- 1Instituto Biomédico, Departamento de Microbiologia e Parasitologia, Universidade Federal do Estado do Rio de Janeiro, Rio de Janeiro, Brazil
- 2Programa de Pós Graduação em Biologia Animal, Universidade Federal Rural do Rio de Janeiro, Rio de Janeiro, Brazil
- 3Programa de Pós-graduação em Ciências Biológicas, Universidade Federal do Estado do Rio de Janeiro, Rio de Janeiro, Brazil
- 4Laboratório de Diptera, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil
The Atlantic Forest encompasses forests, sandbanks, mangroves and high-altitude grasslands. This biome has high biodiversity, including more than 15,000 species of endemic vascular plants. This study aimed to inventory and analyze the Calliphoridae and Mesembrinellidae fauna at Três Picos State Park, in Cachoeiras de Macacu, Rio de Janeiro, Brazil. Two traps containing preserved beef liver bait were installed at each site in a gradient from 0 to 1.000 meters from the edge. Samples of 15 species were collected quarterly, between June 2021 and May 2023. The specimens were euthanized with an ethyl alcohol and ethyl acetate solution, then transferred to the laboratory for identification based on morphological characteristics observed under a stereoscope microscope and consultation of descriptions and taxonomic keys. The species were classified according to abundance and frequency, and diversity indices were calculated. A total of 5,476 dipterans of 15 species were collected, of which five were Calliphoridae (77.1%), with Lucilia eximia (Wiedemann, 1819) (59,3%) and Hemilucilia segmentaria (Fabricius, 1805) (12,9%) being the most abundant species. Ten were Mesembrinellidae (22.9%), with Mesembrinella bellardiana (Aldrich, 1922) (16,3%) and Laneella nigripes (Guimarães, 1977) (4,5%) being the most abundant species. Six species were common and one was rare in terms of abundance, and five were constant and seven accidentals in frequency. Rényi’s diversity profile varied throughout the sampling period, with higher richness and diversity in winter and lower diversity in autumn. We highlight the occurrence of Mesembrinella currani (Guimarães, 1977), a species previously known to be restricted to the Amazon Forest.
Introduction
The Atlantic Rainforest comprises different types of vegetation, including forests, sandbanks, mangroves and high-altitude fields. Covering only 7% of its original territory, this biome is considered one of the world’s 36 hotspots due to its extraordinary diversity of more than 15,000 endemic vascular plant species (1, 2). In addition to its flora, the Atlantic Rainforest if home to one of the world’s greatest concentrations of endemic animal species, This biome extends across 17 Brazilian states but has suffered constant anthropogenical pressure due to resource extraction and intense urbanization (3–5). Biodiversity loss due to human action is one of the primary reasons for establishing environmental protection areas, and has been the subject of extensive debate (6–8). Consequently, the number of environmental protection areas in Atlantic Rainforest fragments increased in recent years (5). However, due to its high biodiversity and intricate biotic interactions, it merits even greater concern (6, 9).
The class Insecta is the most diverse group of animals, containing three times more species than all other groups combined. Insects are ecologically important, as pollinators, carriers of pathogen-causing agents, parasites, economic pests and decomposers (10, 11). Diptera is one of the most diverse insect orders, and is present in almost all environments and niches (12, 13). The Calliphoridae family, commonly known as blowflies (14), is widely distributed around the world, with over 1,000 species and around 150 recognized genera (15). The immature stages of this family can be biontophagous (feeding on healthy tissue from a living host), scavengers (feeding on decomposing organic matter, such as garbage, feces and carcasses), or necrobiontophagous (feeding on necrotic tissue in living hosts). These maggots cause obligate and facultative myiasis, making these dipterans extremely important to animal (16) and human health (17). In forensic entomology, data from their biology has been used to solve criminal cases by identifying suspects through molecular analyses, evidencing the transposition of corpses, detecting ingested chemical substances, and estimating the postmortem interval (PMI) (18–21).
The Mesembrinellidae family, previously considered a subfamily of the Calliphoridae family (22), exhibits similar scavenging habits. This small group of exclusively Neotropical flies is highly related to forest areas, where they are abundant and diverse. They are considered potential biological indicators of preserved forest areas and respond to different types of environmental impacts (6, 19, 20, 23).
However, the forest species of Calliphoridae and Mesembrinellidae are little known. This is largely due to the difficulty of rearing or keeping these insects out of their habitat for extended periods, which makes observing their bionomy difficult (4, 6, 23). Furthermore, the taxonomic classification of these dipterans is still the subject of extensive discussion. Mesembrinellidae has been elevated to family status, ceasing to be a subfamily of Calliphoridae (22, 24). A recent study unified Albuquerquea (Mello, 1967), Eumesembrinella (Towsend, 1931), Henriquella Bonatto in Bonatto and Marinoni, 2005, Huascaromusca (Towsend, 1918), Giovanella Bonatto in Bonatto and Marinoni, 2005 and Thompsoniella (Guimarães, 1977) with Mesembrinella (Giglio-Toss, 1893) (25).
Understanding the Calliphoridae and Mesembrinellidae dipteran fauna in this Atlantic Rainforest unit will help us to better understand species diversity in these families. Therefore, this study aimed to inventory the Calliphoridae and Mesembrinellidae faunain the Três Picos State Park (TPSP), by carrying out a faunal analysis of these insect communities over two year using diversity indices (Rényi, Richness, Shannon diversity, Simpson’s Dominance and Pielou’s evenness) and constancy and frequency analyses of the collected species.
Materials and methods
Ethics statement
All research was conducted in accordance with Scientific License Number 019/2020 (Extension Number 068/2022), which was provided by the State Environmental Institute (INEA). Yellow fever vaccine was administered to all sampling personnel and the risks in the study areas were acknowledged.
Study site
Samples were collected near the headquarters of the Três Picos State Park (TPSP) in Cachoeiras de Macacu (Figure 1). The TPSP was established in 2002 to preserve remnants of the Atlantic Forest, restore degraded areas of Serra do Mar, and promote environmental education. In 2009, its jurisdiction expanded, and it now covers around 58,790 hectares encompassing the following municipalities: Cachoeiras de Macacu, Nova Friburgo, Teresópolis, Guapimirim and Silva Jardim. The TPSP is currently the largest fully protected area in the state and makes up Rio de Janeiro’s central Atlantic Forest ecological corridor. The park’s great diversity of habitats and altitude gradient, ranging from 100 to 2,310 meters, are reflected in its diverse fauna and flora. Experts consider it to be one of the priority areas for Atlantic Forest conservation in Brazil (2).
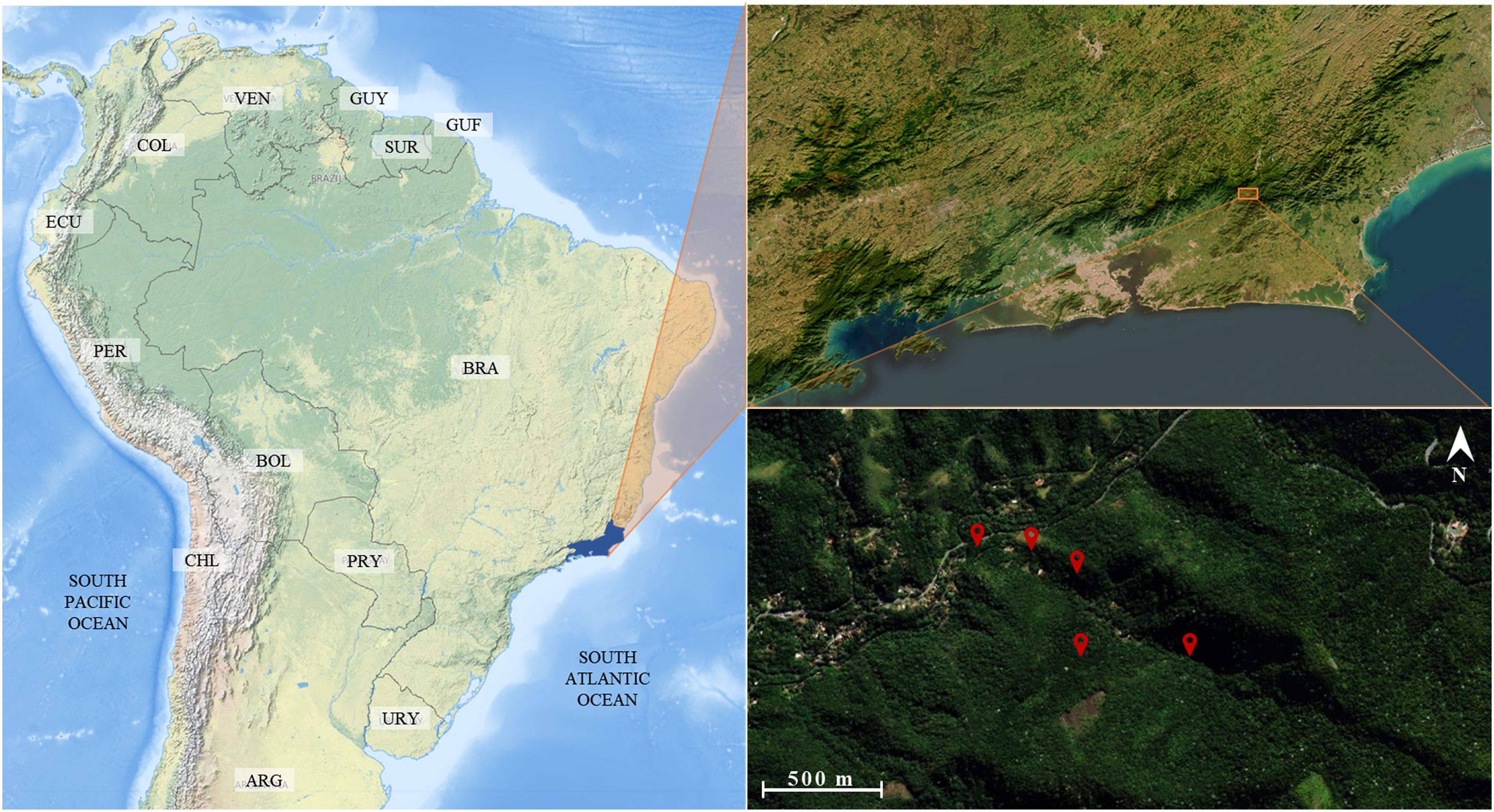
Figure 1. Location of the sampling sites on a gradient of distance (0, 200, 400, 700 and 1,000 meters) from the entrance of the Jequitibá Nucleus of Três Picos State Park, municipality of Cachoeiras de Macacu, State of Rio de Janeiro, Brazil.
The TPSP serves as both an environmental protection area and an ecological corridor between various conservation units. These include the Serra dos Órgãos National Park and the Environmental Protection Areas of Petrópolis, Macaé de Cima and Rio São João. However, in addition to the areas open to visitors, the park faces anthropogenic pressure from private properties for expropriation, irregular occupation, hunting, extraction of plants and forest fires (2).
Sampling methods
Samplings were carried out quarterly between June 2021 and May 2023, with the aim of capturing in the middle of each season. Five sites were selected along an edge gradient at distances of 0, 200, 400, 700, and 1,000 meters from the park entrance (Figure 1; Table 1) to bypass edge-effect biases. Two traps containing preserved bovine liver were exposed for 48 hours at each site. The traps were placed on trees at least five meters apart, at a height of 1,5 meters from the soil. Thus, there was a total of ten traps per sampling and 80 traps in total. The traps followed the description by Mello et al. (26), and consisted of a PVC tube base with lateral holes for insects to enter. The odor-attracting bait was placed inside the base, which was covered by a transparent polyethylene container containing a funnel that retained insects through positive phototropism (Figure 2). After sampling, the specimens were transferred to sequentially numbered polyethylene containers and recorded on field cards with a description of the sampling point and the sampling date. The collected individuals were then sacrificed using cotton wool soaked in a solution of ethyl alcohol and ethyl acetate. The containers with the specimens were transported to the Laboratory for Dipteran Studies at the Federal University of the State of Rio de Janeiro (LED-UNIRIO), where the specimens were kept in a freezer at -5°C until they were screened and identified at the species level.
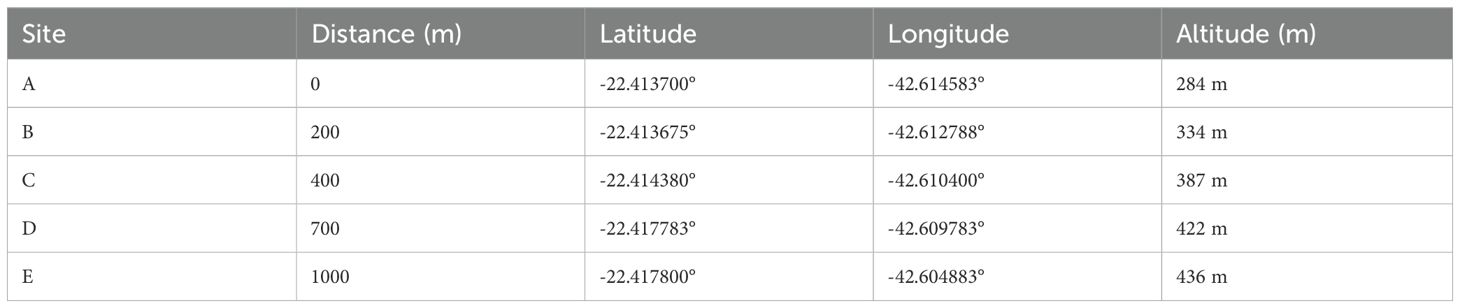
Table 1. Gerorreference of the study sites for samplings of Calliphoridae and Mesembrinellidae at distances of 0, 200, 400, 700 and 1,000 meters from the Jequitibá Nucleus entrance of Três Picos State Park, municipality of Cachoeiras de Macacu, State of Rio de Janeiro, between August 2021 and May 2023.

Figure 2. Trap following Mello et al. (26) description, used for Diptera (Calliphoridae and Mesembrinellidae) sampling at Três Picos State Park, municipality of Cachoeiras de Macacu, State of Rio de Janeiro, between June 2021 and May 2023.
Screening consisted of sorting the insects based on morphological characteristics to separate the Calliphoridae and Mesembrinellidae from other possible species collected in the traps. For taxonomic identification, the insects were dried under incident light on absorbent paper. The insects were pinned, and the species were identified based on direct observation of morphological characteristics visible under a stereoscopic microscope, as well as consultation of respective species descriptions and diagnoses using dichotomous keys developed by Mello (27) and Kosmann et al. (28), with updates by Whitworth and Yussef-Venegas (25). Some of the samples (n = 4 per sampling point, or fewer if there were fewer individuals) were pinned to be sent to the entomological samplings of the LED and the National Museum of the Federal University of Rio de Janeiro (UFRJ) The rest of the material was stored in entomological envelopes with labels containing the sampling and identification information, and kept in the LED collection.
Data on temperature, rainfall, and relative humidity from the sampling days were obtained to characterize the sampling periods. These data were obtained from the National Institute of Meteorology’s Meteorological Database (BDMEP: http://www.inmet.gov.br/) for the Salinas meteorological station, Nova Friburgo (A624). The mean values for each sampling period were computed.
Statistical analysis
Species abundances were tabulated in Excel and imported into RStudio 2023.12.0 + 369 for statistical analyses. A Coleman accumulation curve was produced to assess the sufficiency of the sampling effort. Following the methodology proposed by (29), species were classified according to their frequency as follows: constant (occurring in more than 50% of the samples), accessory (occurring between 25 and 50% of the samples), or accidental (occurring in less than 25% of the samples). According to their abundance, species were classified as common (52 or more individuals), intermediate (three to 51 individuals) and rare (one or two specimens) following the methodology of (29). To compare the abundance of the most abundant species across the studied seasons, we applied the Kruskal-Wallis test, followed by the Conover post hoc test. The Rènyi diversity profile was plotted using the “renyi” and “plot” functions from the “vegan” package. to the plots were used to characterize species diversity, highlighting the richness (S), diversity (Shannon-Wiener index, H’), dominance (inverted Simpson index, 1-D), and evenness (Pielou index, J’) indices.
Results
A total of 5,476 dipterans representing 15 species from the Calliphoridae and Mesembrinellidae families were collected at the TPSP during the two-year study period (Table 2). Of those, 77.1% were representatives of five Calliphoridae species, with Lucilia eximia (Wiedemann, 1819) (Diptera: Calliphoridae) as the most abundant (59.3% of the total collected). The Mesembrinellidae family accounted for 22.9% of the dipterofauna, contributing ten species, with Mesembrinella bellardiana (Aldrich, 1922) (Diptera: Mesembrinellidae) as the most abundant species (16.3% of the total). The sampling effort was sufficient to characterize the dipteran community, as indicated by the asymptotic trend in Coleman’s species accumulation curve. (Figure 3).
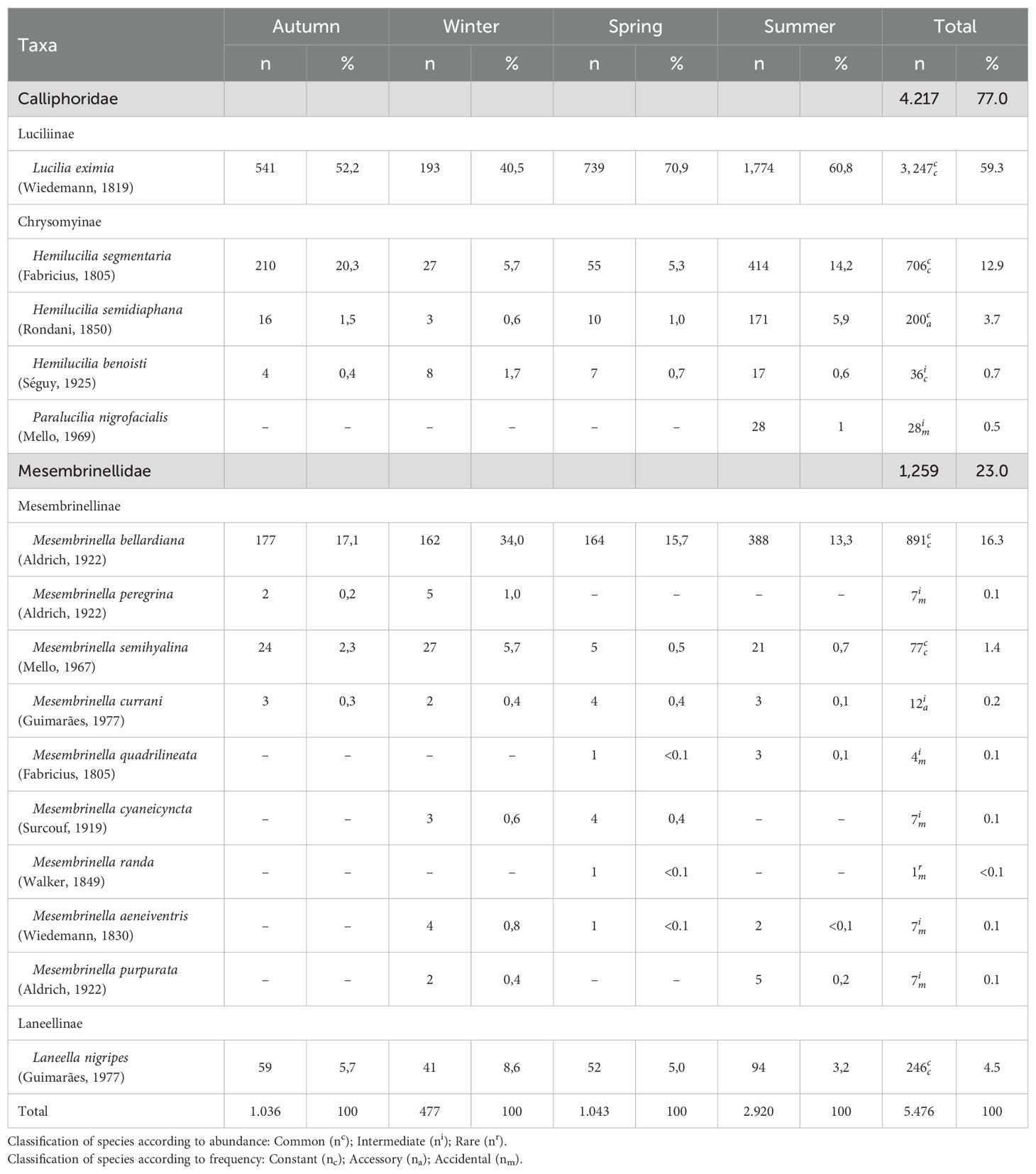
Table 2. Absolute and relative abundance of species of the families Calliphoridae and Mesembrinellidae captured in different seasons at the Jequitibá Nucleus of Três Picos State Park, municipality of Cachoeiras de Macacu, State of Rio de Janeiro, between August 2021 and May 2023.
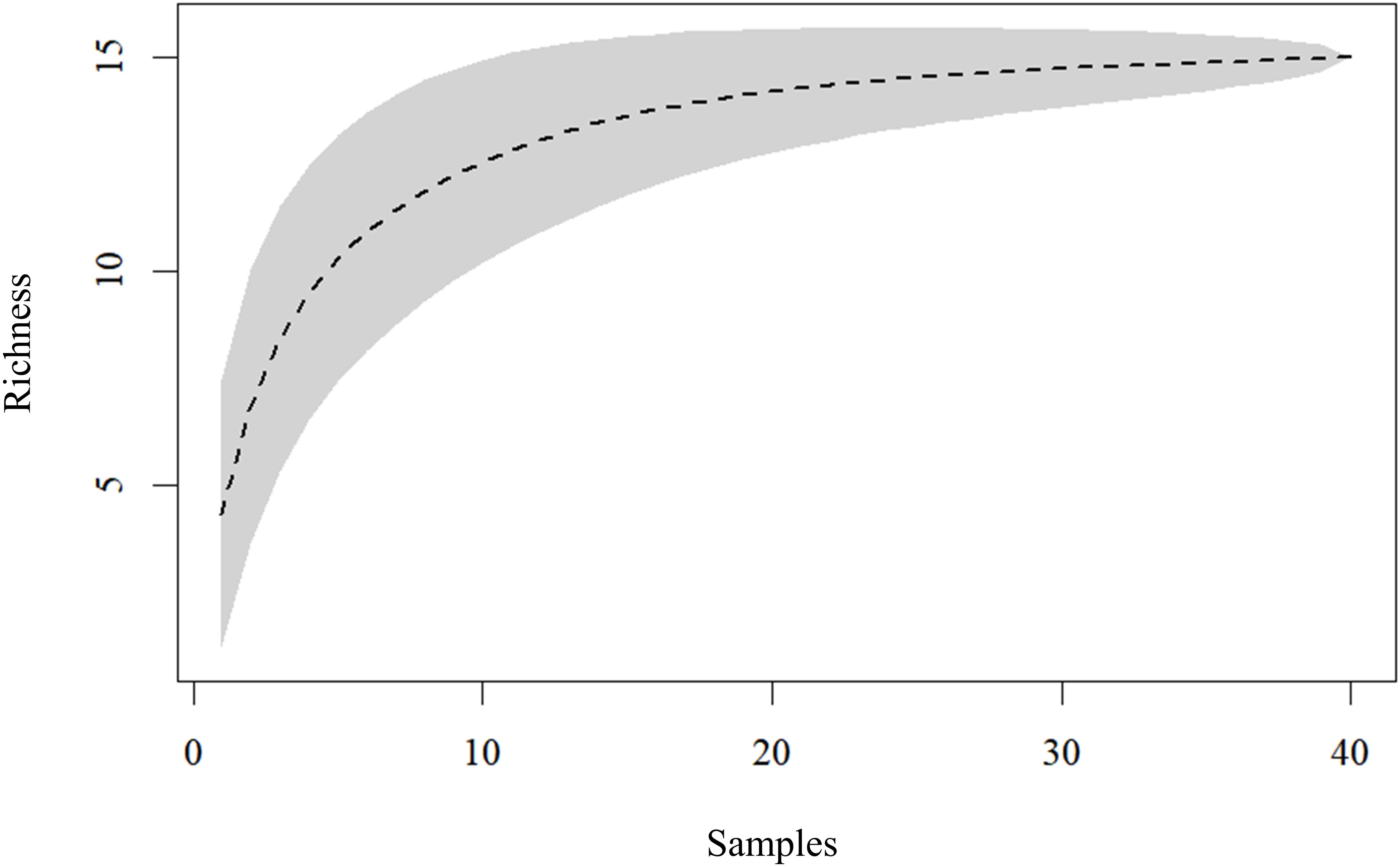
Figure 3. Coleman collector’s curve illustrating the sampling effort of Calliphoridae and Mesembrinellidae collected at Três Picos State Park, municipality of Cachoeiras de Macacu, State of Rio de Janeiro, between June 2021 and May 2023.
Analyzing the fauna in terms of species abundance (Table 2), the following species were classified as common, with abundances greater than 51 specimens: Hemilucilia segmentaria (Fabricius, 1805) (Diptera: Calliphoridae), Hemilucilia semidiaphana (Rondani, 1850) (Diptera: Calliphoridae), L. eximia, Laneella nigripes (Guimarães, 1977) (Diptera: Mesembrinellidae), M. bellardiana and Mesembrinella semihyalina (Mello, 1967) (Diptera: Mesembrinellidae). Eight species were considered intermediate, with abundances between three and 51 individuals: Hemilucilia benoisti (Séguy, 1925), (Diptera: Calliphoridae), Paralucilia nigrofacialis (Mello, 1969) (Diptera: Calliphoridae), Mesembrinella peregrina (Aldrich, 1922) (Diptera: Mesembrinellidae), Mesembrinella currani (Guimarães, 1977) (Diptera: Mesembrinellidae), Mesembrinella quadrilineata (Fabricius, 1805) (Diptera: Mesembrinellidae), Mesembrinella cyaneicyncta (Surcouf, 1919) (Diptera: Mesembrinellidae), Mesembrinella aeneiventris (Wiedemann, 1830) (Diptera: Mesembrinellidae) and Mesembrinella purpurata (Aldrich, 1922) (Diptera: Calliphoridae). Mesembrinella randa (Walker, 1849) (Diptera: Mesembrinellidae) was the only rare species, with only one specimen occurring in the spring.
It is numerically possible to observe the preference of some species for certain seasons. The abiotic variables of temperature, relative humidity, and rainfall are available in Table 3. Paralucilia nigrofacialis occurred exclusively in the summer, while other species, such as Lucilia eximia and H. segmentaria, were more abundant in this season, especially in the summer of 2022. Hemilucilia semidiaphana was abundant in both summers (Figure 4). Mesembrinella bellardiana showed the highest peak in summer 2023 and a lower peak in autumn 2022. Laneella nigripes was most abundant in summer 2022 and did not change during the other seasons. Kruskal-Wallis tests show significant differences in the abundances of the evaluated species across the seasons: L. eximia (p = 0.0046), H. segmentaria (p = 0.0262), H. semidiaphana (p = 0.0109), M. bellardiana (p = 0.0015), and L. nigripes (p = 0.0117). The Conover post-tests highlight the differences across seasons. Lucilia eximia exhibited higher abundance in the summer of 2022 than in winter (p = 0.0002) and autumn (p = 0.0130) of 2021, and winter of 2022 (p = 0.0136). Winter of 2021 also showed lower abundance than the spring of that year (p = 0.0223). Hemilucilia segmentaria also had higher abundance during the summer of 2022 compared to the winter of 2021 (p = 0.032) and spring of 2022 (p = 0.036). Similar patterns were observed for H. semidiaphana, which was more abundant in the summer of 2022 than in the winter of 2021 (p = 0.019) and spring of 2022 (p = 0.019). Mesembrinella bellardiana had lower abundance in the winter of 2021 than in the winter (p = 0.0283) and autumn (p = 0.0092) of 2022, and summer of 2023 (p = 0.0001). The autumn of 2021 exhibited lower abundance than the autumn of 2022 (p = 0.0175) and the summer of 2023 (p = 0.0003). This species also exhibited lower abundance in the spring of 2021 compared to the summer of 2023 (p = 0.0167). Comparing the abundance of L. nigripes across seasons reveals that it was lower in the winter of 2021 compared to the winter (p = 0.0338), autumn (p = 0.0159), and summer (p = 0.0032) of 2022.
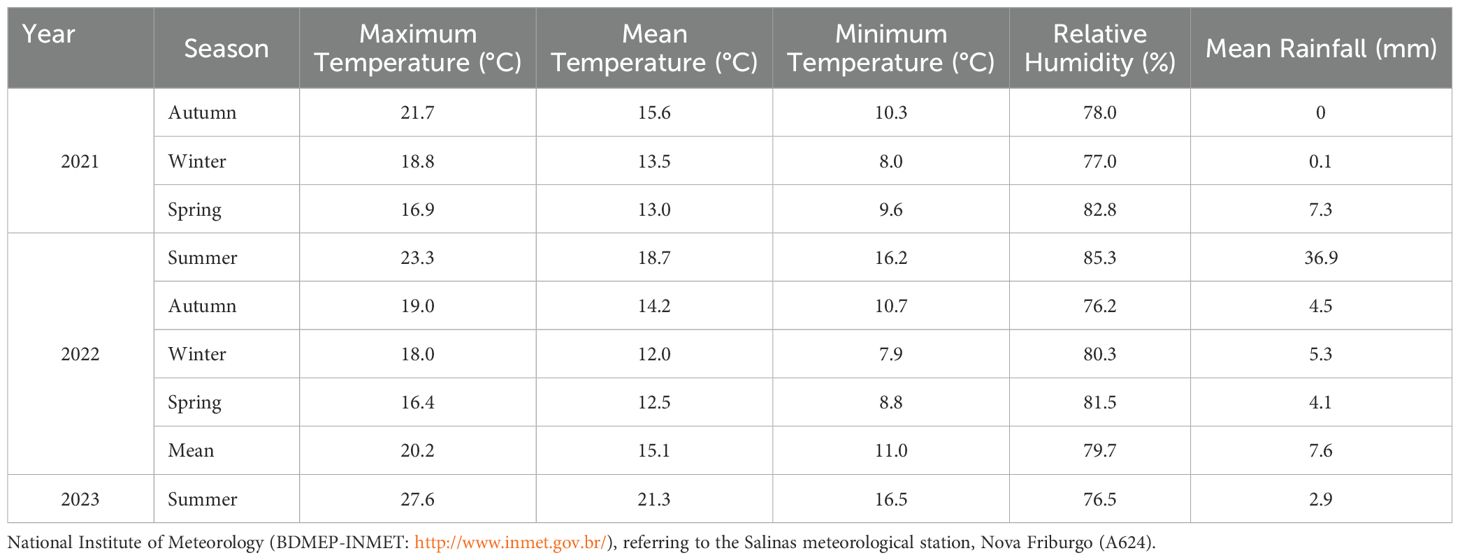
Table 3. Temperature (°C), Relative Humidity (%) and Precipitation (mm) measurements during the sampling periods of Calliphoridae and Mesembrinellidae in the Jequitibá Nucleus of Três Picos State Park, municipality of Cachoeiras de Macacu, State of Rio de Janeiro, between August 2021 and May 2023.
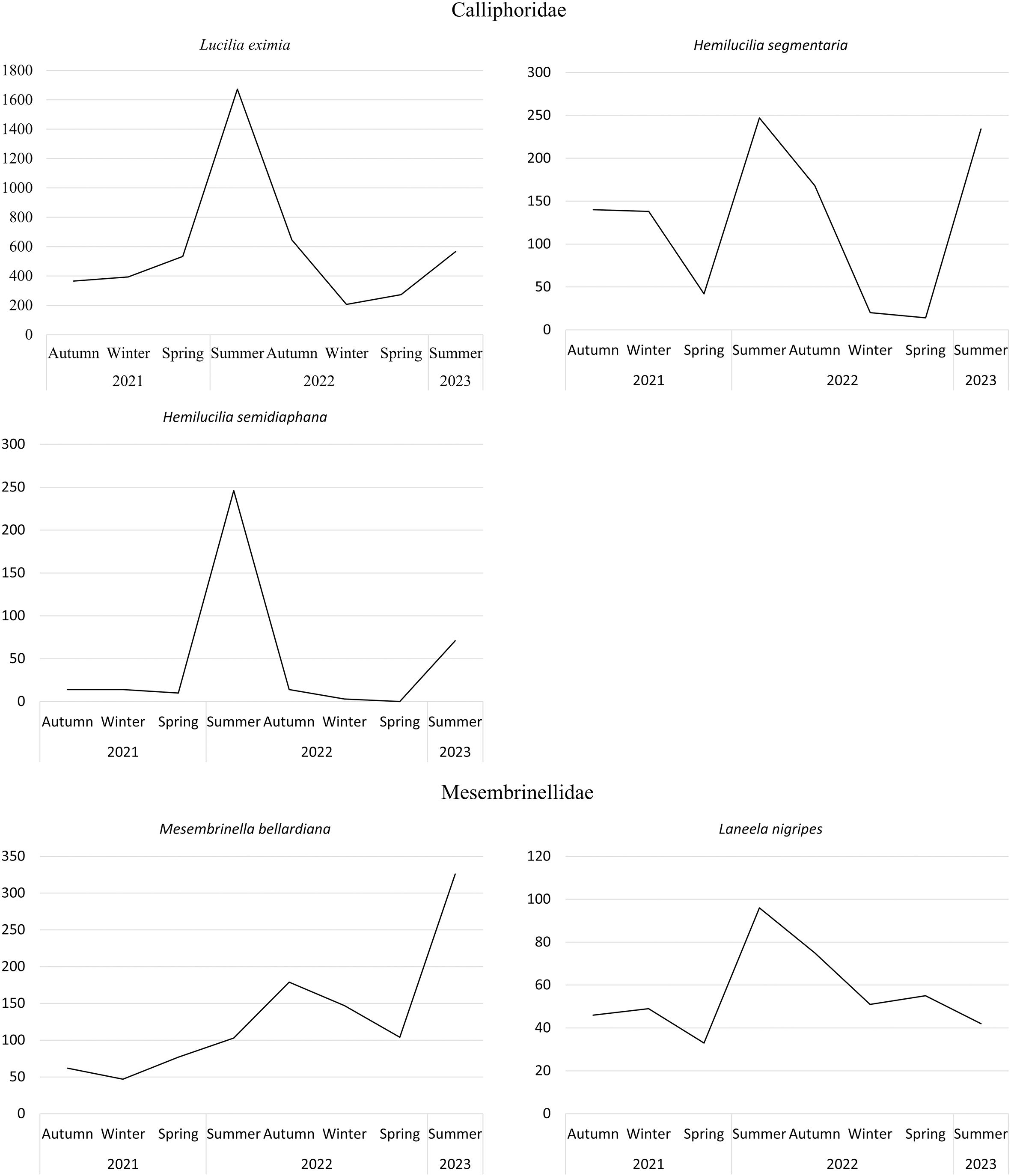
Figure 4. Abundance of the most abundant species collected in the Três Picos State Park, Cachoeiras de Macacu, Rio de Janeiro, Brazil, throughout the seasons between June 2021 and May 2023.
In terms of frequency, H. segmentaria, L. eximia, La. nigripes, M. bellardiana, and M. semihyalina were considered constant species, occurring in more than 50% of the samples. H. semidiaphana, H. benoisti, and M. currani were considered accessory species, occurring in 25-50% of samples. Paralucilia nigrofacialis, M. peregrina, M. quadrilineata, M. cyaneicyncta, M. randa, M. aeneiventris and M. purpurata were considered accidental, occurring in less than 25% of samples.
Analysis of Rényi’s diversity profile by sampling period (Figure 5, Table 4) revealed the highest richness (alpha = 0) during winter (S = 15), while the lowest number of species (S = 11) was observed in autumn. Winter also had the highest diversity (alpha = 1, H = 1.451) and evenness (alpha = inf, J = 0.528). The lowest diversity was observed during the spring (H = 1.066), which also showed the highest dominance (alpha = 2, 1-D = 0.507) and consequently the lowest evenness (alpha = inf, J = 0.416). Overall, the park exhibited a diversity (alpha = 1) of H = 1.304. Dominance (alpha = 2) was 1-D = 0.602, and evenness (alpha = inf) was 0.482.
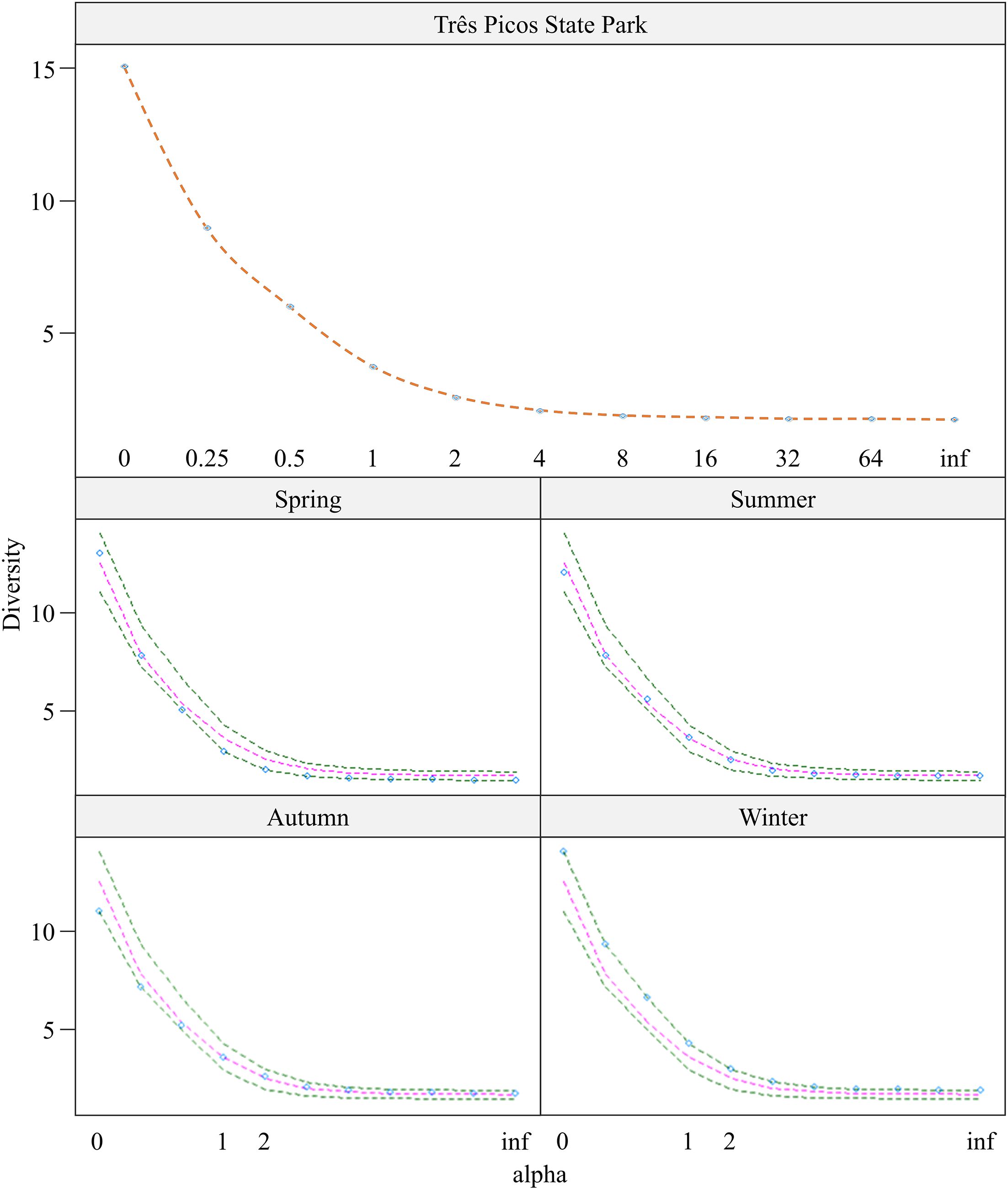
Figure 5. Rényi diversity profile, by climatic season, of the Calliphoridae and Mesembrinellidae samplings captured at different points in the Jequitibá Nucleus of the Três Picos State Park, municipality of Cachoeiras de Macacu, State of Rio de Janeiro, between June 2021 and May 2023.
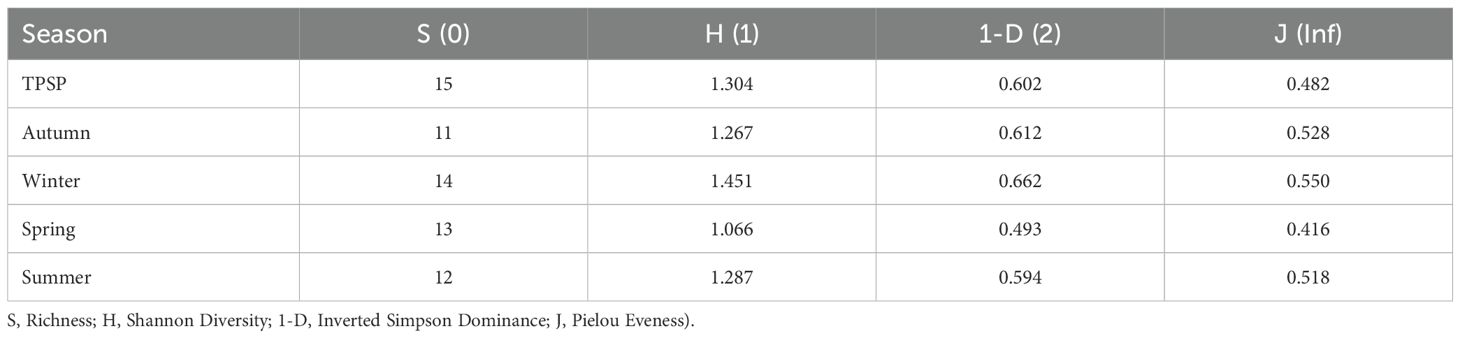
Table 4. Rényi diversity profile, by climatic season, of the Calliphoridae and Mesembrinellidae samplings captured at different sites in the Jequitibá Nucleus of Três Picos State Park (TPSP), municipality of Cachoeiras de Macacu, State of Rio de Janeiro, between August 2021 and May 2023.
Discussion
Scavenging dipterans play a fundamental ecological role in nutrient cycling. The immature stages of some saprophagous species can develop on decaying plant and animal matter, as well as on excrement and other organic waste. Maggots can become contaminated by direct contact with microorganisms living in these substrates. Adults, in turn, frequent these environments to obviate and feed and can carry pathogenic agents when they land on food, our body and surfaces we use, potentially transferring those pathogens and causing diseases and infections. Studies by Förster et al. (30, 31) in rural Germany identified various potentially harmful fungi and bacteria in synanthropic flies, including Enterobacter aerogenes, Enterococcus faecium, Staphylococcus aureus, Mucor sp., and different strains of Escherichia coli. In Thailand, Chaiwong et al. (32) found that the presence of C. megacephala flies contaminated with potentially harmful bacteria was correlated with the frequency of diarrhea cases. A review study by Khamesipour et al. (33) also observed the occurrence of viruses in Musca domestica Lineaus. Taenia spp. eggs have been found on the bodies of Calliphoridae in various studies, as reviewed by Benelli et al. (34). Thus, these insects can carry pathogens and are of great economic and health importance.
In the medical field, these dipterans are also relevant because they cause myiasis, an infestation of living vertebrates that can debilitate or even kill the host (35, 36). Conversely, scavenger species can be used to promote debridement, disinfection, and healing of chronic wounds in a treatment known as biotherapy or larval therapy (37). These insect’s scavenging habits also allow them to be used in forensic entomology, the study of insects and other arthropods to help solve criminal or civil cases (38). Lucilia eximia has been reported to cause human myiasis (39) and exhibits synanthropic tolerance, as observed with H. segmentaria and H. semidiaphana (40). Thus, these species are potentially medically relevant as they can carry pathogens and infest humans and animals. Additionally, they have been used several times as estimators of postmortem intervals, helping to elucidate forensic investigations (20, 41, 42).
On the other hand, these organisms act as pollinators (43), and serve as food for spiders, beetles, amphibians, birds, and mites in the trophic chain (44). Finally, these dipterans can be used as bioindicators. Santos Jr. et al. (45) observed that different species of Chrysomya Robineau-Desvoidy, 1830 were able to detect and bioaccumulate heavy metals present in the soil during larval development. They can also indicate environmental quality because some species have very specific synanthropic preferences (i.e., a preference to human-altered environments) (40). Mesembrinellidae are exclusively forest-dwelling and Neotropical. Mesembrnella bellardiana and La. nigripes are often reported as abundant in well-preserved forest areas. Despite the marked presence of Calliphoridae, the presence of M. bellardiana in forest remnants is common in the literature (46–48), showing that this species has great plasticity. Of particular note, M. currani’ known distribution is expanded by this study since previous records were limited to the Northern and Northeastern regions of Brazil and other countries farther North (40). The occurrence of this species suggests that there is efficient habitat connectivity between the Atlantic and the Amazon forests, or that there was in the past. The distribution may also been underestimated due to a lack of studies. Another possibility is that this species is expanding or rearranging its biogeographical distribution for some reason (e.g., climatic changes, habitat loss). However, further studies are necessary to test these hypotheses.
Calliphoridae species, on the other hand, thrive in warmer environments, finding favorable conditions for reproduction, which may explain their high abundance during the summer. Carmo et al. (49), when studying an Atlantic Forest fragment in Recife, Brazil, found only two species from the Mesembrinellidae family, M. bellardiana and Mesembrinella bicolor (Fabricius, 1805), in low abundance. Meanwhile, species from the Calliphoridae family occurred in high abundance. Another noteworthy finding of this study was the replacement of Calliphoridae species. The genus Hemilucilia (Brauer, 1895), as well as the species Lucilia eximia, are commonly abundant in forest environments but become less abundant as the environment becomes drier and more open. N these environments, species of the genera Cochliomyia (Coquerel, 1858), and Chrysomya begin to dominate.
When studying a 2,000-meter edge gradient of the Tinguá Biological Reserve, in Rio de Janeiro, using sardines as bait, Ferraz et al. (50) and Gadelha et al. (4) found H. semidiaphana, La. Nigripes, and M. bellardiana in high abundance, as well as L. eximia, H. segmentaria, M. semihyalina, M. aeneiventris, M. quadrilineata, P. nigrofacialis, M. randa and M. purpurata in lower abundance. However, these authors did not record H. benoisti, M. currani or M. cyaneicyncta. They also recorded 12 additional species of Calliphoridae and three species of Mesembrinellidae that were not found in the present study, likely due to the difference in bait used.
When studying the fauna of scavenging dipterans using sardines in the Tijuca National Park, Gadelha et al. (23) recorded the occurrence of L. eximia, M. bellardiana, H. segmentaria and La. nigripes, as common and constant species, as well as H. semidiaphana as the fourth most abundant species. They also recorded M. aeneiventris and M. peregrina in low abundance, as well as eight additional species of Calliphoridae and two of Mesembrinellidae that were not recorded in the TPSP. However, they did not record two species of Calliphoridae and five species of Mesembrinellidae that were observed in this study.
Carvalho et al. (19) and Azevedo et al. (20), in studies also carried out in the Tijuca National Park using rat carcasses as bait, found three s Mesembrinellidae species that were also present in the Três Picos State Park, L. eximia, H. segmentaria and H. semidiaphana. The differences in richness may have been due to the baits used. Bovine liver has been used successfully to attract necrophagous and biontophagous flies (40, 51, 52). Bovine liver is sometimes used as bait to collect fertile females of the screwworm Cochliomyia hominivorax, which are used in eradication programs (53). Due to its strong odor, sardine bait proved to be very effective in capturing scavenging dipterans, allowing for a higher richness to be captured. Unlike the liver used in this study, rat carcass bait has a milder odor. Additionally, studies show that using a whole organism can be more attractive, depending on each species’ preferences of each species due to the release of volatile organic compounds from different organs (48, 49, 54). Other factors that may explain the difference in dipterofauna observed between these Atlantic Forest environments include the degree of preservation, variations in climate and microhabitats, and the geographical location of the study sites. Altitude can be a barrier for some species, and other geographical barriers may occur between latitudes and longitudes.
Species constancy likely reflects their biological adaptability to environmental conditions (55), since they persist in environments suffering minimal impact from biotic and abiotic variations. Rare species are most susceptible to extinction, which defines patterns of diversity. These species play important roles in biological communities and perform essential ecological functions and processes, such as nutrient cycling, decomposition, trophic interactions. They influence how species respond to human activities. Organizations such as the IUCN use them as a criterion for identifying key biodiversity areas, covering species that are most appealing to interventions (56, 57). A species’ frequency seems to reflect its population size and its fluctuations. Constant species have stable populations and are sampled throughout most of the study. Accidental species, on the other hand, may undergo fluctuations due environmental variations, experiencing reduced populations in certain periods and consequently decreasing their likelihood of being sampled. Accidental species may also occur due to ineffective traps for a particular taxon.
Although the literature indicates that rare species are the majority in terms of richness, our study revealed only Mesembrinella randa to be rare and accidental. As expected, a few more common and dominant species accounted for nearly all of the observed abundance, demonstrating their high degree of adaptation to this environment. These species have been recorded by other authors in Atlantic Forest environments, as previously discussed.
Thus, the diversity profiles reflect what has already been discussed. Mesembrinellidae species, which are typically forest-dwelling, are adapted to milder climates and more humid environments, while Calliphoridae species benefit from higher temperatures. For this reason, the highest levels of richness, diversity, and evenness were observed during the winter, when the greater abundance of Mesembrinellidae reduces the dominance of Calliphoridae. During the summer and autumn, when Calliphoridae species find more suitable conditions and multiply significantly, becoming dominant and decreasing diversity and evenness. The seasonal variation was not significant for the Mesembrinellidae species, with only a slight increase in abundance during the summer for M. bellardiana and La. nigripes. This suggests that these organisms are well adapted to variations in this environment. A more in-depth analysis of the biotic and abiotic parameters influencing this community during the sampling periods will be presented in another article. Other potential causes of this variation, such as resource availability, could not be measured during this study.
Conclusion
The dipterofauna of the Três Picos State Park consists of five Calliphoridae species and 11 Mesmebrinellidae species Seven of these species are common (occurring in more than 51 individuals), is rare (occurring in one individual), five are constant (occurring in more than 50% of samples) and seven are accidental (occurring in less than 25% of samples). The most abundant Calliphoridae species are Lucilia eximia and Hemilucilia segmentaria. The most abundant Mesembrinellidae species are Mesembrinella bellardiana and Laneella nigripes. Diversity indices vary according to the seasonal climate period of sampling. The greatest richness, diversity, and evenness are recorded during the winter climate period.
Finally, this study reports a unique record of Mesembrinella currani occurring in this biome, updating its current distribution from the Amazon Forest to include the Atlantic Forest. This observation may fill a data gap, since Mesembrinellidae are little studied. However, it may also suggest more serious scenarios, such as habitat loss and climate changes.
Besides providing useful data for identifying species of medical and forensic relevance, knowledge of the diversity of dipterans in different forest remnants, along with knowledge of the biology and ecology of the species, helps us understand the dynamics of these environments and identify the need for protective actions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
WA: Writing – original draft, Writing – review & editing, Formal Analysis, Methodology, Conceptualization. MN: Methodology, Writing – original draft, Writing – review & editing. VMLA: Writing – review & editing, Formal Analysis, Methodology. CL: Conceptualization, Methodology, Writing – review & editing. JA: Conceptualization, Formal Analysis, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. VMA: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, E-26/211.439/2021 (FAPERJ- https://www.faperj.br/-VMA); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, grant number 88882.426021/2019- 01), (CAPES - https://www.gov.br/capes/pt-br/-WTAA); Conselho Nacional de Desenvolvimento Científico e Tecnológico (Grant number: 303286/2021-0, JA) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (E-26/200.956/2002/2022, JA).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. CEPF – Critical Ecosystem Partnership Fund. Perfil do Ecossistema Mata Atlântica, Hotspot de Biodiversidade, Brasil (2001). Available online at: https://www.cepf.net/sites/default/files/atlantic-forest-ecosystem-profile-2001-portuguese.pdf (Accessed October 23, 2024).
2. INEA – Instituto Nacional de Ecologia e Ambiente. Parque Estadual dos Três Picos (2020). Available online at: http://www.inea.rj.gov.br/Portal/Agendas/BIODIVERSIDADEEAREASPROTEGIDAS/UnidadesdeConservacao/INEA_008598/Sobreoparque (Accessed December 13, 2024).
3. Turner RM and Collet RT. The conservation value of small, isolated fragments of lowland tropical rain forest. Trends Ecol Evol. (1996) 11:330–3. doi: 10.1016/0169-5347(96)10046-X
4. Gadelha BQ, Ribeiro AC, Aguiar VM, and Mello-Patiu CA. Edge effects on the blowfly fauna (Diptera, Calliphoridae) of the Tijuca National Park, Rio de Janeiro, Brazil. Braz J Biol. (2015) 75:999–1007. doi: 10.1590/1519-6984.05614
5. ICMBIO - Instituto Chico Mendes de Conservação da Biodiversidade. Mata atlântica (2019). Available online at: http://www.icmbio.gov.br/portal/unidadesdeconservacao/biomas-brasileiros/mata-atlantica (Accessed October 21, 2024).
6. Ferraz ACP, Gadelha BQ, and Aguiar-Coelho VM. Influência climática e antrópica na abundância e riqueza de Calliphoridae (Diptera) em fragmento florestal da Reserva Biológica do Tinguá, RJ. Neotrop Entomol. (2010) 39:476–85:4. doi: 10.1590/S1519-566X2010000400004
7. Battán-Horenstein BLM. Diversity of necrophagous blowfly (Diptera: Calliphoridae) of medical and veterinary importance in urban environments in Córdoba, Argentina. Caldasia. (2016) 38:189–95. doi: 10.15446/caldasia.v38n1.57837
8. Mendes TP, Esposito MC, Carvalho-Filho FS, Juen L, Alvarado ST, and Sousa JRP. Necrophagous flies (Diptera: Calliphoridae and Sarcophagidae) as indicators of the conservation or anthropization of environments in eastern Amazonia, Brazil. J Insect Conserv. (2021) 25:719–32. doi: 10.1007/s10841-021-00338-3
9. Dufek MI, Oscherov EL, Damborsky MP, and Mulieri PR. Calliphoridae (Diptera) in human-transformed and wild habitats: diversity and seasonal fluctuations in the humid chaco ecoregion of south america. J Med Entomol. (2019) 56:725–36. doi: 10.1093/jme/tjy234
10. Serra-Freire NM and Mello RP. Entomologia e acarologia na medicina veterinária. Livros LF, editor. Rio de Janeiro: de Veterinária Ltda. (2006). p. 200.
11. Borghesan TC, Campaner M, Matsumoto TE, Espinosa AO, Razafindranaivo V, Paiva F, et al. Genetic diversity and phylogenetic relationships of coevolving symbiont-harboring insect trypanosomatids, and their Neotropical dispersal by invader African blowflies (Calliphoridae). Front Microbiol. (2018) 9:131. doi: 10.3389/fmicb.2018.00131
12. Skevington JH and Dang PT. Exploring the diversity of flies (Diptera). Biodiversity. (2002) 3:3–27. doi: 10.1080/14888386.2002.9712613
13. Borror DJ, Triplehorn CA, and Johnson NF. An Introduction to the Study of Insects. 7a. Philadelphia: Saunders College Publishing (2005). p. 818.
15. Vargas J and Wood DM. Calliphoridae. In: Brown BV, Borkent A, Cumming JM, Wood DM, Woodley NE, and Zumbado MA, editors. Manual of Central American Diptera. NCR Research Press, Canada (2010). p. 1297–304.
16. Baumhover AH. Erradication of the screw worm fly, an agent of myiasis. J Am Med Assoc. (1966) 166:240–8. doi: 10.1001/jama.1966.03100160090027
17. Azevedo WTA, Figueiredo AL, Carvalho RP, Lemos GA, Silva PF, Miranda TA, et al. Record of the first cases of human myiasis by Lucilia cuprina (Diptera: Calliphoridae), Rio de Janeiro, Brazil. J Med Entomol. (2015) 52:1368–73. doi: 10.1093/jme/tjy234
18. Oliveira-Costa J. Entomologia Forense: quando os insetos são os vestígios. 3. Campinas, São Paulo, Brazil: Millenium (2011). p. 513.
19. Carvalho RP, Azevedo WTA, Figueiredo AL, Lessa CSS, and Aguiar VM. Dipterofauna associated with rat carcasses in the Atlantic Forest, Southeastern Brazil. J Med Entomol. (2017) 54:1498–509. doi: 10.1093/jme/tjx118
20. Azevedo WTA, Carvalho RP, Figueiredo AL, Ross SD, Lessa CSS, Fortes RDR, et al. Calliphoridae (Diptera) associated with Rattus rattus carcasses in the Tijuca National Park, Rio de Janeiro, Brazil. J Med Entomol. (2018) 55:915–22. doi: 10.1093/jme/tjy234
21. Pontes LPP, Frances PAC, and Magalhães LKA. Agentes tóxicos e o desenvolvimento de insetos: uma revisão bibliográfica e sua aplicabilidade em entomotoxicologia. Rev Bras Criminal. (2021) 11:30–7. doi: 10.15260/rbc.vlli2.502
22. Marinho MAT, Wolff M, Ramos-Pastrana Y, Azeredo-Espin AML, and Amorim DS. The first phylogenetic study of Mesembrinellidae (Diptera: Oestroidea) based on molecular data: clades and congruence with morphological characters. Cladistics. (2017) 33:134–52. doi: 10.1111/cla.12157
23. Gadelha BQ, Silva AB, Ferraz ACP, and Aguiar VM. Mesembrinellinae (Diptera: Calliphoridae) to edge effects in the Tinguá Biological Reserve, Rio de Janeiro, Brazil. Braz J Biol. (2015) 75:S196–205. doi: 10.1590/1519-6984.10214
24. Guimarães JHA. A systematic revision of the Mesembrinellidae, stat. nov. (Diptera, Cyclorrhapha). Arquivos Zool. (1977) 29:1–109. doi: 10.11606/issn.2176-7793.v29i1p1-109
25. Whitworth TL and Yussef-Venegas S. A revision of the genera and species of the Neotropical family Mesembrinellidae (Diptera: Oestroidea). Zootaxa. (2019) 4659:001–146. doi: 10.11646/zootaxa.4659.1.1
26. Mello RS, Queiroz MMC, and Guiar-Coelho VM. Population fluctuations of Calliphorid species (Diptera, Calliphoridae) in the Biological Reserve of Tinguá, state of Rio de Janeiro, Brazil. Iheringia Série Zool. (2007) 97:481–5. doi: 10.1590/S0073-47212007000400019
27. Mello RP. Chave para a identificação das formas adultas das espécies da família Calliphoridae (Diptera, Brachycera, Cyclorrhapha) encontradas no Brasil. Entomol y Vect. (2003) 10:255–68.
28. Kosmann C, Mello RP, Harterreiten-Souza ES, and Pujol-Luz JR. A list of current valid blow fly names (Diptera: Calliphoridae) in the Americas South of Mexico with a key to the Brazilian species. EntomoBrasilis. (2013) 6:74–85. doi: 10.12741/ebrasilis.v6i1.266
29. Lima ES. Fauna edáfica em sistemas agroflorestais (tradicional e cabruca) e floresta nativa. Pará, Brazil: Monography (Faculdade de Engenharia Florestal, da Universidade Federal do Pará (2019) p. 13–4. Available at: https://bdm.ufpa.br/jspui/handle/prefix/3539 (Accessed November 7, 2024).
30. Förster M, Klimpel S, Mehlhorn H, Severt K, Messler S, and Pfeffer K. Pilot study on synanthropic flies (e.g. Musca, Sarcophaga, Calliphora, Fannia, Lucilia, Stomoxys) as vectors of pathogenic microorganisms. Parasitol Res. (2007) 101:243–6. doi: 10.1007/s00436-007-0522-y
31. Förster M, Sievert K, Messler S, Klimpel S, and Pfeffer K. Comprehensive study on the occurrence and distribution of pathogenic microorganisms carried by synanthropic flies caught at different rural locations in Germany. J Med Entomol. (2009) 46:1164–6. doi: 10.1603/033.046.0526
32. Chaiwong T, Srivoramas T, Sueabsamran P, Sukontason K, Sanford MR, and Sukontason KL. The blow fly, Chrysomya megacephala, and the house fly, Musca domestica, as mechanical vectors of pathogenic bacteria in Northeast Thailand. Trop Biomed. (2014) 24:336–46.
33. Khamesipour F, Lankarani KB, Honarvar B, and Kwenti TE. A systematic review of human pathogens carried by the housefly (Musca domestica L.). BMC Public Health. (2018) 18:1049. doi: 10.1186/s12889-018-5934-3
34. Benelli G, Wassermann M, and Brattig NW. Insects dispersing taeniid eggs: Who and how? Vet Parasitol. (2021) 295:109450. doi: 10.1016/j.vetpar.2021.109450
35. Azevedo WTA, Rodrigues FT, Nunes MP, Coelho TA, Cardozo MRP, Silva LK, et al. Perfil Socioepidemiológico e diagnóstico entomolígico de pacientes acometidos com miíase no Rio de Janeiro. In: Coletânea nacional sobre entomologia, 3. Ponta Grossa, Paraná, Brazil: Atena (2020) doi: 10.22533/at.ed.399200110
36. Bugelli V, Tarozzi I, Galante N, Bortolini S, and Franceschetti L. Review on forensic importance of myiasis: Focus on medicolegal issues on post-mortem interval estimation and neglect evaluation. Legal Med. (2023) 63:102263. doi: 10.1016/j.legalmed.2023.102263
37. Ferraz. ACP, Gadelha. GBDQ, and Aguiar-Coelho. VM. Análise faunística de calliphoridae (diptera) da reserva biológica do Tinguá, Nova Iguaçu, rio de janeiro. Rev Bras Entomol. (2009) 53:620–8. doi: 10.1590/S0085-56262009000400012
38. Filho CRD and Francez PADC. Introdução à biologia forense. 2a. Campinas, São Paulo, Brazil: Campinas/SP (2018). p. 448.
39. Sanford MR, Whitworth TL, and Phatak DR. Human Wound Colonization by Lucilia eximia and Chrysomya rufifacies (Diptera: Calliphoridae): Myiasis, Perimortem, or Postmortem Colonization? J Med Entomol. (2014) 51:716–9. doi: 10.1603/ME13229
40. Nunes MP, Azevedo WTA, Silva AS, Lessa CSS, Alencar JA, and Aguiar VM. Synanthropy and ecological aspects of Calliphoridae and Mesembrinellidae (Diptera: Oestroidea) in three ecological areas in Rio de Janeiro State, Brazil. PLoS One. (2023) 18:e0285844. doi: 10.1371/journal.pone.0285844
41. Kosmann C, Macedo MP, Barbosa TAF, and Pujol-Luz JR. Chrysomya albiceps (Wiedemann) and Hemilucilia segmentaria (Fabricius) (Diptera, Calliphoridae) used to estimate the postmortem interval in a forensic case in Minas Gerais, Brazil. Rev Bras Entomol. (2011) 55:621–3. doi: 10.1590/S0085-56262011000400022
42. Silva SM and Moura MO. Intrapuparial development of hemilucilia semidiaphana (Diptera: calliphoridae) and its use in forensic entomology. J Med Entomol. (2019) 26:1623–35. doi: 10.1093/jme/tjz118
43. Saeed S, Naqqash MN, and Jaleel W. The effect of blow flies (Diptera: Calliphoridae) on the size and weight of mangos (Mangifera indica L.). PeerJ. (2016) 5:e2076. doi: 10.7717/peerj.2076
44. Marchiori CH. Calliphoridae family (Insecta: diptera) of importance in forensic entomology, larval therapy, medical, veterinary and vector of pathogens. J Modern Agric Biotechnol. (2022) 1:23. doi: 10.53964/jmab.2022020
45. Santos EG Jr., Andrade-Silva J, Rocha LC, Silva CPC, Oliveira JD, and Sousa JRP. Exploring the potential of three species of blowfly, genus Chrysomya, as bioindicators of heavy metals in the Brazilian Cerrado savanna. Entomol Experiment Applic. (2025) 00:1–11. doi: 10.1111/eea.13542
46. Ururahy-Rodrigues A, Rafael JA, and Pujol-Luz JR. Temporal distribution of blowflies of forensic importance (Diptera: calliphoridae), in man-size domestic pigs carcasses, in the forest reserve adolpho ducke, manaus, amazonas, Brazil. EntomoBrasilis. (2013) 6:9–22. doi: 10.12741/ebrasilis.v6i1.242
47. Vasconcelos SD, Barbosa TM, and Oliveira TPB. Diversity of forensically-important dipteran species in different environments in northeastern Brazil, with notes on the attractiveness of animal baits. Florida Entomol. (2015) 98:770–5. doi: 10.1653/024.098.0256
48. Oliveira DL, Soares TF, and Vasconcelos SD. Effect of bait decomposition on the attractiveness to species of Diptera of veterinary and forensic importance in a rainforest fragment in Brazil. Parasitol Res. (2016) 115:449–55. doi: 10.1007/s00436-015-4811-6
49. Carmo RFR, Oliveira DL, Barbosa TM, Soares TF, Souza JRB, and Vasconcelos SD. Visitors versus colonizers: an empirical study on the use of vertebrate carcasses by necrophagous diptera in a rainforest fragment. Ann Entomol Soc Am. (2017) 110:492–500. doi: 10.1093/aesa/sax045
50. Ferraz ACP, Gadelha BQ, Queiroz MMC, Moya-Borja GE, and Aguiar-Coelho VM. Effects of forest fragmentation on dipterofauna (Calliphoridae) at the Reserva Biológica do Tinguá, Nova Iguaçu, RJ. Braz J Biol. (2010) 70:55–63. doi: 10.1590/S1519-69842010000100009
51. Medeiros R, Pédra MG, Fonseca LS, Duarte JLP, Zafalon-Silva Â, Marques R, et al. Effects of burned liver on necrophagous flies in southern Brazil. Rev Bras Entomol. (2024) 68:e20240048. doi: 10.1590/1806-9665-RBENT-2024-0048
52. Gonçalves JDS, Azevedo WTA, Albuquerque VML, Nunes MP, Thomaz GS, Cordioli LA, et al. Faunistic survey and diversity analysis of Calliphoridae (Insecta: Diptera) in Campo de Santana, Rio de Janeiro, Brasil. J Med Entomol. (2025) 62:552–9. doi: 10.1093/jme/tjaf025
53. Skoda SR, Philips PL, and Welch JB. Screwworm (Diptera: calliphoridae) in the United States: response to and elimination of the 2016–2017 outbreak in florida. J Med Ensomol. (2018) 55:777–86. doi: 10.1093/jme/tjy049
54. Oliveira DL and Vasconcelos SD. Diversity, daily flight activity and temporal occurrence of necrophagous diptera associated with decomposing carcasses in a semi-arid environment. Neotrop Entomol. (2018) 47:470–7. doi: 10.1007/s13744-017-0540-0
55. Lemes EM and Garutti V. Ecologia da ictiofauna de um córrego de cabeceira da bacia do alto Rio Paraná, Brasil. Iheringia Série Zool. (2002) 92:69–78. doi: 10.1590/S0073-47212002000300007
56. Dee LE, Cowles J, Isbell F, Pau S, Gaines SD, and Reich PB. When do ecosystem services depend on rare species? Trends Ecol Evol. (2019) 34:746–58. doi: 10.1016/j.tree.2019.03.010
Keywords: asynanthopes, biodiversity, blowflies, neotropical, rainforest
Citation: Azevedo WTA, Nunes MP, Albuquerque VML, Lessa CSS, Alencar J and Aguiar VM (2025) Inventory and faunistic analysis of Calliphoridae and Mesembrinellidae at Três Picos State Park, Brazil. Front. Trop. Dis. 6:1589167. doi: 10.3389/fitd.2025.1589167
Received: 07 March 2025; Accepted: 17 June 2025;
Published: 23 July 2025.
Edited by:
Edwin Michael, University of South Florida, United StatesReviewed by:
Karina Salvatierra, Universidad Nacional de Misiones, ArgentinaStephany Alejandra Rodríguez, National Autonomous University of Mexico, Mexico
Copyright © 2025 Azevedo, Nunes, Albuquerque, Lessa, Alencar and Aguiar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: J. Alencar, amFsZW5jYXJAaW9jLmZpb2NydXouYnI=; V. M. Aguiar, dmFsZXJpYUB1bmlyaW8uYnI=
 W. T. A. Azevedo
W. T. A. Azevedo M. P. Nunes
M. P. Nunes V. M. L. Albuquerque
V. M. L. Albuquerque C. S. S. Lessa
C. S. S. Lessa J. Alencar
J. Alencar V. M. Aguiar
V. M. Aguiar