- 1Institute for Medical Microbiology, Immunology and Parasitology (IMMIP), University Hospital Bonn (UKB), Bonn, Germany
- 2German Center for Infection Research (DZIF), Partner Site Bonn-Cologne, Bonn, Germany
- 3Faculty of Allied Health Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
- 4Kumasi Center for Collaborative Research (KCCR), Kumasi, Ghana
- 5Department of Clinical Microbiology, School of Medicine and Dentistry, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana
- 6Unité de Recherche en Immunologie et Immunomodulation (UR2IM)/Laboratoire de Microbiologie et de Contrôle de Qualité des Denrées Alimentaires (LAMICODA), Ecole Supérieure des Techniques Biologiques et Alimentaires, Université de Lomé, Lomé, Togo
- 7Parasites and Vector Research Unit (PAVRU), Department of Microbiology and Parasitology, University of Buea, Buea, Cameroon
- 8Research Foundation in Tropical Diseases and Environment (REFOTDE), Buea, Cameroon
- 9Drugs for Neglected Diseases initiative (DNDi), Geneva, Switzerland
Filariae are vector borne parasitic nematodes, endemic in tropical and subtropical regions causing avoidable infections ranging from asymptomatic to stigmatizing and disfiguring disease. The filarial species that are the major focus of our institution’s research are Onchocerca volvulus causing onchocerciasis (river blindness), Wuchereria bancrofti and Brugia spp. causing lymphatic filariasis (elephantiasis), Loa loa causing loiasis (African eye worm), and Mansonella spp. causing mansonellosis. This paper aims to showcase the contribution of our institution and our collaborating partners to filarial research and covers more than two decades of research spanning basic research using the Litomosoides sigmodontis animal model to development of drugs and novel diagnostics. Research with the L. sigmodontis model has been extensively useful in elucidating protective immune responses against filariae as well as in identifying the mechanisms of filarial immunomodulation during metabolic, autoimmune and infectious diseases. The Institute for Medical Microbiology, Immunology and Parasitology (IMMIP), University Hospital Bonn (UKB), Bonn, Germany has also been actively involved in translational research in contributing to the identification of new drug targets and pre-clinical drug research with successful and ongoing partnership with sub-Saharan Africa, mainly Ghana (the Kumasi Centre for Collaborative Research (KCCR)), Cameroon (University of Buea (UB)) and Togo (Laboratoire de Microbiologie et de Contrôle de Qualité des Denrées Alimentaires (LAMICODA)), Asia and industry partners. Further, in the direction of developing novel diagnostics that are sensitive, time, and labour saving, we have developed sensitive qPCRs as well as LAMP assays and are currently working on artificial intelligence based histology analysis for onchocerciasis. The article also highlights our ongoing research and the need for novel animal models and new drug targets.
1 Introduction
With extraordinary host manipulative strategies combined with a co-evolutionary history spanning over 5,000 years, parasitic helminths are some of the most successful pathogens. Filariae, vector borne parasitic nematodes (round worms), endemic in tropical and subtropical regions, comprised of Onchocerca volvulus that cause onchocerciasis (river blindness) (1); Wuchereria bancrofti and Brugia spp., causing lymphatic filariasis (elephantiasis) (2); Loa loa causing loiasis (African eye worm) (3); and Mansonella spp, causing mansonellosis (4). The majority of these neglected tropical diseases (NTDs) can cause stigmatizing pathologies leading to considerable socio-economic burden to the affected people and, thus, are serious public health problems to endemic countries. The burden of onchocerciasis is estimated at 21 million affected people, including 14.6 million with skin diseases and around 1.15 million people suffering from vision impairment. In 2017, at least 220 million people required preventive chemotherapy against onchocerciasis (1, 5). Lymphatic filariasis is confined to sub-Saharan Africa and South-East Asia with a few foci in Central and South America, including Brazil, Haiti, Dominican Republic and Guyana. It is estimated that 51 million people were infected in 2018, with 863 million people in 47 countries worldwide living in areas that require mass drug administration (MDA). While around 25 million men are estimated to suffer from hydrocele, lymphedema occurs in around 15 million people (2, 6). Loiasis affects over 13 million individuals (7) and around 600 million people are estimated to be at risk of infection with mansonellosis (3, 8). For onchocerciasis annual community-directed treatment with ivermectin, with or without albendazole, is conducted. MDA with a triple combination of ivermectin, albendazole and diethylcarbamazine has been the mainstay of treatment against lymphatic filariasis that is not co-endemic for onchocerciasis or loiasis (9–11). However, this treatment strategy is potentially accompanied by severe adverse events (SAE) in onchocerciasis and loiasis patients on treatment with diethylcarbamazine citrate (DEC) or ivermectin, suboptimal drug efficacy and the lack of a prominent macrofilaricidal efficacy (12–14). No MDAs are performed for loiasis and mansonellosis. For loiasis, individual therapy consists of daily DEC treatment for 21 days, which cannot be given to patients with high microfilariae loads. In such patients, fatal encephalopathy may occur following DEC or ivermectin treatment, which is the reason that MDAs for lymphatic filariasis or onchocerciasis cannot be performed in areas co-endemic for loiasis.
The WHO targets to end the transmission of onchocerciasis in 12 countries (31%) and eliminate lymphatic filariasis as a public health problem in 58 (81%) endemic countries (defined as infection sustained below transmission assessment survey threshold) by 2030, and has formulated a roadmap to attain this goal, including new alternative treatment strategies and development of macrofilaricidal compounds (15–18). Loiasis and mansonellosis are not included in the list of NTDs so far and are therefore not targeted by the WHO NTD roadmap 2030.
In this context, the present review summarizes the work done at the Institute for Medical Microbiology, Immunology and Parasitology (IMMIP), University Hospital Bonn (UKB), Germany along with its main collaborators in Ghana (the Kumasi Centre for Collaborative Research (KCCR) and the Kwame Nkrumah University of Science and Technology, Kumasi), Cameroon (Research Foundation in Tropical Diseases and Environment (REFOTDE) and the University of Buea (UB)) and Togo (Laboratoire de Microbiologie et de Contrôle de Qualité des Denrées Alimentaires (LAMICODA) and the University of Lomé, Togo) at the forefront of filarial research for the past two decades. The research conducted ranges from basic research and delineating the pathology of these diseases, to development of novel treatment options, pre-clinical research and diagnostics (Figure 1). Of particular emphasis is the support we receive from non-profit organizations like the Bill & Melinda Gates Foundation (BMGF) and Drugs for Neglected Diseases initiative (DNDi), and our collaborations with partners from academia and industry.
Figure 1 Summary of the contributions to filarial research. Created with BioRender.com.
2 Basic research
The rodent filarial nematode infection with Litomosoides sigmodontis has been a model for filariasis basic and translational research for 70 years. The model, which is available in only 4 labs in the world, has contributed to major discoveries in the field (19–21). L. sigmodontis infective third stage larvae (L3) are transmitted by the bite of the hematophagous tropical rat mite Ornithonyssus bacoti to the rodent host. Cotton rats (Sigmodon hispidus) are the natural host, but Mongolian gerbils (Meriones unguiculatus) and BALB/c mice are also susceptible to the infection (20). L3 migration through the skin and lymphatics leads them to the blood circulation and they eventually reach the pleural cavity within one week post infection (22, 23). In BALB/c mice that are susceptible to infection with L. sigmodontis, adult worms develop by 30 days after infection and microfilariae are present in the peripheral blood after about 8 weeks (24). On the other hand, in semi-susceptible C57BL/6 mice, adult worms are cleared shortly after the development into adult worms (~ 45 days after infection) and, therefore, no release of microfilariae occurs (25). Work from our institute and others using the L. sigmodontis model has contributed to a better understanding of immune responses during filarial diseases and is presented in the following section. With the help of the L. sigmodontis model, we, e.g., were the first to describe suppressive regulatory T cell populations during filarial infection and showed that type 1 as well as type 2 immune responses contribute to protective immune responses (26). In this context, the role of an IFN-γ dependent effector mechanism to the function of neutrophils in murine filariasis was shown for the first time (27, 28)
2.1 Protective immune responses against filariae
2.1.1 Role of neutrophils
Neutrophils are the first line of defence against bacteria and are rapidly recruited to inflamed sites and their role in helminth infections is increasingly being recognized. In addition, endosymbiotic Wolbachia bacteria, present in most human pathogenic filariae (except for L. loa) and L. sigmodontis, are known to recruit neutrophils. The major roles of neutrophils in controlling the early stages of infection have been demonstrated by Prof. Coralie Martin's laboratory using Cxcr4+/1013 mice, which carry a gain-of-function mutation of the chemokine receptor CXCR4 leading to profound blood neutropenia. In this mouse model, subcutaneous delivery of the infective L3 larvae decreased the success of L3 larvae dramatically. Further, an oxidative burst response and the release of neutrophil extracellular traps (NET) were found to be mechanisms that directly contribute to the anti-parasitic strategies implemented by the host (29).
Early neutrophil recruitment, along with increased expression of S100A8 and S100A9 proteins, has been shown by the Martin laboratory to be associated with transient lung inflammation, lung haemorrhages and granulomas. This study suggests a mechanistic route via which L3 larvae reach the lung via penetration of pulmonary capillaries, endothelium and then to the pleural cavity (22). Using the L. sigmodontis model, the role of the neutrophil protein S100A8/S100A9 on the protective immune responses against filariae was investigated. The heterodimer formed by S100A8/S100A9, called calprotectin, is responsible for the mitigation of inflammation and tissue damage. In S100A8/A9 deficient mice, a significantly reduced worm burden was observed. Further, lack of S100A8/A9 was shown to trigger inflammatory responses in response to L3 larvae, leading to increased levels of chemokines, stronger recruitment of granulocytes and activation of neutrophils, which arguably impaired the migration of L3 larvae and susceptibility towards filarial infection (30). In IFN-γ knock out mice, neutrophils were found to be decreased in the thoracic cavity, thereby impairing the encapsulation process; and also displayed diminished chemotactic and phagocytic activities in comparison to the neutrophils of the control mice (27).
Wolbachia synthesize the muramyl dipeptide (MDP) containing cell wall precursor lipid II that is recognized by nucleotide-binding oligomerization domain-containing protein 2 (NOD2). Ligand binding triggers NFκB-induced pro-inflammation. Therefore, the role of NOD2 was investigated in the L. sigmodontis model. While neutrophil recruitment was impaired in the skin of NOD2 deficient mice on intra-dermal injection of L3 larvae, the number of neutrophils in blood was also reduced. Depletion of neutrophils before L. sigmodontis infection increased the recovery of worms in the wild type mice, corroborating the importance of neutrophils in fighting invading L3 larvae. This study highlighted the role of NOD like receptors in first line defence against filarial nematodes (31).
Similarly, the importance of IL-6, a cytokine for neutrophil trafficking, in the immune responses to filariae was investigated. In mice deficient in IL-6, infection with L. sigmodontis led to a significantly increased worm burden, which may be due to the observed increased vascular permeability that facilitated larval migration. Analysis of skin samples after exposure to L3 larvae or filarial extract indicated a delayed recruitment of neutrophils and macrophages to the site of infection in the IL-6 deficient mice (32). The occurrence of NETosis in human Onchocerca volvulus infection was demonstrated for the first time by Tamarozzi et al. (33). NETosis was induced by Wolbachia via the direct ligation of lipoprotein peptidoglycan associated lipoprotein to TLR2/TLR6 on neutrophils.
In a mouse model of ocular onchocerciasis, antigen derived from microfilariae harbouring Wolbachia were injected into the corneas of mice. 18 hours after infection, neutrophils were observed to surround the nematodes in close proximity to Wolbachia. Incubation of parasite extracts with and without Wolbachia with peritoneal neutrophils clearly demonstrated that Wolbachia depleted extracts did not induce TNF, CXC chemokines, MIP-2, or KC by neutrophils. Taken together these findings indicate neutrophil activation is a crucial mechanism by which Wolbachia contribute to the pathogenesis of ocular onchocerciasis (34, 35). This could also be shown by immunohistology on O. volvulus nodules, where the strong presence of neutrophils around the adult worms was abolished in nodules from patients that had received a Wolbachia-depleting treatment with doxycycline (36).
2.1.2 Role of Toll like receptor (TLR) signalling in protective responses against filariae
Owing to the fact that most human filarial pathogens harbour Wolbachia bacteria, the possibility of an inflammatory response through TLRs was investigated. When Brugia malayi and Onchocerca volvulus extracts containing Wolbachia were treated on human embryonic kidney cells, stimulation was observed in those expressing TLR2, but not TLR3 or TLR4. Further, Wolbachia containing extracts could not stimulate cytokine production from TLR2 and TLR6 deficient murine macrophages. This report also showed the involvement of the adaptor molecules MyD88 and TIRAP/Mal (37). In the L. sigmodontis model, lack of the central adaptor molecular TRIF was shown to lead to a higher worm burden and reduced thoracic cavity cell numbers 30 days post infection. Further, in the thoracic cavity, increased frequencies of macrophages and lymphocytes and a decreased frequency of eosinophils, CD4 and CD8 cells were found, demonstrating the importance of TRIF on worm recovery and recruitment of immune cells in C57BL/6 mice (38). In a mouse model of chronic filarial infection, triggering TLR2 has been shown to result in a significantly increased patency, while TLR4 has been shown to dampen the CD4 T cell responses (39). In conclusion, TLRs play an important role in the recognition of Wolbachia and protective immune responses against filariae.
2.1.3 Role of eosinophils, IL-4, IL-5, ILC2s, IL-10, and regulatory B and T cell populations
Although filariae are known to induce type 2 responses in their host and eosinophils are the essential part of this protective immune response, the exact role of eosinophils in protective immunity against filariae is still poorly understood. Using eosinophil-deficient dblGATA mice, IL-5-/- mice, IL-4R-/- mice and IL-4R-/-/IL-5-/- mice infected with L. sigmodontis, it was demonstrated that mice lacking IL-4R/IL-5, and therefore alternatively activated macrophages (AAM) and eosinophils, showed the greatest susceptibility to L. sigmodontis infection. All IL-4R/IL-5 knock out mice became microfilaremic, which developed earlier and had increased numbers of microfilariae and adult worms. The adult worms had an extended lifespan, leading to enhanced pathology in the pleural cavity; especially within diaphragm and lung tissue (40, 41). Mice, solely lacking IL-4R (and AAM) had an increased frequency of animals developing microfilaremia, but no significant differences in adult worm burden and microfilariae levels. In the absence of eosinophils (dblGATA mice, IL-5-/- mice), increased microfilariae counts, frequency of animals developing microfilaremia, and adult worm counts with an extended survival were observed (41). In BALB/c mice, that are fully permissive for filarial infection, IL-5 was shown to be essential for protection against L. sigmodontis after vaccination with irradiated L3 larvae (42). Moreover, in eotaxin-1 deficient mice infected with L. sigmodontis, increased survival of the adult filariae was observed, which was associated with a reduced expression of CD80 and CD86 on eosinophils from eotaxin-1 deficient mice (43). To further characterize the role of eosinophilic granule proteins, mice deficient in eosinophil peroxidase (EPO) or major basic protein (MBP) were infected with L. sigmodontis. These mice developed a higher worm burden and increased levels of the anti-inflammatory cytokine IL-10 from thoracic cavity macrophages (44).
Eosinophils have several mechanisms to mediate protective immune responses and our group first described that eosinophils release extracellular DNA traps (EETosis) in response to filariae. Ehrens et al. (45) showed that these DNA traps inhibit the motility of L. sigmodontis microfilariae in a DNA dependent manner. The microfilariae induced EETosis was mediated by dectin-1 signalling and the released DNA was of mitochondrial origin (45). A more recent comprehensive review describes not only the role of eosinophils in the pathology of filarial infections, but also discusses eosinophils as drivers of pathology during tropical pulmonary eosinophilia in lymphatic filariasis and in inducing adverse events in DEC treated patients (46). Work from the Martin group showed that in Meriones unguiculatus infected with L. sigmodontis infection, fibrotic polypoid structures were observed in the pleura and these structures were associated with eosinophils, macrophages and lymphocytes (47).
Given that ILC2s are potent producers of IL-5, which can induce eosinophil responses, ILC2s are potentially also involved in protective immune responses against filariae. To elucidate the role of ILC2 during murine filariasis, susceptible BALB/c and semi-susceptible C57BL/6 mice were infected with L. sigmodontis. At 30 days post infection, the C57BL/6 mice showed higher numbers of ILC2s and increased levels of IL-5 and IL-13; indicating a stronger type 2 immune response. Depletion of ILC2s in T and B cell deficient Rag2-/- mice indicated that ILC2s are involved in the control of microfilaremia in this mouse model, as more microfilariae were present in the absence of ILC2s (48).
Another immune cell subset involved in protective immune responses is the myeloid derived suppressor cell (MDSC) subset, which have been shown to expand during inflammation and have inhibitory effects on T and B cell responses. MDSCs consist of two distinct sub-populations, polymorphonuclear (PMN)-MDSCs and monocytic (Mo)-MDSCs based on morphology and phenotype. However, we have recently shown that, during L. sigmodontis infection, both Mo-MDSCs and PMN-MDSCs accumulate at the site of infection in BALB/c mice and positively correlate with worm burden or microfilariae numbers. However, only Mo-MDSCs were able to suppress the production of IFN-γ and IL-13 by L. sigmodontis-specific CD4+ T cells and neither of the cellular sub-populations had an effect on IL-5 production (49, 50).
In order to survive, helminths establish an immunomodulated milieu in the host via induction of regulatory T cells (Tregs), type 2 responses from Th2 cells, eosinophils, IL-4, IL-5, IL-13, alternatively activated macrophages and anti-inflammatory cytokines like IL-10 and TGF-ß (51–54). To study the role of IL-10, which along with TGF-β is involved in the downregulation of T cell responses, C57BL/6 mice overexpressing IL-10 in CD68+ macrophages were used. L. sigmodontis infection in these mice led to higher adult worm numbers and fully patent infections. Thus, macrophage secretion of IL-10 overcomes resistance from L. sigmodontis infection (55). Additional proof for the immunosuppressive role of IL-10 came from another study wherein IL-4 knockout resulted in increased susceptibility to L. sigmodontis infection in the C57BL/6 background. Double-knockout of IL-4/IL-10 resulted in the reversal of this susceptibility. This finding suggests that IL-10 dependent responses are the major reason for patency and are antagonistic to Th2 responses involved in containing the parasitemia (56).
We also showed that adaptive immune responses involving T, B and NK cell populations are important to prevent development of patent immune responses in the semi-susceptible C57BL/6 mouse strain and that Th17 polarization of the CD4+ T cells plays a crucial role for parasite clearance (57). Figure 2 summarizes the findings on the immune responses against filariae.
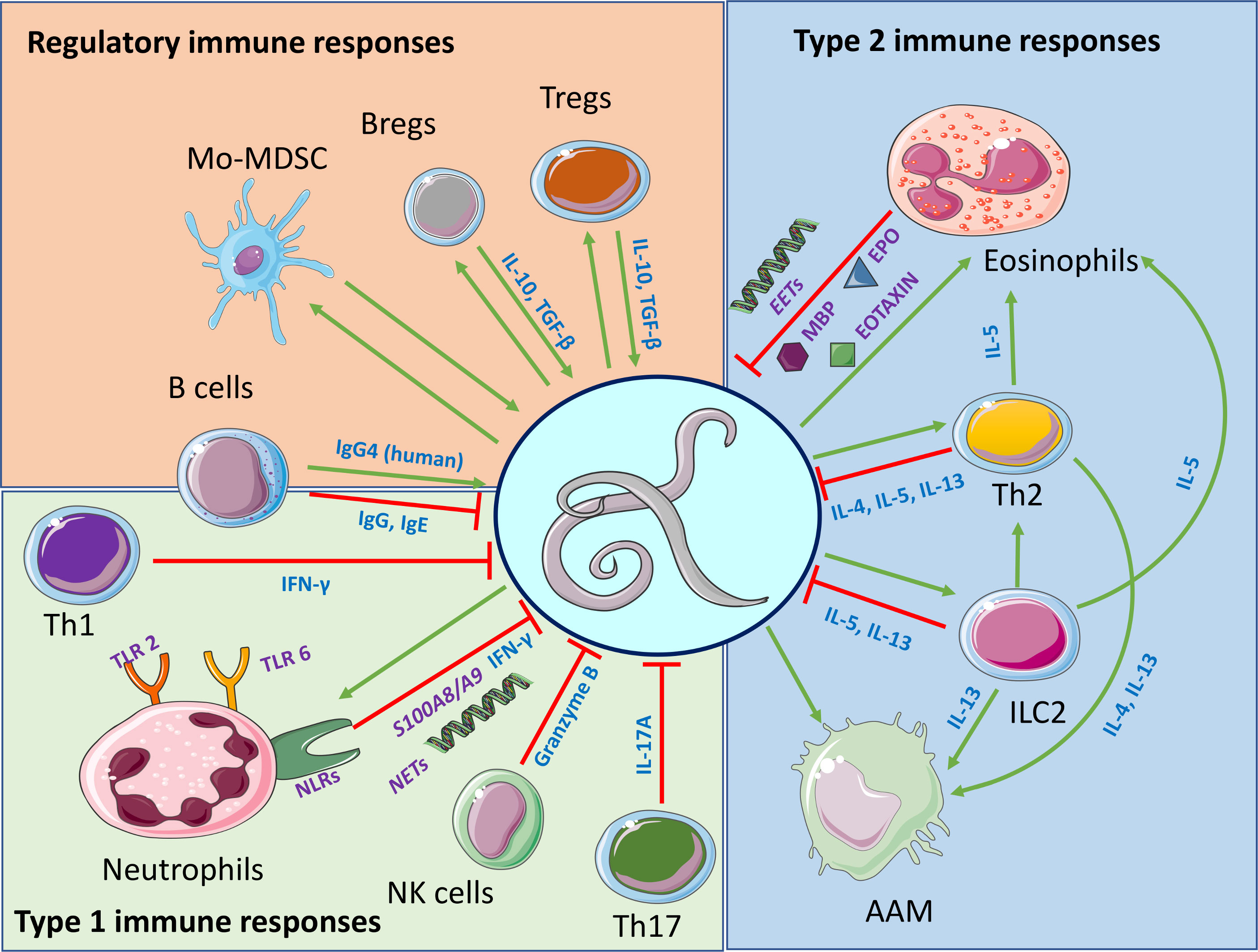
Figure 2 Major findings on protective immune responses against filariae and immune responses induced by the filariae. Regulatory, type 1 and type 2 immune responses are highlighted. Interactions among regulatory, type 1 and type 2 immune responses are not highlighted. Created with BioRender.com.
Further, the role of IL-10 and TGF-ß in mediating hypo-responsiveness to onchocerciasis was studied. PBMCs from individuals with onchocerciasis produced significantly more IL-10, and T cell proliferative hypo-responsiveness in this group could be reversed by the addition of anti-IL-10 and anti-TGF-β antibodies. On the other hand, IL-5 was found to be associated with high cellular O. volvulus specific proliferation (26)
Mansonella perstans-specific immune responses were studied for the first-time using worm antigen extracts. The findings of the study showed that during M. perstans infection, distinct Th2, regulatory B cell and Treg subsets are induced, resulting in a reduced systemic, innate and adaptive immune response and increased levels of filarial-specific IgG4 levels (58). In a study done in Ghanian individuals, an ongoing W. bancrofti infection was shown to induce distinct populations of Breg and Treg populations from the peripheral blood mononuclear cells. These regulatory immune populations might contribute to the regulated state of the host immune system and contribute to the survival and fertility of the nematodes (59). However, the regulative state can be imbalanced; reflected in patients with severe pathology. The majority of these patients are characterized by increased Th1 and Th17 responses and constant immune activation (60, 61). Indeed, we recently showed that constant immune activation by W. bancrofti (62) can lead to CD4 and CD8 T cell exhaustion in patients suffering from lymphedema (63, 64), showing that imbalanced filarial-driven immune modulation, despite clearing the infection, can cause impaired T cell function; which might contribute to a higher risk for bacterial infections and consequently acute dermatolymphangioadenitis (ADLA) attacks.
2.2 Filarial immunomodulation on metabolic, infectious and autoimmune diseases
As described in the previous chapter, filarial nematodes establish a predominance of Tregs, AAMs, TGF-β and IL-10 etc., which results in the creation of an anti-inflammatory environment that helps parasite survival (51–54). Our group has demonstrated a significantly increased worm load in IFN-γ/IL-5 double-knockout mice indicating synergism between IFN-γ and IL-5 in controlling worm infection in the L. sigmodontis model (65). There is also some evidence that there is a “dichotomy” between Tregs and Th2 (+Th17) which is effective during hyperreactive onchocerciasis in humans (66). On one hand this kind of immunomodulation can affect bystander immune responses and can improve the outcome of allergies (67, 68) or autoimmune diseases (69–71), but, on the other hand, efficacy to vaccinations could be reduced due to nematode mediated immunomodulation (72, 73). Further, filarial nematode infections could alter immune responses to unrelated pathogens like bacterial and viral infections. The following section highlights the comprehensive work done to understand how filarial infections could regulate the outcome of a range of metabolic, autoimmune bacterial and viral infections.
2.2.1 Sepsis
Our work with the L. sigmodontis animal model has demonstrated that the exacerbated systemic inflammatory immune response characteristic of sepsis could be contained through helminth immunomodulation. The impact of the different life-cycle stages of L. sigmodontis on mice injected with sublethal doses of LPS was investigated. Infection with female adult worms from prepatent infections protected mice from LPS-induced sepsis. However, microfilariae worsened LPS induced sepsis through induction of pro-inflammatory cytokines, upregulation of granulocytes, NK cells and monocytes in the peripheral blood (74). Mice chronically infected with L. sigmodontis and subsequently challenged with E. coli showed significant protection from hypothermia, improved bacterial clearance and survival. These outcomes were correlated with decreased levels of pro-inflammatory cytokines and chemokines and a diminished inflammatory profile of macrophages. These effects were shown to be mediated in a Wolbachia and TLR2 dependent manner (75).
TGF-ß is one of the major hallmarks of chronic filarial infection and the dependence of TGF-ß on the protective effect of filarial infection on sepsis was investigated. This effect was found to be independent of TGF-ß signalling as depletion of TGF-ß before challenge with E. coli did not lead to changes in pro-inflammatory cytokine or chemokine levels, nor increased bacterial burden and hypothermia. Despite TGF-ß depletion, L. sigmodontis infected mice demonstrated milder hypothermia, reduced bacterial load and pro-inflammatory cytokine levels (76). In addition, the impact of chronic L. sigmodontis infection on T cell responses during the compensatory anti-inflammatory phases following sepsis was studied. Chronic filarial infection did not worsen E. coli-induced impairment of adenovirus-induced CD8 T cell cytotoxicity and adenovirus-specific secretion of IFN-γ, suggesting that filarial immunoregulation does not exacerbate T cell paralysis induced by E. coli (77).
2.2.2 Diabetes and atherosclerosis
In the backdrop of cross-sectional studies from China, India, Indonesia and aboriginal Australian populations showcasing an inverse association between the prevalence of helminth infections and type 2 diabetes, the idea of helminth-induced immune modulation delaying the onset of type 2 diabetes started gaining popularity (78–81). Our group was the first to test the mechanisms underlying this in an experimental mouse model of diet-induced obesity. Berbudi et al. demonstrated that after diet-induced obesity, infection with the filarial nematode L. sigmodontis or administration of crude L. sigmodontis adult worm extract (LsAg) resulted in an improved glucose tolerance and an increase in AAM, ILC2s and eosinophils in the visceral adipose tissue (82).
In another study, a novel role for the adipose tissue secreted adipokine adiponectin was demonstrated in orchestrating the beneficial effects of LsAg administration during diet-induced obesity. While stimulation of T cells with adipocyte conditioned media from LsAg treated mice resulted in decreased frequencies of Th1 and Th17 cell populations, this effect was ameliorated when the adipocyte conditioned media from LsAg treated mice was depleted of adiponectin; highlighting a novel role for adiponectin in mediating some of the beneficial effects of helminth infections during obesity and insulin resistance (83). Regarding type 1 diabetes, we showed that infection with L. sigmodontis or administration of crude LsAg prevented the development of diabetes in non-obese diabetic mice (NOD) (70). This protective effect was dependent on TGF-β (84). A combination therapy of LsAg and pro-insulin was found to prevent the onset of diabetes in NOD mice even after insulitis occurred (85, 86). In addition, administration of LsAg to ApoE knockout mice, a mouse model for atherosclerosis, was found to decrease plaque size with both prophylactic and therapeutic treatment regimens. LsAg administration reduced the expression of Th-1 specific gene expression and intraplaque inflammation. The plaque size showed an inverse correlation with the AAM in the plaques (87).
2.2.3 COVID-19
In the wake of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, causing COVID-19, that has infected more than 600 million people worldwide and caused 6.8 million deaths as of 29th January 2023 (88), helminth-mediated immunomodulation on viral infections has been gaining increased interest, especially in the helminth endemic African sub-continent. This is owing to the fact that the prevalence and mortality of COVID-19 on the African sub-continent have been moderate (89). While factors like younger average age and low testing capacities could partially explain the mortality of COVID-19 and low prevalence, the co-endemicity of helminth infections in this part of the world as a potential contributor to this trend cannot be ruled out.
Infections with SARS-CoV-2 lead to a wide spectrum of clinical manifestations ranging from asymptomatic infection to potentially lethal respiratory complications, including hypoxemic respiratory failure and acute respiratory distress syndrome (ARDS) (90). These severe complications of the disease are associated with the hyperactivation of the immune system and the uncontrolled release of cytokines, including IL-2, IL-6, IFN-γ and TNF (91, 92). On the other hand, helminths are known to trigger a modified Th2 response wherein regulatory cell types (Tregs, B cells and anti-inflammatory cytokines like IL-10 and TGF-ß) balance the pro-inflammatory components. Considering the important roles of CD4 and CD8 T cell responses in controlling the pathology of SARS-CoV-2 infection (93, 94), we recently investigated the impact of helminth antigen co-stimulation on the activation of SARS-CoV-2-specific T cells from convalescent COVID-19 patients (95). The findings of the study showed that helminth antigens from three major helminth parasites B. malayi, O. volvulus, and Ascaris lumbricoides were able to reduce the frequency of SARS-CoV-2-reactive CD4 T helper cells but not CD8 T cells in the PBMCs of COVID-19 patients. Further, increased IL-10 secretion and decreased levels of IFN-γ and TNF were observed in PBMCs stimulated with helminth antigens (95). Moreover, work in progress in our team demonstrates a robust negative correlation between Ascaris-specific antibody expression and hospitalization rate and severe symptoms in COVID-19 patients from Benin. These data suggest that helminth immunomodulation could shift the immune response to SARS-CoV-2 in a manner that SARS-CoV-2-induced hyperactive immune reactions are mitigated similar to our observations in animal models.
2.2.4 Sexually transmitted infections
In another example of helminth mediated immunomodulation, our collaborative work with partners from South Africa, Ghana and Tanzania has demonstrated the influence of helminths on sexually transmitted viral infections (STVIs). Low-middle income countries bear the double burden of parasitic nematode infections as well as STVIs (96, 97). This has led researchers to test the hypothesis that nematode infections, despite not colonizing the female genital tract, cause changes in female fecundity (98) and female genital tract immunity (99). In this respect, Chetty et al. (100) investigated if nematode infections drive altered immunity to herpes simplex virus-2 (HSV-2) infection. Intriguingly, the findings of the study in the mouse model show that acute infection with Nippostrongylus brasiliensis initiates significant expansion of eosinophils in the female genital tract, which in turn leads to increased ulceration of the vaginal epithelium following HSV-2 infection. While our earlier reports have demonstrated a beneficial effect of helminth immunomodulation in sepsis, type 2 diabetes and COIVD-19, this study points towards a deterioration of the disease pathogenesis.
Indeed, our collaboration with Dr. Katawa from the University of Lomé in Togo has shown that Togolese women with systemic helminth infections have altered vaginal immunity and an immune milieu that increases sexually transmitted diseases; especially human papillomavirus (101–103). However, the mechanisms behind these observations remain uncertain. Thus, ongoing research using the L. sigmodontis mouse model aims to elucidate signalling mechanisms of eosinophils and ILCs in the female reproductive tract, which drive the vaginal type 2 immune signature leading to increased pathology and enhanced risk for STVIs. These observations reiterate the fact that the impact of helminths on other diseases may vary from beneficial to detrimental depending on the specific disease, species of helminth, localization of the helminth stages vs. secondary infection, chronicity of infection, and organs affected.
2.3 Genetics
As has been described in previous sections, infection with filarial nematodes has a spectrum of clinical states with two major poles and a range of responses between the poles. One pole is represented by microfilaremic patients with high parasite numbers and down-regulated cell-mediated responses (28), while the second is represented by patients who typically have few or no parasites, but vigorous specific immune reactions that cause immunopathology, e.g. dermatitis for onchocerciasis or lymphedema/hydrocele for lymphatic filariasis (66, 104, 105). The actual causes of the heterogeneity in infection and disease are not well understood, but have been attributed to differences in inflammatory processes that are mediated by host genetic factors as well as to secondary bacterial infections (105–107)
In onchocerciasis, case-control studies on Single Nucleotide Polymorphisms (SNPs) for IL-13 and IL-10 in onchocerciasis patients identified factors that lead to two different responses. The variant form of the pro-inflammatory cytokine IL-13 was associated with the Sowda form of extreme dermatitis in onchocerciasis. Sowda patients have an exacerbated Th2 response with extremely high IgE levels which leads to the killing of skin microfilariae, but at the expense of severe dermatitis in the infected person. The association of the IL-13 SNP with Sowda is stronger than that for allergy, the benchmark for IL-13 in pathology (106). Similarly, individuals with the variant forms of the immunosuppressive cytokine IL-10 promoter SNPs -1082, -819 and -592 are associated with lower IL-10 expression by immune cells from patients, resulting in lower numbers of circulating microfilariae (108, 109).
Although susceptibility to infection, parasite load, and pathology has been shown to cluster in families (110–114) the genetic architecture underlying lymphatic filariasis is poorly understood. Knowledge of genomic loci linked to clinical disease would help in the understanding of disease etiology which could lead to the development of new diagnostic tests, and prevention and treatment strategies.
In order to identify the first genetic risk factors, we have performed case-control studies of SNPs in TGF-β1 and VEGF-A. We have shown that reduced TGF-β1 secretion leads to more inflammatory cells which kill microfilariae but also damage host tissue (26, 115). Because of this strong association of TGF-β1 with filarial disease, we examined Leu10Pro variant SNPs of TGF-β1 in Ghanaian patients with lymphatic filariasis. We found that individuals carrying the Leu/Leu genotype have no circulating microfilariae (116).
VEGF-A is a major mediator of vascular permeability and angiogenesis and promotes extravasation of fluid and plasma proteins from the blood vessels that has been associated with Chyluria, a filarial disease manifestation characterized by lymph fluid in the urine (117). We therefore analyzed VEGF-A promoter SNPs for a possible role in the different manifestations in patients with lymphatic filariasis. The C/C genotype at base pair -460 was significantly higher in hydrocele patients than in non-hydrocele patients. Furthermore, plasma levels of VEGF-A were significantly higher in subjects with the C/C genotype than in those with other genotypes. A positive correlation between plasma VEGF-A and stage of hydrocele was observed, suggesting that the C allele at -460 is a genetic risk factor for hydrocele development in lymphatic filariasis (118).
To elucidate the genetic basis and a possible association of genetic markers to hydrocele development in lymphatic filariasis, we performed a candidate gene analysis with 850 Wuchereria bancrofti-infected volunteers by genotyping 49 single nucleotide polymorphisms (SNPs) in 32 angiogenesis genes by MassARRAY (119). We found variants in four genes of the angiogenesis pathway significantly associated with hydrocele patients compared to infected individuals without pathology (Endothelin-1 rs5370 and rs1800541; Caveolin-1 rs4730748 and rs926198; Collagen Type 1A1 rs2269336 and matix-metalloproteinase-2 rs1030868).
A similar analysis was also done to assess SNPs associated with filarial lymphedema development, a cross-sectional study of unrelated Ghanaian volunteers genotyped for SNPs in 285 lymphedema patients and 682 infected patients without pathology. In all 131 SNPs in 64 genes were genotyped by MassARRAY (manuscript in preparation). Genetic associations were identified for seven SNPs in five genes: vascular endothelial growth factor receptor-3 (VEGFR3; rs75614493), nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor alpha (NFΚBα; rs696), insulin like growth factor-1 (rs7136446), two SNPs in matrix metallopeptidase 2 (rs1030868 and rs2241145), and two SNPs in carcinoembryonic antigen-related cell adhesion molecule -1 (rs8110904 and rs8111171) (119). Interaction analyses revealed an interaction of these genes within the angiogenesis pathway.
A genome wide association study is currently being analyzed on a Ghanaian cohort of 1933 filarial lymphedema cases and 2474 endemic controls that were genotyped for 654,027 SNPs and genetic variations using the Illumina’s HumanOmniExpress Bead Chips. With imputation, the total number of genetic variations detected will total 2.5 million SNPs and genetic variations. With the larger sample size and broader genetic coverage, finding SNPs associated with disease etiology with high significance should be identified. Identification of such genetic markers of filarial pathology could aid in ameliorating pathology other than invasive, dangerous, expensive surgery. Knowing genetic markers for filarial lymphedema and hydrocele could provide a way to identify persons at risk before pathology is seen and might become the basis for development of a rapid screening test that, in the case of filarial lymphedema which develops early in life, might be applied to school-aged children.
3 Drug development
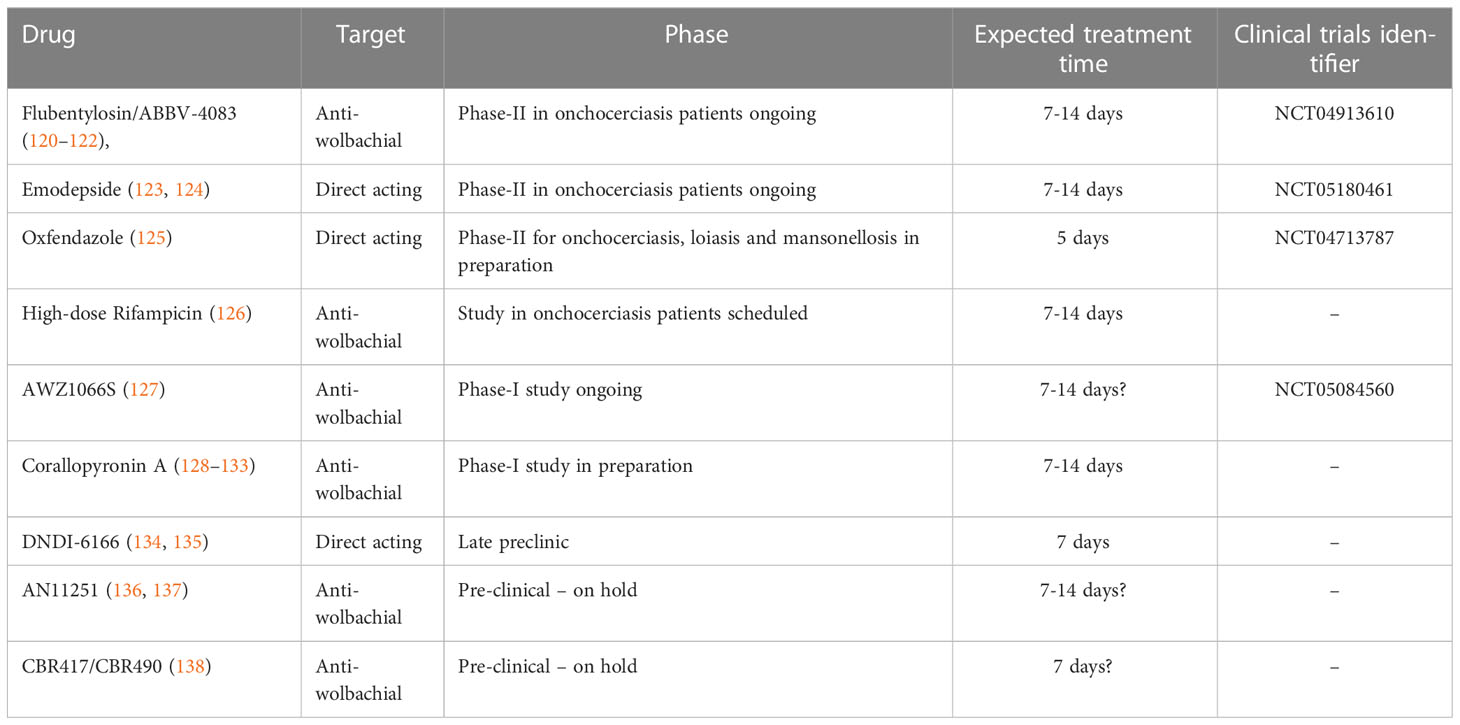
Besides contributing to significant advances in basic science, the institute and its collaborating partners has also been actively involved in translational research in contributing to the identification of new drug targets and pre-clinical drug research (Table 1). Detailed reviews on this topic are available (139–141). As the purpose of this review is to provide a comprehensive overview of the overall contribution of the institution to helminth research, this section covers only selected aspects of our work on drug development. These contributions aim to overcome drawbacks of the standard drugs used for MDA - ivermectin, albendazole and DEC. Ivermectin and DEC are microfilaricidal and inhibit the release of microfilariae by the adult filariae for up to 6-12 months (142), but have no prominent macrofilaricidal efficacy. Albendazole has limited macrofilaricidal adult worm sterilising efficacy in some filarial species, but as a broad-spectrum anthelmintic drug it rather targets other intestinal nematodes and cestodes (143, 144).
3.1 Doxycycline, the first macrofilaricidal drug
Chemotherapy against onchocerciasis and lymphatic filariasis in the past was only discussed within the framework of MDAs which despite their benefits also came with a lot of disadvantages. In this regard, the use of doxycycline against Wolbachia endosymbionts turned a new and promising chapter in the direction of novel treatment options. Doxycycline came to the forefront of filariasis research when earlier studies in animal models showed effectiveness of the antibiotic tetracycline against Wolbachia of the filarial nematode L. sigmodontis leading to inhibition of embryogenesis, female worm sterility and subsequent clearance of microfilariae (145, 146). This effect of tetracycline was also confirmed in infections with O. ochengi (147), B. pahangi and D. immitis (148). Doxycycline is a second-generation tetracycline antibiotic and its use in first clinical trials at a dose of 100mg daily for 6 weeks resulted in Wolbachia depletion from O. volvulus, adult worm killing, and embryogenesis inhibition that resulted in amicrofilaridermia (149). A double-blind randomised field trial of 200mg doxycycline per day for 8 weeks strongly reduced the adult worm burden in lymphatic filariasis (as measured by ultrasonography) and resulted in amicrofilaremia at 8-14 months in the doxycycline group (150). Further testing of this antibiotic in bancroftian filariasis patients also proved its effectiveness in Wolbachia depletion as a dose of 200mg for six weeks resulted in 96% loss of Wolbachia with a 99% reduction of microfilaremia (151). In addition, a single dose of ivermectin after doxycycline treatment led to a fast and complete elimination of microfilariae, since microfilariae eliminated by ivermectin are not replenished due to embryogenesis interruption by doxycycline (151). In lymphedemic patients after 4 months of doxycycline treatment, a single dose of 150-200 mug/kg ivermectin and 400 mg albendazole led to a significant reduction in the mean stage of lymphedema in the doxycycline-treated patients compared to placebo patients (152). As a result of Wolbachia depletion, embryogenesis is blocked leading to a gradual decrease of microfilariae according to its half-life. Ivermectin acts in a complementary manner to target the fully developed microfilariae. Although doxycycline alone resulted in Wolbachia depletion and microfilariae reduction, additional ivermectin treatment advances the killing of microfilariae and doxycycline impeded the reappearance of microfilariae. Combination of 200mg of doxycycline regimens for 6 weeks followed by a single dose of ivermectin and albendazole four months after doxycycline led to a macrofilaricidal effect of 89% after 2 years and significant improvements in lymphedema already after one year (152). When doxycycline treatment duration was reduced to 4 weeks, there was still a macrofilaricidal activity of 83% after 24 months (153). However, a 3-week course of doxycycline followed by ivermectin and albendazole 4 months later did not affect the adult worm burden, although it led to ablation of microfilariae (154). In addition, doxycycline, arguably through its effects on lymphatic dilation was shown to be effective against lymphatic pathology in a study cohort from India (155). However, recent findings from a multicentre clinical trial on lymphedema management indicate that doxycycline has no additional benefit in reducing the progression of lymphedema pathology if strict hygiene measurements are maintained.
In B. malayi-infected persons, a daily 6 week treatment with doxycycline alone or in combination with DEC and albendazole was found to decrease microfilaremia as well as reduce adverse reactions following antifilarial treatment (156). A report on doxycycline-induced changes in gene expression has provided new hints regarding the symbiotic relationship between Wolbachia and B. malayi. The genes in B. malayi essential for reproduction, growth and development were found to be down-regulated on treatment with doxycycline. The changes in the genome are consistent with the effect of doxycycline on filarial embryogenesis (157).
In case of onchocerciasis, a 200mg daily 6 week doxycycline regimen resulted in a gradual Wolbachia decline starting from month 2 and inhibition of embryogenesis observed by 6 months and a microfilariae decline at 11 months after the start of treatment. In the same trial a dosage of doxycycline 200mg for 4 weeks resulted also in Wolbachia depletion and female worm sterility (158). In another trial 100mg doxycycline for 6 weeks was used in patients with persistent microfilaridermia despite many rounds of IVM which led also in this special collective to sterility and enhanced killing of the adult worms (SCOOTT) (159). Further studies also validated and confirmed the effects of doxycycline such as macrofilaricidal activity, female worm sterility, and Wolbachia depletion during O. volvulus infection (160–162). Doxycycline was also found to be safe in areas that are co-endemic for loiasis as L. loa is a Wolbachia lacking nematode. Because of that, and also because anti-wolbachial efficacy affects only adult worm vitality and embryogenesis but does not have a direct effect on microfilariae, it does not induce the serious adverse events observed after microfilaricidal treatment with DEC or IVM (163–166). Based on these compelling evidences, WHO has recommended doxycycline as individual therapy against filariasis (1).
The drawback of doxycycline treatment is that it is contraindicated in pregnant and breast-feeding women as well as in children under the age of eight (167). Although a 6-week course of doxycycline has been administered successfully and with high compliance to a whole community of 12,000 people, it is only recommended for individual therapy because it requires daily treatments over 4-6 weeks. Individual treatment with doxycycline is being used as an end game strategy to clear remaining onchocerciasis in regions like Brazil and Republic of Venezuela (1). Its use as mass drug administration for onchocerciasis or lymphatic filariasis is not considered, due to the 4-6 week daily treatment requirement. Further there is also a risk of potential emergence of antimicrobial resistance if doxycycline ends up being used in MDA like Ivermectin.
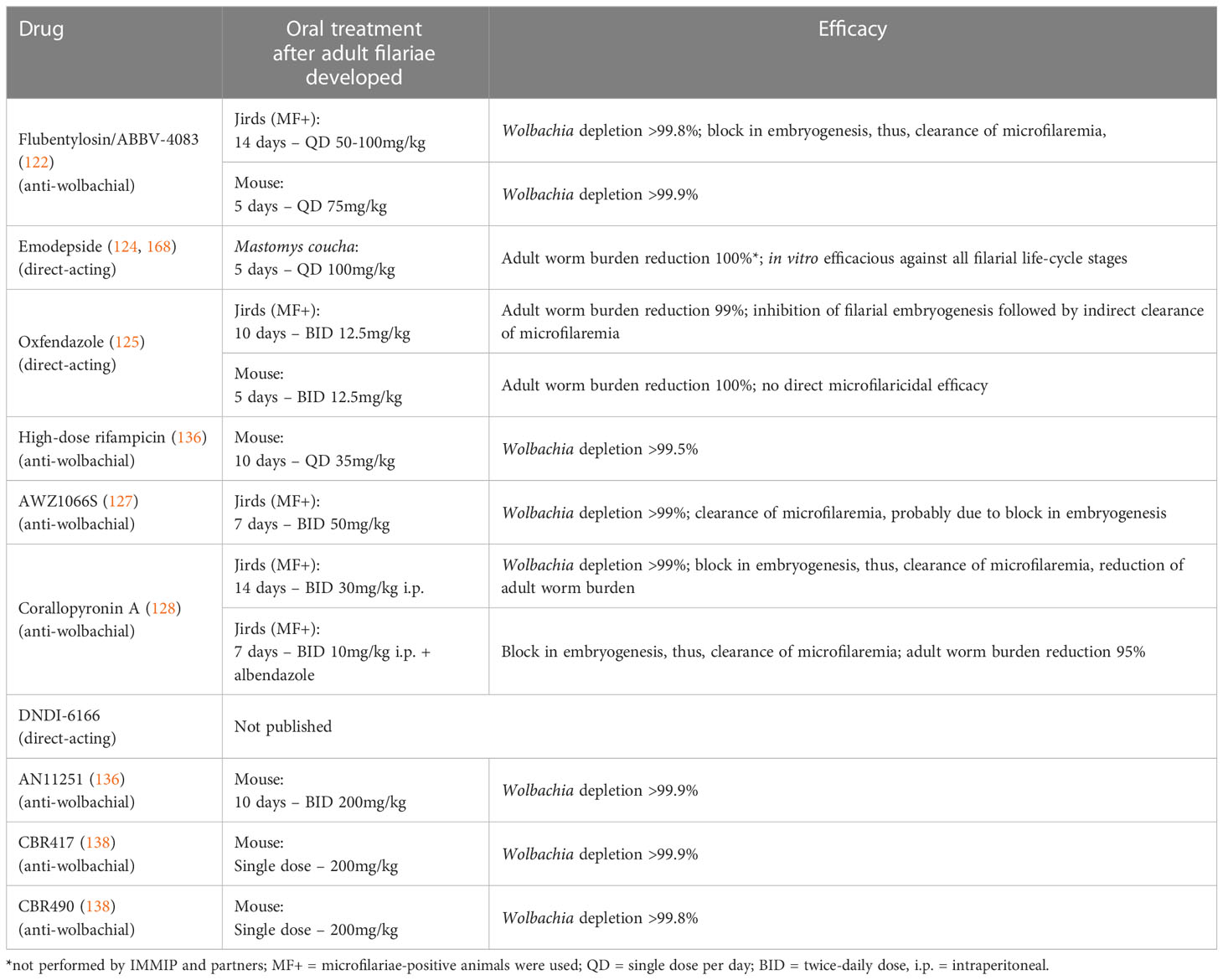
3.2 New, short course anti-wolbachials under development
Considering the long treatment regimen of 4 to 6 weeks necessary for doxycycline and the contraindications for pregnant and breastfeeding women and children, there is an urgent need for short course macrofilaricidal adult worm killing drugs. Several candidates are currently at the early clinical or late pre-clinical stage (140). These drug candidates include emodepside, flubentylosin/ABBV-4083 (both currently tested in phase 2 clinical studies in onchocerciasis patients), oxfendazole and AWZ1066S (completed and ongoing phase 1 clinical study, respectively) (Table 1). Detailed information on these compounds is available in the article by Ehrens et al. (140) and an overview of their efficacy in the L. sigmodontis rodent model is given in Table 2. Next to the testing of those drug candidates in the pre-clinical L. sigmodontis model as well as supporting the clinical studies, our institute along with our partner institutions is developing corallopyronin A (CorA) as a novel candidate for the treatment of filariasis (139). In the L. sigmodontis model, treatment with CorA for 2 weeks has been shown to clear Wolbachia endosymbionts in vivo leading to a macrofilaricidal effect. In addition, combining CorA with the drug albendazole led to the shortening of CorA treatment to 7 days as well as the death of adult filariae (128). CorA is a natural product from Corallococcus coralloides (129) and acts via non-competitively inhibiting the DNA dependent RNA polymerase of the bacteria by targeting the switch region (130–133). It has been shown to be potent against gram-positive bacteria including rifampicin-resistant Staphylococcus aureus (169). Encouraging results from safety and toxicity testing indicate that CorA is safe and non-toxic up to a dose of 500-1000 mg/kg in rats and dogs. Final GLP-tox studies funded by the EU Horizon 2020 HELP consortium are underway, and a phase 1 trial is in filed in 2024 supported by the German Center for Infection Research (DZIF). The standard Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) studies for CorA has been completed and the results of the study show that CorA has an acceptable in vitro early ADMET profile which is supported by the in vivo experiments in mice, mongolian gerbils and rats (170). In addition, CorA has also demonstrated excellent efficacy against other gram-positive and some gram-negative pathogens, e.g., Chlamydia trachomatis, Orientia tsutsugamushi and Staphylococcus aureus. Secondary indications are being developed for these organisms (140, 169).
4 Novel methods
In order to facilitate faster development of drugs against the various developmental stages of the filarial parasite, novel assays that have increased sensitivity, are more objective, or are easy to implement are needed for clinical trials, basic research, and drug development. Additionally, in vitro cultures are needed of the human parasitic filarial nematodes to de-risk drug candidates selected using animal filarial nematode models.
For clinical trials, we have developed a sensitive qPCR tool for the quantification of Wolbachia from few O. volvulus microfilariae in skin biopsies. The ability to monitor the effect of antiwolbachial drug in few microfilariae is essential because: (i) the level of microfilaridermia is decreasing as MDA coverage is being attained, and (ii) effective anitwolbachial drugs lead to a reduction in skin microfilariae. The method allows the analysis of anti-wolbachial drug efficacy from as few as 2 microfilariae and can be used to predict the effect in adult nematodes at less than 6 months post treatment without excising onchocermata, which are limited resource per patient; and the removal of onchocercomata can directly affect end point readouts – removal of onchocercomata also removes adult nematodes, resulting in a concomitant reduction in microfilariae independent from treatment (171). The method is being validated using prospective samples from the flubentylosin phase 2 trial.
The institute, together with its academic collaborating partners and Capgemini is developing artificial intelligence (AI) to evaluate nodules from onchocerciasis patients. Until now, histological analysis was indispensable to assess the effect of a treatment on the adult worm. However, this time-consuming process and the lack of experts worldwide slow clinical trials required to register new drug regimens. With funding from the Bill & Melinda Gates Foundation, the establishment of AI is expected to drastically reduce the time required for the analysis of nodules, replace subjective analysis with objective analysis that can be established by other groups, and provide a constant algorithm of decision making in nodule assessment, an important request by regulatory authorities who have not yet approved antifilarial drugs with adulticidal efficacy. Similar to gold standard microscopic analysis, AI requires the presence of nodules, which prevents the diagnosis of early infection with the infective stage of O. volvulus. For this, improved serological diagnostic tests are required.
For new drug screening, an in vitro system has been developed for the maintenance of O. volvulus L3 larvae, which can develop to the adult stage. This system may provide a platform for the investigation of parameters like mating behaviour and early stage nodulogenesis; which are additional targets for macrofilaricidal drug screening (172). In addition, in vitro culture systems for the filariae M. perstans, L. loa and O. volvulus were established by the group of our long-time partner Prof. Wanji from the University of Buea in Cameroon. These systems will provide platforms for the investigation of larvae, adult worms and mating behaviour as well as drug screening (173–178).
In settings where there is a low prevalence of filariasis, e.g., after successful MDA, rapid and sensitive tests are needed to support serological tests (for those infections that have them) to determine the correct timepoint to stop MDA (179). With our partners, loop mediated isothermal amplification (LAMP) assays for detecting filarial nematodes have been developed and evaluated as an alternative to microscopy. For the detection of loiasis, a LAMP assay of parasite DNA has been developed for the detection in infected Chrysops spp. The method has been validated under experimental and natural field conditions and is superior to the current gold standard, microscopy (178). This study demonstrated LAMP as a potential tool for surveillance with more sensitivity than microscopy for the detection of experimental and natural L. loa infections in Chrysops vectors. LAMP assays have also been developed for the detection of other filarial nematode species (180–182): B. malayi, O. volvulus and W. bancrofti. All the assays have been shown to offer high levels of sensitivity and specificity that are better than microscopy. As M. perstans can also modulate the immune system as described in the preceding sections, a LAMP diagnostic test has been developed for the detection of M. perstans. The M. perstans assay detects up to 0.1 pg, equivalent of 1/1000th of a microfilaria. The assay performed well on infected human and Culicoides vector samples. Thus, it will prove useful for mapping and surveillance of these less-studied filarial infections (181). Although real-time PCR also provides high sensitivity and specificity, the advantages LAMP assays offer are: (i) technical ease of use without expensive equipment, e.g. a thermocycler, and (ii) rapid visual detection when using a colorimetric readout.
5 Ongoing research
Besides our ongoing basic research, drug development and diagnostic research, along with our collaboration partners we are also involved in trials that are carried out to improve the morbidity management for patients suffering from lymphedema or hydroceles due to lymphatic filariasis. In two trials it was shown that doxycycline 200mg for 6 weeks led to halt of progression or even improvement of the lymphedema (152, 183). To confirm these results in other settings and by other research groups, 5 clinical trials were carried out in collaboration with the Taskforce of Global Health (TFGH), Atlanta, USA (184). The IMMIP conducted two of the five trials with the partners in Ghana (https://doi.org/10.1186/ISRCTN14042737) and Tanzania (https://doi.org/10.1186/ISRCTN65756724) as part of the Tackling the obstacles to fight filariasis and podoconiosis (TAKeOFF) consortium. TAKeOFF is one out of five research networks, funded by the German Federal Ministry of Education and Research (BMBF) for health innovations in sub-Saharan Africa. The three trials supported by the TFGH, funded by the USAID, were carried out in Sri Lanka (NCT02929134), Mali (NCT02927496) and India (NCT02929121). The results of these five trials will help to improve the WHO guidelines for the morbidity management of lymphedema patients. Additionally, we are involved in studying podoconiosis, which is a non-filarial lymphedema of lower limbs considered as one of the most neglected diseases affecting the poorest of people (185). Podoconiosis is a geochemical disease endemic in 32 nations of Africa, Latin America and South East Asia; afflicting 4 million individuals. Contact with volcanic red soil, genetic heritability, high altitude, seasonal rainfall and subsistent farming are the known risk factors and research with our collaboration partners is expected to bring a better understanding of the pathogenesis, novel treatment strategies and point-of-care diagnosis (185). A clinical trial with the same design as the trials mentioned above was carried out by the TAKeOFF consortium in Cameroon with the aim to also improve the morbidity management and to test if doxycycline has a beneficial effect for patients suffering from podoconiosis (https://doi.org/10.1186/ISRCTN11881662).
6 Conclusion
A number of exciting developments ranging from novel antifilarial drug candidates and diagnostic tool development to deciphering basic disease mechanisms are exploding in the filarial research field. Our tight network of collaborations throughout the world with filarial researchers in Ghana, Cameroon and Tanzania have contributed immensely to our work. These collaborations are still expanding to other regions like Togo, Benin and South Africa. Further development of novel animal models to study the complete life cycle of human pathogenic filariae and novel drugs with macrofilaricidal capacity will go a long way in achieving the aim of elimination of transmission of onchocerciasis and lymphatic filariasis as a public health problem.
Author contributions
Preparation of the original draft: IK, MR, KP, UK-S, SW, TA, MH, AH. All authors contributed to the article and approved the submitted version.
Funding
MH is funded by the German Center for Infection Research (DZIF Translational Thematic Unit: Novel Antibiotics #09.701) and the Deutsche Forschungsgesellschaft (DFG grant HU2144/3-1). AH, KP and UKS received support from the DZIF (#09.807, #09.806, #09.822 and #09.914), the Deutsche Forschungsgesellschaft (DFG grant PF673/3-1) and the Federal Ministry of Education and Research (BMBF grant 16GW0227K). Additional funding was received by the German Federal Ministry of Education and Research (BMBF) [01KA1611 and 01KA2027] and the German Research Foundation (DFG) [RI 3036/1-1, HO 2009/10-1, HO 2009/11-2, LA2476/1-2, LA 2746/2-1, LA 2476/5-1, HO 2009/14-01, PF 673/2-0, PF 673/4-1, PF 673/6-1 and Germany’s Excellence Strategy – EXC2151 – 390873048). AYD received funding from EDCTP2 grant TMA2018SF-2451, SW is the Senior Fellow Plus of EDCTP2 (TMA2019SFP-2814).
Conflict of interest
KP, SS and AH hold patents for the indication of filariasis of Corallopyronin A. US 9168244 B2, Compounds for use in the treatment of filariasis Therapeutic treatment of filariasis/dirofilariasis in humans/animals Inventors: KP, AH, Gabriele Maria Koenig, SS, Andrea Schiefer, Till Friedrich Schaeberle, Alexander Schmitz, Stefan Kehraus granted 2015-10-27, duration: 2032-05-04, US 9687470 B2, Compounds for use in the treatment of filariasis Prevention of filariasis/dirofilariasis in dogs and humans Inventors: KP, AH, Gabriele Maria Koenig, SS, Andrea Schiefer, Till Friedrich Schaeberle, Alexander Schmitz, Stefan Kehraus granted 2017-06-27, duration: 2032-05-04. EP 2704708 B1, Compounds for use in the treatment of filariasis Prevention and treatment of filariasis/dirofilariasis in humans/animals Inventors: KP, AH, Gabriele Maria Koenig, SS, Andrea Schiefer, Till Friedrich Schaeberle, Alexander Schmitz, Stefan Kehraus 2017-10-11 and validated in: DE, GB, NL, CH, IT, ES, FR and HR, duration: 2032-05-04. Patent-Pending: A European application EP 20 172 409.3 “Solid and liquisolid formulations of Corallopyronin A” has been filed with the EPO on 30.4.2020. A PCT application PCT/EP2021/061310 with the same title, claiming priority to the EP 20 172 409.3, has been filed for patent on 29.04.2021.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO progress in eliminating onchocerciasis in the WHO region of the americas: doxycycline treatment as an end-game strategy. Wkly Epidemiol Rep (2019) 37:415–9.
2. WHO. Lymphatic filariasis (2022). Available at: https://www.who.int/en/news-room/fact-sheets/detail/lymphatic-filariasis (Accessed October 15, 2022).
3. Kamtchum Tatuene JGFR, Nkoa T, Tchateng Mbougua JB, Nana Djeunga HC, Bopda J, Kamgno J. Epidemiology of Loa loa and Mansonella perstans filariasis in the akonolinga health district, centre region, Cameroon. Health Sci Dis (2014) 15(1):3.
4. Akue JP, Nkoghe D, Padilla C, Moussavou G, Moukana H, Mbou RA, et al. Epidemiology of concomitant infection due to Loa loa and Mansonella perstans in Gabon. PloS Negl Trop Dis (2011) 5(10):e1329. doi: 10.1371/journal.pntd.0001329
5. WHO. Onchocerciasis (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/onchocerciasis (Accessed October 15, 2022).
6. WHO. Global programme to eliminate lymphatic filariasis: progress report, (2019). Weekly epidemiological Rep (2020) 95:509–24.
8. Simonsen PE, Onapa AW, Asio SM. Mansonella perstans filariasis in Africa. Acta Trop.,120(Suppl (2011) 1):S109–20. doi: 10.1016/j.actatropica.2010.01.014
9. Taylor MJ, Hoerauf A, Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet (2010) 376(9747):1175–85. doi: 10.1016/S0140-6736(10)60586-7
10. Hoerauf A. Onchocerciasis. In: Guerrant RL, Walker DH, Weller PF, editors. Tropical infectious diseases: Principles, pathogens and practice. Edinburgh: Elsevier (2011). p. 741–9.
11. WHO. Guideline − alternative mass drug administration regimens to eliminate lymphatic filariasis. In: Dr J, editor. King/Lymphatic filariasis. Geneva: World Health Organization (2017).
12. WHO. Progress report on the elimination of human onchocerciasis, 2016–2017. Wkly Epidemiol Rec (2017) 92(45):681–700.
13. Francis H, Awadzi K, Ottesen EA. The mazzotti reaction following treatment of onchocerciasis with diethylcarbamazine: clinical severity as a function of infection intensity. Am J Trop Med Hyg (1985) 34(3):529–36. doi: 10.4269/ajtmh.1985.34.529
14. Wanji S, Chounna Ndongmo WP, Fombad FF, Kengne-Ouafo JA, Njouendou AJ, Longang Tchounkeu YF, et al. Impact of repeated annual community directed treatment with ivermectin on loiasis parasitological indicators in Cameroon: Implications for onchocerciasis and lymphatic filariasis elimination in areas co-endemic with Loa loa in Africa. PloS Negl Trop Dis (2018) 12(9):e0006750. doi: 10.1371/journal.pntd.0006750
15. WHO. Working to overcome the global impact of neglected tropical diseases – first WHO report on neglected tropical diseases. Geneva: WHO (2010).
16. WHO. Elimination of human onchocerciasis: progress report, 2020. Wkly Epidemiol Rec. (2021) 96(41):617–23.
17. WHO. Global programme to eliminate lymphatic filariasis: progress report, 2020. Wkly Epidemiol Rec. (2021) 96(41):497–508.
18. WHO. Ending the neglect to attain the sustainable development goals: A road map for neglected tropical diseases 2021–2030. Geneva: World Health Organization (2020).
19. Chandler AC. New genera and species of nematode worms. Proc US Natl Museum (1931) 78:1–11. doi: 10.5479/si.00963801.78-2866.1
20. Risch F, Ritter M, Hoerauf A, Hübner MP. Human filariasis-contributions of the Litomosoides sigmodontis and Acanthocheilonema viteae animal model. Parasitol Res (2021) 120(12):4125–43. doi: 10.1007/s00436-020-07026-2
21. Allen JE, Adjei O, Bain O, Hoerauf A, Hoffmann WH, Makepeace BL, et al. Of mice, cattle, and humans: the immunology and treatment of river blindness. PloS Negl Trop Dis (2008) 2(4):e217. doi: 10.1371/journal.pntd.0000217
22. Karadjian G, Fercoq F, Pionnier N, Vallarino-Lhermitte N, Lefoulon E, Nieguitsila A, et al. Migratory phase of Litomosoides sigmodontis filarial infective larvae is associated with pathology and transient increase of S100A9 expressing neutrophils in the lung. PloS Negl Trop Dis (2017) 11(5):e0005596. doi: 10.1371/journal.pntd.0005596
23. Kilarski WW, Martin C, Pisano M, Bain O, Babayan SA, Swartz MA. Inherent biomechanical traits enable infective filariae to disseminate through collecting lymphatic vessels. Nat Commun (2019) 10(1):2895. doi: 10.1038/s41467-019-10675-2
24. Hübner MP, Torrero MN, McCall JW, Mitre E. Litomosoides sigmodontis: a simple method to infect mice with L3 larvae obtained from the pleural space of recently infected jirds (Meriones unguiculatus). Exp Parasitol (2009) 123(1):95–8. doi: 10.1016/j.exppara.2009.05.009
25. Petit G, Diagne M, Maréchal P, Owen D, Taylor D, Bain O. Maturation of the filaria Litomosoides sigmodontis in BALB/c mice; comparative susceptibility of nine other inbred strains. Ann Parasitol Hum Comp (1992) 67(5):144–50. doi: 10.1051/parasite/1992675144
26. Doetze A, Satoguina J, Burchard G, Rau T, Loliger C, Fleischer B, et al. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int Immunol (2000) 12:623–30. doi: 10.1093/intimm/12.5.623
27. Saeftel M, Volkmann L, Korten S, Brattig N, Al-Qaoud K, Fleischer B, et al. Lack of interferon-gamma confers impaired neutrophil granulocyte function and imparts prolonged survival of adult filarial worms in murine filariasis. Microbes Infect (2001) 3:203–13. doi: 10.1016/S1286-4579(01)01372-7
28. Hoerauf A, Brattig N. Resistance and susceptibility in human onchocerciasis–beyond Th1 vs. Th2. Trends Parasitol (2002) 18:25–31. doi: 10.1016/S1471-4922(01)02173-0
29. Pionnier N, Brotin E, Karadjian G, Hemon P, Gaudin-Nomé F, Vallarino-Lhermitte N, et al. Neutropenic mice provide insight into the role of skin-infiltrating neutrophils in the host protective immunity against filarial infective larvae. PloS Negl Trop Dis (2016) 10(4):e0004605. doi: 10.1371/journal.pntd.0004605
30. Frohberger SJ, Fercoq F, Neumann AL, Surendar J, Stamminger W, Ehrens A, et al. S100A8/S100A9 deficiency increases neutrophil activation and protective immune responses against invading infective L3 larvae of the filarial nematode Litomosoides sigmodontis. PloS Negl Trop Dis (2020) 14(2):e0008119. doi: 10.1371/journal.pntd.0008119
31. Ajendra J, Specht S, Ziewer S, Schiefer A, Pfarr K, Parčina M, et al. NOD2 dependent neutrophil recruitment is required for early protective immune responses against infectious Litomosoides sigmodontis L3 larvae. Sci Rep (2016) 6:39648. doi: 10.1038/srep39648
32. Muhsin M, Ajendra J, Gentil K, Berbudi A, Neumann AL, Klaas L, et al. IL-6 is required for protective immune responses against early filarial infection. Int J Parasitol (2018) 48(12):925–35. doi: 10.1016/j.ijpara.2018.05.011
33. Tamarozzi F, Turner JD, Pionnier N, Midgley A, Guimaraes AF, Johnston KL, et al. Wolbachia endosymbionts induce neutrophil extracellular trap formation in human onchocerciasis. Sci Rep (2016) 6:35559. doi: 10.1038/srep35559
34. Gillette-Ferguson I, Hise AG, McGarry HF, Turner J, Esposito A, Sun Y, et al. Wolbachia-induced neutrophil activation in a mouse model of ocular onchocerciasis (river blindness). Infect Immun (2004) 72(10):5687–92. doi: 10.1128/IAI.72.10.5687-5692.2004
35. Saint Andre A, Blackwell NM, Hall LR, Hoerauf A, Brattig NW, Volkmann L, et al. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science (2002) 295:1892–5. doi: 10.1126/science.1068732
36. Brattig NW, Büttner DW, Hoerauf A. Neutrophil accumulation around Onchocerca worms and chemotaxis of neutrophils are dependent on Wolbachia endobacteria. Microbes Infect (2001) 3(6):439–46. doi: 10.1016/S1286-4579(01)01399-5
37. Hise AG, Daehnel K, Gillette-Ferguson I, Cho E, McGarry HF, Taylor MJ, et al. Innate immune responses to endosymbiotic Wolbachia bacteria in Brugia malayi and Onchocerca volvulus are dependent on TLR2, TLR6, MyD88, and mal, but not TLR4, TRIF, or TRAM. J Immunol (2007) 178(2):1068–76. doi: 10.4049/jimmunol.178.2.1068
38. Wiszniewsky A, Ritter M, Krupp V, Schulz S, Arndts K, Weighardt H, et al. The central adaptor molecule TRIF influences L. sigmodontis worm development. Parasitol Res (2019) 18(2):539–49. doi: 10.1007/s00436-018-6159-1
39. Rodrigo MB, Schulz S, Krupp V, Ritter M, Wiszniewsky K, Arndts K, et al. Patency of litomosoides sigmodontis infection depends on toll-like receptor 4 whereas toll-like receptor 2 signalling influences filarial-specific CD4(+) T-cell responses. Immunology (2016) 147(4):429–42. doi: 10.1111/imm.12573
40. Ritter M, Tamadaho RS, Feid J, Vogel W, Wiszniewsky K, Perner S, et al. IL-4/5 signalling plays an important role during litomosoides sigmodontis infection, influencing both immune system regulation and tissue pathology in the thoracic cavity. Int J Parasitol (2017) 47(14):951–60. doi: 10.1016/j.ijpara.2017.06.009
41. Frohberger SJ, Ajendra J, Surendar J, Stamminger W, Ehrens A, Buerfent BC, et al. Susceptibility to L. sigmodontis infection is highest in animals lacking IL-4R/IL-5 compared to single knockouts of IL-4R, IL-5 or eosinophils. Parasit Vectors (2019) 12(1):248. doi: 10.1186/s13071-019-3502-z
42. Martin C, Al-Qaoud KM, Ungeheure MN, Paehle K, Vuong PN, Bain O, Fleischer B, et al. IL-5 is essential for vaccine-induced protection and for resolution of primary infection in murine filariasis. Med Microbiol Immunol (2000) 189:67–74. doi: 10.1128/IAI.68.6.3651-3656.2000
43. Gentil K, Lentz CS, Rai R, Muhsin M, Kamath AD, Mutluer O, et al. Eotaxin-1 is involved in parasite clearance during chronic filarial infection. Parasite Immunol (2014) 36(2):60–77. doi: 10.1111/pim.12079
44. Specht S, Saeftel M, Arndt M, Endl E, Dubben B, Lee NA, et al. Lack of eosinophil peroxidase or major basic protein impairs defense against murine filarial infection. Infect Immun (2006) 74(9):5236–43. doi: 10.1128/IAI.00329-06
45. Ehrens A, Lenz B, Neumann AL, Giarrizzo S, Reichwald JJ, Frohberger SJ, et al. Microfilariae trigger eosinophil extracellular DNA traps in a dectin-1-Dependent manner. Cell Rep (2021) 34(2):108621. doi: 10.1016/j.celrep.2020.108621
46. Ehrens A, Hoerauf A, Hübner MP. Eosinophils in filarial infections: Inducers of protection or pathology? Front Immunol (2022) 13:983812. doi: 10.3389/fimmu.2022.983812
47. Fercoq F, Remion E, Vallarino-Lhermitte N, Alonso J, Raveendran L, Nixon C, et al. Microfilaria-dependent thoracic pathology associated with eosinophilic and fibrotic polyps in filaria-infected rodents. Parasit Vectors. (2020) 13(1):551. doi: 10.1186/s13071-020-04428-0
48. Reichwald JJ, Risch F, Neumann A-L, Frohberger SJ, Scheunemann JF, Lenz B, et al. ILC2s control microfilaremia during Litomosoides sigmodontis infection in Rag2-/- mice. Front Immunol (2022) 13:863663. doi: 10.3389/fimmu.2022.863663
49. Tamadaho RSE, Hoerauf A, Layland LE. Immunomodulatory effects of myeloid-derived suppressor cells in diseases: Role in cancer and infections. Immunobiology (2018) 223:4–5, 432-442. doi: 10.1016/j.imbio.2017.07.001
50. Tamadaho RSE, Ritter M, Wiszniewsky A, Arndts K, Mack M, Hoerauf A, et al. Infection-derived monocytic MDSCs require TGF-β to suppress filarial-specific IFN-γ but not IL-13 release by filarial-specific CD4+ T cells In vitro. front. Trop Dis (2022) 2:707100. doi: 10.3389/fitd.2021.707100
51. Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites–masters of regulation. Immunol Rev (2004) 201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x
52. Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol (2011) 11:375–88. doi: 10.1038/nri2992
53. Anthony RM, Rutitzky LI, Urban JF, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol (2007) 7:975–87. doi: 10.1038/nri2199
54. Hoerauf A, Satoguina J, Saeftel M, Specht S. Immunomodulation by filarial nematodes. Parasite Immunol (2005) 27:417–29. doi: 10.1111/j.1365-3024.2005.00792.x
55. Specht S, Taylor MD, Hoeve MA, Allen JE, Lang R, Hoerauf A. Over expression of IL-10 by macrophages overcomes resistance to murine filariasis. Exp Parasitol (2012) 132(1):90–6. doi: 10.1016/j.exppara.2011.09.003
56. Specht S, Volkmann L, Wynn T, Hoerauf A. Interleukin-10 (IL-10) counterregulates IL-4-dependent effector mechanisms in murine filariasis. Infect Immun (2004) 72(11):6287–93. doi: 10.1128/IAI.72.11.6287-6293.2004
57. Layland LE, Ajendra J, Ritter M, Wisniewsky A, Hoerauf A, Hübner MP. Development of patent Litomosoides sigmodontis infections in semi-susceptible C57BL/6 mice in the absence of adaptive immune responses. Parasites Vectors. (2015) 8:396. doi: 10.1186/s13071-015-1011-2
58. Ritter M, Ndongmo WPC, Njouendou AJ, Nghochuzie NN, Nchang LC, Tayong DB, et al. Mansonella perstans microfilaremic individuals are characterized by enhanced type 2 helper T and regulatory T and b cell subsets and dampened systemic innate and adaptive immune responses. PloS Negl Trop Dis (2018) 12(1):e0006184. doi: 10.1371/journal.pntd.0006184
59. Ritter M, Osei-Mensah J, Debrah LB, Kwarteng A, Mubarik Y, Debrah AY, et al. Wuchereria bancrofti-infected individuals harbor distinct IL-10-producing regulatory b and T cell subsets which are affected by anti-filarial treatment. PloS Negl Trop Dis (2019) 13(5):e0007436. doi: 10.1371/journal.pntd.0007436
60. Babu S, Nutman TB. Immunopathogenesis of lymphatic filarial disease. Semin Immunopathol (2012) 34(6):847–61. doi: 10.1007/s00281-012-0346-4
61. Babu S, Nutman TB. Immunology of lymphatic filariasis. Parasite Immunol (2014) 36(8):338–46. doi: 10.1111/pim.12081
62. Kroidl I, Chachage M, Mnkai J, Nsojo A, Berninghoff M, Verweij JJ, et al. Wuchereria bancrofti infection is linked to systemic activation of CD4 and CD8 T cells. PloS Negl Trop Dis (2019) 13(8):e0007623. doi: 10.1371/journal.pntd.0007623
63. Horn S, Borrero-Wolff D, Ritter M, Arndts K, Wiszniewsky A, Debrah LB, et al. Distinct immune profiles of exhausted effector and memory CD8+ T cells in individuals with filarial lymphedema. Front Cell Infect Microbiol (2021) 11:680832. doi: 10.3389/fcimb.2021.680832
64. Horn S, Ritter M, Arndts K, Borrero-Wolff D, Wiszniewsky A, Debrah LB, et al. Filarial lymphedema patients are characterized by exhausted CD4+ T cells. Front Cell Infect Microbiol (2022) 11:767306. doi: 10.3389/fcimb.2021.767306
65. Saeftel M, Arndt M, Specht S, Volkmann L, Hoerauf A. Synergism of gamma interferon and interleukin-5 in the control of murine filariasis. Infect Immun (2003) 71(12):6978–85. doi: 10.1128/IAI.71.12.6978-6985.2003
66. Katawa G, Layland LE, Debrah AY, von Horn C, Batsa L, Kwarteng A, et al. Hyperreactive onchocerciasis is characterized by a combination of Th17-Th2 immune responses and reduced regulatory T cells. PloS Negl Trop Dis (2015) 9(1):e3414. doi: 10.1371/journal.pntd.0003414
67. Dittrich AM, Erbacher A, Specht S, Diesner F, Krokowski M, Avagyan A, et al. Helminth infection with litomosoides sigmodontis induces regulatory T cells and inhibits allergic sensitization, airway inflammation, and hyperreactivity in a murine asthma model. J Immunol (2008) 180:1792–9. doi: 10.4049/jimmunol.180.3.1792
68. Wilson MS, Taylor MD, Balic A, Finney CAM, Lamb JR, Maizels RM, et al. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med (2005) 202:1199–212. doi: 10.1084/jem.20042572
69. Hübner MP, Shi Y, Torrero MN, Mueller E, Larson D, Soloviova K, et al. Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-β. J Immunol (2012) 188:559–68. doi: 10.4049/jimmunol.1100335
70. Hübner MP, Stocker JT, Mitre E. Inhibition of type 1 diabetes in filaria-infected non-obese diabetic mice is associated with a T helper type 2 shift and induction of FoxP3+ regulatory T cells. Immunology (2009) 127:512–22. doi: 10.1111/j.1365-2567.2008.02958.x
71. Summers RW, Elliott DE, Urban JF, Thompson R, Weinstock JV. Trichuris suis therapy in crohn’s disease. Gut (2005) 54:87–90. doi: 10.1136/gut.2004.041749
72. Cooper PJ, Espinel I, Paredes W, Guderian RH, Nutman TB. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10. J Infect Dis (1998) 178:1133–8. doi: 10.1086/515661
73. Stewart GR, Coulson T, Elson L, Nutman T, Bradley JE. Onchocerciasis modulates the immune response to mycobacterial antigens. Clin Exp Immunol (1999) 117:517–23. doi: 10.1046/j.1365-2249.1999.01015.x
74. Hübner MP, Pasche B, Kalaydjiev S, Soboslay PT, Lengeling A, Schulz-Key H, et al. Microfilariae of the filarial nematode litomosoides sigmodontis exacerbate the course of lipopolysaccharide-induced sepsis in mice. Infect Immun (2008) 76(4):1668–77. doi: 10.1128/IAI.01042-07
75. Gondorf F, Berbudi A, Buerfent BC, Ajendra J, Bloemker D, Specht S, et al. Chronic filarial infection provides protection against bacterial sepsis by functionally reprogramming macrophages. PloS Pathog (2015) 11(1):e1004616. doi: 10.1371/journal.ppat.1004616
76. Buerfent BC, Ajendra J, Stamminger W, Gondorf F, Hoerauf A, Hübner MP. TGFβ depletion does neither modulate acute e. coli-induced inflammatory immune responses nor impair the protective effect by chronic filarial infection. GMS Infect Dis (2019) 7:Doc04. doi: 10.3205/id000044
77. Buerfent BC, Gondorf F, Wohlleber D, Schumak B, Hoerauf A, Hübner MP. Escherichia coli-induced immune paralysis is not exacerbated during chronic filarial infection. Immunology (2015) 145(1):150–60. doi: 10.1111/imm.12435
78. Aravindhan V, Mohan V, Surendar J, Muralidhara Rao M, Pavankumar N, Deepa M, et al. Decreased prevalence of lymphatic filariasis among diabetic subjects associated with a diminished pro-inflammatory cytokine response (CURES 83). PloS Negl Trop Dis (2010) 4:e707. doi: 10.1371/journal.pntd.0000707
79. Chen Y, Lu J, Huang Y, Wang T, Xu Y, Xu M, et al. Association of previous schistosome infection with diabetes and metabolic syndrome: a cross-sectional study in rural China. J Clin Endocrinol Metab (2013) 98:E283–7. doi: 10.1210/jc.2012-2517
80. Wiria AE, Hamid F, Wammes LJ, Prasetyani MA, Dekkers OM, May L, et al. Infection with soil-transmitted helminths is associated with increased insulin sensitivity. PloS One (2015) 10:e0127746. doi: 10.1371/journal.pone.0127746
81. Hays R, Esterman A, Giacomin P, Loukas A, McDermott R, Krolewiecki AJ, et al. Does Strongyloides stercoralis infection protect against type 2 diabetes in humans? evidence from Australian aboriginal adults. Diabetes Res Clin Pract (2015) 107:355–61. doi: 10.1016/j.diabres.2015.01.012
82. Berbudi A, Surendar J, Ajendra J, Gondorf F, Schmidt D, Neumann AL, et al. Filarial infection or antigen administration improves glucose tolerance in diet-induced obese mice. J Innate Immun (2016) 8(6):601–16. doi: 10.1159/000448401
83. Surendar J, Frohberger SJ, Karunakaran I, Schmitt V, Stamminger W, Neumann AL, et al. Adiponectin limits IFN-γ and IL-17 producing CD4 T cells in obesity by restraining cell intrinsic glycolysis. Front Immunol (2019) 10:2555. doi: 10.3389/fimmu.2019.02555
84. Hübner MP, Shi Y, Torrero MN, Mueller E, Larson D, Soloviova K, et al. Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-beta. J Immunol (2012) 188:559–68. doi: 10.4049/jimmunol.1100335
85. Ajendra J, Berbudi A, Hoerauf A, Hübner MP. Combination of worm antigen and proinsulin prevents type 1 diabetes in NOD mice after the onset of insulitis. Clin Immunol (2016) 164:119–22. doi: 10.1016/j.clim.2016.02.005
86. Surendar J, Indulekha K, Hoerauf A, Hübner MP. Immunomodulation by helminths: Similar impact on type 1 and type 2 diabetes? Parasite Immunol (2017) 39(5):e12401. doi: 10.1111/pim.12401
87. Kuehn C, Tauchi M, Furtmair R, Urschel K, Raaz-Schrauder D, Neumann AL, et al. Filarial extract of litomosoides sigmodontis induces a type 2 immune response and attenuates plaque development in hyperlipidemic ApoE-knockout mice. FASEB J (2019) 33(5):6497–513. doi: 10.1096/fj.201800947RR
88. WHO. WHO coronavirus (COVID-19) dasboard 2023 (2023). Available at: https://covid19.who.int (Accessed 29 Jan 2023).
89. Mbow M, Lell B, Jochems SP, Cisse B, Mboup S, Dewals BG, et al. COVID-19 in Africa: dampening the storm? Science (2020) 369(6504):624–6. doi: 10.1126/science.abd3902
90. Badraoui R, Alrashedi MM, El-May MV. Acute respiratory distress syndrome: a life threatening associated complication of SARS-CoV-2 infection inducing COVID-19. J Biomol Struct Dyn (2020) 39(17):6842–51. doi: 10.1080/07391102.2020.1803139
91. Melo AKG, Milby KM, Caparroz A, Pinto A, Santos RRP, Rocha AP, et al. Biomarkers of cytokine storm as red flags for severe and fatal COVID-19 cases: A living systematic review and meta-analysis. PloS One (2021) 16(6):e0253894. doi: 10.1371/journal.pone.0253894
92. Mehta P, Fajgenbaum DC. Is severe COVID-19 a cytokine storm syndrome: a hyperinflammatory debate. Curr Opin Rheumatol (2021) 33(5):419–30. doi: 10.1097/BOR.0000000000000822
93. Fong SW, Yeo NK, Chan YH, Goh YS, Amrun SN, Ang N, et al. Robust virus-specific adaptive immunity in COVID-19 patients with saRS-CoV-2 Delta382 variant infection. J Clin Immunol (2022) 42(2):214–29. doi: 10.1007/s10875-021-01142-z
94. Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. (2020) 183(4):996–1012.e19. doi: 10.1016/j.cell.2020.09.038
95. Adjobimey T, Meyer J, Terkeš V, Parcina M, Hoerauf A. Helminth antigens differentially modulate the activation of CD4+ and CD8+ T lymphocytes of convalescent COVID-19 patients in vitro. BMC Med (2022) 20(1):241. doi: 10.1186/s12916-022-02441-x
96. Looker KJ, Elmes JAR, Gottlieb SL, Schiffer JT, Vickerman P, Turner KME, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis (2017) 17(12):1303–16. doi: 10.1016/S1473-3099(17)30405-X
97. WHO. Prevention and control of schistosomiasis and soil-transmitted helminthiasis, world health organization technical report series. (2002), 57.
98. Blackwell AD, Tamayo MA, Beheim B, Trumble BC, Stieglitz J, Hooper PL, et al. Helminth infection, fecundity, and age of first pregnancy in women. Science (2015) 350:970–2. doi: 10.1126/science.aac7902
99. Chetty A, Omondi MA, Butters C, Smith KA, Katawa G, Ritter M, et al. Impact of helminth infections on female reproductive health and associated diseases. Front Immunol (2020) 11:577516. doi: 10.3389/fimmu.2020.577516
100. Chetty A, Darby MG, Vornewald PM, Martín-Alonso M, Filz A, Ritter M, et al. Il4ra-independent vaginal eosinophil accumulation following helminth infection exacerbates epithelial ulcerative pathology of HSV-2 infection. Cell Host Microbe (2021) 29(4):579–593.e5. doi: 10.1016/j.chom.2021.02.004
101. Omondi MA, Kamassa EH, Katawa G, Tchopba CN, Vogelbusch C, Parcina M, et al. Hookworm infection associates with a vaginal type 1/Type 2 immune signature and increased HPV load. Front Immunol (2022) 13:1009968. doi: 10.3389/fimmu.2022.1009968
102. Holali Ameyapoh A, Katawa G, Ritter M, Nguepou Tschopba C, Edlom Tchadie P, Arndts K, et al. Hookworm infections and sociodemographic factors associated with female reproductive tract infections in rural areas of the central region of Togo. Front Microbiol (2021) 12:738894. doi: 10.3389/fmicb.2021.738894
103. Katawa G, Tchopba CN, Ritter M, da Silva M, Ameyapoh AH, Arndts K, et al. Female reproductive tract health: prevalence and risk factors associated with infections in lomé. Clin Res Trials (2021) 7:1–9. doi: 10.15761/CRT.1000342
104. Maizels RM, Sartono E, Kurniawan A, Partono F, Selkirk ME, Yazdanbakhsh M. T-Cell activation and the balance of antibody isotypes in human lymphatic filariasis. Parasitol Today (1995) 11:50–6. doi: 10.1016/0169-4758(95)80116-2
105. Pfarr KM, Debrah AY, Specht S, Hoerauf A. Filariasis and lymphoedema. Parasite Immunol (2009) 31:664–72. doi: 10.1111/j.1365-3024.2009.01133.x
106. Hoerauf A, Kruse S, Brattig NW, Heinzmann A, Mueller-Myhsok B, Deichmann KA. The variant Arg110Gln of human IL-13 is associated with an immunologically hyper-reactive form of onchocerciasis (sowda). Microbes Infect (2002) 4:37–42. doi: 10.1016/S1286-4579(01)01507-6
107. Ottesen E. Immunopathology of lymphatic filariasis in man. Springer Semin Immunopathol (1980) 2:373–85. doi: 10.1007/BF01857174
108. Timmann C, Fuchs S, Thoma C, Lepping B, Brattig NW, Sievertsen J, et al. Promoter haplotypes of the interleukin-10 gene influence proliferation of peripheral blood cells in response to helminth antigen. Genes Immun (2004) 5(4):256–60. doi: 10.1038/sj.gene.6364094
109. Yilmaz V, Yentür SP, Saruhan-Direskeneli G. IL-12 and IL-10 polymorphisms and their effects on cytokine production. Cytokine (2005) 30(4):188–94. doi: 10.1016/j.cyto.2005.01.006
110. Cuenco KT, Halloran ME, Lammie PJ. Assessment of families for excess risk of lymphedema of the leg in a lymphatic filariasis-endemic area. Am J Trop Med Hyg (2004) 70:185–90. doi: 10.4269/ajtmh.2004.70.185
111. Cuenco KT, Halloran ME, Louis-Charles J, Lammie PJ. A family study of lymphedema of the leg in a lymphatic filariasis-endemic area. Am J Trop Med Hyg (2004) 70:180–4. doi: 10.4269/ajtmh.2004.70.180
112. Cuenco KT, Ottesen EA, Williams SA, Nutman TB, Steel C. Heritable factors play a major role in determining host responses to Wuchereria bancrofti infection in an isolated south pacific island population. J Infect Dis (2009) 200:1271–8. doi: 10.1086/605844
113. Terhell AJ, Houwing-Duistermaat JJ, Ruiterman Y, Haarbrink M, Abadi K, Yazdanbakhsh M. Clustering of Brugia malayi infection in a community in south-sulawesi, Indonesia. Parasitology (2000) 120(Pt 1):23–9. doi: 10.1017/S0031182099005247
114. Wahyuni S, Houwing-Duistermaat JJ, Syafruddin, Supali T, Yazdanbakhsh M, Sartono E. Clustering of filarial infection in an age-graded study: genetic, household and environmental influences. Parasitology (2004) 128:315–21. doi: 10.1017/S0031182003004487
115. Satoguina JS, Weyand E, Larbi J, Hoerauf A. T Regulatory-1 cells induce IgG4 production by b cells: role of IL-10. J Immunol (2005) 174:4718–26. doi: 10.4049/jimmunol.174.8.4718
116. Debrah AY, Batsa L, Albers A, Mand S, Toliat MR, Nürnberg P, et al. Transforming growth factor-β 1 variant Leu10Pro is associated with both lack of microfilariae and differential microfilarial loads in the blood of people infected with lymphatic filariasis. Hum Immunol (2011) 72:1143–8. doi: 10.1016/j.humimm.2011.07.305
117. Esterre P, Plichart C, Huin-Blondey MO, Nguyen LN. Soluble cellular adhesion molecules, selectins, VEGF and endothelin-1 in patients with Wuchereria bancrofti infection and association with clinical status. Parasite Immunol (2005) 27:9–16. doi: 10.1111/j.1365-3024.2005.00732.x
118. Debrah AY, Mand S, Toliat MR, Marfo-Debrekyei Y, Batsa L, Nürnberg P, et al. Plasma vascular endothelial growth factor-a (VEGF-a) and VEGF-a gene polymorphism are associated with hydrocele development in lymphatic filariasis. Am J Trop Med Hyg (2007) 77:601–8. doi: 10.4269/ajtmh.2007.77.601
119. Debrah LB, Albers A, Debrah AY, Brockschmidt FF, Becker T, Herold C, et al. Single nucleotide polymorphisms in the angiogenic and lymphangiogenic pathways are associated with lymphedema caused by Wuchereria bancrofti. Hum Genomics (2017) 11:26. doi: 10.1186/s40246-017-0121-7
120. von Geldern TW, Morton HE, Clark RF, Brown BS, Johnston KL, Ford L, et al. Discovery of ABBV-4083, a novel analog of tylosin a that has potent anti-Wolbachia and anti-filarial activity. PloS Negl Trop Dis (2019) 13(2):e0007159. doi: 10.1371/journal.pntd.0007159
121. Taylor MJ, von Geldern TW, Ford L, Hübner MP, Marsh K, Johnston KL, et al. Preclinical development of an oral anti-Wolbachia macrolide drug for the treatment of lymphatic filariasis and onchocerciasis. Sci Transl Med (2019) 11:eaau2086. doi: 10.1126/scitranslmed.aau2086
122. Hübner MP, Koschel M, Struever D, Nikolov V, Frohberger SJ, Ehrens A, et al. In vivo kinetics of Wolbachia depletion by ABBV-4083 in l. sigmodontis adult worms and microfilariae. PloS Negl Trop Dis (2019) 13:e0007636. doi: 10.1371/journal.pntd.0007636
123. Krücken J, Holden-Dye L, Keiser J, Prichard RK, Townson S, Makepeace BL, et al. Development of emodepside as a possible adulticidal treatment for human onchocerciasis-the fruit of a successful industrial-academic collaboration. PloS Pathog (2021) 17(7):e1009682. doi: 10.1371/journal.ppat.1009682
124. Hübner MP, Townson S, Gokool S, Tagboto S, Maclean MJ, Verocai GG, et al. Evaluation of the in vitro susceptibility of various filarial nematodes to emodepside. Int J Parasitol Drugs Drug Resist (2021) 17:27–35. doi: 10.1016/j.ijpddr.2021.07.005
125. Hübner MP, Martin C, Specht S, Koschel M, Dubben B, Frohberger SJ, et al. Oxfendazole mediates macrofilaricidal efficacy against the filarial nematode litomosoides sigmodontis in vivo and inhibits Onchocerca spec. motility in vitro. PloS Negl Trop Dis (2020) 14(7):e0008427.
126. Aljayyoussi G, Tyrer HE, Ford L, Sjoberg H, Pionnier N, Waterhouse D, et al. Short-course, high-dose rifampicin achieves Wolbachia depletion predictive of curative outcomes in preclinical models of lymphatic filariasis and onchocerciasis. Sci Rep (2017) 7:210. doi: 10.1038/s41598-017-00322-5
127. Hong WD, Benayoud F, Nixon GL, Ford L, Johnston KL, Clare RH, et al. AWZ1066S, a highly specific anti-Wolbachia drug candidate for a short-course treatment of filariasis. Proc Natl Acad Sci U.S.A. (2019) 116(4):1414–9. doi: 10.1073/pnas.1816585116
128. Schiefer A, Hübner MP, Krome A, Lämmer C, Ehrens A, Aden T, et al. Corallopyronin a for short-course anti-Wolbachial, macrofilaricidal treatment of filarial infections. PloS Negl Trop Dis (2020) 14(12):e0008930. doi: 10.1371/journal.pntd.0008930
129. Schäberle TF, Schmitz A, Zocher G, Schiefer A, Kehraus S, Neu E, et al. Insights into structure-activity relationships of bacterial RNA polymerase inhibiting corallopyronin derivatives. J Nat Prod (2015) 78(10):2505–9. doi: 10.1021/acs.jnatprod.5b00175
130. Belogurov GA, Vassylyeva MN, Sevostyanova A, Appleman JR, Xiang AX, Lira R, et al. Transcription inactivation through local refolding of the RNA polymerase structure. Nature (2009) 457(7227):332–5. doi: 10.1038/nature07510
131. Mukhopadhyay J, Das K, Ismail S, Koppstein D, Jang M, Hudson B, et al. The RNA polymerase “switch region” is a target for inhibitors. Cell (2008) 135(2):295–307. doi: 10.1016/j.cell.2008.09.033
132. Irschik H, Jansen R, Höfle G, Gerth K, Reichenbach H. The corallopyronins, new inhibitors of bacterial RNA synthesis from myxobacteria. J Antibiot (Tokyo) (1985) 38(2):145–52. doi: 10.7164/antibiotics.38.145
133. Rentsch A, Kalesse M. The total synthesis of corallopyronin a and myxopyronin b. Angew Chem Int Ed Engl (2012) 51(45):11381–4. doi: 10.1002/anie.201206560
134. Hawryluk N, Robinson D, Shen Y, Kyne G, Bedore M, Menon S, et al. Discovery of substituted di(pyridin-2-yl)-1,2,4-thiadiazol-5-amines as novel macrofilaricidal compounds for the treatment of human filarial infections. J Med Chem (2022) 65(16):11388–403. doi: 10.1021/acs.jmedchem.2c00960
135. Hawryluk N, Zhiru L, Carlow C, Gokool S, Townson S, Kreiss T, et al. Filarial nematode phenotypic screening cascade to identify compounds with anti-parasitic activity for drug discovery optimization. Int J Parasitol Drugs Drug Resist (2022) 19:89–97. doi: 10.1016/j.ijpddr.2022.06.002
136. Ehrens A, Lunde CS, Jacobs RT, Struever D, Koschel M, Frohberger SJ, et al. In vivo efficacy of the boron-pleuromutilin AN11251 against Wolbachia of the rodent filarial nematode Litomosoides sigmodontis. PloS Negl Trop Dis (2020) 14(1):e0007957. doi: 10.1371/journal.pntd.0007957
137. Jacobs RT, Lunde CS, Freund YR, Hernandez V, Li X, Xia Y, et al. Boron-pleuromutilins as anti- Wolbachia agents with potential for treatment of onchocerciasis and lymphatic filariasis. J medicinal Chem (2019) 62:2521–40. doi: 10.1021/acs.jmedchem.8b01854
138. Bakowski MA, Shiroodi RK, Liu R, Olejniczak J, Yang B, Gagaring K, et al. Discovery of short-course antiWolbachial quinazolines for elimination of filarial worm infections. Sci Transl Med (2019) 11:eaav3523. doi: 10.1126/scitranslmed.aav3523
139. Krome AK, Becker T, Kehraus S, Schiefer A, Gütschow M, Chaverra-Muñoz L, et al. Corallopyronin a: antimicrobial discovery to pre-clinical development. Nat Prod Rep (2022) 39(9):1705–20. doi: 10.1039/D2NP00012A
140. Ehrens A, Hoerauf A, Hübner MP. Current perspective of new anti-Wolbachial and direct-acting macrofilaricidal drugs as treatment strategies for human filariasis. GMS Infect Dis (2022) 10:Doc02. doi: 10.3205/id000079
141. Miethke M, Pieroni M, Weber T, Brönstrup M, Hammann P, Halby L, et al. Towards the sustainable discovery and development of new antibiotics. Nat Rev Chem (2021) 5(10):726–49. doi: 10.1038/s41570-021-00313-1
142. Ette EI, Thomas WO, Achumba JI. Ivermectin: a long-acting microfilaricidal agent. DICP (1990) 24(4):426–33. doi: 10.1177/106002809002400417
143. Kwarteng A, Ahuno ST, Akoto FO. Killing filarial nematode parasites: role of treatment options and host immune response. Infect Dis Poverty (2016) 5(1):86. doi: 10.1186/s40249-016-0183-0
144. Horton J. Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology. (2000) 121(Suppl):S113–32. doi: 10.1017/S0031182000007290
145. Genchi C, Sacchi L, Bandi C, Venco L. Preliminary results on the effect of tetracycline on the embryogenesis and symbiotic bacteria (Wolbachia) of Dirofilaria immitis. Update discussion. Parassitologia (1998) 40:247–9.
146. Hoerauf A, Nissen-Pähle K, Schmetz C, Henkle-Dührsen K, Blaxter ML, Büttner DW, et al. Tetracycline therapy targets intracellular bacteria in the filarial nematode Litomosoides sigmodontis and results in filarial infertility. J Clin Invest (1999) 103(1):11–8. doi: 10.1172/JCI4768
147. Langworthy NG, Renz A, Mackenstedt U, Henkle-Dührsen K, de Bronsvoort MB, Tanya VN, et al. Macrofilaricidal activity of tetracycline against the filarial nematode Onchocerca ochengi: elimination of Wolbachia precedes worm death and suggests a dependent relationship. Proc Biol Sci (2000) 267(1448):1063–9. doi: 10.1098/rspb.2000.1110
148. Bandi C, McCall JW, Genchi C, Corona S, Venco L, Sacchi L. Effects of tetracycline on the filarial worms Brugia pahangi and Dirofilaria immitis and their bacterial endosymbionts Wolbachia. Int J Parasitol (1999) 29(2):357–64. doi: 10.1016/S0020-7519(98)00200-8
149. Hoerauf A, Volkmann L, Hamelmann C, Adjei O, Autenrieth IB, Fleischer B, et al. Endosymbiotic bacteria in worms as targets for a novel chemotherapy in filariasis. Lancet (2000) 355(9211):1242–3. doi: 10.1016/S0140-6736(00)02095-X
150. Taylor MJ, Makunde WH, McGarry HF, Turner JD, Mand S, Hoerauf A. Macrofilaricidal activity after doxycycline treatment of Wuchereria bancrofti: a double-blind, randomised placebo-controlled trial. Lancet. (2000) 365(9477):2116–21. doi: 10.1016/S0140-6736(05)66591-9
151. Hoerauf A, Mand S, Fischer K, Kruppa T, Marfo-Debrekyei Y, Debrah AY, et al. Doxycycline as a novel strategy against bancroftian filariasis-depletion of Wolbachia endosymbionts from Wuchereria bancrofti and stop of microfilaria production. Med Microbiol Immunol (2003) 192(4):211–6. doi: 10.1007/s00430-002-0174-6
152. Debrah AY, Mand S, Specht S, Marfo-Debrekyei Y, Batsa L, Pfarr K, et al. Doxycycline reduces plasma VEGF-C/sVEGFR-3 and improves pathology in lymphatic filariasis. PloS Pathog (2006) 2(9):e92. doi: 10.1371/journal.ppat.0020092
153. Debrah AY, Mand S, Marfo-Debrekyei Y, Batsa L, Pfarr K, Buttner M, et al. Macrofilaricidal effect of 4 weeks of treatment with doxycycline on Wuchereria bancrofti. Trop Med Int Health (2007) 12(12):1433–41. doi: 10.1111/j.1365-3156.2007.01949.x
154. Turner JD, Mand S, Debrah AY, Muehlfeld J, Pfarr K, McGarry HF, et al. A randomized, double-blind clinical trial of a 3-week course of doxycycline plus albendazole and ivermectin for the treatment of Wuchereria bancrofti infection. Clin Infect Dis (2006) 42(8):1081–9. doi: 10.1086/501351
155. Mand S, Pfarr K, Sahoo PK, Satapathy AK, Specht S, Klarmann U, et al. Macrofilaricidal activity and amelioration of lymphatic pathology in bancroftian filariasis after 3 weeks of doxycycline followed by single-dose diethylcarbamazine. Am J Trop Med Hyg (2009) 81(4):702–11. doi: 10.4269/ajtmh.2009.09-0155
156. Supali T, Djuardi Y, Pfarr KM, Wibowo H, Taylor MJ, Hoerauf A, et al. Doxycycline treatment of Brugia malayi-infected persons reduces microfilaremia and adverse reactions after diethylcarbamazine and albendazole treatment. Clin Infect Dis (2008) 46(9):1385–93. doi: 10.1086/586753
157. Rao RU, Huang Y, Abubucker S, Heinz M, Crosby SD, Mitreva M, et al. Effects of doxycycline on gene expression in Wolbachia and Brugia malayi adult female worms in vivo. J BioMed Sci (2012) 19(1):21. doi: 10.1186/1423-0127-19-21
158. Hoerauf A, Specht S, Büttner M, Pfarr K, Mand S, Fimmers R, et al. Wolbachia endobacteria depletion by doxycycline as antifilarial therapy has macrofilaricidal activity in onchocerciasis: a randomized placebo-controlled study. Med Microbiol Immunol (2008) 197(3):335. doi: 10.1007/s00430-007-0062-1
159. Debrah AY, Specht S, Klarmann-Schulz U, Batsa L, Mand S, Marfo-Debrekyei Y, et al. Doxycycline leads to sterility and enhanced killing of female Onchocerca volvulus worms in an area with persistent microfilaridermia after repeated ivermectin treatment: A randomized, placebo-controlled, double-blind trial. Clin Infect Dis (2015) 61(4):517–26. doi: 10.1093/cid/civ363
160. Specht S, Hoerauf A, Adjei O, Debrah A, Büttner DW. Newly acquired Onchocerca volvulus filariae after doxycycline treatment. Parasitol Res (2009) 106(1):23–31. doi: 10.1007/s00436-009-1624-5
161. Hoerauf A, Specht S, Marfo-Debrekyei Y, Büttner M, Debrah AY, Mand S, et al. Efficacy of 5-week doxycycline treatment on adult Onchocerca volvulus. Parasitol Res (2009) 104(2):437–47. doi: 10.1007/s00436-008-1217-8
162. Klarmann-Schulz U, Specht S, Debrah AY, Batsa L, Ayisi-Boateng NK, Osei-Mensah J, et al. Comparison of doxycycline, minocycline, doxycycline plus albendazole and albendazole alone in their efficacy against onchocerciasis in a randomized, open-label, pilot trial. PloS Negl Trop Dis (2017) 11(1):e0005156. doi: 10.1371/journal.pntd.0005156
163. Debrah AY, Mand S, Marfo-Debrekyei Y, Larbi J, Adjei O, Hoerauf A. Assessment of microfilarial loads in the skin of onchocerciasis patients after treatment with different regimens of doxycycline plus ivermectin. Filaria J (2006) 5:1. doi: 10.1186/1475-2883-5-1
164. Turner JD, Tendongfor N, Esum M, Johnston KL, Langley RS, Ford L, et al. Macrofilaricidal activity after doxycycline only treatment of Onchocerca volvulus in an area of Loa loa co-endemicity: a randomized controlled trial. PloS Negl Trop Dis (2010) 4(4):e660. doi: 10.1371/journal.pntd.0000660
165. Gardon J, Gardon-Wendel N, Demanga N, Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. (1997) 350(9070):18–22. doi: 10.1016/S0140-6736(96)11094-1
166. Wanji S, Tendongfor N, Nji T, Esum M, Che JN, Nkwescheu A, et al. Community-directed delivery of doxycycline for the treatment of onchocerciasis in areas of co-endemicity with loiasis in Cameroon. Parasit Vectors. (2009) 2(1):39. doi: 10.1186/1756-3305-2-39
167. Hoerauf A. Filariasis: new drugs and new opportunities for lymphatic filariasis and onchocerciasis. Curr Opin Infect Dis (2008) 21(6):673–81. doi: 10.1097/QCO.0b013e328315cde7
168. Zahner H, Taubert A, Harder A, von Samson-Himmelstjerna G. Filaricidal efficacy of anthelmintically active cyclodepsipeptides. Int J Parasitol (2001) 31:1515–22. doi: 10.1016/S0020-7519(01)00263-6
169. Balansky J, Pfarr K, Szekat C, Kehraus S, Aden T, Grosse M, et al. The RNA polymerase inhibitor corallopyronin a has a lower frequency of resistance than rifampicin in Staphylococcus aureus. Antibiotics (Basel) (2022) 11(7):920. doi: 10.3390/antibiotics11070920
170. Ehrens A, Schiefer A, Krome AK, Becker T, Rox K, Neufeld H, et al. Pharmacology and early ADMET data of corallopyronin a, a natural product with macrofilaricidal anti-Wolbachial activity in filarial nematodes. Front Trop Dis (2022) 3:983107. doi: 10.3389/fitd.2022.983107
171. Schlabe S, Korir P, Lämmer C, Landmann F, Dubben B, Koschel M, et al. A qPCR to quantify Wolbachia from few Onchocerca volvulus microfilariae as a surrogate for adult worm histology in clinical trials of antiWolbachial drugs. Parasitol Res (2022) 121(4):1199–206. doi: 10.1007/s00436-021-07411-5
172. Gandjui NVT, Njouendou AJ, Gemeg EN, Fombad FF, Ritter M, Kien CA, et al. Establishment of an in vitro culture system to study the developmental biology of Onchocerca volvulus with implications for anti-Onchocerca drug discovery and screening. PloS Negl Trop Dis (2021) 15(2):e0008513. doi: 10.1371/journal.pntd.0008513
173. Fombad FF, Njouendou AJ, Ndongmo PC, Ritter M, Chunda VC, Metuge HM, et al. Effect of flubendazole on developing stages of Loa loa in vitro and in vivo: a new approach for screening filaricidal agents. Parasit Vectors (2019) 12(1):14. doi: 10.1186/s13071-018-3282-x
174. Njouendou AJ, Ritter M, Ndongmo WPC, Kien CA, Narcisse GTV, Fombad FF, et al. Successful long-term maintenance of Mansonella perstans in an in vitro culture system. Parasites Vectors (2017) 10:563. doi: 10.1186/s13071-017-2515-8
175. Njouendou AJ, Ritter M, Kien CA, Esum ME, Ndongmo WPC, Fombad FF, et al. Dataset on in vitro maintenance of Mansonella perstans microfilariae and drug testing. Data Brief (2019) 28:104930. doi: 10.1016/j.dib.2019.104930
176. Njouendou AJ, Kien CA, Esum ME, Ritter M, Chounna Ndongmo WP, Fombad FF, et al. In vitro maintenance of Mansonella perstans microfilariae and its relevance for drug screening. Exp Parasitol (2019) 206:107769. doi: 10.1016/j.exppara.2019.107769
177. Zofou D, Fombad FF, Gandjui NVT, Njouendou AJ, Kengne-Ouafo AJ, Chounna Ndongmo PW, et al. Evaluation of in vitro culture systems for the maintenance of microfilariae and infective larvae of Loa loa. Parasit Vectors (2018) 11(1):275. doi: 10.1186/s13071-018-2852-2
178. Amambo GN, Abong RA, Fombad FF, Njouendou AJ, Nietcho F, Beng AA, et al. Validation of loop-mediated isothermal amplification for the detection of Loa loa infection in chrysops spp in experimental and natural field conditions. Parasit Vectors (2021) 14(1):19. doi: 10.1186/s13071-020-04506-3
179. Won KY, Gass K, Biamonte M, Dagne DA, Ducker C, Hanna C, et al. Diagnostics to support elimination of lymphatic filariasis-development of two target product profiles. PloS Negl Trop Dis (2021) 15(11):e0009968. doi: 10.1371/journal.pntd.0009968
180. Poole CB, Li Z, Alhassan A, Guelig D, Diesburg S, Tanner NA, et al. Colorimetric tests for diagnosis of filarial infection and vector surveillance using non-instrumented nucleic acid loop-mediated isothermal amplification (NINA-LAMP). PloS One (2017) 12(2):e0169011. doi: 10.1371/journal.pone.0169011
181. Poole CB, Sinha A, Ettwiller L, Apone L, McKay K, Panchapakesa V, et al. In silico identification of novel biomarkers and development of new rapid diagnostic tests for the filarial parasites Mansonella perstans and Mansonella ozzardi. Sci Rep (2019) 9(1):10275. doi: 10.1038/s41598-019-46550-9
182. Amambo GN, Innocentia N, Abong RA, Fombad FF, Njouendou AJ, Nietcho F, et al. Application of loop mediated isothermal amplification (LAMP) assays for the detection of Onchocerca volvulus, Loa loa and Mansonella perstans in humans and vectors. Front Trop Dis (2023) 3:1016176. doi: 10.3389/fitd.2022.1016176
183. Mand S, Debrah AY, Klarmann U, Batsa L, Marfo-Debrekyei Y, Kwarteng A, et al. Doxycycline improves filarial lymphedema independent of active filarial infection: a randomized controlled trial. Clin Infect Dis (2012) 55(5):621–30. doi: 10.1093/cid/cis486
184. Horton J, Klarmann-Schulz U, Stephens M, Budge PJ, Coulibaly Y, Debrah A, et al. The design and development of a multicentric protocol to investigate the impact of adjunctive doxycycline on the management of peripheral lymphoedema caused by lymphatic filariasis and podoconiosis. Parasit Vectors (2020) 13(1):155. doi: 10.1186/s13071-020-04024-2
Keywords: Filaria, onchocerciasis, lymphatic filariasis, Litomosoides sigmodontis, eosinophils, immunomodulation, diagnostics, macrofilaricide
Citation: Karunakaran I, Ritter M, Pfarr K, Klarmann-Schulz U, Debrah AY, Debrah LB, Katawa G, Wanji S, Specht S, Adjobimey T, Hübner MP and Hoerauf A (2023) Filariasis research – from basic research to drug development and novel diagnostics, over a decade of research at the Institute for Medical Microbiology, Immunology and Parasitology, Bonn, Germany. Front. Trop. Dis 4:1126173. doi: 10.3389/fitd.2023.1126173
Received: 17 December 2022; Accepted: 14 February 2023;
Published: 02 March 2023.
Edited by:
Alfonso J. Rodriguez-Morales, Fundacion Universitaria Autónoma de las Américas, ColombiaReviewed by:
Vishal Khatri, Sana Biotechnology, United StatesJacob Souopgui, Université libre de Bruxelles, Belgium
Nicolas Pionnier, Manchester Metropolitan University, United Kingdom
Copyright © 2023 Karunakaran, Ritter, Pfarr, Klarmann-Schulz, Debrah, Debrah, Katawa, Wanji, Specht, Adjobimey, Hübner and Hoerauf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marc Peter Hübner, aHVlYm5lckB1bmktYm9ubi5kZQ==
 Indulekha Karunakaran1
Indulekha Karunakaran1 Manuel Ritter
Manuel Ritter Kenneth Pfarr
Kenneth Pfarr Ute Klarmann-Schulz
Ute Klarmann-Schulz Alexander Yaw Debrah
Alexander Yaw Debrah Linda Batsa Debrah
Linda Batsa Debrah Gnatoulma Katawa
Gnatoulma Katawa Samuel Wanji
Samuel Wanji Sabine Specht
Sabine Specht Marc Peter Hübner
Marc Peter Hübner