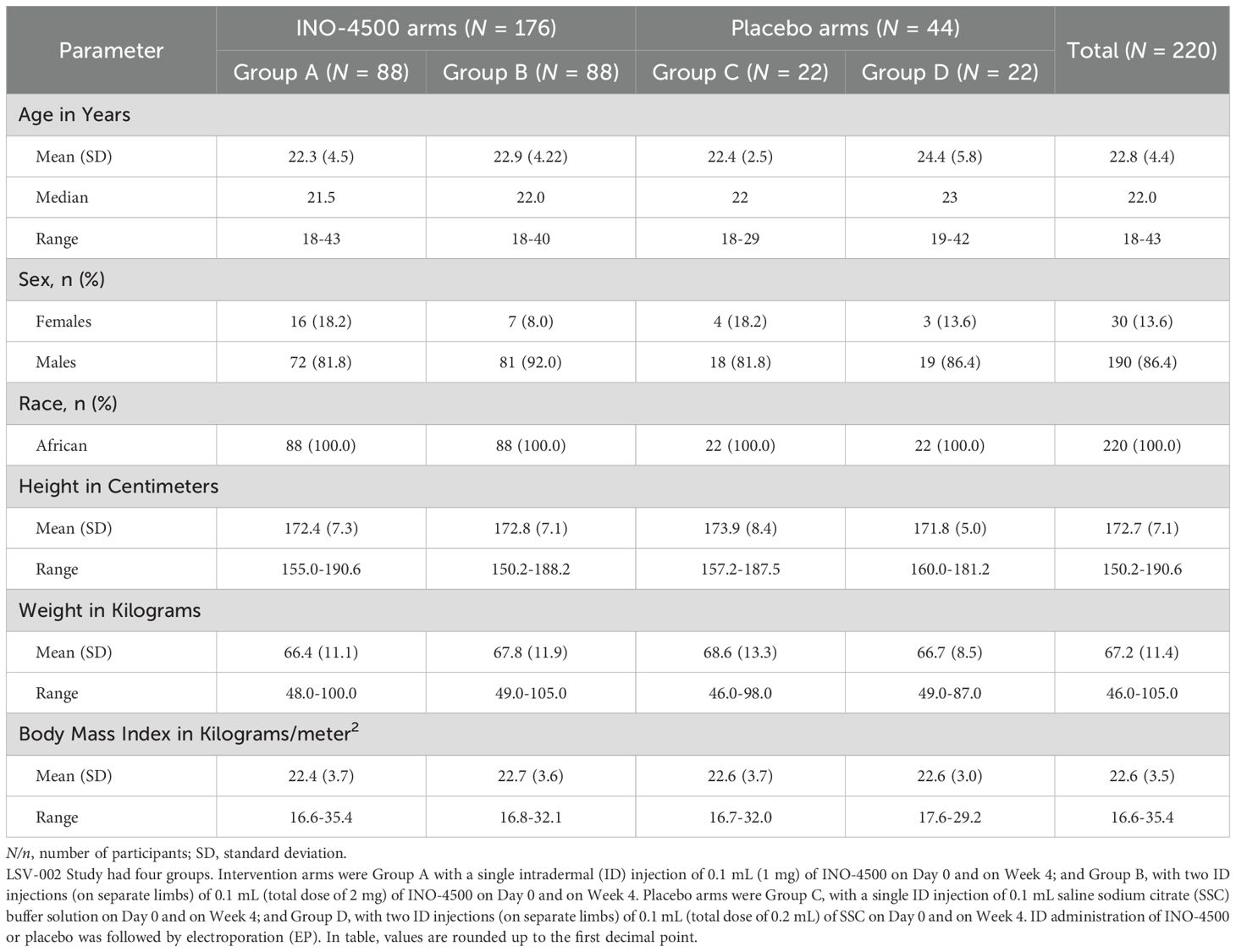
A total of 311 participants were screened for eligibility, with 220 participants subsequently enrolled and randomized at a single clinical trial site in Accra, Ghana, between December 2020 and September 2021 (Figure 1). The 220 study participants were on average 22 years old (range, 18-43), 86% were males, and the mean Body Mass Index was 21.8 kg/m2 (range, 16.6-35.4), very similar across study groups (Table 1).
Study procedure
Of the 220 randomized participants, 88 received 1 mg INO-4500 (Group A), 88 received 2 mg INO-4500 (Group B), 22 received placebo in each of two groups (Groups C and D) (Figure 1). All participants received at least one dose of INO-4500 or placebo followed by EP. Overall, most participants received all planned study doses (215 of 220, 97.7%) and 97.9% (646/660) of planned injections were administered. Of the eighteen participants who did not complete all study visits, ten were lost to follow-up, one participant withdrew, and seven did not complete the study for other reasons.
Safety assessments
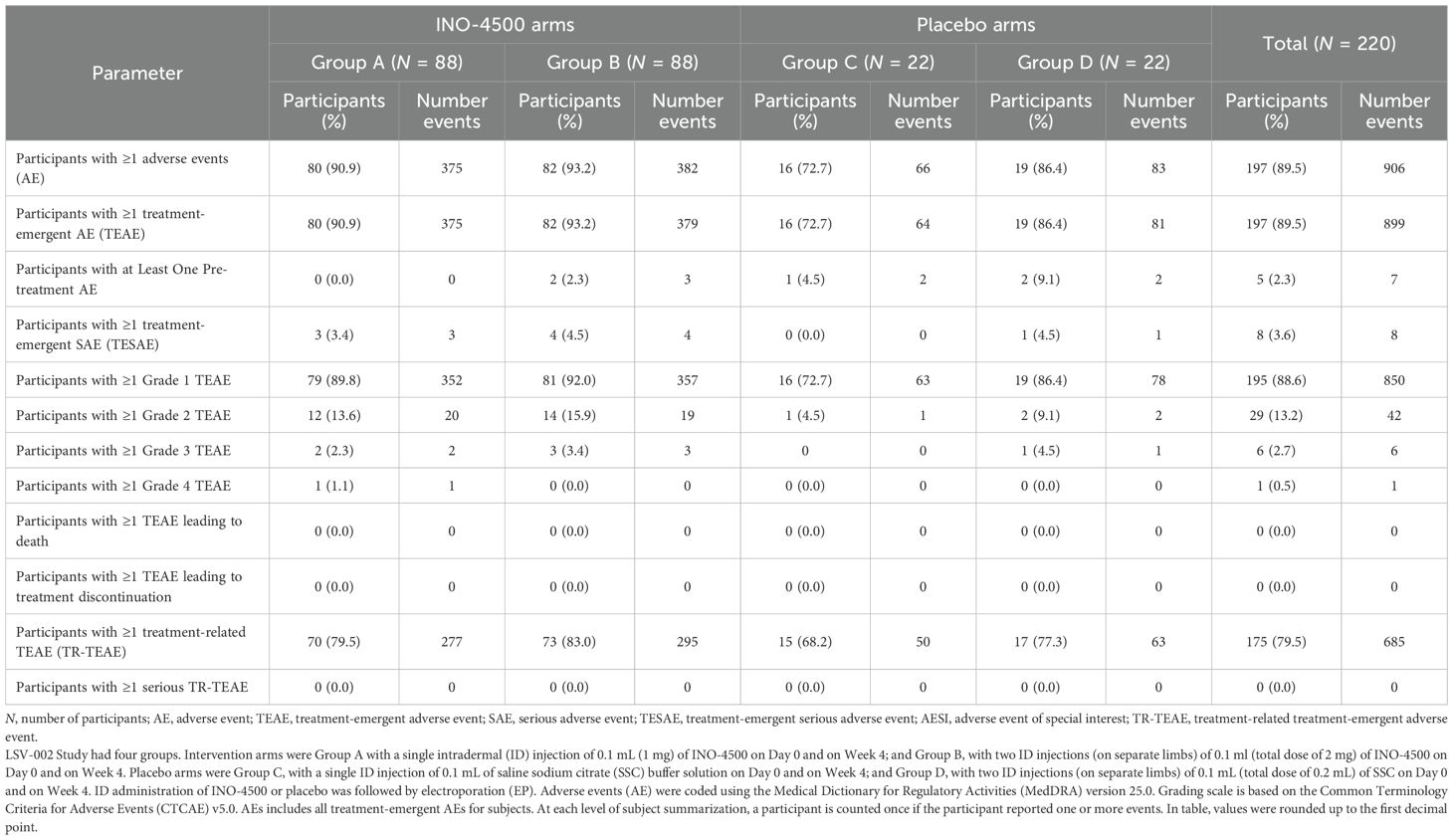
A summary of AEs is provided in Table 2. Of the 220 participants in the study, 197 (89.5%) reported 906 AEs. All but seven AEs (899 AEs) were TEAEs. Among participants, 195 (88.6%), and 29 (13.2%) experienced at least one Grade 1 and Grade 2 TEAE. A single Grade 3 and a single Grade 4 TEAE was experienced by 6 (2.7%) and 1 (0.5%) participants, respectively. Eight participants (3.6%) experienced one SAE each. There were no SAEs preceding initiation of treatment. None of the AEs led to discontinuation or death.
Three participants experienced a single AESI each: thrombocytopenia in a subject in INO-4500 Group A and placebo Group C, and gingival bleeding in a subject in INO-4500 Group B. All three AESIs were assessed as non-serious and not related to clinical trial treatment. No other AESIs including cases of attributable hearing loss, either self-reported or detected by audiometry, were observed during the trial (Supplementary Table S1).
Of the seven Grade ≥3 TEAE, six occurred in participants receiving INO-4500 and consisted of peptic ulcer, gastroenteritis (two), elevated aspartate aminotransferase, headache, and seizure. The only Grade 4 event was a headache. A case of malaria was the one TEAE Grade 3 occurring in a participant receiving placebo (Table 3).
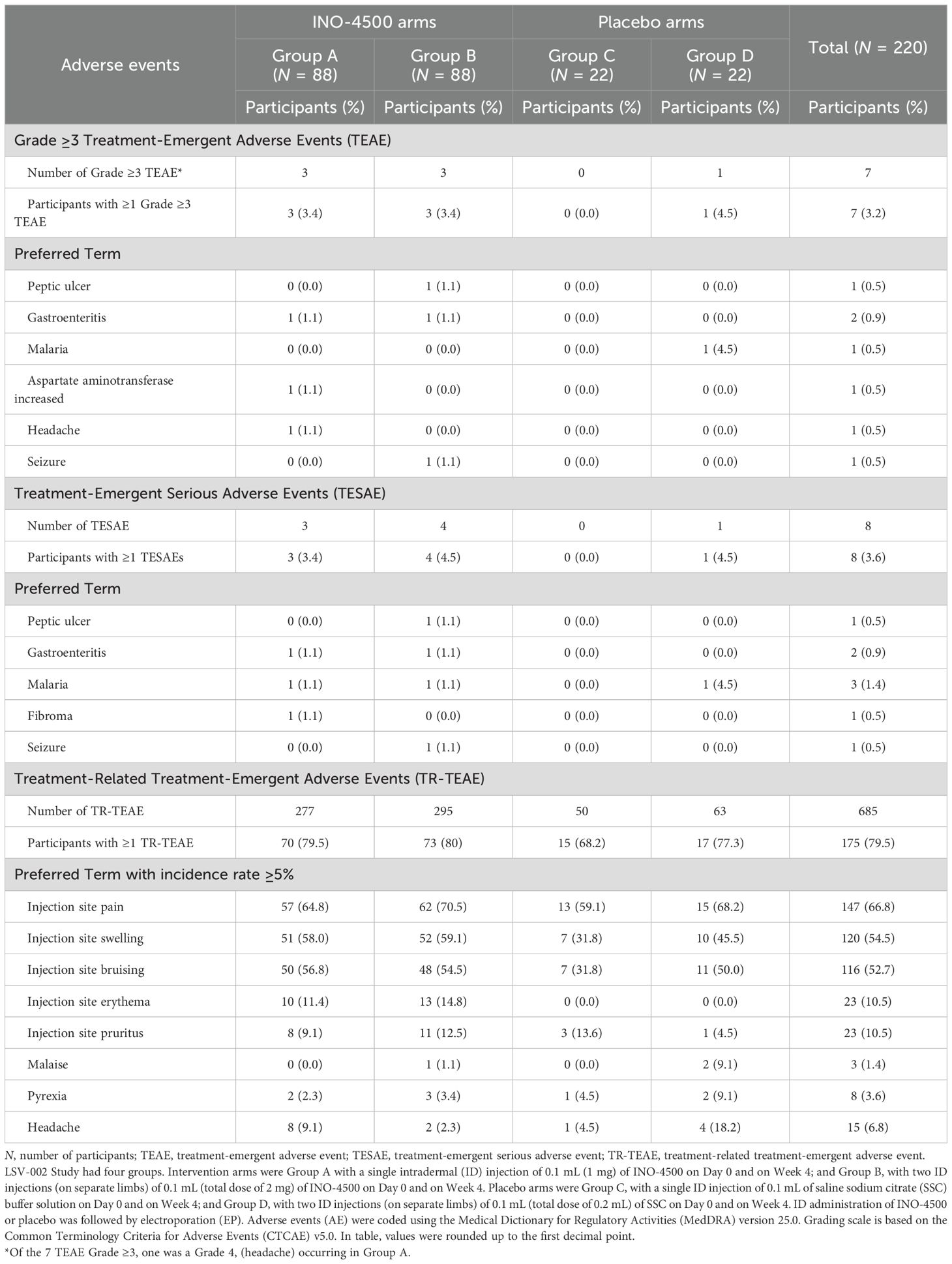
Table 3. Treatment-emergent adverse events by preferred term including high grade, seriousness, and relation to treatment in LSV-002 study safety population.
Of the eight serious TEAEs, seven occurred in participants receiving INO-4500, three in Group A and four in Group B, and included peptic ulcer, gastroenteritis (two), malaria (two), fibroma, and seizure. The self-reported single episode compatible with a seizure occurred 48 days post administration of the second dose of INO-4500 in the context of self-medication with an over-the-counter medicine available in Ghana that can cause seizures. Clinical and paraclinical repeated evaluations found no abnormalities, and the subject evolved favorably. A case of malaria was categorized as a serious TEAE in a participant receiving placebo. None of these TEAEs were considered related to the treatment and none led to study withdrawal (Table 3).
A total of 175 participants (79.5%) experienced a total of 685 TEAEs that were assessed as treatment-related by the investigators. The most frequently reported (≥5%) treatment-related TEAEs overall were localized to the injection site, including pain (66.8%), swelling (54.5%), bruising (52.7%), erythema (10.5%) and pruritus (10.5%). Three systemic treatment-related TEAE had an incidence ≥5% in at least one treatment group: malaise (9.1% in placebo Group D), pyrexia (9.1% in placebo Group D) and headache (9.1% and 18.2% in INO-4500 Group A and placebo Group D, respectively). Table 3 details the incidence of treatment-related TEAE by treatment groups.
No clinically meaningful trends or abnormalities were observed in clinical laboratory measurements or physical examination findings throughout the study.
Humoral immune responses
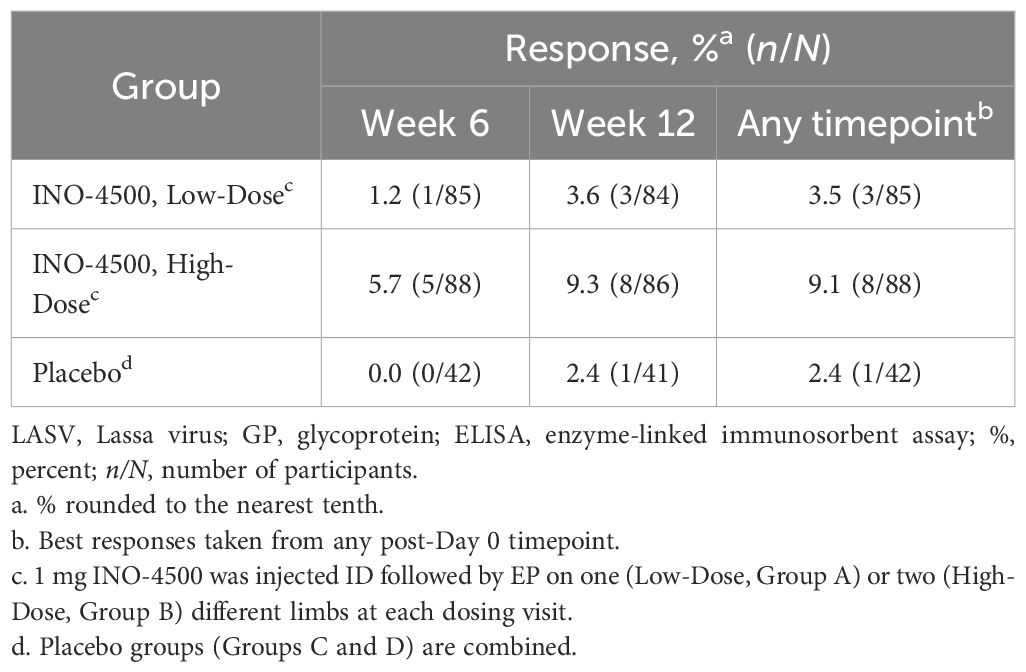
To assess the humoral immune responses induced by INO-4500, LASV GP binding ELISA was performed using sera collected from participants both before and after immunization. Participants treated with either low-dose (Group A, 1 mg) or high-dose (Group B, 2 mg) INO-4500 exhibited statistically significant increases in LASV GP-specific binding antibodies at Week 6 and Week 12 compared to Day 0 levels, while those who received placebo did not (Figure 2A, Table 4). Geometric mean fold rise (GMFR) analysis demonstrated a statistically significant increase in LASV GP-specific binding antibodies in both INO-4500 groups compared to placebo at Week 6 (low-dose, padj = 0.019; high-dose, padj <0.001) and Week 12 (low-dose, padj = 0.050; high-dose, padj <0.001), and the high-dose group also showed significantly greater responses than the low-dose group at both Week 6 (padj = 0.019) and Week 12 (padj = 0.024) (Figure 2B, Table 5). LASV GP-specific binding antibodies peaked at Week 12 in participants receiving either low- or high-dose INO-4500, with 3.6% and 9.3% responders, respectively, defined as two-fold rise over baseline (Table 6). While INO-4500 induced humoral immune responses that were not only detectable but also significantly increased, the magnitude of the antibody response was modest, resulting in few participants that met the threshold for seroreactivity.
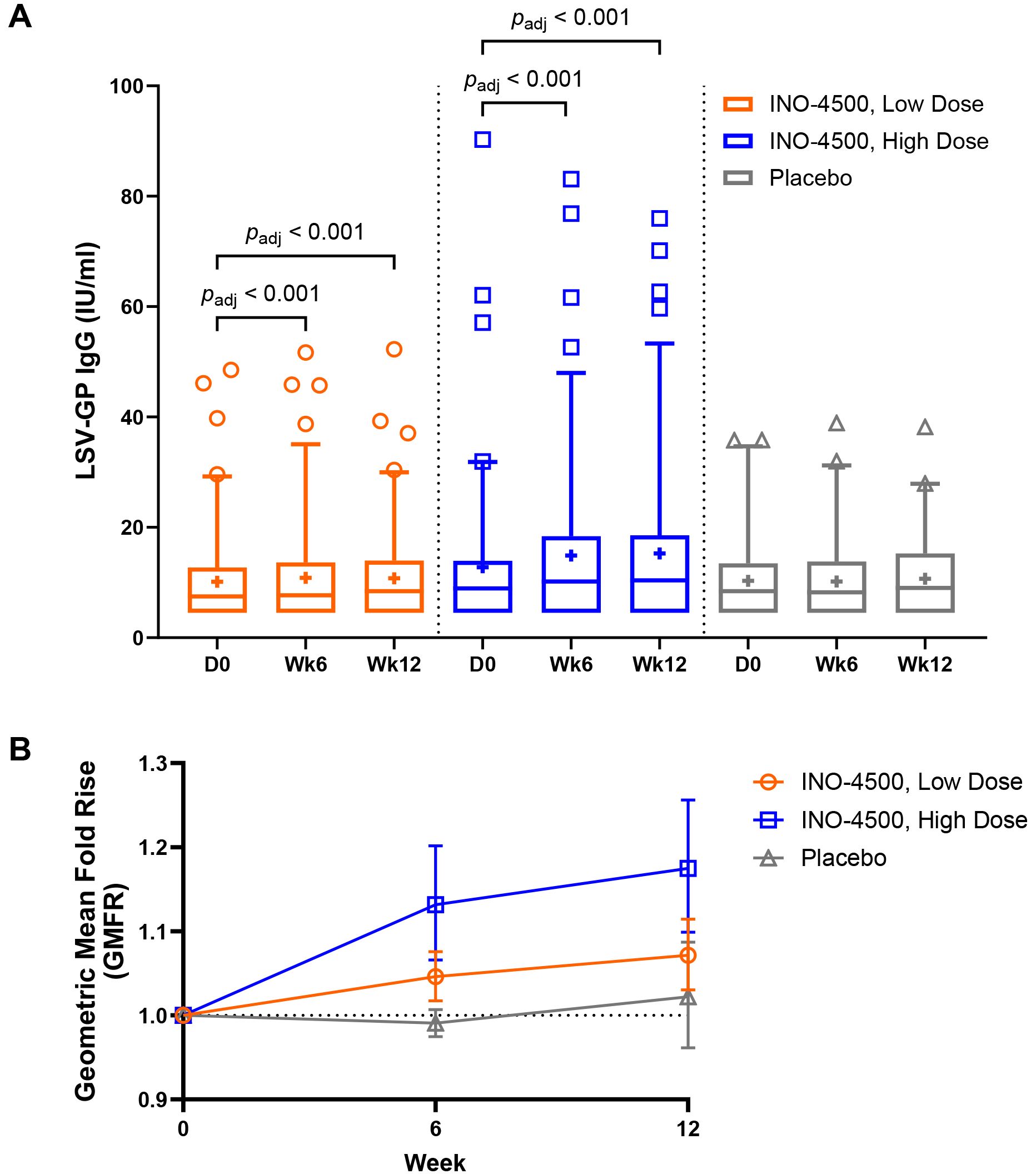
Figure 2. LASV GP-specific binding antibodies as measured by ELISA. LASV GP-specific binding antibodies are shown for each group. Group A (up to n = 85) participants received a total of 1 mg INO-4500 (low-dose) at each dosing visit, whereas Group B (up to n = 88) participants received 2 mg INO-4500 (high-dose). Placebo groups (Groups C and D; up to n = 42) are combined. (A) Box and whiskers extend from 25th to 75th and 5th to 95th percentiles, respectively, with outliers represented by open symbols. Line at the median; + at mean. padj-values were calculated at each study week compared to Day 0 within each group using the Wilcoxon signed-rank test and between groups at each timepoint using the Kruskal-Wallis test; each test was followed by Benjamini-Hochberg adjustment. Only significant padj-values displayed. (B) Geometric Mean Fold Rise (GMFR) of LASV GP-specific binding antibodies in each group represented by open symbols. Error bars show 95% CI. padj-values calculated between groups at each post-vaccination timepoint using the Kruskal-Wallis test followed by Benjamini-Hochberg adjustment are displayed in Table 5. LASV, Lassa virus; GP, glycoprotein; ELISA, enzyme-linked immunosorbent assay; Ig, immunoglobulin; IU, International Unit; mL, milliliters; CI, confidence interval.
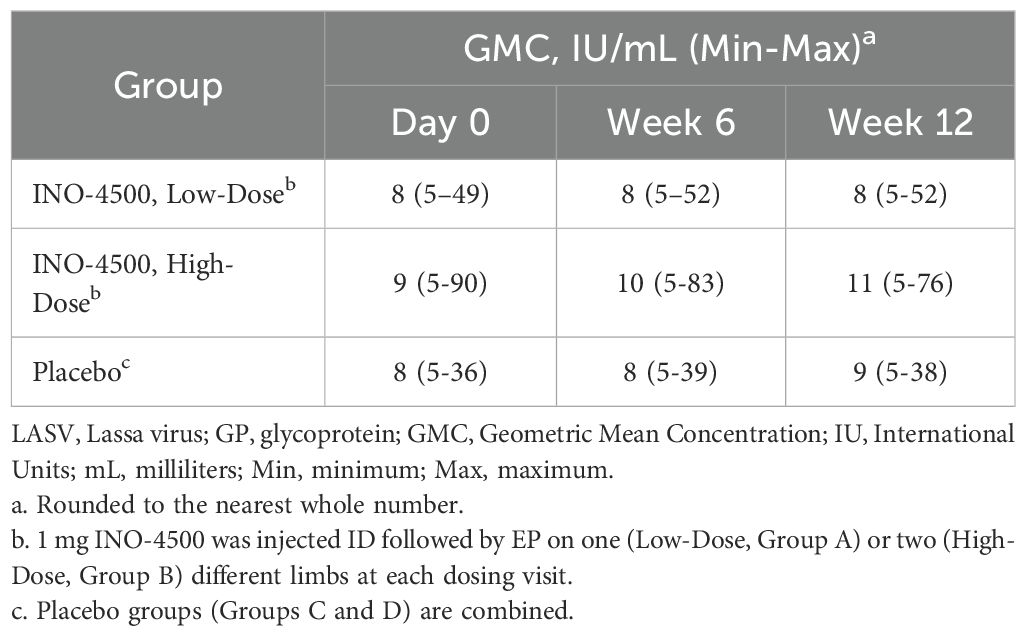
Table 4. Geometric mean concentration (GMC) of LASV GP-specific binding antibodies as measured by ELISA in LSV-002 study.
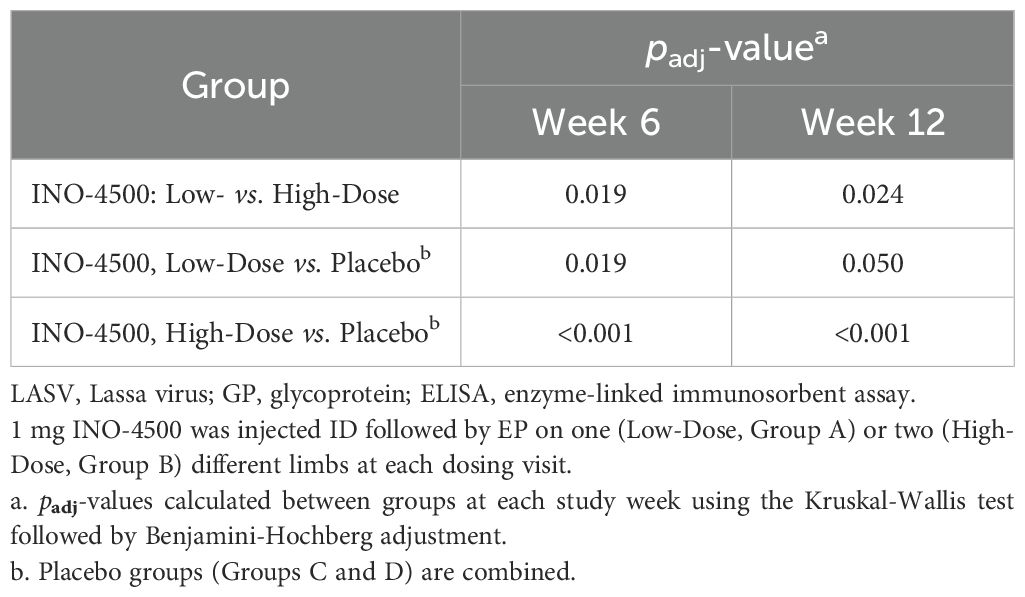
Table 5. Statistical comparison between groups for geometric mean fold rise (GMFR) of LASV GP-specific binding antibodies as measured by ELISA in LSV-002 study.
Cellular immune responses
To evaluate INO-4500-induced cellular immune responses, an IFN-γ ELISpot assay was performed on PBMCs collected pre-vaccination and at multiple timepoints during the study. This assay detected and quantified IFN-γ secreting cells following stimulation with antigens (LASV GP-derived peptide pools GP1 and GP2). Participants who received either low- (Group A) or high-dose (Group B) INO-4500 exhibited increases in LASV GP-specific IFN-γ secreting T cells after vaccination (Figure 3, combined dataset; Supplementary Figure S1 datasets 1 and 2). Additionally, both groups showed a statistically significant increase in LASV GP-specific T cell response at Weeks 4, 6, 12, 24, and 48 as compared to those who received placebo (Figure 3A). The high-dose INO-4500 group exhibited greater responses than the low-dose group from Week 6 through Week 48. T cell response magnitude peaked at Week 6 in the low-dose group and at 12 weeks in the high-dose group, with elevated responses sustained above the Day 0 responses through the end of the study at Week 48 (Figure 3B). Responder rates, defined by positive IFN-γ responses to GP1 and GP2, peaked with 63% at Week 6 for low-dose and 80% at Week 12 for high-dose recipients (Figure 4, combined dataset; Supplementary Figure S2, datasets 1 and 2). The 2 mg dose also resulted in a better response rate for participants than the 1 mg dose at Week 6 (71% versus 63%, respectively). Rates of T cell response at any timepoint were 75% and 86% for participants who received low- and high-dose INO-4500, respectively, compared to 26% for those who received placebo (Table 7, combined dataset; Supplementary Table S2, dataset 1; Supplementary Table S3, dataset 2). Participants from the high-dose INO-4500 group generated the highest mean peak responses to stimulation with total LASV GP (324 SFU/106 PBMCs) followed by low-dose INO-4500 (198 SFU/106 PBMCs) then placebo (103 SFU/106 PBMCs) groups.
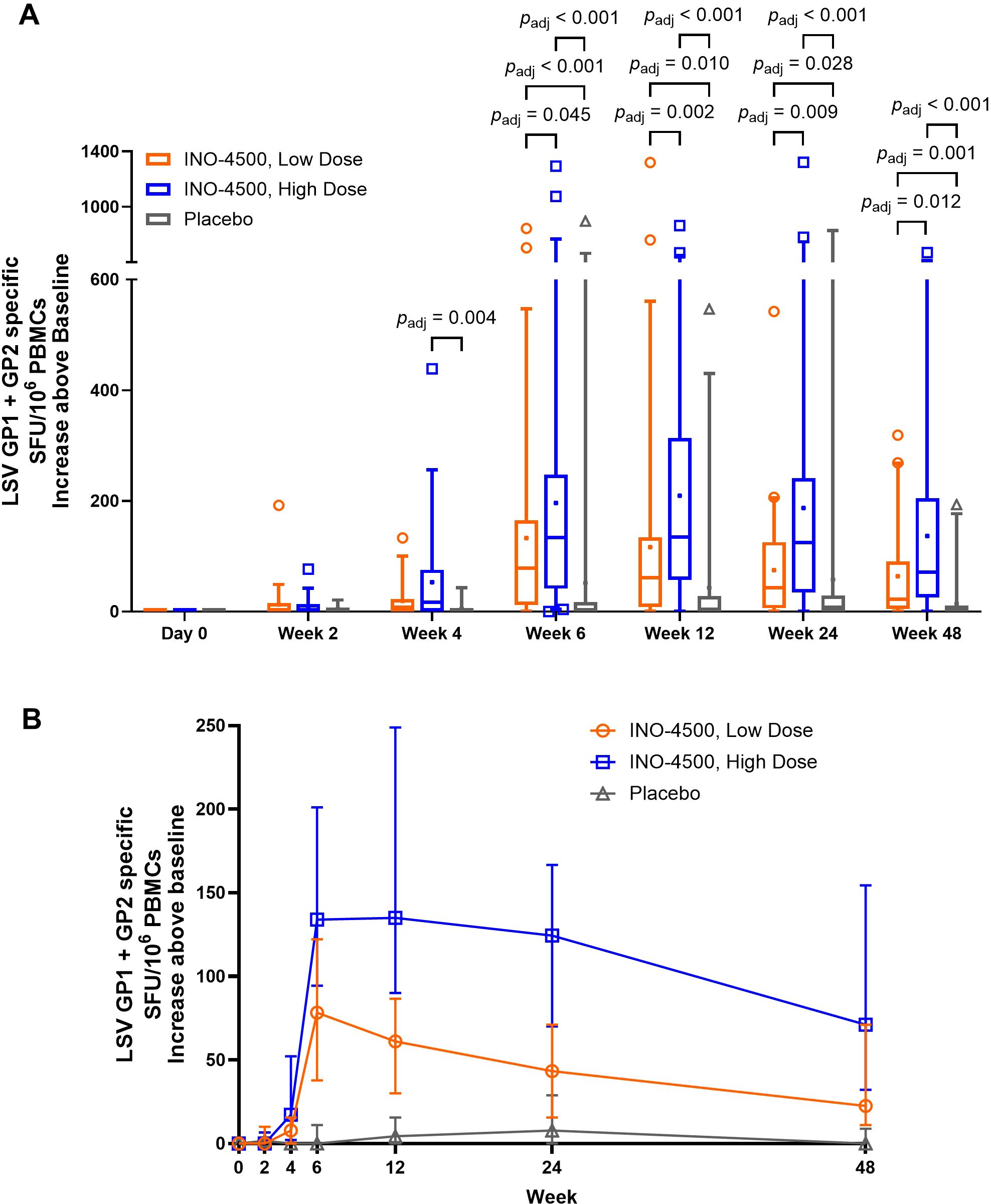
Figure 3. Cellular immune responses to LASV as measured by IFN-γ ELISpot. Baseline subtracted LASV-specific SFU per million PBMCs in response to total LASV (the sum of glycoprotein peptide pools GP1 and GP2) are shown for each group with Datasets 1 (interim analysis) and 2 (long-term follow-up analysis) combined. Placebo groups (Groups C and D) are combined. The larger of two magnitudes above baseline were excluded for six participants with duplicate Week 24 data. Group A, up to n = 59; Group B, up to n = 66; Placebo, up to n = 31. (A) Box and whiskers extend from 25th to 75th and 5th to 95th percentiles, respectively, with outliers represented by open symbols. Line at the median; + at mean. padj-values were calculated at each study week between groups using the Kruskal-Wallis test followed by Benjamini-Hochberg adjustment. Only significant padj-values displayed. (B) Medians with 95% CI are represented by open symbols for each group. LASV, Lassa virus; GP, glycoprotein; IFN-γ, interferon-gamma; ELISpot, Enzyme-linked immunosorbent spot; SFU, spot forming units; PBMCs, peripheral blood mononuclear cells; CI, confidence interval.
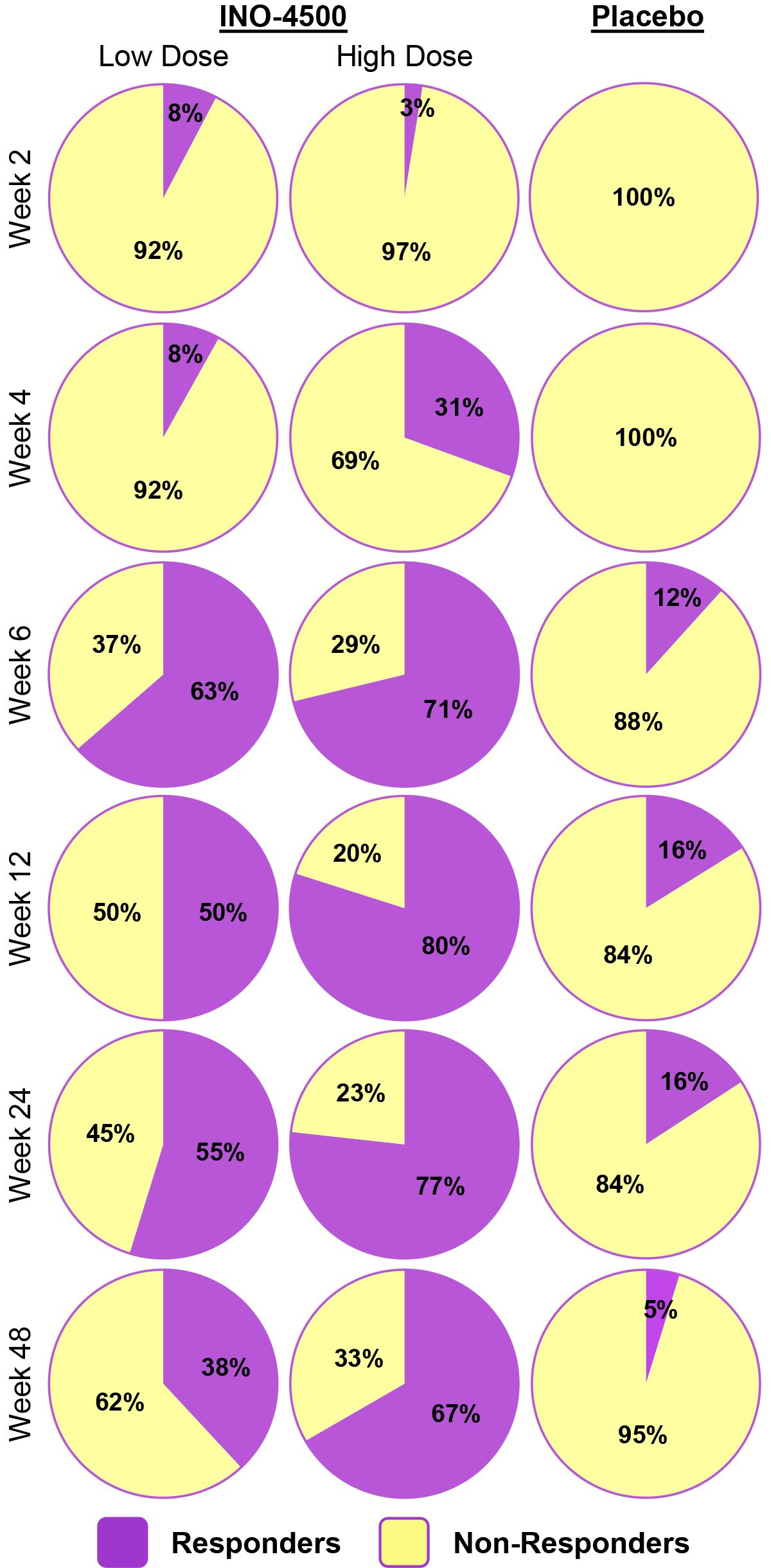
Figure 4. Responder status to total LASV GP as measured by IFN-γ ELISpot by group. Percent participants who displayed a cellular immune response to stimulation with total LASV GP (the sum of glycoprotein peptide pools GP1 and GP2) for each group at each timepoint with Datasets 1 (interim analysis) and 2 (long-term follow-up analysis) combined. Placebo groups (Groups C and D) are combined. Group A, up to n = 59; Group B, up to n = 66; Placebo, up to n = 31. Responder status was assigned to any of the six participants with duplicate Week 24 data only if each were considered responders for both Datasets 1 and 2. LASV, Lassa virus; GP, glycoprotein; IFN-γ, interferon-gamma; ELISpot, Enzyme-linked immunosorbent spot.
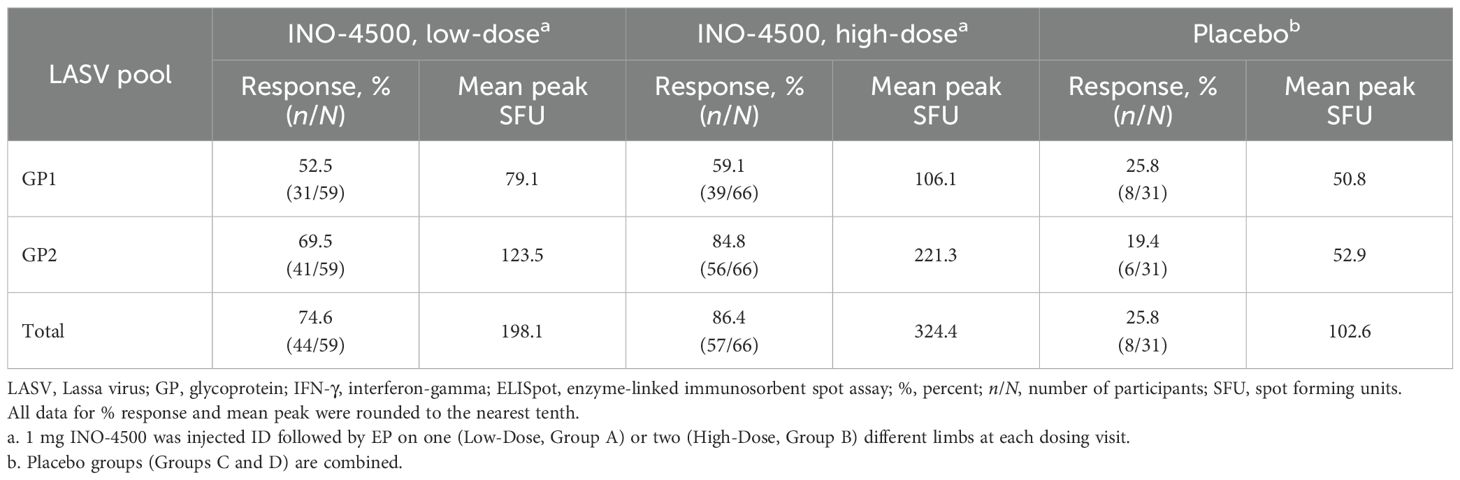
Table 7. Cellular immune responses to LASV GP at any timepoint as measured by IFN-γ ELISpot, combined datasets in LSV-002 study.
Discussion
Lassa fever remains a persistent public health threat in West Africa, yet no licensed vaccine is currently available. Although research began in the 1970s, progress has been limited by the extensive genetic diversity of LASV strains and insufficient surveillance data (12, 36). In more recent years, the disease has gained global attention and was added to the WHO Research and Development Roadmap (9). Since 2015, 34 vaccine candidates have been developed, and four are currently in clinical trials: INO-4500, rVSVΔG-LASV-GPC, MV-LASV, and EBS-LASV (12, 36, 37). This includes a CEPI-supported Phase II clinical trial ongoing in West Africa to evaluate a recombinant vesicular stomatitis virus (rVSV) vector vaccine, rVSVΔG-LASV-GPC (10, 14). INO-4500 is notably the only DNA-based vaccine investigated in clinical trials (37).
One of the scenarios of the WHO’s target product profile (TPP) for development of a LASV vaccine focuses on non-emergency, preventive vaccine campaigns for those living in endemic areas and individuals such as healthcare workers at high risk of exposure (38). This approach is further supported by a recent epidemiological modeling study supported by CEPI and conducted by the University of Liverpool and the University of Oxford. The authors analyzed the projected impact of six LASV vaccination scenarios over a 10-year period. The analysis found that preventative vaccination strategies targeting endemic areas were significantly more effective than reactive vaccination based on estimated rates of infections, hospitalizations, deaths, disability-adjusted life years (DALYs), and societal expenditures. Of the estimated 2.7 million LASV infections occurring annually in West Africa, causing a burden of approximately 200,000 DALYs, population-wide preventative vaccination in a non-emergency setting could potentially save $20.1 million in DALY losses and $128.8 million in societal costs – ten times more than a reactive outbreak response (4). Further, a 2023 survey of eight West African-based LF experts also recommend prioritizing preventative vaccination of endemic areas due to the unclear cost-benefit ratio of reactive campaigns (39).
The Phase 1b study reported here provides important evidence supporting the safety, tolerability and immunogenicity of INO-4500, a synthetic DNA vaccine encoding for LASV GPC, which was administered by ID injection followed by EP. Building on the encouraging results of an US-based Phase 1a trial (NCT03805984), in which we observed no SAEs or AESIs, the current LSV-002 study provides further evidence that INO-4500 administered ID and followed by EP exhibited a well-tolerated safety profile in a target population of healthy Ghanaian adults. The vaccine produced no Grade 3 or higher treatment-related TEAEs, and 88.6% of all TEAEs were Grade 1. Notably, no cases of hearing loss or sensorineural impairment, key concerns for LASV infection, were observed following INO-4500 vaccination in this study. Although sensorineural hearing loss occurs in approximately 30% of LF survivors (32, 33), evidence to date from both clinical trials and preclinical studies evaluating LF vaccines, including one evaluating INO-4500 in an LASV challenge study involving NHPs, has revealed no reports of hearing loss post-LASV vaccination (15, 33, 40).
Our results further support the clinical trial evidence regarding the favorable safety profile of the DNA vaccine platform. Across multiple studies utilizing this technology, the reported AEs were mild to moderate, with no evidence to date that they cause severe or persistent systemic AEs (41–46). Furthermore, the DNA vaccine platform has been shown to have a lower risk of local and systemic AEs following immunization as compared to alternative vaccine modalities (inactivated vaccine, mRNA, viral-based vector, and protein subunit vaccines), as reported in a meta-analysis by Kouhpayeh, et al. (47).
INO-4500 elicited humoral and cellular immune responses that were statistically significant over placebo. While both INO-4500 groups exhibited significant increases in LASV GP-specific binding antibodies at Weeks 6 and 12 compared to placebo, with the high-dose group eliciting the strongest responses, humoral response levels and seroconversion rate remained low (<10%) through Week 12. Negligible IgG and IgM antibody responses have been observed in viremic patients during acute LASV infection (48, 49), which may explain, in part, why low rates of seroconversion were observed after INO-4500 administration during the present study. Moreover, structural properties, such as the glycan shield, obscure the glycoprotein subunits of the LASV GPC (49) and the presence of irrelevant LASV GPC conformations (50) have been reported to mask key regions of the GPC, making it harder for antibodies, including neutralizing, to recognize and bind to their target. In fact, both LASV infection and vaccination have resulted in delayed and/or weak neutralizing antibody responses (49). For these reasons, neutralizing antibody responses were not assessed at the early timepoints indicated in the current study. However, characterization at both early and later timepoints may have offered additional insights into the humoral immune responses to vaccination with INO-4500.
Cellular immune responses were collected across two analytical runs to support informal interim and long-term follow-up analyses. When analyzed individually, responses from these two datasets differed in range and magnitude across time points, perhaps due in part to the range in number of participants tested at different timepoints across both datasets; however, overall trends were similar when analyzed in combination. INO-4500 induced T cell responses in both groups, with the high-dose group eliciting the strongest mean peak response followed by the low-dose group, which was two-fold greater than placebo. 71% and 80% of participants from the high-dose group responded at Week 6 and Week 12, respectively. Notably, T cell responses were durable and remained elevated above baseline through Week 48 in both INO-4500 groups, underscoring the vaccine’s lasting immunogenicity. Robust CD4+ and CD8+ T cell responses have been observed during and following LASV infection in LF survivors and are likely important for control and clearance (19, 51, 52). While cellular immune responses were assessed by ELISpot in the current study, deeper characterization of LASV-specific CD4+ and CD8+ T cell responses may have offered additional insights into the cellular immune responses to vaccination with INO-4500.
Although the 2-dose vaccine regimen examined in this study demonstrated an adequate and durable cellular immune response, the low humoral response reported may be seen as a limit for the use of INO-4500 as a preventative measure. It is worth noticing that INO-4500 vaccinated NHPs exposed to a lethal dose of LASV Josiah strain a year later survived while presenting low to no humoral response at the initiation of the challenge (15). Nevertheless, based on preclinical studies in NHPs with INO-4500 in which 2- and 3-dose regimens were evaluated, seroconversion rates and magnitude in binding, and more importantly, in neutralizing antibody levels, increased to a greater degree with a 3-dose vaccination schedule (Andrade V et al. [manuscript in preparation]). Echoing the clinical developments of other DNA vaccines (43, 45, 50), future clinical studies with INO-4500 could benefit from exploring a 3-dose regimen. Finally, while this study demonstrated durability of immune responses for 12 months, further research is needed to assess longer-term durability and achieve the minimum WHO TPP benchmark of three years.
The DNA medicine platform used as the basis for INO-4500 is uniquely positioned to prevent infectious diseases in non-emergency settings, and has shown promising results as an effective, versatile platform (43, 45, 46, 53, 54). Using this innovative technology, DNA vaccines can be rapidly designed using a common platform to express relevant antigens and readily integrated into large-scale manufacturing (55, 56). They are not constrained by ultra cold-chain requirements due to their thermostability at refrigerated temperatures, making them particularly well suited for deployment in endemic regions with limited infrastructure (57). Unlike viral vector platforms, DNA vaccines are conducive to repeated dosing without eliciting anti-vector immunity, a critical advantage for long-term immunization strategies (58, 59) which could require the use of booster doses, as is now being discussed for the prevention of Ebola Virus Disease (EVD) (60). These features align well with the logistical and public health demands of preparing for LASV outbreaks in endemic regions.
In conclusion, this study demonstrates the safety, tolerability, and immunogenicity of INO-4500 administered by ID injection and followed by EP. Continued development of INO-4500 would potentially provide access to a safe, well tolerated and effective vaccine for the prevention of Lassa fever in healthcare workers and those living in endemic areas ahead of outbreaks. Such a vaccine could substantially reduce the burden of LF in West Africa and mitigate the risk of broader regional or global spread, fulfilling an urgent global health priority.
Data availability statement
The datasets presented in this article are not readily available because restrictions may apply due to privacy reasons, regulatory submissions, and/or ongoing research projects. Requests to access the datasets should be directed to LH, TGF1cmVudC5IdW1lYXVAaW5vdmlvLmNvbQ==.
Ethics statement
The studies involving humans were approved by Noguchi Memorial Institute for Medical Research (NMIMR) Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KKo: Project administration, Investigation, Writing – review & editing, Writing – original draft. KW: Methodology, Project administration, Writing – review & editing. BO: Investigation, Writing – review & editing. IM: Formal Analysis, Writing – review & editing. JB: Formal Analysis, Project administration, Writing – review & editing, Methodology. SY: Conceptualization, Investigation, Writing – review & editing. KB: Funding acquisition, Supervision, Writing – review & editing, Conceptualization, Project administration. KKu: Writing – review & editing, Investigation. EK-B: Investigation, Writing – review & editing. EO: Investigation, Writing – review & editing. AP: Investigation, Writing – review & editing. SA-A: Writing – review & editing, Investigation. MA-C: Investigation, Writing – review & editing. HB: Writing – review & editing, Investigation. BA: Writing – review & editing, Investigation. EB: Writing – review & editing, Investigation. MN: Investigation, Writing – review & editing. LQ: Writing – review & editing, Investigation. MM: Writing – review & editing, Supervision. AS: Data curation, Software, Writing – review & editing. ER: Writing – review & editing, Visualization, Writing – original draft, Formal Analysis. EG: Writing – review & editing, Writing – original draft, Formal Analysis, Visualization. DL: Writing – review & editing, Investigation, Supervision, Writing – original draft, Data curation. LH: Funding acquisition, Writing – review & editing, Project administration, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study received funding from the Coalition for Epidemic Preparedness Innovation (CEPI PRJ-6069).
Acknowledgments
This work was funded by the Coalition for Epidemic Preparedness Innovations (CEPI). We are grateful to all the study participants who dutifully followed the protocol and enabled us to acquire this data. We would like to thank Sophie Biernaux, Renske Hesselink and Lorna Clark at CEPI, and acknowledge Sergio Vazquez, Aditya Patel, Samantha Satyavrata, Laura George, Erin McCaffrey, Jose Suaya, Eduardo Barranco, Hedieh Badie, Keiko Simon and Stefani Slog for their contributions to the study and development of this manuscript. We would like to thank Dana Barberio, MS, CMPP of Edge Bioscience Communications for her contributions in editing this manuscript.
Conflict of interest
AS, ER, EG, and LH are employees of and KW, BO, IM, JB, SY, KB, MM, and DL are previous employees of Inovio Pharmaceuticals and may hold stock and/or stock options.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from Inovio Pharmaceuticals. The funder was involved in the study design, collection, analysis, interpretation of data, the writing of this article, and the decision to submit it for publication.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1658549/full#supplementary-material
References
1. Asogun DA, Gunther S, Akpede GO, Ihekweazu C, and Zumla A. Lassa fever: epidemiology, clinical features, diagnosis, management and prevention. Infect Dis Clin North Am. (2019) 33:933–51. doi: 10.1016/j.idc.2019.08.002
2. Salami K, Gouglas D, Schmaljohn C, Saville M, and Tornieporth N. A Review of Lassa Fever Vaccine Candidates. Curr Opin Virol. (2019) 37:105–11. doi: 10.1016/j.coviro.2019.07.006
3. Reyna RA, Littlefield KE, Shehu N, Makishima T, Maruyama J, and Paessler S. The importance of lassa fever and its disease management in west africa. Viruses. (2024) 16. doi: 10.3390/v16020266
4. Smith DRM, Turner J, Fahr P, Attfield LA, Bessell PR, Donnelly CA, et al. Health and economic impacts of lassa vaccination campaigns in west africa. Nat Med. (2024) 30:3568–77. doi: 10.1038/s41591-024-03232-y
5. Warner BM, Safronetz D, and Stein DR. Current perspectives on vaccines and therapeutics for lassa fever. Virol J. (2024) 21:320. doi: 10.1186/s12985-024-02585-7
6. Purushotham J, Lambe T, and Gilbert SC. Vaccine platforms for the prevention of lassa fever. Immunol Lett. (2019) 215:1–11. doi: 10.1016/j.imlet.2019.03.008
7. World Health Organization. Lassa Fever (2025). Available online at: https://www.who.int/health-topics/lassa-fevertab=tab_1 (Accessed: February 19, 2025).
8. Kayem ND, Benson C, Aye CYL, Barker S, Tome M, Kennedy S, et al. Lassa fever in pregnancy: A systematic review and meta-analysis. Trans R Soc Trop Med Hygiene. (2020) 114:385–96. doi: 10.1093/trstmh/traa011
9. World Health Organization. R&D Blueprint. Lassa Fever (2025). Available online at: https://www.who.int/teams/blueprint/lassa-fever (Accessed February 19, 2025).
10. National Institutes of Health. Clinicaltrials.Gov. A Clinical Trial to Evaluate the Safety and Immunogenicity of rVSVΔG-LASV-GPC Vaccine in Adults in Good General Heath (2024). Available online at: https://clinicaltrials.gov/search?term=NCT04794218 (Accessed June 17, 2025).
11. National Institutes of Health. Clinicaltrials.Gov. A Lassa Fever Vaccine Trial in Adults and Children Residing in West Africa (2024). Available online at: https://clinicaltrials.gov/search?term=NCT05868733%20 (Accessed June 17, 2025).
12. Sulis G, Peebles A, and Basta NE. Lassa Fever vaccine candidates: A scoping review of vaccine clinical trials. Trop Med Int Health. (2023) 28:420–31. doi: 10.1111/tmi.13876
13. Tschismarov R, Van Damme P, Germain C, De Coster I, Mateo M, Reynard S, et al. Immunogenicity, safety, and tolerability of a recombinant measles-vectored lassa fever vaccine: A randomised, placebo-controlled, first-in-human trial. Lancet. (2023) 401:1267–76. doi: 10.1016/S0140-6736(23)00048-X
14. Coalition for Epidemic Preparedness Innovations (CEPI). Vaccine Technology (2025). Available online at: https://cepi.net/vaccine-technology (Accessed June 17, 2025).
15. Andrade VM, Cashman K, Rosenke K, Wilkinson E, Josleyn N, Lynn G, et al. The DNA-based lassa vaccine INO-4500 confers durable protective efficacy in cynomolgus macaques against lethal lassa fever. Commun Med (Lond). (2024) 4:253. doi: 10.1038/s43856-024-00684-8
16. LaVergne SM, Sakabe S, Momoh M, Kanneh L, Bond N, Garry RF, et al. Expansion of CD8+ T cell population in lassa virus survivors with low T cell precursor frequency reveals durable immune response in most survivors. PloS Negl Trop Dis. (2022) 16:e0010882. doi: 10.1371/journal.pntd.0010882
17. Sullivan BM, Sakabe S, Hartnett JN, Ngo N, Goba A, Momoh M, et al. High crossreactivity of human T cell responses between lassa virus lineages. PloS Pathog. (2020) 16:e1008352. doi: 10.1371/journal.ppat.1008352
18. Baize S, Marianneau P, Loth P, Reynard S, Journeaux A, Chevallier M, et al. Early and strong immune responses are associated with control of viral replication and recovery in lassa virus-infected cynomolgus monkeys. J Virol. (2009) 83:5890–903. doi: 10.1128/jvi.01948-08
19. ter Meulen J, Badusche M, Kuhnt K, Doetze A, Satoguina J, Marti T, et al. Characterization of human CD4(+) T-cell clones recognizing conserved and variable epitopes of the lassa virus nucleoprotein. J Virol. (2000) 74:2186–92. doi: 10.1128/jvi.74.5.2186-2192.2000
20. Abreu-Mota T, Hagen KR, Cooper K, Jahrling PB, Tan G, Wirblich C, et al. Non-neutralizing antibodies elicited by recombinant lassa–rabies vaccine are critical for protection against lassa fever. Nat Commun. (2018) 9:4223. doi: 10.1038/s41467-018-06741-w
21. Jiang J, Banglore P, Cashman KA, Schmaljohn CS, Schultheis K, Pugh H, et al. Immunogenicity of a protective intradermal DNA vaccine against lassa virus in cynomolgus macaques. Hum Vaccines immunotherapeutics. (2019) 15:2066–74. doi: 10.1080/21645515.2019.1616499
22. Hallam HJ, Hallam S, Rodriguez SE, Barrett ADT, Beasley DWC, Chua A, et al. Baseline mapping of lassa fever virology, epidemiology and vaccine research and development. NPJ Vaccines. (2018) 3:11. doi: 10.1038/s41541-018-0049-5
23. Murphy H and Ly H. Understanding immune responses to lassa virus infection and to its candidate vaccines. Vaccines (Basel). (2022) 10:1668. doi: 10.3390/vaccines10101668
24. Fisher-Hoch SP, Hutwagner L, Brown B, and McCormick JB. Effective vaccine for lassa fever. J Virol. (2000) 74:6777–83. doi: 10.1128/jvi.74.15.6777-6783.2000
25. Ronk AJ, Lloyd NM, Zhang M, Atyeo C, Perrett HR, Mire CE, et al. A Lassa Virus mRNA Vaccine Confers Protection but Does Not Require Neutralizing Antibody in a Guinea Pig Model of Infection. Nat Commun. (2023) 14:5603. doi: 10.1038/s41467-023-41376-6
26. Mantlo E, Paessler S, and Huang C. Differential immune responses to hemorrhagic fever-causing arenaviruses. Vaccines (Basel). (2019) 7(4):138. doi: 10.3390/vaccines7040138
27. Russier M, Pannetier D, and Baize S. Immune responses and lassa virus infection. Viruses. (2012) 4:2766–85. doi: 10.3390/v4112766
28. Lukashevich IS, Paessler S, and de la Torre JC. Lassa virus diversity and feasibility for universal prophylactic vaccine. F1000Research. (2019) 8:F1000 Faculty Rev-134. doi: 10.12688/f1000research.16989.1
29. Cashman KA, Wilkinson ER, Shaia CI, Facemire PR, Bell TM, Bearss JJ, et al. A DNA vaccine delivered by dermal electroporation fully protects cynomolgus macaques against lassa fever. Hum Vaccines immunotherapeutics. (2017) 13:2902–11. doi: 10.1080/21645515.2017.1356500
30. Bowen MD, Rollin PE, Ksiazek TG, Hustad HL, Bausch DG, Demby AH, et al. Genetic diversity among lassa virus strains. J Virol. (2000) 74:6992–7004. doi: 10.1128/jvi.74.15.6992-7004.2000
31. U.S. Department of Health and Human Services, Food and Drug Administration and Center for Biologics Evaluation and Research. Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials (2025). Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical (Accessed May 29, 2025).
32. Ficenec SC, Percak J, Arguello S, Bays A, Goba A, Gbakie M, et al. Lassa fever induced hearing loss: the neglected disability of hemorrhagic fever. Int J Infect Dis. (2020) 100:82–7. doi: 10.1016/j.ijid.2020.08.021
33. Reed NS, Brewer CC, Akintunde G, Blackie FF, Charles L, Fast P, et al. Report of a SPEAC webinar 22 september 2023: sensorineural hearing loss, lassa virus disease and vaccines. Vaccine. (2025) 43:126525. doi: 10.1016/j.vaccine.2024.126525
34. Liu YC, Ibekwe T, Kelso JM, Klein NP, Shehu N, Steuerwald W, et al. Sensorineural hearing loss (SNHL) as an adverse event following immunization (AEFI): case definition & Guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. (2020) 38:4717–31. doi: 10.1016/j.vaccine.2020.05.019
35. Bangura U, Davis C, Lamin J, Bangura J, Soropogui B, Davison AJ, et al. Spatio-temporal spread of lassa virus and a new rodent host in the mano river union area, west africa. Emerg Microbes Infect. (2024) 13:2290834. doi: 10.1080/22221751.2023.2290834
36. Ballivian J, Berrueta M, Ciapponi A, Sambade JM, Stegelmann K, Mazzoni A, et al. Safety, immunogenicity, efficacy, and effectiveness of lassa fever vaccines in pregnant persons, children, and adolescents: A protocol for a living systematic review and meta-analysis. Reprod Health. (2025) 22:53. doi: 10.1186/s12978-025-02008-y
37. Moore KA, Ostrowsky JT, Mehr AJ, Johnson RA, Ulrich AK, Moua NM, et al. Lassa fever research priorities: towards effective medical countermeasures by the end of the decade. Lancet Infect Dis. (2024) 24:e696–706. doi: 10.1016/S1473-3099(24)00229-9
38. World Health Organziation. WHO Target Product Profile for Lassa Virus Vaccine (2017). Available online at: https://www.who.int/publications/m/item/who-target-product-profile-for-lassa-virus-vaccine (Accessed June 17, 2025).
39. Kaboré L, Pecenka C, and Hausdorff WP. Lassa fever vaccine use cases and demand: perspectives from select west african experts. Vaccine. (2024) 42:1873–7. doi: 10.1016/j.vaccine.2024.02.044
40. Huynh T, Gary JM, Welch SR, Coleman-McCray J, Harmon JR, Kainulainen MH, et al. Lassa virus antigen distribution and inflammation in the ear of infected strain 13/N Guinea pigs. Antiviral Res. (2020) 183:104928. doi: 10.1016/j.antiviral.2020.104928
41. Jia S, Shao C, Cheng X, Pan H, Wang Z, Xia Y, et al. Immunogenicity and safety of a COVID-19 DNA vaccine in healthy adults and elderly: A randomized, observer-blind, placebo-controlled phase 2 trial. Hum Vaccines immunotherapeutics. (2025) 21:2448405. doi: 10.1080/21645515.2024.2448405
42. Colluru VT, Johnson LE, Olson BM, and McNeel DG. Preclinical and clinical development of DNA vaccines for prostate cancer. Urol Oncol. (2016) 34:193–204. doi: 10.1016/j.urolonc.2013.09.014
43. Kraynyak KA, Blackwood E, Agnes J, Tebas P, Giffear M, Amante D, et al. SARS-coV-2 DNA vaccine INO-4800 induces durable immune responses capable of being boosted in a phase 1 open-label trial. J Infect Dis. (2022) 225:1923–32. doi: 10.1093/infdis/jiac016
44. Morrow MP, Gillespie E, Sylvester A, Amin MR, Belafsky PC, Best SR, et al. DNA immunotherapy for recurrent respiratory papillomatosis (RRP): phase 1/2 study assessing efficacy, safety, and immunogenicity of INO-3107. Nat Commun. (2025) 16:1518. doi: 10.1038/s41467-025-56729-6
45. Tebas P, Kraynyak KA, Patel A, Maslow JN, Morrow MP, Sylvester AJ, et al. Intradermal syncon(R) ebola GP DNA vaccine is temperature stable and safely demonstrates cellular and humoral immunogenicity advantages in healthy volunteers. J Infect Dis. (2019) 220:400–10. doi: 10.1093/infdis/jiz132
46. Tebas P, Yang S, Boyer JD, Reuschel EL, Patel A, Christensen-Quick A, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-coV-2: A preliminary report of an open-label, phase 1 clinical trial. EClinicalMedicine. (2021) 31:100689. doi: 10.1016/j.eclinm.2020.100689
47. Kouhpayeh H and Ansari H. Adverse events following COVID-19 vaccination: A systematic review and meta-analysis. Int Immunopharmacol. (2022) 109:108906. doi: 10.1016/j.intimp.2022.108906
48. Yun NE and Walker DH. Pathogenesis of lassa fever. Viruses. (2012) 4:2031–48. doi: 10.3390/v4102031
49. Jahrling PB, Frame JD, Rhoderick JB, and Monson MH. Endemic lassa fever in Liberia. IV. Selection of optimally effective plasma for treatment by passive immunization. Trans R Soc Trop Med Hygiene. (1985) 79:380–4. doi: 10.1016/0035-9203(85)90388-8
50. Modjarrad K, Roberts CC, Mills KT, Castellano AR, Paolino K, Muthumani K, et al. Safety and immunogenicity of an anti-middle east respiratory syndrome coronavirus DNA vaccine: A phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis. (2019) 19:1013–22. doi: 10.1016/S1473-3099(19)30266-X
51. McElroy AK, Akondy RS, Harmon JR, Ellebedy AH, Cannon D, Klena JD, et al. A case of human lassa virus infection with robust acute T-cell activation and long-term virus-specific T-cell responses. J Infect Dis. (2017) 215:1862–72. doi: 10.1093/infdis/jix201
52. Sakabe S, Hartnett JN, Ngo N, Goba A, Momoh M, Sandi JD, et al. Identification of common CD8+ T cell epitopes from lassa fever survivors in Nigeria and Sierra Leone. J Virol. (2020) 94(12):e00153-20. doi: 10.1128/jvi.00153-20
53. Cashman KA, Wilkinson ER, Wollen SE, Shamblin JD, Zelko JM, Bearss JJ, et al. DNA vaccines elicit durable protective immunity against individual or simultaneous infections with lassa and ebola viruses in Guinea pigs. Hum Vaccines immunotherapeutics. (2017) 13:3010–9. doi: 10.1080/21645515.2017.1382780
54. Patel A, Reuschel EL, Xu Z, Zaidi FI, Kim KY, Scott DP, et al. Intradermal delivery of a synthetic DNA vaccine protects macaques from middle east respiratory syndrome coronavirus. JCI Insight. (2021) 6(10):e146082. doi: 10.1172/jci.insight.146082
55. Cai Y, Rodriguez S, Rameswaran R, Draghia-Akli R, Juba RJ Jr., and Hebel H. Production of pharmaceutical-grade plasmids at high concentration and high supercoiled percentage. Vaccine. (2010) 28:2046–52. doi: 10.1016/j.vaccine.2009.10.057
56. Gary EN and Weiner DB. DNA vaccines: prime time is now. Curr Opin Immunol. (2020) 65:21–7. doi: 10.1016/j.coi.2020.01.006
57. Maslow JN, Kwon I, Kudchodkar SB, Kane D, Tadesse A, Lee H, et al. DNA vaccines for epidemic preparedness: SARS-coV-2 and beyond. Vaccines (Basel). (2023) 11(6):1016. doi: 10.3390/vaccines11061016
58. Williams JA. Vector design for improved DNA vaccine efficacy, safety and production. Vaccines (Basel). (2013) 1:225–49. doi: 10.3390/vaccines1030225
59. Kutzler MA and Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. (2008) 9:776–88. doi: 10.1038/nrg2432
Keywords: DNA medicine, Lassa fever, Lassa virus (LASV), safety, immunogenicity, electroporation (EP), vaccine
Citation: Koram KA, Walker KA, Orizu B, Marrero I, Boyer J, Yang S, Broderick KE, Kusi KA, Kyei-Baafour E, Ofori EA, Pobee A, Adu-Amankwah S, Amoakoh-Coleman M, Amoakoh HB, Abuaku B, Badji E, Ntiri M, Quaye L, Morrow MP, Sylvester AJ, Reuschel EL, Gillespie E, Liebowitz D and Humeau LM (2025) Safety, tolerability, and immunogenicity of INO-4500, a synthetic DNA-based vaccine against Lassa virus, in a phase 1b clinical trial in healthy Ghanaian adults. Front. Immunol. 16:1658549. doi: 10.3389/fimmu.2025.1658549
Received: 02 July 2025; Accepted: 08 October 2025;
Published: 24 October 2025.
Edited by:
Slobodan Paessler, University of Texas Medical Branch at Galveston, United StatesReviewed by:
Juan C. De La Torre, The Scripps Research Institute, United StatesJunki Maruyama, University of Texas Medical Branch at Galveston, United States
Nathan Yakubu Shehu, University of Texas Medical Branch at Galveston, United States
Copyright © 2025 Koram, Walker, Orizu, Marrero, Boyer, Yang, Broderick, Kusi, Kyei-Baafour, Ofori, Pobee, Adu-Amankwah, Amoakoh-Coleman, Amoakoh, Abuaku, Badji, Ntiri, Quaye, Morrow, Sylvester, Reuschel, Gillespie, Liebowitz and Humeau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laurent M. Humeau, TGF1cmVudC5IdW1lYXVAaW5vdmlvLmNvbQ==
 Kwadwo Ansah Koram
Kwadwo Ansah Koram Kathleen A. Walker
Kathleen A. Walker Bonaventure Orizu2
Bonaventure Orizu2 Kwadwo Asamoah Kusi
Kwadwo Asamoah Kusi Eric Kyei-Baafour
Eric Kyei-Baafour Ebenezer Addo Ofori
Ebenezer Addo Ofori Benjamin Abuaku
Benjamin Abuaku Michael Ntiri
Michael Ntiri Emma L. Reuschel
Emma L. Reuschel Elisabeth Gillespie
Elisabeth Gillespie David Liebowitz
David Liebowitz Laurent M. Humeau
Laurent M. Humeau