- 1Clinical Operations, Centre de Recherches Médicales de Lambaréné, Lambaréné, Gabon
- 2Fondation pour la Recherche Scientifique, Cotonou, Benin
- 3Institute of Tropical Medicine, University of Tübingen, Tübingen, Germany
- 4African Society of Laboratory Medicine (ASLM), Kirkos Subcity Kebele, Addis Ababa, Ethiopia
- 5Department of Clinical Research, Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany
- 6Internal Department of Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
- 7Institute of Medical Research and Medicinal Plant Studies, Yaoundé, Cameroon
- 8Direction Générale, Nexakili, Paris, France
- 9Institut national de recherche biomédicale (INRB), Kinshasha, Democratic Republic of Congo
- 10Public Health Consultant, Kinshasa, Democratic Republic of Congo
- 11Centre pour le développement des vaccins, Bamako, Mali
- 12Cochrane South Center Africa, South African Medical Research Council, Cape Town and Stellenbosch Universities, Cape Town, South Africa
- 13University of Zambia-University College London Medical School (UNZA-UCLMS) Program, University Teaching Hospital (UTH), Lusaka, Zambia
- 14Division of Infection and Immunity, University College London; NIHR Biomedical Research Centre, University College London Hospitals, London, United Kingdom
- 15Comité National d´Ethique pour la Recherche du Gabon, Libreville, Gabon
- 16Epicentre, Medecins Sans Frontières (MSF), Yaoundé, Cameroon
- 17Fondation congolaise pour la recherche médicale, Brazzaville, Republic of Congo
Introduction
A clinical trial is intrinsically a collaborative undertaking. The complex steps, including identifying the biological mechanisms, discovering products, preclinical studies, and the clinical development from phase 1 to 3 clinical trials allowing market authorisation of a product, are unlikely to be feasible for a single institution or a country alone. Collaboration is therefore necessary to establish and maintain the research and innovation that is a prerequisite to tackle health threats, irrespective of the socioeconomic status of the countries (1).
Clinical trials undertaken in sub-Saharan Africa (SSA) are transnational partnerships, with a substantial involvement of partners being made from Global North scientists and institutions, with them often contributing as sponsors, funders, and investigators. The partnerships also involve a growing community of scientists based in SSA. These partnerships contribute to the international fundraising being done to help build the infrastructure required to perform the clinical trials locally and to support the emergence of skilled human resources necessary to run these types of trials (2–4). However, these efforts are still scattered and fragmented, and their social benefits are not easily perceivable (5). Instead, negative social perceptions often represent the concept of clinical trials and this may be due to poor awareness (6). In the context of SSA, social representations of clinical trials encompass exploiting people for the benefit of people living in wealthy countries of the Global North, the continuity of colonisation, and the promulgation of various complot theories and corruption (7).
SSA bears an enormous global burden of neglected infectious diseases (5), which lack effective, affordable, implementable preventive, and curative pharmaceutical measures (8).
As a coalition of scientists working in Africa, we emphasise the necessity of establishing sustainable clinical trial capacities across all African regions as a public norm. A public norm that will ensure the local ownership of clinical trial capacities and create context-relevant data to inform evidence-based decisions in public health (9).
The evidence-based decisions in public health: the current situation in sub-Saharan African countries
In several African countries, local research institutions design, register, conduct, and report at least one clinical trial annually. The ethics and regulatory environment around these institutions is gradually strengthening (Figure 1). In most countries, the absence of a stringent legal framework governing all the necessary aspects of clinical trials delays or impedes authorisation and the appropriate regulation of the clinical trials. In turn, timely data acquisition by clinical trials is jeopardised, thus leading to poor decision-making in public health (10).
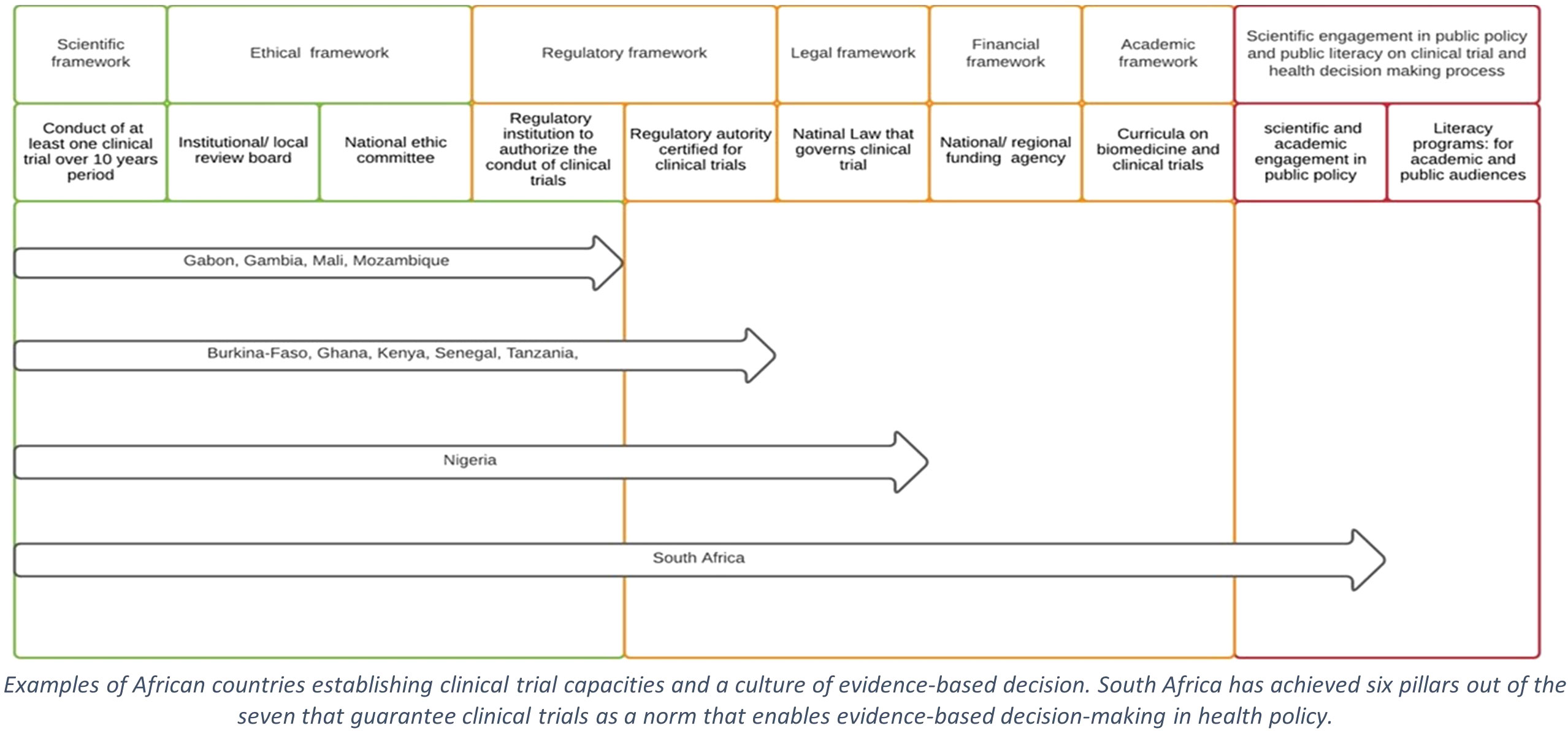
Figure 1 Steps supporting clinical trial as public norm in sub-Saharan Africa. Examples of African countries establishing clinical trial capacities and a culture of evidence-based decision. South Africa has achieved six pillars out of the seven that guarantee clinical trials as a norm that enables evidence-based decision-making in health policy.
Beyond the lack of a legal framework, the requirement of evidence to support decision-making in public health needs to be more consistently shared among the ethics and regulatory decision-makers (10). The poor literacy about the purposes, objectives, procedures, and values of clinical trials is noticeable among the ethics and regulatory decision-makers, decision-makers of national health control programmes, political authorities, and the general public. A weakness that maintains a poor level of evidence-based information among the population drives social misperceptions and results in the rejection of clinical trials in public opinion (11).
An important recent example of such a vicious circle is the debate about evaluating the SARS-CoV-2 vaccine candidates in Africa. In mid-2020, multiple statements from the general public and the political authorities of the African countries claiming to protect populations against the exploitation by the Global North countries spread resentment against the COVID-19 vaccine trials (11, 12). The line of thought was that the vaccines were being trialled in African people for the sole benefit of Global North populations and pharmaceutical companies. This perception led to an essential delay in generating data about the vaccines’ safety, immunogenicity, and efficacy in the epidemiological contexts of Africa, thus in turn severely impeding an evidence-based public health response. An informed discourse based on basic knowledge about the ethics and regulatory requirements for clinical trials in Europe, the USA, and Japan would have provided reassurance that investigational products exclusively tested in Africa would not reach the European and American markets without further evaluation in their local populations. This would have supported the notion that a COVID-19 vaccine trialled in Africa primarily helps public health decisions for African people (13).
South Africa (SA) is arguably the most advanced SSA country in biomedical research, promoting clinical trials as the norm, which in turn supports evidence-based decision-making in public health (14). SA has conducted several clinical trials for various COVID-19 vaccine candidates developed by Pfizer-BioNtech, AstraZeneca, J&J, Novavax, ReiThera, and ImmunityBio (15). Importantly, data from South Africa indicated a sub-optimal protective efficacy of the AstraZeneca vaccine against symptomatic moderate cases following infection in the local population where the beta SARS-CoV-2 variant of concern had become predominant (16). Based on these data, the country decided not to proceed with the rollout of this vaccine.
In contrast to the initial reluctance to participate in the COVID-19 vaccine trials, several African countries have subsequently opted to deploy any available vaccine, even with no or only a paucity of evidence available from clinical trials. Sputnik V, Sinovac, and Covaxin vaccines have been rolled out, but not evaluated, in most SSA populations (15). Coordination of clinical trials across several African countries would have generated locally relevant evidence, reducing uncertainty and guiding decisions for the public use of COVID-19 vaccines. This clearly demonstrates how the vicious cycle of inconsistent knowledge about clinical trials by decision-makers and opinion leaders in African societies results in the misperception of interventional research for the benefit of local populations, which in turn leads to an incomplete evidence base to inform public health decision-making on the African continent. However, the COVID-19 vaccine hesitancy is a global move that needs to be carefully and continuously addressed by reassuring people about their fears about the possibility of infertility, the genetic modification of the mRNA-based vaccines, and any other safety concerns.
A call for action: establishing clinical trials as a public norm
Aligning the field of clinical trials with the policy system is essential to engage the general public and ensure consistent, evidence-based messaging from scientists and public health authorities. In most African countries, the scientific and public policy systems work in parallel and may only have infrequent interactions.
The following steps will contribute to a shared culture of evidence-based decisions in public health, thus providing the critical link between science, the policy system, and the general public.
1 A national legal framework for clinical trials and evidence-based decisions in public health
The juridical apparatus and rules will enforce regulations for the sponsorship, conduct, reporting, and ownership of clinical trials initiated locally or in transnational collaborations. A national law on clinical trials must clarify and contextualise international guidelines, such as the International Conference on Harmonization for Good Clinical Practice (ICH-GCP) guidelines, thus binding, for example, the content of informed consent forms, the age of the assent procedures to specific socioeconomic and cultural contexts. Another essential point to be covered legally is to link public health decisions to context-specific data generated from clinical trials. A national clinical trial law will facilitate the creation, strengthening, and functioning of in-country funding agencies that collect public funds and private donations for basic research, drugs and vaccine discovery, and clinical trial programmes against endemic and emerging diseases. The national or regional funding will add to, and potentially strengthen, the international commitment and funding opportunities.
2 Scientific and academic commitment to the policy system and for public literacy for a shared culture of evidence-based decisions in public health
A cultural shift from intuition-based decision-making towards a system of evidence-based decision-making is essential. The research and academic institutions must establish professorships and develop postgraduate training curricula, thus galvanising the education of future decision-makers and public literacy about clinical research. These curricula would include basic literacy in health-based clinical trials, evidence-based medicine, and evidence-based decision-making in public health. They must be available for citizens through secondary schools, universities, and postgraduate educational institutions, irrespective of the subjects of expertise, thus disseminating education and cultural change to all civil society members who will be the next generation of decision-makers. Advanced courses in the selected topics above may become compulsory for the curricula of those who graduate in biology, science, and health-related subjects, as well as high-level public administration and politics schools. Customised programmes jointly developed by the research institutions and experts in communication would enhance public literacy on health, biomedical research, clinical trials, and evidence-based knowledge. Literacy will reduce the negative impact of fake news and anti-science information spilling through social media. Public literacy will enforce and facilitate understanding of research activities, secure the commitment of decision-makers and citizens in clinical trials, and support evidence-based decisions.
3 The ownership of clinical trials by sub-Saharan African countries: generation of evidence-based data, the availability of clinical trial goods, and African drug and vaccine candidates
Whether transnational or locally initiated, a clinical trial that contributes effectively to building the evidence for public health decisions adds to the country’s social wealth, thus ensuring some level of ownership of clinical trials. However, the issue of the availability of drugs, vaccines, or any other health goods after clinical development for the populations and countries involved in the trials is equally important as the valuable data generated. The lack of genuine African investigational products in the pipeline of worldwide drug and vaccine candidates is the last major component of the ownership of clinical trials.
Following an extensive clinical development programme, which ran from from 1997 to 2021, including a phase 1 trial in adults, a comprehensive phase 2 clinical trial programme, and the largest phase 3 clinical trial ever conducted in African children in approximately 10 SSA countries, the RTS,S/AS01 malaria vaccine was scientifically recommended in 2015 by the European Medical Agency. An implementation study, which assessed the vaccine’s effectiveness in the real world from 2017, led to the prequalification of RTS,S/AS01 by the WHO in 2021 and a recommendation for its use as a preventive measure against Plasmodium falciparum malaria among children living in endemic areas. The clinical development of RTS,S/AS01 was instrumental in augmenting several SSA countries’ capacities to set up and maintain clinical research teams and institutions. It also provided the evidence for data specific to countries, malaria transmission intensity, age-specific responses, and the responses when co-administered with other childhood vaccines. However, the RTS,S/AS01 vaccine doses will likely not be sufficient to cover the needs of malaria control programmes of the countries involved in the vaccine’s clinical development. The limited production capacities of the pharmaceutical company GlaxoSmithKline Biologicals (GSK), the controversies among the international scientific community against or in favour about the potential benefits of the vaccine publicly, the lack of African scientists’ voice endorsing its use or not, and the absence of commitment of the African states to invest in implementing the RTS,S/AS01 vaccine through the national control programmes are among the other major causes that impede the availability of a public health good, which is licensed after having been widely tested among SSA populations. After the implementation studies, eligible countries were invited to apply for Gavi support in January and April 2023. However, because it will take time for the vaccine’s manufacturer, GSK, to scale up production, doses are expected to initially target populations in areas of greatest need. By 2028, vaccine production will still fall short of the demand, so other malaria vaccines are needed to fill the gap (17).
A timely and continuing debate among scientists, product owners, and political authorities must address this issue during the preparatory steps and along with the clinical development of a public health good.
On 12 and 13 April 2021, the African Union and the Africa Centres for Disease Control and Prevention (Africa CDC) convened a conference on African vaccine manufacturing, where African Heads of State expressed a high-level of commitment towards the promotion of domestic manufacturing of vaccines and other health commodities, including the promotion of research and development for improved health security on the continent. This political commitment can be achieved only in synergy with a cultural shift to evidence-based decision-making. The Platform for Harmonized African Health Products Manufacturing (PHAHM) Ministerial Working Group was launched in October 2023 during the first manufacturers’ marketplace for vaccine manufacture in the African Union Member States (18). If the capacities for the manufacture of drugs and vaccines in SSA countries are developed and expanded, they will be mid- to long-term and sustainable solutions to make new public health goods available for populations. They will also certainly boost the discovery of investigational products by African scientists and institutions. Having several drug and vaccine candidates from African institutions and scientists being evaluated in countries outside Africa through a global clinical development will certainly strengthen the literacy of the African public and trust about clinical trials and in turn improve evidence-based decision-making in public health.
Intermediate initiatives such as the African Congress of Clinical Trials, the African Society of Clinical Trials, the clinical trial community, and the African Consortium for Cancer Clinical Trials are instrumental in building the continuing number of encounters and transparency between scientists, decision-makers, and citizens for the advocacy to engage politicians’, policymakers’, and the private sector’s commitment to invest in biomedical research.
The move towards evidence-based decisions and information is a prerequisite for African countries to build genuine, land-efficient solutions, including international collaborations, and the study of the local pharmacopoeia to develop a pharmaceutical sector capable of tackling the intolerable burden of diseases and thus significantly contributing to socioeconomic prosperity.
Author contributions
SA: Conceptualization, Writing – original draft, Writing – review & editing. ML: Writing – original draft, Writing – review & editing. AM: Writing – original draft, Writing – review & editing. FM: Writing – original draft, Writing – review & editing. PE: Writing – review & editing. GM-N: Writing – review & editing. RL: Writing – review & editing. YM: Writing – review & editing. J-JM-T: Writing – review & editing. J-MO-B: Writing – review & editing. SS: Writing – review & editing. CW: Writing – review & editing. AZ: Writing – review & editing. AA: Writing – review & editing. MR: Writing – review & editing. PK: Writing – review & editing. P-BM: Writing – review & editing. YB: Writing – review & editing. FN: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. SA is a senior fellow of the European Developing Countries Clinical Trial Partnership and supported by the grant TMA2017SF-1946.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed are those of the authors and should not be construed to represent the positions of the Comité National d’Ethique pour la Recherche au Gabon.
References
1. Dsouza VS, Kurian JR, Cauvery K, Leyens L, Pattanshetty S, Brand H. Collaborative clinical trials on infectious disease among the G20 nations using scientometric analysis. Perspect Clin Res (2023) 14(4):211–2. doi: 10.4103/picr.picr_242_22
2. Matee MI, Manyando C, Ndumbe PM, Corrah T, Jaoko WG, Kitua AY, et al. European and Developing Countries Clinical Trials Partnership (EDCTP): the path towards a true partnership. BMC Public Health (2009) 9(1):249. doi: 10.1186/1471-2458-9-249
3. Miiro GM, Ouwe Missi Oukem-Boyer O, Sarr O, Rahmani M, Ntoumi F, Dheda K, et al. EDCTP regional networks of excellence: initial merits for planned clinical trials in Africa. BMC Public Health (2013) 13(1):258. doi: 10.1186/1471-2458-13-258
4. Zumla A, Benn CS, Bockarie M, Grewal HMS, Ntoumi F, Gyapong J. Evolution of a strategic, transformative Europe-Africa Global Health partnership-EDCTP3. Lancet Infect Dis (2023) 24(1):16–8. doi: 10.1016/S1473-3099(23)00737-5
5. Franzen SRP, Chandler C, Lang T. Health research capacity development in low and middle income countries: reality or rhetoric? A systematic meta-narrative review of the qualitative literature. BMJ Open (2017) 7(1):e012332. doi: 10.1136/bmjopen-2016-012332
6. Tobiasz-Adamczyk B. Clinical trials from the perspective of the sociology of medicine. Zdrowie Publiczne i Zarządzanie (2018) 16(1):26–34.
7. Egharevba E, Atkinson J. The role of corruption and unethical behaviour in precluding the placement of industry sponsored clinical trials in sub-Saharan Africa: Stakeholder views. Contemp Clin trials Commun (2016) 3:102–10. doi: 10.1016/j.conctc.2016.04.009
8. De Rycker M, Baragaña B, Duce SL, Gilbert IH. Challenges and recent progress in drug discovery for tropical diseases. Nature (2018) 559(7715):498–506. doi: 10.1038/s41586-018-0327-4
9. De Rycker M, Baragaña B, Duce SL, Gilbert IH. Global coalition to accelerate COVID-19 clinical research in resource-limited settings. Lancet (2020) 395(10233):1322–5. doi: 10.1038/s41586-018-0327-4
10. Alemayehu C, Mitchell G, Nikles J. Barriers for conducting clinical trials in developing countries- a systematic review. Int J Equity Health (2018) 17(1):37. doi: 10.1186/s12939-018-0748-6
11. Flint K. Africa isn’t a testing lab”: considering COVID vaccine trials in a history of biomedical experimentation and abuse. J West Afr History (2020) 6(2):126–40. doi: 10.14321/jwestafrihist.6.2.0126
12. Samarasekera U. Feelings towards COVID-19 vaccination in Africa. Lancet Infect Dis (2021) 21(3):324–. doi: 10.1016/S1473-3099(21)00082-7
13. Singh JA. The case for why Africa should host COVID-19 candidate vaccine trials. J Infect Dis (2020) 222(3):351–5. doi: 10.1093/infdis/jiaa303
14. Makoni M. COVID-19 vaccine trials in Africa. Lancet Respir Med (2020) 8(11):e79–80. doi: 10.1016/S2213-2600(20)30401-X
15. Massinga Loembé M, Nkengasong JN. COVID-19 vaccine access in Africa: Global distribution, vaccine platforms, and challenges ahead. Immunity (2021) 54(7):1353–62.
16. Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. New Engl J Med (2021) 384(20):1885–98. doi: 10.1056/NEJMoa2102214
Keywords: clinical trial as a public norm, sub-Saharan Africa, evidence-based decision-making in public health, clinical trial literacy, clinical trial law, international collaborations
Citation: Agnandji ST, Loembe MM, Mbouna AV, Mbadinga F, Essone PN, Mombo-Ngoma G, Leke R, Mburu YK, Muyembe-Tamfum J-J, Okwo-Bele J-M, Sow S, Wiysonge CS, Zumla A, Adegnika AA, Ramharter M, Kremsner PG, Matsiegui P-B, Boum Y and Ntoumi F (2024) Making clinical trials a public norm for health decisions in sub-Saharan Africa. Front. Trop. Dis 4:1297109. doi: 10.3389/fitd.2023.1297109
Received: 19 September 2023; Accepted: 19 December 2023;
Published: 11 January 2024.
Edited by:
Andrea Haselbeck, International Vaccine Institute, Republic of KoreaReviewed by:
Jesse Gitaka, Mount Kenya University, KenyaCopyright © 2024 Agnandji, Loembe, Mbouna, Mbadinga, Essone, Mombo-Ngoma, Leke, Mburu, Muyembe-Tamfum, Okwo-Bele, Sow, Wiysonge, Zumla, Adegnika, Ramharter, Kremsner, Matsiegui, Boum and Ntoumi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Selidji T. Agnandji, YWduYW5kamlzQGNlcm1lbC5vcmc=
 Selidji T. Agnandji
Selidji T. Agnandji Marguerite Massinga Loembe
Marguerite Massinga Loembe Armel V. Mbouna
Armel V. Mbouna Fallowne Mbadinga1
Fallowne Mbadinga1 Paulin N. Essone
Paulin N. Essone Ghyslain Mombo-Ngoma
Ghyslain Mombo-Ngoma Charles Shey Wiysonge
Charles Shey Wiysonge Alimuddin Zumla
Alimuddin Zumla Ayola A. Adegnika
Ayola A. Adegnika Peter G. Kremsner
Peter G. Kremsner Francine Ntoumi
Francine Ntoumi