- 1Department of Medical Parasitology and Infection Biology, Swiss Tropical and Public Health Institute, Allschwil, Switzerland
- 2University of Basel, Basel, Switzerland
Soil-transmitted helminthiases caused by Ascaris lumbricoides, Trichuris trichiura, and hookworm (Ancylostoma duodenale and Necator americanus) are responsible for the infection of approximately 1.5 billion people worldwide, mostly in tropical and subtropical regions. Preventive chemotherapy is the mainstay of control, which is the regular administration of anthelminthic drugs, mainly albendazole and mebendazole to at-risk populations. As benzimidazoles face a risk of developing drug resistance and have shortcomings in their therapeutic profile, efforts have been made to develop alternative anthelminthics. The aim of this review is to provide a state-of-the-art update on available treatments and ongoing efforts in Research and Development (R&D) for the three main soil-transmitted helminth infections. Recent findings on the use of drug combinations and advanced drug candidates such as oxantel pamoate and emodepside and how these drugs fulfill the target product profile will be reviewed. Lastly, progress in drug discovery will be summarized.
Introduction
Soil-transmitted helminthiases are caused by one of the three major soil-transmitted helminths (STHs), Ascaris lumbricoides, Trichuris trichiura, and hookworm (Ancylostoma duodenale and Necator americanus). The fourth species the threadworm Strongyloides stercoralis is largely neglected. STHs are responsible for the infection of approximately 1.5 billion people worldwide, mostly in tropical and subtropical regions (1), and are a considerable global health burden (2). The burden of STH infections includes dietary deficiencies, anemia, physical and cognitive retardation in children, and reduction in work performance in adulthood (1).
In STH endemic areas, the cornerstone of control by the World Health Organization (WHO) is preventive chemotherapy (PC), which is the regular administration of anthelminthic drugs without prior diagnosis to at-risk populations.
Parasitological surveys are conducted to identify the settings where PC is needed, define treatment frequency, and monitor progress. The initial coverage aim of 75% treatment in the high-risk group of school-age children was recently expanded to two other high-risk populations of STH-related morbidity: preschool-age children and women of reproductive age (3).
However, the number of drugs currently recommended to treat and control STH infections is restricted, including four drugs, with two benzimidazoles (albendazole and mebendazole) at the forefront. Between 2010 and 2020, approximately 1.9 billion tablets of albendazole and 1.4 billion tablets of mebendazole were donated for the control of STH in school-age children (3). Levamisole and pyrantel pamoate are rarely used (4). The combination of albendazole-ivermectin was added in 2017 as a recommended treatment to the essential medicine list (5).
A key 2030 target set recently for STH control aims to achieve and maintain the elimination of STH-attributable morbidity in preschool- and school-age children. In order to achieve these targets, the development of additional anthelminthic drugs or a combination of existing anthelminthics was recommended (3). In the past years, increasing efforts in the field of drug discovery and development for STH infections have been witnessed. The aim of this review is to provide a state-of-the-art update on available treatments and ongoing efforts in Research and Development (R&D) for the three main STH infections. A recent review gave an in-depth overview of ivermectin and moxidectin, which are two excellent drugs against Strongyloides stercoralis (6). In this review, I will first summarize recent evidence on the performance of the four recommended drugs. Next, I will highlight recent findings on the use of drug combinations. Advanced drug candidates such as oxantel pamoate and emodepside and how these drugs fulfill the target product profile will be reviewed. Lastly, progress in drug discovery will be summarized.
Recommended treatments for soil-transmitted helminthiasis
Standard drugs on the essential medicine list by WHO for STH infections include the two benzimidazoles (albendazole and mebendazole), levamisole, and pyrantel pamoate. Ivermectin is the recommended treatment for infections with S. stercoralis and is recommended in combination with albendazole for STH infections (5).
Albendazole is the most widely used drug in preventive chemotherapy programs (3). In a systematic review and meta-analysis conducted in 2017, cure rates of 79.5%, 30.7%, and 95.7% for albendazole against hookworm, T. trichiura, and A. lumbricoides were calculated. The corresponding egg reduction rates were 89.6%, 49.9%, and 98.5% (7) (Table 1). While these figures can serve as excellent benchmarks, they are based on studies conducted over several decades using a wide range of trial methodologies. Nonetheless, studies investigating albendazole conducted in the past 5 years, after conducting the systematic review (e.g. 8–15), confirm these findings. Reassuringly, the efficacy of albendazole against A. lumbricoides and hookworm was high in all studies. Recent findings have highlighted the presence of a food effect resulting in differences in cure rates against hookworm within fed and fasted participants in Ethiopia (97.4% vs 74.2%) and confirmed a lower efficacy in moderate versus light infections (43% vs 94.6%) (15) as well as a lower sensitivity of N. americanus versus A. duodenale (13).
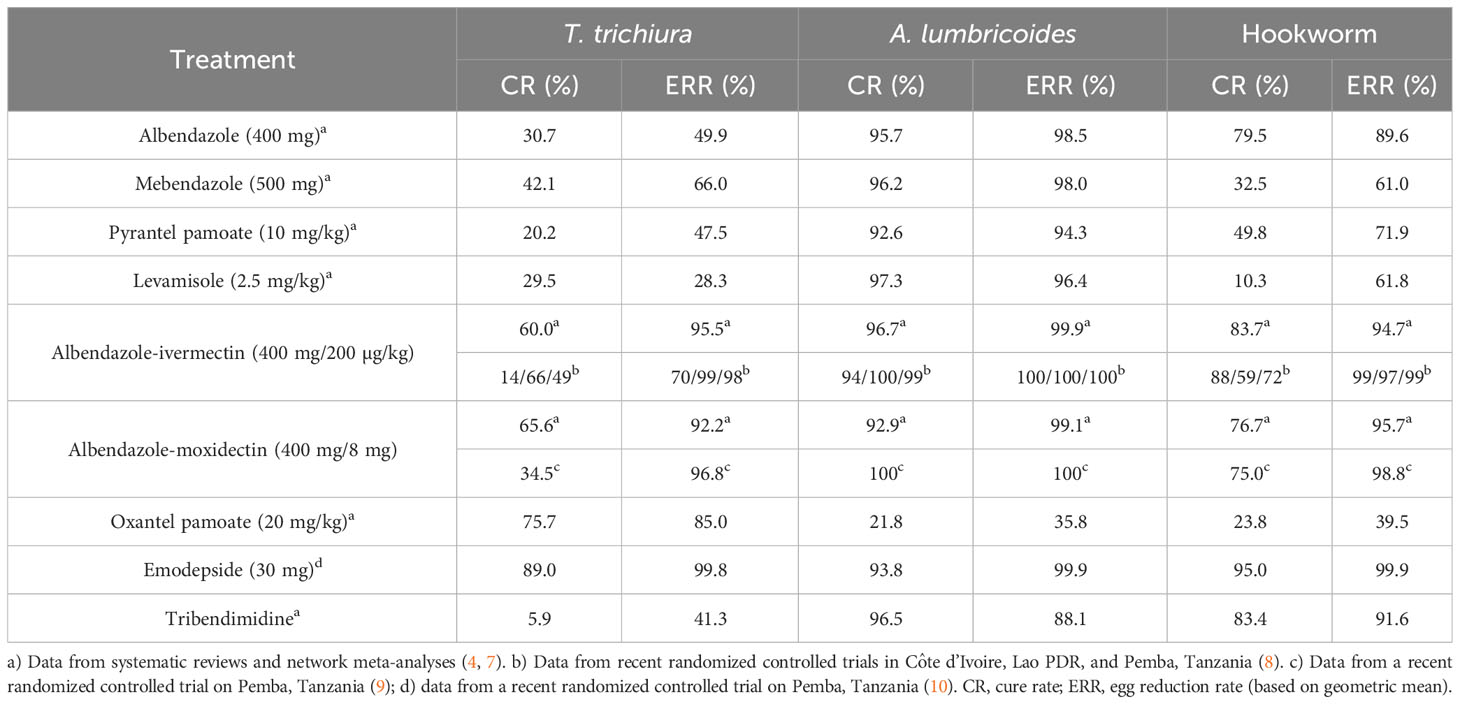
Table 1 Efficacy (geometric mean egg reduction rates and cure rates) of treatments against soil-transmitted helminthiasis.
Cure rates against T. trichiura were very low in the majority of studies, with cure and egg reduction rates as low as 6% and 16%, respectively. The highest cure rates with albendazole against T. trichiura were observed in Indonesia (cure rate of 46.2%) (16) and Timo-Leste (cure rate of 50%), studies, however, had low sample sizes.
Several studies have investigated whether a relationship exists between the presence of putative benzimidazole resistance single-nucleotide polymorphisms (SNPs) in the β-tubulin gene of T. trichiura and other STHs. However, putative benzimidazole resistance SNPs were not found to be higher after treatment (17).
For mebendazole, cure rates put forth by the systematic review were 32.5%, 42.1%, and 96.2% against hookworm, T. trichiura, and A. lumbricoides. Egg reduction rates calculated were 61.0%, 66.0%, and 98.0% (7). Studies conducted in the past 5 years confirmed that mebendazole has low efficacy against hookworm and T. trichiura while being an excellent drug for A. lumbricoides. Recent studies have reported cure rates of 13.0-30.8% against hookworm, 6.8-33.9% against T. trichiura, and 96.9-100% against A. lumbricoides (18–20). The only exception is an alarmingly low cure rate of 59.6% with a single dose of 500 mg of mebendazole against A. lumbricoides in Ethiopia, which should be assessed further (21). Two studies confirmed that a multiple dose of mebendazole reveals a considerably higher efficacy against hookworm and T. trichiura infection (18, 19). However, a treatment scheme consisting of six dosages is incompatible with preventive chemotherapy programs. Lastly, a child-friendly formulation of mebendazole was developed in 2018 (22), which despite different tablet characteristics reveals a nearly identical efficacy profile (23) that will greatly facilitate the treatment of young children.
Levamisole and pyrantel pamoate are not used in routine deworming programs and only a handful of research studies that have used these drugs as monotherapy or in combination and evaluated the efficacy in the past recent years were conducted (16, 24, 25). A combination of albendazole and pyrantel pamoate was found not to improve the cure rate or egg reduction rate in children with T. trichiura infection (24). On the other hand, the combination of albendazole and levamisole showed an improved cure rate for light T. trichiura infection (16); however, the results would need to be confirmed in additional trials.
Drug combinations
In order to increase the spectrum of activity and to be prepared for threatening drug resistance, the use of drug combinations has been suggested by experts and policymakers for the treatment of STH infections. A list of available co-administration treatments has been put forth ranked by the current evidence and whether the drugs are already marketed and available (4). Albendazole-ivermectin is the top-ranked drug combination. As the co-administration of albendazole-ivermectin has been widely used against filarial infections, from a regulatory perspective, the treatment could be integrated into PC programs in a fast manner. It is worth highlighting that ivermectin has excellent efficacy against S. stercoralis and hence the use of ivermectin as a partner drug would also target this neglected STH species (26, 27). Albendazole-ivermectin was recently proposed as an improved treatment strategy for STH infections and placed on the WHO essential medicine list (4, 5, 28). A meta-analysis was conducted to prepare the dossier for submission to the WHO essential medicine list. The findings suggested good tolerability and higher efficacy of albendazole-ivermectin against T. trichiura compared to single-dose albendazole treatment. Based on four studies that were identified, albendazole-ivermectin was significantly associated with lower risk (risk ratio (RR) = 0.44, 95% confidence interval (CI) = 0.31-0.62) for T. trichiura infection after treatment compared to albendazole alone (28). Yet, the authors concluded that large-scale randomized controlled trials are required to confirm these results. In three countries (Pemba, Tanzania; Lao PDR; and Côte d’Ivoire), a randomized, double-blinded, placebo-controlled trial was therefore conducted among 600 participants in each site to compare the efficacy and safety of monotherapy of albendazole to albendazole-ivermectin against T. trichiura to provide further evidence for this new treatment. The coadministration of albendazole-ivermectin was well tolerated with a similar number of adverse events in both treatment arms. However, unexpectedly, we observed a very low efficacy in Côte d’Ivoire (cure rate: 12.9%; egg reduction rate: 69.2%), which is in sharp contrast to the efficacy observed in other countries (8). In more detail, a statistically significant difference was found in cure and egg reduction rates between monotherapy and combination therapy in Pemba, Tanzania and Lao PDR signifying the superiority of the combination treatment. In Côte d’Ivoire, a statistically significant difference between monotherapy and combination therapy was not found for both cure and egg reduction rates.
The underlying reasons for the lack of drug efficacy are yet to be fully elucidated. Possible confounding factors (age, sex, and infection intensity) of the low efficacy of the albendazole-ivermectin combination could not be identified. There were no potential candidate benzimidazole-resistance mutations at codons 167, 198, and 200 in the β-tubulin gene in any of the mapped Amplicon Sequence Variants (ASVs) from Pemba, Tanzania and Lao PDR (unpublished observation).
However, two contributing factors could be determined which are T. trichiura strain differences and the gut microbiome. In more detail, phylogenetic analysis of the ITS-2 rDNA locus revealed that the ASVs from all samples from Pemba, Tanzania and Lao PDR clustered together whereas those from Côte d’Ivoire clustered separately. Primers targeting the mitochondrial nad1, nad4, and the major β-tubulin gene generated ASVs, mapping to the appropriate reference sequences from all samples from Pemba, Tanzania and Lao PDR but not from any samples from Côte d’Ivoire. Phylogenetic analysis of the ribosomal ITS-1 and ITS-2 markers placed the Trichuris from Côte d’Ivoire populations in a clade that includes Trichuris sp. from non-human primates and pigs but is separate from the clade containing T. trichiura from the Laos and Pemba populations (29).
Moreover, using samples from one of the study villages in Lao PDR showed that a large majority of cured patients who received the combination therapy (albendazole-ivermectin) presented a distinct gut microbial composition hinting towards a compositional aspect of the gut microbiome driving decreasing response to albendazole-ivermectin treatment of T. trichiura. These observations require analyzing additional human stool samples collected in Côte d’Ivoire (30).
To facilitate weight-based regimens, which are complex in preventive chemotherapy programs, a fixed-dose formulation of albendazole with a high dose of ivermectin is currently under development (31–33). An adaptive phase II/III randomized controlled trial has been undertaken in STH endemic sites in Ethiopia, Kenya, and Mozambique to evaluate an oral fixed-dose combination of 400 mg albendazole and either 9 or 18 mg ivermectin with the goal of providing a simplified treatment formulation (33).
Another co-administration therapy researched in the past years is albendazole-moxidectin (9, 34, 35). Moxidectin, like ivermectin, is a macrocyclic lactone that was approved in 2018 by the US Food and Drug Administration (FDA) as a treatment for onchocerciasis in patients over the age of 12 years (36). Several exploratory studies suggested that it might be a good partner drug in combination treatment against STH infections while being less effective in monotherapy (35). A recent head-to-head comparison revealed an egg reduction rate of 96.8% for moxidectin-albendazole, which was inferior to ivermectin-albendazole (ERR 99.0%, difference of -2.2%-points (95% CI -4.2 to -1.4)), while as expected, both combination treatments resulted in significantly higher efficacy than albendazole, moxidectin, or ivermectin (9). Like ivermectin, moxidectin has a high efficacy against S. stercoralis infections (37) and, hence, adding a macrocyclic lactone to albendazole does not only increase efficacy against T. trichiura infections but will also be beneficial for infections with S. stercoralis.
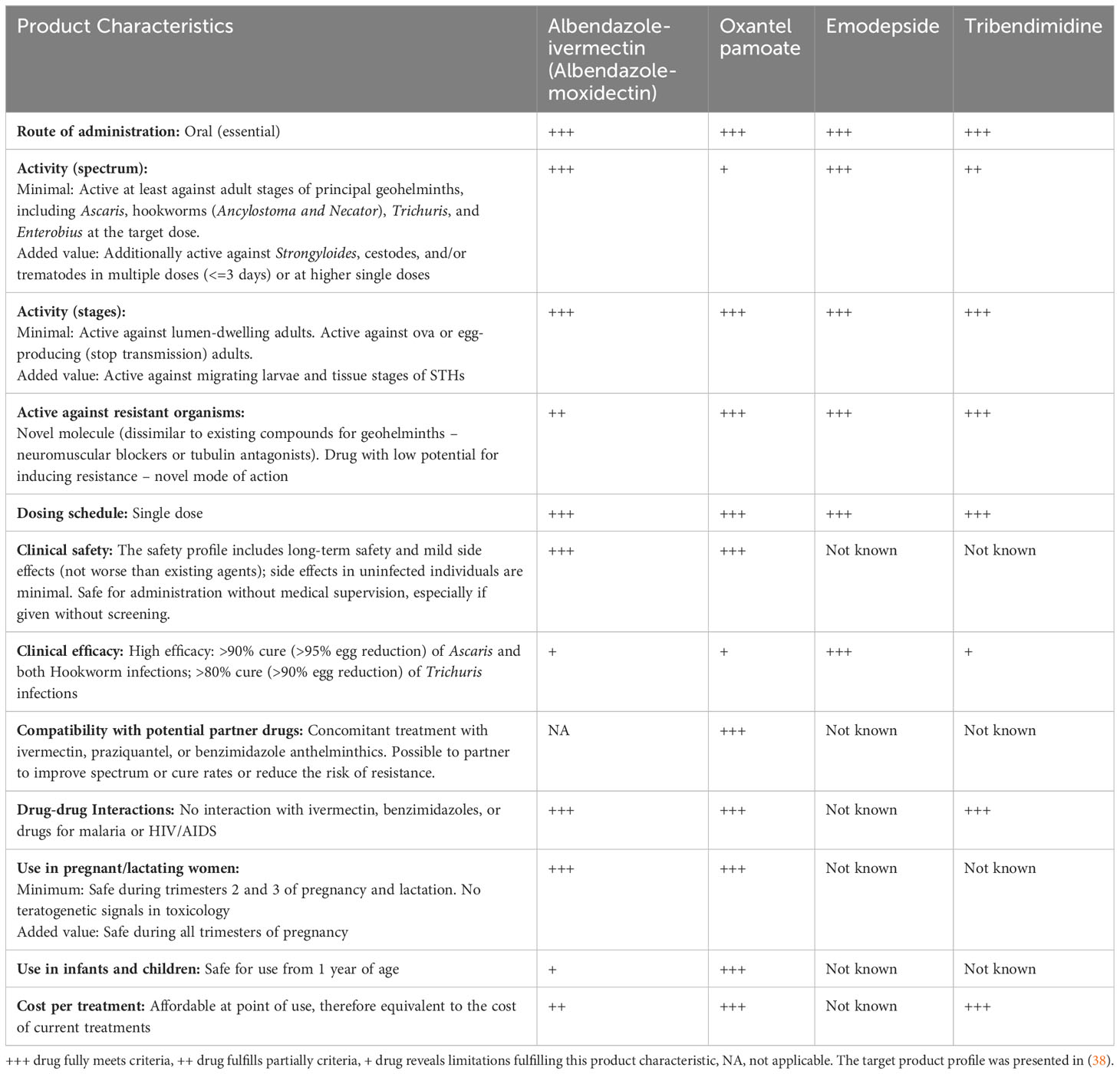
In Table 2, I have summarized how these two drug co-administrations would fulfill the suggested target product profile for soil-transmitted helminthiasis (38).
The essential characteristics of a novel drug against STH infections include a broad spectrum of activity that is safe for preventive chemotherapy programs, offers a simple dosing and ideally a single-dose treatment, reveals no cross-resistance to existing drugs, and is affordable (38).
The two combination treatments (albendazole-ivermectin and albendazole-moxidectin) have two drawbacks; first, the clinical efficacy in terms of cure rate is still below an ideal threshold of 80% against T. trichiura, yet the egg reduction rates, which are the main drivers for morbidity reduction, are high. Second, neither moxidectin nor ivermectin is currently approved for children. Efforts are ongoing to develop a child-friendly formulation of ivermectin (39). Pediatric studies with moxidectin in children from 4 to 11 years are also currently ongoing (https://clinicaltrials.gov/ct2/show/NCT03962062).
Other drug combinations have been researched in the recent past including pyrantel pamoate and oxantel pamoate, which will be discussed further below, as well as the Chinese anthelminthic tribendimidine (4). Several of the drug combinations showed highly promising results with high cure and egg reduction rates against STH infections. For example, a high cure rate of 84% was observed with a triple dose of albendazole, pyrantel pamoate, and oxantel pamoate against T. trichiura (40). Tribendimidine combined with ivermectin revealed a high efficacy against hookworm infections (41). However, as oxantel pamoate and tribendimidine are not marketed yet by stringent regulatory authorities, these combinations will not become available in the near future.
Advanced development candidates
Two drugs can be classified as late-stage candidates, namely, oxantel pamoate and emodepside, as they have already been tested in Phase II clinical trials against STH infections. Tribendimidine is a drug registered in the Chinese market but further studies would be required before the drug would obtain registration outside China.
Oxantel pamoate
Oxantel pamoate is a tetrahydropyrimidine derivative that has been marketed for veterinary use for several decades. Currently, oxantel pamoate is only approved and marketed for human use in some countries of South America and Asia for children from 6 months of age in combination with pyrantel pamoate (Quantrel®). Oxantel pamoate is currently being developed in the European Union-funded project “Establishment of a pan-nematode drug development pipeline”, Helminth Drug Development Platform (HELP, www.eliminateworms.org) in order to register the drug for the treatment of T. trichiura infections at a stringent regulatory authority (42). Oxantel pamoate has high efficacy against T. trichiura but low activity against A. lumbricoides and hookworm infections. Based on a recent network meta-analysis, a 20 mg/kg single dose of oxantel pamoate yields a cure rate of 76% (4). Details on the drug and the suggested clinical development plan for oxantel pamoate endorsed by Swissmedic have been summarized in a recent review (43). The HELP-funded activities include the development of a child-friendly formulation and a regulatory-compliant Phase I study comparing single administration versus single administration on three consecutive days. A two-week repeated-dose toxicity study including pharmacokinetics and local tolerability and reversibility of findings (if any) in in vitro and in vivo genotoxicity testing and one regulatory-compliant Phase III study in T. trichiura-positive patients are required for approval (43).
Emodepside
Emodepside, registered as a veterinary drug for the treatment of gastrointestinal helminths in dogs and cats, was already highlighted a decade ago as a potential drug candidate for STH infections (38). Emodepside belongs to the N-methylated cyclooctadepsipeptides and is a semisynthetic derivative of PF1022A, a fermentation product of a fungus (Rosellinia sp.), which is part of the microflora of the leaves of Camellia japonica (44). Preclinical studies confirmed that the drug has excellent activity against all major nematode species used in the laboratory, i.e., Trichuris muris, Ancylostoma ceylanicum, Necator americanus, and Strongyloides ratti, with a significantly higher activity than the currently recommended treatments (45). Studies using C. elegans and filarial nematodes revealed that the calcium- and voltage-activated potassium channel SLO-1 is the major receptor triggering emodepside’s action (44).
In 2014, Bayer and the Drugs for Neglected Diseases initiative (DNDi) started a collaboration to develop an emodepside for onchocerciasis. Phase I studies were completed and a Phase II study is currently ongoing (44). This development program allowed the conducting of two Phase IIa studies against T. trichiura and hookworm infections. In these studies, emodepside revealed high efficacy against T. trichiura and hookworm infections. At a dose of 15 mg, emodepside cured T. trichiura infections, while 25-30 mg achieved cure rates of 94 and 95%, respectively (10). The further development of emodepside against STH infections has been launched in partnership with Bayer AG.
Tribendimidine
Tribendimidine, inspired by the veterinary drug amidantel, is a drug discovered by Chinese researchers at the National Institute of Parasitic Diseases (NIPD) in Shanghai and developed by Xinhua Co., Ltd. in Shandong, China (46). Tribendimidine has a similar activity profile as albendazole with excellent activity against A. lumbricoides and hookworm infections (4). It also has remarkable activity against O. viverrini and C. sinensis infections (47). Efforts to develop it as a next-generation therapy for hookworm infections were launched with the aim of using the FDA’s Tropical Disease Priority Review Voucher (PRV) program (48) to fund the development. While this program has been a game changer for the development of several drugs (49), tribendimidine did not qualify for obtaining a PRV as the drug would not achieve superiority to the standard of care marketed in the US, i.e., six doses of mebendazole, which has a high efficacy against hookworm infection (18). The development of tribendimidine for registration at a stringent regulatory authority was therefore put on hold.
With regard to the TPP, oxantel and tribendimidine would fulfill many criteria, yet both drugs do not have a spectrum of activity against all STHs and hence would ideally be administered as combination chemotherapy. For emodepside, several characteristics such as affordability are unknown currently as the compound is under development.
Research on novel drugs for soil-transmitted helminth infections
Table 3 summarizes compounds that are in the discovery phase or have been researched against laboratory models of soil-transmitted helminth infections in the past 6 years (2017-2023). Reassuringly, efforts on STH R&D are taking place at different academic institutions. H. polygyrus is the most widely used model. Many innovative compounds was studied, ranging from a wide range of natural compounds to compounds already marketed for other diseases, hence, drug repurposing (67, 70). The most advanced compound entering preclinical studies is the Bacillus thuringiensis Cry5B, which shows a broad spectrum of anthelminthic activity in vitro and in vivo (78, 79).
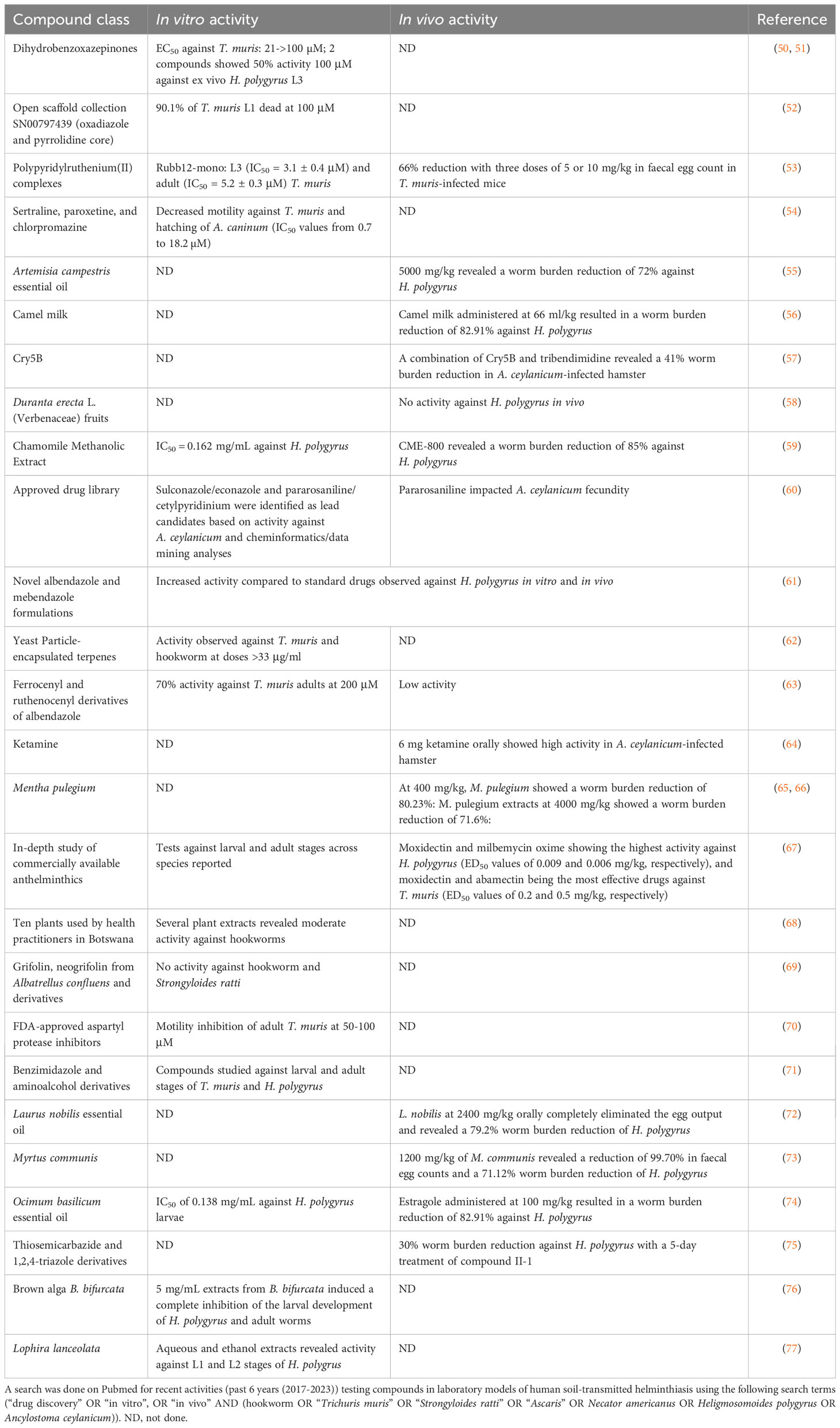
Table 3 Compounds studied in vitro and in vivo in laboratory models for soil-transmitted helminthiasis.
Conclusion
The past decade has witnessed significant efforts in developing alternative treatments for STH infections. Safe and effective treatments are pivotal to making progress towards the elimination of STH infections. A first milestone was reached when the first drug combination for STH infections albendazole-ivermectin was placed on the WHO essential medicine list in 2017. Yet, there is a need to understand the lack of responsiveness of this combination on T. trichiura in Côte d’Ivoire. It is not known whether this is a phenomenon unique to Côte d’Ivoire or common in other settings. Pilot efficacy testing is therefore necessary before countries plan to implement albendazole-ivermectin on a large scale as currently done in Uganda. Oxantel pamoate and emodepside are excellent late-stage candidates, which will hopefully increase the small armamentarium for the treatment of STH infections in the near future. While these drugs are nearing the finish line, access strategies should be carefully considered.
Author contributions
JK: Conceptualization, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by European Research Council (No. 101019223).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jourdan PM, Lamberton PHL, Fenwick A, Addiss DG. Soil-transmitted helminth infections. Lancet (2018) 391(10117):252–65. doi: 10.1016/S0140-6736(17)31930-X
2. Global Burden of Disease Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (2018) 392:1859–922. doi: 10.1016/S0140-6736(18)32335-3
3. Montresor A, Mupfasoni D, Mikhailov A, Mwinzi P, Lucianez A, Jamsheed M, et al. The global progress of soil-transmitted helminthiases control in 2020 and World Health Organization targets for 2030. PloS Negl Trop Dis (2020) 14:e0008505. doi: 10.1371/journal.pntd.0008505
4. Moser W, Schindler C, Keiser J. Drug combinations against soil-transmitted helminth infection. Adv Parasitol (2019) 103:91–115. doi: 10.1016/bs.apar.2018.08.002
6. Hürlimann E, Hofmann D, Keiser J. Ivermectin and moxidectin against soil-transmitted helminth infections. Trends Parasitol (2023) 39:272–84. doi: 10.1016/j.pt.2023.01.009
7. Moser W, Schindler C, Keiser J. Efficacy of recommended drugs against soil transmitted helminths: systematic review and network meta-analysis. BMJ (Clinical Res ed) (2017) 358:j4307. doi: 10.1136/bmj.j4307
8. Hürlimann E, Keller L, Patel C, Welsche S, Hattendorf J, Ali SM, et al. Efficacy and safety of co-administered ivermectin and albendazole in school-aged children and adults infected with Trichuris trichiura in Côte d'Ivoire, Laos, and Pemba Island, Tanzania: a double-blind, parallel-group, phase 3, randomised controlled trial. Lancet Infect Dis (2022) 22:123–35. doi: 10.1016/S1473-3099(21)00421-7
9. Welsche S, Mrimi EC, Hattendorf J, Hürlimann E, Ali SM, Keiser J. Efficacy and safety of moxidectin and albendazole compared with ivermectin and albendazole coadministration in adolescents infected with Trichuris trichiura in Tanzania: an open-label, non-inferiority, randomised, controlled, phase 2/3 trial. Lancet Infect Dis (2023) 23:331–40. doi: 10.1016/S1473-3099(22)00589-8
10. Mrimi EC, Welsche S, Ali SM, Hattendorf J, Keiser J. Emodepside for Trichuris trichiura and hookworm infection. New Engl J Med (2023) 388:1863–75. doi: 10.1056/NEJMoa2212825
11. Aschale Y, Abebaw A, Atnaf A, Mengist A, Kassie B, Yihunie W. Hookworm re-infection rate and efficacy of single-dose albendazole among pregnant women in Debre Elias District, Northwest Ethiopia: A single-arm trial. Trop Doctor (2022) 52:322–24. doi: 10.1177/00494755221080593
12. Hailu T, Abera B, Mulu W, Alemu M, Yizengaw E, Genanew A. Efficacy of single dose albendazole and praziquantel drugs among helminth-infected school children at Rural Bahir Dar, northwest Ethiopia. Trop Doctor (2018) 48:270–2. doi: 10.1177/0049475518786835
13. Vaz Nery S, Qi J, Llewellyn S, Clarke NE, Traub R, Gray DJ, et al. Use of quantitative PCR to assess the efficacy of albendazole against Necator americanus and Ascaris spp. in Manufahi District, Timor-Leste. Parasit Vectors (2018) 11:373. doi: 10.1186/s13071-018-2838-0
14. Vlaminck J, Cools P, Albonico M, Ame S, Ayana M, Cringoli G, et al. Therapeutic efficacy of albendazole against soil-transmitted helminthiasis in children measured by five diagnostic methods. PloS Negl Trop Dis (2019) 13:e0007471. doi: 10.1371/journal.pntd.0007471
15. Bezie W, Aemero M, Tegegne Y, Eshetu T, Addisu A, Birhanie M, et al. In vivo and in vitro efficacy of a single dose of albendazole against hookworm infection in northwest Ethiopia: open-label trial. Trop Med Health (2021) 49:25. doi: 10.1186/s41182-021-00308-0
16. Anto EJ, Nugraha SE. Efficacy of albendazole and mebendazole with or without levamisole for ascariasis and trichuriasis. Open Access Maced J Med Sci (2019) 7:1299–302. doi: 10.3889/oamjms.2019.299
17. Grau-Pujol B, Gandasegui J, Escola V, Marti-Soler H, Cambra-Pellejà M, Demontis M, et al. Single-nucleotide polymorphisms in the beta-tubulin gene and its relationship with treatment response to albendazole in human soil-transmitted helminths in Southern Mozambique. Am J Trop Med Hyg (2022) 107:649–57. doi: 10.4269/ajtmh.21-0948
18. Palmeirim MS, Ame SM, Ali SM, Hattendorf J, Keiser J. Efficacy and safety of a single dose versus a multiple dose regimen of mebendazole against hookworm infections in children: a randomised, double-blind trial. EClinicalMedicine (2018) 1:7–13. doi: 10.1016/j.eclinm.2018.06.004
19. Eshetu T, Aemero M, Zeleke AJ. Efficacy of a single dose versus a multiple dose regimen of Mebendazole against hookworm infections among school children: a randomized open-label trial. BMC Infect Dis (2020) 20:376. doi: 10.1186/s12879-020-05097-1
20. Zeleke AJ, Bayih AG, Afework S, Gilleard JS. Treatment efficacy and re-infection rates of soil-transmitted helminths following mebendazole treatment in schoolchildren, Northwest Ethiopia. Trop Med Health (2020) 48:90. doi: 10.1186/s41182-020-00282-z
21. Ejigu K, Hailu T, Alemu M. Efficacy of Mebendazole and Praziquantel against Soil-Transmitted Helminths and Schistosoma mansoni Infections among Schoolchildren in Northwest Ethiopia. BioMed Res Int (2021) 2021:6682418–6682418. doi: 10.1155/2021/6682418
22. Silber SA, Diro E, Workneh N, Mekonnen Z, Levecke B, Steinmann P, et al. Efficacy and safety of a single-dose mebendazole 500 mg chewable, rapidly-disintegrating tablet for Ascaris lumbricoides and Trichuris trichiura infection treatment in pediatric patients: a double-blind, randomized, placebo-controlled, phase 3 study. Am J Trop Med Hyg (2017) 97:1851–6. doi: 10.4269/ajtmh.17-0108
23. Palmeirim MS, Bosch F, Ame SM, Ali SM, Hattendorf J, Keiser J. Efficacy, safety and acceptability of a new chewable formulation versus the solid tablet of mebendazole against hookworm infections in children: An open-label, randomized controlled trial. EClinicalMedicine (2020) 27:100556. doi: 10.1016/j.eclinm.2020.100556
24. Sapulete EJJ, de Dwi Lingga Utama IMG, Sanjaya Putra IGN, Kanya Wati D, Arimbawa IM, Gustawan IW. Efficacy of albendazole-pyrantel pamoate compared to albendazole alone for Trichuris trichiura infection in children: A double blind randomised controlled trial. Malays J Med Sci (2020) 27:67–74. doi: 10.21315/mjms2020.27.3.7
25. Aribodor OB, Ekwunife CA, Sam-Wobo SO, Aribodor DN, Ejiofor OS, Ugwuanyi IK, et al. Status of intestinal helminth infection in schools implementing the home-grown school feeding program and the impact of the program on pupils in Anambra State, Nigeria. Acta Parasitol (2021) 66:1528–37. doi: 10.1007/s11686-021-00429-w
26. Buonfrate D, Salas-Coronas J, Muñoz J, Maruri BT, Rodari P, Castelli F, et al. Multiple-dose versus single-dose ivermectin for Strongyloides stercoralis infection (Strong Treat 1 to 4): a multicentre, open-label, phase 3, randomised controlled superiority trial. Lancet Infect Dis (2019) 19:1181–90. doi: 10.1016/S1473-3099(19)30289-0
27. Gandasegui J, Onwuchekwa C, Krolewiecki AJ, Doyle SR, Pullan RL, Enbiale W, et al. Ivermectin and albendazole coadministration: opportunities for strongyloidiasis control. Lancet Infect Dis (2022) 22:e341–7. doi: 10.1016/S1473-3099(22)00369-3
28. Palmeirim MS, Hürlimann E, Knopp S, Speich B, Belizario V Jr, Joseph SA, et al. Efficacy and safety of co-administered ivermectin plus albendazole for treating soil-transmitted helminths: A systematic review, meta-analysis and individual patient data analysis. PloS Negl Trop Dis (2018) 12(4):e0006458. doi: 10.1371/journal.pntd.0006458
29. Venkatesan A,R. Exploring the genetic differences among populations of Trichuris trichiura from Laos, Tanzania and Côte d'Ivoire with differing responses to albendazole-ivermectin treatment. Am J Trop Med Hyg (2021) 105:1376.
30. Schneeberger PHH, Gueuning M, Welsche S, Hürlimann E, Dommann J, Häberli C, et al. Different gut microbial communities correlate with efficacy of albendazole-ivermectin against soil-transmitted helminthiases. Nat Commun (2022) 13:1063. doi: 10.1038/s41467-022-28658-1
31. Muñoz J, Ballester MR, Antonijoan RM, Gich I, Rodríguez M, Colli E, et al. Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers. PloS Negl Trop Dis (2018) 12:e0006020. doi: 10.1371/journal.pntd.0006020
32. Algorta J, Krolewiecki A, Pinto F, Gold S, Muñoz J. Pharmacokinetic characterization and comparative bioavailability of an innovative orodispersible fixed-dose combination of ivermectin and albendazole: A single dose, open label, sequence randomized, crossover clinical trial in healthy volunteers. Front Pharmacol (2022) 13:914886. doi: 10.3389/fphar.2022.914886
33. Krolewiecki A, Enbiale W, Gandasegui J, van Lieshout L, Kepha S, Messa Junior A, et al. An adaptive phase II/III safety and efficacy randomized controlled trial of single day or three-day fixed-dose albendazole-ivermectin co-formulation versus albendazole for the treatment of Trichuris trichiura and other STH infections. ALIVE Trial Protocol (2022). [version 1; peer review: 2 approved]. Gates Open Research 6:62. doi: 10.12688/gatesopenres.13615.1
34. Barda B, Ame SM, Ali SM, Albonico M, Puchkov M, Huwyler J, et al. Efficacy and tolerability of moxidectin alone and in co-administration with albendazole and tribendimidine versus albendazole plus oxantel pamoate against Trichuris trichiura infections: a randomised, non-inferiority, single-blind trial. Lancet Infect Dis (2018) 18:864–73. doi: 10.1016/S1473-3099(18)30233-0
35. Keller L, Palmeirim MS, Ame SM, Ali SM, Puchkov M, Huwyler J, et al. Efficacy and safety of ascending dosages of moxidectin and moxidectin-albendazole against Trichuris trichiura in adolescents: a randomized controlled trial. Clin Infect Dis (2020) 70:1193–201. doi: 10.1093/cid/ciz326
36. Tan B, Opoku N, Attah SK, Awadzi K, Kuesel AC, Lazdins-Helds J, et al. Pharmacokinetics of oral moxidectin in individuals with Onchocerca volvulus infection. PloS Negl Trop Dis (2022) 16:e0010005. doi: 10.1371/journal.pntd.0010005
37. Hofmann D, Sayasone S, Sengngam K, Chongvilay B, Hattendorf J, Keiser J. Efficacy and safety of ascending doses of moxidectin against Strongyloides stercoralis infections in adults: a randomised, parallel-group, single-blinded, placebo-controlled, dose-ranging, phase 2a trial. Lancet Infect Dis (2021) 21:1151–60. doi: 10.1016/S1473-3099(20)30691-5
38. Olliaro P, Seiler J, Kuesel A, Horton J, Clark JN, Don R, et al. Potential drug development candidates for human soil-transmitted helminthiases. PloS Negl Trop Dis (2011) 5:e1138. doi: 10.1371/journal.pntd.0001138
39. Jittamala P, Monteiro W, Smit MR, Pedrique B, Specht S, Chaccour CJ, et al. A systematic review and an individual patient data meta-analysis of ivermectin use in children weighing less than fifteen kilograms: Is it time to reconsider the current contraindication? PloS Negl Trop Dis (2021) 15:e0009144. doi: 10.1371/journal.pntd.0009144
40. Moser W, Sayasone S, Xayavong S, Bounheuang B, Puchkov M, Huwyler J, et al. Efficacy and tolerability of triple drug therapy with albendazole, pyrantel pamoate, and oxantel pamoate compared with albendazole plus oxantel pamoate, pyrantel pamoate plus oxantel pamoate, and mebendazole plus pyrantel pamoate and oxantel pamoate against hookworm infections in school-aged children in Laos: a randomised, single-blind trial. Lancet Infect Dis (2018) 18:729–37. doi: 10.1016/S1473-3099(18)30220-2
41. Moser W, Coulibaly JT, Ali SM, Ame SM, Amour AK, Yapi RB, et al. Efficacy and safety of tribendimidine, tribendimidine plus ivermectin, tribendimidine plus oxantel pamoate, and albendazole plus oxantel pamoate against hookworm and concomitant soil-transmitted helminth infections in Tanzania and Côte d'Ivoire: a randomised, controlled, single-blinded, non-inferiority trial. Lancet Infect Dis (2017) 17:1162–71. doi: 10.1016/S1473-3099(17)30487-5
42. Specht S, Keiser J. Helminth infections: enabling the World Health Organization road map. Int J Parasitol (2023) 53:411–4. doi: 10.1016/j.ijpara.2022.10.006
43. Palmeirim MS, Specht S, Scandale I, Gander-Meisterernst I, Chabicovsky M, Keiser J. Preclinical and clinical characteristics of the trichuricidal drug oxantel pamoate and clinical development plans: A review. Drugs (2021) 81:907–21. doi: 10.1007/s40265-021-01505-1
44. Krücken J, Holden-Dye L, Keiser J, Prichard RK, Townson S, Makepeace BL, et al. Development of emodepside as a possible adulticidal treatment for human onchocerciasis-The fruit of a successful industrial-academic collaboration. PloS Pathog (2021) 17:e1009682. doi: 10.1371/journal.ppat.1009682
45. Karpstein T, Pasche V, Haberli C, Scandale I, Neodo A, Keiser J. Evaluation of emodepside in laboratory models of human intestinal nematode and schistosome infections. Parasit Vectors (2019) 12:226. doi: 10.1186/s13071-019-3476-x
46. Lv S, Ding W, Bergquist R, Yang G, Guo J, Zhou XN. Swiss-Chinese cooperation in tropical medicine: the role of professor Marcel Tanner. Diseases (2022) 10:83. doi: 10.3390/diseases10040083
47. Qian MB, Patel C, Palmeirim MS, Wang X, Schindler C, Utzinger J, et al. Efficacy of drugs against clonorchiasis and opisthorchiasis: a systematic review and network meta-analysis. Lancet Microbe (2022) 3:e616–24. doi: 10.1016/S2666-5247(22)00026-X
48. Aerts C, Barrenho E, Miraldo M, Sicuri E. The impact of the priority review voucher on research and development for tropical diseases. Pharmaceut Med (2022) 36:189–97. doi: 10.1007/s40290-022-00427-x
49. Berman J, Radhakrishna T. The tropical disease priority review voucher: A game-changer for tropical disease products. Am J Trop Med Hyg (2017) 96:11–3. doi: 10.4269/ajtmh.16-0099
50. Partridge FA, Murphy EA, Willis NJ, Bataille CJ, Forman R, Heyer-Chauhan N, et al. Dihydrobenz[e][1,4]oxazepin-2(3H)-ones, a new anthelmintic chemotype immobilising whipworm and reducing infectivity in vivo. PloS Negl Trop Dis (2017) 11:e0005359. doi: 10.1371/journal.pntd.0005359
51. Partridge FA, Bataille CJR, Forman R, Marriott AE, Forde-Thomas J, Häberli C, et al. Structural requirements for dihydrobenzoxazepinone anthelmintics: actions against medically important and model parasites: Trichuris muris, Brugia malayi, Heligmosomoides polygyrus, and Schistosoma mansoni. ACS Infect Dis (2021) 7:1260–74. doi: 10.1021/acsinfecdis.1c00025
52. Preston S, Jiao Y, Baell JB, Keiser J, Crawford S, Koehler AV, et al. Screening of the 'Open Scaffolds' collection from Compounds Australia identifies a new chemical entity with anthelmintic activities against different developmental stages of the barber's pole worm and other parasitic nematodes. Int J Parasitol Drugs Drug Resist (2017) 7:286–94. doi: 10.1016/j.ijpddr.2017.05.004
53. Sundaraneedi M, Eichenberger RM, Al-Hallaf R, Yang D, Sotillo J, Rajan S, et al. Polypyridylruthenium(II) complexes exert in vitro and in vivo nematocidal activity and show significant inhibition of parasite acetylcholinesterases. Int J Parasitol Drugs Drug Resist (2018) 8:1–7. doi: 10.1016/j.ijpddr.2017.11.005
54. Weeks JC, Roberts WM, Leasure C, Suzuki BM, Robinson KJ, Currey H, et al. Sertraline, paroxetine, and chlorpromazine are rapidly acting anthelmintic drugs capable of clinical repurposing. Sci Rep (2018) 8:975. doi: 10.1038/s41598-017-18457-w
55. Abidi A, Sebai E, Dhibi M, Alimi D, Rekik M, B'Chir F, et al. Chemical analyses and anthelmintic effects of Artemisia campestris essential oil. Vet Parasitol (2018) 263:59–65. doi: 10.1016/j.vetpar.2018.10.003
56. Alimi D, Abidi A, Sebai E, Rekik M, Maizels RM, Dhibi M, et al. In vivo nematicidal potential of camel milk on Heligmosomoides polygyrus gastro-intestinal nematode of rodents. Helminthologia (2018) 55:112–8. doi: 10.2478/helm-2018-0001
57. Hu Y, Miller M, Zhang B, Nguyen TT, Nielsen MK, Aroian RV. In vivo and in vitro studies of Cry5B and nicotinic acetylcholine receptor agonist anthelmintics reveal a powerful and unique combination therapy against intestinal nematode parasites. PloS Negl Trop Dis (2018) 12:e0006506. doi: 10.1371/journal.pntd.0006506
58. Udobi MI, Nzeakor TA, Eke IG, Ezeh IO, Onyeabor A, Idika IK, et al. Evaluation of the anthelminthic potential of Duranta erecta L. (Verbenaceae) fruits used in Nigerian ethnomedicine as a vermifuge. J Ethnopharmacol (2018) 216:57–62. doi: 10.1016/j.jep.2018.01.030
59. Hajaji S, Jabri MA, Alimi D, Rekik M, Akkari H. Chamomile methanolic extract mitigates small bowel inflammation and ROS overload related to the intestinal nematodes infection in mice. Acta Parasitol (2019) 64:152–61. doi: 10.2478/s11686-019-00027-x
60. Elfawal MA, Savinov SN, Aroian RV. Drug screening for discovery of broad-spectrum agents for soil-transmitted nematodes. Sci Rep (2019) 9:12347. doi: 10.1038/s41598-019-48720-1
61. Buchter V, Priotti J, Leonardi D, Lamas MC, Keiser J. Preparation, physicochemical characterization and in vitro and in vivo activity against Heligmosomoides polygyrus of novel oral formulations of albendazole and mebendazole. J Pharm Sci (2020) 109:1819–26. doi: 10.1016/j.xphs.2020.02.002
62. Mirza Z, Soto ER, Hu Y, Nguyen TT, Koch D, Aroian RV, et al. Anthelmintic activity of yeast particle-encapsulated terpenes. Molecules (2020) 25:2958. doi: 10.3390/molecules25132958
63. Lin Y, Ong YC, Keller S, Karges J, Bouchene R, Manoury E, et al. Synthesis, characterization and antiparasitic activity of organometallic derivatives of the anthelmintic drug albendazole. Dalton Trans (2020) 49:6616–26. doi: 10.1039/D0DT01107J
64. Ferreira SR, MaChado ART, Furtado LF, Gomes JHS, de Almeida RM, de Oliveira Mendes T, et al. Ketamine can be produced by Pochonia chlamydosporia: an old molecule and a new anthelmintic? Parasit Vectors (2020) 13:527. doi: 10.1186/s13071-020-04402-w
65. Sebai E, Serairi R, Saratsi K, Abidi A, Sendi N, Darghouth MA, et al. Hydro-ethanolic extract of mentha pulegium exhibit anthelmintic and antioxidant proprieties in vitro and in vivo. Acta Parasitol (2020) 65:375–87. doi: 10.2478/s11686-020-00169-3
66. Sebai E, Abidi A, Serairi R, Marzouki M, Saratsi K, Darghouth MA, et al. Essential oil of Mentha pulegium induces anthelmintic effects and reduces parasite-associated oxidative stress in rodent model. Exp Parasitol (2021) 225:108105. doi: 10.1016/j.exppara.2021.108105
67. Keiser J, Häberli C. Evaluation of commercially available anthelminthics in laboratory models of human intestinal nematode infections. ACS Infect Dis (2021) 7:1177–85. doi: 10.1021/acsinfecdis.0c00719
68. Dube M, Raphane B, Sethebe B, Seputhe N, Tiroyakgosi T, Imming P, et al. Medicinal plant preparations administered by Botswana traditional health practitioners for treatment of worm infections show anthelmintic activities. Plants (Basel) (2022) 11:2945. doi: 10.3390/plants11212945
69. Dube M, Llanes D, Saoud M, Rennert R, Imming P, Häberli C, et al. Albatrellus confluens (Alb. & schwein.) kotl. & pouz.: natural fungal compounds and synthetic derivatives with in vitro anthelmintic activities and antiproliferative effects against two human cancer cell lines. Molecules (2022) 27:2950. doi: 10.3390/molecules27092950
70. Beld L, Jung H, Bulman CA, Rosa BA, Fischer PU, Janetka JW, et al. Aspartyl protease inhibitors as anti-filarial drugs. Pathog (Basel Switzerland) (2022) 11:707. doi: 10.3390/pathogens11060707
71. Valderas-García E, Häberli C, Álvarez-Bardón M, Escala N, Castilla-Gómez de Agüero V, de la Vega J, et al. Benzimidazole and aminoalcohol derivatives show in vitro anthelmintic activity against Trichuris muris and Heligmosomoides polygyrus. Parasit Vectors (2022) 15:243. doi: 10.1186/s13071-022-05347-y
72. Sebai E, Abidi A, Benyedem H, Dhibi M, Hammemi I, Akkari H. Phytochemical profile and anthelmintic effects of Laurus nobilis essential oil against the ovine nematode Haemonchus contortus and the murine helminth model Heligmosomoides polygyrus. Vet Parasitol (2022) 312:109835. doi: 10.1016/j.vetpar.2022.109835
73. Sebai E, Abidi A, Serairi R, Ghawari B, Dhibi M, Benyedem H, et al. Assessment of anthelmintic potentials of Myrtus communis against Haemonchus contortus and Heligmosomoides polygyrus. Exp Parasitol (2022) 240:108320. doi: 10.1016/j.exppara.2022.108320
74. Alimi D, Hajri A, Jallouli S, Sebai H. Acaricidal and anthelmintic efficacy of Ocimum basilicum essential oil and its major constituents estragole and linalool, with insights on acetylcholinesterase inhibition. Vet Parasitol (2022) 309:109743. doi: 10.1016/j.vetpar.2022.109743
75. Kołodziej P, Wujec M, Doligalska M, Makuch-Kocka A, Khylyuk D, Bogucki J, et al. Synthesis and anthelmintic activity of novel thiosemicarbazide and 1,2,4-triazole derivatives: in vitro, in vivo, and in silico study. J Adv Res (2023) S2090-1232(23)00196-0. doi: 10.1016/j.jare.2023.07.004
76. Miclon M, Courtot É, Guégnard F, Lenhof O, Boudesocque-Delaye L, Matard-Mann M, et al. The brown alga bifurcaria bifurcata presents an anthelmintic activity on all developmental stages of the parasitic nematode Heligmosomoides polygyrus bakeri. Pathog (Basel Switzerland) (2023) 12:540. doi: 10.3390/pathogens12040540
77. Cédric Y, Christelle Nadia NA, Sylvain Raoul SN, Berinyuy S, Azizi MA, Jemimah Sandra TN, et al. Antihelminthic activity of Lophira Lanceolata on Heligmosomoides polygyrus using an automated high-throughput method. J Trop Med (2023) 2023:9504296. doi: 10.1155/2023/9504296
78. Li H, Abraham A, Gazzola D, Hu Y, Beamer G, Flanagan K, et al. Recombinant paraprobiotics as a new paradigm for treating gastrointestinal nematode parasites of humans. Antimicrob Agents Chemother (2021) 65:e01469–20. doi: 10.1128/AAC.01469-20
Keywords: soil-transmitted helminthiasis, drug development, anthelminthics, albendazole, mebendazole
Citation: Keiser J (2023) Present drugs and future perspectives in treating soil-transmitted helminthiasis. Front. Trop. Dis 4:1282725. doi: 10.3389/fitd.2023.1282725
Received: 24 August 2023; Accepted: 31 October 2023;
Published: 22 November 2023.
Edited by:
Alfonso J. Rodriguez-Morales, Fundacion Universitaria Autónoma de las Américas, ColombiaReviewed by:
Charles D. Mackenzie, Task Force for Global Health, United StatesGnatoulma Katawa, Université de Lomé, Togo
Copyright © 2023 Keiser. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer Keiser, amVubmlmZXIua2Vpc2VyQHN3aXNzdHBoLmNo
 Jennifer Keiser
Jennifer Keiser