- Department of Molecular Microbiology and Immunology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA
Lyme disease, caused by Borrelia burgdorferi, is the most common vector-borne disease in the United States and Europe. While the majority of Lyme disease patients can resolve their symptoms if treated promptly, 10–20% of patients suffer from prolonged symptoms called post-treatment Lyme disease syndrome (PTLDS). Although the cause for PTLDS is unclear, one possibility is the presence of bacterial persisters not effectively cleared by the current Lyme antibiotics. Recent studies identified several drug candidates including daptomycin, daunomycin, doxorubicin, and mitomycin C that had good activity against B. burgdorferi persisters. However, their relative activities against B. burgdorferi persisters have not been evaluated under the same conditions. In this study, we tested the anti-persister activities of these drugs against both 7-day and 15-day old stationary phase cultures of B. burgdorferi individually as well as in combination with Lyme antibiotics doxycycline and cefuroxime (Ceftin). Our findings demonstrate daunomycin and daptomycin were more active than mitomycin C in single drug comparison at 10 and 20 μM, as well as in drug combinations with doxycycline and cefuroxime. In addition, daunomycin was more active than doxorubicin which correlated with their ability to stain and accumulate in B. burgdorferi. The two drug combination of doxycycline and cefuroxime was unable to eradicate biofilm-like microcolonies of B. burgdorferi persisters. However, the addition of either daunomycin or daptomycin to the doxycycline + cefuroxime combination completely eradicated the biofilm-like structures and produced no visible bacterial regrowth after 7 and 21 days, while the addition of doxorubicin was unable to prevent regrowth at either 7 or 21 day subculture. Mitomycin C in combination with doxycycline and cefuroxime caused no regrowth at 7 days but visible spirochetal regrowth occurred after 21 day subculture. Furthermore, we found that cefuroxime (Ceftin), the third commonly used and most active antibiotic to treat Lyme disease, could replace cefoperazone (a drug no longer available in the US) in the daptomycin + doxycycline combination with complete eradication of the biofilm-like structures as shown by lack of any regrowth in subcultures. Our findings may have implications for improved treatment of Lyme disease.
Introduction
Borrelia burgdorferi is the causative agent of Lyme disease, which is the most common vector-borne disease in the United States with an estimated 300,000 cases in 2013(CDC, 2015a). The infection is transmitted to humans by tick vectors that feed upon rodents, reptiles, birds and deer (Radolf et al., 2012). In the early stage of Lyme disease, approximately 50% of patients have localized erythema migrans, a target-shaped rash that expands as the bacteria disseminate from the cutaneous infection site (CDC, 2015a). Late stage Lyme disease is a multi-system disorder with symptoms including arthritis, carditis, and neurologic impairment (CDC, 2015a). The majority of Lyme disease patients can resolve their symptoms if treated promptly with doxycycline, amoxicillin, or cefuroxime (Wormser et al., 2006). However, at least 10–20% of Lyme disease patients experience prolonged symptoms such as neurologic impairment, muscular pain, and fatigue 6 months after antibiotic treatment, a collection of symptoms called Post-Treatment Lyme Disease Syndrome (PTLDS; CDC, 2015b).
The cause of PTLDS is unknown, though there are several theories including co-infections (Swanson et al., 2006), autoimmune response (Steere et al., 2001), immune response to continued presence of antigenic debris (Bockenstedt et al., 2012), as well as B. burgdorferi persisters that are not killed by the current antibiotics (Hodzic et al., 2008, 2014; Embers et al., 2012). Using a combination of diagnostic techniques including xenodiagnosis and PCR, studies have found evidence of B. burgdorferi persistence in dogs (Straubinger et al., 1997), mice (Hodzic et al., 2008, 2014), monkeys (Embers et al., 2012), and humans (Marques et al., 2014) after antibiotic treatment, though no viable bacteria could be cultured.
Borrelia burgdorferi develops persisters stochastically in stationary phase which are tolerant to the antibiotics used to treat Lyme disease (Feng et al., 2014a, 2015a; Caskey and Embers, 2015; Sharma et al., 2015). These persister bacteria have been found to have an altered RNA profile, making them phenotypically drug tolerant (Feng et al., 2015c). In log phase cultures (3–5 days old), B. burgdorferi is primarily in motile spirochetal form which is highly susceptible to current Lyme antibiotics doxycycline and amoxicillin, however, in stationary phase cultures (7–15 days old), increased numbers of atypical forms such as round bodies and aggregated biofilm-like microcolonies develop (Feng et al., 2014a, 2015a). These atypical forms have been shown to have increased tolerance to doxycycline and amoxicillin when compared to the growing spirochetal forms (Feng et al., 2014a, 2015a; Caskey and Embers, 2015; Sharma et al., 2015). Therefore, stationary phase cultures (7–15 days old) which are enriched in persisters were used as a model for high-throughput drug screens against persister populations (Feng et al., 2014a, 2015a,b,d).
Drugs with high activity against the B. burgdorferi stationary phase persisters were identified through screens of FDA approved drug library and NCI compound libraries (Feng et al., 2014a, 2015b,d). Among them, daptomycin, a lipopeptide antibiotic targeting bacterial cell membranes, was found from the FDA drug library to have the highest anti-persister activity against B. burgdorferi (Feng et al., 2014a). In addition, anticancer anthracycline antibiotics, such as daunomycin and doxorubicin, and also mitomycin C were found from the NCI compound library screen as having excellent or good activity against B. burgdorferi persisters (Feng et al., 2015b). Daunomycin, doxorubicin and mitomycin C were all isolated from Streptomyces and are used in the treatment of a wide range of cancers. Daunomycin and doxorubicin belong to anthracycline anti-cancer antibiotic and kill the bacteria by inhibiting DNA and RNA synthesis, causing DNA damage and producing reactive oxygen species. Mitomycin C blocks DNA replication and causes cell death by DNA crosslinking. Although the anti-persister drugs such as daptomycin are more active than the current Lyme antibiotics such as doxycycline or amoxicillin against B. burgdorferi persisters (Feng et al., 2014a), they alone could not completely eradicate the more resistant biofilm-like microcolonies and a drug combination approach is required to do so (Feng et al., 2015a). The more effective drug combination approach to eradicate biofilm-like microcolonies is consistent with the drug combination principle for treatment of persistent infections like tuberculosis (Zhang et al., 2012; Zhang, 2014). In a recent study using a relatively young 5 days old culture, mitomycin C was found to have higher activity than daptomycin against B. burgdorferi persisters (Sharma et al., 2015). However, their relative activity against B. burgdorferi persisters has not been compared or evaluated in the same study under the same conditions. In this study, four of the identified drugs with the highest activity against stationary phase B. burgdorferi persisters were tested to determine their anti-persister activity at more clinically achievable levels. In addition, we assessed these persister active drugs in combination with the commonly prescribed Lyme antibiotics doxycycline and cefuroxime, which have high activity against growing log phase cultures, with the aim to increase the activity of these drugs for more effective treatment of Lyme disease.
Materials and Methods
Strain, Media, and Culture Techniques
Borrelia burgdorferi strain B31 (ATCC 35210) was obtained from American Type Tissue Collections (Manassas, VA, USA). B. burgdorferi was grown in BSK-H medium (HiMedia Laboratories, Mumbai, India) and supplemented with 6% rabbit serum (Sigma Aldrich, St. Louis, MO, USA). The medium was filter-sterilized via passage through a 0.22 μM filter. The inoculated medium was incubated in sterile 50 mL conical tubes (BD Biosciences, San Jose, CA, USA) in a 33°C incubator without shaking. The culture was maintained in these conditions for 7 or 15 days until the culture reached stationary phase, when it was transferred to a 96 well plate for evaluation with the drugs or their combinations.
Drugs
The following drugs were obtained from Sigma–Aldrich, St. Louis, MO, USA and dissolved in the solvents suggested by the Clinical and Laboratory Standards Institute to make a stock solution: doxycycline (Dox), cefuroxime (CefU), cefoperazone (CefP), daptomycin (Dap), mitomycin C (MitC), doxorubicin (DoxR), daunomycin (Dau), (Clinical and Laboratory Standards Institute, 2007). The drug stock solutions were filter-sterilized using a 0.22 μM filter and stored at –20°C.
Microscopy
The B. burgdorferi cultures were examined using a Zeiss AxioImager M2 microscope with differential interference contrast and epifluorescent illumination. Pictures were taken using a SPOT slider camera. A SYBR Green I/PI assay was performed as previously described to assess cell viability using the ratio of green:red fluorescence to determine the live:dead cell ratio, respectively, as measured by a plate reader (Feng et al., 2014b). This residual cell viability reading was confirmed by analyzing three representative images of the bacterial culture using epifluorescence microscopy. Image Pro-Plus software was used to quantitatively determine the fluorescence intensity.
Evaluation of Drugs Against Biofilm-Like Structures in B. burgdorferi Stationary Phase Cultures
For single drug evaluation, an aliquot of the drug stock solution was added to each 96 well plate containing 100 μL of 7-day old stationary phase B. burgdorferi culture to obtain the desired drug concentration. The plate was then sealed and was incubated at 33°C without shaking for 7 days. After incubation, the viability of the residual viable cells was assessed using the SYBR Green I/PI viability assay and confirmed using epifluorescence microscopy (Feng et al., 2014b). Each sample was analyzed in triplicate and the mean residual viable cells remaining were calculated.
For assessing the activity of anthracycline compounds and daptomycin and mitomycin C in combination with current Lyme antibiotics against biofilm-like structures, a 15-day old B. burgdorferi stationary phase culture was used. Aliquots of the drugs were added to 96 well plate containing 100 μL of the 15-day old stationary phase B. burgdorferi culture which was enriched in aggregated biofilm-like structures to create a final drug concentration of 10 μg/mL for each drug. This drug concentration was chosen as most drugs evaluated in this study fell within or close to their Cmax values (maximum serum concentration; Table 1). The plate was then sealed and was incubated at 33°C without shaking for 7 days, when the residual viable cells remaining were measured using the SYBR Green I/PI viability assay and confirmed using epifluorescence microscopy as described (Feng et al., 2014b).
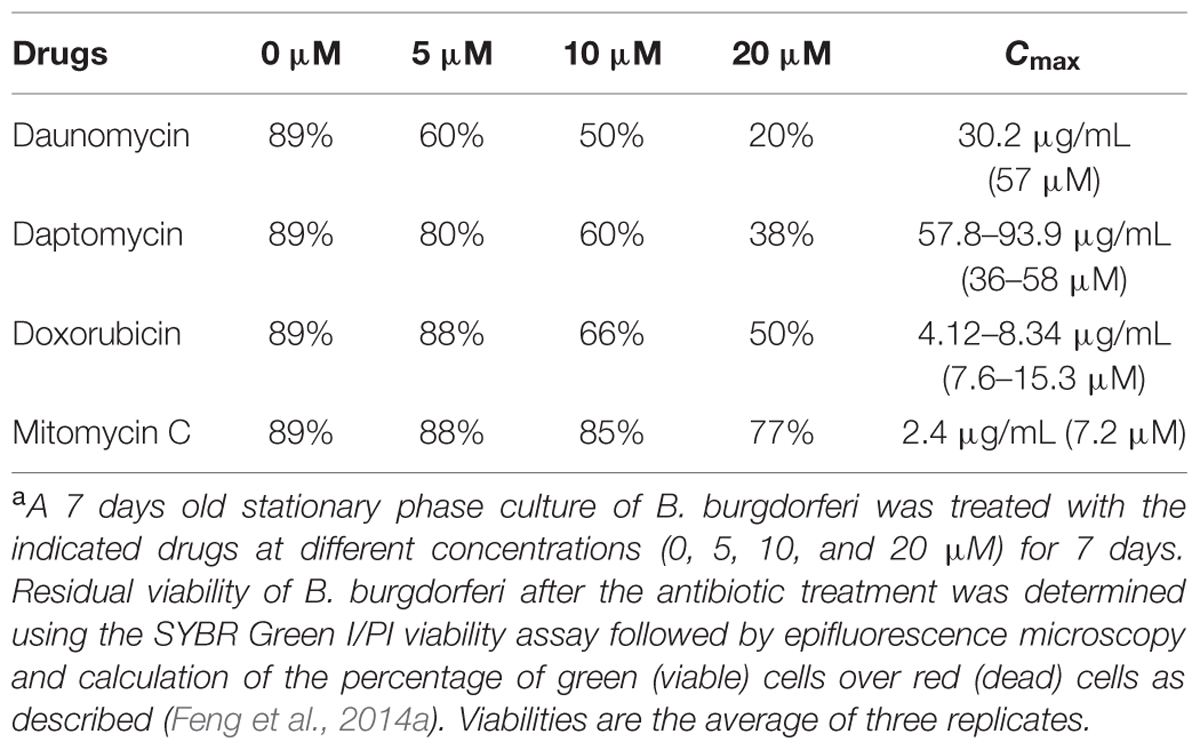
TABLE 1. Relative activity of daunomycin, daptomycin, doxorubicin, and mitomycin C on a 7-day old B. burgdorferi stationary phase culturea.
Subculture Study to Assess the Effect of Drug Combination on the Biofilm-Like Structures in B. burgdorferi Stationary Phase Cultures
A 15-day old B. burgdorferi culture (500 μL of 1 × 107 spirochetes/mL) was exposed to the indicated drug combinations in Eppendorf tubes, and incubated at 33°C for 7 days without shaking. After incubation, the bacteria were spun down and washed with 1 mL fresh BSK-H medium. The cultures were resuspended in 500 μL BSK-H medium, and a 50 μL aliquot was used to inoculate a new tube of 1 mL fresh BSK-H medium for subculture. The cultures were allowed to grow for either 7 or 21 days, when they were evaluated for regrowth with viable cells using the previously described SYBR Green I/PI assay and epifluorescence microscopy (Feng et al., 2015a).
Results and Discussion
Comparison of the Relative Anti-Persister Activity of Daunomycin, Doxorubicin, Daptomycin, and Mitomycin C in Single Drug Exposure Against Stationary Phase B. burgdorferi Culture
Although daptomycin (Feng et al., 2014a), daunomycin (Feng et al., 2015b), doxorubicin (Feng et al., 2015b), and mitomycin C (Feng et al., 2015b; Sharma et al., 2015) were identified to have high activity against B. burgdorferi persisters, their relative activities have not been compared under the same conditions. To do so, we compared them for their activity against the same 7-day old B. burgdorferi stationary phase culture at the same concentrations (5, 10, and 20 μM), using SYBR Green I/PI viability stain followed by epifluorescence microscopy. The anthracycline drug daunomycin was shown to have the highest activity against the stationary phase cultures even at the lowest concentration (5 μM) with a dose-dependent increase in killing activity resulting in a near total clearance of bacteria at the highest concentration (20 μM) as shown by mostly red (dead) cells and dispersed, smaller aggregated microcolony size, revealed by the SYBR Green I/PI viability assay (Figure 1, Table 1). Daptomycin was the second most active drug against the B. burgdorferi stationary phase culture, followed by doxorubicin (Figure 1, Table 1). Mitomycin C was the least active drug among the four persister-active drugs, and even at 20 μM had poor activity against the aggregated biofilm-like microcolony form of B. burgdorferi persisters, as shown by mostly green (live) microcolonies remaining after the drug treatment for 7 days (Figure 1).
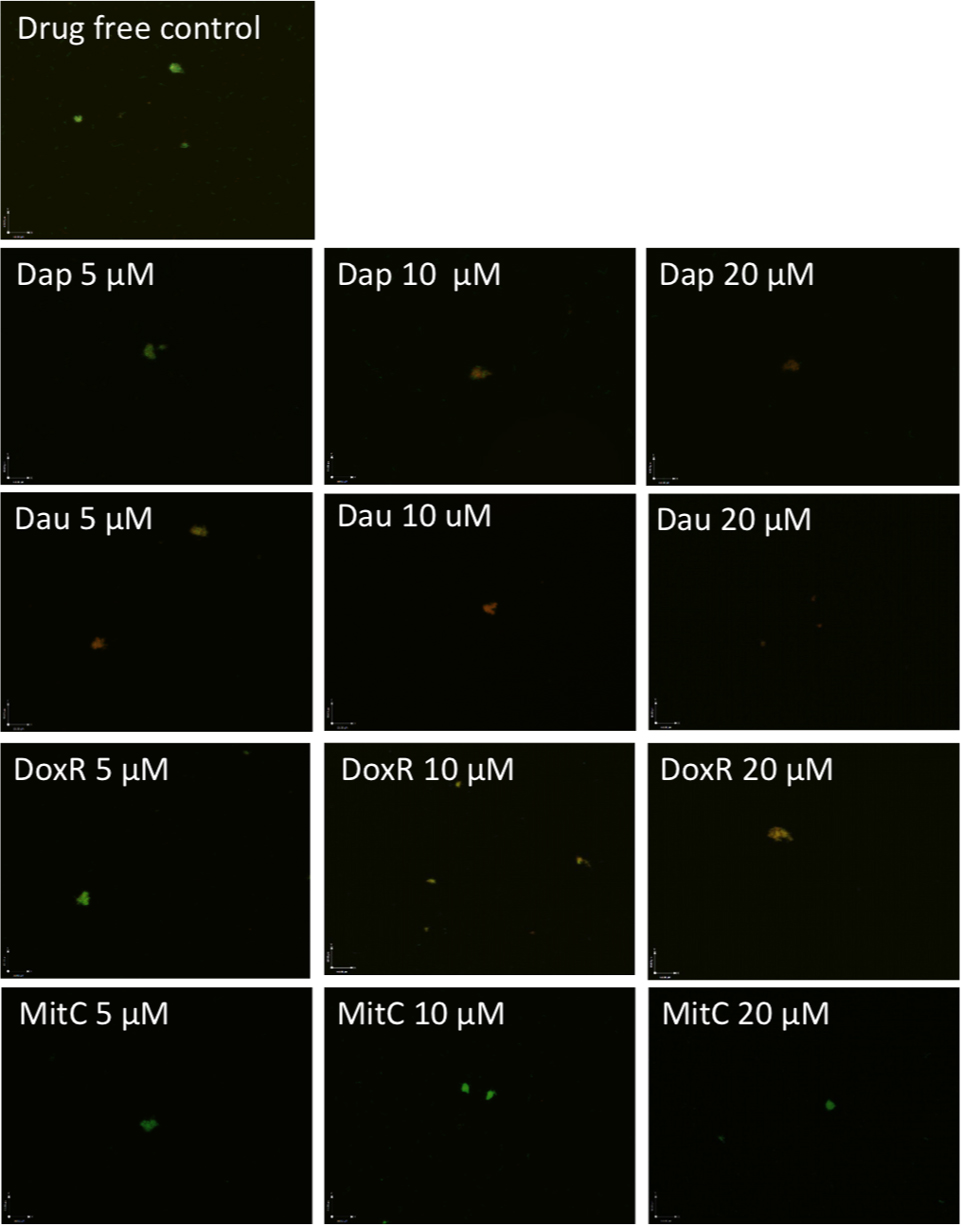
FIGURE 1. Comparison of anti-persister activity of daunomycin, doxorubicin, daptomycin, and mitomycin C. A 7-day old B. burgdorferi stationary phase culture containing aggregated microcolonies was incubated for 7 days with daptomycin (Dap), daunomycin (Dau), doxorubicin (DoxR), or mitomycin C (MitC) at the same drug concentrations of 5, 10, or 20 μM, respectively, followed by viability assessment using the SYBR Green I/PI assay. Representative images were taken using epifluorescence microscopy at 400× magnification. Green cells indicate live cells while red cells indicate dead cells.
Doxorubicin was less active than daunomycin as shown by higher percentage of viable cells remaining after drug exposure (Table 1) despite their both belonging to the same anthracycline class. These results could be explained by structural differences between those compounds (Figure 2A). Doxorubicin possesses a hydroxyl group as opposed to a methyl group in the corresponding position of daunomycin, with the remainder of the anthracycline structure being identical. Interestingly, although doxorubicin and daunomycin both have orange-red color in solution (Figure 2B), we found daunomycin visibly stained the B. burgdorferi cells red as seen in the red cell pellet while doxorubicin only stained the cells rather faintly (Figure 2C). This finding suggests that daunomycin may cross the B. burgdorferi cell membrane more efficiently to accumulate in the cell while doxorubicin may have poor ability to enter or accumulate in B. burgdorferi cells.
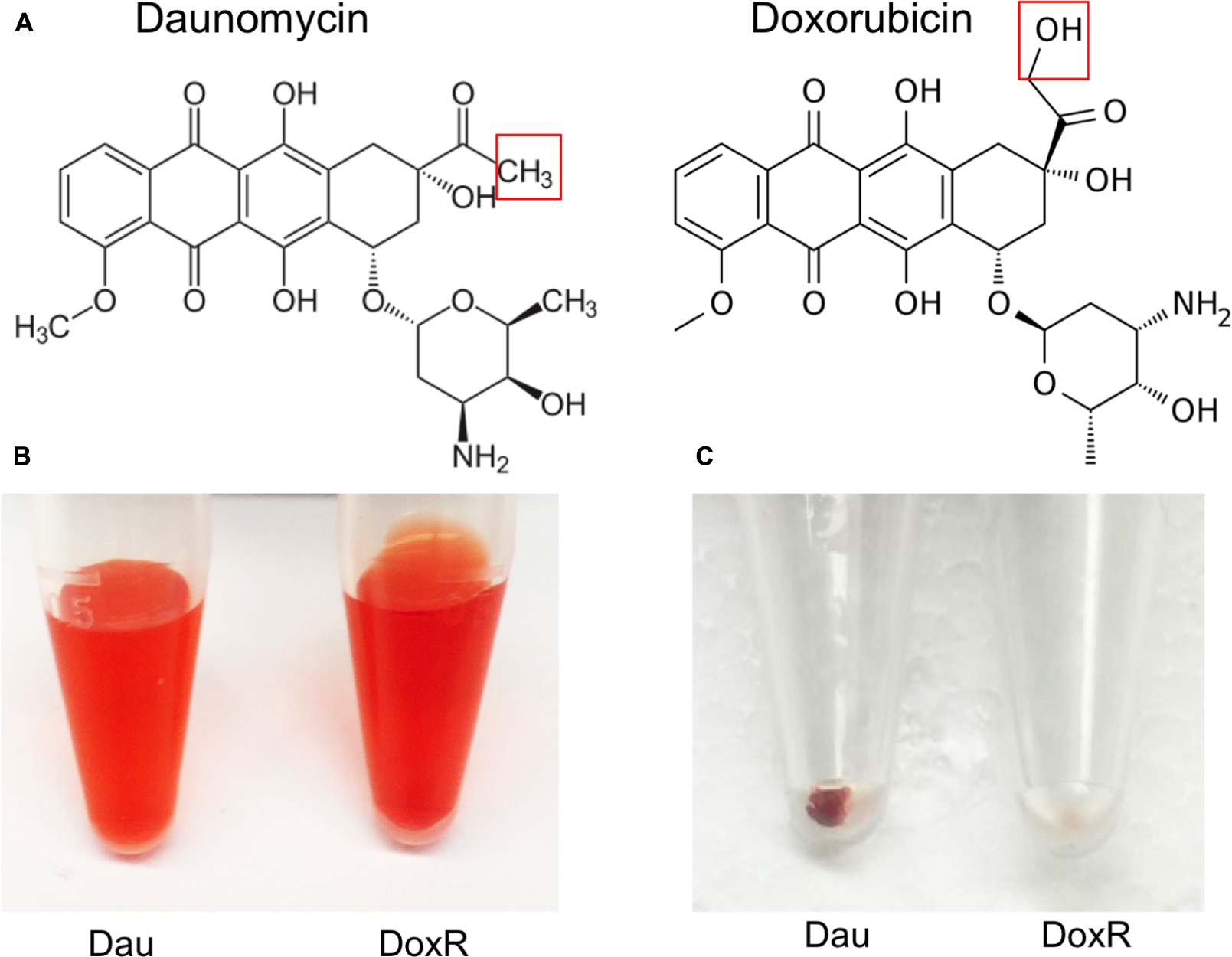
FIGURE 2. Differences in structures of daunomycin and doxorubicin and their ability to accumulate in B. burgdorferi. (A) Chemical structures of daunomycin and doxorubicin. Red box shows the difference between the structures of daunomycin (methyl group) and doxorubicin (hydroxyl group). (B) Daunomycin and doxorubicin show the same orange–red color at 10 mM solution. (C) Cell pellets of 7-day old B. burgdorferi treated with 10 μM daunomycin (left-side tube) 10 μM doxorubicin (right-side tube) for 7 days, where daunomycin stained B. burgdorferi red while doxorubicin hardly stained the organism.
Comparison of the Relative Anti-Persister Activity of Daunomycin, Daptomycin, Doxorubicin, and Mitomycin C in Drug Combinations Using SYBR Green I/PI Viability Assay
Both two-drug combinations doxycycline + cefuroxime and doxycycline + cefoperazone showed poor activity against the 15-day old stationary phase culture, with 67% residual viable (green) cells remaining in comparison to 79% viable cells in the drug-free control (Figure 3, Table 2). Consistent with the single drug exposure experiment (Figure 1), daunomycin, doxorubicin and daptomycin when added to the drug combination doxycycline + cefuroxime had a high anti-persister activity as seen by 12, 18, and 30% viable cells remaining (Table 2) as well as mostly red (dead) cells after treatment (Figure 3). In contrast, when mitomycin C was added to the drug combination doxycycline + cefuroxime, the anti-persister activity of these compounds was moderately increased as shown by 45% residual viable cells remaining (Table 2), but more green (live) cells were seen with the mitomycin C drug combination than with the daunomycin or daptomycin drug combination (Figure 3).
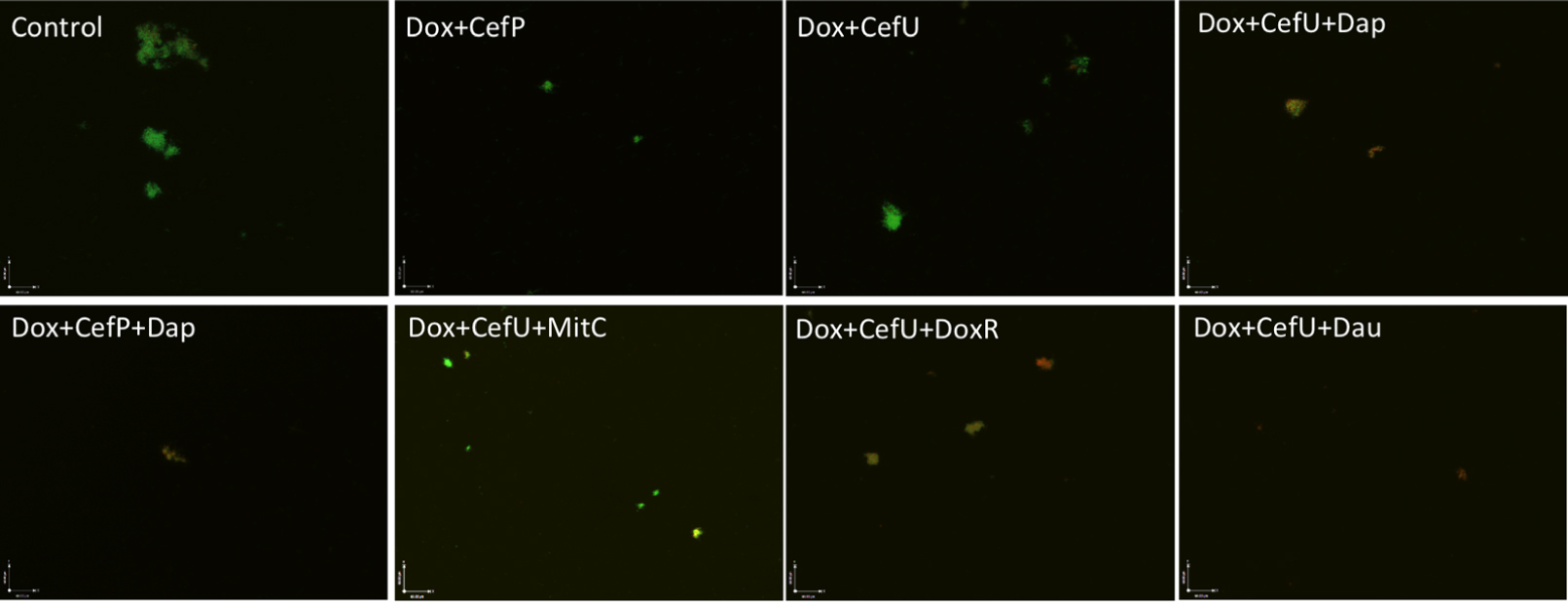
FIGURE 3. Comparison of the activity of daunomycin, daptomycin, and mitomycin C in combination with currently used Lyme antibiotics. A 15-day old B. burgdorferi stationary phase culture was incubated with the indicated drug combinations at a final concentration of 10 μg/mL for each antibiotic for 7 days followed by SYBR Green I/PI stain and epifluorescence microscopy. Abbreviations: Dox, doxycycline; CefU, cefuroxime; CefP, cefoperazone; Dap, daptomycin; MitC, mitomycin C; DoxR, doxorubicin; Dau, daunomycin.
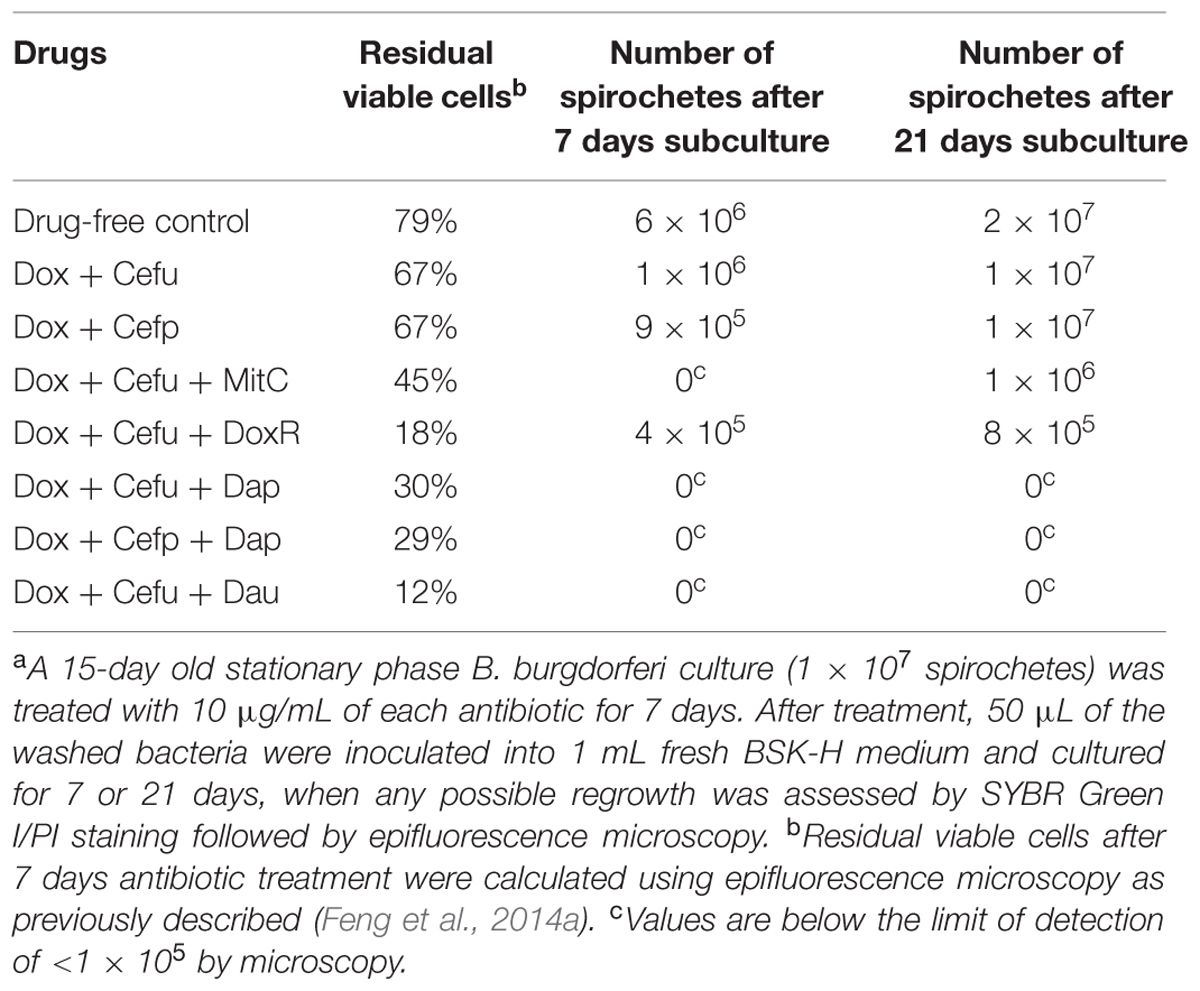
TABLE 2. Viability of stationary phase B. burgdorferi after antibiotic treatmenta assessed by direct SYBR Green I/PI viability assay and subculture.
Subculture Study to Assess the Relative Anti-Persister Activity of Daunomycin, Daptomycin, and Mitomycin C in Drug Combinations
To validate the activity of these drug combinations, samples of the above drug-treated cultures were subjected to subculture after removal of the drugs by washing followed by incubation in fresh BSK medium for 7 or 21 days. A lack of visible regrowth when measured by microscopy suggests that few to no viable cells remain after drug treatment, while visible regrowth of the culture indicates the presence of viable cells after drug treatment. The addition of daunomycin or daptomycin to the doxycycline + cefuroxime drug combination showed no visible regrowth after 7 and 21 days, suggesting no viable B. burgdorferi organisms were left after the treatment (Figure 4). Despite the high anti-persister activity of doxorubicin + doxycycline + cefuroxime in the microscopic analysis (Figure 3), with only 18% residual viable cells after treatment (Table 2), this triple drug combination was unable to prevent bacterial regrowth at either 7 or 21 days subculture, indicating it is not as active as daunomycin or daptomycin (Table 2, Figure 4). However, doxorubicin + doxycycline + cefuroxime was more active than mitomycin C + doxycycline + cefuroxime as shown by less regrowth than the latter combination (Figure 4). This is consistent with the single drug data where doxorubicin was more active than mitomycin C (Figure 1).
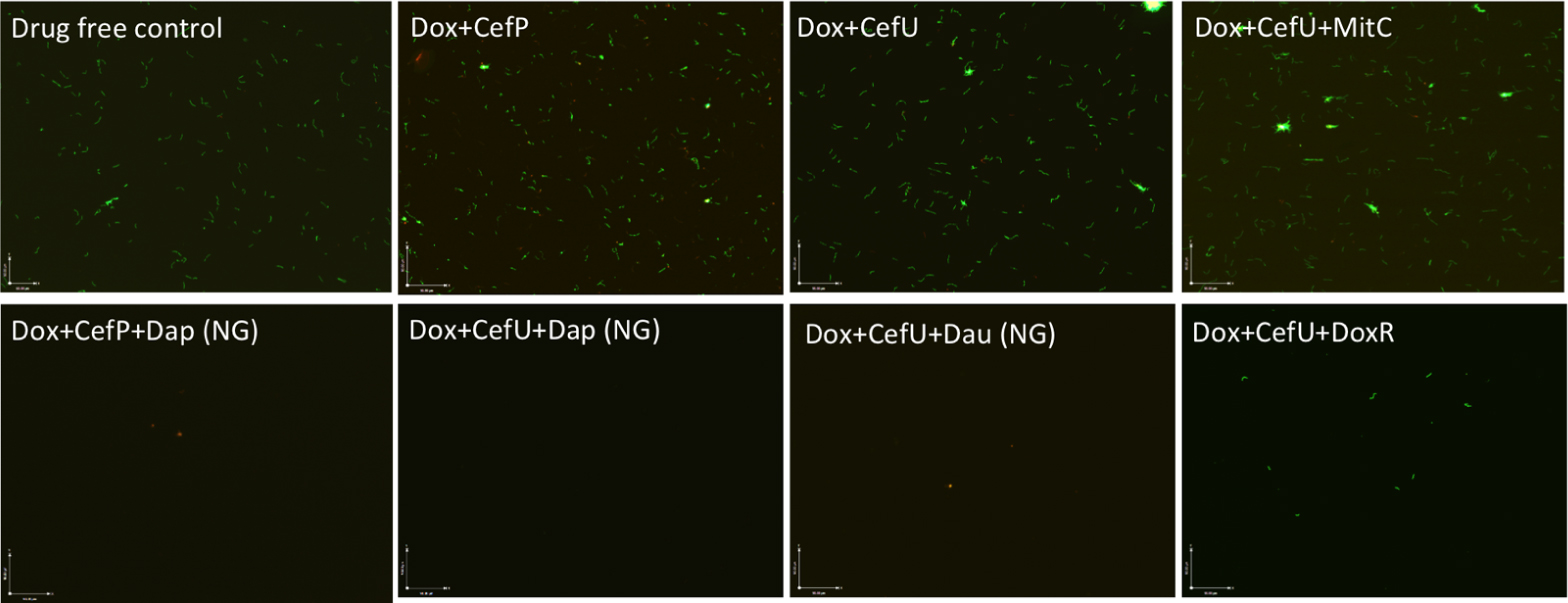
FIGURE 4. Subculture (21 days) of 15-day old B. burgdorferi stationary phase culture treated with different drug combinations. The 15-day old B. burgdorferi culture was incubated with the indicated drug combinations at a final concentration of 10 g/mL for each antibiotic for 7 days followed by washing and resuspension of cells in fresh BSK medium and subcultured for 21 days. The viability of the subculture was examined by SYBR Green I/PI viability assay and epifluorescence microscopy (400 magnification). NG, no growth.
The discrepancy in the activity of doxorubicin in epifluorescence microscopy based viability analysis and subculture study was noted in our previous studies (Feng et al., 2015a,b). This is due to the red orange color of the anthracycline drug doxorubicin, which stains the cells red and could give false impression of a high killing activity. However, subculture studies were able to show the inability of doxorubicin + doxycycline + cefuroxime to eradicate the microcolony form of B. burgdorferi persisters as shown by regrowth after subculture. Thus, the subculture study is crucial in validating the results of other forms of viability assays such as SYBR Green I/PI assay in persister drug evaluations.
When mitomycin C was added to doxycycline + cefuroxime combination treated B. burgdorferi stationary phase culture, there was no regrowth at 7 days, but visible spirochetal regrowth occurred after 21 days subculture (Figure 4). This finding suggests that the addition of mitomycin C to the commonly used Lyme antibiotics doxycycline + cefuroxime is not as active as the addition of daunomycin or daptomycin, but is more active than doxorubicin. Furthermore, this result indicates that 7 days subculture is not sufficient to reveal the small number of residual bacteria remaining after drug treatment and that a prolonged incubation to 21 days is needed to demonstrate the small numbers of viable bacteria for more reliable evaluation of drug combinations against B. burgdorferi persisters.
In a recent study, mitomycin C was shown to be more active than daptomycin and to eradicate all B. burgdorferi persisters (Sharma et al., 2015). This is in contrast to the results of this study which found daptomycin to have higher anti-persister activity than mitomycin C in both single drug exposure (Table 1, Figure 1) and drug combination studies (Figures 3 and 4). Several possibilities exist to explain the discrepancy. First, we used older 7 and 15 days stationary phase cultures containing an increased number of persisters and biofilm-like microcolonies previously shown to have increased tolerance to antibiotics (Feng et al., 2015a), while the other study used a younger culture of 5 days (Sharma et al., 2015), which would have more growing cells and fewer persister cells. The difference in persister numbers in these cultures would result in the bacteria in the younger culture of 5 days being more easily killed by mitomycin C but not by daptomycin. Indeed, daptomycin is known to have relatively high MIC (12.5–25 mg/mL) for growing spirochetes despite its high activity against B. burgdorferi persisters (Feng et al., 2014a), and this may also explain why daptomycin had limited activity in that study as a younger culture was used (Sharma et al., 2015). The use of a younger culture in the other study that contained mainly growing spirochetes is also consistent with their finding that the 5-day old culture was readily killed by even amoxicillin and ceftriaxone (Sharma et al., 2015), which are known to kill mainly growing bacteria. Second, we used different viability assays. In this study, we used SYBR Green I/PI viability stain along with microscopy and subculture in liquid medium to assess the viability of residual bacteria after drug treatment. In contrast, the other study used colony forming unit (CFU) assay on solid agar to determine the viable bacteria after drug exposure (Sharma et al., 2015). Based on studies with other bacteria like M. tuberculosis (Dhillon et al., 2004), the CFU assay favors the detection of more viable organisms and is less sensitive than culture in liquid medium which can detect small numbers of viable cells which may not grow on solid medium after drug exposure. Third, we used BSK-H medium, which is richer than the BSK-II medium used by the other study (Sharma et al., 2015), which might have also contributed to the higher activity of mitomycin C than daptomycin in that study (Sharma et al., 2015).
Cefuroxime (Ceftin) Could Replace Cefoperazone in the Daptomycin + Doxycycline Combination to Completely Eradicate Biofilm-Like Structures
In our previous drug combination study, we found that daptomycin + doxycycline + cefoperazone was able to completely eradicate the most resistant aggregated biofilm-like microcolonies (Feng et al., 2015a). However, since cefoperazone is not available in the US, we replaced it with the current Lyme antibiotic cefuroxime (Ceftin) in the daptomycin + doxycycline combination and found they had equivalent activity as shown by the same 30% residual viable cells after antibiotic treatment using SYBR Green I/PI viability stain and microscopy (Figure 3, Table 2). In subculture studies, we found replacement of cefoperazone with cefuroxime (Ceftin) in the daptomycin + doxycycline combination similarly resulted in complete eradication of the biofilm-like structures as shown by lack of any regrowth in 7 and 21 days subcultures (Figure 4).
Cefuroxime and cefoperazone, which are second and third generation cephalosporins, respectively, function as a highly penetrative beta-lactam antibiotic by disrupting the bacterial cell wall biosynthesis (Barriere and Flaherty, 1984). Despite being reported as the best beta-lactam antibiotic in the 7-day stationary phase persister model (Feng et al., 2014a), cefoperazone did not give any advantage over the use of the commonly prescribed Lyme antibiotic cefuroxime (Ceftin) in the context of drug combination with daptomycin + doxycycline (Figures 3 and 4). This data suggests that replacing cefoperazone with the commonly used cefuroxime (Ceftin) will maintain comparable efficacy against the biofilm-like microcolony form of B. burgdorferi persisters in the drug combination with daptomycin + doxycycline.
In this study, we were able to confirm our previous observations of the high anti-persister activity of daptomycin (Feng et al., 2014a, 2015a) and daunomycin (Feng et al., 2015b) alone and in drug combination with doxycycline + cefuroxime.It is worth noting that the high anti-persister activity of daptomycin and the anthracycline antibiotic daunomycin is due to the unique mechanisms of action through disruption of cell membrane and damage of DNA, respectively (Feng et al., 2014a, 2015b). These observations suggest that bacterial membranes and DNA integrity are important targets for bacterial persister drugs. The complete eradication of biofilm-like structures of B. burgdorferi by daunomycin or daptomycin in drug combination with doxycycline + cefuroxime, again supports the Yin–Yang treatment principle of combining drugs that target growing bacteria (Yang; with doxycycline + cefuroxime) and drugs like daunomycin or daptomycin that target non-growing persisters (Yin) for more effective treatment of persistent infections (Zhang, 2014). This strategy may be generally useful for treatment of persistent infections including biofilm infections, which cannot be eradicated by a single drug alone. Future studies are needed to validate this principle.
Although our findings that daunomycin or daptomycin plus doxycycline + cefuroxime could completely eradicate the biofilm-like structures are encouraging, they are in vitro studies and have limitations and cannot be equated to the clinical situation. Moreover, daunomycin and daptomycin are intravenous drugs and not convenient to administer. Future studies to develop oral regimens as effective as the above combinations are needed for more convenient administration. In addition, the toxicity associated with the anticancer drug daunomycin calls for caution with its use in clinical settings. Further in vivo animal studies are needed to validate the highly active drug combinations identified in this study before they can be used for patient treatment in the clinic.
Conclusion
In summary, we found that daunomycin and daptomycin were more active against B. burgdorferi biofilm-like structures than mitomycin C and doxorubicin in single drug comparisons as well as in drug combinations. Daunomycin or daptomycin when added to doxycycline + cefuroxime completely eradicated the biofilm-like structures, while the two drug combination doxycycline + cefuroxime alone or mitomycin C and doxorubicin when added to the above combination failed to do so. Additionally, we showed that cefuroxime (Ceftin) could replace cefoperazone in the daptomycin + doxycycline combination and caused complete eradication of the biofilm-like structures. Future studies are needed to evaluate these promising drug combinations in vivo in animal models, and if promising, in patients. Our findings may have implications for improved treatment of Lyme disease.
Author Contributions
YZ conceived the experiments; JF, MW, WS, SZ, performed the experiments; JF, MW, and YZ analyzed the data; and MW, JF, YZ wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the support of our work by Global Lyme Alliance, and Johns Hopkins Fisher Center for Environmental Infectious Diseases. YZ was supported in part by NIH grants AI099512 and AI108535.
References
Barriere, S. L., and Flaherty, J. F. (1984). Third-generation cephalosporins: a critical evaluation. Clin. Pharm. 3, 351–373.
Bockenstedt, L. K., Gonzalez, D. G., Haberman, A. M., and Belperron, A. A. (2012). Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J. Clin. Invest. 122, 2652–2660. doi: 10.1172/JCI58813
Caskey, J. R., and Embers, M. E. (2015). Persister development by Borrelia burgdorferi populations in vitro. Antimicrob. Agents Chemother. 59, 6288–6295. doi: 10.1128/AAC.00883-15
CDC (2015a). Lyme Disease. Available at: http://www.cdc.gov/lyme/ [accessed September 13, 2015].
CDC (2015b). Post-Treatment Lyme Disease Syndrome. Available at: http://www.cdc.gov/lyme/postLDS/index.html [accessed September 13, 2015].
Clinical and Laboratory Standards Institute [CLSI] (2007). Performance Standards for Antimicrobial Susceptibility Testing; Seventeenth Informational Supplement. CLSI document M100-S17 27. Wayne, PA: Clinical and Laboratory Standards Institute. 154–161.
Dhillon, J., Lowrie, D. B., and Mitchison, D. A. (2004). Mycobacterium tuberculosis from chronic murine infections that grows in liquid but not on solid medium. BMC Infect. Dis. 4:51. doi: 10.1186/1471-2334-4-51
Embers, M. E., Barthold, S. W., Borda, J. T., Bowers, L., Doyle, L., Hodzic, E., et al. (2012). Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS ONE 7:e29914. doi: 10.1371/journal.pone.0029914
Feng, J., Auwaerter, P. G., and Zhang, Y. (2015a). Drug combinations against Borrelia burgdorferi persisters in vitro: eradication achieved by using daptomycin, cefoperazone and doxycycline. PLoS ONE 10:e0117207. doi: 10.1371/journal.pone.0117207
Feng, J., Shi, W., Zhang, S., and Zhang, Y. (2015b). Identification of new compounds with high activity against Borrelia burgdorferi persisters from the NCI compound collection. Emerg. Microb. Infect. 4:e31. doi: 10.1038/emi.2015.31
Feng, J., Shi, W., Zhang, S., and Zhang, Y. (2015c). Persister mechanisms in Borrelia burgdorferi: implications for improved intervention. Emerg. Microb. Infect. 4, e51. doi: 10.1038/emi.2015.56
Feng, J., Weitner, M., Shi, W., Zhang, S., Sullivan, D., and Zhang, Y. (2015d). Identification of additional anti-persister activity against Borrelia burgdorferi from an FDA drug library. Antibiotics 4, 397–410. doi: 10.3390/antibiotics4030397
Feng, J., Wang, T., Shi, W., Zhang, S., Sullivan, D., Auwaerter, P. G., et al. (2014a). Identification of novel activity against Borrelia burgdorferi persisters using an FDA approved drug library. Emerg. Microb. Infect. 3:e49. doi: 10.1038/emi.2014.1053
Feng, J., Wang, T., Zhang, S., Shi, W., and Zhang, Y. (2014b). An optimized SYBR green I/PI assay for rapid viability assessment and antibiotic susceptibility testing for Borrelia burgdorferi. PLoS ONE 9:e111809. doi: 10.1371/journal.pone.0111809
Hodzic, E., Feng, S., Holden, K., Freet, K. J., and Barthold, S. W. (2008). Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob. Agents Chemother. 52, 1728–1736. doi: 10.1128/AAC.01050-07
Hodzic, E., Imai, D., Feng, S., and Barthold, S. W. (2014). Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS ONE 9:e86907. doi: 10.1371/journal.pone.0086907
Marques, A., Telford, S. R. III, Turk, S. P., Chung, E., Williams, C., Dardick, K., et al. (2014). Xenodiagnosis to Detect Borrelia burgdorferi Infection: a first-in-human study. Clin. Infect. Dis. 58, 937–945. doi: 10.1093/cid/cit939
Radolf, J. D., Caimano, M. J., Stevenson, B., and Hu, L. T. (2012). Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 10, 87–99. doi: 10.1038/nrmicro2714
Sharma, B., Brown, A. V., Matluck, N. E., Hu, L. T., and Lewis, K. (2015). Borrelia burgdorferi, the causative agent of lyme disease, forms drug-tolerant persister cells. Antimicrob. Agents Chemother. 59, 4616–4624. doi: 10.1128/AAC.00864-15
Steere, A. C., Gross, D., Meyer, A. L., and Huber, B. T. (2001). Autoimmune mechanisms in antibiotic treatment-resistant lyme arthritis. J. Autoimmun. 16, 263–268. doi: 10.1006/jaut.2000.0495
Straubinger, R. K., Summers, B. A., Chang, Y. F., and Appel, M. J. (1997). Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J. Clin. Microbiol. 35, 111–116.
Swanson, S. J., Neitzel, D., Reed, K. D., and Belongia, E. A. (2006). Coinfections acquired from ixodes ticks. Clin. Microbiol. Rev. 19, 708–727. doi: 10.1128/CMR.00011-06
Wormser, G. P., Dattwyler, R. J., Shapiro, E. D., Halperin, J. J., Steere, A. C., Klempner, M. S., et al. (2006). The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 43, 1089–1134. doi: 10.1086/508667
Zhang, Y. (2014). Persisters, persistent infections and the yin-yang model. Emerg. Microb. Infect. 3:e3. doi: 10.1038/emi.2014.3
Keywords: Borrelia burgdorferi, persister, anti-persister activity, biofilm, drug combination
Citation: Feng J, Weitner M, Shi W, Zhang S and Zhang Y (2016) Eradication of Biofilm-Like Microcolony Structures of Borrelia burgdorferi by Daunomycin and Daptomycin but not Mitomycin C in Combination with Doxycycline and Cefuroxime. Front. Microbiol. 7:62. doi: 10.3389/fmicb.2016.00062
Received: 08 December 2015; Accepted: 14 January 2016;
Published: 10 February 2016.
Edited by:
Octavio Luiz Franco, Universidade Católica de Brasília, BrazilReviewed by:
César De La Fuente-Núñez, Massachusetts Institute of Technology, USASuzana Meira Ribeiro, Universidade Católica Dom Bosco, Brazil
Copyright © 2016 Feng, Weitner, Shi, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Zhang, eXpoYW5nQGpoc3BoLmVkdQ==
 Jie Feng
Jie Feng Ying Zhang
Ying Zhang