- 1Department of Biochemistry and Biotechnology, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana
- 2Department of Chemistry, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana
- 3Department of Laboratory Technology, Kumasi Technical University, Kumasi, Ghana
- 4Kumasi Centre for Collaborative Research in Tropical Medicine, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana
Filarial lymphedema is a chronic pathophysiological condition initiated by parasitism by lymphatic filarial worms. Although the disease is not immediately fatal, it is a significant social and economic issue, particularly in sub-Saharan Africa. Given the ongoing need for effective therapeutic strategies for filarial lymphedema, several countries have turned to natural products and herbal interventions as promising source for developing anti-filarial agents to manage lymphatic filariasis (LF). This review aims to classify various plant molecules implicated in treating LF, with a focus on their anti-filarial properties. This information can be used to further investigate their efficacy in managing filarial lymphedema.
1 Introduction
Lymphatic filariasis (LF) is one of the deleterious diseases classified as a neglected tropical disease. It is caused by vector-borne nematodes that live in the tissue of infected people (1). Wuchereria bancrofti, Brugia malayi, and Brugia timori transmitted by several mosquito species cause the majority of human LF infections (2). According to WHO estimates from 2019, 893 million people in 49 countries are at risk of the disease and require preventive chemotherapy to stop transmission. About 36 million people worldwide suffer from chronic manifestations of the disease, with 25 million men suffering from hydrocele and millions suffering from filarial lymphedema. Over 1 billion people are estimated to live in endemic regions, where filarial diseases are a public health concern (3). The number of people living with disabilities increased from 850,000 in 1997 to 1.3 million in 2017, necessitating additional management efforts to facilitate the elimination process (4). Filarial infection is primarily associated with host inflammation from adult parasites, which can lead to impaired lymphatic drainage and the accumulation of interstitial fluid in subcutaneous tissues known as lymphedema (5). Patients with lymphedema have a lymphatic system that is highly susceptible to bacterial infections due to their exposure to contaminated environments. Bacteria such as Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia, Streptococcus pyrogens, and Pseudomonas aeruginosa are commonly found to cause infections in these patients (6).
Unfortunately, lymphedema is a disfiguring and debilitating condition characterized by chronic swelling, stiffness, and thickening of tissues in the affected areas (7). Although most visible in the lower limbs, it can also commonly affect the breasts, arms, scrotum, and genitalia. Lymphedema is a significant public health issue associated with lymphatic filariasis due to the impairment and disability it causes (8). While lymphedema is not fatal, it can significantly reduce patients’ quality of life (9). Currently, lymphedema has no cure, and treatment options are limited. However, several preclinical studies have shown that therapeutic lymphangiogenesis treatments can be effective for secondary lymphedema (10). Kwarteng et al. (6) conducted a study in rural Ghana and found that filarial lymphedema patients have a shifting bacterial population that facilitates the entry of pathogenic bacteria and displacement of commensals, which can affect the therapeutic environment and lead to recurrent secondary infections. To prevent filariasis, chemotherapy drugs such as ivermectin, diethylcarbamazine (DEC), and albendazole are administered in various combinations during community mass drug administration (MDA) campaigns (11).
According to Kumar et al. (12), the drugs used for MDA kill microfilaria from various filarial species but have minimal impact on adult worms. Patients’ self-management of late-stage filarial lymphedema complicates the healing process, and promotes antibiotic-drug resistance (13) as observed in the Ahanta West District in the Western Region of Ghana. The search for novel therapeutic strategies for managing the pathological conditions associated with filarial lymphedema has increased in recent years as infectious diseases progress and pathogens develop resistance to existing pharmaceuticals.
The use of medicinal plants to manage symptoms is an integral part of the culture of the majority of the African and Asian populations (14), making it a distinguishing feature. Herbal medicine has gained attention from patients and practitioners as a less expensive and less side effect-prone treatment for LF infections (15). The use of herbal medicines has increased in vascular and lymphatic trials for various reasons, including their potential to inhibit endothelial proliferation and prevent metastasis through angiogenesis (16). To effectively manage lymphedema patients and alleviate their suffering, plant-mediated therapy must address recurrent infections, swellings, acute inflammation, and tumor formation (17). This review aims to evaluate the potential Phyto-constituents for the treatment of filarial lymphedema, paying particular attention to their anti-inflammatory, anti-filarial (nematodicidal), and antimicrobial properties.
2 Pathogenesis of filarial lymphedema disease
The intricate life cycles of all human filarial nematodes require an insect vector; mosquitoes. When a mosquito bites a person, infectious-stage larvae: L3, are deposited in the human host’s skin and move through the skin and enter the lymphatic vessels where they go through molting and development to become L4 larvae and ultimately mature into adult worms as shown in Figure 1. The adult worms live in the lymphatics and lymph nodes, and after mating, they produce live progeny known as microfilariae in the bloodstream (18). For adult men, the preferred site is the scrotal lymphatics. In women and children, the larger lymph vessels and lymph nodes, draining the lower and higher limbs and the inguinal areas have also been described.
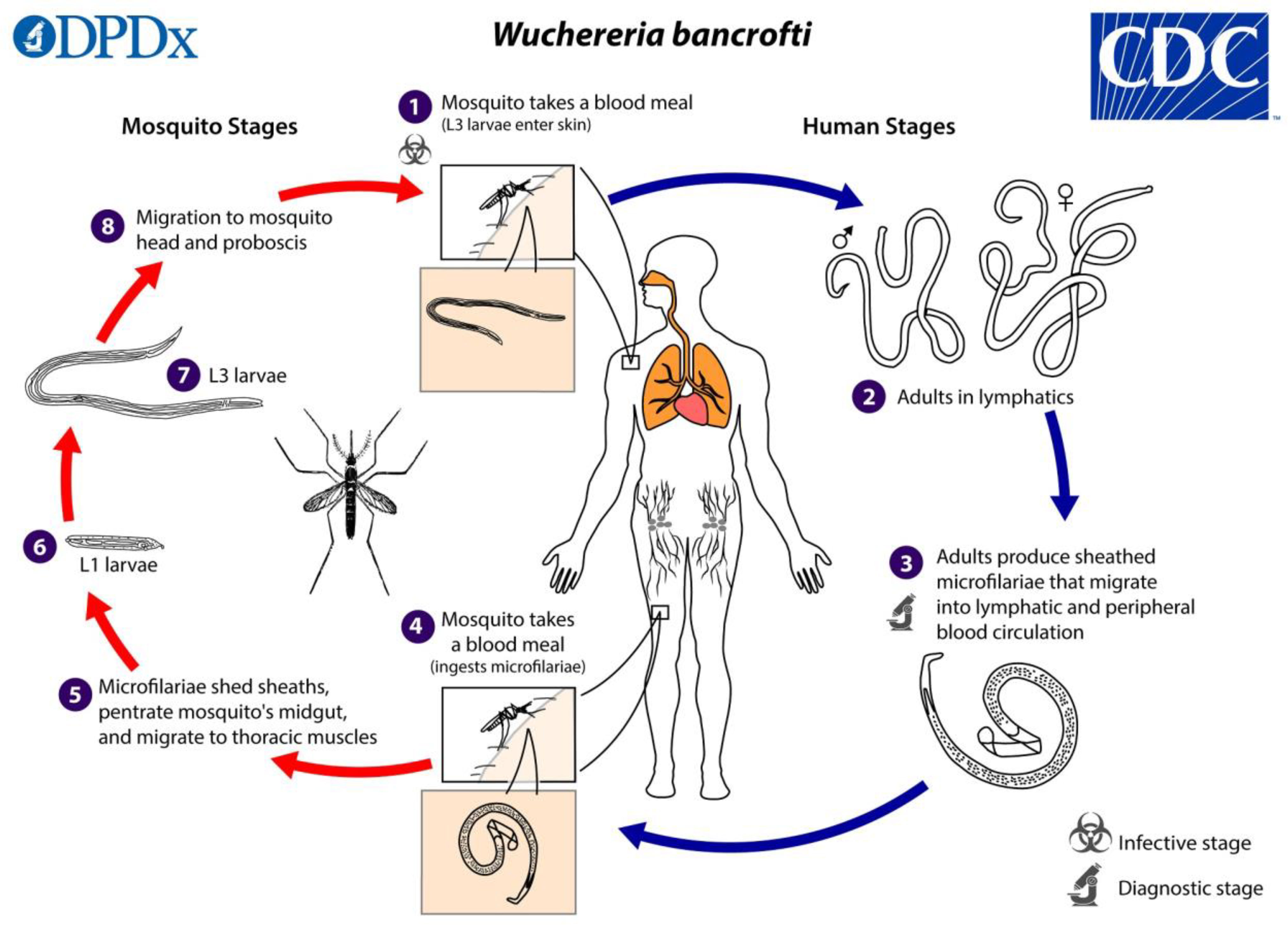
Figure 1 The life cycle of Wuchereria bancrofti parasite in both the human host and vector host. The figure was obtained from the Centers for Disease Control and Prevention (https://www.cdc.gov/parasites/lymphaticfilariasis/biology.html).
Live adult filarial parasites in the lymphatic channels immediately affect lymphatic endothelial cells along with innate and adaptive immune system cells. The combined effect of the persistent inflammatory response, parasite attrition, lymphatic blockage, and other variables all contribute to the pathophysiology and progression of filarial disease (19). Inflammation affects the small lymphatic vessels, limiting their ability to discharge fluid. Lymph stagnation promotes the growth of bacteria invading the area. More so, any interference with the skin integrity of the affected region, such as injuries, fungal or bacterial infections, skin fissuring, and mossy knobs that harden at the lower foot in the late stages of lymphedema, enhances the entry of pathogenic bacteria into the tissues (6).
3 Therapy approaches in the treatment of lymphedema
Complete decongestive therapy (CDT) is considered the standard care for the treatment of lymphedema and aims to reduce swelling, restore tissue homeostasis, and prevent infection to improve the patient’s quality of life (20). CDT involves an intensive daily phase until maximal volume reduction and tissue texture normalization are achieved. This typically includes 60 minutes of manual lymph drainage and specialized massage to stimulate the lymphatic system, followed by multilayer limb bandages, exercises to improve lymphatic pumping, and fitting of appropriate compression garments to maintain the reductions achieved through treatment (21).
Benzopyrones have been proposed as a potential chemotherapy for lymphedema, with the ability to decrease vascular permeability and prevent fibrosis in lymphedematous limbs by enhancing macrophage activity and accelerating protein lysis. However, there is currently a lack of strong evidence to support the efficacy of this treatment, particularly for the management of secondary lymphedema such as filarial lymphedema (22).
4 Phytochemicals used as anti-filarial agents
Plant-based materials have emerged as a novel approach to the management of lymphedema. Phytochemicals possess diverse biological activities that assist the immune system in combating pathogenic microorganisms and mitigating debilitating conditions through their anti-inflammatory and antimicrobial properties (23). These extracts elicit their bioactivity through adjuvant activity, either single or combined, which triggers a Th1/Th2 response via immunomodulation (24). Secondary metabolites, such as flavonoids, terpenoids, coumarins, glycosides, alkaloids, and steroids, have been identified in plants screened for their potential in treating filarial lymphedema (25).
4.1 Flavonoids
Flavonoids are naturally occurring compounds found in the plant kingdom, and they can be classified into three categories: Flavones, Flavonols, and Flavonones (26, 27). These compounds are abundant in fruits and vegetables and are considered an essential component of many pharmaceutical, medicinal, and cosmetic products due to their anti-oxidative, anti-inflammatory, anti-mutagenic, and anti-carcinogenic properties, as well as their ability to regulate critical cellular enzyme function (28).
The use of flavonoids in the ethnobotanical management of LF is supported by their reported anti-filarial and antimicrobial activities. Lakshmi et al. (26) investigated the anti-filarial potential of six flavonoids both in vivo and in vitro. The study, uses an in vitro motility assay with adult worms and microfilariae, a biochemical viability test (MTT)-reduction assay and two animal models, Meriones unguiculatus and Mastomys coucha revealed that all six flavonoids (naringenin, flavone, hesperetin, rutin, naringin, and chrysin) exhibited anti-filarial activity against the human lymphatic filarial parasite B. malayi at concentrations between 250-500 g/ml. Notably, naringenin at 50 g/ml was found to eliminate 73% of microfilariae worms, and naringenin and flavone were identified as the most potent flavonoids among the six screened. Luteolin has been implicated to exhibit immense anti-inflammatory activity by blocking Nuclear Factor-kappa B (NF- κB) (29). Therefore, drugs that block NF-B and reduce chronic inflammation may be effective and safe anti-inflammatory drugs in filarial infection (30).
Kaempferol is a polyphenol antioxidant that is abundant in fruits and vegetables, and it possesses several biological properties, including antitumor, antioxidant, and anti-inflammatory effects (31). Kaempferol also displays antimicrobial activity, with minimum inhibitory concentrations (MIC) ranging from 32 to 512 μg/ml, and antioxidant properties, with an IC50 of 52.48 μg/ml (32). Flavonoids are also known to have potent nematodicidal action on second-stage juveniles of Meloidogyne incognita, a root-knot nematode after 24 hours of incubation, as has been shown in previous studies (33). Flavonoids exhibit anti-filarial and down regulate inflammation process by inhibiting COX, a naturally occurring enzyme responsible for the conversion of arachidonic acid to prostaglandins and thromboxanes to induce inflammation and pain (34).
4.2 Glycosides
Glycosides are natural secondary metabolites found in both plants and animals which contain one or more sugar moieties (glycone) linked together with a non-sugar molecule by a glycosidic linkage. Various glycosides from a variety of plants have been cited to exert anti-filarial actions. Barlerin (8-O-Acetyl shanzhiside methyl ester) is an iridoid glucoside isolated from Barleria cristata (35). Extracts obtained from B. cristata leaves have shown pharmacological activity in both in-vitro and in-vivo, including antimicrobial, anti-inflammatory, antidiabetic, antioxidant, hepatoprotective, and antifungal activity (36). Agnuside and negundoside both glycosides from Vitex negundo, together with vitegnoside effectively help in the management of filarial-associated lymphedema due to their anti-inflammatory and antioxidant properties (37). V. negundo, a herb used to manage filariasis exhibited a substantial decrease in filarial adult worm motility in a dose-dependent manner (38). Elsewhere, vitegnoside, from V. negundo, is known to have medicinal values, such as anti-inflammatory, antibacterial, antifungal, antimicrobial, antioxidant, and anticancer properties (39). Vitegnoside elicits its anti-inflammatory action by rendering p38 MAPK/MK2, JNK/c-Jun inactive, and downstream NF-κB inflammatory transductions (40). These studies suggest that negundoside, agnuside, and vitegnoside are the most abundant secondary metabolites in the plant V. negundo, and they are responsible for their use in managing LF either in synergy or individually (41).
Asperoside and strebloside, two cardiac glycosides, have been identified as anti-filarial agents. Asperoside has shown to be highly effective in eliminating Litomosoides carinii, as well as having a success rate of over 70% against both B. malayi and Acanthocheilonema viteae at a dose of 50 mg/kg administered orally in vivo. Both glycosides have also exhibited in vitro activity against all three filarial species (42). In vitro tests revealed that the two glycosides asperoside and streblosid were active against B. malayi. However, L. carinii showed less susceptibility, possibly due to metabolic differences. In a separate study, the alcoholic extract did not exhibit significant activity against adult Setaria cervi in vitro, suggesting that S. cervi is less susceptible to S. asper. An attempt to separate the antifilarial active principle from the toxic component by hydrolyzing asperoside into aglycon and sugar parts did not yield comparable activity individually. The work therefore indicates that both aglycon and sugar parts of the glycosides are necessary for exerting antifilarial activity. Notably, the study demonstrated that asperoside, a cardiac glycoside from the bark of S. asper, possesses macrofilaricidal activity and gradually reduces peripheral microfilaremia when administered orally. In contrast, the standard drug DEC has no effect on adult L. carinii or A. viteae, but shows some activity against B. malayi in mastomys. Other drugs like ivermectin or mebendazole have minimal or no effect on adult parasites when administered orally (42). The two isolated cardiac glycosides, asperoside, and strebloside, were found to be responsible for the plant’s anti-filarial properties, with asperoside being the most potent and promising agent for drug development (43) due to its ability to disrupt the glutathione metabolism of adult S. cervi.
4.3 Terpenes
Terpenes are naturally occurring compounds found in plants and some animals, and they are responsible for the aromas, flavors, and colors associated with several types of flora. Terpenes are referred to as terpenoids when other functional groups such as alcohols, aldehydes, or ketones are present in their chemical structures. They are considered the largest and most diverse group of naturally occurring compounds and are classified based on the available isoprene units present in their structure, known as mono, di, tri, tetra, and sesquiterpenes (44). Triterpenoids, a subclass of terpenoids, have been found to have various potential biological activities, including anti-inflammatory, hepatoprotective, antimicrobial, antimycotic, analgesic, and immunomodulatory effects (45).
In Calotropis procera flowers, various compounds were evaluated for their bioactivities. A001 and F001 were investigated for antifilarial activity in vitro using motility and 3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyltetrazolium bromide (MTT) tests, as well as in rodent models of B. malayi-Meriones unguiculatus and B. malayi-Mastomys coucha. Isolate F001 from the flowers showed the dose-dependent killing of 12-60% of the B. malayi adult worm at 125-500 mg/kg, and further analysis estimated that 0.61% of F001 was lupeol, 0.50% was β-sitosterol, and 1.50% was triacontanol. The ethanolic crude extract also killed approximately 49-54% of adult filarial parasite (46).
According to Wal et al. (47), lupeol and its derivatives have been evaluated for their potential as anti-inflammatory and anti-cancer agents, showing promising results in cytotoxic studies, as well as in antimicrobial, anti-inflammatory, antitumor, and chemopreventive properties. Kangsamaksin et al. (48) reported that lupeol was able to downregulate TNF-alpha and VEGFR-2, thus exhibiting anti-angiogenic and anti-cancer properties. In addition, Ahmad et al. (49) found lupeol as an effective agent in reducing the infective stage larvae of cyst nematode Heterodera zeae, causing a mortality rate of 91% and 93% at the concentration of 0.5% and 1%, respectively, after 72 hours of exposure in vitro. Lupeol, therefore, is a potential drug candidate to manage LF due to its larvicidal, anti-inflammatory, and antioxidant activities. Further work, however, needs to be done to clarify its anti-filarial action.
Bauhinia racemosa, a plant that is used to treat LF, was found to be rich in lupeol (41). Moreover, Azadirachtin, a limonoid triterpene, has also been shown to be effective in treating LF (41). Mukherjee et al. (50) confirmed the anti-filarial potential of azadirachtin against Setaria cervi, with an LC50 value of 6.28 μg/ml for microfilariae and 9.55 μg/ml for macrofilariae, using the Dye exclusion and MTT assay. The physical damage caused to the parasites after the azadirachtin exposure was remarkable, and its pharmacological effectiveness as a drug against filariasis was demonstrated.
Nimbidin, a tetranortriterpene and a major component of neem seed oil, has been shown to inhibit the movement of macrophages to peritoneal sites and phagocytosis in rats when administered in vivo at a dose of 5-25 mg/kg for three consecutive days (51). It also inhibited PMA-stimulated respiratory bursts in these cells. In vitro, studies on rat peritoneal macrophages have confirmed that nimbidin suppresses the functions of macrophages and neutrophils that are relevant to inflammation (51). Nimbidin, nimbin, and nimbolide, all isolated from neem seed oil, have been found to possess antibacterial, anti-inflammatory, and antioxidant properties, which may explain the extensive use of neem, Azadirachta indica, in the management of LF (52).
Another study demonstrated that the grass plant Cymbopogon martinii, which is also used in the treatment of LF, contains the major terpenes Geraniol, Sabinene, Limonene, and Geranyl acetate (41). In an evaluation of the efficacy of essential oils from various plants against Meloidogyne graminicola (root-knot nematode), the researchers found C. martinii (Roxb) to be a potent nematicide (53). From the study, it cannot be fully admitted that all four compounds are responsible for the activity of C. martinii in managing LF. It can however be said that the compounds contribute to the observed activity.
The crude extract from the Lantana camara stem had strong anti-filarial efficacy, according to researchers. The crude extract killed 43.05% of adult B. malayi parasites and sterilized 76% of surviving female worms when supplied orally to experimentally infected Mongolian gerbils. The extract also demonstrated encouraging efficacy in a transplanted intraperitonially-gerbil model, killing up to 80% of adult worms and sterilizing all remaining female parasites. The extract was also efficient against another filarial species, Acanthocheilonema viteae, in vivo with high microfilaricidal (95.04%) and sterilization (60.66%) effects. The two chemicals obtained from the extract, oleanonic acid, and oleanolic acid, were found to be effective against B. malayi in vitro, LC100 at 31.25 and 62.5 μg/ml, respectively, on B. malayi in vitro (54).
The crude aqueous ethanolic Xylocarpus granatum extract had adulticidal and embryotic effects against B. malayi in vitro. Gedunin and photogedunin, two chemicals isolated from X. granatum had excellent adulticidal effectiveness, respectively killing 80% (IC50 = 0.239 μg/ml, CC50 = 212.5 μg/ml, SI = 889.1) and 70% (IC50 = 0.213 μg/ml, CC50 = 262.3 μg/ml, SI = 1231.4) at 5 × 100 mg/kg of transplanted adult B. malayi transplanted in the peritoneal cavity of the animal models (55).
The majority of terpenes implicated in the management of LF are from the subclass triterpenes. Unfortunately, the mechanism of these compounds is still not understood. This, therefore, presents an area of research for natural product scientists.
4.4 Phenolic acids
Phenolic acids (PAs) are secondary metabolites found throughout the plant kingdom and have been shown to possess varied bioactive properties (56). The PA content of blueberry polyphenol fractions was quantified using HPLC/MS-MS, and their anti-inflammatory activities were investigated by induced lipopolysaccharide-induced macrophages (56). Similarly, in a study by Su et al. (57), the antioxidant capacities of the polyphenol fractions of the blueberries were evaluated by the DPPH assay, and the results proved that the antioxidant and anti-inflammatory activities of the polyphenol fractions of the blueberries agreed with their PA contents.
Cajanus scarabaeoides is a creeping, climbing flowering plant with an abundance of phenolic acids (Gallic acid, caffeic acid, ferulic acid) and some flavonoids. A study on the potential of C. scarabaeoides as a filaricide indicated that polyphenols were effective against all three developmental stages: eggs, microfilariae (mf), and adults. Polyphenol LD50 values were determined to be 2.5, 10, and 35 g/ml, respectively. The HPTLC study revealed that PAs were the major component found in C. scarabaeoides. (58). Therefore, PAs could be the main compounds eliciting the anti-filarial action of C. scarabaeoides or may be doing that in tandem with their flavonoid counterparts (58).
Furthermore, the anti-filarial potential of plant ferulic acid (59) and resveratrol (50) have all been reported in the literature. Significant antifilarial activity was observed in ferulic acid isolated from the ethyl acetate fraction of Hibiscus mutabilis against both micro- and macrofilariae of Setaria cervi. The antifilarial effect of ferulic acid is achieved through the induction of apoptosis, which is facilitated by the generation of oxidative stress. Additionally, the level of certain antioxidants such as glutathione, glutathione–S–transferase, and superoxide dismutase in the filarial nematode S. cervi is downregulated and altered by ferulic acid (59).
5 Family of medicinal plants used to treat lymphatic filariasis
Phytochemicals used in the treatment of LF are derived from a diverse range of plant families, as evidenced by various works reviewed. In an ethnobotanical survey conducted in South Africa by Komoreng et al., a total of 46 plants were sampled, with the highest number of seven (7) plants each belonging to the Asteraceae and Hyacinthaceae families. The Euphorbiaceae family had four (4) plants, Solanaceae had three (3), Fabaceae and Vitaceae had two (2) each, while the remaining 23 plants belonged to other families (8). The Asteraceae family contains essential oils, lignans, saponins, polyphenolic compounds, phenolic acids, sterols, and polysaccharides, with total phenolic content ranging from 8.035 to 90.305 mg GAE/L and total flavonoid content from 18.031 to 185.437 mg QE/L (60). Similarly, the Hyacinthaceae family contains a range of phytochemicals, including alkaloids, tannins, saponins, phytosterols, phlobatinins, terpenoids, cardiac glycosides, phytates, and oxalates (61).
Kumar and colleagues evaluated 17 plants used for managing LF in India, which belonged to various plant families. Among these, one plant each was identified from Apiaceae, Asteraceae-Heliamtheae, Acanthaceae, Piperaceae, Verbenaceae, Plumbaginaceae, Menispermaceae, Meliaceae, Sapindaceae, Mimosaceae, Euphorbiaceae, Umbelliferae, Senecioneae, Solonaceae, and Zingiberaceae, while two plants were from Fabaceae family (12).
Shrivastava et al. (62) conducted research on the use of herbal plants in the treatment of LF, with a focus on non-toxic novel herbal drugs with anti-filarial activity. They identified 78 different plants used to treat LF, belonging to various families. The Fabaceae and Leguminosae families had the highest number of five (5) plants each, followed by Euphorbiaceae and Meliaceae families with four (4) plants each, and Malvaceae, Apocynaceae, Asteraceae, Compositae, Caesalpinaeceae, and Verbenaceae families with three (3) plants each. Other families had two (2) or one (1) plant each.
Plants belonging to the Fabaceae family are known to contain various phytochemicals such as flavonoids, lectins, saponins, alkaloids, carotenoids, and phenolic acids, which are present in every genus of the family (63). Meanwhile, the Leguminosae family has been found to contain alkaloids, flavonoids, terpenoids, tannins, saponins, and phenolic acids among various species (64). The overall findings suggest that Asteraceae was found to be the family with the most plant species used for the management of LF, followed by Fabaceae.
6 Conclusion
Filarial lymphedema has been treated using chemotherapeutic agents, but their use is limited. These agents are effective at reducing the mf load in the blood, alleviating symptoms and interrupting transmission. They do, however, have a minimal direct impact on adult worms. Adult worm persistence is a key difficulty in filarial disease control since it might result in the recurrence of mf and clinical symptoms. Individuals with established lymphatic filariasis (LF) can continue to endure chronic symptoms and long-term problems linked with the existence of adult worms even in the absence of an active infection. To target adult worms directly, alternative approaches such as macrofilaricidal drugs or interventions that affect the viability, fertility, or survival of adult worms must be sought. To overcome this challenge, phytochemical extracts have emerged as a more effective approach for managing this disease. Plant products are readily available and affordable, making them a popular choice in the treatment of LF, especially in tropical regions. We reviewed the available evidence on many plant-derived compounds that have been isolated and used to manage LF. These compounds act individually or in combination with other molecules to produce anti-inflammatory, antimicrobial, and nematodicidal effects. However, the phytochemicals’ method of action was not determined, which may restrict their use in medication development. In the absence of a better understanding of how these chemicals interact with biological targets, optimizing their therapeutic potential based on their mechanisms of action becomes difficult. More research and clarification on the particular molecular pathways by which these chemicals exert their effects will substantially improve their potential as therapeutic options. Although there has been no comparison of the efficacy of phytochemicals to that of existing anti-filarial drugs, which may be more effective or have fewer side effects, it is clear from this review that, natural products offer promising areas for researchers to find precursors for drug development. This study on medicinal plants and their interventions for filarial lymphedema will provide valuable information for further research on the efficacy of phytochemicals in managing chronic wounds associated with filarial lymphedema.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hailu FA, Tafesse G, Hailu TA. Pathophysiology and gastrointestinal impacts of parasitic helminths in human beings. J Pathol Res Rev Rep (2020) 2(2):1–8. doi: 10.47363/jpr/2020(2)122
2. Gyapong JO, Owusu IO, da-Costa vroom FB, Mensah, Ernest O, Gyapong M. Elimination of lymphatic filariasis: current perspectives on mass drug administration. Res Rep Trop Med (2018) 25–33. doi: 10.2147/RRTM.S125204
3. Risch F, Ritter M, Hoerauf A, et al. Human filariasis—contributions of the Litomosoides sigmodontis and Acanthocheilonema viteae animal model. Parasitol Res (2021) 120:4125–43. doi: 10.1007/s00436-020-07026-2
4. Batsa LD, Mohammed A, Osei-Mensah J, Mubarik Y, Agbenyega O, Ayisi-Boateng NK, et al. Morbidity management and surveillance of lymphatic filariasis disease and acute dermatolymphangioadenitis attacks using a mobile phone-based tool by community health volunteers in Ghana. PLOS Negl Trop Dis (2020) 14(11):e0008839. doi: 10.1371/journal.pntd.0008839
5. Grada AA, Phillips TJ. Lymphedema: diagnostic workup and management. J Am Acad Dermatol (2017) 77(6):995–1006. doi: 10.1016/j.jaad.2017.03.021
6. Kwarteng A, Wireko S, Asiedu SO, Kini P, Aglomasa BC, Amewu EKA, et al. Shift in the skin microbiome among individuals presenting with filarial lymphedema compared to nonfilarial healthy individuals in Ghana. Sci Afr (2022) 16:e01237. doi: 10.1016/J.SCIAF.2022.E01237
7. Nurlaila I, Roh K, Yeom C. Acquired lymphedema: molecular contributors and future directions for developing intervention strategies. Front Pharmacol (2022) 13. doi: 10.3389/fphar.2022.873650
8. Komoreng L, Thekisoe O, Lehasa S, Tiwani T, Mzizi N, Mokoena N, et al. An ethnobotanical survey of traditional medicinal plants used against lymphatic filariasis in south Africa. South Afr J Bot (2017) 111:12–6. doi: 10.1016/j.sajb.2017.03.005
9. Morgan PA, Franks PJ, Moffatt CJ. Health-related quality of life with lymphoedema: a review of the literature. Int Wound J (2005) 2(1):47–62. doi: 10.1111/j.1742-4801.2005.00066.x
10. Li J, Chen Y, Zhang L, Xing L, Xu H, Wang Y, et al. Total saponins of panaxnotoginseng promotes lymphangiogenesis by activation VEGF-c expression of lymphatic endothelial cells. J Ethnopharmacol (2016) 193:293–302. doi: 10.1016/J.JEP.2016.08.032
11. Kelly-Hope LA, Blundell HJ, Macfarlane CL, Molyneux DH. Innovative surveillance strategies to support the elimination of filariasis in Africa. Trends Parasitol (2018) 34(8):694–711. doi: 10.1016/J.PT.2018.05.004
12. Kumar K, Kumar M, Verma M, Yadav A, Uddin Q. Anti-wolbachia: a herbal approach to treatment of filariasis. Spatula DD - Peer Reviewed J Complement Med Drug Discovery (2014) 4(2):101. doi: 10.5455/spatula.20140602123033
13. Aglomasa BC, Adu-Asiamah CK, Asiedu SO, Kini P, Amewu EKA, Boahen KG, et al. Multi-drug resistant bacteria isolates from lymphatic filariasis patients in the ahanta West district, Ghana. BMC Microbiol (2022) 22(1):1–16. doi: 10.1186/s12866-022-02624-9
14. Rohini MR. Biotechnological interventions for conservation and multiplication of threatened medicinal plants. In: Conservation and utilization of threatened medicinal plants. Springer International Publishing (2020). p. 135–58. doi: 10.1007/978-3-030-39793-7_6
15. Sheikhi-Mobarakeh Z, Yarmohammadi H. Management: a systematic review Herbs as old potential treatments for lymphedema. In: Therapies in medicine. Elsevier (2020). https://www.sciencedirect.com/science/article/pii/S0965229920318823.
16. Bahmani M, Shirzad H, Shahinfard N, Sheivandi L, Rafieian-Kopaei M. Cancer phytotherapy: recent views on the role of antioxidant and angiogenesis activities. J Evidence-Based Complement Altern Med (2017) 22(2):299–309. doi: 10.1177/2156587215625157
17. Mortimer PS. Therapy approaches for lymphedema. Angiology (1997) 48(1):87–91. doi: 10.1177/000331979704800114
18. Shenoy RK. Clinical and pathological aspects of filarial lymphedema. Korean J Parasitol (2008) 46(3):119–25. doi: 10.3347/kjp.2008.46.3.119
19. Nutman TB. Insights into the pathogenesis of disease in human lymphatic filariasis. Lymphat Res Biol (2013) 11(3):144–8. doi: 10.1089/lrb.2013.0021
20. Poage EG, Rodrick JR, Wanchai A, Stewart BR, Cormier JN, Armer JM. Exploring the usefulness of botanicals as an adjunctive treatment for lymphedema: a systematic search and review. PM R (2015) 7(3):296–310. doi: 10.1016/j.pmrj.2014.09.019
21. Chakraborty S, Gurusamy M, Zawieja DC, Muthuchamy M. Lymphatic filariasis: perspectives on lymphatic remodeling and contractile dysfunction in filarial disease pathogenesis. Microcirculation (2013) 20(5):349–64. doi: 10.1111/micc.12031
22. Badger C, Preston N, Seers K, Mortimer P. Physical therapies for reducing and controlling lymphoedema of the limbs. Cochrane Database Syst Rev (2004) 4):CD003141. doi: 10.1002/14651858.cd003141.pub2
23. Khalid S, Shahzad A, Basharat N, Abubakar M, Anwar P. Phytochemical screening and analysis of selected medicinal plants in gujrat. J Phytochem Biochem (2018) 2(1):2–4.
24. Al-Abd NM, Nor ZM, Al-Adhroey AH, Suhaimi A, Sivanandam S. Recent advances on the use of biochemical extracts as filaricidal agents. Evidence-Based Complement Altern Med (2013) 2013:1–13. doi: 10.1155/2013/986573
25. Bin Emran T. Phytochemical, antimicrobial, cytotoxic, analgesic and antiinflammatory properties of azadirachta indica: a therapeutic study. J Bioanal Biomed (2015) 01(s12):2. doi: 10.4172/1948-593x.s12-007
26. Lakshmi V, Joseph SK, Srivastava S, Verma SK, Sahoo MK, Dube V, et al. Antifilarial activity in vitro and in vivo of some flavonoids tested against brugia malayi. Acta Tropica (2010) 116(2):127–33. doi: 10.1016/j.actatropica.2010.06.006
27. Luo Y, Shang P, Li D, Chapple SJ. Luteolin : a flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Front Pharmacol (2017) 8(October):1–10. doi: 10.3389/fphar.2017.00692
28. Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci (2016) 5. doi: 10.1017/jns.2016.41
29. Taheri Y, Sharifi-Rad J, Antika G, Yilmaz YB, Tumer TB, Abuhamdah S, et al. Paving luteolin therapeutic potentialities and agro-Food-Pharma applications: emphasis on in vivo pharmacological effects and bioavailability traits. Oxid Med Cell Longev (2021). doi: 10.1155/2021/1987588
30. Fordjour FA, Asiedu E, Larbi A, Kwarteng A. The role of nuclear factor kappa b (NF-κB) in filarial pathology. J Cell Commun Signal (2021) 15:185–93. doi: 10.1007/s12079-021-00607-5
31. Wang J, Fang X, Ge L, Cao F, Zhao L, Wang Z, et al. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PloS One (2018) 13(5):e0197563. doi: 10.1371/journal.pone.0197563
32. Tatsimo SJN, Tamokou JDD, Havyarimana L, Csupor D, Forgo P, Hohmann J, et al. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from bryophyllum pinnatum. BMC Res Notes (2012) 5:1–6. doi: 10.1186/1756-0500-5-158
33. Bano S, Iqbal EY, Lubna, Zik-ur-Rehman S, Fayyaz S, Faizi S. Nematicidal activity of flavonoids with structure activity relationship (SAR) studies against root knot nematode meloidogyne incognita. Eur J Plant Pathol (2020) 157(2):299–309. doi: 10.1007/s10658-020-01988-w
34. Stabrauskiene J, Kopustinskiene DM, Lazauskas R, Bernatoniene J. Naringin and naringenin: their mechanisms of action and the potential anticancer activities. Biomedicines (2022) 10(7):1686. doi: 10.3390/biomedicines10071686
35. Hemalatha K, Hareeka N, Sunitha D. Chemical constituents isolated from leaves of barleria cristata Linn. Int J Pharma Bio Sci (2012) 3(1).
36. Kumar H, Agrawal R, Kumar V. Barleria cristata: perspective towards phytopharmacological aspects. J Pharm Pharmacol (2018) 70(4):475–87. doi: 10.1111/jphp.12881
37. Tasduq SA, Kaiser PJ, Gupta BD, Gupta VK, Johri RK. Negundoside, an irridiod glycoside from leaves of vitex negundo, protects human liver cells against calcium-mediated toxicity induced by carbon tetrachloride. World J Gastroenterol (2008) 14(23):3693–709. doi: 10.3748/wjg.14.3693
38. Sahare KN, Singh V. Antifilarial activity of methanolic extract of vitex negundo l. leaves against setaria cervi filarial parasite. Sch Acad J Pharm (SAJP) (2015) 4(410):88–92. Available at: https://saspublishers.com/media/articles/SAJP-4288-92.pdf.
39. Kamal N, Mio Asni NS, Rozlan INA, Mohd Azmi MAH, Mazlan NW, Mediani A, et al. Traditional medicinal uses, phytochemistry, biological properties, and health applications of vitex sp. Plants (2022) 11(15):1–33. doi: 10.3390/plants11151944
40. Wang Q, Jiang H, Wang L, Yi H, Li Z, Liu R. Vitegnoside mitigates neuronal injury, mitochondrial apoptosis, and inflammation in an alzheimer's disease cell model via the p38 MAPK/JNK pathway. J Alzheimers Dis (2019) 72(1):199–214. doi: 10.3233/JAD-190640
41. Anil N, Talluri VR. Lymphatic filariasis: drug targets and nematicidal plants. J Pharm Sci Res (2015) 7(11):928–33.
42. Chatterjee RK, Fatma N, Murthy PK, Sinha P, Kulshrestha DK, Dhawan BN. Macrofilaricidal activity of the stembark of streblus asper and its major active constituents. Drug Dev Res (1992) 26(1):67–78. doi: 10.1002/ddr.430260106
43. Rastogi S, Kulshreshtha DK, Rawat AKS. Streblus asper lour. (Shakhotaka): a review of its chemical, pharmacological and ethnomedicinal properties. Evidence-Based Complement Altern Med (2006) 3(2):217–22. doi: 10.1093/ecam/nel018
44. Cox-Georgian D, Ramadoss N, Dona C, Basu C. Therapeutic and medicinal uses of terpenes. Medicinal Plants: From Farm to Pharm. 1st Ed. (2019), 333–59. doi: 10.1007/978-3-030-31269-5_15
45. Sandeep, Ghosh S. Triterpenoids: structural diversity, biosynthetic pathway, and bioactivity. In: Studies in natural products chemistry, 1st ed, vol. 67. Elsevier Inc (2021). https://www.sciencedirect.com/science/article/abs/pii/B9780128194836000126.
46. Kushwaha V, Rastogi S, Pandey MM, Saxena K, Khatoon S, Rawat AK, et al. In vitro and in vivo antifilarial activity of standardized extract of calotropis procera flowers against brugia malayi. Curr Topics Med Chem (2019) 19(14):1252–62. doi: 10.2174/1568026619666190620154054
47. Wal P, Wal A, Sharma G, Rai AK. Biological activities of lupeol. Syst Rev Pharm (2011) 2(2):96–103. doi: 10.4103/0975-8453.86298
48. Kangsamaksin T, Chaithongyot S, Wootthichairangsan C, Hanchaina R, Tangshewinsirikul C, Svasti J. Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-a. PloS One (2017) 12(12):1–16. doi: 10.1371/journal.pone.0189628
49. Ahmad A, Siddiqui PJA, Fayyaz S, Khan K, Iqbal EY, Rasheed M, et al. Bioassay directed fractionation of petroleum ether extract of aerial parts of ceriops tagal: isolation of lupeol as the nematicidal agent against cyst nematode heterodera zeae. Chem Biodiversity (2022) 19(3):e202100759. doi: 10.1002/cbdv.202100759
50. Mukherjee N, Joardar N, Sinha Babu SP. Antifilarial activity of azadirachtin fuelled through reactive oxygen species induced apoptosis: a thorough molecular study on setaria cervi. J Helminthol (2019) 93(5):519–28. doi: 10.1017/S0022149X18000615
51. Kaur G, Alam MS, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytother Res (2004) 18(5):419–24. doi: 10.1002/ptr.1474
52. Bhowmik D, Yadav J, Tripathi KK, Sampath Kumar KP. Herbal remedies of azadirachta indica and its medicinal application. J Chem Pharm Res (2010) 2(1):62–72.
53. Ajith M, Kaushik P, Shakil NA, Rana VS. ). efficacy of essential oils and their major compounds against meloidogyne graminicola (rice root-knot nematode) in pots and field trials. Indian J Nematol (2021) 51(2):102–8.
54. Misra N, Sharma M, Raj K, Dangi A, Srivastava S, Misra-Bhattacharya S. Chemical constituents and antifilarial activity of lantana camara against human lymphatic filariid brugia malayi and rodent filariid acanthocheilonema viteae maintained in rodent hosts. Parasitol Res (2007) 100:439–48. doi: 10.1007/s00436-006-0312-y
55. Misra S, Verma M, Mishra SK, Srivastava S, Lakshmi V, Misra-Bhattacharya S. Gedunin and photogedunin of xylocarpus granatum possess antifilarial activity against human lymphatic filarial parasite brugia malayi in experimental rodent host. Parasitol Res (2011) 109:1351–60.
56. Kang J, Thakali KM, Jensen GS, Wu X. Phenolic acids of the two major blueberry species in the US market and their antioxidant and anti-inflammatory activities. Plant Foods Hum Nutr (2015) 70(1):56–62. doi: 10.1007/s11130-014-0461-6
57. Su X, Zhang J, Wang H, Xu J, He J, Liu L, et al. Phenolic acid profiling, antioxidant, and anti-inflammatory activities, and miRNA regulation in the polyphenols of 16 blueberry samples from China. Molecules (Basel Switzerland) (2017) 22(2):312. doi: 10.3390/molecules22020312
58. Ray AS, Joardar N, Mukherjee S, Rahaman CH, Sinha Babu SP. Polyphenol enriched ethanolic extract of cajanus scarabaeoides (L.) thouars exerts potential antifilarial activity by inducing oxidative stress and programmed cell death. PloS One (2018) 13(12):e0208201. doi: 10.1371/journal.pone.0208201
59. Saini P, Gayen P, Nayak A, Kumar D, Mukherjee N, Pal BC, et al. Effect of ferulic acid from hibiscus mutabilis on filarial parasite setaria cervi: molecular and biochemical approaches. Parasitol Int (2012) 61(4):520–31. doi: 10.1016/j.parint.2012.04.002
60. Koc S, Isgor BS, Isgor YG, Shomali Moghaddam N, Yildirim O. The potential medicinal value of plants from asteraceae family with antioxidant defense enzymes as biological targets. Pharm Biol (2015) 53(5):746–51. doi: 10.3109/13880209.2014.942788
61. Temikotan T, Akinyele BO, Odiyi AC, Arotupin DJ. Phytochemicals of some members of the family hyacinthaceae and their significance in plant protection. Lecture Notes Eng Comput Sci (2013) 2 LNECS(2008):1345–50.
62. Shrivastava S, Gidwani B, Gupta A, Kaur D. Ethnopharmacological approaches to treat lymphatic filariasis. Int J Pharm Analytical Res (2016) 5(3):455–70.
63. Malik S. Biotechnology and production of anti-cancer compounds. Biotechnol Production Anti-Cancer Compounds (2017) 1–328 1st Ed. doi: 10.1007/978-3-31953880-8
Keywords: lymphedema, lymphatic filariasis (LF), phytochemicals, anti-filarial activity, anti-inflammatory
Citation: Bonnah R, Ayisi F, Wireko S and Kwarteng A (2023) Phytochemical intervention for lymphatic filariasis and filarial lymphedema. Front. Trop. Dis 4:1168668. doi: 10.3389/fitd.2023.1168668
Received: 17 February 2023; Accepted: 23 June 2023;
Published: 03 August 2023.
Edited by:
Joseph Daniel Turner, Liverpool School of Tropical Medicine, United KingdomReviewed by:
Charles D. Mackenzie, Task Force for Global Health, United StatesDr. P. Vivekanandhan, Saveetha Dental College And Hospitals, India
Copyright © 2023 Bonnah, Ayisi, Wireko and Kwarteng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Kwarteng, c2Vua3dhcnRlbmdAeWFob28uY28udWs=
 Rose Bonnah
Rose Bonnah Felix Ayisi2
Felix Ayisi2 Alexander Kwarteng
Alexander Kwarteng