- 1School of Population Health, University of New South Wales, Sydney, NSW, Australia
- 2World Health Organization, Regional Office for the Eastern Mediterranean, Cairo, Egypt
Despite improvements in the detection and control of infectious diseases, many new pathogens are emerging and re-emerging in various parts of the world. Most of these emerging and re-emerging infections are of zoonotic origin, which highlights the importance of the human–animal interface. Similarly, the rate of vector-borne diseases has increased recently due to changes in human habitats, climate change, deforestation, changes in food production practices, and increased population movement. The risk of spread of these zoonotic and vector-borne diseases is higher in the Eastern Mediterranean Region (EMR) of the World Health Organization due to its topography and geopolitical situation, fragile health systems, complex humanitarian emergencies, and, in some countries, other socioeconomic risk factors. Many countries in the region have reported outbreaks of zoonotic and vector-borne diseases over the last few decades, and some of these diseases have spread to other WHO regions as well. Avian influenza A (H5N1) and Middle East respiratory syndrome coronavirus (MERS-CoV) are among the greatest threats to global health security and both viruses are endemic in the EMR. Countries in the EMR have made significant progress toward the control of zoonotic and vector-borne diseases in recent years, and prevention, preparedness, and response capacities have been improved. However, there are still many challenges associated with the control of these diseases in the EMR, particularly in countries facing humanitarian emergencies. In this paper, we present the current situation of emerging and re-emerging infections in the EMR and discuss progress, challenges, and ways forward.
Introduction
Despite advances in the detection and control of infectious diseases, many new infections are emerging and re-emerging globally, including avian influenza viruses H5N1 and H7N9, Middle East Respiratory Syndrome coronavirus (MERS-CoV), severe acute respiratory syndrome coronavirus 1 (SARS-CoV1), and, more recently, severe acute respiratory syndrome coronavirus 2 (SARS-CoV2). The consequences of these emerging infections may be catastrophic, as people have very little or no immunity against these pathogens. Moreover, some existing infections are emerging in new geographical areas or have seen increased incidences recently; these include Ebola, monkeypox, West Nile virus (WNV), dengue, chikungunya, and Zika (1–3). Most of the emerging and re-emerging infections are of zoonotic origin, which highlights the importance of controlling these diseases not only in humans but also in animals (4). Vector-borne diseases (VBDs) are also a major public health issue and more than 80% of the world’s population is at risk of developing one or more VBDs (5). Important factors contributing to the emergence and re-emergence of these zoonotic and vector-borne diseases include changes in human habitat and climate due to urbanization, deforestation, and reforestation; changes in food production and farming practices; increased poultry density and more frequent contact with animals; and an increase in travel, trade, and tourism (1, 6–8).
Rik of zoonotic and vector-borne disease in the Eastern Mediterranean Region
The Eastern Mediterranean Region (EMR) of the World Health Organization (WHO) is sociopolitically diverse and includes countries from the Middle East, North Africa, the Horn of Africa, and Central Asia (9). The emergence risk of zoonotic and vector-borne diseases is high in this region due to its topography, complicated geopolitical situation, mass gatherings for religious and sporting events, and other socioeconomic factors (10–12). Four of the eight migratory birds’ flyways pass through various countries of the EMR: Central Asia–India, East Atlantic, Mediterranean–Black Sea, and West Asia–Africa (13). These migratory wild birds are reservoirs for many avian influenza viruses and may transfer viruses to domestic birds, poultry, and subsequently, to humans as well. Climate change and other environmental factors also affect the survival and distribution of high-risk pathogens and their vectors and hosts. The complicated geopolitical situation and humanitarian emergencies have also resulted in the spread of infections due to huge population displacement, interruptions in health services, poor living conditions, weak surveillance and disease-detection capacities, and the lack of disease control measures (10).
Important outbreaks and epidemics of zoonotic and vector-borne diseases in EMR
Over the last few decades, at least 11 countries in the EMR have reported major outbreaks and epidemics of zoonotic and vector-borne diseases, which have had the potential to spread globally (Table 1) (14). Currently, there are approximately 50 active outbreaks ongoing in the region, with 70% of them related to vector-borne and zoonotic diseases. Avian influenza A (H5N1) and MERS-CoV are considered two of the greatest threats to global health security and both viruses are endemic in the EMR. Sporadic cases and outbreaks of monkeypox, sandfly fever, Alkhurma hemorrhagic fever (AHF) (20–22), and plague are also occurring in a few countries, but there is limited information on other rare zoonotic diseases such as Q fever, and tularemia (23). In addition to these outbreaks and epidemics, many zoonotic and vector-borne infections are endemic in the region, such as rabies, brucellosis, leishmaniases, Ebola, other food-borne zoonotic infections, and malaria.
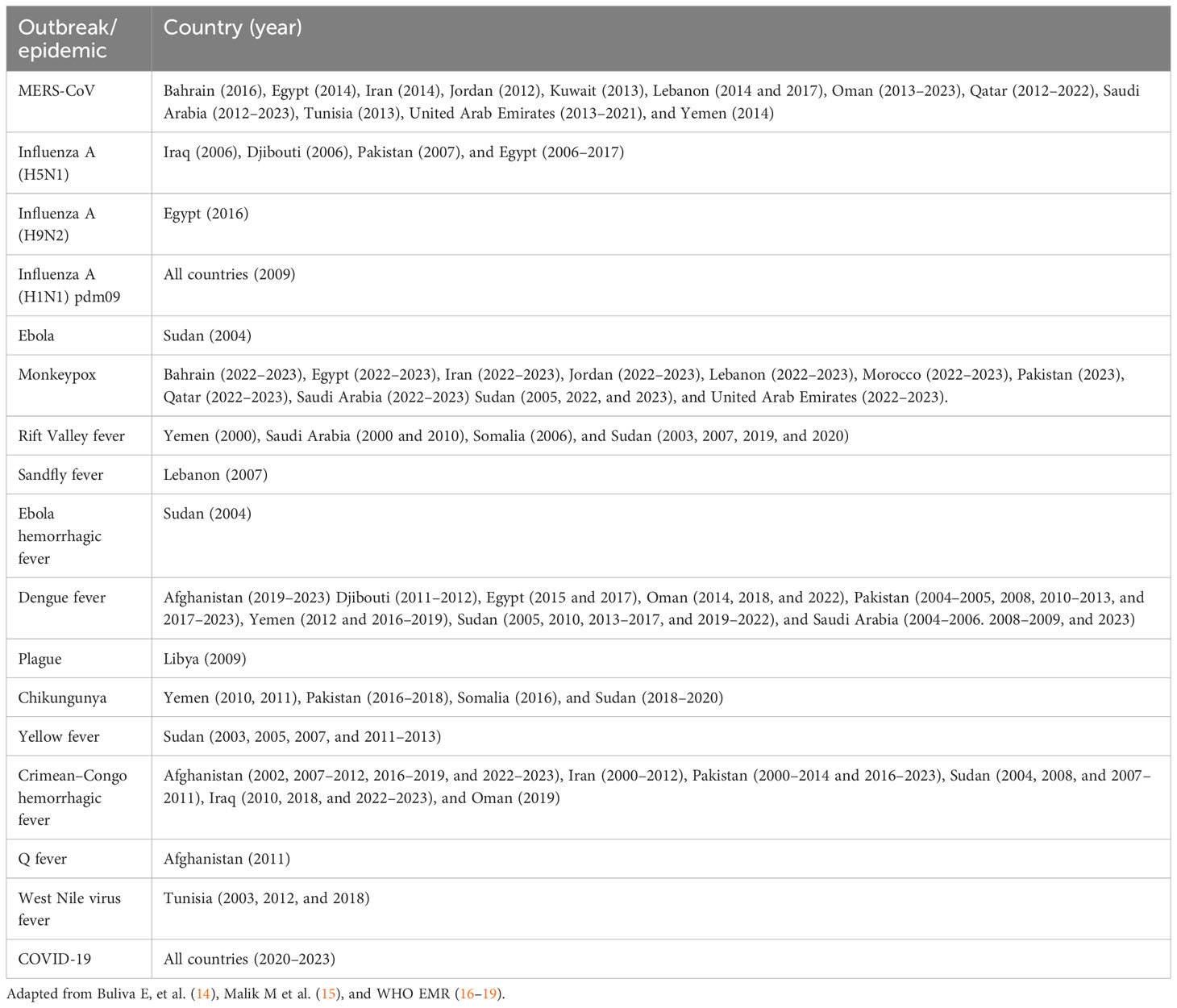
Table 1 Emerging and epidemic-prone zoonotic and vector-borne disease outbreaks in the Eastern Mediterranean Region since 2000.
Avian influenza
Avian influenza A (H5N1) remains a major concern in the EMR and, according to a recent study, approximately 10,000 outbreaks due to various types of avian influenza were reported in birds and other species in the EMR from 2011 to 2021 (24). The majority of these avian outbreaks were reported in Iran, Egypt, and Iraq, whereas most other countries either reported no outbreaks or very few outbreaks in avian species. From January 2003 to 31 May 2023, 876 cases and 458 deaths (CFR of 52%) have been reported worldwide due to avian influenza H5N1 in humans, and of them, 366 cases (42%) and 123 deaths (27%), were reported in the EMR (25, 26). Most of these cases and deaths were reported in Egypt, and a few cases and deaths were also reported in Djibouti, Iraq, and Pakistan (27).
The number of avian influenza H5N1 cases in Egypt is the largest globally, and Egypt is the only country with both a large number of H5N1 outbreaks among poultry and a large number of cases among humans. Some serologic studies showed that H5N1 cases in Egypt are underreported, and the actual number may therefore be larger than this (28–30). One study showed that approximately 2% of Egyptians exposed to poultry had been infected with H5N1 (29). The virus has become enzootic in Egypt and a low level of transmission is reported throughout the year (31). The upsurge in avian influenza H5N1 cases in Egypt had been attributed to uncontrolled poultry farming practices, lack of awareness, and engagement in high-risk behaviors and failure to take adequate personal protection measures while in contact with poultry (32). The risk of a major outbreak of H5N1 in poultry is high in Egypt, owing to the presence of more than 40,000 poultry farms with low-biosecurity and a high prevalence of backyard farming (33). An outbreak of the H5N1 subtype was also reported in a backyard poultry farm in an eastern region of Libya in 2014 (34). Avian influenza H5N1 is still a risk to regional and global health security, given that the virus has recently been detected among many new land and sea mammals including farmed mink, seals, sea lions, and cats in several countries (35). These mammals are biologically closer to humans than birds; therefore, it is likely that the virus might adapt to infect humans more easily in future.
Middle East respiratory syndrome coronavirus
Most MERS cases are reported in EMR countries—those in Saudi Arabia alone constitute more than 84% of the global cases of MERS (36). MERS cases have also been reported in many other countries in the region, including the United Arab Emirates, Jordan, Qatar, Oman, Iran, Kuwait, Tunisia, Yemen, Bahrain, Egypt, and Lebanon (37). The number of cases in the region has decreased since the start of the COVID-19 pandemic, likely due to a reduction in testing and the adoption of various control measures such as mask-wearing, hand hygiene practices, improved ventilation, and social distancing.
However, MERS is still a threat in the region as cases are regularly being reported in Saudi Arabia and, currently, there is no vaccine or MERS-specific treatment available. According to a WHO risk assessment, additional cases of MERS-CoV infection will continue to be reported in countries in the Middle East, particularly when routine surveillance activities resume after the acute phase of the COVID-19 pandemic has passed (38). The risk of MERS spreading to other countries is high due to travel in endemic areas and exposure to dromedaries, animal products, or humans. Moreover, the transmission mechanism from animals to humans is still unclear. Although dromedary camels are considered a major reservoir for MERS-CoV, the role of dromedary camels in MERS transmission is not fully understood. Limited, non-sustained human-to-human transmission of MERS has been reported, mainly in healthcare settings (37). Although MERS surveillance and case detection have been improved in the region, case management, compliance with infection control measures in hospitals, and contact follow-up remain major challenges (39).
Other zoonotic diseases
Rabies has been controlled in most countries of the Americas and Western Europe; however, it is still a public health challenge in the EMR, where only three countries (i.e., Bahrain, Kuwait, and the United Arab Emirates) are rabies-free (40). Rabies is endemic in at least 14 countries in the region, comprising Egypt, Iran, Iraq, Jordan, Lebanon, Morocco, Oman, Palestine, Pakistan, Tunisia, Syria, Saudi Arabia, Sudan, and Yemen (23, 40). During 2017 alone, there were 239,578 cases in humans caused by dog bites reported in 11 countries and 52 reported cases of human rabies in nine countries, although the actual case numbers are thought to be much larger, due to underreporting (41). Most cases of rabies in the region are dog-mediated (42); however, high positivity rates have been reported in foxes in Oman and wolves in Syria (43). The risk of rabies is reported to be higher in children, people with low socioeconomic backgrounds, and those living in rural areas (44). Very little information is available on the rabies situation in conflict-affected countries such as Palestine and Syria (40), although rabies cases have recently increased in some of their neighboring countries (45).
Brucellosis is also a major public health problem in the region, and Brucella spp. is widespread in many countries. Human cases have been reported in Saudi Arabia, Yemen, Iran, Egypt, and Jordan; however, accurate estimates of human cases of brucellosis are lacking, largely because of underreporting and misdiagnosis (46). Risk factors for human brucellosis in the region are the consumption of unpasteurized milk, direct contact with infected animals, and occupational exposure. Although Ebola is currently not circulating in the region and no cases have been reported during recent outbreaks, the risk of contracting it is very high. Ebola outbreaks occurred in Sudan in 1976, 1979, and 2004, resulting in 284, 34, and 17 cases, respectively (47, 48). Finally, outbreaks of RVF have been reported in Saudi Arabia, Yemen, and Sudan (49–51).
Emerging and re-emerging vector-borne diseases
The region is home to several arthropod-borne viruses (arboviruses) and mosquito-borne diseases, which are particularly expanding their range and emerging in new areas. Dengue is one of the fastest-growing mosquito-borne viral diseases in the region and is endemic in at least eight countries in the region. Large outbreaks of dengue have been reported in Pakistan, Afghanistan, Egypt, Sudan, and Yemen (52, 53). The disease has also spread to some neighboring countries including Syria and Kuwait. Chikungunya outbreaks have been reported in Pakistan, Somalia, Yemen, and Sudan (14, 16). Similar to dengue, chikungunya is also spreading to new areas, and Sudan’s first ever outbreak, resulting in more than 20,000 cases, was reported in 2018 (16). In the region, yellow fever outbreaks have been reported in Sudan only (54); however, serologic studies have also shown evidence of the circulation of yellow fever in Djibouti and Somalia (14, 55). According to a systematic review of 35 seroprevalence studies in the region, WNV-specific antibodies were detected in the human population of 11 countries, namely Djibouti, Egypt, Iran, Iraq, Jordan, Lebanon, Libya, Morocco, Pakistan, Sudan, and Tunisia. WNV RNA was also detected among patients in Iran, Pakistan, and Tunisia (56).
In addition to mosquitos, diseases are also spread by other vectors in the region. The incidence of CCHF has increased in many countries in the region, with sporadic human cases and outbreaks of CCHF reported in 10 out of the region’s 22 countries (57–59). CCHF is endemic in Afghanistan, Iran, Iraq, and Pakistan, and several outbreaks have occurred during the last few years, particularly in the border areas of these countries, owing to several factors including population movement and animal trade. Despite the regular reporting of CCHF cases and outbreaks, its true disease burden in the region is still unknown due to limited awareness and reporting of suspected cases and the different surveillance systems used for CCHF in EMR countries. Other major challenges include the delay in diagnosis and lack of awareness among healthcare providers. Leishmaniases are also widespread in the region due to poor awareness and reporting, difficult case management, and a lack of effective control measures (60). Most cases of leishmaniases are reported in disadvantaged communities in the region (61).
Progress toward control of zoonotic and vector-borne diseases in the EMR and lessons learned
Despite many challenges, countries in the EMR have made significant progress toward the control of zoonotic and vector-borne diseases in recent years. Prevention, preparedness, and response capacities to emerging and re-emerging zoonotic and vector-borne diseases have been improved. IHR (2005) core capacities have been strengthened and 17 countries in the region have completed joint external evaluations of IHR (2005) capacities (62, 63). Surveillance systems have been improved and 19 out of 22 countries have sentinel surveillance to collect and analyze data on severe acute respiratory infections (SARIs) and influenza-like illnesses (ILIs) (64). After the emergence of avian influenza virus A (H5N1) in Egypt, the Eastern Mediterranean Acute Respiratory Infection Surveillance (EMARIS) network was established in the region to improve SARI and ILI surveillance and to strengthen countries’ capacities to detect seasonal and new influenza viruses (65, 66). Moreover, the Early Warning Alert and Response Network (EWARN) has been introduced in many countries facing humanitarian emergencies. The laboratory diagnosis capacity for emerging and re-emerging infectious diseases has been enhanced across the region, with more reference laboratories with adequate capacity being built. The Emerging and Dangerous Pathogen Laboratory Network (EDPLN) has also expanded (67). In January 2020, only four countries in the region had the laboratory capacity to test for COVID-19. By mid-February that same year, however, with the support of the WHO and partners, all 22 Member States had built laboratories with the capacity to conduct COVID-19 reverse transcription-polymerase chain reaction (RT-PCR) testing in 2021 (68). By late 2022, with the support of the WHO and partners, 21 Member States could carry out domestic COVID-19 genome sequencing.
Similarly, outbreak response capacity has been improved and most countries in the region have trained rapid response teams at the central level to investigate outbreaks due to emerging and re-emerging pathogens (64). Training has been provided on surveillance, field investigation, and diagnosis. Many new activities were initiated to control the Aedes mosquito in the region, which is the primary vector for many emerging and re-emerging VBDs. The WHO EMRO has also developed a framework for a One Health approach, although the coordination mechanism between human and animal health sectors is still weak in most countries in the region (69). Various activities are ongoing in the region to prevent outbreaks during events with mass gatherings, such as risk assessments, strengthened surveillance and case detection/reporting, case management training, laboratory capacity scale-up, vaccination against common infection, increased awareness, and rapid response. The WHO EMRO has also developed a strategic framework for the prevention and control of emerging and epidemic-prone diseases in the region, with the goal of reducing the burden of emerging and epidemic-prone zoonotic and vector-borne infectious diseases in the WHO Eastern Mediterranean Region by 2024 (70). Countries in other WHO regions may also adopt some of these strategies in a local context to control zoonotic and vector-borne diseases.
Owing to these initiatives, a number of outbreaks of emerging infectious diseases have been successfully contained in the region. The lessons learned from these outbreaks were leveraged to manage the COVID-19 pandemic in several countries. For example, surveillance systems, laboratories, infection prevention and control measures, and clinical care networks for endemic diseases were rapidly utilized during the early phase of the COVID-19 pandemic. Guidelines, policy documents, training modules, standard operating procedures, risk assessment tools, and checklists developed for MERS-CoV and other emerging and re-emerging diseases were used to inform initial technical guidance documents and information products for COVID-19. Similarly, standardized seroepidemiological protocols developed for other emerging and re-emerging infections were quickly adapted for COVID-19 and were implemented across many countries in the region to rapidly identify key epidemiologic parameters of SARS-CoV-2, and also to understand the nature of the infection, the extent of its spread, and risk factors. Reference laboratories in high-resource countries in the region were leveraged, particularly in the early stages of the pandemic, to provide laboratory support and confirmatory testing for affected countries.
Challenges and the way forward
There are many challenges associated with the control of zoonotic and vector-borne diseases in the EMR, most of which are also common in other WHO regions, such as increased population movement, the unavailability of vaccines and specific treatments for many zoonotic and vector-borne diseases, and zoonotic spillover. The challenges specific to the EMR are highlighted in Table 2. Some important challenges, particularly in countries experiencing humanitarian emergencies, are weak detection and response capacities, poor animal source/vector control measures, fragile health systems, the lack of resources, competing priorities, and the unavailability of drugs and vaccines (61). Countries need to address these challenges to control emerging and re-emerging zoonotic and vector-borne diseases and to prevent morbidity and mortality, particularly among vulnerable groups. Table 2 also contains some strategies to address these challenges in the EMR countries and countries in other regions in similar circumstances. These strategies are in line with the WHO strategy for the Eastern Mediterranean Region, 2020–2023 (68), and the strategic framework for the prevention and control of emerging and epidemic-prone infectious diseases in the Eastern Mediterranean Region (70).

Table 2 Important challenges in the control of emerging and re-emerging zoonotic and vector-borne diseases in the Eastern Mediterranean Region and proposed strategies to address these challenges (10, 68–73).
In summary, the incidence of many zoonotic and vector-borne diseases is expected to increase in the future due to climate change, globalization, urbanization, and other factors (74). Therefore, countries should focus on strengthening their capacities to prevent, detect, and respond to outbreaks due to these diseases. Surveillance systems should be strengthened, incorporating an early-warning component for rapid detection and response to outbreaks due to zoonotic and vector-borne diseases. The IHR (2005) should be the key driver for rapid epidemic intelligence and global health security. There is a need to improve laboratory capacities for the timely and accurate detection of emerging pathogens, including point-of-care testing, with implications for regional and global health security.
The majority of zoonotic and vector-borne diseases spread due to close contact with animal hosts or vectors; therefore, countries should foster effective collaboration between the animal, human, and environmental health sectors to prevent the emergence and spread of these diseases. Intersectoral collaboration and coordination are important for the timely sharing of epidemiological and laboratory surveillance data and for exercising integrated responses. To achieve this, the One Health approach should be adopted and a system should be in place to oversee all One Health activities, including governance, policy and legislative frameworks, advocacy and communication, collaboration among various sectors, training and capacity building, data sharing, integrated surveillance and response systems, research, and monitoring and evaluation (75, 76). Countries in the EMR are at different stage of implementing the One Health framework. Countries without One Health initiatives in place should develop a national strategy and operational plan in consultation with relevant stakeholders, endorsed by relevant ministries. Countries with existing One Health plans should enhance their key activities, such as risk assessment, prioritization of zoonotic diseases, preparedness and response activities, assessment of existing capacities, identification of research priorities, and coordination with partners (75). Many countries in the region may lack the capacity to implement the One Health approach, thus international organizations, non-governmental organizations, academic institutes, and private sectors can be engaged (76).
Response capacity should also be strengthened to contain outbreaks of zoonotic and vector-borne diseases early; otherwise, they may cause significant morbidity and mortality. Tailored strategies should be developed for countries experiencing humanitarian emergencies, as in addition to poor surveillance and testing capacities, these countries do not have adequate healthcare infrastructure and basic healthcare services such as immunization against common diseases. Therefore, rapid detection of and response to epidemics should be a high priority among affected populations. Finally, as there is limited information on the burdens, trends, and risks of zoonotic and vector-borne diseases in the region, more research is needed.
Data availability statement
Publicly available data sets were analyzed in this study. These data can be found here: https://www.emro.who.int/entity/about-us/index.html.
Author contributions
AC prepared the first draft of the manuscript. CK, MT, and MP provided data from regional offices and reviewed the manuscript; RJ and AA reviewed the manuscript and gave critical input. All authors approved the final version.
Acknowledgments
We acknowledge the support of the WHO’s Regional Office for the Eastern Mediterranean Region in providing data around emerging and re-emerging zoonotic and vector-borne diseases.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Morens DM, Fauci AS. Emerging infectious diseases: threats to human health and global stability. PloS pathogens. (2013) 9(7):e1003467. doi: 10.1371/journal.ppat.1003467
2. World Health Organisation (WHO). Global Alert and Response (GAR). Ebola Situation reports. Available at: http://www.who.int/csr/disease/ebola/situation-reports/en/.
3. Huntington MK, Allison J, Nair D. Emerging vector-borne diseases. Am Family Physician (2016) 94(7):551–7.
4. Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. (2004) 430(6996):242. doi: 10.1038/nature02759
5. World Health Organisation (WHO). Global vector control response 2017–2030. Geneva: World Health Organization (2017). Licence: CC BY-NC-SA 3.0 IGO.
6. Wilson ME. Travel and the emergence of infectious diseases. Emerging Iinfectious Diseases. (1995) 1(2):39. doi: 10.3201/eid0102.950201
7. Patz JA, Graczyk TK, Geller N, Vittor AY. Effects of environmental change on emerging parasitic diseases. Int J Parasitol (2000) 30(12-13):1395–405. doi: 10.1016/S0020-7519(00)00141-7
8. van Doorn HR. Emerging infectious diseases. Medicine. (2014) 42(1):60–3. doi: 10.1016/j.mpmed.2013.10.014
9. Eastern Mediterranean Region World health Organisation (WHO). Countires in Eastern Mediterranean Region of World Health Organisation. Available at: http://www.emro.who.int/countries.html.
10. Raad II, Chaftari A-M, Dib RW, Graviss EA, Hachem R. Emerging outbreaks associated with conflict and failing healthcare systems in the Middle East. Infection Control Hosp Epidemiol (2018) 39(10):1230–6. doi: 10.1017/ice.2018.177
11. Memish ZA, Al-Rabeeah AA. Public health management of mass gatherings: the Saudi Arabian experience with MERS-CoV. SciELO Public Health (2013) 91(12):899–899A. doi: 10.2471/BLT.13.132266
12. World Health Organisation (WHO). Communicable disease alert and response for mass gatherings. Key Considerations (2008). Available at: https://www.who.int/publications/i/item/public-health-for-mass-gatherings-key-considerations (Access year 2023).
13. Kayali G, Webby RJ, Samhouri D, Mafi AR, Bassili A. Influenza research in the Eastern Mediterranean Region: the current state and the way forward. Influenza Other Respir Viruses. (2013) 7(6):914–21. doi: 10.1111/irv.12136
14. Buliva E, Elhakim M, Minh T, Nguyen N, Elkholy A, Mala P, et al. Emerging and reemerging diseases in the World Health Organization (WHO) Eastern Mediterranean Region—progress, challenges, and WHO initiatives. Front Public Health (2017) 5:276. doi: 10.3389/fpubh.2017.00276
15. Malik M, Mafi A, Mahjour J, Opoka M, Elhakim M, Muntasir M. Novel coronavirus infection in the Eastern Mediterranean Region: time to act. EMHJ - Eastern Mediterranean Health J (2013) 19(supp. 1):S31–8. doi: 10.26719/2013.19.supp1.S31
16. Eastern Mediterranean Region World health Organisation (WHO). Infectious disease outbreaks reported in the Eastern Mediterranean Region in 2018. Available at: http://www.emro.who.int/pandemic-epidemic-diseases/news/infectious-disease-outbreaks-reported-in-the-eastern-mediterranean-region-in-2018.html.
17. Intercountry meeting on the strategic framework for prevention and control of emerging and epidemic-prone diseases in the Eastern Mediterranean Region. Amman, Jordan 16–19 December 2018. Cairo: WHO Regional Office for the Eastern Mediterranean (2019). Licence: CC BY-NC-SA 3.0 IGO.
18. World health Organisation (WHO). Epidemic and pandemic-prone diseases. Emerging infectious disease outbreaks reported in the Eastern Mediterranean Region in 2017. Available at: http://www.emro.who.int/pandemic-epidemic-diseases/news/emerging-infectious-disease-outbreaks-reported-in-the-eastern-mediterranean-region-in-2017.html.
19. Eastern Mediterranean Region World health Organisation (WHO). Epidemic and pandemic-prone diseases. Current outbreaks in the WHO Eastern Mediterranean Region. Available at: http://www.emro.who.int/pandemic-epidemic-diseases/outbreaks/index.html.
20. Madani TA, Azhar EI, Abuelzein E-TM, Kao M, Al-Bar HM, Abu-Araki H, et al. Alkhumra (Alkhurma) virus outbreak in Najran, Saudi Arabia: epidemiological, clinical, and laboratory characteristics. J Infection. (2011) 62(1):67–76. doi: 10.1016/j.jinf.2010.09.032
21. Carletti F, Castilletti C, Di Caro A, Capobianchi MR, Nisii C, Suter F, et al. Alkhurma hemorrhagic fever in travelers returning from Egypt, 2010. Emerging Infect Diseases. (2010) 16(12):1979. doi: 10.3201/eid1612101092
22. Memish ZA, Fagbo SF, Osman Ali A, AlHakeem R, Elnagi FM, Bamgboye EA. Is the epidemiology of alkhurma hemorrhagic fever changing?: a three-year overview in Saudi Arabia. PloS One (2014) 9(2):e85564. doi: 10.1371/journal.pone.0085564
23. Mostafavi E, Ghasemian A, Abdinasir A, Nematollahi Mahani SA, Rawaf S, Salehi Vaziri M, et al. Emerging and re-emerging infectious diseases in the WHO Eastern Mediterranean region, 2001-2018. Int J Health Policy Management. (2022) 11(8):1286–300. doi: 10.34172/ijhpm.2021.13
24. Badra R, Abubakar A, Tempia S, Alam N, ElNaja HA, Kayali G, et al. State and situation of avian influenza in the Eastern Mediterranean Region. Influenza Other Respir Viruses. (2023) 17(4):e13137. doi: 10.1111/irv.13137
25. World Health Organisation (WHO). Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003-2023, 5 January 2023. Available at: https://www.who.int/publications/m/item/cumulative-number-of-confirmed-human-cases-for-avian-influenza-a(h5n1)-reported-to-who-2003-2022-5-jan-2023.
26. World Health Organisation (WHO). Avian influenza weekly update number 904. Available at: https://cdn.who.int/media/docs/default-source/wpro—documents/emergency/surveillance/avian-influenza/ai_20230714.pdf?sfvrsn=5f006f99_117#:~:text=The%20last%20cases%20in%20the,23%20and%2024%20February%202023.&text=Globally%2C%20from%20January%202003%20to,of%2052%25)%20(Source).
27. World Health Organisation (WHO). Cumulative number of confirmed human cases of avian influenza A(H5N1) reported to WHO. Available at: http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/.
28. Gomaa MR, El Rifay AS, Zeid DA, Elabd MA, Elabd E, Kandeil A, et al. Incidence and seroprevalence of avian influenza in a cohort of backyard poultry growers, Egypt, August 2015–March 2019. Emerging Infect diseases. (2020) 26(9):2129. doi: 10.3201/eid2609.200266
29. Gomaa MR, Kayed AS, Elabd MA, Zeid DA, Zaki SA, El Rifay AS, et al. (H5N1) and A (H9N2) seroprevalence and risk factors for infection among Egyptians: a prospective, controlled seroepidemiological study. J Infect Diseases. (2015) 211(9):1399–407. doi: 10.1093/infdis/jiu529
30. Samaha H, Ibrahim MS, Ayoub M, Shaaban SI. Seroepidemiology of avian influenza viruses H5 and H9 in Beheira Governorate. Alexandria J Veterinary Sci (2015) 44(1):86–92. doi: 10.5455/ajvs.161061
31. Kayali G, Kandeil A, El-Shesheny R, Kayed AS, Maatouq AM, Cai Z, et al. (H5N1) virus in Egypt. Emerging Infect Diseases. (2016) 22(3):379. doi: 10.3201/eid2203.150593
32. World Health Organisation (WHO). Regional Office for Eastern Mediterranean. Egypt: upsurge in H5N1 human and poultry cases but no change in transmission pattern of infection. Available at: http://www.emro.who.int/egy/Egypt-news/upsurge-h5n1-human-poultry-cases-may-2015.html.
33. Eastern Mediterranean Region World health Organisation (WHO). Egypt. Pandemic influenza. Available at: http://www.emro.who.int/egy/programmes/influenza.html.
34. Kammon A, Heidari A, Dayhum A, Eldaghayes I, Sharif M, Monne I, et al. Characterization of avian influenza and Newcastle disease viruses from poultry in Libya. Avian Diseases. (2015) 59(3):422–30. doi: 10.1637/11068-032215-ResNote.1
35. World Health Organisation (WHO). Ongoing avian influenza outbreaks in animals pose risk to humans (2023). Available at: https://www.who.int/news/item/12-07-2023-ongoing-avian-influenza-outbreaks-in-animals-pose-risk-to-humans.
36. Eastern Mediterranean Region World health Organisation (WHO). Epidemic and pandemic-prone diseases. MERS situation update (2019). Available at: http://www.emro.who.int/pandemic-epidemic-diseases/mers-cov/mers-situation-update-april-2019.html.
37. World Health Organisation (WHO). Middle East respiratory syndrome coronavirus (MERS-CoV). Available at: https://www.who.int/health-topics/middle-east-respiratory-syndrome-coronavirus-mers#tab=tab_1.
38. World Health Organisation (WHO). Middle East respiratory syndrome: global summary and assessment of risk (2022). Available at: https://www.who.int/publications/i/item/WHO-MERS-RA-2022.1.
39. World health Organisation (WHO). Middle East respiratory syndrome coronavirus (MERS-CoV). WHO MERS Global Summary and Assessment of Risk (2018). Available at: https://www.who.int/csr/disease/coronavirus_infections/risk-assessment-august-2018.pdf?ua=1.
40. Bannazadeh Baghi H, Alinezhad F, Kuzmin I, Rupprecht CE. A perspective on rabies in the Middle East—Beyond Neglect. Veterinary Sci (2018) 5(3):67. doi: 10.3390/vetsci5030067
41. World Health Organisation (WHO). Rabies. Available at: https://www.who.int/data/gho/data/themes/topics/rabies.
42. Horton DL, McElhinney LM, Freuling CM, Marston DA, Banyard AC, Goharrriz H, et al. Complex epidemiology of a zoonotic disease in a culturally diverse region: phylogeography of rabies virus in the Middle East. PloS Negl Trop Diseases. (2015) 9(3):e0003569. doi: 10.1371/journal.pntd.0003569
43. Al Abaidani I, Al Abri S, Prakash K, Hussain MH, Hussain MH, Al Rawahi A. Epidemiology of rabies in Oman: a retrospective study [1991-2013]. EMHJ-Eastern Mediterr Health J (2015) 21(8):591–7. doi: 10.26719/2015.21.8.591
44. World Health Organisation (WHO). Fact sheet. Rabies. Available at: https://www.who.int/en/news-room/fact-sheets/detail/rabies.
45. Kassir M, El Zarif T, Kassir G, Berry A, Musharrafieh U, Bizri A. Human rabies control in Lebanon: a call for action. Epidemiol Infection. (2019) 147:e46. doi: 10.1017/S095026881800300X
46. Musallam I, Abo-Shehada M, Hegazy Y, Holt H, Guitian F. Systematic review of brucellosis in the Middle East: disease frequency in ruminants and humans and risk factors for human infection. Epidemiol Infection. (2016) 144(4):671–85. doi: 10.1017/S0950268815002575
47. World Health Organisation (WHO). WHO announces end of Ebola outbreak in southern Sudan. Available at: https://www.who.int/mediacentre/news/releases/2004/pr54/en/.
48. World health Organisation (WHO). Ebola virus disease. Available at: https://www.who.int/news-room/fact-sheets/detail/ebola-virus-disease.
49. Madani TA, Al-Mazrou YY, Al-Jeffri MH, Mishkhas AA, Al-Rabeah AM, Turkistani AM, et al. Rift Valley fever epidemic in Saudi Arabia: epidemiological, clinical, and laboratory characteristics. Clin Infect Diseases. (2003) 37(8):1084–92. doi: 10.1086/378747
50. Centers for Disease Control and Prevention (CDC). Outbreak of Rift Valley fever--Saudi Arabia, August-October, 2000. MMWR. Morbidity Mortality Weekly Rep (2000) 49(40):905–8.
51. Hassan OA, Ahlm C, Evander M. A need for one health approach–lessons learned from outbreaks of Rift Valley fever in Saudi Arabia and Sudan. Infection Ecol Epidemiol (2014) 4(1):20710. doi: 10.3402/iee.v4.20710
52. Seidahmed OM, Hassan SA, Soghaier MA, Siam HA, Ahmed FT, Elkarsany MM, et al. Spatial and temporal patterns of dengue transmission along a Red Sea coastline: a longitudinal entomological and serological survey in Port Sudan city. PloS Negl Trop Diseases. (2012) 6(9):e1821. doi: 10.1371/journal.pntd.0001821
53. Humphrey JM, Cleton NB, Reusken CB, Glesby MJ, Koopmans MP, Abu-Raddad LJ. Dengue in the Middle East and North Africa: a systematic review. PloS Negl Trop Diseases. (2016) 10(12):e0005194. doi: 10.1371/journal.pntd.0005194
54. Markoff L. Yellow fever outbreak in Sudan. New Engl J Med (2013) 368(8):689–91. doi: 10.1056/NEJMp1300772
55. Andayi F, Charrel RN, Kieffer A, Richet H, Pastorino B, Leparc-Goffart I, et al. A sero-epidemiological study of arboviral fevers in Djibouti, Horn of Africa. PloS Negl Trop Diseases. (2014) 8(12):e3299. doi: 10.1371/journal.pntd.0003299
56. Eybpoosh S, Fazlalipour M, Baniasadi V, Pouriayevali MH, Sadeghi F, Vasmehjani AA, et al. Epidemiology of West Nile Virus in the Eastern Mediterranean Region: a systematic review. PloS Negl Trop Diseases. (2019) 13(1):e0007081. doi: 10.1371/journal.pntd.0007081
57. Al-Abri SS, Al Abaidani I, Fazlalipour M, Mostafavi E, Leblebicioglu H, Pshenichnaya N, et al. Current status of Crimean-Congo haemorrhagic fever in the World Health Organization Eastern Mediterranean Region: issues, challenges, and future directions. Int J Infect Diseases. (2017) 58:82–9. doi: 10.1016/j.ijid.2017.02.018
58. Al-Abri SS, Hewson R, Al-Kindi H, Al-Abaidani I, Al-Jardani A, Al-Maani A, et al. Clinical and molecular epidemiology of Crimean-Congo hemorrhagic fever in Oman. PLoS Neglected Trop Dis (2019) 13(4):e0007100.
59. Wasfi F, Dowall S, Ghabbari T, Bosworth A, Chakroun M, Varghese A, et al. Sero-epidemiological survey of Crimean-Congo hemorrhagic fever virus in Tunisia. Parasite (2016) 23. doi: 10.1051/parasite/2016010
60. Gradoni L. The leishmaniases of the Mediterranean Region. Curr Trop Med Rep (2017) 4(1):21–6. doi: 10.1007/s40475-017-0099-1
61. Fazaludeen Koya S, Abdalla SM, Kodama C, Keita M, Abubakar A. Vector-borne and zoonotic diseases in the Eastern Mediterranean Region: a systematic review. J Epidemiol Global Health (2023) 13(1):105–14. doi: 10.1007/s44197-023-00091-7
62. Eastern Mediterranean Region World health Organisation (WHO). The work of WHO in the Eastern Mediterranean Region annual report of the regional director. (2016). Available at: https://apps.who.int/iris/handle/10665/259257 (Access year 2023).
63. World Health Organisation (WHO). Joint external evaluation tool: International Health Regulations (2005). Available at: https://apps.who.int/iris/handle/10665/204368.
64. Abubakar A, Elkholy A, Barakat A, Shrestha B, Elhakim M, Malik MR, et al. Pandemic influenza preparedness (PIP) framework: progress challenges in improving influenza preparedness response capacities in the Eastern Mediterranean Region, 2014–2017. J Infection Public Health (2019) 13(3):446–50. doi: 10.1016/j.jiph.2019.03.006
65. Horton KC, Dueger EL, Kandeel A, Abdallat M, El-Kholy A, Al-Awaidy S, et al. Viral etiology, seasonality and severity of hospitalized patients with severe acute respiratory infections in the Eastern Mediterranean Region, 2007–2014. PloS One (2017) 12(7):e0180954. doi: 10.1371/journal.pone.0180954
66. Malik M, Mahjour J, Khan W, Alwan A. Influenza in the Eastern Mediterranean Region: identifying the unknowns for detection and control of epidemic and pandemic threats. EMHJ-Eastern Mediterr Health J (2016) 22(7):428–9. doi: 10.26719/2016.22.7.428
67. Eastern Mediterranean Region World health Organisation (WHO). Epidemic and pandemic-prone diseases Pandemic and epidemic diseases: 2018 in retrospect. Available at: http://www.emro.who.int/pandemic-epidemic-diseases/information-resources/ped-2018-in-retrospect.html.
68. WHO’s strategy for the Eastern Mediterranean Region, 2020–2023: Turning Vision 2023 into action. Cairo: WHO Regional Office for the Eastern Mediterranean (2019). Licence: CC BY-NC-SA 3.0 IGO.
69. World Health Organisation (WHO). Implementation of the pandemic influenza preparedness (PIP) framework in the Eastern Mediterranean Region, july 2014 - august 2016. Cairo: WHO Regional Office for the Eastern Mediterranean (2017). Licence: CC BY-NC-SA 3.0 IGO.
70. Strategic framework for the prevention and control of emerging and epidemic-prone infectious diseases in the Eastern Mediterranean Region 201920203. Cairo: WHO Regional Office for the Eastern Mediterranean (2020). Licence: CC BY-NC-SA 3.0 IGO.
71. Eastern Mediterranean Region of WHO. Civil registration and vital statistics. Available at: https://www.emro.who.int/civil-registration-statistics/assesment/crvs-assessments.html.
72. The FAO–OIE–WHO collaboration. Sharing responsibilities and coordinating global activities to address health risks at the animal–human–ecosystems interfaces. A Tripartite Concept Note (2010). Available at: http://www.who.int/foodsafety/zoonoses/final_concept_note_Hanoi.pdf.
73. Eastern Mediterranean Region of WHO. Zoonotic disease: emerging public health threats in the Region. Available at: https://www.emro.who.int/about-who/rc61/zoonotic-diseases.html.
74. Colón-González FJ, Sewe MO, Tompkins AM, Sjödin H, Casallas A, Rocklöv J, et al. Projecting the risk of mosquito-borne diseases in a warmer and more populated world: a multi-model, multi-scenario intercomparison modelling study. Lancet Planetary Health (2021) 5(7):e404–e14. doi: 10.1016/S2542-5196(21)00132-7
75. Mahrous H, Redi N, Nguyen TMN, Al Awaidy S, Mostafavi E, Samhouri D. One health operational framework for action for the Eastern Mediterranean Region, focusing on zoonotic diseases. Eastern Mediterr Health J (2020) 26(6):720–5. doi: 10.26719/emhj.20.017
Keywords: zoonotic diseases, vector-borne diseases, Eastern Mediterranean Region, emerging infections, MERS-CoV, avian influenza H5N1, COVID-19
Citation: Chughtai AA, Kodama C, Joshi R, Tayyab M, Paiman MA and Abubakar A (2023) Control of emerging and re-emerging zoonotic and vector-borne diseases in countries of the Eastern Mediterranean Region. Front. Trop. Dis 4:1240420. doi: 10.3389/fitd.2023.1240420
Received: 15 June 2023; Accepted: 25 August 2023;
Published: 15 September 2023.
Edited by:
Alfonso J. Rodriguez-Morales, Fundacion Universitaria Autónoma de las Américas, ColombiaReviewed by:
Juan Pablo Escalera-Antezana, Secretaria Municipal de Salud, BoliviaCopyright © 2023 Chughtai, Kodama, Joshi, Tayyab, Paiman and Abubakar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiori Kodama, a29kYW1hY0B3aG8uaW50; Abrar Ahmad Chughtai, YWJyYXIuY2h1Z2h0YWlAdW5zdy5lZHUuYXU=
 Abrar Ahmad Chughtai
Abrar Ahmad Chughtai Chiori Kodama
Chiori Kodama Rohina Joshi1
Rohina Joshi1 Muhammad Tayyab
Muhammad Tayyab Abdinasir Abubakar
Abdinasir Abubakar