- 1Unit of Animal Infectious Diseases, National Key Laboratory of Agricultural Microbiology, College of Veterinary Medicine, Huazhong Agricultural University, Wuhan, China
- 2Key Laboratory of Development of Veterinary Diagnostic Products, Ministry of Agriculture, Wuhan, China
Streptococcus suis 2 is an important swine pathogen and an emergent zoonotic pathogen. Excessive inflammation caused by S. suis is responsible for the high levels of early mortality observed in septic shock-like syndrome cases. However, the mechanisms through which S. suis 2 (SS2) causes excessive inflammation remain unclear. Thus, this study aimed to identify novel pro-inflammatory mediators that play important roles in the development of therapies against SS2 infection. In this study, the novel pro-inflammatory protein HP0459, which was encoded by the SSUSC84_0459 gene, was discovered. The stimulation of RAW 264.7 macrophages with recombinant HP0459 protein induced the expression of pro-inflammatory cytokines (IL-1β, MCP-1 and TNF-α). Compared with the wild-type (WT) strain, the isogenic knockout of HP0459 in SS2 led to reduced production of pro-inflammatory cytokines in RAW264.7 macrophages and in vivo. The pro-inflammatory activity of HP0459 was significantly reduced by an antibody against Toll-like receptor 2 (TLR2) in RAW264.7 macrophages and was lower in TLR2-deficient (TLR2-/-) macrophages than in WT macrophages. Furthermore, specific inhibitors of the extracellular signal-regulated kinase 1/2 (ERK1/2) pathways significantly decreased the HP0459-induced pro-inflammatory cytokine production, and a western blot assay showed that HP0459 stimulation induced the activation of the ERK1/2 pathway. Taken together, our data indicate that HP0459 is a novel pro-inflammatory mediator of SS2 and induces TLR2-dependent pro-inflammatory activity in RAW264.7 macrophages through the ERK1/2 pathway.
Introduction
Streptococcus suis is a major pathogen responsible for important economic losses to the swine industry worldwide, particularly during the past 20 years (Gottschalk et al., 2010). It causes meningitis, septicemia, pneumonia, endocarditis, arthritis, and other diseases in swine (Wei et al., 2009). Among the 33 serotypes based on capsular antigens that have been described, serotype 2 is the most frequently isolated from diseased pigs, particularly in Europe and Asia (Wisselink et al., 2000). Since the first human case was reported in Denmark in Perch et al. (1968), more S. suis infections in humans have been documented in several European and Asian countries as well as in North and South America, Australia, and New Zealand (Wertheim et al., 2009; Gottschalk et al., 2010). For a long time, it has been considered to be a fact that S. suis only infects people working with pigs or pork-derived products (Arends and Zanen, 1988); however, S. suis infections in the general population were recently reported in Southeast and East Asia (Gottschalk et al., 2010). Although most reports concern sporadic cases of infection, a large series of 151 S. suis meningitis cases was recently reported in southern Vietnam (Mai et al., 2008). Furthermore, an important outbreak, which involved 215 cases and 38 deaths, occurred in China in during 2005 (Yu et al., 2006). In addition, S. suis is considered one of the most important causes of meningitis in humans in various locations, including Vietnam, Thailand, and Hong Kong (Suankratay et al., 2004; Hui et al., 2005; Ip et al., 2007; Mai et al., 2008). These findings emphasize the importance of S. suis as an emerging zoonosis and indicate that S. suis represents a significant public health concern (Fittipaldi et al., 2012). The increased severity of S. suis infection in humans underscores the critical need to better understand the factors associated with the pathogenesis of S. suis infection (Gottschalk and Segura, 2000).
Although several virulence-related molecules have been proposed, only the capsular polysaccharide (CPS) has been proven to play a critical role in the virulence of S. suis (Charland et al., 1998; Smith et al., 1999; Segura et al., 2004). Some putative virulence factors have also been reported in S. suis, such as suilysin, muramidase-released protein (MRP), subtilisin-like protease (SspA), and LTA D-alanylation (DltA; Smith et al., 1992; Lun et al., 2003; Fittipaldi et al., 2008; Bonifait et al., 2011); however, the current knowledge on the pathogenesis of S. suis infection remains limited (Baums and Valentin-Weigand, 2009). To cause disease, S. suis must breach epithelial barriers, reach and survive in the bloodstream, invade different organs and cause exaggerated inflammation (Fittipaldi et al., 2012). The upregulated expression of several pro-inflammatory cytokines and chemokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6 and monocyte chemotactic protein-1 (MCP-1), has been reported in S. suis infection (Gottschalk and Segura, 2000; Segura et al., 2002; Al-Numani et al., 2003). Furthermore, inflammation is thought to be responsible for most clinical signs of meningitis, septicemia and sudden death (Segura et al., 2006). S. suis sequence type (ST) 7 was recognized as the causative agent for the Sichuan outbreak, and streptococcal toxic shock-like syndrome (STSLS) was observed for the first time in this large outbreak (Ye et al., 2006). A previous study showed that the increased virulence of S. suis ST7 is associated with an increased ability to stimulate excessive pro-inflammatory cytokines that may be responsible for the shock syndrome (Zheng et al., 2008). In addition, the most important clinical feature associated with S. suis is meningitis in pigs (Gottschalk and Segura, 2000); however, the mechanisms of S. suis crossing the blood–brain barrier (BBB) to cause meningitis are poorly understood. Even so, some mechanisms, such as the up-regulation of pro-inflammatory cytokines and increased leukocyte trafficking, have been proposed to contribute to the breakdown of the BBB (Vadeboncoeur et al., 2003; Adam et al., 2004; Jobin et al., 2005; Tenenbaum et al., 2005). The activation of the innate immune response depends on the recognition of pathogen-associated molecular patterns (PAMPs). Toll-like receptors (TLRs) are critical sensors that activate the innate immune response (Beutler, 2009; Kawai and Akira, 2010). For example, TLR2 can form heterodimers with TLR1 or TLR6 to recognize bacterial lipoprotein, lipoteichoic acid (LTA), peptidoglycans (PGNs) and zymosan and induce the release of many cytokines and chemokines responsible for inflammation (Akira and Hemmi, 2003; Beutler, 2004; Lachance et al., 2013). Many previous studies have reported that TLR2 is the major (but not exclusive) immune receptor involved in S. suis recognition (Graveline et al., 2007; Li et al., 2010; Lecours et al., 2012).
As mentioned above, inflammation has been thought to be a hallmark of S. suis infection (Gottschalk et al., 2007). However, the research on inflammation induced by S. suis remains limited. Therefore, it is important to identify novel pro-inflammatory mediators of S. suis in order to improve our understanding of the mechanism of inflammation induced by this pathogen. In our previous study, more than 50 extracellular proteins of S. suis were expressed, including membrane proteins, secreted proteins and cell wall proteins. And several pro-inflammatory proteins were identified, of which a novel protein HP0459 displayed rather robust pro-inflammatory activity (data not shown). In this study, through measuring the IL-1β and MCP-1 levels by relative quantitative polymerase chain reaction (qPCR) and enzyme-linked immunosorbent assay (ELISA), the pro-inflammatory ability of HP0459 was examined. We investigated the recognition receptor and signal transduction pathway through which HP0459 induces IL-1β, TNF-α and MCP-1 in RAW264.7 macrophages. As a result, we elucidated the mechanism through which HP0459 stimulation induces pro-inflammatory cytokine production.
Materials and Methods
Bacterial Strains, Plasmids and Growth Conditions
Streptococcus suis serotype 2 strain SC-19, which was isolated from the brain of a dead pig during the epidemic outbreak in Sichuan province of China in 2005, was selected as the wild-type (WT) strain. SC-19 was grown in Tryptic Soy Broth (TSB) or on Tryptic Soy Agar (TSA) plates (Difco, MI, USA) with 5% newborn bovine serum (Sijiqing Biological Engineering Materials Co., Ltd., Hangzhou, China) at 37°C (Li et al., 2013). A temperature-sensitive S. suis–Escherichia coli shuttle vector (pSET4s) was used to construct the Δhp0459 mutant, which carries a spectinomycin resistance gene (spcr). pSET2, a S. suis–E. coli shuttle vector carrying spcr, was used in the construction of the complementary bacterium (Takamatsu et al., 2001).
Cell Culture
RAW 264.7 macrophages were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum in a 5% CO2 atmosphere at 37°C (Kang et al., 2009). The primary mouse macrophages were isolated from TLR2-deficient (TLR2-/-; JAX® Mice) and WT mice. The mice were injected intraperitoneally (i.p.) with 4% thioglycolate, and peritoneal exudate cells were harvested 4 days later (van Lint et al., 2010). More than 90% of the exudate cells were identified as macrophages by microscopic analysis and non-specific esterase staining (Sodhi et al., 2005). The macrophages were plated at a density of 106 cells per well in 12-well plates.
Cloning, Expression, Purification and Endotoxin Removal of HP0459 Protein
The HP0459 protein, which was reported as a secreted protein in a previous study, was encoded by SSUSC84_0459 (Liu et al., 2009). The HP0459 protein was cloned and purified according to published methods (Liu et al., 2012). Briefly, the hp0459 gene was amplified from the chromosomal DNA of SC-19 by PCR using the primers listed in Table 1. The purified PCR product was inserted into pET-28a and harbored in E. coli BL21 (DE3) cells. HP0459 was induced with 0.5 mM isopropyl-b-D-thiogalactopyranoside (IPTG) and purified by ultrasonication and Ni-NTA agarose chromatography. The endotoxin in the purified recombinant protein was removed using an Endotoxin Removal Kit (Genmed Scientifics Inc. USA), and the endotoxin level was tested using a Quantitative Chromogenic Tachypleus Amebocyte Lysate For Endotoxin Detection kit (Chinese Horseshoe Crab Reagent Manufactory Co., Ltd., Xiamen, China; Liu et al., 2011). The protein was then treated using a 0.22-μm filter. After the above-mentioned treatment, the HP0459 protein was stored at -80°C.
RNA Extraction and qPCR Assay
After the treatment of RAW 264.7 cells with HP0459 at 10 μg ml-1 for 10 h, the total RNA of the cells was extracted with the TRIzol® reagent (Invitrogen, Paisley, UK), according to the manufacturer’s guidelines. The RNA pellets were suspended in RNase-Free water, and the DNA contamination of the RNA was removed by DNase treatment (Promega, Madison, WI, USA). cDNA was obtained from 4 μg of RNA by reverse transcription using AMV reverse transcriptase (TAKARA, Japan) and the oligo-dT primer (300 pmol) in a total reaction volume of 40 μl (Moore et al., 2005). Relative quantitative PCR (qPCR) was performed to measure the mRNA levels of pro-inflammatory cytokines (IL-1β, MCP-1 and TNF-α) using a SYBR green PCR Kit (Roche) and the ABI ViiA7 instrument. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene, and all of the primers used in the qPCR assay are listed in Table 1. The data were analyzed using the ViiA7 software (Applied Biosystems; Zhao et al., 2014).
Enzyme-Linked Immunosorbent Assay for Cytokines
After treatment of RAW 264.7 cells with HP0459 at 10 μg ml-1 for 10 h, the protein levels of IL-1β, MCP-1 and TNF-α in the cell culture supernatants were determined using commercially available ELISA kits (Biolegend) according to the manufacturer’s instructions.
Generation of an Isogenic hp0459 Deletion Mutant and Complemented Strains
The construction of the Δhp0459-knockout mutant was performed using a previously described procedure (Takamatsu et al., 2001). Briefly, DNA fragments were amplified from the genomic DNA of SC-19 by PCR using two pairs of specific primers, namely hp0459-L1/hp0459-L2 and hp0459-R1/hp0459-R2 (Table 1), which carry EcoRI/SalI and SalI/HindI restriction enzyme sites, respectively. The fragments were digested with the corresponding restriction enzymes and sequentially ligated into the temperature-sensitive S. suis–E. coli shuttle vector pSET4s to generate the hp0459-knockout vector pSET4sΔhp0459. To obtain the isogenic mutant Δhp0459, competent cells of SC-19 were subjected to electrotransformation with pSET4sΔhp0459 as described previously (Takamatsu et al., 2001). The suspected mutant was verified by PCR using three pairs of primers: P1/P2 (to identify hp0459), P3/P4 (to identify gdh) and P5/P6 (to identify the pSET4s).
The complemented strain of hp0459 was constructed as described previously (Zhang et al., 2012). Briefly, a DNA fragment that contained the hp0459 gene and its predicted upstream promoter was amplified by PCR using the primers chp0459-1/chp0459-2 (Table 1), which carry SphI/EcoRI restriction enzyme sites, respectively. To generate the recombinant plasmid pSET2Chp0459, the fragment was digested with the appropriate restriction enzymes and was cloned into the E. coli–S. suis shuttle vector pSET2 carrying the same cohesive terminus. The plasmid was then electrotransformed into Δhp0459 to obtain the complemented CΔhp0459 strain.
Experimental Infections In Vitro and In Vivo
In vitro, RAW 264.7 cells were infected with 5 × 106 CFU of the WT (SC-19), Δhp0459 or CΔhp0459 strains in the logarithmic phase of growth. The supernatants were collected for western blot analysis after 10 h at 37°C.
All of the animal studies were performed according to the experimental protocols approved by the Laboratory Animal Monitoring Committee of Hubei Province, China. A total of 125 6-weeks-old female C57BL/6 mice were randomly divided into three groups with 40 mice per group, and the remaining five mice were used as controls. The three groups of mice were challenged intraperitoneally (i.p.) with 5 × 108 CFU log-phase WT (SC-19), Δhp0459 or CΔhp0459 strains, respectively. At certain times post infection (3, 6, 9, and 12 h), an equal number of mice in each group were killed. Bacteriological isolation from the blood or part of the spleen was performed essentially as described previously (Zhang et al., 2011), and the lungs or another part of the spleen were used to determine the IL-1β and TNF-α levels by qPCR.
Investigating the Recognition Receptor of HP0459
Antibody blocking assays were performed to investigate the recognition receptor of HP0459 in RAW264.7 cells using the anti-TLR2 (eBioscience) and anti-TLR4 (BioLegend) antibodies. Briefly, RAW264.7 cells were pretreated using 8 μg of anti-TLR2 and anti-TLR4 antibody for 30 min respectively and then incubated with 10 μg ml-1 HP0459 for 10 h. The expression levels of various cytokines were determined by ELISA. According to the conditions of cytokine activation, the recognition receptor of the HP0459 was analyzed. In addition, TLR2-/- macrophages were isolated from TLR2-/- mice to verify the results of the blocking assays.
Analysis of HP0459-Induced Cell Signal Transduction Pathways
RAW264.7 macrophages (1 × 106 cells ml-1) were seeded into 12-well tissue culture plates. The cells were pretreated with the following specific inhibitors for 30 min prior to the addition of HP0459 (Liu et al., 2008): U0126 (for ERK1/2; 10 μM), SP600125 (for JNK; 10 μM), pyrrolidine dithiocarbamate (PDTC; for NF-κB; 20 μM) and LY294002 (for PI3K; 20 μM). All inhibitors were purchased from Cayman Chemical. Culture supernatants were collected at the indicated times and stored at -80°C until assayed.
SDS-PAGE and Western Blot Analysis
To confirm the HP0459-induced phosphorylation of signal transduction molecules, a western blot analysis was performed. To extract the cytosolic protein, after stimulation with HP0459 (10 μg ml-1) for 10 h, RAW264.7 cells were washed with cold PBS and harvested by centrifugation. The pellets were then suspended in RIPA lysis buffer with phosphatase inhibitor (Roche) for 15 min on ice. The protein concentrations in the lysates were quantified with the Bradford protein assay, and 40 μg of proteins were subjected to 12% SDS-PAGE and transferred onto a 0.22-μm nitrocellulose membrane. Subsequently, these proteins were probed with specific Abs against the phosphorylated forms of ERK1/2 and NF-κB p65 (Cell Signaling Technology, Beverly, MA, USA), and β-actin was assessed as an internal control using anti-β-actin antibody (Wuhan PMK Biotechnology Co., Ltd.). The detection of the bands was performed using HRP-conjugated secondary antibody and an enhanced chemiluminescence (ECL) system (Amersham Life Science, Arlington Heights, IL, USA).
Statistical Analysis
The statistical significance of the data was determined using Student’s t test with GraphPad Prism software (San Diego, CA, USA), and all the assays were repeated at least three times. For all tests, a value of P < 0.05 was considered as the threshold for significance.
Results
Cytokine Secretion from RAW264.7 Cells Stimulated with HP0459
After purification by Ni-NTA agarose chromatography, the SDS-PAGE (Figure 1A) and western blot analysis (Figure 1B) of HP0459 revealed that the HP0459 protein was successfully purified. The average endotoxin level in HP0459 was ∼0.05 endotoxin units per milliliter via endotoxin removal. After the above-mentioned treatment, to determine the pro-inflammatory role of HP0459, RAW264.7 cells were stimulated with HP0459 at a concentration of 10 μg ml-1 for 10 h and analyzed by qPCR and ELISA respectively. As shown in Figure 2, it was confirmed that HP0459 stimulation significantly increased the expression levels of IL-1β, MCP-1, and TNF-α (P <0.01) by qPCR (Figure 2A and ELISA (Figure 2B) analyses.
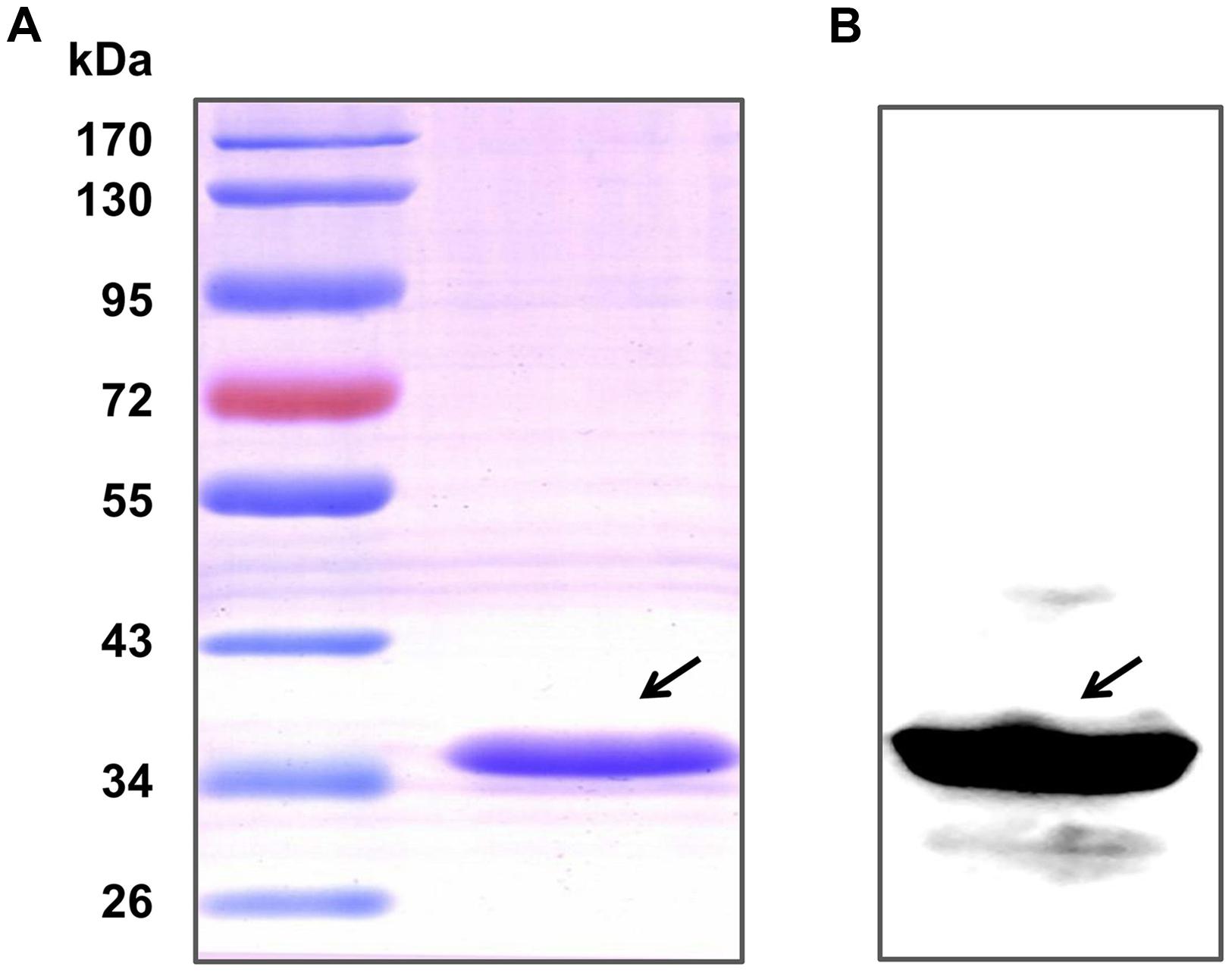
FIGURE 1. Purification of the recombinant Hp0459 protein. (A) SDS-PAGE. (B) Western blot analysis. The blot was probed with his tag monoclonal antibody (Cali-Bio).
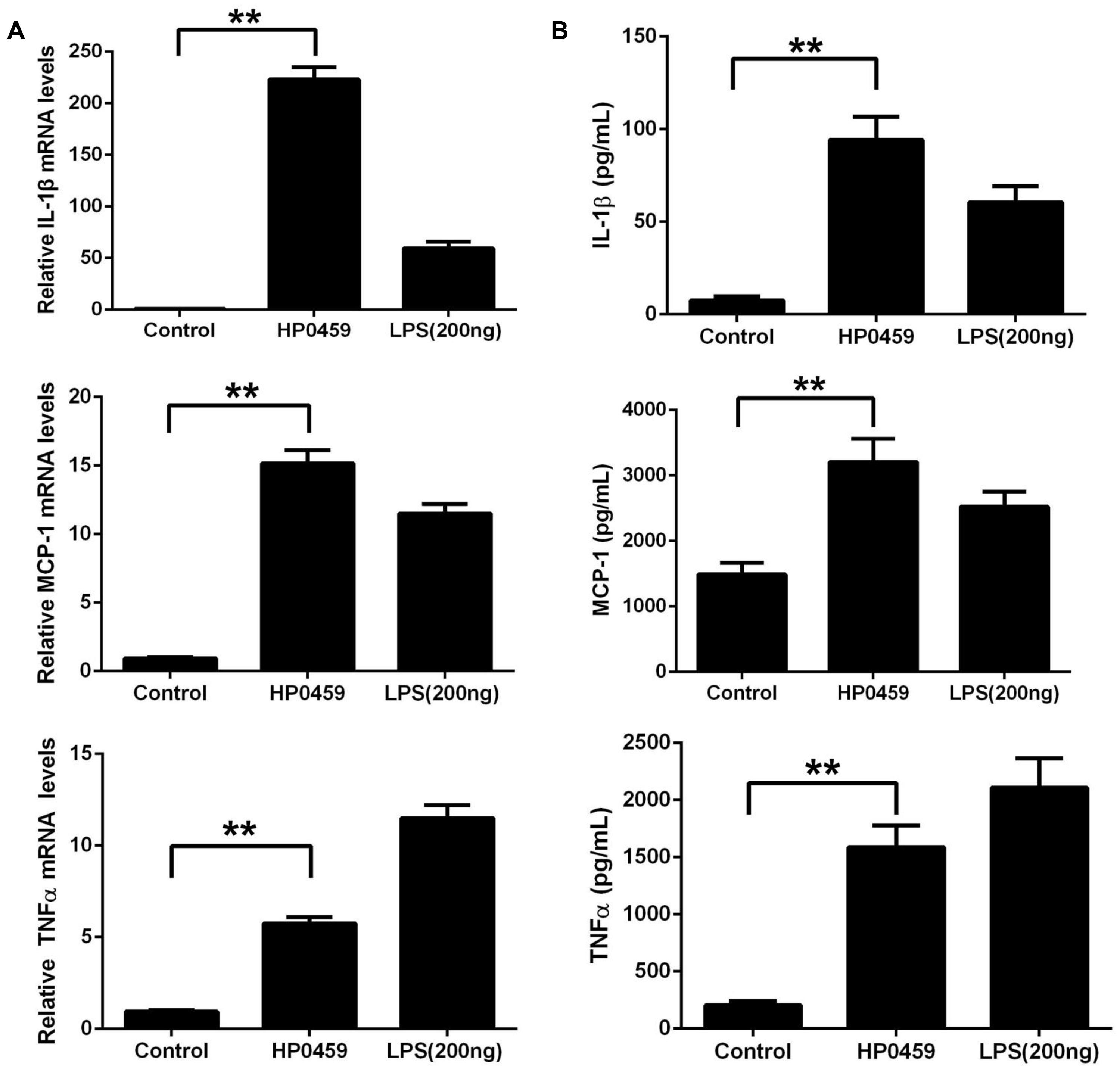
FIGURE 2. Induction of cytokine mRNA and protein in RAW 264.7 macrophages by recombinant HP0459 stimulation. RAW 264.7 macrophages were incubated with 10 μg ml-1 HP0459 and 200 ng ml-1 LPS (positive control) for 10 h, as well as single culture media (negative control), (A) the cytokine mRNA levels were then determined by qPCR, (B) and the protein levels of IL-1β, MCP-1 and TNF-α in the culture supernatants were determined by ELISA. The bars represent the standard errors of the means, based on three independent experiments. **P <0.01.
Construction and Characterization of the Mutant Strain Δhp0459
To study the role of HP0459 in the S. suis-induced pro-inflammatory response, the HP0459-knockout mutant Δhp0459 was constructed by homologous recombination, and the double-crossover event was confirmed by PCR (Figure 3A). To examine the growth characteristics of the mutants in vitro, the OD600 values of cultures of the SC-19, Δhp0459 and CΔhp0459 strains in TSB containing 10% newborn bovine serum at 37°C were determined (Figure 3B), and Gram-staining of these three strains was also performed (Figure 3C). No significant difference in growth was found between the SC-19, Δhp0459 and CΔhp0459 strains.
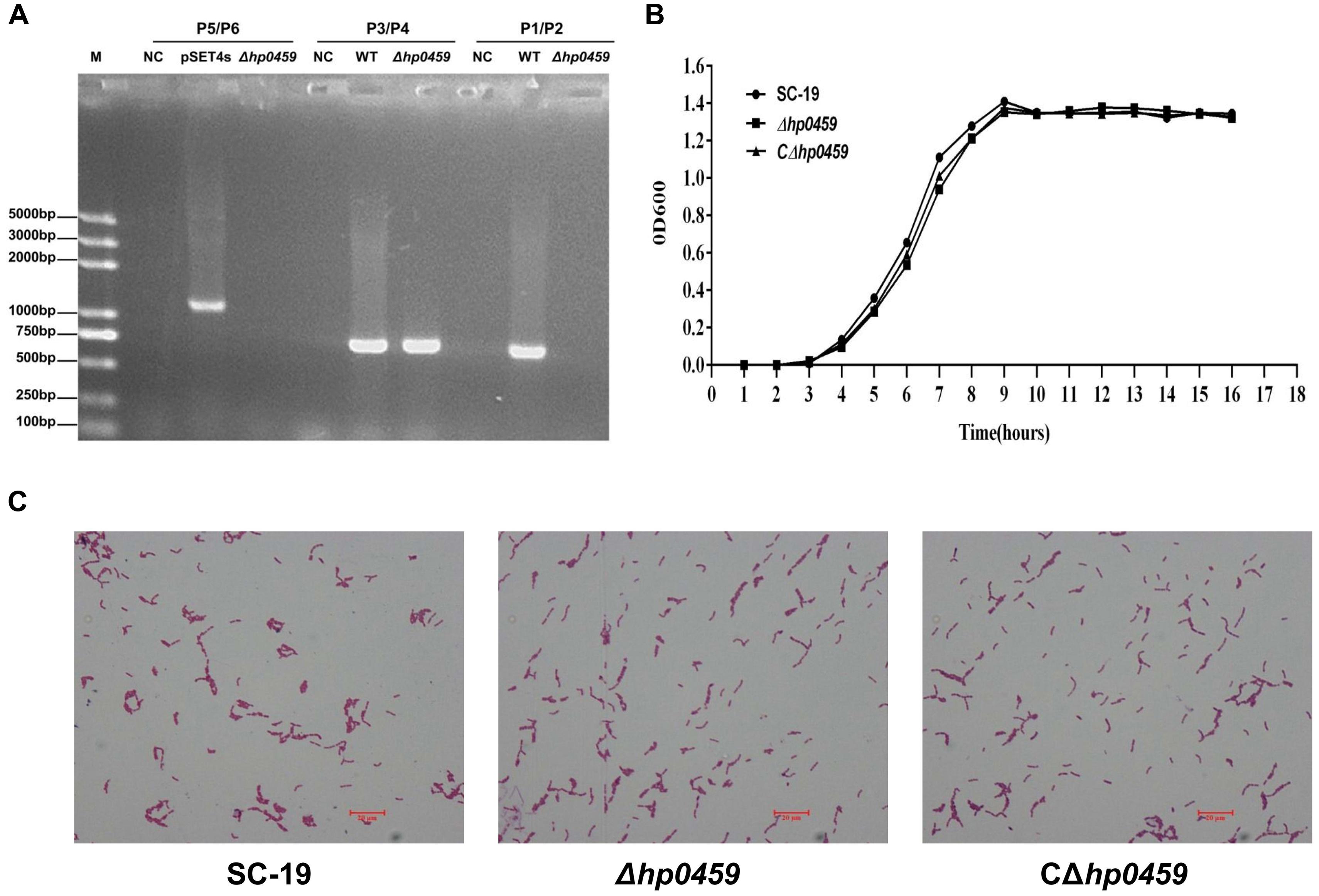
FIGURE 3. Construction and confirmation of Δhp0459 and the complementary strain CΔhp0459. (A) Confirmation of the Δhp0459 mutant by PCR using the primers pairs P1/P2 (to identify hp0459), P3/P4 (to identify gdh), and P5/P6 (to identify the pSET4s). (B) Growth curves of the SC-19, Δhp0459 and CΔhp0459 strains. The bacteria were cultured in TSB containing 5% newborn bovine serum at 37°C. The absorbance at 600 nm was measured at intervals of 1 h. Results shown are representative of three independent experiments. (C) Light microscope morphology of the SC-19, Δhp0459 and CΔhp0459 strains using Gram staining. The bar indicates the magnification size.
Pro-inflammatory Attenuation Induced by the Δhp0459 Strain In Vitro and In Vivo
After construction of the Δhp0459 strain, the role of HP0459 in the S. suis-induced pro-inflammatory response was assessed in RAW264.7 cells in vitro. The culture supernatants of RAW264.7 cells incubated with the SC-19, Δhp0459 and CΔhp0459 strains were harvested, and the levels of IL-1β and TNF-α in these supernatants were measured by ELISA. The results showed that the pro-inflammatory activity of the Δhp0459 strain was significantly lower than those of the SC-19 and CΔhp0459 strains (Figure 4). This finding suggested that HP0459 plays an important role in the S. suis-induced pro-inflammatory response in vitro.
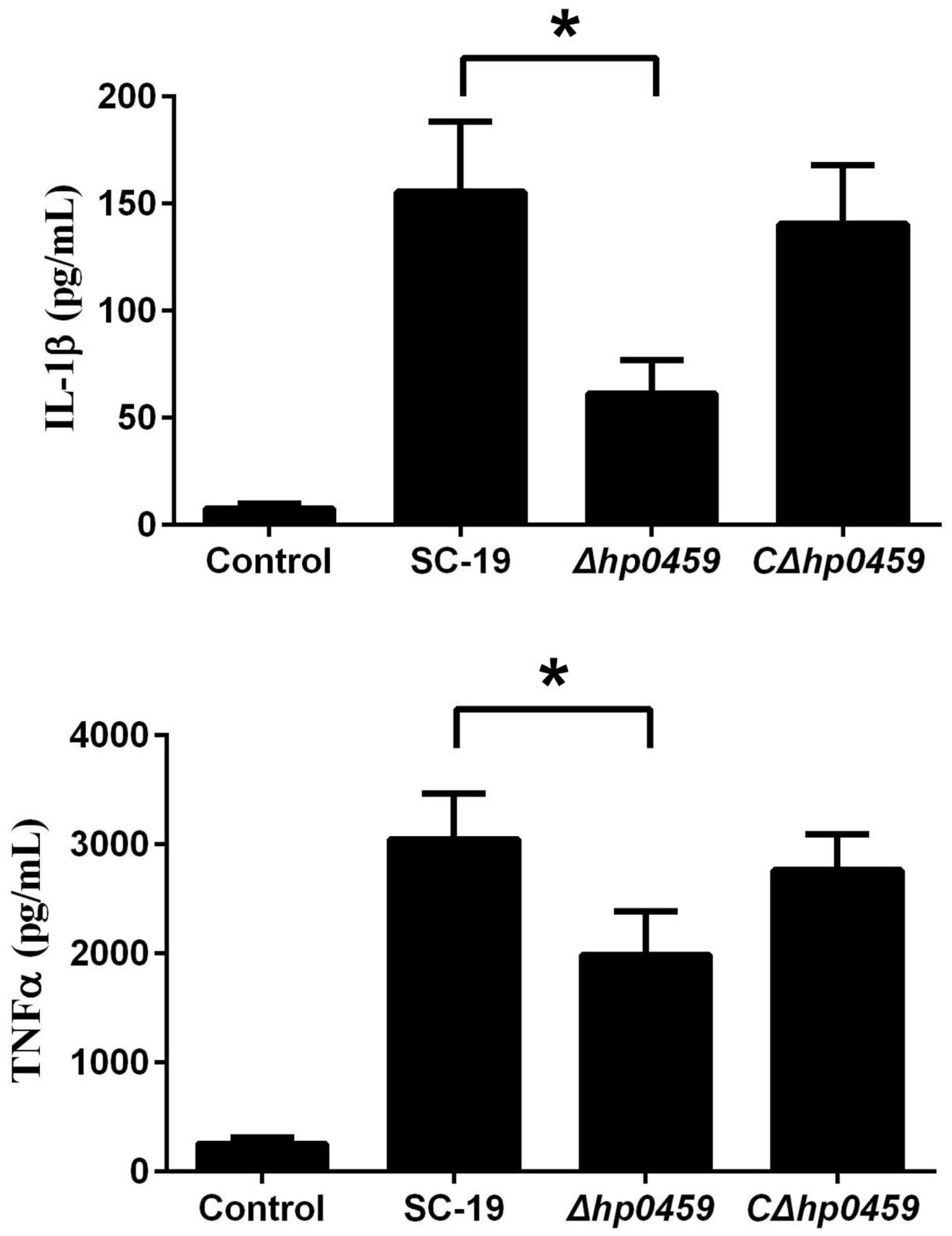
FIGURE 4. Induction of IL-1βandTNF-α by SS2 strains in RAW 264.7 macrophages. RAW 264.7 macrophages were incubated with the SC-19, Δhp0459 and CΔhp0459 strains for 10 h. The levels of IL-1β and TNF-α in the supernatants were measured by ELISA. The bars represent the SEs of the means, based on three independent experiments. *P <0.05.
The role of HP0459 in vivo in the S. suis-induced pro-inflammatory response was then assessed using an experimental infection model in C57BL/6 mice. Bacteriological isolation from the blood and part of the spleen showed that, compared with the SC-19 and CΔhp0459 strains, the bacterial content of the Δhp0459 strain was significantly increased (Figure 5). At the same time, the lungs and another part of the spleen from infected and control mice were separated to extract the total RNA, and the mRNA levels of IL-1β and TNF-α were measured by qPCR. The results indicated that the mRNA levels of IL-1β and TNF-α induced by the Δhp0459 strain in vivo were significantly lower than those induced by the SC-19 and CΔhp0459 strains (Figure 6). Thus, the HP0459 protein plays an important role in the S. suis-induced pro-inflammatory response.
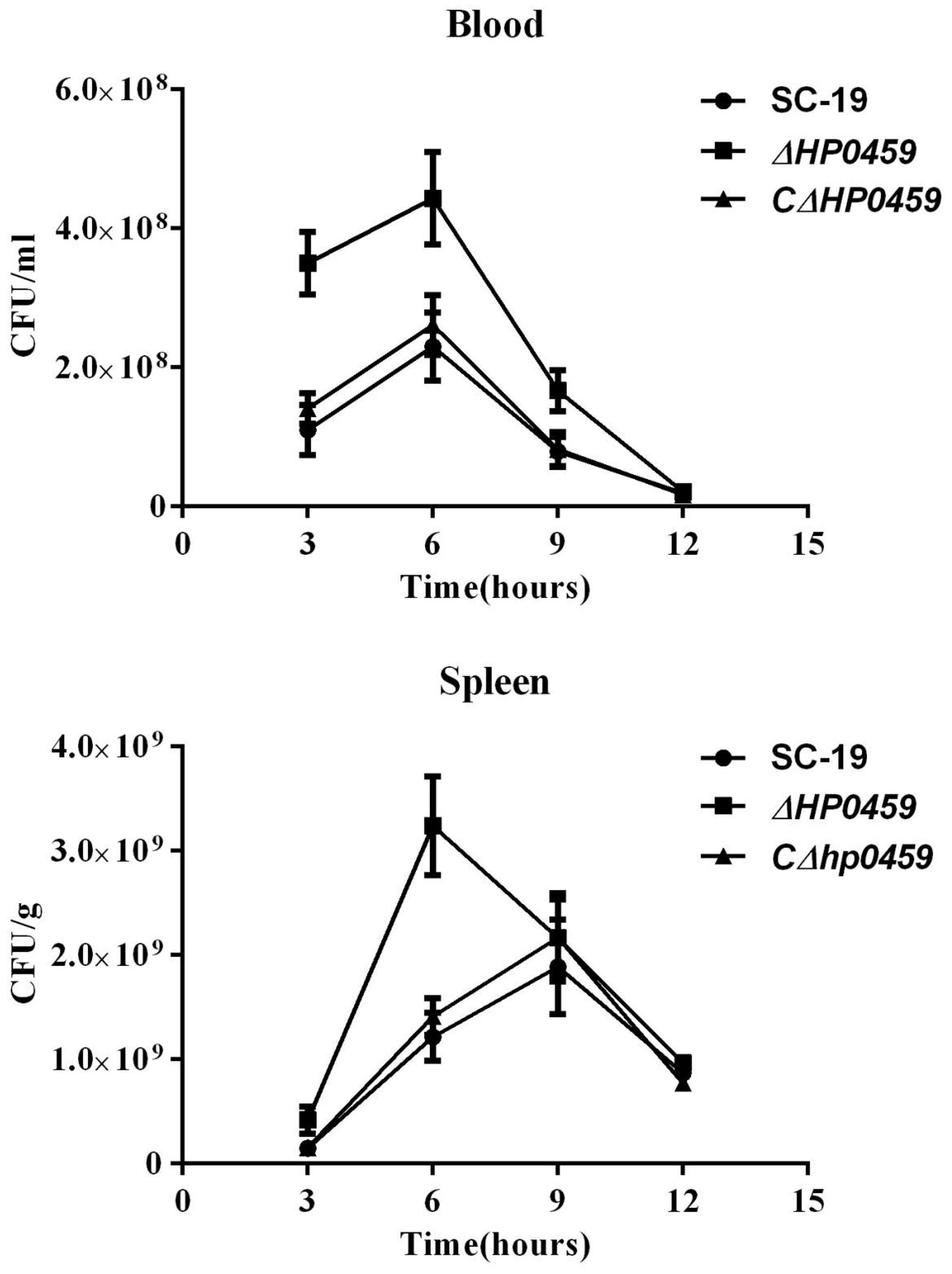
FIGURE 5. Bacteriological isolation from blood and spleen. C57BL/6 mice were challenged with 5 × 108 CFU of the log-phase SC-19, Δhp0459 or CΔhp0459 strains. After infection for 3, 6, 9, and 12 h, bacteriological isolation from the blood and spleens was performed. Results shown are representative of three independent experiments.

FIGURE 6. Induction of IL-1βandTNF-α mRNA in vivo by stimulation with SS2 strains. C57BL/6 mice were challenged with 5 × 108 CFU of the log-phase SC-19, Δhp0459 or CΔhp0459 strains. After infection for 3, 6, 9, and 12 h, the mRNA levels of IL-1β and TNF-α in the lungs and spleens were measured by qPCR. Results shown are representative of three independent experiments.
HP0459 Protein Induced the Expression of IL-1β, MCP-1 and TNF-α by TLR2
To determine the recognition receptor responsible for the HP0459-mediated induction of cytokines, antibody blocking assays were performed. Compared with the positive control, anti-TLR2 antibody could significantly reduce the expression of IL-1β, MCP-1 and TNF-α induced by HP0459, whereas anti-TLR4 antibody could not (Figure 7A). The results showed that the HP0459-induced cytokine secretion may depend on TLR2. To verify this result, TLR2-/- and WT macrophages were isolated from TLR2-/- and WT mice, respectively, and were incubated with HP0459. The results show that HP0459 could significantly induce cytokine secretion in WT macrophages but not in TLR2-/- cells (Figure 7B), demonstrating that the HP0459 protein can induce a TLR2-dependent pro-inflammatory response.
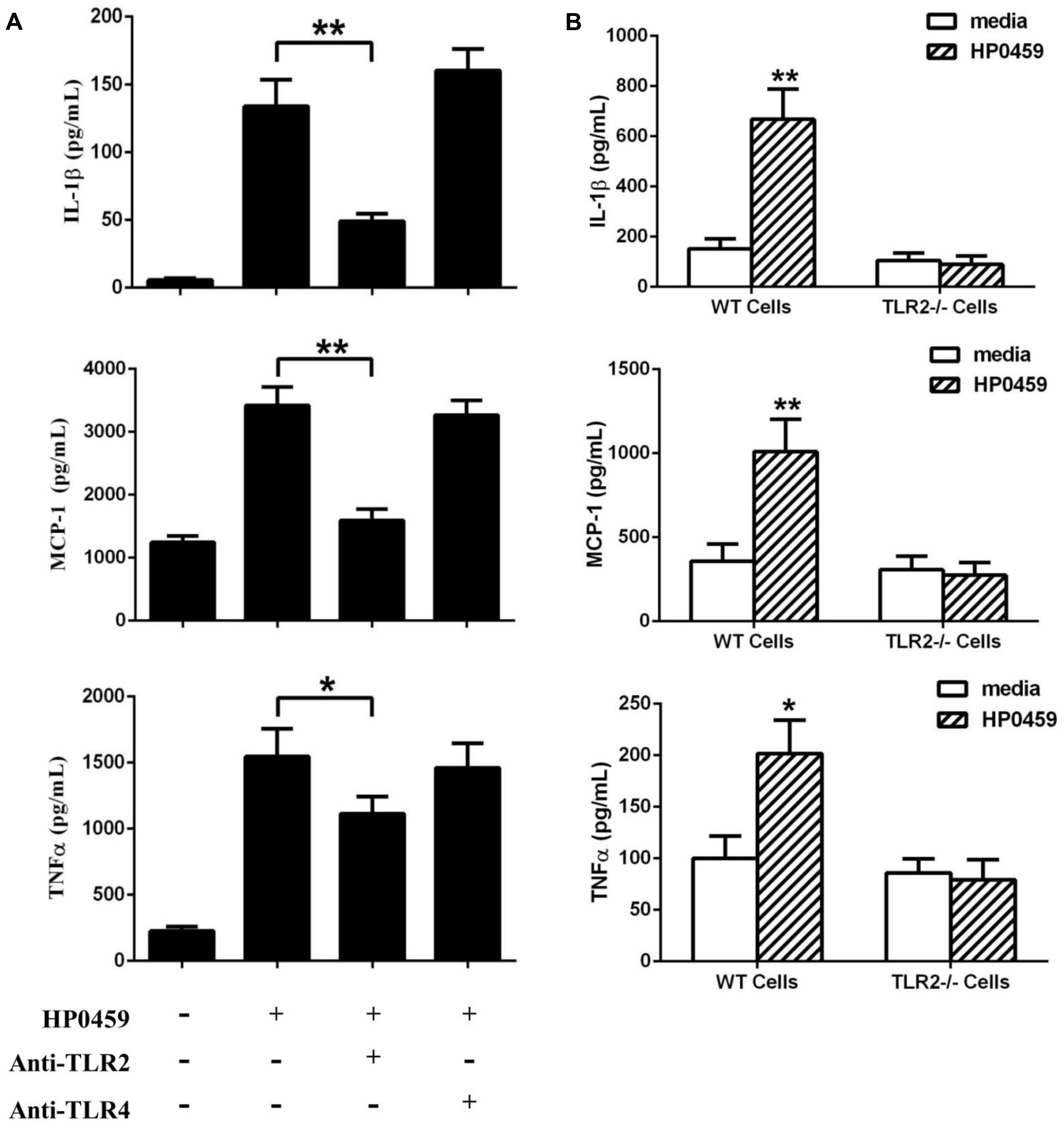
FIGURE 7. Recognition receptor of the HP0459-stimulated pro-inflammatory response. (A) Antibody blocking assays. After pretreatment with 8 μg of anti-TLR2 and anti-TLR4 antibody for 30 min, RAW 264.7 macrophages were incubated with 10 μg ml-1 HP0459 for 10 h. The expression levels of various cytokines were then determined by ELISA. (B) Primary peritoneal macrophages were isolated from TLR2-/- and WT mice and incubated with 10 μg ml-1 HP0459 for 10 h. The induction of IL-1β, MCP-1 and TNF-α in the supernatants was then determined by ELISA. The bars represent the SEs of the means, based on three independent experiments. *P <0.05, **P <0.01.
P0459-Induced Cell Signal Transduction Pathways in RAW264.7 Cells
Next, to further elucidate the mechanisms through which the HP0459 protein induced cytokine secretion, we investigated the cell signal transduction pathways in HP0459-stimulated RAW264.7 cells. RAW264.7 cells were pretreated with specific inhibitors of several cell signal pathways for 30 min and were then were incubated with HP0459 for 10 h. The levels of IL-1β, MCP-1, and TNF-α in the supernatants were quantified by ELISA. As shown in Figure 8A, the ERK 1/2 MAPK inhibitor (U0126) significantly decreased the HP0459-induced cytokine production, and the NF-κB inhibitor (PDTC) induced a lower degree of reduction. This result suggested that the HP0459-induced cytokine production likely primarily depends on the phosphorylation of ERK 1/2 MAPK. To verify this hypothesis, we performed a western blotting analysis to measure the phosphorylation of ERK 1/2 MAPK and NF-κB in RAW264.7 cells induced by HP0459 stimulation (Figure 8B). The results showed that the phosphorylation of ERK 1/2 MAPK was significantly enhanced, whereas the phosphorylation of NF-κB was slight. In addition, a western blot analysis with anti-actin antibody was used to confirm that equivalent amounts of the samples were loaded into the gels. These results suggested that signal transduction pathway ERK 1/2 MAPK play a primary role in the pro-inflammatory response induced by HP0459 stimulation in RAW264.7 cells.
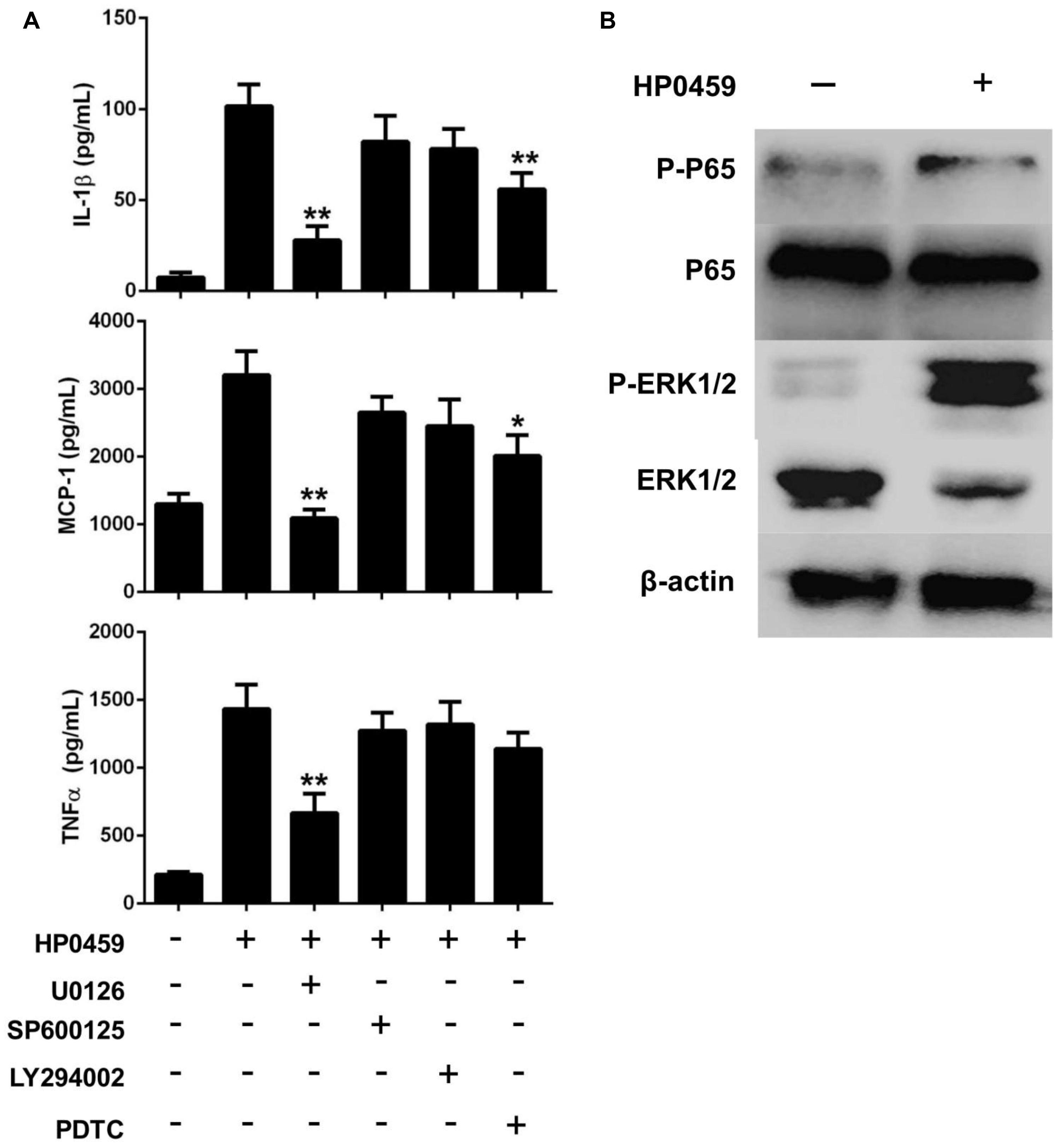
FIGURE 8. Signal transduction pathways of the HP0459-stimulated pro-inflammatory response in RAW 264.7 macrophages. (A) Effect of ERK 1/2 (U0126), JNK (SP600125), PI3K (LY294002) and NF-κB (PDTC) inhibitors on cytokine production during HP0459 stimulation. After incubation with the inhibitors for 30 min, RAW 264.7 macrophages were stimulated with 10 μg ml-1 HP0459 for 10 h. The cytokine levels were then determined by ELISA. Data are expressed as the mean ± SD of three independent experiments. (B) HP0459-induced phosphorylation of ERK 1/2 MAPK in RAW264.7 macrophages. RAW264.7 macrophages were stimulated with HP0459 (10 μg ml-1) for 10 h. The cell lysates were analyzed by western blotting using specific antibodies against ERK 1/2 MAPK, phospho-ERK 1/2 MAPK, NF-κB and phospho-NF-κB. At the same time, β-actin was assessed as an internal control using anti-β-actin antibody. Results shown are representative of three independent experiments. *P <0.05, **P <0.01.
Discussion
Due to the high prevalence of S. suis-induced diseases in humans in Southeast and East Asia, pathogen has been increasingly investigated (Fittipaldi et al., 2012). Although the pathogenesis of S. suis infection is not entirely known at present, several viewpoints have been generally recognized, and one of these is that inflammation plays an important role in S. suis infection (Segura et al., 2006). After pathogen invasion into a host, the innate immune system of the host will recognize key molecular signatures borne by PAMPs to activate the inflammatory response in order to clear pathogens (Martinon and Tschopp, 2005). However, during S. suis infection, the inflammatory response is excessively activated and thus plays an important role in most clinical signs of S. suis disease, including meningitis, septicemia and sudden death (Dominguez-Punaro Mde et al., 2008). Thus, it is significant to identify the pro-inflammatory molecules of S. suis in order to understand its pathogenesis. In this study, it was found that HP0459 could significantly activate inflammatory response in RAW264.7 cells, but heat-killed HP0459 couldn’t (data not shown). Further, we elucidated the mechanism of HP0459 inducing inflammation. This result contributes to the understanding of the excessive inflammation induced by S. suis.
During S. suis infection, this pathogen can be recognized by some receptors, including CD14, TLR2, TLR6, and TLR9. CD14 has been considered one of the recognition receptors of LPS, the major component of the outer membrane of Gram-negative bacteria (Wright et al., 1990), and CD14 has been shown to be important in the recognition of cell wall constituents of Gram-positive bacteria (Wright, 1995; Moreillon and Majcherczyk, 2003). A previous study proved that S. suis can mediate CD14-dependent cytokine and chemokine production by human monocytes (Segura et al., 2002). Because CD14 lacks transmembrane and intracellular domains, it is not able to transduce the signal by itself. Thus, to activate the CD14-dependent inflammatory response, additional transmembrane receptors, such as TLR2, are required (Manukyan et al., 2005). TLR2, as a major pattern recognition receptor for ligands derived from Gram-positive bacteria (Kawai and Akira, 2005), has been shown to play an important role during S. suis ST1 strain interactions with mouse, swine and human cells (Graveline et al., 2007; Zheng et al., 2011, 2012). In addition, it has been indicated that not only TLR2 but also TLR6 and TLR9 play an important role on cell activation through in vitro studies carried out with the whole cells of the epidemic ST7 strain and human peripheral blood cells (Zheng et al., 2012). In the present study, using antibody blocking and TLR2-/- mouse macrophages assays, we found that TLR2 plays an important role in the inflammatory response activated by HP0459. This is consistent with S. suis-induced pro-inflammatory response, which is primary TLR2-dependent. However, LipoP prediction of HP0459 displayed that the signal peptide of HP0459 belonged to SpI (lipoprotein signal peptide belonged to SpII; Hutchings et al., 2009). This result suggested that the recognition of HP0459 may be different from bacterial lipoproteins. So, the mechanisms of HP0459 recognized by TLR2 need further research. We have proved that HP0459 contributes to pro-inflammatory response during S. suis infection. And it is known that there is a close link between excessive inflammation and the development of Streptococcus toxic shock syndrome (STSS; Zhao et al., 2011). This implies that HP0459 may be responsible for the pathogenesis of STSS caused by S. suis 2.
Compared with the SC-19 strains, the pro-inflammatory activation of the Δhp0459 strain in vivo was found to be significantly reduced in the present study. However, the mouse experiment showed that the lethality of the SC-19 and Δhp0459 strains were not significant different after challenge with the same CFUs (data not shown). To explain this result, we performed bacteriological isolation from the blood and spleen, and compared with the SC-19 and CΔhp0459 strains, the bacteria content of the Δhp0459 strain was significantly increased (Figure 5). It is known that higher bacterial counts of pathogenic bacteria in vivo may be an important cause leading to disease worsening (Sullivan et al., 1982). Thus, this finding suggests that inflammation is mainly but not exclusively responsible for the pathology of S. suis and that the bacteria content also plays an important role.
Finally, our data identified a novel pro-inflammatory protein denoted HP0459 from S. suis. Further, we demonstrated that HP0459 induces a TLR2-dependent pro-inflammatory response in RAW 264.7 macrophages via activation of the ERK1/2 pathway. These findings could be important for improving our understanding of the excessive inflammation induced by S. suis and may aid the development of therapies against SS2 infection.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to the State Key Laboratory of Agricultural Microbiology (Wuhan, China) for supporting this study. This work was supported by the High-Tech Research and Development Program (863) of China (2011AA10A210), the National Basic Research Program (973) of China (2012CB518805), the Chinese Major Special Science and Technology Project (2012ZX10004214-005) the Special Fund for Public Welfare Industry of Chinese Ministry of Agriculture (201303041), and the Fundamental Research Funds for the Central Universities (2011PY006).
References
Adam, R. A., Tenenbaum, T., Valentin-Weigand, P., Laryea, M., Schwahn, B., Angelow, S.,et al. (2004). Porcine choroid plexus epithelial cells induce Streptococcus suis bacteriostasis in vitro. Infect. Immun. 72, 3084–3087. doi: 10.1128/IAI.72.5.3084-3087.2004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Akira, S., and Hemmi, H. (2003). Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 85, 85–95. doi: 10.1016/S0165-2478(02)00228-6
Al-Numani, D., Segura, M., Dore, M., and Gottschalk, M. (2003). Up-regulation of ICAM-1, CD11a/CD18 and CD11c/CD18 on human THP-1 monocytes stimulated by Streptococcus suis serotype 2. Clin. Exp. Immunol. 133, 67–77. doi: 10.1046/j.1365-2249.2003.02189.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Arends, J. P., and Zanen, H. C. (1988). Meningitis caused by Streptococcus suis in humans. Rev. Infect. Dis. 10, 131–137. doi: 10.1093/clinids/10.1.131
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baums, C. G., and Valentin-Weigand, P. (2009). Surface-associated and secreted factors of Streptococcus suis in epidemiology, pathogenesis and vaccine development. Anim. Health Res. Rev. 10, 65–83. doi: 10.1017/S146625230999003X
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Beutler, B. (2004). Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430, 257–263. doi: 10.1038/nature02761
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Beutler, B. (2009). Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol. Rev. 227, 248–263. doi: 10.1111/j.1600-065X.2008.00733.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bonifait, L., Vaillancourt, K., Gottschalk, M., Frenette, M., and Grenier, D. (2011). Purification and characterization of the subtilisin-like protease of Streptococcus suis that contributes to its virulence. Vet. Microbiol. 148, 333–340. doi: 10.1016/j.vetmic.2010.09.024
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Charland, N., Harel, J., Kobisch, M., Lacasse, S., and Gottschalk, M. (1998). Streptococcus suis serotype 2 mutants deficient in capsular expression. Microbiology 144(Pt 2), 325–332. doi: 10.1099/00221287-144-2-325
Dominguez-Punaro Mde, L., Segura, M., Radzioch, D., Rivest, S., and Gottschalk, M. (2008). Comparison of the susceptibilities of C57BL/6 and A/J mouse strains to Streptococcus suis serotype 2 infection. Infect. Immun. 76, 3901–3910. doi: 10.1128/IAI.00350-08.
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fittipaldi, N., Segura, M., Grenier, D., and Gottschalk, M. (2012). Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol. 7, 259–279. doi: 10.2217/fmb.11.149
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fittipaldi, N., Sekizaki, T., Takamatsu, D., Harel, J., Dominguez-Punaro Mde, L., Von Aulock, S.,et al. (2008). D-alanylation of lipoteichoic acid contributes to the virulence of Streptococcus suis. Infect. Immun. 76, 3587–3594. doi: 10.1128/IAI.01568-07.
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gottschalk, M., and Segura, M. (2000). The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet. Microbiol. 76, 259–272. doi: 10.1016/S0378-1135(00)00250-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gottschalk, M., Segura, M., and Xu, J. (2007). Streptococcus suis infections in humans: the Chinese experience and the situation in North America. Anim. Health Res. Rev. 8, 29–45. doi: 10.1017/S1466252307001247.
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gottschalk, M., Xu, J., Calzas, C., and Segura, M. (2010). Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiol. 5, 371–391. doi: 10.2217/fmb.10.2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Graveline, R., Segura, M., Radzioch, D., and Gottschalk, M. (2007). TLR2-dependent recognition of Streptococcus suis is modulated by the presence of capsular polysaccharide which modifies macrophage responsiveness. Int. Immunol. 19, 375–389. doi: 10.1093/intimm/dxm003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hui, A. C., Ng, K. C., Tong, P. Y., Mok, V., Chow, K. M., Wu, A.,et al. (2005). Bacterial meningitis in Hong Kong: 10-years’ experience. Clin. Neurol. Neurosurg. 107, 366–370. doi: 10.1016/j.clineuro.2004.10.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hutchings, M. I., Palmer, T., Harrington, D. J., and Sutcliffe, I. C. (2009). Lipoprotein biogenesis in Gram-positive bacteria: knowing when to hold ‘em, knowing when to fold ‘em. Trends Microbiol. 17, 13–21. doi: 10.1016/j.tim.2008.10.001
Ip, M., Fung, K. S., Chi, F., Cheuk, E. S., Chau, S. S., Wong, B. W.,et al. (2007). Streptococcus suis in Hong Kong. Diagn. Microbiol. Infect. Dis 57, 15–20. doi: 10.1016/j.diagmicrobio.2006.05.011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jobin, M. C., Fortin, J., Willson, P. J., Gottschalk, M., and Grenier, D. (2005). Acquisition of plasmin activity and induction of arachidonic acid release by Streptococcus suis in contact with human brain microvascular endothelial cells. FEMS Microbiol. Lett. 252, 105–111. doi: 10.1016/j.femsle.2005.08.044
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kang, E. H., Gebru, E., Kim, M. H., Cheng, H., and Park, S. C. (2009). EstA protein, a novel virulence factor of Streptococcus pneumoniae, induces nitric oxide and pro-inflammatory cytokine production in RAW 264.7 macrophages through NF-kappaB/MAPK. Microb. Pathog. 47, 196–201. doi: 10.1016/j.micpath.2009.07.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kawai, T., and Akira, S. (2005). Pathogen recognition with Toll-like receptors. Curr. Opin. Immunol 17, 338–344. doi: 10.1016/j.coi.2005.02.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kawai, T., and Akira, S. (2010). The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384. doi: 10.1038/ni.1863
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lachance, C., Segura, M., Gerber, P. P., Xu, J., and Gottschalk, M. (2013). Toll-like receptor 2-independent host innate immune response against an epidemic strain of Streptococcus suis that causes a toxic shock-like syndrome in humans. PLoS ONE 8:e65031. doi: 10.1371/journal.pone.0065031
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lecours, M. P., Segura, M., Fittipaldi, N., Rivest, S., and Gottschalk, M. (2012). Immune receptors involved in Streptococcus suis recognition by dendritic cells. PLoS ONE 7:e44746. doi: 10.1371/journal.pone.0044746
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Li, R., Zhang, A., Chen, B., Teng, L., Wang, Y., Chen, H.,et al. (2010). Response of swine spleen to Streptococcus suis infection revealed by transcription analysis. BMC Genomics 11:556. doi: 10.1186/1471-2164-11-556
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Li, W., Wan, Y., Tao, Z., Chen, H., and Zhou, R. (2013). A novel fibronectin-binding protein of Streptococcus suis serotype 2 contributes to epithelial cell invasion and in vivo dissemination. Vet. Microbiol. 162, 186–194. doi: 10.1016/j.vetmic.2012.09.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liu, B., Teng, D., Wang, X., Yang, Y., and Wang, J. (2012). Expression of the soybean allergenic protein P34 in Escherichia coli and its indirect ELISA detection method. Appl. Microbiol. Biotechnol. 94, 1337–1345. doi: 10.1007/s00253-012-4006-3.
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liu, D., Yumoto, H., Hirota, K., Murakami, K., Takahashi, K., Hirao, K.,et al. (2008). Histone-like DNA binding protein of Streptococcus intermedius induces the expression of pro-inflammatory cytokines in human monocytes via activation of ERK1/2 and JNK pathways. Cell Microbiol 10, 262–276. doi: 10.1111/j.1462-5822.2007.01040.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liu, L., Cheng, G., Wang, C., Pan, X., Cong, Y., Pan, Q.,et al. (2009). Identification and experimental verification of protective antigens against Streptococcus suis serotype 2 based on genome sequence analysis. Curr. Microbiol. 58, 11–17. doi: 10.1007/s00284-008-9258-x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liu, N., Liu, J. T., Ji, Y. Y., and Lu, P. P. (2011). Rosiglitazone regulates c-reactive protein-induced inflammatory responses via glucocorticoid receptor-mediated inhibition of p38 mitogen-activated protein kinase-toll-like receptor 4 signal pathway in vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 57, 348–356. doi: 10.1097/FJC.0b013e31820a0e67
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lun, S., Perez-Casal, J., Connor, W., and Willson, P. J. (2003). Role of suilysin in pathogenesis of Streptococcus suis capsular serotype 2. Microb. Pathog. 34, 27–37. doi: 10.1016/S0882-4010(02)00192-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mai, N. T., Hoa, N. T., Nga, T. V., Linh Le, D., Chau, T. T., Sinh, D. X.,et al. (2008). Streptococcus suis meningitis in adults in Vietnam. Clin. Infect. Dis. 46, 659–667. doi: 10.1086/527385
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Manukyan, M., Triantafilou, K., Triantafilou, M., Mackie, A., Nilsen, N., Espevik, T.,et al. (2005). Binding of lipopeptide to CD14 induces physical proximity of CD14, TLR2 and TLR1. Eur. J. Immunol. 35, 911–921. doi: 10.1002/eji.200425336
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Martinon, F., and Tschopp, J. (2005). NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 26, 447–454. doi: 10.1016/j.it.2005.06.004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moore, L. J., Pridmore, A. C., Lee, M. E., and Read, R. C. (2005). Induction of pro-inflammatory cytokine release by human macrophages during exposure of Streptococcus pneumoniae to penicillin is influenced by minimum inhibitory concentration ratio. Int. J. Antimicrob. Agents 26, 188–196. doi: 10.1016/j.ijantimicag.2005.06.006
Moreillon, P., and Majcherczyk, P. A. (2003). Proinflammatory activity of cell-wall constituents from gram-positive bacteria. Scand. J. Infect. Dis. 35, 632–641. doi: 10.1080/00365540310016259
Perch, B., Kristjansen, P., and Skadhauge, K. (1968). Group R streptococci pathogenic for man. Two cases of meningitis and one fatal case of sepsis. Acta Pathol. Microbiol. Scand. 74, 69–76. doi: 10.1111/j.1699-0463.1968.tb03456.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Segura, M., Gottschalk, M., and Olivier, M. (2004). Encapsulated Streptococcus suis inhibits activation of signaling pathways involved in phagocytosis. Infect. Immun. 72, 5322–5330. doi: 10.1128/IAI.72.9.5322-5330.2004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Segura, M., Vadeboncoeur, N., and Gottschalk, M. (2002). CD14-dependent and -independent cytokine and chemokine production by human THP-1 monocytes stimulated by Streptococcus suis capsular type 2. Clin. Exp. Immunol 127, 243–254. doi: 10.1046/j.1365-2249.2002.01768.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Segura, M., Vanier, G., Al-Numani, D., Lacouture, S., Olivier, M., and Gottschalk, M. (2006). Proinflammatory cytokine and chemokine modulation by Streptococcus suis in a whole-blood culture system. FEMS Immunol. Med. Microbiol. 47, 92–106. doi: 10.1111/j.1574-695X.2006.00067.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Smith, H. E., Damman, M., Van Der Velde, J., Wagenaar, F., Wisselink, H. J., Stockhofe-Zurwieden, N.,et al. (1999). Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect. Immun. 67, 1750–1756.
Smith, H. E., Vecht, U., Gielkens, A. L., and Smits, M. A. (1992). Cloning and nucleotide sequence of the gene encoding the 136-kilodalton surface protein (muramidase-released protein) of Streptococcus suis type 2. Infect. Immun. 60, 2361–2367.
Sodhi, A., Sharma, R. K., and Batra, H. V. (2005). Yersinia rLcrV and rYopB inhibits the activation of murine peritoneal macrophages in vitro. Immunol. Lett. 99, 146–152. doi: 10.1016/j.imlet.2005.02.009
Suankratay, C., Intalapaporn, P., Nunthapisud, P., Arunyingmongkol, K., and Wilde, H. (2004). Streptococcus suis meningitis in Thailand. Southeast Asian J. Trop. Med. Public Health 35, 868–876.
Sullivan, T. D., Lascolea, L. J. Jr., and Neter, E. (1982). Relationship between the magnitude of bacteremia in children and the clinical disease. Pediatrics 69, 699–702.
Takamatsu, D., Osaki, M., and Sekizaki, T. (2001). Thermosensitive suicide vectors for gene replacement in Streptococcus suis. Plasmid 46, 140–148. doi: 10.1006/plas.2001.1532
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tenenbaum, T., Adam, R., Eggelnpohler, I., Matalon, D., Seibt, A., Ge, K. N.,et al. (2005). Strain-dependent disruption of blood-cerebrospinal fluid barrier by Streptoccocus suis in vitro. FEMS Immunol. Med. Microbiol. 44, 25–34. doi: 10.1016/j.femsim.2004.12.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vadeboncoeur, N., Segura, M., Al-Numani, D., Vanier, G., and Gottschalk, M. (2003). Pro-inflammatory cytokine and chemokine release by human brain microvascular endothelial cells stimulated by Streptococcus suis serotype 2. FEMS Immunol. Med. Microbiol. 35, 49–58. doi: 10.1111/j.1574-695X.2003.tb00648.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
van Lint, A. L., Murawski, M. R., Goodbody, R. E., Severa, M., Fitzgerald, K. A., Finberg, R. W.,et al. (2010). Herpes simplex virus immediate-early ICP0 protein inhibits Toll-like receptor 2-dependent inflammatory responses and NF-kappaB signaling. J. Virol. 84, 10802–10811. doi: 10.1128/JVI.00063-10
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wei, Z., Li, R., Zhang, A., He, H., Hua, Y., Xia, J.,et al. (2009). Characterization of Streptococcus suis isolates from the diseased pigs in China between 2003 and 2007. Vet. Microbiol. 137, 196–201. doi: 10.1016/j.vetmic.2008.12.015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wertheim, H. F., Nghia, H. D., Taylor, W., and Schultsz, C. (2009). Streptococcus suis: an emerging human pathogen. Clin. Infect. Dis. 48, 617–625. doi: 10.1086/596763
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wisselink, H. J., Smith, H. E., Stockhofe-Zurwieden, N., Peperkamp, K., and Vecht, U. (2000). Distribution of capsular types and production of muramidase-released protein (MRP) and extracellular factor (EF) of Streptococcus suis strains isolated from diseased pigs in seven European countries. Vet. Microbiol. 74, 237–248. doi: 10.1016/S0378-1135(00)00188-7
Wright, S. D., Ramos, R. A., Tobias, P. S., Ulevitch, R. J., and Mathison, J. C. (1990). CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249, 1431–1433. doi: 10.1126/science.1698311
Ye, C., Zhu, X., Jing, H., Du, H., Segura, M., Zheng, H.,et al. (2006). Streptococcus suis sequence type 7 outbreak, Sichuan, China. Emerg. Infect. Dis 12, 1203–1208. doi: 10.3201/eid1208.060232
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yu, H., Jing, H., Chen, Z., Zheng, H., Zhu, X., Wang, H.,et al. (2006). Human Streptococcus suis outbreak, Sichuan, China. Emerg. Infect. Dis. 12, 914–920. doi: 10.3201/eid1206.051194
Zhang, A., Chen, B., Yuan, Z., Li, R., Liu, C., Zhou, H.,et al. (2012). HP0197 contributes to CPS synthesis and the virulence of Streptococcus suis via CcpA. PLoS ONE 7:e50987. doi: 10.1371/journal.pone.0050987
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, A., Mu, X., Chen, B., Han, L., Chen, H., and Jin, M. (2011). IgA1 protease contributes to the virulence of Streptococcus suis. Vet. Microbiol. 148, 436–439. doi: 10.1016/j.vetmic.2010.09.027
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhao, Y., Liu, G., Li, S., Wang, M., Song, J., Wang, J.,et al. (2011). Role of a type IV-like secretion system of Streptococcus suis 2 in the development of streptococcal toxic shock syndrome. J. Infect. Dis. 204, 274–281. doi: 10.1093/infdis/jir261
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhao, Z., Yi, C., Zhao, L., Wang, S., Zhou, L., Hu, Y.,et al. (2014). PB2-588I enhances 2009 H1N1 pandemic influenza virus virulence by increasing viral replication and exacerbating PB2 inhibition of beta interferon expression. J. Virol. 88, 2260–2267. doi: 10.1128/JVI.03024-13
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zheng, H., Luo, X., Segura, M., Sun, H., Ye, C., Gottschalk, M.,et al. (2012). The role of toll-like receptors in the pathogenesis of Streptococcus suis. Vet. Microbiol. 156, 147–156. doi: 10.1016/j.vetmic.2011.10.014
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zheng, H., Punaro, M. C., Segura, M., Lachance, C., Rivest, S., Xu, J.,et al. (2011). Toll-like receptor 2 is partially involved in the activation of murine astrocytes by Streptococcus suis, an important zoonotic agent of meningitis. J. Neuroimmunol. 234, 71–83. doi: 10.1016/j.jneuroim.2011.02.005
Zheng, H., Ye, C., Segura, M., Gottschalk, M., and Xu, J. (2008). Mitogenic effect contributes to increased virulence of Streptococcus suis sequence type 7 to cause streptococcal toxic shock-like syndrome. Clin. Exp. Immunol 153, 385–391. doi: 10.1111/j.1365-2249.2008.03722.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: Streptococcus suis 2, inflammation, pro-inflammatory protein, Toll-like receptor 2, ERK1/2 pathway
Citation: Zhang Q, Yang Y, Yan S, Liu J, Xu Z, Yu J, Song Y, Zhang A and Jin M (2015) A novel pro-inflammatory protein of Streptococcus suis 2 induces the Toll-like receptor 2-dependent expression of pro-inflammatory cytokines in RAW 264.7 macrophages via activation of ERK1/2 pathway. Front. Microbiol. 6:178. doi: 10.3389/fmicb.2015.00178
Received: 10 November 2014; Paper pending published: 09 January 2015;
Accepted: 18 February 2015; Published online: 09 March 2015
Edited by:
Denise Monack, Stanford University School of Medicine, USAReviewed by:
David Weiss, Emory University, USAHeinrich Korner, Menzies Research Institute Tasmania, Australia
Copyright © 2015 Zhang, Yang, Yan, Liu, Xu, Yu, Song, Zhang and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meilin Jin, Unit of Animal Infectious Diseases, National Key Laboratory of Agricultural Microbiology, College of Veterinary Medicine, Huazhong Agricultural University, No.1 Shizishan Street, Wuhan, Hongshan District, Hubei 430070, China e-mail:amlubWVpbGluQG1haWwuaHphdS5lZHUuY24=
†These authors have contributed equally to this work.
 Qiang Zhang
Qiang Zhang Yujie Yang
Yujie Yang Shuxian Yan1
Shuxian Yan1 Junping Yu
Junping Yu Meilin Jin
Meilin Jin