- 1Institutes of Health Central Plains, Henan Medical University, Xinxiang, Henan, China
- 2Xinxiang Key Laboratory for Tumor Drug Screening and Targeted Therapy, Xinxiang, Henan, China
- 3College of Life Science, Institute of Biomedical Science, Henan Normal University, Xinxiang, Henan, China
- 4State Key Laboratory of Cell Differentiation and Regulation, Xinxiang, Henan,, China
- 5Department of Anesthesiology, Xinxiang First People’s Hospital, The Affiliated People’s Hospital of Xinxiang Medical University, Xinxiang, Henan, China
Trained immunity (TI) represented a unique state of innate immune activation, characterized primarily by persistent epigenetic modifications in immune cells. This phenomenon was first observed during pathogen infections and vaccinations, where it manifested as enhanced defensive responses in innate immune effector cells—such as those of the mononuclear phagocyte system and natural killer cells—upon re-stimulation. Cancer was a disease with complex mechanisms, marked by the loss of normal growth regulation in cells due to genetic mutations or epigenetic dysregulation, leading to abnormal proliferation and dissemination. With hundreds of subtypes, cancer could arise in virtually any human tissue or organ. The primary cause of cancer-related mortality was metastasis, which referred to the spread of cancer cells from their original site to distant organs and accounted for approximately 90% of cancer deaths worldwide. The induction of TI involved multiple immune components including myeloid cells, natural killer cells, pattern recognition receptors, and various cytokines. Notably, the enhanced response observed during secondary stimulation remained non-specific to particular pathogens. Compared to conventional therapeutic approaches, TI demonstrated superior systemic immune activation. Simple pharmacological stimuli such as β-glucan or Bacillus Calmette-Guérin (BCG) not only triggered innate immune responses but also conferred benefits to adaptive immunity, resulting in more rapid immune activation and enhanced efficacy. TI enhanced the capacity of immune cells to recognize and eliminate cancer cells, playing a critical role in countering metastasis. In this review, we summarized existing knowledge in the field, focusing on the mechanisms underlying TI induction and its significance in combating cancer.
1 Introduction
The immune system was classically divided into two categories (1): innate immunity and acquired immunity. Innate immunity, which developed through long-term evolutionary processes, was present from birth and provided broad-spectrum, non-specific protection against pathogens. In contrast, acquired immunity represented an antigen-specific response that was individualized, non-heritable, and characterized by immune memory. TI described the epigenetic reprogramming of bone marrow progenitor cells induced by infection or vaccination, which conferred enhanced non-specific protection against secondary infections (2).This phenomenon differed fundamentally from classical immune memory in several aspects. First, TI primarily involved innate immune components including bone marrow progenitor cells, natural killer cells (NK cells), innate lymphoid cells (ILCs), and pattern recognition receptors, rather than the adaptive immune cells characteristic of classical memory responses. Second, TI-mediated responses to secondary challenges were not antigen-specific but rather driven by transcriptional regulation and epigenetic modifications. Furthermore, the functional enhancements in innate immune cells following TI typically persisted for weeks to months after clearance of the initial stimulus, in contrast to the years-long persistence of classical immune memory (3).The mechanistic foundation of TI was closely associated with metabolic reprogramming in immune cells (4). This process could occur either in bone marrow progenitor cells (central TI) or in terminally differentiated innate immune cells and peripheral tissues (peripheral TI) (5).TI played significant roles in both disease prevention and treatment strategies. For example, it enhanced vaccine efficacy and offered novel therapeutic opportunities for cancer and inflammatory disorders. Among innate immune cells, neutrophils emerged as crucial mediators of TI. As the most abundant innate immune population derived from bone marrow, neutrophils served as primary responders to infectious or inflammatory insults (6).Induction of TI in lung-resident alveolar macrophages (AMs) enhanced their capacity for cellular debris clearance and tissue repair (7). However, TI could also contribute to disease pathogenesis in certain contexts. In chronic metabolic disorders, autoimmune conditions, and neurological diseases, TI might promote persistent or maladaptive immune activation. Under these circumstances, TI represented a potential driver of disease progression through dysregulation of innate immune responses (8).
Cancer represents a class of malignant tumors whose incidence has been rising steadily in the 21st century. Current statistics indicate approximately one in four individuals faces cancer risk (9). In 2020, cancer claimed nearly 600,000 American lives. While children and young adults demonstrate relatively low cancer incidence and mortality rates, these figures increase exponentially with age after adulthood. Among adults, lung, colorectal, and breast cancers account for the highest diagnosis rates (10). Emerging evidence suggests a strong correlation between cancer incidence and obesity, with data indicating overweight conditions elevate risk for at least 13 cancer types, including esophageal, pancreatic, colorectal, and thyroid cancers (11). Cancer research faces significant challenges due to intertumoral heterogeneity and dynamic cellular characteristics, complicating the development of effective treatments (12). Notably, cancer cells exhibit substantial signaling pathway alterations that profoundly impact cellular growth, proliferation, metastasis, and apoptosis. These pathway changes coincide with metabolic reprogramming, where both proto-oncogenes and tumor suppressor genes exert substantial metabolic influence (13).Current therapeutic research explores multiple avenues. Epigenetic modulation has emerged as a potential intervention strategy by targeting epigenetic genes involved in carcinogenesis (14). Existing medications like metformin have demonstrated cancer risk reduction (15). Certain vaccines, including recombinant BCG, have shown capacity to enhance immunity by upregulating pro-inflammatory factors, inducing epigenetic and metabolomic changes, and improving both immune function and anti-tumor efficacy (16). Growing evidence indicates that TI system generated anti-tumor responses (17).
TI enhanced pathogen resistance and strengthened immune surveillance. Research showed that induced TI activated immune cells effectively. The induction of TI in cancer contexts may represent a novel therapeutic approach (5). This review summarizes various cancer types and mechanisms while analyzing the role of TI in oncogenesis, potentially offering new perspectives for cancer treatment strategies.
2 Trained immunity
The body’s immune system consists of two main types: innate immunity and acquired immunity (18). Phylogenetically, lower animals possessed only innate immunity while vertebrates developed specific immunity. The innate immune system comprised barrier structures, innate immune cells and innate immune molecules. Barrier structures included three types: interspecific barriers, skin and mucous membrane barriers, and internal barriers. The interspecific barrier, also known as species immunity, represented genetic resistance of certain species to specific pathogens (19). The skin and mucous membrane barrier formed from external body coverings and cavity linings (20), while internal barriers included the blood-brain barrier (21), blood-fetal barrier and blood-thymus barrier. Key innate immune molecules involved defensins, complement system, cytokines, lysozyme and other effector factors. Innate immune cells consisted of phagocytes, innate lymphoid cells, natural killer cells, and dendritic cells. The recognition mechanism of innate immunity differed from adaptive immunity by targeting conserved microbial patterns rather than specific antigens, exhibiting relatively limited specificity (22). Adaptive immune responses were MHC-restricted and involved coordinated actions of various immune cells and molecules, with antigen specificity being their most distinctive feature (23).
The concept of TI emerged when researchers discovered that innate immunity could enhance protection against reinfection in organisms lacking adaptive immunity, challenging the conventional view that only adaptive immunity could establish immune memory (24). This innate immune memory phenomenon was termed TI, which was mediated through epigenetic reprogramming. Unlike mutations or genetic recombination in adaptive immunity, TI induced transient changes in gene expression and cellular physiology without permanent genetic alterations (3).
The molecular basis of TI involved metabolic rewiring, where epigenetic modifications interacted with cellular metabolism to regulate gene transcription (25). When occurring in bone marrow precursors of innate immune cells, it was called central TI, while peripheral TI referred to changes in tissue-resident immune or non-immune cells (5).
2.1 TI and monocytes
In TI, monocytes serve as crucial functional effector cells. Their involvement in TI is primarily manifested through their epigenetic features, functional mechanisms, regulatory pathways, and long-term in vivo memory effects. A key characteristic of trained monocytes is the lactylation (H3K18la) of the lysine residue at position 18 on histone H3. This modification predominantly occurs in distal regulatory regions and exhibits a positive correlation with both active chromatin states and gene transcription. Notably, it persists even after the removal of the training stimulus. Moreover, when monocytes encounter a secondary stimulus, H3K18la is closely linked to the transcription of “training-associated” genes—ultimately sustaining the enhanced response traits of the TI system. From a regulatory mechanism perspective, the elevated lactic acid production induced during TI promotes the generation of pro-inflammatory cytokines, a process that depends on histone lactylation. If lactic acid production or histone lactylation is pharmacologically inhibited, the TI response is abrogated. Additionally, genetic polymorphisms in LDHA (a gene involved in lactic acid production) and EP300 (a gene potentially involved in lactic acid regulation) can also modulate the efficacy of TI. At the in vivo level, following BCG vaccination, histone lactylation modifications associated with monocyte function persist in the body for 90 days. This finding not only confirms that H3K18la is an epigenetic marker of innate immune memory, but also underscores the role of monocytes in the long-term maintenance of TI (26) (Table 1). Studies have shown that following Plasmodium infection, its metabolic product hemozoin (Hz) accumulates continuously in the bone marrow, a process that can induce TI. Specifically, Hz enhances the accessibility of granulocyte-macrophage progenitors (GMPs) via the MyD88-dependent pathway, thereby laying the groundwork for monocyte generation and functional remodeling. In mice that have recovered from Plasmodium infection, the number of Ly6C+ monocytes in the peripheral blood is significantly increased—and this elevated count can be sustained for up to 6 months post-infection. Additionally, certain distinct monocyte subtypes emerge during this period. Notably, the recovered mice exhibit enhanced functional capacities of these monocytes, including increased reactive oxygen species (ROS) production, as well as improved bactericidal and phagocytic activities (27) (Table 1).When human monocytes are stimulated with β-G, BCG, or Candida albicans, histone modifications—including H3K4me1 and H3K4me3—occur in the promoter regions of inflammatory cytokine genes. These modifications collectively form the epigenetic signatures underlying TI. Following training, human monocytes undergo alterations in cellular metabolic pathways, which are characterized by a marked increase in glycolysis, glutamine catabolism, and cholesterol biosynthesis (28) (Table 1). In studies where a TI model was established via BCG inoculation, monocyte-like THP-1 cells were employed as a research subject to mimic the characteristics of monocytes. Transcriptome analysis revealed that the Akt-HIF-mTOR signaling pathway was activated in these cells (29) (Table 1). The TI of the human body causes epigenetic reprogramming of monocytes, presenting an enhanced and persistent pro-inflammatory phenotype. This phenotype can provide non-specific protection for the body and help resist reinfection (30).This study identified a combination therapy involving β-G-induced TI and irreversible electroporation (IRE) enhancement, confirming that monocytes serve as the core effector cells of TI. In the mechanism of action: First, β-G acts as the core inducer and is recognized by monocytes via intraperitoneal injection (IP) or oral administration. The Dectin-1 receptor on the monocyte surface is the primary binding target for β-G; upon their binding, downstream signaling cascades are triggered. Subsequently, IRE releases damage-associated molecular patterns (DAMPs), which function as “secondary stimuli.” These DAMPs bind to the surface receptors of monocytes that have already been trained by β-G, significantly boosting the trained response of the monocytes. Notably, the intensity of this response increases in a dose-dependent manner with the elevation of the secondary stimulus concentration (31) (Table 1). Monocytes and macrophages are the main cell populations in trained immunity research, and more recent advances are most likely to come from a focus on monocytes.
2.2 TI and macrophages
Macrophages played a central role in TI as their phenotypes and functions dynamically adapted to environmental stimuli. Classically activated M1 macrophages promoted inflammation while alternatively activated M2 macrophages mediated anti-inflammatory responses (32).During TI induction, macrophages underwent significant metabolic shifts from oxidative phosphorylation to aerobic glycolysis (the Warburg effect) when exposed to stimuli like β-G (33). Concurrent epigenetic remodeling occurred not only in tissue macrophages but also in bone marrow myeloid precursors, enabling long-lasting effects (34). Following TI macrophages exhibited enhanced effector functions including improved phagocytosis, increased pathogen clearance, and elevated production of pro-inflammatory cytokines like IL-1β, IL-6 and TNF-α. These cytokines not only directly combated infections but also recruited and activated other immune cells, amplifying the overall immune response. In TI, circulating monocytes serve as the core cells that undergo functional reprogramming. Studies have demonstrated that stimulants such as β-G can induce substantial metabolic and epigenetic changes in human monocytes—for example, an increase in the methylation of the histone marker H3K4me3. Even after these monocytes differentiate into macrophages, this “trained” imprint persists, resulting in a more robust inflammatory cytokine response to secondary stimuli (35). Furthermore, this reprogramming effect extends to monocyte precursors in the bone marrow, thereby exerting a sustained influence on myeloid cells (36).
2.3 TI and NK cells
NK cells were identified as another crucial component of the innate immune system capable of developing TI (37). When initially exposed to cytokine stimulation, NK cells rapidly activated intracellular epigenetic modification programs, particularly showing significant histone modifications at the INF-γ gene locus. These epigenetic changes occurred not only in mature NK cells but were also transmitted to daughter cells, establishing a stable “memory imprint.” During TI, NK cells underwent substantial metabolic reprogramming, with glycolytic flux increasing 3-5-fold and lactate dehydrogenase activity enhancing significantly. Mitochondrial alterations included elevated ROS production and improved ATP synthesis efficiency. Functionally, trained NK cells demonstrated approximately threefold increases in perforin and granzyme expression, substantially enhanced cytotoxic capacity, and markedly elevated production of cytokines including INF-γ and TNF-α. This trained resulted in more potent and rapid anti-infection responses (38). Clinical observations revealed that MMR (Measles Mumps Rubella) vaccines could induce long-term immune memory, with transcriptomic analyses showing changes in both CD14+ monocytes and NK cells, though the most pronounced effects were observed in γδT cells (39).
2.4 TI and ILCs
TI and ILCs are both key components in the field of innate immunity. They engage in cross-functional collaboration and work together to enhance the body’s defensive capacity against threats such as pathogens and tumors. In the human immune system, innate immunity and adaptive immunity work in concert to fend off pathogen invasion. ILCs can be activated without antigen presentation and thus mount rapid responses to signals from pathogens or tumors (40).
Emerging evidence highlights the potential of TI in conferring protection against diverse pathogens. A pivotal study demonstrated that replication-deficient human adenovirus vectors can effectively TI system, resulting in protective immunity in mice against a broad spectrum of influenza virus strains, including H1N1, H3N2, H5N2, H7N9, and H9N2. Notably, bovine and chimpanzee adenoviruses were also capable of activating human ILCs and provided protection against H3N2 influenza challenge in mice. A critical finding was that this protection occurred independently of the conventional influenza-specific adaptive immune response, as evidenced by the absence of hemagglutination-inhibiting antibodies, neutralizing antibodies, and influenza nucleoprotein-specific CD8+T cells. The underlying protective mechanisms were identified as the enhanced activation of ILC1, ILC2, and ILC3 populations, increased expression of interferon-stimulated genes (ISGs), upregulation of antiviral signaling pathways, and metabolic reprogramming within ILC subsets. This research establishes a novel strategy for bolstering innate antiviral immunity, offering a promising approach not only for epidemic preparedness against known influenza strains but also as a countermeasure against emerging infectious diseases (41).Clinical investigations into allergen-specific immunotherapy (AIT) have revealed its profound impact on the innate immune compartment. Observational clinical studies indicate that AIT induces significant changes in the composition and heterogeneity of circulating innate immune cells. Remarkably, these alterations shift the innate immune profile toward a state that mirrors that observed in healthy individuals, suggesting a normalization or resetting of innate immune homeostasis as a component of AIT’s therapeutic mechanism (42).The strategic design of vaccine antigens can critically influence the engagement of innate immunity at mucosal sites, a key battleground for many pathogens. Research on SIV and HIV vaccines revealed that envelope V1-deleted (ΔV1) vaccines, when delivered systemically via the DNA/ALVAC/gp120 platform, conferred a superior reduction in the risk of mucosal SIV or SHIV acquisition compared to their V1-replete counterparts. This enhanced protection was linked to the effective activation of specific innate lymphoid cell populations in the mucosal environment, namely IFN-γ ILC1s and IL-13+ ILC2s. This finding underscores the importance of antigen design in eliciting protective mucosal innate immune responses against viral challenges (43). The traditional paradigm of immunological memory as an exclusive feature of adaptive immunity is being challenged by findings in innate lymphoid cells. It has been discovered that stimulation with IL-33 or IL-25, or exposure to the allergen papain, induces the expression of the transcription factor c-Maf in murine group ILC2s. This program enables ILC2s to develop memory-like properties, thereby contributing to the pathophysiology of type 2 inflammatory diseases such as allergies. This phenomenon, often termed “trained immunity” or “innate immune memory” in ILC2s, represents a significant expansion of our understanding of how innate mechanisms can perpetuate and exacerbate chronic inflammatory conditions (44).
2.5 TI and γδT cells
Vaccines have long been shown to protect beyond their target antigen through induction of TI via regulating epigenetic, transcriptional, and functional reprogramming of innate immune cells (3, 45, 46). γδT cells represent a unique subset of the immune system, exhibiting characteristics of both innate and adaptive immunity. These cells constitute only 1% to 5% of T cells in peripheral blood, however, when pathogens invade the human body, γδT cells respond with heightened sensitivity, serving as the immune system’s first and faster-acting line of defense. Importantly, γδT cells could combat tumors through multiple pathways, including directly eliminating tumor cells via NK cell receptors and inducing tumor cell apoptosis through apoptosis-related factor ligands (47). A clinical trial using single-cell RNA-Seq has verified that MMR vaccination induces TI via functional and metabolic reprogramming of γδT cells. It is manifested as higher production of TNF and IFN-γ, as well as upregulation of cellular metabolic pathways (39). It is also reported that BCG vaccination has impact on the repertoire of human γδT cell receptors (48). BCG-activation of leukocytes is sufficient for the generation of innate anti-tumor NK and γδT cells (49). And intravenously BCG vaccination and aerosol BCG revaccination could induce mycobacteria-responsive γδT cells immune memory and protect against bacterial infection (50). Interestingly, subcutaneous BCG administration in pre-weaned calves can induce TI in γδT cells. This trained memory presents as increased expression of innate immune response-related genes, thereby inducing a functional TI response evidenced by elevated IL-6 and TNF-α cytokine production (51). Suen TK and their teamwork also confirmed this phenomenon in humans that γδ T cells could serve as key target cells for TI through metabolic and epigenetic regulation, TI can enhance the effector functions of γδ T cells and even extend the duration of their “memory-like state” (52). Similarly, another recent study confirmed that respiratory syncytial virus infection confers heterologous protection against SARS-CoV-2 via induction of γδ T cell-mediated TI (53). It means that TI could provide a long-term cross-protection against various infections.
2.6 TI and neutrophils
As mentioned earlier, neutrophils are the most abundant leukocytes which have multiple functions during immune response and the first responders when challenged with infection or inflammation diseases (54, 55). In addition, neutrophils have been recently identified as vital effectors of peripheral TI, they may exert beneficial effects in infection and cancer diseases (56). It is reported that the absolute number of neutrophils increases signally following BCG vaccination in healthy humans and these neutrophils remain in a trained state after 3 months. More importantly, the capacity of degranulation and phagocytic as well as IL-8 and ROS production in these trained neutrophils have all elevated when facing with the secondary stimulus (57). Research also indicated that TI participated in the progression of diabetes by regulating the formation of extracellular trap in neutrophils (58). Another study has confirmed that BCG and β-G could reprogram the hematopoietic stem and progenitor cells in innate immune cells, especially neutrophils, these trained neutrophils showed higher ROS production and tumor core infiltration to restrain the vascularization and tumor progression (59, 60). Meanwhile, BCG-trained neutrophils could reshape the tumor microenvironment to drive the adaptive anti-tumor immune responses and augment the effect of immune checkpoint blockade therapy (61). All above foundation suggests that TI maybe beneficial to the solid cancer therapy. For further, BCG vaccination could amplify the granulocytic-macrophage progenitors and enhance their oxidative metabolism as well as the ability which stimulates T cell proliferation to improve neonates’ survival to polymicrobial sepsis (62). In a clinical trial, researchers have indicated that the frequency of eosinophils, neutrophils, NK cells and donor-unrestricted T cells all increased 7 days after aerosol BCG infection (63). It is worth noting that the innate immunity elicited by BCG could be better control the SARS-CoV-2 replication and this inhibition is related to higher frequencies of lung infiltrating neutrophils and higher CD14+ cells (64). Likewise, prior low dose of lipopolysaccharide (LPS) exposure-induced TI could upregulate the expression of antibacterial protein, galectin-3 in neutrophils to protects mice from a lethal bacterial infection (65). All above reviewed findings indicated that trained-neutrophils participate in the progression of multiple infection and cancer diseases, manipulating TI in neutrophils may enhance the function of neutrophil and provide a strategy to treat infection and cancers.
3 Research on TI in cancer
TI played an indispensable role in many diseases, though not all manifestations of TI were beneficial to health. For example, in atherosclerotic cardiovascular disease (ASCVD), TI was identified as a potential driver of chronic inflammation. Studies suggested that endogenous pro-atherosclerotic factors could induce TI, triggering epigenetic reprogramming in immune cells. This implied that TI might activate cardiovascular disease mechanisms through systemic modulation of bone marrow hematopoietic progenitor cells (66). While TI exhibited detrimental effects in ASCVD, it demonstrated protective roles in oncology. In tumors, TI enhanced immune responses, suppressed metastasis, and prolonged survival. β-G-induced TI elicited stronger responses to LPS and amplified reactivity to tumor-derived factors. TI also showed therapeutic potential in chronic disease recovery, muscle injury repair, and tissue regeneration. For instance, β-G stimulation modulated gut microbiota, improved lipid/glucose metabolism, reduced cholesterol, and conferred protection against microbial infections—effects relevant to metabolic syndrome and gastrointestinal disease management (67). Further evidence revealed that WGP(whole beta-glucan particle) induced TI inhibited lung metastasis and boosted anti-tumor activity. Nano-biologics and β-G-induced TI were reported to exert anti-tumor effects in primary subcutaneous tumors. WGP-trained macrophages exhibited a “trained” response upon exposure to tumor cells or tumor-derived factors, suggesting a role in immune surveillance against tumor progression and metastasis (68) (Table 1).
3.1 Lung cancer
Lung cancer represented a heterogeneous pulmonary malignancy with diverse clinicopathological characteristics, broadly categorized as small cell lung cancer (SCLC) or non-small cell lung cancer (NSCLC), the latter further divisible into squamous and non-squamous subtypes (69). As the leading cause of cancer mortality in many developed countries, its primary etiology was tobacco smoking - in high-smoking prevalence nations, over 80% of lung cancer cases were smoking-related (70). Statistical data showed lung cancer deaths in Canada exceeded combined mortality from colorectal, pancreatic, and breast cancers (71). Projections indicated the global cancer burden would double by 2050 compared to 2020 levels, with lung cancer remaining predominant. The disease’s high mortality stemmed from frequent late-stage diagnosis and elevated risk among populations exposed to smoke, occupational hazards (e.g., oil fields), and tobacco use. However, advances in screening techniques and novel therapies had begun reducing lung cancer mortality rates. Studies demonstrated that lung-resident alveolar macrophages which underwent TI exhibited enhanced resistance to pathogen-induced cell death. These cells effectively prevented excessive tissue inflammation and rapidly restored homeostasis following repeated pathogen exposure (7). Relevant research confirmed that TI significantly bolstered anti-lung cancer defenses by promoting M1-like macrophage polarization within tumor tissues (72). In murine models, β-G-induced TI enabled neutrophils to suppress lung tumor growth through a ROS-dependent mechanism (73) (Figure 1, Table 1). The induction of macrophage-mediated TI emerged as a promising strategy for cancer prevention and treatment. For lung cancer specifically, BCG-disguised macrophage membranes demonstrated selective tumor-targeting capabilities (74) (Table 1).Intravenous BCG administration synergistically enhanced immune checkpoint-blocked anti-tumor activity without systemic toxicity, highlighting TI’s exceptional anti-tumor potential. Notably, influenza-trained mucosal alveolar macrophages established long-term pulmonary anti-tumor immunity. In murine models of influenza and lung metastasis, these TI-activated macrophages infiltrated tumor lesions, exhibiting potent phagocytic and cytotoxic effects (75) (Table 1). Sepsis-trained lung macrophages stimulated CCR2 and CXCR6 chemokine release, triggering T-cell tissue residency - an immune mechanism that reduced post-sepsis tumor recurrence risk. This suggested sepsis-induced TI conferred protective effects against lung cancer development (76). The incorporation of β-G into alginate hydrogel-based nanovaccine formulations facilitates targeted delivery to lymph nodes and dendritic cells (DCs). This specific targeting significantly promotes antigen uptake and maturation of DCs, accompanied by enhanced production of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α. In murine models, this nanovaccine strategy resulted in the complete suppression of TC-1 tumor growth and, notably, the eradication of established lung tumors (77) (Table 1). A complex clinical interplay exists between lung cancer and active tuberculosis (TB), wherein certain anticancer agents can independently promote TB progression. For instance, the chemotherapeutic drug Gemcitabine (Gem) induces granulocyte-biased hematopoiesis in the bone marrow via G-CSF signaling, leading to neutrophilic pulmonary inflammation. However, pre-existing Mycobacterium tuberculosis-specific T cell responses, such as those elicited by BCG vaccination, can normalize granulopoiesis by restricting G-CSF production. This immunomodulatory effect consequently suppresses lung cancer development (78).
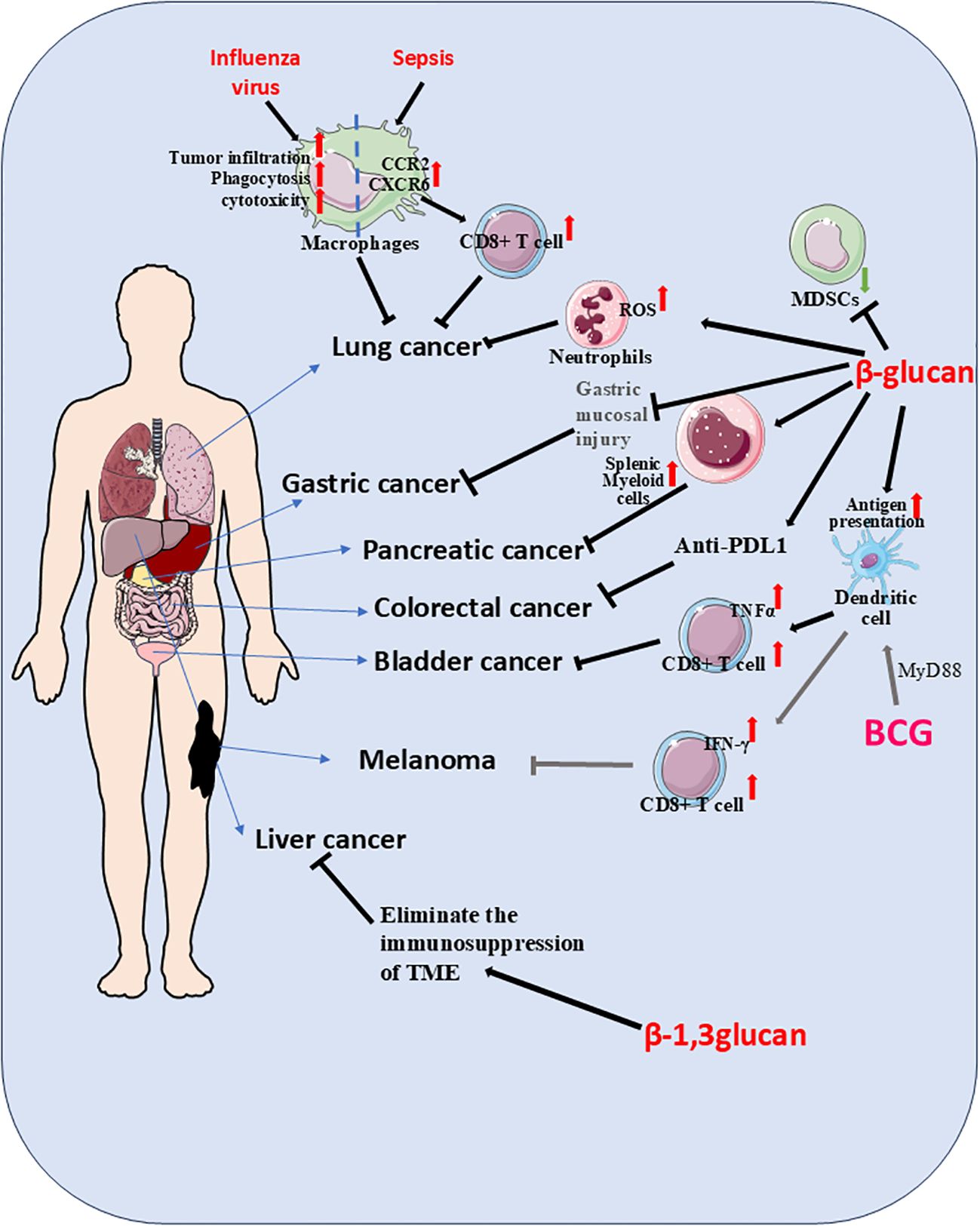
Figure 1. Stimulation with trained immunity (TI) inducers such as β-G or BCG not only activated innate immunity but also enhanced adaptive immune responses. During secondary challenges, TI potentiated the capacity of immune cells to recognize and eliminate malignant cells. In lung cancer models, β-G-stimulated neutrophils suppressed tumor growth through reactive oxygen species (ROS)-dependent mechanisms, while influenza virus-primed TI macrophages infiltrated tumor sites, exhibiting potent phagocytic and cytotoxic activity. Pulmonary macrophages in TI states following sepsis released CCR2 and CXCR6 chemokines, promoting T-cell tissue residency and subsequent tumor inhibition. For pancreatic cancer, nanobiologic targeting of splenic myeloid cells induced peripheral TI, establishing robust anti-tumor immunity. Intravenous BCG administration fostered a bladder tumor-suppressive microenvironment characterized by elevated cytotoxic T lymphocyte (CTL)/TNFα ratios and reduced myeloid-derived suppressor cell (MDSC) populations. TI-conditioned dendritic cells demonstrated enhanced antigen capture and presentation capabilities, significantly amplifying CTL proliferation. BCG-induced TI played a pivotal role in bladder cancer immunotherapy, where MyD88 signaling emerged as critical for mediating anti-melanoma effects. Through the MyD88 pathway, BCG not only generated tumor-specific immunity but also promoted dendritic cell-mediated TI, ultimately enhancing CD8+ T cell anti-tumor responses. The combined treatment of β-G and anti-PD-L1 can significantly inhibit the growth of CRC. β-1, 3-glucan can eliminate the immunosuppression of the tumor microenvironment, thereby slowing down the progression of liver cancer. When β-G is used in combination with drugs as a carrier, it can reduce gastric mucosal damage and more effectively alleviate precancerous lesions.
3.2 Colorectal cancer
Colorectal cancer (CRC) represents a significant global health burden as the fourth leading cause of cancer mortality worldwide, accounting for 9.2% of all cancer-related deaths. This malignancy exhibits notable gender disparities, ranking as the second most common cancer in women and third in men, with male incidence and mortality rates consistently 25% higher than females. The epidemiology of CRC further reveals substantial racial variations, with non-Hispanic Black populations experiencing the highest disease burden while Asian Americans/Pacific Islanders demonstrate the lowest prevalence. Although incidence rates have historically declined in individuals over 50 years old, recent epidemiological data indicates a concerning reversal of this trend in this age group (79). The development of CRC involves a complex interplay between environmental factors and genetic predisposition, with clinical manifestations categorized into hereditary, familial, and sporadic forms (80). Dietary patterns, particularly fiber consumption, have been strongly associated with disease risk. Mounting scientific evidence has elucidated the critical role of intestinal microbiota composition in CRC pathogenesis, establishing specific microbial profiles as potential diagnostic biomarkers (81).The human gastrointestinal tract hosts an extraordinarily diverse microbial ecosystem consisting of over 1000 species encompassing 1014 distinct microbial types (82). These commensal microorganisms perform essential physiological functions including maintaining intestinal homeostasis (83), regulating energy metabolism (84), reinforcing epithelial barrier integrity, and enhancing host defense mechanisms against pathogenic invaders (85). Contemporary CRC treatment strategies include radiotherapy, which has been demonstrated to significantly alter gut microbiota composition, particularly through the depletion of beneficial symbiotic bacteria such as Bifidobacterium and Clostridium species (86). Beyond conventional approaches, emerging therapeutic modalities in regenerative medicine, particularly stem cell therapy, have shown considerable promise in the management of CRC (87).
Of note, Dectin-1 (encoded by the gene Clec7a), a known receptor for β-G, plays a pivotal role in host defense against fungi and the maintenance of intestinal immune homeostasis. Intervention with the short-chain β-G laminarin, a Dectin-1 antagonist, has been demonstrated to suppress the development of colorectal tumors in murine models. Mechanistically, Dectin-1 signaling upregulates the expression of prostaglandin E2 (PGE2) synthases, and subsequently, PGE2 inhibits the expression of IL22RA2 in human CRC-infiltrating cells. These observations collectively suggest the existence of a Dectin-1–PGE2–IL-22BP axis that modulates intestinal tumorigenesis, thereby positioning Dectin-1 as a potential therapeutic target for CRC (88). Building upon the immunomodulatory potential of β-G, a novel strategy involving the conjugation of an anti-PD-L1 antibody with β-G has been developed. This construct is designed to promote interactions between tumor cells and DCs, thereby augmenting the benefits of immunotherapy. In the MC38 murine model of colorectal cancer, this conjugated agent exhibited a potent antitumor effect, achieving a remarkable tumor suppression rate of 86.7% (89) (Table 1). Oat-derived β-G, a soluble dietary fiber, possesses a broad spectrum of bioactivities, including anti-inflammatory and anti-tumor properties. Research indicates that its suppressive effect on colorectal cancer development in rats is mediated through the stimulation of both autophagy and the extrinsic apoptosis pathway (90). Lentinan (LNT), a β-D-glucan purified from the cultured fruiting body of Lentinula edodes mushrooms, also exhibits significant antitumor efficacy. In cervical cancer, LNT exerts its inhibitory effects by activating the tumor suppressor p53, leading to cell proliferation arrest and apoptosis via the p21 and mitochondrial-dependent pathways (91) (Table 1). Furthermore, molecular size reduction of LNT through specific processing enhances its bioactivity against colorectal cancer, enabling the suppression of cancer cell proliferation, induction of apoptosis, and reduction of inflammation (92).
3.3 Breast cancer
Breast cancer represents a complex and heterogeneous malignancy influenced by both genetic predisposition and environmental factors. As the most prevalent life-threatening cancer among women in Western nations, it ranks as the second leading cause of female cancer mortality, surpassed only by lung cancer (93). The aggressive nature of breast cancer stems largely from cancer stem cells, which drive tumor invasiveness and metastatic potential (94). Epidemiologically, the disease affects approximately 1 in 20 women globally (95), and holds the distinction of being the most frequently diagnosed malignancy during pregnancy (96). While predominantly affecting women, breast cancer incidence in men has shown a steady increase worldwide. Key risk factors for male breast cancer include obesity, testicular disorders, other primary tumors, and particularly BRCA2 gene mutations, with carriers facing an 80-fold greater risk compared to the general population (97). Histologically, invasive lobular carcinoma constitutes the second most common subtype after invasive ductal carcinoma, representing 10-15% of all cases. These tumors typically exhibit luminal molecular features with estrogen and progesterone receptor positivity and HER2 negativity, contributing to their unpredictable response to neoadjuvant treatments (98). Among the most aggressive variants, metaplastic breast cancer accounts for less than 1% of invasive cases. This rare subtype demonstrates distinct pathological characteristics, including adenocarcinoma Tous differentiation with spindle cell, squamous epithelial, and/or mesenchymal components. Clinically, it shows marked chemotherapy resistance and carries a particularly poor prognosis with low survival rates (99). The evolution of breast cancer management has progressed significantly from radical mastectomy to modified radical procedures and currently favors breast-conserving approaches incorporating chemotherapy and radiotherapy (100). However, the emergence of drug resistance in advanced disease poses a major therapeutic challenge, primarily driven by endocrine dysfunction and hormonal imbalances (101). Studies have demonstrated that the number of γδT cells in breast tissue is closely associated with the survival duration of breast cancer patients: the higher the number of these cells, the longer the patients’ survival time. This finding also provides a novel immunotherapeutic strategy for intervening in triple-negative breast cancer. Recent investigations highlight the significant potential of particulate β-1,3-glucans in modulating tumor biology and enhancing chemotherapeutic efficacy, particularly in the context of breast cancer. One such agent, a granular β-1,3-glucan extracted from the spore wall of Ganoderma lucidum (GPG), has been identified as a biological response modifier. Notably, research demonstrates that GPG can reverse the immunosuppressive tumor microenvironment (TME) induced by Gem chemotherapy. This reversal is characterized by the mitigation of GEM-induced reductions in anti-tumor T cells and the suppression of increases in both myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) (102) (Table 1). Concurrently, strategies to ameliorate the dose-limiting cytotoxicity of conventional chemotherapeutics, such as doxorubicin (DOX), have leveraged the unique properties of yeast-derived β-G particles (YGPs). YGPs possess a hollow, porous vesicular structure, making them excellent candidates for drug delivery. For instance, DOX encapsulated within YGPs has shown potent cytotoxic effects against various breast cancer cell lines, including MCF-7 and 4T1, while simultaneously reducing the associated physiological toxicity of the free drug (103). Further advancing this concept, β-G-based nanotubes have been engineered as promising nanocarriers for the delivery of hydrophobic DOX. This formulation has demonstrated superior efficacy compared to free DOX, achieving a 74.5% inhibition rate in breast cancer models. It promotes tumor cell apoptosis, blocks proliferation, and results in significantly suppressed tumor growth both in vitro and in vivo, alongside a marked reduction in adverse effects (104).
The therapeutic application of β-G-based drug delivery systems can be further potentiated by combination therapies. A related study indicates that a similar β-G nanoplatform, when co-administered with photothermal therapy, can effectively block the synthesis of essential nutrients required for tumor DNA replication. This combinatorial approach significantly induces oxidative stress and ultimately exacerbates DNA damage in breast cancer cells, leading to enhanced therapeutic outcomes (105) (Table 1).
3.4 Bladder cancer
Bladder cancer represents the most prevalent and lethal malignancy of the urinary tract system, with lymphatic spread serving as the predominant metastatic pathway (106). Annually, this disease is diagnosed in over 430000 individuals globally and accounts for approximately 170000 deaths, underscoring its significant public health impact. Epidemiological studies reveal pronounced gender disparities in incidence patterns, with emerging evidence suggesting androgen receptor signaling plays a pivotal role in driving tumor development, disease progression, and clinical recurrence (107). Among established risk factors, tobacco smoking remains the most consistently identified environmental contributor to bladder carcinogenesis (108). The standard immunotherapy for patients with non-muscle-invasive bladder cancer involves intravesical instillations of BCG. However, the precise contribution of TI to BCG’s mechanism of action in suppressing bladder cancer progression remains to be rigorously quantified and warrants further careful investigation (59). BCG, a live attenuated vaccine derived from Mycobacterium bovis and primarily used against tuberculosis, also serves as an immunotherapeutic agent in oncology. A significant clinical limitation is its requirement for repeated administrations and its failure to elicit a response in approximately 50% of patients (59). Intriguingly, preclinical studies using an orthotopic bladder cancer model have demonstrated that a single intravesical dose of BCG, when combined with β-G, can lead to the eradication of invasive tumors with a 100% survival rate. In this context, reprogrammed neutrophils exhibited enhanced ROS production and infiltrated the tumor core, resulting in reduced tumor vascularization and growth. The concept of TI appears to play a pivotal role in remodeling the tumor immune microenvironment. For instance, macrophages trained with BCG, upon co-administration with ovalbumin-expressing bladder tumor cells, were shown to increase the proportion of tumor-specific cytotoxic T lymphocytes (CTLs) (109) (Table 1). Furthermore, BCG-trained dendritic cells demonstrate enhanced antigen uptake and presentation, subsequently promoting the proliferation of CTLs (109) (Table 1). The systemic immunomodulatory effects of BCG are also evident at the level of hematopoiesis. While intravenous administration of BCG is known to reprogram hematopoietic stem and progenitor cells (HSPCs) to confer heterologous protection against infections, intravesical administration can also lead to BCG colonization of the bone marrow. In both murine models and humans, this reprograms HSPCs to alter and expand myelopoiesis. These BCG-reprogrammed HSPCs are sufficient to enhance anti-bladder tumor immunity by generating neutrophils, monocytes, and dendritic cells (61).The combination of BCG therapy with molecular interventions, such as Mcl-1 shRNA, has been shown to shift macrophage polarization towards an anti-tumor M1 phenotype. This is characterized by elevated expression of M1 markers (TNF-α, CD86, iNOS) and reduced expression of M2 markers (IL-10, CD206, Arg-1), ultimately leading to significantly increased apoptosis of bladder cancer cells and diminished proliferative, migratory, and invasive capacities (110) (Table 1).Concurrently, numerous studies are exploring the development of novel BCG-based composites to augment its anti-tumor potential. For example, innovative drug delivery strategies have been employed, such as a “biotin-streptavidin strategy” to coat live BCG with nanoparticles loaded with the chemotherapeutic agent DOX. This approach has proven effective in inhibiting cancer progression and prolonging survival in rat/mouse models of orthotopic bladder cancer. Notably, these constructs also demonstrated improved tolerability, biosafety, and the establishment of a potent anti-tumor immune response within the tumor microenvironment (111).The tumor microenvironment in bladder cancer is notably enriched with tumor-associated macrophages (TAMs), which represent one of the most abundant immune cell populations infiltrating these malignancies. While TAM-targeted therapies have shown promising results in other cancer types, this approach remains under investigated in bladder cancer (112).Molecular characterization of bladder cancer has identified several recurrent genetic alterations, including mutations in TERT promoter regions, FGFR3, TP53, PIK3CA, and STAG2 genes, along with various chromatin-modifying genes (113). For patients with high-risk non-metastatic disease, ranging from high-grade non-muscle invasive to muscle-invasive presentations, radical cystectomy with lymph node dissection continues to serve as the gold standard therapeutic approach (114). The BCG vaccine induced stronger TI, which was characterized by an enhanced response to pro-inflammatory factors, greater reprogramming of myeloid cells toward inflammatory and activated states, and more significant epigenetic and metabolomic alterations. In bladder cancer models, the BCG vaccine was found to boost immune activity and exert anti-tumor effects (16). Another study demonstrated that intravenous administration of the BCG vaccine promoted an anti-tumor bladder microenvironment, evidenced by an increased proportion of CTLs and a decreased proportion of MDSCs (Figure 1). This TI-mediated remodeling of the tumor immune microenvironment played a crucial role in driving anti-tumor immunity (109). Intravenous injection of the BCG vaccine created an anti-tumor bladder microenvironment, specifically marked by a rise in CTLs and a reduction in MDSCs (115). Following either intravenous or intravesical BCG administration, systemic anti-tumor immunity became polarized, with TI contributing to the reshaping of the tumor immune microenvironment. When TI-primed macrophages were co-cultured with ovalbumin-expressing bladder tumor cells, an increase in tumor-specific CTLs was observed. Furthermore, dendritic cells conditioned by TI displayed stronger antigen uptake and presentation capabilities, enhancing CTL proliferation. These findings collectively indicate that BCG-induced TI plays a significant role in mediating anti-tumor immune responses in bladder cancer (109).
3.5 Pancreatic cancer
Pancreatic cancer remains one of the most aggressive malignancies, with a dismal prognosis characterized by a median survival of just 4 months and a 5-year survival rate hovering around 10% (116). The disease demonstrates distinct geographic variation, with the highest incidence rates observed in North America, Europe, and Australia. The rising global incidence is largely attributable to population aging, with additional risk factors including tobacco use, obesity, diabetes mellitus, and excessive alcohol consumption (117).While exceptionally rare in pediatric populations, childhood cancers - particularly renal cell carcinoma - may metastasize to the pancreas, sometimes manifesting more than two decades after initial diagnosis (118). The overwhelming majority of patients present with advanced disease, leaving few viable treatment options and contributing to the characteristically poor outcomes (119). Those with familial pancreatic cancer syndromes or specific genetic mutations face substantially elevated risks of developing the disease. Emerging research has identified long non-coding RNAs (lncRNAs) - including MACC1-AS1, LINC00976, LINC00462, LINC01559, HOXA-AS2, LINC00152, TP73-AS1, XIST, SNHG12, LUCAT1 and UCA1 - as key regulators of pancreatic cancer progression (120). Current projections suggest pancreatic cancer will become the second leading cause of cancer mortality by 2030. Clinical staging categorizes disease into three main groups: resettable, borderline resettable/locally advanced, and metastatic (121). The tumor’s characteristic immunologically “cold” microenvironment renders it particularly refractory to immunotherapy approaches (122), with similarly poor responses observed to targeted therapies, chemotherapy, and radiation (123). Current surgical management may involve neoadjuvant therapy followed by procedures such as pancreaticoduodenectomy, extended pancreatectomy, and lymphadenectomy (124). Emerging evidence highlights the promising role of systemic β-G administration in reprogramming innate and adaptive immunity to combat pancreatic ductal adenocarcinoma (PDAC). In a murine model resistant to checkpoint inhibition, concurrent activation of Dectin-1 via β-G and CD40 using an agonist antibody drives T cell–mediated IFN-γ signaling. This synergistic signaling cascade reprograms distinct macrophage subsets to promote antitumor responses, leading to the eradication of established tumors and the induction of long-term immunological memory (125). Furthermore, β-G has been demonstrated to activate liver-resident macrophages (Kupffer cells) in murine cancer models, promoting their inflammatory polarization. This activation not only suppresses the proliferation of pancreatic cancer cells but also elicits a potent T cell–dependent inhibitory response against hepatic metastases of pancreatic cancer (126). Another innovative approach involves combining β-G–induced TI in peripheral monocytes with non-thermal tumor ablation via IRE. Studies showed that yeast-derived β-G effectively induced TI, leading to a significant reduction in pancreatic tumor burden in murine models. When administered in combination with irreversible electroporation, β-G treatment substantially enhanced TI-mediated anti-tumor responses against pancreatic cancer and prolonged survival in tumor-bearing mice (31). In an orthotopic pancreatic cancer model, this combination therapy significantly reduced both local and distant tumor burden and extended survival, underscoring the potential of integrating innate immune training with physical ablation techniques (31). Beyond irreversible electroporation, pancreatic cancer recurrence was also prevented through nano-biologic targeting of splenic myeloid cells, which induced peripheral TI to mount an effective anti-tumor defense (127) (Figure 1).
3.6 Melanoma
Melanoma, a malignant tumor arising from melanocytes, developed either de novo or within pre-existing moles, frequently demonstrating cutaneous and subcutaneous metastatic potential during disease progression (128). In subcutaneous melanoma models, BCG vaccine stimulation of murine melanoma cells demonstrated the critical role of MyD88 signaling in mediating BCG-induced immunotherapy. The absence of MyD88 in knockout models prevented TI-mediated inflammatory cell recruitment within the tumor microenvironment, consequently impairing tumor suppression and T cell-derived INF-γ production, collectively establishing the essential role of the MyD88 pathway in BCG’s anti-tumor immunity (129) (Figure 1). Investigations into squalene cyclooxygenase, a pivotal TI-regulating enzyme, revealed its significant contribution to TI-mediated anti-tumor responses (130). Complementary studies demonstrated that cholera toxin B subunit-induced TI in dendritic cells effectively potentiated CD8+ T cell anti-melanoma immunity in murine models (131). Cancer vaccines hold significant potential in clinical oncology by eliciting T cell-mediated immunity. However, their efficacy is often severely compromised by several key limitations: a restricted number of antigen-presenting cells (APCs) at the injection site, insufficient phagocytosis of tumor antigens by APCs, and the potent immunosuppressive tumor microenvironment (72). Emerging strategies to overcome these barriers involve the use of β-G to induce TI. For instance, engineered β-G formulations are highly accumulated and phagocytosed by macrophages at the injection site. This uptake initiates a state of TI, leading to a robust adaptive anti-tumor immune response and the establishment of long-term immunological memory. This approach has demonstrated potent prophylactic and therapeutic efficacy, effectively inhibiting melanoma growth in vivo (72). The promise of β-G-induced TI extends to the control of metastasis, a leading cause of cancer-related mortality where myeloid cells play a critical role. Studies show that WGP can reprogram macrophages, enhancing their reactivity not only to lipopolysaccharide but also to tumor-derived factors. This trained phenotype resulted in reduced lung metastasis of melanoma and prolonged survival in multiple murine metastatic models (68). Similarly, intraperitoneal administration of β-G in combination with IFN-γ was found to expand a novel subset of immunostimulatory IL27+ macrophages, inducing potent tumor regression in a clinically relevant model of metastatic ovarian cancer (132). It is important to note that the efficacy of TI may be modulated by systemic metabolites. Recent evidence indicates that trimethylamine N-oxide (TMAO), a gut microbiota-derived metabolite, can augment β-G-induced TI. This interaction suggests new therapeutic targets not only for cancer but also for a range of conditions including cardiovascular diseases (CVD), CKD-promoted CVD, inflammation, transplantation, and aging (133). Further supporting the therapeutic application of these molecules, synthetic, water-soluble β-G (molecular weight~60 kDa) have been developed. These compounds significantly inhibit the migration of highly metastatic melanoma B16F10 cells in vitro. Notably, in vivo administration substantially reduced and eliminated tumor nodules, achieving a remarkable inhibition rate of 86.7% (134). Moreover, with the deepening of melanoma research, TI holds promising potential for more effective combination with other treatment modalities, which will in turn enhance therapeutic outcomes to a greater extent.
3.7 Liver cancer, gastric cancer and other cancers
Primary liver cancer represents the third most prevalent cause of cancer-related mortality globally, characterized by aggressive tumor progression and alarmingly high fatality rates (135). The dismal prognosis primarily stems from late-stage diagnosis, with the majority of cases identified at advanced phases when therapeutic options become severely limited (136). Ranking as the sixth most common primary cancer site in humans, the liver’s distinctive immunosuppressive microenvironment coupled with its unique tissue architecture makes it particularly susceptible to metastatic colonization from other malignancies, especially colorectal cancers (137). β-G, particularly those with specific structural modifications, have emerged as promising immunomodulatory agents in cancer therapy. For instance, surface-modified β-1,3-glucans demonstrate potent immunostimulatory properties and the ability to repolarize macrophages, thereby counteracting immunosuppressive conditions within the tumor microenvironment and suppressing the progression of advanced hepatocellular carcinoma (HCC) (138).
In addition, Photothermal therapy (PTT) and photodynamic therapy (PDT) represent promising modalities; however, their efficacy is often suboptimal when applied individually. Innovative approaches are exploring combinatorial strategies, such as utilizing the photothermal and photosensiting properties of iron diselenide (FeSe2). Recent work has evaluated β-G nanotubes loaded with BFP-FeSe2 nanocomposites, demonstrating that the combination of PTT and PDT can significantly enhance therapeutic outcomes in HCC (139).
Gastric cancer, a primary epithelial malignancy of stomach origin, presented as a complex heterogeneous disease (140), ranking among the top five most prevalent cancers globally and top three cancer-related deaths. Major risk factors included Helicobacter pylori infection, high salt intake, aging, and low fruit/vegetable consumption (141). In gastric cancer, encapsulating DOX within a mushroom-derived β-G-based carrier mitigates gastric mucosal injury and more effectively alleviates precancerous lesions. This protective and therapeutic effect is achieved through modulation of the p53 and PI3K pathways, leading to reduced oxidative stress and inflammation (142). The translational potential of β-G is further supported by clinical investigations. A preliminary clinical study in patients with advanced gastric adenocarcinoma evaluated a regimen of β-G combined with camrelizumab (an immune checkpoint inhibitor) and SOX chemotherapy (oxaliplatin and oral S-1), administered every three weeks. This combination therapy showed promising efficacy and a manageable safety profile, warranting further validation in larger studies (143).
Beyond gastric cancer, β-G exhibit broad anticancer activity. LNT, a β-D-glucan derived from cultured Lentinula edoda mushrooms, disrupts cellular homeostasis in HeLa cervical cancer cells by significantly increasing intracellular Ca2+ levels and promoting autophagic flux. This effect is mediated, at least partially, via the PI3K/Akt/mTOR pathway, characterized by upregulation of LC3-II and downregulation of p62, PI3K, phospho-Akt, and mTOR. These molecular changes correlate with inhibited proliferation of HeLa cell xenografts in BALB/c nude mice (144). Interestingly, early-life immune modulation may also influence cancer risk. Epidemiological evidence suggests that childhood BCG vaccination is associated with a significantly reduced incidence of childhood leukaemia (145), hinting at the long-term, systemic immunoprotective effects that certain biological response modifiers can confer.
In summary, β-G demonstrate multifaceted antitumor activities, ranging from direct immunomodulation and sensitization of cancer cells to death pathways, to serving as versatile drug delivery platforms and enhancing the efficacy of combination therapies in clinical settings.
4 Future perspectives
The emerging field of TI represents a transformative approach in cancer immunotherapy, offering novel therapeutic strategies through the epigenetic and metabolic reprogramming of innate immune cells. Future investigations should focus on elucidating the precise molecular mechanisms underlying TI’s antitumor effects, particularly the epigenetic modifications (including histone acetylation, methylation patterns, and DNA methylation) and metabolic regulation (especially through mTOR, HIF-1α, and AMPK pathways) that mediate long-term immune memory in various tumor microenvironments. Current advancements in immunomodulatory nanomaterials, particularly β-G-based nanoparticles and innovative nano biotic formulations, demonstrate significant potential to enhance tumor-specific immune responses by improving antigen presentation and T cell activation (146). In the future, it is expected that the existing research bottlenecks will be broken through by optimizing drug delivery strategies (such as using nanoparticle systems to enhance the targeting of inducers), developing new TI agonists, and integrating artificial intelligence technology to analyze the laws of immune regulation. Meanwhile, the single-cell multi-omics approach is expected to provide deeper insights into TI’s cross-organ immune regulatory network, helping to reveal the collaborative regulatory mechanisms among different immune cells. These developments position TI as a highly promising strategy that can not only enhance the efficacy of existing immunotherapies but also overcome tumor drug resistance and prevent recurrence. The research breakthroughs are expected to drive the development of a stronger and more durable framework for tumor immunotherapy, providing new treatment hope for cancer patients. In addition, the development of novel agonists capable of inducing TI represents a major research direction in this field. Current trends indicate a growing focus on combinatorial strategies, such as conjugating glucan with novel biomaterials or targeting antibodies, as well as encapsulating anticancer agents within glucan-based vacuolar structures to achieve sustained release and enhanced targeting. These approaches constitute a promising frontier for therapeutic applications.
It is also important to note that BCG, a well‐established inducer of TI, has long been used as a standard treatment for certain cancers, such as bladder cancer. However, whether the antitumor effects of BCG can be attributed specifically to TI requires careful discrimination and further mechanistic investigation.
Another critical avenue for future research involves the application of high‐throughput sequencing technologies to dissect the impact of TI at the level of specific cell populations or even single cells. Such approaches will help elucidate the fundamental signaling pathways underlying TI and identify more robust biomarkers for its induction and maintenance.
At the same time, the potential of TI to exacerbate or precipitate autoimmune pathologies, as a consequence of its broad‐spectrum immunostimulatory effects, remains a significant concern in the context of cancer therapy. Understanding how to harness its benefits while minimizing such risks represents a key translational challenge.
Nonetheless, as an important functional extension of innate immunity, TI has demonstrated considerable clinical potential and offers a promising outlook for future therapeutic development.
5 Conclusion
TI, as an immune memory model formed based on the epigenetic and metabolic reprogramming of innate immune cells, has opened up a brand-new direction for tumor immunotherapy. Its core value lies in establishing long-term and systematic anti-tumor immune memory by inducing epigenetic and metabolic reprogramming of innate immune cells, effectively addressing the main limitations of traditional immunotherapy, such as insufficient improvement of the immunosuppressive state of the tumor microenvironment and short duration of immune memory.
At the cellular level, innate immune cells such as macrophages, NK cells, ILCs and γδT cells undergo metabolic conversion from oxidative phosphorylation to aerobic glycolysis after being stimulated by inducers like β-G and BCG, accompanied by epigenetic remodeling such as histone modifications (such as H3K4me3) and changes in chromatin accessibility. Form a long-term “training imprint”, significantly enhancing phagocytic ability, cytotoxicity and the secretion capacity of pro-inflammatory cytokines such as IL-1β and TNF-α; In cancer applications, TI inducers represented by BCG vaccines have performed outstandingly. They not only enhance the antigen-presenting ability of macrophages and dendritic cells but also reshape the tumor microenvironment. For instance, in bladder cancer models, BCG can promote the infiltration of CTLS and reduce MDSCs. In lung cancer models, β-G can induce pulmonary interstitial macrophages to produce reactive oxygen species to inhibit tumor metastasis. In melanoma studies, the central TI induced by nanobiators can enhance the efficacy of PD-1/CTLA-4 inhibitors - ultimately building a bridge between innate immunity and adaptive immune responses. It exerts anti-tumor effects on various cancers such as lung cancer, gastric cancer, colorectal cancer, liver cancer, breast cancer, bladder cancer, pancreatic cancer and melanoma. Moreover, some induction strategies (such as β-G nanocaterals and recombinant BCG) can synergize with immune checkpoint inhibitors, irreversible electroporation and other therapies.
Most studies are based on animal models, and their clinical transformation effects in different subtypes of human cancers and the screening criteria for applicable populations still need to be verified by more clinical trials.
Author contributions
JQ: Funding acquisition, Methodology, Writing – original draft. XG: Methodology, Writing – original draft. XW: Validation, Writing – review & editing. HM: Validation, Writing – review & editing. PL: Methodology, Writing – review & editing. ZS: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by grants from National Natural Science Foundation youth fund of China (82403252); Natural Science Foundation of Henan Province (242300421535); Xinxiang Medical University Research Launch Foundation (505501) to JQ; National Natural Science Foundation youth fund of China (32200714); Henan Normal University Research Launch Foundation (20220099) to ZS. Henan Provincial Medical Science and Technology Research Joint Venture Project (LHGJ20250871).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
β-G, β-glucan; AIT, allergen-specific immunotherapy; Ams, alveolar macrophages; APCs, antigen-presenting cells; ASCVD, atherosclerotic cardiovascular disease; BCG, Bacillus Calmette-Guérin; CRC, Colorectal cancer; CTLs, cytotoxic T lymphocytes; CVD, cardiovascular diseases; DAMPs, damage-associated molecular patterns; DCs, dendritic cells; DOX, doxorubicin; Gem, Gemcitabine; GMPs, granulocyte-macrophage progenitors; GPG, Ganoderma lucidum; HCC, hepatocellular carcinoma; HSPCs, hematopoietic stem and progenitor cells; Hz, hemozoin; ILCs, innate lymphoid cells; IP, intraperitoneal injection; IRE, irreversible electroporation; ISGs, interferon-stimulated genes; lncRNAs, long non-coding RNAs; LNT, Lentinan; LPS, lipopolysaccharide; MDSCs, myeloid-derived suppressor cells; MMR, Measles Mumps Rubella; NK cells, natural killer cells; NSCLC, non-small cell lung cancer; PDAC, pancreatic ductal adenocarcinoma; PDT, photodynamic therapy; PGE2, prostaglandin E2; PTT, Photothermal therapy; ROS, reactive oxygen species; SCLC, small cell lung cancer; TAMs, tumor-associated macrophages; TB, tuberculosis; TI, Trained immunity; TMAO, trimethylamine N-oxide; TME, tumour microenvironment; Tregs, regulatory T cells; WGP, whole beta-glucan particle; YGPs, yeast-derived β-G particles; ΔV1, V1-deleted.
References
1. Primorac D, Vrdoljak K, Brlek P, Pavelić E, Molnar V, Matišić V, et al. Adaptive immune responses and immunity to SARS-coV-2. Front Immunol. (2022) 13:848582. doi: 10.3389/fimmu.2022.848582
2. Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. (2014) 345:1250684. doi: 10.1126/science.1250684
3. Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, et al. Trained immunity: A program of innate immune memory in health and disease. Science. (2016) 352:aaf1098. doi: 10.1126/science.aaf1098
4. Bhargavi G and Subbian S. The causes and consequences of trained immunity in myeloid cells. Front Immunol. (2024) 15:1365127. doi: 10.3389/fimmu.2024.1365127
5. Ziogas A, Bruno M, van der Meel R, Mulder WJM, and Netea MG. Trained immunity: Target for prophylaxis and therapy. Cell Host Microbe. (2023) 31:1776–91. doi: 10.1016/j.chom.2023.10.015
6. Kalafati L, Hatzioannou A, Hajishengallis G, and Chavakis T. The role of neutrophils in trained immunity. Immunol Rev. (2023) 314:142–57. doi: 10.1111/imr.13142
7. Chakraborty S, Singh A, Wang L, Wang X, Sanborn MA, Ye Z, et al. Trained immunity of alveolar macrophages enhances injury resolution via KLF4-MERTK-mediated efferocytosis. J Exp Med. (2023) 220:e20221388. doi: 10.1084/jem.20221388
8. Wlodarczyk M, Druszczynska M, and Fol M. Trained innate immunity not always amicable. Int J Mol Sci. (2019) 20:2565. doi: 10.3390/ijms20102565
9. Roy PS and Saikia BJ. Cancer and cure: A critical analysis. Indian J Cancer. (2016) 53:441–2. doi: 10.4103/0019-509X.200658
11. Avgerinos KI, Spyrou N, Mantzoros CS, and Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. (2019) 92:121–35. doi: 10.1016/j.metabol.2018.11.001
12. Cordani M, Dando I, Ambrosini G, and González-Menéndez P. Signaling, cancer cell plasticity, and intratumor heterogeneity. Cell Commun Signal. (2024) 22:255. doi: 10.1186/s12964-024-01643-5
13. Vaghari-Tabari M, Ferns GA, Qujeq D, Andevari AN, Sabahi Z, Moein S, et al. Signaling, metabolism, and cancer: An important relationship for therapeutic intervention. J Cell Physiol. (2021) 236:5512–32. doi: 10.1002/jcp.30276
14. Ghasemi S. Cancer’s epigenetic drugs: where are they in the cancer medicines? Pharmacogenomics J. (2020) 20:367–79. doi: 10.1038/s41397-019-0138-5
15. Gillies RJ, Pilot C, Marunaka Y, and Fais S. Targeting acidity in cancer and diabetes. Biochim Biophys Acta Rev Cancer. (2019) 1871:273–80. doi: 10.1016/j.bbcan.2019.01.003
16. Singh AK, Praharaj M, Lombardo KA, Yoshida T, Matoso A, Baras AS, et al. Re-engineered BCG overexpressing cyclic di-AMP augments trained immunity and exhibits improved efficacy against bladder cancer. Nat Commun. (2022) 13:878. doi: 10.1038/s41467-022-28509-z
17. Hajishengallis G, Netea MG, and Chavakis T. Trained immunity in chronic inflammatory diseases and cancer. Nat Rev Immunol. (2025) 25:497–514. doi: 10.1038/s41577-025-01132-x
18. Kaur BP and Secord E. Innate immunity. Immunol Allergy Clin North Am. (2021) 41:535–41. doi: 10.1016/j.iac.2021.07.003
19. Fujii S, Yamamoto E, Ito S, Tangpranomkorn S, Kimura Y, Miura H, et al. SHI family transcription factors regulate an interspecific barrier. Nat Plants. (2023) 9:1862–73. doi: 10.1038/s41477-023-01535-5
20. Classic articles in colonic and rectal surgery. John Miller Turpin Finney 1863-1942. Gastro-enterostomy for cicatrizing ulcer of the pylorus. Dis Colon Rectum. (1986) 29:218–21. doi: 10.1007/BF02555032
21. Kadry H, Noorani B, and Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. (2020) 17:69. doi: 10.1186/s12987-020-00230-3
22. Carpenter S and O’Neill LAJ. From periphery to center stage: 50 years of advancements in innate immunity. Cell. (2024) 187:2030–51. doi: 10.1016/j.cell.2024.03.036
23. Sun L, Wang X, Saredy J, Yuan Z, Yang X, Wang H, et al. Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biol. (2020) 37:101759. doi: 10.1016/j.redox.2020.101759
24. Bekkering S, Domínguez-Andrés J, Joosten LAB, Riksen NP, and Netea MG. Trained immunity: reprogramming innate immunity in health and disease. Annu Rev Immunol. (2021) 39:667–93. doi: 10.1146/annurev-immunol-102119-073855
25. Fanucchi S, Domínguez-Andrés J, Joosten LAB, Netea MG, and Mhlanga MM. The intersection of epigenetics and metabolism in trained immunity. Immunity. (2021) 54:32–43. doi: 10.1016/j.immuni.2020.10.011
26. Ziogas A, Novakovic B, Ventriglia L, Galang N, Tran KA, Li W, et al. Long-term histone lactylation connects metabolic and epigenetic rewiring in innate immune memory. Cell. (2025) 188:2992–3012.e16. doi: 10.1016/j.cell.2025.03.048
27. Zhu Y, Gao Q, Zhang J, Cheng Y, Yang S, Xu R, et al. Persistent bone marrow hemozoin accumulation confers a survival advantage against bacterial infection via cell-intrinsic Myd88 signaling. Cell Rep. (2024) 43:114850. doi: 10.1016/j.celrep.2024.114850
28. Zhou H, Lu X, Huang J, Jordan P, Ma S, Xu L, et al. Induction of trained immunity protects neonatal mice against microbial sepsis by boosting both the inflammatory response and antimicrobial activity. J Inflammation Res. (2022) 15:3829–45. doi: 10.2147/JIR.S363995
29. Xu JC, Wu K, Ma RQ, Li JH, Tao J, Hu Z, et al. Establishment of an in vitro model of monocyte-like THP-1 cells for trained immunity induced by bacillus Calmette-Guerin. BMC Microbiol. (2024) 24:130. doi: 10.1186/s12866-024-03191-x
30. Zarzour A, Kim HW, and Weintraub NL. Epigenetic regulation of vascular diseases. Arterioscler Thromb Vasc Biol. (2019) 39:984–90. doi: 10.1161/ATVBAHA.119.312193
31. Woeste MR, Shrestha R, Geller AE, Li S, Montoya-Durango D, Ding C, et al. Irreversible electroporation augments beta-glucan induced trained innate immunity for the treatment of pancreatic ductal adenocarcinoma. J Immunother Cancer. (2023) 11:351–61. doi: 10.1097/XCS.0000000000001291
32. Yunna C, Mengru H, Lei W, and Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. (2020) 877:173090. doi: 10.1016/j.ejphar.2020.173090
33. Chen J, Gao L, Wu X, Fan Y, Liu M, Peng L, et al. BCG-induced trained immunity: history, mechanisms and potential applications. J Transl Med. (2023) 21:106. doi: 10.1186/s12967-023-03944-8
34. Jeyanathan M, Vaseghi-Shanjani M, Afkhami S, Grondin JA, Kang A, D'Agostino MR, et al. Parenteral BCG vaccine induces lung-resident memory macrophages and trained immunity via the gut-lung axis. Nat Immunol. (2022) 23:1687–702. doi: 10.1038/s41590-022-01354-4
35. Saeed S, Quintin J, Kerstens HHD, Rao NA, Aghajanirefah A, Matarese F, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. (2014) 345:1251086. doi: 10.1126/science.1251086
36. Mitroulis T, Ruppova K, Wang B, Chen LS, Grzybek M, Grinenko T, et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell. (2018) 172:147–161.e12. doi: 10.1016/j.cell.2017.11.034
37. Torcellan T, Friedrich C, Doucet-Ladevèze R, Ossner T, Visaconill Solé V, Riedmann S, et al. Circulating NK cells establish tissue residency upon acute infection of skin and mediate accelerated effector responses to secondary infection. Immunity. (2024) 57:124–140.e7. doi: 10.1016/j.immuni.2023.11.018
38. Myers JA and Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. (2021) 18:85–100. doi: 10.1038/s41571-020-0426-7
39. Röring RJ, Debisarun PA, Botey-Bataller J, Suen TK, Bulut Ö, Kilic G, et al. MMR vaccination induces trained immunity via functional and metabolic reprogramming of gammadelta T cells. J Clin Invest. (2024) 134:e170848. doi: 10.1172/JCI170848
40. Su X, Deng Z, Lan Y, Liu B, and Liu C. Helper ILCs in the human hematopoietic system. Trends Immunol. (2025) 46:244–57. doi: 10.1016/j.it.2025.01.009
41. Mboko WP, Wang Y, Cao W, Sayedahmed EE, Mishina M, Kumar A, et al. Trained ILCs confer adaptive immunity-independent protection against influenza. J Virol. (2025) 99:e0053225. doi: 10.1128/jvi.00532-25
42. Eljaszewicz A, Ruchti F, Radzikowska U, Globinska A, Boonpiyathad T, Gschwend A, et al. Trained immunity and tolerance in innate lymphoid cells, monocytes, and dendritic cells during allergen-specific immunotherapy. J Allergy Clin Immunol. (2021) 147:1865–77. doi: 10.1016/j.jaci.2020.08.042
43. Rahman MA, de Castro IS, Schifanella L, Bissa M, and Franchini G. Vaccine induced mucosal and systemic memory NK/ILCs elicit decreased risk of SIV/SHIV acquisition. Front Immunol. (2024) 15:1441793. doi: 10.3389/fimmu.2024.1441793
44. Trabanelli S, Ercolano G, Wyss T, Gomez-Cadena A, Falquet M, Cropp D, et al. c-Maf enforces cytokine production and promotes memory-like responses in mouse and human type 2 innate lymphoid cells. EMBO J. (2022) 41:e109300. doi: 10.15252/embj.2021109300
45. Caron J, Ridgley LA, and Bodman-Smith M. How to train your dragon: harnessing gamma delta T cells antiviral functions and trained immunity in a pandemic era. Front Immunol. (2021) 12:666983. doi: 10.3389/fimmu.2021.666983
46. Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. (2020) 20:375–88. doi: 10.1038/s41577-020-0285-6
47. Hayday A, Dechanet-Merville J, Rossjohn J, and Silva-Santos B. Cancer immunotherapy by gammadelta T cells. Science. (2024) 386:eabq7248. doi: 10.1126/science
48. Xia M, Blazevic A, Fiore-Gartland A, and Hoft DF. Impact of BCG vaccination on the repertoire of human gammadelta T cell receptors. Front Immunol. (2023) 14:1100490. doi: 10.3389/fimmu.2023.1100490
49. Esteso G, Felgueres MJ, García-Jiménez ÁF, Reyburn-Valés C, Benguría A, Vázquez E, et al. BCG-activation of leukocytes is sufficient for the generation of donor-independent innate anti-tumor NK and gammadelta T-cells that can be further expanded in vitro. Oncoimmunology. (2023) 12:2160094. doi: 10.1080/2162402X.2022.2160094
50. Morrison AL, Sarfas C, Sibley L, Williams J, Mabbutt A, Dennis MJ, et al. IV BCG Vaccination and Aerosol BCG Revaccination Induce Mycobacteria-Responsive gammadelta T Cells Associated with Protective Efficacy against M. tb Challenge. Vaccines (Basel). (2023) 11:1604. doi: 10.3390/vaccines11101604
51. Samuel BER, Diaz FE, Maina TW, Corbett RJ, Tuggle CK, McGill JL, et al. Evidence of innate training in bovine gammadelta T cells following subcutaneous BCG administration. Front Immunol. (2024) 15:1423843. doi: 10.3389/fimmu.2024.1423843
52. Suen TK, Moorlag SJCFM, Li W, de Bree LCJ, Koeken VACM, Mourits VP, et al. BCG vaccination induces innate immune memory in gammadelta T cells in humans. J Leukoc Biol. (2024) 115:149–63. doi: 10.1093/jleuko/qiad103
53. Adam A, Wu W, Jones MC, Hao H, Aditi, Samir P, et al. Respiratory syncytial virus infection confers heterologous protection against SARS-CoV-2 via induction of gammadelta T cell-mediated trained immunity and SARS-CoV-2 reactive mucosal T cells. bioRxiv. (2025). doi: 10.1101/2025.07.02.662833
54. Scharf P, Aschner M, and Farsky S. Neutrophils in toxicology: a forgotten field. J Toxicol Environ Health B Crit Rev. (2024) p:1–32. doi: 10.1080/10937404
55. Adrover JM, Han X, Sun L, Fujii T, Sivetz N, Daßler-Plenker J, et al. Neutrophils drive vascular occlusion, tumour necrosis and metastasis. Nature. (2025) 645:484–95. doi: 10.1038/s41586-025-09278-3
56. Moorlag S, Rodriguez-Rosales YA, Gillard J, Fanucchi S, Theunissen K, Novakovic B, et al. BCG vaccination induces long-term functional reprogramming of human neutrophils. Cell Rep. (2020) 33:108387. doi: 10.1016/j.celrep.2020.108387
57. Cirovic B, de Bree LCJ, Groh L, Blok BA, Chan J, van der Velden WJFM, et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe. (2020) 28:322–334.e5. doi: 10.1016/j.chom.2020.05.014
58. Shrestha S, Lee YB, Lee H, Choi YK, Park BY, Kim MJ, et al. Diabetes primes neutrophils for neutrophil extracellular trap formation through trained immunity. Res (Wash D C). (2024) 7:0365. doi: 10.34133/research.0365
59. Jurado LF, Daman AW, Li Z, Ross VMS, Nikolaou K, Tran KA, et al. A fungal-derived adjuvant amplifies the antitumoral potency of Bacillus Calmette-Guerin via reprogramming granulopoiesis. Immunity. (2025) 58:1984–2001.e6. doi: 10.1016/j.immuni.2025.05.026
60. Darroch H, Keerthisinghe P, Sung YJ, Rolland L, Prankerd-Gough A, Crosier PS, et al. Infection-experienced HSPCs protect against infections by generating neutrophils with enhanced mitochondrial bactericidal activity. Sci Adv. (2023) 9:eadf9904. doi: 10.1126/sciadv.adf9904
61. Daman AW, Antonelli AC, Redelman-Sidi G, Paddock L, Khayat S, Ketavarapu M, et al. Microbial cancer immunotherapy reprograms hematopoiesis to enhance myeloid-driven anti-tumor immunity. Cancer Cell. (2025) 43:1442–1459.e10. doi: 10.1016/j.ccell.2025.05.002
62. Rincon JC, Wang D, Polcz VE, Barrios EL, Dirain ML, Ungaro RF, et al. Innate immune training in the neonatal response to sepsis. Mol Med. (2025) 31:159. doi: 10.1186/s10020-025-01179-5
63. Marshall JL, Satti I, Surakhy M, Harris SA, Morrison H, Wittenberg RE, et al. Early mucosal responses following a randomised controlled human inhaled infection with attenuated Mycobacterium bovis BCG. Nat Commun. (2025) 16:4989. doi: 10.1038/s41467-025-60285-4
64. Rahman MA, Goldfarbmuren KC, Sarkis S, Bissa M, Gutowska A, Schifanella L, et al. BCG immunization mitigates SARS-CoV-2 replication in macaques via monocyte efferocytosis and neutrophil recruitment in lungs. JCI Insight. (2025) 10:e194633. doi: 10.1172/jci.insight.194633
65. Das S, Kaminski TW, Schlegel BT, Bain W, Hu S, Patel A, et al. Neutrophils and galectin-3 defend mice from lethal bacterial infection and humans from acute respiratory failure. Nat Commun. (2024) 15:4724. doi: 10.1038/s41467-024-48796-y
66. Riksen NP, Bekkering S, Mulder WJM, and Netea MG. Trained immunity in atherosclerotic cardiovascular disease. Nat Rev Cardiol. (2023) 20:799–811. doi: 10.1038/s41569-023-00894-y
67. Renke G, Baesso T, Paes R, and Renke A. beta-glucan “Trained immunity” Immunomodulatory properties potentiate tissue wound management and accelerate fitness recover. Immunotargets Ther. (2022) 11:67–73. doi: 10.2147/ITT.S381145
68. Ding C, Shrestha R, Zhu X, Geller AE, Wu S, Woeste MR, et al. Inducing trained immunity in pro-metastatic macrophages to control tumor metastasis. Nat Immunol. (2023) 24:239–54. doi: 10.1038/s41590-022-01388-8
69. Thai AA, Solomon BJ, Sequist LV, Gainor JF, and Heist RS. Lung cancer. Lancet. (2021) 398:535–54. doi: 10.1016/S0140-6736(21)00312-3
70. Herbst RS, Morgensztern D, and Boshoff C. The biology and management of non-small cell lung cancer. Nature. (2018) 553:446–54. doi: 10.1038/nature25183
71. Nooreldeen R and Bach H. Current and future development in lung cancer diagnosis. Int J Mol Sci. (2021) 22:8661. doi: 10.3390/ijms22168661
72. Chen Z, Yong T, Wei Z, Zhang X, Li X, Qin J, et al. Engineered probiotic-based personalized cancer vaccine potentiates antitumor immunity through initiating trained immunity. Adv Sci (Weinh). (2024) 11:e2305081. doi: 10.1002/advs.202305081
73. Kalafati L, Kourtzelis I, Schulte-Schrepping J, Li X, Hatzioannou A, Grinenko T, et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell. (2020) 183:771–785.e12. doi: 10.1016/j.cell.2020.09.058
74. Zhang L, Xiao Z, Zhang D, Yang L, Yuan Z, Wang G, et al. Targeted initiation of trained immunity in tumor-associated macrophages with membrane-camouflaged bacillus calmette-guerin for lung carcinoma immunotherapy. ACS Nano. (2024) 18:34219–34. doi: 10.1021/acsnano.4c11658
75. Wang T, Zhang J, Wang Y, Li Y, Wang L, Yu Y, et al. Influenza-trained mucosal-resident alveolar macrophages confer long-term antitumor immunity in the lungs. Nat Immunol. (2023) 24:423–38. doi: 10.1038/s41590-023-01428-x
76. Broquet A, et al. Author Correction: Sepsis-trained macrophages promote antitumoral tissue-resident T cells. Nat Immunol. (2024) 25:2167. doi: 10.1038/s41590-024-02000-x
77. Li D, Li W, Zheng P, Yang Y, Liu Q, Hu Y, et al. A “trained immunity” inducer-adjuvanted nanovaccine reverses the growth of established tumors in mice. J Nanobiotechnology. (2023) 21:74. doi: 10.1186/s12951-023-01832-3
78. Kwon KW, Kang TG, Lee JB, Choi E, Kim H, Park MC, et al. Mycobacterium tuberculosis-specific T cells restrain anti-cancer drug-induced neutrophilic lung inflammation in tuberculosis. Nat Commun. (2025) 16:8875. doi: 10.1038/s41467-025-63930-0
79. Li J, Ma X, Chakravarti D, Shalapour S, and DePinho RA. Genetic and biological hallmarks of colorectal cancer. Genes Dev. (2021) 35:787–820. doi: 10.1101/gad.348226.120
80. Eng C, Jácome AA, Agarwal R, Hayat MH, Byndloss MX, Holowatyj AN, et al. A comprehensive framework for early-onset colorectal cancer research. Lancet Oncol. (2022) 23:e116–28. doi: 10.1016/S1470-2045(21)00588-X
81. Kim J and Lee HK. Potential role of the gut microbiome in colorectal cancer progression. Front Immunol. (2021) 12:807648. doi: 10.3389/fimmu.2021.807648
82. Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R, et al. Bacterial community variation in human body habitats across. space time. Sci. (2009) 326:1694–7. doi: 10.1126/science.1177486
83. Thursby E and Juge N. Introduction to the human gut microbiota. Biochem J. (2017) 474:1823–36. doi: 10.1042/BCJ20160510
84. Weersma RK, Zhernakova A, and Fu J. Interaction between drugs and the gut microbiome. Gut. (2020) 69:1510–9. doi: 10.1136/gutjnl-2019-320204
85. Busnelli M, Manzini S, and Chiesa G. The gut microbiota affects host pathophysiology as an endocrine organ: A focus on cardiovascular disease. Nutrients. (2019) 12:79. doi: 10.3390/nu12010079
86. Wang C, Gu Y, Chu Q, Wang X, Ding Y, Qin X, et al. Gut microbiota and metabolites as predictors of biologics response in inflammatory bowel disease: A comprehensive systematic review. Microbiol Res. (2024) 282:127660. doi: 10.1016/j.micres.2024.127660
87. Tape CJ. Plastic persisters: revival stem cells in colorectal cancer. Trends Cancer. (2024) 10:185–95. doi: 10.1016/j.trecan.2023.11.003
88. Tang C, Sun H, Kadoki M, Han W, Ye X, Makusheva Y, et al. Blocking Dectin-1 prevents colorectal tumorigenesis by suppressing prostaglandin E2 production in myeloid-derived suppressor cells and enhancing IL-22 binding protein expression. Nat Commun. (2023) 14:1493. doi: 10.1038/s41467-023-37229-x
89. Wang Q, Jiang H, Zhang H, Lu W, Wang X, Xu W, et al. beta-Glucan-conjugated anti-PD-L1 antibody enhances antitumor efficacy in preclinical mouse models. Carbohydr Polym. (2024) 324:121564. doi: 10.1016/j.carbpol.2023.121564
90. Kopiasz Ł, Dziendzikowska K, Oczkowski M, Harasym J, and Gromadzka-Ostrowska J. Low-molar-mass oat beta-glucan impacts autophagy and apoptosis in early stages of induced colorectal carcinogenesis in rats. Int J Biol Macromol. (2024) 254:127832. doi: 10.1016/j.ijbiomac.2023.127832
91. Hu S, Xu H, Xie C, Meng Y, and Xu X. Inhibition of human cervical cancer development through p53-dependent pathways induced by the specified triple helical beta-glucan. Int J Biol Macromol. (2023) 251:126222. doi: 10.1016/j.ijbiomac.2023.126222
92. Liu N, Zou S, Xie C, Meng Y, and Xu X. Effect of the beta-glucan from Lentinus edodes on colitis-associated colorectal cancer and gut microbiota. Carbohydr Polym. (2023) 316:121069. doi: 10.1016/j.carbpol.2023.121069
93. Grabinski VF and Brawley OW. Disparities in breast cancer. Obstet Gynecol Clin North Am. (2022) 49:149–65. doi: 10.1016/j.ogc.2021.11.010
94. Barzaman K, Karami J, Zarei Z, Hosseinzadeh A, Kazemi MH, Moradi-Kalbolandi S, et al. Breast cancer: Biology, biomarkers, and treatments. Int Immunopharmacol. (2020) 84:106535. doi: 10.1016/j.intimp.2020.106535
95. Britt KL, Cuzick J, and Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. (2020) 20:417–36. doi: 10.1038/s41568-020-0266-x
96. Boere I, Lok C, Poortmans P, Koppert L, Painter R, Heuvel-Eibrink MMV, et al. Breast cancer during pregnancy: epidemiology, phenotypes, presentation during pregnancy and therapeutic modalities. Best Pract Res Clin Obstet Gynaecol. (2022) 82:46–59. doi: 10.1016/j.bpobgyn.2022.05.001
97. Fox S, Speirs V, and Shaaban AM. Male breast cancer: an update. Virchows Arch. (2022) 480:85–93. doi: 10.1007/s00428-021-03190-7
98. Corso G, Fusco N, Guerini-Rocco E, Leonardi MC, Criscitiello C, Zagami P, et al. Invasive lobular breast cancer: Focus on prevention, genetics, diagnosis, and treatment. Semin Oncol. (2024) 51:106–22. doi: 10.1053/j.seminoncol.2024.05.001
99. Yan Q, Deng Y, and Zhang Q. A comprehensive overview of metaplastic breast cancer: Features and treatments. Cancer Sci. (2024) 115:2506–14. doi: 10.1111/cas.16208
100. Huang ML, Tomkovich K, Lane DL, Katta R, Candelaria RP, Santiago L, et al. Breast cancer cryoablation fundamentals past and present: technique optimization and imaging pearls. Acad Radiol. (2023) 30:2383–95. doi: 10.1016/j.acra.2023.05.019
101. Khan MM, Yalamarty SSK, Rajmalani BA, Filipczak N, and Torchilin VP. Recent strategies to overcome breast cancer resistance. Crit Rev Oncol Hematol. (2024) 197:104351. doi: 10.1016/j.critrevonc.2024.104351
102. Bu Y, Liu Q, Shang Y, Zhao Z, Sun H, Chen F, et al. Ganoderma lucidum spores-derived particulate beta-glucan treatment improves antitumor response by regulating myeloid-derived suppressor cells in triple-negative breast cancer. Int J Biol Macromol. (2024) 270:131949. doi: 10.1016/j.ijbiomac.2024.131949
103. He F, Xie C, and Xu X. Hyaluronic acid-modified yeast beta-glucan particles delivering doxorubicin for treatment of breast cancer. Carbohydr Polym. (2023) 314:120907. doi: 10.1016/j.carbpol.2023.120907
104. Lyu F, Xie C, Zhang L, and Xu X. Nanotubes fabricated from a triple helix polysaccharide as a novel carrier delivering doxorubicin for breast cancer therapy. Int J Biol Macromol. (2023) 242:124153. doi: 10.1016/j.ijbiomac.2023.124153
105. Cai L, Yang Q, Shao X, Wang S, Fu Y, Mao X, et al. Dual-responsive triple-helix beta-glucan-based self-assembled supramolecule for combination chemo-photothermal therapy against lung metastatic triple negative breast cancer. Int J Biol Macromol. (2025) 306:141370. doi: 10.1016/j.ijbiomac.2025.141370
106. Liu S, Chen X, and Lin T. Lymphatic metastasis of bladder cancer: Molecular mechanisms, diagnosis and targeted therapy. Cancer Lett. (2021) 505:13–23. doi: 10.1016/j.canlet.2021.02.010
107. Chen J, Huang CP, Quan C, Zu X, Ou Z, Tsai YC, et al. The androgen receptor in bladder cancer. Nat Rev Urol. (2023) 20:560–74. doi: 10.1038/s41585-023-00761-y
108. Patel VG, Oh WK, and Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin. (2020) 70:404–23. doi: 10.3322/caac.21631
109. Atallah A, Grossman A, Nauman RW, Paré JF, Khan A, Siemens DR, et al. Systemic versus localized Bacillus Calmette Guerin immunotherapy of bladder cancer promotes an anti-tumoral microenvironment: Novel role of trained immunity. Int J Cancer. (2024) 155:352–64. doi: 10.1002/ijc.34897
110. Tan C, Li C, Ge R, Zhang W, Wu Z, Wang S, et al. Mcl-1 downregulation enhances BCG treatment efficacy in bladder cancer by promoting macrophage polarization. Cancer Cell Int. (2025) 25:48. doi: 10.1186/s12935-025-03676-3
111. Liu K, Wang L, Peng J, Lyu Y, Li Y, Duan D, et al. Drug-loaded bacillus calmette-guerin bacteria for immuno-chemo combo therapy in bladder cancer. Adv Mater. (2024) 36:e2310735. doi: 10.1002/adma.202310735
112. Ma Y, Sun Y, Guo H, and Yang R. Tumor-associated macrophages in bladder cancer: roles and targeted therapeutic strategies. Front Immunol. (2024) 15:1418131. doi: 10.3389/fimmu.2024.1418131
113. Dyrskjøt L, Hansel DE, Efstathiou JA, Knowles MA, Galsky MD, Teoh J, et al. Bladder cancer. Nat Rev Dis Primers. (2023) 9:58. doi: 10.1038/s41572-023-00468-9
114. Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. (2021) 79:82–104. doi: 10.1016/j.eururo.2020.03.055
115. Luo Y and Knudson MJ. Mycobacterium bovis bacillus Calmette-Guerin-induced macrophage cytotoxicity against bladder cancer cells. Clin Dev Immunol 2010. (2010) p:357591. doi: 10.1155/2010/357591
116. Cai J, Chen H, Lu M, Zhang Y, Lu B, You L, Zhang T, et al. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. (2021) 520:1–11. doi: 10.1016/j.canlet.2021.06.027
117. Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. (2021) 18:493–502. doi: 10.1038/s41575-021-00457-x
118. Diaconescu S, Gîlcă-Blanariu GE, Poamaneagra S, Marginean O, Gabriela Paduraru G, Stefanescu G, et al. Could the burden of pancreatic cancer originate in childhood? World J Gastroenterol. (2021) 27:5322–40. doi: 10.3748/wjg.v27.i32.5322
119. Stoffel EM, Brand RE, and Goggins M. Pancreatic cancer: changing epidemiology and new approaches to risk assessment, early detection, and prevention. Gastroenterology. (2023) 164:752–65. doi: 10.1053/j.gastro.2023.02.012
120. Ghafouri-Fard S, Fathi M, Zhai T, Taheri M, and Dong P. LncRNAs: novel biomarkers for pancreatic cancer. Biomolecules. (2021) 11:1665. doi: 10.3390/biom11111665
121. Valente R, Coppola A, Scandavini CM, and Arnelo U. Endoscopic workup in pancreatic cancer. Int J Surg. (2024) 110:6064–9. doi: 10.1097/JS9.0000000000000777
122. Mukherji R, Debnath D, Hartley ML, and Noel MS. The role of immunotherapy in pancreatic cancer. Curr Oncol. (2022) 29:6864–92. doi: 10.3390/curroncol29100541
123. Espona-Fiedler M, Patthey C, Lindblad S, Sarró I, and Öhlund D. Overcoming therapy resistance in pancreatic cancer: New insights and future directions. Biochem Pharmacol. (2024) 229:116492. doi: 10.1016/j.bcp.2024.116492
124. Karunakaran M and Barreto SG. Surgery for pancreatic cancer: current controversies and challenges. Future Oncol. (2021) 17:5135–62. doi: 10.2217/fon-2021-0533
125. Wattenberg MM, Coho H, Herrera VM, Graham K, Stone ML, Xue Y, et al. Cancer immunotherapy via synergistic coactivation of myeloid receptors CD40 and Dectin-1. Sci Immunol. (2023) 8:eadj5097. doi: 10.1126/sciimmunol.adj5097
126. Thomas SK, Wattenberg MM, Choi-Bose S, Uhlik M, Harrison B, Coho H, et al. Kupffer cells prevent pancreatic ductal adenocarcinoma metastasis to the liver in mice. Nat Commun. (2023) 14:6330. doi: 10.1038/s41467-023-41771-z
127. Wu S, Xu W, Shan X, Sun L, Liu S, Sun X, et al. Targeting splenic myeloid cells with nanobiologics to prevent postablative pancreatic cancer recurrence via inducing antitumor peripheral trained immunity. Adv Sci (Weinh). (2025) 12:e2413562. doi: 10.1002/advs.202413562
128. Borzillo V and Muto P. Radiotherapy in the treatment of subcutaneous melanoma. Cancers (Basel). (2021) 13:5859. doi: 10.3390/cancers13225859
129. Borges VM, Marinho FV, Caldeira CVA, de Queiroz NMGP, and Oliveira SC. Bacillus Calmette-Guerin immunotherapy induces an efficient antitumor response to control murine melanoma depending on MyD88 signaling. Front Immunol. (2024) 15:1380069. doi: 10.3389/fimmu.2024.1380069
130. Liu Y, Wang Z, Jin H, Cui L, Huo B, Xie C, et al. Squalene-epoxidase-catalyzed 24(S),25-epoxycholesterol synthesis promotes trained-immunity-mediated antitumor activity. Cell Rep. (2024) 43:114094. doi: 10.1016/j.celrep.2024.114094
131. Tepale-Segura A, Gajón JA, Muñoz-Cruz S, Castro-Escamilla O, and Bonifaz LC. The cholera toxin B subunit induces trained immunity in dendritic cells and promotes CD8 T cell antitumor immunity. Front Immunol. (2024) 15:1362289. doi: 10.3389/fimmu.2024.1362289
132. Murphy B, Miyamoto T, Manning BS, Mirji G, Ugolini A, Kannan T, et al. Myeloid activation clears ascites and reveals IL27-dependent regression of metastatic ovarian cancer. J Exp Med. (2024) 221:e20231967. doi: 10.1084/jem.20231967
133. Saaoud F, Liu L, Xu K, Cueto R, Shao Y, Lu Y, et al. Aorta- and liver-generated TMAO enhances trained immunity for increased inflammation via ER stress/mitochondrial ROS/glycolysis pathways. JCI Insight. (2023) 8:e158183. doi: 10.1172/jci.insight.158183
134. Li J, Zheng M, Wang Z, Liu Y, Niu Q, Zhou H, et al. Anti-tumor and anti-metastasis of water-soluble sulfated beta-glucan derivatives from Saccharomyces cerevisiae. Carbohydr Polym. (2024) 344:122466. doi: 10.1016/j.carbpol.2024.122466
135. Lin S and Kuang M. RNA modification-mediated mRNA translation regulation in liver cancer: mechanisms and clinical perspectives. Nat Rev Gastroenterol Hepatol. (2024) 21:267–81. doi: 10.1038/s41575-023-00884-y
136. Yang WS, Zeng XF, Liu ZN, Zhao QH, Tan YT, Gao J, et al. Diet and liver cancer risk: a narrative review of epidemiological evidence. Br J Nutr. (2020) 124:330–40. doi: 10.1017/S0007114520001208
137. Li X, Ramadori P, Pfister D, Seehawer M, Zender L, Heikenwalder M, et al. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat Rev Cancer. (2021) 21:541–57. doi: 10.1038/s41568-021-00383-9
138. Cui X, Cao C, Hao W, Pan X, Cao Y, Fu Y, et al. A nanoplatform of reversing tumor immunosuppressive microenvironment based on the NIR-II gold hollow nanorod for the treatment of hepatocellular carcinoma. Small. (2025) 21:e2500144. doi: 10.1002/smll.202500144
139. Ning Y, Yang A, Zhou S, Wang Y, Lin Z, Xu X, et al. beta-glucan nanotubes loaded with iron diselenide for enhanced photothermal and photodynamic therapy in hepatocellular carcinoma. Acta Biomater. (2025) 8:45358–68. doi: 10.1016/j.actbio.2025.10.005
140. Yang WJ, Zhao HP, Yu Y, Wang JH, Guo L, Liu JY, et al. Updates on global epidemiology, risk and prognostic factors of gastric cancer. World J Gastroenterol. (2023) 29:2452–68. doi: 10.3748/wjg.v29.i16.2452
142. Zhang S, Feng X, Yang S, Shi X, Chen J, Zhu R, et al. Acid-triggered rattan ball-like beta-glucan carrier embedding doxorubicin to synergistically alleviate precancerous lesions of gastric cancer via p53 and PI3K pathways. Int J Biol Macromol. (2024) 281:136540. doi: 10.1016/j.ijbiomac.2024.136540
143. Chu Y, He X, Xue Y, Jiang H, Zhu C, Qi C, et al. An exploratory clinical study of beta-glucan combined with camrelizumab and SOX chemotherapy as first-line treatment for advanced gastric adenocarcinoma. Front Immunol. (2024) 15:1448485. doi: 10.3389/fimmu.2024.1448485
144. Hu S, Meng Y, Guo L, and Xu X. A novel strategy to enhance inhibition of Hela cervical cancer by combining Lentinus beta-glucan and autophagic flux blockage. Int J Biol Macromol. (2024) 281:136309. doi: 10.1016/j.ijbiomac.2024.136309
145. Singh S, Kishore D, and Singh RK. Trained Immunity” from Mycobacterium spp. exposure (BCG vaccination and environmental) may have an impact on the incidence of early childhood leukemia. Front Immunol. (2023) 14:1193859. doi: 10.3389/fimmu.2023.1193859
Keywords: trained immunity, lung cancer, gastric cancer, liver cancer, colorectal cancer
Citation: Qu J, Guo X, Wang X, Meng H, Li P and Sun Z (2025) The significance of trained immunity in cancer. Front. Immunol. 16:1665099. doi: 10.3389/fimmu.2025.1665099
Received: 13 July 2025; Accepted: 17 November 2025; Revised: 12 October 2025;
Published: 01 December 2025.
Edited by:
Mary Poupot-Marsan, INSERM U1037 Centre de Recherche en Cancérologie de Toulouse, FranceReviewed by:
Yongjun Sui, National Cancer Institute (NIH), United StatesFenghua Xu, Chinese PLA General Hospital, China
Copyright © 2025 Qu, Guo, Wang, Meng, Li and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peizhi Li, eHhody4wODA2QDE2My5jb20=; Zhiheng Sun, c3VuemhpaGVuZ0BodHUuZWR1LmNu
 Junxing Qu
Junxing Qu Xinya Guo3,4
Xinya Guo3,4 Zhiheng Sun
Zhiheng Sun