- 1Department of Urology, The First Hospital of Jilin University, Changchun, China
- 2Organ Transplant Center, First Hospital of Jilin University, Changchun, China
Environmental exposure to heavy metals, such as cadmium, lead, arsenic, and copper, represents a significant yet underappreciated threat to the long-term survival of kidney transplants. Accumulating epidemiological evidence consistently links even low-level exposure to a substantially elevated risk of late graft failure. The transplanted kidney is particularly vulnerable due to its heightened susceptibility to oxidative stress, compounded by immunosuppressive therapy and often impaired excretory function. The core pathophysiological mechanism involves the accumulation of heavy metals in the renal cortex, where they disrupt mitochondrial function and catalyze the generation of reactive oxygen species (ROS) via Fenton-like reactions. This oxidative surge depletes antioxidant defenses, triggering a deleterious cascade of inflammation, apoptosis, and fibrosis, which accelerates the progression of chronic allograft injury. Recognizing this modifiable environmental risk factor is paramount for improving outcomes. This review synthesizes the current evidence and proposes a multi-pronged management strategy, encompassing rigorous biomonitoring, targeted dietary interventions, and novel therapeutic approaches, such as mitochondrial transplantation and the use of natural antioxidants, to mitigate heavy metal toxicity and enhance graft longevity.
1 Introduction
With the rapid advancement of global industrialization and urbanization, heavy metal pollutants pose an increasingly severe threat to ecological environments and human health. Heavy metals such as cadmium, lead, mercury, and arsenic exhibit high toxicity, environmental persistence, non-biodegradability, and bioaccumulation (1–3). They resist degradation in natural environments yet accumulate continuously through food chains, thereby causing long-term harm to ecosystems and human health (1). These pollutants primarily originate from human activities, including mining, smelting, industrial wastewater discharge, pesticide application, electronic waste disposal, and various emissions generated during urbanization (4–6). This leads to their widespread distribution in soil, water bodies, and air (3, 6, 7). Heavy metals enter the environment through multiple pathways, including wastewater, exhaust gases, and solid waste. They not only accumulate near their emission points but also migrate to previously uncontaminated areas, forming complex pollution and exacerbating ecological risks (8–10). The accumulation of heavy metals is particularly pronounced in coastal waters, lake sediments, and urban environments, severely impacting the environmental balance and sustainable human habitats (11–13). When humans ingest heavy metals through contaminated air, drinking water, or the food chain, these elements gradually accumulate in the body (7, 14, 15). This accumulation can trigger multi-system health damage, including neurological disorders, kidney failure, immune system dysfunction, digestive system disorders, skin diseases, reproductive abnormalities, respiratory diseases, and potential carcinogenic risks (14–16). Long-term exposure to heavy metals, even at low concentrations, may cause progressive, irreversible damage to multiple organs, posing a significant global public health challenge.
The kidneys serve as the body’s primary excretory and metabolic organs. Due to their high blood flow and the reabsorption and concentration functions of the renal tubules, they are more prone to accumulating heavy metal elements, making them a key target for heavy metal toxicity (17–19). Consequently, they become key targets for heavy metal toxicity. After entering the kidneys via the bloodstream, heavy metals exert nephrotoxic effects through multiple mechanisms, including induction of oxidative stress, mitochondrial dysfunction, inflammatory responses, apoptosis, and direct damage to renal tubular epithelial cells (20–24). Chronic low-level exposure to heavy metals and the resulting subclinical kidney injury have become a focal point in public health. Such damage often presents insidiously in its early stages yet exhibits persistent progression, significantly increasing the risk of chronic kidney disease and end-stage renal disease while severely compromising renal transplant outcomes (25–28). Therefore, against the backdrop of widespread heavy metal contamination, elucidating the nephrotoxic mechanisms of these metals is crucial for developing early biomarkers, identifying high-risk populations, and formulating targeted prevention strategies. This review summarizes the sources and exposure pathways of several major heavy metal pollutants, with a focus on their core molecular mechanisms that induce nephrotoxicity. It aims to provide theoretical foundations for toxicity prevention research and to reduce the risk of kidney transplant failure.
2 Epidemiological evidence indicates a strong association between heavy metal exposure and kidney transplant failure
Recent epidemiological studies have identified a significant association between heavy metal exposure and kidney transplant failure. Environmental exposure to heavy metals poses a serious threat to kidney graft function, substantially increasing the risk of graft failure and renal decline. Recognizing heavy metal exposure as a controllable risk factor in the long-term management of transplanted kidneys and implementing corresponding exposure prevention strategies holds significant potential clinical implications for improving graft survival.
2.1 Increased risk of graft failure associated with heavy metal exposure
Heavy metals are ubiquitous in the environment and workplace, and their nephrotoxicity is widely recognized as a potential contributor to the onset and progression of chronic kidney disease (CKD). In recent years, epidemiological research has increasingly focused on the impact of heavy metal exposure on the specific population of kidney transplant recipients (KTRs), particularly its association with graft failure risk. Multiple prospective cohort studies indicate that even at levels typical of environmental exposure, heavy metals significantly increase the risk of graft failure in KTRs. A Dutch prospective cohort study suggested that a doubling of plasma lead concentration in KTRs was associated with a 59% increase in the risk of late graft failure, implying that controlling lead exposure may represent a novel approach to improving long-term graft survival (29). This finding remained significant after multivariable adjustment for major clinical and cardiovascular risk factors, confirming lead exposure as an independent risk factor (29). Separately, a study of 693 KTRs found that doubling urinary copper excretion increased the risk of transplant failure by 57%, demonstrating a dose-response relationship. This mechanism may relate to copper-induced oxidative damage to renal tubules (30). Additionally, elevated plasma cadmium concentrations were independently associated with long-term graft failure and declining renal function (31). Similarly, among 665 KTRs, plasma arsenic concentrations were independently associated with an 80% increased risk of late graft failure, with fish consumption identified as the primary source of arsenic exposure (32). In summary, existing epidemiological evidence consistently indicates that even low-level environmental heavy metal exposure, which is conventionally considered safe, is significantly associated with an increased risk of long-term graft failure in KTRs (33, 34). These findings are consistent with a causal relationship between heavy metal exposure and graft failure and highlight environmental heavy metal exposure as a critical, potentially modifiable risk factor for graft failure in KTRs. However, definitive confirmation of causality awaits support from intervention studies or quasi-experimental evidence. Although prospective cohort studies control major confounding factors through methods such as multivariable adjustment and reveal exposure-response relationships of public health significance, caution is still needed in causal inferences. Potential residual confounding, exposure misclassification, time-varying exposures, and competing risks may still affect the accuracy of effect estimates.
2.2 Summary of epidemiological evidence on heavy metal exposure and kidney transplant failure
Existing epidemiological evidence consistently indicates that even low-level environmental heavy metal exposure is significantly associated with an increased risk of long-term graft failure in KTRs. However, while current studies reveal these associations, they also expose several key issues. First, low-dose, long-term cumulative, and mixed exposures to multiple heavy metals represent the main realistic risk scenarios faced by transplant populations, and their combined toxic effects may be more complex. Second, exposure levels measured in biological matrices, such as blood or urine, may differ from the actual heavy metal load in transplant kidney tissue. Additionally, observational studies cannot entirely rule out reverse causation, in which declining kidney function reduces heavy metal excretion, leading to elevated blood concentrations. These factors limit the causal inference of the exposure-outcome relationship. Therefore, it is urgently necessary to elucidate the exact molecular pathways of heavy metal-induced nephrotoxicity and, on this basis, to conduct targeted interventional studies to consolidate the causal chain of this association and provide a solid foundation for the development of subsequent clinical prevention strategies.
3 Mechanisms by which heavy metal exposure affects kidney transplant outcomes
Although current observational studies have revealed an association between heavy metal exposure and adverse outcomes in kidney transplantation, and these studies’ differences in characterization provide targeted strategies for prevention and treatment, the specific mechanisms of action require further clarification. This section will elaborate on the potential mechanisms linking heavy metal exposure to kidney transplant failure and how such exposure influences the development of transplanted kidneys. The complex relationship between heavy metal exposure and adverse kidney transplant outcomes involves multiple pathways, including nephrotoxic effects, oxidative stress, and cumulative effects.
3.1 Heavy metal accumulation and specific vulnerabilities in transplanted organs
Heavy metals such as cadmium, lead, and arsenic exhibit a significant affinity for the kidneys (35). Following environmental or occupational exposure, they preferentially accumulate in the renal cortex (35). By inducing oxidative stress and mitochondrial dysfunction, they directly damage renal tubular epithelial cells and glomerular structures (31, 36). This accumulation phenomenon also occurs in transplanted kidneys, and because the kidneys are frequently hyper filtrated, the toxic effects may be further amplified. Even when circulating heavy metal concentrations remain within conventional detection ranges, their long-term accumulation in transplanted kidney tissue can persistently induce oxidative damage (29, 37). When multiple heavy metals co-expose, they may synergistically activate oxidative stress pathways, thereby accelerating the progression of renal injury (38, 39). Transplant recipients constitute a specialized group exhibiting heightened sensitivity to heavy metal toxicity. Long-term immunosuppressive therapy weakens the body’s antioxidant defenses, making transplanted kidneys more susceptible to oxidative stress attacks (34, 40). Additionally, certain immunomodulatory drugs may interfere with heavy metal metabolism or inhibit renal self-repair mechanisms, thereby increasing susceptibility to toxicity (37). Although total heavy metal accumulation in transplanted kidneys may be lower than in kidneys removed due to tumors, their intrinsic compensatory mechanisms against heavy metal toxicity are significantly weakened by prolonged subclinical inflammation (37). Transplanted kidneys often carry preexisting pathological foundations from ischemia-reperfusion injury or rejection-related conditions. Heavy metal exposure can further exacerbate oxidative damage, breaching physiological compensation thresholds (31, 40, 41). Moreover, with partial functional impairment in transplanted kidneys, the efficiency of heavy metal excretion decreases, leading to higher accumulation levels compared to normal kidneys (42–44). This accelerates the progression of renal fibrosis and chronic transplant nephropathy.
Exposure to heavy metals in the environment typically occurs as mixtures, which may produce additive or interactive effects when inducing nephrotoxicity (38, 39). For example, cadmium and arsenic may synergistically inhibit the Nrf2 antioxidant pathway, making cells more susceptible to oxidative damage (45, 46). However, the role of Nrf2 in heavy metal-induced nephrotoxicity is complex and dual. While chronic low-dose exposure to heavy metals is generally associated with Nrf2 suppression, recent evidence suggests that acute or high-intensity exposure may trigger persistent overactivation of Nrf2 signaling (47, 48). This abnormal sustained activation is not protective; it may lead to harmful consequences, including the induction of autophagy blockage and a vicious cycle of oxidative stress-autophagy inhibition, further weakening the cell’s antioxidant defenses (47, 48). Such overactivation can cause ‘transcriptional exhaustion,’ meaning that after long-term efforts to combat oxidative stress, the cell can no longer maintain subsequent antioxidant gene expression, leading to the collapse of the antioxidant defense system and autophagy dysfunction (47, 48). Moreover, persistent activation of Nrf2 can reprogram cellular metabolism and promote the expression of profibrotic factors, directly exacerbating renal tubular interstitial inflammation and fibrosis (49). This mechanism involves Nrf2 interfering with normal cellular signaling pathways, which under pathological conditions can transform into a pro-fibrotic effect (49). In the specific context of transplanted kidneys, the inherent oxidative stress state interacts with heavy metal exposure, making the Nrf2 pathway more prone to shift from early protective activation to decompensated dysfunction (50, 51). And lead and copper may have cumulative effects at the mitochondrial level, jointly aggravating disturbances in the electron transport chain and increasing ROS bursts (52). This complex interaction makes risk assessments based on a single metal likely to underestimate the actual health risk. Therefore, in future mechanistic and epidemiological studies, the use of advanced statistical models, such as weighted quantile regression and Bayesian kernel machine regression, is crucial for clarifying the overall effects of mixed heavy metal exposure on transplant kidney injury, identifying key driving components, and understanding potential interactions (53, 54).
3.2 Oxidative stress
Heavy metals are ubiquitous in the environment and occupational settings, entering the human body through multiple pathways and specifically accumulating in the renal cortex, thereby inducing significant nephrotoxic effects. Their core toxic mechanism is closely associated with the induction of oxidative stress (Figure 1). Heavy metals can disrupt the mitochondrial electron transport chain in renal tubules, leading to an increased production of superoxide anion (O2-) and hydrogen peroxide (H2O2) (55–58). Metal ions catalyze the Fenton reaction to generate large amounts of reactive oxygen species (ROS) (52, 59, 60). This process disrupts intracellular redox balance, continuously depleting endogenous antioxidants, such as glutathione, and directly inhibits the activity of key antioxidant enzymes, including superoxide dismutase (SOD) and glutathione peroxidase (GPx) (45, 58, 61). Furthermore, heavy metal exposure inhibits the activation of nuclear factor E2-related factor 2 (Nrf2), thereby obstructing the expression of downstream antioxidant genes (45, 46). In transplanted kidneys, heavy metal-induced ROS surges further lead to the depletion of antioxidant enzymes and the accumulation of lipid peroxides, directly damaging renal cell membrane structures and compromising DNA integrity (31, 38, 39, 43, 62). Oxidative damage ultimately triggers cellular harm, including lipid peroxidation, protein modification, and DNA breaks. This oxidative stress response not only directly damages tubular epithelial cells and glomerular structures, causing acute or chronic kidney injury, but also further activates inflammatory signaling pathways, including NF-κB and MAPK (60, 63, 64).In addition, different members of the mitogen-activated protein kinase (MAPK) signaling pathway play specific roles in heavy metal-associated kidney injury. This family mainly includes extracellular signal-regulated kinase (Erk), c-Jun N-terminal kinase (JNK), and p38 MAPK. The Erk pathway exhibits a dual function in heavy metal nephrotoxicity: while it primarily participates in cell proliferation and survival under physiological conditions, its sustained abnormal activation under heavy metal stress may mediate excessive proliferation and phenotypic transformation of intrinsic kidney cells, thereby promoting the progression of renal interstitial fibrosis (65, 66). Specifically, uranium can promote proliferation signals by enhancing Erk phosphorylation, whereas chromium inhibits its activity, weakening the cell’s self-repair ability; using an Erk inhibitor can effectively alleviate cadmium-triggered inflammatory responses (67–69). In contrast, the JNK and p38 pathways are generally considered core mediators of stress responses and are highly sensitive to oxidative stress caused by heavy metals. The JNK pathway primarily regulates apoptosis. Metals like cadmium and uranium induce a burst of reactive oxygen species (ROS), significantly increasing the p-JNK/JNK ratio, thereby activating the mitochondrial apoptosis pathway and leading to renal tubular epithelial cell death through a caspase-9/3 cascade reaction (67, 70–72). The p38 pathway primarily governs the initiation and amplification of inflammatory responses. Cadmium and lead promote p38 phosphorylation, further activating NF-κB and the NLRP3 inflammasome, leading to massive release of key inflammatory factors such as TNF-α and IL-1β (60, 69). Moreover, p38 can form a positive feedback loop with signaling pathways like endoplasmic reticulum stress, synergistically aggravating tissue damage (60, 73). It is noteworthy that there is close interaction among these MAPK sub-pathways. For instance, cadmium and chromium can jointly activate JNK and p38, synergistically enhancing apoptotic signals (72, 74).Different heavy metals also show pathway preferences, such as lead being more inclined to activate JNK (75). Upstream oxidative stress events collectively regulate all these pathways. ROS induced by cadmium, chromium, uranium, and other metals can directly phosphorylate and activate Erk, JNK, and p38, forming a core ROS-MAPK signaling axis (76). In summary, under heavy metal exposure, a sharp increase in ROS can simultaneously or preferentially activate distinct MAPK signaling pathways. This promotes the release of pro-inflammatory factors, such as TNF-α and IL-6, which drive localized chronic inflammation in the transplanted kidney and amplify its effects (60, 63, 64). Concurrently, ROS activates the caspase-dependent apoptosis pathway and induces TGF-β expression, leading to tubular epithelial cell apoptosis and subsequent interstitial fibrosis (31, 42, 58). In summary, oxidative stress-mediated cellular injury not only disrupts functional recovery in transplanted kidneys but may also contribute to acute rejection. Studies confirm that post-transplant oxidative stress levels serve as key biomarkers for acute rejection, inducing renal cell inflammation and necrosis that delay graft functional recovery or cause long-term dysfunction.
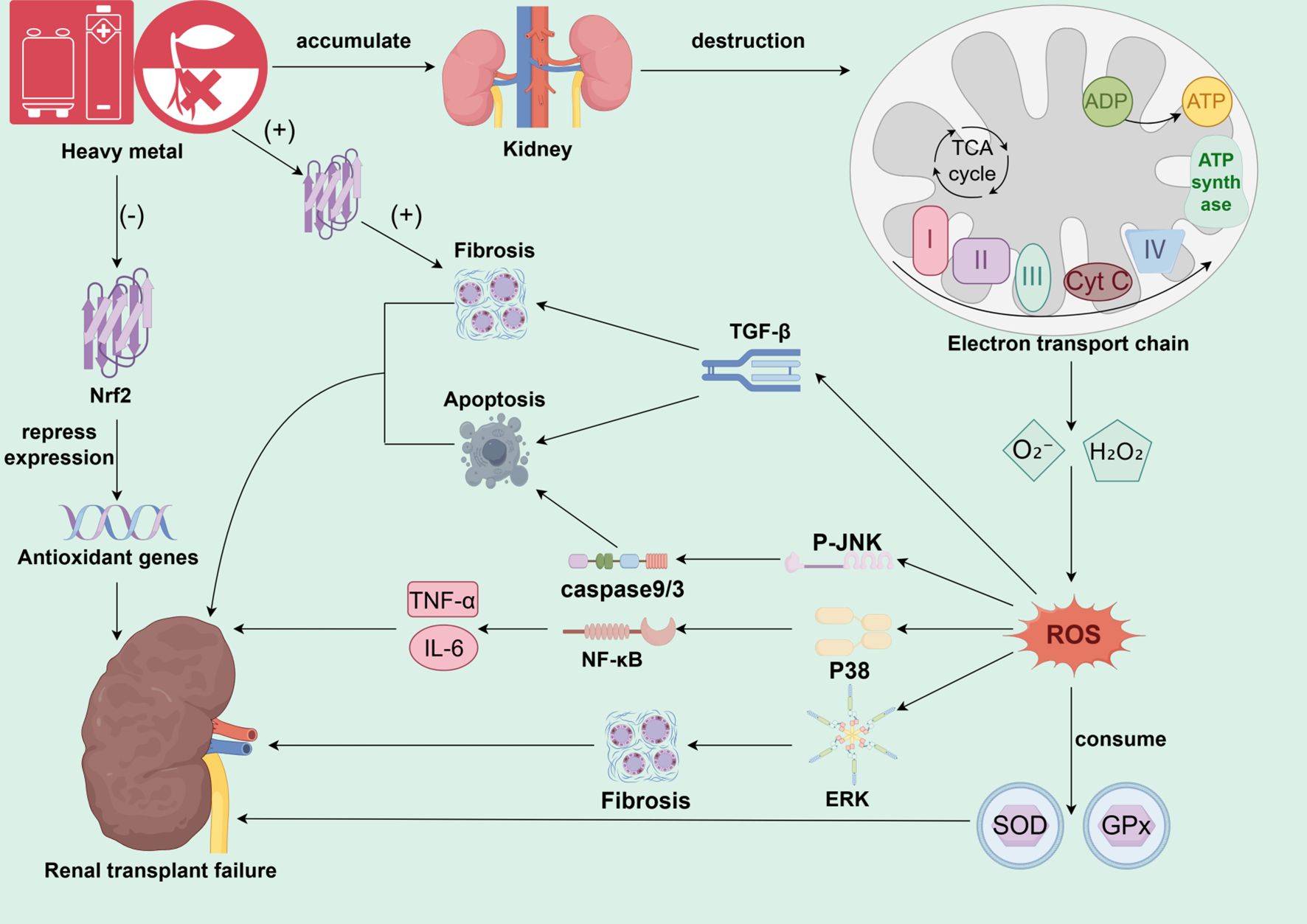
Figure 1. The core mechanism of heavy metal-induced oxidative stress and chronic injury in the transplanted kidney. This figure illustrates the core molecular pathways involved in environmental heavy metal exposure leading to transplanted kidney failure. Upon accumulation of heavy metals in the renal cortex, the primary target is to disrupt renal tubular mitochondrial function, inducing a burst of ROS generation by interfering with the electron transport chain and catalyzing the Fenton reaction. The dramatically elevated ROS disrupts cellular redox homeostasis, depletes endogenous antioxidants such as GSH, and inhibits key antioxidant enzymes, such as SOD and GPx, on the one hand. On the other hand, it leads to a comprehensive dysregulation of cellular antioxidant defense system by inhibiting or abnormally activating the Nrf2 pathway. Dysregulation of the cellular antioxidant defense system. Sustained oxidative damage triggers direct cellular damage such as lipid peroxidation, protein modification, and DNA breakage, and in turn activates three key MAPK signaling pathways (JNK, p38, Erk), which dominate apoptosis, inflammation, and fibrosis, respectively. Meanwhile, ROS also activate the NF-κB inflammatory pathway and TGF-β pro-fibrotic pathway. These pathways are intertwined, and together they lead to renal tubular epithelial cell death, chronic inflammation and interstitial fibrosis, which ultimately drive the progression of chronic transplant kidney injury until the loss of transplant kidney function.
3.3 Genetic susceptibility
Beyond the direct pathophysiological mechanisms, individual genetic susceptibility plays a pivotal role in modulating the risk and severity of heavy metal nephrotoxicity in KTRs. Genetic polymorphisms can influence an individual’s ability to metabolize, detoxify, and excrete heavy metals, as well as the resilience of renal tissue to the ensuing oxidative stress and inflammation (77). As highlighted by Glicklich et al. in their review on heavy metal toxicity in chronic kidney disease and cardiovascular disease, genetic variations are key determinants of inter-individual differences in toxicant handling and disease manifestation (77). This principle is highly relevant to the transplant population. Variations in genes involved in the glutathione (GSH) synthesis and conjugation pathways, such as glutathione S-transferases (GSTs), can significantly impact cellular antioxidant capacity and the elimination of heavy metals, thereby modifying the extent of oxidative damage (78, 79). Specific polymorphisms also illustrate gene-environment interactions that heighten susceptibility to renal injury. The AA genotype of rs13244925 in the epidermal growth factor receptor (EGFR) gene has been associated with higher estimated glomerular filtration rate (eGFR) in cadmium-exposed individuals, suggesting a protective role against cadmium nephrotoxicity that is absent in non-exposed groups (80). Similarly, single-nucleotide polymorphisms (SNPs) in the tumor necrosis factor-α (TNF-α) gene increase susceptibility to metal-induced CKD by upregulating transmembrane TNF-α expression, particularly worsening renal injury through chronic inflammation and fibrosis in advanced CKD (81). Polymorphisms in the vascular endothelial growth factor A (VEGFA) gene exacerbate the risk of renal dysfunction under co-exposure to lead and cadmium (81). In both additive and recessive genetic models, these SNPs—combined with urinary cadmium and blood lead levels—significantly modulate renal dysfunction risk, underscoring their role in altering baseline renal function and amplifying heavy metal toxicity. Furthermore, variants in inflammation-related genes, such as NLRP3, enhance fibrosis propensity in response to metal exposure, significantly elevating CKD risk by amplifying chronic inflammatory responses (82). Certain allelic combinations exert multiplicative effects, further increasing the risk of graft failure and highlighting how genetic variants promote susceptibility via renal interstitial fibrotic pathways. In kidney transplantation, this genetic predisposition interacts with the allograft’s unique milieu. The recipient’s genetic background controls systemic metal metabolism and immune-inflammatory responses, whereas the donor’s genetic makeup influences the kidney’s intrinsic resilience after transplantation. The two interact in a complex manner that ultimately determines the outcome of grafts under heavy metal exposure. Therefore, omitting genetic susceptibility from risk assessments may lead to an incomplete understanding and underestimation of threats faced by vulnerable KTR subpopulations.
4 Potential prevention and treatment strategies
To reduce the risk of graft failure in KTR due to heavy metal exposure, the researchers proposed a set of multi-level integrated intervention strategies (Figure 2). The system is based on environmental control and biomonitoring to reduce exposure at the source and achieve early warning. Based on this system, mitochondrial transplantation to restore cellular energy metabolism, probiotic interventions to minimize absorption and enhance antioxidant defense through the intestinal-renal axis, and natural antagonists to activate the endogenous antioxidant pathway are employed to address the key aspects of heavy metal-induced mitochondrial damage, oxidative stress, and intestinal absorption, thus synergizing to reduce secondary damage such as inflammation and fibrosis. It is important to note that several of the proposed strategies—namely mitochondrial transplantation, probiotic supplementation, and the use of natural antagonists—currently rest largely on preclinical evidence derived from animal studies and in vitro models. Although they represent promising therapeutic avenues, their safety, efficacy, and practical feasibility in the immunocompromised KTR population remain to be validated in future clinical trials. The following sections detail the mechanistic rationale and experimental support for each strategy, and address the challenges associated with their clinical translation.
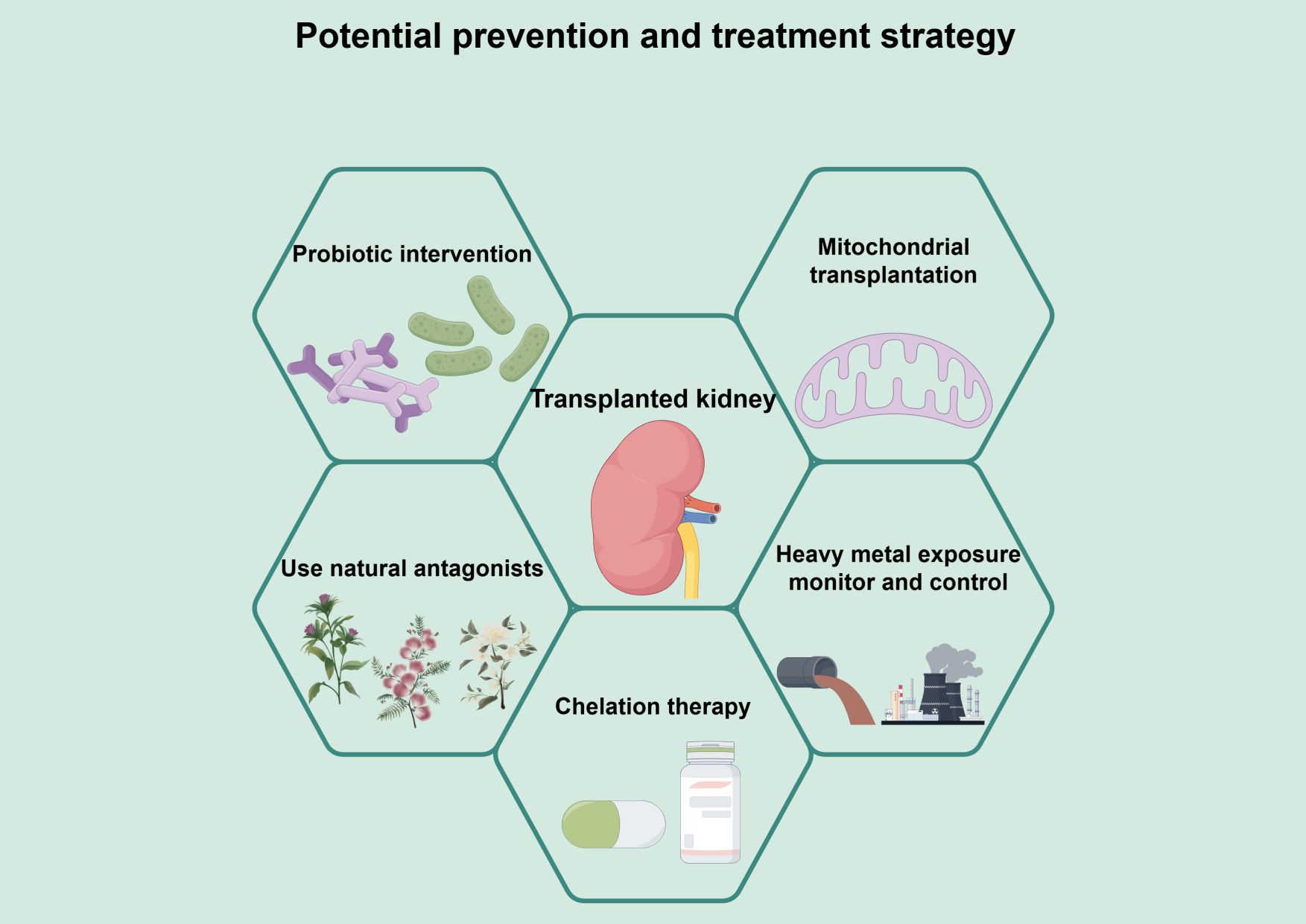
Figure 2. A multi-pronged management framework for mitigating heavy metal-associated injury in kidney transplant recipients. This figure presents a framework for a comprehensive intervention strategy to mitigate heavy metal toxicity and protect kidney function after transplantation. The framework is based on environmental control and biomonitoring to limit exposure through source reduction and early warning. On this basis, multiple intervention strategies are integrated. Mitochondrial transplantation can directly repair heavy metal-induced mitochondrial dysfunction and restore energy metabolism from the root. Probiotic intervention reduces the bioavailability of heavy metals through adsorption and chelation, enhances systemic antioxidant capacity, and improves intestinal barrier function through the intestinal-renal axis. Natural antagonists activate endogenous antioxidant pathways, such as Nrf-2/HO-1, and inhibit inflammation, thereby improving the function of transplanted kidneys. Natural antagonists activate endogenous antioxidant pathways such as Nrf-2/HO-1, which in turn inhibit inflammatory and apoptotic signaling. Chelation therapy may be considered to promote heavy metal excretion after cautious evaluation of patients with clearly excessive body burdens. These strategies target the key components of heavy metal toxicity - mitochondrial damage, intestinal absorption, oxidative stress, and body load - synergistically, and work together to mitigate secondary damage, such as inflammation and fibrosis, to decrease heavy metal accumulation, alleviating oxidative stress, and thus enhancing renal transplantation function.
4.1 Mitochondrial transplantation
To address the risk of renal toxicity and transplant failure associated with heavy metal exposure, mitochondrial transplantation has emerged as a promising but experimental therapeutic strategy in preclinical research. Heavy metals primarily damage renal tubes by inducing oxidative stress, disrupting mitochondrial function, and triggering apoptosis, thereby significantly increasing the risk of graft loss (83–85). Further research indicates that heavy metals specifically damage highly metabolically active mitochondria in the kidney, disrupting their energy supply and waste-clearance capabilities, thereby accelerating post-transplant renal deterioration (42, 44, 85). Mitochondrial transplantation directly repairs heavy metal-induced mitochondrial dysfunction by exogenously supplying healthy mitochondria, effectively reversing oxidative stress and cellular damage (86–89). Preclinical studies in a cadmium-induced nephrotoxicity model have shown that this therapy significantly elevated intracellular reduced glutathione levels, enhanced Bcl-2 anti-apoptotic protein expression, and inhibited caspase-3 activity (86). This promoted ATP synthesis recovery and renal tubular structural regeneration, improving renal function indicators and reducing histopathological damage (86–88). Additionally, mitochondrial transplantation mitigates immunosuppressant-related mitochondrial toxicity and alleviates postoperative oxidative stress, thereby contributing to a comprehensive reduction in the risk of graft dysfunction (90, 91). Although still far from clinical application, mitochondrial transplantation theoretically offers a novel intervention approach in kidney transplantation. However, the promising evidence for mitochondrial transplantation currently derives primarily from preclinical animal studies and in vitro models. Its clinical translation faces several bottlenecks, including the standardized sourcing of viable mitochondria, the risk of immune reactions to allogeneic organelles, the development of safe and efficient in vivo delivery methods to the kidneys, and navigating the substantial regulatory and ethical hurdles associated with such a novel cellular therapy.
4.2 Probiotic intervention
Experimental research, primarily in animal models, indicates that probiotics, such as Lactobacillus and Bacillus, can significantly reduce markers of oxidative damage in kidney tissue and restore antioxidant enzyme activity through their antioxidant properties. For instance, probiotic supplementation after nickel exposure upregulates protective gene expression in the kidneys, reversing the suppression of gene expression caused by heavy metals (63). This antioxidant effect is particularly crucial for immunosuppressed kidneys post-transplantation, mitigating secondary oxidative damage (63). Heavy metal exposure disrupts gut microbiota, exacerbating renal burden through the leaky gut effect. Probiotics directly reduce intestinal heavy metal absorption through mechanisms such as biosorption and bioaccumulation, while repairing intestinal barrier functions to block the migration of inflammatory factors to the kidneys (92–94). Clinical studies in occupationally exposed populations demonstrate that probiotic-containing yogurt reduces urinary cadmium levels by 60%-72% in occupationally exposed populations, while promoting the proliferation of the beneficial gut bacterium Bifidobacterium (95, 96). The specific probiotic strain Lactobacillus acidophilus exhibits a high-affinity binding capacity for cadmium, mercury, and other heavy metals, converting them into less toxic forms for excretion via surface proteins or metabolic enzymes (96–98). For instance, probiotic strains isolated from kimchi significantly reduced renal cadmium accumulation and alleviated tubular necrosis in mice (97). This targeted detoxification reduces heavy metal burden in transplanted kidneys. Moreover, probiotics modulate the Th1/Th2 immune balance to mitigate chronic inflammation induced by heavy metals (92, 99). In KTRs, this immunoregulation may help mitigate synergistic damage from transplant rejection and heavy metal toxicity. Furthermore, synergistic supplementation with probiotics and minerals, such as calcium/iron can further reduce cadmium bioavailability, offering novel strategies for post-transplant nutritional support (94). In summary, preclinical evidence suggests that probiotics may serve as an adjunctive therapy to mitigate the risks of heavy metal exposure in transplanted kidneys through multi-pathway actions involving intestinal detoxification, systemic antioxidant effects, and renal protection (92, 100). While the efficacy of probiotics against heavy metals is supported by studies in occupationally exposed and general populations, direct evidence in KTRs is lacking. Critical safety considerations in this immunocompromised cohort include the risk of probiotic-derived infections and potential interactions with immunosuppressive drugs. Future research should prioritize randomized feasibility trials with endpoints focusing on probiotic tolerability, alongside pharmacokinetic monitoring of immunosuppressants and indicators of metal excretion.
4.3 The use of natural antagonists
Exposure to heavy metals constitutes a significant risk factor for graft failure in KTRs, with its toxic effects primarily mediated through inducing oxidative stress and precipitating renal cellular damage (31, 34). Natural antagonists, including plant extracts, polyphenols, and hormonal substances, have shown potential in experimental settings to alleviate oxidative stress and delay the decline in transplanted kidney function by activating endogenous antioxidant pathways. For instance, melatonin not only suppresses the production of reactive oxygen species (ROS) and enhances superoxide dismutase (SOD) activity, but also reduces heavy metal accumulation in the kidneys, thereby exerting renal protective effects (101). Polyphenolic extracts derived from seaweed improve renal function indicators by directly counteracting heavy metal-induced oxidative stress (102). Furthermore, the compound OOP isolated from traditional Chinese medicine has demonstrated pivotal value in preventing and treating transplant-associated renal injury by inhibiting tubular epithelial cell apoptosis and mitigating oxidative damage through the activation of the Nrf2/HO-1 signaling pathway (103). The mechanisms of action for these natural compounds include enhancing systemic antioxidant capacity and directly chelating heavy metals to reduce their bioavailability, thereby achieving multi-pathway renal protection (104, 105). At the therapeutic strategy level, natural antagonists have demonstrated efficacy in preclinical models. For instance, certain plant extracts, acting as potent antioxidants, significantly alleviate cadmium-induced oxidative stress and renal structural damage. Modulating key signaling pathways further amplifies antioxidant responses, offering potential intervention targets for renal transplant recipients (45, 104, 106). Despite bioavailability limitations, experimental delivery strategies, such as nanomedicines, are being explored in early-stage research to enhance their in vivo efficacy and applicability (107, 108). In summary, natural antagonists demonstrate promising prospects for counteracting heavy metal-related transplanted kidney injury through their multi-mechanism synergistic effects. The translation of natural antagonists is constrained by questions regarding optimal dosing, bioavailability of conventional formulations, and their intricate pharmacokinetic profiles. The development of novel delivery systems, such as nanomedicines, must therefore prioritize the validation of their safety profile and exclusion of any adverse interference with the transplant pharmacotherapy.
However, when considering any interventional strategy to promote heavy metal excretion, a critical clinical paradox must be acknowledged. The excretion process itself may transiently increase the renal toxicant load, potentially exacerbating kidney injury (109). This is particularly evident in the treatment of cadmium poisoning. Although chelating agents are conventional methods for cadmium removal, clinical observations indicate that mobilization of cadmium from storage sites during treatment can lead to a temporary surge in blood and renal cadmium concentrations (110). This surge may precipitate a wave of “secondary injury” in individuals with pre-existing renal impairment (111). Therefore, for KTRs, a vulnerable group with limited functional reserve and whose transplanted kidneys often exhibit subclinical injury, any proactive detoxification treatment must be approached with great caution. Future research should focus on developing gentler, more controlled detoxification strategies and on incorporating close monitoring of early kidney injury biomarkers during treatment. The paramount principle must be the absolute priority of protecting graft function throughout the detoxification process.
4.4 Chelation therapy
For KTRs whose body loads of specific heavy metals have been definitively exceeded, chelation therapy is a way to reduce toxic metal loads through medication directly. As comprehensively reviewed by Glicklich and Frishman, there is a strong case for screening for heavy metal exposure in at-risk populations, including those with chronic kidney disease, as elevated levels of heavy metals are associated with a poorer prognosis (112). Chelating agents (e.g., EDTA) act by forming stable, water-soluble complexes with metal ions in the bloodstream, thereby promoting their excretion through the urine (110, 112, 113). Evidence from studies of non-transplanted patients with chronic kidney disease and confirmed lead toxicity suggests that chelation therapy improves renal function and slows disease progression (77, 112). This provides a rationale for considering this therapy in selected KTRs. However, the application of chelation therapy in KTRs requires extreme caution due to the vulnerability of transplanted organs. The process of mobilizing metals from tissue stores may temporarily increase the concentration of nephrotoxic substances flowing through the renal tubules, thus posing a risk of “secondary injury” to the graft (110, 114, 115). Transplanted kidneys, which often have a reduced functional reserve and are already subclinical compromised, may be particularly vulnerable to such damage. Therefore, the decision to use chelation therapy for KTR must be individualized and implemented only after careful risk-benefit assessment. It should be limited to those cases where there is clear evidence that high heavy metal loads are associated with progressive decline in graft function and where environmental exposures have been clearly controlled. Treatment must begin at low doses and be administered under close clinical supervision with strict monitoring of graft function, electrolytes, and renal injury biomarkers. Collaboration between transplant nephrologists and clinical toxicologists is essential to navigate this complex therapeutic area. Future studies are needed to develop specific safety and efficacy protocols for chelation therapy for the KTR population.
4.5 Environmental heavy metal exposure control and monitoring
Environmental heavy metal exposure is widely recognized as a significant risk factor for kidney damage, particularly for KTRs, where even exposure levels within the normal range may increase the risk of graft failure. Research indicates that heavy metal nephrotoxicity arises from their accumulation in renal tissue following environmental exposure, triggering oxidative stress and inflammatory responses that reduce glomerular filtration rate and lead to graft dysfunction (31, 42, 116). Prospective cohort analyses demonstrate that elevated plasma cadmium levels are significantly associated with an increased risk of kidney transplant failure, independent of other clinical factors, with a progressive increase in risk corresponding to longer exposure duration (31). In the general population, mixed heavy metal exposure may also accelerate chronic kidney injury progression, posing greater threats to transplant recipients with pre-existing renal impairment (53, 116, 117). Environmental exposure control is a core strategy for mitigating risks. This includes reducing heavy metal concentrations in ecological media through regulating pollution sources and implementing environmental protection policies. Strict management of drinking water sources, air particulate matter, and soil contaminants can significantly lower ingestion exposure risks. Research confirms that air pollution exposure is significantly and positively correlated with mortality, graft failure, and rejection risks among KTRs, underscoring the importance of pollution control as a modifiable environmental risk factor (117–119). Furthermore, assessing total environmental exposure levels is crucial for developing targeted interventions, such as using biomarkers to monitor heavy metal concentrations or conducting exposure assessments in polluted areas (120–122). Concurrently, monitoring exposure levels forms the foundation of risk management. Systematic monitoring involves real-time evaluation of heavy metal concentrations in environmental and biological samples to identify high-risk populations and regions. For instance, epidemiological studies recommend using urinary cadmium and lead levels as exposure indicators, combined with early biomarkers of kidney injury, to enable stratified monitoring of susceptibility in KTRs (53, 116, 123, 124). This monitoring not only aids in identifying exposure sources but also supports the development of combined exposure risk models to predict graft failure trends and optimize prevention strategies (54, 122). Finally, comprehensive prevention measures must integrate control and monitoring, including reducing individual exposure, strengthening occupational and community regulations, and promoting mechanism-based interventions such as drug development targeting metal excretion pathways (44, 122). Future efforts should involve multidisciplinary collaboration to optimize pollution management, thereby reducing the burden of heavy metal-related nephrotoxicity and improving long-term outcomes in kidney transplantation.
5 Clinical management stratification and process recommendations
Given the risks above, it is crucial to integrate management of environmental heavy metal exposure into the long-term follow-up system for KTRs, and a stratified management strategy is recommended. For high-risk recipients living in highly polluted areas or engaged in related occupations, baseline assessments should be conducted upon enrollment. Exposure biomarkers, such as urinary cadmium and blood lead, should be monitored regularly every 6–12 months during follow-up, along with early kidney injury markers, including kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, and β2-microglobulin, to capture subclinical toxicity sensitively (125–127). Early indicators of heavy metal-induced kidney injury are key to identifying this injury and predicting graft outcomes in KTRs. Oxidative stress-related biomarkers, such as 8-hydroxy-2’-deoxyguanosine (8-OHdG), protein carbonyls, and the redox ratio (GSH/GSSG), can directly reflect free-radical damage induced by heavy metal exposure (128, 129). Elevated urinary 8-OHdG is closely associated with heavy metal exposure and, as a reliable marker of DNA oxidative damage, can be used to assess the risk of early subclinical kidney injury (128, 129). F2-isoprostanes and oxidized albumin profiling enhance the monitoring of lipid peroxidation and protein modification, supplementing the prediction of tubular interstitial injury. Meanwhile, quantifying inflammatory cytokine profiles, such as IL-6 and TNF-α, can reveal chronic inflammatory responses triggered by heavy metals, which are related to graft rejection and long-term functional decline (34, 130). The combined use of these indicators, such as KIM-1 and NGAL, has been shown to effectively distinguish between exposed and non-exposed groups, providing early warning signals (128, 131, 132). Regarding lifestyle, specific guidance should be provided, including reducing the intake of seafood known to be high in heavy metals, being aware of heavy metals contamination risks in rice and drinking water, and supplementing sufficient minerals such as selenium and calcium to reduce intestinal absorption of heavy metals through competitive inhibition (133–138). When monitoring indicates elevated exposure levels, a structured intervention pathway should be initiated. First, potential sources of exposure in personal life and work environments should be actively investigated. Then, supportive measures can be taken, such as nutritional interventions, probiotic supplementation, or the use of natural antioxidants. Additionally, consider consulting with occupational or environmental health experts to identify and control source exposure. Throughout the process, the most critical aspect is maintaining close coordination with the core management of the transplant team, ensuring that any interventions do not compromise blood concentrations or the efficacy of immunosuppressive drugs, thereby reducing heavy metal toxicity while safeguarding the transplanted kidney’s long-term survival.
6 Future research directions
Future research should focus on deepening our understanding of the association between heavy metal exposure and kidney transplant outcomes across multiple levels, while advancing clinical translation. At the mechanistic level, multi-omics technologies should be employed to elucidate interactions among core pathways within the unique microenvironment of transplanted kidneys, such as oxidative stress, inflammation, and fibrosis, with particular emphasis on the amplified effects of these processes under immunosuppression. Regarding exposure assessment and risk prediction, establish comprehensive risk models for mixed heavy metal exposure using large-scale prospective cohorts, integrating factors such as genetic susceptibility and immune status to enable precise risk stratification. Simultaneously develop novel biomarkers capable of early detection of heavy metal accumulation and subclinical injury within transplanted kidneys. At the level of genetic susceptibility, future studies should integrate genetic and epigenetic analyses to identify variants that confer heightened risk from heavy metal exposure in KTRs. Building polygenic risk scores that incorporate key polymorphisms in metal-handling and antioxidant pathways could enable more precise stratification of patients and personalized management strategies. At the clinical translation level, the critical task is to design rigorous intervention trials to validate novel therapeutic strategies that target antioxidant defense, maintain mitochondrial function, and modulate the effects of probiotics. The feasibility of incorporating environmental exposure control into comprehensive post-transplant management should be evaluated, ultimately translating research findings into clinical guidelines and public health policies that effectively enhance graft long-term survival.
7 Conclusions
This review systematically elucidates that environmental and occupational heavy metal exposure constitutes a significant threat to the long-term prognosis of KTRs. Through accumulation in the renal cortex, induction of mitochondrial dysfunction, and catalysis of Fenton reactions, these metals trigger bursts of reactive oxygen species that disrupt intracellular redox homeostasis. This cascade activates inflammatory signaling pathways, promotes the progression of fibrosis, and induces apoptosis, ultimately accelerating the progression of chronic transplant kidney injury. Given the graft’s inherent susceptibility, immunosuppressed state, and potential impaired excretory function, these toxic effects are further amplified. Therefore, recognizing environmental heavy metal exposure as a key modifiable risk factor and incorporating targeted monitoring and protective strategies into clinical management are crucial for improving long-term graft survival.
Author contributions
SL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. HC: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. BW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YQ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. BY: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. WW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. WU JIEPING Medical Foundation (NO.3D4240299428).
Acknowledgments
All figures in this article were drawn by Figdraw (https://www.figdraw.com/#/).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ROS, reactive oxygen species; Nrf2, nuclear factor erythroid 2–related factor 2; TGF-β, transforming growth factor-β; KTRs, kidney transplant recipients; GSH, glutathione; SOD, superoxide dismutase; WQS, weighted quantile sum regression; BKMR, Bayesian kernel machine regression; eGFR, estimated glomerular filtration rate; EGFR, epidermal growth factor receptor; GSTs, glutathione s-transferases.
References
1. Parida L and Patel TN. Systemic impact of heavy metals and their role in cancer development: a review. Environ Monit Assess. (2023) 195:766. doi: 10.1007/s10661-023-11399-z
2. Misra SK, Kumar A, Pathak K, Kumar G, and Virmani T. Role of genetically modified microorganisms for effective elimination of heavy metals. BioMed Res Int. (2024) 2024:9582237. doi: 10.1155/2024/9582237
3. Chen YG, He XL, Huang JH, Luo R, Ge HZ, Wołowicz A, et al. Impacts of heavy metals and medicinal crops on ecological systems, environmental pollution, cultivation, and production processes in China. Ecotoxicol Environ Saf. (2021) 219:112336. doi: 10.1016/j.ecoenv.2021.112336
4. Wu B, Ga L, Wang Y, and Ai J. Recent advances in the application of bionanosensors for the analysis of heavy metals in aquatic environments. Molecules. (2023) 29:34. doi: 10.3390/molecules29010034
5. Alotaibi BS, Khan M, and Shamim S. Unraveling the underlying heavy metal detoxification mechanisms of bacillus species. Microorganisms. (2021) 9:1628. doi: 10.3390/microorganisms9081628
6. Wang B, Lan J, Bo C, Gong B, and Ou J. Adsorption of heavy metal onto biomass-derived activated carbon: review. RSC Adv. (2023) 13:4275–302. doi: 10.1039/d2ra07911a
7. Kotnala S, Tiwari S, Nayak A, Bhushan B, Chandra S, Medeiros CR, et al. Impact of heavy metal toxicity on the human health and environment. Sci Total Environ. (2025) 987:179785. doi: 10.1016/j.scitotenv.2025.179785
8. Zhao K, Wang Q, Qian S, and Li F. Spatial and temporal distribution characteristics of antibiotics and heavy metals in the Yitong River basin and ecological risk assessment. Sci Rep. (2023) 13:4202. doi: 10.1038/s41598-023-31471-5
9. Li S, Lin Q, Shen JW, Wei Y, and Wang C. Co-adsorption and co-removal of cu(II), ciprofloxacin, and norfloxacin from water by a modified three-component covalent organic framework. Langmuir. (2025) 13:4202. doi: 10.1021/acs.langmuir.5c02394
10. Blagojev N, Vasić V, Kukić D, Šćiban M, Prodanović J, and Bera O. Modelling and efficiency evaluation of the continuous biosorption of Cu(II) and Cr(VI) from water by agricultural waste materials. J Environ Manage. (2021) 281:111876. doi: 10.1016/j.jenvman.2020.111876
11. Li G, Chen R, Li Z, Wu X, Xiang K, Wang C, et al. Ecological risk and human health assessment of heavy metals in sediments of datong lake. Toxics. (2025) 13:560. doi: 10.3390/toxics13070560
12. Rong S, Wu J, Cao X, and Sun Y. Comprehensive ecological risk assessment of heavy metals based on species sensitivity distribution in aquatic of coastal areas in hong kong. Int J Environ Res Public Health. (2022) 19:13376. doi: 10.3390/ijerph192013376
13. Uysal E, Dursun HN, Güler R, Takmaz U, Küt A, Çeri M, et al. Waste refractory brick material added chitosan/oxidized pullulan complex gel production and removal of heavy metals from waste water. Sci Rep. (2024) 14:26229. doi: 10.1038/s41598-024-72187-4
14. Panghal A, Thakur A, Deore MS, Goyal M, Singh C, and Kumar J. Multimetal exposure: Challenges in diagnostics, prevention, and treatment. J Biochem Mol Toxicol. (2024) 38:e23745. doi: 10.1002/jbt.23745
15. Paduraru E, Iacob D, Rarinca V, Plavan G, Ureche D, Jijie R, et al. Zebrafish as a potential model for neurodegenerative diseases: A focus on toxic metals implications. Int J Mol Sci. (2023) 24:3428. doi: 10.3390/ijms24043428
16. Yüce Y and Can Eke B. Investigation of some metal levels in people using electronic cigarettes and IQOS. Toxicol Mech Methods. (2025) 35:1023–38. doi: 10.1080/15376516.2025.2506796
17. Gu J, Dai S, Liu Y, Liu H, Zhang Y, Ji X, et al. Activation of Ca(2+)-sensing receptor as a protective pathway to reduce Cadmium-induced cytotoxicity in renal proximal tubular cells. Sci Rep. (2018) 8:1092. doi: 10.1038/s41598-018-19327-9
18. Ge J, Huang Y, Lv M, Zhang C, Talukder M, Li J, et al. Cadmium induced Fak -mediated anoikis activation in kidney via nuclear receptors (AHR/CAR/PXR)-mediated xenobiotic detoxification pathway. J Inorg Biochem. (2022) 227:111682. doi: 10.1016/j.jinorgbio.2021.111682
19. Ou YC, Li JR, Wu CC, Yu TM, Chen WY, Liao SL, et al. Cadmium induces the expression of Interleukin-6 through Heme Oxygenase-1 in HK-2 cells and Sprague-Dawley rats. Food Chem Toxicol. (2022) 161:112846. doi: 10.1016/j.fct.2022.112846
20. Deng P, Li J, Lu Y, Hao R, He M, Li M, et al. Chronic cadmium exposure triggered ferroptosis by perturbing the STEAP3-mediated glutathione redox balance linked to altered metabolomic signatures in humans. Sci Total Environ. (2023) 905:167039. doi: 10.1016/j.scitotenv.2023.167039
21. Chou X, Ding F, Zhang X, Ding X, Gao H, and Wu Q. Sirtuin-1 ameliorates cadmium-induced endoplasmic reticulum stress and pyroptosis through XBP-1s deacetylation in human renal tubular epithelial cells. Arch Toxicol. (2019) 93:965–86. doi: 10.1007/s00204-019-02415-8
22. Osukoya OA, Oyinloye BE, Ajiboye BO, Olokode KA, and Adeola HA. Nephroprotective and anti-inflammatory potential of aqueous extract from Persea americana seeds against cadmium-induced nephrotoxicity in Wistar rats. Biometals. (2021) 34:1141–53. doi: 10.1007/s10534-021-00333-w
23. Cui J, Liu Y, Hao Z, Liu Y, Qiu M, Kang L, et al. Cadmium induced time-dependent kidney injury in common carp via mitochondrial pathway: Impaired mitochondrial energy metabolism and mitochondrion-dependent apoptosis. Aquat Toxicol. (2023) 261:106570. doi: 10.1016/j.aquatox.2023.106570
24. Alanazi ST, Harisa GI, and Salama SA. Modulating SIRT1, Nrf2, and NF-κB signaling pathways by bergenin ameliorates the cadmium-induced nephrotoxicity in rats. Chem Biol Interact. (2024) 387:110797. doi: 10.1016/j.cbi.2023.110797
25. Akinleye A, Oremade O, and Xu X. Exposure to low levels of heavy metals and chronic kidney disease in the US population: A cross sectional study. PloS One. (2024) 19:e0288190. doi: 10.1371/journal.pone.0288190
26. Farkhondeh T, Naseri K, Esform A, Aramjoo H, and Naghizadeh A. Drinking water heavy metal toxicity and chronic kidney diseases: a systematic review. Rev Environ Health. (2021) 36:359–66. doi: 10.1515/reveh-2020-0110
27. Sorensen CJ, Butler-Dawson J, Dally M, Krisher L, Griffin BR, Johnson RJ, et al. Risk factors and mechanisms underlying cross-shift decline in kidney function in Guatemalan sugarcane workers. J Occup Environ Med. (2019) 61:239–50. doi: 10.1097/jom.0000000000001529
28. Satarug S, Gobe GC, Ujjin P, and Vesey DA. A comparison of the nephrotoxicity of low doses of cadmium and lead. Toxics. (2020) 8:18. doi: 10.3390/toxics8010018
29. Sotomayor CG, Giubergia F, Groothof D, Ferreccio C, Nolte IM, Navis GJ, et al. Plasma lead concentration and risk of late kidney allograft failure: findings from the transplantLines biobank and cohort studies. Am J Kidney Dis. (2022) 80:87–97.e81. doi: 10.1053/j.ajkd.2021.10.009
30. Yepes-Calderon M, Kremer D, Post A, Sotomayor CG, Seidel U, Huebbe P, et al. Urinary copper excretion is associated with long-term graft failure in kidney transplant recipients. Am J Nephrol. (2023) 54:425–33. doi: 10.1159/000531147
31. Sotomayor CG, Groothof D, Vodegel JJ, Eisenga MF, Knobbe TJ, J IJ, et al. Plasma cadmium is associated with increased risk of long-term kidney graft failure. Kidney Int. (2021) 99:1213–24. doi: 10.1016/j.kint.2020.08.027
32. Sotomayor CG, Groothof D, Vodegel JJ, Gacitúa TA, Gomes-Neto AW, Osté MCJ, et al. Circulating arsenic is associated with long-term risk of graft failure in kidney transplant recipients: A prospective cohort study. J Clin Med. (2020) 9:417. doi: 10.3390/jcm9020417
33. Roels HA, Hoet P, and Lison D. Usefulness of biomarkers of exposure to inorganic mercury, lead, or cadmium in controlling occupational and environmental risks of nephrotoxicity. Ren Fail. (1999) 21:251–62. doi: 10.3109/08860229909085087
34. Kremer D, Riemersma NL, Groothof D, Sotomayor CG, Eisenga MF, Post A, et al. Plasma thallium concentration, kidney function, nephrotoxicity and graft failure in kidney transplant recipients. J Clin Med. (2022) 11:1970. doi: 10.3390/jcm11071970
35. Lentini P, Zanoli L, Granata A, Signorelli SS, Castellino P, and Dell'Aquila R. Kidney and heavy metals - The role of environmental exposure (Review). Mol Med Rep. (2017) 15:3413–9. doi: 10.3892/mmr.2017.6389
36. Kosiba AA, Wang Y, Chen D, Wong CKC, Gu J, and Shi H. The roles of calcium-sensing receptor (CaSR) in heavy metals-induced nephrotoxicity. Life Sci. (2020) 242:117183. doi: 10.1016/j.lfs.2019.117183
37. Wilk A, Kalisińska E, Kosik-Bogacka DI, Romanowski M, Różański J, Ciechanowski K, et al. Cadmium, lead and mercury concentrations in pathologically altered human kidneys. Environ Geochem Health. (2017) 39:889–99. doi: 10.1007/s10653-016-9860-y
38. Yin G, Zhao S, Zhao M, Xu J, Ge X, Wu J, et al. Complex interplay of heavy metals and renal injury: New perspectives from longitudinal epidemiological evidence. Ecotoxicol Environ Saf. (2024) 278:116424. doi: 10.1016/j.ecoenv.2024.116424
39. Tian X, Shan X, Ma L, Zhang C, Wang M, Zheng J, et al. Mixed heavy metals exposure affects the renal function mediated by 8-OHG: A cross-sectional study in rural residents of China. Environ pollut. (2023) 317:120727. doi: 10.1016/j.envpol.2022.120727
40. Tanaka R, Imafuku T, Suzuki Y, Nishida K, Matsusaka K, Shin T, et al. Changes in redox state of albumin before and after kidney transplantation in patients with end-stage renal disease. Clin Biochem. (2020) 81:20–6. doi: 10.1016/j.clinbiochem.2020.04.010
41. Rodriguez-Sanchez E, Aceves-Ripoll J, Mercado-García E, Navarro-García JA, Andrés A, Aguado JM, et al. Donor-dependent variations in systemic oxidative stress and their association with one-year graft outcomes in kidney transplantation. Am J Nephrol. (2024) 55:509–19. doi: 10.1159/000539509
42. Pócsi I, Dockrell ME, and Price RG. Nephrotoxic biomarkers with specific indications for metallic pollutants: implications for environmental health. biomark Insights. (2022) 17:11772719221111882. doi: 10.1177/11772719221111882
43. Kribi-Boukhris SE, Boughattas I, Zitouni N, Helaoui S, Sappin-Didier V, Coriou C, et al. Ecotoxicity of trace elements to chicken GALLUS gallus domesticus exposed to a gradient of polymetallic-polluted sites. Environ pollut. (2020) 265:114831. doi: 10.1016/j.envpol.2020.114831
44. Ren H and Shen X. Molybdenum exposure causes kidney damage related to the excretion of heavy metals. Ecotoxicol Environ Saf. (2025) 302:118635. doi: 10.1016/j.ecoenv.2025.118635
45. Chen J, Guo G, Wang X, Li Z, Ji T, Li Y, et al. BRD4 mediates cadmium-induced oxidative stress and kidney injury in mice via disruption of redox homeostasis. Toxics. (2025) 13:258. doi: 10.3390/toxics13040258
46. Muthusamy S, Peng C, and Ng JC. Effects of multi-component mixtures of polyaromatic hydrocarbons and heavy metal/loid(s) on Nrf2-antioxidant response element (ARE) pathway in ARE reporter-HepG2 cells. Toxicol Res (Camb). (2016) 5:1160–71. doi: 10.1039/c6tx00024j
47. Fan RF, Tang KK, Wang ZY, and Wang L. Persistent activation of Nrf2 promotes a vicious cycle of oxidative stress and autophagy inhibition in cadmium-induced kidney injury. Toxicology. (2021) 464:152999. doi: 10.1016/j.tox.2021.152999
48. Lian CY, Chu BX, Xia WH, Wang ZY, Fan RF, and Wang L. Persistent activation of Nrf2 in a p62-dependent non-canonical manner aggravates lead-induced kidney injury by promoting apoptosis and inhibiting autophagy. J Adv Res. (2023) 46:87–100. doi: 10.1016/j.jare.2022.04.016
49. Bian Y, Dong J, Zhou Z, Zhou H, Xu Y, Zhang Q, et al. The spatiotemporal and paradoxical roles of NRF2 in renal toxicity and kidney diseases. Redox Biol. (2025) 79:103476. doi: 10.1016/j.redox.2024.103476
50. Ng C, Kim M, Yanti, and Kwak MK. Oxidative stress and NRF2 signaling in kidney injury. Toxicol Res. (2025) 41:131–47. doi: 10.1007/s43188-024-00272-x
51. Nezu M, Suzuki N, and Yamamoto M. Targeting the KEAP1-NRF2 system to prevent kidney disease progression. Am J Nephrol. (2017) 45:473–83. doi: 10.1159/000475890
52. Paithankar JG, Saini S, Dwivedi S, Sharma A, and Chowdhuri DK. Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction. Chemosphere. (2021) 262:128350. doi: 10.1016/j.chemosphere.2020.128350
53. An Q, Wang Q, Liu R, Zhang J, Li S, Shen W, et al. Analysis of relationship between mixed heavy metal exposure and early renal damage based on a weighted quantile sum regression and Bayesian kernel machine regression model. J Trace Elem Med Biol. (2024) 84:127438. doi: 10.1016/j.jtemb.2024.127438
54. Liu J, Liu R, Zhang Z, Zhang H, Cai Y, Yang Z, et al. Copula-based exposure risk dynamic simulation of dual heavy metal mixed pollution accidents at the watershed scale. J Environ Manage. (2021) 277:111481. doi: 10.1016/j.jenvman.2020.111481
55. Rawee P, Kremer D, Nolte IM, Leuvenink HGD, Touw DJ, De Borst MH, et al. Iron deficiency and nephrotoxic heavy metals: A dangerous interplay? Int J Mol Sci. (2023) 24:5315. doi: 10.3390/ijms24065315
56. Tahir I and Alkheraije KA. A review of important heavy metals toxicity with special emphasis on nephrotoxicity and its management in cattle. Front Vet Sci. (2023) 10:1149720. doi: 10.3389/fvets.2023.1149720
57. Yang Z, He Y, Ma Q, Wang H, and Zhang Q. Alleviative effect of melatonin against the nephrotoxicity induced by cadmium exposure through regulating renal oxidative stress, inflammatory reaction, and fibrosis in a mouse model. Ecotoxicol Environ Saf. (2023) 265:115536. doi: 10.1016/j.ecoenv.2023.115536
58. Riaz MA, Nisa ZU, Mehmood A, Anjum MS, and Shahzad K. Metal-induced nephrotoxicity to diabetic and non-diabetic Wistar rats. Environ Sci pollut Res Int. (2019) 26:31111–8. doi: 10.1007/s11356-019-06022-z
59. He Y, Zou L, Luo W, Yi Z, Yang P, Yu S, et al. Heavy metal exposure, oxidative stress and semen quality: Exploring associations and mediation effects in reproductive-aged men. Chemosphere. (2020) 244:125498. doi: 10.1016/j.chemosphere.2019.125498
60. Upamalika S, Wannige CT, Vidanagamachchi SM, Gunasekara SC, Kolli RT, De Silva P, et al. A review of molecular mechanisms linked to potential renal injury agents in tropical rural farming communities. Environ Toxicol Pharmacol. (2022) 92:103850. doi: 10.1016/j.etap.2022.103850
61. Zeng Y, Song Z, Song G, Li S, Sun H, Zhang C, et al. Oxidative stress and antioxidant biomarker responses in fish exposed to heavy metals: a review. Environ Monit Assess. (2025) 197:892. doi: 10.1007/s10661-025-14376-w
62. Nayak S, Herold A, Shiny M, Vedula GS, Soundharrajan I, Almutairi BO, et al. Therapeutic potential of methylindoline derivative in ameliorating cadmium-induced nephritis experimented in zebrafish model. J Biochem Mol Toxicol. (2025) 39:e70312. doi: 10.1002/jbt.70312
63. Beglari S, Rezaie N, Jouriani FH, Khiavi E, Aghamohammad S, and Rohani M. Novel native probiotics with protective effects against nickel-induced kidney injury: a strategy for heavy metal toxicity. Biometals. (2025) 38:863–71. doi: 10.1007/s10534-025-00681-x
64. Skalny AV, Lima TRR, Ke T, Zhou JC, Bornhorst J, Alekseenko SI, et al. Toxic metal exposure as a possible risk factor for COVID-19 and other respiratory infectious diseases. Food Chem Toxicol. (2020) 146:111809. doi: 10.1016/j.fct.2020.111809
65. Zhou J, Liu S, Guo L, Wang R, Chen J, and Shen J. NMDA receptor-mediated CaMKII/ERK activation contributes to renal fibrosis. BMC Nephrol. (2020) 21:392. doi: 10.1186/s12882-020-02050-x
66. Overstreet JM, Wang Y, Wang X, Niu A, Gewin LS, Yao B, et al. Selective activation of epidermal growth factor receptor in renal proximal tubule induces tubulointerstitial fibrosis. FASEB J. (2017) 31:4407–21. doi: 10.1096/fj.201601359RR
67. P SV, P DR, and A BA. Role of PI3K-akt and MAPK signaling in uranyl nitrate-induced nephrotoxicity. Biol Trace Elem Res. (2019) 189:405–11. doi: 10.1007/s12011-018-1505-9
68. Ruiz-Ramirez C, Antaño-Martinez AR, Robles J, Gallegos-Corona MA, Gallegos-Reyes MA, Avila EE, et al. Correlation between urinary KIM-1 and kidney protein expression of p-ERK following damage in rats exposed to gentamicin or lead acetate. J Biochem Mol Toxicol. (2021) 35:e22875. doi: 10.1002/jbt.22875
69. Phuagkhaopong S, Ospondpant D, Kasemsuk T, Sibmooh N, Soodvilai S, Power C, et al. Cadmium-induced IL-6 and IL-8 expression and release from astrocytes are mediated by MAPK and NF-κB pathways. Neurotoxicology. (2017) 60:82–91. doi: 10.1016/j.neuro.2017.03.001
70. Peng L, Chen X, Wang AQ, Xie G, Zhang B, and Feng JF. Insulin like growth factor binding protein 7 activate JNK/ERK signaling to aggravate uranium-induced renal cell cytotoxicity. Naunyn Schmiedebergs Arch Pharmacol. (2025) 398:10645–56. doi: 10.1007/s00210-025-03923-4
71. Yang Z, Wang S, Liu H, and Xu S. MAPK/iNOS pathway is involved in swine kidney necrosis caused by cadmium exposure. Environ pollut. (2021) 274:116497. doi: 10.1016/j.envpol.2021.116497
72. Cao X, Fu M, Bi R, Zheng X, Fu B, Tian S, et al. Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere. (2021) 263:128346. doi: 10.1016/j.chemosphere.2020.128346
73. Li S, Baiyun R, Lv Z, Li J, Han D, Zhao W, et al. Exploring the kidney hazard of exposure to mercuric chloride in mice:Disorder of mitochondrial dynamics induces oxidative stress and results in apoptosis. Chemosphere. (2019) 234:822–9. doi: 10.1016/j.chemosphere.2019.06.096
74. Cha S, Lee J, Jang J, Kim Y, Han D, Lee HP, et al. Sodium chromate induces acute lung injury via mitogen-activated protein kinase-mediated oxidative stress and inflammation. Ecotoxicol Environ Saf. (2025) 304:119090. doi: 10.1016/j.ecoenv.2025.119090
75. Yin J, Wang AP, Li WF, Shi R, Jin HT, Wei JF, et al. Sensitive biomarkers identification for differentiating Cd and Pb induced toxicity on zebrafish embryos. Environ Toxicol Pharmacol. (2017) 56:340–9. doi: 10.1016/j.etap.2017.10.010
76. Xue M, Sun H, Xu R, Wang Y, Guo J, Li X, et al. GADD45B Promotes Glucose-Induced Renal Tubular Epithelial-Mesenchymal Transition and Apoptosis via the p38 MAPK and JNK Signaling Pathways. Front Physiol. (2020) 11:1074. doi: 10.3389/fphys.2020.01074
77. Glicklich D, Shin CT, and Frishman WH. Heavy metal toxicity in chronic renal failure and cardiovascular disease: possible role for chelation therapy. Cardiol Rev. (2020) 28:312–8. doi: 10.1097/crd.0000000000000304
78. Yohannes YB, Nakayama SMM, Yabe J, Toyomaki H, Kataba A, Nakata H, et al. Glutathione S-transferase gene polymorphisms in association with susceptibility to lead toxicity in lead- and cadmium-exposed children near an abandoned lead-zinc mining area in Kabwe, Zambia. Environ Sci pollut Res Int. (2022) 29:6622–32. doi: 10.1007/s11356-021-16098-1
79. Medina Pérez OM, Flórez-Vargas O, Rincón Cruz G, Rondón González F, Rocha Muñoz L, and Sánchez Rodríguez LH. Glutathione-related genetic polymorphisms are associated with mercury retention and nephrotoxicity in gold-mining settings of a Colombian population. Sci Rep. (2021) 11:8716. doi: 10.1038/s41598-021-88137-3
80. Lin CT, Chen TH, Yang CC, Luo KH, Chen TH, and Chuang HY. Epidermal growth factor receptor (EGFR) gene polymorphism may be a modifier for cadmium kidney toxicity. Genes (Basel). (2021) 12:1573. doi: 10.3390/genes12101573
81. El-Gedamy MS, Elnagar SS, Shabana EE, and Derbala SA. Association of tumor necrosis factor alpha gene polymorphism (TNF-α 308 G/A) with enhanced severity of metal-induced chronic kidney disease via upregulation of transmembrane protein expression. Biol Trace Elem Res. (2025). doi: 10.1007/s12011-025-04650-w
82. Hsueh YM, Chen WJ, Lin YC, Huang YL, Shiue HS, Lin YF, et al. Combined effects of nucleotide-binding domain-like receptor protein 3 polymorphisms and environmental metals exposure on chronic kidney disease. Sci Rep. (2022) 12:6307. doi: 10.1038/s41598-022-10098-y
83. Pavón N, Buelna-Chontal M, Macías-López A, Correa F, Uribe-Álvarez C, Hernández-Esquivel L, et al. On the oxidative damage by cadmium to kidney mitochondrial functions. Biochem Cell Biol. (2019) 97:187–92. doi: 10.1139/bcb-2018-0196
84. Seydi E, Nambani AK, Khorasani A, Kamranfar F, Arjmand A, and Pourahmad J. Mitochondrial administration alleviates lead- and cadmium-induced toxicity in rat renal cells. Cell Biol Int. (2024). doi: 10.1002/cbin.12165
85. Turkington RE, Hukriede NA, Ho J, Jayasundara N, and Sanders AP. Metal mechanisms of mitochondrial toxicity: recent review of arsenic, cadmium, and lead-induced nephrotoxicity. Environ Sci pollut Res Int. (2025) 32:14439–51. doi: 10.1007/s11356-025-36538-6
86. Hernández-Cruz EY, Amador-Martínez I, Aranda-Rivera AK, Cruz-Gregorio A, and Pedraza Chaverri J. Renal damage induced by cadmium and its possible therapy by mitochondrial transplantation. Chem Biol Interact. (2022) 361:109961. doi: 10.1016/j.cbi.2022.109961
87. Kubat GB, Ozler M, Ulger O, Ekinci O, Atalay O, Celik E, et al. The effects of mesenchymal stem cell mitochondrial transplantation on doxorubicin-mediated nephrotoxicity in rats. J Biochem Mol Toxicol. (2021) 35:e22612. doi: 10.1002/jbt.22612
88. Arjmand A, Shiranirad S, Ameritorzani F, Kamranfar F, Seydi E, and Pourahmad J. Mitochondrial transplantation against gentamicin-induced toxicity on rat renal proximal tubular cells: the higher activity of female rat mitochondria. In Vitro Cell Dev Biol Anim. (2023) 59:31–40. doi: 10.1007/s11626-022-00743-1
89. Kubat GB, Kartal Y, Atalay O, Ulger O, Ekinci O, Celik E, et al. Investigation of the effect of isolated mitochondria transplantation on renal ischemia-reperfusion injury in rats. Toxicol Appl Pharmacol. (2021) 433:115780. doi: 10.1016/j.taap.2021.115780
90. Swanson KJ, Muth B, Aziz F, Garg N, Mohamed M, Bloom M, et al. Kidney delayed graft function after combined kidney-solid organ transplantation: A review. Transplant Rev (Orlando). (2022) 36:100707. doi: 10.1016/j.trre.2022.100707
91. Kadatz M, Gill JS, Gill J, Formica RN, and Klarenbach S. Economic evaluation of extending medicare immunosuppressive drug coverage for kidney transplant recipients in the current era. J Am Soc Nephrol. (2020) 31:218–28. doi: 10.1681/asn.2019070646
92. Giri SS, Kim HJ, Jung WJ, Bin Lee S, Joo SJ, Gupta SK, et al. Probiotics in addressing heavy metal toxicities in fish farming: Current progress and perspective. Ecotoxicol Environ Saf. (2024) 282:116755. doi: 10.1016/j.ecoenv.2024.116755
93. Dahiya P, Kumari S, Behl M, Kashyap A, Kumari D, Thakur K, et al. Guardians of the gut: harnessing the power of probiotic microbiota and their exopolysaccharides to mitigate heavy metal toxicity in human for better health. Probiot Antimicrob Proteins. (2024) 16:1937–53. doi: 10.1007/s12602-024-10281-9
94. Xi JF, Zhou L, Zhang YS, Lin XY, Chen S, Xue RY, et al. Consuming probiotics protects against cadmium exposure from rice consumption while promotes gut health: An assessment based on a mouse model. Sci Total Environ. (2025) 958:177997. doi: 10.1016/j.scitotenv.2024.177997
95. Feng P, Yang J, Zhao S, Ling Z, Han R, Wu Y, et al. Human supplementation with Pediococcus acidilactici GR-1 decreases heavy metals levels through modifying the gut microbiota and metabolome. NPJ Biofilms Microbiomes. (2022) 8:63. doi: 10.1038/s41522-022-00326-8
96. Jiang X, Gu S, Liu D, Zhao L, Xia S, He X, et al. Lactobacillus brevis 23017 relieves mercury toxicity in the colon by modulation of oxidative stress and inflammation through the interplay of MAPK and NF-κB signaling cascades. Front Microbiol. (2018) 9:2425. doi: 10.3389/fmicb.2018.02425
97. Darwish AM, Khattab AEE, Almehizia AA, Alkahtani HM, Ghabbour HA, and Kalmouch A. Mitigating cadmium toxicity: the therapeutic potential of lactobacillus acidophilus pro4 in mice. Probiot Antimicrob Proteins. (2025). doi: 10.1007/s12602-025-10581-8
98. Zhai Q, Tian F, Zhao J, Zhang H, Narbad A, Chen W, et al. Oral administration of probiotics inhibits absorption of the heavy metal cadmium by protecting the intestinal barrier. Appl Environ Microbiol. (2016) 82:4429–40. doi: 10.1128/aem.00695-16
99. Tang C and Lu Z. Health promoting activities of probiotics. J Food Biochem. (2019) 43:e12944. doi: 10.1111/jfbc.12944
100. Miranda MV, González FC, Paredes-Godoy OS, Maulén MA, Vásquez CC, and Díaz-Vásquez WA. Characterization of metal(loid)s and antibiotic resistance in bacteria of human gut microbiota from chronic kidney disease subjects. Biol Res. (2022) 55:23. doi: 10.1186/s40659-022-00389-z
101. Yu Y, Teng Z, Mou Z, Lv Y, Li T, Chen S, et al. Melatonin confers heavy metal-induced tolerance by alleviating oxidative stress and reducing the heavy metal accumulation in Exophiala pisciphila, a dark septate endophyte (DSE). BMC Microbiol. (2021) 21:40. doi: 10.1186/s12866-021-02098-1
102. Nabil-Adam A and Shreadah MA. Ameliorative role of Ulva extract against heavy metal mixture-induced cardiovascular through oxidative/antioxidant pathways and inflammatory biomarkers. Environ Sci pollut Res Int. (2021) 28:27006–24. doi: 10.1007/s11356-020-11994-4
103. Qiu T, Wang ZS, Liu XH, Chen H, Zhou JQ, Chen ZY, et al. Effect of ozone oxidative preconditioning on oxidative stress injury in a rat model of kidney transplantation. Exp Ther Med. (2017) 13:1948–55. doi: 10.3892/etm.2017.4193
104. Lv D, Zhou Q, Xia Y, You X, Zhao Z, Li Y, et al. The association between oxidative stress alleviation via sulforaphane-induced nrf2-HO-1/NQO-1 signaling pathway activation and chronic renal allograft dysfunction improvement. Kidney Blood Press Res. (2018) 43:191–205. doi: 10.1159/000487501
105. Yang H, Xing R, Liu S, Yu H, and Li P. Role of Fucoxanthin towards Cadmium-induced renal impairment with the antioxidant and anti-lipid peroxide activities. Bioengineered. (2021) 12:7235–47. doi: 10.1080/21655979.2021.1973875
106. Agha F, Batool Z, Batool TS, Nisar R, Naqvi F, Saleem S, et al. Tree nuts supplementation instigates the oxidative status and improves brain performance in male rats. Pak J Pharm Sci. (2020) 33:2785–91.
107. Guan Q and Du C. Antioxidant nanozymes for prevention of diseased kidney from failure. Kidney Int. (2022) 102:961–3. doi: 10.1016/j.kint.2022.08.003
108. Guo Q, Liu Y, Jia Q, Zhang G, Fan H, Liu L, et al. Ultrahigh sensitivity multifunctional nanoprobe for the detection of hydroxyl radical and evaluation of heavy metal induced oxidative stress in live hepatocyte. Anal Chem. (2017) 89:4986–93. doi: 10.1021/acs.analchem.7b00306
109. Satarug S, Vesey DA, Gobe GC, and Phelps KR. Estimation of health risks associated with dietary cadmium exposure. Arch Toxicol. (2023) 97:329–58. doi: 10.1007/s00204-022-03432-w
110. Schilling K, Ujueta F, Gao S, Anderson WA, Escolar E, Mon A, et al. Pharmacokinetics of metal excretion following different doses of sodium EDTA infusion. Metallomics. (2025) 17:mfaf010. doi: 10.1093/mtomcs/mfaf010
111. Yan LJ and Allen DC. Cadmium-induced kidney injury: oxidative damage as a unifying mechanism. Biomolecules. (2021) 11:1575. doi: 10.3390/biom11111575
112. Glicklich D and Frishman WH. The case for cadmium and lead heavy metal screening. Am J Med Sci. (2021) 362:344–54. doi: 10.1016/j.amjms.2021.05.019
113. Arenas IA, Navas-Acien A, Ergui I, and Lamas GA. Enhanced vasculotoxic metal excretion in post-myocardial infarction patients following a single edetate disodium-based infusion. Environ Res. (2017) 158:443–9. doi: 10.1016/j.envres.2017.06.039
114. Taher AT, Saliba AN, Kuo KH, Giardina PJ, Cohen AR, Neufeld EJ, et al. Safety and pharmacokinetics of the oral iron chelator SP-420 in β-thalassemia. Am J Hematol. (2017) 92:1356–61. doi: 10.1002/ajh.24914
115. Layne KA, Wood DM, and Dargan PI. Gadolinium-based contrast agents - what is the evidence for ‘gadolinium deposition disease’ and the use of chelation therapy? Clin Toxicol (Phila). (2020) 58:151–60. doi: 10.1080/15563650.2019.1681442
116. Smereczański NM and Brzóska MM. Current levels of environmental exposure to cadmium in industrialized countries as a risk factor for kidney damage in the general population: A comprehensive review of available data. Int J Mol Sci. (2023) 24:8413. doi: 10.3390/ijms24098413
117. Guldan M, Ozbek L, Fidan DG, Gulmaliyev I, Rustamov A, Kanbay M, et al. Association between air pollution and transplant outcomes in kidney transplant recipients: a systematic review and meta-analysis. Clin Kidney J. (2025) 18:sfaf222. doi: 10.1093/ckj/sfaf222
118. Lao XQ, Bo Y, Chen D, Zhang K, and Szeto CC. Environmental pollution to kidney disease: an updated review of current knowledge and future directions. Kidney Int. (2024) 106:214–25. doi: 10.1016/j.kint.2024.04.021
119. Hosseinpoor S, Habibi S, and Mohammadi A. Understanding heavy metal contamination in the vicinity of Lake Urmia, NW Iran: Environmental and health Perspectives. Heliyon. (2024) 10:e34198. doi: 10.1016/j.heliyon.2024.e34198
120. Zhao X, Li Z, Wang D, Li J, Zou B, Tao Y, et al. Assessment of residents’ total environmental exposure to heavy metals in China. Sci Rep. (2019) 9:16386. doi: 10.1038/s41598-019-52649-w
121. Danziger J, Dodge LE, Hu H, and Mukamal KJ. Susceptibility to environmental heavy metal toxicity among americans with kidney disease. Kidney360. (2022) 3:1191–6. doi: 10.34067/kid.0006782021
122. Xu X, Nie S, Ding H, and Hou FF. Environmental pollution and kidney diseases. Nat Rev Nephrol. (2018) 14:313–24. doi: 10.1038/nrneph.2018.11
123. Chen H, Zou Y, Leng X, Huang F, Huang R, Wijayabahu A, et al. Associations of blood lead, cadmium, and mercury with resistant hypertension among adults in NHANES, 1999-2018. Environ Health Prev Med. (2023) 28:66. doi: 10.1265/ehpm.23-00151
124. Jin Y, Lu Y, Li Y, Zhao H, Wang X, Shen Y, et al. Correlation between environmental low-dose cadmium exposure and early kidney damage: A comparative study in an industrial zone vs. a living quarter in Shanghai, China. Environ Toxicol Pharmacol. (2020) 79:103381. doi: 10.1016/j.etap.2020.103381
125. Strader M and Kant S. Novel biomarkers for rejection in kidney transplantation: A comprehensive review. J Clin Med. (2025) 14:5489. doi: 10.3390/jcm14155489
126. Leibler JH, Ramirez-Rubio O, Velázquez JJA, Pilarte DL, Obeid W, Parikh CR, et al. Biomarkers of kidney injury among children in a high-risk region for chronic kidney disease of uncertain etiology. Pediatr Nephrol. (2021) 36:387–96. doi: 10.1007/s00467-020-04595-3
127. Ortega-Romero M, Jiménez-Córdova MI, Barrera-Hernández Á, Sepúlveda-González ME, Narvaez-Morales J, Aguilar-Madrid G, et al. Relationship between urinary biomarkers of early kidney damage and exposure to inorganic toxins in a pediatric population of Apizaco, Tlaxcala, Mexico. J Nephrol. (2023) 36:1383–93. doi: 10.1007/s40620-023-01598-9
128. Hanser O, Martin Remy A, Melczer M, Grova N, and Ndaw S. Early-effect biomarkers and hazard index approaches to assessing risks from multiple exposure to metals among battery recyclers in France. Environ Toxicol Pharmacol. (2025) 119:104819. doi: 10.1016/j.etap.2025.104819
129. Al-Saleh I, Al-Rouqi R, Elkhatib R, Abduljabbar M, and Al-Rajudi T. Risk assessment of environmental exposure to heavy metals in mothers and their respective infants. Int J Hyg Environ Health. (2017) 220:1252–78. doi: 10.1016/j.ijheh.2017.07.010
130. Ortiz F, Nylund KM, Ruokonen H, Meurman JH, Furuholm J, Bostanci N, et al. Salivary biomarkers of oral inflammation are associated with cardiovascular events and death among kidney transplant patients. Transplant Proc. (2020) 52:3231–5. doi: 10.1016/j.transproceed.2020.07.007
131. Tabernero G, Pescador M, Ruiz Ferreras E, Morales AI, and Prieto M. Evaluation of NAG, NGAL, and KIM-1 as prognostic markers of the initial evolution of kidney transplantation. Diagn (Basel). (2023) 13:1843. doi: 10.3390/diagnostics13111843
132. Ortega-Romero M, Rojas-Lima E, Rubio-Gutiérrez JC, Aztatzi-Aguilar OG, Narváez-Morales J, Esparza-García M, et al. Associations among environmental exposure to trace elements and biomarkers of early kidney damage in the pediatric population. Biometals. (2024) 37:721–37. doi: 10.1007/s10534-024-00603-3
133. Luvonga C, Rimmer CA, Yu LL, and Lee SB. Organoarsenicals in seafood: occurrence, dietary exposure, toxicity, and risk assessment considerations - A review. J Agric Food Chem. (2020) 68:943–60. doi: 10.1021/acs.jafc.9b07532
134. Biswas B, Chakraborty A, Chatterjee D, Pramanik S, Ganguli B, Majumdar KK, et al. Arsenic exposure from drinking water and staple food (rice): A field scale study in rural Bengal for assessment of human health risk. Ecotoxicol Environ Saf. (2021) 228:113012. doi: 10.1016/j.ecoenv.2021.113012
135. Notario-Barandiaran L, Irizar A, Begoña-Zubero M, Soler-Blasco R, Riutort-Mayol G, Fernández-Somoano A, et al. Association between mediterranean diet and metal(loid) exposure in 4-5-year-old children living in Spain. Environ Res. (2023) 233:116508. doi: 10.1016/j.envres.2023.116508
136. Kong S, Jia C, Liu R, Wei X, Bai B, Song H, et al. Mechanisms and health implications of calcium in reducing arsenic bioaccessibility via. Vitro Gastrointest Digest Sci Total Environ. (2025) 978:179410. doi: 10.1016/j.scitotenv.2025.179410
137. Krohn RM, Raqib R, Akhtar E, Vandenberg A, and Smits JE. A high-selenium lentil dietary intervention in Bangladesh to counteract arsenic toxicity: study protocol for a randomized controlled trial. Trials. (2016) 17:218. doi: 10.1186/s13063-016-1344-y
Keywords: heavy metals, kidney transplant, environmental pollution, graft failure, oxidative stress
Citation: Li S, Cao H, Wang B, Huang G, Qu Y, Yuan B and Wei W (2025) Effects of heavy metal exposure on kidney transplant recipients: mechanisms and clinical implications for graft failure risk. Front. Immunol. 16:1718695. doi: 10.3389/fimmu.2025.1718695
Received: 04 October 2025; Accepted: 17 November 2025; Revised: 14 November 2025;
Published: 28 November 2025.
Edited by:
Cheng Yang, Fudan University, ChinaReviewed by:
Daniel Glicklich, Westchester Medical Center, United StatesZijiang Yang, Xinxiang Medical University, China
Copyright © 2025 Li, Cao, Wang, Huang, Qu, Yuan and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Yuan, amx1eXVhbmJvQGpsdS5lZHUuY24=; Wei Wei, d2Vpd0BqbHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Shuxin Li
Shuxin Li Hongliang Cao
Hongliang Cao Binbin Wang
Binbin Wang Gengchen Huang
Gengchen Huang Yongliang Qu2
Yongliang Qu2 Bo Yuan
Bo Yuan Wei Wei
Wei Wei