- Department of Laboratory Medicine, Shangyu People’s Hospital of Shaoxing, Shaoxing University, Shaoxing, China
Human papillomavirus (HPV) is a highly prevalent virus that primarily infects human epithelial cells, resulting in a significant health burden by causing conditions such as anogenital warts, cervical cancers, and head and neck squamous cell carcinoma. Although vaccination has been implemented for cancer prevention, a thorough understanding of anti-HPV immunity remains of critical importance for HPV-related disease management. The cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway forms a key signaling cascade within the innate immune system, which is activated by cytosolic DNA and functions through the production of type I interferon (IFN-I). Accumulating evidence indicates a correlation between the cGAS-STING pathway and HPV infection, as well as HPV-related malignancies, suggesting its potential as a promising therapeutic target. This review discusses the role of the cGAS-STING signaling pathway in HPV infection and HPV-related cancers, as well as potential therapeutic strategies that target this pathway.
1 Introduction
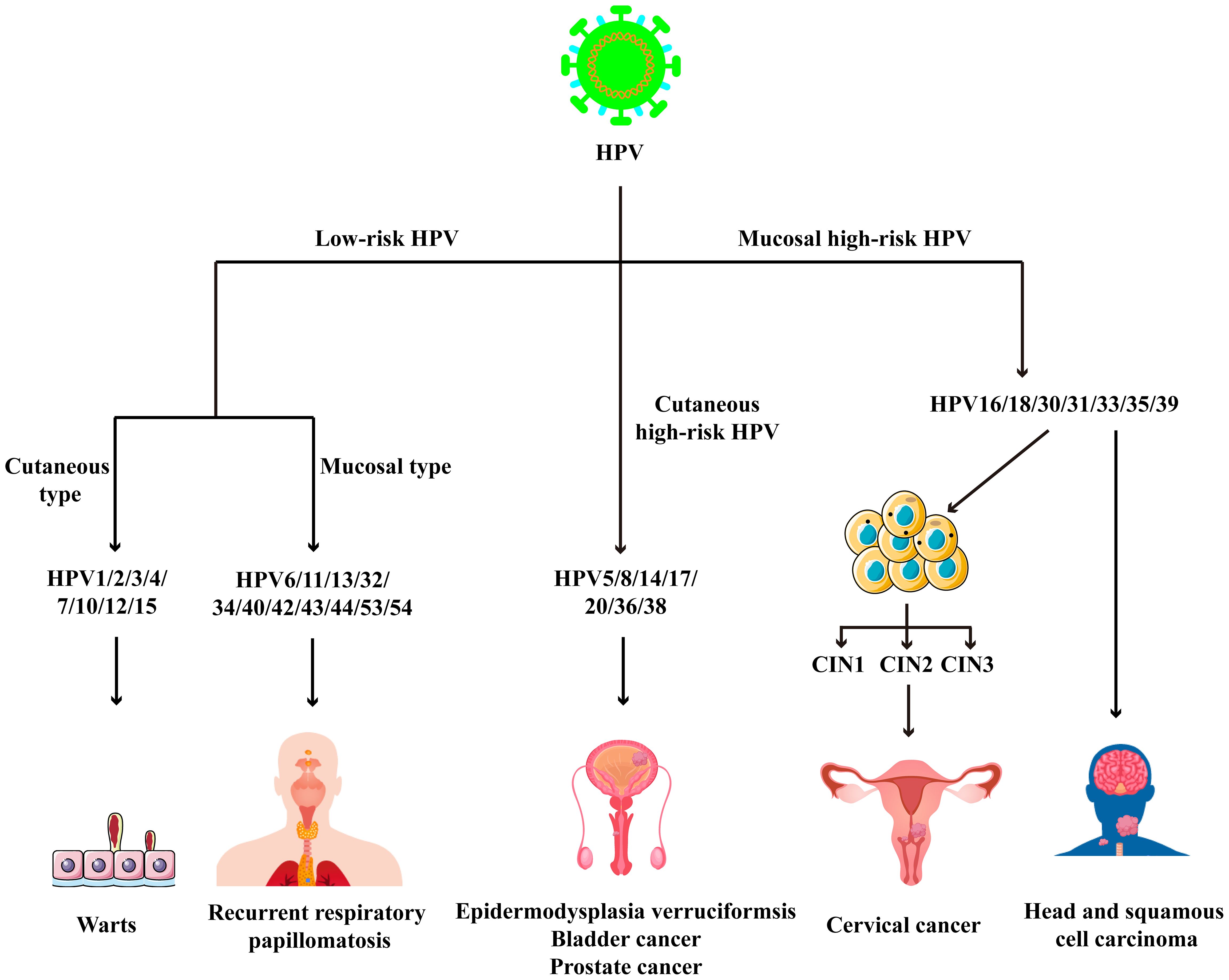
Human papillomavirus (HPV) is a small DNA virus that belongs to the Papillomaviridae family and poses a significant threat to global public health (1–3). Based on the disease outcomes, HPV strains are categorized into low-risk and high-risk types. Low-risk HPV types, such as HPV 6 and 11, are primarily associated with benign lesions, including genital warts and common warts (4). In contrast, high-risk HPV types, including HPV 16 and 18, are strongly linked to the pathogenesis of head and neck squamous cell carcinoma (HNSCC)and cervical cancer (Figure 1) (5). The innate immunity serves as the frontline against viruses, employing pattern recognition receptors (PRRs) to detect pathogen-associated molecular patterns (PAMPs). This recognition triggers the production of a wide array of cellular and molecular factors to counteract the viral infection (6, 7). Additionally, innate immunity also plays a pivotal role in HPV-related cancers (8, 9).
The cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) signaling pathway serves as a crucial axis for type I interferon (IFN-I) induction upon cell-free DNA stimulation (10). Researchers have discovered that the cGAS-STING signaling pathway is closely related to the innate immunity against HPV infection and HPV-related cancers. For instance, HPV E2 attenuates the STING-IFN response to facilitate viral replication, whereas STING activation inhibits cervical cancer growth by augmenting the anti-tumor response (11, 12). The loss of HPV16 E7 restores cGAS-STING responses in HPV-positive oropharyngeal squamous cell carcinomas (13). Also, the cGAS-STING signaling pathway has been considered as a therapeutic target because its activation suppresses tumor growth and overcomes resistance to anti-PD-1 therapy (14, 15).
In this review, we present a concise overview of the cGAS-STING pathway, highlighting its significance in HPV infection and HPV-related cancers. Additionally, we discuss the therapeutic potential of this signaling pathway in the treatment of HPV-related diseases.
2 Concise view of cGAS-STING signaling pathway
The cGAS-STING signaling pathway functions as the primary mechanism for cells to sense and respond to double-stranded DNA (dsDNA) in the cytoplasm, thereby establishing a robust innate immune response by inducing the expression of IFN-I (16). In detail, cytoplasmic dsDNA, whether originating from pathogens or cellular components, can be recognized by cGAS. Then, cGAS is activated to catalyze the synthesis of cGAMP from GTP and ATP (17). cGAMP binds to STING, triggering its conformational change and activation. Meanwhile, STING translocates from the endoplasmic reticulum (ER) to the Golgi apparatus through the ER-Golgi intermediate compartment (ERGIC) (18, 19). STING then recruits TANK-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3), resulting in their phosphorylation (20). Phosphorylated IRF3 undergoes dimerization and translocates into the nucleus to induce the expression of IFN-I (Figure 2) (20). In addition to the classical pathway, studies have identified non-classical cGAS-STING signaling mechanisms, including cGAS-STING-induced pyroptosis, cGAS-STING-PERK-eIF2α pathway, and STING-induced autophagy (21–23).
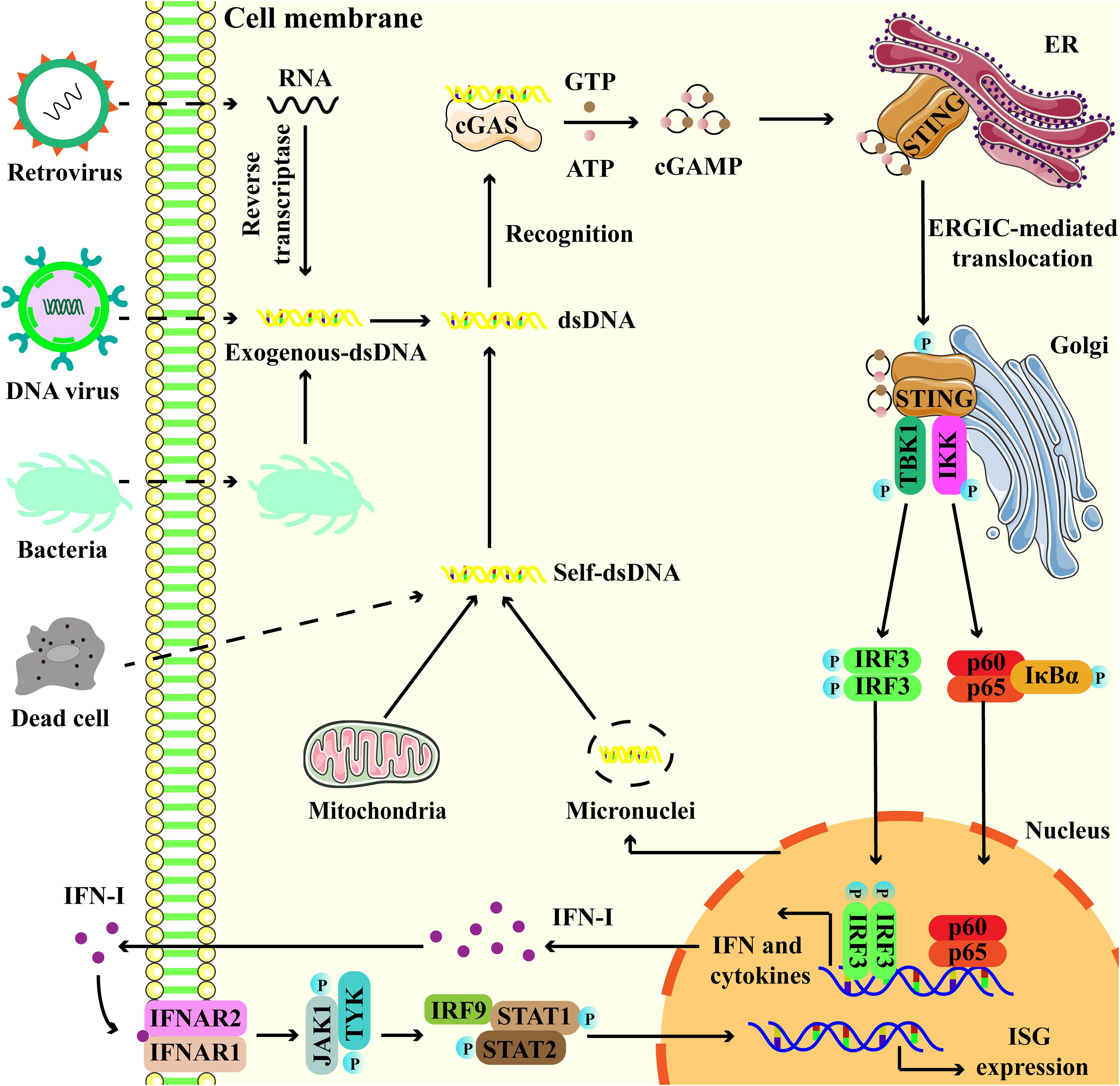
Figure 2. Overview of the cGAS-STING signaling pathway. Exogenous DNA from dead cell, virus and bacteria, and endogenous DNA leakage from micronuclei and mitochondria, interact with cGAS, promoting the activation of cGAS to catalyze the production of 2′,3′-cyclic GMP-AMP (cGAMP) from ATP and GTP. cGAMP binds to endoplasmic reticulum (ER)-located STING, then STING undergoes endoplasmic reticulum (ER)-to-Golgi trafficking via ER-Golgi intermediate compartment (ERGIC). Subsequently, STING serves as a platform to recruit TANK binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3), as well as their phosphorylation. Phosphorylated IRF3 translocates to the nucleus and turns on the expression of type I interferons (IFN-I). Besides, STING also interacts and activates IKK, triggering the transcriptional activation of NF-κB. Binding of IFN to IFNAR1-IFNAR2 activates JAK1 and STAT, leading to the induction of interferon-stimulated genes (ISGs).
The activation of the cGAS-STING signaling pathway is regulated by multiple factors, such as viral components, protein modifications, and other epigenetic mechanisms, thereby influencing a series of physiological and pathological processes (20, 24). The cGAS-STING signaling plays a crucial role in safeguarding the host against viral and bacterial infections (25, 26). In the context of cancer, the cGAS-STING signaling exhibits a dual nature, exerting anti-tumor effects through the induction of IFNs while, paradoxically, fostering tumor progression in certain scenarios via the promotion of chronic inflammation (14, 27).
3 cGAS-STING signaling in HPV infection
Unlike other DNA viruses, HPV follows a distinct vesicular trafficking pathway that may interfere with the activation of cGAS-STING DNA-sensing pathway (28). In this study, it was observed that HPV16 pseudovirus evaded cGAS-STING responses during the initial infection, as evidenced by rare transcriptional activation of IFNs and interferon-stimulated genes (ISGs) (28). Furthermore, experimental induction of premature viral penetration of vesicular membranes using membrane-disrupting cationic lipids demonstrated that circumventing natural trafficking pathway of HPV could trigger the activation of the cGAS-STING pathway (28). Therefore, targeting the trafficking process of HPV during the early infection presents a potential strategy to restrict its infection.
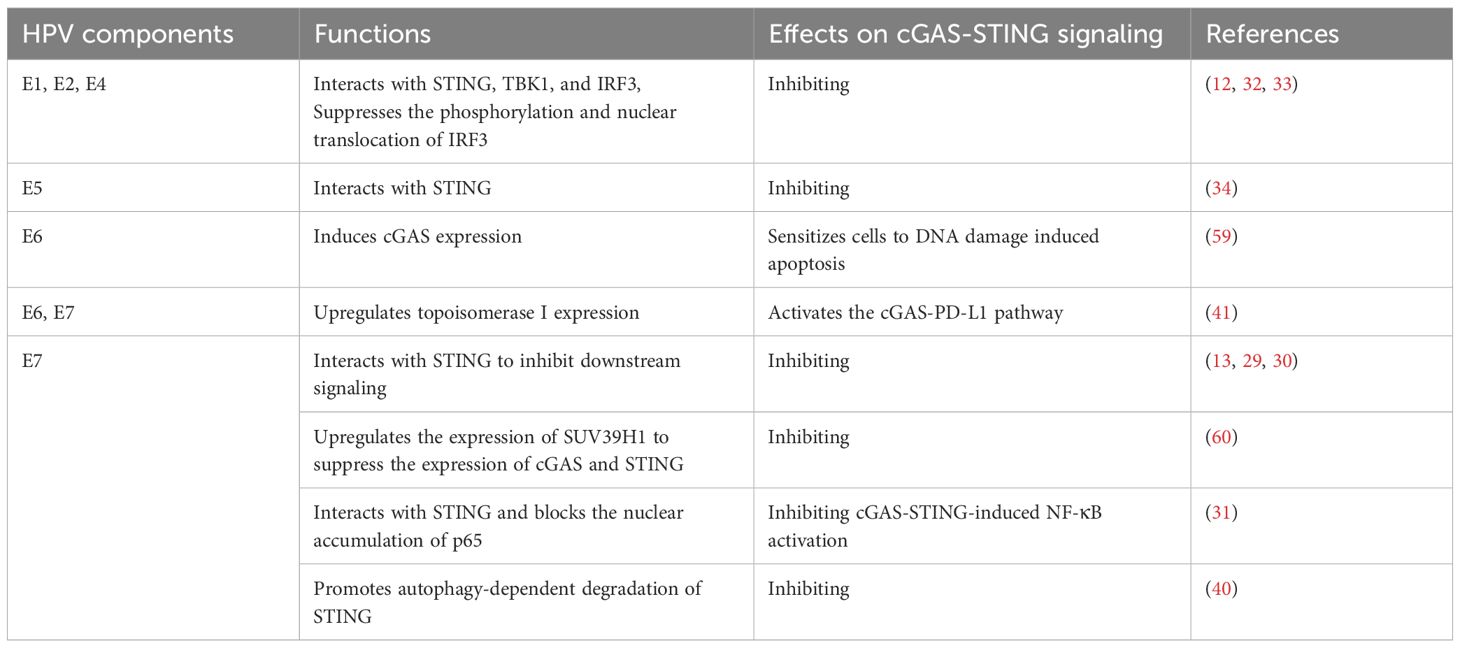
E7, originating from HPV, antagonized the cGAS-STING DNA-sensing pathway (Table 1) (29). Specifically, it was discovered that the transduction of HPV E7 into primary mouse embryonic fibroblasts inhibited DNA-activated Ifnb mRNA expression (29). Further investigations revealed that the LXCXE motif within E7 played a crucial role in antagonizing DNA sensing by interacting with STING (29). Recurrent respiratory papillomatosis (RRP) represents an uncommon benign tumor primarily triggered by HPV6/11 infection in respiratory tract epithelial cells, with the IFN-β response to RRP being subject to regulation by HPV11 E7 (30). A negative correlation was observed between IFN-β levels in localized neoplasms and the HPV load, indicating the disruptive effects of IFN-β on viral replication (30). Moreover, this study demonstrated that HPV11 E7 downregulated IFN-β responses by inhibiting the phosphorylation of STING, as evidenced by the reduction of IFN-β expression in cells expressing HPV11 E7 (30). In vitro, macrophages with activated STING exhibited increased production of IFN-β, which subsequently induced the death of HPV-infected epithelial cells and reduced the expression of HPV proteins (30). Interestingly, HPV18 E7 exhibited selective antagonism towards cGAS‐STING-induced NF‐κB activation. In detail, HPV18 E7 interacted with STING, which blocked the nuclear accumulation of p65 and impeded NF‐κB signaling (31). Moreover, E7 inhibition of STING‐triggered NF‐κB activation was related to its HPV pathogenicity (31).
Other HPV proteins, including E1, E2, E4 and E5, have also been demonstrated to downregulate the activation of cGAS-STING pathway (32–35). By mimicking HPV infection, elevated viral titers were detected in HPV16 E2-expressing cells compared to the controls (12). This study demonstrated that E2 interacted with STING, TBK1, and IRF3 to inhibit IRF3 phosphorylation and nuclear translocation, thereby attenuating IFN expression (12). Clinically, STING expression was found to be downregulated in HPV-positive low-grade squamous intraepithelial lesions compared to HPV-negative controls, accompanied by the upregulated HPV E2 transcriptional levels (35). Recently, HPV E1 and HPV E4 have been demonstrated to inhibit IFN responses induced by multiple stimuli, which is mediated by the suppression of RIG-I/MDA5-MAVS, TLR3-TRIF, and cGAS-STING pathways (32, 33). For the cGAS-STING pathway, HPV E1 and E4 interacted with STING, TBK1, and IRF3, leading to the inhibition of both the phosphorylation and nuclear translocation of IRF3 (Table 1) (32, 33).
Similar to isoforms of key IFN signaling molecules, the STING isoforms that function as negative regulators of the cGAS-STING pathway have been reported (36–39). STING-ΔC was generated by retention of intron 6, which shared its N-terminal domain with full-length STING but contained a distinct C-terminal sequence (39). Functionally, STING-ΔC interacted with the full-length STING, inhibiting its oligomerization and its assembly with TBK1, which was a crucial component of the STING-TBK1-IRF3 signalosome. This interaction impeded the phosphorylation and nuclear translocation of IRF3, consequently blocking IFN production. Upon HPV16 infection, STING-ΔC downregulated the expression of IFN-β and ISGs (39). STING-ΔN was a shortened STING isoform that lacked 1 to 3 N-terminal transmembrane domains by exon 3 skipping and a new start codon introducing in exon 4, while the C-terminal domain remained intact. STING-ΔN reduced HPV-induced IFN-I and IFN-III production by disrupting the formation of the 2′3′-cGAMP-STING and STING-TBK1 complexes (38).
4 cGAS-STING signaling in HPV-related cancers
Increasing evidence has described the role of the cGAS-STING pathway in HPV-related cancers, such as cervical cancer and head and neck squamous cell carcinoma (40–42). The activation of cGAS-STING signaling pathway suppressed cancer progression, which was associated with the apoptosis of cancer cells and infiltration of immune cells within the tumor microenvironment (TME) (14, 43). However, the activation of cGAS-STING signaling can be regulated through different mechanisms.
4.1 Cervical cancer
Cervical cancer is the most prevalent cancer type associated with HPV infection, and a growing body of evidence underscores the critical role of the cGAS-STING signaling pathway in cervical cancer. The presence of homozygous variants HAQ/HAQ and R232H/R232H of STING1 was found to correlate with an earlier age at diagnosis and an elevated recurrence rate of cervical cancer, accompanied by poorer survival. Furthermore, patients carrying HAQ/HAQ and R232H/R232H genotypes exhibited a dysfunctional cGAS-STING pathway that failed to stimulate efficient anti-cancer immunity (44). Another study found that advanced-stage cervical cancers were characterized by reduced levels of STING, suggesting that low STING expression could serve as a predictor of worse survival outcomes (45). Moreover, multivariate analyses revealed that STING acted as an independent prognostic indicator for survival in cervical cancer. Interestingly, high STING levels combined with CD103-positive tumor infiltrating lymphocytes were strongly associated with improved prognosis (45). STING-mediated response was inhibited in cervical cancer cells, as evidenced by the reduction of cGAS, STING, and IFN-β expression (11). The knockdown of STING increased the viability and migration of cervical cancer cells, accompanied by the reduction of IFN-β and IL-6 mRNA expression following dsDNA stimulation (11). In vitro, ADU-S100 (a STING agonist)-treated cervical cancer cells exhibited reduced cell viability in a dose-dependent manner, accompanied by the upregulation of STING/IFN-β/IL-6 expression (11). In vivo, administration of ADU-S100 markedly reduced the volume and weight of xenograft tumors. Concurrently, there was a substantial increase in the number of tumor-infiltrating CD8+ T cells and CD103+ dendritic cells (11). These findings indicate that the activation of STING enhances the anti-tumor immunity not only through the production of cytokines but also the recruitment of tumor infiltrating lymphocytes.
The presence of PD-L1 downregulates immune response and promotes cancer immune escape (46). IFI16, an interferon-stimulated gene, promoted cervical cancer progression by upregulating PD-L1 expression through the activation of STING-TBK1-NF-κB pathway (47). Compared with HPV-negative cervical cancer cells, the expression levels of IFI16 and PD-L1 were higher in HPV-positive samples. Moreover, IFI16 promoted the expression of PD-L1 and facilitated the oncogenic behaviors of cervical cancer cells (47). Mechanistically, IFI16 activated the STING-TBK1-NF-κB signaling cascade, thereby upregulating PD-L1 expression by promoting the binding between NF-κB and PD-L1 promoter (47). In vivo, knockdown of PD-L1 or IFI16 significantly restrained the growth of SiHa-derived tumors (47). A recent study demonstrated that HPV oncoproteins E6 and E7 upregulated topoisomerase I (TOP1) expression to activate the cGAS-PD-L1 pathway, consequently promoting cervical cancer development (41). Specifically, the expression of TOP1 was upregulated in cervical cancer and correlated with poor prognosis. In vitro, TOP1 knockdown suppressed cervical cancer cell growth, reduced their migration, and limited their invasion. Moreover, TOP1 inhibition disrupted DNA damage repair and caused cell death. In vivo, downregulation of TOP1 markedly suppressed xenograft tumor growth in mice (41).
The downstream genes of STING pathway, such as CCL5, CXCL9, and CXCL10, were found to be closely associated with the prognosis of cervical cancer, as patients with high expression of these genes exhibited improved overall survival and relapse-free survival (15). Moreover, this study demonstrated a correlation between STING downstream genes and immune cell infiltration within the TME of cervical cancer, including CD8+ T cells, M1 macrophages, and NK cells (15). The in vivo experiment unveiled that MSA-2, a STING agonist, was capable of significantly suppressing the growth of subcutaneous cervical tumors when administered either as a monotherapy or in combination with anti-PD-1 therapy. Furthermore, the combination approach markedly enhanced therapeutic efficacy relative to anti-PD-1 monotherapy (15).
Post-translational modifications (PTMs), including ubiquitination and SUMOylation, play crucial roles in the regulation of cervical cancer (48, 49). It was found that Bcl2-associated athanogene 2 (BAG2) suppressed the progression of cervical cancer by stabilizing STING. Specifically, BAG2 formed a complex with STIP1 homology and U-box-containing protein 1 (STUB1). This complex prevented STUB1 from attaching the K48-linked ubiquitin chains at K338 and K370 of STING, thereby activating the STING-dependent IFN-I pathway to suppress cervical cancer (49). Clinically, there was a positive correlation between BAG2 and STING levels in cervical cancer, with low BAG2 expression strongly linked to advanced disease and poor prognosis (49). A research reported that PIN1 promoted the proliferation and invasion of cervical cancer cells by inhibiting ferroptosis, which was mediated by the inhibition of the cGAS-STING pathway (48). In addition, knockdown of PIN1 significantly downregulated the survival rate of cervical cancer cells. Subsequent investigation suggested that USP34 enhanced the expression of PIN1 via SUMOylation in cervical cancer cells, thereby achieving the tumor-promoting effects (48). However, the role of other PTMs, including glycosylation, acetylation, and palmitoylation, remains to be fully explored preclinically and clinically.
4.2 Head and neck cancer
HNSCC represents the predominant histological subtype of head and neck cancers, and HPV16 is the most common subtype associated with HNSCC (50). A previous study revealed that the expression level of STING protein was elevated in HPV+ (p16+) HNSCC tumor specimens when compared to the HPV- specimens, but only a weak association was observed between high STING expression and improved cancer-specific survival (51). Interestingly, STING activation enhanced cetuximab-mediated NK cell activation and subsequent DC maturation, as indicated by increased expression of CD86, CD83, HLA-DR and PD-L1 on DCs. Moreover, tumor cell STING downregulation diminished cetuximab-mediated NK-DC crosstalk (51). Another study demonstrated that the expression levels of cGAS, TBK1, and IRF3 were similar across all HNSCC cell lines regardless of HPV status, while HPV− cells displayed higher expression of STING compared with low or absent levels in the HPV+ cell lines (52). However, both STING-intact HPV− HNSCC cells and STING-overexpressing HPV+ HNSCC cell lines exhibited a functional dysregulation of this pathway (52). As opposed to the cell lines, the expression of STING was enhanced in HPV-positive HNSCC patient tissue, and elevated intratumoral STING expression correlated with improved overall survival (52). Another study also indicated that elevated STING expression was associated with improved overall survival in young patients (aged under 60) with HNSCC (40). The different findings regarding STING expression and prognosis in HNSCC among these studies may be attributed to variations in endpoint definition, sample size, and patient status.
Similar to the above findings, it was found that HPV16 E7 was responsible for the inhibition of the cGAS-STING response in HNSCC cells (53). HPV16 E7 shared low homology with HPV18 E7 and employed mechanisms that were distinct from those utilized by HPV18 E7 (40). In detail, HPV16 E7 specifically interacted with NLRX1, resulting in the autophagic degradation of STING. This degradation contributed to the inhibition of cGAS-STING response in HNSCC cells. Moreover, knockdown of HPV16 E7 or NLRX1 deficiency restored the STING signaling and IFN-I induction, as evidenced by increased p-TBK1 expression and downstream molecule expression. In vivo, NLRX1-mediated inhibition of anti-tumor immunity was IFN-I-dependent, which was evidenced by the comparable levels of tumor volumes and STING signature genes between groups in Ifnar1–/– hosts (40).
In HPV-positive oropharyngeal squamous cell carcinomas (OPSCC) cells, knockdown of HPV16 E7 led to a notable restoration of calf thymus DNA-induced IFN-β expression (13). Among patients with squamous cell carcinomas of oral cavity and oropharynx, the proportion of cancers with tumor STING immunoexpression (TSI) was significantly higher in patients with regression of disease than that in patients with progression of disease (54).
In HPV-related carcinogenesis of tongue squamous cell carcinoma (TSCC), it was found that the activation of STING augmented Treg infiltration through the c-jun/CCL22 signaling (27). Interestingly, STING activation promoted the production of immunosuppressive cytokines, such as CCL22, IDO, and IL-10 that played important roles in the development of HPV+ TSCC (27). Furthermore, STING activation-induced CCL22 promoted the recruitment of Foxp3+ Tregs in HPV+ TSCC (27). Mechanistically, c-jun was required for the STING activation-induced CCL22 expression, as silencing of c-jun inhibited the induction of CCL22 at both mRNA and protein levels. Further investigation demonstrated that miR-27 could impair CCL22 production and subsequent Tregs infiltration induced by STING activation, as evidenced by the reduced CCL22 expression and Treg migration in vitro (27).
5 cGAS-STING signaling: a potential therapeutic target for HPV-related diseases
STING ligands induced rapid regression of murine papillomas, accompanied by enhanced T cell infiltration, IFN-β and TNF-α production (55). Importantly, STING ligands exhibited significantly greater efficacy when compared to Imiquimod (an immunotherapy for papilloma) (55). Activating STING with ligands induced local inflammation and immune responses that specifically targeted HPV-infected and dysplastic cells, potentially resulting in the regression of premalignant lesions and aiding in the control of malignancies (55). Interestingly, this study also found that STING ligands enhanced the survival of mice bearing STING-deficient SCCVII tumors, indicating the therapeutic potential of STING agonists regardless of STING expression by the cancer cells (55). The mutant type of HPV 16 E7 (E7GRG) protein was formulated with 2’-3’cGAMP CDN and/or CpG-C ODN adjuvants to evaluate its immunogenic response and anti-tumor activity (14). In E7GRG + 2’-3’cGAMP+CpG-C-treated tumor-bearing mice, the tumor growth was markedly suppressed, accompanied by the enhanced lymphocyte proliferation response, Th1 cytokine profile, CD8+ T-cell responses, granzyme B production, and antibody responses (14).
Studies have demonstrated that STING activation restricted cervical cancer progression, including diABZI, MSA-2, and other STING agonists (15, 43). In vitro, a significant downregulation of HPV16/18 E7 protein expression was observed when cervical cancer cells treated with diABZI, accompanied by the reduced cell proliferation. In addition, other STING agonists and radiotherapy (RT) also suppressed its production by activating STING signaling. Interestingly, the STING agonist enhanced the inhibitory effects of radiation on HPV16 E7 protein expression, cell proliferation and clone formation (43). Moreover, this study revealed that activated STING-TBK1 signaling induced the ubiquitin-proteasome degradation of HPV16/18 E7 proteins in cells, which was mediated by E3 ligase HUWE1. Further exploration found that TBK1-mediated phosphorylation of HPV16/18 E7 promoted their ubiquitination and degradation (43). In vivo, the STING-TBK1 activation led to a marked suppression of tumor growth, as indicated by reduced tumor volume and tumor weight in tumor-bearing mice (43).
A study revealed the potential of gas-amplified metalloimmunotherapy with dual activation of pyroptosis and the STING pathway for the regulation of immunosuppressive microenvironment in cervical cancer (56). Specifically, PEGylated manganese-doped calcium sulfide nanoparticles (MCSP) were developed, which exhibited the capability to release Ca²+, Mn²+, and H2S in the tumor microenvironment. Besides the calcium overload-induced pyroptosis, H2S-induced mitochondrial dysfunction facilitated the release of mtDNA, thereby augmenting the activating effect of Mn²+ on the cGAS-STING signaling pathway and consequently activating immunosuppressed dendritic cells (56). In vivo, MCSP efficiently triggered the anti-tumor responses, including the upregulation of DC maturation, CD8+ T cell percentages, and IFN-γ production. Importantly, the combination of MCSP nanoparticles and PD-1 immunotherapy exhibited synergistic anti-tumor effects and effectively suppressed tumor growth, as evidenced by the pronounced reduction of tumor volume and improved survival rate (56).
Nanoparticles have also been developed to deliver the caffeic acid (CA) for cervical cancer treatment (57). The nanoparticles based on fucoidan (Fu/CA NPs) significantly suppressed the proliferation of cervical cancer HeLa cells, accompanied by the induction of apoptosis. These effects were achieved by the accumulation of reactive oxygen species and mitochondrial damage, which could elicit the activation of cGAS-STING pathway (57). In vivo, Fu/CA NPs suppressed solid tumor growth, with even more pronounced anti-tumor effects observed when Fu/CA NPs was combined with cisplatin. The enhanced anti-tumor activity was associated with the activation of the cGAS-STING pathway. Importantly, the combination alleviated cisplatin-induced nephrotoxicity, suggesting the potential of Fu/CA NPs in treating cervical cancer by targeting the cGAS-STING pathway (57). A recent study demonstrated mRNA-encoded STING adjuvant enhanced the efficacy of HPV E6/E7 vaccination in suppressing murine HPV+ TC-1 tumor growth, as evidenced by the reduced tumor volume and prolonged survival (58). Moreover, the effects of STINGV155M mRNA were dependent on the presence of CD8+ T cells, as these effects were abolished when CD8+ T cells were depleted. Notably, the production of IFN-γ and TNF-α by CD8+ T cells was upregulated when mice were immunized with mRNA-lipid nanoparticles (LNPs) coformulated with HPV E6/E7 and STINGV155M (58).
Although these modulators exhibit potential in inhibiting HPV infection and HPV-associated cancers, enhancing their safety and efficacy remains a crucial endeavor. Furthermore, exploring strategies to extend the release and utilization of these modulators (such as the use of nanoparticles or hydrogel systems) represents future directions for clinical research.
6 Discussion
Hitherto, it has been clear that innate immunity holds a pivotal position in the development of infectious diseases, autoimmune diseases, and cancers. In the past few years, there has been a significant increase in interest and understanding surrounding the role of cGAS-STING signaling pathway in HPV-related diseases. For instance, HPV18 E7 bound to STING in a region critical for NF-κB activation and then blocked the nuclear accumulation of p65, suppressing the production of inflammatory cytokines (31). Moreover, E5 and E2 have also been reported to inhibit STING-mediated responses, resulting immune evasion (34, 35). Notably, studies pointed out that activation of STING inhibited cervical cancer tumor growth by enhancing the anti-tumor immune response (11). However, some studies confirmed the roles of cGAS-STING signaling in HPV-related cancer development through the upregulation of PD-L1 and expansion of Tregs, indicating the tumor-promoting effects of cGAS-STING activation under certain contexts (11, 47).
Considering the role of the cGAS-STING pathway in HPV infection and HPV-related cancers, targeting the cGAS-STING signaling pathway is promising for their treatments. For example, it has been reported that activation of STING by ADU-S100 reduced the cell viability of cervical cancer cells (11). Notably, STING agonist has been demonstrated to activate the cervical cancer immune microenvironment and overcome anti-PD-1 therapy resistance (15). Moreover, nanoparticles have also been developed for the treatment (57, 58). Due to challenges in medicinal chemistry, rare cGAS-STING modulators have been tested in HPV-related clinical trials. Collectively, future explorations should pay more attention to the following fields: 1) molecular mechanisms regulating the activation of cGAS-STING signaling during HPV infection and HPV-related cancers; 2) the development of novel drugs targeting the cGAS-STING signaling pathway, with emphasis on clinical applications. These efforts may provide new insights into the therapies for HPV infection and HPV-related cancers.
Author contributions
QZ: Software, Writing – original draft, Writing – review & editing. SY: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HPV, human papillomavirus; IFN-I, type I interferon; cGAS, cyclic GMP-AMP synthase; STING, stimulator of interferon gene; CIN, cervical intraepithelial neoplasia; PAMPs, pathogen-associated molecular patterns; PRRs, pattern recognition receptors; ER, endoplasmic reticulum; TBK1, TANK-binding kinase 1; IRF3, interferon regulatory factor 3; ISGs, interferon-stimulated genes; RRP, recurrent respiratory papillomatosis; PTMs, post-translational modifications; TME, tumor microenvironment; HNSCC, head and neck squamous cell carcinoma; OPSCC, oropharyngeal squamous cell carcinomas.
References
1. Gong X, Chi H, Xia Z, Yang G, and Tian G. Advances in hpv-associated tumor management: therapeutic strategies and emerging insights. J Med Virol. (2023) 95:e28950. doi: 10.1002/jmv.28950
2. McBride AA. Human papillomaviruses: diversity, infection and host interactions. Nat Rev Microbiol. (2022) 20:95–108. doi: 10.1038/s41579-021-00617-5
3. Kombe Kombe AJ, Li B, Zahid A, Mengist HM, Bounda GA, Zhou Y, et al. Epidemiology and burden of human papillomavirus and related diseases, molecular pathogenesis, and vaccine evaluation. Front Public Health. (2020) 8:552028. doi: 10.3389/fpubh.2020.552028
4. Wolf J, Kist LF, Pereira SB, Quessada MA, Petek H, Pille A, et al. Human papillomavirus infection: epidemiology, biology, host interactions, cancer development, prevention, and therapeutics. Rev Med Virol. (2024) 34:e2537. doi: 10.1002/rmv.2537
5. Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. (2003) 16:1–17. doi: 10.1128/cmr.16.1.1-17.2003
6. Amador-Molina A, Hernández-Valencia JF, Lamoyi E, Contreras-Paredes A, and Lizano M. Role of innate immunity against human papillomavirus (Hpv) infections and effect of adjuvants in promoting specific immune response. Viruses. (2013) 5:2624–42. doi: 10.3390/v5112624
7. Dogan S, Terzioglu E, and Ucar S. Innate immune response against hpv: possible crosstalking with endocervical Γδ T cells. J Reprod Immunol. (2021) 148:103435. doi: 10.1016/j.jri.2021.103435
8. Fu K, Yang X, Zhang M, and Yin R. The role of innate immunity triggered by hpv infection in promoting cervical lesions. J Mol Med (Berlin Germany). (2025) 103:739–54. doi: 10.1007/s00109-025-02553-w
9. De Martino S, Capasso B, Cis L, D'Orsi L, Canali G, Capasso P, et al. Role of innate immunity in tumor microenvironment of hpv-associated anal cancer: the hypothetical beneficial role of Γδ T cells. Crit Rev oncology/hematology. (2025) 212:104771. doi: 10.1016/j.critrevonc.2025.104771
10. Sun L, Wu J, Du F, Chen X, and Chen ZJ. Cyclic gmp-amp synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Sci (New York NY). (2013) 339:786–91. doi: 10.1126/science.1232458
11. Shi F, Su J, Wang J, Liu Z, and Wang T. Activation of sting inhibits cervical cancer tumor growth through enhancing the anti-tumor immune response. Mol Cell Biochem. (2021) 476:1015–24. doi: 10.1007/s11010-020-03967-5
12. Li JX, Zhang J, Li CH, Zhang Q, Kong B, and Wang PH. Human papillomavirus E2 proteins suppress innate antiviral signaling pathways. Front Immunol. (2025) 16:1555629. doi: 10.3389/fimmu.2025.1555629
13. Bortnik V, Wu M, Julcher B, Salinas A, Nikolic I, Simpson KJ, et al. Loss of hpv type 16 E7 restores cgas-sting responses in human papilloma virus-positive oropharyngeal squamous cell carcinomas cells. J microbiology immunology infection = Wei mian yu gan ran za zhi. (2021) 54:733–9. doi: 10.1016/j.jmii.2020.07.010
14. Dorostkar F, Arashkia A, Roohvand F, Shoja Z, Navari M, Mashhadi Abolghasem Shirazi M, et al. Co-administration of 2'3'-cgamp sting activator and cpg-C adjuvants with a mutated form of hpv 16 E7 protein leads to tumor growth inhibition in the mouse model. Infect Agents Cancer. (2021) 16:7. doi: 10.1186/s13027-021-00346-7
15. Li T, Zhang W, Niu M, Wu Y, Deng X, and Zhou J. Sting agonist inflames the cervical cancer immune microenvironment and overcomes anti-pd-1 therapy resistance. Front Immunol. (2024) 15:1342647. doi: 10.3389/fimmu.2024.1342647
16. Decout A, Katz JD, Venkatraman S, and Ablasser A. The cgas-sting pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. (2021) 21:548–69. doi: 10.1038/s41577-021-00524-z
17. Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Rohl I, et al. Cgas produces a 2'-5'-linked cyclic dinucleotide second messenger that activates sting. Nature. (2013) 498:380–4. doi: 10.1038/nature12306
18. Shang G, Zhang C, Chen ZJ, Bai XC, and Zhang X. Cryo-em structures of sting reveal its mechanism of activation by cyclic gmp-amp. Nature. (2019) 567:389–93. doi: 10.1038/s41586-019-0998-5
19. Gao P, Ascano M, Zillinger T, Wang W, Dai P, Serganov AA, et al. Structure-function analysis of sting activation by C[G(2',5')Pa(3',5')P] and targeting by antiviral dmxaa. Cell. (2013) 154:748–62. doi: 10.1016/j.cell.2013.07.023
20. Chen Q, Sun L, and Chen ZJ. Regulation and function of the cgas-sting pathway of cytosolic DNA sensing. Nat Immunol. (2016) 17:1142–9. doi: 10.1038/ni.3558
21. Gui X, Yang H, Li T, Tan X, Shi P, Li M, et al. Autophagy induction via sting trafficking is a primordial function of the cgas pathway. Nature. (2019) 567:262–6. doi: 10.1038/s41586-019-1006-9
22. Zhang D, Liu Y, Zhu Y, Zhang Q, Guan H, Liu S, et al. A non-canonical cgas-sting-perk pathway facilitates the translational program critical for senescence and organ fibrosis. Nat Cell Biol. (2022) 24:766–82. doi: 10.1038/s41556-022-00894-z
23. Xiao L, Ai YL, Mi XY, Liang H, Zhi X, Wu LZ, et al. Cgas activation converges with intracellular acidification to promote sting aggregation and pyroptosis in tumor models. J Clin Invest. (2025) 1353:e188872. doi: 10.1172/jci188872
24. Xie F and Zhu Q. The regulation of cgas-sting signaling by rna virus-derived components. Virol J. (2024) 21:101. doi: 10.1186/s12985-024-02359-1
25. Liu N, Pang X, Zhang H, and Ji P. The cgas-sting pathway in bacterial infection and bacterial immunity. Front Immunol. (2021) 12:814709. doi: 10.3389/fimmu.2021.814709
26. Yu XF, Wang S, Ye R, and Wei W. Viral evasion of cgas-sting pathway: opportunities for intervention. Trends Pharmacol Sci. (2025) 46:989–1003. doi: 10.1016/j.tips.2025.08.009
27. Liang D, Xiao-Feng H, Guan-Jun D, Er-Ling H, Sheng C, Ting-Ting W, et al. Activated sting enhances tregs infiltration in the hpv-related carcinogenesis of tongue squamous cells via the C-jun/ccl22 signal. Biochim Biophys Acta. (2015) 1852:2494–503. doi: 10.1016/j.bbadis.2015.08.011
28. Uhlorn BL, Jackson R, Li S, Bratton SM, Van Doorslaer K, and Campos SK. Vesicular trafficking permits evasion of cgas/sting surveillance during initial human papillomavirus infection. PloS Pathog. (2020) 16:e1009028. doi: 10.1371/journal.ppat.1009028
29. Lau L, Gray EE, Brunette RL, and Stetson DB. DNA tumor virus oncogenes antagonize the cgas-sting DNA-sensing pathway. Sci (New York NY). (2015) 350:568–71. doi: 10.1126/science.aab3291
30. Chen L, Hu H, Pan Y, Lu Y, Zhao M, Zhao Y, et al. The role of hpv11 E7 in modulating sting-dependent interferon B Response in recurrent respiratory papillomatosis. J Virol. (2024) 98:e0192523. doi: 10.1128/jvi.01925-23
31. Lou M, Huang D, Zhou Z, Shi X, Wu M, Rui Y, et al. DNA virus oncoprotein hpv18 E7 selectively antagonizes cgas-sting-triggered innate immune activation. J Med Virol. (2023) 95:e28310. doi: 10.1002/jmv.28310
32. Chen HM, Zhang J, Li JX, Li CH, Li YF, Zhang Q, et al. The viral early protein 4 of human papillomavirus type 16 suppresses innate antiviral immunity. Int J Biol macromolecules. (2025) 315:144542. doi: 10.1016/j.ijbiomac.2025.144542
33. Li JX, Zhang J, Li CH, Li YF, Chen HM, Li T, et al. Human papillomavirus E1 proteins inhibit rig-I/mda5-mavs, tlr3-trif, cgas-sting, and jak-stat signaling pathways to evade innate antiviral immunity. Front Immunol. (2025) 16:1549766. doi: 10.3389/fimmu.2025.1549766
34. Miyauchi S, Kim SS, Jones RN, Zhang L, Guram K, Sharma S, et al. Human papillomavirus E5 suppresses immunity via inhibition of the immunoproteasome and sting pathway. Cell Rep. (2023) 42:112508. doi: 10.1016/j.celrep.2023.112508
35. Sunthamala N, Thierry F, Teissier S, Pientong C, Kongyingyoes B, Tangsiriwatthana T, et al. E2 proteins of high risk human papillomaviruses down-modulate sting and ifn-K Transcription in keratinocytes. PloS One. (2014) 9:e91473. doi: 10.1371/journal.pone.0091473
36. Carpenter S, Ricci EP, Mercier BC, Moore MJ, and Fitzgerald KA. Post-transcriptional regulation of gene expression in innate immunity. Nat Rev Immunol. (2014) 14:361–76. doi: 10.1038/nri3682
37. Wang PH, Fung SY, Gao WW, Deng JJ, Cheng Y, Chaudhary V, et al. A novel transcript isoform of sting that sequesters cgamp and dominantly inhibits innate nucleic acid sensing. Nucleic Acids Res. (2018) 46:4054–71. doi: 10.1093/nar/gky186
38. Deng J, Zheng SN, Zhang J, Li CH, Li T, and Wang PH. Sting-Δn, a novel splice isoform of sting, modulates innate immunity and autophagy in response to DNA virus infection. Cell communication signaling: CCS. (2025) 23:299. doi: 10.1186/s12964-025-02305-w
39. Zheng SN, Zhang J, Li T, Li CH, Deng J, Li JX, et al. Sting-ΔC, a novel splice isoform of sting, inhibits DNA virus-induced innate immunity and autophagy. Int J Biol macromolecules. (2025) 311:143894. doi: 10.1016/j.ijbiomac.2025.143894
40. Luo X, Donnelly CR, Gong W, Heath BR, Hao Y, Donnelly LA, et al. Hpv16 drives cancer immune escape via nlrx1-mediated degradation of sting. J Clin Invest. (2020) 130:1635–52. doi: 10.1172/jci129497
41. Luo Y, Niu M, Liu Y, Zhang M, Deng Y, Mu D, et al. Oncoproteins E6 and E7 upregulate topoisomerase I to activate the cgas-pd-L1 pathway in cervical cancer development. Front Pharmacol. (2024) 15:1450875. doi: 10.3389/fphar.2024.1450875
42. Ni H, Zhang H, Li L, Huang H, Guo H, Zhang L, et al. T cell-intrinsic sting signaling promotes regulatory T cell induction and immunosuppression by upregulating foxp3 transcription in cervical cancer. J Immunother Cancer. (2022) 10:e005151. doi: 10.1136/jitc-2022-005151
43. Huang X, Huo L, Xiao B, Ouyang Y, Chen F, Li J, et al. Activating sting/tbk1 suppresses tumor growth via degrading hpv16/18 E7 oncoproteins in cervical cancer. Cell Death Differ. (2024) 31:78–89. doi: 10.1038/s41418-023-01242-w
44. Lubbers JM, Koopman B, de Klerk-Sluis JM, van Rooij N, Plat A, Pijper H, et al. Association of homozygous variants of sting1 with outcome in human cervical cancer. Cancer Sci. (2021) 112:61–71. doi: 10.1111/cas.14680
45. Kol A, Lubbers JM, Terwindt ALJ, Workel HH, Plat A, Wisman GBA, et al. Combined sting levels and cd103+ T cell infiltration have significant prognostic implications for patients with cervical cancer. Oncoimmunology. (2021) 10:1936391. doi: 10.1080/2162402x.2021.1936391
46. Yi M, Niu M, Xu L, Luo S, and Wu K. Regulation of pd-L1 expression in the tumor microenvironment. J Hematol Oncol. (2021) 14:10. doi: 10.1186/s13045-020-01027-5
47. Cai H, Yan L, Liu N, Xu M, and Cai H. Ifi16 promotes cervical cancer progression by upregulating pd-L1 in immunomicroenvironment through sting-tbk1-nf-kb pathway. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. (2020) 123:109790. doi: 10.1016/j.biopha.2019.109790
48. Liao D, Cui Y, Shi L, Zeng S, and Wang H. Usp34 regulates pin1-cgas-sting axis-dependent ferroptosis in cervical cancer via sumoylation. Int Immunopharmacol. (2025) 147:113968. doi: 10.1016/j.intimp.2024.113968
49. Yao S, Chen S, Wang A, Liang Z, Liu X, Gao Y, et al. Bag2 inhibits cervical cancer progression by modulating type I interferon signaling through stabilizing sting. Advanced Sci (Weinheim Baden-Wurttemberg Germany). (2025) 12:e70005. doi: 10.1002/advs.202414637
50. Marur S and Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clinic Proc. (2016) 91:386–96. doi: 10.1016/j.mayocp.2015.12.017
51. Lu S, Concha-Benavente F, Shayan G, Srivastava RM, Gibson SP, Wang L, et al. Sting activation enhances cetuximab-mediated nk cell activation and dc maturation and correlates with hpv(+) status in head and neck cancer. Oral Oncol. (2018) 78:186–93. doi: 10.1016/j.oraloncology.2018.01.019
52. Saulters EL, Kennedy PT, Carter RJ, Alsufyani A, Jones TM, Woolley JF, et al. Differential regulation of the sting pathway in human papillomavirus-positive and -negative head and neck cancers. Cancer Res Commun. (2024) 4:118–33. doi: 10.1158/2767-9764.Crc-23-0299
53. Shaikh MH, Bortnik V, McMillan NA, and Idris A. Cgas-sting responses are dampened in high-risk hpv type 16 positive head and neck squamous cell carcinoma cells. Microbial pathogenesis. (2019) 132:162–5. doi: 10.1016/j.micpath.2019.05.004
54. Biesaga B, Smolarczyk R, Mucha-Małecka A, Czapla J, Ryś J, and Małecki K. Prognostic significance of sting immunoexpression in relation to hpv16 infection in patients with squamous cell carcinomas of oral cavity and oropharynx. Biomedicines. (2022) 10:2538. doi: 10.3390/biomedicines10102538
55. Baird JR, Feng Z, Xiao HD, Friedman D, Cottam B, Fox BA, et al. Sting expression and response to treatment with sting ligands in premalignant and Malignant disease. PloS One. (2017) 12:e0187532. doi: 10.1371/journal.pone.0187532
56. Liu L, Lei H, Hou G, Zhang L, Chen Y, Lu Y, et al. Gas-amplified metalloimmunotherapy with dual activation of pyroptosis and the sting pathway for remodeling the immunosuppressive cervical cancer microenvironment. ACS nano. (2024) 18:12830–44. doi: 10.1021/acsnano.4c00017
57. Gao X, Jiang T, Wu X, Li Y, Xiao J, Long L, et al. The fucoidan delivery system enhanced the anti-cervical cancer effect of caffeic acid. Int J Biol macromolecules. (2025) 307:141976. doi: 10.1016/j.ijbiomac.2025.141976
58. Tse SW, McKinney K, Walker W, Nguyen M, Iacovelli J, Small C, et al. Mrna-encoded, constitutively active sting(V155m) is a potent genetic adjuvant of antigen-specific cd8(+) T cell response. Mol therapy: J Am Soc Gene Ther. (2021) 29:2227–38. doi: 10.1016/j.ymthe.2021.03.002
59. Gusho E and Laimins LA. Human papillomaviruses sensitize cells to DNA damage induced apoptosis by targeting the innate immune sensor cgas. PloS Pathog. (2022) 18:e1010725. doi: 10.1371/journal.ppat.1010725
Keywords: HPV, cGAS, STING, infection, cancers, therapeutic target
Citation: Zhu Q and Yu S (2025) The role of cGAS-STING signaling in HPV infection and HPV-related cancers. Front. Immunol. 16:1709613. doi: 10.3389/fimmu.2025.1709613
Received: 20 September 2025; Accepted: 29 September 2025;
Published: 09 October 2025.
Edited by:
Sheefa Mirza, University of Witwatersrand, South AfricaReviewed by:
Lekh N. Dahal, University of Liverpool, United KingdomCopyright © 2025 Zhu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiyi Yu, eXN5c3lybUAxNjMuY29t
 Qiugang Zhu
Qiugang Zhu Shiyi Yu
Shiyi Yu