- 1Institute for Chemistry and Biology of the Marine Environment (ICBM), Oldenburg, Germany
- 2Department of Ecological Chemistry, Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany
- 3Institute of Toxicology and Pharmacology for Natural Scientists, University Medical School Schleswig-Holstein, Kiel, Germany
- 4Flanders Marine Institute (VLIZ), Oostende, Belgium
The environmental risks associated with dumped munitions, unexploded ordnance (UXO) and sunken war ships is gaining more and more attention nowadays, since these warfare materials may start leaking, posing a threat to marine wildlife. This study aims to assess the effects of pollution by explosives for marine fauna associated with sunken war ships still loaded with munitions at the time of sinking. For this purpose, transplanted blue mussels (Mytilus edulis) and passive samplers were exposed for several weeks on two WWII warship wrecks (HMS Basilisk and V1302, formerly named John Mahn) to detect leakage of explosives and to characterize the effects of those substances on mussel health. In addition, fish (Trisopterus luscus) dwelling at V1302 were caught and investigated following the same approach as used with the mussels. The hazardous potential of dissolved explosives was assessed using multi-biomarker analysis, which includes the enzyme activity of catalase (CAT), glutathione S-transferase (GST) and acetylcholinesterase (AChE), as well as histochemical biomarkers like lysosomal membrane stability (LMS), lipofuscin (LIPF), neutral lipids (NL) and glycogen (GLY) as an indicator of mussel’s energy reserve. Chemical analysis of passive samplers as well as mussel and fish tissue indicated leakage of explosives at both wrecks and a subsequent uptake by exposed organisms. The leakage of explosives was correlated with membrane impairments and signs of oxidative stress measured in exposed mussels and fish.
Highlights
● Investigated wrecks are leaking energetic compounds from corroding munition.
● Blue mussels and fish take up energetic compounds.
● Concentrations of munition compounds are in the order of nanograms per gram tissue.
● Mussels respond to concentrations of dissolved explosive showing signs of oxidative stress.
1 Introduction
Since World War I (WWI) the production of conventional munitions and chemical warfare agents (CWA) increased significantly (Bełdowski et al., 2014), as well as their entry into marine waters (Beddington and Kinloch, 2005). There were two main ways of how warfare materials got into the oceans: (1) By war activities including training, mine laying, failed detonations, emergency launches and sunken wrecks loaded with warfare materials (Böttcher et al., 2011) and (2) by intentional dumping operations especially after World War II (WWII) (Böttcher et al., 2011; HELCOM, 2013), chosen as fast and cheap method of demilitarization (HELCOM, 2013).
Throughout WWII, the world experienced the single, largest loss of shipping in any period of time (Gilbert, 2005). For instance, in the East Asian Pacific alone more than 3,800 vessels were sunk during WWII, and it is likely that many of these ships were still loaded with munitions at the time of sinking (Monfils et al., 2006). Also the North Sea was a so-called watery grave for many ships (Bellamy, 1991). Data from the German Maritime Agency, Hamburg record approx. 120 military shipwrecks (Grassel et al., 2021) from WWI and II within the German Exclusive Economic Zones (EEZ) alone. In the Belgian part of the North Sea (BPNS) there are 140 known and identified wrecks that were sunk during war activities. About one hundred of these shipwrecks may still contain munitions (Deutsches Schifffahrtsmuseum, n.d).
Shipwrecks may pose severe risks to the aquatic environment due to their remaining fuel, their cargo or both. Especially military wrecks may contain considerable amounts of munition shells, mines, depth charges and other explosives, as well as chemical warfare agents (Gilbert, 2005). Hence, shipwrecks are of particular importance for environmental risk assessments. For the present study two identified wrecks, the HMS Basilisk and the V1302 formerly named John Mahn, were selected. Main reasons for selection were the confirmation of remaining munitions onboard, the existence of archive information regarding the type of munition and the accessibility of the wrecks for scientific divers. Further information about the ships and their sinking scenarios are provided in the supplement of this study (chapter S.1 and Supplementary Figure S1).
Lost or dumped underwater munitions of the World Wars still endanger the marine environment and human health nowadays. This threat can be divided into three categories: (1) risk of spontaneous detonation and the spreading of unexploded material e.g. during underwater works or when washed onshore (Appel et al., 2019; Beddington and Kinloch, 2005; Böttcher et al., 2011); (2) direct physical contact with toxic chemicals or munitions and the resulting health risks (Beddington and Kinloch, 2005) and (3) contamination of the marine environment by leaking chemicals through corroded munition shells (Appel et al., 2018; Beck et al., 2019; Beddington and Kinloch, 2005; Böttcher et al., 2011; Strehse et al., 2017). Since both CWAs and explosives are bio-available for organisms (Beck et al., 2022; Höher et al., 2019; Schuster et al., 2021; Strehse et al., 2017) there is also a risk that the released chemicals enter the marine food web (Böttcher et al., 2011; Ek et al., 2006, 2008; Maser and Strehse, 2021).
One of the most commonly used explosives is the nitroaromatic compound 2,4,6-trinitrotoluene (TNT) (Juhasz and Naidu, 2007; Lotufo et al., 2013). TNT and other munition constituents are known to be metabolized in the marine environment: The main metabolic pathway of TNT is the reduction to 2-amino-4,6-dinitrotoluol (2-ADNT) and 4-amino-2,6-dinitrotoluol (4-ADNT) via biotic processes, mainly by bacteria or enzymes of or within marine organisms (Beck et al., 2018; Juhasz and Naidu, 2007). During the manufacturing of TNT, several other nitroaromatic compounds are created as by-products. Most of them get removed or destroyed by the purification processes but some of them remain as contaminants in the munitions (Wellington and Mitchell, 1991). This includes 2,4-dinitrotoluene (2,4-DNT) and 1,3-dinitrobenzene (1,3-DNB), which are only slowly biodegradable (Wexler, 2014). The chemical structure of the mentioned explosives is shown in Figure 1.
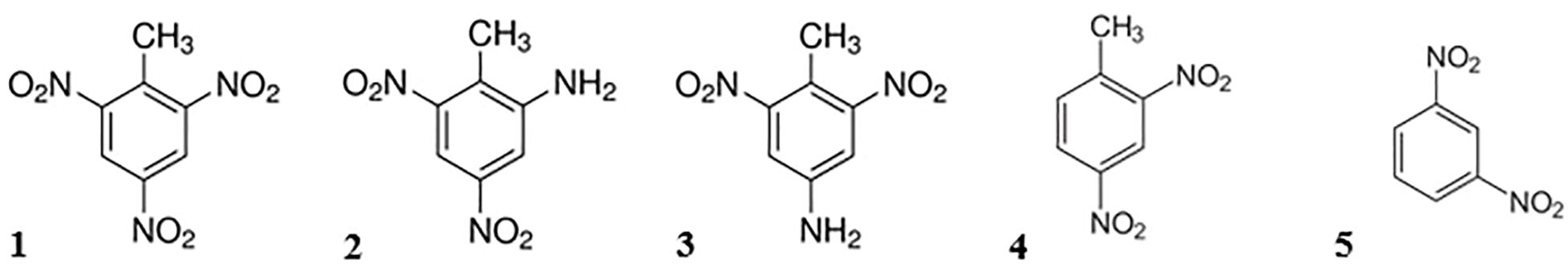
Figure 1. Chemical structure of selected explosives. (1) 2,4,6-trinitrotoluene (TNT), (2) 2-amino-4,6-dinitrotoluene (2-ADNT), (3) 4-amino-2,6-dinitrotoluene (4-ADNT), (4) 2,4-dinitrotoluene (2,4-DNT) and (5) 1,3-dinitrobenzene (1,3-DNB).
TNT, its metabolites 2-ADNT and 4-ADNT, as well as the impurities 2,4-DNT and 1,3-DNB all cause adverse health effects, and are harmful to the aquatic environment (Wexler, 2014). The toxic effects of TNT and other explosives have been shown in several studies using different toxicological endpoints (e.g. Nipper et al., 2001, 2009). However, there is still a scientific controversy whether TNT is more or less toxic than its metabolites (Sims and Steevens, 2008).
To assess the effects of pollutants, mussels have often been used as test organisms (Strehse and Maser, 2020). Blue mussels have several properties which make them suitable bioindicators as summarized by Beyer et al. (2017) and Farrington and Quinn (1973) (e.g. potential of bioconcentrating chemicals, robust organisms, abundant in all world oceans, common prey species, represent the environmental conditions of a specific area due to sessile lifestyle, can be easily transplanted) Mussels, especially Mytilus spp., are well established bioindicators which have already been used to monitor dissolved energetic compounds (Strehse et al., 2017) or to detect effects of dumped CWAs (Lastumäki et al., 2020).
In addition to mussels, fish have also been used as bioindicators, because these organisms fulfil the general requirements of a bioindicator as reviewed by Kuklina et al. (2013): they are ecologically relevant because they play an important role in the marine food web, they are generally sensitive to stressors, and fish have a wide distribution, etc. Fish are also known to bioaccumulate toxic substances (de Andrade et al., 2003) and may be especially suitable to assess the toxic effects of TNT, as it has been shown that they are sensitive to TNT exposure with LC50-values ranging from 0.8 to 3.7 mg/L (Talmage et al., 1999). For the present study, pout (Trisopterus luscus) was chosen as fish model, since the species is known to live around hard substrates like reef structures, stones and wrecks (Mallefet et al., 2007; Zintzen et al., 2006).
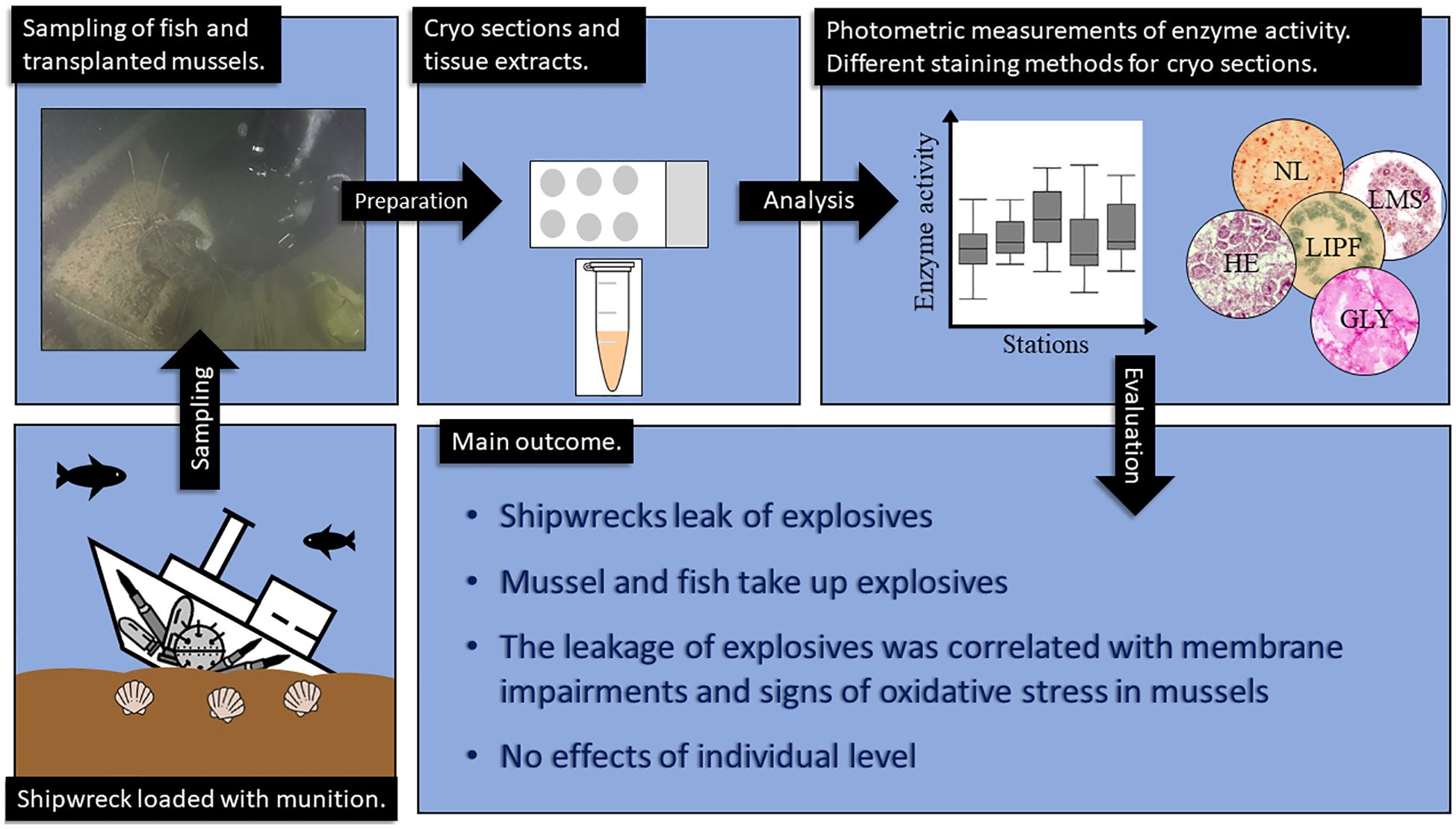
TNT and its metabolites are suspected to cause oxidative stress in cells and tissues of exposed organisms (Adomako-Bonsu et al., 2024). In addition, pre-investigations at the wreck sites revealed low exposure concentrations for dissolved TNT. Therefore, biomarkers were chosen which proved already their response to dissolved TNT in lab studies (Schuster et al., 2021) and are capable to function as early warning markers, showing their effects or endpoints on sub-cellular or cellular levels. Further, preference was given to biomarker which can be applied using both fish and mussels as bioindicators. At the conclusion, for the present study the following biomarkers were selected: Lysosomal membrane stability (LMS), enzyme activity of Catalase (CAT), glutathione S-transferase (GST) and acetylcholinesterase (AChE), as well as the biomarkers lipofuscin (LIPF), neutral lipids (NL) and glycogen (GLY). Further information about the biomarkers is available in the Supplementary Material (chapter S.2).
The aim of the present study was to assess the hazard potential of pollution by explosives and its ecological effects on exposed fauna at two World War II shipwrecks located near the Belgian coast, the British Destroyer HMS Basilisk and the patrol boat V1302. The objectives were to determine (1) whether the selected shipwrecks emit hazardous energetic compounds and, if so, (2) whether this contamination affects the health condition of the transplanted mussels of the species Mytilus edulis and the health of caught fish (Trisopterus luscus) by using a multi-biomarker approach including enzyme activities.
2 Material and methods
2.1 Study areas
Subjects of the present study were the two wrecks V1302 (formally named John Mahn) and the HMS Basilisk (also known as H11), as well as the wind park Belwind which served as a reference area. All three stations are located in the Belgian part of the North Sea (BPNS), in front of the Belgian coast near the cities Nieuwpoort, Ostend and Zeebrugge (Figures 2, 3).
Figure 2. Southern part of the North Sea including the economic zones. The area enclosed by green lines represent the Southern Bight. The white shaded area marked with “B” represents the Belgian economic zone (Maes et al., 2005 modified).
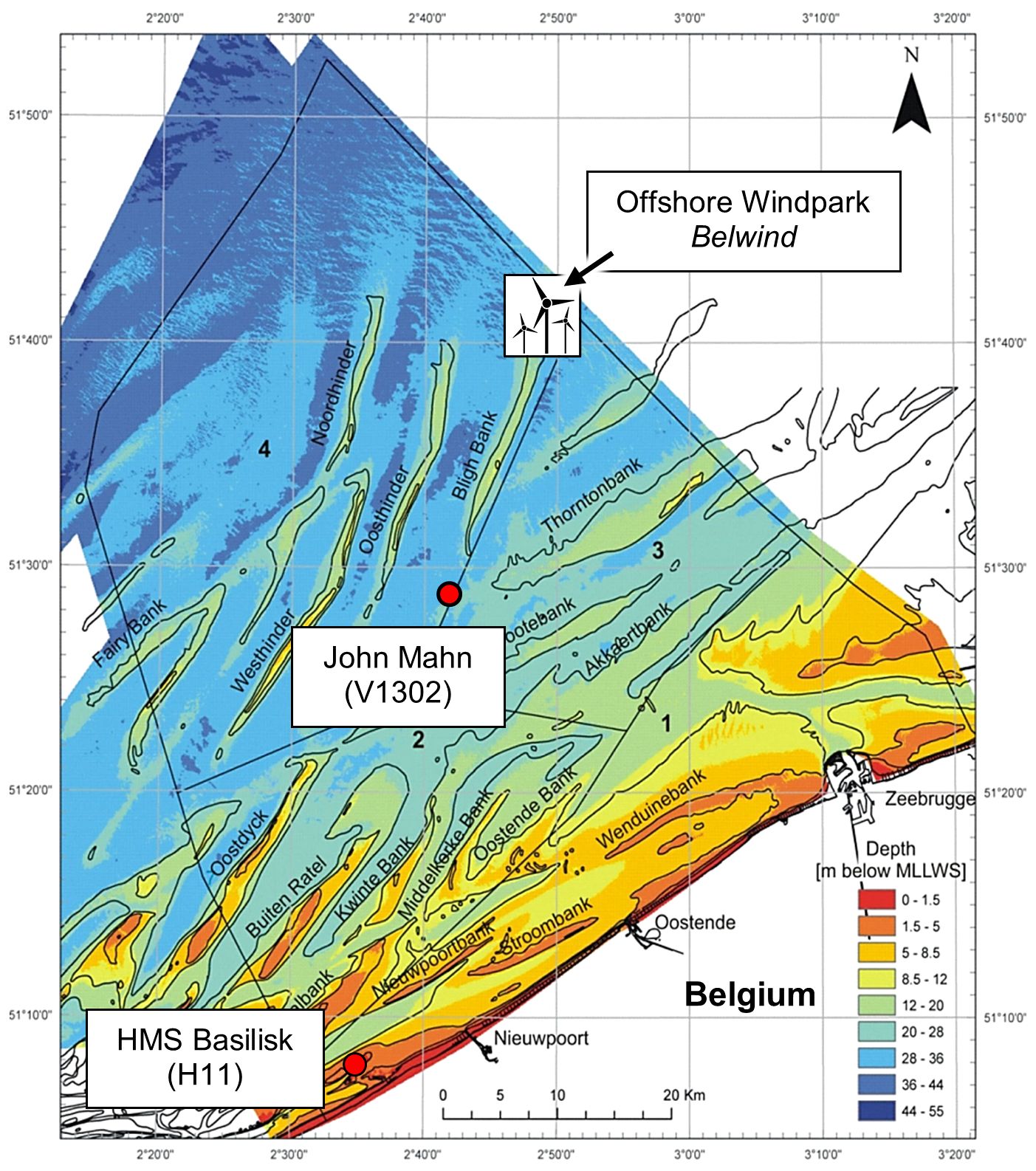
Figure 3. Map of the Belgian coast. Positions of the shipwrecks John Mahn/V1302 and HMS Basilisk are indicated by red dots. The wind park reference area Belwind is indicated by windmills. The colour code represents depth in m below mean lower low water spring tides (MLLWS) (Mathys, 2009 modified).
The BPNS is criss-crossed by several sandbanks (Figure 3). The current in this area can be highly variable due to the tidal cycles, but two main currents can be identified: Near the coast there is a north-easterly current coming from the English Channel into the North Sea. The other current flows further away from the coast in south south-westerly direction parallel to the Westhinder Bank (Maes et al., 2005). Within the BPNS there is a tidal range from approximately 2 to 5 m, with the highest tidal action near the English Channel (Sündermann and Pohlmann, 2011).
The two wrecks are located in different geographical and hydrographical locations. HMS Basilisk lies in 10 m depth at 51° 8′ 16″ N, 2° 35′ 6″ E, on the Trapegeer sandbank in front of the Belgian coast (Figure 3). The seabed near the shore consists mostly of fine sand (Maes et al., 2005). The front part of the wreck was separated from the main part close to the engine room and lies to port side (Figure 4).
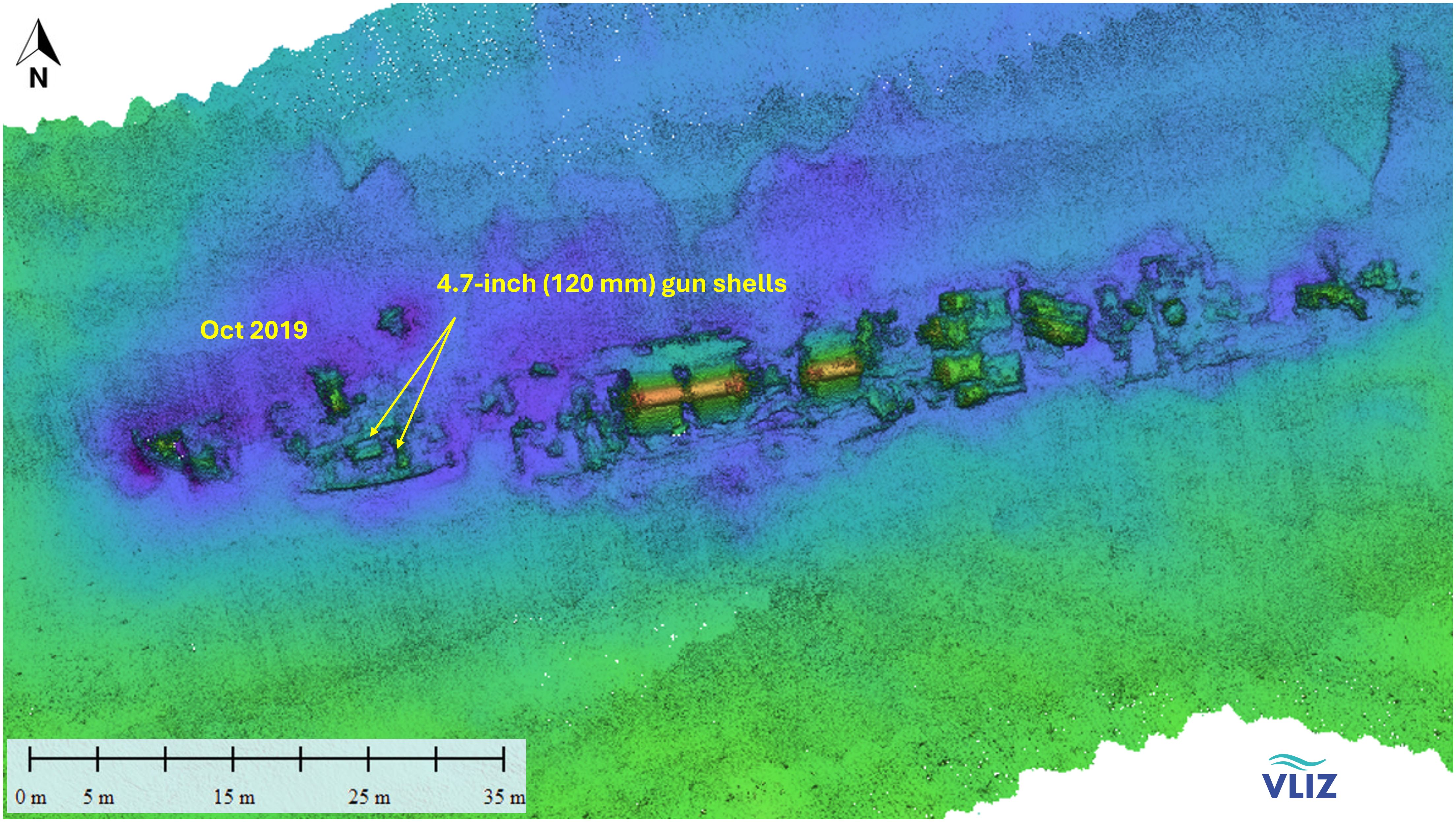
Figure 4. Multibeam image of HMS Basilisk including the location where mussels were transplanted in 2019.
The V1302 lies approx. 37 km northwest of Zeebrugge at 51° 28′ 42″ N; 2° 43′ 18″E (Figure 3) in 35 meter depth (Gröner et al., 1993; Lettens, 2002). The wreck is positioned on its keel with a slight slope to starboard side (25°) in east-west direction (Figure 5). Below the former water line close to the bridge a big hole from one of the bomb impacts is visible. Depth charges can be found on the stern. The wreck is entangled with nets, trawls and fishing lines (Lettens, 2002).
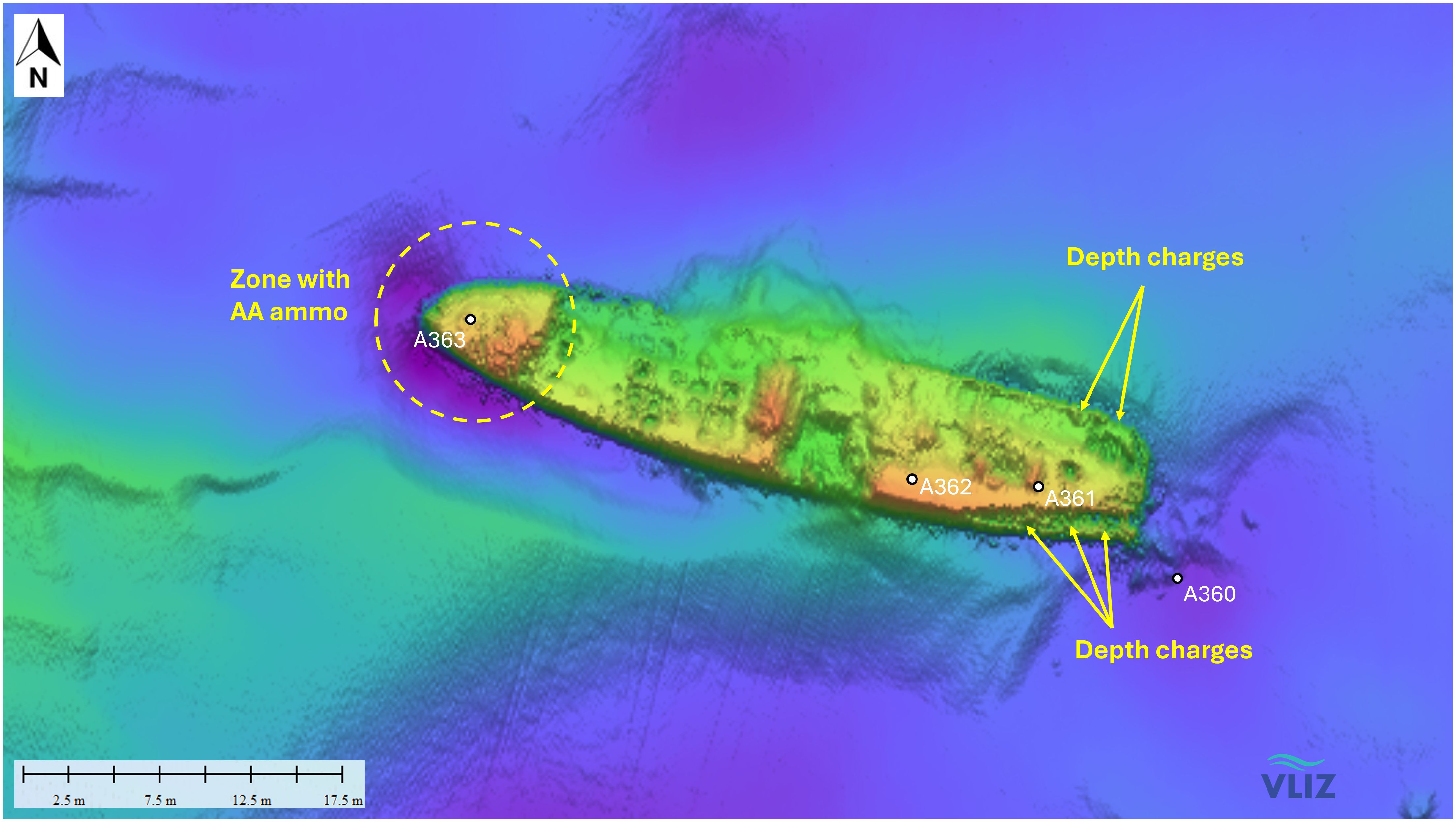
Figure 5. Multibeam image of munitions onboard the V1302 and the locations for the transplanted mussels (A360-A363).
The offshore wind park Belwind is located in the BPNS on top of the Bligh Bank (51° 39′ 36″ N, 2° 48′ 0″ E) and is one of several wind farms operated by Parkwind. It covers a surface area of 17 km2 and is located 46 km off the coast of Zeebrugge (Figure 3). Water depth at the site is between 20 and 37 m. The wind farm contains 56 turbines at distances of 500 to 650 m and has been operating since 2010 (Belwind, 2017; BOP, n.d).
2.2 Experimental design
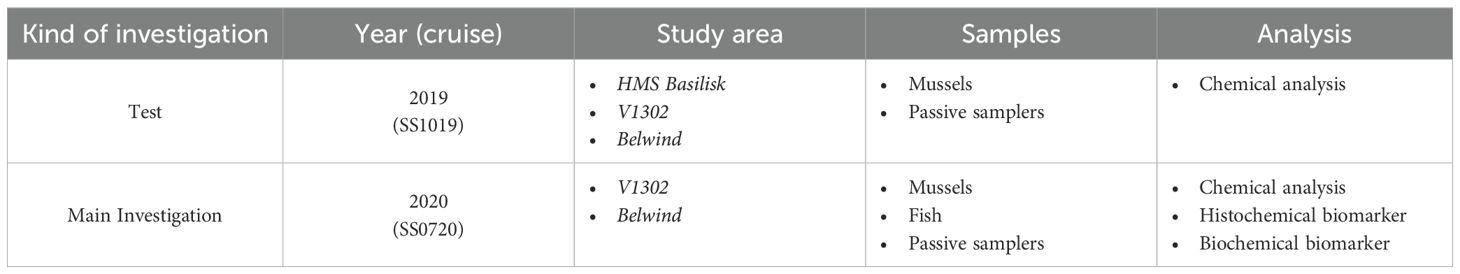
The investigation was conducted during two cruises of the RV Simon Stevin: One cruise in 2019 (labelled as SS1019) to both wrecks, and a second in 2020 (labelled as SS0720) to the V1302, during which the main investigation took place. The cruise SS1019 served as test for the feasibility of the experimental setup and to investigate whether any leakage of explosive chemicals occurs at one of the wrecks. For the main investigation the V1302 was chosen because the wreck is easier to access by divers, since it is further outside the tidal zone and the water is less turbid. During the main experiment, mussels were exposed and fish were caught to determine any potential biological effects as a response to the exposure with dissolved explosives from the wreck. A tabular overview about the experimental setup can be seen in Table 1.
Mussels (Mytilus edulis) for transplantation on the wrecks were collected by divers from pylons at the reference area Belwind from approx. 10 m depth. Collected mussels were measured and only mussels with a shell length of 6 to 8 cm were kept for the experiment. Nylon net bags were filled with mussels and a set of two passive samplers (Chemcatcher®) to detect energetic compounds during the exposure. The use of passive samplers allows a qualitative detection of energetic compounds within the water column as well as the estimation of their ratio. Transplantation and retrieval of the mussels were conducted by scientific divers.
Mussels at the HMS Basilisk were placed at 8 m depth. Due to this wreck’s deeper location, mussels at the V1302 were placed at 30 m depth. During both field experiments passive samplers were also installed at the reference site Belwind. In addition, reference mussels were also obtained from the pylons at 10 m depth at the end of the experiments.
During the field experiment in 2019 (cruise SS1019) mussels were exposed at both wrecks: For each wreck one site was chosen close to munition items where mussels were exposed for 3 months (from August until October) together with passive samplers.
The main experiment was conducted in 2020 (cruise SS0720). Here, only the V1302 was investigated, and the mussel nets were placed along the entire length of the wreck (Figure 5). The different stations were labelled A360, A361, A362 and A363. At each station 30 mussels (20 mussel for biomarker analysis, 3 for chemical analysis and the rest as reserve) were exposed for 4 months (from March 13th 2020 until July 30th 2020).
2.3 Mussel and fish sampling, dissection, and morphometric measurements
After the exposure phase mussels were collected and directly processed in the wet lab of the research vessel. Shell length was measured using a Vernier calliper (± 0.1 mm). Total wet weight and shell wet weight were determined using a laboratory scale to calculate the condition index (CI) (Equation 1).
To prepare the dissection of mussels, the posterior adductor muscle was first cut. Then gill, mantle and digestive gland tissue were dissected and transferred to cryo-vials. Tissues were snap frozen in liquid nitrogen and stored in nitrogen vapour on board. Back on land, samples were kept at -80°C until further processing. 20 mussels per station were used for biomarker analysis. Three additional mussels per station were used for the chemical analysis of tissues. For chemical tissue analysis the whole soft body was used, which were also snap frozen and subsequently stored at -20°C. Since more than half of the mussels at station A363 had died, only 9 mussels were available for biomarker analysis, and 1 mussel for chemical tissue analysis.
Pouting (T. luscus) were caught directly above the wreck at slack water using commercially available sports fishing gear. Twenty specimens of a length above 25 cm were caught at the wreck and the reference site. Caught fish were processed directly on board. Fish were weighed (wet weight), the total length measured and anesthetized by a blow on the head, followed by decapitation prior to the subsequent dissection. The bile was collected by puncture of the gall bladder with needles (0.15 mm × 35 mm) and disposable syringes (1 mL) and transferred into a cryo-vial before snap frozen in liquid nitrogen. In addition, fish livers were investigated macroscopically for nodules and tumours. Pieces of liver were dissected for anti-oxidative-defence measurement and cryo-sectioning, placed in cryo-vials and snap frozen as described above. Another piece of liver was placed in aluminium foil and frozen at -20°C for chemical analysis. Finally, approx. 20 g of skin free muscle tissue from the fish’s filet was dissected and stored in a 15 mL Falcon tube kept at -20°C for chemical analysis. The biometric data were used to determine the Fulton’s condition factor (K) as an indicator of the general fish fitness status (Equation 2).
2.4 Chemical analysis
The preparation of passive samplers as well as chemical analyses by GC-MS/MS were performed according to Bünning et al. (2021) and Maser et al. (2023). In brief: The PES and HLB membranes of the passive samplers were freeze-dried separately, extracted with acetonitrile, and the extracts were analyzed using GC-MS/MS, consisting of a TSQ 8000 Evo mass spectrometer and a Trace 1310 gas chromatograph with a TG-5MS Amine column (15 m * 0.25 mm * 0.25 µm) (Thermo Fisher Scientific Inc., Waltham, MA, USA). Mussels and fish muscle were freeze-dried, ground, extracted with ACN, purified using 3 mL SPE columns, eluted with ACN, and also analyzed using GC-MS/MS. The bile samples were incubated with glucuronidase for 20 hours, extracted using 1 mL SPE columns, eluted with ACN, and analyzed using GC-MS/MS. All measurements were performed with splitless injections and quantified using external calibration curves.
Detailed descriptions of the methodology, instrument parameters and detection limits used for the GC-MS/MS measurements of the samples from the V1302 are published in Maser et al. (2023). Sample preparation and analysis of the samples from the HMS Basilisk and the reference site are carried out in the same way as described in Maser et al. (2023).
2.5 Biomarker analysis
2.5.1 Biochemical biomarkers
2.5.1.1 Homogenisation of mussel digestive gland, mussel gill tissue and fish liver
Homogenisation of mussel digestive gland tissue and fish liver was conducted using a modified version of the method described by Ahvo (2020a). Samples were individually homogenised in 100 mM potassium phosphate buffer with a pH of 7.0. Mussel digestive gland tissue was diluted at a ratio of 1:10 (tissue in mg, buffer in µL) in 2 mL vials for soft tissue homogenisation and kept on ice. Samples of fish liver were diluted in a ratio of 1:20 (tissue in mg, buffer in µL). Homogenisation of mussel gill tissue was conducted as described by Ahvo (2020d) using a 20 mM sodium phosphate buffer with a pH of 7.0. Buffer used for gill tissue was freshly mixed with 0.1% Triton-X100 on each day. Tissue samples of gill were diluted at a ratio of 1:3 (tissue in mg, buffer in µL) in 2 mL vials for soft tissue homogenisation and kept on ice. Homogenisation of tissue samples was executed using the precellys lysing kit Tissue homogenizing CK Mix (Cat.: P000918-LYSK0-A) and the Precellys® 24 homogenizer (Bertin Technology) by 5000 rpm, 2 x 15 sec with 15 sec break between shakings. To keep temperature at 4°C during homogenisation, a Cryolys® (Bertin Technology) was used and (re)filled with liquid nitrogen. Samples were then centrifuged with an Eppendorf centrifuge 5430R for 20 min at 4°C at 10000 g. Supernatants were transferred into 1.5 mL Eppendorf tubes and kept on ice until subsequent enzyme analysis.
Enzyme activity measurements for catalase (CAT) and glutathioneS-transferase (GST) activity were conducted using 96-well UV microplates. Acetylcholinesterase (AChE) activity was measured by using non-UV 96-well microplates. Measurements were done with a microplate reader (Infinite 200, TECAN) and analysed with i-control software 2.0. The volume of supernatants, reaction buffer and reagents in the wells for photometric measurements were adjusted to the used tissue samples in the present study. Samples were measured directly after homogenisation. Leftover extracts were frozen at -20°C for the determination of total proteins. Enzyme activity was adjusted to protein concentration in samples according to Bradford (1976). Protein analyses were conducted using the same microplate reader and non-UV 96-well microplates.
2.5.1.2 Catalase activity
Catalase (CAT) activity was determined by measuring the decrease in absorbance at 240 nm. The used method was based on Claiborne (1985/2018) and modified for mussel and fish tissue by Ahvo (2020b). CAT activity was measured with a final concentration of 10 mM H2O2.
2.5.1.3 Glutathione S-transferase activity
Glutathione S-transferase (GST) activity was measured using the method based on Habig et al. (1974) and modified for mussel and fish samples by Ahvo (2020c). GST was measured at 340 nm as increase in absorbance when adding a mixture of GSH (glutathione), CDNB (1-chloro-2,4 dinitrobenzene) and Dulbecco’s buffer with a final concentration of 2 mM GSH and 1 mM CDNB.
2.5.1.4 Acetylcholinesterase activity
The method used for determining acetylcholinesterase (AChE) activity was based on Bocquené and Galgani (1998) and modified by Ahvo (2020d) for microplates. AChE was measured as increase in absorbance at 412 nm in a mixture containing 0.57 mM DTNB (5,5’-dithiobis 2-nitrobenzoic acid) and 2.86 mM ACTC (acetylthiocholine) in phosphate buffer.
2.5.1.5 Determination of proteins in extract and calculation of activity per protein
Enzyme activity was determined as activity per protein (Equations 3–5). To do so total protein content in extracts were defined according to Bradford (1976) using a bovine serum albumin standard (BSA, Sigma A-6003). Protein analyses were executed by measuring the absorbance at 590 nm. Enzyme activity per protein [U/mg protein] was calculated using the following formulas (used molar coefficients : H2O2: 43,6 M-1cm-1, CDNB: 9.6 M−1 cm−1, DTNB: 1.36 * 104 M−1 cm−1):
2.5.2 Histochemical biomarkers
2.5.2.1 Tissue preparation for histochemical biomarkers
For determination of sex and gonad status of mussels, as well as for lysosomal membrane stability (LMS) and histochemical biomarker analysis, cryostat sections were prepared by gluing (Thermo Scientific™ Richard-Allan Scientific™ Neg-50™) frozen samples of mussel digestive gland and mantel on pre-frozen aluminium chucks for cryostat sectioning (three samples on one chuck). Tissue sections of 10 µm were obtained using a cryotome (Thermo Scientific™, Cryo Star NX70) with chamber/knife temperature of -25/-23°C. Per chuck a duplicate section was made. The sections were stored at -80°C until further processing for LMS, lipofuscin, glycogen, and neutral lipid staining (only digestive gland tissue) as well as for sex and gonad status determination (digestive gland and mantel tissue). Sections for LMS analysis were stored for a maximum of 24 h by -20°C before treatment.
2.5.2.2 Determination of sex and gonad status
Cryostat sections of mussel digestive gland and mantel were stained with Gill’s Hematoxylin and counterstained using an Eosine-Phloxin solution (HE method) as described e.g. in Brenner et al. (2014). First, cryo-sections were fixed 5 min in Baker’s Formalin, rinsed with Milli-Q water for 1-2 min and stained in Gill’s Hematoxylin for 15 sec. Sections were then counterstained in Eosine-Phloxin solution for 30 sec. Finally, the stained samples were dipped 6 times in 80% ethanol and dried briefly before mounted with Euparal.
2.5.2.3 Lysosomal membrane stability
The lysosomal membrane stability test was performed according to Moore et al. (2004). First, serial cryostat sections were treated at 37°C for increasing time intervals (2 to 50 min) using a mixture of 0.1 M citrate buffer with a pH of 4.5 and containing 3% NaCl to destabilize the membrane. Subsequently section incubation took place for 15 min at 37°C in a solution containing Naphthol AS-BI Nacetyl b-D-glucosamide (Sigma) dissolved in 2-methoxy ethanol and low-viscosity polypeptide (Polypep, Sigma) dissolved in 0.1 M citrate buffer, pH 4.5 with 3% NaCl. Then sections were treated with Fast Violet B (Sigma) dissolved in 0.1 phosphate buffer with a pH of 7.4 to alter a post-coupling reaction resulting in a violet colour change. After 10 min samples were rinsed with running tap water and fixed in Baker’s Formalin. Finally, slides dried overnight in the dark at room temperature and were than mounted with Kaiser’s glycerine-gelatine.
2.5.2.4 Glycogen
The glycogen (GLY) content was used to assess mussel fitness and nutritional status. To determine GLY quantities, duplicate sections were stained using the Perjod-Acid-Schiff-Method (PAS) based on Culling (1974). Duplicate sections were fixed 10 min in Carnoy’s fixative rinsed with Milli-Q water, placed in 1% periodic acid, and again rinsed in Milli-Q water. Sections were then stained for 5 min in Schiff’s reagent and rinsed with Milli-Q water again. Stained sections were rinsed in a series of different ethanol solutions (50%, 70%, 80%, 96%, 100%) for 1 min each and cleaned with Appliclear until no more colours arises in the water. After a short period of drying, the stained sections were mounted with Permount.
2.5.2.5 Lipofuscin
Lipofuscin (LIPF) accumulation in the lysosomes served as indicator for oxidative stress and was determined using the Schmorl’s reaction after Pearse (1985), and modified by Brenner et al. (2014). Duplicate cryostat sections were fixed for 15 min in 4% Baker’s formalin, rinsed in Milli-Q water and then stained in a 1% hydrous ferric chloride/potassium ferricyanide (1:1) solution. Afterwards, stained sections were washed in 1% acetic acid for 2 min and rinsed under tap water for 10 min. Finally, sections were rinsed 3 times in Milli-Q water and mounted with Euparal after a short drying period.
2.5.2.6 Neutral lipids
The accumulation of neutral lipids (NL) was determined using the Oil-Red-O method (ORO) after Lillie and Ashburn (1943) modified by Brenner et al. (2014). First, duplicated sections were fixed in Baker’s formalin for 15 min, and dipped 15 times in Milli-Q water, followed by washing in 60% Triethylphosphate for 1 min. Afterwards, the sections were stained for 15 min in Oil-Red-O solution containing 1% Oil Red O and 60% Triethylphosphate (solution was pre-cooked for 5 min and filtered hot and cold once) and rinsed again for 30 sec in 60% Triethylphosphate. Finally, the stained sections were rinsed with Milli-Q water, dried and mounted using Kaiser’s glycerine-gelatine.
2.5.2.7 Microscopic image analyses
To determine lysosomal membrane stability as well as the lipofuscin, glycogen, and neutral lipid amount in the digestive gland of mussel tissue, a computer assisted image analysis program (camera: MRc, ZEISS; software: AxioVision SE64 Rel. 4.9, ZEISS) combined with a light microscope (Zeiss, Axioskop) at 400-fold magnification were used. For each staining method and sample, three black and white pictures were taken and analysed (3 measurements per individual).
To assess the lysosomal membrane stability the staining intensity of lysosomes was measured. The time needed for destabilisation of the membrane is reflected by the maximal staining intensity in the lysosomes represented by a “peak”. In general two peaks are present which represent different membrane properties of two sub-populations of lysosomes (Moore et al., 2004). The earlier the peaks occur, the more unstable the membrane was which indicates pre-damage. To determine the labilisation period only the first peak is used (Moore et al., 2004).
For lipofuscin and neutral lipids, image analysis was conducted on selected areas of the image, using the microscope-camera system described above. Suitable areas for image analyses were areas with intact tissue and visible tubules. Within this selected image the areas containing the respective staining was chosen. For glycogen the entire images were analysed because tubules were hard to identify.
Sex and gonadal status were determined using the same light microscope and camera like described earlier at 100-fold magnification. Gonadal status was separated into 3 categories according to Brenner et al. (2014): (1) Recovering (non-discriminable), (2) premature (discriminable) and (3) mature gonads (discriminable). Pictures showing examples of the different categories are given in Supplementary Figure S2 in the supplementary chapter S.3. Numbers of individuals assigned to a specific category were counted and displayed as percentages.
2.6 Statistics
Data was arranged using Microsoft Excel 365, and visualized as well as analysed using R (Version 4.1.0, The R Foundation for Statistical Computing). Data is mostly presented as boxplot. A boxplot visualises the data distribution using quantiles including the median, minimum and maximum values and also outliers. The Shapiro-Wilk test and Levene’s test were used to test the data for normal distribution and homogeneity of variance. To determine significant differences between the mussel stations a one-way ANOVA was performed, and the Tukey Test was applied as post hoc test when data was normally distributed and showed homogeneity in variances. If this was not the case the Kruskal-Wallis ANOVA and the Dunn’s Test were used as non-parametric alternatives. Fish samples were tested with the independent t-test when data were normally distributed and homogenic in variances. As non-parametric alternative the Mann–Whitney U-test was used. Significant levels were set to p< 0.05, p< 0.005 and p< 0.0005. In each graphic, significance levels of wreck station compared to the reference are displayed with stars (p< 0.05 = *, p< 0.005 = ** and p< 0.0005 = ***).
3 Results
3.1 Cruise 2019 – chemical analysis
For the cruise in 2019 passive samplers, TNT, 4-ADNT and 2-ADNT were targeted (Figure 6). Results revealed the presence of explosives at both the V1302/John Mahn (JM) and the HMS Basilisk (BA) in the order of ng per sampler. The passive samplers at V1302 had a higher total concentration of explosives (JM: Average concentration of 22.5 ng/sampler. BA: Average concentration of 16.5 ng/sampler). In general, TNT was found at the highest concentration (JM S1: 8.2 ng/sampler, JM S2: 15.1 ng/sampler, BA S1: 5.2 ng/sampler, BA S2: 7.9 ng/sampler), followed by 2-ADNT (JM S1: 5.2 ng/sampler, JM S2: 6.8 ng/sampler, BA S1: 3.1 ng/sampler, BA S2: 9.1 ng/sampler) and 4-ADNT (JM S1: 4.5 ng/sampler, JM S2: 5.2 ng/sampler, BA S1: 2.5 ng/sampler, BA S2: 5.2 ng/sampler).
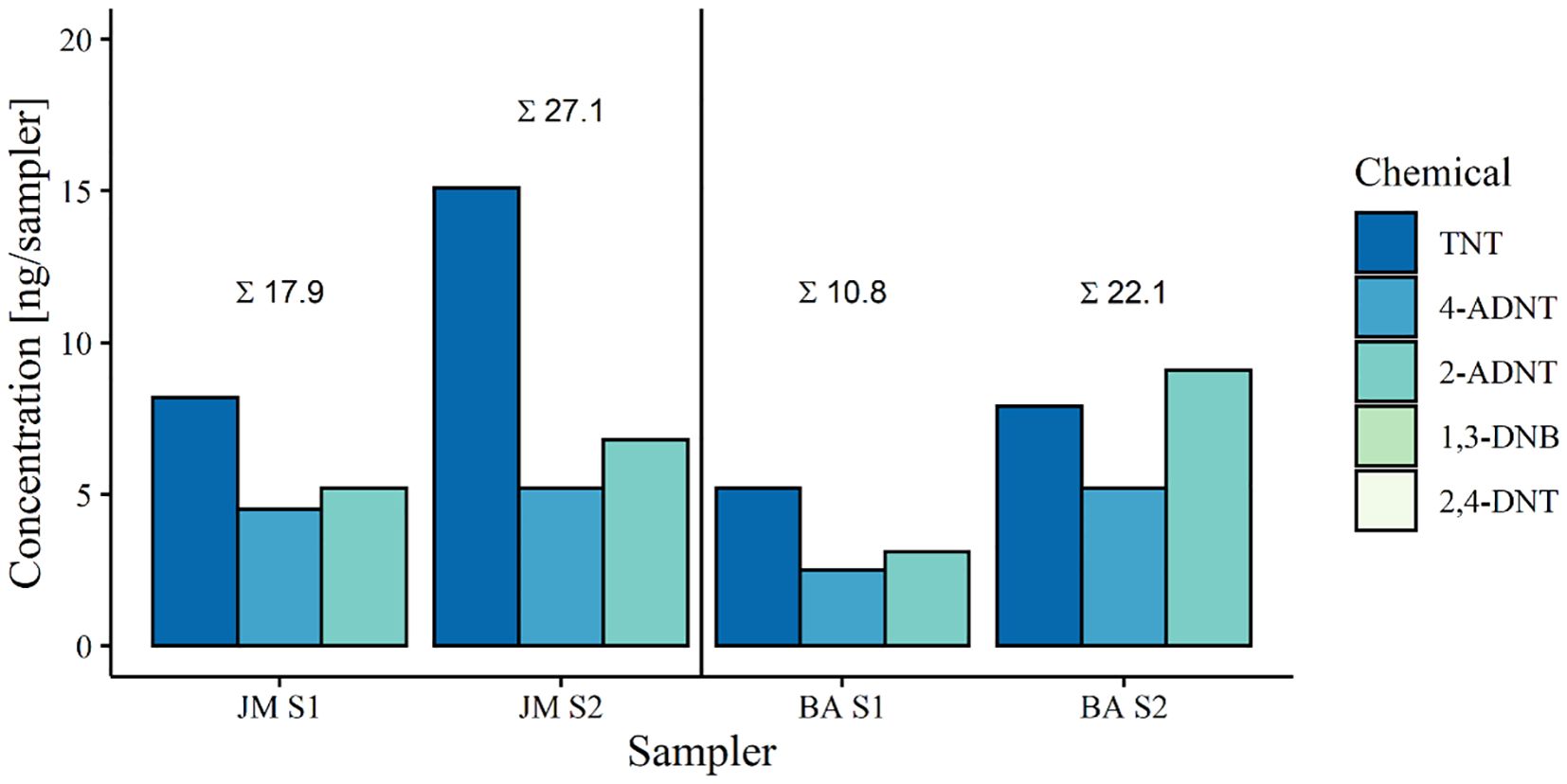
Figure 6. Energetic compounds detected in passive samplers during the exposure phase in 2019. Targeted chemicals were TNT (2,4,6-trinitrotoluene), 4-ADNT (4-amino-2,6-dinitrotoluene) and 2-ADNT (2-amino-4,6-dinitrotoluene). JM = V1302/John Mahn, BA = HMS Basilisk.
3.2 Cruise 2020
3.2.1 Chemical analysis
Concentration of energetic compounds measured within passive samplers at the different stations revealed the presence of explosive chemicals within the water column in the order of ng per sampler (Table 2). Dissolved explosives were not only detected at the wreck sites of the V1302 (average explosive concentration 28.6 ng/sampler. A361: 25.4 ng/sampler, A362: 50.7 ng/sampler, A363: 9.6 ng/sampler) but also at the reference site (average explosive concentration: 16.1 ng/sampler). Except for station A362, TNT concentrations were highest at the exposure locations. The highest TNT concentration was detected at station A361 with 19.1 ng/sampler, followed by A362 with 17.3 ng/sampler, the reference area with 8.7 ng/sampler and the lowest concentration was found at A363 with 7.6 ng/sampler. At station A362, 1,3-DNB showed the highest concentration (28.7 ng/sampler), whereas this chemical was not detectable at the other stations. For station A360, no data were available because the passive samplers vanished during the exposure. It should be noted here that passive sampler data give only a rough indication on the leakage of explosives from corroding munitions.
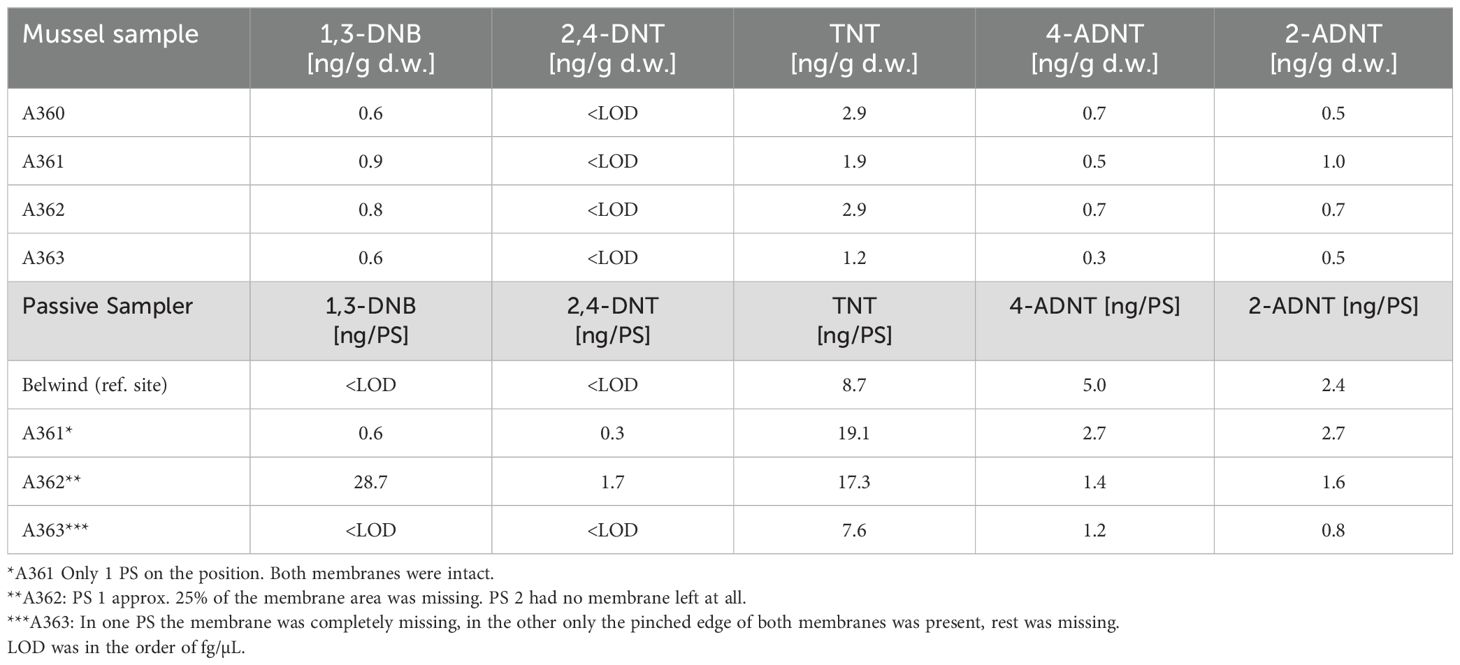
Table 2. Mussel and passive sampler chemical analysis of the 2020 cruise to V1302 (Maser et al., 2023).
The concentrations of explosives in freeze-dried mussel tissues were an order of magnitude lower relative to the concentrations found in the passive samplers (Table 2). The overall amount of energetic compounds within mussel tissue was similar at the different stations at the V1302 wreck site (Table 2). The highest amount of energetic compounds was measured in mussels collected at station A362 with 5.1 ng/g dry weight (d. w.) followed by A360 (4.7 ng/g d. w.), A361 (4.3 ng/g d. w) and A363 (2.3 ng/g d. w.). Results from the reference site are not available as no samples were taken for chemical analysis. At all stations, TNT concentration was consistently highest (A360: 2.9 ng/g d. w., A361: 1.9 ng/g d. w., A362: 2.9 ng/g d. w., A363: 1.2 ng/g d. w.). Concentration of other chemicals were comparable with a level of< 1 ng/g d. w.
3.2.2 Biomarker studies at the V1302 using blue mussels
3.2.2.1 Condition index
Condition indices (CI) of mussels at the wreck and the reference area did not show significant differences (p = 0.117), although the CI of mussels at the reference site was generally slightly lower (72.08 ± 21.78) compared to the CI of the wreck (88.63 ± 26.78) (Figure 7). CI of mussels collected at the wreck were similar (A360: 83.26 ± 24.1, A361: 91.45 ± 26.2, A362: 91.81 ± 27.74, A362: 87.48 ± 33.89).
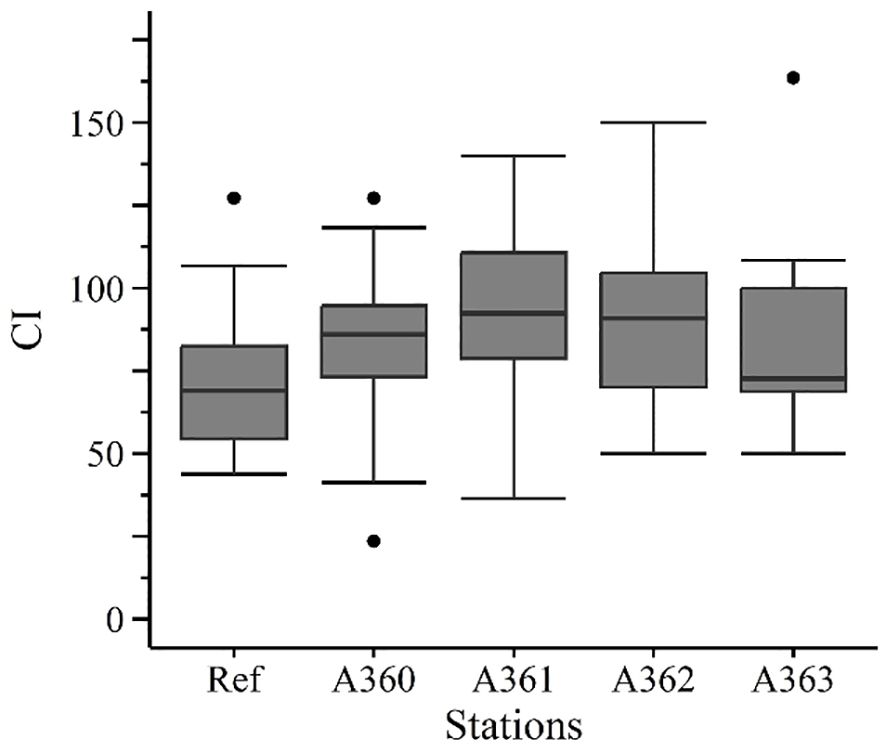
Figure 7. Condition index of mussel from the cruise in 2020. Ref = Reference area Belwind, A360-A363 = Stations at the wreck V1302, [·] statistical outlier. Differences between the groups were not significant (p< 0.05).
3.2.2.2 Glycogen content
Glycogen (GLY) contents in the mussel digestive gland cells were similar between the different stations except for station A362 (Figure 8): Station A362 had a GLY level of 19 ± 3.52% whereas the other stations had a similar GLY level of 13.02 up to 13.96% (A360: 13.36 ± 1.69%, A361: 13.06 ± 1.74%, A363: 13.02 ± 1.46%, Ref: 13.96 ± 1.83%). Mussels at station A362 showed significant higher values than at station A360 (p = 0.011) and A361 (p = 0.013).
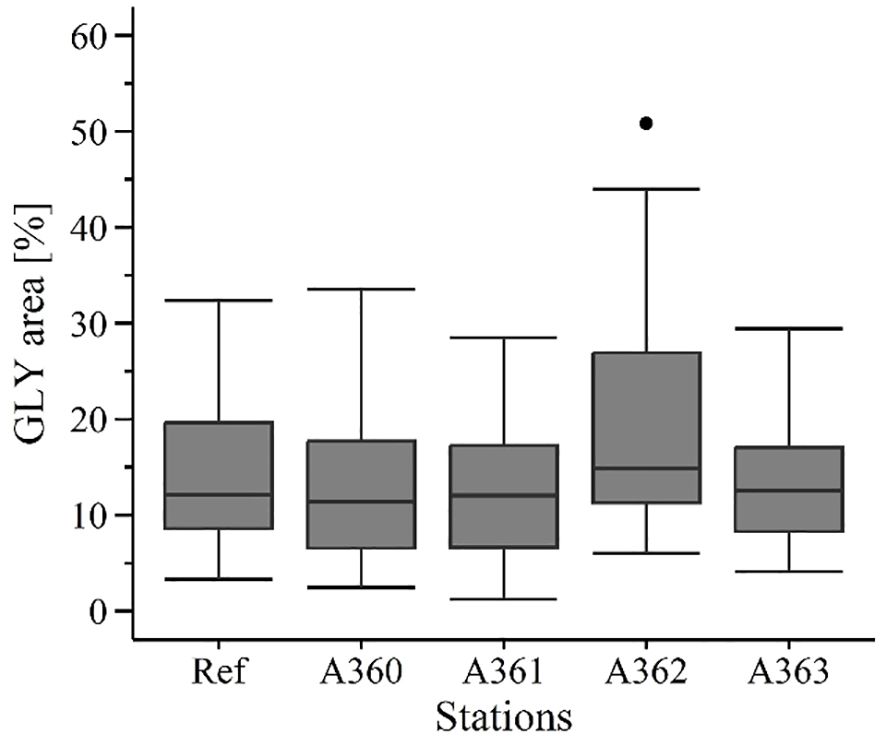
Figure 8. Glycogen content [%] in digestive gland tissue of mussels from the cruise in 2020. Ref = Reference area Belwind, A360-A363 = Stations at the wreck V1302, [·] statistical outlier. Differences between the groups were not significant (p< 0.05).
3.2.2.3 Gonad status
Gonad status between the stations showed no significant differences (Figure 9). Most of the mussels were immature or recovering (45 to 65%), followed by premature (22 to 40%) and mature (5 to 33%). In general, half of the collected mussels had differentiable gonads (premature + mature) while the other half of mussel individuals could not be determined sexually.
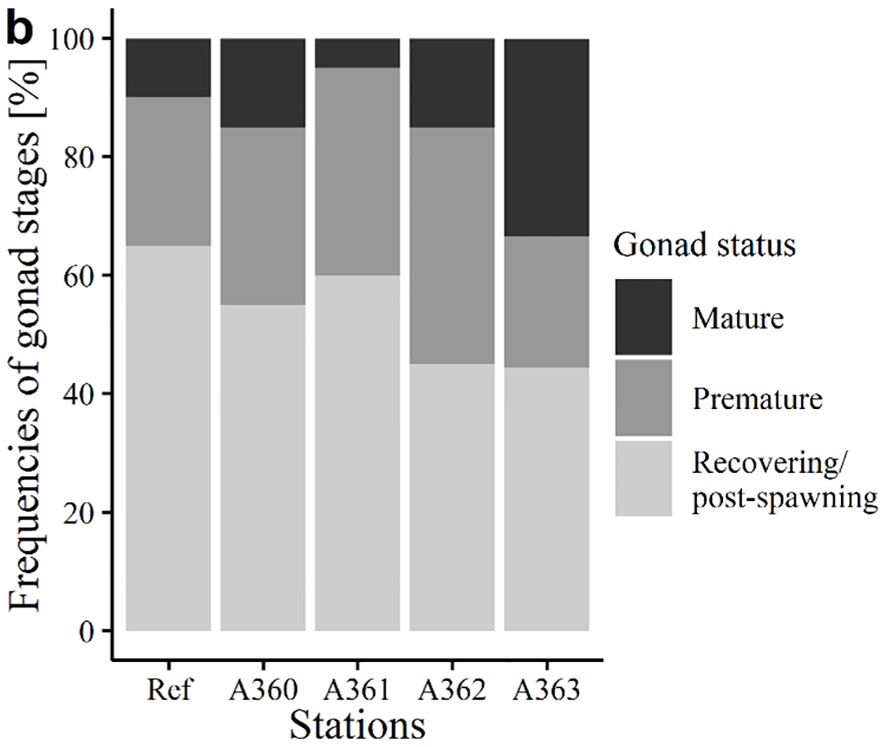
Figure 9. Gonad status of mussels [%] from the cruise in 2020. Ref = Reference area Belwind, A360-A363 = Stations at the wreck V1302.
3.2.2.4 Biochemical biomarkers
Catalase (CAT) activity was higher in mussel tissue of the wreck sites (average: 25.72 ± 9.24 U/mg protein) in comparison to the reference area (20.74 ± 7.87 U/mg protein) but only station A361 showed significance over the reference (p = 0.017) with an activity of 29.79 ± 9.87 U/mg protein (Figure 10a).
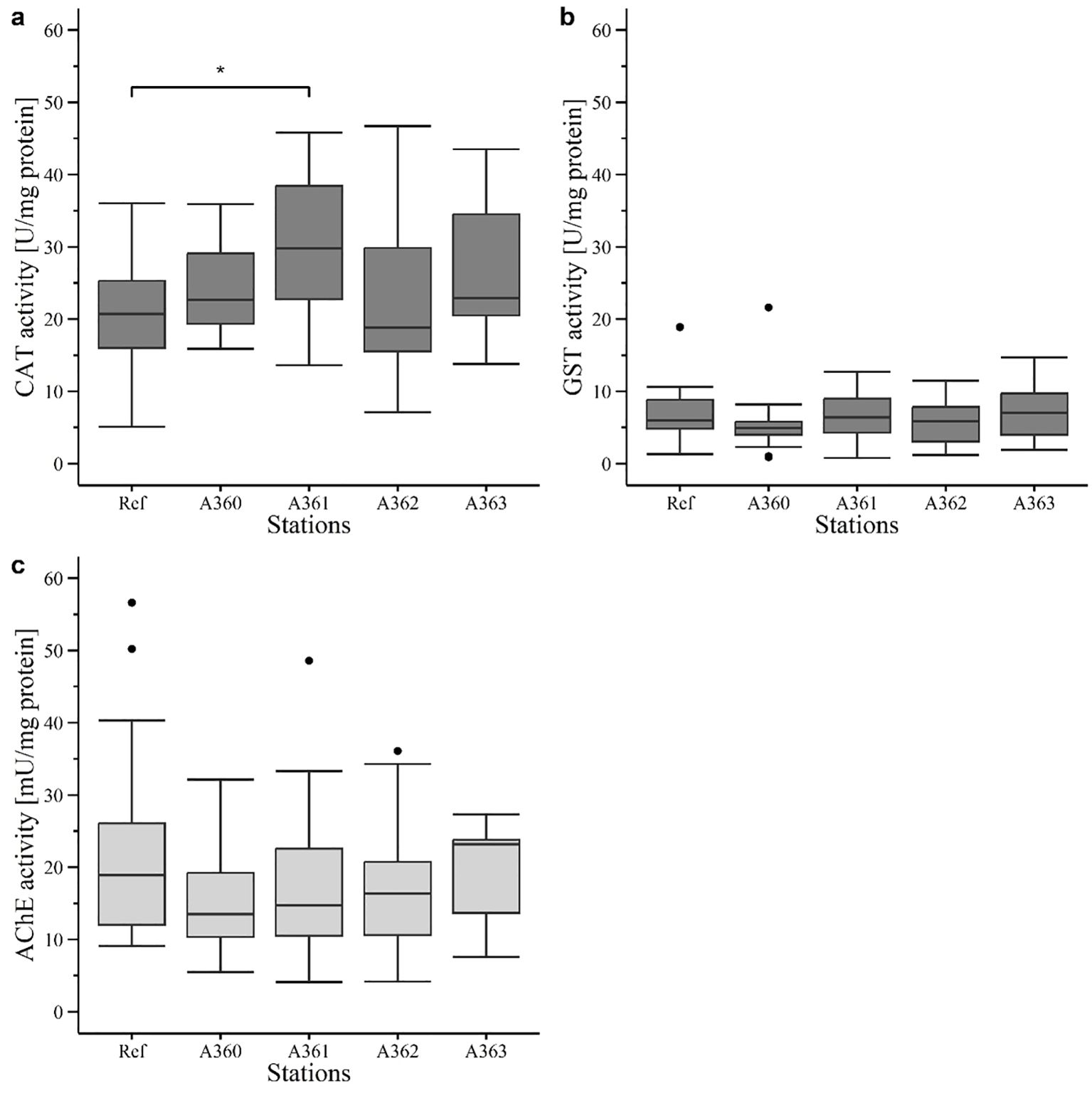
Figure 10. Enzyme activity in digestive gland (dark grey) and gill tissue (light grey) of mussels in 2020: (a) Catalase (CAT) activity (U/mg protein). (b) Glutathione S-transferase (GST) activity (U/mg protein). (c) Acetylcholinesterase (AChE) activity (mU/mg protein). Ref = Reference area Belwind, A360 – A363 = Stations at V1302, [·] statistical outlier. Significant differences to the reference are displayed with stars (p< 0.05 = *).
Activity of glutathione S-transferase (GST) was generally lower than CAT activity. GST activity showed similar levels for mussels at wreck sites (average: 6.16 ± 3.63 U/mg protein) and the reference site (7 ± 3.76 U/mg protein) (Figure 10b). Activity levels were ranging from 5.36 ± 4.23 U/mg protein (A360) up to 7.16 ± 4.09 U/mg protein (A363) with no significances.
Acetylcholinesterase (AChE) activity was in general one dimension lower than CAT and GST activity (Figure 10c). It must be noted that AChE activity was measured in gill tissue and not in digestive gland as the other enzymes. AChE in mussel tissue of the wreck sites had only slightly lower activity (average of all wreck stations: 17.16 ± 8.53 mU/mg protein) compared to the reference (19.53 ± 13.14 mU/mg protein). Station A360 had the lowest activity with 14.67 ± 7.11 mU/mg protein. However, differences were not significant.
3.2.2.5 Histochemical biomarkers
Results of the lysosomal membrane stability (LMS) analysis showed a similar pattern for peak 1 and 2 (Figure 11). For both, the peaks of the reference mussels occurred significantly later than compared to wreck mussels. The first peak of LMS in reference mussels and mussels from station A362 occurred mainly after 20 min, whereas mussels of the other stations showed the first peak between 10 and 15 min. Also, for the second peak reference mussels and mussels from station A362 showed a later response with a peak after 40 min compared to the other station which had a peak after 35 min. Furthermore, both peaks showed significant differences between the stations (first peak: Ref vs A360: p< 0.0005, Ref vs A361: p< 0.005, Ref vs A363: p< 0.05, A360 vs A362: p< 0.005, A361 vs A362: p< 0.005, A362 vs A363: p = 0.04; Second peak: Ref vs A360: p< 0.005, Ref vs A361: p< 0.0005, Ref vs A363: p< 0.005 A361 vs A362: p = 0.01).
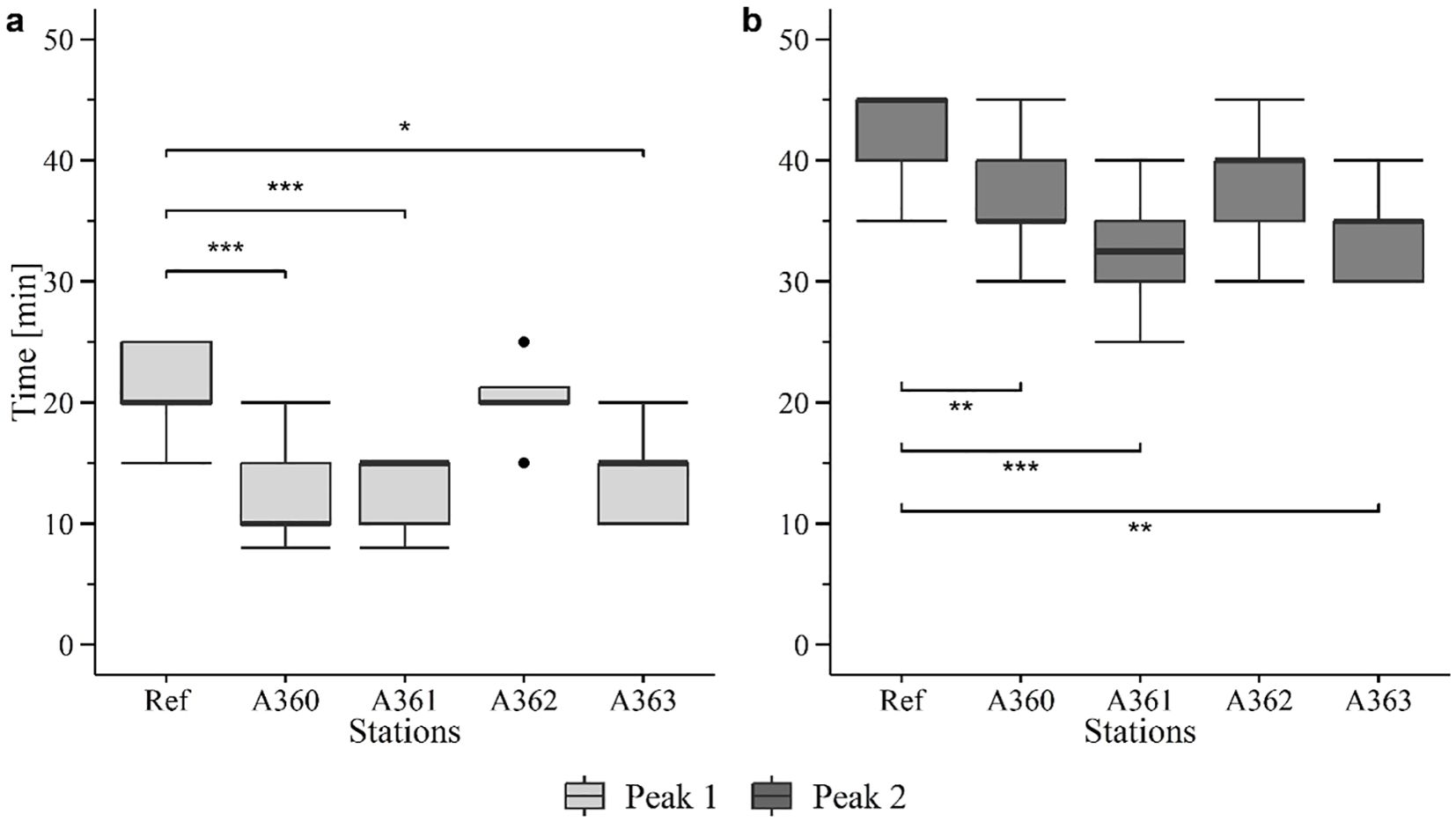
Figure 11. Boxplots of lysosomal membrane stability (LMS) of peak 1 (a) and peak 2 (b) in cells of mussel digestive gland tissue from the cruise in 2020. Ref = Reference area Belwind, A360 – A363 = Stations at V1302, [·] statistical outlier. Significant differences to the reference are displayed with stars (p< 0.05 = *, p< 0.005 = ** and p< 0.0005 = ***).
Lipofuscin (LIPF) level in digestive gland cells was measured between 8 and 12.5% with significant differences between stations (p< 0.0005) (Figure 12a). Significant differences were detected between reference and A361 (p = 0.033) as well as between reference and A362 (p< 0.0005). Further, differences with statistical significance could also be detected between station A360 and A361 (p = 0.006), A362 (p< 0.0005) and A363 (p = 0.031). The highest LIPF content in average could be detected in mussels from the station A360 (12.53 ± 0.65%) and from the reference (12.20 ± 1.10%). The lowest content was found in mussels from station A362 (8.63 ± (0.75%).
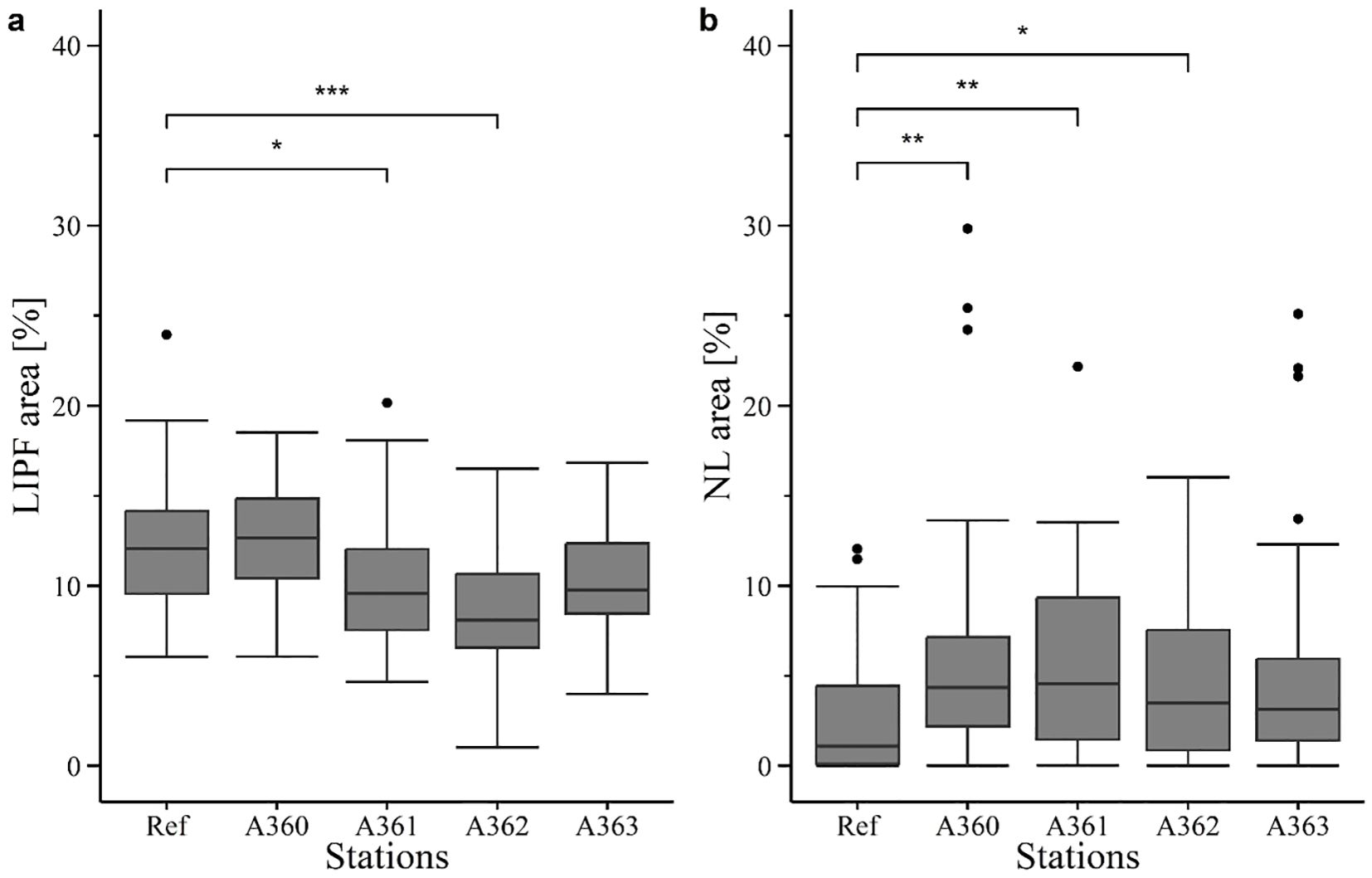
Figure 12. Histochemical biomarkers determined in tissue cells of digestive gland in mussels in 2020. Ref = Reference area at Belwind, A360 – A363 = Stations at V1302, [·] statistical outlier. Significant Differences to the reference are displayed with stars (p< 0.05 = *, p< 0.005 = ** and p< 0.0005 = ***) Used biomarkers were (a) lipofuscin content (LIPF) and (b) neutral lipids (NL).
Neutral lipid (NL) content in mussel tissue was slightly lower in mussels of the reference (2.63 ± 1.42) compared to wreck mussels (average of wreck: 5.54 ± 1.43%). (Figure 12b). Significant differences were detected for reference compared with station A360 (p = 0.0006), A361(p = 0.0015) and A362 (p = 0.014).
3.2.3 Exposed fish
Fish liver samples were taken from both the V1302 wreck and the reference site to assess the biomarker response within these tissues according to the exposure to dissolved TNT and its metabolites deriving from corroding munition onboard the wreck. However, the fat content of the liver samples was relatively high. Hence, samples remained soft and smeary even after being stored at -80°C, making it impossible to cut and process the samples with a cryotome. Therefore, no LMS and histochemical biomarker analysis could be conducted. The remaining tissues were used for the assessment of enzyme activities (see below).
3.2.3.1 Chemical analysis
Chemical analysis of the fish filets revealed the presence of TNT and its degradation products within fish (Table 3). The average detected TNT concentration was 4.2 ng/g d.w. Two individuals showed a clearly higher concentration, up to 3 times higher than average (12.8 and 10.3 ng/g d.w.). The lowest TNT concentration was 1.7 ng/g d.w. In general, the average detected TNT concentration was 4 times higher than the degradation products 4-ADNT and 2-ADNT, whereas 2-ADNT was slightly higher than 4-ADNT (average 2-ADNT: 1.6 ng/g d.w. 4-ADNT: 1.1 ng/g d.w.). 2-ADNT concentrations ranged from 0.7 up to 2.1 ng/g d.w. and 4-ADNT ranged from 0.9 up to 3.2 ng/g d.w. 1,3-DNB and 2,4-DNT were mostly beneath the limit of detection. 1,3-DNB (0.2 ng/g d.w.) and 2,4-DNT (0.6 ng/g d.w.) were only detected in the filet with the highest TNT concentration. 2,4-DNT could also be detected in one additional filet with a concentration of 1.1 ng/g d.w. The fish bile contained only very low concentrations of 2-ANDT (up to 0.5 ng/g d.w.) and 4-ADNT (up to 0.6 ng/g d.w.) whereas TNT could not be detected.
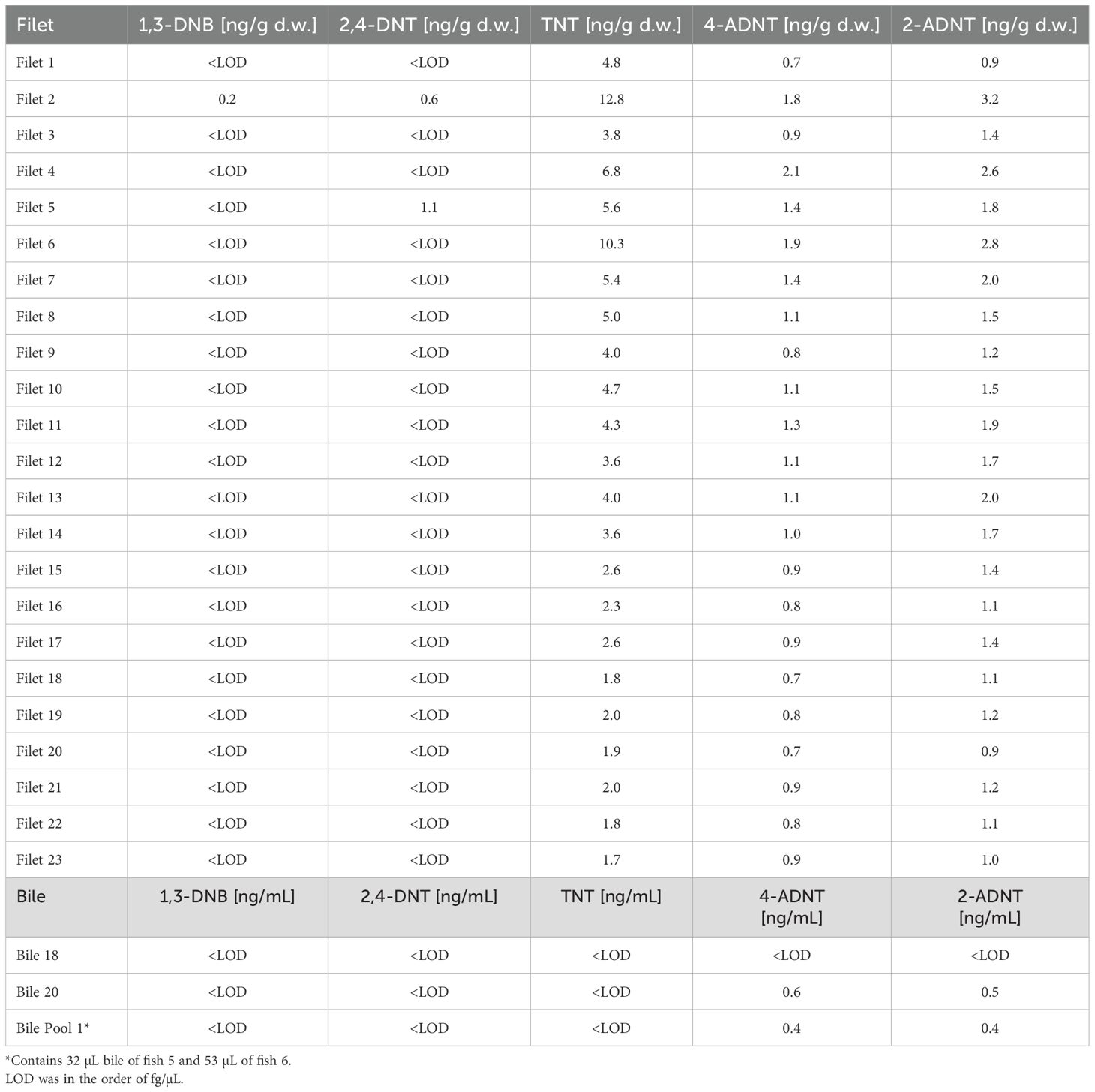
Table 3. Chemical analysis of fish filet samples and bile caught during the 2020 cruise at V1302 (Maser et al., 2023).
3.2.3.2 Fulton’s condition factor
The condition factor (K) between caught fish at the reference site (1.49 ± 0.26) and at the V1302 wreck (1.54 ± 0.17) showed no significant differences.
3.2.3.3 Biochemical biomarkers
Catalase (CAT) activity in fish liver were very similar between the fish caught at the reference area (165 ± 78.85 U/mg protein in average) and the wreck site (165.1 ± 62.52 U/mg protein in average) (Figure 13a). Therefore, no significant differences could be detected between the stations. The glutathione S-transferase (GST) activity also showed no significant differences between the reference and wreck sites (Figure 13b), even though the fish caught at the wreck had a general higher enzyme activity (110.41 ± 51.98 U/mg protein in average) than the reference fish (87.77 ± 40.33 U/mg protein in average).
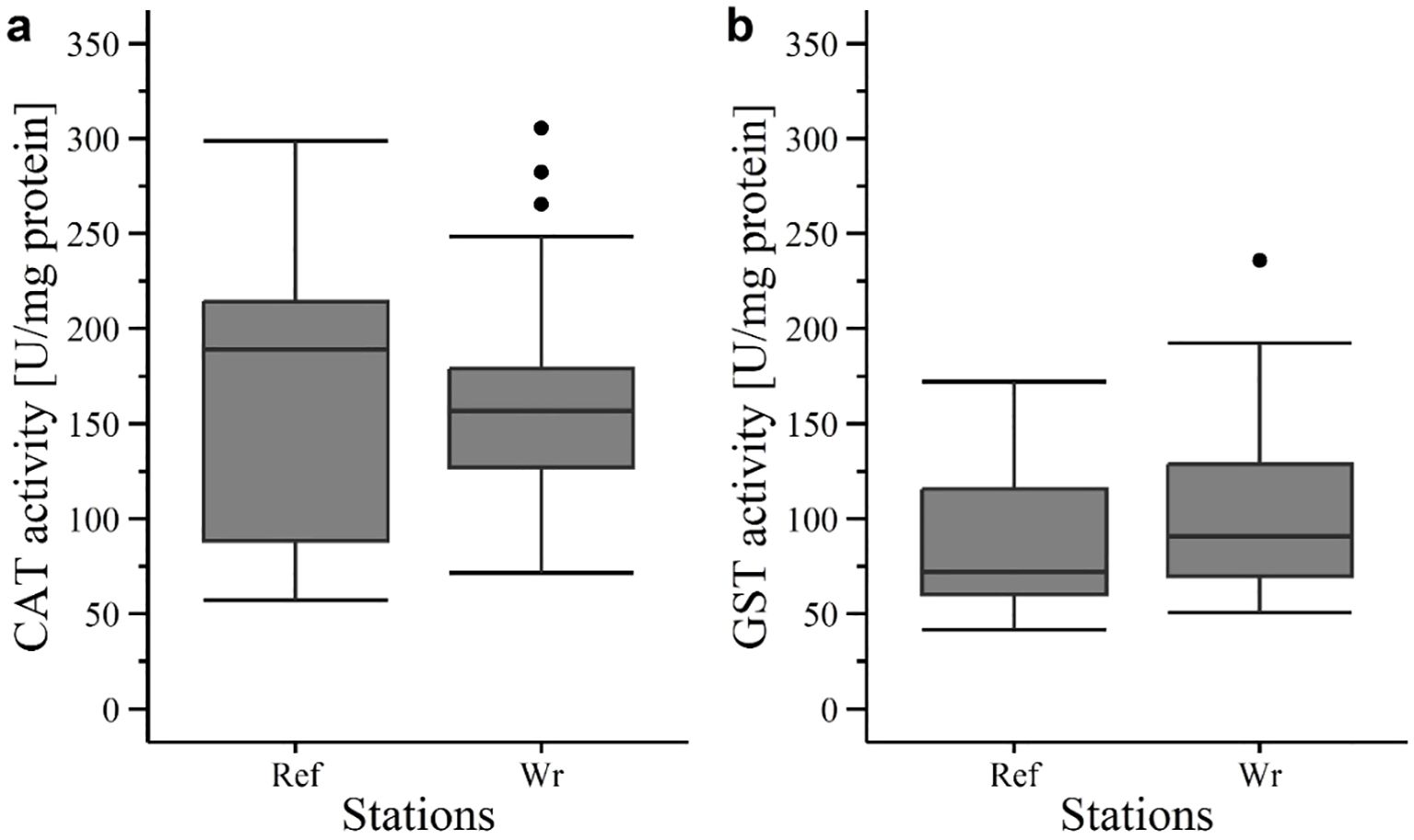
Figure 13. Enzyme activity in liver tissue of fish in 2020 at V1302: (a) Catalase (CAT) and (b) glutathione S-transferase (GST) activity. Ref, reference; Wr, wreck; [·] statistical outlier. Differences between the groups were not significant (p< 0.05).
4 Discussion
Wrecks can be a source of many pollutants depending of their cargo, armament and the vessel’s propulsion. The wrecks investigated for this study were coal or fuel-oil driven. To keep the engine running also lubricants and smears sometimes based on mineral oil derivates were used. In addition, steel ships were protected against sea water driven corrosion by covering the hull with lead containing coatings. Finally, the ships were loaded with munition filled with toxic explosives. Thus, making the wrecks a point source of a pollutant mixtures most probably unique for a specific wreck. For remaining munition investigations using archive information and battle reports were done to estimate amount of munition still on the wrecks at least until the time of sinking. Further, in case of V1302 and HMS Basilisk the remaining munition was visually confirmed by divers. Overall, munition is assumed to be the prominent source of pollutants on the investigated wrecks, knowing, that other pollutants my add to the responses of biomarker analysed for this study.
It is well established that explosives are taken up, metabolized and accumulated in marine organisms of different types (Ballentine et al., 2015; Lotufo et al., 2009; Rosen and Lotufo, 2007b). Several studies have shown that exposure to explosives poses a threat to a variety of marine species. For instance, Nipper et al. (2001) showed negative effects of TNT, tetryl and hexogen on different marine species like algae, polychaetes, opossum shrimps and redfish, with a lowest effective concentration ranging from 0.26 to 7.6 mg/L. It is also known that exposure to munition compounds has varying degrees of effects on different life stages. Rosen and Lotufo (2007a), for instance, showed a higher sensitivity of juvenile mussels (Mytilus galloprovincialis) against exposure to explosives compared to adults. Exposure can also trigger a change in behaviour as reported by Schuster et al. (2021). They found higher numbers of mussels with closed valves when Baltic blue mussels of the Mytilus family were exposed to higher TNT concentrations. They also reported effects of TNT exposure on different stress biomarkers. Most of the available studies are laboratory experiments using concentrations of munitions compounds that are much higher than those found in the environment. Knowledge about effects of dissolved explosives under field conditions is still scarce, but studies are now being published that show a response of organisms and benthic communities to dissolved explosives from dump and wreck sites of conventional munitions (Van Landuyt et al., 2022; Vedenin et al., 2023).
To assess the environmental risks of sunken warships and their cargo, two research expeditions to preselected wrecks were conducted, one in October 2019 (SS1019) and one in July 2020 (SS0720). The aim of the cruise SS1019 was to determine if the wrecks exhibit leakage of chemicals, and whether the exposure setup and sampling methods were suitable for the planned experiments. It turned out that both wrecks showed leakage of dissolved explosives. The V1302 was finally selected as the main research site in 2020, because the wreck site of the HMS Basilisk is under strong tidal influence. This results in high current velocities and turbid water, restricting the diver-based operations necessary to transplant and retrieve the mussels.
Due to the relatively short exposure time of 4 months possible responses of mussels were expected to be on low organisational levels, e.g. on subcellular, cellular and tissue levels. Therefore, the present study used several biomarkers which are known for potential alternation at low concentrations of pollutants. This includes histochemical biomarkers and the measurements of the energy status as well as the assessment of enzyme activities of the Anti-Ox-Defence-System. The same biomarker approach was then used to assess also the fish caught at the wreck and reference area.
4.1 Bioavailability of warfare chemical substances in the marine environment
Chemical analyses revealed leakage of explosives at the V1302 and HMS Basilisk as well as uptake by transplanted mussels and wild fish. Traces of explosives could be detected within the water and sediment, as well as mussel and fish tissue (for results of water and sediment samples, see Maser et al. (2023). Detected explosives within the various samples were on the order of ng per unit. This fits with the usually detected concentrations of munition compounds within environmental samples which is on the order of ng/L or µg/kg as reviewed by Beck et al. (2018). Recent studies already showed the potential of explosive leakage through corroded munition shells. For instance, Strehse et al. (2017) detected leakage of munition compounds at a bulk of explosives and Appel et al. (2018) near a pile of corroded mines in Kiel Bight, Baltic Sea. Both studies used transplanted mussels for assessing the leakage. Dissolved TNT leaking out of the munition shells can be metabolised, mainly by bacteria (Juhasz and Naidu, 2007; Rosen and Lotufo, 2007b) or by enzymes within the marine biota (Koske et al., 2020). Munition compounds as well as their metabolites are taken up and further metabolized by mussels (e.g. Appel et al., 2018; Schuster et al., 2021; Strehse et al., 2017). For instance, Appel et al. (2018) and Strehse et al. (2017) could detect explosive concentrations within mussel tissue in the order of ng/g mussel tissue. This is in accordance with the findings of the present study where mussel tissue also contained explosive concentrations in the order of ng/g mussel tissue. It is known that M. edulis contains several enzyme systems like cytochrome P-450 monooxygenase which may play a role in TNT metabolism (Livingstone and Pipe, 1992). Lab exposure experiments using blue mussels provided further evidence for the ability of mussels to metabolise TNT, since after the exposure to dissolved TNT mostly TNT metabolites were found in the investigated mussel tissues (Schuster et al., 2021).
4.2 Comparability of physiological status of mussel individuals
Species reproduction is an energy consuming and stressful process. Mussels also invest a lot of energy into their gametes and into spawning (Mredul et al., 2024). Individuals which have recently spawned are most probably not comparable, regarding their energy and stress level, to individuals that are still developing their gonads. To ensure that mussels used in the experiments were in a similar physical condition, the gonad status was determined. Further, also the condition index of all investigated mussels was tested for differences. As result no significant differences between mussel from the two different sites were detectable, suggesting that site specific geo- and hydrographical conditions did not influence mussel condition.
Mussels transplanted to the V1302 and HMS Basilisk were first collected from the reference area and pre-sorted according to their size before caged and transplanted. After the exposure mantle tissue of all exposed specimen was analysed for the gonad status. The results show that mussels from the different exposure stations of the V1302 and from the reference site were in a comparable physiological state as indicated by a similar gonad status. Approximately, half of the mussels had mature/premature gonads and the other half were still developing, which fits with the seasonal time of mussel sampling. The general spawning period of Mytilus edulis extends from April until the end of June and in Autumn gonads begin to build up again (Sprung, 1983).
4.3 Effects of leaked explosives on mussels and fish
Leaked munition compounds show a slow dissolution (Beck et al., 2018) and are usually found in ng/L concentrations in the aquatic environment (Beck et al., 2019; Kammann et al., 2024; Koske et al., 2020). It has therefore been suggested that those compounds are unlikely to cause acute toxicity or even lethal effects in organisms living at or in the vicinity from munition dumping sites (Beck et al., 2018, 2019). This appears to be true for the present study. Lethal concentrations of dissolved TNT are in the range of mg/L for many marine organisms, but at the two wreck sites investigated here measured concentrations in the water column were in the range of ng/L. This means that the caged mussels were exposed to concentrations of dissolved TNT reflecting a chronic toxicity level.
To measure the effects of chronic toxicity a multi-biomarker approach focussing on early warning marker on sub-cellular and cellular levels was used. Enzymes of the Anti-Ox-Defence system, such as catalase (CAT) and glutathione S-transferase (GST) were tested in both exposed fish and mussels. In mussels, CAT activity tended to be higher in individuals exposed at the wreck site. However, there was only a single, low significant difference detected for one wreck station (A361) relative to the reference site. This is in line with findings from Schuster et al. (2021) who conducted an exposure experiment under laboratory conditions in which mussels (Mytilus spp.) were exposed for 96 h to 10 mg TNT/L (acute exposure experiment) and 21 d to 2.5 mg TNT/L (chronic exposure). In this study, the authors could not detect significant increased CAT activity during the acute exposure. During the chronic exposure experiment CAT activity was only increased in gill tissue but not in the digestive gland. It has been suggested that CAT activity in gill tissue is more sensitive to environmental pollutants than other tissues due to their physiological role in respiration (Regoli and Principato, 1995). For future studies, it might be of interest to detect CAT activity in mussel gills as well.
GST activity in mussels of the present study was similar between wreck stations and the reference area and showed no significant differences. This is in line with another study conducted by Höher et al. (2019) who exposed M. trossulus for 96 h hours to CWA’s in the order of µg/L in a lab-controlled experiment. In this study the chemical concentration used was too low to trigger GST adaptation. TNT exposure has previously been shown to increase GST activity in mussels, as reported by Schuster et al. (2021). The authors could detect a significant increase of GST activity in the chronic exposure experiment in mussels exposed to 1.25 and 2.5 mg/L TNT. Combined, these studies suggest that the exposure concentrations of dissolved TNT in our study were too low to induce significant GST activity.
Results of the neurotoxic biomarker acetylcholinesterase (AChE) exhibited a lower activity in mussel tissue of the wreck sites than those found in the reference area. In general, a lowered AChE activity is a sign of reaction to pollution, but the differences in AChE activity were not significant. This finding matches the results found by Lastumäki et al. (2020) and Schuster et al. (2021). Both, the field experiment conducted by Lastumäki et al. (2020) and the two laboratory experiments conducted by Schuster et al. (2021) did not reveal a significant increase in AChE when mussels were exposed to munition compounds. Further, it is suggested that AChE inhibition and the alteration of antioxidant enzymes like CAT are reversely correlated as reviewed by Lionetto et al. (2011). However, this phenomenon could not be observed in the present study.
Results of the lysosome membrane stability (LMS) in mussels show a clear pattern. Membrane stability was significantly higher in cells of the digestive gland tissue at the reference site than at all exposed mussels except for station A362. This is true for both peak 1 and 2. A possible explanation is that labialisation of membranes is caused by oxyradicals, developed due to the presence of TNT and its metabolites within the cells. Other studies showed that low LMS values are negatively correlated with high values of neutral lipids (NL) and lipofuscin (LIPF) (Krishnakumar et al., 1994, 1997; Lastumäki et al., 2020). In the present study, the results showed an increase in NL in tissue of wreck mussels but a decrease in LIPF.
LIPF content in wreck mussels were generally lower compared to reference mussels, but only two stations showed significant differences (A361 and A362). In addition, the significant level of station A361 was only on low level. In general, a downregulation of biomarkers could indicate a bell-shaped response (Regoli, 2000; Viarengo et al., 2007). If that would be the case for our data, then LIPF of all wreck stations should be significant lower compared to the reference and CAT activity should show a significant negative correlation to LIPF. Since these trends were not observed, a bell-shaped response seems unlikely. However, the exposure concentration might be too low to trigger a response. For instance, Lastumäki et al. (2020) conducted a field experiment with transplanted mussels (Mytilus trossulus) were mussels were exposed 2.5 month at dumping sites of chemical warfare agents (CWA) in the Baltic Sea. The concentrations of munition compounds found in mussel tissue was on the order of ng/L and they could not detect an increase in LIPF content either. In contrast, an increase in LIPF content can be observed when mussels are exposed to high TNT concentrations (10 mg/L conducted over 96 h), as shown by the laboratory exposure experiment conducted by Schuster et al. (2021).
An accumulation of NL in digestive gland cells of mussels is associated with stress induced by toxic chemicals indicating lipidosis (Krishnakumar et al., 1994; Lowe, 1988; Viarengo et al., 2007). In the present study significantly higher NL levels were found in wreck mussels compared to the reference. Accordingly, Schuster et al. (2021) could detect an increase in NL when mussels were exposed to higher TNT concentration up to 2.5 mg/L during the chronic exposure experiment. In the present study the detected explosive concentrations at the sampling sites were only on the order of ng/L, but there is still an effect visible. It should, however, be noted that the highest NL content was not necessarily found at stations were mussels had the highest TNT concentration in their tissues.
Glycogen (GLY) is the primary energy reserve in most bivalves (Patterson et al., 1999). The GLY storage is affected by various environmental factors including chemical pollution which induces stress in the organisms. This often leads to an increased metabolism and a reduced GLY storage. Therefore, in the present study a lower amount of GLY was expected in mussels located near to munition due to the increased energy requirements caused by increased stress. However, this could not be observed. All stations showed a similar GLY level with exception of station A362 which had an increased GLY content compared to the other wreck stations A360 and A363 but not to the reference site. Schuster et al. (2021) also, could not detect a decrease in GLY of digestive gland cells when mussels were exposed to TNT, despite using a much higher concentration than what was detected at the wreck stations of the present study. Even though some of the tested biomarkers in the present study showed a response to chemical pollution, the effects were not prominent enough to change the energy storage of mussels. This is also true for the individual level, since the Condition Index CI did not differ between mussels exposed at the wrecks site and those from the reference. However, considering the available literature, this seems to be plausible given the low exposure concentrations and the relatively short time of exposure.
In addition to the mussel caging experiment, wild fish were also caught at the wreck and reference sites and tested for biomarker responses. Fish samples showed no significant alteration in Fulton’s Condition Factor (K) and enzyme activity when exposed to dissolved explosives at the wreck site compared to the reference area. Only GST activity showed an increasing trend in fish from the wreck site, although the difference was not statistically significant. In contrast, Ek et al. (2005) already proofed that TNT can have an influence on GST activity in fish: They could detect a significant higher GST activity in fish (Oncorhynchus mykiss) intraperitoneally injected with TNT when compared to a control. Della Torre et al. (2008) also found increased GST activity in European eel (Anguilla anguilla) when exposed to higher TNT concentrations in a laboratory experiment. Further, Niemikoski et al. (2020) could detect a significant change in CAT and GST activity in Atlantic cod (Gadus morhua) from chemical munition dumpsites in the Baltic Sea when exposed to CWAs. However, the test organisms used in those studies were exposed to higher concentrations of munition compounds (lab studies) or to different chemicals (Niemikoski et al., 2020).
The acute and chronic toxicity of TNT and its metabolites has, nevertheless, already been reported for many fish species by older studies like those reviewed by Talmage et al. (1999). Recent studies also proved that fish take up TNT (Maser et al., 2023) and CWA (Niemikoski et al., 2020). While Koske et al. (2020) and Kammann et al. (2024) found munition compounds in the bile of common dab (Limanda limanda, L.) living near dump sites in the North and Baltic Sea, Maser et al. (2023) and Maser et al. (in press) could even show that TNT and it’s metabolites occurred in the edible part (fillet) of fish (pouting; Trisopterus luscus) living at a wreck site in the Belgian North Sea, as well as in flounders (Platichthys flesus) caught in the German North Sea at the coast of Lower Saxony, respectively.
Overall, the comparison of the biomarker results of the present study with findings of former studies is difficult, since most existing knowledge is derived from laboratory studies, where higher concentrations of explosives were used or different chemicals like CWAs were applied. For instance, in comparison with Schuster et al. (2021), there is a difference in exposure concentration of ng/L detected in the present study whereas Schuster et al. (2021) used mg/L in the laboratory experiment with mussels. Even though they used much higher explosive concentration, not all biomarkers were significantly increased during exposure to higher TNT concentration. The same is true for fish. Studies referring to the enzyme activity as a response to higher TNT exposure concentrations used much higher concentrations of explosives then the present study (Della Torre et al., 2008; Ek et al., 2005).
Another problem regarding the assessment of the biomarker results is the fact that also traces of dissolved TNT were found at the reference site. Concentrations measured in the passive sampler from the reference site are lower than those from two wrecks stations (A361 & A362) but comparable to the values measured at A360. As the area has been cleared of lost explosives before the construction phase of the windmill farm, we had assumed that no TNT would be present at this site. At a true reference area, however, the background concentrations of the target chemical used as exposure agent should of course be zero or close to zero. In our study, the tested biomarkers have probably also responded to the TNT traces at the reference site levelling the expected responses between exposure and reference sites. Future studies should put more effort in pre-investigations of the planned reference area, to make sure that the area is free of dissolved explosives.
In addition, there is increasing evidence of sublethal and chronic effects of marine organisms when exposed to munition compounds, especially for immobile organisms living in or on the sediment (Juhasz and Naidu, 2007; Lotufo et al., 2013; Talmage et al., 1999). Those chronic effects can manifest in various forms, including reduced growth and reproduction, impaired development, and damage to the nervous, immune and blood systems (Gong et al., 2007). However, such investigations would need extended exposure times. Alternatively, histological investigations of organs, such as the liver of long-living non-migrating fish could help to assess chronic effects of low concentrated chemicals, such as dissolved explosives.
5 Conclusion
Results of the present study proved the leakage of energetic chemicals at the wrecks of HMS Basilisk and the V1302/John Mahn, as well as the uptake of those compounds by investigated mussels and fish. In mussels, the leakage of explosives is correlated with membrane impairments of cell organelles and signs of oxidative stress. However, in fish no correlation between uptake of explosives and biomarker response could be detected. If responses of a biomarker occurred, they were restricted to the sub-cellular and cellular levels. No effects on the individual level were visible. However, as corrosion of munition shells and the leakage of explosives proceeds, the situation may worsen, since more TNT will get dissolved. Therefore, further monitoring is recommended.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Binder et al., 2023; https://doi.org/10.1594/PANGAEA.962697.
Ethics statement
The animal study was approved by Prof. Dr. Thomas Brey, E-Mail: VGhvbWFzLkJyZXlAYXdpLmRl, Phone: +49 471 48 31 1300. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
FB: Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. LB: Formal Analysis, Investigation, Methodology, Validation, Writing – original draft. JS: Formal Analysis, Investigation, Methodology, Writing – original draft. SV: Conceptualization, Investigation, Methodology, Writing – original draft. MD: Conceptualization, Investigation, Methodology, Writing – original draft. EM: Funding acquisition, Resources, Supervision, Writing – original draft. MB: Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was co-funded by the European Union (European Regional Development Fund) under the North Sea Interreg Programme 2014-2020. Research was conducted, within the framework of the project “North Sea Wrecks -An Opportunity for Blue Growth: Healthy Environment, Shipping, Energy Production and -transmission (NSW). Publication costs were financed by the Alfred Wegener Institute’s Open Access Fund.
Acknowledgments
We would like to thank the captain and the crew of the RV Simon Stevin for all the support during the two expeditions for the North Sea Wrecks project. In addition, we would like to thank the scientific divers from VLIZ, Oostende, for their support in placing and recovering the mussels at the wreck sites. Last but not least we thank the reviewers for their valuable comments, which helped to improve the present publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1456409/full#supplementary-material
References
Adomako-Bonsu A. G., Jacobsen J., and Maser E. (2024). Metabolic activation of 2,4,6-trinitrotoluene; a case for ROS-induced cell damage. Redox Biol. 72, 103082. doi: 10.1016/j.redox.2024.103082
Ahvo A. (2020a). DAIMON 2 PROJECT SOPs: Homogenisation of fish liver and mussel digestive gland tissues. version 1.1. (SYKE) (Finland: F. E. I. Helsinki).
Ahvo A. (2020b). DAIMON 2 PROJECT SOPs: Catalase activity. version 1.1. (SYKE) (Helsinki: F. E. I. Finland).
Ahvo A. (2020c). DAIMON 2 PROJECT SOPs: Glutathione-S-transferase activity. version 1.1. (SYKE) (Helsinki: F. E. I. Finland).
Ahvo A. (2020d). DAIMON 2 PROJECT SOPs: Acetylcholinesterase. version 1.1. (SYKE) (Helsinki: F. E. I. Finland).
Appel D., Beck A. J., Eggert A., Gräwe U., Kampmeier M., Martin H.-J., et al. (2019). Practical Guide for Environmental Monitoring of Conventional Munitions in the Seas - Results from the BMBF funded project UDEMM “Umweltmonitoring für die Delaboration von Munition im Meer” Version 1.1. Ed. Greinert J. (Kiel, Germnay: GEOMAR Helmholtz-Zentrum für Ozeanforschung).
Appel D., Strehse J. S., Martin H. J., and Maser E. (2018). Bioaccumulation of 2,4,6-trinitrotoluene (TNT) and its metabolites leaking from corroded munition in transplanted blue mussels (M. edulis). Mar. pollut. Bull. 135, 1072–1078. doi: 10.1016/j.marpolbul.2018.08.028
Ballentine M., Tobias C., Vlahos P., Smith R., and Cooper C. (2015). Bioconcentration of TNT and RDX in coastal marine biota. Arch. Environ. Contamination Toxicol. 68, 718–728. doi: 10.1007/s00244-014-0104-9
Beck A. J., Gledhill M., Kampmeier M., Feng C., Schlosser C., Greinert J., et al. (2022). Explosives compounds from sea-dumped relic munitions accumulate in marine biota. Sci. Total Environ. 806, 151266. doi: 10.1016/j.scitotenv.2021.151266
Beck A. J., Gledhill M., Schlosser C., Stamer B., Böttcher C., Sternheim J., et al. (2018). Spread, behavior, and ecosystem consequences of conventional munitions compounds in coastal marine waters. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00141
Beck A. J., van der Lee E. M., Eggert A., Stamer B., Gledhill M., Schlosser C., et al. (2019). In situ measurements of explosive compound dissolution fluxes from exposed munition material in the Baltic Sea. Environ. Sci. Technol. 53, 5652–5660. doi: 10.1021/acs.est.8b06974
Beddington J. and Kinloch A. J. (2005). Munitions dumped at sea: A literature review (London: Imperial College London Consultants).
Bełdowski J., Potrykus J., Szubska M., Klusek Z., Anu L., Lehtonen K. K., et al. (2014). CHEMSEA Findings: Results from the CHEMSEA project - Chemical munitions search and assessment (Sopot, Poland: Institute of Oceanology of the Polish Academy of Sciences).
Bellamy C. (1991). The military uses of the North Sea. Ocean Shoreline Manag. 16 (3-4). 275–289. doi: 10.1016/0951-8312(91)90008-P
Belwind (2017). Belwind - Wind farm in the North Sea Press Information. Available online at: https://arquivo.pt/wayback/20160118141207/http://www.belwind.eu/files/604739_belwind%20persdossier_ENG.pdf (Accessed May 25, 2021).
Beyer J., Green N. W., Brooks S., Allan I. J., Ruus A., Gome T., et al. (2017). Blue mussels (Mytilus edulis spp.) as sentinel organisms in coastal pollution monitoring: A review. Mar. Environ. Res. 130, 338–365. doi: 10.1016/j.marenvres.2017.07.024
Binder F. I., Marx U., De Rijcke M., Van Haelst S., and Brenner M. (2023). Enzyme activity and histochemical biomarkers of mussels and fish caught at two shipwrecks at the Belgian coast (PANGAEA). doi: 10.1594/PANGAEA.962697
Bocquené G. and Galgani F. (1998). Biological Effects of Contaminants: Cholinesterase Inhibition by Organophosphate and Carbamate Compounds Vol. 22 (Denmark: Copenhagen International Council for the Exploration of the Sea Copenhagen).
BOP The Belwind offshore wind project. Available online at: https://www.belgianoffshoreplatform.be/en/projects/belwind/ (Accessed May 25 2021).
Böttcher C., Knobloch T., Rühl N.-P., Sternheim J., Wichert U., and Wöhler J. (2011). Munitionsbelastung der deutschen Meeresgewässer - Bestandsaufnahme und Empfehlungen (Stand 2011) (Hamburg: Bundesamt für Seeschifffahrt und Hydrographie).
Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Brenner M., Broeg K., Frickenhaus S., Buck B. H., and Koehler A. (2014). Multi-biomarker approach using the blue mussel (Mytilus edulis L.) to assess the quality of marine environments: season and habitat-related impacts. Mar. Environ. Res. 95, 13–27. doi: 10.1016/j.marenvres.2013.12.009
Bünning T. H., Strehse J. S., Hollmann A. C., Botticher T., and Maser E. (2021). A toolbox for the determination of nitroaromatic explosives in marine water, sediment, and biota samples on femtogram levels by GC-MS/MS. Toxics 9. doi: 10.3390/toxics9030060
Claiborne A. (1985/2018). “Catalase activity,” in Handbook of methods for oxygen radical research. Ed. Greenwald R. A. (CRC Press, Boca Raton), 283–284.
Culling C. F. A. (1974). Handbook of Histopathological and Histochemical Techniques. 3rd ed (Butterworth, London: Including Museum Techniques).
de Andrade V. M., da Silva J., da Silva F. R., Heuser V. D., Dias J. F., Yoneama M. L., et al. (2003). Fish as bioindicators to assess the effects of pollution in two southern Brazilian rivers using the comet assay and micronucleus test. Environ. Mol. Mutagenesis 44, 459–468. doi: 10.1002/em.20070
Della Torre C., Corsi I., Arukwe A., Valoti M., and Focardi S. (2008). Interactions of 2,4,6-trinitrotoluene (TNT) with xenobiotic biotransformation system in European eel Anguilla Anguilla (Linnaeus 1758). Ecotoxicology Environ. Saf. 71, 798–805. doi: 10.1016/j.ecoenv.2008.03.003
Deutsches Schifffahrtsmuseum Toxic Legacies of War - North Sea Wrecks. Available online at: https://www.dsm.museum/ausstellung/ausstellungen/toxic-legacies-of-war-north-sea-wrecks (Accessed August 27 2021).
Ek H., Dave G., Nilsson E., Sturve J., and Birgersson G. (2006). Fate and effects of 2,4,6-trinitrotoluene (TNT) from dumped ammunition in a field study with fish and invertebrates. Arch. Environ. Contamination Toxicol. 51, 244–252. doi: 10.1007/s00244-005-0117-5
Ek H., Dave G., Sturve J., Almroth B. C., Stephensen E., Forlin L., et al. (2005). Tentative biomarkers for 2,4,6-trinitrotoluene (TNT) in fish (Oncorhynchus mykiss). Aquat. Toxicol. 72, 221–230. doi: 10.1016/j.aquatox.2005.01.001
Ek H., Nilsson E., and Dave G. (2008). Effects of TNT leakage from dumped ammunition on fish and invertebrates in static brackish water systems. Ecotoxicology Environ. Saf. 69, 104–111. doi: 10.1016/j.ecoenv.2006.12.016
Farrington J. W. and Quinn J. G. (1973). Petroleum hydrocarbons in Narragansett Bay. 1. Survey of hydrocarbons in sediments and clams (Mercenaria mercenaria). Estuar. Coast. Mar. Sci. 1, 71–79. doi: 10.1016/0302-3524(73)90059-5
Gilbert R. (2005). The global risk of marine pollution from WWII shipwrecks: examples from the seven seas. Int. Oil Spill Conf. Proc. 1, 1049–1054. doi: 10.7901/2169-3358-2005-1-1049
Gong P., Guan X., Inouye L. S., Pirooznia M., Indest K. J., Athow R. S., et al. (2007). Toxicogenomic analysis provides new insights into molecular mechanisms of the sublethal toxicity of 2,4,6-trinitrotoluene in Eisenia fetida. Envionmental Sci. Technol. 41, 8195–8202. doi: 10.1021/es0716352
Grassel P., Otte F., and Bergmann S. (2021). “Militärische Altlasten im Meer: ein gefährliches Erbe. Das Projekt North Sea Wrecks,” in Nachrichten des Marschenrates, vol. 58, (Wilhelmshaven, Germany: Marschenrat zur Förderung der Forschung im Küstengebiet der Nordsee e. V), 13–17.
Gröner E., Maass J., and Maas M. (1993). “Flußfahrzeuge, ujäger, vorpostenboote, hilfminensucher, küstenschutzverbände (Teil 1),” in Die deutschen Kriegsschiffe 1815-1945, vol. 8/1. (Bernard & Graefe Verlag, Bonn), 611.
Habig W. H., Pabst M. J., and Jakoby W. B. (1974). Glutathione S-transferases. J. Biol. Chem. 249, 7130–7139. doi: 10.1016/s0021-9258(19)42083-8
HELCOM (2013). “Chemical munitions dumped in the Baltic Sea,” in Report of the ad hoc Expert Group to Update and Review the Existing Information on Dumped Chemical Munitions in the Baltic Sea Baltic Sea Environment Proceeding (BSEP) (Helsinki, Finland: Baltic Marine Environment Protection Commission (HELCOM) HELCOM), vol. 142. , 128.
Höher N., Turja R., Brenner M., Nyholm J. R., Ostin A., Leffler P., et al. (2019). Toxic effects of chemical warfare agent mixtures on the mussel Mytilus trossulus in the Baltic Sea: A laboratory exposure study. Mar. Environ. Res. 145, 112–122. doi: 10.1016/j.marenvres.2019.02.001
Juhasz A. L. and Naidu R. (2007). Explosives: Fate, dynamics, and ecological impact in terrestrial and marine environments. Rev. Environ. Contamination Toxicol. 191, 163–215. doi: 10.1007/978-0-387-69163-3_6
Kammann U., Topker V., Schmidt N., Rodiger M., Aust M.-O., Gabel M., et al. (2024). Explosives leaking from dumped munition contaminate fish from German coastal waters: a reason for chronic effects? Environ. Sci. Europe 36, 116. doi: 10.1186/s12302-024-00942-5
Koske D., Straumer K., Goldenstein N. I., Hanel R., Lang T., and Kammann U. (2020). First evidence of explosives and their degradation products in dab (Limanda limanda L.) from a munition dumpsite in the Baltic Sea. Mar. pollut. Bull. 155, 111131. doi: 10.1016/j.marpolbul.2020.111131
Krishnakumar P. K., Casillas E., and Varanasi U. (1997). Cytochemical responses in the digestive tissue of Mytilus edulis complex exposed to microencapsulated PAHs or PCBs. Part C Pharmacol. Toxicol. Endocrinol. Comp. Biochem. Physiol. 118C, 11–18. doi: 10.1016/S0742-8413(97)00076-5
Kuklina I., Kouba A., and Kozák P. (2013). Real-time monitoring of water quality using fish and crayfish as bio-indicators: a review. Environ. Monit. Assess. 185, 5043–5053. doi: 10.1007/s10661-012-2924-2
Lastumäki A., Turja R., Brenner M., Vanninen P., Niemikoski H., Butrimaviciene L., et al. (2020). Biological effects of dumped chemical weapons in the Baltic Sea: A multi-biomarker study using caged mussels at the Bornholm main dumping site. Mar. Environ. Res. 161, 105036. doi: 10.1016/j.marenvres.2020.105036
Lettens J. (2002). V-1302 (John Mahn) [+1942]. Available online at: https://www.wrecksite.eu/wreck.aspx?20 (Accessed May 21 21).
Lillie R. D. and Ashburn L. L. (1943). Supersaturated solutions of fat stains in dilute isopropanol for demonstration of acute fatty degeneration not shown by Herxheimer’s technique. Arch. Pathol. 36, 432–440.
Lionetto M. G., Caricato R., Calisi A., and Schettino T. (2011). “Acetylcholinesterase inhibition as a relevant biomarker in environmental biomonitoring: New insights and perspectives,” in Ecotoxicology around the globe. Ed. Visser J. E. (Nova Science Publisher Inc, New York, United States), 87–115.
Livingstone D. R. and Pipe R. K. (1992). “Mussels and environmental contaminants: molecular and cellular aspects,” in The mussel Mytilus; ecology, physiology, genetics, and culture. Ed. Gosling E. G. (Elsevier, Amsterdam), 425–464.
Lotufo G. R., Lydy M., Rorrer G. L., Cruz-Uribe O., and Cheney D. P. (2009). “Bioconcentration, bioaccumulation, and biotransformation of explosives and related compounds in aquatic organisms,” in Ecotoxicology of Explosives. Eds. Sunahara G. I., Lotufo G., Kuperman R. G., and Hawari J. (CRC Press, Boca Raton, London, New York), 135–155.
Lotufo G. R., Rosen G., Wild W., and Carton G. (Eds.) (2013). Summary Review of the Aquatic Toxicology of Munitions Constituents (Vicksburg, Mississippi, USA: Environmental Laboratory, U.S. Army Engineer Research and Development Cente).
Lowe D. M. (1988). Alterations in cellular structure of Mytilus edulis resulting from exposure to environmental contaminants under field and experimental conditions. Mar. Ecol. - Prog. Ser. 46, 91–100. doi: 10.3354/meps046091
Maes F., Schrijvers J., Van Lancker V., Verfaillie E., Degraer S., Derous S., et al. (2005). “Towards a spatial structure plan for sustainable management of the sea,” in Research in the framework of the BSP programme “Sustainable Management of the Sea” – PODO II (Brussels, Belgium: Belgian Science Policy (BELSPO)), 539.
Mallefet J., Zintzen V., Massin C., Norro A., Vincx M., De Maersschalck V., et al. (2007). Belgian Shipwreck: Hotspots for Marine Biodiversity (BEWREMABI) (Brüssel: Belgian Science Policy Office).
Maser E., Bünning T. H., Brenner M., Van Haelst S., De Rijcke M., Müller P., et al. (2023). Warship wrecks and their munition cargos as a threat to the marine environment and humans: The V 1302 “JOHN MAHN” from World War II. Sci. Total Environ. 857, 159324. doi: 10.1016/j.scitotenv.2022.159324
Maser E., Bünning T. H., and Strehse J. S. (2024). How contaminated is flatfish living near World Wars’ munition dumping sites with energetic compounds. Arch. Toxicol. 98, 3825–3836. doi: 10.1007/s00204-024-03834-y
Maser E. and Strehse J. S. (2021). Can seafood from marine sites of dumped World War relicts be eaten? Arch. Toxicol. 95, 2255–2261. doi: 10.1007/s00204-021-03045-9
Mathys M. (2009). The Quaternary geological evolution of the Belgian Continental Shelf, southern North Sea (Gent, Belgium: (PhD thesis), University Gent).
Monfils R., Gilbert T., and Nawadra S. (2006). Sunken WWII shipwrecks of the Pacific and East Asia: the need for regional collaboration to address the potential marine pollution threat. Ocean Coast. Manage. 49, 779–788. doi: 10.1016/j.ocecoaman.2006.06.011
Moore M. N., Lowe D., and Köhler A. (2004). “Biological effects of contaminants: Measurement of lysosomal membrane stability,” in ICES Techniques in Marine Environmental Sciences (TIMES) Copenhagen: ICES, vol. 36. (ICES, Copenhagen), 31.
Mredul M. M. H., Sokolov E. P., Kong H., and Sokolova I. M. (2024). Spawning acts as a metabolic stressor enhanced by hypoxia and independent of sex in a broadcast marine spawner. Sci. Total Environ. 909, 168419. doi: 10.1016/j.scitotenv.2023.168419
Niemikoski H., Straumer K., Ahvo A., Turja R., Brenner M., Rautanen T., et al. (2020). Detection of chemical warfare agent related phenylarsenic compounds and multibiomarker responses in cod (Gadus morhua) from munition dumpsites. Mar. Environ. Res. 162, 105160. doi: 10.1016/j.marenvres.2020.105160
Nipper M., Carr R. S., Biedenbach J. M., Hooten R. L., Miller K., and Saepoff S. (2001). Development of marine toxicity data for ordnance compounds. Arch. Environ. Contamination Toxicol. 41, 308–318. doi: 10.1007/s002440010253
Nipper M., Carr R. S., and Lotufo G. R. (2009). “Aquatic toxicology of explosives,” in Ecotoxicology of Explosives. Eds. Sunahara G. I., Lotufo G., Kuperman R. G., and Hawari J. (CRC Press, Boca Raton, London, New York), 77–115.
Patterson M. A., Parker B. C., and Neves R. J. (1999). Glycogen concentration in the mantle tissue of freshwater mussels (Bivalvia: Unionidae) during starvation and controlled feeding. Am. Malacological Bull. 15, 47–50.
Pearse A. G. E. (1985). “Histochemistry: theoretical and applied,” in Analytical Technology, 4th ed, vol. 2. (Churchill, Livingstone, Edinburgh, London, Melbourne and New York).
Regoli F. (2000). Total oxyradical scavenging capacity (TOSC) in polluted and translocated mussels: a predictive biomarker of oxidative stress. Aquat. Toxicol. 50, 351–361. doi: 10.1016/S0166-445X(00)00091-6
Regoli F. and Principato G. (1995). Glutathione, glutathione-dependent and antioxidant enzymes in mussel, Mytilus galloprovincialis, exposed to metals under field and laboratory conditions: implications for the use of biochemical biomarkers. Aquat. Toxicol. 31, 143–164. doi: 10.1016/0166-445X(94)00064-W
Rosen G. and Lotufo G. R. (2007a). Toxicity of explosive compounds to the marine mussel, Mytilus galloprovincialis, in aqueous exposures. Ecotoxicology Environ. Saf. 68, 228–236. doi: 10.1016/j.ecoenv.2007.03.006
Rosen G. and Lotufo G. R. (2007b). Bioaccumulation of explosive compounds in the marine mussel, Mytilus galloprovincialis. Ecotoxicology Environ. Saf. 68, 237–245. doi: 10.1016/j.ecoenv.2007.04.009
Schuster R., Strehse J. S., Ahvo A., Turja R., Maser E., Bickmeyer U., et al. (2021). Exposure to dissolved TNT causes multilevel biological effects in Baltic mussels (Mytilus spp.). Mar. Environ. Res. 167, 105264. doi: 10.1016/j.marenvres.2021.105264
Sims J. G. and Steevens J. A. (2008). The role of metabolism in the toxicity of 2,4,6-trinitrotoluene and its degradation products to the aquatic amphipod Hyalella azteca. Ecotoxicology Environ. Saf. 70, 38–46. doi: 10.1016/j.ecoenv.2007.08.019
Sprung M. (1983). Reproduction and fecundity of the mussel Mytilus edulis at Helgoland (North Sea). Helgoländer Meeresuntersuchungen 36, 243–255. doi: 10.1007/BF01983629
Strehse J. S., Appel D., Geist C., Martin H. J., and Maser E. (2017). Biomonitoring of 2,4,6-trinitrotoluene and degradation products in the marine environment with transplanted blue mussels (M. edulis). Toxicology 390, 117–123. doi: 10.1016/j.tox.2017.09.004
Strehse J. S. and Maser E. (2020). Marine bivalves as bioindicators for environmental pollutants with focus on dumped munitions in the sea: A review. Mar. Environ. Res. 158, 105006. doi: 10.1016/j.marenvres.2020.105006
Sündermann J. and Pohlmann T. (2011). A brief analysis of North Sea physics. Oceanologia 53, 663–689. doi: 10.5697/oc.53-3.663
Talmage S. S., Opresko D. M., Maxwell C. J., Welsh C. J., Cretella F. M., Reno P. H., et al. (1999). Nitroaromatic munition compounds: environmental effects and screening values. Rev. Environ. Contamination Toxicol. 161, 1–156. doi: 10.1007/978-1-4757-6427-7_1
Van Landuyt J., Kundu K., Van Haelst S., Neyts M., Parmentier K., De Rijcke M., et al. (2022). 80 years later: Marine sediments still influenced by an old war ship. Front. Mar. Sci. 9, 1017136. doi: 10.3389/fmars.2022.1017136
Vedenin A., Kröncke I., and Greinert J. (2023). Impact of munition dumpsites on the benthic macrofauna in the western Baltic Sea (Vienna, Austria: Paper presented at the EGU General Assembly 2023).
Viarengo A., Lowe D., Bolognesi C., Fabbri E., and Koehler A. (2007). The use of biomarkers in biomonitoring: a 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comp. Biochem. Physiology Part C 146, 281–300. doi: 10.1016/j.cbpc.2007.04.011
Wellington D. R. and Mitchell W. R. (1991). In vitro cytotoxicity of certain munition nitroaromatic compounds. Chemosphere 23, 363–373. doi: 10.1016/0045-6535(91)90190-O
Keywords: multi-biomarker approach, enzyme activity, dissolved TNT, biological effects, warship wrecks
Citation: Binder FI, Bünning LTH, Strehse JS, Van Haelst S, De Rijcke M, Maser E and Brenner M (2025) Biological effects of munition left on sunken war ships in the North Sea: a multi-biomarker study using caged blue mussels and fish caught at WWII wreck sites at the Belgian coast. Front. Mar. Sci. 12:1456409. doi: 10.3389/fmars.2025.1456409
Received: 28 June 2024; Accepted: 03 April 2025;
Published: 17 June 2025.
Edited by:
Xuchun Qiu, Jiangsu University, ChinaReviewed by:
Manu Soto, University of the Basque Country, SpainDario Savoca, University of Palermo, Palermo, Italy
Copyright © 2025 Binder, Bünning, Strehse, Van Haelst, De Rijcke, Maser and Brenner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthias Brenner, TWF0dGhpYXMuQnJlbm5lckBhd2kuZGU=
 Franziska Isabell Binder
Franziska Isabell Binder Lillian Tabea Hannah Bünning
Lillian Tabea Hannah Bünning Jennifer Susanne Strehse
Jennifer Susanne Strehse Sven Van Haelst4
Sven Van Haelst4 Maarten De Rijcke
Maarten De Rijcke Edmund Maser
Edmund Maser Matthias Brenner
Matthias Brenner