- 1The Rheumatology Department of the First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, China
- 2The First Clinical College of Henan University of Chinese Medicine, Zhengzhou, China
- 3The Second Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, China
Primary Sjögren’s disease (SjD) is a chronic systemic autoimmune disorder whose pathogenesis remains incompletely understood. Current clinical interventions demonstrate limited efficacy, yielding suboptimal therapeutic outcomes. microRNAs (miRNAs)–critical regulators of transcriptional networks–participate in SjD pathogenesis through multifaceted mechanisms. Dysregulated miRNA expression during SjD progression directly influences disease prognosis, establishing miRNAs as promising therapeutic targets. Evidence implicates macrophage polarization, apoptosis dysregulation, Th17/Treg imbalance, T/B lymphocyte dysfunction, glandular impairment, and aberrant type I interferon responses in SjD development. Notably, miR-216a-3p, miR-31-5p, and miR-155-5p modulate key signaling pathways (NF-κB, JAK/STAT, PI3K/AKT) to optimize macrophage polarization, suppress apoptosis, restore Th17/Treg equilibrium, regulate T/B lymphocyte activity, enhance glandular function, normalize type I interferon responses,thereby exerting potent anti-SjD effects. This review synthesizes recent literature to elucidate SjD pathogenesis and miRNA-mediated therapeutic mechanisms, providing a theoretical foundation for novel SjD management strategies.
Primary Sjögren’s disease (SjD) is a chronic autoimmune disease characterized by exocrine gland dysfunction, leading to symptoms such as dry mouth and dry eyes that significantly impair patients’ quality of life, and severe complications may even be life-threatening (1, 2). The pathogenesis of SjD is highly complex, involving multiple factors such as genetics, environment, host factors, and immune function; however, the exact mechanisms remain elusive (3). In SjD, lymphocyte infiltration occurs in the salivary and lacrimal glands. During early disease stages, CD4+ T cell infiltration predominates; as the disease progresses, B cell infiltration increases and becomes more prominent (4). Currently, there are still no disease-modifying drugs specifically for SjD. Treatment primarily aims to relieve clinical symptoms; however, drug-related adverse effects often limit its overall efficacy (5). Consequently, there is an urgent need to develop safer and more effective therapies.
1 Overview of microRNA
microRNA (miRNA) is an endogenous, non-protein-coding RNA, primarily transcribed in the nucleus, with a length of approximately 20–22 nucleotides. Functionally, miRNA regulates gene expression post-transcriptionally, primarily through gene silencing, and plays a significant role in modulating disease-related genes (6). Through diverse molecular pathways, miRNA exerts regulatory effects on critical physiological and pathological processes, including cell growth, development, differentiation, proliferation, and apoptosis (7). Mature miRNA is released into the receptor cells to participate in transcription and gene expression, and simultaneously regulates various cellular and molecular pathways (8).
1.1 The biological occurrence process of miRNA
miRNA regulates gene expression by targeting the 3’ untranslated region (3’ UTR) of mRNA. Its biogenesis involves tightly regulated steps, starting with transcription by RNA polymerase II or III. The resulting primary transcript (pri-miRNA) is processed through either classical or non-classical pathways (9). In the classical pathway, pri-miRNA is cleaved by a complex containing DGCR8 and the RNase III enzyme Drosha. DGCR8 recognizes specific motifs such as “GGAC” and m6A modifications in pri-miRNA (10). Drosha then trims the pri-miRNA into a precursor miRNA (pre-miRNA) with a 2–3 nucleotide 3’ overhang (11). Pre-miRNA is exported to the cytoplasm via exportin-5/RanGTP and further processed by Dicer, which removes the terminal loop to produce a miRNA duplex (12). One strand (5p or 3p) is selected as the guide strand based on 5’ end stability or the presence of a 5’ uridine, and loaded into an AGO protein (AGO1–4 in humans) (13, 14). The passenger strand is typically degraded-cleaved by AGO2 if perfectly complementary, or passively removed if mismatched (15). Non-classical pathways enhance the diversity of miRNA regulation and rely on alternative combinations of processing factors (16). These include DGCR8/Drosha-independent routes, such as Mirtrons (derived from introns) and m7G-capped pre-miRNAs that are exported via exportin-1 without Drosha cleavage (17). Dicer-independent pathways involve shRNA-derived pre-miRNAs processed by Drosha and matured in the cytoplasm by AGO2, where the 3’ strand is trimmed to facilitate 5’ strand maturation (18).
1.2 The mechanism of miRNA transcriptional action
The transcriptional regulatory mechanism of miRNA mainly includes two ways: translation inhibition and mRNA degradation. These two mechanisms can work together and are closely related to the degree of complementarity between miRNA and its target (19). In animal cells, translation inhibition is the most important and widely existing mechanism: when miRNA binds to the 3’ untranslated region (3’ UTR) of the target mRNA but does not have a complete complementary match, it mainly inhibits the translation process (20). while when the degree of complementarity is higher, it causes mRNA degradation (21). This regulatory mechanism enables cells to rapidly and flexibly adjust the protein composition without changing the DNA sequence of the gene and the quantity of the transcript, in response to developmental signals, environmental pressures, and various physiological and pathological processes (22).
In conclusion, miRNA plays a pivotal role in regulating immune responses and its dysregulation is implicated in metabolic disorders, tumors, and autoimmune diseases (23). miRNA plays a crucial role in almost all cellular activities and can exert biological functions through multiple pathways (24). By specifically binding to target genes, miRNA regulates critical processes such as inflammatory responses, macrophage polarization, and cell apoptosis. Consequently, miRNA represents a major focus in biological research and an emerging therapeutic target for numerous diseases.
2 Future treatment strategies for SjD based on miRNA
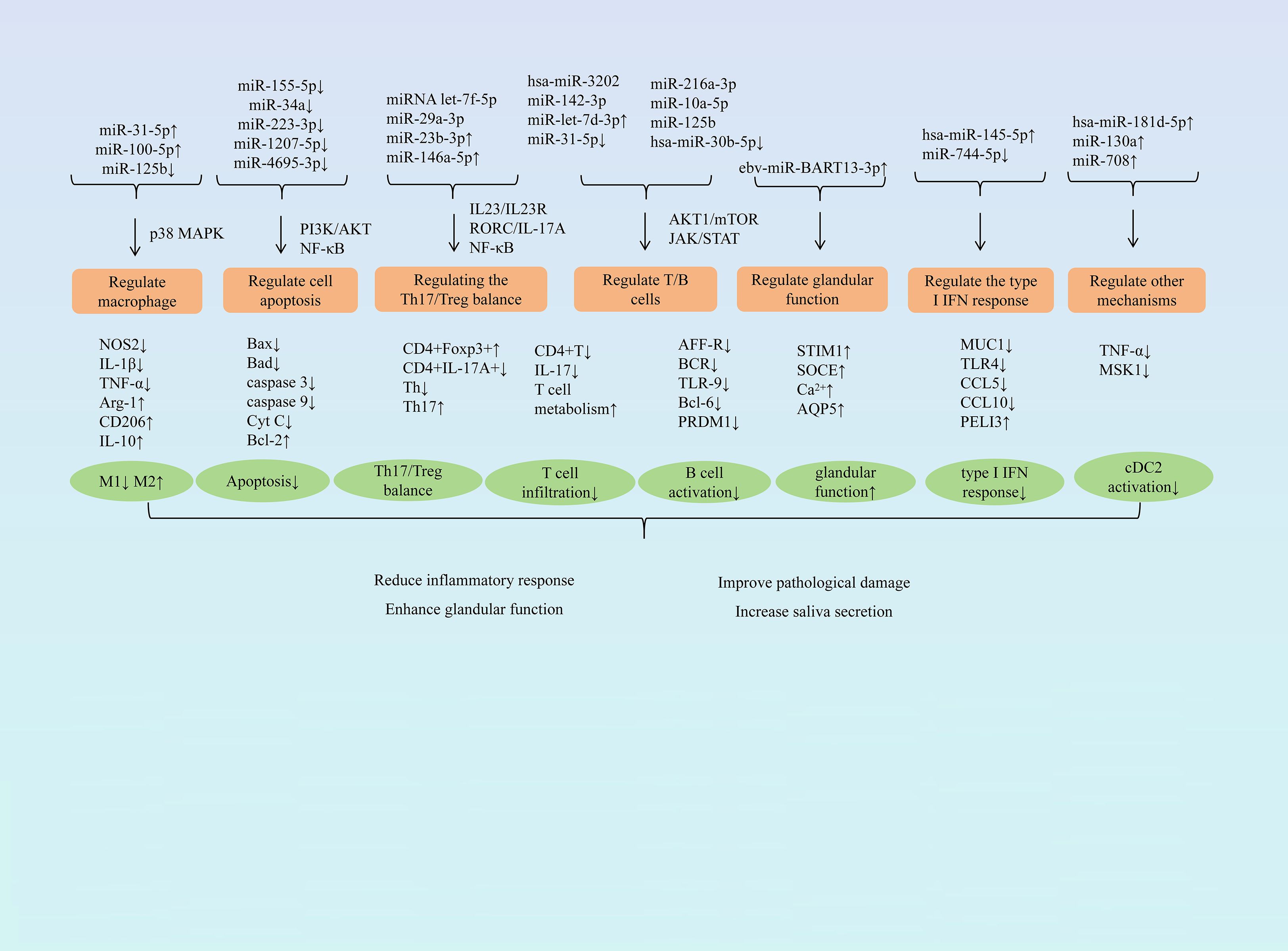
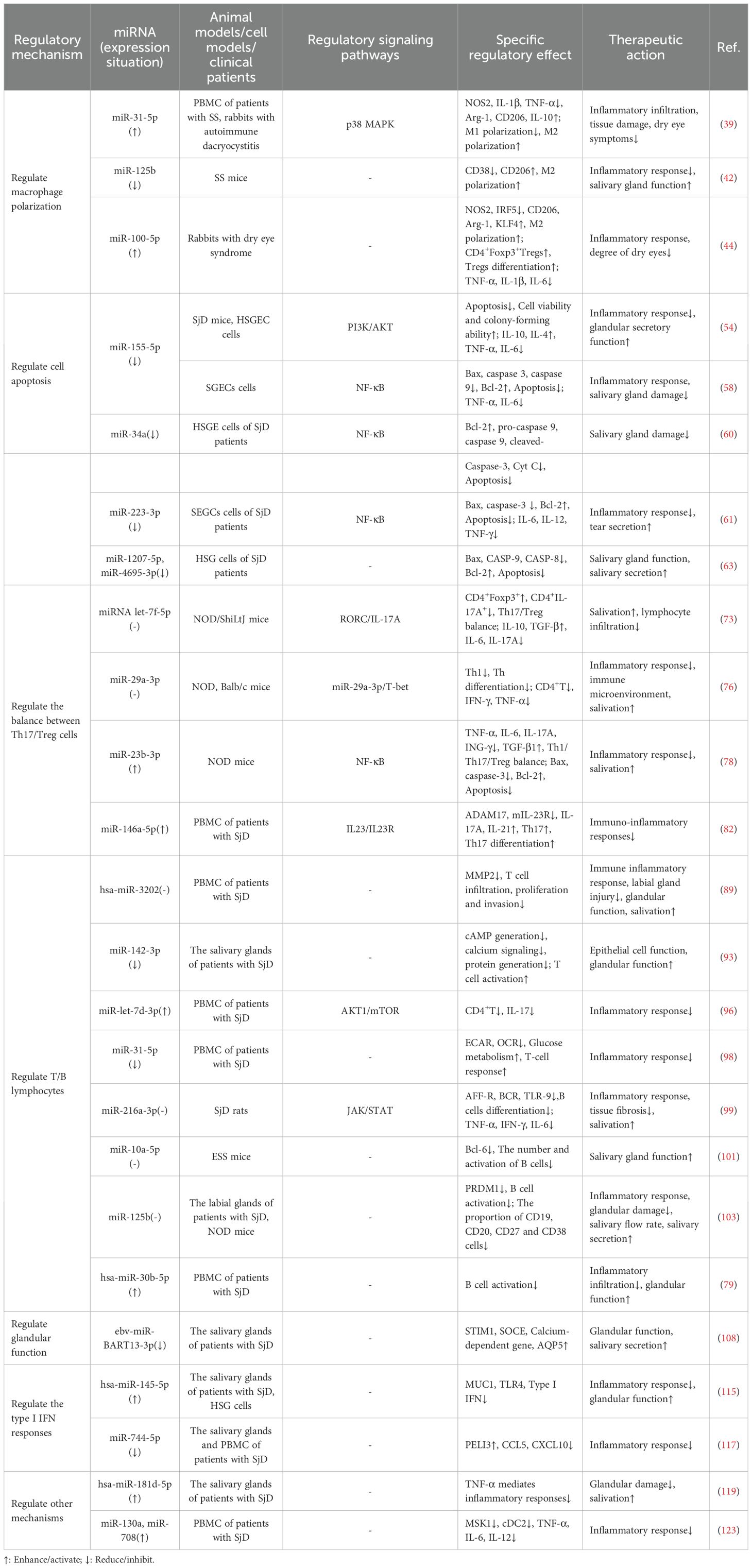
Research has demonstrated that miRNA exerts protective effects against SjD through multiple pathways, including modulating macrophage polarization, influencing cell apoptosis, balancing the Th17/Treg ratio, regulating T and B lymphocyte activity, restoring glandular function, and regulating type I IFN responses. (Figure 1 summarizes the mechanism of miRNA in treating SjD).
2.1 miRNA regulation of macrophage polarization
As key components of the innate immune system, macrophages reside in all tissues and organs, performing critical functions including immune regulation, tissue repair, and maintaining immune homeostasis (25). Dysfunctional macrophages contribute to impaired tissue repair/regeneration and excessive inflammatory factor production in various diseases (26–28). Conversely, M2 macrophages, activated by IL-4 or IL-13, secrete anti-inflammatory cytokines (e.g., IL-10, TGF-β) which generally suppress inflammation; however, sustained M2 activity can promote the progression of certain chronic inflammatory conditions (29, 30). In early SjD, M1 macrophage polarization increases, accompanied by elevated secretion of inflammatory factors (TNF-α, IL-6, IL-1β, IL-12), exacerbating submandibular gland inflammation (31). As SjD progresses, chronic inflammation transitions to irreversible salivary gland fibrosis, a process predominantly mediated by M2 macrophages (32). This imbalance, characterized by upregulated M1 and downregulated M2 polarization, is a hallmark of SjD (33). Targeting this imbalance offers therapeutic potential. Myeloid-derived growth factor (MYDGF) alleviates SjD symptoms and improves submandibular gland function by inhibiting M1 macrophage infiltration and promoting M2 polarization via suppression of the CX3CL1/CX3CR1 axis (34). Therefore, modulating macrophage polarization represents a significant strategy for ameliorating SjD.
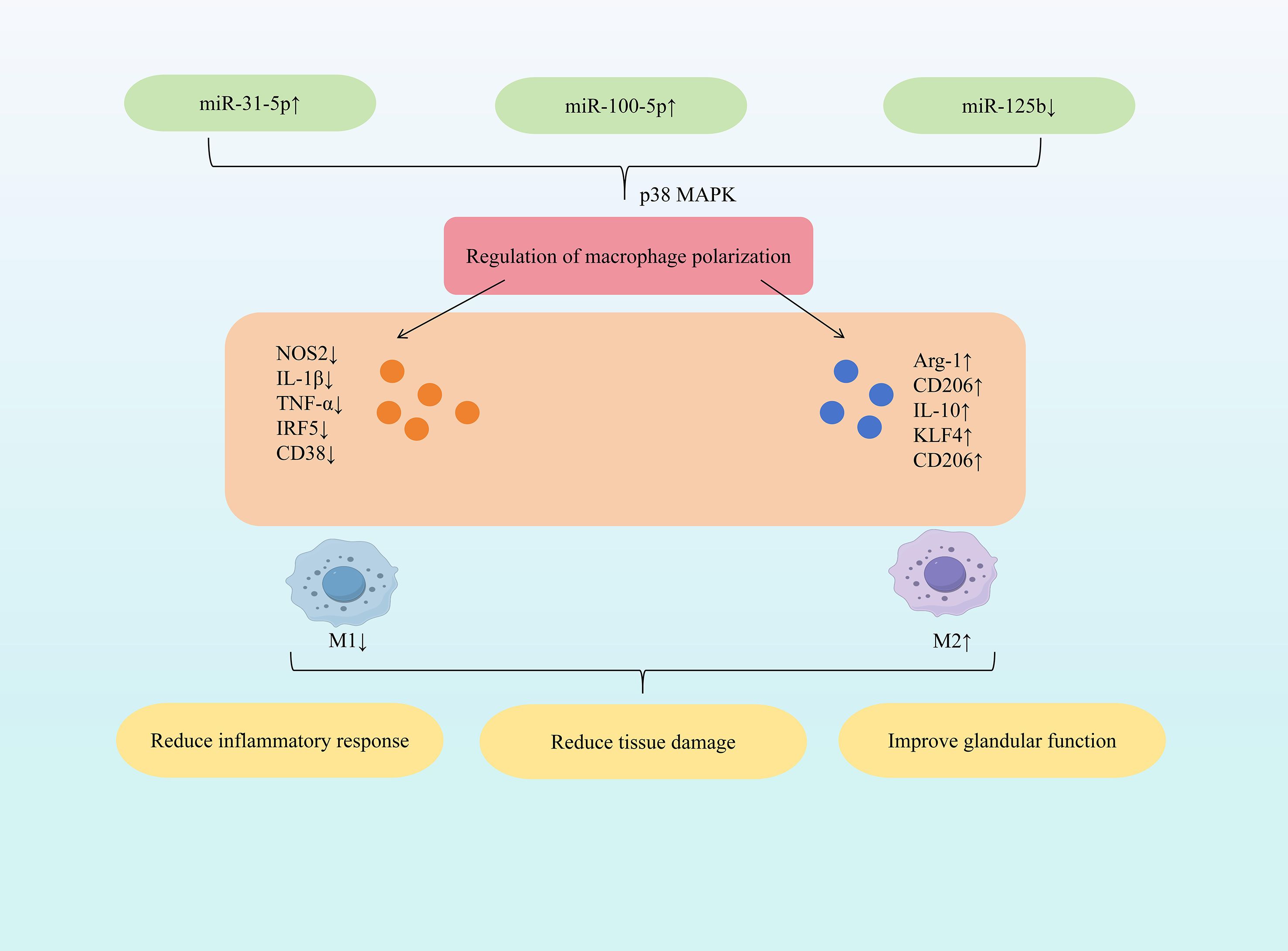
Research indicates that specific miRNAs (e.g., increased miR-31-5p, miR-100-5p; decreased miR-125b) can regulate macrophage cytokine and marker expression. This promotes polarization towards the anti-inflammatory M2 phenotype, dampens inflammatory responses, and modulates Th17/Treg balance, ultimately exerting protective effects against SjD. Among all the modifications, N6-methyladenosine (m6A) is the one that has been most extensively studied and is the most commonly found internal chemical modification on mRNA and non-coding RNA (35). m6A is a key upstream regulatory factor in miRNA biogenesis, capable of modifying various components in the miRNA generation pathway. It regulates RNA transcription, translation and stability, thereby widely influencing the level and function of miRNA (36). Dysregulation of m6A regulatory factors is implicated in the pathogenesis of Sjögren’s syndrome (SS) (37, 38). Study demonstrated that increased expression of m6A-modified miR-31-5p directly targets the P2X7 receptor, downregulating p38 MAPK expression and inhibiting the p38 MAPK signaling pathway. This cascade reduces NOS2, IL-1β, and TNF-α expression while increasing Arg-1, CD206, and IL-10 expression. Consequently, M1 macrophage activation is suppressed, M2 macrophage activation is promoted, restoring the M1/M2 balance. This shift alleviates inflammatory cell infiltration and tissue damage, thereby reducing dry eye symptoms (39). Mesenchymal stem cells (MSCs), pluripotent stem cells isolated from bone marrow and other tissues, primarily promote regeneration and modulate immune responses via paracrine mechanisms (40). Extracellular vesicles (EVs) derived from early-passage induced MSCs (iMSCs) effectively inhibited salivary gland inflammation onset in SS mouse models, with efficiency comparable to EVs from young iMSCs and bone marrow MSCs (41). Further research demonstrated that inhibiting miR-125b expression in senescence-associated iMSC-EVs reduced CD38 expression and increased CD206 expression, lowering the CD38/CD206 ratio and promoting M2 macrophage polarization. This intervention also reduced the expression of IL-17a and IL-21, inhibited the differentiation of Th17 cells, and prevented the occurrence of salivary gland inflammation (42). Mesenchymal stem cell-derived small extracellular vesicles (MSC-sEVs) exhibit potent immunomodulatory properties and have therapeutic potential for various autoimmune diseases (43). Specifically, miR-100-5p within human umbilical cord MSC-sEVs (hUC-MSC-sEVs) exerts significant anti-SS effects. Elevating miR-100-5p expression reduced NOS2 and IRF5 expression while increasing CD206, Arg-1, and KLF4 expression, promoting M2 macrophage polarization. Furthermore, it increased the proportion of CD4+Foxp3+Tregs and promoted Treg differentiation. These changes decreased TNF-α, IL-1β, and IL-6 expression, mitigating inflammation. Consequently, this approach alleviated dry eye disease severity and ameliorated inflammation in both the tear film and conjunctiva (44). (Figure 2 illustrates miRNA-mediated macrophage polarization mechanisms in SjD therapy).
2.2 miRNA modulation of cell apoptosis
Apoptosis, an autonomous and orderly programmed cell death process, is essential for organismal development and tissue homeostasis (45). It is primarily regulated through endogenous and exogenous pathways. The endogenous pathway is chiefly controlled by Bcl-2 family proteins, with Bax and Bcl-2 playing pivotal roles as key components (46). The caspase family of highly conserved cysteine proteases also significantly contributes to apoptosis (47). Exogenous pathways are typically activated by death receptors or Toll-like receptors (TLRs), leading to receptor-interacting protein kinase 1 formation, caspase cascade initiation, and subsequent apoptosis (48). When immune function is impaired, apoptosis protects tissues from damage; dysregulation of this process may contribute to SjD pathogenesis (15). Apoptosis of exocrine gland cells represents a key early pathological change in SjD, promoting innate immune system activation and lymphocyte-mediated autoimmune responses (49). Specific apoptotic factors further participate in submandibular gland dysfunction in SjD (50). Exogenous and endogenous apoptotic pathways can be involved in the entire process of the occurrence and development of SjD (51, 52). Consequently, modulating apoptosis may alleviate immune-inflammatory responses and delay SjD advancement.
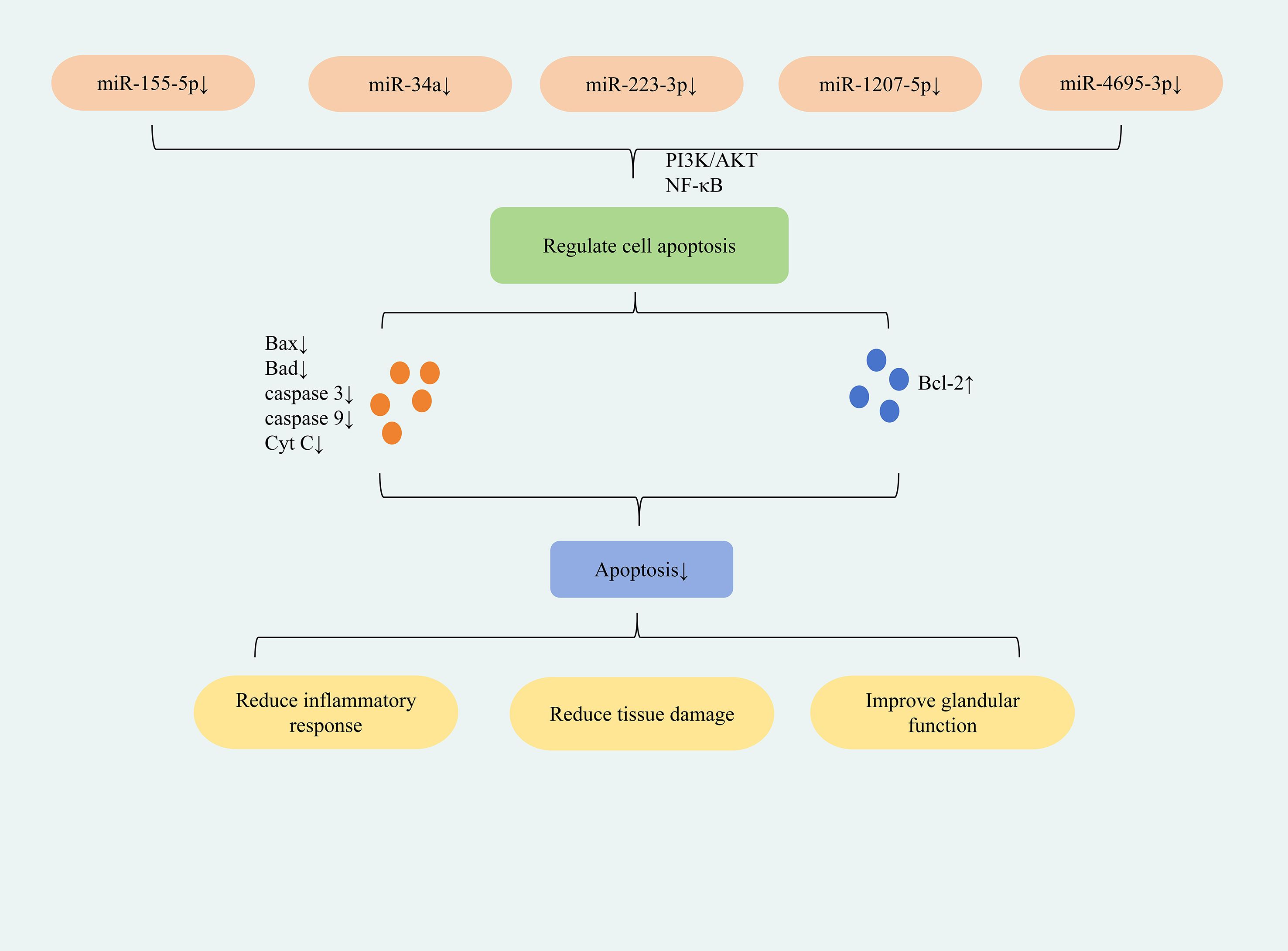
The study found that down-regulation of miR-155-5p expression can inhibit apoptosis in HSGEC and SGECs cells; down-regulation of miR-34a expression can inhibit apoptosis in HSGE cells; down-regulation of miR-223-3p expression can inhibit apoptosis in SEGCs cells; down-regulation of miR-1207-5p and miR-4695-3p expression can inhibit apoptosis in HSG cells. This reduces inflammation, restores glandular secretion, and ultimately ameliorates SjD symptoms. The differences in the roles of specific miRNAs in different tissues or cells mainly result from mechanisms such as the cell type specificity of target gene expression, the diversity of signaling pathway networks, and the different binding affinities between miRNAs and their targets. Located on human chromosome 21, miR-155 is a well-characterized miRNA that regulates immune cell development, differentiation, and function (e.g., in B cells, T cells, and dendritic cells), playing critical roles in immune-inflammatory responses (53). Downregulation of miR-155-5p was found to upregulate PIK3R1 expression, lower the p-PI3K/PI3K and p-AKT/AKT ratios, and thereby inhibit the PI3K/AKT signaling pathway. This suppressed apoptosis while promoting proliferation, viability, and colony formation in SjD submandibular gland epithelial cells (HSGECs). Concomitantly, it elevated anti-inflammatory cytokines (IL-10, IL-4and reduced pro-inflammatory cytokines (TNF-α, IL-6), mitigating inflammation. Ultimately, this restored glandular secretory function and ameliorated symptoms in SjD mice (54). miR-155-5p exhibits multifaceted functions, including regulation of tumorigenesis, immunomodulation, and oxidative stress (55, 56). Its immunoregulatory role further implicates it in multiple immune-related disorders, including SS (57). ARRB2 functions as a downstream target gene of miR-155-5p. The study demonstrated that downregulation of miR-155-5p expression directly targets ARRB2, leading to reduced expression of NF-κB p65 and a decreased phospho-IκBα-to-IκBα ratio. This consequently suppresses the NF-κB signaling pathway. This inhibition decreased the expression of pro-apoptotic proteins Bax, caspase-3, and caspase-9 while concomitantly increasing anti-apoptotic Bcl-2 expression, ultimately suppressing apoptosis in SGEC cells. Furthermore, NF-κB pathway suppression lowered TNF-α and IL-6 expression, mitigating the inflammatory response and reducing salivary gland damage (58). miR-34a is implicated in the development of various inflammatory diseases and is upregulated in the tears of patients with SS (59). In patients with severe SjD, NF-κB p65 expression was significantly elevated, whereas IκBα expression was reduced, collectively indicating activation of the NF-κB signaling pathway. Concurrently, key pro-apoptotic factors associated with the mitochondrial pathway—including pro-caspase-9, caspase-9, cleaved-caspase-3, and Cyt C—were markedly upregulated. This upregulation signifies mitochondrial damage and activation of apoptotic responses within the salivary glands. Downregulation of miR-34a expression effectively suppressed NF-κB pathway activation, upregulated Bcl-2 expression, and downregulated pro-apoptotic factors including Bad, pro-caspase-9, caspase-9, cleaved-caspase-3, and cytochrome c. Collectively, these changes inhibited the mitochondrial apoptosis pathway and alleviated salivary gland damage in NOD mice (60). ITPR3 is a direct downstream target of miR-223-3p and is negatively regulated by it. In samples from SS patients and in IFNγ-induced submandibular gland epithelial cells (SGECs), miR-223-3p expression was upregulated, whereas ITPR3 expression was downregulated.The study demonstrated that inhibiting miR-223-3p expression reduced Bax and caspase-3 levels while elevating Bcl-2 expression via the NF-κB pathway, thereby suppressing apoptosis in SGECs. This inhibition also decreased the expression of pro-inflammatory cytokines IL-6, IL-12, and TNF-α, thus mitigating inflammatory responses. Furthermore, this intervention enhanced SGEC viability under IFNγ stimulation and ultimately alleviated ocular dryness in SS patients (61). Through miRNA analysis, two miRNAs targeting TRIM21 were identified, namely miR-1207-5p and miR-4695-3p (62). These two miRNAs mainly exerted their effects through the apoptotic pathway and were also specific anti-apoptotic miRNAs. Further research demonstrated that downregulating miR-1207-5p and miR-4695-3p expression could reduce Bax, CASP-9, and CASP-8 expression and increased Bcl-2 expression. This suppressed apoptosis in HSG cells, improved salivary gland function, and enhanced saliva secretion in SS patients (63). (Figure 3 depicts miRNA-regulated apoptotic mechanism for SjD treatment).
2.3 miRNA maintenance of Th17/Treg balance
Helper T cell 17 (Th17) and regulatory T cells (Treg) play key roles in the pathogenesis of various autoimmune diseases (64). Transforming growth factor-β (TGF-β) is a pivotal regulator of the interaction and balance between Th17 and Treg cells. TGF-β induces the expression of the lineage-defining transcription factors retinoic acid receptor-related orphan receptor γt (RORγt) and forkhead box P3 (Foxp3), primarily by activating signal transducer and activator of transcription 3 (STAT3) (65). Th17 cells can promote inflammatory responses by secreting pro-inflammatory factors such as IL-17A, IL-17F, and IL-21 (66). Treg cells are a type of negatively regulatory cells that can alleviate inflammatory responses by releasing anti-inflammatory factors such as IL-10 (67). Th17/Treg immune imbalance contributes to glandular damage in SjD. Platelet-derived peptides suppress Th17 cell activation, promote Treg differentiation, restore Th17/Treg balance, and thereby ameliorate SS-related dry eye syndrome and autoimmune inflammation (68). In SS model mice, elevated Th17 cells and IL-17 levels were observed in exocrine glands, accompanied by significant reductions in Treg cells and TGF-β (69). Targeted downregulation of IL-6, TNF-α, and IL-17 reduced the Th17/Treg ratio, expanded Treg cell populations, enhanced Treg functionality, and thereby restored immune balance. This rebalancing significantly attenuated salivary gland inflammation (70).Accordingly, restoring and maintaining the Th17/Treg immune balance is a key therapeutic measure for SjD.
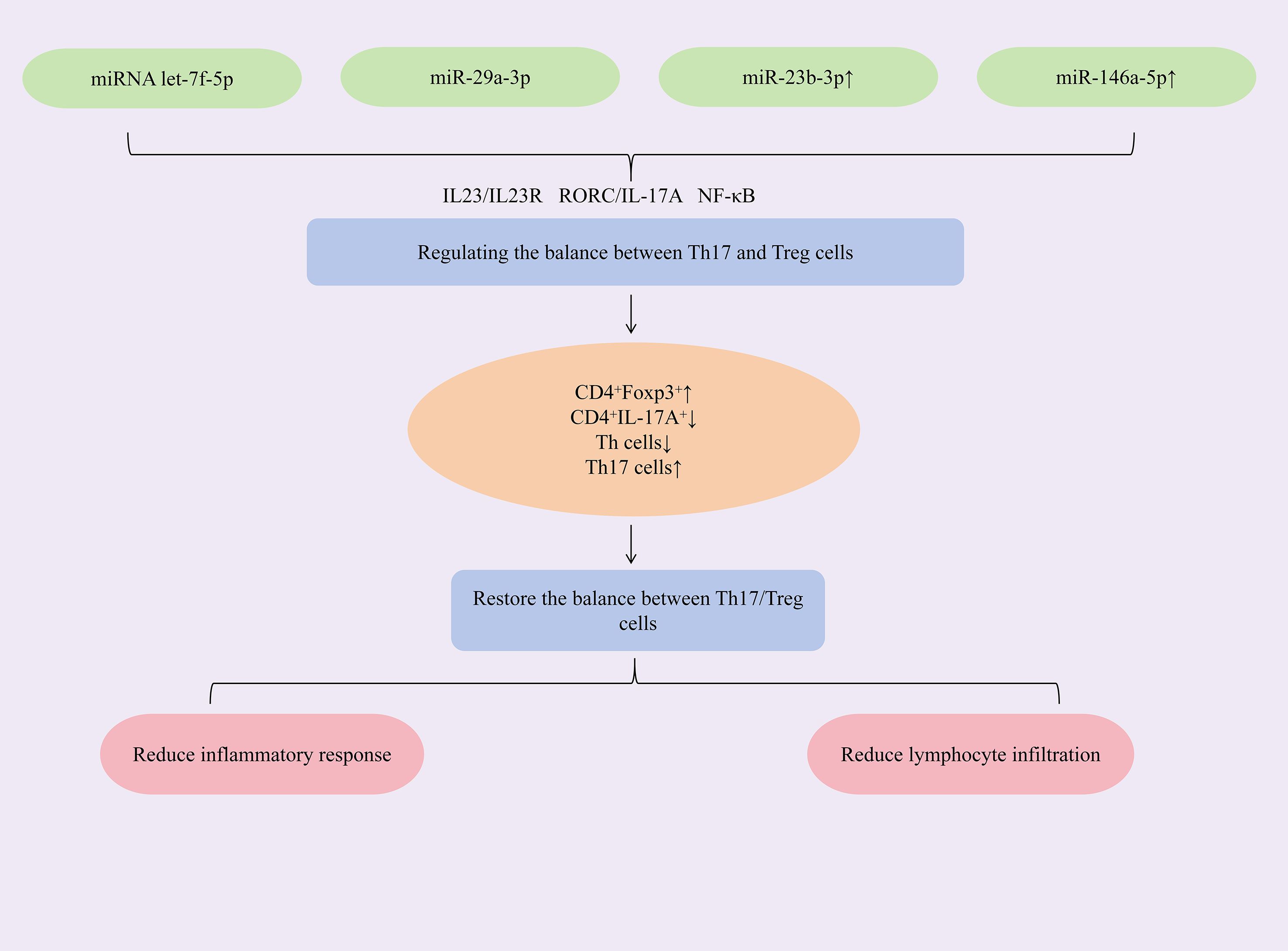
Researches have found that increasing the expression of miRNAs such as let-7f-5p, miR-29a-3p, miR-23b-3p, and miR-146a-5p could restore the Th17/Treg immune balance, alleviate immune-inflammatory responses, and act synergistically to inhibit cell apoptosis and reduce CD4+ T lymphocyte infiltration, thereby exerting therapeutic effects against SjD. Mesenchymal stem cells (MSCs) and their extracellular vesicles (EVs) exhibit potent immunomodulatory properties and hold significant therapeutic potential for autoimmune diseases (71, 72). To augment the efficacy of labial gland MSCs (LGMSCs) and their EVs (LGMSC-EVs), researchers loaded exogenous miRNA let-7f-5p into LGMSC-EVs. The study revealed that miRNA let-7f-5p encapsulated in LGMSC-EVs could suppress the RORγt/IL-17A signaling axis. This increased the proportion of CD4+Foxp3+Treg cells while reduced the proportion of CD4+IL-17A+Th17 cells, thereby lowering the Th17/Treg ratio and restoring Th17/Treg immune balance. Additionally, it elevated IL-10 and TGF-β expression, reduced IL-6 and IL-17A expression, mitigated inflammation, increased salivary flow rate, and alleviated lymphocytic infiltration in submandibular glands of NOD/ShiLtJ mice (73). Exosomes, natural nanoparticles derived from the endosomal system, demonstrate therapeutic potential in Sjögren’s syndrome. Specifically, human deciduous tooth stem cell-derived exosomes (SHAp-exos) inhibit inflammation-induced epithelial barrier disruption and modulate apoptotic responses through suppression of p-ERK1/2 activation. This dual action consequently enhances salivary secretion and ameliorates SS symptoms (74, 75). The study demonstrated that SHED-exo nanoparticles modulate the miR-29a-3p/T-bet axis, reducing Th1 cell polarization and inhibiting T helper cell differentiation. This attenuated Th1/Th2/Th17-mediated immune-inflammatory responses and restored the glandular immune microenvironment. Critically, treatment decreased CD4+ T-cell infiltration in submandibular glands of NOD mice, suppressed pro-inflammatory cytokines (IFN-γ, TNF-α), alleviated inflammation, and enhanced saliva secretion (76). Dysregulated miRNA expression is closely associated with Sjögren’s syndrome pathogenesis. Specifically, miR-23b-3p expression is significantly reduced in the NOD mouse model of SS, and this miRNA demonstrates broad protective effects against multiple autoimmune disorders (77). The study demonstrated that upregulating miR-23b-3p expression targets SOX6, downregulating NF-κB p65 expression and reducing the phospho-IκBα/IκBα ratio, thereby inhibiting the NF-κB signaling pathway. This cascade suppressed pro-inflammatory cytokines (TNF-α, IL-6, IL-17A, IFN-γ) while elevating TGF-β1 expression, rebalancing the Th1/Th17/Treg immune equilibrium. Concurrently, it reduced pro-apoptotic Bax and cleaved caspase-3 levels, increased anti-apoptotic Bcl-2 expression, and attenuated submandibular gland epithelial cell apoptosis—ultimately mitigating glandular inflammation and restoring salivary flow in NOD mice (78). miR-146a-5p is significantly upregulated in peripheral blood mononuclear cells (PBMCs) of primary Sjögren’s syndrome (SjD) patients (79, 80), with meta-analyses supporting its utility as a diagnostic biomarker (81). Mechanistically, upregulation of miR-146a-5p reduces ADAM17 protein levels, thereby decreasing membrane-bound IL-23R (mIL-23R) shedding and activating the IL-23/IL-23R signaling pathway. This cascade elevates IL-17A and IL-21 production, expands the Th17 cell population, enhances Th17 differentiation, and ultimately mitigates immune inflammation in SjD (82). (Figure 4 demonstrates miRNA modulation of Th17/Treg balance in SjD therapy).
2.4 miRNA regulation of T and B lymphocytes
T and B lymphocytes—key effectors of the adaptive immune system—play central roles in autoimmune pathogenesis. Specifically, T lymphocytes orchestrate both pro-inflammatory and immunoregulatory responses, with T-cell-mediated autoimmunity recognized as a critical driver of SjD pathology (83). Patients with SjD exhibit significant infiltration of CD4+ and CD8+ T lymphocytes in salivary and lacrimal glands. Additionally, CD8+ T lymphocytes show abnormal activation and proliferation both in peripheral circulation and around acinar epithelial cells (84). Follicular helper T cells (Tfh) promote B lymphocyte responses within ectopic germinal centers (GC) by secreting IL-21, thereby facilitating B cell activation and differentiation. This process disrupts the formation and function of GC-like structures in the salivary glands of SjD patients (85). In patients with SjD, peripheral B lymphocytes undergo significant proliferation, while substantial B lymphocyte infiltration occurs in salivary gland tissue. This aberrant B cell activity drives excessive autoantibody secretion, contributing to autoimmune pathology (86). Marginal zone B cells (MZB) contribute to the pathogenesis of SjD. In BAFF transgenic mice, reduced MZB cell numbers alleviate SjD manifestations: preserved salivary function, absence of autoantibodies, and normalized histology in salivary and lacrimal glands (87). B-cell activating factor (BAFF) promotes the formation of ectopic GC-like structures and shows elevated serum expression in SjD patients. This elevation positively correlates with anti-Ro/SSA and anti-La/SSB antibody titer (88). Therefore, both T lymphocytes and B lymphocytes critically contribute to the pathogenesis and progression of SjD, directly impacting its treatment and prognosis.
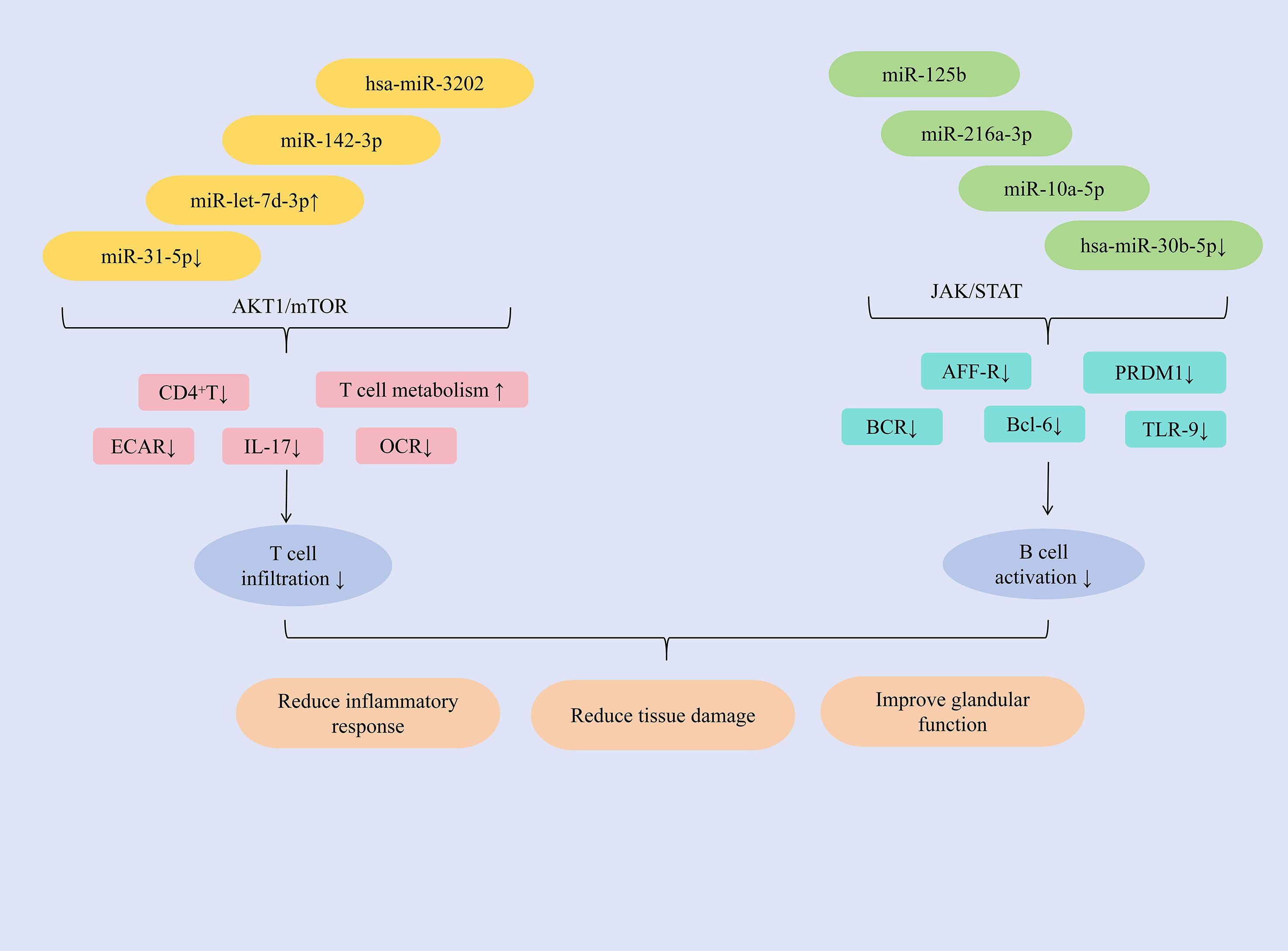
Studies have shown that hsa-miR-3202 can inhibit the proliferation and invasion of Jurkat cells, miR-142-3p promotes T cell activation, upregulation of miR-let-7d-3p expression can reduce the proportion of CD4+ T cells, and downregulation of miR-31-5p expression can promote glycolysis in CD4+ T cells, inducing an autoimmune T cell response, ultimately exerting an anti-SjD effect. MMP2 is highly expressed in PBMCs and salivary gland tissues of patients with SjD. The study found that hsa-miR-3202 can reduce the expression of MMP2 in Jurkat cells, inhibit T lymphocyte infiltration, improve immune inflammatory responses; and inhibit the proliferation and invasion of Jurkat cells, alleviate salivary gland tissue damage, improve gland function, and increase saliva secretion (89). T-cell exosome-derived miR-142-3p functions as a pathogenic driver of SS immunopathology. This miRNA exhibits predominant expression within immune compartments and critically regulates T-cell functionality (90, 91). Key predictive targets of miR-142-3p include adenylate cyclase 9 (AC9), sarcoplasmic reticulum Ca²+-ATPase 2b (SERCA2b), and ryanodine receptor 2 (RyR2)–all essential regulators of salivary gland secretion (92). miR-142-3p impairs salivary gland function through multiple mechanisms: restricting cAMP production, altering calcium signaling, and reducing acinar protein secretion. Concurrently, it promotes T-cell activation and directly disrupts epithelial cell function, collectively exacerbating glandular dysfunction in SS patients. Consequently, targeted reduction of miR-142-3p represents a promising therapeutic strategy for SS (93). The abnormal expression of IL-17 can run through the entire process of the occurrence and development of SjD and is regarded as an important target for anti-SjD treatment (94). The AKT1/mTOR signaling pathway regulates IL-17 function in a variety of autoimmune diseases (95). miR-let-7d-3p is regarded as the miRNA with the most significant inhibition in expression in patients with SjD (79). Research has found that in SjD patients, the expression of miR-let-7d-3p is negatively correlated with the expression of IL-17. Furthermore, upregulating miR-let-7d-3p expression could modulate the AKT1/mTOR signaling pathway, regulate the proportion of CD4+ T cells, reduce IL-17 expression, and alleviate inflammation (96). Previous study has shown that the decreased level of miR-31-5p in CD3+T cells of SS patients was closely related to the decreased level of miR-31-5p in CD3+T cells of SLE patients (97). Further research has found that by inhibiting the expression of miR-31-5p, the expression of basal extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) of CD4+T cells could be reduced, and the OCR/ECAR ratio could be regulated, the glycolytic process could be promoted, the basal level of glucose metabolism could be increased, and ultimately the autoimmune T cell response could be promoted (98).
In addition, miR-216a-3p, miR-10a-5p, miR-125b, and the downregulation of hsa-miR-30b-5p expression can inhibit B cell activation, reduce the number and activity of B cells, and thereby alleviate immune infiltration. miRNA expression dysregulation can disrupt immune tolerance and lead to the development of autoimmune diseases (57). Bioinformatic screening of miRNAs targeting STAT1 identified miR-216a-3p as a key regulator. This miRNA is significantly downregulated in SjD and exhibits inverse correlation with STAT1 expression. Functionally, miR-216a-3p downregulates JAK1, JAK2, and STAT1 expression, thereby inhibiting JAK/STAT signaling. Consequently, this reduces protein levels of B-cell activation markers (APR-R, BCR, TLR-9), suppresses B-cell activation, and mitigates immune infiltration. The pathway inhibition concurrently decreases TNF-α, IFN-γ, and IL-6 production–alleviating inflammation and tissue fibrosis. Ultimately, miR-216a-3p restoration restores salivary flow rates and ameliorates xerostomia in SjD rat models (99). Extracellular vesicles derived from myeloid-derived suppressor cells (MDSC-EVs) inhibit autoimmune disease progression by impairing T lymphocyte function (100). Further study has found that miR-10a-5p, delivered by MDSC-EVs, downregulates Bcl-6 expression. This reduces the proportion of germinal center (GC) B cells in experimental Sjögren’s syndrome (ESS) mice, suppresses B cell activation, and consequently improves salivary gland function while significantly inhibiting SS progression (101). Mesenchymal stem cells (MSCs) produce microvesicles and exosomes, which are regarded as the primary bioactive vesicles mediating their paracrine effects. These vesicles serve as effectors of cell signal transduction and intercellular communication, exhibiting significant immunomodulatory functions (102). The study revealed that miR-125b, delivered via labial gland mesenchymal stem cell-derived exosomes (LGMSC-Exos), could reduce PRDM1 (BLIMP-1) expression in B lymphocytes, and inhibit their activation. It decreased the proportion of CD19+CD20+CD27+CD38+plasma cells, ameliorated inflammatory responses, and reduced glandular damage in NOD mice (103). B-cell activating factor (BAFF) was upregulated in salivary gland B lymphocytes of patients with SjD. Hsa-miR-30a-3p was found to negatively regulate BAFF expression, likely through binding to the 3′-UTR of BAFF mRNA (104). Study has shown that inhibiting the expression of hsa-miR-30b-5p could significantly increase the expression of BAFF and promote the activation of B cells. Therefore, in summary, BAFF expression is negatively correlated with hsa-miR-30b-5p levels. Increasing hsa-miR-30b-5p expression represents a key strategy to inhibit B cell activation, reduce inflammatory infiltration, and thereby improve glandular function (79). (Figure 5 presents the regulatory mechanism of miRNAs on T/B lymphocytes in SjD treatment).
2.5 miRNA modulation of glandular function
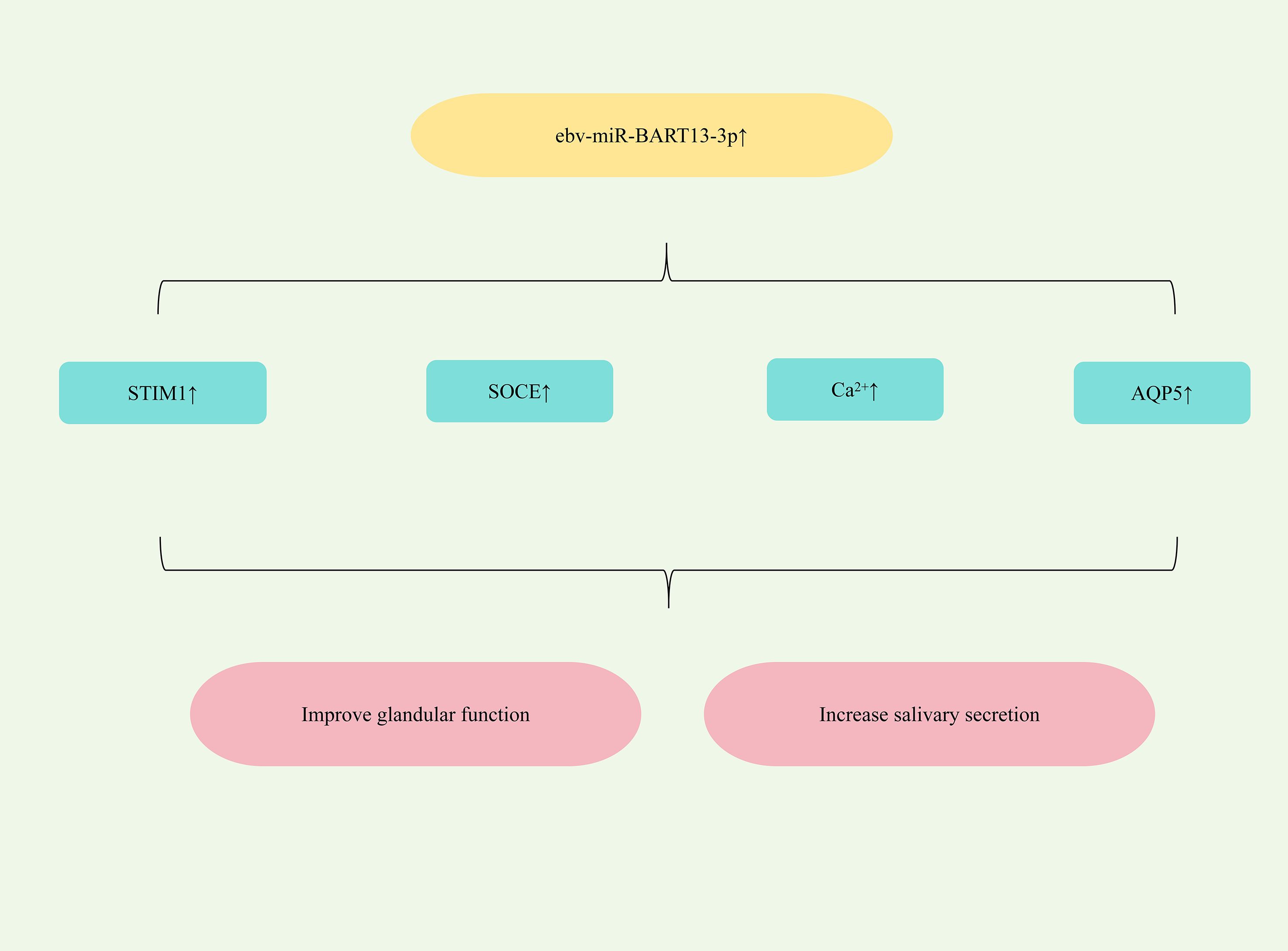
All patients with SjD experience glandular dysfunction. This dysfunction arises, in part, from abnormal acinar cell function, which can impair duct cell activation and disrupt progenitor cell homeostasis, ultimately contributing to salivary gland impairment (105). Furthermore, Epstein-Barr virus (EBV) exploits exosomes to transfer its miRNAs from infected B lymphocytes to non-B lymphocytes, promoting salivary dysfunction in SjD (106). Significantly elevated levels of the EBV-specific miRNA ebv-miR-BART13-3p are detected in the salivary glands (SG) of SjD patients (107). Study has found that ebv-miR-BART13-3p could down-regulate the expression of matrix-interacting molecule 1 (STIM1), damage calcium reservoir manipulated calcium entry (SOCE), reduce the expression of Ca2+ -dependent genes regulated by SOCE, and thereby impair SG function. It could also down-regulate the expression of AQP5, damage glandular function and reduce saliva secretion (108). Therefore, targeting the upregulation of ebv-miR-BART13-3p represents a potential therapeutic strategy for alleviating dry mouth symptoms in SS patients. (Figure 6 shows miRNA-mediated restoration of glandular function for SjD therapy).
2.6 miRNA regulation of type I interferon response
Study has shown that activation of the type I interferon (IFN) response plays a key role in the pathogenesis of SS (109). The expression of type I IFN response-related proteins in PBMCS derived from SjD patients was significantly increased (110). Crucially, the level of type I IFN response correlates with both glandular and extra-glandular manifestations of SS, and its downregulation can prevent SjD onset and progression (111). Mechanistically, the type I IFN response stimulates excessive B-cell activating factor (BAFF) production in the peripheral blood and salivary glands of SjD patients, thereby promoting B lymphocyte maturation and differentiation (112). Furthermore, this response modulates regulatory T cell (Treg) function, leading to a significantly increased proportion of Treg cells in the serum of type I IFN-positive SjD patients (113). Therefore, type I IFN response is a potential target for the treatment of SjD.
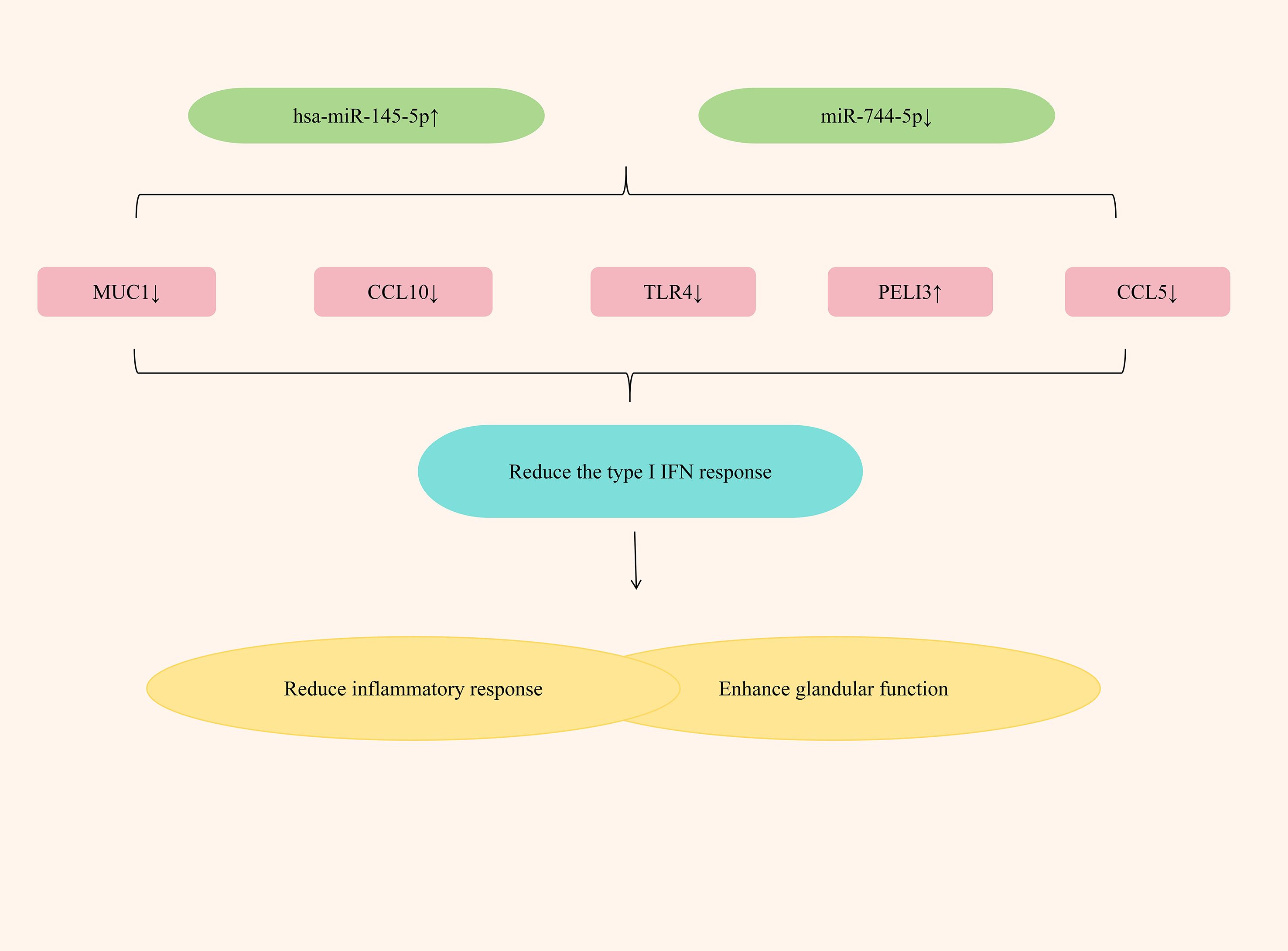
Studies have found that by up-regulating the expression of hsa-miR-145-5p and down-regulating the expression of miR-744-5p, the type I IFN response can be significantly alleviated, exerting an anti-SjD effect. Hsa-miR-145-5p, an anti-inflammatory miRNA, is significantly downregulated in the salivary glands (SG) of SjD patients. This suppression is mediated by type I interferon (IFN), which reduces its expression level (114). Mucin 1 (MUC1) and Toll-like receptor 4 (TLR4) are two related targets of hsa-miR-145-5p. The research has found that in patients with Sjögren’s disease (SjD), by inhibiting the expression of hsa-miR-145-5p, the expressions of MUC1 and TLR4 can be increased, thereby causing salivary gland inflammation and gland dysfunction; if the expression of hsa-miR-145-5p is upregulated, the expressions of MUC1, TLR4 and type I interferons can be reduced, significantly alleviating the inflammatory response, enhancing gland function, and improving dryness symptoms (115). PELI3, a member of the Pellino E3 ubiquitin ligase family, is a recognized negative regulator of inflammation that functions through its interaction with Toll-like receptors (TLRs) (116). In patients with SjD, the expression of miR-744-5p significantly increased, while the expression of PELI3 significantly decreased (48). Further research has found that by down-regulating the expression of miR-744-5p, the expression of PELI3 could be increased, the expression of IFN-dependent chemokines Rantes (CCL5) and CXCL10 could be reduced, the inflammatory response could be significantly alleviated, and the dry eye symptoms of SS patients could be improved (117). (Figure 7 elucidates miRNA regulation of type I interferon responses in SjD treatment).
2.7 miRNA involvement in additional mechanisms
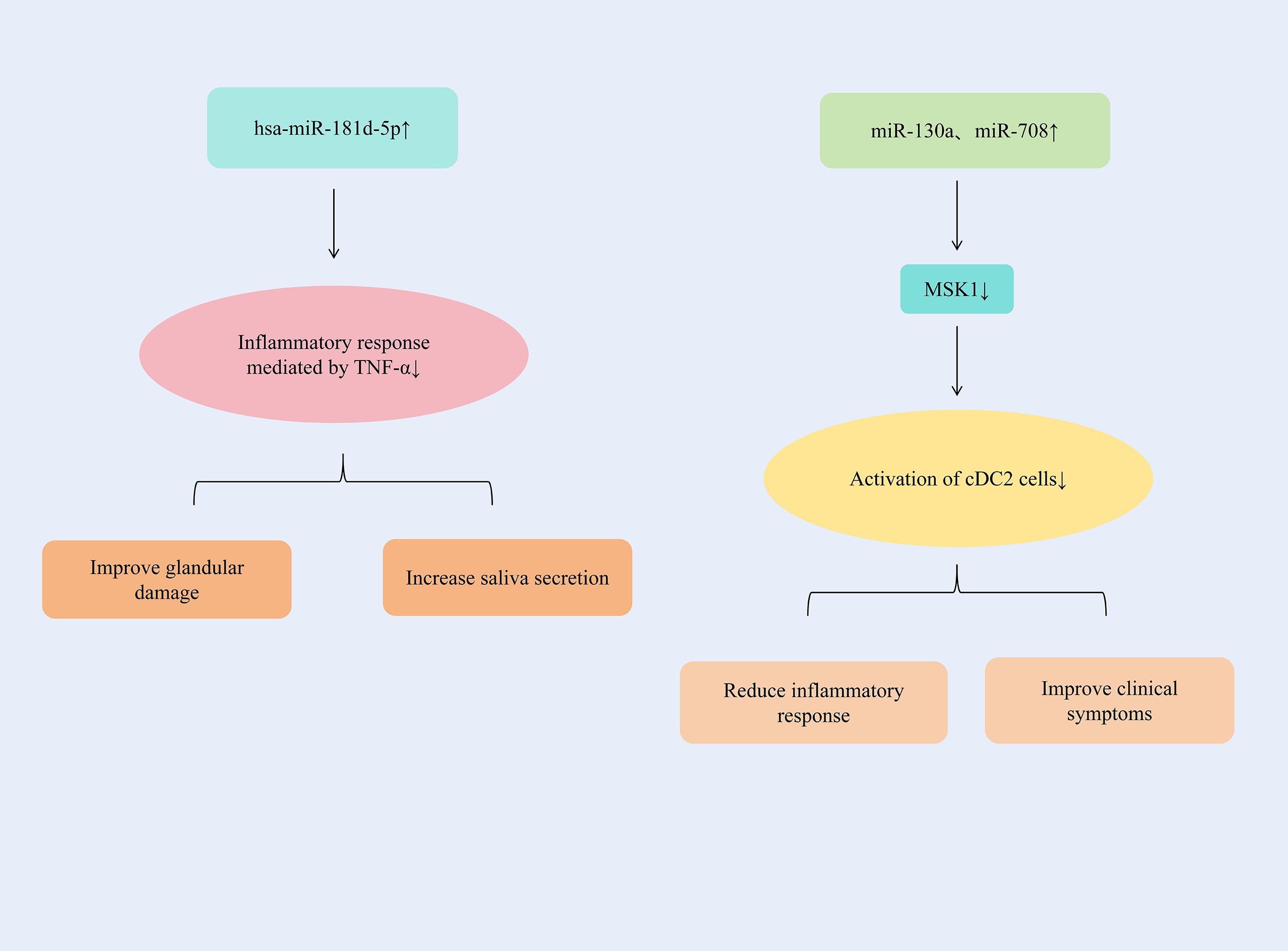
By up-regulating the expression of hsa-miR-181d-5p, the inflammatory response mediated by TNF-α could be significantly alleviated. The up-regulation of miR-130a and miR-708 expression could inhibit the activation of cDC2 cells and alleviate the inflammatory response of SjD.
TNF-α contributes to autoimmune disease progression by facilitating pro-inflammatory cytokine expression, inflammatory cell recruitment, and organ damage. Specifically in the labial salivary glands (LSG) of Sjögren’s syndrome (SS) patients, TNF-α is produced by glandular epithelial cells, infiltrating CD4+ T lymphocytes, and monocytes (118). In the miRNA-seq analysis, it was determined that the level of this miRNA had decreased, and one of its main targets was TNFα, which was highly expressed in SS. TNF-α in patients with SjD could increase significantly and was negatively correlated with the presence of hsa-miR-181d-5p. By increasing the expression of hsa-miR-181d-5p, the inflammatory response mediated by TNF-α could be significantly alleviated, glandular damage could be improved, and salivary secretion could be increased (119). Conventional dendritic cells (CDCS) are effective antigen-presenting cells and play a significant role in the initiation and control of immune responses. CDCS could be classified into two subgroups with different phenotypes and functions, namely CD141 (cDC1) and CD1c (cDC2). The main target cells of cDC2, CD4+T cells, are considered to play a crucial role in the immunopathology of SjD (120). There was an increased number of DCS in the salivary glands of patients with SjD (121); Therefore, it is suspected that cDC2 plays an important role in driving salivary gland inflammation (122). The expressions of miR-130a and miR-708 continued to decrease in cDC2 of patients with SjD, which might be related to cell activation. MSK1 is an important mediator upstream of NF-κB, controlling cDC2 to produce pro-inflammatory cytokines. By increasing the expressions of miR-130a and miR-708 in cDC2, the expression of MSK1 could be significantly reduced and the activation of cDC2 cells could be inhibited. And it reduced the expressions of TNF-α, IL-6 and IL-12, alleviated the inflammatory response, and improved the symptoms of patients with SjD (123). (Figure 8 summarizes additional miRNA regulatory mechanisms in SjD therapy.) (Table 1 summarizes the therapeutic mechanisms of miRNAs in SjD).
3 Discussion
miRNAs critically regulate the pathogenesis, progression, and prognosis of primary Sjögren’s disease (SjD). As a diagnostic biomarker for pSS, miRNAs possess the characteristics of tissue specificity, high stability, and ease of detection. For instance, miR-17-5p, miR-146a-5p and miR-155-5p, etc. show highly specific expression in the serum of patients with pSS and are related to the disease activity. They are expected to become highly specific diagnostic markers. However, their clinical application still needs to address issues such as standardization, validation and cost. In pSS, miRNAs interact with lncRNAs (such as XIST) and circRNAs through the ceRNA mechanism, and target epigenetic modification enzymes (such as miR-29 inhibiting DNMTs). They jointly form a regulatory network that affects DNA methylation, NF-κB pathways, breaks immune tolerance, promotes lymphocyte infiltration and glandular damage, and drives disease progression.
Summary and analysis are as follows: 1) signaling pathways: miRNAs predominantly modulate the NF-κB, JAK/STAT, and PI3K/AKT pathways. For example, downregulation of miR-155-5p, miR-223-3p, and miR-34a, or upregulation of miR-23b-3p, inhibits NF-κB signaling; 2) mechanistic targets: key regulatory functions include macrophage polarization, apoptosis, Th17/Treg balance, T/B lymphocyte activity, glandular function, and type I interferon (IFN) responses; 3) expression dynamics: most studies report directional miRNA alterations (up-/down-regulation). Apoptosis is typically regulated through miRNA downregulation, while macrophage polarization, T/B lymphocytes, and type I IFN responses exhibit bidirectional miRNA regulation. However, some studies lack explicit directionality; 4) research models: clinical therapeutic potential is demonstrated for miRNAs like miR-223-3p and miR-125b, while other evidence derives from in vivo (NOD mice) or in vitro (e.g., SGECs) models; 5) Certain miRNAs exhibit multi-mechanistic regulatory roles in SjD pathogenesis. For instance: miR-125b modulated macrophage polarization and promotes Th17 activation; miR-100-5p regulated macrophage polarization while enhancing Treg activation; miR-23b-3p balanced Th17/Treg ratios and inhibits apoptosis. Notably, individual miRNAs could target distinct signaling pathways. For example, down-regulation of miR-155-5p can inhibit apoptosis by regulating the PI3K/AKT and NF-κB signaling pathways respectively.
However, several deficiencies persist in current research: 1) Some studies have not fully elucidated the specific signaling pathways involved in the role of miRNA. For instance, miR-125b, miR-100-5p, miR-1207-5p, etc. still lack the exploration and clarification of signaling pathways. This might be determined by the inherent multi-target characteristics of miRNAs, the complexity of the cross-interaction of signaling pathways, the difficulty in validating the targets, and the limitations of the SjD disease model. 2) The regulatory role of miRNA expression itself remains unclear. For instance, miR-29a-3p, hsa-miR-3202, miR-216a-3p, etc. lack regulatory effects. This might be related to the influence of transcription factors, epigenetics and other factors on the abnormal expression of specific miRNAs in SjD, and further research and clarification are needed. 3) The interactions of miRNA in the pathogenesis of SjD and their common and unique roles are not yet fully understood. For instance, miR-1207-5p and miR-4695-3p jointly regulate cell apoptosis, while miR-130a and miR-708 jointly regulate cDC2. However, it is unclear how they interact, cooperate or antagonize to regulate the pathological and physiological processes of SjD. Further research is needed to strengthen this understanding. 4) Various miRNAs such as miR-125b, miR-100-5p, and miR-23b-3p possess multi-target and multi-pathway regulatory properties. They can regulate macrophage polarization, cell apoptosis, and Treg differentiation, etc. However, the relative importance and causal relationship of these miRNAs in different mechanisms of SjD pathology remain unclear and require further research and clarification. 5). Therapeutic strategies based on miRNAs (such as agomiR, antagomiR, viral vectors, and EV delivery) all face challenges in terms of delivery efficiency, safety, and production. Their multi-target characteristics lead to high off-target risks. Although theoretically a miRNA can target hundreds of mRNAs, in the actual cellular environment, its regulatory effect is selective and is mainly influenced by factors such as binding affinity, target abundance, and the expression level of the miRNA itself. The mimics may excessively inhibit unintended targets, while the antagonists may abnormally activate pathways, causing severe adverse reactions, resulting in the termination of clinical trials such as MRX34 and Miravirsen.
Therefore, future efforts should focus on: 1) Advancing miRNA extraction and utilization technologies to enhance safety and maturity; 2) Expanding research on miRNA-related signaling pathways; 3) Validating and elucidating miRNA regulatory mechanisms; 4) Deepening investigations into miRNA interrelationships and their regulatory roles in SjD; 5) Conducting large-scale, rigorous clinical research aligned with clinical needs. Concurrently, clinical translation frameworks for miRNA-based SjD therapies should be strengthened to accelerate novel drug R&D. Emphasis must be placed on improving targeting precision to ensure miRNAs directly engage SjD pathological tissues and maximize therapeutic efficacy.
Author contributions
WC: Writing – original draft, Writing – review & editing. LS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. LY: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. LH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Negrini S, Emmi G, Greco M, Borro M, Sardanelli F, Murdaca G, et al. Sjögren’s syndrome: a systemic autoimmune disease. Clin Exp Med. (2022) 22:9–25. doi: 10.1007/s10238-021-00728-6
2. Longhino S, Chatzis LG, Dal Pozzolo R, Peretti S, Fulvio G, La Rocca G, et al. Sjögren’s syndrome: one year in review 2023. Clin Exp Rheumatol. (2023) 41:2343–56. doi: 10.55563/clinexprheumatol/255qsx
3. Xuan J, Ji Z, Wang B, Zeng X, Chen R, He Y, et al. Serological evidence for the association between epstein-barr virus infection and sjögren’s syndrome. Front Immunol. (2020) 11:590444. doi: 10.3389/fimmu.2020.590444
4. Tian Y, Yang H, Liu N, Li Y, and Chen J. Advances in pathogenesis of sjögren’s syndrome. J Immunol Res. (2021) 2021:5928232. doi: 10.1155/2021/5928232
5. Ramos-Casals M, Brito-Zerón P, Bombardieri S, Bootsma H, De Vita S, Dörner T, et al. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann Rheumat Dis. (2020) 79:3–18. doi: 10.1136/annrheumdis-2019-216114
6. Guo X, Dang W, Li N, Wang Y, Sun D, Nian H, et al. PPAR-α Agonist fenofibrate ameliorates sjögren syndrome-like dacryoadenitis by modulating th1/th17 and treg cell responses in NOD mice. Invest Ophthalmol Visual Sci. (2022) 63:12. doi: 10.1167/iovs.63.6.12
7. Masi L, Capobianco I, Magrì C, Marafini I, Petito V, Scaldaferri F, et al. MicroRNAs as innovative biomarkers for inflammatory bowel disease and prediction of colorectal cancer. Int J Mol Sci. (2022) 23:7991. doi: 10.3390/ijms23147991
8. Diener C, Keller A, and Meese E. Emerging concepts of miRNA therapeutics: from cells to clinic. Trends Genet: TIG. (2022) 38:613–26. doi: 10.1016/j.tig.2022.02.006
9. Ha M and Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. (2014) 15:509–24. doi: 10.1038/nrm3838
10. Alarcón CR, Lee H, Goodarzi H, Halberg N, and Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature. (2015) 519:482–5. doi: 10.1038/nature14281
11. Denli AM, Tops BBJ, Plasterk RHA, Ketting RF, and Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. (2004) 432:231–5. doi: 10.1038/nature03049
12. Zhang H, Kolb FA, Jaskiewicz L, Westhof E, and Filipowicz W. Single processing center models for human Dicer and bacterial RNase III. Cell. (2004) 118:57–68. doi: 10.1016/j.cell.2004.06.017
13. Khvorova A, Reynolds A, and Jayasena SD. Functional siRNAs and miRNAs exhibit strand bias. Cell. (2003) 115(2):209–16. doi: 10.1016/s0092-8674(03)00801-8
14. Yoda M, Kawamata T, Paroo Z, Ye X, Iwasaki S, Liu Q, et al. ATP-dependent human RISC assembly pathways. Nat Struct Mol Biol. (2010) 17:17–23. doi: 10.1038/nsmb.1733
15. Ainola M, Porola P, Takakubo Y, Przybyla B, Kouri VP, Tolvanen TA, et al. Activation of plasmacytoid dendritic cells by apoptotic particles - mechanism for the loss of immunological tolerance in Sjögren’s syndrome. Clin Exp Immunol. (2018) 191:301–10. doi: 10.1111/cei.13077
16. Babiarz JE, Ruby JG, Wang Y, Bartel DP, and Blelloch R. Mouse ES cells express endogenous shRNAs, siRNAs, and other Microprocessor-independent, Dicer-dependent small RNAs. Genes Dev. (2008) 22:2773–85. doi: 10.1101/gad.1705308
17. Cheloufi S, Dos Santos CO, Chong MMW, and Hannon GJ. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature. (2010) 465:584–9. doi: 10.1038/nature09092
18. Yang J-S, Maurin T, Robine N, Rasmussen KD, Jeffrey KL, Chandwani R, et al. Conserved vertebrate mir-451 provides a platform for Dicer-independent, Ago2-mediated microRNA biogenesis. Proc Natl Acad Sci United States America. (2010) 107:15163–8. doi: 10.1073/pnas.1006432107
19. Menon A, Abd-Aziz N, Khalid K, Poh CL, and Naidu R. miRNA: A promising therapeutic target in cancer. Int J Mol Sci. (2022) 23:11502. doi: 10.3390/ijms231911502
20. Shen Y, Cheng L, Xu M, Wang W, Wan Z, Xiong H, et al. SGLT2 inhibitor empagliflozin downregulates miRNA-34a-5p and targets GREM2 to inactivate hepatic stellate cells and ameliorate non-alcoholic fatty liver disease-associated fibrosis. Metabolism. (2023) 146:155657. doi: 10.1016/j.metabol.2023.155657
21. Truesdell SS, Mortensen RD, Seo M, Schroeder JC, Lee JH, LeTonqueze O, et al. MicroRNA-mediated mRNA translation activation in quiescent cells and oocytes involves recruitment of a nuclear microRNP. Sci Rep. (2012) 2:842. doi: 10.1038/srep00842
22. Ørom UA, Nielsen FC, and Lund AH. MicroRNA-10a binds the 5’UTR of ribosomal protein mRNAs and enhances their translation. Mol Cell. (2008) 30:460–71. doi: 10.1016/j.molcel.2008.05.001
23. Yao Q, Song Z, Wang B, and Zhang J-A. Emerging roles of microRNAs in the metabolic control of immune cells. Cancer Lett. (2018) 433:10–7. doi: 10.1016/j.canlet.2018.06.024
24. Ferragut Cardoso AP, Banerjee M, Nail AN, Lykoudi A, and States JC. miRNA dysregulation is an emerging modulator of genomic instability. Semin Cancer Biol. (2021) 76:120–31. doi: 10.1016/j.semcancer.2021.05.004
25. Kolliniati O, Ieronymaki E, Vergadi E, and Tsatsanis C. Metabolic regulation of macrophage activation. J Innate Immun. (2022) 14:51–68. doi: 10.1159/000516780
26. Wang N, Liang H, and Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. (2014) 5. doi: 10.3389/fimmu.2014.00614
27. Sun Y, Li J, Xie X, Gu F, Sui Z, Zhang K, et al. Macrophage-osteoclast associations: origin, polarization, and subgroups. Front Immunol. (2021) 12:778078. doi: 10.3389/fimmu.2021.778078
28. Wynn TA and Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. (2016) 44:450–62. doi: 10.1016/j.immuni.2016.02.015
29. Boutilier AJ and Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. (2021) 22:6995. doi: 10.3390/ijms22136995
30. van der Heide D, Weiskirchen R, and Bansal R. Therapeutic targeting of hepatic macrophages for the treatment of liver diseases. Front Immunol. (2019) 10:2852. doi: 10.3389/fimmu.2019.02852
31. Baturone R, Soto MJ, Márquez M, Macías I, de Oca MM, Medina F, et al. Health-related quality of life in patients with primary Sjögren’s syndrome: relationship with serum levels of proinflammatory cytokines. Scand J Rheumatol. (2009) 38:386–9. doi: 10.1080/03009740902973821
32. Raes G, Brys L, Dahal BK, Brandt J, Grooten J, Brombacher F, et al. Macrophage galactose-type C-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukocyte Biol. (2005) 77:321–7. doi: 10.1189/jlb.0304212
33. Chen X, Zhu L, and Wu H. The role of M1/M2 macrophage polarization in primary Sjogren’s syndrome. Arthritis Res Ther. (2024) 26:101. doi: 10.1186/s13075-024-03340-7
34. Yang Z, Liu M, Chang Z, Du C, Yang Y, Zhang C, et al. Myeloid-derived growth factor promotes M2 macrophage polarization and attenuates Sjögren’s syndrome via suppression of the CX3CL1/CX3CR1 axis. Front Immunol. (2024) 15:1465938. doi: 10.3389/fimmu.2024.1465938
35. Moon JS, Siddiqui A, and Kim GW. Regulation of viral hepatitis by N6-methyladenosine RNA methylation. Emerging Microbes Infect. (2025) 14:2544726. doi: 10.1080/22221751.2025.2544726
36. Yi Q, Liao Y, Sun W, Li J, Yang D, Shang H, et al. m6A modification of non−coding RNA: Mechanisms, functions and potential values in human diseases (Review). Int J Mol Med. (2025) 56:164. doi: 10.3892/ijmm.2025.5605
37. Ma S, Chen C, Ji X, Liu J, Zhou Q, Wang G, et al. The interplay between m6A RNA methylation and noncoding RNA in cancer. J Hematol Oncol. (2019) 12:121. doi: 10.1186/s13045-019-0805-7
38. Huang Y, Xue Q, Chang J, Wang Y, Cheng C, Xu S, et al. M6A methylation modification in autoimmune diseases, a promising treatment strategy based on epigenetics. Arthritis Res Ther. (2023) 25:189. doi: 10.1186/s13075-023-03149-w
39. Zhao L, Li X, Gao M, Liu L, Ma B, Liu X, et al. M6A modified miR-31-5p suppresses M1 macrophage polarization and autoimmune dry eye by targeting P2RX7. Adv Sci (Weinheim Baden-Wurttemberg Germany). (2025) 12:e2415341. doi: 10.1002/advs.202415341
40. Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V, et al. Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cells. (2019) 8:467. doi: 10.3390/cells8050467
41. Kim H, Zhao Q, Barreda H, Kaur G, Hai B, Choi JM, et al. Identification of molecules responsible for therapeutic effects of extracellular vesicles produced from iPSC-derived MSCs on sjo¨gren’s syndrome. Aging Dis. (2021) 12:1409–22. doi: 10.14336/AD.2021.0621
42. Zhao Q, Bae EH, Zhang Y, et al. Inhibitory Effects of Extracellular Vesicles from iPS-Cell-Derived Mesenchymal Stem Cells on the Onset of Sialadenitis in Sjögren’s Syndrome Are Mediated by Immunomodulatory Splenocytes and Improved by Inhibiting miR-125b. Int J Mol Sci. (2023) 24:5258. doi: 10.3390/ijms24065258
43. Cai JX, Wu JY, Wang JM, Li YJ, Hu XB, Luo SF, et al. Extracellular vesicles derived from different sources of mesenchymal stem cells: therapeutic effects and translational potential. Cell Biosci. (2020) 10:69. doi: 10.1186/s13578-020-00427-x
44. Li N, Gao Z, Zhao L, Du B, Ma B, Nian H, et al. MSC-Derived Small Extracellular Vesicles Attenuate Autoimmune Dacryoadenitis by Promoting M2 Macrophage Polarization and Inducing Tregs via miR-100-5p. Front Immunol. (2022) 13:888949. doi: 10.3389/fimmu.2022.888949
45. Cheng X and Ferrell JE. Apoptosis propagates through the cytoplasm as trigger waves. Sci (New York NY). (2018) 361:607–12. doi: 10.1126/science.aah4065
46. Vandenabeele P, Bultynck G, and Savvides SN. Pore-forming proteins as drivers of membrane permeabilization in cell death pathways. Nat Rev Mol Cell Biol. (2023) 24:312–33. doi: 10.1038/s41580-022-00564-w
47. Man SM and Kanneganti TD. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol. (2016) 16:7–21. doi: 10.1038/nri.2015.7
48. Bertheloot D, Latz E, and Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. (2021) 18:1106–21. doi: 10.1038/s41423-020-00630-3
49. Voulgarelis M and Tzioufas AG. Pathogenetic mechanisms in the initiation and perpetuation of Sjögren’s syndrome. Nat Rev Rheumatol. (2010) 6:529–37. doi: 10.1038/nrrheum.2010.118
50. Huang L, Liu Q, Zhou T, Zhang J, Tian Q, Zhang Q, et al. Deficiency of β-arrestin2 alleviates apoptosis through GRP78-ATF6-CHOP signaling pathway in primary Sjögren’s syndrome. Int Immunopharmacol. (2021) 101:108281. doi: 10.1016/j.intimp.2021.108281
51. Kang Y, Sun Y, Zhang Y, and Wang Z. Cytochrome c is important in apoptosis of labial glands in primary Sjogren’s syndrome. Mol Med Rep. (2018) 17:1993–7. doi: 10.3892/mmr.2017.8083
52. Treviño-Talavera BA, Palafox-Sánchez CA, Muñoz-Valle JF, Orozco-Barocio G, Navarro-Hernández RE, Vázquez-Del Mercado M, et al. FAS -670A>G promoter polymorphism is associated with soluble Fas levels in primary Sjögren’s syndrome. Genet Mol Res: GMR. (2014) 13:4831–8. doi: 10.4238/2014.July.2.12
53. Mahesh G and Biswas R. MicroRNA-155: A master regulator of inflammation. J Interferon Cytokine Res. (2019) 39:321–30. doi: 10.1089/jir.2018.0155
54. Zhang Y, Wan L, Fang H, Li F, Wang L, Li K, et al. Inhibiting miR-155-5p promotes proliferation of human submandibular gland epithelial cells in primary Sjogren’s syndrome by negatively regulating the PI3K/AKT signaling pathway via PIK3R1. Nan Fang Yi Ke Da Xue Xue Bao = J South Med Univ. (2025) 45:65–71. doi: 10.12122/j.issn.1673-4254.2025.01.09
55. Wang F, Shan S, Huo Y, Xie Z, Fang Y, Qi Z, et al. MiR-155-5p inhibits PDK1 and promotes autophagy via the mTOR pathway in cervical cancer. Int J Biochem Cell Biol. (2018) 99:91–9. doi: 10.1016/j.biocel.2018.04.005
56. Jiang K, Hu J, Luo G, Song D, Zhang P, Zhu J, et al. miR-155-5p promotes oxalate- and calcium-induced kidney oxidative stress injury by suppressing MGP expression. Oxid Med Cell Longevity. (2020) 2020:5863617. doi: 10.1155/2020/5863617
57. Reale M, D’Angelo C, Costantini E, Laus M, Moretti A, Croce A, et al. MicroRNA in sjögren’s syndrome: their potential roles in pathogenesis and diagnosis. J Immunol Res. (2018) 2018:7510174. doi: 10.1155/2018/7510174
58. Zhang J, Zhu L, Shi H, and Zheng H. Protective effects of miR-155-5p silencing on IFN-γ-induced apoptosis and inflammation in salivary gland epithelial cells. Exp Ther Med. (2021) 22:882. doi: 10.3892/etm.2021.10314
59. Kim YJ, Yeon Y, Lee WJ, Shin YU, Cho H, Sung Y-K, et al. Comparison of microRNA expression in tears of normal subjects and sjögren syndrome patients. Invest Ophthalmol Visual Sci. (2019) 60:4889–95. doi: 10.1167/iovs.19-27062
60. He F, Yu J, Ma S, Zhao W, Wang Q, He H, et al. MiR-34a promotes mitochondrial pathway of apoptosis in human salivary gland epithelial cells by activating NF-κB signaling. Arch Biochem Biophys. (2024) 758:110063. doi: 10.1016/j.abb.2024.110063
61. Qi X, Wang RH, Jin L, Tian Y, Jin HT, Han YX, et al. MiR-223-3p aggravates ocular inflammation in sjögren’s syndrome. Endocrine Metab Immune Disord Drug Targets. (2023) 23(8):1087–95. doi: 10.2174/1871530323666230103123831
62. Yang Y, Peng L, Ma W, Yi F, Zhang Z, Chen H, et al. Autoantigen-targeting microRNAs in Sjögren’s syndrome. Clin Rheumatol. (2016) 35:911–7. doi: 10.1007/s10067-016-3203-3
63. Yang Y, Hou Y, Li J, Zhang F, and Du Q. Characterization of antiapoptotic microRNAs in primary Sjögren’s syndrome. Cell Biochem Funct. (2020) 38:1111–8. doi: 10.1002/cbf.3569
64. Xin X, Wang Q, Qing J, Song W, Gui Y, Li X, et al. Th17 cells in primary Sjögren’s syndrome negatively correlate with increased Roseburia and Coprococcus. Front Immunol. (2022) 13:974648. doi: 10.3389/fimmu.2022.974648
65. Zhan Q, Zhang J, Lin Y, Chen W, Fan X, Zhang D, et al. Pathogenesis and treatment of Sjogren’s syndrome: Review and update. Front Immunol. (2023) 14:1127417. doi: 10.3389/fimmu.2023.1127417
66. Yang J, Su J, Chai K, and Liu H. The role of Th9 CD4+ T cells and IL-9 during primary Sjogren’s syndrome. J Clin Lab Anal. (2022) 36:e24646. doi: 10.1002/jcla.24646
67. Blinova VG, Vasilyev VI, Rodionova EB, and Zhdanov DD. The role of regulatory T cells in the onset and progression of primary sjögren’s syndrome. Cells. (2023) 12:1359. doi: 10.3390/cells12101359
68. Contreras Ruiz L, Mir FA, Turpie B, and Masli S. Thrombospondin-derived peptide attenuates Sjögren’s syndrome-associated ocular surface inflammation in mice. Clin Exp Immunol. (2017) 188:86–95. doi: 10.1111/cei.12919
69. Yao G, Qi J, Liang J, Shi B, Chen W, Li W, et al. Mesenchymal stem cell transplantation alleviates experimental Sjögren’s syndrome through IFN-β/IL-27 signaling axis. Theranostics. (2019) 9:8253–65. doi: 10.7150/thno.37351
70. Kim J-W, Kim S-M, Park J-S, Hwang S-H, Choi J, Jung K-A, et al. Metformin improves salivary gland inflammation and hypofunction in murine Sjögren’s syndrome. Arthritis Res Ther. (2019) 21:136. doi: 10.1186/s13075-019-1904-0
71. Zaripova LN, Midgley A, Christmas SE, Beresford MW, Pain C, Baildam EM, et al. Mesenchymal stem cells in the pathogenesis and therapy of autoimmune and autoinflammatory diseases. Int J Mol Sci. (2023) 24:16040. doi: 10.3390/ijms242216040
72. Pharoun J, Berro J, Sobh J, Abou-Younes M-M, Nasr L, Majed A, et al. Mesenchymal stem cells biological and biotechnological advances: Implications for clinical applications. Eur J Pharmacol. (2024) 977:176719. doi: 10.1016/j.ejphar.2024.176719
73. Xie Y, Chai M, Xing Y, Zhou P, Wei P, Hua H, et al. miRNA let-7f-5p-encapsulated labial gland MSC-derived EVs ameliorate experimental Sjögren’s syndrome by suppressing Th17 cells via targeting RORC/IL-17A signaling axis. J Nanobiotechnol. (2025) 23:228. doi: 10.1186/s12951-025-03308-y
74. Du Z-H, Ding C, Zhang Q, Zhang Y, Ge X-Y, Li S-L, et al. Stem cells from exfoliated deciduous teeth alleviate hyposalivation caused by Sjögren syndrome. Oral Dis. (2019) 25:1530–44. doi: 10.1111/odi.13113
75. Chu W-X, Ding C, Du Z-H, Wei P, Wang Y-X, Ge X-J, et al. SHED-exos promote saliva secretion by suppressing p-ERK1/2-mediated apoptosis in glandular cells. Oral Dis. (2024) 30:3066–80. doi: 10.1111/odi.14776
76. Du Z-H, Chu W-X, Peng X, Wu L-L, Liu Y, Yu G-Y, et al. SHED-Derived Exosomes Ameliorate Sjögren’s Syndrome-Induced Hyposalivation by Suppressing Th1 Cell Response via the miR-29a-3p/T-bet Axis. ACS Appl Mater Interfaces. (2025) 17:5752–61. doi: 10.1021/acsami.4c16595
77. Chen J-Q, Papp G, Szodoray P, and Zeher M. The role of microRNAs in the pathogenesis of autoimmune diseases. Autoimmun Rev. (2016) 15:1171–80. doi: 10.1016/j.autrev.2016.09.003
78. Cai Y, Zhang Y, Wang S, and Changyong E. MiR-23b-3p alleviates Sjögren’s syndrome by targeting SOX6 and inhibiting the NF-κB signaling. Mol Immunol. (2024) 172:68–75. doi: 10.1016/j.molimm.2024.06.002
79. Wang-Renault S-F, Boudaoud S, Nocturne G, Roche E, Sigrist N, Daviaud C, et al. Deregulation of microRNA expression in purified T and B lymphocytes from patients with primary Sjögren’s syndrome. Ann Rheumat Dis. (2018) 77:133–40. doi: 10.1136/annrheumdis-2017-211417
80. Chen J-Q, Papp G, Póliska S, Szabó K, Tarr T, Bálint BL, et al. MicroRNA expression profiles identify disease-specific alterations in systemic lupus erythematosus and primary Sjögren’s syndrome. PloS One. (2017) 12:e0174585. doi: 10.1371/journal.pone.0174585
81. Sun HY, Lv AK, and Yao H. Relationship of miRNA-146a to primary Sjögren’s syndrome and to systemic lupus erythematosus: a meta-analysis. Rheumatol Int. (2017) 37:1311–6. doi: 10.1007/s00296-017-3756-8
82. Wang X, Xin S, Wang Y, Ju D, Wu Q, Qiu Y, et al. MicroRNA-146a-5p enhances T helper 17 cell differentiation via decreasing a disintegrin and metalloprotease 17 level in primary sjögren’s syndrome. Bioengineered. (2021) 12:310–24. doi: 10.1080/21655979.2020.1870321
83. Yao Y, Ma J-F, Chang C, Xu T, Gao C-Y, Gershwin ME, et al. Immunobiology of T cells in sjögren’s syndrome. Clin Rev Allergy Immunol. (2021) 60:111–31. doi: 10.1007/s12016-020-08793-7
84. van der Heijden EHM, Blokland SLM, Hillen MR, Lopes APP, and van Vliet-Moret FM. Leflunomide-hydroxychloroquine combination therapy in patients with primary Sjögren’s syndrome (RepurpSS-I): a placebo-controlled, double-blinded, randomised clinical trial. Lancet Rheumatol. (2020) 2:e260–9. doi: 10.1016/S2665-9913(20)30057-6
85. Verstappen GM, Kroese FGM, and Bootsma H. T cells in primary Sjögren’s syndrome: targets for early intervention. Rheumatol (Oxford England). (2021) 60:3088–98. doi: 10.1093/rheumatology/kez004
86. Baer AN, Gottenberg J-E, St Clair EW, Sumida T, Takeuchi T, Seror R, et al. Efficacy and safety of abatacept in active primary Sjögren’s syndrome: results of a phase III, randomised, placebo-controlled trial. Ann Rheumat Dis. (2021) 80:339–48. doi: 10.1136/annrheumdis-2020-218599
87. Fogel O, Rivière E, Seror R, Nocturne G, Boudaoud S, Ly B, et al. Role of the IL-12/IL-35 balance in patients with Sjögren syndrome. J Allergy Clin Immunol. (2018) 142:258–268.e5. doi: 10.1016/j.jaci.2017.07.041
88. Zhou H, Yang J, Tian J, and Wang S. CD8+ T lymphocytes: crucial players in sjögren’s syndrome. Front Immunol. (2020) 11:602823. doi: 10.3389/fimmu.2020.602823
89. Gao Q, Ye Z, Liu T, Jiang J, Jiang Z, Cao D, et al. Hsa-miR-3202 attenuates Jurkat cell infiltration via MMP2 in primary Sjögren’s syndrome. J Oral Pathol Med. (2022) 51:818–28. doi: 10.1111/jop.13355
90. Lu X, Li X, He Q, Gao J, Gao Y, Liu B, et al. miR-142-3p regulates the formation and differentiation of hematopoietic stem cells in vertebrates. Cell Res. (2013) 23:1356–68. doi: 10.1038/cr.2013.145
91. Sun Y, Oravecz-Wilson K, Mathewson N, Wang Y, McEachin R, Liu C, et al. Mature T cell responses are controlled by microRNA-142. J Clin Invest. (2015) 125:2825–40. doi: 10.1172/JCI78753
92. Ahuja M, Jha A, Maléth J, Park S, and Muallem S. cAMP and Ca2+ signaling in secretory epithelia: crosstalk and synergism. Cell Calcium. (2014) 55:385–93. doi: 10.1016/j.ceca.2014.01.006
93. Cortes-Troncoso J, Jang S-I, Perez P, Hidalgo J, Ikeuchi T, Greenwell-Wild T, et al. T cell exosome-derived miR-142-3p impairs glandular cell function in Sjögren’s syndrome. JCI Insight. (2020) 5:e133497, 133497. doi: 10.1172/jci.insight.133497
94. Zhang L-W, Zhou P-R, Wei P, Cong X, Wu L-L, Hua H, et al. Expression of interleukin-17 in primary Sjögren’s syndrome and the correlation with disease severity: A systematic review and meta-analysis. Scand J Immunol. (2018) 87:e12649. doi: 10.1111/sji.12649
95. Koga T, Ichinose K, Kawakami A, and Tsokos GC. The role of IL-17 in systemic lupus erythematosus and its potential as a therapeutic target. Expert Rev Clin Immunol. (2019) 15:629–37. doi: 10.1080/1744666X.2019.1593141
96. Wang-Renault S-F, Boudaoud S, Nocturne G, Roche E, Sigrist N, Daviaud C, et al. MiR-let-7d-3p regulates IL-17 expression through targeting AKT1/mTOR signaling in CD4+ T cells. In Vitro Cell Dev Biol Anim. (2020) 56:67–74. doi: 10.1007/s11626-019-00409-5
97. Wang J, Wang X, Wang L, Sun C, Xie C, Li Z, et al. Identification of microRNA-31 as a novel regulator contributing to impaired interleukin-2 production in T cells from patients with systemic lupus erythematosus. Arthritis Rheumat. (2012) 64:3715–25. doi: 10.1002/art.34596
98. Fan W, Liang D, Tang Y, Qu B, Cui H, Luo X, et al. miR-31 regulates energy metabolism and is suppressed in T cells from patients with Sjögren’s syndrome. Eur J Immunol. (2019) 49:313–22. doi: 10.1002/eji.201747416
99. Johansson A, Nyberg WA, Sjöstrand M, Moruzzi N, Bergman P, Khademi M, et al. miR-216a-3p alleviates primary Sjögren’s syndrome by regulating the STAT1/JAK signaling pathway. Biochem Biophys Res Commun. (2025) 758:151647. doi: 10.1016/j.bbrc.2025.151647
100. Reale M, D’Angelo C, Costantini E, Laus M, Moretti A, Croce A, et al. Crucial role of granulocytic myeloid-derived suppressor cells in the regulation of central nervous system autoimmune disease. J Immunol (Baltimore Md: 1950). (2012) 188:1136–46. doi: 10.4049/jimmunol.1101816
101. Liu Y, Guo L, Cao J, Ning X, Zheng X, Li F, et al. Extracellular vesicle-encapsulated miR-10a-5p derived from MDSCs restrains germinal center B cells in experimental Sjögren’s syndrome. Immunol Res. (2023) 71:760–70. doi: 10.1007/s12026-023-09390-4
102. Ioannou M, Alissafi T, Lazaridis I, Deraos G, Matsoukas J, Gravanis A, et al. MSC-exosome: A novel cell-free therapy for cutaneous regeneration. Cytotherapy. (2018) 20:291–301. doi: 10.1016/j.jcyt.2017.11.002
103. Zhou H, Zhu Q, Mao Z, Li M, Zhang Y, Yang J, et al. Labial gland mesenchymal stem cell derived exosomes-mediated miRNA-125b attenuates experimental sjogren’s syndrome by targeting PRDM1 and suppressing plasma cells. Front Immunol. (2022) 13:871096. doi: 10.3389/fimmu.2022.871096
104. Wu P, Zhang B, Shi H, Qian H, and Xu W. MiR-30a-3p negatively regulates BAFF synthesis in systemic sclerosis and rheumatoid arthritis fibroblasts. PloS One. (2014) 9:e111266. doi: 10.1371/journal.pone.0111266
105. Xing Y, Li B, He J, and Hua H. Expression of BAFF and BAFF receptors in primary Sjögren’s syndrome patients with ectopic germinal center-like structures. Clin Exp Med. (2020) 20:871096. doi: 10.3389/fimmu.2022.871096
106. Alsaleh G, François A, Philippe L, Gong Y-Z, Bahram S, Cetin S, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci United States America. (2010) 107:6328–33. doi: 10.1073/pnas.0914843107
107. Wang-Renault S-F, Boudaoud S, Nocturne G, Roche E, Sigrist N, Daviaud C, et al. MicroRNA expression profiles as biomarkers of minor salivary gland inflammation and dysfunction in Sjögren’s syndrome. Arthritis Rheumat. (2011) 63:535–44. doi: 10.1002/art.30131
108. Carrillo-Ballesteros FJ, Palafox-Sánchez CA, Franco-Topete RA, and Muñoz-Valle JF. Targeting the ca(2+) sensor STIM1 by exosomal transfer of ebv-miR-BART13-3p is associated with sjögren’s syndrome. EBioMedicine. (2016) 10:216–26. doi: 10.1016/j.ebiom.2016.06.041
109. Thorlacius GE, Wahren-Herlenius M, and Rönnblom L. An update on the role of type I interferons in systemic lupus erythematosus and Sjögren’s syndrome. Curr Opin Rheumatol. (2018) 30:471–81. doi: 10.1097/BOR.0000000000000524
110. Alevizos I, Alexander S, Turner RJ, and Illei GG. LncRNA and mRNA expression profile of peripheral blood mononuclear cells in primary Sjögren’s syndrome patients. Sci Rep. (2020) 10:19629. doi: 10.1038/s41598-020-76701-2
111. Gallo A, Jang S-I, Ong HL, Perez P, Tandon M, Ambudkar I, et al. Type I interferon receptor deficiency prevents murine Sjogren’s syndrome. J Dental Res. (2013) 92:444–9. doi: 10.1177/0022034513483315
112. Thorlacius GE, Wahren-Herlenius M, and Rönnblom L. Type I interferon signature in Sjögren’s syndrome: pathophysiological and clinical implications. Clin Exp Rheumatol. (2019) 37 Suppl 118:185–91. doi: 10.1097/BOR.0000000000000524
113. Peng Y, Luo X, Chen Y, Peng L, Deng C, Fei Y, et al. Association of increased treg cell levels with elevated indoleamine 2,3-dioxygenase activity and an imbalanced kynurenine pathway in interferon-positive primary sjögren’s syndrome. Arthritis Rheumatol (Hoboken NJ). (2016) 68:1688–99. doi: 10.1002/art.39629
114. Szczerba BM, Rybakowska PD, Dey P, Payerhin KM, Peck AB, Bagavant H, et al. Type I IFN inhibits innate IL-10 production in macrophages through histone deacetylase 11 by downregulating microRNA-145. J Immunol (Baltimore Md: 1950). (2013) 191:3896–904. doi: 10.4049/jimmunol.1203450
115. Marketos N, Cinoku I, Rapti A, and Mavragani CP. Type I interferon dependent hsa-miR-145-5p downregulation modulates MUC1 and TLR4 overexpression in salivary glands from sjögren’s syndrome patients. Front Immunol. (2021) 12:685837. doi: 10.3389/fimmu.2021.685837
116. Moynagh PN. The Pellino family: IRAK E3 ligases with emerging roles in innate immune signalling. Trends Immunol. (2009) 30:33–42. doi: 10.1016/j.it.2008.10.001
117. Lin L, Hou J, Ma F, Wang P, Liu X, Li N, et al. miR-744-5p contributes to ocular inflammation in patients with primary Sjogrens Syndrome. Sci Rep. (2020) 10:7484. doi: 10.1038/s41598-020-64422-5
118. Jara D, Carvajal P, Castro I, Barrera M-J, Aguilera S, González S, et al. Tumor necrosis factor-alpha and receptors for it in labial salivary glands in Sjögren’s syndrome. Clin Exp Rheumatol. (2001) 19:131–7. doi: 10.3389/fimmu.2021.685837
119. Moynagh PN. Small RNA expression profiling reveals hsa-miR-181d-5p downregulation associated with TNF-α Overexpression in sjögren’s syndrome patients. Front Immunol. (2022) 13:870094. doi: 10.3389/fimmu.2022.870094
120. Kiripolsky J, McCabe LG, and Kramer JM. Innate immunity in Sjögren’s syndrome. Clin Immunol (Orlando Fla). (2017) 182:4–13. doi: 10.1016/j.clim.2017.04.003
121. Koski H, Janin A, Humphreys-Beher MG, Sorsa T, Malmström M, Konttinen YT, et al. Decrease of blood dendritic cells and increase of tissue-infiltrating dendritic cells are involved in the induction of Sjögren’s syndrome but not in the maintenance. Clin Exp Immunol. (2010) 159:315–26. doi: 10.1111/j.1365-2249.2009.04071.x
122. Castro I, Carvajal P, Jara D, Aguilera S, Heathcote B, Barrera M-J, et al. Dendritic cells, T-cells and epithelial cells: a crucial interplay in immunopathology of primary Sjögren’s syndrome. Expert Rev Clin Immunol. (2014) 10:521–31. doi: 10.1586/1744666X.2014.878650
Keywords: microRNA, primary Sjögren’s disease, regulatory networks, therapeutic perspectives, macrophage polarization, apoptosis, Th17/Treg balance
Citation: Chengzhi W, Songwei L, Yifan L, Mengmeng D and Huan L (2025) The role of microRNAs in primary Sjögren’s disease: deciphering regulatory networks and assessing current therapeutic perspectives. Front. Immunol. 16:1669382. doi: 10.3389/fimmu.2025.1669382
Received: 19 July 2025; Accepted: 29 September 2025;
Published: 16 October 2025.
Edited by:
Ioannis Parodis, Karolinska Institutet (KI), SwedenReviewed by:
Sheng-Yan Lin, Huazhong University of Science and Technology, ChinaPatricia Carvajal, University of Chile, Chile
Copyright © 2025 Chengzhi, Songwei, Yifan, Mengmeng and Huan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Songwei, cnlhbnp6ZHhAeWVhaC5uZXQ=
 Wang Chengzhi
Wang Chengzhi Li Songwei1,2*
Li Songwei1,2* Liu Yifan
Liu Yifan Du Mengmeng
Du Mengmeng