- 1Department of Microbiology and Immunology, University of Health and Allied Sciences, Ho, Volta Region, Ghana
- 2School of Basic Medical Sciences, Saint James School of Medicine, Park Ridge, IL, United States
- 3Office of Graduate Studies, Universidad Especializada de Las Américas UDELAS, Panamá City, Panama
- 4School of Public Health and Health Sciences, College of Health, Human Services and Nursing, California State University Dominguez Hills, Carson, CA, United States
- 5Department of Biomedical Sciences, School of Health Professions, D’Youville University, Buffalo, NY, United States
Parasites depend on complex life cycles that involve multiple hosts and different environmental elements. Human and animal feces provide a medium for some of these parasites, especially those responsible for some Neglected Tropical Diseases (NTDs), to be transmitted between their intermediate and definitive hosts. Open defecation has been identified as a menace that contributes significantly to the spread of these parasites. While preventive strategies like the WASH initiatives have shown promising results, improper feces disposal has offset these achievements by contaminating the environment, especially soil, water, and crops. Furthermore, the poor attention to open defecation of animals has allowed these parasites to contaminate and pollute the environment and transmit diseases with relative ease, with no environmental legislation. As a result, certain NTDs-Open defecation driven-NTDS such as Soil-Transmitted Helminthiasis, Schistosomiasis, Taeniasis, Cysticercosis, and Echinococcosis have become increasingly successful in their spread, a situation driven by both human and animal open defecation. This article explores how the practice of open defecation by both humans and animals aids in spreading these NTDs, and it highlights how a One Health integrated approach might provide a lasting solution for these Open defecation driven NTDs in Sub-Saharan Africa.
1 Introduction
Open defecation is the practice of defecating in open spaces, usually in bushes or open fields, in waterways, or any other place outside a designated modern or traditional toilet facility (1). It is a serious public health problem that necessitated its inclusion in the Millennium and Sustainable Development Goals (SDGs). Millennium Development Goal (MDG) number 7 was specific for the control of open defecation. It also targets reducing by half the population of people without access to sustainable water and basic sanitation, including toilet facilities, by 2015 (2), but this was not attained. SDGs were developed in 2015 to renew the efforts to achieve targets that were not met with the MDGs, and controlling open defecation was aptly included in SDG number 6, which focused on providing sustainable access to clean drinking water and basic sanitation for all (2, 3). Projections show that, as 2030 is just a few years away, this target might not be attained in many countries, especially in sub-Saharan Africa (3).
There is historical evidence that open defecation is also a nagging problem in many developed countries, but civilization coupled with technological advancement helped in eradicating the menace (4). Unfortunately, the same cannot be said of countries in the developing world, especially those in sub–Saharan Africa, as many are still grappling with providing drinking water and basic sanitation amenities for their citizens (1). Open defecation is a nagging problem in low- and middle-income countries of the world located in sub-Saharan Africa, South, Central, and East Asia, and others (5). According to the United Nations Children’s Fund (UNICEF), countries would need to triple their current pace to be able to eliminate open defecation by 2030, and only 18 countries are successful in that track (5). Approximately 1.5 billion people lack basic sanitation amenities in most developing countries (6), and as a result, over 400 million people still practice open defecation because of limited toilet facilities. However, the practice of open defecation cannot only be attributed to a lack of latrines, but factors such as dysfunctional or collapsed toilets and the unsanitary nature of the public toilet facilities, especially during times of water scarcity, contribute to the problem (6).
Improper disposal of human feces is another huge sanitation problem in many developing countries. The improper disposal of feces of children highlights the pervading problem of improper disposal of feces in limited resources developing countries already battling with open defecation (7, 8). Aside from the disposal of feces of children, it is important to note that improper feces disposal constitutes a huge problem even in areas and populations that use toilet facilities (9). Improper disposal of human feces is a spectrum of open defecation, as both practices ultimately achieve the same unwanted outcome of fecal contamination of the environment with its attendant health problems (7). However, the academic distinction between the two terms is not the focus of this review; rather, the common impact of contamination of the environment with the resultant hazardous health consequences. Open defecation in this review refers to both direct open defecation and improper disposal of feces, and thus, we define open defecation as the unsanitary disposal of human feces either by direct open defecation or improper disposal of collected feces into the environment resulting in the fecal contamination of soil, water, and food, which poses a danger to human health. Fecal contamination of the environment is a serious public health hazard as human feces contains many pathogenic organisms ranging from millions of viruses and bacteria to several thousand eggs and cysts of parasites (2, 9). This has resulted in feco-orally transmitted diseases such as diarrheal diseases, enteric fever, hepatitis B, poliomyelitis, and several other diseases (2, 9). Control and prevention of open defecation is an active component of the Water, Sanitation, and Hygiene (WASH) strategy, which has contributed significantly to the reduction of waterborne and water-related diseases, including some Neglected Tropical Diseases NTDs (10–12).
2 Wash-related and open defecation-driven NTDs
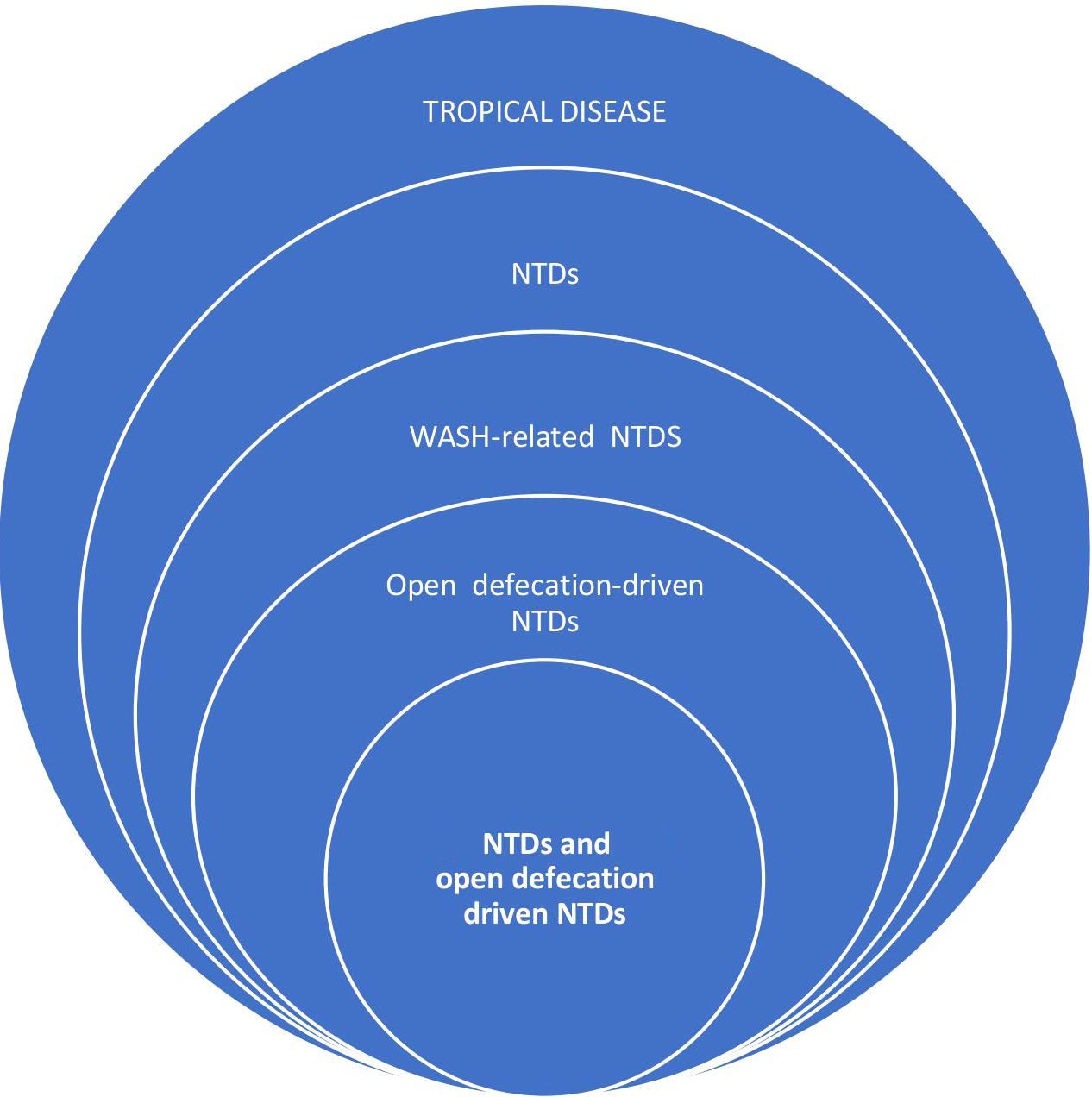
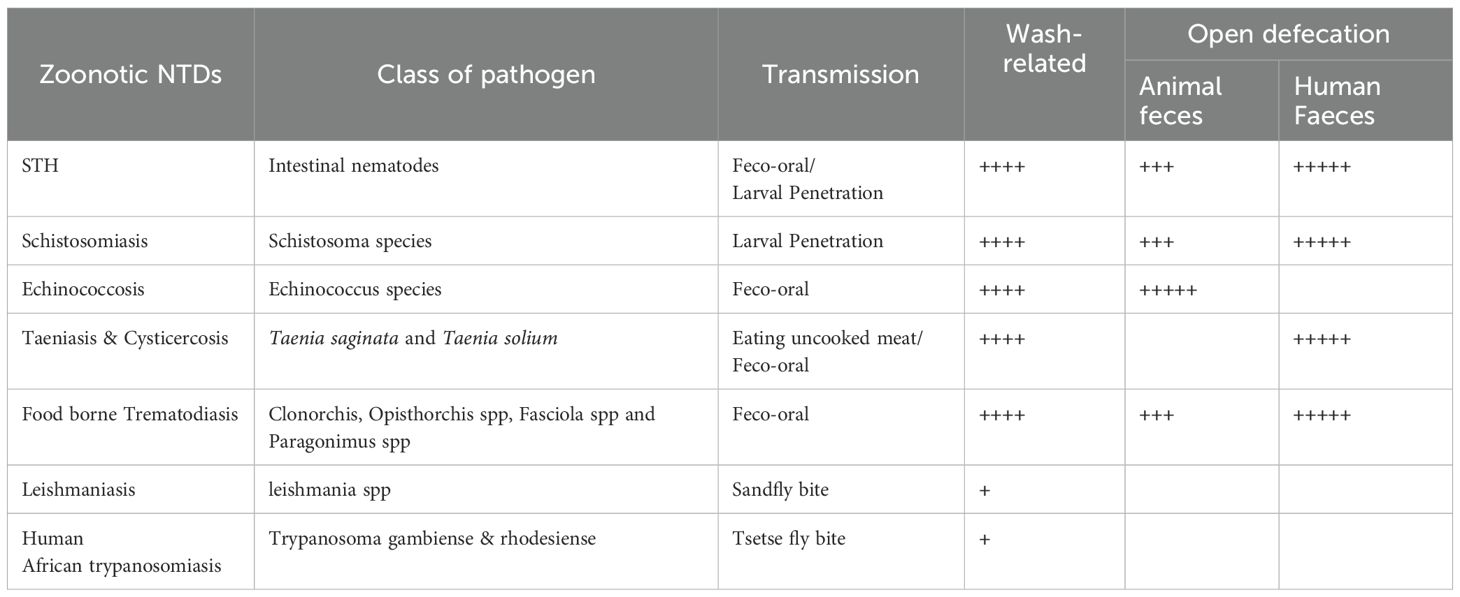
Neglected Tropical Diseases (NTDs) are a subset of tropical diseases (Figure 1), characterized by their preponderance and negative impact in poverty-stricken endemic tropical areas of the world, especially in sub-Saharan Africa (10, 11). NTDs are so named to put a spotlight on them in an attempt to make up for the years of inattention in terms of control interventions from important local and international stakeholders (13). Currently, 20 diseases comprise this list, including viral, parasitic, bacterial, and fungal diseases, and others like snake bites, yaws, and Buruli ulcers (10, 11). Of all the NTDs, some are caused by a lack of clean water, poor sanitation, and poor personal hygiene and, thus, are controlled by the WASH intervention initiative (12). WASH initiative, through the adequate provision and access to drinking water and sanitation, is believed to have a direct and indirect positive impact on the prevention and management of all NTDs including zoonotic parasitic NTDs (Table 1) (12, 14). The WASH intervention activities directly prevent some NTDs, such as Soil-transmitted helminthiasis (STH), Taeniasis and Cysticercosis, Schistosomiasis, dracunculiasis, Foodborne Trematodiasis, and Trachoma, which can be termed WASH-related NTDs (Figure 1). While the provision of WASH can interrupt the transmission route of these WASH-related NTDs (12), some of them are directly driven by open defecation (pathogens found in feces) such as STH, Taeniasis, and Cysticercosis, Foodborne Trematodiasis, and Schistosomiasis, and hence, can be referred to as Open Defecation Driven NTDs (Figure 1).
WASH has recorded some successes in controlling these NTDs; however, this accomplishment has been uneven as many endemic areas, especially in sub-Saharan Africa, still have a high burden of open defecation driven NTDS (10, 11). The Unfortunate expectation of not meeting the SDGs targets of 2030, coupled with the projected human population explosion, and increasing poverty in many developing nations, makes it highly likely that the problem of open defecation will worsen (3, 15). This foreboding projection calls for a more pragmatic, integrated, and holistic approach to dealing with this problem. One Health provides a platform to help control, prevent open defecation, and provide lasting solutions to WASH-related and open defecation-driven NTDs (16, 17). This is particularly important since open defecation not only poses a threat to human health, as environmental contamination of human feces also affects animals and other biotic components of the environment (18).
3 One Health and open defecation-driven NTDs
One Health is the science that looks at the interconnectedness of human, animal, and environmental health (19–21), and in essence, prevention of diseases involving humans, animals, and the environment is at the center of One Health (20). It provides a holistic, integrated, and cost-effective preventive approach after assessing and understanding the magnitude and ramifications of interconnected health problems of humans, animals, and the environment (19). All these require a multidisciplinary and interdisciplinary collaborative effort involving multiple sectors with a unifying agenda of simultaneously improving the health of humans and animals, as well as the health of the environment in which they live (19).
The One Health approach has been applied to understand the epidemiology of NTDs, as well as their control, especially to help achieve the NTDs 2030 road map goals (10, 11, 21, 22). The One Health concept has been applied to WASH interventions where the provision of clean water and sanitation has been extended to animals and livestock (10, 11, 22). One Health has aptly broadened the scope of open defecation to include animal feces, a welcome development since animal feces are known to cause a variety of human diseases (17). Thus, a One Health definition of open defecation is the unsanitary disposal of human feces either by direct open defecation or improper disposal of collected feces into the environment, resulting in the fecal contamination of soil, water, and food, posing a danger to the health of both humans and animals. For this reason, we added Echinococcosis (primarily driven by dog feces) to STH, Taeniasis and Cysticercosis, Foodborne Trematodiasis, and Schistosomiasis on the list of Open defecation driven NTDs. There is a vicious cycle between animals and humans involving fecal contamination of the environment with subsequent contamination of food, water, and soil (Figure 2). All these Open defecation-driven NTDs are endemic in sub–Saharan Africa except foodborne trematodiasis, which is a group of trematodes, flukes acquired through the ingestion of seafood and water vegetables contaminated by the larvae of the parasites as a result of the fecal contamination of water bodies.
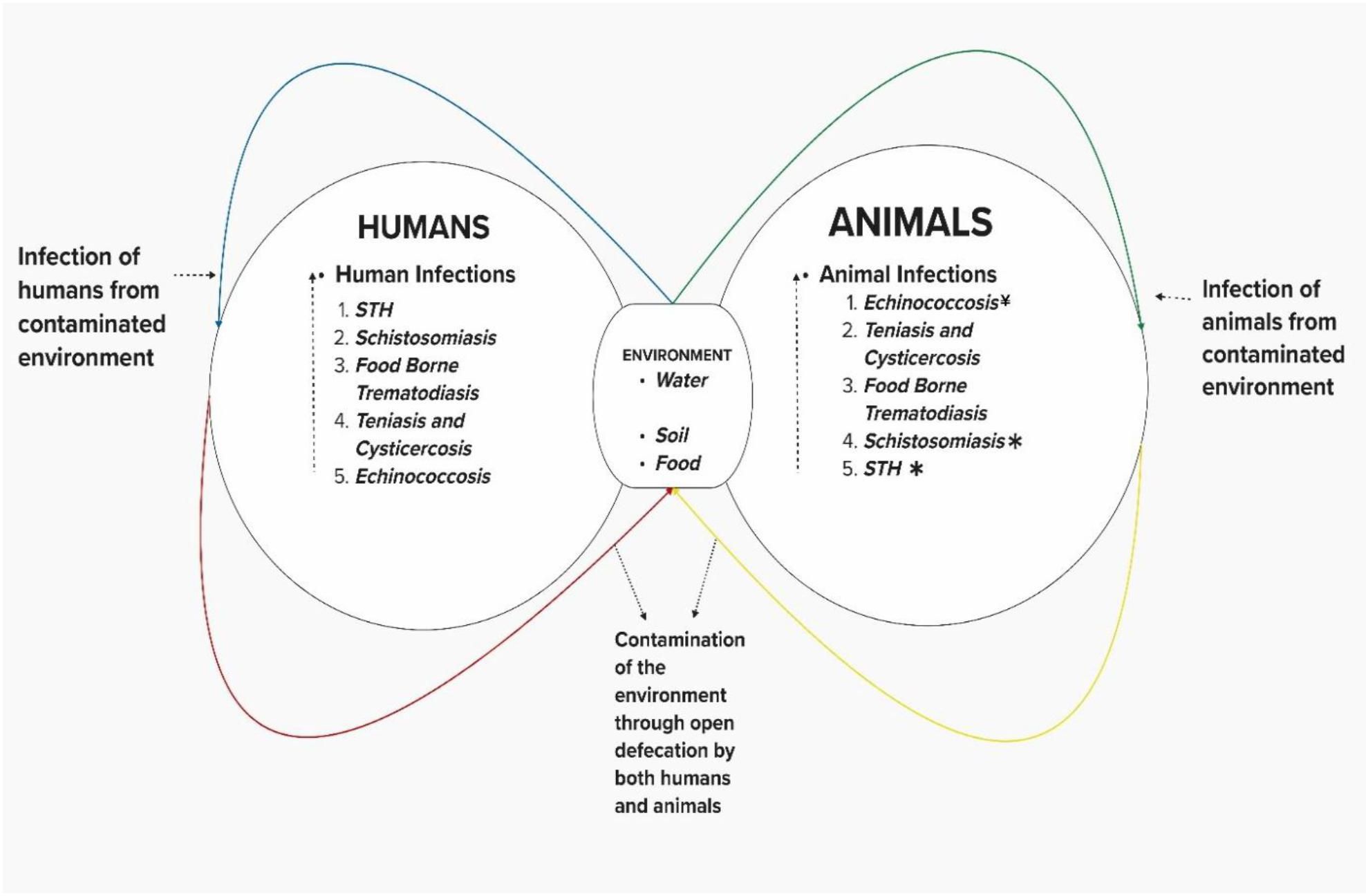
Figure 2. Open defecation-driven NTDs * Transmitted by both animal and human feces ¥ Only transmitted by animal Faeces.
3.1 Soil-transmitted helminthiasis
STH is an open defecation driven NTD predominantly seen in areas of poverty and poor sanitation, and in areas where there are pervading practices of open defecation (10, 11, 22–24). They are caused by parasitic nematodes, namely Ascaris lumbricoides, Necator americanus/Acylostoma duodenale, Trichuris trichiura, and Strongyloides stercoralis (25, 26). Fecal contamination of the soil (Figure 2) is critical in the transmission to humans and animals, hence the name soil-transmitted helminthiasis. Fecal-oral transmission is the main mode of transmission through the inadvertent ingestion of eggs of the nematodes in soil or from food such as vegetables and fruits. However, some nematode helminths such as Necator americanus/Acylostoma duodenale and Strongyloides stercoralis are transmitted via larva penetration of the skin, larvae released from eggs in feces deposited in the soil (25, 26). There are over 1.5 billion people infected worldwide, especially children, causing malnutrition and significant Disability-Adjusted Life Years (DALY) (27). The major control strategies for soil-transmitted helminthiasis include the periodic deworming exercise, especially among school-going children, and WASH strategies involving the provision of clean water for proper sanitation and education on proper hand-washing technique, personal hygiene practices, and avoiding open defecation.
Animal feces can transmit soil-transmitted helminthiasis to humans and vice versa (Figure 2), involving cross-transmission either from 100% distinct parasite species of animal or human (28–30), or from hybrid species (31, 32). These identified transmission routes call for One Health control measures that will simultaneously include humans and animals in WASH programs, especially the aggressive control of open defecation (17) One Health-WASH approach, especially one that will focus on the control and prevention of open defecation, is particularly important because the indiscriminate chemotherapy in animals is driving anti-helminthic resistance (33).
Human or animal feces should not be disposed of in domestic and livestock environments; WASH should be extended to animals, especially those living close to humans, either as pets or livestock (17). Also, education should be given to livestock farmers on the most appropriate ways to handle animal and human feces. Farmers should be educated on the proper and safe use of human and animal feces as fertilizers (34). Open defecation should be prohibited and sanctioned by law in places where humans and animals recreate, like playgrounds and beaches (35, 36) or on topsoil used for purposes of horticulture or gardening (37).
3.2 Schistosomiasis
Schistosomiasis is another open defecation-driven NTD transmitted through larva (cercaria) penetration of the skin of humans who come in contact with feces and urine-infested freshwater bodies (10, 11, 22–24). All seven species of Schistosoma, including the ones found in sub–Saharan Africa, such as S. mansoni, S. intercalatum, and S. guineensis, are transmitted through human feces, except S. haematobium responsible for urogenital schistosomiasis and is transmitted through urine (38). Although the eggs of S. haematobium are found in the urine, studies have reported a high prevalence of urogenital schistosomiasis in areas of high prevalence of open defecation, probably because unsanitary practice of open defecation facilitates contamination of water bodies either through direct discharge of the urine together with feces or through runoffs (39–42). Fecal or urine deposition in freshwater bodies such as rivers, dams, and streams introduces the eggs of schistosomes, which eventually hatch, releasing the miracidia, which subsequently infect the intermediate host, the water snail of the genera Biomphalaria, Bulinus, and Oncomelania, depending on the species of Schistosoma. The snail releases the infective form of the parasite, the cercaria, which penetrates the skin of people who spend time in the water in activities like swimming, bathing, and washing. There are close to 250 million cases of schistosomiasis globally, resulting in the loss of 2.5 million DALYs (43, 44). In sub–Saharan Africa, schistosomiasis is second to malaria in terms of endemic diseases (43, 44). The control strategy for schistosomiasis includes periodic mass chemotherapy with praziquantel, especially among school-going children, provision of clean water and improved sanitation, including the use of toilets and proper personal hygiene, and vector control strategies targeting the snail intermediate host (10, 11, 22–24).
Vector control using the molluscicide, which targets the intermediate host snail, seems to be favored over control of open defecation in combating the transmission of schistosomiasis (45). The arguments are that miracidia in feces stored in sewerage systems or latrine pits are not capable of infecting snails to continue the life cycles of schistosomiasis, and regarding the eggs, the longer they stay in the sewerage or latrine pits, the less likely they will hatch to release miracidia (46). However, direct discharge of feces in water bodies or the sewage system that opens directly into water bodies will successfully infect snails (46). Again, it is argued that fecal contamination of water does not need to happen continuously for transmission to be maintained because only a few eggs are necessary to cause a sustained production of cercaria by the infected snails suggesting that the control of open defecation will contribute little to the control of schistosomiasis (23, 46). However, controlling open defecation, despite its purported minimal contribution to the control of schistosomiasis, is an integrated One Health approach since it prevents the contamination of water bodies, preventing other open defecation driven NTD and other diseases that pose a health threat to humans and animals (23). More so, molluscicide can be toxic to humans and other animals (47), which is obviously against the principle of One Health (22).
Animals are known to be involved in the transmission of schistosomiasis, with the zoonotic species S. mekongi and S. japonicum in Asia, and recent reports suggest that animal Schistosoma species are infecting humans (48). Reports suggest that livestock and rodents can be reservoirs for Schistosoma mansoni and haematobium (22). Like STH, hybrids of these species have also been reported to be responsible for the cross-transmission between humans and animals (49–51). This calls for a One Health approach by extending WASH, especially controlling open defecation in animals by preventing their feces and urine from reaching water bodies (22).
3.3 Taeniasis and cysticercosis
Taeniasis and cysticercosis are parasitic diseases caused by the cestode tapeworm Taenia solium. They are open defecation driven NTDs because human feces infects both animals(pigs) and humans (23, 25, 26). Pigs ingest the eggs of Taenia Solium in the feces of infected persons; the eggs hatch in the intestines, releasing larvae that penetrate the intestine into the bloodstream to various tissues, forming a cyst called cysticerci (25, 26). Humans become infected after eating the cysticerci in poorly cooked pork, forming adult worms in the intestine (25, 26). This adult Taenia solium eventually releases eggs in feces that infect the pig to continue the cycle. The Adult taenia solium tapeworm in the intestines of humans results in Taeniasis, a usually mild and asymptomatic disease. Taeniasis is also caused by other cestode species, such as Taenia saginata and Taenia asiatica (23). Taenia solium is the only taeniasis-causing cestode that can cause life-threatening cysticercosis in humans because its eggs, besides infecting pigs, can also infect humans via feco-oral transmission of the eggs in food and water. Open defecation is responsible for human infection of T. solium directly through feco-oral transmission of the eggs and indirectly through the ingestion of poorly cooked pork from pigs infected through feco-oral transmission of eggs in human feces (25, 26, 52, 53). More than 5 million people are infected in more than 75 endemic countries, including those in sub–Saharan Africa, causing 28 thousand deaths in 2015 with a loss of 2.8 million DALYs (10, 11). Control measures against taeniasis and cysticercosis include the use of either Praziquantel, Niclosamide, or Albendazole in humans and animals for preventive chemotherapy and treatment; WASH intervention chiefly focuses on sanitation, on safe disposal of human feces; and education of pig farmers to prevent their pigs from roaming (10, 11). Most recently, an anti-cysticercosis vaccine for pigs (Cysvax) has been developed with 99–100% protection of vaccinated pigs from tapeworm infection (54).
Pig feces plays no role in human or animal infection of T. solium; thus, control of open defecation by humans is key in controlling taeniasis and cysticercosis. The fact that pigs eat human feces as part of their diet is quite troubling, as in some communities where taeniasis/cysticercosis is endemic and open defecation is practiced widely, pigs are used to rid the environment of feces (55). Provision of toilet facilities and encouraging people to use them will most certainly decrease the prevalence of taeniasis and cysticercosis (56, 57).
3.4 Echinococcosis
Echinococcosis is a parasitic disease caused by a cestode in the genus Echinococcus, which is primarily a parasite of canines (25). There are several species under the genus, but the most common ones are Echinococcus granulosus and Echinococcus multilocularis, which cause Cystic Echinococcosis and Alveolar Echinococcosis, respectively (10, 11, 25). The life cycle of these diseases involves dogs as the definitive host and ungulates, usually sheep, goat, and other livestock, as intermediate hosts in the case of Echinococcus granulosus, while the definitive host of Echinococcus multilocularis is usually wild canids and sometimes domestic dogs, with rodents as intermediate hosts (25). The intermediate host gets infected after ingesting the eggs of the parasite in the feces of the definitive host, then the definitive host gets infected after eating the uncooked flesh and organs of the intermediate host (25). Humans are accidental hosts in this cycle involving only animals, getting infected through feco-oral transmissions of the eggs of Echinococcus spp. Present in the feces of the definitive host. The eggs hatch in the intestines of humans, releasing the larvae, which migrate out of the intestine into the bloodstream and settle in various organs such as the liver and lungs (25). Human feces play no role in this life cycle, as human infections are a dead end for the parasite. However, when infected, human diseases are quite serious and life-threatening (58). Echinococcosis is driven by the open defecation of dogs and other canines with fecal contamination of the environment, including food and water, affecting other animals and humans (59). In 2011, it was reported that 1 million people worldwide were infected, with 19 thousand deaths and 870 thousand DALY reported in 2015 (10, 11). Control measures include prompt diagnosis and adequate case management with appropriate treatment of humans and livestock, cooking of livestock carcasses when provided as food for dogs, WASH involving avoidance of dog feces, especially by livestock, and proper hand washing and others (10, 11, 60).
Controlling the open defecation of dogs is key to controlling Echinococcosis, as in most developing countries, dogs roam freely in proximity to livestock (59). Scaling up WASH among livestock farmers, coupled with education on how to safely use dog feces on their farms, would help curb this issue (59). Feces of dogs should be safely handled on farms and removed from markets to avoid contamination of farm produce such as vegetables, fruits, and other foods (59).
4 Challenges in the prevention and control of open defecation
The World Health Organization is strongly advocating for an integrated approach in the control of NTDs, especially in the race to meet the 2030 targets (10, 11), and such an integrated approach will oversee several NTDs involving multi-sectoral and inter-disciplinary collaborations (10, 11).
The control of open defecation of humans and animals can be an integrated One Health approach that can simultaneously control multiple NTDs. Feces from animals or humans should be considered a biohazard, a harbinger of disease and death, and their proper management should be a priority for all stakeholders (61). However, this is not easy owing to the complex problems influencing open defecation in endemic areas, especially in Sub-Saharan Africa, such as poverty, poor education, lack of infrastructure, and lack of political will (62).
The major control effort against open defecation has targeted human feces, probably because humans produce the largest amount of feces, with an individual producing an average of 250 grams of feces daily (63). In poor developing countries, preventing open defecation is a Gordian knot due to a lack of infrastructure, poor finances, population expansion, poor perception of the dangerous effects of open defecation practices, and lack of awareness of the negative impacts of open defecation on the community (64). Some of these adverse community practices are so entrenched in places that control programs, even with proper community engagement, have trouble initiating or achieving success in open defecation control campaigns (22). Also, the fact that it took developed countries several years with millions of dollars invested in improving sanitation and stopping open defecation, proves that this will be a daunting task for developing countries, especially in sub–Saharan Africa, where there is little to no resources and political will to achieve it (62).
The control of open defecation of animals has not enjoyed the same attention as that of humans, even though animal feces contribute significantly to environmental contamination with attendant health consequences for both animals and humans (61, 65, 66). In many developing countries, humans are more exposed to animal feces than human feces, and they pose a greater threat to human health. This is because animals, especially domestic animals, live with humans in households, providing the opportunity for possible health problems (61, 65, 66).
By and large, humans and animals produce about 4 trillion tons of feces per year, so massive that it is metaphorically likened to filling 1.6 million Olympic-sized swimming pools (61). A significant amount of these feces contributes to the menace of open defecation driven NTDs and other diseases resulting from open defecation. While WASH interventions should be intensified and further expanded to include control of open defecation from animals, harnessing the usefulness of these feces vis-à-vis their use in manure and other by-products might be the way to go (67). It is now important that a relatively cheaper alternative means of recycling feces is a venture that should be considered, as has already been done in some countries like Kenya, Madagascar, and other places where feces have been converted to fertilizer, fuel, and biogas (68). Safe recycling of human and animal feces to benefit humans, animals, crops, and the ecosystem is the ultimate One Health strategy in the control and prevention of open defecation (68, 69).
5 Conclusion
Open defecation of both animal and human feces directly impacts some NTDs, termed Open defecation-driven NTDs, a category of WASH-related NTDs affecting millions in endemic areas. A One Health evaluation of the burden of open defecation, highlighting both human and animal feces, provides a platform for an integrated and holistic approach to the control of open defecation. Despite the challenges, this might be a useful inclusion in the WASH interventions strategy in the fight against NTDs, providing a sustainable control that might contribute significantly to the prevention of open defecation driven NTDs and other diseases related to fecal contamination of the environment.
Author contributions
VO: Conceptualization, Supervision, Writing – original draft. IA: Writing – original draft. DA: Writing – original draft. AO: Writing – original draft. MA: Writing – original draft. AM: Writing – review & editing. TG: Writing – review & editing. RI: Writing – review & editing. RP: Writing – review & editing. CO: Writing – review & editing. AS: Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO. Population practicing open defecation. Indicator metadata registry details (2024). Available online at: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/4823 (Accessed December 4, 2024).
2. Saleem M, Burdett T, and Heaslip V. Health and social impacts of open defecation on women: a systematic review. BMC Public Health. (2019) 19(1):158. doi: 10.1186/s12889-019-6423-z
3. Abubakari SW, Oppong B, Wiru K, Manu G, Apraku A, Abukari M, et al. Open defecation and attainment of Sustainable Development Goal Six: evidence from Kintampo Surveillance System, Ghana. Ghana Med J. (2021) 55:273–7. doi: 10.4314/gmj.v55i4.7
4. IWA Publishing. A brief history of water and health from ancient civilizations to modern times. UK London: IWA Publishing (2012). Available online at: https://www.iwapublishing.com/news/brief-history-water-and-health-ancient-civilizations-modern-times. (Accessed December 4, 2024).
5. UNICEF. UNICEF’s game plan to end open defecation UNICEF’s game plan to end open defecation (2018). Available online at: https://www.unicef.org/media/91316/file/Game-plan-to-end-open-defecation-2018.pdf (Accessed December 4, 2024).
6. WHO. Sanitation (2024). Available online at: https://www.who.int/news-room/fact-sheets/detail/sanitation:~:text=Over%201.5%20billion%20people%20still (Accessed December 4, 2024).
7. Azage M and Haile D. Factors associated with safe child feces disposal practices in Ethiopia: evidence from the demographic and health survey. Arch Public Health. (2015) 73(1):40. doi: 10.1186/s13690-015-0090-z
8. Demissie GD, Zerihun MF, Ekubagewargies DT, Yeshaw Y, Jemere T, Misganaw B, et al. Associated factors of safe child feces disposal in sub-Saharan Africa: Evidence from recent demographic and health surveys of 34 sub-Saharan countries. PloS One. (2023) 18:e0281451. doi: 10.1371/journal.pone.0281451
9. Bauza V, Ye W, Liao J, Majorin F, and Clasen T. Interventions to improve sanitation for preventing diarrhea. Cochrane Database Systematic Rev. (2023) 2023(1). doi: 10.1002/14651858.cd013328.pub2
10. World Health Organization. Ending the neglect to attain the Sustainable Development Goals: A road map for neglected tropical diseases 2021–2030 (2021). Available online at: https://www.who.int/publications/i/item/9789240010352 (Accessed December 4, 2024).
11. World Health Organization. Ending the neglect to attain the sustainable development goals. A Global Strategy on Water, Sanitation and Hygiene to Combat Neglected Tropical Diseases 2021–2030 (2021). Available online at: https://www.who.int/publications/i/item/9789240022782 (Accessed December 4, 2024).
12. InfoNTD. WASH and NTDs | InfoNTD (2024). Available online at: https://www.infontd.org/cross-cutting-issues/wash-and-ntds (Accessed December 4, 2024).
13. Hotez PJ, Aksoy S, Brindley PJ, and Kamhawi S. What constitutes a neglected tropical disease? PloS Negl Trop Dis. (2020) 14:e0008001. doi: 10.1371/journal.pntd.0008001
14. Boisson S, Engels D, Gordon BA, Medlicott KO, Neira MP, Montresor A, et al. Water, sanitation, and hygiene for accelerating and sustaining progress on neglected tropical diseases: a new Global Strategy 2015–20. Int Health. (2016) 8:i19–21. doi: 10.1093/inthealth/ihv073
15. Vermeulen LC, de Kraker J, Hofstra N, Kroeze C, and Medema G. Modelling the impact of sanitation, population growth, and urbanization on human emissions of Cryptosporidium to surface waters—a case study for Bangladesh and India. Environ Res Lett. (2015) 10:94017. doi: 10.1088/1748-9326/10/9/094017
16. Kahn LH. Developing a one-health approach by using a multi-dimensional matrix. One Health. (2021) 13:100289. doi: 10.1016/j.onehlt.2021.100289
17. Yasobnat S, Tadvi R, Patel K, and Saxena D. Water, sanitation, and hygiene from One Health perspective. One Health Bull. (2022) 2:10. doi: 10.4103/2773-0344.350691
18. Fagunwa O, Mthiyane T, Oluwasanmi Fagunwa A, Idowu Olayemi K, Alozie A, Onyeaka H, et al. Priority regions for eliminating open defecation in Africa: implications for antimicrobial resistance. Environment Dev Sustainability. (2023) 27(1):2675–99. doi: 10.1007/s10668-023-03992-6
19. Prata JC, Ribeiro AI, and Rocha-Santos T. Chapter 1 - An introduction to the concept of One Health. In: Prata JC, Ribeiro AI, and Rocha-Santos T, editors. ScienceDirect. Academic Press (2022). Available online at: https://www.sciencedirect.com/science/article/abs/pii/B9780128227947000046 (Accessed December 7, 2024).
20. Promoting the science of One Health. Promoting the science of one health. Nat Commun. (2023) 14:4735. doi: 10.1038/s41467-023-40293-y
21. Pitt SJ and Gunn A. The one health concept. Br J Biomed Sci. (2024) 81:12366. doi: 10.3389/bjbs.2024.12366
22. World Health Organization. Ending the neglect to attain the sustainable development goals. One health: approach for action against neglected tropical diseases 2021-2030 (2022). Available online at: https://www.who.int/publications/i/item/9789240042414 (Accessed December 7, 2024).
23. World Health Organization. Neglected tropical diseases: Taeniasis and cysticercosis (2022). Available online at: https://www.who.int/news-room/questions-and-answers/item/taeniasis-and-cysticercosis (Accessed December 7, 2024).
24. World Health Organization. WASH and snail control interventions. Geneva Switzerland: World Health Organization (2022). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK578381/.
25. Centers for Disease Control and Prevention. CDC - DPDx - echinococcosis. CDC USA, Atlanta GA: Centers for Disease Control and Prevention (2019). Available online at: https://www.cdc.gov/dpdx/echinococcosis/index.html (Accessed December 7, 2024).
26. Centers for Disease Control and Prevention. CDC - DPDx - taeniasis (2019). Available online at: https://www.cdc.gov/dpdx/taeniasis/index.html (Accessed December 7, 2024).
27. PAHO/WHO. (2020) Soil-transmitted helminthiasis - PAHO/WHO. US, Washington (DC): Pan American Health Organization. Available online at: https://www.paho.org/en/topics/soil-transmitted-helminthiasis (Accessed December 7, 2024).
28. Kajero OT, Janoušková E, Bakare EA, Belizario V, Divina B, Alonte AJ, et al. Co-infection of intestinal helminths in humans and animals in the Philippines. Trans R Soc Trop Med Hygiene. (2022) 116(8):727–35. doi: 10.1093/trstmh/trac002
29. Sadaow L, Sanpool O, Phosuk I, Rodpai R, Thanchomnang T, Wijit A, et al. Molecular identification of Ascaris lumbricoides and Ascaris suum recovered from humans and pigs in Thailand, Lao PDR, and Myanmar. Parasitol Res. (2018) 117:2427–36. doi: 10.1007/s00436-018-5931-6
30. Izurieta R, Reina-Ortiz M, and Ochoa-Capello T. Trichuris trichiura. Water and Sanitation for the 21st Century: Health and Microbiological Aspects of Excreta and Wastewater Management (Global Water Pathogen Project). (Michigan State University USA) (2019). doi: 10.14321/waterpathogens.43.
31. Peng W and Criscione CD. Ascariasis in people and pigs: New inferences from DNA analysis of worm populations. Infection Genet Evol. (2012) 12:227–35. doi: 10.1016/j.meegid.2012.01.012
32. Easton A, Gao S, Lawton SP, Bennuru S, Khan A, Dahlstrom E, et al. Molecular evidence of hybridization between pig and human Ascaris indicates an interbred species complex infecting humans. ELife. (2020) 9:e61562. doi: 10.7554/elife.61562
33. Ng’etich AI, Dennis Amoah I, Bux F, and Kumari S. Anthelmintic resistance in soil-transmitted helminths: One-Health considerations. Parasitol Res. (2023) 123(1):62. doi: 10.1007/s00436-023-08088-8
34. Häfner F, Monzon Diaz OR, Tietjen S, Schröder C, and Krause A. Recycling fertilizers from human excreta exhibits high nitrogen fertilizer value and results in low uptake of pharmaceutical compounds. Front Environ Sci. (2023) 10:1038175. doi: 10.3389/fenvs.2022.1038175
35. Mohd Zain SN, Rahman R, and Lewis JW. Stray animal and human defecation as sources of soil-transmitted helminth eggs in playgrounds of Peninsular Malaysia. J Helminthol. (2014) 89:740–7. doi: 10.1017/s0022149x14000716
36. Tylkowska A, Mocha N, Kołnierzak MM, and Szenejko M. Risk factors associated with soil-transmitted helminths in dog feces that contaminate public areas of Warsaw, Poland. Anim (Basel). (2024) 14:450–0. doi: 10.3390/ani14030450
37. Yawson DO, Kudu IBY, and Adu MO. Soil-transmitted helminths in topsoils used for horticultural purposes in cape coast, Ghana. J Environ Public Health. (2018) 2018:1–5. doi: 10.1155/2018/5847439
38. Orish VN, Morhe EKS, Azanu W, Alhassan RK, and Gyapong M. The parasitology of female genital schistosomiasis. Curr Res Parasitol Vector-Borne Dis. (2022) 2:100093. doi: 10.1016/j.crpvbd.2022.100093
39. Orish VN, Ofori-Amoah J, Amegan-Aho KH, Lennox M-A, Ibrahim Jamfaru I, Afeke I, et al. Low prevalence of helminth infections among primary school children in the volta region of Ghana. Asian J Med Health. (2017) 5:1–9. doi: 10.9734/ajmah/2017/34393
40. Ntajal J, Evers M, Kistemann T, and Falkenberg T. Influence of human–surface water interactions on the transmission of urinary schistosomiasis in the Lower Densu River basin, Ghana. Soc Sci Med. (2021) 288:113546. doi: 10.1016/j.socscimed.2020.113546
41. Ahmed H, Cha S, Jin Y, and Hong S-T. Programmatic implications for schistosomiasis elimination based on community-based survey in the Blue Nile, North Kordofan, and Sennar states, Sudan. Life. (2023) 13:1049–9. doi: 10.3390/life13041049
42. Bishop HG, Inabo HI, Ella EE, and Bello M. Urinary schistosomiasis: risk factors and symptoms among school adolescents in Kaduna State, Nigeria. EUREKA: Life Sci. (2023) 2:56–62. doi: 10.21303/2504-5695.2023.002905
43. World Health Organization. Schistosomiasis: progress report 2001–2011, strategic plan 2012–2020 (2013). Available online at: https://www.who.int/publications/i/item/978941503174 (Accessed December 11, 2024).
44. Ross A, Inobaya M, Olveda R, Chau T, and Olveda D. Prevention and control of schistosomiasis: a current perspective. Res Rep Trop Med. (2014) 5:65. doi: 10.2147/rrtm.s44274
45. Muhsin MA, Wang X, Kabole FM, Zilabumba J, and Yang K. The indispensability of snail control for accelerating schistosomiasis elimination: evidence from zanzibar. Trop Med Infect Dis. (2022) 7:347. doi: 10.3390/tropicalmed7110347
46. Grimes JE, Croll D, Harrison WE, Utzinger J, Freeman MC, and Templeton MR. The roles of water, sanitation, and hygiene in reducing schistosomiasis: a review. Parasites Vectors. (2015) 8(1):156. doi: 10.1186/s13071-015-0766-9
47. King CH and Bertsch D. Historical perspective: snail control to prevent schistosomiasis. PloS Negl Trop Dis. (2015) 9:e0003657. doi: 10.1371/journal.pntd.0003657
48. Díaz AV, Lambert S, Inês Neves M, Borlase A, Léger E, Diouf ND, et al. Modeling livestock test-and-treat: A novel One Health strategy to control schistosomiasis and mitigate drug resistance. Front Trop Dis. (2022) 3:893066. doi: 10.3389/fitd.2022.893066
49. Léger E, Garba A, Hamidou AA, Webster BL, Pennance T, Rollinson D, et al. Introgressed Animal Schistosomes Schistosoma curation and S. bovis Naturally Infecting Humans. Emerging Infect Dis. (2016) 22:2212–4. doi: 10.3201/eid2212.160644
50. Onyekwere AM, Rey O, Nwanchor MC, Alo M, Angora EK, Allienne JF, et al. Prevalence and risk factors associated with urogenital schistosomiasis among primary school pupils in Nigeria. Parasite Epidemiol Control. (2022) 18:e00255. doi: 10.1016/j.parepi.2022.e00255
51. Inceboz T. One Health Concept against Schistosomiasis: An Overview (2022). Available online at: https://www.intechopen.com/chapters/83441 (Accessed December 11, 2024).
52. Pray IW, Muro C, Gamboa R, Vilchez P, Wakeland W, Pan W, et al. Seasonal patterns in risk factors for Taenia solium transmission: a GPS tracking study of pigs and open human defecation in northern Peru. Parasites Vectors. (2019) 12:352. doi: 10.1186/s13071-019-3614-5
53. Kusolsuk T, Chaisiri K, Poodeepiyasawad A, Sa-Nguankiat S, Homsuwan N, Yanagida T, et al. Risk factors and prevalence of taeniasis among the Karen people of Tha Song Yang District, Tak Province, Thailand. Parasite. (2021) 28:53. doi: 10.1051/parasite/2021041
54. Nsadha Z, Rutebarika C, Ayebazibwe C, Aloys B, Mwanja M, Poole EJ, et al. Control trial of porcine cysticercosis in Uganda using a combination of the TSOL18 vaccination and oxfendazole. Infect Dis poverty. (2021) 10:34. doi: 10.1186/s40249-021-00823-6
55. Aviles-Rosa EO, Rakhshandeh A, and McGlone JJ. Preliminary Study: Depriving Piglets of Maternal Feces for the First Seven Days Post-Partum Changes Piglet Physiology and Performance before and after Weaning. Animals. (2019) 9:268. doi: 10.3390/ani9050268
56. Mwang’onde BJ. Taenia solium cysticercosis in sub-Saharan Africa: perspectives for a better control. Adv Infect Dis. (2019) 09:105–21. doi: 10.4236/aid.2019.92008
57. Chege B, Ndambuki G, Owiny M, Kiyong’a A, and Fèvre EM. & Elizabeth. Improved latrine coverage may reduce porcine cysticercosis: a comparative cross-sectional study, Busia County, Kenya, 2021. Front Veterinary Sci. (2023) 10:1155467. doi: 10.3389/fvets.2023.1155467
58. Yu Q, Xiao N, Han S, Tian T, and Zhou X-N. Progress on the national echinococcosis control programme in China: analysis of humans and dogs population intervention during 2004–2014. Infect Dis Poverty. (2020) 9(05):69–80. doi: 10.1186/s40249-020-00747-7
59. Gharbi M and Giraudoux P. Cystic echinococcosis (Echinococcus granulosus sensu lato infection) in Tunisia, a One Health perspective for a future control program. Parasite. (2024) 31:30. doi: 10.1051/parasite/2024029
60. Macpherson CN. Human behaviour and the epidemiology of parasitic zoonoses. Int J Parasitol. (2005) 35:1319–31. doi: 10.1016/j.ijpara.2005.06.004
61. Kahn LH. How human and animal excrement harms the planet’s ecosystem. USA Chicago, IL: Bulletin of the Atomic Scientists (2019). Available online at: https://thebulletin.org/2019/11/how-human-and-animal-excrement-harm-the-planets-ecosystem/ (Accessed December 15, 2024).
62. Ahmad J. How to eliminate open defecation by 2030. Canada Ottawa: Devex (2014). Available online at: https://www.devex.com/news/how-to-eliminate-open-defecation-by-2030-84634 (Accessed December 15, 2024).
63. Britannica. Feces | biology | Britannica. In: Encyclopædia britannica. (UK London: Encyclopædia britannica) (2019). Available online at: https://www.britannica.com/science/feces (Accessed December 15, 2024).
64. Adugna D. Challenges of sanitation in developing countries - Evidenced by a study of fourteen towns in Ethiopia. Heliyon. (2023) 9:e12932. doi: 10.1016/j.heliyon.2023.e12932
65. Headey D. Chickens don’t use toilets: Why managing animal feces helps children grow taller. USA Washington, DC: World Bank Blogs (2017). Available online at: https://blogs.worldbank.org/en/water/chickens-dont-use-toilets-why-we-should-deal-animal-feces-better-sanitation (Accessed December 15, 2024).
66. Penakalapati G, Swarthout J, Delahoy MJ, McAliley L, Wodnik B, Levy K, et al. Exposure to animal feces and human health: A systematic review and proposed research priorities. Environ Sci Technol. (2017) 51:11537–52. doi: 10.1021/acs.est.7b02811
67. Purvis K. 10 steps to ending open defecation by 2030. UK London: The Guardian (2015). Available online at: https://www.theguardian.com/global-development-professionals-network/2015/nov/24/10-steps-to-ending-open-defecation-by-2030 (Accessed December 15, 2024).
68. Zeldovich L. A history of human waste as fertilizer | JSTOR daily. USA NY: JSTOR Daily (2019). Available online at: https://daily.jstor.org/a-history-of-human-waste-as-fertilizer/ (Accessed December 15, 2024).
Keywords: open defecation, parasites, helminths, NTDs, one health
Citation: Orish VN, Addei IB, Adzah DE, Oteng AG, Ayaaba MA, Marinkovic A, Gardellini T, Izurieta R, Pandit R, Okorie C and Sanyaolu A (2025) One Health approach for the prevention of open defecation: a panacea for open defecation-driven neglected tropical diseases in sub-Saharan Africa. Front. Trop. Dis. 6:1630115. doi: 10.3389/fitd.2025.1630115
Received: 16 May 2025; Accepted: 11 July 2025;
Published: 29 July 2025.
Edited by:
Lucas Sousa Magalhães, Federal University of Alagoas, BrazilReviewed by:
Sammuel Shahzad, United States Department of Agriculture (USDA), United StatesCopyright © 2025 Orish, Addei, Adzah, Oteng, Ayaaba, Marinkovic, Gardellini, Izurieta, Pandit, Okorie and Sanyaolu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Verner N. Orish, b3Jpc2h2QHlhaG9vLmNvbQ==
 Verner N. Orish
Verner N. Orish Isaac B. Addei1
Isaac B. Addei1 David E. Adzah
David E. Adzah Chuku Okorie
Chuku Okorie Adekunle Sanyaolu
Adekunle Sanyaolu