Abstract
Ticks and tick-borne diseases (TBDs) are escalating health and veterinary threats in the Global South, driven by environmental change, human activity, and socioeconomic vulnerability. Ticks transmit bacterial, viral, and protozoal pathogens, causing significant public health and economic burdens. Climate shifts and land-use changes have expanded tick habitats, intensifying disease transmission. This review examines the distribution of major tick species in the Global South and explores how ecological disruptions influence disease dynamics. Regional case studies from Africa, the Middle East, and South Asia highlight the impact on human health, livestock productivity, and food security. Addressing this growing threat requires integrated One Health strategies, improved public awareness, enhanced veterinary services, and investment in surveillance and vaccine development. International cooperation and strong policy frameworks are vital to mitigate the spread and impact of TBDs.
Introduction
Ticks are blood-feeding ectoparasites that affect both humans and animals by transmitting a variety of infectious agents, including bacteria, viruses, and protozoa. These pathogens cause tick-borne diseases (TBDs), such as Lyme disease, tick-borne encephalitis, Crimean-Congo hemorrhagic fever (CCHF), babesiosis, anaplasmosis, and ehrlichiosis (1, 2). Globally, TBDs present a serious threat to public health, animal welfare, and the economy. In livestock, these diseases lead to reduced milk yield, impaired growth, reproductive losses, and mortality. It is estimated that TBDs affect approximately 80% of the 1.2 billion cattle population worldwide, contributing to economic losses of up to $7 billion annually (3). The burden is especially severe in the Global South, where limited access to diagnostic tools, veterinary services, and effective therapeutics impedes disease control. Compounding these challenges are inadequate research funding and limited pharmaceutical interest in vaccine development. These issues threaten not only animal productivity but also public health and food security in vulnerable communities. Environmental changes, particularly rising global temperatures, shifting precipitation patterns, deforestation, and land-use alterations, have further expanded the geographic range of ticks and TBDs (4–6). These drivers enable ticks to establish in previously unaffected areas, increasing human and animal exposure to pathogens.
In light of these challenges, a robust One Health approach—integrating human, animal, and environmental health—remains central to reducing the burden of TBDs in the Global South. However, additional efforts are needed to complement this approach. These include strengthening local diagnostic capacity, establishing region-specific disease surveillance systems, promoting cross-border collaborations, and integrating technological tools such as GIS for real-time monitoring. Climate-resilient agricultural practices and public-private partnerships can also help mitigate disease emergence.
This paper reviews the impact of climate and environmental changes on the prevalence and distribution of ticks and TBDs in the Global South, their implications for human and animal health, and regional economies.
Distribution and prevalence of ticks and tickborne diseases in the Global South
The Global South—which includes countries in Africa, Latin America, Asia, and Oceania—is characterized by diverse socio-economic and ecological conditions that support a wide range of tick species. Many of these ticks act as vectors for diseases affecting human and animal health, posing significant public health and veterinary challenges.
Tick-borne diseases (TBDs) remain a major constraint to livestock production in the region due to the transmission of bacterial, protozoal, and viral pathogens (7–9). Both hard ticks (Ixodidae) and soft ticks (Argasidae) are prevalent, thriving in favorable tropical and subtropical climates (7, 10). A review of literature spanning 1901 to 2020 identified 55 tick species across eight genera—Amblyomma, Dermacentor, Haemaphysalis, Hyalomma, Ixodes, Ornithodoros, Otobius, and Rhipicephalus—with Rhipicephalus being the most dominant genus (7, 11).
Common TBDs in the Global South include anaplasmosis, babesiosis, theileriosis, cowdriosis, and Lyme disease (12, 13). The most significant causative agents—Babesia, Anaplasma, Theileria, and Ehrlichia species—pose serious threats to animal productivity and human health (7, 14). Of the 116 tick species known to transmit TBDs globally, 84 belong to the Ixodidae family and 32 to Argasidae (15, 16). The highest burden of these diseases is observed in Africa and Asia, where environmental and ecological conditions support tick survival and proliferation (11).
In Sudan, several tick species—including Hyalomma, Rhipicephalus, and Amblyomma—are known to transmit babesiosis, anaplasmosis, and theileriosis, all of which contribute to substantial veterinary and economic losses (7). Similarly, in Egypt, Hyalomma and Rhipicephalus species are primary vectors of Crimean-Congo hemorrhagic fever (CCHF) and babesiosis (17, 18). In Mauritania, Hyalomma rufipes and Rhipicephalus evertsi dominate the tick population and are associated with multiple TBDs (19).
In the Middle East, Hyalomma dromedarii has been reported as the major vector of CCHF in camel-rearing regions of Saudi Arabia and the United Arab Emirates (20). Further east, countries such as Yemen, Jordan, and Oman continue to contribute to the regional TBD burden. In these areas, Hyalomma impeltatum, Rhipicephalus sanguineus, and Hyalomma anatolicum are among the key species implicated in the transmission of babesiosis and theileriosis (7).
The epidemiology of TBDs in the Global South is shaped by distinct ecological, climatic, and socio-environmental factors that vary widely across regions. In Southeast Asia (SEA), a tropical region comprising 11 countries and over 620 million people, at least 97 tick species have been identified—Haemaphysalis being the most diverse genus (3). A broad spectrum of pathogens, including Anaplasma, Borrelia, Babesia, Coxiella, Ehrlichia, Rickettsia, and Theileria, circulate in the region (21). Despite its high ecological suitability for vector-borne diseases, limited surveillance systems and diagnostic infrastructure, particularly in rural areas, contribute to underreporting and misdiagnosis (3). The relationship between tick species, pathogens, and hosts varies across regions of the Global South and is summarized in Table 1.
Table 1
| Region | Tick Species / Genus | Pathogens | Disease(s) | Primary Host(s) | References |
|---|---|---|---|---|---|
| Southeast Asia | Haemaphysalis spp., Ixodes spp. | Anaplasma, Borrelia, Babesia, Coxiella, Ehrlichia, Rickettsia, Theileria | Multiple febrile illnesses (e.g., rickettsiosis, babesiosis) | Livestock, wildlife, humans | (3, 21) |
| South Asia (India) | Hyalomma anatolicum, H. marginatum | Kyasanur Forest Disease Virus (KFDV) | Kyasanur Forest Disease (KFD) | Monkeys, small mammals, humans | (24) |
| East Africa | Hyalomma spp. | Rift Valley Fever Virus (RVFV) | Rift Valley Fever (RVF) | Cattle, goats, sheep, humans | (25) |
| Sub-Saharan Africa | Amblyomma spp., Hyalomma spp., Rhipicephalus spp. | Rickettsia, Anaplasma, Ehrlichia, Babesia, Theileria, Dugbe virus, Bhanja virus, CCHFV | Ehrlichiosis, babesiosis, theileriosis, CCHF | Livestock (cattle, goats), wildlife, humans | (22) |
| Southern Africa (South Africa) | Rhipicephalus spp., Amblyomma spp. | Rickettsia, Anaplasma, Ehrlichia, Babesia, Theileria | Tick-borne bacterial and protozoal diseases | Cattle, small ruminants | (23) |
Summary of tick-borne diseases (TBDs) by region, tick species, pathogens, diseases, and hosts.
As shown in Figure 1, In Sub-Saharan Africa (SSA), nine tick genera—including Amblyomma, Hyalomma, and Rhipicephalus—play critical roles in transmitting both zoonotic and non-zoonotic diseases, such as babesiosis, ehrlichiosis, rickettsiosis, borreliosis, and anaplasmosis, as well as viral infections like Crimean-Congo hemorrhagic fever, Dugbe virus, and Bhanja virus (22). In South Africa, Rickettsia, Anaplasma, Ehrlichia (formerly Cowdria), Babesia, and Theileria have been found at high prevalence in cattle across multiple provinces (23), reflecting strong endemic transmission cycles in livestock.
Figure 1
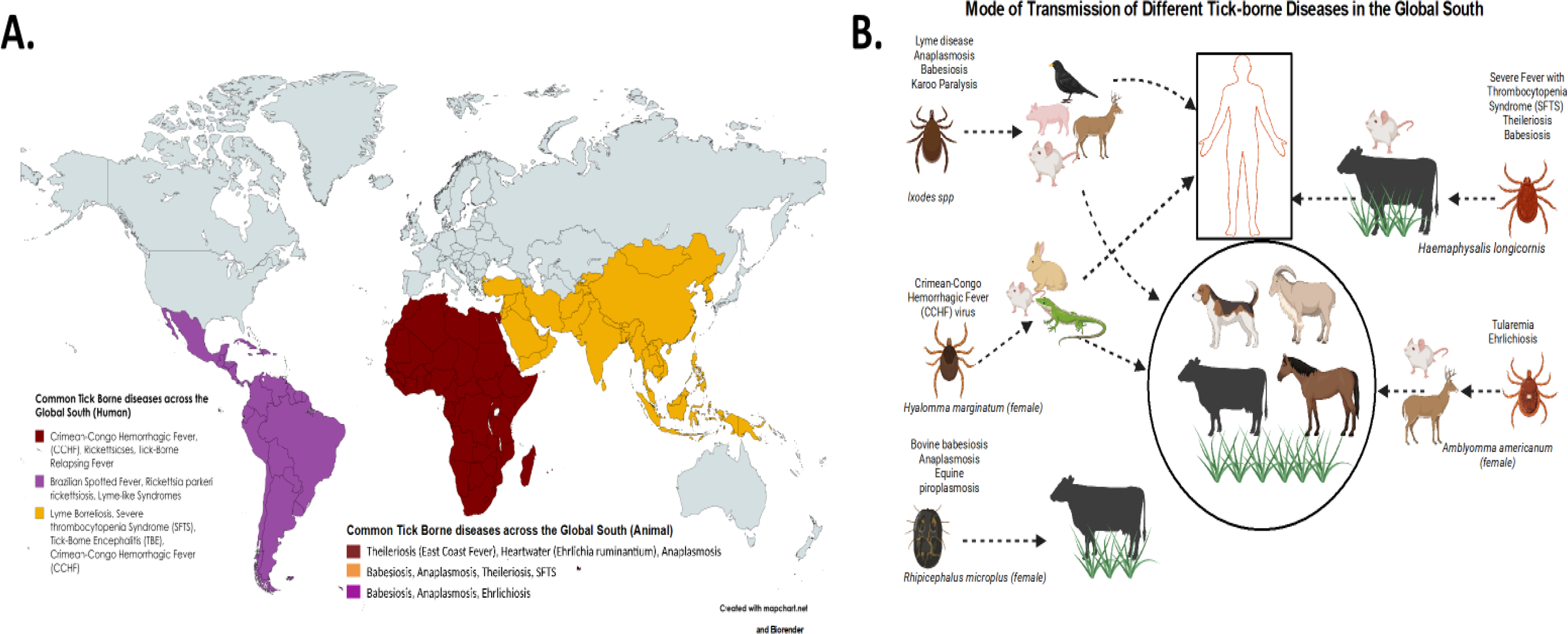
Geographic distribution of medically important tick species and tick-borne diseases (TBDs) in selected countries of the Global South (A). The map highlights regions with documented tick diversity, vector–host-pathogen interactions, and reported TBD outbreaks, including Rift Valley Fever (RVF), Kyasanur Forest Disease (KFD), and Crimean-Congo Hemorrhagic Fever (CCHF). Countries represented include: Uganda, Kenya, Tanzania, Rwanda, Burundi, South Sudan, Nigeria, South Africa, Sudan, Egypt, Mauritania, Saudi Arabia, India, Pakistan, Bangladesh, Sri Lanka, Malaysia, Thailand, Vietnam, Myanmar, Cambodia, Laos, and Indonesia. Ixodes spp. Uses birds, ungulates, small rodents, and Suidae as intermediate hosts to infect humans and domestic animals with Lyme disease, anaplasmosis, babesiosis, and Karo paralysis. Hyalomma marginatum females use leporids, members of Lacertidae, and rodents as intermediate hosts to infect humans and domestic animals with Crimean-Congo Hemorrhagic Fever (CCHF) virus. Rhipicephalus microplus female ticks infect domestic animals with bovine babesiosis, anaplasmosis, and equine piroplasmosis. Amblyomma americanum female ticks use rodents and ungulates to infect domestic animals with tularemia and ehrlichiosis. Haemaphysalis longicornis causes babesiosis, theileriosis in domestic animals (B).
Regional case studies further illustrate the variation in vector–host-pathogen dynamics. In East Africa, for example, Hyalomma species are involved in the complex ecology of Rift Valley fever (RVF), where 67 outbreaks were recorded between 2010 and 2024 across Uganda, Rwanda, Kenya, Tanzania, Burundi, and South Sudan (25). These outbreaks are closely linked to climatic conditions such as heavy rainfall and flooding, which create ideal breeding environments for RVF-transmitting mosquitoes and promote interactions between infected livestock and humans. Conversely, in the dry forested regions of southern India, Hyalomma anatolicum and H. marginatum are primary vectors of Kyasanur Forest Disease (KFD), a viral hemorrhagic fever maintained in a sylvatic cycle involving monkeys and small mammals. A case-control study in Thirthahalli, Shivamogga, identified residences near recent monkey deaths and household tick exposure as significant risk factors for human infection (24). The limited awareness among residents further exacerbates the spread of the disease, emphasizing the importance of community education and localized vector surveillance. These show that there is ecological heterogeneity of TBDs in the Global South and the need for geographically tailored prevention strategies. While tropical zones support a high diversity of vectors and pathogens, arid and forested environments can also sustain unique transmission cycles that pose severe public health risks.
The Global South comprises countries primarily in Africa, Latin America, Asia, and Oceania, characterized by diverse socio-economic and ecological conditions. This region is home to numerous tick species, many of which are vectors for human and animal diseases, posing significant public health and veterinary challenges.
Environmental changes and tick ecology
Environmental and climate changes significantly influence tick ecology and the transmission of tick-borne diseases (TBDs). Rising temperatures, shifts in rainfall, and humidity changes affect tick survival, reproduction, and geographic distribution (26–30). Warmer climates accelerate tick development and expand their range into higher altitudes and latitudes, as seen with Ixodes scapularis establishing in southern Canada (27). However, extreme heat and prolonged drought may hinder tick survival due to desiccation stress (31). Moisture levels, particularly high humidity, support tick activity by enhancing host-seeking behavior (32). In addition, land-use changes such as deforestation alter tick-host dynamics by bringing wildlife reservoirs (e.g., deer, rodents) into closer proximity with humans, thereby increasing TBD transmission risk (33, 34). These interconnected environmental shifts highlight the complex drivers of TBD emergence and spread. Urbanization fragments habitats and creates ecotones where ticks and their hosts can thrive. Similarly, agricultural expansion increases the availability of livestock hosts, promoting the persistence of pathogens such as Babesia and Anaplasma (33). Additionally, globalization and human mobility contribute to the spread of ticks and TBDs. International trade and cross-border livestock movement have introduced ticks like Rhipicephalus microplus to new regions, while Hyalomma marginatum has been transported via agricultural goods (35, 36). Companion animals such as dogs also play a role, as ticks like Rhipicephalus sanguineus can be transported over long distances when pets travel with their owners (7).
Implications of ticks and tick-borne diseases on human health
Tick-borne diseases (TBDs) pose a significant public health challenge in the Global South due to the interplay of environmental, biological, human, and socioeconomic factors (37–40). Limited healthcare infrastructure, high levels of tick exposure, and poor public awareness contribute to higher morbidity and mortality compared to high-income regions.
The region’s biodiversity creates a complex web of interactions between humans, domestic animals, and wildlife. Wildlife species serve as natural reservoirs for tick-borne pathogens, while domestic animals often act as bridges for transmission between wildlife and human populations (41). In many parts of Africa, extensive agriculture and transhumance—where farmers move with their livestock—facilitate frequent contact with ticks, increasing disease transmission risk (36). These practices also contribute to the cross-border spread of emerging pathogens, complicating regional disease control efforts (42).
Clinically, TBDs often present with nonspecific symptoms such as fever, chills, fatigue, and body pain (43). Some infections, like Lyme disease, may be associated with rashes, while others, such as Crimean-Congo hemorrhagic fever (CCHF), can lead to rapid deterioration involving hemorrhaging and organ failure (44). Beyond acute symptoms, patients may suffer from long-term effects such as joint pain, neurological complications, and cardiovascular issues, which can evolve into life-threatening conditions like meningoencephalitis, myocarditis, and acute respiratory distress syndrome (45). These chronic sequelae contribute to sustained public health burdens (46).
These burdens disproportionately affect vulnerable groups. Rural populations, which depend heavily on livestock for food and income, are at heightened risk due to close contact with potentially infected animals (23). Children are also more susceptible, as their developing immune systems offer less protection and they spend more time outdoors. In countries like South Africa, smallholder farmers experience high infection rates worsened by limited access to preventive resources and health information.
Implications of ticks and tick-borne diseases on animal health
Ticks are among the most significant ectoparasites affecting livestock in tropical and subtropical regions, particularly in the Global South. They cause substantial economic losses both through direct effects such as blood-feeding and through their role as vectors of debilitating pathogens (47). These impacts reduce livestock productivity and threaten food security, especially in communities that heavily rely on animal products for sustenance and income.
Tick-borne diseases (TBDs) impair livestock performance by decreasing growth rates, reducing milk yield and quality, lowering reproductive efficiency, and, in severe cases, causing mortality (48, 49). Additionally, tick infestations lead to conditions such as "tick worry," where repeated bites and irritation cause stress and reduced feed intake. Other effects include toxin injection, hide damage, blood loss, and increased susceptibility to secondary infections (47, 50). Collectively, these effects result in reduced animal productivity and poor-quality animal products.
Socioeconomic factors such as poverty, weak healthcare systems, and limited public health education increase vulnerability to tick-borne diseases (TBDs) (51). Many individuals are unaware of exposure risks during activities like farming, hunting, and mining (52). Inadequate veterinary care worsens the situation, as untreated livestock can harbor pathogens and serve as sources of infection (36). These challenges place significant pressure on both public health systems and household economies.
The economic impact of TBDs is considerable. Direct costs—including diagnostics, treatment, and hospitalizations—strain already limited healthcare resources, while indirect costs such as lost productivity, long-term disability, and premature death further burden national economies (53). For example, Uganda loses an estimated USD 1.1 billion annually due to TBDs, and Lyme disease cost the United States approximately USD 968 million in 2016 alone (54, 55). Globally, the economic burden of TBDs in developing countries is estimated at USD 14–19 billion each year (22, 56). Rhipicephalus microplus alone accounts for significant annual losses—USD 32.4 million in Brazil, USD 573 million in Mexico, and USD 168 million in Colombia (57–59). Comparable figures are reported in Australia (USD 26 million) and India (USD 499 million) (47, 57). These statistics highlight the global scope of the issue, with more severe consequences in the Global South due to limited veterinary infrastructure and restricted access to control measures.
Beyond production losses, the financial burden includes treatment, prevention, and replacement of animals lost to disease. In regions with poor veterinary services and few food alternatives, outbreaks can drastically reduce livestock populations, worsening poverty and malnutrition, and impeding agricultural and economic development (60).
The control of animal diseases, including TBDs, is essential for livestock development in Latin America, Asia, the Caribbean, and Africa. Alongside optimal nutrition, management, and market access, disease prevention remains a cornerstone of agricultural sustainability (56). TBDs also directly impact food security by reducing the availability of meat, milk, and other animal-derived foods. Infected animals may lose 10–15% of their body weight annually (47), while dairy cows can produce up to 20% less milk than their healthy counterparts (58).
The financial burden includes not only production losses but also the cost of treatment, preventive measures, and the replacement of animals lost to disease. In regions with limited access to veterinary care and alternative food sources, outbreaks of TBDs can drastically reduce animal populations. This in turn worsens poverty and malnutrition, trapping communities in a cycle that hinders agricultural growth and economic development.
Role of animals in maintaining tick populations and tick-borne disease transmission
Ticks are obligate blood-feeding ectoparasites that rely entirely on vertebrate hosts to complete their life cycle. Globally, they are the most significant arthropod vectors of pathogens affecting animals (61). These hosts not only provide necessary blood meals but also serve as reservoirs and amplifiers of tick-borne pathogens (TBPs), thereby playing a vital role in sustaining endemic transmission cycles (39).
Because ticks have limited independent movement, their distribution is largely influenced by host behavior and mobility. Migratory and wide-ranging animal species, especially birds, are instrumental in dispersing ticks across long distances and introducing them into new geographical regions. For example, migratory birds are estimated to carry between 50 and 175 million Ixodes scapularis ticks each spring, demonstrating their critical role in tick population expansion and TBP transmission (62). Increased interactions between wildlife, migratory birds, livestock, and domestic animals elevate the risk of human exposure to infected ticks. Wildlife species in urban and peri-urban settings often act as both maintenance hosts and pathogen reservoirs. Their movement within these environments sustains local tick populations and enhances the likelihood of contact between infected ticks and human populations.
The ecological relationships among ticks, their hosts, and the environment are fundamental to the persistence and spread of TBPs. Factors such as host density, migratory patterns, and interactions between domestic and wild animals contribute to a complex network that facilitates tick survival and disease transmission. Understanding these dynamics is essential for designing effective mitigation strategies, especially in both endemic and emerging regions (63). TBDs also pose serious threats to animal health. In livestock and companion animals, diseases such as babesiosis and ehrlichiosis can cause significant morbidity and mortality. In dogs, these infections often result in severe clinical symptoms. Many TBPs are zoonotic, including Lyme disease, Rocky Mountain spotted fever, and Crimean-Congo hemorrhagic fever (CCHF), which are transmitted from animal reservoirs to humans through tick bites. As a result, the global incidence of TBDs is rising, posing increasing challenges to both public health and veterinary medicine (64).
Ticks also transmit the widest variety of zoonotic pathogens among all arthropod vectors, and while these pathogens often primarily affect animals, their zoonotic potential complicates disease control efforts (47). Moreover, ticks can maintain TBPs across generations via transovarial and transstadial transmission, ensuring pathogen persistence in the environment. Effective tick and TBD control demands integrated strategies. These include promoting host resistance, applying targeted acaricide treatments, and implementing strategic interventions that consider the seasonal dynamics of tick activity. A multifaceted, sustainable approach is necessary to reduce the overall burden of TBDs in both animals and humans (13).
Control and prevention strategies
A variety of strategies have been employed to control ticks and tick-borne diseases (TBDs), including Integrated Pest Management (IPM), acaricides, biological control, public health education, and veterinary interventions. IPM offers an eco-friendly framework that is particularly suitable for regions with limited resources and infrastructure (65).
Acaricides remain the most widely used method for tick control and are effective when applied appropriately. However, concerns about resistance and environmental impact necessitate the development of complementary strategies (66). Biological control, which utilizes natural entomopathogenic fungi such as Metarhizium anisopliae and Beauveria bassiana, has shown promise as a sustainable alternative to synthetic chemicals (67).
Public health education is critical in raising awareness about tick biology, disease transmission, and prevention practices. In the Global South, where TBD prevalence is increasing, educating both the public and healthcare professionals can facilitate early detection and prompt treatment. Veterinary interventions, especially anti-tick vaccines, play a vital role in reducing tick populations and disease transmission in livestock. For example, the BM86 vaccine, derived from Rhipicephalus microplus, has demonstrated moderate efficacy in reducing tick infestations and the transmission of associated TBDs in cattle (68). However, its effectiveness can vary depending on the tick species and regional genetic diversity (69, 70) and it offers limited protection against non-Rhipicephalus species, highlighting the need for broader-spectrum or species-specific vaccine development.
Recommendations for future research
Future research should focus on a deeper investigation of tick ecology and the transmission dynamics of TBDs—similar to advances made in other vector-borne disease systems (71, 72). This includes examining how environmental changes, host availability, and pathogen interactions drive the emergence and spread of TBDs. Moreover, targeted actions are required to address the growing burden of TBDs through improved surveillance, research, and policy implementation.
Robust and responsive surveillance systems are vital for early detection and control of tick and TBD outbreaks. Real-time monitoring using advanced technologies such as GIS and remote sensing can enhance preparedness and inform rapid interventions of TBDs and other vector-borne diseases (73). Additionally, strengthening laboratory capacity for accurate and timely diagnosis is essential, especially in under-resourced settings. Cross-border and regional collaboration in disease monitoring will support more effective data sharing, risk assessment, and coordinated response in the Global South.
Further research is needed to advance our understanding of tick ecology and disease transmission dynamics, particularly in the face of climate and environmental changes. Studies should explore the interactions among hosts, vectors, and pathogens, and how these are influenced by factors such as habitat change and host density. Moreover, investigating the genetic and molecular mechanisms that contribute to tick survival, resistance to acaricides, and vector competence is crucial for developing innovative interventions (74). Research should also prioritize investigating the genetic and molecular mechanisms that enable tick survival, adaptation, and resistance to control measures. Exploring these pathways could inform the development of novel, more effective interventions. Research should also examine the roles of wildlife and livestock in maintaining tick populations and spreading TBDs across rural, urban, and peri-urban interfaces. Additionally, investment in the development of sustainable control measures—such as anti-tick vaccines, biological control agents, and genetic approaches—should be prioritized to reduce reliance on chemical acaricides.
Furthermore, effective control of TBDs requires supportive policy frameworks that integrate One Health principles and promote collaboration across human, animal, and environmental health sectors. Governments and non-governmental organizations should work together to expand access to tick control tools, strengthen veterinary and public health services, and fund research and innovation. Policies should also support public education campaigns, workforce training, and incentives for the adoption of integrated control strategies. International cooperation will be critical in facilitating technology transfer, knowledge exchange, and the development of regional research hubs. A long-term commitment to evidence-based policy implementation will be instrumental in reducing the burden of TBDs and safeguarding health, food security, and economic development in the Global South.
Conclusion
Climate change, human activities, and socioeconomic factors are key drivers exacerbating the burden of TBDs in the Global South. These factors threaten both human and animal health by undermining public health systems, reducing livestock productivity, and straining national and regional economies.
Challenges such as inadequate veterinary infrastructure, limited public awareness, and gaps in disease surveillance hinder effective TBD prevention and control. Addressing these challenges requires a comprehensive One Health approach that integrates human, animal, and environmental health. Global collaboration is essential to mitigate the impact of TBDs. Governments and non-governmental organizations must work together to implement effective tick control measures, strengthen disease surveillance, and invest in vaccine development research.
Statements
Author contributions
SS: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. OA: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. II: Writing – original draft, Writing – review & editing. PD: Writing – original draft, Writing – review & editing. DA: Writing – original draft, Writing – review & editing. VA: Writing – original draft, Writing – review & editing. MO: Writing – original draft, Writing – review & editing. CU: Writing – original draft, Writing – review & editing. CU: Writing – original draft, Writing – review & editing. SA: Writing – original draft, Writing – review & editing. BO: Writing – original draft, Writing – review & editing. CI: Writing – original draft, Writing – review & editing. SYS: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Estrada-Peña A . Ticks as vectors: taxonomy, biology and ecology. Rev Sci Tech. (2015) 34:53–65. doi: 10.20506/rst.34.1.2345
2
Madison-Antenucci S Kramer LD Gebhardt LL Kauffman E . Emerging tick-borne diseases. Clin Microbiol Rev. (2020) 33:e00083–18. doi: 10.1128/CMR.00083-18
3
Sharifah N Heo CC Ehlers J Houssaini J Tappe D . Ticks and tick-borne pathogens in animals and humans in the island nations of Southeast Asia: a review. Acta Trop. (2020) 209:105527. doi: 10.1016/j.actatropica.2020.105527
4
Tsoumani ME Papailia SI Papageorgiou EG Voyiatzaki C . Climate change impacts on the prevalence of tick-borne diseases in Europe. Environ Sci Proc. (2023) 12:18. doi: 10.3390/environsciproc2023026018
5
Gregory N Fernandez MP Diuk-Wasser M . Risk of tick-borne pathogen spillover into urban yards in New York City. Parasites Vectors. (2022) 15:1. doi: 10.1186/s13071-022-05416-2
6
Sonenshine D . Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease. Int J Environ Res Public Health. (2018) 15:478. doi: 10.3390/ijerph15030478
7
Perveen N Muzaffar SB Ali M . Ticks and tick-borne diseases of livestock in the Middle East and North Africa: a review. Insects. (2020) 12:83. doi: 10.3390/insects12010083
8
Food and Agriculture Organization of the United Nations (FAO) . Livestock in South East Asia: notes on development and growth (2012). Available online at: http://www.fao.org/ag/againfo/home/en/news_archive/2011_Livestock_S-Asia.html (Accessed March 5, 2025).
9
Dantas-Torres F Martins TF . Ecology and epidemiology of ticks and tick-borne diseases in Brazil. Parasite. (2007) 14:244–51. doi: 10.1007/s00436-007-0696-3
10
Alanazi AD Abdullah S Helps C Wall R Puschendorf R ALHarbi SA et al . Tick-borne pathogens in ticks and blood samples collected from camels in Riyadh Province, Saudi Arabia. Int J Zool Res. (2018) 14:30–6. doi: 10.3923/ijzr.2018.30.36
11
Tan LP Hamdan RH Hassan BN Reduan MF Okene IA Loong SK et al . Rhipicephalus tick: a contextual review for Southeast Asia. Pathogens. (2021) 10:821. doi: 10.3390/pathogens10070821
12
Gayle A Ringdahl E . Tick-borne diseases. Am Fam Phys. (2001) 64:461–6.
13
Jongejan F Uilenberg G . The global importance of ticks. Parasitology. (2004) 129:S3–S14. doi: 10.1017/S0031182004005967
14
Ali A Khan MA Zahid H Yaseen PM Qayash Khan M Nawab J et al . Seasonal dynamics, record of ticks infesting humans, wild and domestic animals and molecular phylogeny of Rhipicephalus microplus in Khyber Pakhtunkhwa Pakistan. Front Physiol. (2019) 10:793. doi: 10.3389/fphys.2019.00793
15
El Kammah KM Oyoun LM El Kady GA Shafy SA . Investigation of blood parasites in livestock infested with argasid and ixodid ticks in Egypt. J Egypt Soc Parasitol. (2001) 31:365–71.
16
Hassan MI Gabr HS Abdel-Shafy S Hammad KM Mokhtar MM . Prevalence of tick-vectors of Theileria annulata infesting the one-humped camels in Giza, Egypt. J Egypt Soc Parasitol. (2017) 47:425–32. doi: 10.21608/jesp.2017.77797
17
Abdel-Shafy S Allam NAT Mediannikov O Parola P Raoult D . Molecular detection Tanof spotted fever group Rickettsiae associated with ixodid ticks in Egypt. Vector-Borne Zoonotic Dis. (2012) 12:346–59. doi: 10.1089/vbz.2010.0241
18
Asmaa NM ElBably MA Shokier KA . Studies on prevalence, risk indicators, and control options for tick infestation in ruminants. Beni-Suef Univ J Basic Appl Sci. (2014) 3:68–73. doi: 10.1016/j.bjbas.2014.02.009
19
Nabeth P Cheikh DO Lo B Faye O Ould I Vall M . Crimean-Congo hemorrhagic fever, Mauritania. Emerg Infect Dis. (2004) 10:2143–9. doi: 10.3201/eid1012.040535
20
Scrimgeour EM Mehta FR Suleiman AJM . Infectious and tropical diseases in Oman: a review. Am J Trop Med Hyg. (1999) 61:920–5. doi: 10.4269/ajtmh.1999.61.920
21
Yean S Prasetyo DB Marcombe S Hadi UK Kazim AR Tiawsirisup S et al . Challenges for ticks and tick-borne diseases research in Southeast Asia: Insight from the first international symposium in Cambodia. PLoS Negl Trop Dis. (2024) 18:e0012269. doi: 10.1371/journal.pntd.0012269
22
Djiman TA Biguezoton AS Saegerman C . Tick-borne diseases in Sub-Saharan Africa: a systematic review of pathogens, research focus, and implications for public health. Pathogens. (2024) 13:697. doi: 10.3390/pathogens13080697
23
Monakale KS Ledwaba MB Smith RM Gaorekwe RM Malatji DP . A systematic review of ticks and tick-borne pathogens of cattle reared by smallholder farmers in South Africa. Curr Res Parasitol Vector Borne Dis. (2024) 6:100205. doi: 10.1016/j.crpvbd.2024.100205
24
Vedachalam SK Rajput BL Choudhary S Narayanaswamy D Chandra S Pallavi DM et al . Kyasanur Forest Disease: An epidemiological investigation and case-control study in Shivamogga, Karnataka, India—2022. Int J Public Health. (2024) 69:1606715. doi: 10.3389/ijph.2024.1606715
25
Ndishimye P Umuhoza T Umutoni B Zakham F Ndayambaje M Hewins B et al . Rift Valley fever outbreaks in the East African Community: Insights from ProMed data (2010–2024). Front Public Health. (2024) 12:1298594. doi: 10.3389/fpubh.2024.1298594
26
Bouchard C Dibernardo A Koffi J Wood H Leighton PA Lindsay LR . Climate change and infectious diseases: the challenges: N increased risk of tick-borne diseases with climate and environmental changes. Can Commun Dis Rep. (2019) 45:83. doi: 10.14745/ccdr.v45i04a02
27
Ogden NH Ben Beard C Ginsberg HS Tsao JI . Possible effects of climate change on ixodid ticks and the pathogens they transmit: predictions and observations. J Med Entomol. (2021) 58:1536–45. doi: 10.1093/jme/tjaa220
28
Nuttall PA . Climate change impacts on ticks and tick-borne infections. Biologia. (2022) 77:1503–12. doi: 10.1007/s11756-021-00927-2
29
Mark L . Effect of climate variability on Lyme disease cases in the United States – a retrospective study. Capstone Exp. University of Nebraska Medical Center, College of Public Health (2022) 204. Available online at: https://digitalcommons.unmc.edu/coph_slce/204 (Accessed March 25, 2025).
30
Randolph SE . Is expert opinion enough? a critical assessment of the evidence for potential impacts of climate change on tick-borne diseases. Anim Health Res Rev. (2013) 14:133–7. doi: 10.1017/S1466252313000091
31
Gasmi S Bouchard C Ogden NH Adam-Poupart A Pelcat Y Rees EE et al . Evidence for increasing densities and geographic ranges of tick species of public health significance other than Ixodes scapularis in Québec, Canada. PLoS One. (2018) 13:e0201924. doi: 10.1371/journal.pone.0201924
32
Bacon EA Kopsco H Gronemeyer P Mateus-Pinilla N Smith RL . Effects of climate on the variation in abundance of three tick species in Illinois. J Med Entomol. (2022) 59:700–9. doi: 10.1093/jme/tjab189
33
Diuk-Wasser MA VanAcker MC Fernandez MP . Impact of land use changes and habitat fragmentation on the eco-epidemiology of tick-borne diseases. J Med Entomol. (2021) 58:1546–64. doi: 10.1093/jme/tjaa209
34
Ortiz DI Piche-Ovares M Romero-Vega LM Wagman J Troyo A . The impact of deforestation, urbanization, and changing land use patterns on the ecology of mosquito and tick-borne diseases in Central America. Insects. (2021) 13:20. doi: 10.3390/insects13010020
35
Kilpatrick AM Dobson AD Levi T Salkeld DJ Swei A Ginsberg HS et al . Lyme disease ecology in a changing world: consensus, uncertainty and critical gaps for improving control. Philos Trans R Soc B. (2017) 372:20160117. doi: 10.1098/rstb.2016.0117
36
Nimo-Paintsil SC Mosore M Addo SO Lura T Tagoe J Ladzekpo D et al . Ticks and prevalence of tick-borne pathogens from domestic animals in Ghana. Parasit Vectors. (2022) 15(1):86. doi: 10.1186/s13071-022-05208-8
37
Jensen BB Bruun MT Jensen PM Pedersen AK Fournier PE Skarphedinsson S et al . Evaluation of factors influencing tick bites and tick-borne infections: a longitudinal study. Parasit Vectors. (2021) 14:289. doi: 10.1186/s13071-021-04751-0
38
Noden BH Roselli MA Loss SR . Factors influencing abundance of 3 tick species across a gradient of urban development intensity in the US Great Plains. J Med Entomol. (2024) 61:233–44. doi: 10.1093/jme/tjad132
39
Pfäffle M Littwin N Muders SV Petney TN . The ecology of tick-borne diseases. Int J Parasitol. (2013) 43:1059–77. doi: 10.1016/j.ijpara.2013.06.009
40
Slatculescu AM Duguay C Ogden NH Sander B Desjardins M Cameron DW et al . Spatiotemporal trends and socioecological factors associated with Lyme disease in eastern Ontario, Canada from 2010–2017. BMC Public Health. (2022) 22:736. doi: 10.1186/s12889-022-13167-z
41
Githaka NW Bishop RP Šlapeta J Emery D Nguu EK Kanduma EG . Molecular survey of Babesia parasites in Kenya: first detailed report on occurrence of Babesia bovis in cattle. Parasites & Vectors. (2022) 15:161. doi: 10.1186/s13071-022-05279-7
42
Ouedraogo AS Zannou OM Biguezoton AS Yao KP Belem AMG Farougou S et al . Cross border transhumance involvement in ticks and tick-borne pathogens dissemination and first evidence of Anaplasma centrale in Burkina Faso. Ticks Tick Borne Dis. (2021) 12:101781. doi: 10.1016/j.ttbdis.2021.101781
43
Pace EJ O’Reilly M . Tickborne diseases: diagnosis and management. Am Fam Physician. (2020) 101:530–40.
44
Rochlin I Toledo A . Emerging tick-borne pathogens of public health importance: a mini-review. J Med Microbiol. (2020) 69:781. doi: 10.1099/jmm.0.001206
45
Garber B Glauser J . Tick-borne illness for emergency medicine providers. Curr Emerg Hosp Med Rep. (2019) 7:74–82. doi: 10.1007/s40138-019-00187-0
46
de la Fuente J Estrada-Peña A Rafael M Almazán C Bermúdez S Abdelbaset AE et al . Perception of ticks and tick-borne diseases worldwide. Pathogens. (2023) 12:1258. doi: 10.3390/pathogens12101258
47
Desta BGEAH . Review on the impact of ticks on livestock health and productivity. J Bio Agr Health. (2016) 6:1–7.
48
Sansoucy R . Livestock—a driving force for food security and sustainable development. World Anim Rev. (1997) 5–17.
49
Jabbar A Abbas T Sandhu ZU Saddiqi HA Qamar MF Gasser RB . Tick-borne diseases of bovines in Pakistan: major scope for future research and improved control. Parasites Vectors. (2015) 8:283. doi: 10.1186/s13071-015-0894-2
50
Taylor MA Coop RL Wall R . Veterinary parasitology. John Wiley & Sons (2015).
51
United Nations Conference on Trade and Development (UNCTAD) . Forging a path beyond borders: the Global South. Available online at: https://unctad.org/system/files/official-document/osg2018d1_en.pdf (Accessed March 8, 2025).
52
Stefanoff P Rosinska M Samuels S White DJ Morse DL Randolph SE . A national case-control study identifies human socio-economic status and activities as risk factors for tick-borne encephalitis in Poland. PLoS One. (2012) 7(9):e45511. doi: 10.1371/journal.pone.0045511
53
Paddock CD Lane RS Staples JE et al . Changing paradigms for tick-borne diseases in the Americas. In: Forum on Microbial Threats; Board on Global Health; Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine. Global health impacts of vector-borne diseases: workshop summary. National Academies Press (US, Washington (DC (2016). Available at: https://www.ncbi.nlm.nih.gov/books/NBK390439/ (Accessed May 17, 2025).
54
Kasaija P Estrada-Peña A Contreras M Kirunda H de la Fuente J . Cattle ticks and tick-borne diseases: a review of Uganda's situation. Ticks Tick Borne Dis. (2021) 12:101756. doi: 10.1016/j.ttbdis.2021.101756
55
Kasaija PD Estrada-Peña A Contreras M Kirunda H de la Fuente J . Economic burden of reported Lyme disease in high-incidence areas, United States, 2014–2016. Emerg Infect Dis. (2022) 28:1170–9. doi: 10.3201/eid2806.211335
56
De Castro JJ . Sustainable tick and tickborne disease control in livestock improvement in developing countries. Vet Parasitol. (1997) 71(2-3):77–97. doi: 10.1016/S0304-4017(97)00033-2
57
Grisi L Leite RC Martins JRDS Barros ATMD Andreotti R Cançado PHD et al . Reassessment of the potential economic impact of cattle parasites in Brazil. Rev Bras Parasitol Vet. (2014) 23:150–6. doi: 10.1590/S1984-29612014042
58
Singh K Kumar S Sharma AK Jacob SS RamVerma M Singh NK et al . Economic impact of predominant ticks and tick-borne diseases on Indian dairy production systems. Exp Parasitol. (2022) 243:108408. doi: 10.1016/j.exppara.2022.108408
59
Rodríguez-Vivas RI Grisi L Pérez de León AA Villela HS Torres-Acosta JFDJ Fragoso Sánchez H et al . Potential economic impact assessment for cattle parasites in Mexico. Rev Mex Cienc Pecu. (2017) 8:61–74.
60
Akinyera B Maimadu AA Akinsulie OC Olabode MP Sabo A . Microbial loads of beef and hygienic practice of butchers in Jos municipal abattoir. Asian J. Res. Anim. Vet. Sci. (2018) 1:151–159.
61
Šimo L Kazimirova M Richardson J Bonnet SI . The essential role of tick salivary glands and saliva in tick feeding and pathogen transmission. Front Cell Infect Microbiol. (2017) 7:281. doi: 10.3389/fcimb.2017.00281
62
Ogden NH Lindsay LR Hanincová K Barker IK Bigras-Poulin M Charron DF et al . Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl Environ Microbiol. (2008) 74:1780–90. doi: 10.1128/AEM.01982-07
63
Rizzoli A Silaghi C Obiegala A Rudolf I Hubálek Z Földvári G et al . Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: new hazards and relevance for public health. Front Public Health. (2014) 2:251. doi: 10.3389/fpubh.2014.00251
64
Kazimírová M Mangová B Chvostáč M Didyk YM de Alba P Mira A et al . The role of wildlife in the epidemiology of tick-borne diseases in Slovakia. Curr Res Parasitol Vector Borne Dis. (2024) 6:100195. doi: 10.1016/j.crpvbd.2024.100195
65
Thaware VH . Integrated pest management: an eco-friendly approach to control and manage ticks in cattle. Acta Entomol Zool. (2023) 4:33–6. doi: 10.33545/27080013.2023.v4.i1a.93
66
Drummond RO . Tick-borne livestock diseases and their vectors. 4. Chem control ticks. (1976). Available at: https://www.fao.org/4/x6538e/x6538e03.htm (Accessed June 17, 2025)
67
Samish M Ginsberg H Glazer I . Biological control of ticks. Parasitology. (2004) 129:S389–403. doi: 10.1017/S0031182004005219
68
Merino O Alberdi P Pérez de la Lastra JM de la Fuente J . Tick vaccines and the control of tick-borne pathogens. Front Cell Infect Microbiol. (2013) 3:30. doi: 10.3389/fcimb.2013.00030
69
Pereira DFS Ribeiro HS Gonçalves AAM da Silva AV Lair DF de Oliveira DS et al . Rhipicephalus microplus: An overview of vaccine antigens against the cattle tick. Ticks Tick-borne Dis. (2022) 13:101828. doi: 10.1016/j.ttbdis.2021.101828
70
Asadollahi Z Nabian S Taheri M Ebrahimzadeh E . Introducing a new anti-Rhipicephalus (Boophilus) microplus tick recombinant vaccine candidate using cathepsin and tropomyosin multi-epitope gene. Vet Res Forum. (2021) 12:445–50. doi: 10.30466/vrf.2021.113699.2704
71
Akinsulie OC Idris I . Global re-emergence of dengue fever: The need for a rapid response and surveillance. Microbe. (2024) 4:100107. doi: 10.1016/j.microb.2024.100107
72
Akinsulie OC Adesola RO Aliyu VA Oladapo IP Hamzat A . Epidemiology and transmission dynamics of viral encephalitides in west africa. Infect Dis Rep. (2023) 15:504–17. doi: 10.3390/idr15050050
73
Daniel M Kolar J Zeman P . GIS tools for tick and tick-borne disease occurrence. Parasitology. (2004) 129:S329–52. doi: 10.1017/S0031182004006080
74
Maimadu AA Akinsulie OC Olabode MP Bata SI Waziri IA Sabo JA et al . Prevalence of Gastrointestinal Parasites and their Impact in Sheep in Riyom Local Government Area of Plateau State. J. Bacteriol. Parasitol. (2020) 11:375.
Summary
Keywords
ticks, tick-borne diseases, One Health, tick ecology, climate-driven disease spread, Global South
Citation
Shahzad S, Akinsulie OC, Idris I, Devnath P, Ajagbe D, Aliyu VA, Oladoye MJ, Ukauwa C, Ugwu CE, Ajulo S, Oyeleye BS, Ikele CG and Shelly SY (2025) Ticks and tickborne diseases in Global South countries: impact and implications of environmental changes. Front. Trop. Dis. 6:1597236. doi: 10.3389/fitd.2025.1597236
Received
20 March 2025
Accepted
06 June 2025
Published
02 July 2025
Volume
6 - 2025
Edited by
Daba Abdissa, Jimma University, Ethiopia
Reviewed by
Zubaidah Ya'cob, University of Malaya, Malaysia
Updates
Copyright
© 2025 Shahzad, Akinsulie, Idris, Devnath, Ajagbe, Aliyu, Oladoye, Ukauwa, Ugwu, Ajulo, Oyeleye, Ikele and Shelly.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sammuel Shahzad, sammuel.shahzad@wsu.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.