Abstract
Objective:
Race and region-specific variables influence lupus nephritis clinical features and prognosis. We examined the clinicopathological presentation and long-term kidney outcomes of lupus nephritis in Indonesia.
Methods:
This was a retrospective cohort study conducted from 1 January 2011 to 31 December 2021 on biopsy-proven lupus nephritis patients, corresponding to the International Society of Nephrology/Renal Pathology Society 2018 classification. Patients were followed until death, development of end-stage kidney disease, initiation of kidney replacement therapy, or end of study. The association between lupus nephritis class and kidney outcomes was analyzed using cumulative incidence plots. A linear mixed-model analysis was performed to assess the association between lupus nephritis class and kidney function decline.
Results:
This study included 268 patients, with a mean age of 28.7 + 8.5 years, and 94.8% were female. The main histopathological diagnosis was class IV (39.6%). The prescription rate of renin–angiotensin–aldosterone system (RAAS) inhibitors ranged from 0.5% in class VI to 37.4% in class IV (p = 0.138), while that of hydroxychloroquine usage ranged from 0% in class VI to 37.7% in class IV (p = 0.845). Class IV was associated with higher chronic and active lesions, including global (42.6%, p = 0.073) and segmental (41.1%, p = 0.009) glomerulosclerosis; segmental (43.1%, p < 0.001) and global (74.1%, p = 0.004) endocapillary hypercellularity; and sub-endothelial deposit (59.5%, p = 0.007). Over a median follow-up of 26 (IQR = 6.0–48.0) months, 16.4% of patients died, and 3.7% developed end-stage kidney disease or initiated kidney replacement therapy. Infection, including tuberculosis (9.1%), was the leading cause of death. Class IV was associated with a high mortality risk (HR 1.94, p = 0.028), a lower baseline estimated glomerular filtration rate (eGFR) compared with class I/II (β = −51.3, SE = 12.3, p < 0.001), and a less steep decline or even an increase in eGFR over time (β = 15.7, SE = 7.0, p = 0.026).
Conclusions:
This cohort demonstrated a high prevalence of chronic lesions, low use of renin–angiotensin–aldosterone system inhibitors and immunosuppressive medications, and notable mortality. This study highlights the importance of timely detection on kidney involvement in SLE patients, routine use of renin–angiotensin–aldosterone system inhibitors, optimal prescription of immunosuppressive medications, and aggressive screening and prophylactic measures of infectious diseases should be encouraged to improve kidney outcomes in lupus nephritis patients in Indonesia.
1 Introduction
Lupus nephritis (LN) is a severe organ manifestation in systemic lupus erythematosus (SLE) affecting 40%–60% of patients (1–4). LN leads to significant morbidity and mortality due to progression to kidney failure requiring kidney replacement therapy (KRT) (5, 6). Our recent study revealed that LN leading to end-stage kidney disease (ESKD) accounted for 91% of all secondary glomerulonephritis cases, which comprised 13% of the total population of dialysis and kidney transplant patients (7). Race has a strong influence on the incidence of LN where an elevated likelihood is observed in Asian populations (8–10). Asian patients with SLE typically present at a younger age, are more likely to be autoantibody-positive, and experience a more severe disease course and organ damage compared with Caucasian patients (8–13). Additionally, racial and ethnic differences are associated with variations in pharmacogenomics/kinetics, treatment response, and disease- or treatment-related complications (14).
We recognize that various disparities could negatively impact the long-term prognosis of LN patients in Indonesia. Ensuring lupus patients have access to proper care is a challenge in Indonesia due to the unequal distribution of resources across the vast country. In large urban areas and advanced medical facilities, treatments such as immunosuppressants are more readily accessible, but not all of these drugs are covered by national insurance. For instance, cyclophosphamide is primarily used as a chemotherapy drug (15). The unequal distribution of specialized healthcare facilities across different regions in Indonesia is also a problem that needs to be addressed. Several concerns were also identified including adherence to treatment plans influenced by socioeconomic and educational backgrounds and regional disparities in infection risks, including both subacute infections such as tuberculosis and chronic infections including hepatitis B or C (7, 8, 13). Providing a sufficient pathological diagnosis of kidney disease in Indonesia remains a challenge (16). Several issues in performing kidney biopsies include the large number of kidney patients needing specific pathologic evaluation while the number of centers and pathologists qualified to perform kidney biopsies is limited (17). As a result, we face lengthy waiting lists for kidney biopsies, sometimes extending to several weeks. Furthermore, the lack of pathological diagnostic tools, such as the availability of reagents necessary for immunohistochemistry and the absence of an electron microscope, constitutes a significant challenge in establishing a complete pathological diagnosis of the kidney. A comprehensive evaluation of the influence of various factors, including genetic predispositions, environmental influences, and access to treatment in the development of LN and its implications for long-term outcomes, particularly in the context of Indonesia, remains insufficiently explored. Based on the above observations, it is reasonable to postulate that LN patients in Indonesia may have a more severe phenotype than generally described in the literature. An accurate estimation of the disease burden allows for better planning and provision of healthcare resources. Therefore, it is crucial to carefully study the clinical characteristics, histopathological features, and prognosis of LN patients in Indonesia. The present study was performed to examine the clinicopathological manifestation of LN and its progression toward CKD.
1.1 Study aims
The primary aim is to evaluate the clinical and histopathological features of LN and their relationship to classes of LN. The secondary aim is to examine the association of LN classes with kidney outcomes defined as progression to ESKD/initiation of chronic dialysis or kidney transplantation or death and to provide the rate of kidney function decline across LN classes during the study period.
2 Materials and methods
2.1 Study design and setting
This is a retrospective cohort study on biopsy-proven lupus nephritis. The study was conducted at Dr. Cipto Mangunkusumo National General Hospital, Jakarta from 1 January 2011 to 31 December 2021. This center is one of the referral centers in Indonesia for the diagnosis and treatment of patients with kidney disease. All kidney biopsy data performed during the study period were recorded. Patients were followed until they reached the study outcome or the end of the follow-up period (31 December 2023), whichever comes first.
2.2 Participants
We included all patients with a first kidney biopsy-confirmed LN. Exclusion criteria were (1) kidney transplant-related biopsy, (2) biopsies containing less than five glomeruli regarded as inadequate specimens, (3) repeat kidney biopsy, and (4) incomplete medical records.
2.3 Variables
Demographic data including gender (male vs. female) and age (years) at the time of biopsy were identified.
Clinical and laboratory data were collected, including comorbidities [diabetes mellitus, congestive heart failure (CHF), stroke, hypertension, and others], previous diagnosis of SLE, period of SLE onset and time to biopsy (months), disease duration (calculated from time of kidney biopsy to study outcome or the end of study and expressed in months), use of renin–angiotensin–aldosterone system (RAAS) inhibitors, immunosuppressive therapy, clinical indication for kidney biopsy, mean arterial pressure (MAP, mmHg), erythrocyturia, 24 h proteinuria (mg/24 h), urine albumin to creatinine ratio (uACR, mg/g Cr), estimated glomerular filtration rate (eGFR, ml/min/1.73 m2), hemoglobin (g/dl), albumin (mg/dl), antinuclear antibody (ANA, positivity), anti-double-stranded DNA (anti-dsDNA, IU/ml), Complement 3 (mg/dl), Complement 4 (mg/dl), and anticardiolipin antibody (ACA, mg/dl) IgM and IgG levels in plasma.
Disease activity was assessed using the renal domain of the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI-R) (18), a subscore derived from the SLEDAI-2000 (19). The SLEDAI-R ranges from 0 to 16, with a score of 0 indicating inactive lupus nephritis. It comprises four components—proteinuria (>0.5 g/day), hematuria (>5 RBCs/HPF), pyuria (>5 WBCs/HPF), and the presence of cellular casts—each assigned a score of 4 if present. The SLEDAI-R score was calculated for 250 patients with complete clinical data.
Pathology reports were reviewed for the following features:
: mesangial matrix expansion, mesangial hypercellularity, ischemic glomeruli, segmental and global endocapillary hypercellularity, cellular/fibrocellular and fibrous crescents, wire loops, adhesions, fibrinoid necrosis, nodular sclerosis, karyorrhexis, double contour, spikes/vacuoles, mesangiolysis, hyaline thrombi, sub-endothelial deposit, segmental and global glomerulosclerosis.
Tubulointerstitial compartment: interstitial fibrosis and tubular atrophy
Vascular compartment: arterial hyalinosis
Lupus nephritis activity and chronicity indexes were evaluated using the modified NIH activity and chronicity index scoring system (
20). Components of the activity index (AI) included endocapillary hypercellularity, neutrophils or karyorrhexis, fibrinoid necrosis, hyaline deposits, cellular or fibrocellular crescents, and interstitial inflammation.
Scoring was based on the extent of the lesion: not present (0), <25% (1), 25%–50% (2), >50% (3). Chronicity index (CI) included total glomerulosclerosis, fibrous crescents, interstitial fibrosis, and tubular atrophy. Scoring was based on the proportion of the lesions: present in <10% (0), 10%–25% (1), 25%–50% (2), and >50% (3). Crescents and fibrinoid necrosis scores were weighted twice, and the total activity and chronicity indices became 24 and 12, respectively. The score was then differentiated into three categories, i.e., high (AI, 18–24; CI, 8–12), moderate (AI, 6–17; CI, 4–7), and low (AI, 0–5; CI, 0–3).
Predictors of study outcomes were documented as LN classes, which were further classified according to the ISN/RPS 2018 classification (20). Overall, we classified seven classes of LN, including LN class I/II, III, III + V, IV, IV + V, V, and VI.
Study outcomes were defined as progression to ESKD (eGFR <15 ml/min/1.73 m2) or initiation of chronic dialysis or kidney transplantation or death. Time to ESKD/initiation of KRT or death was calculated analogously. Progression of eGFR decline was analyzed over 1, 5, and 10 years, in comparison with its baseline eGFR at the time of biopsy (eGFR0).
Diagnostic criteria used for the diagnosis of LN are consistent with the ISN/RPS 2018 classification (20). Full-house immunofluorescence criteria were not used because they are not always available as the diagnostic tool.
2.4 Data sources and measurement
Clinical and laboratory parameters were gathered from electronic medical records within 3 months, before or after kidney biopsy. Pathology reports were identified from the pathology archives at Dr. Cipto Mangunkusumo National General Hospital, Jakarta.
The eGFR was estimated using the CKD-EPI formula based on serum creatinine (21).
2.5 Bias
All kidney biopsies conducted throughout the study period were reviewed to mitigate the risk of selection bias. Two renal pathologists reviewed the pathology reports for glomerular, tubulointerstitial, vascular compartments, activity, and chronicity indexes.
2.6 Study size
This study did not involve a formal sample size calculation; rather, we utilized an opportunistic approach by including all kidney biopsy data collected at our center throughout the duration of the study.
2.7 Quantitative variables
Baseline data were reported as mean ± standard deviation, skewed variables as median and interquartile ranges, and categorical variables as numbers and proportions. Prevalence of LN classes was assessed and presented in a bar chart. Distribution of histopathology lesions including activity and chronicity indexes, proportion of histopathological lesions based on percentage of affected glomeruli, and proportion of antibody deposits across LN classes were analyzed using chi-square tests and presented in bar charts.
2.8 Statistical methods
The relationship between clinical and histopathological variables (including light microscope findings and immunofluorescence studies) with classes of LN was examined using ANOVA one-way test (for numerical normal data), Kruskall–Wallis tests (for numerical non-normal data), and chi-square tests (for categorical data).
To assess the association of LN classes with kidney outcomes, we first present cumulative incidence plots (cumulative incidence competing risk/CICR analysis) of the competing outcomes death of any cause and ESKD/initiation of KRT adjusted for age stratified by LN class. Censoring was performed when a participant's event (e.g., death or ESKD/KRT) was not observed due to being lost to follow-up. Participants who dropped out or were lost to follow-up were treated as censored at the last time they were known to be alive or in the study.
In addition, a linear mixed-model analysis was used to assess the association of LN class and kidney function decline over time, adjusted for age and gender. Subjects with a minimum of one eGFR measurement were included in the analysis.
Missing data were addressed using multiple imputation by Markov Chain Monte Carlo (MCMC). The details on the missing data management are provided in Supplementary Materials. Sensitivity analysis was conducted by comparing the results of before and after multiple imputation with the MCMC method to ensure the robustness of the findings (Supplementary Table S1).
We conducted our analysis using IBM SPSS Statistic Version 27 and STATA software version 17 (StataCorp, Texas, USA). A p-value of <0.05 was considered statistically significant. This study was reported in accordance with STROBE cohort reporting guidelines (22) (Supplementary Material).
2.9 Ethical consideration
This study was approved by the Ethics Committee of the Faculty of Medicine, Universitas Indonesia—Dr. Cipto Mangunkusumo Hospital, number KET-1065/UN2.F1/ETIK/PPM.00. 02/2021 by 1 November 2021 and was conducted in accordance with the principles of the Declaration of Helsinki.
3 Results
3.1 Baseline demography
We collected 1,221 kidney biopsies for review and included 268 (22%) biopsy-confirmed LN in the final analysis (flow diagram of biopsy review can be found in Supplementary Figure S1). The median age of our subjects was 28.7 ± 8.5 years, where approximately 7.1% were childhood-onset lupus. The majority of our cohort was female (94.8%; male-to-female ratio = 1:18), and hypertension was the most common comorbid condition, affecting 39.2% of the patients. The main clinical indication for renal biopsy was a suspected renal involvement in patients diagnosed with SLE (86.7%). Other major clinical findings at the presentation of biopsy were erythrocyturia, proteinuria, ANA positive, and anti-dsDNA positive. A high SLEDAI-R score (mean + SD = 7.2 + 3.3) was observed in 98% of patients indicating a high level of renal disease activity within the cohort. The baseline eGFR was reasonably preserved among patients with low serum albumin. The baseline clinical and laboratory findings are presented in Table 1.
Table 1
| Clinical characteristics | n = 268 |
|---|---|
| Age, year (mean + SD) | 28.7 + 8.5 |
| Age <18 years (n, %) | 19 (7.1) |
| Female (n, %) | 254 (94.8) |
| Comorbidities (n, %) | |
| Diabetes mellitus | 7 (2.6) |
| Congestive heart failure | 3 (1.1) |
| Stroke | 1 (0.4) |
| Hypertension | 105 (39.2) |
| Other | 9 (3.4) |
| Previous diagnosis of SLE (n, %) | 82 (30.6) |
| Onset of SLE to kidney biopsy, months (median; IQR) | 24.0; 11.3–48.0 |
| Missing data (n, %) | 192 (71.6) |
| Disease duration, months (median; IQR) | 60 (41–90) |
| Use of renin–angiotensin–aldosterone system inhibitors (n, %) | 181 (80.4) |
| Missing data (n, %) | 43 (16.0) |
| Use of immunosuppressive medication (n, %) | |
| Oral glucocorticoids | 41 (14.9) |
| Intravenous glucocorticoids | 5 (1.8) |
| Glucocorticoids + mycophenolate mofetil or mycophenolic acid | 122 (44.2) |
| Glucocorticoids + cyclosporin A | 5 (1.8) |
| Glucocorticoids + azathioprine | 11 (4.0) |
| Glucocorticoids + tacrolimus | 1 (0.4) |
| Hydroxychloroquine | 62 (22.5) |
| Mycophenolate mofetil or mycophenolic acid only | 7 (2.5) |
| None | 22 (8.0) |
| Missing data | 41 (15.3) |
| Indication for biopsy (n, %) | |
| Nephrotic syndrome | 29 (11.6) |
| Nephritic syndrome | 3 (1.2) |
| SLE with hematuria/proteinuria/declining kidney function | 216 (86.7) |
| Persistent proteinuria | 1 (0.4) |
| Mean arterial pressure, mmHg (mean + SD) | 101.6 + 16.6 |
| Missing data (n, %) | 95 (35.4) |
| Laboratory values | |
| Erythrocyturia (n, %) | 164 (77.4) |
| Missing data | 56 (20.9) |
| Proteinuria (n, %) | 176 (98.9) |
| Missing data | 90 (33.6) |
| 24 h proteinuria, gram/day (median; IQR) | 2.9; 1.4–5.9 |
| Urine albumin to creatinine ratio, mg/g Cr (median; IQR) | 2,648.3; 1,388.0–7,607.2 |
| eGFR, ml/min/1.73 m2, (median; IQR) | 100.5; 66.0–126.9 |
| Missing data (n, %) | 24 (8.9) |
| Hemoglobin, g/dl; (median; IQR) | 10.5; 9.3–12.1 |
| Missing data (n, %) | 25 (9.3) |
| Serum albumin, mg/dl (mean + SD) | 2.8 + 0.7 |
| Missing data (n, %) | 56 (20.9) |
| Antinuclear antibody positive (n, %) | 131 (97.0) |
| Missing data (n, %) | 133 (49.6) |
| Anti-double-stranded DNA positive, IU/ml (n, %) | 155 (77.1) |
| Missing data (n, %) | 67 (25) |
| Anti-double-stranded DNA level, IU/ml (median; IQR) | 404.6; 87.6–808.9 |
| Complement 3 level, mg/dl, (median; IQR) | 56.1; 38.8–78.0 |
| Missing data (n, %) | 76 (28.4) |
| Complement 4 level, mg/dl, (median; IQR) | 12.0; 6.0–19.3 |
| Missing data (n, %) | 82 (30.6) |
| Anticardiolipin antibody IgM, IU/L, (median; IQR) | 22.5; 14.3–30.2 |
| Anticardiolipin antibody IgG, IU/L, (median; IQR) | 9.0; 7.5–10.7 |
| SLEDAI-R score (mean ± SD) | 7.2 + 3.3 |
| Score 0 (inactive) (n, %) | 5 (2) |
| Score ≥4 (active) (n, %) | 245 (98) |
| Chronic kidney disease stage (n, %) | |
| 1 (eGFR >90) | 137 (51.1) |
| 2 (eGFR 60–89) | 54 (22.1) |
| 3A (eGFR 45–49) | 14 (5.7) |
| 3B (eGFR 30–44) | 19 (7.8) |
| 4 (eGFR 15–29) | 15 (6.1) |
| 5 (eGFR <15) | 5 (2.0) |
Baseline characteristics of lupus nephritis at the time of biopsy.
eGFR, estimated glomerular filtration rate; IQR, interquartile range; SD, standard deviation; SLE, systematic lupus erythematosus.
Complement 3 normal value, 90–180 mg/dl; Complement 4 normal value, 10–40 mg/dl.
A substantial proportion of patients exhibited the presence of chronic lesions within their biopsy specimens, including both global and segmental glomerulosclerosis (51.7% and 44.8%, respectively), as well as interstitial fibrosis and tubular atrophy (83.4% and 90.4%, respectively. The chronicity index predominantly presented with low to moderate category (52.6% and 44%). The complete distribution of histopathological variables is presented in Table 2.
Table 2
| Variables | n (%) | Missing data, n (%) |
|---|---|---|
| Global glomerulosclerosis | 136 (51.7) | 5 (1.87) |
| Segmental glomerulosclerosis | 117 (44.8) | 7 (2.61) |
| Ischemic | 3 (1.1) | 4 (1.49) |
| Mesangial hypercellularity | 237 (95.6) | 20 (7.46) |
| Mesangial matrix expansion | 76 (28.8) | 4 (1.49) |
| Endocapillary hypercellularity | ||
| Segmental | 126 (47.7) | 4 (1.49) |
| Global | 27 (10.2) | 4 (1.49) |
| Crescents | ||
| Cellular/fibrocellular | 36 (13.4) | 0 (0) |
| Fibrous | 5 (1.9) | 0 (0) |
| Wire loops | 12 (4.5) | 4 (1.49) |
| Adhesions | 17 (6.5) | 5 (1.87) |
| Fibrinoid necrosis | 67 (25.5) | 5 (1.87) |
| Nodular sclerosis | 0 (0) | 5 (1.87) |
| Karyorrhexis | 28 (10.6) | 4 (1.49) |
| Double contours | 19 (7.2) | 4 (1.49) |
| Spikes/vacuoles | 26 (9.8) | 4 (1.49) |
| Hyalin thrombi | 29 (11) | 4 (1.49) |
| Mesangiolysis | 1 (0.4) | 4 (1.49) |
| Sub-endothelial deposit | 39 (14.6) | 0 (0) |
| Interstitial fibrosis | 221 (83.4) | 3 (1.12) |
| Tubular atrophy | 103 (90.4) | 154 (57.46) |
| Arterial hyalinosis | 9 (3.6) | 21 (7.84) |
| Activity index total score (0–24) | ||
| High (18–24) | 4 (1.5) | 0 (0) |
| Moderate (6–17) | 100 (37.3) | |
| Low (0–5) | 164 (61.2) | |
| Chronicity index total score (0–12) | ||
| High (8–12) | 9 (3.4) | 0 (0) |
| Moderate (4–7) | 118 (44) | |
| Low (0–3) | 141 (52.6) | |
| Immunofluorescence study | ||
| IgG | 141 (64.4) | 49 (18.28) |
| IgM | 127 (58.3) | 50 (18.66) |
| IgA | 128 (58.4) | 49 (18.28) |
| C3 | 139 (63.5) | 49 (18.28) |
| C1q | 134 (61.2) | 49 (18.28) |
| Fibrinogen | 126 (57.3) | 48 (17.91) |
The distribution of histopathological variables in lupus nephritis.
Based on a histopathological study, class IV LN was the most common diagnosis (39.6%), followed by class III (20.5%), class V (14.2%), class I/II (13.4%), class IV + V (7.5%), class III + V (4.5%), and class VI (0.4%) LN. The distribution of histopathological diagnosis of LN is illustrated in Figure 1.
Figure 1
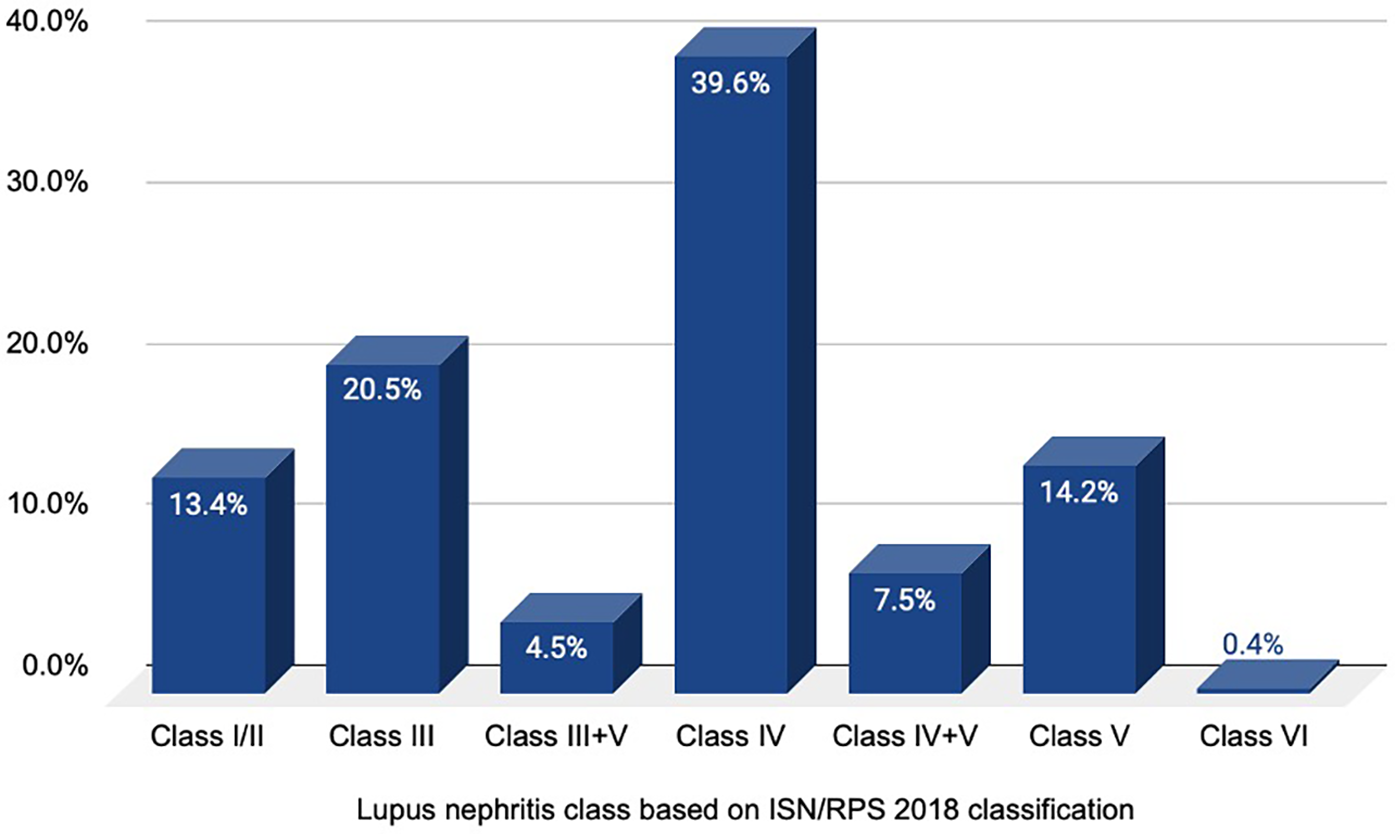
Distribution of histopathological diagnoses of lupus nephritis.
3.2 Distribution of clinical features across lupus nephritis classes
The association of various clinical parameters within LN classes was further studied. Compared to class I/II LN, class IV was significantly associated with a larger proportion of hypertensive population (43.8%, p = 0.004), the highest rate of not receiving any immunosuppressive medications at the time of biopsy (31.8%, p = 0.038), the lowest baseline kidney function (eGFR 77.8 ± 43.1, p < 0.001), the highest rate of CKD (eGFR <60 ml/min/1.73 m2: 61.3%, p < 0.001), and the lowest hemoglobin level (9.8 ± 2.6 g/dl, p = 0.022). Additionally, class IV was also linked to the largest proportion of nephrotic presentation (44.8%, p = 0.405), proteinuria (38.9%, p = 0.709), erythrocyturia (41.2%, p = 0.307), and ANA positivity (35.5%, p = 0.096), although most of these differences were not statistically significant. The combination therapy of glucocorticoids and azathioprine was mostly observed in class I/II and V LN (45.5% and 27.3%, respectively; p = 0.032). We studied that the prescription of hydroxychloroquine within LN classes was low: 0% in class VI, 3.3% in class III + V, 4.9% in class IV, 11.5% in class I/II, 18% in class V, 24.6% in class III, and 37.7% in class IV (p = 0.845). A similar observation was found with the prescription of RAAS inhibitors: 0.5% in class VI, 5.1% in class III + V, 5.6% in class IV + V, 12.1% in class I/II, 16.7% in class V, 22.7% in class III, and 37.4% in class IV (p = 0.138). The lowest serum albumin level was observed in class IV + V LN (2.6 ± 0.6 mg/dl, p = 0.008), while the lowest complement level was studied in class III + V (C3, 41.1 ± 32.0 mg/dl, p = 0.003; C4, 6.9 ± 5.3 mg/dl, p = 0.037). Table 3 summarizes the clinical characteristics of the population among classes of lupus nephritis.
Table 3
| Clinical variables | Class of lupus nephritis based on ISN/RPS 2018 classification | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| I/II | III | III + V | IV | IV + V | V | VI | |||
| Age, years; n (%) | <28 | 21 (16.8) | 27 (21.6) | 5 (4) | 46 (36.8) | 7 (5.6) | 19 (15.2) | 0 (0) | 0.626 |
| ≥28 | 13 (10.4) | 25 (20) | 7 (5.6) | 50 (40) | 11 (8.8) | 18 (14.4) | 1 (0.8) | ||
| Gender, n (%) | Female | 34 (14.3) | 49 (20.7) | 11 (4.6) | 90 (38) | 18 (7.6) | 34 (14.3) | 1 (0.4) | 0.673 |
| Male | 0 (0) | 3 (23.1) | 1 (7.7) | 6 (46.2) | 0 (0) | 3 (23.1) | 0 (0) | ||
| Hypertension, n (%) | 4 (3.8) | 28 (26.7) | 5 (4.8) | 46 (43.8) | 7 (6.7) | 14 (13.3) | 1 (1) | 0.004* | |
| Previous diagnosis of SLE, n (%) | 9 (11) | 18 (22) | 5 (6.1) | 34 (41.5) | 4 (4.9) | 11 (13.4) | 1 (1.2) | 0.604 | |
| Nephrotic presentation, n (%) | 2 (6.9) | 3 (10.3) | 1 (3.4) | 13 (44.8) | 4 (13.8) | 6 (20.7) | 0 (0) | 0.405 | |
| Immunosuppressant use at biopsy, n (%) | |||||||||
| Oral glucocorticoids | 7 (17.1) | 7 (17.1) | 3 (7.3) | 15 (36.6) | 3 (7.3) | 6 (14.6) | 0 (0) | 0.949 | |
| IV glucocorticoids | 1 (20) | 1 (20) | 0 (0) | 2 (40) | 0 (0) | 1 (20) | 0 (0) | 0.990 | |
| Glucocorticoids + MMF or MPA | 12 (9.9) | 27 (22.3) | 6 (5) | 50 (41.3) | 6 (5) | 20 (16.5) | 0 (0) | 0.404 | |
| Glucocorticoids + cyclosporin A | 1 (20) | 2 (40) | 1 (0) | 2 (40) | 0 (0) | 0 (0) | 0 (0) | 0.874 | |
| Glucocorticoids + azathioprine | 5 (45.5) | 2 (18.2) | 0 (0) | 1 (9.1) | 0 (0) | 3 (27.3) | 0 (0) | 0.032* | |
| –Glucocorticoids + tacrolimus | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0.952 | |
| Mycophenolate mofetil or mycophenolic acid only | 0 (0) | 1 (14.3) | 0 (0) | 3 (42.9) | 1 (14.3) | 2 (28.6) | 0 (0) | 0.813 | |
| None | 4 (18.2) | 6 (27.3) | 0 (0) | 7 (31.8) | 2 (9.1) | 2 (9.1) | 1 (4.5) | 0.038* | |
| Hydroxychloroquine | 7 (11.5) | 15 (24.6) | 2 (3.3) | 23 (37.7) | 3 (4.9) | 11 (18) | 0 (0) | 0.845 | |
| Use of RAAS inhibitors, n (%) | 24 (12.1) | 45 (22.7) | 10 (5.1) | 74 (37.4) | 11 (5.6) | 33 (16.7) | 1 (0.5) | 0.138 | |
| Laboratory value at biopsy | |||||||||
| Proteinuria, n (%) | 32 (13.1) | 50 (20.5) | 12 (4.9) | 95 (38.9) | 18 (7.4) | 36 (14.8) | 1 (0.4) | 0.709 | |
| Erythrocyturia, n (%) | 22 (11.8) | 41 (21.9) | 10 (5.3) | 77 (41.2) | 12 (6.4) | 24 (12.8) | 1 (0.5) | 0.307 | |
| eGFR, ml/min/1.73 m2; mean ± SD | 110.6 ± 32.6 | 96.9 ± 32.4 | 90.7 ± 32.2 | 77.8 ± 43.1 | 84.2 ± 31.3 | 107.2 ± 38.0 | – | <0.001* | |
| eGFR, ml/min/1.73 m2; | ≥60 | 31 (16.3) | 44 (23.2) | 10 (5.3) | 59 (31.1) | 14 (7.4) | 32 (16.8) | 0 (0) | |
| n (%) | <60 | 3 (5) | 8 (13.3) | 2 (3.3) | 37 (61.3) | 4 (6.7) | 5 (8.3) | 1 (1.7) | |
| Hemoglobin, g/dl; mean ± SD Hemoglobin, g/dl; n (%) | 10.9 ± 2.5 | 10.9 ± 2.4 | 9.4 ± 3.4 | 9.8 ± 2.6 | 9.9 ± 2.6 | 10.9 ± 2.0 | – | 0.019* | |
| ≥10 | 26 (16.9) | 38 (24.7) | 7 (4.5) | 47 (30.5) | 11 (7.1) | 25 (16.2) | 0 (0) | ||
| <10 | 8 (8.3) | 14 (4.6) | 5 (5.2) | 49 (51) | 7 (7.3) | 12 (12.5) | 1 (1) | ||
| Serum albumin, mg/dl; mean ± SD | 3.1 ± 0.9 | 3.1 ± 0.6 | 2.7 ± 0.7 | 2.8 ± 0.9 | 2.6 ± 0.6 | 2.7 ± 0.7 | – | 0.008* | |
| Serum albumin, mg/dl; n (%) | ≥3.5 | 15 (30.6) | 14 (28.6) | 2 (4.1) | 10 (20.4) | 2 (4.1) | 5 (10.2) | 1 (2) | <0.001* |
| <3.5 | 19 (9.5) | 38 (18.9) | 10 (5) | 86 (42.8) | 16 (8) | 32 (15.9) | 0 (0) | ||
| ANA positive, n (%) | 29 (14.3) | 42 (20.7) | 10 (4.9) | 72 (35.5) | 17 (8.4) | 33 (16.3) | 0 (0) | 0.096 | |
| Anti-dsDNA, IU/ml; mean ± SD | 895.2 ± 1,996.5 | 601.3 ± 452.5 | 716.8 ± 411.4 | 665.4 ± 611.9 | 689.7 ± 513.5 | 720.2 ± 1,537.6 | – | 0.443 | |
| Anti-dsDNA positive, n (%) | 26 (12.8) | 43 (21.2) | 11 (5.4) | 81 (39.9) | 14 (6.9) | 27 (13.3) | 1 (0.5) | 0.666 | |
| C3, mg/dl; mean ± SD | 69.8 ± 36.6 | 48.4 ± 27.5 | 41.1 ± 32.0 | 52.9 ± 33.4 | 43.9 ± 32.4 | 65.9 ± 34.0 | – | 0.003* | |
| C3; mg/dl; n (%) | ≥90 | 10 (28.6) | 5 (14.3) | 1 (2.9) | 10 (28.6) | 1 (2.9) | 8 (22.9) | 0 (0) | 0.064 |
| <90 | 24 (11.2) | 47 (21.9) | 11 (5.1) | 86 (40) | 17 (7.9) | 29 (13.5) | 1 (0.5) | ||
| C4, mg/dl; mean ± SD | 14.6 ± 10.5 | 12.3 ± 8.6 | 6.9 ± 5.3 | 12.8 ± 9.5 | 11.3 ± 7.0 | 16.8 ± 12.4 | – | 0.037* | |
| C4, mg/dl; n (%) | ≥10 | 20 (14.6) | 27 (19.7) | 4 (2.9) | 55 (40.1) | 8 (5.8) | 22 (16.1) | 1 (0.7) | 0.570 |
| <10 | 14 (12.4) | 25 (22.1) | 8 (7.1) | 41 (36.3) | 10 (8.8) | 15 (13.3) | 0 (0) | ||
| SLEDAI-R score (mean ± SD) | 6.2 ± 3.4 | 6.3 ± 3.0 | 7.3 ± 3.0 | 7.7 ± 3.4 | 8.2 ± 2.9 | 7.2 ± 3.2 | – | 0.114 | |
| Score 0 (inactive) (n, %) | 2 (40) | 2 (40) | 0 (0) | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 0.401 | |
| Score ≥4 (active) (n, %) | 32 (13.1) | 50 (20.4) | 12 (4.9) | 96 (39.2) | 18 (7.3) | 36 (14.7) | 1 (0.4) | ||
The association of clinical variables and classes of lupus nephritis.
Reference: class I/II LN.
ANA, antinuclear antibody; anti-dsDNA, anti-double-stranded DNA, C3, Complement 3; C4, Complement 4; eGFR, estimated glomerular filtration rate; MMF, mycophenolate mofetil; MPA, mycophenolic acid; RAAS inhibitors, renin–angiotensin–aldosterone system inhibitors; SLE, systematic lupus erythematosus.
A p-value less than 0.05 is considered statistically significant.
3.3 Distribution of histopathological lesions across lupus nephritis classes
We studied the histopathological characteristics in association with different classes of lupus nephritis (Table 4). When compared with class I/II LN, class IV showed a higher presentation of both chronic and acute lesions. Class IV was linked to several forms of chronic lesions, including global and segmental glomerulosclerosis (42.6%, p = 0.073, and 41.1%, p = 0.009, respectively), fibrous crescent (80%, p = 0.572), interstitial fibrosis (38.7%, p = 0.791), and tubular atrophy (38.9%, p = 0.710). Other forms of chronic lesions were more prevalent in class III LN, such as glomerular adhesion (43.8%, p = 0.083) and arterial hyalinosis (55.6%, p = 0.183), while spike/vacuole formation was more common in class V (48%, p < 0.001). Additionally, most active lesions were predominantly found in class IV, including mesangial hypercellularity (38.2%, p = 0.704), mesangial matrix expansion (37%, p = 0.388), segmental and global endocapillary hypercellularity (43.1%, p < 0.001, and 74.1%, p = 0.004, respectively), cellular/fibrocellular crescent (62.9%, p = 0.068), wire loop formation (25%, p = 0.207), fibrinoid necrosis (49.2%, p = 0.341), karyorrhexis (38.5%, p = 0.878), double contours (73.7%, p = 0.059), and sub-endothelial deposit (59.5%, p = 0.007).
Table 4
| Histopathological characteristic | Class of lupus nephritis based on ISN/RPS 2018 | p-value | ||||||
|---|---|---|---|---|---|---|---|---|
| I/II | III | III + V | IV | IV + V | V | VI | ||
| Global glomerulosclerosis | 10 (7.8) | 28 (21.7) | 6 (4.7) | 55 (42.6) | 12 (9.3) | 17 (13.2) | 1 (0.8) | 0.073 |
| Segmental glomerulosclerosis | 9 (8) | 23 (29.5) | 3 (2.7) | 46 (41.1) | 9 (8) | 12 (10.7) | 0 (0) | 0.009* |
| Ischemic | 0 (0) | 0 (0) | 1 (33.3) | 1 (33.3) | 0 (0) | 1 (33.3) | 0 (0) | 0.266 |
| Mesangial hypercellularity | 32 (14.5) | 46 (20.9) | 10 (4.5) | 84 (38.2) | 17 (7.7) | 31 (14.1) | - | 0.704 |
| Mesangial matrix expansion | 9 (12.3) | 19 (26) | 5 (6.8) | 27 (37) | 2 (2.7) | 11 (15.1) | 0 (0) | 0.388 |
| Endocapillary hypercellularity | ||||||||
| Segmental | 4 (3.3) | 40 (32.5) | 10 (8.1) | 53 (43.1) | 8 (6.5) | 8 (6.5) | 0 (0) | <0.001* |
| Global | 0 (0) | 5 (18.5) | 0 (0) | 20 (74.1) | 1 (3.7) | 1 (3.7) | 0 (0) | 0.004 |
| Crescents | ||||||||
| Cellular/fibrocellular | 1 (2.9) | 6 (17.1) | 1 (2.9) | 22 (62.9) | 2 (5.7) | 3 (8.6) | 0 (0) | 0.068 |
| Fibrous | 0 (0) | 0 (0) | 0 (0) | 4 (80) | 0 (0) | 1 (20) | 0 (0) | 0.572 |
| Wire loops | 1 (8.3) | 6 (50) | 1 (8.3) | 3 (25) | 1 (8.3) | 0 (0) | 0 (0) | 0.207 |
| Adhesions | 2 (12.5) | 7 (43.8) | 2 (12.5) | 5 (31.3) | 0 (0) | 0 (0) | 0 (0) | 0.083 |
| Fibrinoid necrosis | 5 (7.7) | 14 (21.5) | 3 (4.6) | 32 (49.2) | 3 (4.6) | 8 (12.3) | 0 (0) | 0.341 |
| Karyorrhexis | 2 (7.7) | 7 (26.9) | 1 (3.8) | 10 (38.5) | 3 (11.5) | 3 (11.5) | 0 (0) | 0.878 |
| Double contours | 1 (5.3) | 1 (5.3) | 1 (5.3) | 14 (73.7) | 0 (0) | 2 (10.5) | 0 (0) | 0.059 |
| Spikes/vacuoles | 0 (0) | 3 (12) | 5 (20) | 2 (8) | 3 (12) | 12 (48) | 0 (0) | <0.001* |
| Hyalin thrombi | 2 (7.1) | 10 (35.7) | 1 (3.6) | 10 (35.7) | 3 (10.7) | 2 (7.1) | 0 (0) | 0.371 |
| Mesangiolysis | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.395 |
| Sub-endothelial deposit | 1 (2.7) | 8 (21.6) | 4 (10.8) | 22 (59.5) | 1 (2.7) | 1 (2.7) | 0 (0) | 0.007* |
| Interstitial fibrosis | 27 (12.7) | 46 (21.7) | 11 (5.2) | 82 (38.7) | 15 (7.1) | 30 (14.2) | 1 (0.5) | 0.791 |
| Tubular atrophy | 32 (13.4) | 49 (20.5) | 12 (5) | 93 (38.9) | 16 (6.7) | 36 (15.1) | 1 (0.4) | 0.710 |
| Arterial hyalinosis | 1 (11.1) | 5 (55.6) | 0 (0) | 2 (22.2) | 1 (11.1) | 0 (0) | 0 (0) | 0.183 |
The association of histopathological lesions and classes of lupus nephritis.
Reference: class I/II lupus nephritis.
A p-value less than 0.05 is considered statistically significant.
A more detailed analysis of the association between the activity and chronicity index and the classes of LN is presented in Supplementary Figure S2. Class IV LN was significantly associated with higher activity and chronicity index compared with class I/II LN. Furthermore, renal disease activity as measured by the SLEDAI-R score demonstrated a significant linear correlation with the histopathological activity index (p < 0.001) (Supplementary Table S2).
3.4 Immunofluorescence study
The largest expression of all antibodies was found in class IV LN, although these antibodies were present in <50% of kidney biopsies (Figure 2). The presence of IgM and C1q antibodies was significantly associated with class IV LN (44%, p = 0.028% and 45%, p = 0.015; respectively), compared with class I/II LN.
Figure 2
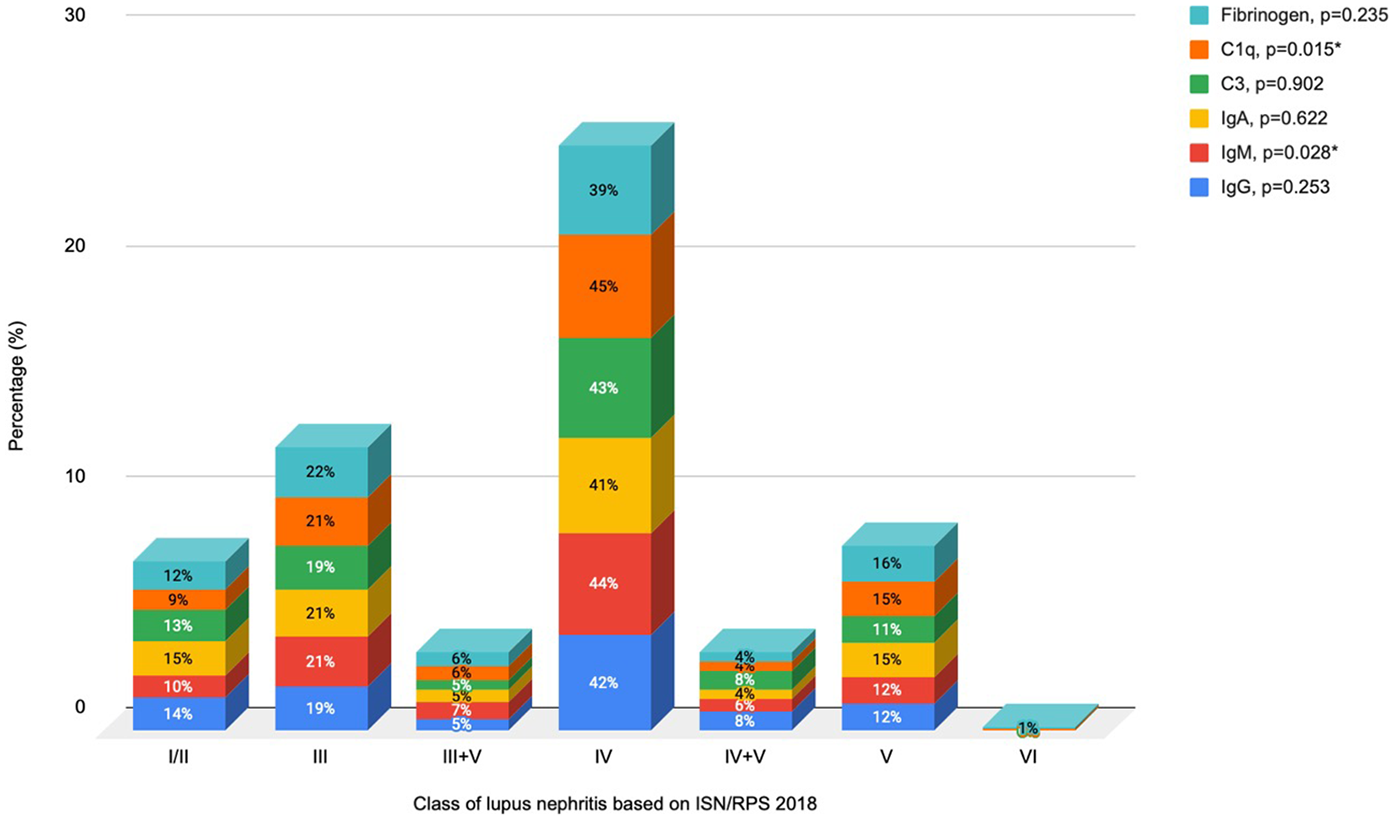
Association between antibody expression and classes of lupus nephritis. *Reference: class I/II lupus nephritis.
3.5 Cumulative incidence of kidney outcomes
We examined two kidney outcomes, i.e., death from any cause and ESKD/KRT initiation, among all subjects during an average median follow-up of 26 months (IQR 6.0–48.0). Overall, 16.4% of patients died during follow-up, and 3.7% of patients developed ESKD/initiated KRT (Figure 3). Most of the patients died due to sepsis (45.5%), followed by tuberculosis infection and respiratory failure of any cause (9.1%, individually). The percentage of lost to follow-up cases was approximately 24.6%, primarily comprised of individuals from class IV and class III LN (51.5% and 13.6%, respectively; Supplementary Table S3).
Figure 3
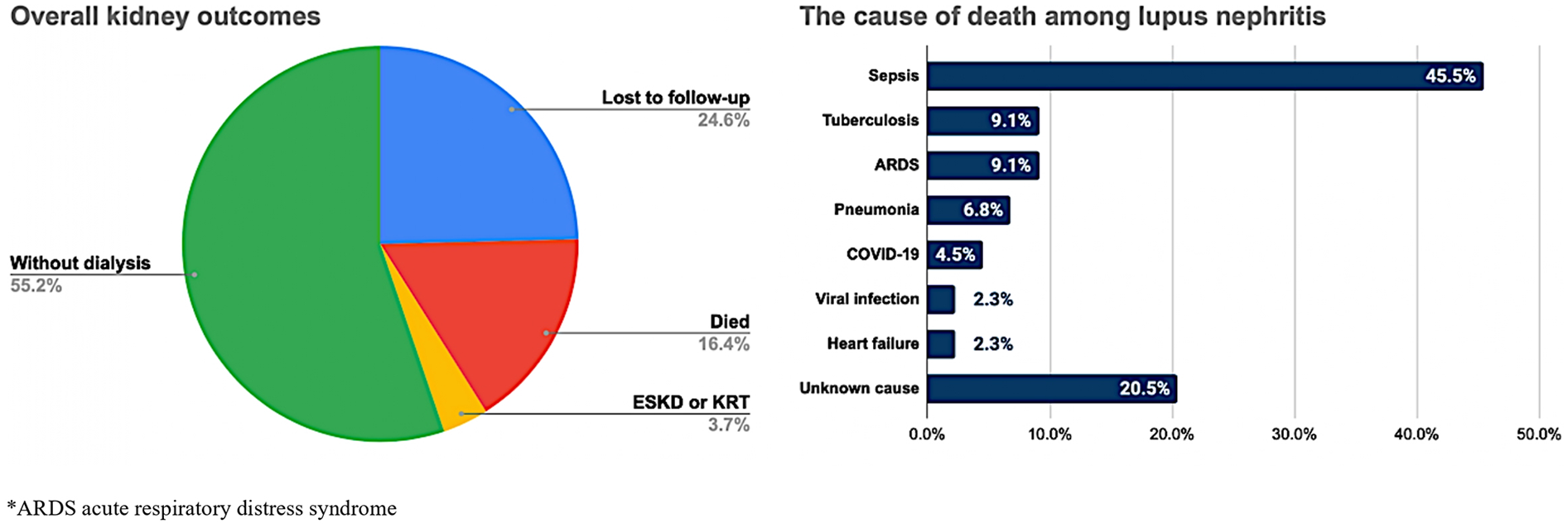
Proportion of kidney outcomes and causes of death among the lupus nephritis population.
Given the high mortality rate observed in our cohort, we further examined the relationship between clinical and treatment-related variables with the study outcomes (Supplementary Table S4). Although the trend shows that more severe classes (especially class IV and IV + V) were associated with worse outcomes, this was not statistically significant (p = 0.195). The presence of CKD appears to worsen outcomes, but the difference does not reach statistical significance (p = 0.173). There is a clinical trend suggesting worse outcomes with active renal disease (SLEDAI-R > 4), but the sample size for inactive cases was too small (n = 5) for statistical validation. Moderate activity or chronicity index may be associated with worse outcomes, but numbers were too small to confirm (p = 0.346 and 0.580, respectively).
CKD status was found to be associated with different causes of death (p = 0.042); in particular, heart failure was a more frequent cause in patients with CKD, while ARDS and infections dominate in those without CKD (Supplementary Table S5).
The results in Figure 4 allow us to visualize that the total probability of death was higher compared with the risk being in KRT across all classes of LN, except class III + V. Kidney outcomes of ESKD/KRT initiation were not observed in class III LN, as well as both death and ESKD/KRT initiation were not observed within class VI.
Figure 4
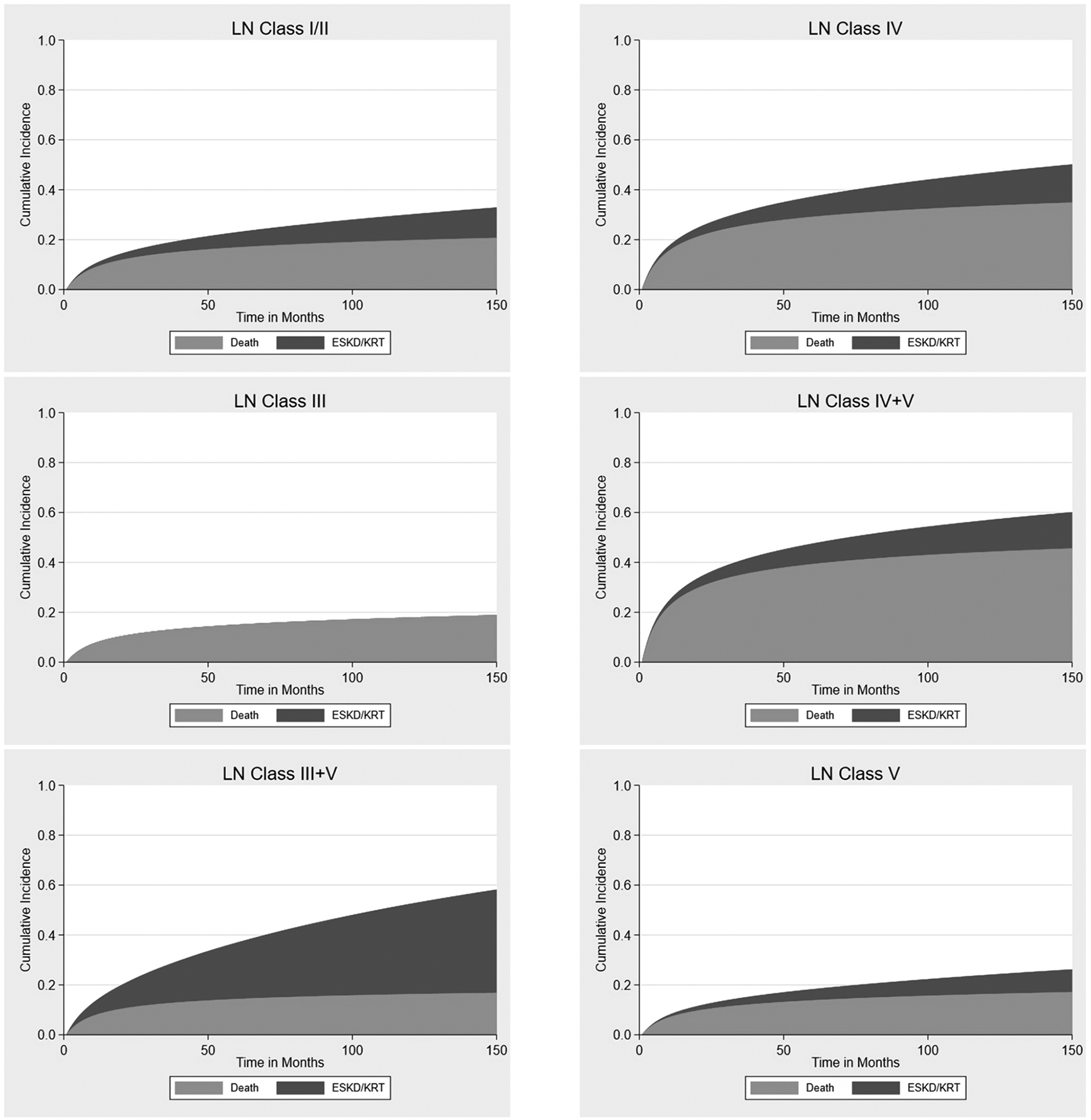
Cumulative incidence of death and end-stage kidney disease or initiation of kidney replacement therapy across lupus nephritis classes, adjusted for age.
The cumulative incidence for the probability of death while accounting for the competing risk of ESKD/KRT initiation was further detailed in Table 5. Class IV LN had a higher risk of death compared with the risk of acquiring ESKD/KRT across all classes (HR 1.94, 95% CI 1.07–3.50, p = 0.028).
Table 5
| Class of lupus nephritis | Death | End-stage kidney disease or kidney replacement therapy initiation | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% confidence interval | p-value | Hazard ratio | 95% confidence interval | p-value | |
| I/II | 0.74 | 0.31–1.77 | 0.498 | 1.00 | 0.21–4.78 | 0.998 |
| III | 0.58 | 0.25–1.49 | 0.229 | |||
| III + V | 0.68 | 0.09–4.95 | 0.704 | 4.33 | 0.54–35.10 | 0.169 |
| IV | 1.94 | 1.07–3.50 | 0.028* | 2.05 | 0.60–7.07 | 0.258 |
| IV + V | 2.50 | 0.96–6.46 | 0.059 | 1.99 | 0.25–16.04 | 0.518 |
| V | 0.57 | 0.20–1.59 | 0.284 | 0.66 | 0.08–5.22 | 0.695 |
The cumulative incidence of kidney outcomes, adjusted for age and stratified by lupus nephritis classes.
A p-value less than 0.05 is considered statistically significant.
3.6 Progression of kidney function over time
We included 247 patients with a minimum of one eGFR measurement during the study period to assess kidney function decline over time, adjusted for age and gender (Table 6).
Table 6
| Parameter | Estimate (β) | Standard error (SE) | p-value | 95% confidence interval | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| Intercept | 112.1 | 32.6 | <0.001* | 48.1 | 176.1 |
| Time | −4.7 | 25.9 | 0.855 | −55.6 | 46.1 |
| Male | Reference | ||||
| Female | −7.0 | 25.2 | 0.781 | −56.5 | 42.5 |
| Age ≥28 years | Reference | ||||
| Age <28 years | 25.6 | 8.5 | 0.003* | 8.8 | 42.4 |
| LN class I/II | Reference | ||||
| LN class III | −17.4 | 13.5 | 0.197 | −43.9 | 9.1 |
| LN class III + V | −48.1 | 26.7 | 0.072 | −100.5 | 4.3 |
| LN class IV | −51.3 | 12.3 | <0.001* | −75.5 | −27.2 |
| LN class IV + V | −30.6 | 18.2 | 0.094 | −66.4 | 5.2 |
| LN class V | −13.7 | 15.4 | 0.374 | −44.0 | 16.5 |
| LN class VI | −89.7 | 78.3 | 0.252 | −243.5 | 64.2 |
| Male × time | Reference | ||||
| Female × time | 7.0 | 17.8 | 0.692 | −27.9 | 42.0 |
| Age ≥28 years × time | Reference | ||||
| Age <28 years × time | −9.0 | 5.2 | 0.085 | −19.3 | 1.3 |
| LN class I/II × time | Reference | ||||
| LN class III × time | 2.1 | 7.5 | 0.78 | −12.7 | 16.9 |
| LN class III + V × time | 24.5 | 18.4 | 0.183 | −11.6 | 60.6 |
| LN class IV × time | 15.7 | 7.0 | 0.026* | 1.8 | 29.5 |
| LN class IV + V × time | 4.4 | 10.9 | 0.683 | −16.9 | 25.8 |
| LN class V × time | 4.5 | 9.2 | 0.626 | −13.6 | 22.6 |
| LN class VI × time | 2.3 | 49.4 | 0.963 | −94.8 | 99.4 |
The effect of lupus nephritis on kidney function during study follow-up.
Dependent variable: eGFR at baseline.
A p-value less than 0.05 is considered statistically significant.
The mean baseline kidney function for the reference group LN class I/II was reasonably high (β 112.1, SE 32.6, p < 0.001) and declined by 4.7 (SE 25.9) ml/min/1.73 m2 per year, although this was not statistically significant (p = 0.855). Compared to LN class I/II, all classes showed a lower baseline kidney function, with the lowest eGFR observed in class VI, although this difference was not statistically significant (β = −89.7, SE = 78.3, p = 0.252). Class IV LN showed a significant difference in eGFR of −51.3 (SE = 12.3, p < 0.001) at baseline, compared with class I/II LN.
Over time, kidney function declined less or even increased within all LN classes compared with LN class I/II, although most effects were not significant. A significant difference in eGFR decline over time was observed in class IV (β = 15.7, SE = 7.0, p = 0.026), compared with class I/II LN, reflecting an effective eGFR increase over time in class IV.
4 Discussion
Our study demonstrated that LN patients in Indonesia share clinical characteristics with those in other countries but show notably lower use of RAAS inhibitors and hydroxychloroquine. Class IV was the most prevalent diagnosis and was associated with a higher prevalence of hypertension, lower baseline eGFR, higher rate of CKD, lower hemoglobin level, and the highest proportion of patients not receiving immunosuppressive medications at the time of biopsy. Class IV also had the greatest burden of both acute and chronic histopathological lesions. Overall mortality was high, with infections—particularly tuberculosis—being the leading cause of death. CKD status was significantly associated with different causes of death. Across all LN classes except class III + V, the risk of death exceeded the risk of requiring kidney replacement therapy (KRT). Class IV LN, in particular, showed significantly lower baseline eGFR than class I/II, but demonstrated a less steep decline or even improvement in eGFR over time.
Renal involvement was identified in 22% of our SLE patients, a prevalence comparable to findings in other Asian populations. The prevalence of SLE is higher in Asian populations than in White populations (23, 24), often accompanied by more severe organ involvement, particularly affecting the kidneys (7, 12, 23–28), thus suggesting disparities in population demographics, genetic predisposition, environmental exposures, and socioeconomic status. In line with prior reports, our cohort was predominantly female, with most patients presenting with hypertension, hematuria, and proteinuria (29–34). Similarly, proliferative glomerulonephritis, specifically class III or IV, was the most frequently observed histopathological subtype (35, 36).
Our findings reflected a low usage of RAAS inhibitors and hydroxychloroquine across all classes of LN. Although both agents are typically included in the Indonesian National Health Insurance System formulary (15), the access and availability can depend on the specific treatment protocols and regional healthcare infrastructure with larger cities often having better access to these medications compared with more rural or remote areas. The potential side effects of RAAS inhibitors (such as hyperkalemia) and hydroxychloroquine (such as retinopathy), which require long-term monitoring and better healthcare resources, may result in clinical inertia and hesitancy among patients to adhere to the prescribed regimen. There may be inconsistent or outdated treatment guidelines in some regions of Indonesia regarding the management of lupus. Inadequate adherence to evidence-based guidelines or unfamiliarity with the benefits of hydroxychloroquine may result in healthcare providers underprescribing or even overlooking its role in lupus management.
In our cohort, kidney biopsy was performed approximately 2 years after SLE diagnosis, in contrast to a Chinese study reporting a median interval of 0 months between SLE and LN diagnoses (37). This delayed referral may contribute to the higher prevalence of chronic lesions observed. Contributing factors include limited availability of trained personnel and appropriate facilities, with renal biopsy and histopathology services (e.g., immunofluorescence) restricted to tertiary centers and no access to electron microscopy nationwide. Delays may also result from prolonged waiting times between biopsy indication and pathological diagnosis, which in our experience often exceeded 3 months, although precise data were not recorded. Financial barriers due to high costs and partial insurance coverage further limit access. Clinician reluctance—stemming from procedural concerns or reliance on empirical management—along with limited awareness of the diagnostic and prognostic value of biopsy, particularly among general practitioners, also plays a role. The lack of national guidelines and robust local data further impedes the routine use of renal biopsy in clinical practice.
Class IV was present in nearly 40% of kidney biopsies, despite over half of our cohort exhibiting an eGFR of >60 ml/min/1.73 m2, which was higher than reported in other studies (37–39). This finding is intriguing since we anticipated a higher proportion of patients with earlier LN stages (i.e., class I/II). We hypothesize that the high prevalence of class IV reflects the clinical indication for biopsy rather than the true distribution of renal pathology. The underrepresentation of class I/II may be due to fewer biopsies performed in this subgroup, further supporting the notion of delayed referrals, potentially arising from low awareness of kidney involvement in SLE.
Kidney biopsy has become an integral component of lupus nephritis management in our center, guiding both treatment decisions and prognostic assessment based on histopathological findings. However, due to the retrospective design and limited data availability, we could not assess how biopsy results influenced treatment choices or patient response rates. Additionally, follow-up was incomplete for some patients who were referred back to their local hospitals, did not return for further care due to non-adherence, financial, or access barriers, or opted for alternative therapies.
Although most recommended treatments for lupus nephritis are available at our center, fewer than half of the patients received the standard regimen of glucocorticoids combined with mycophenolate mofetil (MMF)/mycophenolic acid (MPA). The remainder were treated with alternative immunosuppressants (e.g., cyclosporin A or azathioprine) or glucocorticoid monotherapy. This rate is considerably lower than reported in neighboring countries (40–43). These findings may be influenced by incomplete medical records and limited treatment documentation for patients referred from other centers.
The all-cause mortality rate in our cohort was high, aligning with previous studies showing increased mortality among patients with class IV lupus nephritis (43, 44). In contrast, studies from the USA report standardized mortality ratios (SMRs) of 6.3–7.9, while European cohorts demonstrate more favorable outcomes, with an 8-year survival rate of 89% and an SMR of 2.65 (95% CI: 2.13–3.26), despite a 6.7% progression to ESK D (2, 45, 46). Compared to these figures, as well as mortality rates reported in other Asian countries (9.1%–14.8%) (35, 40, 41), our cohort exhibited a slightly higher mortality rate, suggesting a relatively poorer prognosis. Contributing factors may include delayed presentation, limited access to optimal therapy, and inadequate follow-up and monitoring. Additionally, a high rate of infections—particularly severe respiratory infections such as tuberculosis—was a leading cause of death, consistent with patterns observed in other developing countries (40). Being situated in an endemic area, tuberculosis remains a substantial cause of death in our population, particularly among those at higher risk due to their immunocompromised status. Therefore, this study suggests performing an appropriate antituberculosis prophylactic and aggressive tuberculosis screening in the lupus nephritis population. Additionally, renal function monitoring, especially in patients at risk of CKD, should be performed routinely.
The strength of this study lies in the involvement of two renal pathologists reviewing biopsy results, reducing the risk of measurement bias. To our knowledge, this is the first study to report long-term renal outcomes in a large lupus nephritis cohort in Indonesia. However, its retrospective design led to incomplete clinical data and a high loss to follow-up (24.6%), addressed through multiple imputation, sensitivity analyses, and appropriate censoring to reduce attrition bias. Variability in immunosuppressive regimens, due to the absence of standardized treatment protocols, may have introduced bias. Additionally, data on treatment duration, adherence, and response were largely unavailable, limiting further analysis.
In conclusion, despite similarities with cohorts from other countries, our population showed more chronic lesions, higher mortality, and lower use of RAAS inhibitors and immunosuppressive therapy. Biopsies were primarily performed for diagnostic confirmation rather than early screening. These findings highlight the need for earlier detection of kidney involvement in SLE, timely nephrology referral, optimized use of RAAS inhibitors and immunosuppressants, and proactive infection management. Further cohort studies are needed to clarify the impact of patient phenotypes and varied treatment approaches on long-term outcomes in lupus nephritis in Indonesia.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Faculty of Medicine, Universitas Indonesia—Dr. Cipto Mangunkusumo Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. We collected kidney biopsy records from 1 January 2011 to 31 December 2021 at Dr. Cipto Mangunkusumo Hospital, Jakarta, Indonesia. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
NH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. ES: Conceptualization, Supervision, Writing – review & editing. MM: Validation, Writing – review & editing. MS: Validation, Writing – review & editing. YT: Writing – review & editing. MD: Methodology, Supervision, Writing – review & editing. JR: Conceptualization, Supervision, Writing – review & editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. Ni Made Hustrini receiving funding as part of graduate school program from Leiden University Medical Center (LUMC) (Agreement Number: 91/AOI/FK/UI/2021).
Acknowledgments
We would like to express our gratitude to Ms. Gitri Syiamil Awali and Ms. Avissa Sakina for their study coordination and data management.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/flupu.2025.1604644/full#supplementary-material
Abbreviations
ACA, anticardiolipin antibody; ANA, antinuclear antibody; anti-dsDNA, anti-double-stranded DNA; C3, Complement 3; C4, Complement 4; CHF, congestive heart failure; CKD-EPI, chronic kidney disease epidemiology collaboration; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; HPF, high power field; ISN/RPS, International Society of Nephrology/Renal Pathology Society; KRT, kidney replacement therapy; LN, lupus nephritis; MAP, mean arterial pressure; RAAS, inhibitors renin–angiotensin–aldosterone system inhibitors; RBC, red blood cell; SLE, systemic lupus erythematosus; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; SLEDAI-R, Systemic Lupus Erythematosus Disease Activity Index—Renal Domain; SMRs, standardized mortality ratios; uACR, urine albumin to creatinine ratio; WBC, white blood cell.
References
1.
HocaogluMValenzuela-AlmadaMODabitJYOsei-OnomahSAChevetBGiblonREet alIncidence, prevalence, and mortality of lupus nephritis: a population-based study over four decades using the lupus midwest network. Arthritis Rheumatol. (2023) 75:567. 10.1002/art.42375
2.
PryorKBarbhaiyaMCostenbaderKFeldmanC. Disparities in lupus and LN care and outcomes among U.S. Medicaid Beneficiaries. Rheum Dis Clin North Am. (2021) 47(1):41–53. 10.1016/j.rdc.2020.09.004
3.
SalamaADCaplinB. LN and chronic kidney disease. J Rheumatol. (2020) 47:1303–4. 10.3899/jrheum.200566.
4.
HooverPJCostenbaderKH. Insights into the epidemiology and management of LN from the U.S. rheumatologist’s perspective. Kidney Int. (2016) 90:487. 10.1016/j.kint.2016.03.042
5.
CrocaSCRodriguesTIsenbergDA. Assessment of a LN cohort over a 30-year period. Rheumatology. (2011) 50:1424–30. 10.1093/rheumatology/ker101
6.
FaurschouMDreyerLKamperALStarklintHJacobsenS. Long-term mortality and renal outcome in a cohort of 100 patients with LN. Arthritis Care Res (Hoboken). (2010) 62(6):873–80. 10.1002/acr.20116
7.
HustriniNMSusalitELydiaAMarbunMBHSyafiqMSarwonoJet alThe etiology of kidney failure in Indonesia: a multicenter study in tertiary-care centers in Jakarta. Ann Glob Health. (2023) 89(1):36. 10.5334/aogh.4071
8.
YapDYHChanTM. Lupus nephritis in Asia: clinical features and management. Kidney Dis. (2015) 1(2):100–9. 10.1159/000430458
9.
PetriMFangCGoldmanDW. East-Asian lupus nephritis in the Hopkins lupus cohort. Rheumatol and Immunol Res. (2023) 4(3):157–61. 10.2478/rir-2023-0022
10.
PatelMClarkeAMBruceINSymmonsDPM. The prevalence and incidence of biopsy-proven LN in the UK evidence of an ethnic gradient. Arthritis Rheum. (2006) 54(9):2963–9. 10.1002/art.22079
11.
DeQuattroKTrupinLMurphyLBRushSCriswellLALanataCMet alHigh disease severity among Asians in a US multiethnic cohort of individuals with systemic lupus erythematosus. Arthritis Care Res (Hoboken). (2022) 74(6):896–903. 10.1002/acr.24544
12.
ThumbooJWeeHL. Systemic lupus erythematosus in Asia: is it more common and more severe?APLAR J Rheumatol. (2006) 9:320–6. 10.1111/j.1479-8077.2006.00235.x
13.
TesarVHruskovaZ. LN: a different disease in European patients?Kidney Dis (2015) 1(2):110–8. 10.1159/000438844
14.
YapDYHChanTM. Treatment of LN: practical issues in Asian countries. Int J Rheum Dis. (2015) 18(2):138–45. 10.1111/1756-185X.12423
15.
Sadikin BG - Ministry of Health Republic of Indonesia. Keputusan Menteri Kesehatan Republik Indonesia Nomor HK.01.07/Menkes/2197/2023 tentang Formularium Nasional (2023). Available at:https://www.jdih.kemkes.go.id(Accessed August 15, 2024).
16.
SaraswatiMHustriniNMPurnamaY. Keterbatasan metode diagnostik patologi anatomik dalam bidang transplantasi ginjal di Indonesia. J Indon Med Assoc. (2022) 72(4):151–6. 10.47830/jinma-vol.72.4-2022-879
17.
LydiaA. Merawat Kesehatan Ginjal Generasi Muda Indonesia: Peran Deteksi Dini Glomerulonefritis. Inauguration Speech as Professor in Internal Medicine Faculty of Medicine, University of Indonesia. Depok Jawa Barat: UI Publishing (2023).
18.
MinaRAbulabanKKlein-GitelmanMEberhardAArdoinSSingerNet alValidation of the lupus nephritis clinical indices in childhood-onset systemic lupus erythematosus. Arthritis Care Res. (2016) 68(2):195–202. 10.1002/acr.22651
19.
GladmanDDIbanezDUrowitzMB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. (2002) 29(2):288–91.
20.
BajemaIMWilhelmusSAlpersCEBruijnJAColvinRBCookHTet alRevision of the International Society of Nephrology/Renal Pathology Society classification for LN: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. (2018) 93:789–96. 10.1016/j.kint.2017.11.023
21.
LeveyASStevensLA. Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. (2010) 55(4):622–7. 10.1053/j.ajkd.2010.02.337
22.
von ElmEAltmanDGEggerMPocockSJGotzschePCVandenbrouckeJP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies.
23.
BarberMRDrenkardCFalasinnuTHoiAMakAKowNYet alGlobal epidemiology of systemic lupus erythematosus. Nat Rev Rheumatol. (2021) 17:515–32. 10.1038/s41584-021-00668-1
24.
Osio-SalidoEManapat-ReyesH. Epidemiology of systemic lupus erythematosus in Asia. Lupus. (2010) 19(12):1365–73. 10.1177/0961203310374305
25.
TianJZhangDYaoXHuangYLuQ. Global epidemiology of systemic lupus erythematosus: a comprehensive systematic analysis and modelling study. Ann Rheum Dis. (2023) 82(3):351–6. 10.1136/ard-2022-223035
26.
WangHRenYChangJGuLSunLY. A systematic review and meta-analysis of prevalence of biopsy-proven LN. Arch Rheumatol. (2018) 33(1):17. 10.5606/ArchRheumatol.2017.6127
27.
O’ShaughnessyMMHoganSLThompsonBDCoppoRFogoABJennetteJC. Glomerular disease frequencies by race, sex and region: results from the international kidney biopsy survey. Nephrol Dial Transplant. (2018) 33(4):661–9. 10.1093/ndt/gfx189
28.
MokCCTengYKOSaxenaRTanakaY. Treatment of LN: consensus, evidence and perspectives. Nat Rev Rheumatol. (2023) 19(4):227–38. 10.1038/s41584-023-00925-5
29.
WangYFXuYXTanYYuFZhaoMH. Clinicopathological characteristics and outcomes of male LN in China. Lupus. (2012) 21(13):1472–81. 10.1177/0961203312458467
30.
HwangJLeeJAhnJKParkEJChaHSKohEM. Clinical characteristics of male and female Korean patients with systemic lupus erythematosus: a comparative study. Korean J Intern Med. (2015) 30(2):242. 10.3904/kjim.2015.30.2.242
31.
BoseMJefferiesC. Sex bias in systemic lupus erythematosus: a molecular insight. Immunometabolism (Cobham). (2022) 4(3):e00004. 10.1097/IN9.0000000000000004
32.
ShayakulCOng-aj-yoothLChirawongPNimmannitSParichatikanondPLaohapandTet alLupus nephritis in Thailand: clinicopathologic findings and outcome in 569 patients. Am J Kidney Dis. (1995) 26:300–7. 10.1016/0272-6386(95)90650-9
33.
SulaimanWZukyNSFNAAliMFMAzamMFHMFaridNNMLoongLCet alClinical characteristics of patients with Lupus Nephritis in a tertiary care hospital in Malaysia– A cross sectional study. Asian J Med Health Sci. (2021) 4(2):11–9.
34.
SeligmanVALumRFOlsonJLLiHCrisswellLA. Demographic differences in the development of lupus nephritis: a retrospective analysis. Am J Med. (2002) 112(9):726–9. 10.1016/S0002-9343(02)01118-X
35.
YapDYTangCSMaMKLamMFChanTM. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant. (2012) 27:3248–54. 10.1093/ndt/gfs073
36.
LeeSSLiCSLiPCK. Clinical profile of Chinese patients with systemic lupus erythematosus. Lupus. (1993) 2(2):105–9. 10.1177/096120339300200207
37.
SongKLiuXLiuJYinZChenPCaiGet alAnalysis of clinical and laboratory characteristics and pathology of LN-based on 710 renal biopsies in China. Clin Rheumatol. (2020) 39(11):3353–63. 10.1007/s10067-020-05115-2
38.
SatirapojBTasanavipasPSupasyndhO. The effects of simvastatin on proteinuria and renal function in patients with chronic kidney disease. Int J Nephrol. (2015) 2015:1–6. 10.1155/2015/485839
39.
KwonOCParkJHParkHCJungSMLeeSWSongJJet alNon-histologic factors discriminating proliferative LN from membranous LN. Arthritis Res Ther. (2020) 22:1–12. 10.1186/s13075-020-02223-x
40.
TehCLPhuiVELingGRNguLSWanSATanCHH. Causes and predictors of mortality in biopsy-proven LN: the Sarawak experience. Clin Kidney J. (2018) 11(1):56–61. 10.1093/ckj/sfx063
41.
SuilanKEASalidoEO. Outcomes of patients newly-diagnosed with systemic lupus erythematosus managed in a tertiary training and referral hospital in the Philippines. Acta Med Philipp. (2024) 58(3):15. 10.47895/amp.vi0.5896
42.
ShaharirSSHusseinHRajalinghamSSaidMSMGaforAHAMohdRet alDamage in the multiethnic Malaysian systemic lupus erythematosus (SLE) cohort: comparison with other cohorts worldwide. PLoS One. (2016) 11(11):e0166270. 10.1371/journal.pone.0166270
43.
YokoyamaHWadaTHaraAYamahanaJNakayaIKobayashiMet alThe outcome and a new ISN/RPS 2003 classification of LN in Japanese. Kidney Int. (2004) 66(6):2382–8. 10.1111/j.1523-1755.2004.66027.x
44.
ObriscaBJurubitaRAndronesiASorohanBAchimCBobeicaRet alHistological predictors of renal outcome in LN: the importance of tubulointerstitial lesions and scoring of glomerular lesions. Lupus. (2018) 27:1455–63. 10.1177/0961203318776109
45.
ZenMSalmasoLAmideiCBFedeliUBellioSIaccarinoLet alMortality and causes of death in systemic lupus erythematosus over the last decade: data from a large population-based study. Eur J Intern Med. (2023) 112:45–51. 10.1016/j.ejim.2023.02.004
46.
LuoWFarinhaFIsenbergDARahmanA. Survival analysis of mortality and development of LN in patients with systemic lupus erythematosus up to 40 years of follow-up. Rheumatology. (2023) 62(1):200–8. 10.1093/rheumatology/keac218
Summary
Keywords
lupus nephritis, chronic kidney disease, kidney biopsy, long-term outcome, disease progression
Citation
Hustrini NM, Susalit E, Miranda ME, Saraswati M, Teng YKO, van Diepen M and Rotmans JI (2025) Clinical and histopathological features of lupus nephritis and the risk of long-term kidney outcomes in Indonesia. Front. Lupus 3:1604644. doi: 10.3389/flupu.2025.1604644
Received
02 April 2025
Accepted
28 May 2025
Published
19 June 2025
Volume
3 - 2025
Edited by
Matteo Piga, University of Cagliari, Italy
Reviewed by
Massimo Radin, University of Turin, Italy
Elisabetta Chessa, Azienda Ospedaliero-Universitaria Cagliari, Italy
Updates
Copyright
© 2025 Hustrini, Susalit, Miranda, Saraswati, Teng, van Diepen and Rotmans.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joris I. Rotmans j.i.rotmans@lumc.nl
ORCID Ni Made Hustrini orcid.org/0000-0001-7748-550X Monik Ediana Miranda orcid.org/0000-0002-3716-5808 Meilania Saraswati orcid.org/0000-0001-8227-0108 Y. K. Onno Teng orcid.org/0000-0001-9920-2195 Merel van Diepen orcid.org/0000-0003-3211-9500 Joris I. Rotmans orcid.org/0000-0001-9682-6234
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.