- 1State Key Laboratory of Mariculture Biobreeding and Sustainable Goods, Key Laboratory of South China Sea Fishery Resources Exploitation & Utilization, Ministry of Agriculture and Rural Affairs, South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Guangzhou, China
- 2Ocean College, Hebei Agricultural University, Qinhuangdao, China
- 3Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao, Shandong, China
- 4Key Laboratory of Efficient Utilization and Processing of Marine Fishery Resources of Hainan Province, Sanya Tropical Fisheries Research Institute, Sanya, China
In saline-alkaline water, carbonate alkalinity (CA) stands as the predominant detrimental environmental element impacting aquatic fauna. As a multifunctional organ, the gill plays a key role in the regulation of energy metabolism in shrimp. In this study, the low-salinity cultured Litopenaeus vannamei was exposed to CA stress for a period of 7 days, and then the stress was relieved and recovered for 7 days. The study examined the alterations in the gill energy metabolism following exposure to CA stress and subsequent recovery, analyzing these changes through different biological functional aspects. The results demonstrated that CA stress led to alterations in the gill histomorphology and disrupted the balance of energy metabolism-related parameters. In detail, after CA stress, carbohydrate metabolism related indexes, the pyruvate (PYR) content showed increases, as did the relative expression of the hk, pk, and pdh genes, while the glucose (GLU) and lactate (LAC) content and the expression of the idh gene were slightly decreased; lipid metabolism related indexes, such as the triglycerides (TG) content and the expression of the ampk gene were slightly increased, and the expressions of the srebp, acc, and fas genes were increased significantly; tricarboxylic acid (TCA) cycle related indexes, such as the expressions of the cs, odh, and sdh genes were up-regulated, whereas the expressions of the mdh and idh genes were significantly down-regulated, the expression of the fh gene was slightly decreased in regulation; electron transfer chain related indexes, such as the expressions of the ndh, cytc, coi, cco, and atph genes were significantly increased. Collectively, these alterations jointly affected the energy metabolism homeostasis. After the CA stress was relieved, while certain physiological parameters demonstrated improvement, they did not completely revert to the levels seen in the control group. The findings indicated that CA stress exerted an adverse effect on the histomorphology and energy metabolism in the gills of shrimp, likely by disrupting the functions of glycolysis, lipid metabolism, TCA cycle, and electron transport chain, which may further affect the growth and survival of the shrimp.
1 Introduction
Litopenaeus vannamei, commonly known as the Pacific white shrimp, holds significant economic value globally, which can provide high-quality protein for human diets (Duan et al., 2020; Liu et al., 2024). The L. vannamei is characterized by rapid growth, high stress resistance, and broad salinity tolerance, attributes that facilitate its cultivation across diverse environments (Zhang et al., 2024a). Currently, the resources for shrimp farming are becoming increasingly strained, and it is urgent to expand the culture space. In which, the saline-alkali aquaculture of shrimp has great development potential. The global saline-alkaline land area is 950 million hectares, accounting for one third of the land area (Sumner and Naidu, 1998), and it is currently in the initial phase of development and utilization, and developing saline-alkaline aquaculture is an important way to expand the aquaculture space. The water quality of saline-alkaline water is complex, which can be divided into carbonate, sulfate, and chloride types according to ion content and ratio (Liu et al., 2022). Among them, carbonate alkalinity (CA), as a significant environmental stress factor in saline-alkaline water, jeopardizes the growth, development, and survival of shrimp, and the life activities of shrimp are subject to compounded toxic effects (Yao et al., 2010a; Xu et al., 2024). For example, under low salinity conditions (3.29-4.84‰), the safe concentration of CA for L. vannamei larvae was reported to be 2.90 mmol/L (Yang et al., 2004). Reports indicate that CA stress significantly impacts the survival rate and metabolic functions of shrimp (Yao et al., 2010b; Zhang et al., 2024b, c; Duan et al., 2023; Yang et al., 2006; Song et al., 2024). Therefore, exploring the metabolic strategy of shrimp to cope with CA stress is helpful to develop anti-stress methods for shrimp saline-alkaline aquaculture.
The energy metabolism plays an important role in aquatic animals coping with stress. CA stress induces glycogen decomposition, inhibits lipid synthesis, and interferes with glycerol phospholipid metabolism in L. vannamei (Zhang et al., 2024b, c). CA stress can activate mTOR and AMPK signaling (Shi et al., 2024) and induce transcriptome changes in catabolism, immune response, circulatory function, and lipid metabolism of L. vannamei (Zhang et al., 2023; Song et al., 2024). CA stress triggers changes in substance transport, metabolic processes, and amino acid synthesis in the hepatopancreas of L. vannamei (Duan et al., 2023) and causes L. vannamei to have aberrant lipid metabolism (Shi et al., 2023; Huang et al., 2019). When Exopalaemon carinicauda is under CA stress, its hepatopancreas mainly responds to stress through linoleic acid and glycerophosphate metabolism, and cAMP signaling (Qin et al., 2023), and obtains energy support by enriching lipolysis, amino acid and carbohydrate metabolism (Qin et al., 2021). In Fenneropenaeus chinensis, it can counteract the osmotic imbalance induced by CA stress through the augmentation of energy metabolism in the hepatopancreas (Gao et al., 2024). However, there are few studies on the energy metabolism changes of L. vannamei under CA stress, it is of great significance to explore how the shrimp regulates energy metabolism to cope with CA stress and recovery process.
Gills are very important for the regulation of respiration and osmosis, which are closely related to energy metabolism (Duan et al., 2018; Zhu et al., 2024). In the saline-alkali aquaculture of shrimp, the gills are inevitably subjected to stress due to their direct exposure to a highly alkaline environment. Our previous study found that CA had a negative effect on the physiological homeostasis of L. vannamei (Xiao et al., 2024). The stress of aquatic organisms is a highly energy-consuming process; therefore, we speculate that CA stress will affect the energy metabolism in the gills of L. vannamei. As the main organ for gas exchange, the gills are exposed to water with a large surface area. When faced with CA stress, ion transport in the gills is affected, which affects the penetration and acid-base balance of shrimp. In order to restore this balance, the gills need to consume more energy. Consequently, in this study, after being cultivated in low-salinity circumstances and after being exposed to CA stress for 7 days, L. vannamei had a 7-day recovery period. The energy metabolism characteristics of the gills of L. vannamei were then carefully investigated at various biological function levels during CA stress and recovery, including the carbohydrate metabolism, lipid metabolism, tricarboxylic acid (TCA) cycle, and electron transfer chain. By exploring the energy metabolism strategy of shrimp to cope with CA stress, the result of this study is helpful to develop anti-stress methods for shrimp aquaculture in saline-alkali water, and provides an important scientific basis for shrimp farming.
2 Materials and methods
2.1 Shrimp and culture conditions
In this study, the healthy L. vannamei were obtained from an indoor pond at the Shenzhen Base of South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences (Shenzhen, China), averaging weight was 9.6 ± 0.4 g. The shrimp were acclimated for 7 days in a 300 L experimental tank before the stress test. The water temperature in the tank was 25 ± 0.5°C, the pH was kept at 8.2 ± 0.2, and the salinity was kept at 3‰, and for 24 h, the aeration was maintained. Every day, fresh water was added. Shrimp were fed 5% of their body weight according to the feeding condition every day. Uneaten feed and waste were promptly removed to ensure water cleanliness.
2.2 CA stress exposure and sample collection
This study encompassed a 7-day CA stress experiment, followed by a 7-day recovery period. After 7 days of acclimatization to the experimental conditions, the shrimp were randomly divided into one of two groups: the CA group or the control (CK) group, the pH of the CK group was 8.2 ± 0.2, and that of the CA group was 8.4 ± 0.2. Each group had 3 parallel tanks, each with 50 shrimp. The CA stress experiment then proceeds for 7 days, during which the CK group was maintained in normal water with a salinity of 3‰ without sodium bicarbonate addition; the CA group was added sodium bicarbonate to 3‰ low salinity rearing water to make its concentration as 5 mmol/L. The water of all the tanks was changed every day. Before water changes, the necessary experimental water for the CK and CA groups was pre-prepared. Throughout the stress period, all culture conditions remained identical to those during the acclimation period, with the sole exception being the varying CA concentration in the water. According to the basic research and experimental data of Duan et al. (2023) and Song et al. (2024), we chose 7 days to carry out the short-term reaction and recovery process experiment for the organisms. After 7 days of CA stress, the CA stress was relieved and a new RCA group was set up (that is, the culture water of the CA group was replaced by normal 3‰ low salinity water). Subsequently, 15 shrimp from each tank in the CA group were cultured under normal conditions for an additional 7 days.
On day 7 of the exposure to stress and day 7 of the recuperation, the gills of samples were collected from each tank. Specifically, the gills of three shrimp from each tank were collected and fixed in 4% paraformaldehyde (Beijing Labgic Technology Co. Ltd., Beijing, China) for histological analysis. The gills of three shrimp from each tank were collected and mixed in RNA protection solution (RNAFollow, New Saimei Biotech Co., Ltd., Suzhou, China) at 4°C for 24 h and then stored at -80°C for gene expression analysis. The gills of five shrimp from each tank were collected, mixed, and stored at -80°C for the determination of biochemical indexes. The sampling method and sample number in CA stress and recovery stages were consistent.
2.3 Histomorphological analysis
The gill tissue samples underwent fixation in a 4% paraformaldehyde solution for a period of 24 h. Subsequently, the fixed tissue was placed in 70%, 80%, 90%, and 100% alcohol solutions in turn for gradient dehydration. Subsequently, the dehydrated gill tissue was immersed in xylene to achieve transparency, after which the transparent tissue block was embedded in paraffin for further processing. After that, the embedded tissue was cut into 4 μm pieces using a Leica RM2016 microtome (Shanghai, China); subsequently, H&E was used to stain the samples. Finally, a microscope was used to view and take pictures of the stained sections (Nikon, Tokyo, Japan).
2.4 Biochemical indexes determination
After thawing the frozen gill tissue at -80°C, the tissue weight was about 0.1 g, and the tissue homogenate was prepared according to the ratio of m (tissue): V (physiological saline) = 1:9, which was mechanically homogenized under the condition of an ice-water bath and centrifuged for 15 minutes at 3500 rpm using a centrifuge. The supernatant was then moved to a test tube and stored at -80°C for later use. The identical kit made by Nanjing Jiancheng Bioengineering Institute, China (Nanjing, China), was used to measure all biochemical indexes, including glucose (GLU), pyruvate (PYR), lactate (LAC), triglycerides (TG), and total ATPase (ATPase). The measurement procedure was carried out following the steps of the kit.
2.5 Gene expression analysis
Total RNA was extracted from gill tissues using the TRIzol reagent. The residual genomic DNA was then removed using RQ1 RNase-Free DNase. A Nanodrop 2000 spectrophotometer was used to assess the concentration and purity of the extracted RNA (MPBIO, Irvine, CA, USA). In the meanwhile, 1% agarose gel electrophoresis was performed to verify the integrity of the RNA. Following purification, the Servicebio® RT First Strand cDNA Synthesis Kit (Servicebio, Wuhan, China) was used to reverse-transcribe the RNA into cDNA. After that, the produced cDNA was kept for further examination at -80°C.
To measure gene expression changes, the fluorescent real-time quantitative PCR (qPCR) approach was adopted. The NCBI database was used to get the nucleotide sequences of the target genes in L. vannamei, with β-actin serving as the internal reference gene. The qPCR primers were designed using Primer Premier 5.0 software (Supplementary Table S1). Melting curve and amplification studies were used to confirm their effectiveness and specificity. Using the SGExcel Fast SYBR qPCR Mix (Sangon Biotech, Shanghai, China) and a real-time quantitative PCR instrument (CG-02 Heal Force, Shanghai, China), the qPCR experiments were conducted. 7.5 μL of SYBR Green mix, 1.0 μL of cDNA, 0.6 μL of each primer at a concentration of 10 μmol/L, and 5.3 μL of sterile deionized water were all included in the qPCR reaction system. After an initial 30-second denaturation step at 95°C, the thermal cycling protocol consisted of 40 cycles of 5-second exposure at 95°C followed by 30-second incubation at 60°C. The relative levels of mRNA were calculated according to Livak and Schmittgen (2001) and presented as the fold change relative to the CK group.
2.6 Statistical analysis
The mean ± standard error (SE) was used to express all data. Using the LSD and Duncan post-hoc tests, a one-way analysis of variance (ANOVA) was carried out using the statistical analysis program SPSS 26.0. Significant differences were defined as those with P < 0.05.
3 Results
3.1 The histomorphological changes of the gills
In the CK group, the gill tissue was orderly arranged, and the cuticle appeared smooth (Figure 1A). In the stress phase, compared to the CK group, the gill tissue boundaries became blurred. The diaphragm widened, the subcutaneous space became narrowed, and the number of hemocytes decreased. Additionally, cavity vacuolation increased; the gill tissue was severely constricted, and the gill tissue blood vessels were deformed (Figure 1B).

Figure 1. Changes in the histomorphology of the gills of L. vannamei after CA stress and recovery. (A) The CK group; (B) The CA group; (C) The RCA group. a: cuticle; b: subcutaneous space; c: epithelial cells; d: in-gill blood vessels; e: out-gill blood vessels; f: septum; g: hemocytes; h: vesicles; 400× magnification. Notes: CK, control; CA, 5 mmol/L CA stress; RCA, recovery.
In the recovery phase, relative to the CA group, the gill boundary was clear, and the subcutaneous space was narrowed. However, the gill blood vessels remained deformed, and the diaphragm was narrowed. Moreover, the number of blood cells increased, although the cuticle showed damage (Figure 1C).
3.2 Alterations in carbohydrate metabolism-related biochemical indicators in the gills
In the stress phase, compared to the CK group, the content of the GLU exhibited no significant variation in the CA group (P > 0.05); however, the content of the PYR increased significantly (P < 0.05). The content of the LAC decreased, however, this change was not statistically significant (P > 0.05) (Figure 2).
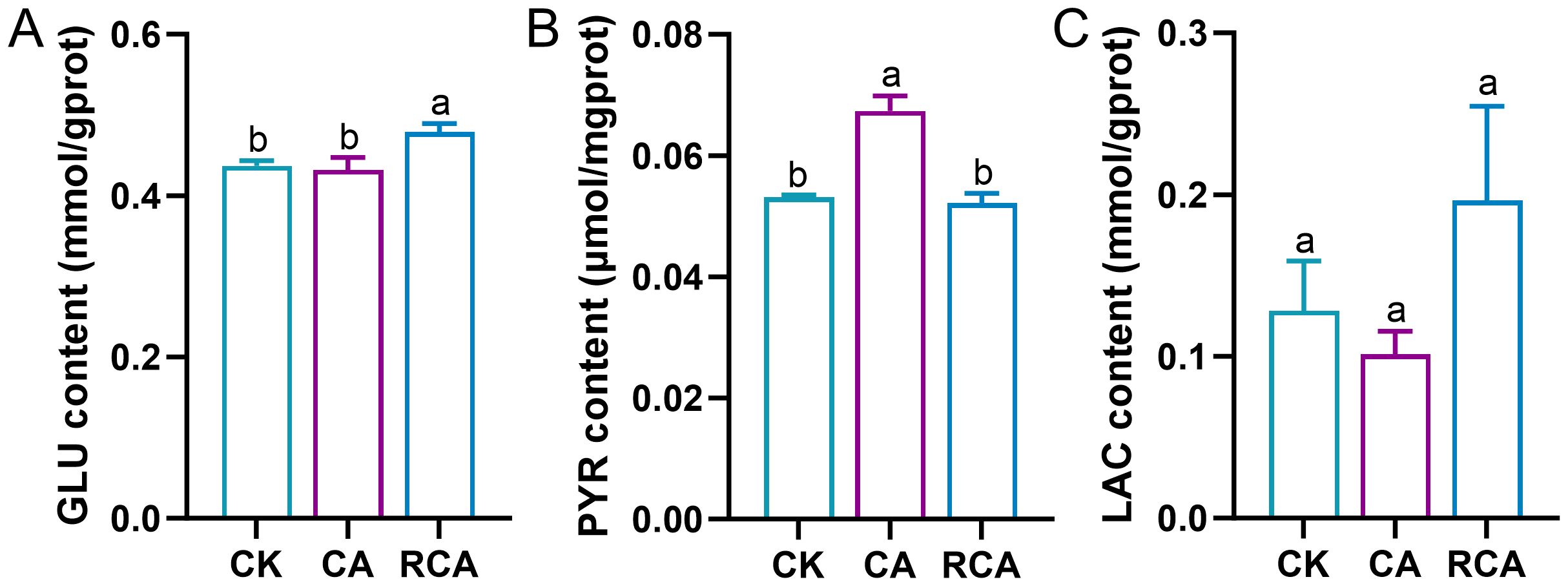
Figure 2. Changes in the carbohydrate metabolism related biochemistry indexes in the gills of L. vannamei after CA stress and recovery. (A) Glucose (GLU) content; (B) Pyruvate (PYR) content; (C) Lactate (LAC) content. Different letters on the bar show significant differences (P < 0.05) between different groups.
In the recovery phase, relative to the CA group, the content of the GLU was significantly increased in the RCA group (P < 0.05) and significantly higher than that in the CK group (P < 0.05); the content of the PYR was decreased significantly in the RCA group (P < 0.05) and returned to the level of the CK group; the content of the LAC was increased in the RCA group and was higher than that in the CK group, but there was no significant difference (P > 0.05).
3.3 Alterations in the expression levels of genes related to carbohydrate metabolism in the gills
In the stress phase, compared to the CK group, the mRNA relative expression levels of the carbohydrate metabolism related genes, such as the pyruvate kinase (pk) and pyruvate dehydrogenase (pdh) genes were increased significantly in the CA group (P < 0.05); the mRNA relative expression of the hexokinase (hk) gene was slightly increased in the CA group, but they was no significant difference (P > 0.05); while that of the lactate dehydrogenase (ldh) gene was decreased slightly in the CA group, but they was no significant difference (P > 0.05) (Figure 3).
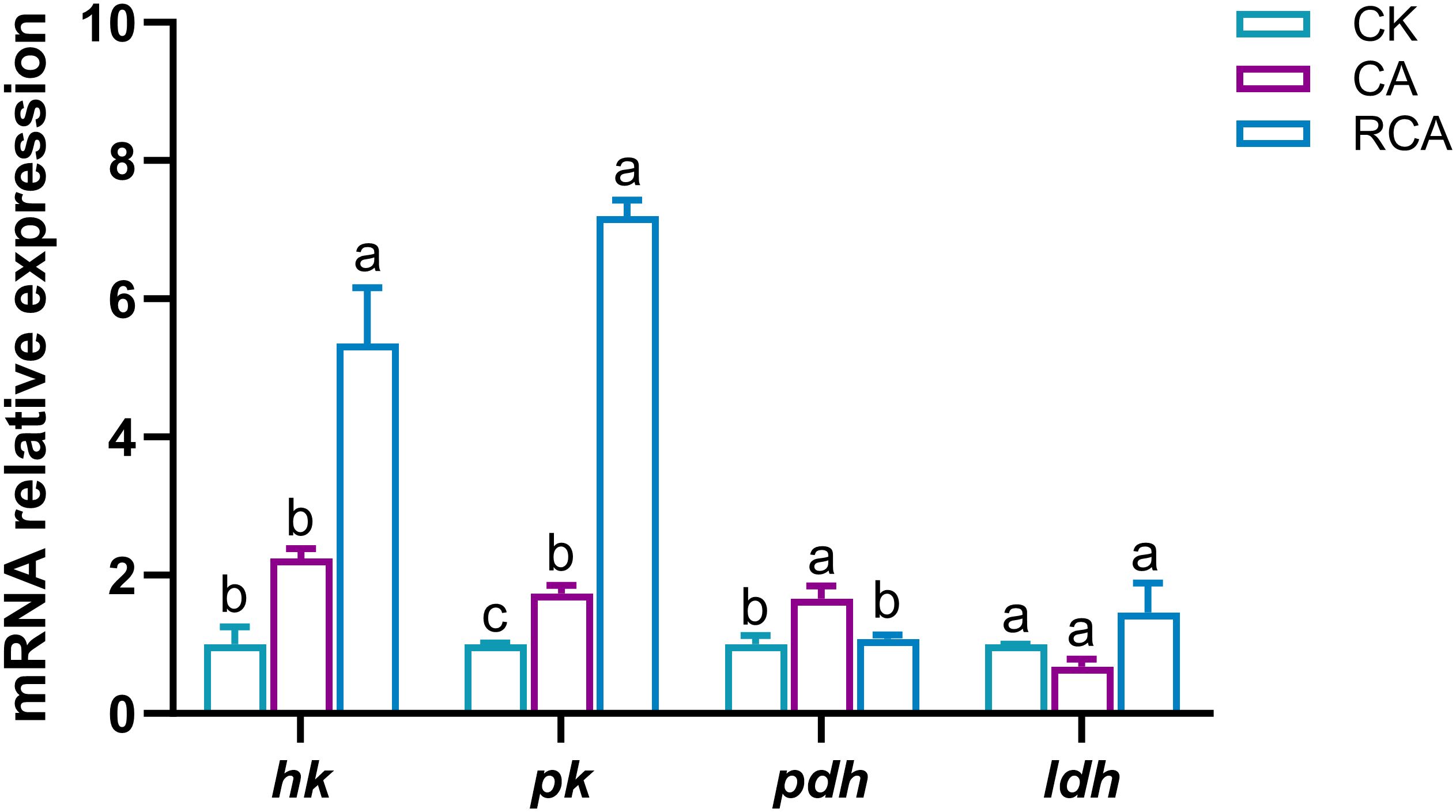
Figure 3. Changes in the mRNA relative expression levels of carbohydrate metabolism related genes in the gills of L. vannamei after CA stress and recovery. Different letters on the bar show significant differences (P < 0.05) between different groups. Hexokinase (hk); pyruvate kinase (pk); pyruvate dehydrogenase (pdh); lactate dehydrogenase (ldh).
In the recovery phase, relative to the CA group, the mRNA relative expression levels of the pk and hk genes were increased significantly in the RCA group (P < 0.05) and higher than that in the CK group (P < 0.05); the mRNA relative expression of the pdh gene was decreased significantly in the RCA group (P < 0.05) and returned to the level of the CK group; the mRNA relative expression of the ldh gene was increased slightly in the RCA group, but they was no significant difference (P > 0.05) and returned to the level of the CK group (P > 0.05).
3.4 Alterations in lipid metabolism-related indices in the gills
In the stress phase, compared to the CK group, in the CA group, the content of the TG and the relative mRNA expression level of the adenosine 5’-monophosphate (AMP)-activated protein kinase (ampk) gene was slightly increased, however, no significant differences were noted (P > 0.05) (Figures 4A, B); the mRNA relative expressions of the cholesterol regulatory element binding protein (srebp), acetyl-CoA carboxylase (acc), and fatty acid synthetase (fas) genes were significantly increased in the CA group (P < 0.05) (Figure 4B).
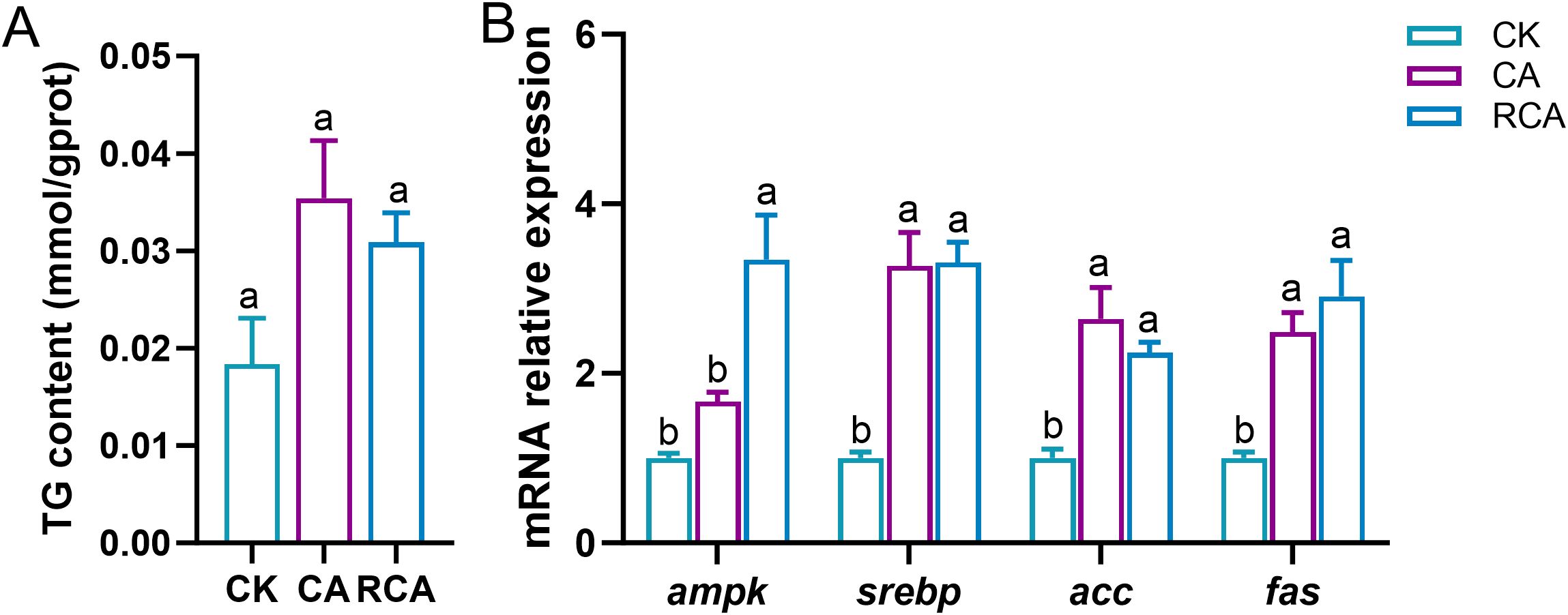
Figure 4. Changes in the lipid metabolism related indices in the gills of L. vannamei after CA stress and recovery. (A) Triglyceride (TG) content; (B) The mRNA relative expression levels of lipid metabolism related gene expression. Different letters on the bar show significant differences (P < 0.05) between different groups. Adenosine 5’-monophosphate (AMP)-activated protein kinase (ampk); cholesterol regulatory element binding protein (srebp); acetyl-CoA carboxylase (acc); fatty acid synthetase (fas).
In the recovery phase, relative to the CA group, in the RCA group, the content of TG showed no significant change and stayed higher than in the CK group, but there was no significant difference (P > 0.05). However, a significant difference (P < 0.05) was observed in the relative mRNA expression level of the ampk gene between the RCA and CK groups. While the RCA group showed no significant change in the srebp, acc, and fas gene expression levels (P > 0.05), these were still significantly higher compared to the CK group (P < 0.05).
3.5 Alterations in the expression levels of genes related to tricarboxylic acid cycle in the gills
In the stress phase, compared to the CK group, the mRNA relative expression levels of the malate dehydrogenase (mdh) and isocitrate dehydrogenase (idh) genes were decreased significantly in the CA group (P < 0.05), while the mRNA relative expressions of the citrate synthase 1 (cs), oxoglutarate dehydrogenase (odh), and succinate dehydrogenase (sdh) genes were increased significantly (P < 0.05); the mRNA relative expression level of the fumarase (fh) gene was decreased slightly in the CA group, but there was no significant difference (P > 0.05) (Figure 5).
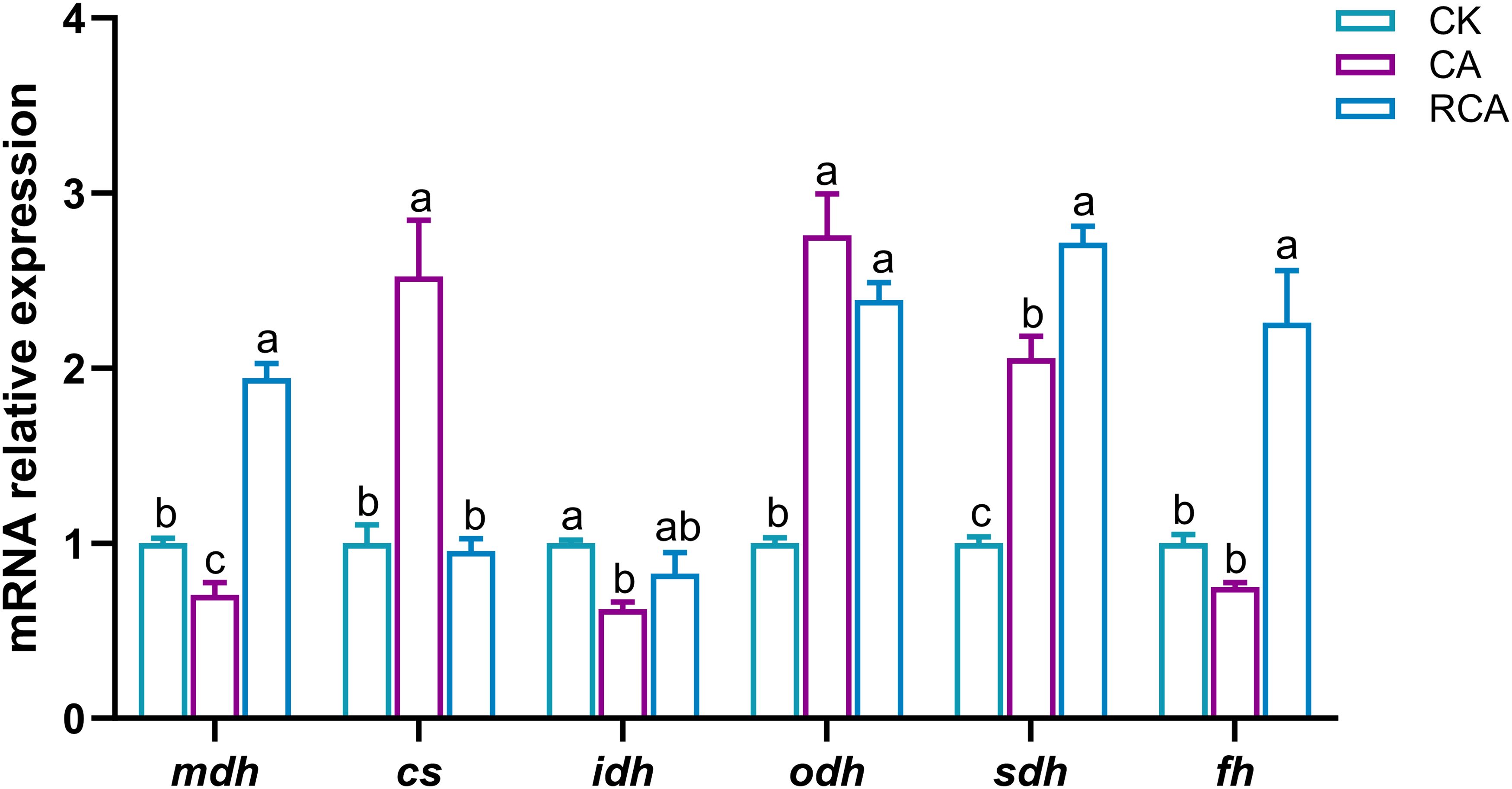
Figure 5. Changes in the mRNA relative expression levels of tricarboxylic acid cycle related genes in the gills of L. vannamei after CA stress and recovery. Different letters on the bar show significant differences (P < 0.05) between different groups. Malate dehydrogenase (mdh); citrate synthase 1 (cs); isocitrate dehydrogenase (idh); oxoglutarate dehydrogenase (odh); succinate dehydrogenase (sdh); fumarase (fh).
In the recovery phase, relative to the CA group, the mRNA relative expression levels of the mdh, sdh, and fh genes were increased significantly in the RCA group, and higher than those in the CK group (P < 0.05); the mRNA relative expression of the cs gene was decreased significantly in the RCA group (P < 0.05), and returned to the level of the CK group; the mRNA relative expression of the idh gene was increased slightly in the RCA group and returned to the level of the CK group, but there was no significant difference (P > 0.05); the mRNA relative expression of the odh gene was not obviously change in the RCA group, but it was still significantly higher than that in the CK group (P < 0.05).
3.6 Alterations in the electron transport chain related indexes in the gills
In the stress phase, compared to the CK group, the mRNA relative expression levels of the NADH dehydrogenase (ndh), cytochrome C (cytc), cytochrome oxidase I (coi), cytochrome c oxidase (cco), and ATP synthase (atph) genes were significantly increased in the CA group (P < 0.05); the activity of ATPase showed an upward trend in the CA group, but there was no significant difference (P > 0.05) (Figure 6).
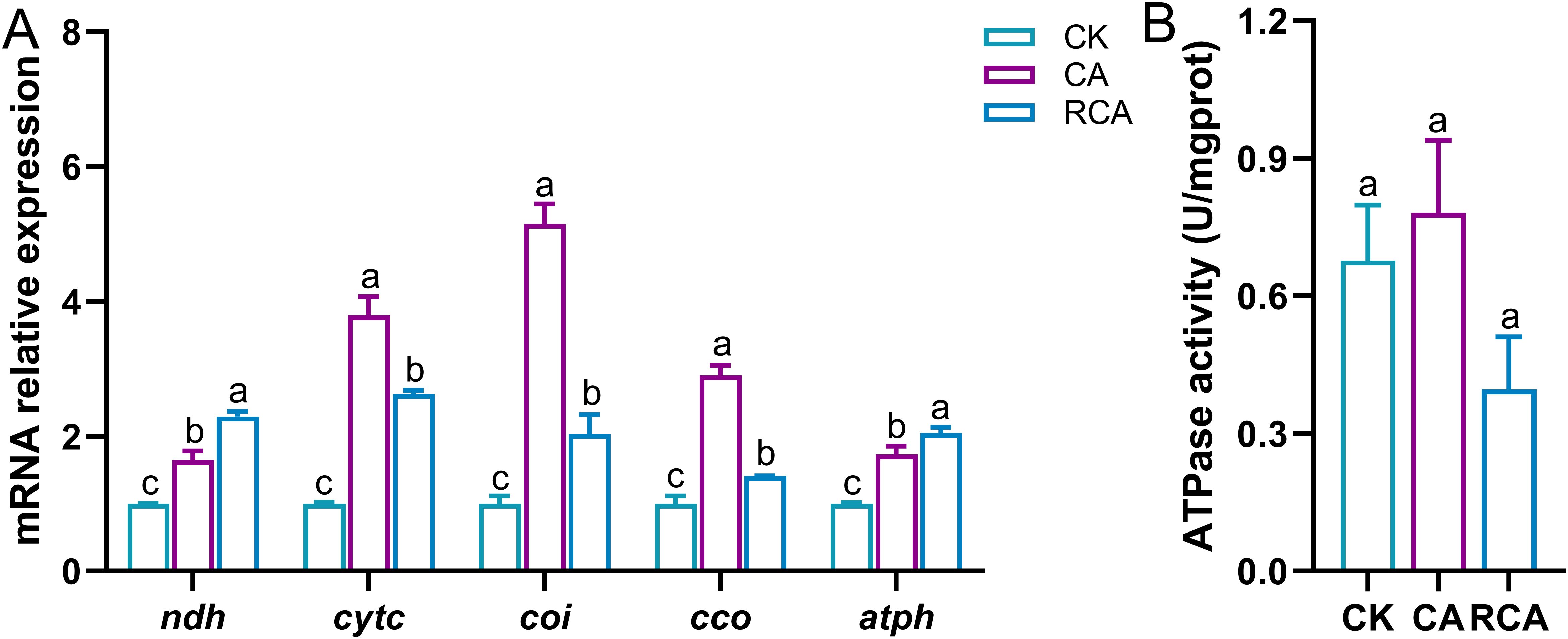
Figure 6. Changes in the electron transport chain related indexes in the gills of L. vannamei after CA stress and recovery. (A) The mRNA relative expression levels of electron transport chain-related genes; (B) Total ATPase (ATPase) activity. Different letters on the bar show significant differences (P < 0.05) between different groups. NADH dehydrogenase (ndh); cytochrome C (cytc); cytochrome oxidase I (coi); cytochrome c oxidase (cco); ATP synthase (atph).
In the recovery phase, relative to the CA group, the activity of the ATPase was decreased slightly in the RCA group and lower than that in the CK group, but there was no significant difference (P > 0.05). The relative mRNA expression levels of the ndh and atph genes increased further, surpassing those in the CK group (P < 0.05). The relative mRNA expressions of the cytc, coi, and cco genes were decreased significantly in the RCA group (P < 0.05), but they were still significantly higher than those in the CK group (P < 0.05).
4 Discussion
Around the world, saline-alkaline water is common, and one of its essential components is CA. Shrimp metabolic pathways may be impacted by CA stress, which may potentially result in mortality (Yao et al., 2010b; Song et al., 2024; Zhang et al., 2024b, c; Duan et al., 2023). It was found that the elevated level of the LAC in the hepatopancreas of L. vannamei indicated that the organism may use anaerobic respiration and metabolism to generate energy to cope with CA stress (Duan et al., 2023). It was reported that acute alkalinity stress damaged the gill structure of L. vannamei (Zhang et al., 2024b). Similar in the present study, under low-salinity conditions, the morphological structure of the gills in L. vannamei was affected by CA stress. In the recovery phase, the gill of shrimp began to repair itself; although there were positive changes, it still did not fully return to normal state. The histomorphological changes of the gills would further affect its physiological homeostasis. Cell stress in organisms is a highly energy-consuming process. However, the effects of CA stress on energy metabolism in the gills of L. vannamei are not well understood. Therefore, we carried out a thorough analysis to look at the changes in energy metabolism in the gills of L. vannamei following CA stress and during the recovery phase, assessing these changes across multiple dimensions of biological function.
Energy metabolism is a process in which nutrients in the organism are catalyzed to release energy through various enzymes. Glycolysis functions as the main energy provider for the physiological activities of organisms (Chen et al., 2023; Jenkins et al., 2011). The hk starts glycolysis by phosphorylating hexose, and the pk catalyzes the last step of glycolysis, producing PYR and ATP, while the pdh is capable of catalyzing the conversion of PYR to acetyl-CoA through decarboxylation (Godoy-Lugo et al., 2019; Li et al., 2024). The ldh is an important coenzyme in carbohydrate metabolism that can catalyze the conversion of PYR to LAC (Shan et al., 2019). It was found that the carbohydrate catabolism of L. vannamei was enhanced under calcium stress (Zhang et al., 2024b). In the present study, after CA stress, the increased PYR content and higher expression of the hk, pk, and pdh genes indicated activation of glycolysis. Specifically, the up-regulation of the hk and pk accelerated the conversion of GLU to PYR, while the pdh promoted the conversion of PYR to acetyl coenzyme A, thus producing Adenosine triphosphate (ATP), with the PYR content being directed towards alternative metabolic pathways instead of being converted to the LAC content. This redirection likely provided the energy necessary for shrimp to withstand CA stress. The activation of glycolysis allowed for a rapid supply of ATP, which was crucial for maintaining cellular functions and supporting stress responses. In the recovery phase, the content of GLU and the expression of the hk and pk genes higher than in the CK group, indicating that glycolysis continued to provide energy, while the content of PYR and LAC and the expression level of the pdh gene recovered to those of the CK group, indicating that the gluconeogenesis pathway was relatively stable in the recovery phase, which was helpful to maintain the balance between the gluconeogenesis pathway and the glycolytic metabolism pathway. This balance ensured that energy production remains sufficient to support cellular repair and normal physiological functions. It has been found that the over-activation of glycolysis may lead to decrease in energy utilization efficiency and affect the growth and immune response of shrimp (Cruz-Moreno et al., 2024).
Lipid metabolism can supply energy for physiological activities, thereby facilitating adaptation to environmental stress (Lee et al., 2018; Qin et al., 2023; Duan et al., 2024b). The ampk can promote fatty acid oxidation and facilitate ATP production (Krishan et al., 2014). The enrichment of the ampk signaling pathway in E. carinicauda after CA stress for 24 h is helpful to maintain energy stability (Qin et al., 2023). In the present study, after CA stress, the slightly increased expression of the ampk gene suggested that the function of fatty acid oxidative catabolism was starting to activate. In the recovery phase, the expression level of the ampk gene was upregulated and exceeded the CK level, indicated that fatty acid oxidative catabolism was activated and had not yet returned to a normal state. This sustained activation suggested that lipid metabolism continued to contribute to the energy supply of the shrimp during recovery, supporting their ability to cope with prolonged stress effects. The TG is mainly used to store lipid energy (Lu et al., 2023). The srebp, acc, and fas are key regulatory enzymes in fatty acid biosynthesis (Shi et al., 2021). Under acute alkalinity stress, the lipid anabolism of L. vannamei was found to be suppressed (Zhang et al., 2024b). CA affects differentially expressed genes related to intestinal lipid metabolism in L. vannamei (Song et al., 2024). In the present study, after CA stress, the increase of TG content and the expression levels of genes linked to lipid metabolism, including srebp, acc, and fas, suggested that CA stress might promote the expression of the acc and fas by activating SREBP signaling pathway, thus enhancing fatty acid synthesis. In the recovery phase, the TG content and the expression levels of srebp, acc and fas genes were still higher than those in the CK group, indicating that the lipid anabolic activity of the organism was still functioning and had not returned to normal. Excessive activation of lipid metabolism would lead to abnormal accumulation of lipid in shrimp, thus disrupting its normal physiological function.
The TCA cycle is the final metabolic pathway of the nutrients and the hub of substance metabolism, which provides energy for life activities through the oxidation of acetyl-CoA (Arnold and Finley, 2023). The mdh, cs, idh, sdh, and fh proteins are key enzymes of the TCA cycle (Nunes-Nesi et al., 2013; Nan et al., 2024). It was found that the malic acid level of Eriocheir sinensis increased under CA stress (Yang et al., 2019). Similarly, CA stress inhibited the TCA cycle in the gills of E. sinensis (Wang et al., 2024). In addition, the TCA cycle was enhanced in the gill of L. vannamei under CA stress (Zhang et al., 2024b). In the present study, after CA stress, the mdh and idh genes had lower expression levels, while the cs, odh, and sdh genes displayed higher expression levels. Down-regulation of key genes such as mdh and idh would reduce the efficiency of circulation and lead to the decrease of ATP production. This implied that CA stress disrupted the functional balance of the TCA cycle in the gills of shrimp, thereby affecting the energy source required to withstand CA stress, insufficient energy supply would affect the growth, immune response and overall health of shrimp. It was found that the expression changes of key enzymes in TCA cycle can affect the metabolic pattern and energy production of shrimp (Duan et al., 2024b). In addition, the disorder of TCA cycle may also lead to the increase of intracellular oxidative stress levels, further weakening the antioxidant capacity and immune function of shrimp (Duan et al., 2024a). In the recovery phase, the relative expression levels of the cs and idh genes recovered to the level of the CK group, while the levels of the mdh, odh, sdh, and fh genes were still significantly higher than those of the CK group, indicating that the function of the TCA cycle in the gills recovered to some extent, but the whole was still not restored to a normal state.
Electron transfer is a key step in converting energy to support cellular activity. The electron donors and acceptors form electron transport chains that pass through redox potentials (Yu et al., 2024). In the electron transport chain, ndh acts as the starting enzyme, and cco serves as the final enzyme (Maclean et al., 2022); the atph is capable of catalyzing ATP synthesis (Fernández-Vizarra and Ugalde, 2022); two important proteins that act as electron transporters are coi and cytc (Fernandez-Vizarra et al., 2022). In the present study, after CA stress, the up-regulation of the ndh and cco genes after CA stress indicated that the initiation and termination steps of electron transport chain may be enhanced and the electron flow may be increased. The high expression of the cytc, coi, and atph genes and slowly increasing ATPase activity further ensured effective electron transfer, thus activating the electron transfer chain of gill to generate energy to cope with CA stress. In the recovery phase, the expression levels of these genes remained noticeably higher than those of the CK group, while ATPase activity was downregulated. This indicated that the electron transport chain in the shrimp gills was still highly active and did not normalize. This might be due to the negative effects caused by CA stress not having been completely eliminated and the body still needing an energy supply to defend against stress. In the future, we can continue to study extending the recovery period and monitoring key physiological and metabolic indicators for a longer time to determine the time required for complete recovery.
5 Conclusions
This study revealed that CA stress could affect the histomorphology structure and the energy metabolism homeostasis of the gills of L. vannamei and could not be completely recovered to the normal state within a short time. The specific performance was as follows (Figure 7): In the process of CA stress, the activation of carbohydrate metabolism and fatty acid synthesis in the gills of shrimp, along with the stimulation of the electron transport chain function, facilitated the generation of energy substances. Conversely, CA stress disrupted the equilibrium of the TCA cycle function in the gills, thus interfering with energy metabolism. After relieving stress, although some indexes could be restored to the control level, glycolysis, the TCA cycle, lipid metabolism, and the electron transfer chain function had not returned to a normal state, indicating that energy metabolism was still in a state of high expression. The results showed that energy metabolism played an important role in the response of shrimp gill tissue to CA stress.
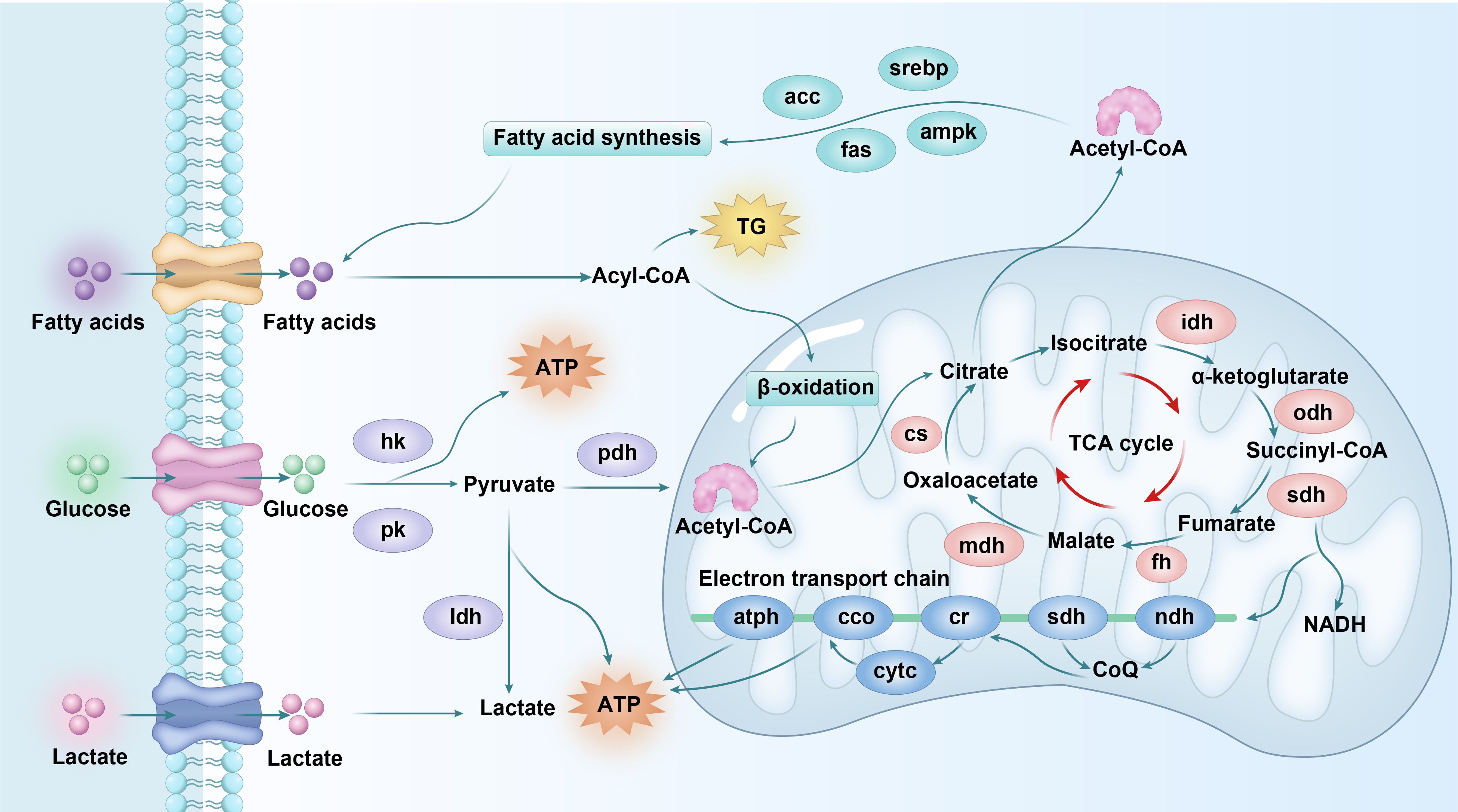
Figure 7. The deduced possible mechanism of the detrimental effects of CA stress on the energy metabolism homeostasis in the gills of L. vannamei. Cr, cytochrome C reductase.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Animal Care and Use Committee of the South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences (nhdf2023-18). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MX: Data curation, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YN: Data curation, Investigation, Methodology, Software, Writing – review & editing. JL: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing. YW: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – review & editing. RZ: Data curation, Investigation, Methodology, Software, Visualization, Writing – review & editing. YD: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Key R & D Program of China (2023YFD2401001); Central Public-interest Scientific Institution Basal Research Fund, South China Sea Fisheries Research Institute, CAFS (2022RC01); Guangzhou Science and Technology Plan Project (2025D04J0016); the Foundation of State Key Laboratory of Mariculture Biobreeding and Sustainable Goods (BRESG202404); Guangdong Basic and Applied Basic Research Foundation (2024A1515030047); Agricultural Research Outstanding Talents Training Program (13210308); Hainan Provincial Natural Science Foundation of China (323MS126); Central Public-interest Scientific Institution Basal Research Fund, CAFS (2023TD97).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1571396/full#supplementary-material
References
Arnold P. K. and Finley L. W. (2023). Regulation and function of the mammalian tricarboxylic acid cycle. J. Biol. Chem. 299, 102838. doi: 10.1016/j.jbc.2022.102838
Chen Z. Z., Zhu T., Lei C. X., Jiang P., Du J. X., Zhu J. J., et al. (2023). Effects of high-sugar diet on growth and liver glucose metabolism of Ctenopharyngodon idellus. South China Fish. Sci. 5, 75–85. doi: 10.12131/20230020
Cruz-Moreno D. G., Hernández-Aguirre L. E., Peregrino-Uriarte A. B., Leyva-Carrillo L., Gómez-Jiménez S., Contreras-Vergara C., et al. (2024). Changes of glycolysis and gluconeogenesis key enzymes in the muscle of the shrimp Penaeus vannamei in response to hypoxia and reoxygenation. J. Exp. Mar. Biol. Ecol. 580, 152052. doi: 10.1016/j.jembe.2024.152052
Duan Y., Wang Y., Dong H., Li H., Liu Q., Zhang J., et al. (2018). Physiological and immune response in the gills of Litopenaeus vannamei exposed to acute sulfide stress. Fish Shellf. Immun. 81, 161–167. doi: 10.1016/j.fsi.2018.07.018
Duan Y. F., Xing Y. F., Zhu X. Y., Li H., Wang Y., and Nan Y. X. (2023). Integration of transcriptomic and metabolomic reveals carbonate alkalinity stress responses in the hepatopancreas of Litopenaeus vannamei. Aquat. Toxicol. 260, 106569. doi: 10.1016/j.aquatox.2023.106569
Duan Y., Xiong D., Wang Y., Dong H., Huang J., and Zhang J. (2020). Effects of Microcystis aeruginosa and microcystin-LR on intestinal histology, immune response, and microbial community in Litopenaeus vannamei. Environ. Pollut. 265, 114774. doi: 10.1016/j.envpol.2020.114774
Duan Y., Zhong G., Nan Y., Yang Y., Xiao M., and Li H. (2024a). Effects of nitrite stress on the antioxidant, immunity, energy metabolism, and microbial community status in the intestine of Litopenaeus vannamei. Antioxidants 13, 1318. doi: 10.3390/antiox13111318
Duan Y., Zhong G., Xiao M., Yang Y., Wang Y., and Nan Y. (2024b). Integrated physiological, energy metabolism, and metabonomic responses indicate the stress response in the hepatopancreas of Litopenaeus vannamei to nitrite stress. Aquat. Toxicol. 277, 107164. doi: 10.1016/j.aquatox.2024.107164
Fernandez-Vizarra E., Lopez-Calcerrada S., Sierra-Magro A., Pérez-Pérez R., Formosa L. E., Hock D. H., et al. (2022). Two independent respiratory chains adapt OXPHOS performance to glycolytic switch. Cell Metab. 34, 1792–1808. doi: 10.1016/j.cmet.2022.09.005
Fernández-Vizarra E. and Ugalde C. (2022). Cooperative assembly of the mitochondrial respiratory chain. Trends Biochem. Sci. 47, 999–1008. doi: 10.1016/j.tibs.2022.07.005
Gao T., Wang Q., Sun H., Liu Y., Li J., and He Y. (2024). Physiological adaptation of Fenneropenaeus chinensis in response to saline-alkaline stress revealed by a combined proteomics and metabolomics method. Biology 13, 488. doi: 10.3390/biology13070488
Godoy-Lugo J. A., Miranda-Cruz M. M., Rosas-Rodríguez J. A., Adan-Bante N. P., Icedo-García R., and Soñanez-Organis J. G. (2019). Hypoxia inducible factor-1 regulates WSSV-induced glycolytic genes in the white shrimp Litopenaeus vannamei. Fish Shellf. Immun. 92, 165–171. doi: 10.1016/j.fsi.2019.05.040
Huang M. X., Dong Y. F., Zhang Y., Chen Q. S., Xie J., Xu C., et al. (2019). Growth and lipidomic responses of juvenile pacific white shrimp Litopenaeus vannamei to low salinity. Front. Physiol. 10, 1087. doi: 10.3389/fphys.2019.01087
Jenkins C. M., Yang J., Sims H. F., and Gross R. W. (2011). Reversible high affinity inhibition of phosphofructokinase-1 by acyl-CoA: a mechanism integrating glycolytic flux with lipid metabolism. J. Biol. Chem. 286, 11937–11950. doi: 10.1074/jbc.M110.203661
Krishan S., Richardson D. R., and Sahni S. (2014). Adenosine monophosphate activated kinase (AMPK) and its key role in catabolism: structure, regulation, biological activity and pharmacological activation. Mol. Pharmacol. 87, 363–377. doi: 10.1124/mol.114.095810
Lee M. C., Park J. C., and Lee J. S. (2018). Effects of environmental stressors on lipid metabolism in aquatic invertebrates. Aquat. Toxicol. 200, 83–92. doi: 10.1016/j.aquatox.2018.04.016
Li J. T., Zhang Y. Q., Zhang H., Liu C., Qiu X. L., Chen M., et al. (2024). Effects of density stress on swimming behavior and muscle energy metabolism in largemouth bass, Peromyscus nigra. South China Fish. Sci. 2, 102–110. doi: 10.12131/20230176
Liu G., Hu X. J., Su H. C., Xu W. J., Xu Y., Wen G. L., et al. (2024). Antagonism of Lactobacillus acidophilus against three Vibrio species and its influence on gut microbiota of Litopenaeus vannamei. South China Fish. Sci. 2, 83–91. doi: 10.12131/20230191
Liu Y. J., Yao M. Z., Li S. W., Wei X. F., Ding L., Han S. C., et al. (2022). Integrated application of multi-omics approach and biochemical assays provides insights into physiological responses to saline-alkaline stress in the gills of crucian carp (Carassius auratus). Sci. Total. Environ. 822, 153622. doi: 10.1016/j.scitotenv.2022.153622
Livak K. J. and Schmittgen T. D. (2001). Analysis of relative gene expression data using realtime quantitative PCR and the 2-ΔΔC(T) method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lu M. X., Liu R. Z., Chen Z. F., Su C., and Pan L. Q. (2023). Effects of dietary dihydromyricetin on growth performance, antioxidant capacity, immune response and intestinal microbiota of shrimp (Litopenaeus vannamei). Fish Shellf. Immun. 142, 109086. doi: 10.1016/j.fsi.2023.109086
Maclean A. E., Hayward J. A., Huet D., van Dooren G. G., and Sheiner L. (2022). The mystery of massive mitochondrial complexes: the apicomplexan respiratory chain. Trends Parasitol. 38, 1041–1052. doi: 10.1016/j.pt.2022.09.008
Nan Y. X., Xiao M., Duan Y. F., and Yang Y. K. (2024). Toxicity of ammonia stress on the physiological homeostasis in the gills of Litopenaeus vannamei under seawater and low-salinity conditions. Biology 13, 281. doi: 10.3390/biology13040281
Nunes-Nesi A., Araújo W. L., Obata T., and Fernie A. R. (2013). Regulation of the mitochondrial tricarboxylic acid cycle. Curr. Opin. Plant Biol. 16, 335–343. doi: 10.1016/j.pbi.2013.01.004
Qin Z., Ge Q., Wang J., Li M., Liu P., Li J., et al. (2021). Comparative transcriptomic and proteomic analysis of Exopalaemon carinicauda in response to alkalinity stress. Front. Mar. Sci. 8, 759923. doi: 10.3389/fmars.2021.759923
Qin Z., Ge Q. Q., Wang J. J., Li M. D., Zhang X. H., Li J., et al. (2023). Metabolomic responses based on transcriptome of the hepatopancreas in Exopalaemon carinicauda under carbonate alkalinity stress. Ecotox. Environ. Safe. 268, 115723. doi: 10.1016/j.ecoenv.2023.115723
Shan H. W., Geng Z. X., Ma S., and Wang T. (2019). Comparative study of the key enzymes and biochemical substances involved in the energy metabolism of Pacific white shrimp, Litopenaeus vannamei, with different ammonia-N tolerances. Comp. Biochem. Phys. C. 221, 73–81. doi: 10.1016/j.cbpc.2019.04.001
Shi B., Lu J., Hu X., Betancor M. B., Zhao M., Tocher D. R., et al. (2021). Dietary copper improves growth and regulates energy generation by mediating lipolysis and autophagy in hepatopancreas of Pacific white shrimp (Litopenaeus vannamei). Aquaculture 537, 736505. doi: 10.1016/j.aquaculture.2021.736505
Shi X., Zhang R., Liu Z., Sun J., Li L., Zhao G., et al. (2023). Combined analysis of mRNA and miRNA reveals the mechanism of pacific white shrimp (Litopenaeus vannamei) under acute alkalinity stress. PloS One 18, e0290157. doi: 10.1371/journal.pone.0290157
Shi X., Zhang R. Q., Liu Z., Zhao G. Y., Guo J. T., Mao X., et al. (2024). Alternative splicing reveals acute stress response of Litopenaeus vannamei at high alkalinity. Mar. Biotechnol. 26, 103–115. doi: 10.1007/s10126-023-10281-w
Song Z. L., Li K., and Li K. J. (2024). Integrated characterizations of intestinal bacteria and transcriptomics revealed the acute stress response to carbonate alkalinity in white shrimp Penaeus vannamei. Fish Shellf. Immun. 146, 109420. doi: 10.1016/j.fsi.2024.109420
Sumner M. E. and Naidu R. (1998). Sodic soils: distribution, properties, management and environmental consequences. Oxford Univ. Press 32, 1069–1093. doi: 10.1071/SR9930681
Wang C., An L., Dong X. S., Xu X., Feng X. Y., Wang Z. Z., et al. (2024). The tricarboxylic acid cycle is inhibited under acute stress from carbonate alkalinity in the gills of Eriocheir sinensis. Comp. Biochem. Phys. D. 51, 101245. doi: 10.1016/j.cbd.2024.101245
Xiao M., Nan Y., Yang Y., Li H., and Duan Y. (2024). Changes in physiological homeostasis in the gills of Litopenaeus vannamei under carbonate alkalinity stress and recovery conditions. Fishes 9, 463. doi: 10.3390/fishes9110463
Xu H. X., Zhu H. G., Zhang J. M., Zhu M., Wang H. H., and Chen J. H. (2024). Effects of carbonate alkalinity on embryonic development and larval vitality of Paraloach macrolepis. South China Fish. Sci. 2, 56–62. doi: 10.12131/20230149
Yang F., Li X., Sun L., and Yang X. (2006). Adaptability of Litopenaeus vannamei to saline-alkali water in west Jilin Province. J. Appl. Ecol. 17, 315–319. doi: 10.13287/j.1001-9332.2006.0064
Yang F. Y., Sun L. M., and Yang X. Q. (2004). Toxicity of carbonate alkalinity to Penaeus vannamei juveniles. J. Fish. China 23, 3–6. doi: 10.16378/j.cnki.1003-1111.2004.09.001
Yang Z., Zhou J., Wei B., Cheng Y., Zhang L., and Zhen X. (2019). Comparative transcriptome analysis reveals osmotic-regulated genes in the gill of Chinese mitten crab (Eriocheir sinensis). PloS One 14, e0210469. doi: 10.1371/journal.pone.0210469
Yao Z. L., Lai Q. F., Zhou K., Rizalita R. E., and Wang H. (2010a). Developmental biology of medaka fish (Oryzias latipes) exposed to alkalinity stress. J. Appl. Ichthyol. 26, 397–402. doi: 10.1111/j.1439-0426.2009.01360.x
Yao Z. L., Wang H., Zhou K., Ying C., and Lai Q. (2010b). Effects of water carbonate alkalinity and pH on survival rate of post-larval Litopenaeus vannamei. Chin. J. Ecol. 29, 945–950. doi: 10.13292/j.1000-4890.2010.0160
Yu L., Min Z. Z., Liu M. H., Xin Y. Y., Liu A. K., Kuang J., et al. (2024). A cytochrome c551 mediates the cyclic electron transport chain of the anoxygenic phototrophic bacterium Roseiflexus castenholzii. Plant Commun. 5, 100715. doi: 10.1016/j.xplc.2023.100715
Zhang C., Guo C. Y., Shu K. H., Xu S. L., and Wang D. L. (2024a). Comparative analysis of the growth performance, vitality, body chemical composition and economic efficiency of the main cultivated strains of Pacific white shrimp (Litopenaeus vannamei) in coastal areas of China. Aquaculture 587, 740856. doi: 10.1016/j.aquaculture.2024.740856
Zhang R. Q., Shi X., Guo J. T., Mao X., and Fan B. Y. (2024b). Acute stress response in gill of Pacific white shrimp Litopenaeus vannamei to high alkalinity. Aquaculture 586, 740766. doi: 10.1016/j.aquaculture.2024.740766
Zhang R. Q., Shi X., Guo J. T., Mao X., and Fan B. Y. (2024c). Acute stress response in hepatopancreas of Pacific white shrimp Litopenaeus vannamei to high alkalinity. Aquacult. Rep. 35, 101981. doi: 10.1016/j.aqrep.2024.101981
Zhang R. Q., Shi X., Liu Z., Sun J., Sun T. Z., and Lei M. Q. (2023). Histological, physiological and transcriptomic analysis reveal the acute alkalinity stress of the gill and hepatopancreas of Litopenaeus vannamei. Mar. Biotechnol. 25, 588–602. doi: 10.1007/s10126-023-10228-1
Keywords: saline-alkali, alkalinity, shrimp, gills, energy metabolism
Citation: Xiao M, Nan Y, Li J, Wang Y, Zhu R and Duan Y (2025) Changes in the energy metabolism of the gills of Litopenaeus vannamei under carbonate alkalinity stress and recovery conditions. Front. Mar. Sci. 12:1571396. doi: 10.3389/fmars.2025.1571396
Received: 05 February 2025; Accepted: 08 May 2025;
Published: 30 May 2025.
Edited by:
Yuan Fang, Jiangsu Agri-animal Husbandry Vocational College, ChinaReviewed by:
Xianyong Bu, Ocean University of China, ChinaSofia Priyadarsani Das, National Taiwan Ocean University, Taiwan
Copyright © 2025 Xiao, Nan, Li, Wang, Zhu and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yafei Duan, ZHVhbnlhZmVpODlAMTYzLmNvbQ==
 Meng Xiao1,2
Meng Xiao1,2 Jitao Li
Jitao Li Yun Wang
Yun Wang Yafei Duan
Yafei Duan