- 1Ethiopian Pharmaceuticals Supply Agency (EPSA), Addis Ababa, Ethiopia
- 2School of Pharmacy, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
- 3Department of Medical Parasitology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 4Department of Epidemiology and Biostatistics, Institute of Public Health, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
- 5Department of Environmental and Occupational Health and Safety, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Background: Visceral leishmaniasis (VL) remains a major public health challenge in East Africa, particularly in Ethiopia, where poor treatment outcomes contribute to high mortality rates in resource-limited settings. This study aimed to evaluate treatment outcomes and to identify factors associated with these outcomes among pediatric patients with VL at the University of Gondar Comprehensive Specialized Hospital.
Methods: A retrospective study was conducted among pediatric patients with VL admitted to the pediatric hospital ward between September 2013 and August 2019. Patient records were reviewed to collect data on demographics, clinical presentations, treatment regimens, and outcomes. Data were entered into Epi-Info version 7.2 and subsequently analyzed using SPSS version 20. Logistic regression was employed to identify factors associated with treatment outcomes, with a statistical significance threshold set at p< 0.05.
Results: Of the 222 pediatric admissions for VL, 200 complete records were included in the analysis. A clinical cure was achieved in 77.5% of patients. Poor treatment outcomes were observed in 22.5% of cases, which included a partial response in 15%, death in 5%, and relapse in 2.5%. Children under 5 years of age had an 80% lower likelihood of clinical cure compared to those aged 11–15 years (AOR 0.2; 95% CI: 0.04–0.6). Additionally, patients from rural areas exhibited a cure rate four times higher than their urban counterparts (AOR, 4; 95% CI: 2–11). Those hospitalized for 11–20 days had a four-fold increased chance of cure compared to those hospitalized for more than 21 days (AOR, 4; 95% CI: 1–16). Immunocompetent children were three times more likely to be cured than immunocompromised children (AOR, 3; 95% CI: 1–9). Furthermore, adherence to international treatment guidelines correlated with a 26-fold increase in cure rates compared to local guidelines (AOR, 26; 95% CI: 8–82).
Conclusion: The findings indicate a high rate of clinical cure among pediatric patients with VL, underscoring the importance of understanding the factors that influence treatment success. Targeted healthcare interventions addressing these determinants may enhance cure rates and improve the overall management of pediatric patients with VL.
1 Introduction
Visceral leishmaniasis (VL) is a neglected tropical disease caused by protozoan parasites of the Leishmania donovani complex, transmitted through the bite of infected female sandflies of the genera Phlebotomus (Old World) and Lutzomyia (New World) (1). VL is endemic in over 98 countries and territories, predominantly affecting developing regions, including 72 developing countries and 13 least developed countries (2). VL is particularly deadly if undiagnosed and untreated, and it disproportionately affects impoverished communities in regions such as India, Brazil, and East Africa (3). Globally, over 90% of VL cases occur in six countries, including Ethiopia, which accounts for an estimated 4,500 to 5,000 cases annually and places over 3.2 million people at risk (4–6). VL can be classified as anthroponotic visceral leishmaniasis (AVL) or zoonotic visceral leishmaniasis (ZVL), which is transmitted between humans via vector carriers. It is primarily caused by L. donovani throughout East Africa and the Middle East, particularly Sudan, Somalia, Yemen, and Saudi Arabia [3]. Children under 15 account for approximately 65% of VL cases in East Africa, highlighting the vulnerability of this age group (7). The disease primarily affects impoverished populations in Africa, Asia, and Latin America due to factors such as malnutrition, population displacement, poor housing, weak immune systems, and lack of resources (8). In endemic areas like Ethiopia, the migration of non-immune workers into these regions further contributes to the epidemiology of VL (9, 10). VL and malnutrition share a bidirectional relationship; high nutritional status favors disease alleviation, while malnourished people are at the highest risk of contracting VL and undergoing early vascularization (11–14). Furthermore, HIV infection increases the risk of developing visceral leishmaniasis (VL) by a factor of 100 to 2,320 in endemic areas (15). It also reduces the likelihood of successful treatment outcomes and greatly increases the risk of relapse (16). Ethiopia has the highest number of VL cases in sub-Saharan Africa, followed by Sudan, with over 2,000–4,500 VL cases recorded annually. More than 85% of the cases were from the Amhara and Tigray regions. It was found to be far more devastating in Northern Ethiopia, especially in the districts of Libokemkem, Metema, and Humera (17, 18). In Northwest Ethiopia, the pooled prevalence of HIV infection among individuals with VL was reported to be 20.88% (19). This comorbidity leads to delayed diagnosis and is often accompanied by notable biochemical abnormalities, such as elevated serum AST, ALT, total bilirubin, albumin, and total protein levels, which significantly disrupt liver function and contribute to disease complications (20). Adding to these challenges is the problem of growing drug resistance, which negatively affects VL treatment in pediatric patients and may result in severe complications or even death (9, 21, 22).
According to the 2016 guidelines of the Infectious Diseases Society of America (IDSA), first-line treatments for VL include liposomal amphotericin B (L-Amb), pentavalent antimonials such as sodium stibogluconate (SSG) and meglumine antimoniate (MA), and miltefosine. Second-line agents include amphotericin-B deoxycholate and paromomycin (PM). Additionally, in some cases, combination therapies such as L-Amb with miltefosine may enhance therapeutic effectiveness (23). The World Health Organization (WHO) has recommended SSG plus PM as the first-line treatment for VL in East Africa since 2010. These treatment guidelines also specified that first-line regimens for primary VL consist of SSG and PM, SSG or MA (monotherapy), and L-Amb, while the second-line treatment for primary VL consists of L-Amb, PM, and Miltefosine (24, 25). L-Amb is often the preferred treatment for immunocompromised patients; however, its use is limited by cost and the need for inpatient administration (23, 26–29). Combination therapies such as SSG and PM are being explored to improve efficacy and reduce drug resistance (23, 27, 30). Despite the burden of VL, particularly among children, there is limited information regarding treatment outcomes such as cure rates, partial responses, mortality, and relapse, especially in the context of long-term follow-up (31–33). Addressing this gap could improve our understanding of how different variables impact treatment efficacy and patient prognosis. Ultimately, these findings may guide healthcare providers in optimizing treatment protocols and enhancing overall patient care, thereby reducing morbidity and mortality associated with VL in pediatric patients.
2 Materials and methods
2.1 Study design, period, and area
A retrospective analysis was conducted on pediatric patients with VL admitted to the University of Gondar Comprehensive Specialized Hospital (UOGCSH) pediatric ward from 2013 to 2019. UOGCSH is a government referral hospital that serves a population of over 5 million and is equipped with specialized wards, including a pediatric intensive care unit, and provides inpatient treatment to pediatric patients with VL. The Leishmaniasis Research and Treatment Center (LRTC), established in 2004 by the Drugs for Neglected Diseases Initiative (DNDi), is integral to the diagnosis and treatment of VL. The center has 24 inpatient beds and treats over 300 patients with VL annually, utilizing diagnostic methods such as microscopy, dried direct agglutination test, polymerase chain reaction, culture, and the rk39 immunochromatographic test. In this study, VL diagnosis was confirmed through specific tests, emphasizing the importance of understanding whether all patients had parasitological confirmations or if alternative criteria were used. Accurate records of diagnostic results were maintained through both computer systems and manual logbooks, which were crucial for assessing treatment outcomes (24, 32) (Figure 1).
2.2 Study population
All pediatric patients (≤15 years) diagnosed with VL at the UOGCSH between September 2013 and August 2019.
2.3 Inclusion criteria
All pediatric patients aged 15 years or younger who were diagnosed with VL and admitted to the pediatric ward of UOGCSH between September 2013 and August 2019 were included in the study.
2.4 Exclusion criteria
Patients with incomplete or ambiguous sociodemographic data or unclear treatment outcomes (e.g., cure or failure) were excluded from the study.
2.5 Sample size determination and sampling techniques
The sample size was calculated by using a single population proportion formula i.e.
Where n= the required sample size for the study.
D2= the margin of tolerable sampling error, which is 0.05.
Z = confidence interval, the most common one is 1.96 for 95%.
p= proportion of prevalence of treatment outcome, which was unknown in Ethiopia, so 50% was used.
Given that the total population of pediatric patients with VL was limited, the sample size was adjusted using the finite population correction formula to gain the minimum sample size.
2.6 Operational definitions
Our operational definitions were based on the IDSA/ASTMH and WHO Guidelines (23, 24).
Clinical Cure: The absence of clinical features of VL after completing the full course of the recommended treatment, confirmed by clinical assessment or negative parasitological test. Parasitological confirmation was through both microscopic and serological diagnostic methods, specifically using the rK39 test and spleen biopsy.
Treatment Failure: Defined as partial response, relapse, death, or documented nonadherence during the initial course of VL treatment.
Partial response: Failure to fully resolve clinical signs despite receiving antileishmanial treatment, including incomplete therapeutic adherence.
Non-adherence to treatment: This refers to a patient’s failure to follow the prescribed treatment plan, including medications, lifestyle changes, or other recommendations from a healthcare provider.
Relapse: This refers to the reappearance of VL signs and symptoms after the initial clinical improvement and discharge, requiring readmission (27).
Nutritional status: Pediatrics categorized as well-nourished, mildly malnourished, or severely malnourished based on clinical assessment.
Immunity status: Pediatric patients with documented diagnoses of HIV, Tuberculosis, malnutrition, cancer, malaria, pneumonia, or sepsis.
Intravenous (IV) injections: Administration of drug to pediatric patients either by using IV fluids such as normal saline 0.9%, dextrose 5% in water, or Intramuscular injections once daily.
Treatment Guideline adherence: Adherence to either the 2016 clinical practice guidelines from the IDSA (23) or the WHO guideline for the management of VL, established in 2013 (24). The 2016 IDSA guidelines recommend treating VL with L-Amb at 3–5 mg/kg for 5–7 days. In contrast, the 2013 WHO guidelines suggest either a combination of SSG at 20 mg/kg and PM at 15 mg/kg daily for 21 days, or SSG alone at the same dosage for the same duration. These differences highlight the importance of following evidence-based international protocols for improved treatment outcomes.
2.7 Study variables
2.7.1 Dependent variables
Treatment outcome (cure or failure)
2.7.2 Independent variables
Age, sex, residence, comorbidities, history of hospitalization, time elapsed before diagnosis, antileishmanial treatment regimen, nutritional status, immunity status, adherence to guidelines, and methods of drug administration
2.8 Sampling procedure
Data on VL treatment outcomes from 2013 to 2019 were compiled from the pediatric ward of the UOGCSH. A chart review was conducted to gather information on patients diagnosed with VL during this period. Laboratory personnel or physicians confirmed positive cases using both microscopic and serological diagnostic methods, based on the patients’ signs and symptoms. The collected data included patient demographics such as age, sex, year of examination, and residency, as well as clinical information including blood film results, serological results, comorbid conditions, history of hospitalization, time elapsed prior to diagnosis, antileishmanial treatment received, immunity status, adherence to treatment guidelines, methods of drug administration, and treatment outcomes (cure or failure). Records with incomplete or ambiguous sociodemographic information or treatment outcomes were excluded from the analysis. A total of 222 patient charts were initially reviewed; however, 22 were excluded due to noncompliance with the inclusion criteria or loss to follow-up (Figure 2).
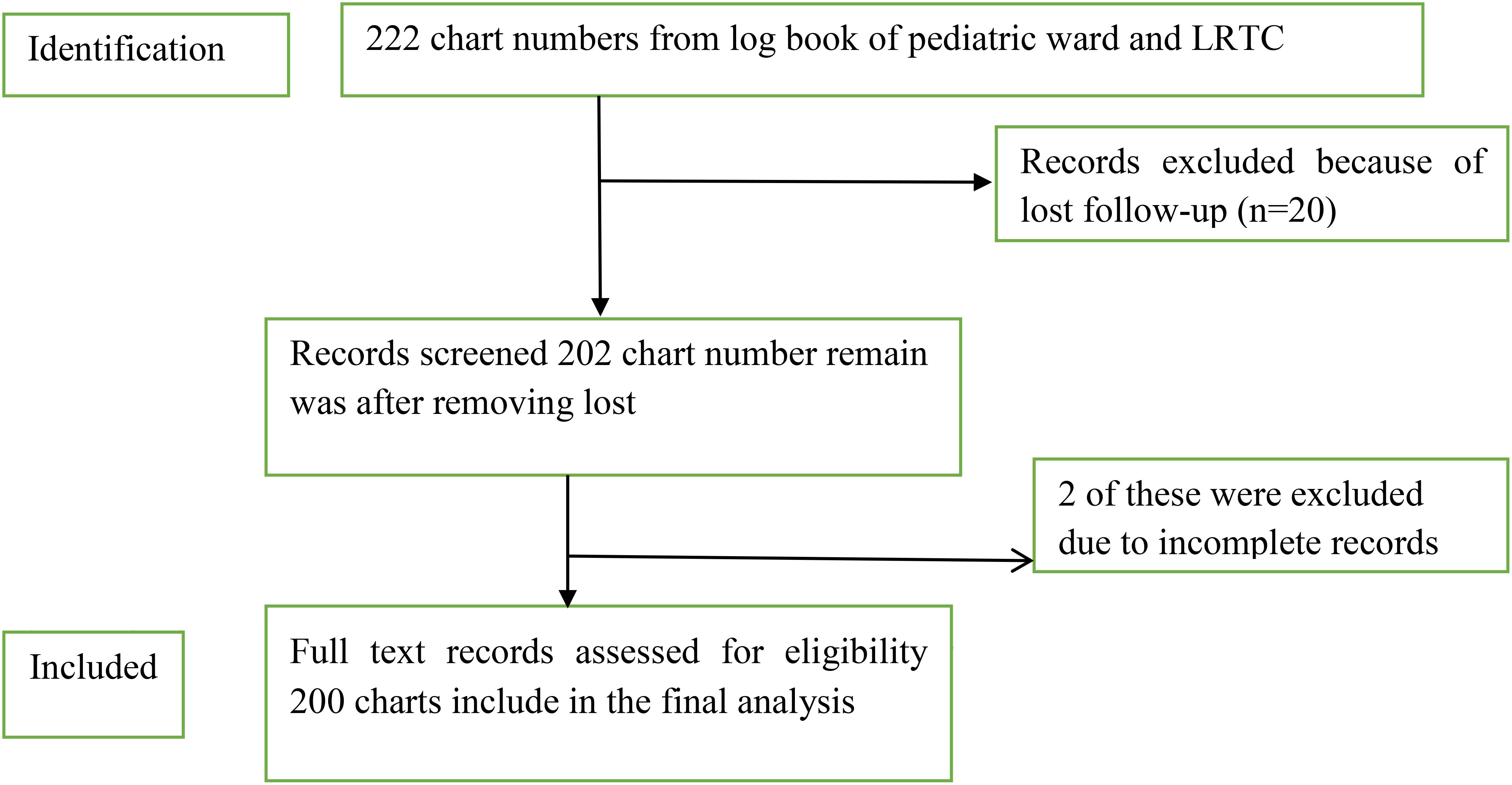
Figure 2. Sampling procedure and sampling technique in pediatric ward at Compressive Specialized Hospital, University of Gondar from September 2013 to August 2019.
2.9 Data collection, quality control, and analysis
Data were obtained from a specialized VL database that was regularly updated and monitored. The study was conducted within a DNDi setting, where the staff had extensive experience in managing VL cases. Patient charts and laboratory records were collected using a meticulously designed checklist that was specifically created for this data collection process. To ensure data quality, the data collection instrument was preliminarily reviewed by one of the co-authors, and the collected data were checked for completeness, accuracy, and clarity before analysis. Data were entered into Epi-info version 7.2 and exported to SPSS version 20 for cleaning, editing, and analysis. Any logical and consistent errors identified during data entry were corrected after revising the completed questionnaires. Descriptive statistics (frequency, percentage, mean, and range) are used to present the data and evaluate VL treatment outcomes. A chi-square test was conducted to examine the association between VL treatment outcomes and variables such as sex, age, year of diagnosis, comorbid conditions, and hospitalization history. A statistically significant association was set at a P value of<0.05, at a 95% confidence interval. All variables that were valid in the assumption of the chi-square test were analyzed using both bivariable and multivariable logistic regression to assess the association between treatment outcomes and explanatory variables. Factors with a p-value ≤0.2 were selected as candidates for the multivariable model. In the multivariable regression, a p-value<0.05 was considered statistically significant. The model’s goodness of fit was evaluated using the Homer-Lemeshow test, which was checked before performing any statistical analyses (P = 0.266). The results are presented as tables, figures, and text, using frequencies and summary statistics to describe the study population in relation to the relevant variables.
2.10 Ethical considerations
Ethical approval was obtained from the Research and Ethics Review Committee of the School of Pharmacy at the University of Gondar (Reference Number: SOPS/023; Date: March 16, 2019). A permission letter was obtained from the pediatric ward of UOGCSH, Ethiopia. Given that this was a retrospective cohort study involving no direct contact with patients or interventions requiring written consent, and since there was no anticipated risk to participants, a waiver of informed consent was granted.
3 Results
3.1 Demographic characteristics of pediatric VL patients
Of the 222 pediatric patients admitted to the hospital pediatric ward with a diagnosis of VL, 20 medical records were missing and two were incomplete. Therefore, a total of 200 patient records was included in the final analysis. These patients received one of three antileishmanial treatments: L-Amb, SSG + PM, or SSG monotherapy. More than two-thirds of the patients were male (67.5%). Ages ranged from 1 month to 15 years, with a mean (± SD) age of 8.81 (± 3.984). The majority of the study participants were residents in rural areas (81.5%). Nearly half of the patients were included in the 6–10 year age group (47.5%) (Table 1).
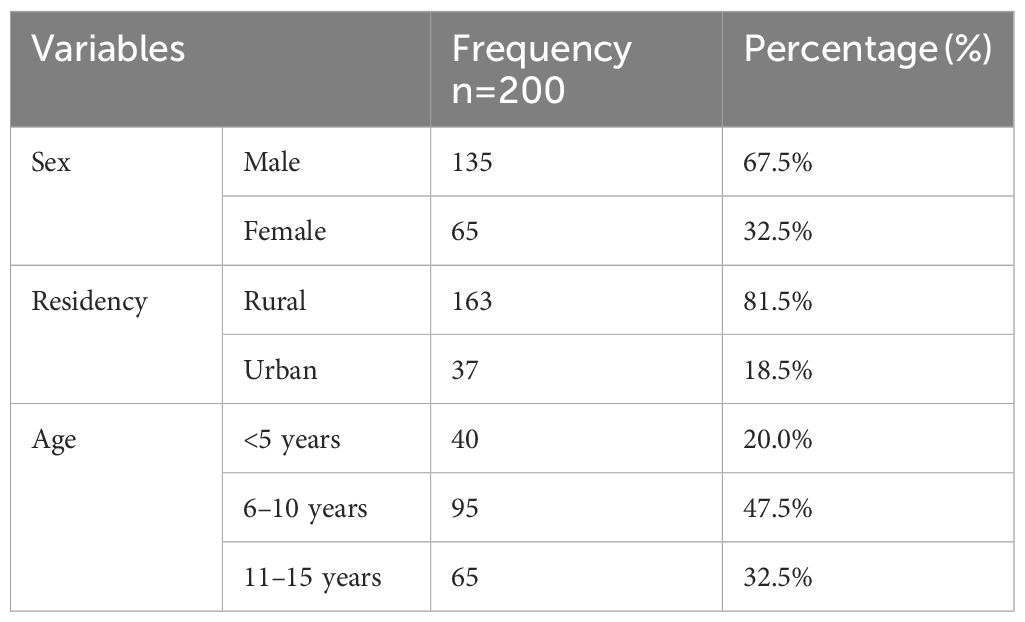
Table 1. Sociodemographic characteristics of pediatric VL patients in a pediatric ward at the University of Gondar Comprehensive Specialized Hospital, from September 2013 to August 2019 (n=200).
3.2 Clinical history and co-infection of pediatric VL patients
Most of the pediatric patients were immunocompetent (72%). The time interval between symptom onset and diagnosis ranged from 18 to 36.5 days. The median duration of hospitalization was 11.5 to 43.5 days. Malaria (49%) and pneumonia (39%) were the most common co-infections. Less frequent co-infections included sepsis and HIV, both occurring in 2.2% of patients (Table 2).
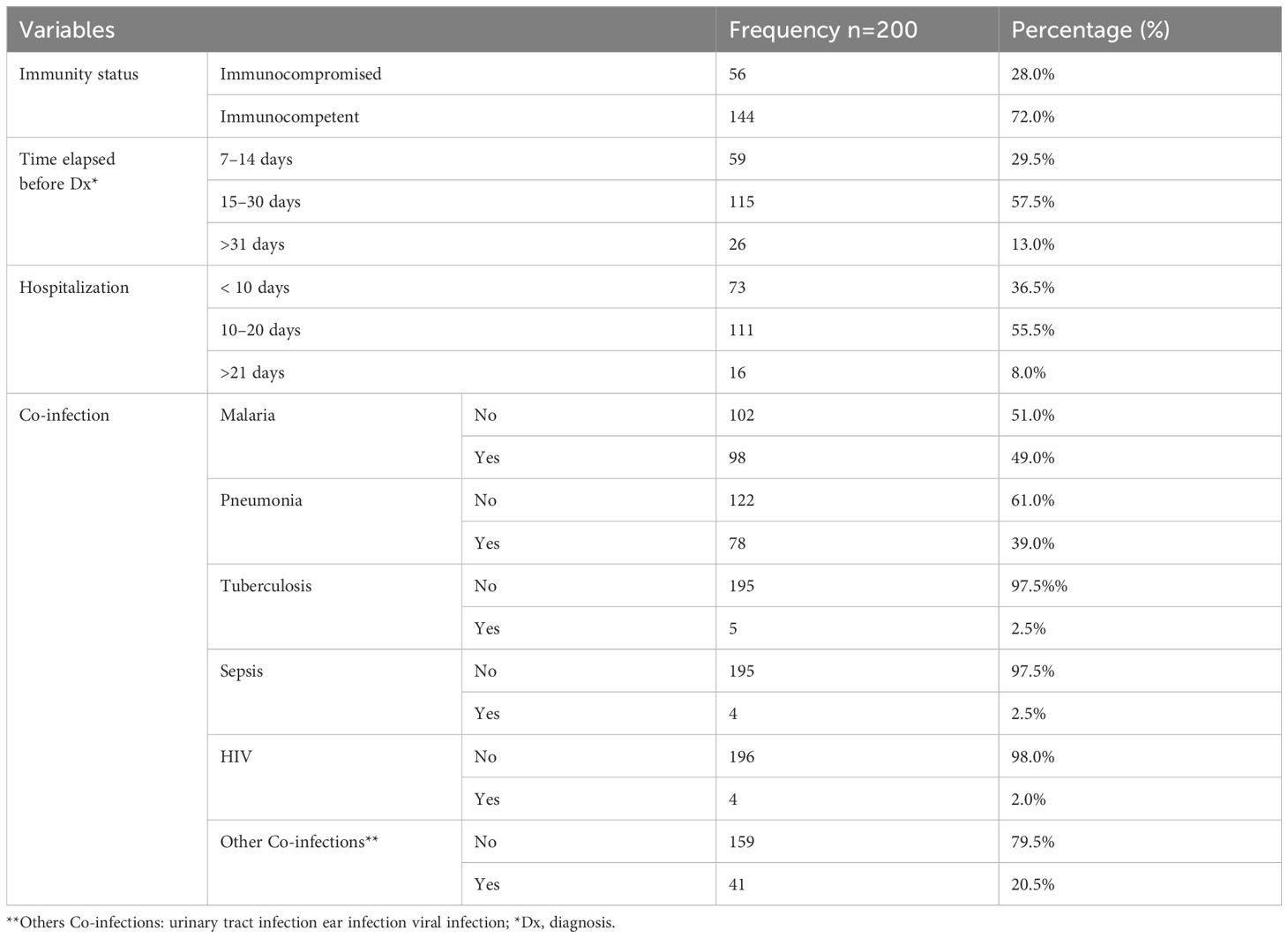
Table 2. Clinical history and co-infection of pediatric VL patients at the University of Gondar Comprehensive Specialized Hospital, from September 2013 to August 2019 (n=200).
3.3 Medication use and administration of pediatric VL patients
Patients admitted to the pediatric ward received the following antileishmanial medications: L-Amb (77.5%), SSG + PM (19%), and SSG alone (3.5%). Most patients received treatment for 6 consecutive days (77.5%). Intravenous administration using 5% dextrose in water was the predominant route (51.5%). Most patients were treated according to the IDSA guidelines (79%). The most frequently prescribed antibiotics were ceftriaxone (38%) and ceftazidime plus vancomycin (10.5%) (Table 3).
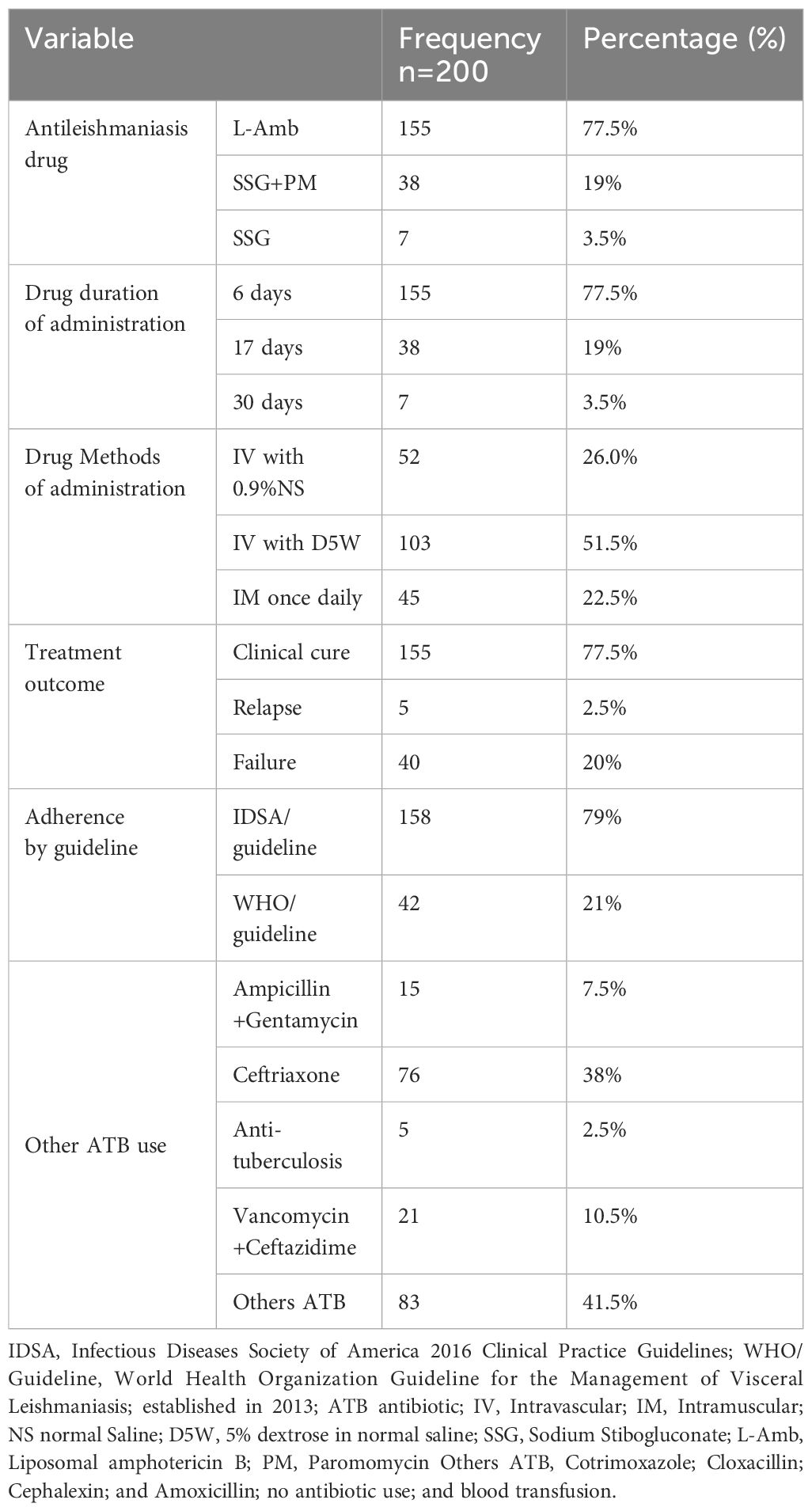
Table 3. Medication use and administration in pediatric VL patients at the University of Gondar Comprehensive Specialized Hospital, from September 2013 to August 2019 (n=200).
3.4 Medication use and treatment outcome of pediatric VL patients
Of the total treatment results, 155 cases (77.5%) achieved a clinical cure, while 22.5% experienced treatment failure. This finding suggests that a significant proportion of patients did not respond effectively to treatment. Among those that experienced treatment failures, relapses accounted for 2.5%, partial responses for 15%, and the mortality rate was 5%, which was particularly alarming. Among the 200 pediatric patients, the majority received L-Amb, with 135 (87.7%) of them achieving clinical cure. In contrast, most patients treated with SSG+PM and SSG alone were in the treatment failure group, five patients (13.2%) and 15 patients (214.3%), respectively. The number of deaths recorded was eight (5.16%) in the L-Amb group and two (5.26%) in the SSG+PM group (Table 4).
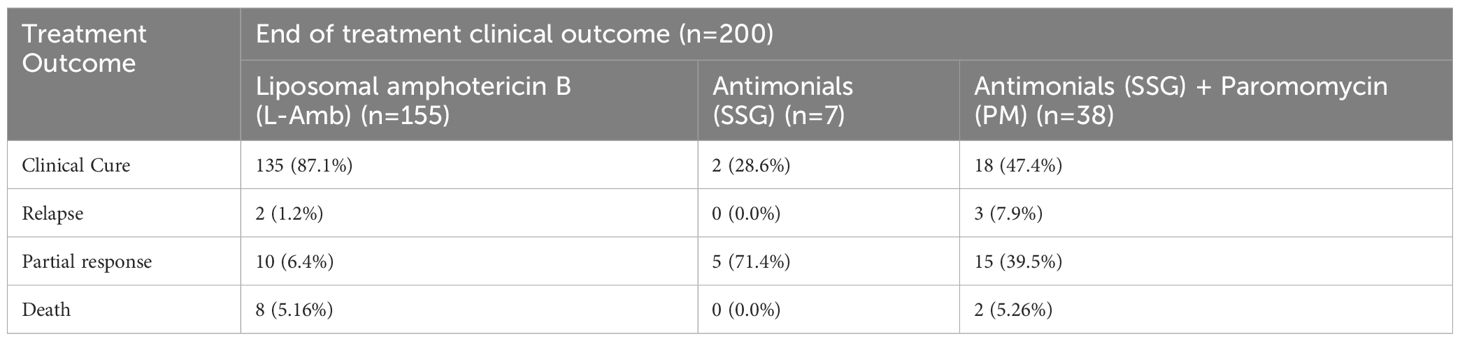
Table 4. Overall initial treatment outcomes of Antileishmania medication among pediatric VL Patients at the University of Gondar Comprehensive Specialized Hospital, from September 2013 to August 2019 (n=200).
3.5 Overall treatment outcome and annual trend of pediatric visceral leishmaniasis patients
In a seven-year period from 2013 to 2019, 200 pediatric patients with VL were treated at the UOGCSH. Annual variations in cure rates were observed. The clinical cure ranged from 3.2% in 2019 (25 cases) to 20.6% in 2018 (33 cases), with a statistically significant variation between years (P< 0.000) (Table 5).
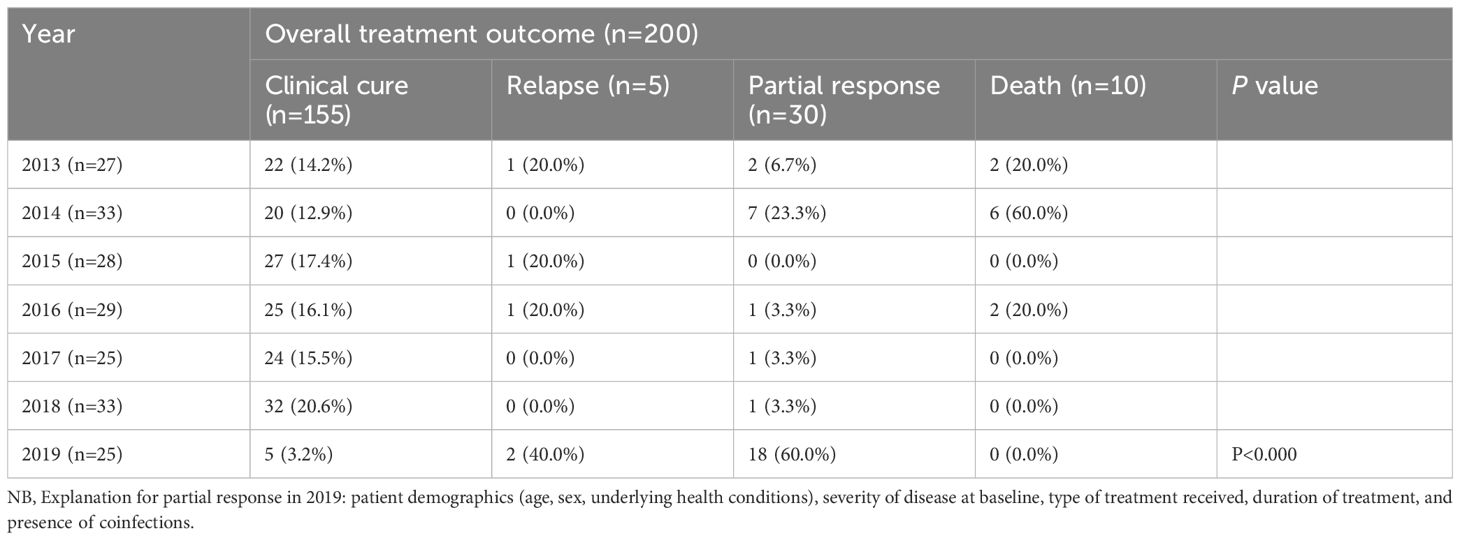
Table 5. Overall treatment outcome and annual trend of among pediatric VL Patients at the University of Gondar Comprehensive Specialized Hospital, from September 2013 to August 2019 (n=200).
3.6 Risk factors associated with treatment outcomes in pediatric visceral leishmaniasis patients
Variables including sex, age, residency, jaundice, lymphadenopathy, malaria, hospitalization, methods of drug administration, immunity status, and adherence to treatment guidelines were included in both bivariate and multivariate binary logistic regression analyses. These variables showed p-values less than or equal to 0.25 in the bivariate analysis and were then used for multivariable analysis. On multivariate analysis, age, residence, hospitalization, immunity status, and adherence to treatment guidelines were significantly associated with treatment outcomes.
The odds of achieving a cure among pediatric patients with VL who were less than five years old decreased by 80% (AOR 0.2: 95% CI: 0.04–0.6) compared to pediatric patients with VL aged 11–15 years. The odds of achieving a cure among pediatric patients with VL residing in rural areas were four times higher than those residing in the urban areas (AOR 4: 95% CI 2-11). Pediatric patients with VL hospitalized for 11 to 20 days were four times more likely to be cured compared to those hospitalized for more than 21 days (AOR, 4; 95% CI: 1–16). The odds of achieving a cure in immunocompetent pediatric patients with VL were three times higher than those with VL who were immunocompromised (AOR 3: 95% CI: 1.1–9). Patients treated according to international guidelines had 26 times higher odds of achieving a cure (AOR 26, 95% CI: 8–82) compared with pediatric patients with VL who were managed using local guidelines (Table 6).
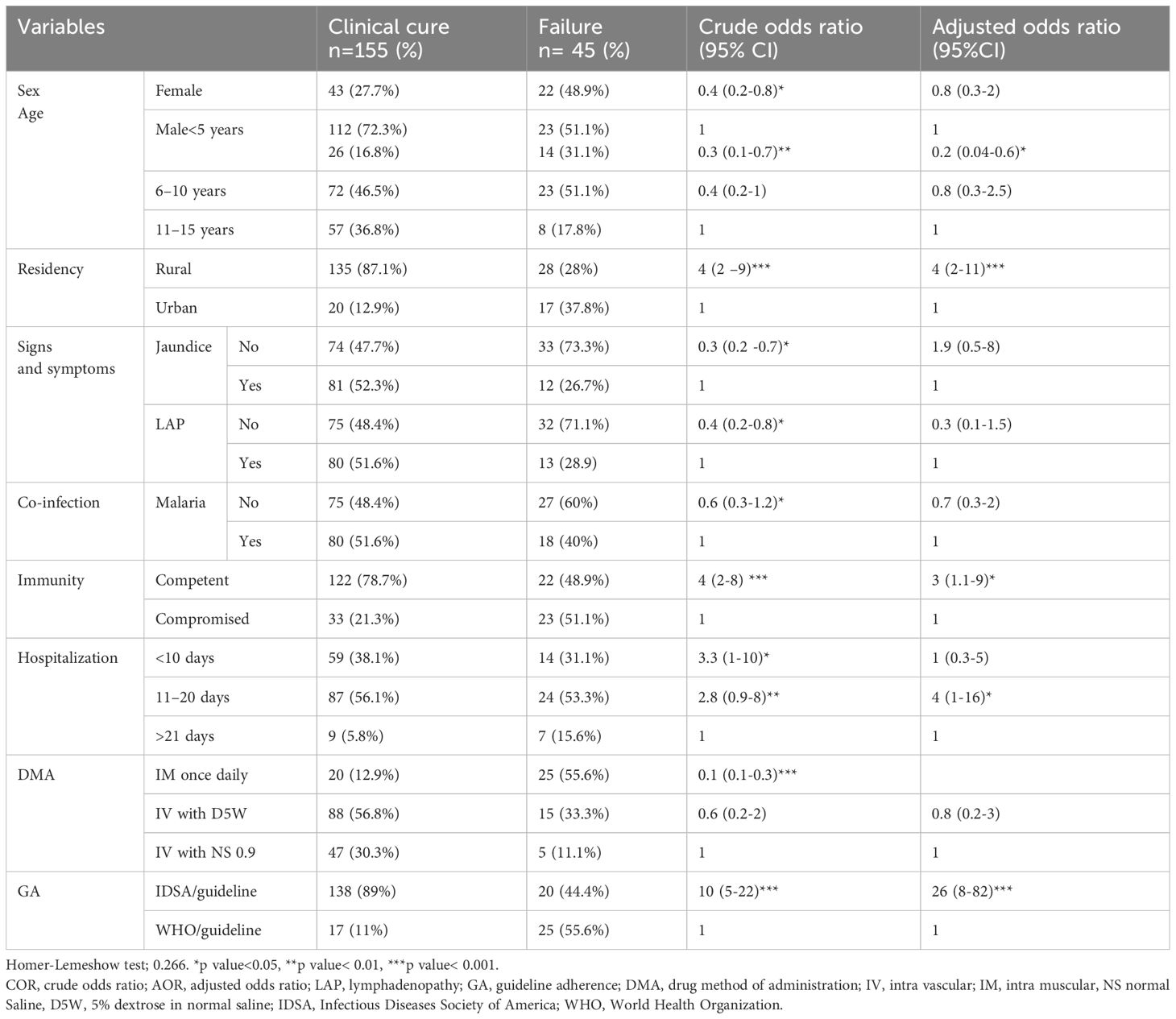
Table 6. Bivariate and multivariable logistic regression analysis of factors associated with treatment outcome of pediatric VL patients at the University of Gondar Comprehensive Specialized Hospital, from September 2013 to August 2019 (n=200).
4 Discussion
This study presents the first published data on VL among pediatric patients in Gondar, located in Northwest Ethiopia, a region with a population of approximately 5 million. Rapid urbanization and human migration are well-documented risk factors that contribute to the spread of the disease. Northwest Ethiopia shares borders with Sudan and Eritrea and is characterized by widespread poverty, a high proportion of nomadic people, and refugees. These factors contribute to the prevalence of VL observed in this region.
To better understand the treatment outcomes in this vulnerable population, a retrospective cohort study was conducted using medical records of pediatric patients with VL who received treatment with L-Amb, SSG, and PM for durations of 6, 17, and 30 days, respectively. Previous review studies have shown that multiple doses of L-Amb can achieve treatment success rates of 96.7% at the end of therapy and between 71% and 100% at the six-month follow-up. Meanwhile, the combination of SSG with PM achieved success rates of up to 90.1% upon completion of treatment. Various studies have assessed the efficacy and safety of these combination therapies within routine clinical settings in Ethiopia (33).
In this study, 155 out of 200 pediatric patients with VL (77.5%) achieved clinical cure. However, a failure rate of 22.5% was observed, which included 2.5% relapses, 15% partial responses, and 5% mortality rates. This high rate of treatment failure emphasizes the severity of VL in pediatric populations and the need for improved treatment strategies to reduce these adverse outcomes.
The overall clinical cure rate in this study was 77.5% (95% CI: 72.5–83.5), indicating that the treatment protocols employed were effective for most pediatric patients with VL. This finding is consistent with the 82.6% clinical cure rate reported in a systematic review and meta-analysis conducted among the general population in northwest Ethiopia (33). This similarity suggests that the current treatment outcome in the pediatric and general population may have comparable efficacy.
In this study, we observed clinical cure rates of 87% for patients treated with L-AMB, 47% for those treated with SSG combined with PM, and 28% for those treated with SSG alone. These outcomes are lower than those reported in previous studies, which include 86% in Sudan (34), 98.8% in Albania (35), 100% in Spain (36), 88% in the Northeast of Brazil (37), 93% in eastern Sudan (38), 95.8% in Nepal (39), 94.7% in southeastern Ethiopia (40), and 98.3% in Northwest Ethiopia (32). Variations in study populations, clinical settings, and treatment regimens may contribute to differing treatment outcomes. There is a critical need for further drug development and clinical trials in East Africa to identify a more effective first-line treatment for VL other than SSG/PM, comparable to the success achieved in South Asia with single-dose L-Amb (41). However, in East Africa, single or low cumulative doses of L-Amb have demonstrated limited efficacy. Additionally, disparities in health outcomes are likely influenced by the socioeconomic status of families, which may contribute to longer hospital stays and an increased risk of nosocomial infections in young children.
The 22.5% treatment failure rate observed in this study indicates that a considerable number of pediatric patients do not respond effectively to treatment. This finding is consistent with findings from a study carried out in a general population in Northwest Ethiopia (42). Notably, this study showed that the prevalence of partial response was 15%, which is higher than the reported rates of 1.6% in Northwest Ethiopia (32), 5.7% in Northwest Ethiopia (43), and 3% in Northwest Ethiopia (42). The elevated rate of partial response among pediatric patients in this study may have significant socioeconomic implications, including increased cost of hospitalization and prolonged recovery time. Several factors may contribute to these differences, including variations in study design, drug resistant patterns, parasite virulence, poor adherence to medication, nutritional status, and immune competence of pediatric patients (43).
In terms of mortality, our findings reveals a death rate of 5%, which is comparatively similar to the 4% reported in two studies conducted in Brazil (44) and 3.7% in Uganda (45). However, it exceeds the 0.8% mortality rates reported in Northwest Ethiopia (32), the no death reported in Spain (36), the 0.16% in Albania (35), 0.7% in Eastern Sudan at diagnosis and 1% after six-month follow-up (34), and 0.3% in Bihar, India (46). Conversely, the observed mortality rate is lower than those documented in other regions, including 10% in Brazil (7), 8.5% in Yemen (47), 6.2% in Southwestern Iran (48), 6.12% in Bangladesh (49), 10% in Greece (50), and 12.4% in Ethiopia (43). These differences may be attributed to variations in study design and study population. In addition, this may be due to dissimilarity of immunity states of the pediatric patients, poor nutritional status, economic background, and national policies on disease prevention and treatment (7).
This study’s findings revealed that the prevalence of relapse was 2.5%, which is lower than 6% reported in Sudan (34), 26.7% in Southern Sudan during the diagnosis (34), 10% in Greece and 6% after six months follow-up (50), 7% in Georgia (51), 10.8% and 20%, respectively, in Nepal at 6 and 12 months (39), and 9.6% in Brazil (37). However, this rate was slightly higher than that reported in Bihar, India (1.4%) (46). The lower relapse rate observed in this study suggests relatively favorable treatment durability; however, relapses still pose clinical challenges, as they are associated with increased hospitalization rates, healthcare costs, and risk of secondary infections. These variations across studies may be attributed to differences in study design, population demographics, immune states, nutritional states of participants, drug resistance patterns, and treatment adherence (46).
Additionally, our findings revealed that 72% of male pediatric patients achieved clinical cure. This rate is higher than those reported in Brazil (50%) (7), Italy (65%) (52), Yemen (64.2%) (47), Southwestern Iran (51%) (48), and Spain (47.4%) (36). These findings suggest a higher treatment success rate among male pediatric patients in this cohort. The observed differences may reflect variations in study populations, gender-related exposure risks, or biological and immunological factors that influence disease progression and response to treatment (53).
This study identified several factors associated with treatment outcomes in pediatric patients with VL. These included age less than five years, rural residency, immunocompetent patients, hospitalization for 11–20 days, and adherence to international treatment guidelines. Identifying such factors is crucial, as it can inform clinical and policy interventions aimed at improving treatment outcomes (43).
These findings showed that the odds of achieving cure among pediatrics patients with VL who were less than 5 years decreased by 80% (AOR 0.2: 95% CI: 0.04–0.6). This finding aligns with studies from Gadarif, Sudan (53), and Georgia (51). The reduced cure rates among younger children may be attributed to immaturity of their immune systems, particularly the underdeveloped production of B and T lymphocyte cells in the bone marrow. In this age group, the lack of acquired immunity and the higher likelihood of being immunocompromised may impair the ability to mount an effective immune response against Leishmania parasites (54).
Moreover, the odds of achieving a cure were four times higher in pediatric patients with VL residing in rural areas than those residing in urban areas (AOR 4: 95% CI: 2–11). This finding may be influenced by differences in environmental exposures, stress levels, and socioeconomic conditions. Urban environments are often associated with increased psychological stress, exposure to pollutants, and limited microbial diversity—all of which can negatively affect immune function. Conversely, children in rural settings may benefit from early exposure to diverse environmental microbes, which can enhance immune system development and responsiveness (55).
Interestingly, the odds of achieving a cure among immunocompetent pediatric patients with VL were three times higher than in pediatrics who were immunocompromised (AOR 3: 95% CI: 1–9). This finding is consistent with a study conducted in Italy, although the association was not statically significant (52). The higher cure rates among immunocompetent pediatrics can be attributed to their stronger immune response. Leishmania species infect macrophages throughout the viscera, and parasites are found in the spleen, liver, and bone marrow, and if left untreated may be fatal (54). Pediatrics that are immunocompromised, especially those with severe immunosuppression, poor nutritional status, or coexisting helminthic infections, have a significantly diminished ability to recover (16). Moreover, host genetic factors and immune status are known to influence both disease progression and treatment outcome (56).
This study demonstrated that pediatric patients with VL who were hospitalized for 11 to 20 days had a four times higher likelihood of being cured than those who were hospitalized for more than 21 days (AOR 4: 95% CI: 1–16). This suggests that pediatrics with longer hospital stay had a decreased cure rate because long-term hospital stay exposed them to other complex chronic conditions, which can lead to death due to exposure to nosocomial infections. Evidence supports that prolonged hospitalizations are associated with increased risk for in-hospital complications, including infections (57).
Furthermore, this study showed that pediatric patients who were treated using the international guideline had 26 times higher odds of achieving a cure than those who were treated using the local guidelines (AOR 26: 95% CI: 8–82). This suggests that pediatrics treated with L-Amb, which is the FDA-approved first-line therapy for both immunocompetent and immunocompromised pediatric patients in the USA, had a greater cure rate than pediatrics treated with SSG+PM and SSG alone (57).
However, this study is not without its limitations, as it was conducted in a single pediatric ward at UOGCSH, which may limit the generalizability of the findings. Additionally, missing or incomplete data in some patients’ records during the study may have influenced the results. Furthermore, the retrospective nature of the study limited access to consent data and follow-up outcomes, such as long-term relapse or mortality beyond the hospital stay.
In conclusion, our findings highlight the significant burden of VL in pediatric populations and emphasize the urgent need for enhanced treatment strategies. By addressing the identified determinants through targeted healthcare interventions, it may be possible to increase cure rates and improve the overall management of pediatric VL. Based on these findings, several recommendations are proposed. First, collaboration with the Department of Pediatrics is essential to conduct a multi-center study with a larger sample size to enhance the generalizability of the findings across the Ethiopian population. Additionally, pediatric ward physicians, nurses, and pharmacists should focus on identifying and addressing factors that contribute to poor treatment outcomes and failures. The University of Gondar is encouraged to perform drug sensitivity tests to mitigate these issues. Finally, policymakers should establish strategies aimed at reducing poor treatment outcomes and failures.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval (Reference Number: SOPS/023; Date: March 16, 2019) was obtained from the Research and Ethics Review Committee of School of Pharmacy, University of Gondar. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
HA: Methodology, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Project administration, Resources, Software, Supervision, Validation. AA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DA: Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. GM: Conceptualization, Methodology, Software, Writing – original draft, Writing – review & editing. TC: Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. MA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MK: Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank the staff of the pediatric ward at the Compressive Specialized Hospital, University of Gondar and Leishmaniasis Research and Treatment Center (LRTC) for their support during data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Reithinger R. Xenodiagnosis leads the way: elimination of visceral leishmaniasis from the Indian subcontinent is feasible and sustainable. Lancet Microbe. (2021) 2:e2–3. doi: 10.1016/s2666-5247(20)30222-6
2. Steverding D. The history of leishmaniasis. Parasites vectors. (2017) 10:1–10. doi: 10.1186/s13071-017-2028-5
3. King JD, Buolamwini J, Cromwell EA, Panfel A, Teferi T, Zerihun M, et al. A novel electronic data collection system for large-scale surveys of neglected tropical diseases. PloS One. (2013) 8:e74570. doi: 10.1371/journal.pone.0074570
4. Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PloS One. (2012) 7:e35671. doi: 10.1371/journal.pone.0035671
5. Alvar J, den Boer M, and Dagne DA. Towards the elimination of visceral leishmaniasis as a public health problem in east Africa: reflections on an enhanced control strategy and a call for action. Lancet Global Health. (2021) 9:e1763–e9. doi: 10.1016/s2214-109x(21)00392-2
6. Jones CM and Welburn SC. Leishmaniasis beyond east Africa. Front Veterinary Science. (2021) 8:618766. doi: 10.3389/fvets.2021.618766
7. de Queiroz Sampaio MJA, Cavalcanti NV, Alves JGB, Fernandes Filho MJC, and Correia JB. Risk factors for death in children with visceral leishmaniasis. PloS Negl Trop diseases. (2010) 4:e877. doi: 10.1371/journal.pntd.0000877
8. Wamai RG, Kahn J, McGloin J, and Ziaggi G. Visceral leishmaniasis: a global overview. J Global Health Sci. (2020) 2(1). doi: 10.35500/jghs.2020.2.e3
9. Gelaglie AK. Visceral leishmaniasis in Ethiopia: transmission and variability. PhD dissertation (2015).
10. Guerin PJ, Olliaro P, Sundar S, Boelaert M, Croft SL, Desjeux P, et al. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect diseases. (2002) 2:494–501. doi: 10.1016/s1473-3099(02)00347-x
11. Oliveira-Sena IV and Werneck GL. Risk factors for in-hospital mortality from visceral leishmaniasis: A case-control study. J infection Public Health. (2020) 13:538–43. doi: 10.1016/j.jiph.2019.10.003
12. Feleke BE. Nutritional status of visceral leishmaniasis patients: a comparative cross-sectional study. Clin Nutr ESPEN. (2019) 33:139–42. doi: 10.1016/j.clnesp.2019.06.005
13. Akuffo H, Costa C, van Griensven J, Burza S, Moreno J, and Herrero M. New insights into leishmaniasis in the immunosuppressed. PloS Negl Trop diseases. (2018) 12:e0006375. doi: 10.1371/journal.pntd.0006375
14. Anstead GM, Chandrasekar B, Zhao W, Yang J, Perez LE, and Melby PC. Malnutrition alters the innate immune response and increases early visceralization following Leishmania donovani infection. Infection Immunity. (2001) 69:4709–18. doi: 10.1128/IAI.69.8.4709–4718.2001
15. Lopez-Velez R, Perez-Molina JA, Guerrero A, Baquero F, Villarrubia J, Escribano L, et al. Clinicoepidemiologic characteristics, prognostic factors, and survival analysis of patients coinfected with human immunodeficiency virus and Leishmania in an area of Madrid, Spain. Am J Trop Med hygiene. (1998) 58:436–43. doi: 10.4269/ajtmh.1998.58.436
16. Sundar S and Agarwal D. Visceral leishmaniasis—Optimum treatment options in children. Pediatr Infect Dis J. (2018) 37:492–4. doi: 10.1097/inf.0000000000001885
17. Ferede G, Diro E, Getie S, Getnet G, Takele Y, Amsalu A, et al. Visceral leishmaniasis-malaria coinfection and their associated factors in patients attending Metema Hospital, Northwest Ethiopia: suggestion for integrated vector management. Malaria Res Treat. (2017) 2017:6816913. doi: 10.1155/2017/6816913
18. Leta S, Dao THT, Mesele F, and Alemayehu G. Visceral leishmaniasis in Ethiopia: an evolving disease. PloS Negl Trop diseases. (2014) 8:e3131. doi: 10.1371/journal.pntd.0003131
19. Mohebali M and Yimam Y. Prevalence estimates of human immunodeficiency virus (HIV) infection among visceral leishmaniasis infected people in Northwest Ethiopia: a systematic review and meta-analysis. BMC Infect Diseases. (2020) 20:1–10. doi: 10.1186/s12879-020-4935-x
20. Endale HT, Mengstie TA, Dawit DD, Mohammed R, Dessie G, and Tesfa KH. Assessment of liver function test and associated factors among visceral leishmaniasis patients attending university of gondar leishmaniasis research and treatment center, Northwest Ethiopia. PloS One. (2021) 16:e0260022. doi: 10.1371/journal.pone.0260022
21. Motuma K, Abera E, Wondu B, and Negash A. Visceral leishmaniasis in Ethiopia: A review. Eur J Biol Sci. (2016) 8:70–8. 10.5829/idosi.ejbs.2016.70.78
22. Agrawal Y, Sinha A, Upadhyaya P, Kafle S, Rijal S, and Khanal B. Hematological profile in visceral leishmaniasis. Int J Infection Microbiol. (2013) 2:39–44. doi: 10.3126/ijim.v2i2.8320
23. Aronson N, Herwaldt BL, Libman M, Pearson R, Lopez-Velez R, Weina P, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect diseases. (2016) 63:e202–e64. doi: 10.1093/cid/ciw742
24. Organization WH. Guideline for diagnosis, treatment & prevention of leishmaniasis in Ethiopia. Addis Ababa: FMoH (2013).
25. Alves F, Bilbe G, Blesson S, Goyal V, Monnerat S, Mowbray C, et al. Recent development of visceral leishmaniasis treatments: successes, pitfalls, and perspectives. Clin Microbiol Rev. (2018) 31:10–128. doi: 10.1128/cmr.00048-18
26. Kimutai R, Musa AM, Njoroge S, Omollo R, Alves F, Hailu A, et al. Safety and effectiveness of sodium stibogluconate and paromomycin combination for the treatment of visceral leishmaniasis in eastern Africa: results from a pharmacovigilance programme. Clin Drug Invest. (2017) 37:259–72. doi: 10.1007/s40261-016-0481-0
27. Ministry of Health Ethiopia. Guideline for Diagnosis, Treatment and Prevention of Leishmaniasis in Ethiopia. In: Directorate AHPaDP, editor. 2nd Edition ed. Addis Ababa: FMoH (2013)
28. Sundar S, Chakravarty J, Agarwal D, Rai M, and Murray HW. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. New Engl J Med. (2010) 362:504–12. doi: 10.1056/nejmoa0903627
29. Goyal V, Mahajan R, Pandey K, Singh SN, Singh RS, Strub-Wourgaft N, et al. Field safety and effectiveness of new visceral leishmaniasis treatment regimens within public health facilities in Bihar, India. PloS Negl Trop diseases. (2018) 12:e0006830. doi: 10.1371/journal.pntd.0006830
30. van Griensven J, Balasegaram M, Meheus F, Alvar J, Lynen L, and Boelaert M. Combination therapy for visceral leishmaniasis. Lancet Infect diseases. (2010) 10:184–94. doi: 10.1016/s1473-3099(10)70011-6
31. Tekalign S, Adera C, Den Boer M, Miecha H, Zewde A, Mulugeta D, et al. Clinical features and treatment outcomes of visceral leishmaniasis patients admitted to three centers in Oromia, Ethiopia. J Infection Developing Countries. (2020) 14(06.1):42S–7S. doi: 10.3855/jidc.11731
32. Diro E, Lynen L, Gebregziabiher B, Assefa A, Lakew W, Belew Z, et al. Clinical aspects of paediatric visceral leishmaniasis in N orth-west E thiopia. Trop Med Int Health. (2015) 20:8–16. doi: 10.1111/tmi.12407
33. Gebreyohannes EA, Bhagvathula AS, Abegaz TM, and Seid MA. Treatment outcomes of visceral leishmaniasis in Ethiopia from 2001 to 2017: a systematic review and meta-analysis. Infect Dis poverty. (2018) 7:1–9. doi: 10.1186/s40249-018-0491-7
34. Atia AM, Mumina A, Tayler-Smith K, Boulle P, Alcoba G, Elhag MS, et al. Sodium stibogluconate and paromomycin for treating visceral leishmaniasis under routine conditions in eastern Sudan. Trop Med Int Health. (2015) 20:1674–84. doi: 10.1111/tmi.12603
35. Petrela R, Kuneshka L, Foto E, Zavalani F, and Gradoni L. Pediatric visceral leishmaniasis in Albania: a retrospective analysis of 1,210 consecutive hospitalized patients (1995–2009). PloS Negl Trop diseases. (2010) 4:e814. doi: 10.1371/journal.pntd.0000814
36. Ramos JM, Clavijo A, Moral L, Gavilan C, Salvador T, and González de Dios J. Epidemiological and clinical features of visceral leishmaniasis in children in Alicante Province, Spain. Paediatrics Int Child Health. (2018) 38:203–8.
37. Naufal Spir PR, Prestes-Carneiro LE, Fonseca ES, Dayse A, Giuffrida R, and D’Andrea LAZ. Clinical characteristics and spatial distribution of visceral leishmaniasis in children in São Paulo state: an emerging focus of visceral leishmaniasis in Brazil. Pathog Global Health. (2017) 111:91–7. doi: 10.1080/20477724.2017.1289666
38. Gorski S, Collin SM, Ritmeijer K, Keus K, Gatluak F, Mueller M, et al. Visceral leishmaniasis relapse in Southern Sudan (1999–2007): a retrospective study of risk factors and trends. PloS Negl Trop diseases. (2010) 4:e705. doi: 10.1371/journal.pntd.0000705
39. Rijal S, Ostyn B, Uranw S, Rai K, Bhattarai NR, Dorlo TP, et al. Increasing failure of miltefosine in the treatment of Kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin Infect Diseases. (2013) 56:1530–8. doi: 10.1093/cid/cit102
40. Tekalign S, Adera C, den Boer M, Miecha H, Zewde A, Mulugeta D, et al. Clinical features and treatment outcomes of visceral leishmaniasis patients admitted to three centers in Oromia, Ethiopia. J Infection Developing Countries. (2020) 14:42S-7S. doi: 10.3855/jidc.11731
41. Sundar S, Singh A, Agrawal N, and Chakravarty J. Effectiveness of single-dose liposomal amphotericin B in visceral leishmaniasis in Bihar. Am J Trop Med Hygiene. (2019) 101:795. doi: 10.4269/ajtmh.19-0179
42. Tamiru A, Mohammed R, Atnafu S, Medhin G, and Hailu A. Efficacy and safety of a combined treatment of sodium stibogluconate at 20mg/kg/day with upper maximum daily dose limit of 850mg and Paromomycin 15mg/kg/day in HIV negative visceral leishmaniasis patients. A retrospective study, northwest Ethiopia. PloS Negl Trop Diseases. (2021) 15:e0009713. doi: 10.1371/journal.pntd.0009713
43. Welay GM, Alene KA, and Dachew BA. Visceral leishmaniasis treatment outcome and its determinants in northwest Ethiopia. Epidemiol Health. (2017) 39:e2017001. doi: 10.4178/epih.e2017001
44. ASdC B, ACdC TJ, and Rabello A. Factors of poor prognosis of visceral leishmaniasis among children under 12 years of age. A retrospective monocentric study in Belo Horizonte, State of Minas Gerais, Brazil, 2001-2005. Rev da Sociedade Bras Medicina Tropical. (2013) 46:55–9. doi: 10.1590/0037-868216432013
45. Mueller Y, Mbulamberi DB, Odermatt P, Hoffmann A, Loutan L, and Chappuis F. Risk factors for in-hospital mortality of visceral leishmaniasis patients in eastern Uganda. Trop Med Int Health. (2009) 14:910–7. doi: 10.1111/j.1365-3156.2009.02305.x
46. Burza S, Sinha PK, Mahajan R, Lima MA, Mitra G, Verma N, et al. Risk factors for visceral leishmaniasis relapse in immunocompetent patients following treatment with 20 mg/kg liposomal amphotericin B (Ambisome) in Bihar, India. PloS Negl Trop diseases. (2014) 8:e2536. doi: 10.1371/journal.pntd.0002536
47. Al-Selwi AAM, Sherei A, Ghaleb A, and Almagrami AAS. Clinical and epidemiological features of visceral leishmaniasis among children in Yemen: One referral hospital Review. Sudan Med J. (2016) 11:7–16.
48. Al-Ghazaly J, Al-Dubai W, Abdullah M, and Al-Gharasi L. Hematological characteristics of Yemeni adults and children with visceral leishmaniasis. Could eosinopenia be a suspicion index? Mediterr J Hematol Infect diseases. (2017) 9(1):e2017056. doi: 10.4084/mjhid.2017.056
49. Huda MM, Chowdhury R, Ghosh D, Dash AP, Bhattacharya SK, and Mondal D. Visceral leishmaniasis-associated mortality in Bangladesh: a retrospective cross-sectional study. BMJ Open. (2014) 4:e005408. doi: 10.1136/bmjopen-2014-005408
50. Georgiadou SP, Stefos A, Spanakos G, Skrimpas S, Makaritsis K, Sipsas NV, et al. Current clinical, laboratory, and treatment outcome characteristics of visceral leishmaniasis: results from a seven-year retrospective study in Greece. Int J Infect Diseases. (2015) 34:46–50. doi: 10.1016/j.ijid.2015.02.021
51. Kajaia M, Morse DL, Kamkamidze G, Butsashvili M, Chubabria G, Zenaishvili O, et al. Risk factors for relapse of visceral leishmaniasis in Georgia. Trop Med Int Health. (2011) 16:186–92. doi: 10.1111/j.1365-3156.2010.02694.x
52. Di Masi F, Ursini T, Iannece MD, Chianura L, Baldasso F, Foti G, et al. Five-year retrospective Italian multicenter study of visceral leishmaniasis treatment. Antimicrobial Agents chemotherapy. (2014) 58:414–8. doi: 10.1128/aac.00840-13
53. Ahmed MAA, Ahmed AA, Omar SM, Adam GK, Abdallah TM, and Ali AA. Epidemiology of visceral leishmaniasis among children in Gadarif hospital, eastern Sudan. BMC Public Health. (2016) 16:1234. doi: 10.1186/s12889-016-3875-2
54. Nozzi M, Del Torto M, Chiarelli F, and Breda L. Leishmaniasis and autoimmune diseases in pediatric age. Cell Immunol. (2014) 292:9–13. doi: 10.1016/j.cellimm.2014.08.004
55. Böbel TS, Hackl SB, Langgartner D, Jarczok MN, Rohleder N, Rook GA, et al. Less immune activation following social stress in rural vs. urban participants raised with regular or no animal contact, respectively. Proc Natl Acad Sci. (2018) 115:5259–64. doi: 10.1073/pnas.1719866115
56. World Health Organization. Visceral leishmaniasis: control strategies and epidemiological situation update in East Africa: report of a WHO bi-regional consultation Addis Ababa, Ethiopia, 9–11 March 2015. World Health Organization. p. 2015. Available online at: https://iris.who.int/handle/10665/190168 (Accessed March 9–11, 2015)
Keywords: visceral leishmaniasis, treatment outcomes, pediatrics, clinical features, Gondar, Ethiopia
Citation: Abere H, Abere A, Abebaw D, Mekonnen GA, Cherkos T, Alemayehu M and Kassa M (2025) Visceral leishmaniasis treatment outcome and associated factors among pediatric patients in Northwest Ethiopia: a seven-year retrospective cohort data analysis (2013–2019). Front. Trop. Dis. 6:1566971. doi: 10.3389/fitd.2025.1566971
Received: 26 January 2025; Accepted: 23 May 2025;
Published: 30 July 2025.
Edited by:
Alfred Kwesi Manyeh, University of Health and Allied Sciences, GhanaReviewed by:
Eugenia Carrillo, Carlos III Health Institute (ISCIII), SpainElham Kazemirad, Tehran University of Medical Sciences, Iran
Copyright © 2025 Abere, Abere, Abebaw, Mekonnen, Cherkos, Alemayehu and Kassa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aberham Abere, YWJlcmhhbWFiZXIyMUBnbWFpbC5jb20=
 Haregewoyn Abere1,2
Haregewoyn Abere1,2 Aberham Abere
Aberham Abere Dessie Abebaw
Dessie Abebaw Gizework Alemnew Mekonnen
Gizework Alemnew Mekonnen