- 1CAS Key Laboratory of Tropical Marine Bio-Resources and Ecology, Guangdong Provincial Key Laboratory of Applied Marine Biology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, China
- 2College of Marine Sciences, University of Chinese Academy of Sciences, Beijing, China
- 3Xisha Marine Environmental National Observation and Research Station, Chinese Academy of Sciences, Sansha, China
- 4Tropical Marine Biological Research Station in Hainan, Chinese Academy of Sciences and Hainan Key Laboratory of Tropical Marine Biotechnology, Sanya, China
Introduction: Coral restoration efforts increasingly focus on enhancing larval settlement and post-settlement survival. However, the species-specific efficacy of different settlement inducers remains inadequately understood, limiting optimization of restoration protocols.
Methods: This study systematically assessed the effectiveness of three settlement inducers—Crustose coralline algae (CCA), Chemical (CaCl2), and Microbial (Metabacillus sp. cB07)—across seven coral species, including both brooders and broadcast spawners. Larvae were exposed to gradient concentrations of each inducer to determine optimal concentrations and treatment durations. Effects on larval survivorship, metamorphosis, and settlement were measured. Post-settlement recruits treated with optimized procedures were further evaluated for metabolic rates, morphology, survival, and growth.
Results: Optimal inducer concentrations and treatment durations varied significantly among coral species, with CaCl2 (10–60 mmol/L) and cB07 (3 × 106–3 × 107 cfu/mL) showing broad-spectrum activity comparable to CCA. CCA induced the highest settlement rates (43.3%–93.3%) within 1–2 days, CaCl2 showed moderate induction (23.3%–60.3%) within 0.5–4 days, and cB07 exhibited similar efficacy (26.7%–60.0%) within 2–4 days. Biological effects differed: CaCl2 accelerated metamorphosis but lowered survival in sensitive species, while cB07 delayed metamorphosis and suppressed respiratory rates, indicating higher toxicity. Post-settlement, recruits induced by CCA and CaCl2 had higher survival and calcification rates than those induced by cB07.
Discussion: These findings underscore the necessity of tailoring settlement inducer protocols to the species-specific life histories and physiological responses of corals. Integrating metabolic and ecological insights offers practical guidelines to enhance coral restoration success amid growing environmental pressures.
1 Introduction
Coral reefs, widely acknowledged as among the most diverse and productive ecosystems on the planet (Mumby et al., 2008; Brandl et al., 2019), are currently facing persistent challenges driven by anthropogenic factors, notably climate change (Fezzi et al., 2023), pollution (Nalley et al., 2021), and overfishing (Milne et al., 2022). Consequently, the degradation of coral reefs has intensified, resulting in a significant loss of biodiversity and ecosystem services (Bellwood et al., 2004). In response to these challenges, coral restoration efforts have been initiated to mitigate the detrimental effects and restore ecosystem vitality (Boström-Einarsson et al., 2020; Suggett and van Oppen, 2022). A fundamental aspect of coral restoration is understanding the factors influencing coral larval settlement, a crucial phase in the coral life cycle for successful reef rehabilitation (Barton et al., 2017; Pollock et al., 2017; Omori, 2019). Settlement inducers play a critical role in this restoration process, as they activate or enhance the settlement of coral larvae on appropriate substrates (Gómez-Lemos and García-Urueña, 2022; Rodd et al., 2022).
The literature on coral larval settlement has explored various natural and artificial cues (Barton et al., 2017), including crustose coralline algae (CCA) (Grasso et al., 2011; Gómez-Lemos and García-Urueña, 2022), inorganic material (Levenstein et al., 2022), calcium chloride (Yang et al., 2022), tetrabromopyrrole (TBP) (Sneed et al., 2014, Sneed et al., 2024), GLW-amide neuropeptide (Iwao et al., 2002; Erwin and Szmant, 2010), and microbial agents like Roseobacter sp (Sharp et al., 2015), Pseudoalteromonas sp (Tebben et al., 2011), and Metabacillus sp (Zhang et al., 2021). CCA, a natural inducer, has long been acknowledged for its role in coral larval settlement through biochemical and structural cues (Morse et al., 1988). However, its practical applications are limited by environmental sensitivity and variability in bioactive compound production (Kuffner et al., 2008). In contrast, synthetic chemical inducers (e.g., calcium chloride) and microbial agents (e.g., Metabacillus sp.) offer advantages in consistency, productivity, and stability, making them promising alternatives for standardized restoration protocols (Zhang et al., 2021; Yang et al., 2022). These innovations highlight the potential to overcome the limitations of natural inducers in large-scale restoration efforts.
Despite significant progress in identifying individual cues affecting settlement, the broader spectrum of inducers and their effects across various coral species, including both brooding and spawning corals, remain significantly understudied. Most of the current studies have focused on single species or narrow inducer categories, with limited cross-species comparisons (Pollock et al., 2017; Levenstein et al., 2022). This gap impedes the scalability of large-scale coral restoration, particularly in regions with high coral diversity such as the Coral Triangle, where over 600 coral species coexist (Barton et al., 2017; Boström-Einarsson et al., 2020). Without understanding species-specific inducer efficacy, large-scale restoration protocols risk inefficiency or failure when applied to multi-species reefs (Barton et al., 2017). For example, broadcast spawners such as Acropora spp. dominate Indo-Pacific reefs but exhibit lower post-settlement survival under generic protocols compared with brooders like Pocillopora damicornis (Bellwood et al., 2004; Pollock et al., 2017; Omori, 2019). Besides, standardized protocols often overlook interspecific physiological thresholds, and a “one-size-fits-all” inducer approach could waste resources or harm sensitive species (Omori, 2019; Boström-Einarsson et al., 2020; Suggett and van Oppen, 2022). For example, prolonged exposure to CaCl2 and strain cB07 increased their toxicity, resulting in decreased survival rates of coral larvae (Zhang et al., 2021; Yang et al., 2022). The identification of broad-spectrum inducers and the delineation of species-specific toxicity thresholds will provide actionable frameworks to improve the precision and cost-effectiveness of restoration workflows, particularly in regions with high coral diversity. Furthermore, evaluations often prioritize settlement rates alone, neglecting post-settlement physiological responses such as metabolism, morphology, and long-term survival (Boström-Einarsson et al., 2020). A comprehensive understanding of inducer effects could therefore transform restoration strategies by aligning protocols with the ecological niches and physiological limits of species, ultimately improving the resilience of reef ecosystems under escalating climatic and anthropogenic pressures.
In our previous research, we identified calcium chloride (CaCl2) and Metabacillus sp. cB07 as effective settlement inducers for P. damicornis, a brooding stony coral species (Zhang et al., 2021; Yang et al., 2022). However, their settlement-inducing effect on other coral species, especially the spawning corals, remains unknown. The current study aims to address a pivotal question: How do the representative chemical and microbial inducers compare to CCA in their efficacy and physiological impacts across multiple coral species with distinct life-history strategies? We systematically evaluated CaCl2, strain cB07, and CCA on seven coral species, including both brooders and broadcast spawners, quantifying larval settlement rates, metabolic responses, morphological changes, and post-settlement survival and growth. By integrating multi-species and multi-indicator analyses, this study advances a framework for selecting inducer protocols tailored to species-specific needs, ultimately improving the scalability and success of coral reef restoration.
2 Materials and methods
2.1 Settlement inducers preparation
Three settlement inducers used in this study—CCA (Hydrolithon reinboldii), Metabacillus sp. cB07, and CaCl2—were collected and prepared as previously reported (Zhang et al., 2021; Yang et al., 2022). CCA was collected from the Luhuitou fringing reef (18.23°N, 109.47°E), located in Sanya, China. Maintenance conditions involved natural light and open-running sand-filtered seawater, as described in previous studies (Jiang et al., 2018; Yang et al., 2022). Seawater was filtered using a 0.22-µm-pore-size polyethersulfone (PES) membrane to remove particulates and microorganisms before use in experiments. The CaCl2 stock solution was prepared by dissolving 1 mol of CaCl2·2H2O in filtered seawater to make up a final volume of 1 L. Strain cB07 was inoculated into 2216E liquid medium (Hopebio, China) and cultured in a shaker at 28°C and 180 rpm for 16–20 hours. The medium was removed by centrifugation (8,000 rpm for 5 minutes) and then resuspended in filtered seawater. Absorbance at 600 nm was measured using a spectrophotometer (BioTek, USA). A standard curve of absorbance versus concentration for strain cB07 was established, as shown in the Supplementary Material (Supplementary Table S8). Both CaCl2 and bacterial stock solutions were further gradient diluted to different concentrations using filtered seawater.
2.2 Coral planula larvae collection
Between March–May 2024, seven coral species including one brooder and six broadcast spawners were collected from the same fringing reef as the CCA in this study, and maintained under the same conditions as the CCA. Five colonies of each species were collected, with each colony spaced at least 5 meters apart (Meixia et al., 2008). The collection in this study was approved by the Hainan Sanya Coral Reef National Nature Reserve.
The collection and cultivation methods for brooding coral larvae (P. damicornis in this study) followed the procedures described in previous studies (Yang et al., 2022). Planula larvae, collected on the same day as spawning, were pooled and randomly selected for subsequent experiments. Sample collection of different spawning coral species (Acropora hyacinthus, Acropora digitifera, Acropora nana, Galaxea fascicularis, Acropora formosa, and Acropora gemmifera) in the Luhuitou fringing reef was conducted based on records of spawning activity (Sun et al., 2024). During the spawning periods, the colonies of the same coral species were temporarily transferred to a tank containing 70 L of sand-filtered seawater. After spawning, the seawater was gently stirred to ensure adequate fertilization. Post-fertilization embryo culture conditions were identical to those for P. damicornis larvae. After 4 to 5 days of development, planula larvae with high swimming ability were randomly selected for further experiments.
2.3 Settlement inducers treatment
Gradient concentrations of CaCl2 (10–70 mmol/L) and strain cB07 (1 × 106–1 × 108 cfu/mL) were tested separately to determine the optimal concentration and species’ broad-spectrum of settlement inducers. Here, “broad-spectrum inducers” refer to compounds or agents capable of inducing larval settlement across phylogenetically diverse coral species, including both broadcast spawners and brooders, thus providing scalable solutions for reef restoration. Culture groups using 0.5 × 0.5 cm squares of CCA and filtered seawater were established as positive and negative controls, respectively. Ten larvae and 10 mL of culture solution were added to each well of a sterile polystyrene six-well plate. Three replicates of 10 larvae per treatment (a total of 30 per treatment) were used. The culture conditions were maintained as described above for larval cultivation. The culture solution in each well was replaced every 24 hours with fresh solution of the same treatment. Settlement, metamorphosis, and survival were recorded every 24 hours for 7 days.
The determination of settlement, metamorphosis, and survival of larvae was based on methods described in previous studies (Heyward and Negri, 1999; Edmunds et al., 2001; Yang et al., 2022). Larval survival refers to the proportion of surviving larvae (including unsettled, settled, and metamorphosed individuals) relative to the initial count. The optimal induction conditions for settlement inducers on coral larvae of seven species were determined as follows: for each inducer concentration, larval settlement rates were compared with the seawater control using one-way ANOVA. The first concentration and time point showing a statistically significant increase in settlement rate (p < 0.05) relative to the control was selected as the optimal condition for each species. Based on the evaluation results, the optimal induction times and concentrations of CaCl2 and strain cB07 solutions for seven coral species larvae were used in subsequent experiments.
2.4 Larval toxicity, stress, and developmental assessment
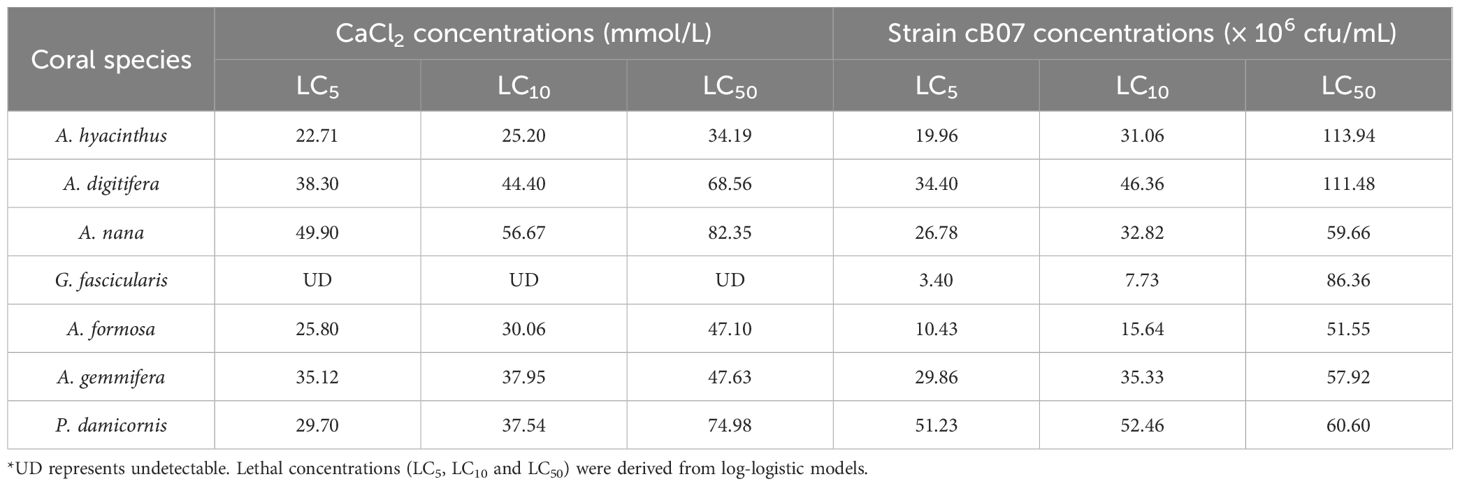
Coral larvae from seven species were exposed to varying concentrations of CaCl2 and strain cB07 for 3 days to determine their lethal concentrations (LCx). Dose-response curves were fitted using the Log-logistic 3-parameter model (LL.3) to describe the relationship between substance concentration and larval survival (Gupta et al., 1999). Lethal concentrations (LC5, LC10, and LC50) represent the inducer concentrations at which 5%, 10%, and 50% of larvae, respectively, die within three days of exposure. For each species, the estimated lethal concentrations were calculated using the “ED” function (Knezevic et al., 2007). Undetectable lethal concentrations (UD) were reported when no toxicity was observed within the tested concentration ranges. Statistical analyses were performed in R to ensure model convergence and reliable parameter estimation. The results were tabulated for each species and concentration type to facilitate comprehensive comparison.
The optimal induction concentrations of CaCl2 and strain cB07 solutions for the coral larvae of seven species were prepared separately in a 1 L beaker. Approximately 1,000 coral larvae were placed in the beaker with continuous aeration. The CCA was cut into 5 × 5 cm squares and fixed above the air stone. The experimental culture conditions were maintained consistent with those for P. damicornis larvae as described in Yang et al. (2022). Larvae were randomly sampled for metabolic and developmental measurements at 1, 12, and 24 hours of treatment.
Coral larvae were placed in a dark environment for 1 hour before measuring their dark respiration rate (Edmunds et al., 2011; Haryanti and Hidaka, 2015). Three groups of 10 larvae were randomly selected from each beaker and placed in a 2 mL respiration bottle containing filtered seawater. Different subsets of larvae were sampled at each time point (1, 12, and 24 hours) to avoid repeated measurements on the same individuals. A micro-stirrer (3 × 6 mm) was placed inside the bottle. The bottle was sealed (air bubbles were discharged) and placed in a water bath at 26°C. The stirring speed (200 rpm) was adjusted to allow larvae to rotate in the bottle. An optical oxygen measurement system (PreSens, Germany) was used to record the change in oxygen content in the bottle for approximately 10 minutes in the dark, with data collected every 3 seconds (Jiang et al., 2022). The dark respiration rate was normalized to time and the number of larvae, expressed in nmol O2 larvae–1 min–1 (Edmunds et al., 2011).
Larval developmental stages and characteristics were quantified and described using aspect ratios (Grasso et al., 2011). Thirty larvae were randomly selected and imaged using a dissecting microscope with a digital camera (Nikon, Japan). Separate larvae were used for each time point. Changes in the length and width of larvae in photographs were measured using Adobe Photoshop 2025 software, and aspect ratios were calculated.
2.5 Post-settlement recruits tracking
500 mL of CaCl2 and strain cB07 solutions with optimal concentrations, along with 2.5 × 2.5 cm squares of CCA and 500 mL of filtered seawater, were prepared in 20 cm diameter glass petri dishes. Approximately 600 planula larvae of both P. damicornis and A. formosa were placed in the dishes, respectively. For each species and inducer combination, one petri dish was used. After the optimal induction times, the culture solution and unsettled larvae were removed, and the newly settled recruits were cultured for 14 days in sand-filtered seawater. The culture conditions were maintained as described above, with filtered seawater changed every 24 hours. After 14 days, the survival rate, lateral growth, and calcification rate of the recruits were measured and calculated using methods described in previous studies (Jiang et al., 2018; Yang et al., 2022).
2.6 Statistical analysis
All statistical analyses were performed using R software (version 4.1.2). The following R packages were used: “dplyr” for data manipulation, “agricolae” for performing the Least Significant Difference (LSD) test, and “drc” for dose-response analysis. The Shapiro-Wilk test was used to assess data normality, and Levene’s test was applied to check for homogeneity of variance. Non-parametric tests were used when both assumptions were violated, if necessary. One-way analysis of variance (ANOVA) was used to explore the effects of the different treatments (seawater, CCA, CaCl2, and Metabacillus sp. cB07) on the response variables (for larvae: survival rate, settlement rate, metamorphosis rate, dark respiration rate, aspect ratio; for recruits: survival rate, lateral growth, calcification rate). Significant differences were considered at p < 0.05, and data were expressed as mean ± standard error (SE). When significant differences were found, LSD post-hoc analyses were conducted, and the analysis results were adjusted by Bonferroni correction. The charts were produced using R, Adobe Photoshop 2025, and Adobe Illustrator 2023 software. The detailed statistical results and original data of this study were provided in the Supplementary Material.
3 Results
3.1 Settlement-inducing effects
CaCl2 and strain cB07 were as broad-spectrum as CCA in inducing coral larvae to settle (Figure 1). This was demonstrated by the one-way ANOVA results, which showed significant differences (p < 0.05) in the settlement rates of seven coral species under the 7-day treatment (Supplementary Tables S1-Supplementary Table S3). Further analysis using LSD post hoc comparisons revealed that at least one concentration (both CaCl2 and strain cB07) was significantly higher (p < 0.05) compared to the control group. This indicates that all three settlement inducers (including CCA) significantly increased the settlement rate of the seven coral species.
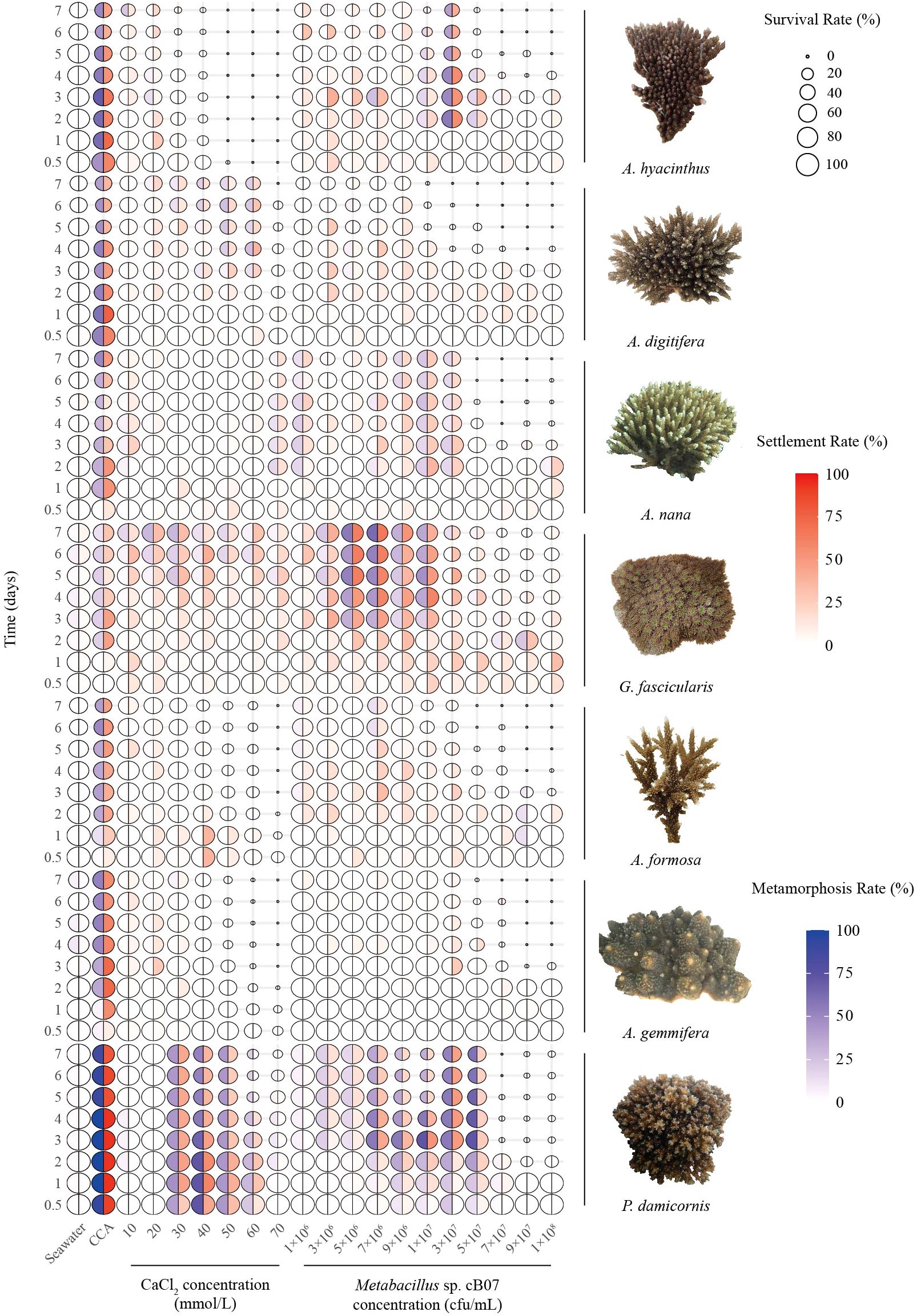
Figure 1. Effects of CCA, CaCl2, and Metabacillus sp. cB07 concentrations on coral larvae survival, settlement, and metamorphosis across different species and time intervals (statistical data shown in Supplementary Tables S1–S3, original data see Supplementary Table S8). Each bubble is divided into two sectors: the left sector (red gradient) represents the settlement rate, and the right sector (blue gradient) represents the metamorphosis rate. Bubble size is proportional to survival rate.
The optimal induction times and concentration ranges for CCA, strain cB07, and CaCl2 were similar across coral species. Specifically, the optimal settlement induction time for CCA was 1–2 days; strain cB07 was most effective at 2–4 days, with a concentration range of 3 × 106–3 × 107 cfu/mL; and CaCl2 was most effective at 0.5–4 days, with concentrations of 10–60 mmol/L (Supplementary Table S4).
The optimal inducer conditions (time and concentration) varied significantly across species, leading to differential effects on survival and metamorphosis (Figure 2). A higher concentration of CaCl2 (60 mmol/L) significantly reduced the survival of A. digitifera larvae (46.67 ± 8.82%, p < 0.05), while CCA (p = 0.11) and strain cB07 (p = 0.07) showed no significant effect on larval survival rates (larval survival defined as the proportion of live individuals, including settled and metamorphosed larvae). Conversely, statistical results showed that only CaCl2 treatment produced significant differences (p < 0.05) in larval settlement rates, while CCA and strain cB07 showed no differences in induction effects among species. In addition, all settlement inducers showed highly significant differences (p < 0.01) in metamorphosis induction among species. P. damicornis exhibited significantly higher metamorphosis rates than four other species under CCA and CaCl2 treatments (p < 0.05) and higher settlement rates than two species under CaCl2.
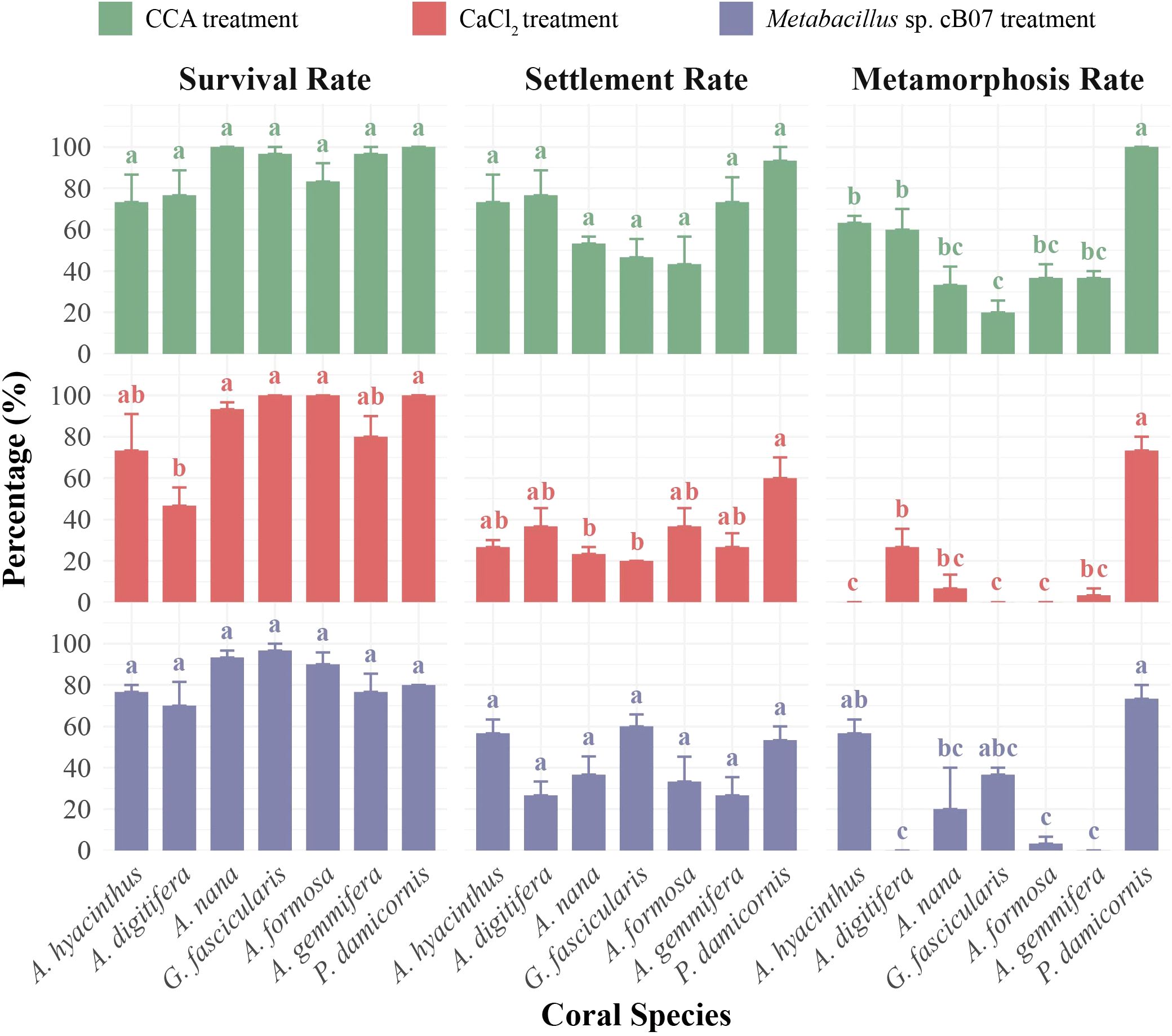
Figure 2. Comparison of interspecific differences in survival rate, settlement rate, and metamorphosis rate among different coral species under optimal treatment conditions (shown in Supplementary Table S4): CCA (1–2 days), CaCl2 (10–60 mmol/L, 0.5–4 days), strain cB07 (3×106–3×107 cfu/mL, 2–4 days). All bar plots show mean ± SE, with n = 3 per group. The letters above the bars indicate significant interspecific differences, as determined by one-way ANOVA followed by LSD post hoc tests at a significance level of 0.05.
3.2 Larval toxicity, stress, and development
Dose-response relationships for CaCl2 and strain cB07 were quantified across larvae of seven coral species using lethal concentration (LC) thresholds after 3 days of exposure (Table 1). LC values were extrapolated using the log-logistic model, as some concentrations exceeded the tested ranges. For CaCl2, LC50 values ranged from 34.19 mmol/L (A. hyacinthus) to 82.35 mmol/L (A. nana), with A. digitifera (68.56 mmol/L) and P. damicornis (74.98 mmol/L) exhibiting intermediate tolerance. Notably, G. fascicularis larvae showed no detectable mortality (LC values undetermined, UD) within the tested CaCl2 concentration range (10–70 mmol/L), indicating exceptional tolerance. In contrast, strain cB07 displayed broader toxicity variability, with A. hyacinthus exhibiting the highest tolerance to strain cB07 (LC50 = 1.14 × 108 cfu/mL), while A. formosa was the most sensitive (LC50 = 5.16 × 107 cfu/mL). Significant interspecific variation was also observed: A. nana demonstrated the highest CaCl2 resistance (LC50 = 82.35 mmol/L) but moderate sensitivity to strain cB07 (LC50 = 5.97 × 107 cfu/mL), whereas G. fascicularis showed extreme divergence, with undetectable CaCl2 toxicity but intermediate susceptibility to strain cB07 (LC50 = 8.64 × 107 cfu/mL). These results highlight species-specific physiological thresholds and underscore the necessity of tailoring inducer concentrations to minimize mortality risks in restoration workflows.
The effects of different settlement inducers on the dark respiration rates of coral larvae were assessed across seven coral species at 1, 12, and 24 hours (Figure 3, Supplementary Table S5). For A. hyacinthus, A. nana, G. fascicularis, A. formosa, and A. gemmifera, no significant differences were observed in respiration rates at any time point (p > 0.05), indicating that respiration rates remained stable across the four treatments (including the seawater control). In contrast, A. digitifera exhibited a significant difference at 24 hours (F = 14.85, p < 0.01), with CCA (0.30 ± 0.03 nmol O2/larva/min) and seawater (0.28 ± 0.01) treatments showing similar respiration rates, while both strain cB07 (0.16 ± 0.02) and CaCl2 (0.17 ± 0.01) resulted in lower rates. A significant effect was also observed for P. damicornis at 1 hour (F = 11.53, p < 0.01), with respiration rates being highest under CaCl2 treatment (3.71 ± 0.2), compared to lower rates under seawater (1.99 ± 0.38), CCA (2.27 ± 0.16), and strain cB07 treatments (2.1 ± 0.1). At 12 and 24 hours, the effects were not significant (p > 0.05), indicating a potential short-term response to CaCl2 treatment.
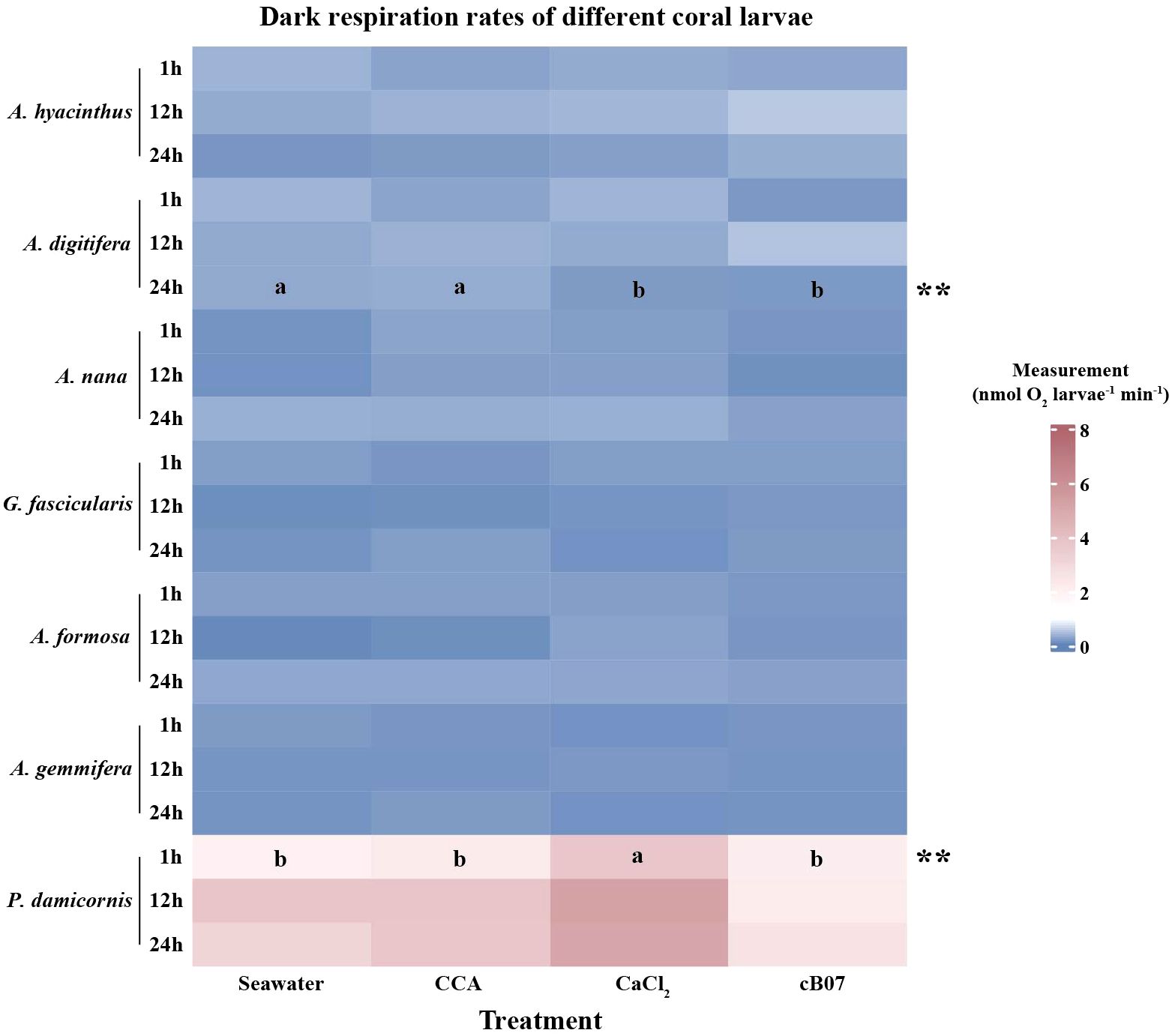
Figure 3. Dark respiration rates of different coral larvae under four treatment conditions (statistical and original data shown in Supplementary Tables S5, S9, respectively). Measurement data were presented as mean values (n = 3 per group). Asterisks (*, **, ***) denote significance levels based on one-way ANOVA, with * indicating p < 0.05, ** indicating p < 0.01, and *** indicating p < 0.001. Letters (a, b, c, etc.) represent group differences determined by post hoc LSD tests (significance level 0.05).
The aspect ratios of coral larvae were significantly affected by treatment with different settlement inducers across all species and time points (Figure 4, Supplementary Table S6). First, CaCl2 promoted the highest elongation and advanced metamorphosis. Across most coral species, CaCl2 consistently led to the highest aspect ratios during the elongation phase (competent larvae stage, rod-shaped, aspect ratio approximately 3–5). For example, in A. hyacinthus, CaCl2 induced an aspect ratio of 4.76 ± 0.34 at 24 hours, which was the highest observed among the treatments (F = 5.24, p < 0.01). Additionally, in the metamorphosis stage, CaCl2 induced the lowest aspect ratio in P. damicornis (1.03 ± 0.02), indicating the most advanced metamorphosis (F = 5.37, p < 0.01). Second, strain cB07 promoted early elongation but delayed metamorphosis. In many species, strain cB07 treatment resulted in higher aspect ratios during the initial (planula larvae stage, pear-shaped, aspect ratio approximately 2–3) and elongation stages. For instance, in A. formosa, strain cB07 produced the highest aspect ratio (3.69 ± 0.22) at 1 hour, compared to other treatments such as CaCl2 (2.50 ± 0.12) and CCA (1.57 ± 0.17, F = 25.79, p < 0.01). However, in the metamorphosis stage at 24 hours, cB07-treated larvae showed less rounding, with an aspect ratio of 2.20 ± 0.21, compared to CCA (1.63 ± 0.10) and CaCl2 (1.84 ± 0.22, F = 7.08, p < 0.01). This indicates that while strain cB07 enhanced elongation, it delayed the completion of metamorphosis compared to CaCl2 and CCA. Finally, CCA treatment usually results in the lowest aspect ratios during the metamorphosis larvae stage (rounded, aspect ratio ~1). In A. hyacinthus, CCA induced the lowest aspect ratio (1.65 ± 0.19) at 1 hour, which was significantly lower than other treatments such as strain cB07 (2.72 ± 0.27) and CaCl2 (3.50 ± 0.25, F = 14.26, p < 0.01). Similarly, in A. formosa, CCA produced the lowest aspect ratio (1.57 ± 0.17) at 1 hour compared to strain cB07 (3.69 ± 0.22) and CaCl2 (2.50 ± 0.12, F = 25.79, p < 0.01). This suggests that CCA accelerates the transition to the metamorphosis stage, with larvae reaching more rounded forms earlier.
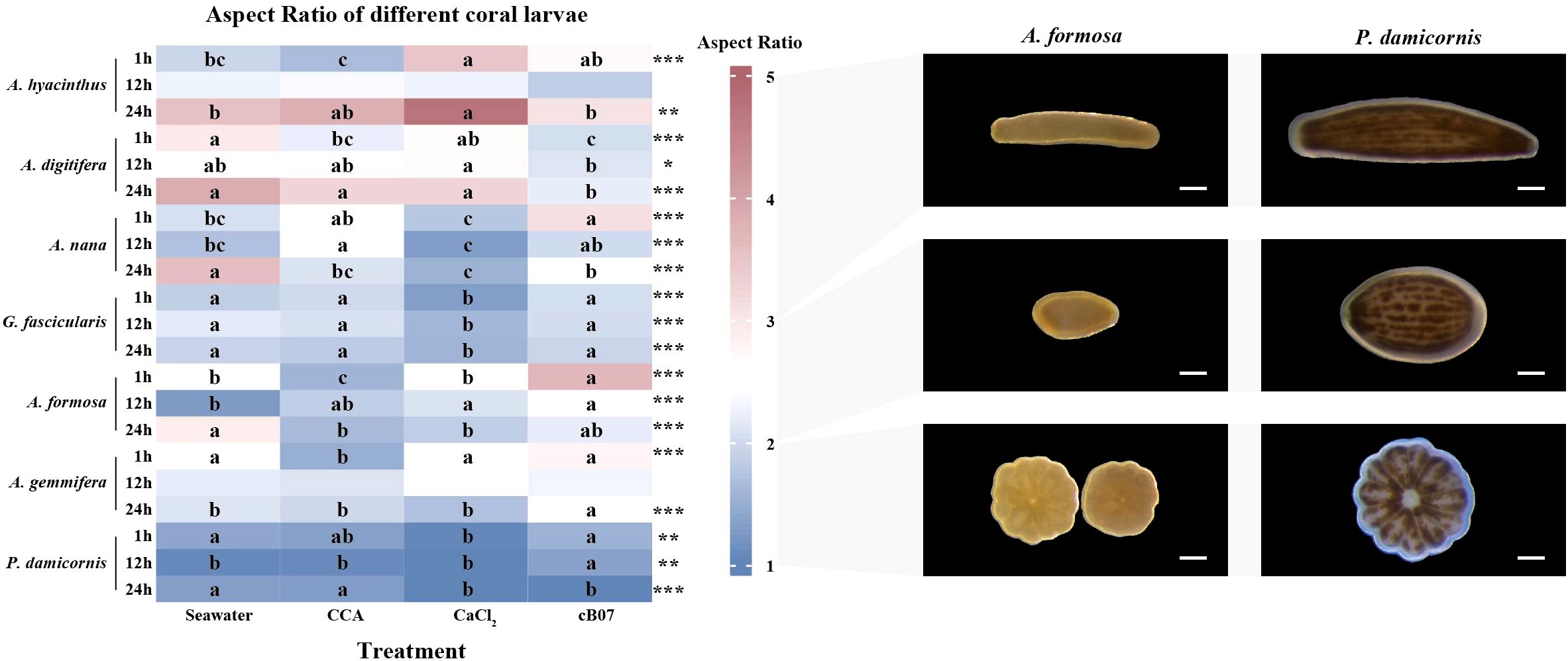
Figure 4. The aspect ratios of different coral larvae under four treatment conditions (statistical and original data shown in Supplementary Tables S6, S10, respectively). The heatmap visualizes the aspect ratios (mean values, n = 30 per group) for each treatment. Asterisks denote significance levels based on one-way ANOVA, with * indicating p < 0.05, ** indicating p < 0.01, and *** indicating p < 0.001. Letters represent group differences determined by post hoc LSD tests (significance level 0.05). Representative images of A. formosa and P. damicornis larvae illustrated morphological differences corresponding to varying aspect ratio values. The scale bar in all images represents 200 µm.
3.3 Effects on newly settled recruits
The effects of different settlement inducers on post-settlement growth and survival of coral recruits were evaluated for A. formosa and P. damicornis after 14 days of settlement (Figure 4, Supplementary Table S7). Relevant experiments could not be performed on the other coral species in this study due to an insufficient number of samples. For A. formosa, survival rates showed significant differences (F = 6.02, p < 0.05), with strain cB07 treatment resulting in significantly lower survival rates (76.4 ± 2.57%). Lateral growth in A. formosa was also significantly different (F = 23.46, p < 0.01), with CCA treatment resulting in the highest growth (0.07 ± 0 mm2/ind/day). However, calcification showed no significant differences (F = 0.39, p = 0.76), with similar values across all treatments (~6 units). In P. damicornis, survival rates (F = 0.36, p = 0.78) and lateral growth (F = 1.6, p = 0.19) showed no significant differences. However, calcification rates showed significant differences (F = 8.88, p < 0.01), with CCA treatment resulting in the highest calcification (42.62 ± 2.57 μg CaCO3/ind/day), followed by CaCl2 (41.14 ± 2.16), while strain cB07 (26.43 ± 4.88) and seawater (26.69 ± 0.52) showed the lowest values. These results suggest that CCA and CaCl2 treatments promote higher survival in A. formosa, with CCA enhancing lateral growth, while calcification was highest under CCA and CaCl2 treatments in P. damicornis. On the other hand, strain cB07 treatment generally resulted in lower survival and calcification, indicating that it may have less beneficial effects on post-settlement development in both species.
4 Discussion
The degradation of coral reefs under escalating environmental and anthropogenic pressures has necessitated the development of restoration strategies that prioritize both larval settlement and post-settlement survival (Boström-Einarsson et al., 2020; Suggett and van Oppen, 2022). This study systematically evaluated three broad-spectrum settlement inducers—CaCl2, the microbial strain Metabacillus sp. cB07, and CCA—across seven coral species. Our findings reveal species-specific responses to these inducers, highlight their differential impacts on larval settlement, metabolism, and post-settlement development, and emphasize their potential utility in optimizing coral restoration protocols.
4.1 Broad-spectrum and efficacy of inducers
A key advancement of this work is the systematic comparison of artificial inducers across phylogenetically diverse species, including brooders (P. damicornis) and spawners (e.g., A. digitifera). Natural inducers like CCA have been well documented to exhibit broad-spectrum settlement-inducing activity on several coral species, including spawning species (Acropora sp., Leptastrea purpurea, Pseudodiploria strigose, and Orbicella faveolata) and brooding species (Agaricia agaricites, Favia fragum, Porites astreoides, and P. damicornis) (Grasso et al., 2011; Tebben et al., 2015; Ritson-Williams et al., 2016; Jorissen et al., 2021; Gómez-Lemos and García-Urueña, 2022). However, artificial inducers like CaCl2 and microbial agents (especially Metabacillus sp.) have been validated on only a single coral species (P. damicornis) (Zhang et al., 2021; Yang et al., 2022; Zhang et al., 2024). This study demonstrated that CaCl2 and strain cB07 exhibit broad-spectrum efficacy comparable to CCA in inducing larval settlement across seven coral species (Figure 1, Supplementary Tables S1-Supplementary Table S3). CCA achieved the highest settlement rates (43.3%–93.3%) within 1–2 days, consistent with its role as a natural inducer through biochemical and structural cues (Grasso et al., 2011; Gómez-Lemos and García-Urueña, 2022). Similarly, artificial inducers like CaCl2 (10–60 mmol/L) and strain cB07 (3 × 106–3 × 107 cfu/mL) showed comparable consistency, particularly in brooding species such as P. damicornis. This aligns with previous studies emphasizing the reliability of synthetic inducers in standardized protocols (Zhang et al., 2021; Yang et al., 2022). Notably, CaCl2’s rapid induction (optimal at 0.5–4 days) and promotion of advanced metamorphosis highlight its utility in accelerating restoration workflows.
Additionally, our findings extend this knowledge by demonstrating that inducer effectiveness is not universal but is modulated by species-specific physiological thresholds. For instance, P. damicornis exhibited higher settlement rates than most tested species under CaCl2, likely due to its brooding reproductive strategy, which equips larvae with pre-adapted metabolic reserves (Ritson-Williams et al., 2016; Wang et al., 2023). In contrast, A. digitifera survival (46.67 ± 8.8%) was reduced at high CaCl2 concentrations (60 mmol/L), whereas other spawners showed no significant decline. One explanation for this phenomenon is that broadcast spawners, which release eggs and sperm into the water column, may rely more heavily on external environmental cues for timely metamorphosis than brooders (Petersen et al., 2021; Longley et al., 2024). These findings bridge a critical gap in coral restoration literature, demonstrating that while synthetic inducers are broadly applicable, their optimization must consider life-history strategies and physiological thresholds.
4.2 Toxicity and trade-offs
The dose-response relationships for CaCl2 and strain cB07 further revealed pronounced interspecific variability in coral larval tolerance, offering critical insights into the risks and limitations of inducer applications in restoration workflows (Table 1). Our results show that CaCl2 toxicity (LC50) ranged from 34.19–82.35 mmol/L, with brooding species P. damicornis larvae (LC50 = 74.98 mmol/L) buffering metabolic stress more effectively than four tested spawners in this study (e.g., A. hyacinthus, LC50 = 34.19 mmol/L), which rely heavily on external calcium uptake for calcification (Guest, 2008). In contrast, strain cB07 exhibited broader toxicity variability (LC50: 51.55–113.94 × 106 cfu/mL), with A. formosa being more sensitive (LC50 = 51.55 × 106 cfu/mL) than P. damicornis (LC50 = 60.60 × 106 cfu/mL). This divergence may stem from species-specific interactions with microbial communities: P. damicornis, a brooder with vertically transmitted symbionts, likely possesses pre-adapted mechanisms to mitigate microbial stress, whereas three tested broadcast spawners in this study like A. formosa may lack such defenses (Ceh et al., 2013; Damjanovic et al., 2020; Kitchen et al., 2020). In this case, the delayed metamorphosis observed in cB07-treated larvae could further exacerbate mortality risks, as prolonged exposure to sublethal concentrations (e.g., LC10 = 15.64 × 106 cfu/mL for A. formosa) may deplete larval energy reserves. The stark contrast in G. fascicularis’s response underscores the complexity of inducer-coral interactions—undetectable CaCl2 toxicity versus intermediate susceptibility to strain cB07 (LC50 = 86.36 × 106 cfu/mL). Such interspecific variability reinforces the need for species-specific protocols, as generalized inducer concentrations risk either inefficacy (e.g., subthreshold CaCl2 for A. nana) or unintended mortality (e.g., high concentration of strain cB07 for A. formosa). These dose-response data also contextualize the metabolic trade-offs: high CaCl2 concentrations, while effective in accelerating settlement, impose survival costs. This aligns with energy allocation theory, where larvae prioritize immediate settlement over long-term viability under inducer-induced stress (Richmond, 1987). For restoration practitioners, these results emphasize the importance of balancing rapid settlement induction with concentration thresholds that minimize mortality.
The identification of optimal concentration ranges for CaCl2 (10–60 mmol/L) and strain cB07 (3 × 106–3 × 107 cfu/mL) provides actionable guidelines for coral restoration practitioners (Figure 2, Supplementary Table S4). These ranges balance efficacy with minimal toxicity, a critical consideration given the observed survival trade-offs. For example, high CaCl2 concentrations (60 mmol/L) reduced A. digitifera survival to 46.67 ± 8.82% (73.33 ± 6.67% in seawater control), while strain cB07 exhibited no significant survival impacts (p > 0.05, compared to seawater control) despite delayed metamorphosis. This aligns with studies showing that calcium ions enhance larval attachment but require precise dosing to avoid metabolic stress (Yang et al., 2022). Similarly, metabolic trade-offs were evident across treatments (Figure 3, Supplementary Table S5). CaCl2 transiently elevated dark respiration in P. damicornis (3.71 vs. 1.99 nmol O2/larva/min at 1 hour, compared to the control), suggesting rapid activation of calcium-dependent signaling pathways (Tran and Hadfield, 2012; Siboni et al., 2014; Strader et al., 2018). Conversely, strain cB07 suppressed respiration in A. digitifera (0.16 vs. 0.28 at 24 hours, compared to the control), potentially indicating metabolic depression or resource reallocation toward elongation. These results echo findings in Porites lobata, where microbial community shifts under stress correlated with altered energy allocation (Shore et al., 2021). Such trade-offs underscore the need to balance short-term settlement success with long-term recruit viability.
Post-settlement outcomes further differentiated settlement inducers. CaCl2 and CCA emerged as the most reliable and effective inducers, supporting both settlement (broad-spectrum efficacy) and post-settlement growth (Figure 5, Supplementary Table S7). Both brooding (A. formosa) and spawning coral species (P. damicornis), CCA-treated recruits exhibited the highest calcification rates and lateral growth, likely due to its dual role as a natural substrate and sustained source of chemical cues (Lei et al., 2021; Wang et al., 2024). Similarly, the ability of CaCl2 to enhance calcification rates and lateral growth in both two coral species is nearly as effective as CCA, without continuous calcium enrichment. These results align with studies on Dipsastrea speciosa, where heterotrophic plasticity under environmental stress correlated with improved resilience (Wang et al., 2024). In contrast, strain cB07 underperformed post-settlement, with lower survival and calcification rates. This may reflect transient microbial activity or competition with beneficial microbiota, as observed in Endozoicomonas-dominated communities under ocean acidification (Shore et al., 2021). These results emphasize that inducer selection must prioritize not only settlement success but also long-term recruit health, a factor often overlooked in restoration protocols (Barton et al., 2017; Boström-Einarsson et al., 2020).
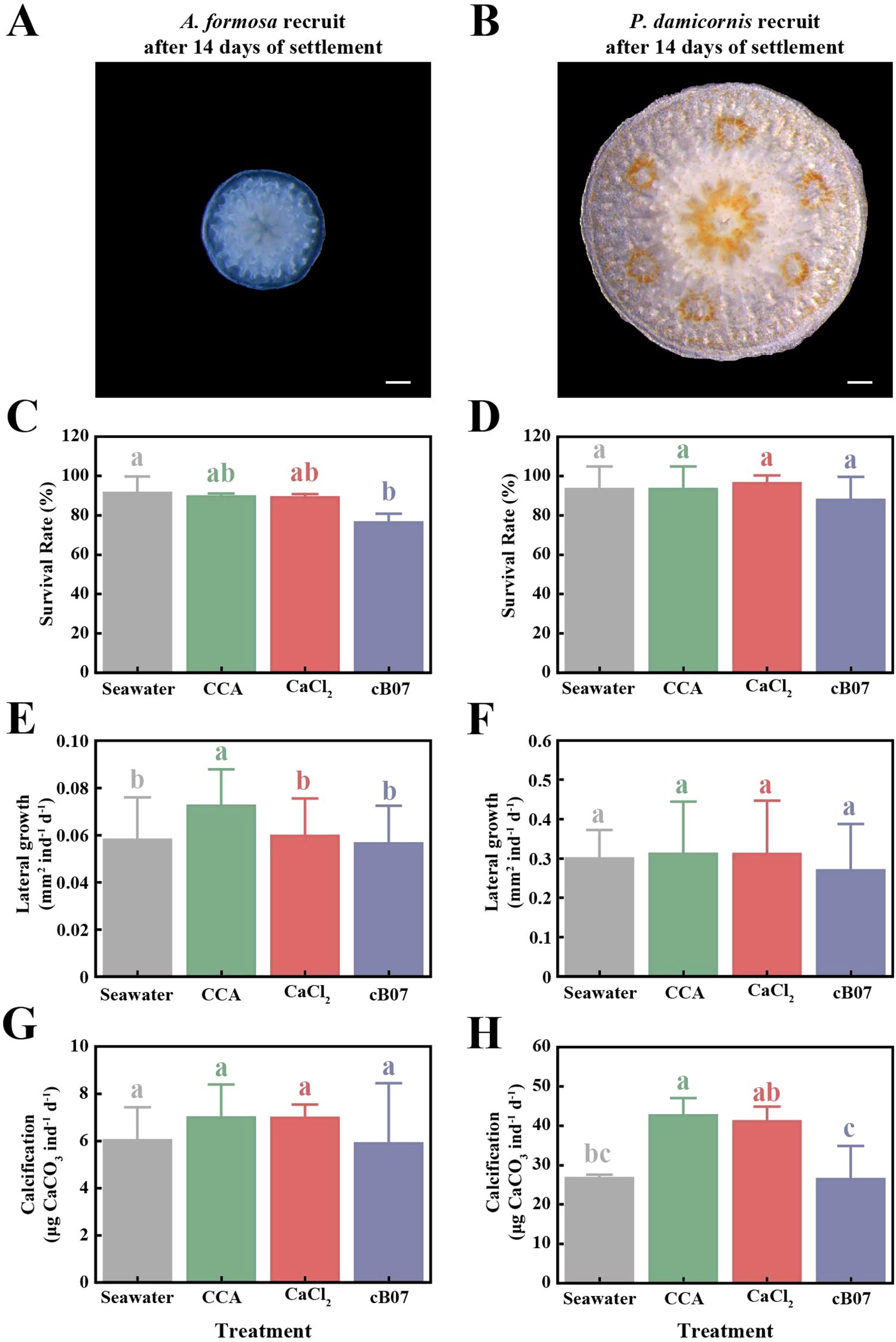
Figure 5. Morphological features and growth parameters of A. formosa (A, C, E, G) and P. damicornis (B, D, F, H) recruits after 14d of settlement, the settlement of which was induced by CaCl2, CCA, Metabacillus sp. cB07, or under natural conditions (Seawater). Representative images of A. formosa (A) and P. damicornis (B) recruits. The scale bar in both images represents 200 µm. Survival rate (C, D), lateral growth rate (E, F), and calcification rate (G, H) of A. formosa and P. damicornis recruit. Error bars represent SE (n > 30 replicates per treatment, see Supplementary Tables S7, S11). Groups with different letters indicate statistically significant differences based on LSD post hoc tests (p < 0.05).
4.3 Settlement-inducing mechanisms
This study provides novel insights into how inducers shape larval morphology and developmental trajectories. CaCl2 promoted the highest elongation and advanced metamorphosis, particularly in A. hyacinthus, A. digitifera, and P. damicornis, likely through Ca2+-mediated activation of adhesion pathways (Tran and Hadfield, 2012). In contrast, strain cB07 promoted early elongation but delayed metamorphosis, particularly in A. formosa and A. nana, possibly due to incomplete activation of developmental pathways or competition for resources between elongation and tissue differentiation (Sneed et al., 2014; Huggett and Apprill, 2019; Sneed et al., 2024). CCA generally led to the lowest aspect ratios during the planula and elongation stages, suggesting that it accelerated the transition to the post-metamorphic stage, allowing larvae to reach their final form earlier, likely through synergistic biochemical and topographic signals (Lei et al., 2021; Abdul Wahab et al., 2023).
While this study provides critical insights, its limitations include the short-term observational window and controlled laboratory conditions, which may not fully replicate natural reef dynamics. For example, while this study evaluates short-term physiological responses, synthetic inducers like CaCl2 may alter coral-associated microbial communities or disrupt symbiont recruitment, potentially affecting long-term reef health. As observed in ocean acidification studies, calcium enrichment could promote pathogenic bacteria or reduce diversity in coral microbiomes (Shore et al., 2021). These ecological risks necessitate further investigation.
Future research should prioritize long-term field trials to validate inducer durability and explore multi-inducer synergies. For example, combining CaCl2 with microbial consortia or CCA-derived metabolites could better mimic natural settlement environments and enhance outcomes (Jorissen et al., 2021; Quinlan et al., 2023). Similarly, strain cB07’s species-specific toxicity (e.g., high sensitivity in A. formosa) may reflect strain-specific immunomodulatory effects, warranting metagenomic or transcriptomic analyses, including calcium signaling pathways or microbial metabolite production (Petersen et al., 2021). Furthermore, current LC50 assessments were conducted under static laboratory conditions, whereas field applications involve dynamic environments where inducer concentrations fluctuate (Barton et al., 2017; Boström-Einarsson et al., 2020). Future studies should adopt tiered toxicity frameworks incorporating sublethal endpoints (e.g., oxidative stress markers and microbiome shifts) to better predict ecological risks (Flores et al., 2020).
The mechanisms by which inducers promote larval settlement remain unresolved. While CaCl2 is hypothesized to activate calcium-dependent signaling pathways (Morse et al., 1988; Tran and Hadfield, 2012; Yang et al., 2022), the specific molecular cascades—such as ion channel regulation or adhesion protein expression—remain to be mapped. Similarly, microbial inducers like strain cB07 likely modulate larval behavior through biofilm-derived metabolites (e.g., dimethylsulfoniopropionate, DMSP; Tebben et al., 2015), but the biochemical identity of these cues and their receptors in coral larvae remains unknown. Additionally, interspecific differences in inducer responsiveness (e.g., P. damicornis vs. A. digitifera) suggest evolutionary divergence in cue recognition systems, potentially linked to life-history strategies or symbiotic microbiome composition (Barton et al., 2017; Longley et al., 2024). Resolving these mechanisms requires integrative approaches, such as single-cell transcriptomics to identify settlement-associated gene networks or metabolomics to characterize inducer-derived bioactive compounds. Furthermore, the long-term ecological implications of inducer use—such as altered microbiome stability or reduced genetic diversity in restored populations—remain critical but unaddressed questions. Bridging these gaps will not only refine inducer protocols but also illuminate fundamental aspects of coral larval ecology, ultimately advancing both restoration science and conservation practice.
5 Conclusion
This study demonstrates that CaCl2 and Metabacillus sp. cB07 exhibit broad-spectrum efficacy similar to CCA in inducing larval settlement across seven coral species, with their effectiveness and safety profiles being species-specific. Compared with the control (up to 13.3% within 7 days), in optimal conditions, CCA induced the highest settlement rates (43.3%–93.3%) with rapid induction (1–2 days), accelerating the transition to the metamorphosis stage. Similarly, CaCl2 at 10–60 mmol/L induced settlement rates ranging from 23.3%–60.3% within 0.5–4 days, promoting the highest elongation and advanced metamorphosis. In contrast, strain cB07 at 3 × 106–3 × 107 cfu/mL induced settlement rates ranging from 26.7%–60.0% within 2–4 days, promoting early elongation but delaying metamorphosis. Toxicity thresholds varied markedly: CaCl2 exhibited moderate lethality (LC50: 34.19–82.35 mmol/L), while cB07 showed higher interspecific variability (LC50: 5.16 × 107–1.14 × 108 cfu/mL), emphasizing the necessity of species-tailored protocols. Post-settlement performance further differentiated inducers, with CCA and CaCl2 enhancing recruit survival and calcification, while strain cB07 lagged in supporting long-term development. These findings highlight the importance of balancing induction efficiency, physiological safety, and post-settlement viability in coral restoration. Future work should integrate molecular analyses to elucidate mechanistic pathways and establish dynamic toxicity frameworks, ultimately advancing scalable, ecologically resilient reef restoration strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
HS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. QY: Funding acquisition, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. JD: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. JiL: Supervision, Writing – review & editing. CC: Supervision, Writing – review & editing. XT: Investigation, Methodology, Writing – review & editing. YZ: Investigation, Methodology, Writing – review & editing. JuL: Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the National Key Research and Development Program of China (2022-20), the National Natural Science Foundation of China (42276160, U23A2036, 42106197), Science and Technology Planning Project of Guangdong Province of China (2023B1212060047), and Science and Technology Projects in Guangzhou (2023A04J0201).
Acknowledgments
We thank all editors and reviewers for their valuable suggestions and comments on the manuscript. We also thank the staff of the Tropical Marine Biological Research Station in Hainan for providing technical assistance and facilities for conducting this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fmars.2025.1670431.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1581753/full#supplementary-material
References
Abdul Wahab M. A., Ferguson S., Snekkevik V. K., McCutchan G., Jeong S., Severati A., et al. (2023). Hierarchical settlement behaviours of coral larvae to common coralline algae. Sci. Rep. 13, 5795. doi: 10.1038/s41598-023-32676-4
Barton J. A., Willis B. L., and Hutson K. S. (2017). Coral propagation: a review of techniques for ornamental trade and reef restoration. Rev. Aquaculture 9, 238–256. doi: 10.1111/raq.12135
Bellwood D. R., Hughes T. P., Folke C., and Nyström M. (2004). Confronting the coral reef crisis. Nature 429, 827–833. doi: 10.1038/nature02691
Boström-Einarsson L., Babcock R. C., Bayraktarov E., Ceccarelli D., Cook N., Ferse S. C. A., et al. (2020). Coral restoration – A systematic review of current methods, successes, failures and future directions. PloS One 15, e0226631. doi: 10.1371/journal.pone.0226631
Brandl S. J., Rasher D. B., Côté I. M., Casey J. M., Darling E. S., Lefcheck J. S., et al. (2019). Coral reef ecosystem functioning: eight core processes and the role of biodiversity. Front. Ecol. Environ. 17, 445–454. doi: 10.1002/fee.2088
Ceh J., van Keulen M., and Bourne D. G. (2013). Intergenerational transfer of specific bacteria in corals and possible implications for offspring fitness. Microbial Ecol. 65, 227–231. doi: 10.1007/s00248-012-0105-z
Damjanovic K., Menéndez P., Blackall L. L., and van Oppen M. J. H. (2020). Early life stages of a common broadcast spawning coral associate with specific bacterial communities despite lack of internalized bacteria. Microbial Ecol. 79, 706–719. doi: 10.1007/s00248-019-01428-1
Edmunds P. J., Cumbo V., and Fan T.-Y. (2011). Effects of temperature on the respiration of brooded larvae from tropical reef corals. J. Exp. Biol. 214, 2783–2790. doi: 10.1242/jeb.055343
Edmunds P., Gates R., and Gleason D. (2001). The biology of larvae from the reef coral Porites astreoides, and their response to temperature disturbances. Marine Biol. 139, 981–989. doi: 10.1007/s002270100634
Erwin P. M. and Szmant A. M. (2010). Settlement induction of Acropora palmata planulae by a GLW-amide neuropeptide. Coral Reefs 29, 929–939. doi: 10.1007/s00338-010-0634-1
Fezzi C., Ford D. J., and Oleson K. L. L. (2023). The economic value of coral reefs: Climate change impacts and spatial targeting of restoration measures. Ecol. Economics 203, 107628. doi: 10.1016/j.ecolecon.2022.107628
Flores F., Kaserzon S., Elisei G., Ricardo G., and Negri A. P. (2020). Toxicity thresholds of three insecticides and two fungicides to larvae of the coral Acropora tenuis. PeerJ 8, e9615. doi: 10.7717/peerj.9615
Gómez-Lemos L. A. and García-Urueña R. (2022). Induction of Staghorn coral settlement and early post-settlement survival in laboratory conditions. Aquat. Ecol. 56, 685–696. doi: 10.1007/s10452-022-09948-8
Grasso L. C., Negri A. P., Fôret S., Saint R., Hayward D. C., Miller D. J., et al. (2011). The biology of coral metamorphosis: Molecular responses of larvae to inducers of settlement and metamorphosis. Dev. Biol. 353, 411–419. doi: 10.1016/j.ydbio.2011.02.010
Guest J. (2008). How reefs respond to mass coral spawning. Science 320, 621–623. doi: 10.1126/science.1155285
Gupta R. C., Akman O., and Lvin S. (1999). A study of log-logistic model in survival analysis. Biometrical J. 41, 431–443. doi: 10.1002/(SICI)1521-4036(199907)41:4<431::AID-BIMJ431>3.0.CO;2-U
Haryanti D. and Hidaka M. (2015). Temperature Dependence of Respiration in Larvae and Adult Colonies of the Corals Acropora tenuis and Pocillopora damicornis. J. Marine Sci. Eng. 3, 509–519. doi: 10.3390/jmse3030509
Heyward A. J. and Negri A. P. (1999). Natural inducers for coral larval metamorphosis. Coral Reefs 18, 273–279. doi: 10.1007/s003380050193
Huggett M. J. and Apprill A. (2019). Coral microbiome database: Integration of sequences reveals high diversity and relatedness of coral-associated microbes. Environ. Microbiol. Rep. 11, 372–385. doi: 10.1111/1758-2229.12686
Iwao K., Fujisawa T., and Hatta M. (2002). A cnidarian neuropeptide of the GLWamide family induces metamorphosis of reef-building corals in the genus Acropora. Coral Reefs 21, 127–129. doi: 10.1007/s00338-002-0219-8
Jiang L., Sun Y.-F., Zhou G.-W., Tong H.-Y., Huang L.-T., Yu X.-L., et al. (2022). Ocean acidification elicits differential bleaching and gene expression patterns in larval reef coral Pocillopora damicornis under heat stress. Sci. Total Environ. 842, 156851. doi: 10.1016/j.scitotenv.2022.156851
Jiang L., Zhang F., Guo M.-L., Guo Y.-J., Zhang Y.-Y., Zhou G.-W., et al. (2018). Increased temperature mitigates the effects of ocean acidification on the calcification of juvenile Pocillopora damicornis, but at a cost. Coral Reefs 37, 71–79. doi: 10.1007/s00338-017-1634-1
Jorissen H., Galand P. E., Bonnard I., Meiling S., Raviglione D., Meistertzheim A.-L., et al. (2021). Coral larval settlement preferences linked to crustose coralline algae with distinct chemical and microbial signatures. Sci. Rep. 11, 14610. doi: 10.1038/s41598-021-94096-6
Kitchen R. M., Piscetta M., de Souza M. R., Lenz E. A., Schar D. W. H., Gates R. D., et al. (2020). Symbiont transmission and reproductive mode influence responses of three Hawaiian coral larvae to elevated temperature and nutrients. Coral Reefs 39, 419–431. doi: 10.1007/s00338-020-01905-x
Knezevic S. Z., Streibig J. C., and Ritz C. (2007). Utilizing R software package for dose-response studies: the concept and data analysis. Weed Technol. 21, 840–848. doi: 10.1614/WT-06-161.1
Kuffner I. B., Andersson A. J., Jokiel P. L., Rodgers K., and Mackenzie F. T. (2008). Decreased abundance of crustose coralline algae due to ocean acidification. Nat. Geosci. 1, 114–117. doi: 10.1038/ngeo100
Lei X., Jiang L., Zhang Y., Sun Y., Zhou G., Lian J., et al. (2021). Coral larval settlement and post-settlement survival facilitated by crustose coralline algae with or without living tissue. Marine Biol. 168, 128. doi: 10.1007/s00227-021-03943-7
Levenstein M. A., Marhaver K. L., Quinlan Z. A., Tholen H. M., Tichy L., Yus J., et al. (2022). Composite substrates reveal inorganic material cues for coral larval settlement. ACS Sustain. Chem. Eng. 10, 3960–3971. doi: 10.1021/acssuschemeng.1c08313
Longley R., Benucci G. M. N., Pochon X., Bonito G., and Bonito V. (2024). Species-specific coral microbiome assemblages support host bleaching resistance during an extreme marine heatwave. Sci. Total Environ. 906, 167803. doi: 10.1016/j.scitotenv.2023.167803
Meixia Z., Kefu Y., Qiaomin Z., and Qi S. (2008). Spatial pattern of coral diversity in Luhuitou fringing reef, Sanya, China. Acta Ecologica Sin. 28, 1419–1428. doi: 10.1016/S1872-2032(08)60051-7
Milne R., Bauch C. T., and Anand M. (2022). Local overfishing patterns have regional effects on health of coral, and economic transitions can promote its recovery. Bull. Math. Biol. 84, 46. doi: 10.1007/s11538-022-01000-y
Morse D. E., Hooker N., Morse A. N. C., and Jensen R. A. (1988). Control of larval metamorphosis and recruitment in sympatric agariciid corals. J. Exp. Marine Biol. Ecol. 116, 193–217. doi: 10.1016/0022-0981(88)90027-5
Mumby P. J., Broad K., Brumbaugh D. R., Dahlgren C. P., Harborne A. R., Hastings A., et al. (2008). Coral reef habitats as surrogates of species, ecological functions, and ecosystem services. Conserv. Biol. 22, 941–951. doi: 10.1111/j.1523-1739.2008.00933.x
Nalley E. M., Tuttle L. J., Barkman A. L., Conklin E. E., Wulstein D. M., Richmond R. H., et al. (2021). Water quality thresholds for coastal contaminant impacts on corals: A systematic review and meta-analysis. Sci. Total Environ. 794, 148632. doi: 10.1016/j.scitotenv.2021.148632
Omori M. (2019). Coral restoration research and technical developments: what we have learned so far. Marine Biol. Res. 15, 377–409. doi: 10.1080/17451000.2019.1662050
Petersen L.-E., Moeller M., Versluis D., Nietzer S., Kellermann M. Y., and Schupp P. J. (2021). Mono- and multispecies biofilms from a crustose coralline alga induce settlement in the scleractinian coral Leptastrea purpurea. Coral Reefs 40, 381–394. doi: 10.1007/s00338-021-02062-5
Pollock F. J., Katz S. M., van de Water J. A. J. M., Davies S. W., Hein M., Torda G., et al. (2017). Coral larvae for restoration and research: a large-scale method for rearing Acropora millepora larvae, inducing settlement, and establishing symbiosis. PeerJ 5, e3732. doi: 10.7717/peerj.3732
Quinlan Z. A., Bennett M.-J., Arts M. G. I., Levenstein M., Flores D., Tholen H. M., et al. (2023). Coral larval settlement induction using tissue-associated and exuded coralline algae metabolites and the identification of putative chemical cues. Proc. R. Soc. B: Biol. Sci. 290, 20231476. doi: 10.1098/rspb.2023.1476
Richmond R. H. (1987). Energetics, competency, and long-distance dispersal of planula larvae of the coral Pocillopora damicornis. Marine Biol. 93, 527–533. doi: 10.1007/BF00392790
Ritson-Williams R., Arnold S. N., and Paul V. J. (2016). Patterns of larval settlement preferences and post−settlement survival for seven Caribbean corals. Marine Ecol. Prog. Ser. 548, 127–138. doi: 10.3354/meps11688
Rodd C., Whalan S., Humphrey C., and Harrison P. L. (2022). Enhancing coral settlement through a novel larval feeding protocol. Front. Marine Sci. 9. doi: 10.3389/fmars.2022.918232
Sharp K. H., Sneed J. M., Ritchie K. B., McDaniel L., and Paul V. J. (2015). Induction of larval settlement in the reef coral porites astreoides by a cultivated marine roseobacter strain. Biol. Bull. 228, 98–107. doi: 10.1086/BBLv228n2p98
Shore A., Day R. D., Stewart J. A., and Burge C. A. (2021). Dichotomy between regulation of coral bacterial communities and calcification physiology under ocean acidification conditions. Appl. Environ. Microbiol. 87, e02189–e02120. doi: 10.1128/aem.02189-20
Siboni N., Abrego D., Motti C. A., Tebben J., and Harder T. (2014). Gene Expression Patterns during the Early Stages of Chemically Induced Larval Metamorphosis and Settlement of the Coral Acropora millepora. PloS One 9, e91082. doi: 10.1371/journal.pone.0091082
Sneed J. M., Demko A. M., Miller M. W., Yi D., Moore B. S., Agarwal V., et al. (2024). Coral settlement induction by tetrabromopyrrole is widespread among Caribbean corals and compound specific. Front. Marine Sci. 10. doi: 10.3389/fmars.2023.1298518
Sneed J. M., Sharp K. H., Ritchie K. B., and Paul V. J. (2014). The chemical cue tetrabromopyrrole from a biofilm bacterium induces settlement of multiple Caribbean corals. Proc. R. Soc. B: Biol. Sci. 281, 20133086. doi: 10.1098/rspb.2013.3086
Strader M. E., Aglyamova G. V., and Matz M. V. (2018). Molecular characterization of larval development from fertilization to metamorphosis in a reef-building coral. BMC Genomics 19, 17. doi: 10.1186/s12864-017-4392-0
Suggett D. J. and van Oppen M. J. H. (2022). Horizon scan of rapidly advancing coral restoration approaches for 21st century reef management. Emerging Topics Life Sci. 6, 125–136. doi: 10.1042/ETLS20210240
Sun Y., Zhang Y., Jiang L., Yu X., Huang L., Yuan T., et al. (2024). Coral spawning patterns on the Luhuitou fringing reef in Hainan Island of the northern South China Sea. Front. Marine Sci. 11. doi: 10.3389/fmars.2024.1418942
Tebben J., Motti C. A., Siboni N., Tapiolas D. M., Negri A. P., Schupp P. J., et al. (2015). Chemical mediation of coral larval settlement by crustose coralline algae. Sci. Rep. 5, 10803. doi: 10.1038/srep10803
Tebben J., Tapiolas D. M., Motti C. A., Abrego D., Negri A. P., Blackall L. L., et al. (2011). Induction of larval metamorphosis of the coral acropora millepora by tetrabromopyrrole isolated from a pseudoalteromonas bacterium. PloS One 6, e19082. doi: 10.1371/journal.pone.0019082
Tran C. and Hadfield M. G. (2012). Are G-protein-coupled receptors involved in mediating larval settlement and metamorphosis of coral planulae? Biol. Bull. 222, 128–136. doi: 10.1086/BBLv222n2p128
Wang C., Zheng X., Kvitt H., Sheng H., Sun D., Niu G., et al. (2023). Lineage-specific symbionts mediate differential coral responses to thermal stress. Microbiome 11, 211. doi: 10.1186/s40168-023-01653-4
Wang Q., Zhou X., Wang J., Zhang H., Fang H., Cai F., et al. (2024). Heterotrophy confers corals with resistance but limits their range expansion: A case of marginal coral communities. Ecosystem Health Sustainability 10, 246. doi: 10.34133/ehs.0246
Yang Q., Zhang W., Zhang Y., Tang X., Ling J., Zhang Y., et al. (2022). Promoting larval settlement of coral Pocillopora damicornis by calcium. Coral Reefs 41, 223–235. doi: 10.1007/s00338-021-02216-5
Zhang Y., Zhang Y., Tang X., Guo X., Yang Q., Sun H., et al. (2024). A transcriptome-wide analysis provides novel insights into how Metabacillus indicus promotes coral larvae metamorphosis and settlement. BMC Genomics 25, 840. doi: 10.1186/s12864-024-10742-z
Keywords: coral restoration, larval settlement, post-settlement survival, broad-spectrum inducers, species-specificity, metabolic trade-offs
Citation: Sun H, Yang Q, Dong J, Li J, Chen C, Tang X, Zhang Y and Ling J (2025) Comprehensive assessment of chemical and microbial inducers for coral larval settlement across diverse coral species. Front. Mar. Sci. 12:1581753. doi: 10.3389/fmars.2025.1581753
Received: 23 February 2025; Accepted: 12 May 2025;
Published: 30 May 2025; Corrected: 07 August 2025.
Edited by:
Cliff Ross, University of North Florida, United StatesReviewed by:
Ann I. Larsson, University of Gothenburg, SwedenXiubao Li, Hainan University, China
Kaidian Zhang, Xiamen University, China
Copyright © 2025 Sun, Yang, Dong, Li, Chen, Tang, Zhang and Ling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingsong Yang, cXN5YW5nQHNjc2lvLmFjLmNu; Juan Ling, bGluZ2p1YW5Ac2NzaW8uYWMuY24=
 Huiming Sun
Huiming Sun Qingsong Yang
Qingsong Yang Junde Dong1,4
Junde Dong1,4 Jie Li
Jie Li Chang Chen
Chang Chen Ying Zhang
Ying Zhang Juan Ling
Juan Ling