- 1Department of Oncology, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University & Clinical Cancer Institute of Nanjing University, Nanjing, China
- 2Department of Oncology, Nanjing Drum Tower Hospital, Clinical College of Nanjing Drum Tower Hospital, Nanjing University of Chinese Medicine, Nanjing, China
- 3Department of Oncology, Nanjing Drum Tower Hospital, Clinical College of Nanjing Drum Tower Hospital, Nanjing Medical University, Nanjing, China
- 4Department of Oncology, Nanjing Drum Tower Hospital, Clinical College of Nanjing Drum Tower Hospital, Jiangsu University, Nanjing, China
Pancreatic Acinar Cell Carcinoma (PACC) is a rare subtype of pancreatic cancer, and its systematic treatment protocol is still pending for further exploration. There have been a few reports that PACC patients benefited from immunotherapy-integrated treatment. Here, we present a metastatic PACC patient who had new hepatic lesions but lowered serum AFP and CA19–9 after 2 cycles of integrated treatment that included chemotherapy, PD-1 blockade and antiangiogenic therapy, which was later confirmed to be pseudoprogression (PsP) by pathological diagnosis. So far as we know, this is the first reported PsP case of PACC. This case highlighted the value of biopsy in distinguishing PsP from authentic tumor progression of PACC.
Introduction
Pancreatic Cancer is one of the most malignant cancers and ranks 6th among the cancer-related mortality (1), with Pancreatic Ductal Adenocarcinoma (PDAC) being its most common subtype. By contrast, originating from acinar cells, Pancreatic Acinar Cell Carcinoma (PACC) is a rare subtype and only accounts for 1~2% of overall pancreatic cancer cases (2). From a general view, the prognosis of PACC is better than PDAC but worse than pancreatic neuroendocrine tumor, with a rough median overall survival of 47 months (3). Although radical resection at localized or even oligometastatic stage has been confirmed to be correlated with improved survival of PACC (4, 5), its tendency of recurrence and metastasis renders it important to explore novel whole-body systematic treatment regimen. Interestingly, a limited number of recent cases have revealed the potential superiority of comprehensive therapy that includes chemotherapy, immunotherapy and antiangiogenic targeted therapy over chemotherapy alone in PACC treatment (6–8), which is believed to have synergistic effect on reconstituting tumor immune microenvironment by multiple means, which ultimately result in cytotoxic T lymphocyte infiltration in tumor microenvironment and enhanced therapeutic effect of immunotherapy (9).
In terms of solid tumors, pseudoprogression (PsP) refers to the phenomenon in which initial increase in tumor sizes occurs or new lesions emerge followed by a decrease in tumor burden (10), usually after Immune Checkpoint Inhibitor (ICI) treatment or other forms of immunotherapy. Despite controversy on its detailed definition, PsP is closely associated with elevated immunocompetent cell level and intensified inflammatory reaction in tumor microenvironment (11), which are potential indicators of therapeutic response and lengthened survivals (12, 13). Immunotherapy-related pseudoprogression is most common in Melanoma, followed by Renal Cell Carcinoma (RCC), Urothelial Carcinoma and Non-Small Cell Lung Carcinoma (NSCLC) (10). By comparison, ICIs are rarely applied in the treatment of pancreatic cancer owing to its disappointing survival outcomes in previous clinical trials (14, 15), which is largely attributed to its nature of immune desert. Thus, there is scarcely any cases of PsP in pancreatic cancer patients.
Here, we present a case of PACC patient who rapidly underwent hepatic metastasis shortly after radical resection. PsP of metastatic hepatic cancer was observed after 2 cycles of comprehensive treatment including Nab-paclitaxel and Gemcitabine chemotherapy, Penpulimab (a PD-1 blockade) and Anlotinib (an antiangiogenic targeted Tyrosine Kinase Inhibitor). This case highlights a clinical scenario where, despite radiologic tumor progression, patients receiving ICI-integrated therapy for PACC may exhibit improved serum tumor markers and quality of life. In such cases, biopsy remains the gold standard to distinguish true disease progression from PsP.
Case presentation
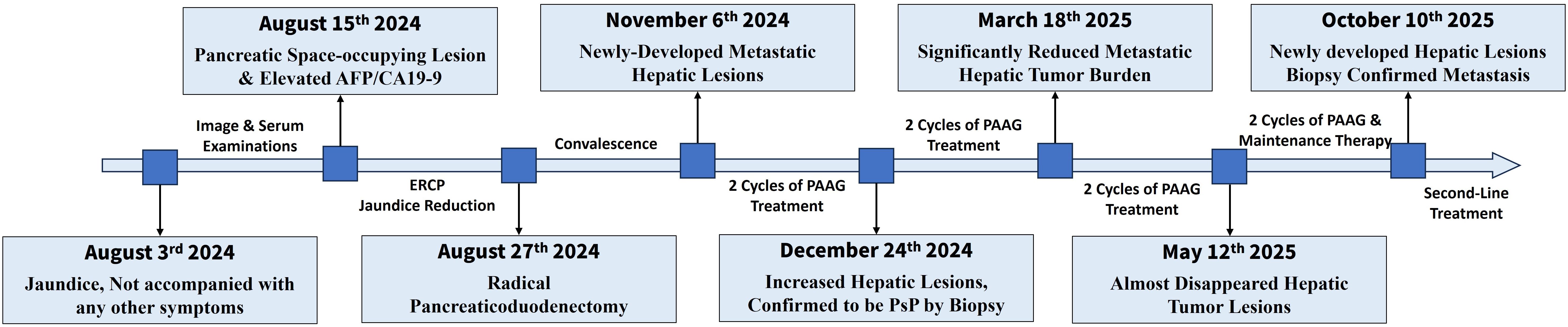
The overview of treatment process of the patient is depicted in Figure 1. A 61-year-old male patient came to our hospital for treatment in August, 2024 due to jaundice for 10 days, not accompanied with any other digestive symptoms. He was normally in good health, with no chronic diseases except stable-controlled hypertension and had no family history of cancer. An elevated level of serum Alpha Fetoprotein (AFP, 247.10ng/ml, Figure 2B) and Carbohydrate Antigen 19-9 (CA19-9, 64.10U/ml, Figure 2B) was detected, and imaging studies found a pancreatic lesion (Figure 2A). No metastatic sites were found. After his symptom of jaundice was controlled by stent implementation and medical treatment, the patient was evaluated as resectable pancreatic cancer by Multidisciplinary Team (MDT) discussion. After exclusion of contraindications, the patient underwent radical pancreaticoduodenectomy. Postoperative pathology (Figure 2C): poorly-differentiated acinic cell carcinoma, R0 margin, vascular invasion (-), perineural invasion (-), lymph node metastasis (3/11), pathological stage (pT2N1cM0, IIB). Immunohistochemical: Cytokeratin (CK) (++), Ki67 (approximately 60%+), Synaptophysin (Syn) (+), Chromogranin A (CgA) (-), CD56(-), D2-40 (Vascular negative), CD31 (Vascular positive), Insulinoma-Associated Protein 1 (INSM1) (-), Somatostatin Receptor 2A (SSTR2A) (-), Alpha-Thalassemia/X-Linked Intellectual Disability Syndrome Gene (ATRX) (+), P53 (Positive at different intensity), Thyroid Transcription Factor-1 (TTF-1) (-), Trypsin (+++), B-Cell Lymphoma/Leukemia 10 (Bcl10) (-), CD10 (-), β-catenin (membrane positive), AFP (partially positive). The patient developed postoperative complications but soon recovered from them shortly after radical surgery, which included pancreatic fistula, intra-abdominal infection and COVID-19 infection.
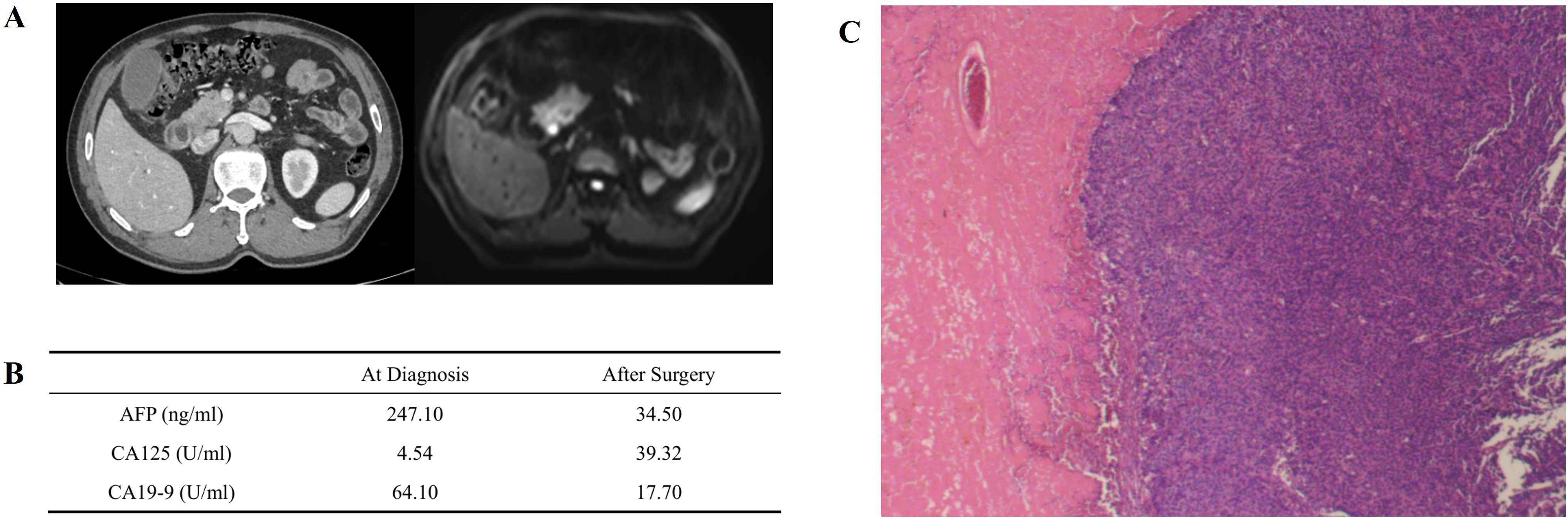
Figure 2. Primary lesion, preoperaitve/postoperative serum tumor marker and pathologic image. (A) Primary pancreatic lesion view under contrast CT (left) and contrast MR (right). An obvious lesion locates at head of pancreas can be found. (B) Serum tumor marker before and after surgery. AFP and CA19–9 decreased significantly after surgery, and slightly increased CA125 may be caused by surgery-associated ascitic fluid. (C) Pathological image of primary lesion, demonstrating poorly differentiated carcinoma with necrosis.
Because of the patient’s diminished fitness and personal reluctancy to further adjuvant therapy, the first postsurgical radiomic follow-up was not performed until November 6th, 2024. Unfortunately, new hepatic lesions (Figure 3A) and elevated serum AFP & CA19-9 (Figure 3C) were observed, indicating that rapid distant metastasis occurred shortly after surgery. NGS and PD-L1 expression were implemented in seek for potential benefit from immunotherapy or targeted therapy, result: Tumor Mutation Burden (TMB): 1.42Muts/Mb, Microsatellite Stability (MSS), KRAS wild type, BCL6/RPS6KA3/GATA6/TCF7L2/GNAS/FANCC/RICTOR mutation, Tumor Proportion Score (TPS): 3%, Combined Positive Score (CPS): 5%.
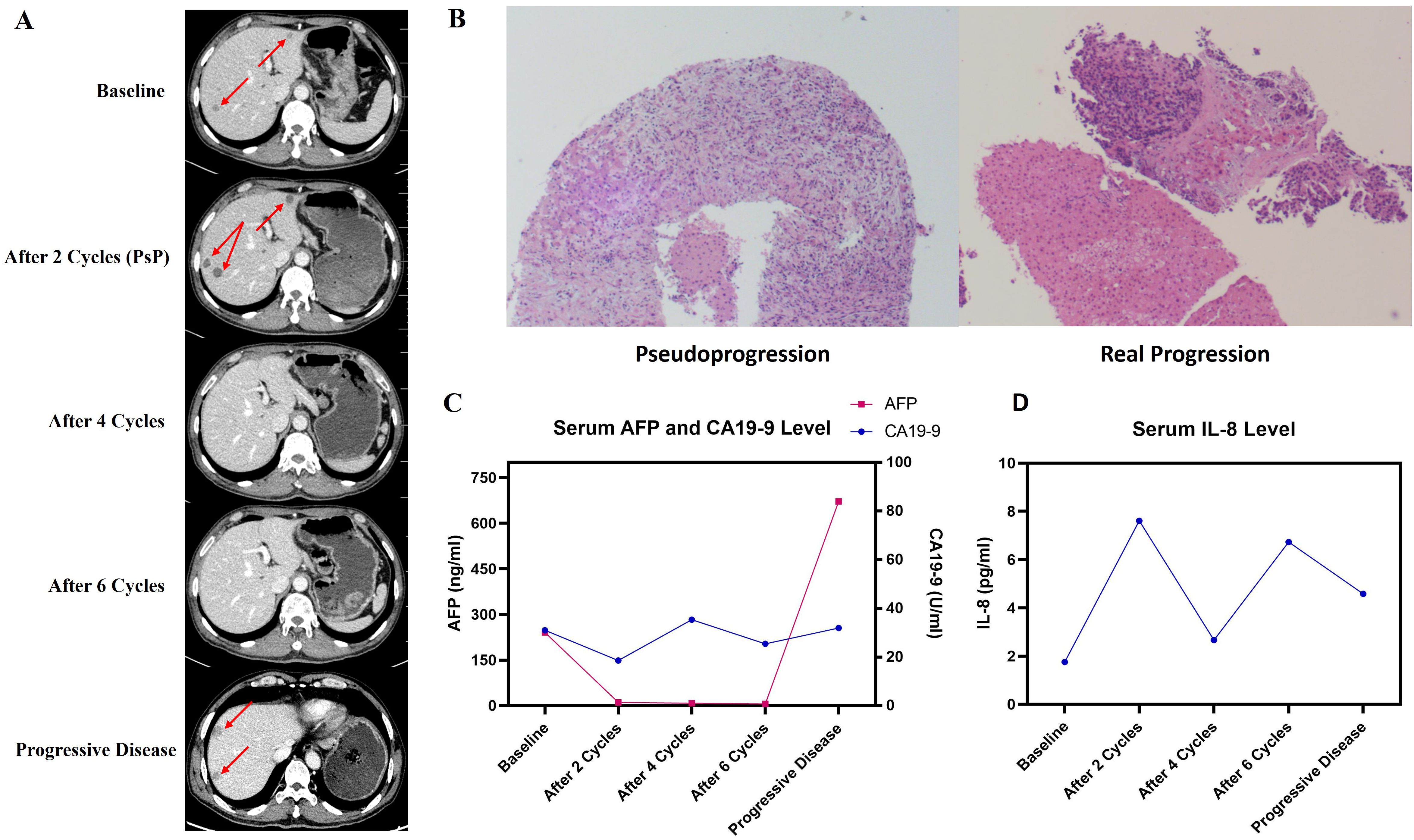
Figure 3. Efficacy of PAAG regimen by follow-up. (A) Outcome of radiologic follow-up by contrast CT. (B) Pathological images of PsP (left) and real progression (right) by hepatic biopsy. (C) Serum AFP and CA19–9 level at different follow-up points. (D) Serum IL-8 level at different follow-up points.
Two senior oncologists systematically reviewed the patient’s overall course of disease. Given the previous PACC cases benefiting from comprehensive treatment, the patient was advised to participate in the Investigator Initiated Trial Penpulimab Combined With Anlotinib and Nab-paclitaxel Plus Gemcitabine as First-line Treatment for Advanced Metastatic Pancreatic Cancer (RCT-PAAG) (NCT06051851) and was successfully enrolled in the experimental group. Starting on November 8th, the patient received treatment regimen of Penpulimab (a PD-1 blockade), Anlotinib (an antiangiogenic targeted drug), Nab-paclitaxel and Gemcitabine (PAAG) as first-line regimen. Follow-up were carried out every 2 cycles, containing contrast-enhanced CT, serum tumor marker and serum cytokine. Intriguingly, after 2 cycles of treatment, newly-developed and increased hepatic lesions were found by contrast CT, but his serum AFP and CA19–9 decreased to the novel range. The patient also stated that his abdominal and digestive symptoms has relieved after 2 cycles of PAAG therapy, which was contradictive to his radiomic result. The patient complained of rash and itching after the first cycle of therapy, and these symptoms soon vanished after treated with Loratadine tablets.
Another MDT discussion considered that possibility of PsP should not be excluded. To clarify the nature of new hepatic lesions, the patient underwent hepatic biopsy under color Doppler ultrasound guidance. Histopathology: focal hepatocyte necrosis accompanied by lymphocyte infiltration and fibrocyte reaction, no evidence of malignant tumor (Figure 3B). Pathological outcomes confirmed PsP of hepatic lesions, which are response to PD-1 Inhibitors. Therefore, the patient continued to receive PAAG treatment for another 2 cycles. In subsequent follow-ups, vanished lesions and decreased AFP further confirmed the nature of PsP.
By June 17th, 2025, the patient has finished 8 cycles of PAAG regimen and was in good health. According to the protocol of the trial, he then entered the stage of chemotherapy-free maintenance therapy (i.e. Penpulimab plus Anlotinib only). During the whole process, the patient did not exhibit treatment-related adverse events except tolerable anemia and neurotoxicity, and no severe adverse event was reported. His scores of Eastern Cooperative Oncology Group Performance Status (ECOG-PS) and Nutritional Risk Screening 2002 (NRS-2002) remained at 0 throughout the PAAG treatment, and always had complete self-care ability. On October 10th 2025, however, newly-developed hepatic lesions (Figure 3A) and significantly elevated AFP (Figure 3C) were observed. The patient insisted on another biopsy to exclude the probability of another PsP, but following pathological outcomes confirmed its nature of metastatic tumor (Figure 3B). The second-line regimen was Liposomal Irinotecan plus Fluorouracil and Calcium Folinate, which referenced the standard second-line regimen of PDAC. By last follow-up conducted on October 31st, 2025, the patient was in good condition, with a Progression-Free Survival of 11.1 months and is still undergoing second-line treatment.
Discussion
This article describes one rare instance of pseudoprogression (PsP) in a patient with metastatic Pancreatic Acinar Cell Carcinoma (mPACC) following first-line treatment regimen of Penpulimab (a PD-1 blockade), Anlotinib (an antiangiogenic targeted drug), Nab-Paclitaxel and Gemcitabine chemotherapy (PAAG). His radiomic follow-up after two cycles revealed new hepatic lesions, which met RECIST criteria for disease progression but were eventually confirmed to be PsP through pathological biopsy and subsequent radiomic follow-ups. To the best of our knowledge, this is the first reported PACC case of immunotherapy-induced PsP. Given that PACC constitutes <2% of pancreatic malignancies (2) and reports of PsPs in this subtype are exceedingly scarce, this case underscores a critical diagnostic pitfall and therapeutic opportunity.
PsP following Immune Checkpoint Inhibitor (ICI) treatment is largely attributed to transient lymphocyte-inflammatory infiltration, which radiologically resembles tumor progression (i.e. increased or newly-merged lesions). While PsP is well-documented in melanoma or NSCLC, its occurrence in PACC is novel. In our case, the combination of chemotherapy, antiangiogenic targeted therapy and PD-1 blockade together successfully transformed one metastatic hepatic site into ‘tumor immune-graveyard’, which can be inferred from immunohistochemical outcomes: The strong positivity of CK and negativity of Trypsin is are indicators of tumor exocrine impotency, suggesting that metabolic-active tumor cells of this lesion have been eradicated after combined PAAG treatment. Besides, scattered positive expression of CD4+, CD8+, CD20+ and MPO are signals of CTL, Th cell, lymphocyte B cell and neutrophil cell infiltration triggered by immunological response, respectively. This case suggests that even in “cold” tumors like PACC, robust immune responses can manifest as PsP, hinting at latent immunogenicity.
Distinguishing PsP from real progressive disease in PACC is challenging yet critical, since such confusions would result in premature abortion of effective treating measures and worsened prognosis. As for melanoma or NSCLC where PsP occurs relatively more frequently, reported methods of differentiation includes radiomic features and peripheral blood results (16, 17). For instance, declined IL-8 or cell-free DNA (ctDNA) levels are latent biomarkers of ICI-treatment responses (18, 19), and thus may function as convenient approaches for distinction. In this case, however, PsP were observed with a transiently increased level of IL-8 (Figure 3D). We predict that this intriguing phenomenon is because that chemotherapy, PD-1 blockade and antiangiogenic targeted therapy together induced tumor cell necrosis, which triggered release of tumor neoantigen and subsequent local inflammation (20), where elevated IL-8 and neutrophil infiltration serve as responders (21). Also, in this case, serum AFP levels were more parallel to tumor burdens than CA19-9 (Figure 3C), and we speculate that serum AFP plays a significant role in efficacy monitoring of PACC if positive. Anyway, biopsy, as the gold standard, should always be considered when confronting such perplexing situation, especially for “cold” tumors such as pancreatic or biliary tract cancer where PsP scarcely occurs.
In this case, the patient benefitted from the combination treatment of chemotherapy, immunotherapy and targeted therapy. Similar strategies also revealed potentially improved efficacy over chemotherapy alone in late-stage non-small-cell lung carcinoma, gastric cancer and colorectal cancer (22–26). The synergistic effect of immune checkpoint inhibitor and antiangiogenic targeted therapy can be viewed from several different perspectives. Firstly, antiangiogenic therapy is capable of normalizing abnormal tumor vessel. As a result, the infiltration of immunologic effector cell (NK cell, cytotoxic T cell, etc.) and delivery of drugs (including ICIs) are promoted (27–29). Normalized tumor vessel also transformed the oxygen-deficient tumor microenvironment, which inhibits immune escape so as to prevent ICI drug resistance (30, 31). In addition, VEGF has been confirmed to abrogate dendritic cell activation (32) and enhance expression of PD-1 and other inhibitory checkpoints involved in CD8+T cell exhaustion (33), and antiangiogenic drugs targeting VEGF remove the inhibition of immune killing (34). In this sense, the integrated therapy of ICI and antiangiogenic drug is worth attempting for “cold” tumors such as pancreatic or biliary cancer based on standard chemotherapy regimens. Meanwhile, the additional risk by immunotherapy and antiangiogenic therapy should not be overlooked.
In conclusion, we reported an immunotherapy-related pseudoprogression case of metastatic PACC patient. This case stressed the importance of comprehensive outlook when evaluating efficacy of immunotherapy for pancreatic cancer patients, especially when increased or newly-developed lesions come along with decreased tumor marker and improved quality of life. In addition, biopsy, as the gold standard, should always be implemented when possible and necessary.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XB: Writing – original draft, Writing – review & editing, Investigation, Project administration. DY: Writing – review & editing, Investigation. JC: Writing – review & editing, Investigation. YL: Investigation, Writing – review & editing. HT: Investigation, Writing – review & editing. XK: Writing – review & editing, Investigation. BL: Writing – original draft, Writing – review & editing. HS: Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing. JD: Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declared financial support was received for this work and/or its publication. This study was funded by Fundings for Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University (2023-LCYJ-PY-29), Medical Science and technology development Foundation, Nanjing Department of Health (ZKX24021) and Medical Research Project of Jiangsu Provincial Health Commission (K2024038).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1663514/full#supplementary-material
References
1. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, and Jemal A. Cancer statistics, 2025. CA Cancer J Clin. (2025) 75:10–45. doi: 10.3322/caac.21871
2. Klimstra DS. Nonductal neoplasms of the pancreas. Mod Pathol. (2007) 20 Suppl 1:S94–112. doi: 10.1038/modpathol.3800686
3. Calimano-Ramirez LF, Daoud T, Gopireddy DR, Morani AC, Watrs R, Gumus K, et al. Pancreatic acinar cell carcinoma: A comprehensive review. World J Gastroenterol. (2022) 28:5827–44. doi: 10.3748/wjg.v28.i40.5827
4. Holen KD, Klimstra DS, Hummer A, Gonen M, Conlon K, Brennan M, et al. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol. (2002) 20:4673–8. doi: 10.1200/JCO.2002.02.005
5. Blair AB, Radomski SN, Chou J, Liu M, Howell TC, Park W, et al. Survival outcomes and genetic characteristics of resected pancreatic acinar cell carcinoma. Ann Surg Oncol. (2025) 32:1869–78. doi: 10.1245/s10434-024-16331-4
6. Qin L, Shen J, Yang Y, and Zou Z. Rapid response to the combination of lenvatinib and sintilimab in a pancreatic acinar cell carcinoma patient with elevated alpha-fetoprotein: A case report. Front Oncol. (2021) 11:692480. doi: 10.3389/fonc.2021.692480
7. Zhao F, Yang D, Xu T, He J, Guo J, and Li X. New treatment insights into pancreatic acinar cell carcinoma: case report and literature review. Front Oncol. (2023) 13:1210064. doi: 10.3389/fonc.2023.1210064
8. Wu G, Fang Y, Bi D, Yang W, and Sun Y. Case report: Immunotherapy in rare high TMB pancreatic acinar carcinoma. Front Oncol. (2024) 14:1357233. doi: 10.3389/fonc.2024.1357233
9. Yi M, Jiao D, Qin S, Chu Q, Wu K, and Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. (2019) 18:60. doi: 10.1186/s12943-019-0974-6
10. Jia W, Gao Q, Han A, Zhu H, and Yu J. The potential mechanism, recognition and clinical significance of tumor pseudoprogression after immunotherapy. Cancer Biol Med. (2019) 16:655–70. doi: 10.20892/j.issn.2095-3941.2019.0144
11. Frelaut M, du Rusquec P, de Moura A, Le Tourneau C, and Borcoman E. Pseudoprogression and hyperprogression as new forms of response to immunotherapy. BioDrugs. (2020) 34:463–76. doi: 10.1007/s40259-020-00425-y
12. Pires da Silva I, Lo S, Quek C, Gonzalez M, Carlino MS, Long GV, et al. Site-specific response patterns, pseudoprogression, and acquired resistance in patients with melanoma treated with ipilimumab combined with anti-PD-1 therapy. Cancer. (2020) 126:86–97. doi: 10.1002/cncr.32522
13. Ahmed FS, Dercle L, Goldmacher GV, Yang H, Connors D, Tang Y, et al. Comparing RECIST 1.1 and iRECIST in advanced melanoma patients treated with pembrolizumab in a phase II clinical trial. Eur Radiol. (2021) 31:1853–62. doi: 10.1007/s00330-020-07249-y
14. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. (2012) 366:2455–65. doi: 10.1056/NEJMoa1200694
15. Weiss GJ, Waypa J, Blaydorn L, Coats J, McGahey K, Sangal A, et al. A phase Ib study of pembrolizumab plus chemotherapy in patients with advanced cancer (PembroPlus). Br J Cancer. (2017) 117:33–40. doi: 10.1038/bjc.2017.145
16. Chen MY and Zeng YC. Pseudoprogression in lung cancer patients treated with immunotherapy. Crit Rev oncology/hematology. (2022) 169:103531. doi: 10.1016/j.critrevonc.2021.103531
17. Basler L, Gabryś HS, Hogan SA, Pavic M, Bogowicz M, Vuong D, et al. Radiomics, tumor volume, and blood biomarkers for early prediction of pseudoprogression in patients with metastatic melanoma treated with immune checkpoint inhibition. Clin Cancer research: an Off J Am Assoc Cancer Res. (2020) 26:4414–25. doi: 10.1158/1078-0432.CCR-20-0020
18. Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol Off J Eur Soc Med Oncol. (2017) 28:1988–95. doi: 10.1093/annonc/mdx190
19. Mcmahon JT, Studer M, Ulrich B, Revuelta Barbero JM, Pradilla I, Palacios-Ariza MA, et al. Circulating tumor DNA in adults with glioma: A systematic review and meta-analysis of biomarker performance. Neurosurgery. (2022) 91:231–8. doi: 10.1227/neu.0000000000001982
20. Meier P, Legrand AJ, Adam D, and Silke J. Immunogenic cell death in cancer: targeting necroptosis to induce antitumour immunity. Nat Rev Cancer. (2024) 24:299–315. doi: 10.1038/s41568-024-00674-x
21. Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, and Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J leukocyte Biol. (1994) 56:559–64. doi: 10.1002/jlb.56.5.559
22. Passaro A, Wang J, Wang Y, Lee SH, Melosky B, Shih JY, et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: primary results from the phase III MARIPOSA-2 study. Ann Oncol Off J Eur Soc Med Oncol. (2024) 35:77–90. doi: 10.1016/j.annonc.2023.10.117
23. Zhang L, Wang W, Ge S, Li H, Bai M, Duan J, et al. Sintilimab Plus Apatinib and Chemotherapy as Second−/Third-Line treatment for Advanced Gastric or Gastroesophageal Junction Adenocarcinoma: a prospective, Single-Arm, phase II trial. BMC Cancer. (2023) 23:211. doi: 10.1186/s12885-023-10661-4
24. Wang Y, Lu J, Chong X, Wang C, Chen X, Peng Z, et al. PD-1 antibody camrelizumab plus apatinib and SOX as first-line treatment in patients with AFP-producing gastric or gastro-esophageal junction adenocarcinoma (CAP 06): a multi-center, single-arm, phase 2 trial. Signal Transduct Target Ther. (2025) 10:100. doi: 10.1038/s41392-025-02193-z
25. Pan QZ, Zhao JJ, Liu L, Zhang DS, Wang LP, Hu WW, et al. XELOX (capecitabine plus oxaliplatin) plus bevacizumab (anti-VEGF-A antibody) with or without adoptive cell immunotherapy in the treatment of patients with previously untreated metastatic colorectal cancer: a multicenter, open-label, randomized, controlled, phase 3 trial. Signal Transduct Target Ther. (2024) 9:79. doi: 10.1038/s41392-024-01788-2
26. Kurtz JE, Pujade-Lauraine E, Oaknin A, Belin L, Leitner K, Cibula D, et al. Atezolizumab combined with bevacizumab and platinum-based therapy for platinum-sensitive ovarian cancer: placebo-controlled randomized phase III ATALANTE/ENGOT-ov29 trial. J Clin Oncol. (2023) 41:4768–78. doi: 10.1200/JCO.23.00529
27. Qian C, Zhou Y, Zhang T, Dong G, Song M, Tang Y, et al. Targeting PKM2 signaling cascade with salvianic acid A normalizes tumor blood vessels to facilitate chemotherapeutic drug delivery. Acta Pharm Sin B. (2024) 14:2077–96. doi: 10.1016/j.apsb.2024.02.003
28. Kim SJ, Jung KH, Son MK, Park JH, Yan HH, Fang Z, et al. Tumor vessel normalization by the PI3K inhibitor HS-173 enhances drug delivery. Cancer Lett. (2017) 403:339–53. doi: 10.1016/j.canlet.2017.06.035
29. Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. (2017) 544:250–4. doi: 10.1038/nature21724
30. Ding XC, Wang LL, Zhang XD, Xu JL, Li PF, Liang H, et al. The relationship between expression of PD-L1 and HIF-1α in glioma cells under hypoxia. J Hematol Oncol. (2021) 14:92. doi: 10.1186/s13045-021-01102-5
31. Viallard C and Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. (2017) 20:409–26. doi: 10.1007/s10456-017-9562-9
32. Hong CH, Lee CH, Chen GS, Chang KL, and Yu HS. STAT3-dependent VEGF production from keratinocytes abrogates dendritic cell activation and migration by arsenic: a plausible regional mechanism of immunosuppression in arsenical cancers. Chemico-biological Interact. (2015) 227:96–103. doi: 10.1016/j.cbi.2014.12.030
33. Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. (2015) 212:139–48. doi: 10.1084/jem.20140559
34. Torrens L, Montironi C, Puigvehí M, Mesropian A, Leslie J, Haber PK, et al. Immunomodulatory effects of lenvatinib plus anti-programmed cell death protein 1 in mice and rationale for patient enrichment in hepatocellular carcinoma. Hepatol (Baltimore Md). (2021) 74:2652–69. doi: 10.1002/hep.32023
Keywords: pseudoprogression, pancreatic acinar cell carcinoma, PD-1 blockade, antiangiogenic therapy, integrated treatment, case report
Citation: Bai X, Yang D, Chen J, Liu Y, Tang H, Kong X, Liu B, Sha H and Du J (2025) Case Report: Pseudoprogression of pancreatic acinar cell carcinoma after PD-1 blockade integrated treatment. Front. Immunol. 16:1663514. doi: 10.3389/fimmu.2025.1663514
Received: 10 July 2025; Accepted: 19 November 2025; Revised: 18 November 2025;
Published: 02 December 2025.
Edited by:
Ayon Chakraborty, Research and Education in Cancer (ACTREC), IndiaReviewed by:
Ming Yang, UCONN Health, United StatesXiaojuan Wu, Huazhong University of Science and Technology, China
Copyright © 2025 Bai, Yang, Chen, Liu, Tang, Kong, Liu, Sha and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baorui Liu, YmFvcnVpbGl1QG5qdS5lZHUuY24=; Huizi Sha, c2hhaHVpemluanVAMTI2LmNvbQ==; Juan Du, ZHVqdWFuZ2x5eUAxNjMuY29t
 Xinyuan Bai1,2
Xinyuan Bai1,2 Xiang Kong
Xiang Kong Baorui Liu
Baorui Liu Huizi Sha
Huizi Sha Juan Du
Juan Du