- 1Department of Pharmacy, Personalized Drug Research and Therapy Key Laboratory of Sichuan Province, Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Vascular Surgery, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
Venous thromboembolism (VTE) is an exceedingly rare occurrence in pediatric patients with Behçet’s syndrome (BS). Currently, the clinical anticoagulation treatment regimen for this condition in the pediatric population remains inadequately defined. Here, we reported the treatment process of a child with Behçet’s disease complicated by VTE and reviewed the relevant literature to study comprehensive diagnosis and treatment strategies, as well as the latest progress for this specific type of disease. A 15-year-old boy who presented BS with pulmonary embolism, pulmonary aortic aneurysm, and recurrent deep vein thrombosis was described. Following a comprehensive strategy of systematic immunotherapy and anticoagulation treatment, the patient’s condition has shown significant improvement, with marked relief from pulmonary embolism and lower extremity venous thrombosis. In addition to the systematic immunotherapy, anticoagulant therapy plays a significant role in the diagnosis and treatment of these patients. The literature review shows that 16 of the 17 patients underwent heparin anticoagulant therapy, while 6 were additionally treated with vitamin K antagonists (VKs). Our case study also indicated that vitamin K antagonists can be applied in this population. Moreover, it is imperative to conduct extensive long-term follow-up studies to better evaluate the outcomes of anticoagulation therapy as these patients transition into adulthood.
Introduction
BS is a chronic, recurrent autoimmune and inflammatory disorder characterized by a range of clinical manifestations, including oral and genital ulcers, skin lesions, and potential involvement of the eyes, blood vessels, joints, gastrointestinal tract, reproductive system, and other organs (1). This condition predominantly affects young adults aged 20 to 40; however, it is also diagnosed in pediatric populations, with 4% to 26% of cases occurring in individuals under 16 years of age, a condition referred to as pediatric Behçet’s disease (2, 3). VTE represents the most prevalent form of vascular involvement in Behçet’s disease. The incidence of vascular involvement in pediatric patients with Behçet’s disease ranges from 1.8% to 21.0% (4). Thromboembolic events in these patients may present in atypical anatomical locations and can extend to the pulmonary artery and multiple veins (5). Given the rarity of pediatric Behçet’s disease, thrombotic complications associated with the syndrome are also uncommon (6). Currently, diagnostic and therapeutic approaches are primarily based on guidelines developed for adults, resulting in a lack of pediatric-specific pharmacological treatment experience, particularly concerning anticoagulation therapy for BS and associated thrombosis. An examination of the current literature reveals that it predominantly consists of case reports and small case series. The diagnosis, treatment, particularly the anticoagulation management, and outcomes for pediatric patients with BS complicated by VTE are insufficiently documented. This article presents a rare case of pediatric Behçet’s syndrome with venous thromboembolism and undertakes an literature review to investigate and elucidate the prevailing anticoagulation strategies for children with BS and VTE.
Methods
A child with BS and multiple venous thromboses was presented. A comprehensive literature search was conducted in PubMed from its inception to 30 April 2025. The keywords used in the search were “Behcet’s syndrome,” “Behcet’s disease,” “child,” “children,” “pediatric,” “venous thromboembolism,” “venous thrombus,” “venous thrombosis,” “deep vein thrombosis,” and “pulmonary embolism,” associated with AND/OR. References for the retrieved studies were reviewed to identify additional reports. Only pediatric cases (aged 16 years or younger) that provided sufficient detail for individual analysis were included.
Results
Case report
A 15-year-old male patient presented with PE and a pulmonary aortic aneurysm secondary to hemoptysis, as well as recurrent DVT of the left lower extremity, diagnosed one week later. Upon inquiry into his medical history, the patient reported experiencing recurrent oral ulcers for three years prior to admission, occurring more than three times annually, and occasional erythema in the lower limbs, which resolved spontaneously. He denied any abnormal vision, headaches, arthralgia, nausea, vomiting, or other symptoms of discomfort. Physical examination upon admission revealed multiple scattered oral mucosal ulcers, each less than 1 cm in diameter, and superficial thrombophlebitis in the left lower limb, accompanied by varicose veins. Additionally, there was erythema surrounding the superficial venous skin, mild edema of the left lower limb, a diminished dorsalis pedis pulse, and a slightly elevated skin temperature compared to the right lower limb. No significant abnormalities were observed in the upper limbs, and there were no genital ulcers. The patient reported no relevant family medical history. Laboratory tests showed elevated C-reactive protein (31.56 mg/L), D-dimer (1.61 μg/mL), and platelet counts (581 × 109/L). During hospitalization, hematologic examinations, including a bone marrow smear and abnormal cell screening, were performed, and hematologic tumors were excluded. Further examination revealed an erythrocyte sedimentation rate of 24 mm/h, increased coagulation factor VIII activity (153.4%), normal plasma protein C and S activity, antinuclear antibody, anticardiolipin antibody, and ferritin levels. Chest enhanced CT demonstrated pulmonary artery malformation with thrombosis in the upper dorsal segment of the left lower lobe, aneurysmal dilatation of the main pulmonary artery in the right lower lobe with embolus formation, and emboli in the branches of the pulmonary arteries in both lower lobes. Echocardiographic evaluation revealed the presence of mild tricuspid valve insufficiency. Following a consultation with a rheumatologist and based on the 2015 classification criteria for pediatric Behçet’s disease (PEDBD) (7, 8), the patient, who exhibited recurrent oral ulcers, venous thrombosis, pulmonary aneurysm, and superficial thrombophlebitis of the lower extremities, was diagnosed with BS.
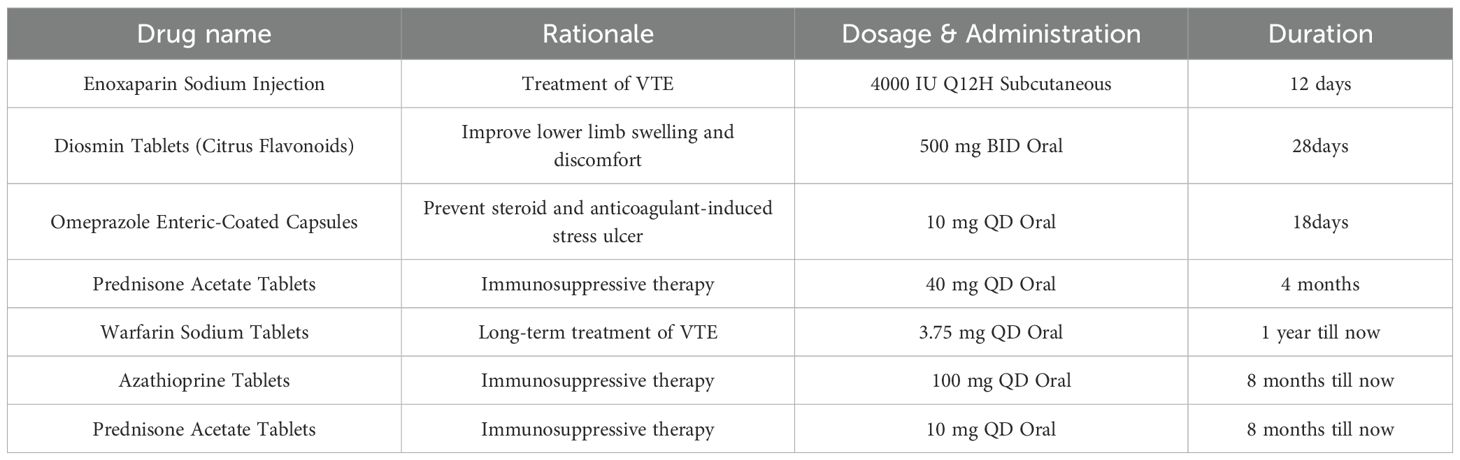
Hemoptysis was treated with bronchial embolization. Medical treatment was initiated with subcutaneous enoxaparin, high-dose oral prednisone (40mg qd), and diosmin to improve the leg heaviness. During treatment, color Doppler ultrasound showed reduced thrombosis in the left external iliac and common femoral veins with partial recanalization. Warfarin monotherapy continued after the INR reached the target range. At discharge, the rash and oral ulcers had resolved. After that, low-dose prednisone and long-term warfarin anticoagulation were administered. Two months following discharge, the patient consulted with a rheumatologist, who strongly recommended the administration of adalimumab, an anti-tumor necrosis factor (TNF) agent. However, this recommendation, along with the suggestion to use cyclophosphamide, was declined by the patient’s guardian. After extensive discussions with the patient’s family, azathioprine was selected as an alternative for immunotherapy and has been administered continuously to date. A comprehensive overview of the medical regimen is detailed in Table 1. Throughout the six-month follow-up period, the child’s daily academic and extracurricular activities remained unaffected. A computed tomography angiography (CTA) of the lungs conducted four months post-discharge revealed the resolution of the thrombus in the main pulmonary artery, with only minor embolisms persisting in a few small branches. A subsequent CT scan performed one year later indicated the complete resolution of the pulmonary embolism, with no significant alterations observed in the venous thrombosis of the left lower extremity (Figure 1).
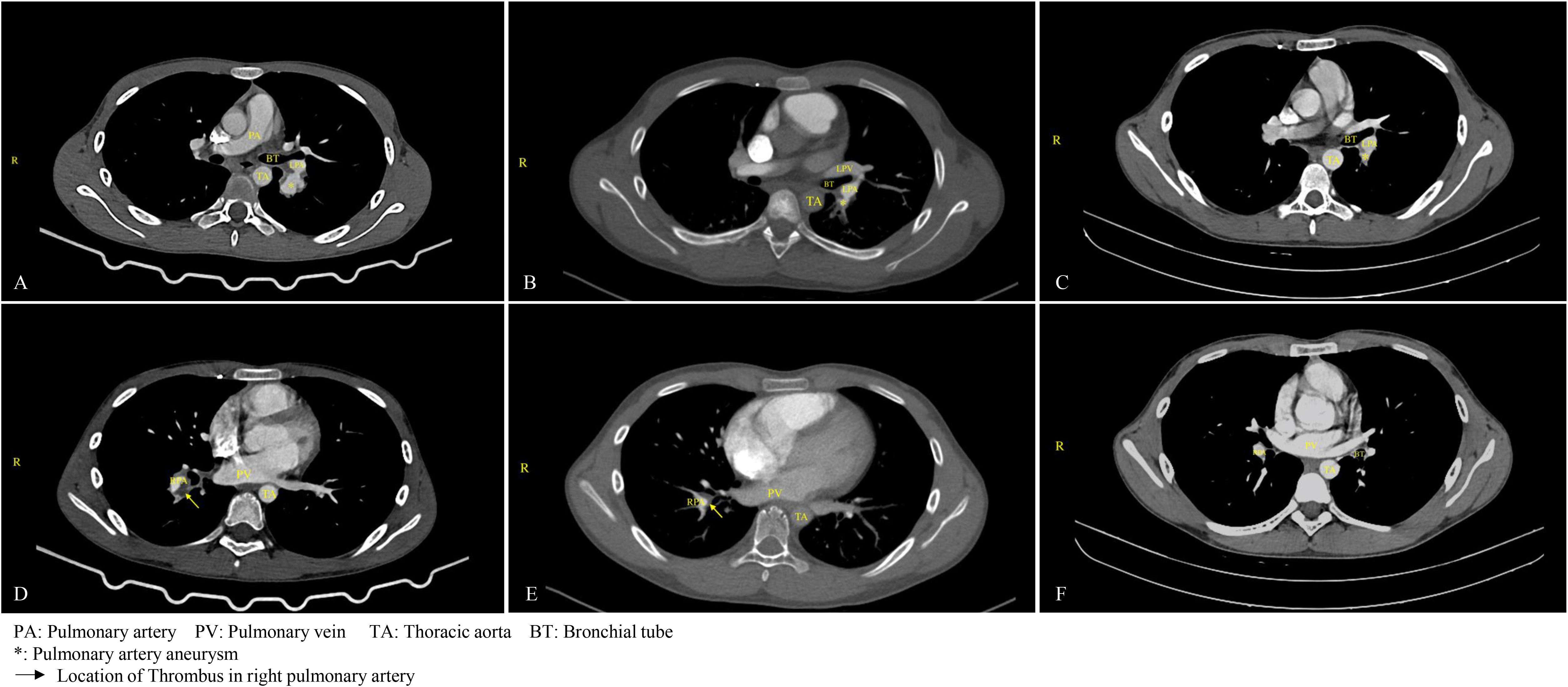
Figure 1. Changes in pulmonary aneurysm and thrombus before and after treatment. (A) On March 18, 2024, the patient was diagnosed with a pulmonary aneurysm. (B) A follow-up examination on August 1, 2024, indicated no significant change in the patient's pulmonary aneurysm compared to the previous assessment. (C) By August 4, 2025, a follow-up examination of the child revealed a significant reduction in the aneurysm. (D) On March 18, 2024, thrombosis was identified in the upper posterior segment of the left lower lobe, along with aneurysmal dilation of the pulmonary artery in the right lower lobe accompanied by thrombus formation, and thrombus formation in the branches of the pulmonary arteries in both lower lobes. (E) After four months of anticoagulation treatment, as of August 1, 2024, the thrombus in the main pulmonary artery had resolved, although embolization persisted in a small branch of the pulmonary artery in both lower lobes. (F) By August 4, 2025, a follow-up examination of the child confirmed the resolution of the pulmonary thrombus.
Literature review
In addition to our case, 18 reports of VTE in children with BS were identified, and one case involving death was excluded. The remaining 17 patients are presented in Table 2. The median age of the patients was 12 years (range, 4–15 years), and 82.3% (n=14) were male. Among the 17 patients, only two were diagnosed with BS first and thrombosis later. Nine patients (52.9%) had DVT, two (11.7%) had PE, two (11.7%) had inferior vena cava thrombosis (IVCT), and four (23.5%) had both DVT and PE. Only 1 patient with a pulmonary aortic aneurysm and PE also had DVT. Six patients had cerebral venous sinus thrombosis (CVST) involvement; in some cases, CVST was the first manifestation of thrombosis. In addition, hepatic vein thrombosis and right atrial thrombosis occurred in two patients. In these patients, the most common manifestation was oral ulcer (11 patients, 64.7%), followed by arthritis or joint pain (8 patients, 47%), and erythema nodosum (6 patients, 35.3%). One patient had a complete loss of vision in the right eye. Colchicine was administered to 82.3% (n=14) of patients, and corticosteroids were administered in the same proportion. Cyclophosphamide was used in 47% (n=8) of the patients, infliximab in 23.5% (n=4), methotrexate in 5.8% (n=1), and mycophenolate mofetil in 5.8% (n=1). Of the 17 patients, 16 underwent definitive anticoagulation therapy, and all received heparin anticoagulants. Additionally, 6 patients were treated with vitamin K antagonists. Detailed records of anticoagulation duration were available for 8 patients, with treatment durations ranging from 3 months to 2 years. The follow-up period for these patients ranged from five months to six years. Two patients experienced thrombus resolution; however, their follow-up periods were relatively short (five and six months, respectively). Recurrent thrombosis occurred in three patients.
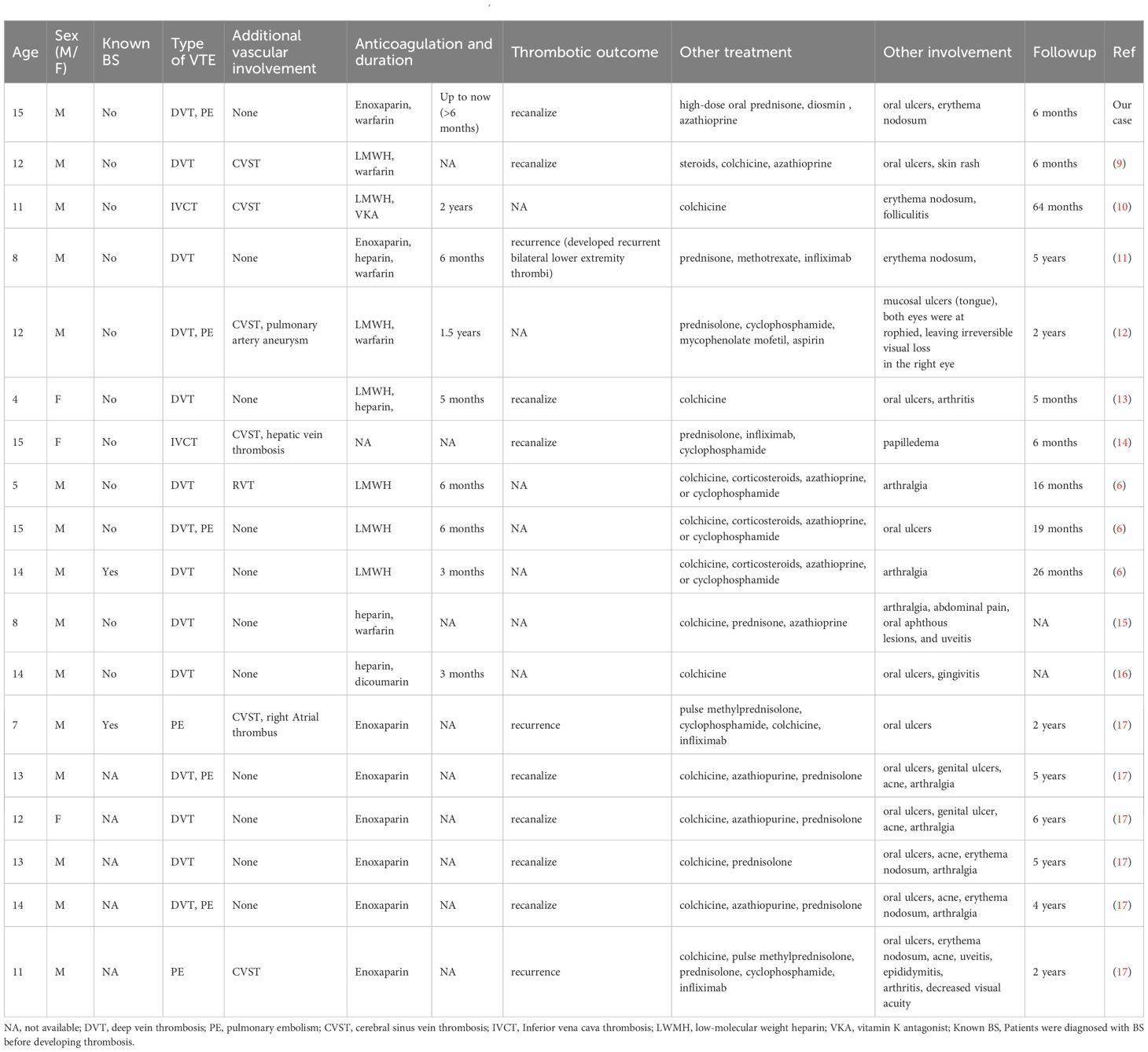
Table 2. Clinical characteristics of reported cases of VTE associated with paediatric Behçet’s disease.
Discussion
BS is a rare multisystem inflammatory disorder characterized by diverse clinical manifestations, recurrent episodes, and chronic progression, with a tendency toward more severe progression in males and young adults (18–20). Male patients are more likely to develop vascular complications (21), particularly affecting cerebral and lower extremity vessels (22). Pediatric BS demonstrates distinct phenotypic clusters compared to adult-onset disease, and a Chinese cohort study identified three subtypes in children (23). Vascular and neurological manifestations are generally rare in children (24). A prospective cohort reported venous thrombosis in 9.6% of pediatric BS cases, with arterial thrombosis or aneurysms occurring in only 1.8% (25). However, a Turkish study noted a higher incidence of mixed vascular disease, which can lead to severe complications, including death (26). According to the 2024 EUROFEVER registry, venous thromboses accounted for 70% of events in pediatric BS, with cerebral venous sinus thrombosis (44%) and lower extremity DVT (23%) being the most common (27). But it’s uncommon to diagnose a pediatric BS patient accompanied by PE, a pulmonary aortic aneurysm, and DVT at the same time. Among 17 documented pediatric cases, only one other patient, aged 12, demonstrated similar vascular complications, which also became symptom-free by anticoagulation therapy (12).
Since thrombosis in BS is primarily driven by vascular inflammation, immunosuppressive therapy leads to resolution or regression of aneurysms or thrombosis in approximately 70% of patients (28), making it the cornerstone of acute venous thrombosis management (29). The role of anticoagulation remains controversial (30). In this review, all but one patient received anticoagulants, and only three experienced recurrent thrombosis. A meta-analysis cited in current guidelines found no significant benefit from adding anticoagulation to immunosuppression in preventing relapse (31). Nonetheless, French guidelines recommend full anticoagulation for DVT in both adult and pediatric BS (32). A retrospective study showed that combined anticoagulant and immunosuppressive therapy significantly reduced thrombosis recurrence compared to immunosuppression alone (33), a finding supported in another study focusing on PE (34). Our case also used anticoagulation therapy combined with immunosuppressive therapy, which achieved a great effect. In all reported cases, patients received heparin or warfarin; no direct oral anticoagulants (DOACs) were used. However, experience in adults suggests that DOACs may reduce recurrent thrombosis risk and synergize with disease-modifying antirheumatic drugs (DMARDs) (35). Although dabigatran and rivaroxaban are approved for some pediatric indications, their use in BS remains undocumented and may represent a future therapeutic avenue. In our case, based on previous guidelines and literature reviews, we also chose warfarin as long-term anticoagulant therapy.
The primary treatment goals in BS are to control inflammation rapidly, prevent relapses and irreversible organ damage, and slow disease progression. Management must be individualized according to age, gender, and organ involvement type and severity (36). First-line agents include corticosteroids, immunomodulators, and immunosuppressants (37). Since thrombosis in BS is primarily driven by vascular inflammation, immunosuppressive therapy leads to resolution or regression of aneurysms or thrombosis in approximately 70% of patients (28), making it the cornerstone of acute venous thrombosis management (29). The 2018 EULAR recommendations advise using azathioprine, cyclophosphamide, or cyclosporine for vascular involvement, including acute DVT, with anti-TNF agents reserved for refractory cases (38). Although glucocorticoids and cyclophosphamide are first-line, anti-TNF agents have shown efficacy in refractory cases (39). In a study examining the application of biological agents in pediatric patients with Behçet’s disease, anti-TNF agents emerged as the most frequently utilized biologic agents. The primary indication for employing these biologic agents was ocular involvement, followed by active multisystem disease. While both agents have demonstrated efficacy in treating Behçet’s syndrome in children and possess an acceptable safety profile, there remains a need for controlled studies to further investigate the appropriate indications for biotherapy in this population (40). In the present case, azathioprine was selected after shared decision-making with the family, considering the high cost and lack of reimbursement for anti-TNF agents in China for pediatric BS. Combined with warfarin, this regimen resulted in regression of pulmonary thrombosis and favorable outcomes during over one year of follow-up. Notably, nearly one-third of pediatric BS patients may develop new major organ involvement or relapse in adulthood (25), underscoring the need for long-term follow-up. Another barrier to anti-TNF use is that neither infliximab nor adalimumab is approved in China for pediatric BS, further limiting treatment options. Currently, no specific guidelines exist for managing vascular BS in children, and large-scale studies are lacking. The potential for recurrent thrombosis remains a concern.
Although the efficacy of enoxaparin and warfarin in preventing thrombotic events remains uncertain, this case is particularly noteworthy and valuable due to the ongoing follow-up and significant improvement in the patient’s thrombotic condition. This case emphasizes the importance of screening for rheumatologic diseases—including Behçet’s, antiphospholipid syndrome, and systemic lupus erythematosus—in children with unprovoked DVT, even when mild thrombophilia is detected (11, 41). Screening should include a detailed history, physical examination for rheumatic features, and inflammatory and immune markers (e.g., ESR, CRP, ANA) (11). In our case, only anti-cardiolipin antibody (aCL) was tested; lupus anticoagulant (LA) was not performed—a study limitation. Simultaneous testing for both LA and aCL is recommended for more comprehensive evaluation in future cases.
Conclusions
The occurrence of BS complicated by DVT and PE is uncommon in pediatric populations, and the combination of DVT, PE, and pulmonary aneurysm is even rarer. The use of anticoagulants in such cases remains controversial and complicates treatment strategies. This case may serve as a reference for clinical management of similar pediatric presentations. Future multicenter controlled trials are needed to provide further insights and evaluations.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
QY: Writing – original draft, Writing – review & editing. YB: Writing – review & editing. FN: Funding acquisition, Writing – original draft. MZ: Methodology, Writing – original draft. SC: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Sichuan Provincial Department of Science and Technology -Sichuan Provincial Natural Science Foundation -Youth Fund (2024NSFSC1704), Sichuan Provincial Medical Research Youth Innovation Project (Q22058), and Sichuan Academy of Medical Sciences -Sichuan Provincial People’s Hospital -Youth Talent Fund (2022QN42), and the Sichuan Science and Technology Plan Project (2022NSFSC0818).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Leccese P and Alpsoy E. Behcet’s disease: an overview of etiopathogenesis. Front Immunol. (2019) 10:1067. doi: 10.3389/fimmu.2019.01067
2. Tugal-Tutkun I and Urgancioglu M. Childhood-onset uveitis in Behcet disease: a descriptive study of 36 cases. Am J Ophthalmol. (2003) 136:1114–9. doi: 10.1016/S0002-9394(03)00791-8
3. Kone-Paut I. Behcet’s disease in children, an overview. Pediatr Rheumatol Online J. (2016) 14:10. doi: 10.1186/s12969-016-0070-z
4. Gungorer V, Polat MC, Çelikel E, Ekici Tekin Z, Kurt T, Tekgöz N, et al. Factors associated with the development of thrombosis in pediatric behcet disease. J Clin Rheumatol. (2023) 29:e19–24. doi: 10.1097/RHU.0000000000001930
5. Bettiol A, Alibaz-Oner F, Direskeneli H, Hatemi G, Saadoun D, Seyahi E, et al. Vascular Behcet syndrome: from pathogenesis to treatment. Nat Rev Rheumatol. (2023) 19:111–26. doi: 10.1038/s41584-022-00880-7
6. Guzelkucuk Z, Gurlek Gokcebay D, Arman Bilir O, Basaran O, Celikel Acar B, Ozbek NY, et al. Children with behcet disease-associated thrombosis: A single-center experience. J Pediatr Hematol Oncol. (2021) 43:e15–8. doi: 10.1097/MPH.0000000000001877
7. Kone-Paut I, Shahram F, Darce-Bello M, Cantarini L, Cimaz R, Gattorno M, et al. Consensus classification criteria for paediatric Behcet’s disease from a prospective observational cohort: PEDBD. Ann Rheum Dis. (2016) 75:958–64. doi: 10.1136/annrheumdis-2015-208491
8. Matucci-Cerinic C, Palluy H, Al-Mayouf SM, Brogan PA, Cantarini L, Gul A, et al. Validation of the PEDiatric Behcet’s Disease classification criteria: an evidence-based approach. Rheumatol (Oxford). (2024) 63:3422–31. doi: 10.1093/rheumatology/kead609
9. Rottenstreich A, Machol K, Eisenstein EM, Padeh S, Klar A, Livneh A, et al. Behcet’s disease and cerebral sinus vein thrombosis in children: a case study and review of the literature. Clin Exp Rheumatol. (2015) 33:S163–8.
10. Metreau-Vastel J, Mikaeloff Y, Tardieu M, Koné-Paut I, and Tran TA. Neurological involvement in paediatric Behcet’s disease. Neuropediatrics. (2010) 41:228–34. doi: 10.1055/s-0030-1269909
11. Gurunathan A, Teachey DT, Chikwava KR, Witmer C, and Desai AV. Behcet disease initially presenting as deep venous thrombosis: A case report. J Pediatr Hematol Oncol. (2017) 39:410–2. doi: 10.1097/MPH.0000000000000830
12. Zhuang LL, Liu HM, Li GM, Shen J, Liu F, Ye M, et al. Dural sinus thrombosis and giant pulmonary artery aneurysm in paediatric Behcet’s disease. Clin Exp Rheumatol. (2020) 38:558–66.
13. Ihle J, Kümmerle-Deschner T, Orlikowsky T, Albert E, Niethammer D, and Dannecker GE. Factor V Leiden and venous thrombosis in a 4-yr-old girl with Behcet’s syndrome. Rheumatol (Oxford). (2000) 39:209–10. doi: 10.1093/rheumatology/39.2.209
14. Seyahi E, Hamuryudan V, Hatemi G, Melikoglu M, Celik S, Fresko I, et al. Infliximab in the treatment of hepatic vein thrombosis (Budd-Chiari syndrome) in three patients with Behcet’s syndrome. Rheumatol (Oxford). (2007) 46:1213–4. doi: 10.1093/rheumatology/kem103
15. Ozen S, Bilginer Y, Besbas N, Ayaz NA, and Bakkaloglu A. Behcet disease: treatment of vascular involvement in children. Eur J Pediatr. (2010) 169:427–30. doi: 10.1007/s00431-009-1040-y
16. Escobosa SO, Moreno Pascual P, Montes de Oca F, Milano Manso G, and Jurado Ortiz A. Deep venous thrombosis: an early manifestation of Behcet’s disease in childhood. Pediatr (Barc). (2004) 61:266–9. doi: 10.1016/s1695-4033(04)78807-4
17. Coskun S, Ekici Tekin Z, Güngörer V, Çelikel E, Kurt T, Polat MC, et al. A case series of intracardiac thrombi and vascular involvement in pediatric Behcet’s disease. Rheumatol Int. (2023) 43:1161–71. doi: 10.1007/s00296-023-05292-8
18. Tekgoz N, Tekgöz E, Çolak S, Sezer M, Çelikel E, Tekin Z, et al. Age-related differences in the clinical phenotypes of Behcet’s disease: The experience of two referral centres. Mod Rheumatol. (2023) 34:194–200. doi: 10.1093/mr/road012
19. Seyahi E and Yurdakul S. Behcet’s syndrome and thrombosis. Mediterr J Hematol Infect Dis. (2011) 3:e2011026. doi: 10.4084/mjhid.2011.026
20. Emmi G, Bettiol A, Hatemi G, and Prisco D. Behcet’s syndrome. Lancet. (2024) 403:1093–108. doi: 10.1016/S0140-6736(23)02629-6
21. Demir F, Sönmez HE, Bağlan E, Akgün Ö, Coçkuner T, Yener GO, et al. Cluster analysis of paediatric Behcet’s disease: Data from The Pediatric Rheumatology Academy-Research Group. Mod Rheumatol. (2023) 33:574–8. doi: 10.1093/mr/roac044
22. Chen Y, Cai JF, Lin CH, and Guan JL. Demography of vascular Behcet’s disease with different gender and age: an investigation with 166 Chinese patients. Orphanet J Rare Dis. (2019) 14:88. doi: 10.1186/s13023-019-1061-1
23. Zou J, Luo JF, Shen Y, and Guan JL. Distinct clinical characteristics of pediatric Behcet’s syndrome: A study from a referral center in China. Mod Rheumatol. (2021) 31:1158–63. doi: 10.1080/14397595.2021.1891670
24. Pain CE. Juvenile-onset Behcet’s syndrome and mimics. Clin Immunol. (2020) 214:108381. doi: 10.1016/j.clim.2020.108381
25. Bozkurt T, Yildiz M, Deniz R, Yazici A, Karabacak M, Karatas H, et al. Clinical course of paediatric-onset Behçet’s disease in young adulthood. Rheumatol (Oxford England). (2025) 64:2876–81. doi: 10.1093/rheumatology/keae624
26. Sarica-Kucukoglu R, Akdag-Kose A, KayabalI M, Yazganoglu KD, Disci R, Erzengin D, et al. Vascular involvement in Behcet’s disease: a retrospective analysis of 2319 cases. Int J Dermatol. (2006) 45:919–21. doi: 10.1111/j.1365-4632.2006.02832.x
27. Mastrolia MV, Matucci-Cerinic C, Ozen S, Kasapcopur O, Gaggiano C, Koné-Paut I, et al. Thrombotic manifestations in pediatric Behcet syndrome: A multicenter comparative study from the EUROFEVER registry. Semin Arthritis Rheum. (2024) 66:152454. doi: 10.1016/j.semarthrit.2024.152454
28. Seyahi E, Melikoglu M, Akman C, Hamuryudan V, Ozer H, Hatemi G, et al. Pulmonary artery involvement and associated lung disease in Behçet disease: a series of 47 patients. Medicine. (2012) 91:35–48. doi: 10.1097/MD.0b013e318242ff37
29. Giani T, Luppino AF, and Ferrara G. Treatment options in pediatric behçet’s disease. Paediatric Drugs. (2023) 25:165–91. doi: 10.1007/s40272-022-00548-5
30. Yildiz M, Koker O, and Kasapcopur O. Juvenile Behçet syndrome: a contemporary view and differential diagnosis in pediatric practice. Curr Opin Rheumatol. (2025) 37:3–14. doi: 10.1097/BOR.0000000000001057
31. Hatemi G, Christensen R, Bang D, Bodaghi B, Çelik AF, Fortune F, et al. 2018 update of the EULAR recommendations for the management of Behcet’s syndrome. Ann Rheum Dis. (2018) 77:808–18. doi: 10.1136/annrheumdis-2018-213225
32. Kone-Paut I, Barete S, Bodaghi B, Deiva K, Desbois AC, Galeotti C, et al. French recommendations for the management of Behçet’s disease. Orphanet J rare Dis. (2021) 16:352. doi: 10.1186/s13023-020-01620-4
33. Erol S, Gürün Kaya A, Arslan F, Hasanzade H, Daş̧tan AO, Çiledağ A, et al. Does anticoagulation in combination with immunosuppressive therapy prevent recurrent thrombosis in Behcet’s disease? J Investig Med. (2024) 72:387–91. doi: 10.1177/10815589241232368
34. Abacar KY, Boncukcuoglu AE, Aksoy A, Kocakaya D, Cimsit C, Direskeneli H, et al. Anticoagulant treatment may decrease the relapse rate of pulmonary arterial involvement in behcet’s disease. J Clin Rheumatol. (2024) 30:303–8. doi: 10.1097/RHU.0000000000002137
35. Vautier M, Gallien Y, Emmi G, Bettiol A, Karadag O, Bolek EC, et al. Direct oral anticoagulant for venous thrombosis in Behçet’s syndrome. Autoimmun Rev. (2021) 20:102783. doi: 10.1016/j.autrev.2021.102783
36. Zheng WJ, Zhang N, Zhu XC, Chi SS, Zhang W, Wei W, et al. Zhonghua nei ke za zhi. Chin J Intern Med. (2021) 60:860–7. doi: 10.3760/cma.j.cn112138-20210604-00398
37. Karadag O and Bolek EC. Management of behcet’s syndrome. Rheumatol (Oxford). (2020) 59:iii108–17. doi: 10.1093/rheumatology/keaa086
38. Aksoy A, Yazici A, Omma A, Cefle A, Onen F, Tasdemir U, et al. Efficacy of TNFα inhibitors for refractory vascular Behçet’s disease: A multicenter observational study of 27 patients and a review of the literature. Int J rheumatic Dis. (2020) 23:256–61. doi: 10.1111/1756-185X.13778
39. Coşkun S, Ekici Tekin Z, Güngörer V, Çelikel E, Kurt T, Polat MC, et al. A case series of intracardiac thrombi and vascular involvement in pediatric Behçet’s disease. Rheumatol Int. (2023) 43:1161–71. doi: 10.1007/s00296-023-05292-8
40. Batu ED, Sener S, Cam V, Aktay Ayaz N, and Ozen S. Treatment with biologic drugs in pediatric behçet’s disease: A comprehensive analysis of the published data. BioDrugs. (2023) 37:813–28. doi: 10.1007/s40259-023-00613-6。
Keywords: Behçet’s syndrome, child, venous thrombus embolism, anticoagulation, treatment
Citation: Yin Q, Bian Y, Niu F, Zhang M and Chen S (2025) Case Report: Behçet’s syndrome and venous thrombosis in children: a case study and review of the literature. Front. Immunol. 16:1664004. doi: 10.3389/fimmu.2025.1664004
Received: 11 July 2025; Accepted: 11 September 2025;
Published: 29 September 2025.
Edited by:
Ozgur Kasapcopur, Istanbul University-Cerrahpasa, TürkiyeReviewed by:
Oya Koker, Istanbul University, TürkiyeVildan Güngörer, University of Health Sciences, Türkiye
Copyright © 2025 Yin, Bian, Niu, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siyu Chen, Y2hlbnNpeXVAbWVkLnVlc3RjLmVkdS5jbg==
†These authors share first authorship
 Qinan Yin
Qinan Yin Yuan Bian
Yuan Bian Fang Niu
Fang Niu Mao Zhang2
Mao Zhang2 Siyu Chen
Siyu Chen