- Kewalo Marine Laboratory, University of Hawai`i at Mānoa, Honolulu, HI, United States
Coral reef ecosystems, especially those in Hawai`i, are increasingly threatened by marine plastic pollution, which may impair coral reproduction. Microplastics contain toxic, persistent, and endocrine-disrupting chemicals. While negative effects of microplastic ingestion have been observed on adult coral viability and health, very few studies have explored impacts on reproduction. Recent studies found that microplastic leachate negatively affects coral fertilization likely due to plastic additives incorporated into products during manufacturing. This study explored the effects of microplastic leachate on coral planula larvae settlement and survival. Planula larvae of the broadcast spawning species, Montipora capitata, and the brooding species, Harbor Porites, were exposed to microplastic leachate from four plastic types [nylon, polypropylene (PP), high-density polyethylene (HDPE), or low-density polyethylene (LDPE)] at three concentrations (50, 100, or 200 particles/L) for 7 days. Settlement and survival were affected by leachate concentration and polymer type strongly on Days 5 and 7 indicating delayed and/or potentially cumulative effects. The most pronounced negative effects on survival were observed with HDPE—100 and LDPE—200 treatments. One treatment unexpectedly promoted settlement (HDPE—200) likely due to attractive chemical cues released by the leachates. This is particularly concerning, as it suggests that planula larvae may be drawn to settle on degraded or suboptimal substrates affecting reef recruitment and replenishment. Species-specific responses were also found, with Harbor Porites exhibiting higher survival and variable settlement. Overall, microplastic leachate significantly impacted survival and settlement of both Montipora capitata and Harbor Porites planula larvae with complex temporal, species-specific, polymer-type, and concentration-dependent effects. Microplastic leachate presents an additional stressor to already threatened coral species making addressing both local and global stressors critical for the protection of coral reef ecosystems.
1 Introduction
Coral reef ecosystems are among the most biodiverse ecosystems on our planet providing important ecological, economic, and cultural resources to millions of people (Cinner et al., 2016; Woodhead et al., 2019). These vital ecosystems are threatened by both global and local level stressors, including ocean warming and ocean acidification, pollution, habitat destruction, disease, eutrophication, and overfishing (Hughes et al., 2017; Good and Bahr, 2021). An additional threat of concern is marine plastic pollution, a prevalent contaminant on reefs, which can affect the health of multiple marine organisms in the ecosystem including corals (Lamb et al., 2018; Pantos, 2022; Pinheiro et al., 2023). Within the Hawaiian Archipelago, corals are especially threatened by plastic pollution because some of the highest rates of marine debris accumulation can be found here due to ocean circulation patterns (Brignac et al., 2019). A combination of the physical (size, shape, and color), chemical (polymer type, additives, and sorbed), and biological characteristics (biofilms, hitchhiking organisms, etc.) of plastics influences their effects on corals. Corals can not only be smothered or covered by larger macroplastics (Yoshikawa and Asoh, 2004; de Carvalho-Souza et al., 2018) but also experience adhesion (Martin et al., 2019; Corona et al., 2020; Kim et al., 2024), ingestion (Hall et al., 2015; Reichert et al., 2018; Rotjan et al., 2019), and incorporation of micro- and nanoplastics (Corinaldesi et al., 2021; Hierl et al., 2021; Jandang et al., 2024).
Previous studies focused on the effects of the ingestion of microplastics on coral health and resilience have shown a variety of effects due to the plastic concentration, type, size, and shape. Ingestion of microplastics has been found to have species-specific effects on health (growth and algal symbiosis), cellular/molecular responses (antioxidant and immune enzyme expression, detoxification, photosynthetic performance, and metabolite profiles), and stress responses (mucus production, bleaching, and tissue inflammation) (Chapron et al., 2018; Tang et al., 2018; Axworthy and Padilla-Gamiño, 2019; Mouchi et al., 2019; Reichert et al., 2019; Syakti et al., 2019; Lanctôt et al., 2020; Savinelli et al., 2020; Tang et al., 2021). Most of the effects seen were due to physical characteristics (e.g., size, shape) raising questions about the effects of microplastic-associated chemicals.
Microplastics also pose a chemical threat to coral health due to both the toxic chemicals added during manufacturing (additives) and chemicals absorbed from the environment (sorbed) (Seidensticker et al., 2018; Caruso, 2019; Campanale et al., 2020). These chemicals include a variety of toxicants that are cancer causing, persistent, bioaccumulative, and endocrine disrupting (Gallo et al., 2018; Yu and Singh, 2023). Of the studies that explore the effects of microplastic-associated chemicals on coral health, bioaccumulation has been seen from hexabromocyclododecanes in polystyrene (Aminot et al., 2020). Microplastic-associated chemicals have also been observed inhibiting energy metabolism enzymes from chromium [Cr(III)] in polyethylene and apoptosis of the Symbiodiniaceae (Xiao et al., 2023). Additionally, the effects of Cr(III) were more obvious than the effects of polyethylene alone further suggesting that the physical threats of microplastic interactions are less severe than the effects of the chemical contaminants.
Although most of the current research has focused on microplastic effects on coral health, sublethal effects on coral reproduction could also be occurring. Corals have two main modes of sexual reproduction: brooding and spawning (Richmond, 1997). Brooding coral species internally produce eggs and sperm, which fertilize and develop into planula larvae within the coral. Alternatively, spawning coral species release egg and sperm into the water column where external fertilization and development occur. Planula larvae undergo settlement and metamorphosis where they change from a planktonic to benthic stage. The process of site selection and development is heavily mediated by chemical cues providing attractive substrates. Since corals are sessile after settlement, they likely encounter sinking particles or less dense, biofouled particles. Conversely, coral planula larvae move throughout the water column and likely encounter a variety of particles with different densities (i.e., sinking and floating plastics).
Only a few studies to date have explored the effects of microplastics on coral reproduction. Berry et al. (2019) found limited effects of weathered polypropylene on fertilization of the broadcast spawning coral, Acropora tenuis, but no significant effects on embryo development and larval settlement (Berry et al., 2019). There was a significant effect of particle size on abnormalities of the fertilized eggs exposed to large-weathered plastics resulting in significantly lower fertilization success. The mechanism for this decline in fertilization was assumed to be due to physical contact with the particles. More recently, Wilkins et al. (2024) found negative effects of microplastic leachate on fertilization and fatty acid quantity and composition of Montipora capitata eggs likely due to the endocrine-disruptive nature of many plastic additives (Wilkins et al., 2024). This study also tested the effects of microplastics alone and found more negative effects on fertilization due to the microplastic leachate likely containing associated chemicals. These two studies (Berry et al., 2019; Wilkins et al., 2024) focused on the effects of microplastic pollution on the gametes of broadcast spawning coral species and highlighted the need for further studies exploring chemical effects on coral reproduction. Based on information from these studies, chemical effects on planula larvae development have been unstudied leaving a gap in understanding the full potential effects on coral reproduction.
The purpose of this study was to test the effects of microplastic leachate from common polymer types (nylon, polypropylene, low-density polyethylene, and high-density polyethylene) at different concentrations (50, 100, and 200 particles/L) on coral settlement and development using planula larvae of the Hawaiian broadcast spawning coral Montipora capitata (Dana, 1846) and the Hawaiian brooding coral Harbor Porites (Spies, 2021). These polymer types were selected based on their environmental relevance, as they are commonly found in the Main Hawaiian Islands: polypropylene, high-density polyethylene, and low-density polyethylene are frequently found on windward beaches and sea surface samples, while nylon is most common on the seafloor (Brignac et al., 2019; Axworthy et al., 2024). The selected concentrations followed those of Berry et al. (2019) and represent a conservative estimate of microplastic concentrations in surface waters by 2100 (Koelmans et al., 2016). Harbor Porites is a brooding coral species with no known lunar reproductive cycle, while M. capitata is a broadcast spawning coral species that reproduces around the new moon from May to August in Hawai`i. Harbor Porites is a recently described coral believed to be resilient due to its ability to withstand harsh environmental conditions and its widespread occurrence in low-water quality Honolulu Harbor, where it thrives with high levels of urban runoff, low circulation, sedimentation, sewage, and oil spills (AECOS, Inc 2014; Spies, 2021). M. capitata is a common coral species found throughout Hawai`i. We hypothesized that planula larvae of both M. capitata and Harbor Porites would exhibit reduced survival and settlement due to exposure to increasing concentrations of microplastic leachate likely containing microplastic-associated chemicals.
2 Materials and methods
Coral planula larvae from two species of Hawaiian corals with different sexual reproductive strategies were used: Montipora capitata and Harbor Porites. For M. capitata, positively buoyant gamete bundles (egg and sperm) were collected on the evenings around the June and July 2023 new moon from Lilipuna Pier in Kāneohe Bay, O’ahu (21.42973°N, 157.79220°W) by placing mesh traps over five adult colonies and collecting additional gamete bundles on the ocean surface. After bringing bundles back to Kewalo Marine Laboratory (Honolulu, HI), they were mixed and kept at ambient temperature in large coolers to fertilize and develop into planula larvae. After 7 days of development, they were used in experiments. For Harbor Porites, adult colonies were collected in June and September 2023 from Sand Island, transported to Kewalo Marine Laboratory, and maintained in outdoor water tables with filtered seawater (30 µm). Any produced planula larvae were collected and separated into 1-L beakers with filtered seawater (30 µm) until use. Planula larvae from both species were exposed to microplastic leachates for 7 days. Each solution consisted of a leachate that contained virgin, recently manufactured microspheres or resin balls (4.76 mm) of one of four polymer types [nylon, low-density polyethylene (LDPE), polypropylene (PP), or high-density polyethylene (HDPE)] at one of three concentrations (50, 100, and 200 particles/L). Microspheres (PP) were purchased from McMaster-Carr, USA, while the remaining resin balls were purchased from Plastic Ball Supply, USA. Each leachate was created by leaving these plastics in 2 L beakers with 2 L of filtered seawater (30 µm) for 1 month prior to the experiment. A leachate control was also created with just filtered seawater and no additional plastics (Control—Leachate). Due to differences in reproductive cycles of the two species, M. capitata experiments took place in June and July, while Harbor Porites experiments took place in June and September.
For both species, treatment solutions of 100-ml were added to 120-ml glass jars. A control with fresh filtered seawater was included in the experiment (Control—Fresh). A total of five swimming planula larvae were added to each jar along with a pre-conditioned ceramic settlement plug conditioned by leaving it in an unfiltered seawater table to allow a biofilm to grow. All jars were placed into an outdoor, free-flowing water table to maintain ambient seawater temperature and light. The number of swimming, settled, and dead planula larvae were recorded every 2 days (Day 0, 1, 3, 5, and 7). Due to their size, sometimes planulae larvae could not be visualized and were initially labeled as “missing.” In case they were observed on later days, they were assigned as alive/swimming for the earlier measurements. If missing planula larvae were not subsequently found, they were recorded as dead. The experiments differed slightly between the species in terms of pre-conditioning of the settlement plugs and age of the planula. M. capitata planula larvae (7 days old) were used for the experiments, and the settlement plugs had been conditioned for 7 days. For Harbor Porites, planula larvae (0–6 days old) were used for the experiment, and settlement plugs had been conditioned for 11–12 days. Additionally, the number of replicates per species varied from month to month due to variability in production of planula larvae (refer to Supplementary Table S1).
2.1 Chemical analysis
The polymers used in this study to make the leachates were the same as those used by Wilkins et al. (2024) (Table 1). Based on the previous analysis using double-shot pyrolysis-gas chromatography/mass spectrometry, we know that several chemical additives were present in the plastics, but we cannot confirm that all these chemicals leached into the seawater.
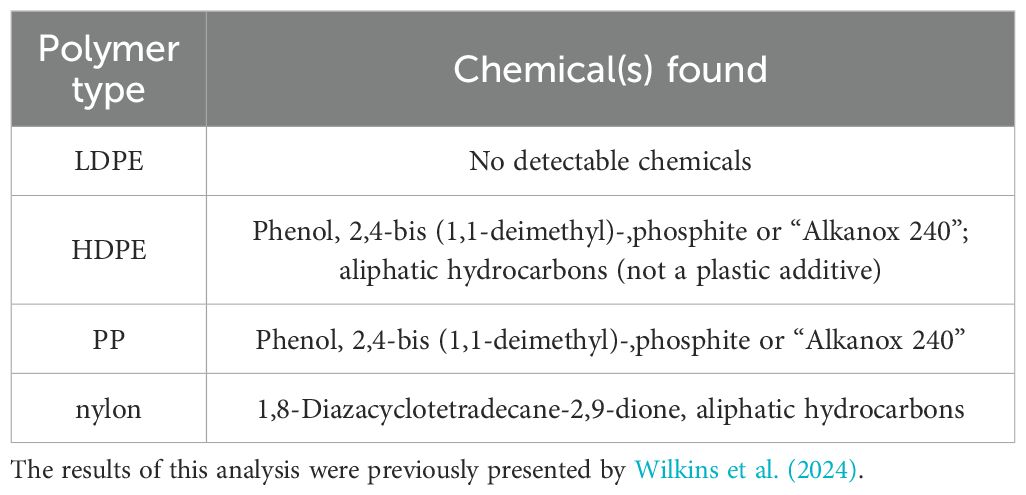
Table 1. Plastic additives found in the polymers through double-shot pyrolysis-gas chromatography/mass spectrometry.
2.2 Statistical analysis
Statistical analyses were performed to examine species-specific differences throughout the experiment to understand how different polymer types and concentrations affected survival and settlement for both species of coral planula larvae. The number of individuals per replicate was converted to a proportion for the analyses. Separate models were created for the survival and settlement analysis. For survival, the proportion of live individuals, which included both the swimming and settled planula larvae, was used as the dependent variable. For settlement, the proportion of settled individuals only was used as the dependent variable. To reduce boundary issues with beta regressions, the response variables were adjusted to avoid values equal to 0 and 1 changing the range from 0–1 to 0.0005–0.9999.
Initially, two full models were created to determine the effects of month, species, day, and treatment on planula larvae survival and settlement. However, these models showed issues with the diagnostics (e.g., quantile deviations, patterns in the residuals, and issues with variance structure). Therefore, to better meet the model assumptions, individual Generalized Linear Mixed Models (GLMM) with beta distributions and a logit link function using the glmmTMB package in R were used. All models used survival or settlement as the response variable, with Treatment and Day interactions as fixed effects and Replicate as a random effect as needed. Model selection was determined by the Akaike Information Criterion. For survival, M. capitata and Harbor Porites—June used Treatment and Day as fixed effects with Replicate as a random effect. For Harbor Porites—September, a random slope model was used with the same predictors. For settlement, M. capitata used a similar model, which added Replicate as a random effect. For Harbor Porites, adding replicate in was unnecessary due to the small variability in replicates. Post-hoc comparisons were carried out as appropriate using Tukey’s honestly significant difference (HSD) tests. All analyses were carried out in R studio (version 2024.12.0 + 467) and validated with Julius AI. Some M. capitata replicates could not be analyzed due to flooding of some of the jars on Day 6, so some counts on Day 7 are missing (see Supplementary Table S1). The data from the previous 5 days were still included in the analysis.
3 Results
3.1 Survival
July M. capitata experiments were removed from the analysis because the Control—Fresh treatment mean survival on Day 7 was very low (0.30 ± 0.30). All other experimental trials had higher planula larvae survival (>0.60, see Table 2). The Control—Leachate treatment mean survival percentages were consistently higher compared to the Control—Fresh treatments (Table 2).
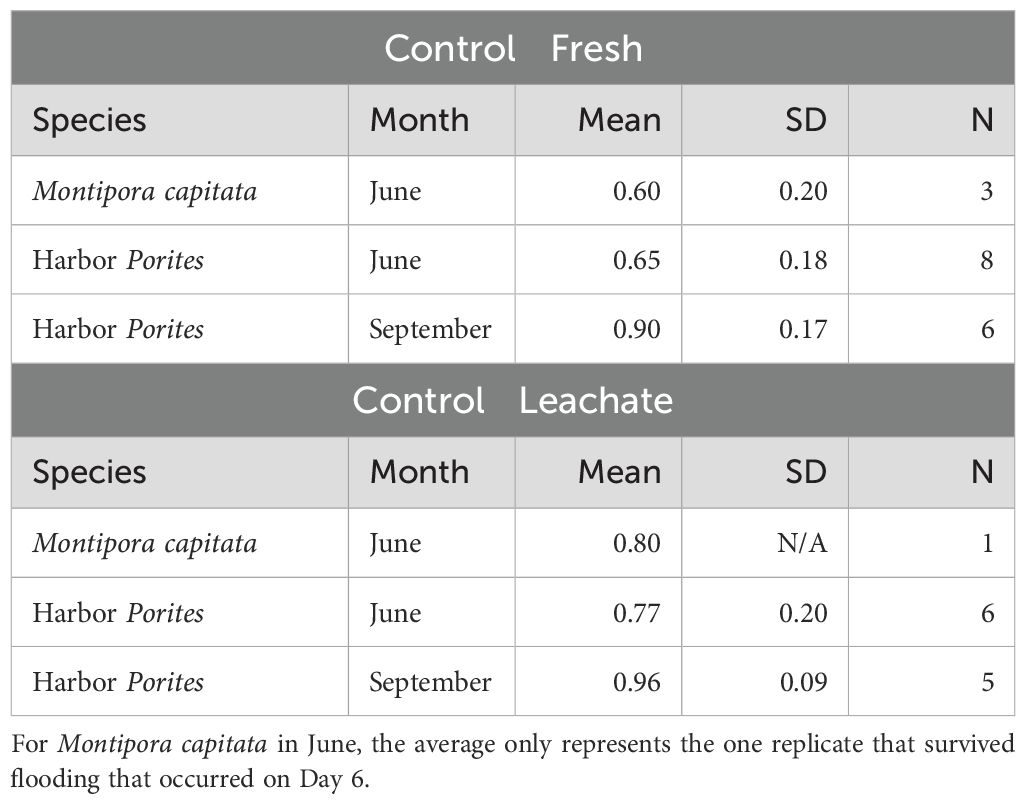
Table 2. The proportion of average survival on Day 7 of the experiments for the control treatments (Control‐Fresh and Control‐Leachate) for each month and species.
Harbor Porites planula larvae showed consistently higher average survival for most treatments when compared to those of M. capitata (Figure 1). Additionally, results from each of the months seemed to have different trends and within days of the experiment (hereon referred to as Day).
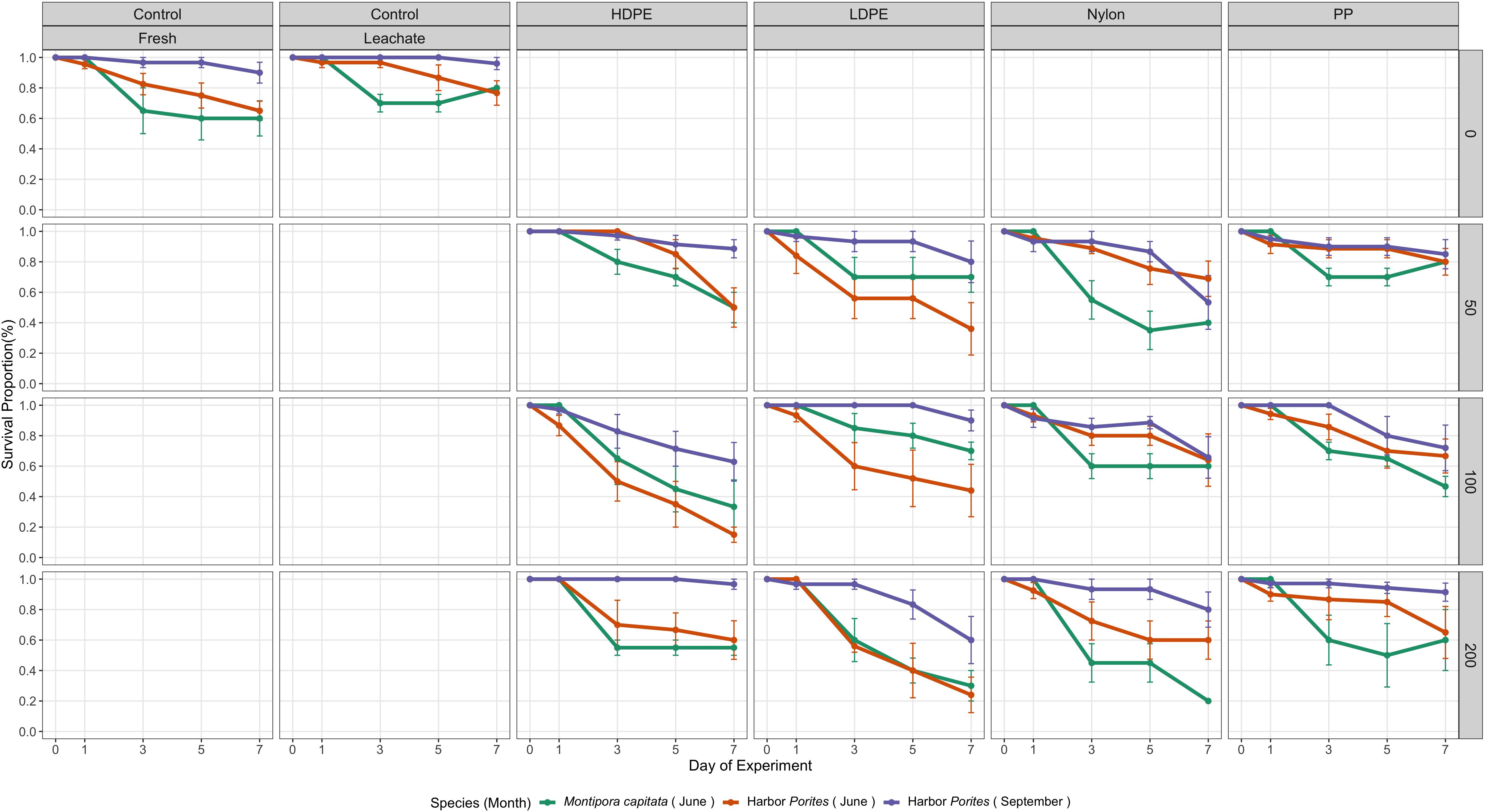
Figure 1. Average proportion of live planula larvae by treatment within each species and month over time. Error bars indicate standard error. Polymer abbreviations: PP, polypropylene; HDPE, high-density polyethylene; LDPE, low-density polyethylene.
3.1.1 M. capitata
Planula larvae showed a strong significant decrease in survival over time that varied by treatment, not within. There was no significant difference between any treatment and the controls. Since flooding occurred on Day 6, several treatments had less replicates on Day 7 likely resulting in post-hoc comparisons showing significant differences only on Day 5. Planula larvae in the LDPE—100 treatment showed significantly higher survival than those in the HDPE—100 (p-value = 0.034) and LDPE—200 (p-value = 0.021) treatments on Day 5 (Table 3).
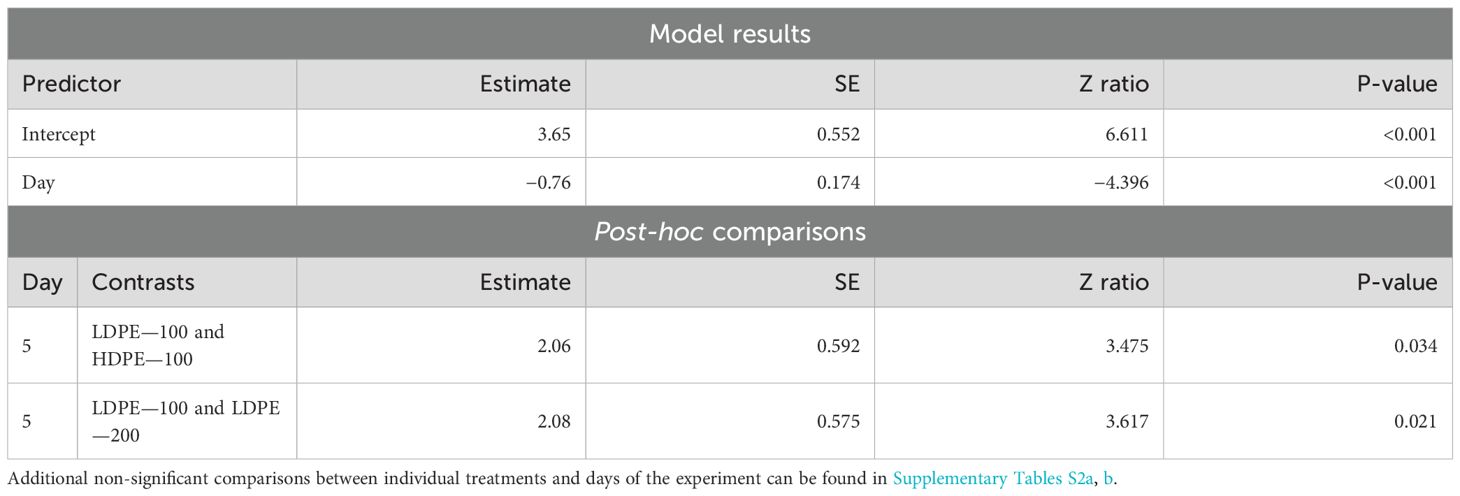
Table 3. Summary of significant model results and all post-hoc comparisons of M. capitata planula larvae survival across all days of the experiment.
3.1.2 Harbor Porites—June
Planula larvae survival showed a strong decrease over time (p-value < 0.001) and across the HDPE—100 (p-value = 0.017) and LDPE—200 (p-value = 0.006) treatments. Significant effects in survival were seen between treatments for Day 3, 5, and 7 (Table 4). For Day 3, planula larvae in the HDPE—100 treatment showed significantly lower survival than those in both control treatments (Control—Fresh p-value = 0.021; Control—Leachate p-value < 0.001). Planula larvae in the HDPE—100 treatment also showed lower survival than those exposed to HDPE at the lowest concentration (HDPE—50 p-value = 0.028) and a different polymer type at the same concentration (PP—100 p-value = 0.039). LDPE—100 and LDPE—200 treatment exposed planula larvae also showed lower survival compared to those in the Control—Leachate treatment (LDPE—100 p-value = 0.024; LDPE—200 p-value = 0.004). For Day 5, similar trends were seen within several treatments, with planula larvae having significantly higher survival than those in the HDPE—100 treatment, including both control treatments (Control—Fresh p-value = 0.015; Control—Leachate p-value < 0.001) and PP—100 treatment (p-value = 0.022). Planula larvae in the LDPE—200 treatment had significantly lower survival than those in both control treatments (Control—Fresh p-value = 0.012; Control—Leachate p-value < 0.001) and PP at the same concentration (PP—200 p-value = 0.003). Planula larvae in the LDPE—100 treatment also showed lower survival than those in the Control—Leachate treatment (p-value = 0.021). For Day 7, more significant differences were seen between treatments and HDPE and LDPE. Again, in the LDPE—200 treatment, planula larvae showed lower survival than those in both control treatments (Control—Fresh p-value = 0.034; Control—Leachate p-value = 0.001) and the PP treatment at the same concentration (PP—200 p-value = 0.007). Lastly, planula larvae in the HDPE—100 treatment showed lower survival compared to those in the Control—Leachate treatment (p-value = 0.002).
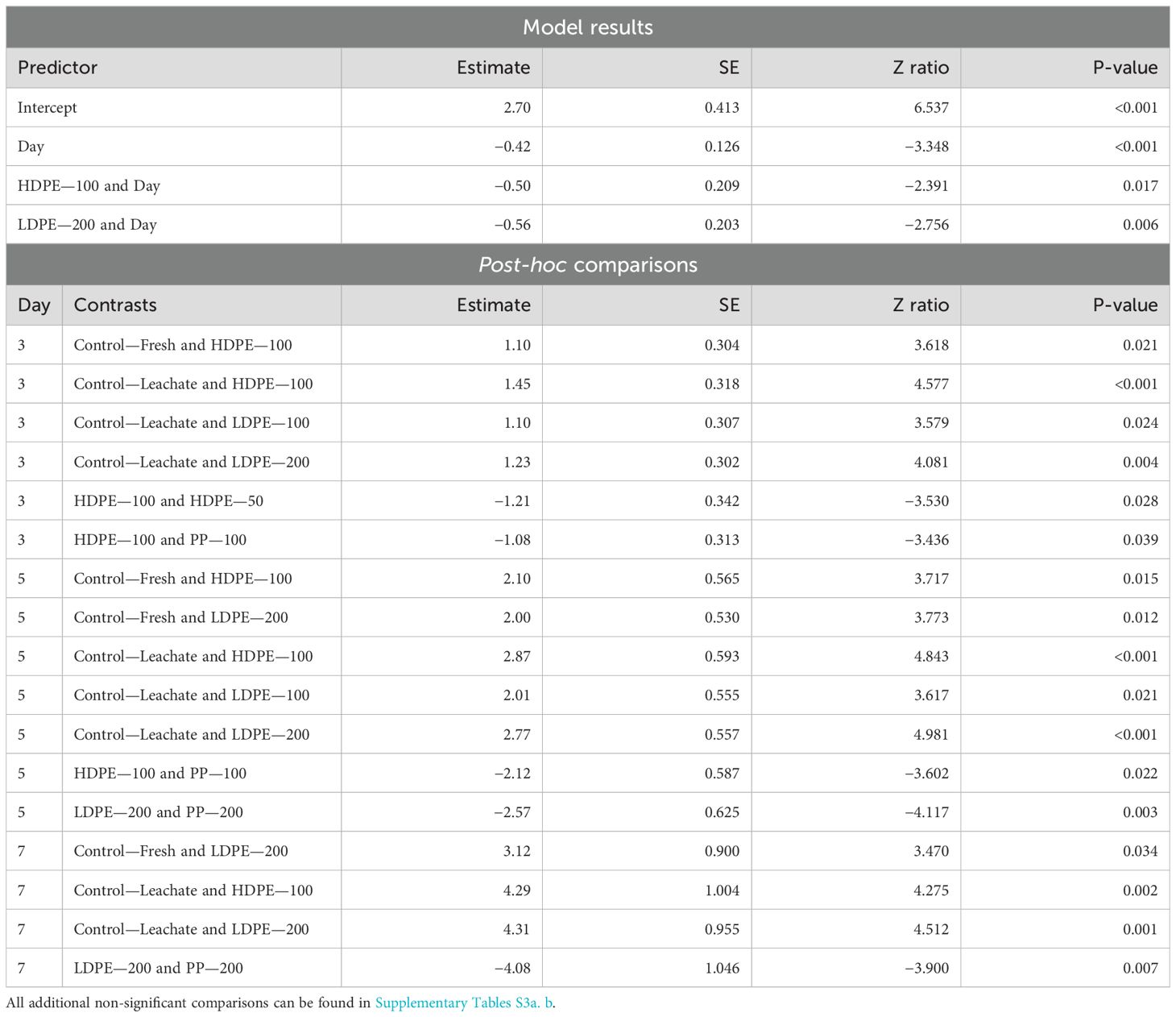
Table 4. Summary of significant model results and post-hoc comparisons of Harbor Porites planula larvae survival across all days of the experiment in June.
3.1.3 Harbor Porites—September
Like what was seen in June for Harbor Porites, significant declines in planula larvae survival were seen over time across the HDPE—100 (p-values = 0.005) and nylon—50 treatments (p = 0.024). Between treatments, significant effects were only seen for Day 5 and 7 (Table 5). Planula larvae exposed to several polymer types and concentrations had significantly lower survival than the controls. For both Day 5 and 7, HDPE—100 treatment exposed planula larvae had significantly lower survival than both control treatments. Nylon—50 treatment-exposed planula larvae also had significantly lower survival than those exposed to Control—Leachate on Day 5 only (p-value = 0.042). HDPE—100 treatment-exposed planula larvae had significantly lower survival than those in several other treatments on both Day 5 and 7 including the other two HDPE concentrations and the LDPE—100 treatment.
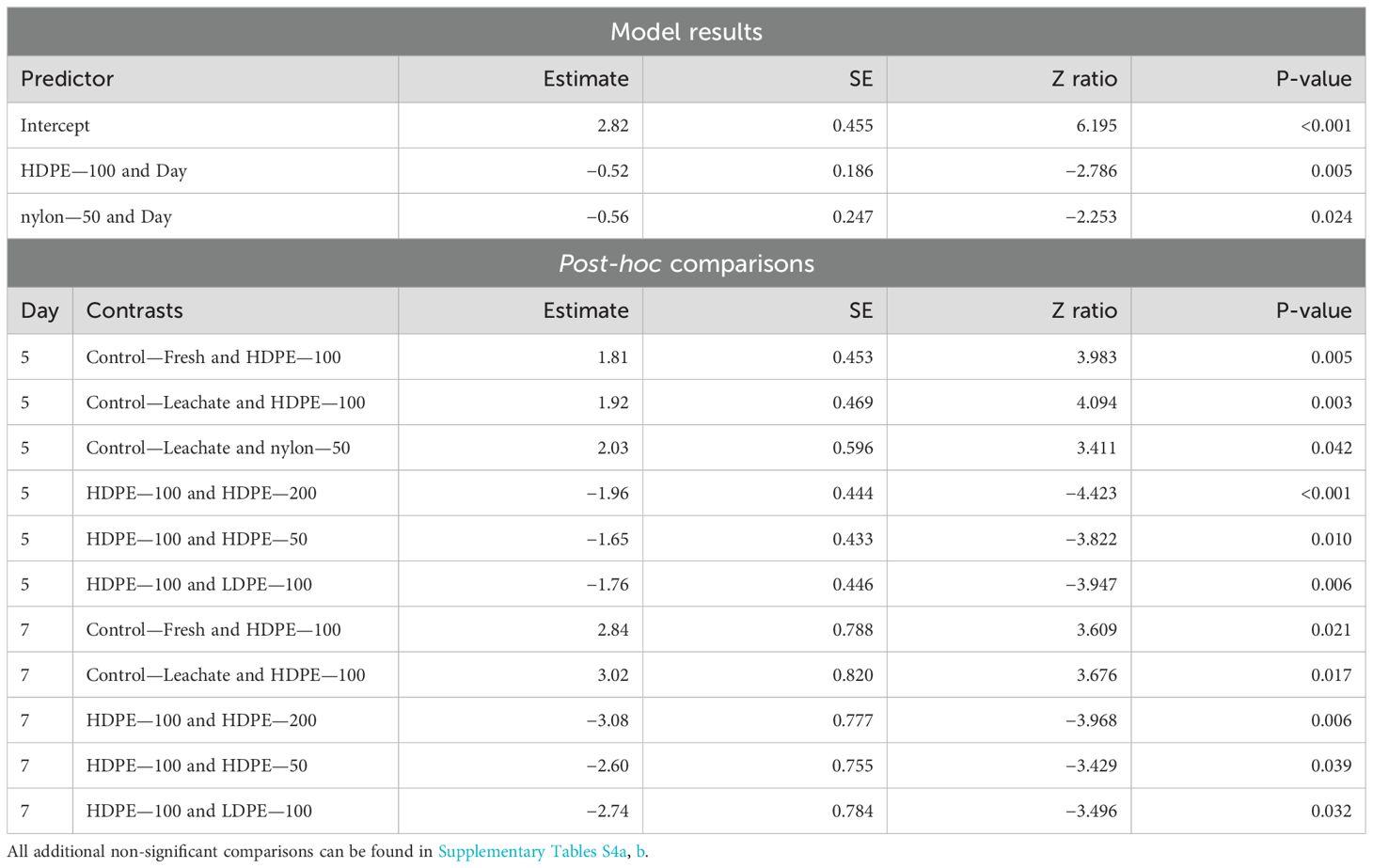
Table 5. Summary of significant model results and post-hoc comparisons of Harbor Porites planula larvae survival across days of the experiment in September.
3.2 Settlement
The June M. capitata experiment showed the highest mean settlement proportion in the Control—Fresh treatment (0.40 ± 0.20) (Table 6). The Control—Leachate treatment had the same average settlement proportion. For Harbor Porites, the June settlement proportions were higher for both controls. Interestingly, the June Control—Leachate treatment mean survival proportion (0.60) was higher than that of any other control or month for either species.
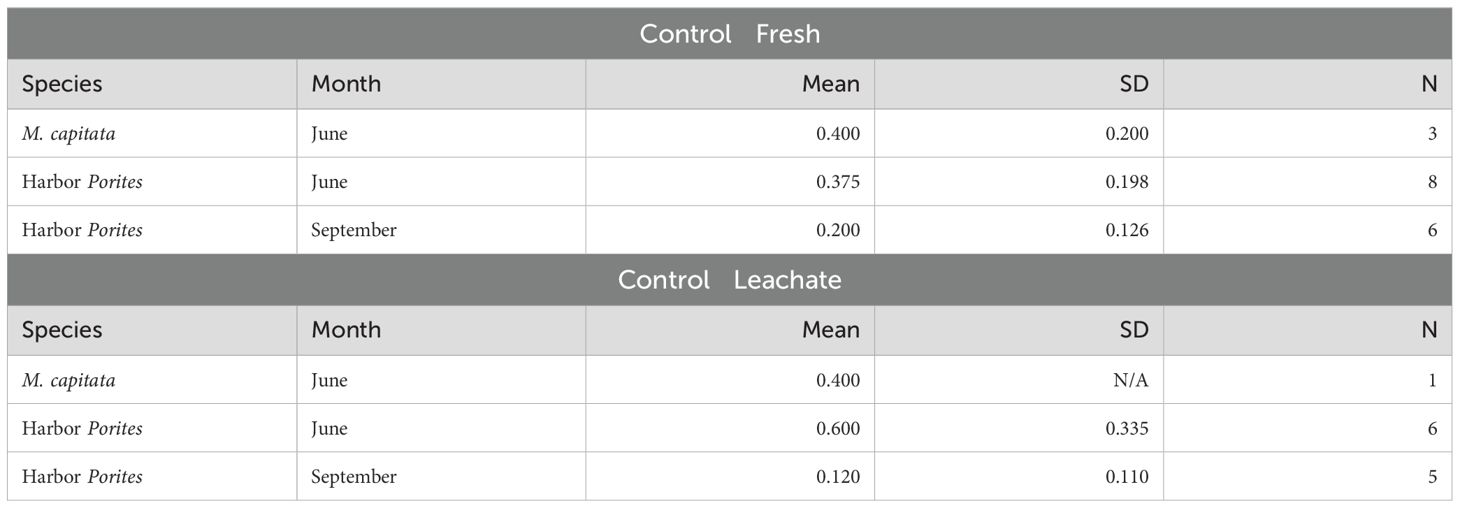
Table 6. Proportion of average settlement on Day 7 for the control treatments (Control‐Fresh and Control‐Leachate) for each month and species.
Each species showed very different trends in the proportion of settled planula larvae with M. capitata showing generally higher settlement proportions (Figure 2). Additionally, each of the months and days within the experiment showed different trends.
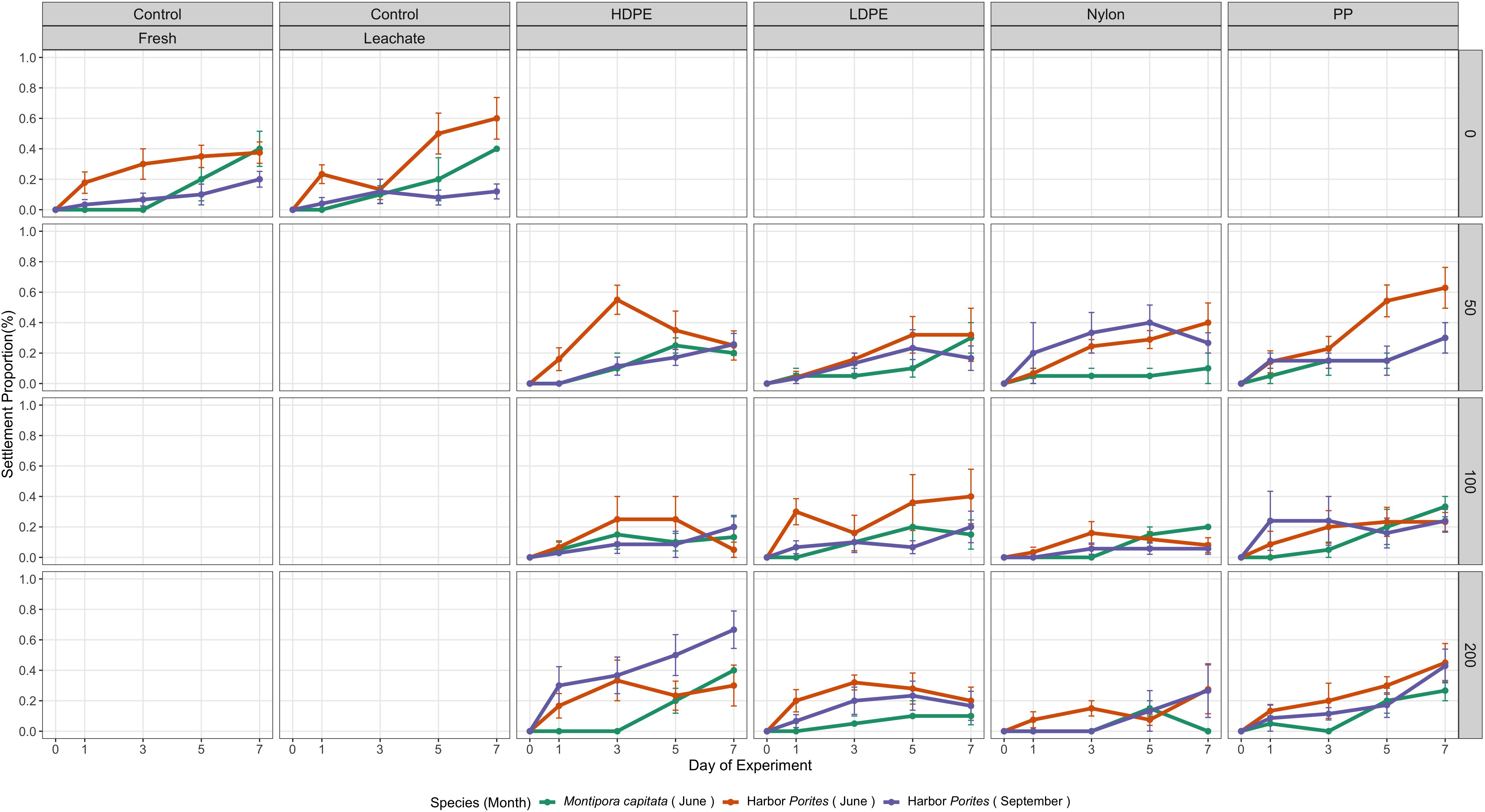
Figure 2. Average proportion of settled planula larvae by treatment within each species and month over time. Error bars indicate standard error. Polymer abbreviations: PP, polypropylene; HDPE, high-density polyethylene; LDPE, low-density polyethylene.
3.2.1 M. capitata
Settlement proportions significantly increased over time (p-value = 0.001), and so did their variability (Table 7). Settlement significantly increased over time for planula larvae in both the HDPE—100 (p-value = 0.045) and nylon—50 (p-value = 0.052) treatments. Planula larvae in the Control—Fresh treatment showed the highest mean settlement proportion (0.395) on Day 7. Planula larvae in the HDPE—100 and nylon—50 treatments showed significant interactions with Day, but post-hoc comparisons did not find any significant treatment differences.
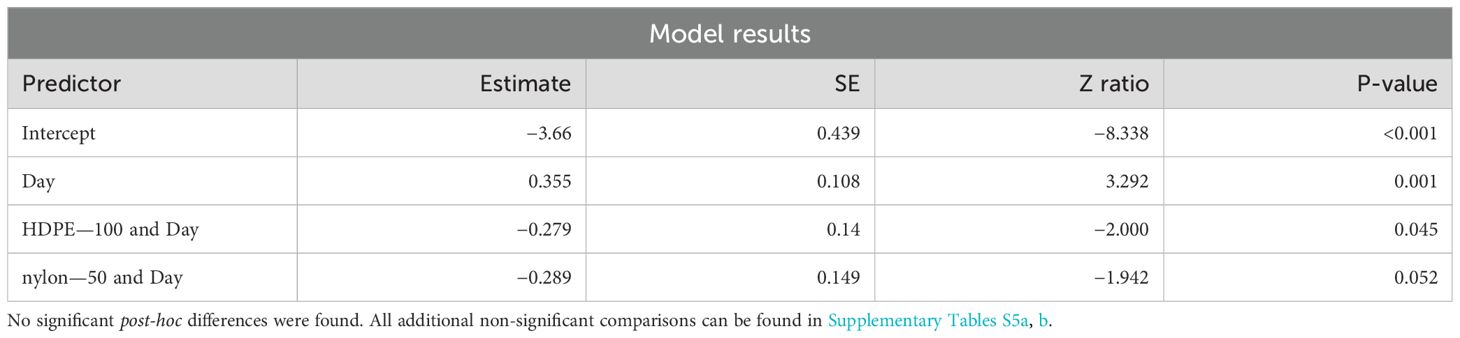
Table 7. Summary of significant model results of M. capitata planula larvae settlement across all days of the experiment.
3.2.2 Harbor Porites—June
Settlement significantly increased over time (p-value = 0.001). There was no significant difference in planula larvae settlement between treatments and the Control—Fresh treatment (Table 8). The positive estimate and significant days suggest an increase in settlement as the experiment went on. Two treatments showed significantly lower planula larvae settlement rates on Day 5 compared to the Control—Leachate treatment: HDPE—100 (p-value = 0.043) and nylon—100 (p-value = 0.043) treatments.
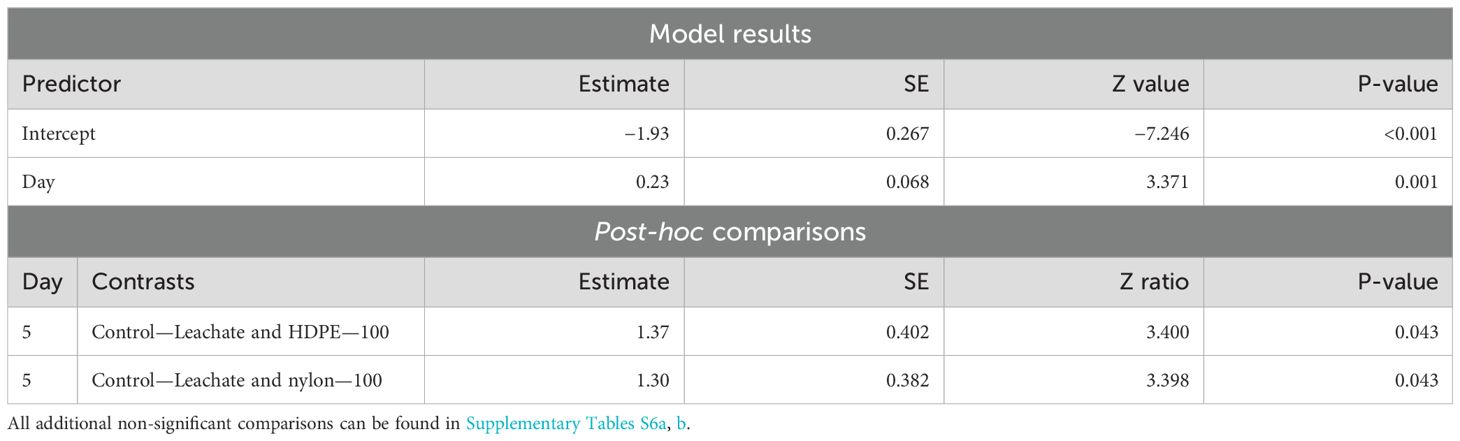
Table 8. Summary of significant model results and post-hoc comparisons of Harbor Porites planula settlement across all days of the experiment in June.
3.2.3 Harbor Porites—September
Settlement significantly increased over time (p-value = 0.049) and within the HDPE—200 treatment (p-value = 0.004) (Table 9). Following post-hoc analysis, significant differences between treatments were seen for Day 3, 5, and 7. On Day 3, the planula larvae in the HDPE—200 treatment had significantly higher settlement than a lot of treatments including both control treatments (Control—Fresh p-value = 0.001, Control—Leachate p-value = 0.002) and the HDPE—100 treatment (p-value < 0.000). Compared to other polymers, planula larvae in the HDPE—200 treatment also had significantly higher settlement than other polymers at 200 particles/L concentration on Day 5 (LDPE—200 p-value = 0.012; nylon—200 p-value = 0.005). On Day 5, the differences between treatments increased with planula larvae in the HDPE—200 treatment again showing the strongest differences compared to other treatments including both control treatments (Control—Fresh and Control—Leachate p-value < 0.0001) and the other HDPE concentrations (HDPE—50 p-value = 0.001; HDPE—100 p-value < 0.0001). Between polymers, planula larvae in the HDPE—200 treatment had higher settlement than all other polymer types at the same concentration: the LDPE—200 treatment (p-value < 0.0001), the PP—200 treatment (p-value = 0.043), and the nylon—200 treatment (p-value < 0.000) like what was seen for Day 3. The nylon—50 treatment-exposed planula larvae also showed significantly higher settlement than those in the nylon—100 treatment (p-value = 0.015). On Day 7, planula larvae in the HDPE—200 treatment again showed higher settlement than those in both control treatments (Control—Fresh p-value < 0.0001; Control—Leachate p-value < 0.0001), the other HDPE treatments (HDPE—50 p-value = 0.002; HDPE—200 p-value < 0.0001) and LDPE—200 (p-value < 0.0001).
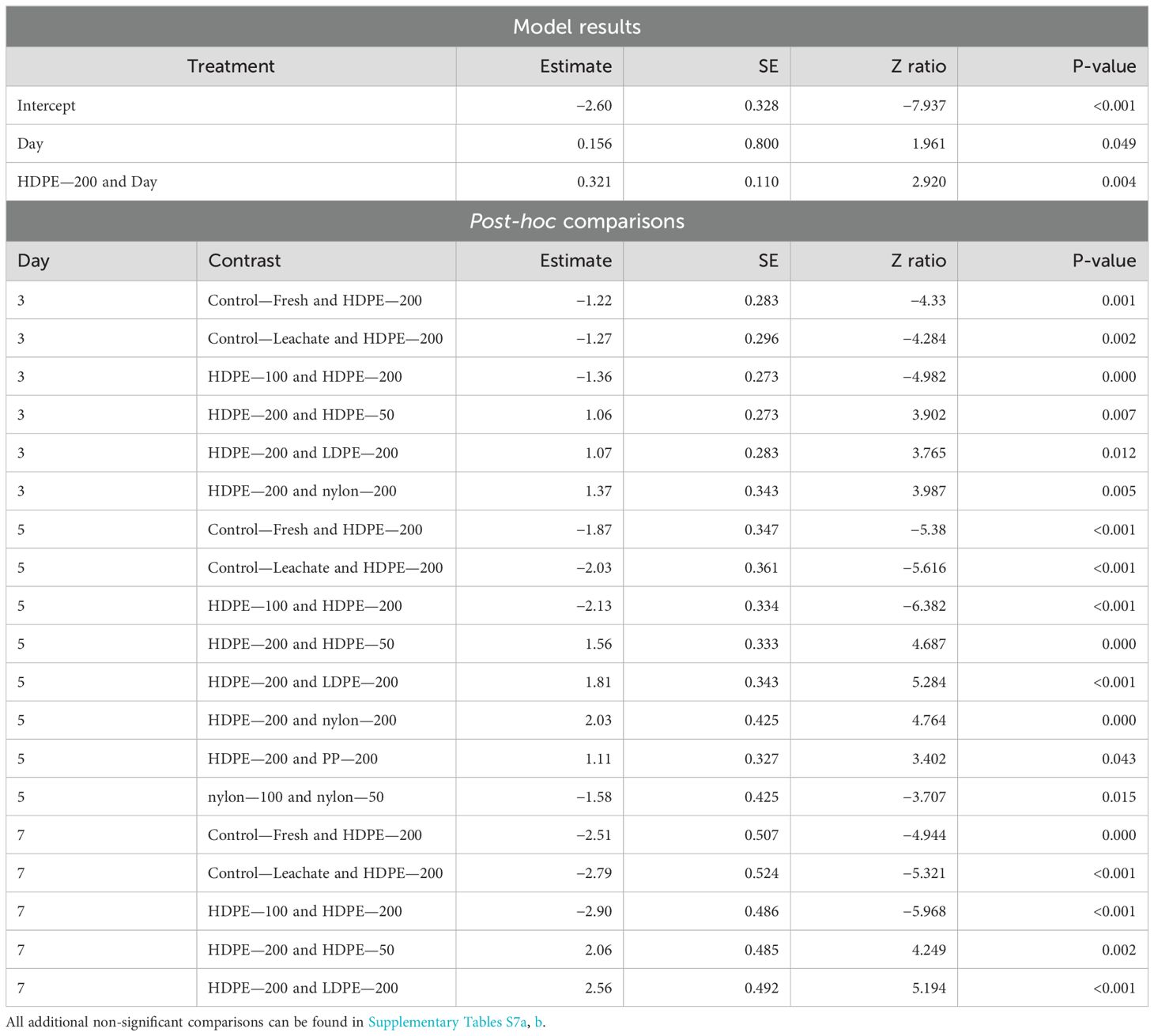
Table 9. Summary of significant model results and post-hoc comparisons of Harbor Porites planula settlement across all days of the experiment in September.
4 Discussion
Microplastic leachate had negative effects on survival and settlement of Montipora capitata and Harbor Porites planula larvae with species-specific and time-dependent effects seen. Harbor Porites planula larvae had higher survival and settlement compared to those of M. capitata in many circumstances. The effects seen vary by months and days of the experiment emphasizing the importance of time in survival and settlement analyses. Polymer type and concentration had significant effects with high-density polyethylene (HDPE) and low-density polyethylene (LDPE) treatments having the most significant effects across species and experiments. Overall, results from this study highlight that the relationship between microplastic leachate and coral planula larvae survival and settlement is complex with species-specific differences, temporal variability, and treatment effects.
Species-specific differences were seen in survival and settlement potentially due to biological differences between M. capitata and Harbor Porites. M. capitata is a broadcast spawning coral and is generally considered more sensitive to environmental stressors. In contrast, Harbor Porites is a brooding coral that thrives and reproduces in a harbor habitat with wide salinity and water quality ranges that few other coral species have been seen in (Spies, 2021). There is very little current information about Harbor Porites in terms of genetic variability and its unique reproductive strategy as Porites corals are generally spawning corals. These differences in reproductive strategy, colony and larval physiology, and life history may have contributed to overall effects seen under certain leachate exposures.
Survival varied significantly by species, with Harbor Porites showing greater resilience than M. capitata. However, Harbor Porites planula larvae in the HDPE—100 treatment had consistently lower survival in both June and September with September showing the most negative effects compared to those of the other HDPE concentrations. Planula larvae in the LDPE—200 treatment showed lower survival than in several treatments in June for Harbor Porites. Between species, negative effects were seen in planula larvae survival of both species in the HDPE—100 and LDPE—200 treatments with more significant effects seen in Harbor Porites. Both the polymer type and concentration influenced survival with significant effects seen on the final days of the experiment (Day 5 and 7) suggesting a delayed or cumulative negative effect. A longer experiment could parse out the specific effects and any delayed effects seen. There may be a non-linear relationship or non-monotonic responses to concentration as commonly seen in studies on endocrine-disrupting chemicals due to the complex way in which hormones are mimicked within organisms (Vandenberg et al., 2012; Lagarde et al., 2015).
Settlement responses were not as consistent with Harbor Porites having generally higher settlement rates, while M. capitata had more variability in treatments and time. There were no matching effects seen between species or months. Unexpectedly, the most pronounced effects were seen in September for Harbor Porites with higher settlement seen in HDPE—200 treatment-exposed planula larvae over time and compared to the control treatments on Days 3, 5, and 7 suggesting that leachate may provide attractive chemical cues and/or induce a stress response. This is supported by the idea that microplastics give off chemical cues that make them attractive for ingestion by adult corals (i.e., phagostimulants) (Allen et al., 2017).
Planula larvae viability due to environmental conditions of the adult colonies also likely played a role in the different survival and settlement rates seen between months. During the summer of 2023, when these experiments took place, global sea surface temperatures recorded the warmest year since 1850 now referred to as a super-marine heatwave (Huang et al., 2024). The global mean sea surface temperatures reached record high levels in April 2023 and were broken again in July and August 2023 with high temperatures persisting throughout the remainder of 2023 (Huang et al., 2024). This high temperature corresponds with the time when the experiments took place, which could explain the month-specific responses seen, particularly for June. Planula larvae that are already impacted by other global or local stressors are more likely to be susceptible to microplastic pollution. This has been previously suggested for adult colonies exposed to multiple stressors (Reichert et al., 2021; Bove et al., 2023). Microplastics present an additional stressor of concern, but likely do not have as significant of an impact on their own as suggested by the September survival data. This finding further supports the management strategy of focusing on mitigating local stressors to buy time to address the global stressors.
The findings of this study are not particularly surprising due to the negative effects of microplastic leachate seen on coral fertilization and fatty acids (Wilkins et al., 2024). Specifically, eggs from the HDPE—100 treatment showed significantly lower fertilization rates consistent with the survival and settlement trends seen in this study. However, this study did not look at fatty acids, so it is possible that sublethal effects may have been missed. Additionally, although microplastic leachate can negatively affect fertilization, survival, and settlement, these are only two points in the coral reproduction process. Exposure of either adults during the development of gametes or eggs and sperm into planula larvae may result in very different effects.
The settlement effects seen in this study are likely not representative of effects at other stages of reproduction. Since planula larvae were only tracked for 7 days, effects on metamorphosis and growth could not be fully assessed. If we simplify the life cycle of a coral into four key stages, adults, gametes, planula larvae, and juveniles, effects of microplastic-associated chemicals could have very different impacts at each life history stage. For example, ingestion of microplastics has a wide variety of negative effects on adult coral health and growth with implications for increased energy allocation to survival and, thus, likely reduced energy allocation to reproduction (Hankins et al., 2021). Exposure of adults to microplastic-associated chemicals through ingestion could impact the development of gametes and brooded planula larvae quality and production. Given the endocrine-disrupting and lipophilic nature of microplastics and their accumulation in the gastrovascular cavity where egg production occurs, these contaminants could accumulate in eggs and again affect quality and colony fecundity. Beyond gametogenesis, external fertilization during spawning has already been seen to be affected along with fatty acid quantity and composition, which are critical for early development (Wilkins et al., 2024). At the larval stage, planula larvae may settle in unfavorable and degraded conditions due to attractive chemical cues given off by chemicals or biofilms. In addition to effects in the water column, plastic can also be a misleading substrate that attracts settlement over natural substrates of corals (Bergami et al., 2021) and other species of marine larvae (Pinochet et al., 2020; Audrézet et al., 2022) due to its accumulation of attractive biofilms, which mimic natural settlement cues. While this may be seen as a beneficial aspect of plastic pollution, especially as a settlement promoter in degraded environments, plastics degrade over time, are easily transported, and may expose vulnerable settled larvae to new stressors. Finally, given that juveniles could also be ingesting nano and microplastics, the effects seen may affect growth and development like what has already been seen in adults.
While this study provides valuable insights, some limitations include the lack of direct, laboratory-based control testing of chemicals in the leachate and consideration of additional environmental variables. Of the chemicals found within the particles, there is no known toxicity information (Dopico-García et al., 2007). However, only chemicals within the specific plastics were tested, and none were tested within the leachate to confirm that they had leached and to determine their specific concentrations. Given our results, the concentrations used may also be misleading as an increased number of particles does not necessarily mean an increase in chemicals in the leachate. This is due to the complex leaching behavior of plastics, which depends on water parameters like pH, water temperature, and characteristics of the plastic, including age and texture (Koelmans et al., 2014; Li and Tang, 2023; Iftikhar et al., 2024). Additionally, the plastics used in this study were virgin plastics, but weathered plastics leach more additives (Luo et al., 2022) and can contain sorbed chemicals, which would likely have very different effects. Size of the particles might also be an important factor. Using smaller particles may have produced more dramatic effects due to more leaching of endocrine-disrupting compounds as seen in previous studies (Chen et al., 2019). Additionally, the microbial growth within the leachate was not considered as a potential influence on some of the positive or negative settlement effects seen. Taken altogether, future research should include a detailed chemical analysis of any microplastic leachates used, the use of environmental plastics, and the investigation of leachate from smaller particles for more realistic conditions.
The vulnerability of coral planula larvae to microplastic leachates seen here is consistent with effects seen for other marine animals. Microplastic leachate has negative effects on embryo development of the brown mussel, Perna perna (e Silva et al., 2016), the sea urchins, Paracentrotus lividus (Martínez-Gómez et al., 2017; Rendell-Bhatti et al., 2021) and Strongylocentrotus purpuratus (Paganos et al., 2023), the sea cucumber, Apostichopus japonicus (Wang et al., 2023), and the survival and settlement of barnacle nauplii, Amphibalanus amphitrite (Li et al., 2016). The complex effects of microplastic leachate demand more research and emphasize a knowledge gap on the most vulnerable stages of marine organism life cycles.
5 Conclusions
This is the first study to provide evidence of the effects of microplastic leachate on coral planula larvae survival and settlement. These species-specific, polymer-type, and time-dependent effects suggest potential delayed or cumulative effects. One leachate promoted settlement suggesting that attractive chemical cues from microplastics may be a concern. The findings from this study highlight the complex effects that marine plastic pollution could be having on the most vulnerable life stages of corals. Given the vulnerability of the early life stages of corals and the many other stressors that they currently face, a greater understanding of microplastic leachate effects on different life history stages of corals is needed to understand potential effects on recruitment and replenishment of reefs. Future research must consider environmental plastics containing sorbed pollutants, different sizes of particles, and a better understanding of the leaching behavior of particles combined with a broader species analysis to truly understand the extent of marine microplastic pollution effects on corals.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
KW: Investigation, Data curation, Visualization, Methodology, Resources, Conceptualization, Funding acquisition, Project administration, Writing – original draft, Formal Analysis, Software, Writing – review & editing. RR: Validation, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. KW is supported by the National Oceanic and Atmospheric Administration’s Dr. Nancy Foster Scholarship for educational expenses.
Acknowledgments
The authors would like to thank Mackenzie Jahnke and Maya Singh for support in the creation of the leachate solutions. A special thank you to Dr. Meredith Seeley for the Py-GC/MS analysis. Lastly, the authors would like to thank Dr. Jessica Reichert (Haver) for her feedback on this manuscript. Harbor Porites were collected under Hawaii Division of Aquatic Resources Special Activity Permit (SAP No SAP 2023-12).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1596594/full#supplementary-material
References
AECOS, Inc (2014). Water Quality and Biological Surveys (HM 2007, Appendix E), 37 and surveys in 2012 by Marine Research Consultants, Inc., entitled Assessment of the Marine Biological Draft Environmental Assessment – Honolulu Harbor Piers 24–29 Subdivision 5-21–1 Community Structure in the Vicinity of the Proposed Kapalama Container Terminal, Honolulu, Hawai’i, 2 which was completed in conjunction with an EIS prepared for the Kapālama Container Terminal and 3 Tenant Relocations project (DOT-H 2014, Appendix E). Available online at: http://oeqc.doh.hawaii.gov/Shared%20Documents/EA_and_EIS_Online_Library/Oahu/2010s/2013-01-23-DEA-5B-Honolulu-Harbor-Pier-12-15-Improvements.pdf.
Allen A. S., Seymour A. C., and Rittschof D. (2017). Chemoreception drives plastic consumption in a hard coral. Mar. pollut. Bull. 124, 198–205. doi: 10.1016/j.marpolbul.2017.07.030
Aminot Y., Lanctôt C., Bednarz V., Robson W. J., Taylor A., Ferrier-Pagès C., et al. (2020). Leaching of flame-retardants from polystyrene debris: Bioaccumulation and potential effects on coral. Mar. pollut. Bull. 151, 110862. doi: 10.1016/j.marpolbul.2019.110862
Audrézet F., Zaiko A., Cahill P., Champeau O., Tremblay L. A., Smith D., et al. (2022). Does plastic type matter? Insights into non-indigenous marine larvae recruitment under controlled conditions. PeerJ 10, e14549. doi: 10.7717/peerj.14549
Axworthy J. B., Lasdin K. S., and Padilla-Gamiño J. L. (2024). Low incidence of microplastics in coral reefs of Kāneohe Bay, Hawaii, USA. Mar. pollut. Bull. 208, 116996.
Axworthy J. B. and Padilla-Gamiño J. L. (2019). Microplastics ingestion and heterotrophy in thermally stressed corals. Sci. Rep. 9, 18193. doi: 10.1038/s41598-019-54698-7
Bergami E., Caroselli E., Vaccari L., Corsi I., Semenov A., and Macali A. (2021). Pioneer settlement of the cold-water coral Desmophyllum dianthus (Espe) on plastic. Coral Reefs 40, 1355–1360. doi: 10.1007/s00338-021-02131-9
Berry K. L. E., Epstein H. E., Lewis P. J., Hall N. M., and Negri A. P. (2019). Microplastic contamination has limited effects on coral fertilisation and larvae. Diversity 11, 228. doi: 10.3390/d11120228
Bove C. B., Greene K., Sugierski S., Kriefall N. G., Huzar A. K., Hughes A. M., et al. (2023). Exposure to global change and microplastics elicits an immune response in an endangered coral. Front. Mar. Sci. 9, 1037130. doi: 10.3389/fmars.2022.1037130
Brignac K. C., Jung M. R., King C., Royer S.-J., Blickley L., Lamson M. R., et al. (2019). Marine debris polymers on main hawaiian island beaches, sea surface, and seafloor. Environ. Sci. Technol. 53, 12218–12226. doi: 10.1021/acs.est.9b03561
Campanale M., Savino L., and Uricchio (2020). A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Public Health 17, 1212. doi: 10.3390/ijerph17041212
Caruso G. (2019). Microplastics as vectors of contaminants. Mar. pollut. Bull. 146, 921–924. doi: 10.1016/j.marpolbul.2019.07.052
Chapron L., Peru E., Engler A., Ghiglione J. F., Meistertzheim A. L., Pruski A. M., et al. (2018). Macro- and microplastics affect cold-water corals growth, feeding and behaviour. Sci. Rep. 8, 15299. doi: 10.1038/s41598-018-33683-6
Chen Q., Allgeier A., Yin D., and Hollert H. (2019). Leaching of endocrine disrupting chemicals from marine microplastics and mesoplastics under common life stress conditions. Environ. Int. 130, 104938. doi: 10.1016/j.envint.2019.104938
Cinner J. E., Huchery C., MacNeil M. A., Graham N. A., McClanahan T. R., Maina J., et al. (2016). Bright spots among the world’s coral reefs. Nature 535, 416–419. doi: 10.1038/nature18607
Corinaldesi C., Canensi S., Dell’Anno A., Tangherlini M., Di Capua I., Varrella S., et al. (2021). Multiple impacts of microplastics can threaten marine habitat-forming species. Commun. Biol. 4, 431. doi: 10.1038/s42003-021-01961-1
Corona E., Martin C., Marasco R., and Duarte C. M. (2020). Passive and active removal of marine microplastics by a mushroom coral (Danafungia scruposa). Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00128
Dana J. D. (1846). “Zoophytes”, in United States Exploring Expedition. During the years 1838, 1839, 1840, 1841, 1842. Under the command of Charles Wilkes, U.S.N. (Philadelphia: Lea and Blanchard), 7:1-740. (For species details see pp. 504-505 and Plate 47, Fig. 4)
de Carvalho-Souza G. F., Llope M., Tinôco M. S., Medeiros D. V., Maia-Nogueira R., and Sampaio C. L. (2018). Marine litter disrupts ecological processes in reef systems. Mar. Pollut. Bull. 133, 464–471.
Dopico-García M. S., López-Vilariñó J. M., and González-Rodríguez M. (2007). Antioxidant content of and migration from commercial polyethylene, polypropylene, and polyvinyl chloride packages. J. Agric. Food Chem. 55, 3225–3231. doi: 10.1021/jf070102+
e Silva P. P. G., Nobre C. R., Resaffe P., Pereira C. D. S., and Gusmão F. (2016). Leachate from microplastics impairs larval development in brown mussels. Water Res. 106, 364–370. doi: 10.1016/j.watres.2016.10.016
Gallo F., Fossi C., Weber R., Santillo D., Sousa J., Ingram I., et al. (2018). Marine litter plastics and microplastics and their toxic chemicals components: the need for urgent preventive measures. Environ. Sci. Europe 30, 1–14. doi: 10.1186/s12302-018-0139-z
Good A. M. and Bahr K. D. (2021). The coral conservation crisis: interacting local and global stressors reduce reef resiliency and create challenges for conservation solutions. SN Appl. Sci. 3, 312. doi: 10.1007/s42452-021-04319-8
Hall N. M., Berry K. L. E., Rintoul L., and Hoogenboom M. O. (2015). Microplastic ingestion by scleractinian corals. Mar. Biol. 162, 725–732. doi: 10.1007/s00227-015-2619-7
Hankins C., Moso E., and Lasseigne D. (2021). Microplastics impair growth in two atlantic scleractinian coral species, Pseudodiploria clivosa and Acropora cervicornis. Environ. pollut. 275, 116649. doi: 10.1016/j.envpol.2021.116649
Hierl F., Wu H. C., and Westphal H. (2021). Scleractinian corals incorporate microplastic particles: identification from a laboratory study. Environ. Sci. pollut. Res. 28, 37882–37893. doi: 10.1007/s11356-021-13240-x
Huang B., Yin X., Carton J. A., Chen L., Graham G., Hogan P., et al. (2024). Record high sea surface temperatures in 2023. Geophysical Res. Lett. 51, e2024GL108369. doi: 10.1029/2024GL108369
Hughes T. P., Barnes M. L., Bellwood D. R., Cinner J. E., Cumming G. S., Jackson J. B., et al. (2017). Coral reefs in the anthropocene. Nature 546, 82–90. doi: 10.1038/nature22901
Iftikhar A., Qaiser Z., Sarfraz W., Ejaz U., Aqeel M., Rizvi Z. F., et al. (2024). Understanding the leaching of plastic additives and subsequent risks to ecosystems. Water Emerging Contaminants Nanoplastics 3. doi: 10.20517/wecn.2023.58
Jandang S., Alfonso M. B., Nakano H., Phinchan N., Darumas U., Viyakarn V., et al. (2024). Possible sink of missing ocean plastic: Accumulation patterns in reef-building corals in the Gulf of Thailand. Sci. Total Environ. 954, 176210. doi: 10.1016/j.scitotenv.2024.176210
Kim A.-R., Mitra S. K., and Zhao B. (2024). Unlocking passive collection of microplastics in coral reefs by adhesion measurements. ACS ES&T Water 4, 5653–5659. doi: 10.1021/acsestwater.4c00650
Koelmans A. A., Bakir A., Burton G. A., and Janssen C. R. (2016). Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 50, 3315–3326. doi: 10.1021/acs.est.5b06069
Koelmans A. A., Besseling E., and Foekema E. M. (2014). Leaching of plastic additives to marine organisms. Environ. pollut. 187, 49–54. doi: 10.1016/j.envpol.2013.12.013
Lagarde F., Beausoleil C., Belcher S. M., Belzunces L. P., Emond C., Guerbet M., et al. (2015). Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment. Environ. Health 14, 1–15. doi: 10.1186/1476-069X-14-13
Lamb J. B., Willis B. L., Fiorenza E. A., Couch C. S., Howard R., Rader D. N., et al. (2018). Plastic waste associated with disease on coral reefs. Science 359, 460–462. doi: 10.1126/science.aar3320
Lanctôt C. M., Bednarz V. N., Melvin S., Jacob H., Oberhaensli F., Swarzenski P. W., et al. (2020). Physiological stress response of the scleractinian coral Stylophora pistillata exposed to polyethylene microplastics. Environ. pollut. 263, 114559. doi: 10.1016/j.envpol.2020.114559
Li H.-X., Getzinger G. J., Ferguson P. L., Orihuela B., Zhu M., and Rittschof D. (2016). Effects of toxic leachate from commercial plastics on larval survival and settlement of the barnacle amphibalanus amphitrite. Environ. Sci. Technol. 50, 924–931. doi: 10.1021/acs.est.5b02781
Li C. and Tang K. H. D. (2023). Effects of pH and temperature on the leaching of di (2-ethylhexyl) phthalate and di-n-butyl phthalate from microplastics in simulated marine environment. Biointerface Res. Appl. Chem. 13, 269. doi: 10.33263/briac133.269
Luo H., Liu C., He D., Xu J., Sun J., Li J., et al. (2022). Environmental behaviors of microplastics in aquatic systems: A systematic review on degradation, adsorption, toxicity and biofilm under aging conditions. J. Hazardous Materials 423, 126915. doi: 10.1016/j.jhazmat.2021.126915
Martin C., Corona E., Mahadik G. A., and Duarte C. M. (2019). Adhesion to coral surface as a potential sink for marine microplastics. Environ. pollut. 255, 113281. doi: 10.1016/j.envpol.2019.113281
Martínez-Gómez C., León V. M., Calles S., Gomáriz-Olcina M., and Vethaak A. D. (2017). The adverse effects of virgin microplastics on the fertilization and larval development of sea urchins. Mar. Environ. Res. 130, 69–76. doi: 10.1016/j.marenvres.2017.06.016
Mouchi V., Chapron L., Peru E., Pruski A. M., Meistertzheim A.-L., Vétion G., et al. (2019). Long-term aquaria study suggests species-specific responses of two cold-water corals to macro-and microplastics exposure. Environ. pollut. 253, 322–329. doi: 10.1016/j.envpol.2019.07.024
Paganos P., Ullmann C. V., Gaglio D., Bonanomi M., Salmistraro N., Arnone M. I., et al. (2023). Plastic leachate-induced toxicity during sea urchin embryonic development: Insights into the molecular pathways affected by PVC. Sci. Total Environ. 864, 160901. doi: 10.1016/j.scitotenv.2022.160901
Pantos O. (2022). Microplastics: impacts on corals and other reef organisms. Emerging Topics Life Sci. 6, 81–93. doi: 10.1042/ETLS20210236
Pinochet J., Urbina M. A., and Lagos M. E. (2020). Marine invertebrate larvae love plastics: Habitat selection and settlement on artificial substrates. Environ. pollut. 257, 113571. doi: 10.1016/j.envpol.2019.113571
Reichert J., Arnold A. L., Hoogenboom M. O., Schubert P., and Wilke T. (2019). Impacts of microplastics on growth and health of hermatypic corals are species-specific. Environ. pollut. 254, 113074. doi: 10.1016/j.envpol.2019.113074
Reichert J., Schellenberg J., Schubert P., and Wilke T. (2018). Responses of reef building corals to microplastic exposure. Environmental Pollution. 237, 955–960. doi: 10.1016/j.envpol.2017.11.006
Reichert J., Tirpitz V., Anand R., Bach K., Knopp J., Schubert P., et al. (2021). Interactive effects of microplastic pollution and heat stress on reef-building corals. Environ. pollut. 290, 118010. doi: 10.1016/j.envpol.2021.118010
Rendell-Bhatti F., Paganos P., Pouch A., Mitchell C., D’Aniello S., Godley B. J., et al. (2021). Developmental toxicity of plastic leachates on the sea urchin Paracentrotus lividus. Environ. pollut. 269, 115744. doi: 10.1016/j.envpol.2020.115744
Rotjan R. D., Sharp K. H., Gauthier A. E., Yelton R., Lopez E. M. B., Carilli J., et al. (2019). Patterns, dynamics and consequences of microplastic ingestion by the temperate coral, Astrangia poculata. Proc. R. Soc. B 286, 20190726. doi: 10.1098/rspb.2019.0726
Savinelli B., Vega Fernández T., Galasso N. M., D’Anna G., Pipitone C., Prada F., et al. (2020). Microplastics impair the feeding performance of a Mediterranean habitat-forming coral. Mar. Environ. Res. 155, 104887. doi: 10.1016/j.marenvres.2020.104887
Seidensticker S., Grathwohl P., Lamprecht J., and Zarfl C. (2018). A combined experimental and modeling study to evaluate pH-dependent sorption of polar and non-polar compounds to polyethylene and polystyrene microplastics. Environ. Sci. Europe 30, 1–12. doi: 10.1186/s12302-018-0155-z
Spies N. P. (2021). Characterization of larval settlement and stress responses in two resilient Hawaiian coral species (Doctoral dissertation (University of Hawai’i at Manoa).
Syakti A. D., Jaya J. V., Rahman A., Hidayati N. V., Raza’i T. S., Idris F., et al. (2019). Bleaching and necrosis of staghorn coral (Acropora formosa) in laboratory assays: Immediate impact of LDPE microplastics. Chemosphere 228, 528–535. doi: 10.1016/j.chemosphere.2019.04.156
Tang J., Ni X., Zhou Z., Wang L., and Lin S. (2018). Acute microplastic exposure raises stress response and suppresses detoxification and immune capacities in the scleractinian coral Pocillopora damicornis. Environ. pollut. 243, 66–74. doi: 10.1016/j.envpol.2018.08.045
Tang J., Wu Z., Wan L., Cai W., Chen S., Wang X., et al. (2021). Differential enrichment and physiological impacts of ingested microplastics in scleractinian corals in situ. J. Hazardous Materials 404, 124205. doi: 10.1016/j.jhazmat.2020.124205
Vandenberg L. N., Colborn T., Hayes T. B., Heindel J. J., Jacobs D. R. Jr., Lee D. H., et al. (2012). Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocrine Rev. 33, 378–455. doi: 10.1210/er.2011-1050
Wang H., Liu H., Zhang Y., Zhang L., Wang Q., and Zhao Y. (2023). The toxicity of microplastics and their leachates to embryonic development of the sea cucumber Apostichopus japonicus. Mar. Environ. Res. 190, 106114. doi: 10.1016/j.marenvres.2023.106114
Wilkins K. W., Yew J. Y., Seeley M., and Richmond R. H. (2024). Microplastic leachate affects fertilization in the coral Montipora capitata. Integr. Comp. Biol. 64, 1131–1140. doi: 10.1093/icb/icae143
Woodhead A. J., Hicks C. C., Norström A. V., Williams G. J., and Graham N. A. (2019). Coral reef ecosystem services in the Anthropocene. Funct. Ecol. 33, 1023–1034. doi: 10.1111/1365-2435.13331
Xiao B., Li D., Liao B., Zheng H., Yang X., Xie Y., et al. (2023). Effects of microplastic combined with Cr (III) on apoptosis and energy pathway of coral endosymbiont. Environ. Sci. pollut. Res. 30, 39750–39763. doi: 10.1007/s11356-022-25041-x
Yoshikawa T. and Asoh K. (2004). Entanglement of monofilament fishing lines and coral death. Biol. Conserv. 117, 557–560. doi: 10.1016/j.biocon.2003.09.025
Keywords: microplastic leachate, Montipora capitata, Hawaii, Harbor Porites, plastic additives, coral planula settlement, marine plastic pollution, coral reproduction
Citation: Wilkins KW and Richmond RH (2025) Unseen threats: negative effects of microplastic leachate on coral planulae settlement. Front. Mar. Sci. 12:1596594. doi: 10.3389/fmars.2025.1596594
Received: 19 March 2025; Accepted: 30 June 2025;
Published: 24 July 2025.
Edited by:
Cheryl Hankins, U.S. Environmental Protection Agency, United StatesReviewed by:
Guilherme V. B. Ferreira, Federal University of Rio de Janeiro, BrazilAbigail S. Clark, Boy Scouts of America - Florida Sea Base: Brinton Environmental Center, United States
Copyright © 2025 Wilkins and Richmond. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keiko W. Wilkins, a2Vpa293MkBoYXdhaWkuZWR1
 Keiko W. Wilkins
Keiko W. Wilkins Robert H. Richmond
Robert H. Richmond