- 1School of Microbiology, University College Cork, Cork, Ireland
- 2Teagasc Food Research Centre, Moorepark, Fermoy, Ireland
- 3APC Microbiome Institute, University College Cork, Ireland
- 4College of Science, Engineering and Food Science, University College Cork, Cork, Ireland
Bacteriocins are ribosomally synthesized antimicrobial peptides produced by bacteria, which have the ability to kill or inhibit other bacteria. Many bacteriocins are produced by food grade lactic acid bacteria (LAB). Indeed, the prototypic bacteriocin, nisin, is produced by Lactococcus lactis, and is licensed in over 50 countries. With consumers becoming more concerned about the levels of chemical preservatives present in food, bacteriocins offer an alternative, more natural approach, while ensuring both food safety and product shelf life. Bacteriocins also show additive/synergistic effects when used in combination with other treatments, such as heating, high pressure, organic compounds, and as part of food packaging. These features are particularly attractive from the perspective of controlling sporeforming bacteria. Bacterial spores are common contaminants of food products, and their outgrowth may cause food spoilage or food-borne illness. They are of particular concern to the food industry due to their thermal and chemical resistance in their dormant state. However, when spores germinate they lose the majority of their resistance traits, making them susceptible to a variety of food processing treatments. Bacteriocins represent one potential treatment as they may inhibit spores in the post-germination/outgrowth phase of the spore cycle. Spore eradication and control in food is critical, as they are able to spoil and in certain cases compromise the safety of food by producing dangerous toxins. Thus, understanding the mechanisms by which bacteriocins exert their sporostatic/sporicidal activity against bacterial spores will ultimately facilitate their optimal use in food. This review will focus on the use of bacteriocins alone, or in combination with other innovative processing methods to control spores in food, the current knowledge and gaps therein with regard to bacteriocin-spore interactions and discuss future research approaches to enable spores to be more effectively targeted by bacteriocins in food settings.
Introduction
Control and eradication of Bacillus and Clostridium spores is one of the most challenging aspects of microbial control faced by the modern food industry. Traditionally, spores have been controlled using extreme treatments such as high heat alone or in combination with chemical additives. However, modern consumers are more conscious than previous generations of the negative health effects associated with the consumption of certain chemical preservatives and of the significant effects of heat on the nutritional value and flavor of many foods. With ready-to-eat and minimally processed foods becoming a staple of the modern diet, the food industry is faced with an unprecedented challenge to provide food that is: (i) low in synthetic chemical additives, (ii) low in salt/sugar, (iii) nutritionally beneficial, and (iv) stable and safe, from a microbial perspective, over an extended period of time. As a result, the food industry is under pressure to employ innovative processing methods to meet consumer and regulatory demands. One potential innovation that has been intensively researched over the last number of decades, and is well positioned to provide a safe and effective alternative to existing processing technologies, involves the use of bacteriocins. This review will examine the efficacy of bacteriocins alone, and in combination with other processing technologies, to control spores in food.
The Bacterial Spore
Metabolically dormant spores of Gram-positive Clostridium and Bacillus species are formed during sporulation. This sporulation process is typically a response to cellular nutrient starvation and involves a complex cascade of enzyme reactions. This process of sporulation has been extensively described over the last number of decades in the model spore former B. subtilis (see review by: Tan and Ramamurthi (2014). Spores consist of a core surrounded by a coat and/or endosporium. The spore core consists of DNA, enzymes, and dipicolinic acid (DPA). DPA plays a role in maintaining spore dormancy, providing resistance to DNA damaging substances and is usually bound to divalent cations such as Ca2+ at a 1:1 ratio in the core (Setlow, 2014b). The composition and structure of the metabolically inactive, dehydrated, spore confers resistance to changes in pH (Blocher and Busta, 1983), wet and dry heat, UV radiation, desiccation (Nicholson et al., 2000), and various toxic chemicals (Russell, 1990; Cortezzo and Setlow, 2005). A spore may be viable after extended periods of dormancy (Cano and Borucki, 1995), monitoring its environment for favorable growth conditions and when suitable, germination and outgrowth occur, ultimately resulting in a vegetative cell (Figure 1). Endospore-forming bacteria vary considerably with respect to genotype and phenotype and, with respect to phenotype, consist of aerobic, facultative anaerobic, and obligate anaerobic, psychrophilic, mesophilic, thermophilic, psychotropic and thermotolerant strains (see review by: Doyle et al., 2015). This phenotypic heterogeneity of spore-forming bacteria means that virtually all types of food are potential targets for spore contamination and spore outgrowth, with potentially severe consequences with respect to food quality and safety.
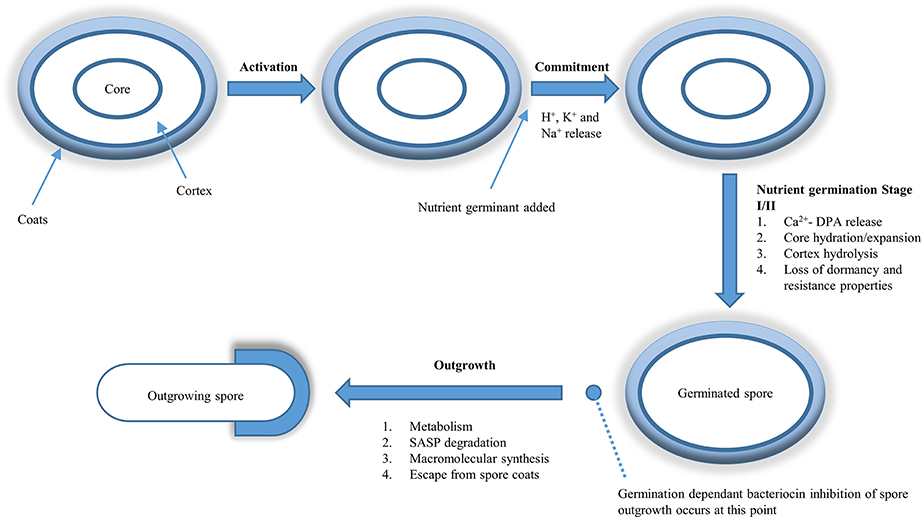
Figure 1. Germination dependent inhibition of spore outgrowth by bacteriocins. Dormant spores may germinate after being activated by a variety of means; most commonly sub-lethal heat being used. Heat is believed to activate the dormant spores by making the germinant receptors (GR) more accessible to nutrient germinants. Once the GR-nutrient binding occurs, the spore is now committed to germination even if the germinant is removed. Stage 1 of germination consists of H+, K+, and Na+ ion release followed by Ca2+-DPA release. This release of Ca2+-DPA triggers stage II of germination where the cortex is degraded, allowing the germ cell wall to expand and take up water. At the end of stage II the spore core is hydrated and has expanded along with the cortex. This rise in water content signals the end of stage II of germination and the beginning of the outgrowth phase. At this point bacteriocins that are not active against dormant spores become active, inhibit outgrowth and reduce viable counts from the germinated spore population. This figure is adapted from Setlow (2014a).
There are many pathways via which spores can gain access to the food chain. Food products are composed of multiple ingredients, potentially from different international origins, each contributing their own specific quantity and diversity of spores into the final formulation. Factors such as microbial ecology, farming practices, the local climate, hygiene of the processing facility and animal feeding practices determine the spore composition of an ingredient. Spores are also highly adhesive and may remain on the surfaces of equipment and contribute to problems long after their initial contamination of the facility. Reducing these initial spore loads is critical in avoiding problems downstream. However, it is important to note that spores are often selected for in food processing as their thermal resistance allows them to endure any heating steps (see review by: Carlin, 2011).
As early as 1956 (Stuy, 1956), the induction of spore germination was identified as a strategy that could facilitate spore eradication. When threshold levels of nutrients (such as amino acids, sugars, and nucleosides) are present, they bind to Ger complexes, located on the inner membrane of the spore. This strategy takes advantage of the loss of the resistance properties that a dormant spore possesses. It has been shown that once spores have germinated, they become more sensitive than dormant spores to: heat (Durban et al., 1970), X-Ray and UV radiation (Stuy, 1956; Munakata, 1974), and copper (Wheeldon et al., 2008). Interestingly the process of spore germination is not 100% efficient, due to the heterogeneity in germination rates among members of the spore population in response to a particular nutrient germinant. Previous studies have highlighted the specificity of germinant receptors (GRs): showing that GerA will respond to L-alanine and L-valine, while GerB and GerK will respond to a mixture of L-asparagine, D-glucose, D-fructose, and potassium ions (Moir et al., 1994; Atluri et al., 2006). The binding of the nutrients to their appropriate GRs results in the irreversible commitment of the spore to germination.
Commonly spores termed superdormant have been isolated from populations of B. subtilis following saturation with nutrient germinant. This super dormancy is attributed to the lag in initiation of the rapid loss of Ca2+-DPA stage in spore germination. Following the initiation of rapid loss of Ca2+-DPA from its core, the spore is no longer superdormant and its germination will proceed in a similar manner as dormant spores (Figure 1). This superdormancy may be an issue for antimicrobials (e.g. nisin) whose effect is only exhibited on those spores that have reached the end of stage II of germination (Figure 1; Chen et al., 2014). Superdormant spores may, however germinate, in response to an alternative germinant that utilizes an alternative GR. A different strategy, which can be used to increase germination of super dormant spores, is by using higher heat activation temperatures than is required for those non-superdormant spores (Ghosh et al., 2009). Treatment of spores with sublethal heat (also called heat activation) has been shown to increase the rate of germination of a number of spore species. Luu et al. (2015) suggested that although the main target of heat activation is the spore's GRs, this may only be indirect and that the sublethal heat is having a more direct effect on the inner membrane of the spore in which the GRs are situated, ultimately resulting in increased spore germination. Therefore decisive triggering of the spore germination process, will allow food processors to render spores sensitive to a variety of inactivation methods that are ineffective against highly resistant dormant spores.
Bacteriocins
Bacteriocins are a class of ribosomally synthesized antimicrobial peptides (AMPs) produced by bacteria. These small and naturally produced peptides can kill other bacteria, which are closely (narrow spectrum) or distantly (broad spectrum) related to the producing bacteria (Cotter et al., 2005). It is hypothesized that the production of bacteriocins is a strategy to control competing bacteria in the hunt for nutrients and space in an environmental niche. Therefore, it is not surprising that it has been estimated that many bacteria produce at least one bacteriocin (Riley and Wertz, 2002), which may help them to influence the surrounding population dynamics. Although many bacteriocin-producing bacteria in the biosphere have been investigated, it is still the case that there remain many are that are still to be discovered (Yang et al., 2014). Indeed, bioinformatic mining of publically available genomes, along with other rapid techniques, are beginning to bridge this gap in initial discovery, by overcoming the previous dependence on the expensive, time consuming, culture-dependent nature of bacteriocin discovery and purification (Sandiford, 2015). BAGEL3 (BActeriocin Genome mining tooL) (van Heel et al., 2013) and antiSMASH 3.0 (antibiotics and Secondary Metabolite Analysis Shell) (Weber et al., 2015) are examples of web based genome mining tools that detect putative bacteriocin biosynthetic gene clusters. Liquid chromatography/mass spectrometry has also been used to rapidly detect bacteriocins in as little as 25 μl of culture supernatant and is sensitive enough to distinguish between variants of the same bacteriocin e.g., nisins A, Z, and Q (Zendo et al., 2008). High throughput, culture-based screens can also be valuable (Rea et al., 2010).
Bacteriocins from the LAB Are Suitable for Food Preservation
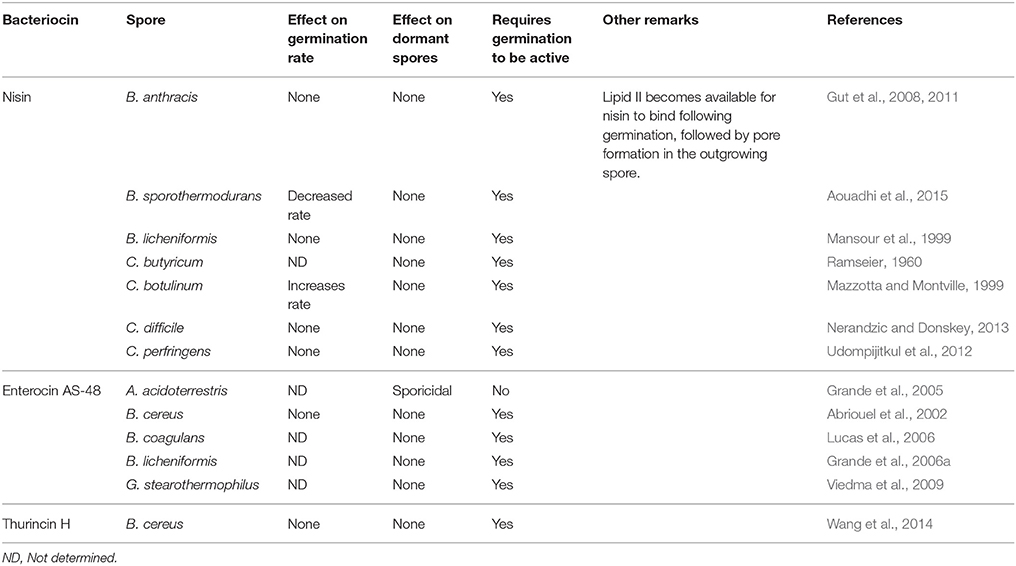
Although there are many Gram-negative and Gram-positive microorganisms which produce bacteriocins, those produced by the lactic acid bacteria (LAB) are of particular interest to the food industry. Many of these bacteria already play a crucial role in a variety of food fermentations by converting lactose to lactic acid, as well as producing a variety of additional antimicrobial molecules such as other organic acids, diacetyl, acetoin, hydrogen peroxide, antifungal peptides, and bacteriocins. The best known LAB genera are Lactococcus, Streptococcus, Lactobacillus, Pediococcus, and Enterococcus, though a number of other, generally regarded as more peripheral and less frequently applied from an industrial perspective, genera also exist. LAB offer several key properties which make their bacteriocins highly desirable for use in food: (i) the LAB are Generally Regarded As Safe (GRAS) and there are perceived by the public as having health promoting features, (ii) their bacteriocins are sensitive to digestive proteases such as pancreatin complex, trypsin and chymotrypsin, and thus don't impact negatively on the gut microbiota, (iii) they are non-toxic to eukaryotic cells (iv) they are often active across a range of pH values and are, in many cases, not temperature sensitive (Table 1), (v) they are gene encoded and therefore highly amenable to genetic manipulation where desired (Field et al., 2015), (vi) not all of the bacteriocins produced by the LAB have similar/the same mode of action, and (vii) they are active against a range of food pathogenic and spoilage bacteria.
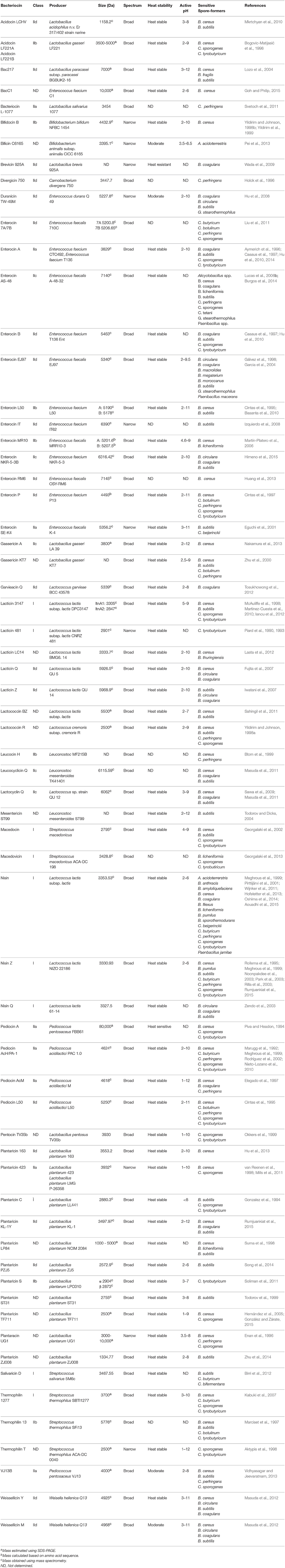
Table 1. Bacteriocins that are active against vegetative cells of Gram-positive spore-forming bacteria.
This review will focus solely on bacteriocins produced by the LAB because these bacteriocins possess the greatest promise with respect to use in the food industry.
Classification of Bacteriocins Produced by the LAB
LAB bacteriocins may be classified into two separate classes based on their modification status: Modified (class I), and minimally modified or cyclic (class II; Rea et al., 2011; Cotter et al., 2013).
Class I are comprised of all peptides that undergo post-translational modification during biosynthesis and include the subclass of lantibiotics among others. While several other subclasses within class I have been described (Arnison et al., 2013; Cotter et al., 2013), this review will focus mainly on those with relevance to the food industry. The commercially important bacteriocin nisin is produced by L. lactis and is the prototypical member of the class I lantibiotics. Nisin is currently used in over 50 countries to improve food safety and extend shelf life. Other important members of this class include: the two peptide lantibiotic lacticin 3147 produced by L. lactis DPC 3147 (Suda et al., 2012), subtilin produced by Bacillus subtilis ATCC 6633 (Lee and Kim, 2011), and lacticin 481 produced by L. lactis CNRZ 481 (Piard et al., 1993). Lantibiotics undergo extensive post-translational modifications, resulting in the presence of unusual amino acids such as lanthionine, β-methyllanthionine, dehydrobutyrine, and dehydroalanine. Covalent bonds are formed between these non-standard residues, resulting in internal rings which are important for its potent activity (Rink et al., 2007).
Class II bacteriocins are < 10 kDa, heat stable and non-modified that can be further subdivided into four subgroups: IIa pediocin like, IIb two peptide bacteriocins, IIc cyclic bacteriocins, and IId single linear non-pediocin bacteriocins. Members of class IIa are Listeria-active peptides which contain a conserved amino acid consensus sequence across all members of this group: Y-G-N-G-V-X1-C-X2-K/N-X3-X4-C (where X is any amino acid) (Cui et al., 2012). This consensus sequence is often referred to as the “pediocin box” and is present at the N-terminal region of the class IIa bacteriocins. Class IIb bacteriocins are unmodified two peptide bacteriocins, which interact to give full activity; having little or no activity in isolation. Class IIc bacteriocins are covalently linked from their N to C termini during post-translational modification resulting in a circular backbone. Class IId are a heterogeneous group, made up of bacteriocins which are linear, do not contain a pediocin box and do not require another peptide for full activity.
Using Bacteriocins Produced by Enterococcus in Food
The bacteriocins produced by Enterococcus species are diverse, both in terms of their classification and inhibitory spectrum (Table 1). While most LAB are GRAS, and thus their associated bacteriocins can be considered for food applications, the status of enterococci is less clear. Indeed, many strains are clearly not food grade. Although Enterococcus species have been used as artisanal cultures in a variety of foods, their suitability for use in food is questionable as they have been sometimes associated with pathogenicity. Indeed, cases of urinary tract infections, bacteremia and endocarditis have been associated with Enterococcus species (Franz et al., 1999; Kayser, 2003). De Vuyst et al. (2003) suggested that Enterococcus species could be safely used in food if virulence genes are absent (cytolysin, vancomycin resistance, etc.). However, in a review by Franz et al. (2011), the ability of Enterococcus to acquire virulence and antibiotic resistance genes on mobile genetic elements was identified as a significant barrier to their use in food. Recently, Jaouani et al. (2015) examined the safety of previously identified bacteriocinogenic enterococci, by examining the presence of virulence and antibiotic resistance genes. Using these criteria, it was concluded that 22/55 of the strains tested were safe for use in food. Ultimately, Enterococcus are an important reservoir for bacteriocin discovery and therefore developing a comprehensive set of guidelines/considerations for their safe use would be highly valuable when considering their suitability for use in food.
Bacteriocin Mode of Action against Vegetative Cells
Mechanistically, bacteriocin molecules produced by the LAB act by one, or both, of two different mechanisms: (i) inhibition of cell wall biosynthesis, and (ii) pore formation.
At the cell envelope, lipid II plays a key role in the synthesis of peptidoglycan as it transports cell wall subunits across the bacterial cytoplasmic membrane. Lipid II delivers its peptidoglycan subunit cargo from the cytosol to an exterior multi-enzyme complex which is responsible for polymerization of that subunit into the peptidoglycan cell wall. The halting of cell wall biosynthesis by sequestering lipid II is a strategy employed by a number of antimicrobial compounds which results in cell death (see review by: Oppedijk et al., 2015). The important clinical antibiotic vancomycin also targets lipid II, though its lipid II binding site is distinctly different to the lantibiotic nisin. The alternative binding site for nisin results in the ability of nisin to kill bacterial cells which are resistant to vancomycin (Gut et al., 2011). Other bacteriocins that exert their bactericidal mechanism of action by inhibition of cell wall biosynthesis are mersacidin, which inhibits transglycosylation (Brötz et al., 1995), and lactococcin 972 which targets septum biosynthesis via lipid II (Martínez et al., 2008). While lipid II is an important receptor for certain bacteriocins, there are however other receptors to which bacteriocins bind on the Gram-positive cell such as: the mannose PTS system, the maltose ABC transporter, Zn-dependent metallopeptidase, and undecaprenyl pyrophosphatase phosphatase (see review by: Cotter, 2014). Indeed these bacteriocin-receptor complexes play an important role in specifying a bacteriocins spectrum of activity. The outer cell membrane of Gram-negative bacteria provides an effective barrier to bacteriocins from binding their lipid II targets. However, Gram-negative bacteria can be sensitized toward bacteriocins if treated with agents or chemicals that destabilize the outer cell membrane (such as sodium phosphate buffer or EDTA).
Bacteriocins may also kill or damage cells by pore formation in the cell membrane. This pore formation is achieved by insertion of the bacteriocin into the cell membrane, forming a membrane pore. This pore results in depolarization of the membrane potential and diffusion of low molecular cytosolic compounds out of the cell; ultimately rendering the bacterial cell non-viable. Enterocin AS-48 is predicted to form aggregates which insert into the bacterial membrane, resulting in accumulation of positive charge along the cell surface, destabilizing the membrane potential, leading to pore formation and cellular leakage. Other bacteriocins that form pores include: streptococcin SA-FF22, lacticin F, and lactococcin A (Héchard and Sahl, 2002).
There are a number of members of the bacteriocins that exhibit dual modes of antimicrobial action by both: forming pores and inhibiting cell wall biosynthesis. The ability of such bacteriocins to act through two mechanisms of action can prevent the development of bacteriocin resistance. Moreover, it is worth noting that microorganisms that are resistant to antibiotics generally do not display cross-resistance to bacteriocins (Jordan et al., 2014). Nisin (Wiedemann et al., 2001), pediocin PA-1 (Diep et al., 2007), lacticin 3147 (Wiedemann et al., 2006), epidermin (Götz et al., 2014), and gallidermin (Götz et al., 2014) are examples of bacteriocins that display a dual mode of action, making their activity particularly potent against their targets.
Bacteriocin-Spore Interactions
In comparison to the vast knowledge available with respect to bacteriocin interactions with vegetative cells, it is safe to say that there is considerably less known about bacteriocin/spore interactions. However, a small number of bacteriocins (Table 2) for which activity against a variety of bacterial spores has been demonstrated. Phase contrast microscopy can be utilized to determine at what stage in the spore cycle (Figure 1) the bacteriocin exhibits its anti-spore activity by combining the bacteriocins with dormant (phase bright) and germinated (phase dark) spores. Spore viability can then be examined following the treatment with bacteriocin to determine the bacteriocins effect on the spore. Two outcomes may ensue: the bacteriocin (i) does not require germination and will be sporicidal against dormant spores, or (ii) will be sporostatic to dormant and germinated spores but requires germination to inhibit spore outgrowth. Bacteriocins can also affect the germination rate of the spore, which can be examined by measuring the drop in absorbance (OD600nm) of a dormant spore suspension as it transitions to a germinated spore suspension over a time period. These outcomes are however heterogeneous (Table 3), with differences occurring at species level where the same bacteriocin was used, and will be further discussed below.
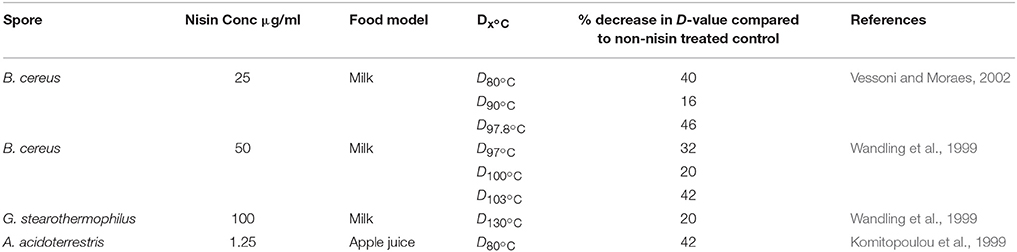
Nisin
Previous studies have shown that for B. anthracis (Gut et al., 2008, 2011), B. licheniformis (Mansour et al., 1999), C. difficile (Nerandzic and Donskey, 2013), and C. perfringens (Udompijitkul et al., 2012), nisin had no impact on the process of germination, as it neither initiated, inhibited, or altered the rate of germination, as examined on the basis of spore refractility, with or without nisin. Conversely, the presence of 25 μg/ml of nisin has been shown to have a progerminant activity for C. botulinum spores, as when it was present in the germination medium, the germination rate was doubled. However, the presence of nisin (125 μg/ml) has been shown to decrease the germination rate of B. sporothermodurans spores (Aouadhi et al., 2015).
With respect to anti-B. anthracis activity, it has been reported that nisin exerts its inhibitory effect after germination initiation, where nisin binds lipid II in the germinating spore and prevents it from becoming metabolically active by interfering with the establishment of a membrane potential and oxidative metabolism. Germination initiation is required for this lipid II binding to occur, as nisin is unable to associate with the dormant spore due to the absence of lipid II on the exterior of the spore (Gut et al., 2011). When investigating the effects of nisin on C. perfringens spores, it was observed that, as for studies involving B. anthracis and C. butyricum, nisin exhibited its inhibitory action during the stage of spore outgrowth (Udompijitkul et al., 2012). Using a truncated nisin derivative consisting of rings A, B and C (which could bind lipid II but not form pores) and fluorescently labeled unmodified nisin, it was shown that lipid II binding alone was insufficient to inhibit spore outgrowth. This was further investigated using the double mutants N20P/M21P and M21P/K22P, which were unable to form pores, but could bind lipid II. These nisin mutants were again shown to be unable to inhibit spore outgrowth. Through the use of the double mutant and truncated nisin, it is clear that pore formation is the essential mechanism by which nisin inhibits spore outgrowth while lipid II is the target for nisin (acting as a receptor for nisin) to inhibit outgrowth in the germinating spore (Gut et al., 2008, 2011). While it has been shown that truncated nisin consisting of rings A, B, and C does not inhibit spore outgrowth in B. anthracis, it has been reported elsewhere that this peptide does inhibit outgrowth of B. subtilis (Rink et al., 2007). While the mechanisms underlying these differing results have not yet been completely elucidated, some possible explanations given were (i) differences in outgrowth measurement methods and (ii) potential spore structure variations (Gut et al., 2011). Nisin however displays sporicidal activity against dormant B. sporothermodurans (Aouadhi et al., 2013), in contrast with the sporostatic activity against other targets described above.
The ability of microorganisms to develop resistance mechanisms to bacteriocins is a concern that could impede their widespread use in food (see review by: Draper et al., 2015). Nisin resistance has been reported for toxigenic spores of C. botulinum which had the ability to germinate and grow in levels of nisin that reduced levels of sensitive germinating spores by 7–8 logs10/ml (Mazzotta and Montville, 1999). The exact mechanism by which these spores exhibited nisin resistance is unknown but, interestingly it has been noted that nisin resistant strains have an altered fatty acid composition, which is consistent with a more rigid membrane. It has also been observed that nisin resistant strains of C. botulinum display cross-resistance to class II bacteriocins (Mazzotta et al., 1997).
Enterocin AS-48
Enterocin AS-48 produced by Enterococcus faecalis A-48-32 is a class IIc cyclic bacteriocin that is active against a number of Bacillus and Clostridium sp. (Table 1). Unlike nisin, the exact molecular mechanism by which enterocin AS-48 interacts with bacterial spores is unknown. It was observed that spores of B. cereus became more sensitive to enterocin AS-48 gradually after germination and were sensitive to 25–50 μg/ml 10 min after germination initiation. The greatest effect of enterocin AS-48 was observed 90–120 min after germination initiation, when cellular growth occurred (Abriouel et al., 2002). Enterocin AS-48 has also been shown to be effective in inhibiting spore outgrowth using heat activated spores of B. cereus. In a boiled rice substrate, 25 μg/ml of enterocin AS-48 reduced heat activated spores incubated at 37 and 15°C, below the level of detections after 3 days, whereas at 6°C, this reduction took 14 days. A higher concentration of 35 μg/ml of enterocin AS-48, reduced the heat activated spores below the level of detection in rice gruel after 24 h at three different temperatures (6, 15, and 37°C; Grande et al., 2006b).
Outgrowth inhibition of the important thermophilic spore-former Geobacillus stearothermophilus has also been shown using enterocin AS-48. G. stearothermophilus is regularly identified as a spoilage agent in low acid canned food, being highly heat resistant with a D121°C value of 1 min, so its removal from canned products require an extensive heat treatment (Durand et al., 2015). Viedma et al. (2009) tested the efficacy of enterocin AS-48 in inhibiting spore outgrowth of G. stearothermophilus using three food models, canned corn, canned peas and coconut milk, using a cocktail of two G. stearothermophilus strains. Here it was shown that AS-48, used at 1.75 μg/ml, reduced the viable counts of heat treated spores below the level of detection after 24 h. B. licheniformis was controlled in a commercial cider by AS-48 at a level of 5 μg/ml at 30°C. A significant reduction was observed in a population of germinated spores following treatment with AS-48 (Grande et al., 2006a).
The genus Alicyclobacillus has in recent years become a problem in the food industry. Members of this genus have an ability to grow at high temperatures (50–70°C), and at low pH values (3.0–3.5), which makes their eradication from certain foods problematic. A. acidoterrestris is a particular problem in acidic juice products such as apple, tomato and orange, amongst others (Steyn et al., 2011). Inhibition of A. acidoterrestris spores by enterocin AS-48 has been observed at concentrations as low as 2.5 μg/ml. At this concentration a reduction of 6 Log10 spores/ml was achieved. Using electron microscopy it was observed that the enterocin AS-48 treated spore structures sustained substantial damage supporting the hypothesis that the bacteriocin adsorbs to the spores negatively charged surface groups. This interaction with A. acidoterrestris would suggest a sporicidal rather than the sporostatic mechanism of action that is suggested for B. cereus (Grande et al., 2005).
Lacticin 3147
Lacticin 3147, produced by L. lactis subsp. lactis DPC3147, has been shown to inhibit spores of C. tyrobutyricum in milk. This Clostridium species is responsible for late blowing in hard cheese, as their spores can survive heat treatments and germinate in the ripening cheese. Previously nitrate was used to combat clostridia but has been banned by the European Food Safety authority (EFSA) in an effort to reduce nitrosamines in food (Bassi et al., 2015). When used at a concentration of 45 μg/ml, lacticin 3147 was also able to completely inactivate 4–5 Log10 spores/ml over a 24 h period. Additionally, when lacticin 3147 was added following a 24 h incubation of the spores, total inactivation 6 days post addition of the bacteriocin was observed. In situ production of lacticin 3147, in a model curd system, has also been shown to significantly reduce (3 Log10 spores/g) the number of Clostridium spores after 13 days, when compared to a non-bacteriocin producing control. After 60 days of ripening, lacticin 3147 produced in situ was shown to be effective in reducing the levels of artificially contaminated clostridia (introduced prior to ripening) from 8 to 2 Log10 spores/g (Carmen Martínez-Cuesta et al., 2010).
Bificin C6165
Bificin C6165 produced by Bifidobacterium animalis subsp. animalis CICC 6165 was shown to inhibit species such as Lactobacillus, Bifidobacterium, Enterococcus, Staphylococcus, and Alicyclobacillus acidoterrestris. Indeed, from an anti-sporeformer perspective, it is notable that bificin C6165 inhibited 20/20 strains of A. acidoterrestris tested. Bificin C6165 could also reduce a population of A. acidoterrestris spores and was more effective as the concentration of the bacteriocin increased (Pei et al., 2013). Another important characteristic of bificin C6165 which makes it an ideal candidate for inhibition of A. acidoterrestris is its activity at acidic pH 3.5–6.5 (Pei et al., 2014).
Plantaricin TF711
Plantaricin TF711, produced by Lactobacillus plantarum TF711, is active over a broad pH range and is active against vegetative cells of B. cereus and C. sporogenes (Hernández et al., 2005). C. sporogenes acts as a research surrogate for proteolytic C. botulinum as these two species are closely related. This species has also been associated with late blowing of hard cheese (Bassi et al., 2015). Plantaricin TF711 was shown to reduce C. sporogenes spore counts significantly from 7 days onwards when introduced in the form of an adjunct culture producing the bacteriocin in situ. The bacteriocin was shown to be present at highest levels at day 21, after which its activity declined. This decline in activity could be due to loss of stability, depletion of the bacteriocin in the cheese, or reduced production of the bacteriocin (González and Zárate, 2015).
Thurincin H
Thurincin H produced by B. thuringiensis SF361 has been shown to be sporostatic against dormant B. cereus spores and sporicidal against germinated B. cereus spores. Similarly to other bacteriocins, thurincin H displays sporicidal activity after germination, while it was sporostatic to dormant spores. Although not an LAB bacteriocin, it has been suggested that Thurincin H may have potential for use in food (Wang et al., 2014).
Other Bacteriocins Active against Bacterial Spores
There are a number of other bacteriocins that have shown potential. Some of these are described here. Soria and Audisio (2014) revealed that heat activated B. cereus spores could be inhibited by the cell free supernatant of E. faecium SM21 containing an enterocin which produced a bacteriostatic effect at both pH 5 and pH 6. Bacteriocin production by Streptococcus thermophilus 580 was capable of inhibiting C. tyrobutyricum gas production in a ripening curd model for up to 14 days, when compared to controls which produced gas after 14 days (Mathot et al., 2003). Pentocin L and pentocin S, are produced by Pediococcus pentosaceus L and S, respectively. Both of these bacteriocins are inhibitory against a variety of vegetative Bacillus and Clostridium strains (Table 1). Furthermore, these bacteriocins were shown to be sporostatic by inhibiting the germination of three different strains of non-heat activated B. cereus spores. These active proteins are larger than typical bacteriocins which suggests that these peptides may in fact be bacteriolysins (Yin et al., 2003).
Comparing the Sensitivity of Spores and Vegetative Cells to Bacteriocins
To date there has been conflicting reports as to whether germinated spores are more or less resistant to bacteriocins than vegetative cells. Heat activated spores of B. sporothermodurans are less sensitive to nisin (1.25 μg/ml), than vegetative cells of B. sporothermodurans (Aouadhi et al., 2015). The Minimum Inhibitory Concentration (MIC) of nisin for vegetative cells of C. butyricum, C. perfringens, C. sporogenes, and C. tyrobutyricum was found to be 0.17, 0.75, 38.4, and 4.8 μg/ml, respectively. However, 23 μg/ml of nisin prevented outgrowth of heat activated Clostridium spores for up to 10 days. Unfortunately in this study it is unclear whether the vegetative cells were more or less resistant than their spores to the nisin treatment as no MIC for spores was carried out (Meghrous et al., 1999). Another study found that vegetative cells of C. sporogenes were less resistant to nisin than heat activated spores, yielding MICs of 0.23 and 1.11 μg/ml, respectively. In contrast, it was revealed that heat activated C. beijerinckii spores were less resistant with an MIC of 1.09 μg/ml while their vegetative cells exhibited an MIC of 1.3 μg/ml (Hofstetter et al., 2013). At odds with these findings, however, were the results obtained by Ávila et al. (2014), which compared the sensitivity of spores and vegetative cells of four clostridia: C. tyrobutyricum, C. butyricum, C. beijerinckii, and C. sporogenes. Using four representatives of each species, they showed that spores had a higher MIC, and thus were more resistant to nisin, than their vegetative counterparts in 15 of the 16 strains tested. The only exception was displayed by C. tyrobutyricum CET 4011 strain where the vegetative and spore MIC values were equal at 0.39 μg/ml. It is also important to note that in this case all the MIC values were below the maximum permissible limit for nisin, which is 12.5 μg/ml in Europe.
Spores of A. acidoterrestris were found to be more sensitive to nisin than their vegetative cells. The MIC values for both spores and vegetative cells were carried out in mYPGA at two different pH values (pH 3.4 and pH 4.2). Interestingly, at pH 3.4, all spores were more sensitive (7/7) than their vegetative cells. However, at pH 4.2 (3/7) spores had equal MIC-values to their vegetative cells (Yamazaki et al., 2000). Whether this is due to the (i) enhanced activity of nisin at lower pH, (ii) negative effects of pH on the spore or (iii) a combined activity of both, has yet to be determined. These findings were further confirmed by Ruiz et al. (2013), who found the MIC of spores and vegetative cells of A. acidoterrestris to be 7.81 and 31.25 μg/ml, respectively.
Inhibition of Spore Outgrowth Prevents Toxin Formation
Toxin formation is an important feature of a number Clostridium and Bacillus species. There are two types of toxin with which B. cereus strains are frequently associated: (i) heat labile diarrheal enterotoxin and/or (ii) heat-stable emetic enterotoxin. Beuchat et al. (1997) showed that the production of diarrheal enterotoxin produced in beef gravy inoculated with B. cereus spores could be inhibited by addition of nisin. Enterotoxin production normally occurred after 3 and 9 days for heat activated B. cereus spores incubated at 15 and 8°C, respectively. Addition of 1 μg/ml of nisin inhibited enterotoxin production completely at 8°C, whereas a higher concentration of 5 μg/ml was needed to inhibit enterotoxin production at 15°C over a 14 day period. The levels of nisin required to prevent enterotoxin production from a spore inoculum also ensured that the final cell numbers did not exceed 4.03 and 6.23 Log10 CFU/g at 8 and 15°C, respectively. Without nisin, enterotoxin was produced when cell numbers exceeded 6.78 and 7.1 Log10 CFU/ml at 15 and 8°C, respectively. This is in agreement with the strategy of keeping the B. cereus population below ~7 Log10 CFU/g to prevent enterotoxin production (Christiansson et al., 1989). It would be interesting to see if the cell numbers in the presence of nisin were allowed to exceed these numbers would enterotoxin be still be produced or would the enterotoxin production cease due to the presence of nisin.
Enterocin AS-48 was also shown to have an effect on enterotoxin production by psychrotrophic vegetative cells of B. cereus. Enterocin AS-48 completely inhibited enterotoxin production and bacterial growth for at least 72 h when used at 7.5 μg/ml. When enterocin AS-48 was used at subinhibitory concentrations (2.5 or 5 μg/ml) the growth of the cells were severely subdued and enterotoxin titres were 10-fold lower than non-bacteriocin treated controls (Abriouel et al., 2002).
Combining Bacteriocins with Other Hurdles
Bacteriocins in Combination with Heating
The thermal resistance of bacterial spores makes their eradication from food by heat a major problem during food processing. Nisin at various concentrations has been shown to reduce the decimal reduction times (D-values) and thus the thermal resistance of bacterial spores. Therefore, nisin has been described as a compound with a “two-fold beneficial effect”: (i) it enhances the heat sensitivity of the bacterial spore (Table 4) and (ii) it prevents the outgrowth of spores which survive the heat treatment (Komitopoulou et al., 1999). Pre-exposing heat activated G. stearothermophilus spores to nisin (50 μg/ml) at 4°C in chocolate milk for 15 and 24 h, resulted in significantly reduced D130°C values of 20.5 and 25.1%, respectively, compared to those spores not pretreated with nisin. When the nisin pretreatment was raised to 100 μg/ml this did not cause a significant reduction over the lower concentration of 50 μg/ml (Beard et al., 1999). B. amyloliquefaciens spores were rapidly inactivated when treated with 90°C and 16 μg/ml of nisin, in contrast to the results when a 90°C treatment was used, alone, where there was no inactivation of spores (Hofstetter et al., 2013).
A reduction of 2 Log10 spores/ml was observed when spores of C. sporogenes spores were subjected to a heat treatment of 90°C for 2 h in the presence of 16 μg/ml nisin vs. a 90°C heat treatment without nisin. Additionally there was 30% greater DPA release when spores of C. sporogenes were heat treated at 90°C with nisin than those spores which were not treated in any way. However, when C. beijerinckii was subjected to the same conditions (90°C for 2 h and 16 μg/ml nisin), no increased inactivation was observed. The ability of nisin to increase the permeability of resting spores of C. sporogenes and C. beijerinckii was observed using DAPI staining. Fluorescence was observed after a treatment at 90°C with nisin, whereas a heat treated spore without nisin that did not fluoresce (Hofstetter et al., 2013). These findings are consistent with the hypothesis that nisin lowers the heat resistance of spores by permeabilizing their exterior.
Response surface technology (RSM) is an empirical modeling technique that can be used to examine and predict the relationship between the response variable and the test variable. RSM can be used to predict optimum processing conditions to achieve a pre-determined reduction in spores (Table 5).
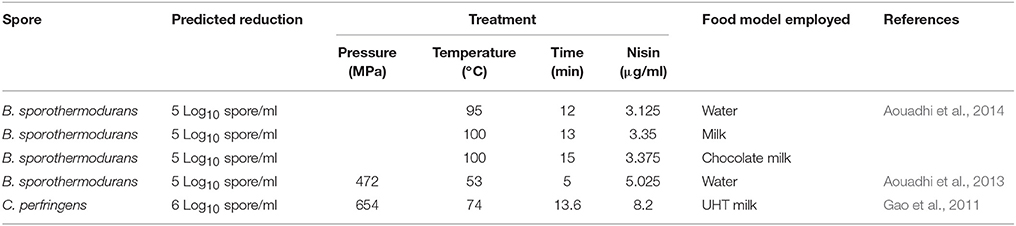
Table 5. RSM models can be used to predict a treatment to achieve a specific spore reduction in food.
Dormant B. coagulans spores were shown to be resistant to enterocin AS-48 in that use of 6 μg/ml bacteriocin resulted in an approximately one log reduction in the number of viable cells when dormant spores were treated with the bacteriocin in three food models: (i) tomato paste, (ii) syrup from canned peach, and (iii) juice from canned pineapple. However, using enterocin AS-48 at 3 and 6 μg/ml in combination with heat treatments (5 min at a minimum of 80°C) showed a significant reduction in the number of viable cells in both food models. When spores were incubated at 22°C for 48 h with bacteriocin, then heat treated at both 80 and 95°C, there was a significant difference in the number of viable cells obtained following both treatments relative to the non-heat treated controls or those that were heat treated without bacteriocin (Lucas et al., 2006). A relationship between heat temperature and survivors was observed, showing that viable counts in samples supplemented with bacteriocin decreased as the temperature was increased. This relationship was further evidenced by the significant reduction in viable counts obtained from bacteriocin treated spores heat treated at 95°C over those heat treated at 80°C. This relationship was observed in all three food models previously discussed (Lucas et al., 2006). Ultimately, this study nicely highlights the efficacy of bacteriocins to (i) reduce the severity of heat treatments and (ii) increase the effectiveness of heat treatments, when used to inactivate spores in food.
Another bacteriocin discussed previously, bificin C6165, has been shown to reduce the D90°C value of A. acidoterrestris as the bacteriocin concentration increased from 0 to 160 μg/ml. Addition of 80 and 160 μg/ml of bificin C6165 was shown to reduce the D90°C A. acidoterrestris CFD1 by 32.7 and 42.7%, respectively (Pei et al., 2014).
Bacteriocins in Combination with High Pressure
High-pressure processing (HPP) is a “non-thermal” food preservation technique that inactivates harmful pathogens and vegetative spoilage microorganisms by using pressure rather than heat to effect pasteurization. HPP utilizes intense pressure (about 400–600 MPa or 58,000–87,000 psi) at chilled or mild process temperatures (< 45°C), allowing most foods to be preserved with minimal effects on taste, texture, appearance, or nutritional value. Microorganisms do however display a variability in their sensitivity to HHP in the order: Gram-negative bacteria > Gram-positive bacteria > bacterial spores. While HPP is an effective method used for the destruction of microorganisms in food, it is not sufficient alone to inactivate spores and therefore must be combined with other hurdles, such as bacteriocins, to increase its efficacy. Indeed, treating food with bacteriocins may be an excellent combination as HHP can induce germination, which can facilitate the germination-dependent sporicidal activity of bacteriocins. Black et al. (2008) showed that treatment of 8 Log10 spores/ml of B. subtilis with low pressure (100 MPa i.e., not HHP) at 40°C in milk resulted in germination and inactivation of 4 and 1 Log10 spores/ml, respectively. A similar level of germination, but without inactivation, was observed in milk when a higher treatment of 500 MPa was used. When spores were treated with a combination of HP (500 MPa) and nisin (12.5 μg/ml), spore germination and inactivation increased to 6 and 3 Log10 spores/ml, respectively. When cycled twice with nisin there was a further increase in spore germination and inactivation of 8 and 6 Log10 spores/ml, respectively. High pressure-induced germination is known not to require the presence of nutrient receptors and is characterized by a rapid release in DPA-Ca2+ from the core. Nisin can be characterized as a potent pro-germinant in the presence of germinants (naturally present in milk) such as L-alanine and L-cysteine. Interestingly, nisin doubled the rate of germination in C. botulinum spores, while it had no effect on nisin resistant spores (Mazzotta and Montville, 1999). It was hypothesized that inactivation of spores by HHP and nisin could be due to (i) nisin and HP acting synergistically to inactivate spores or (ii) HP inducing germination after which nisin exerts its lethal effect on the germinated spore (Black et al., 2008). C. sporogenes spores were also shown to be inhibited rapidly by a treatment of nisin and 600 MPa at 90°C, relative to a treatment of 90°C alone (Hofstetter et al., 2013).
More recently, several studies have used response surface methodology (RSM) to test the effectiveness of high pressure, heat and nisin. Aouadhi et al. (2013) used RSM to investigate the effects of high pressure, in combination with moderate heat and nisin treatment, on B. sporothermodurans spores. The authors showed that spore inactivation was concentration dependent and that 1.25 and 125 μg/ml caused an inactivation of 0.4 and 4 Log10 spores/ml, respectively. Aouadhi et al. (2014) and Gao et al. (2011) showed that RSM (Table 5) could be effectively implemented to design an optimum treatment, involving multiple parameters to reduce spores loads by a predetermined amount.
Interestingly, superdormant spores of B. cereus and B. subtilis have been shown to germinate similarly to dormant spores when treated with pressure of 150MPa regardless of whether they were heat-activated or non-heat-activated. There have, however, been conflicting reports regarding the ability of pressure treatments to cause germination of an entire spore population. This uncertainty has impeded the widespread use of high pressure. It has been hypothesized that spores which remain superdormant after high pressure may do so via a distinct mechanism from that involved in making some spores superdormant to nutrient germinants (Wei et al., 2010).
Bacteriocin in Combination with Pulsed Electric Field
Pulsed electric field (PEF) is an innovative food preservation method, which may be suitable for reducing spore loads in liquid food. One of the distinct advantages of PEF is that the thermal impacts on food are minimized as this treatment is relatively non-thermal. Any heat produced is directly influenced by the energy input of the treatment. While it is known that vegetative cells of B. cereus are sensitive to PEF and nisin (Pol et al., 2000) and that this combination is sporostatic but this treatment did not initiate germination nor did they affect the viability of the dormant spores. After germination, B. cereus immediately became sensitive to nisin (1.25 μg/ml) but it was longer (50 min) before they became sensitive to PEF (27 kV/cm, 302-μs pulses; flow rate, 10 ml/min). Unlike the synergistic activity of nisin and PEF against vegetative cells (Pol et al., 2000), when spores when treated with both PEF and nisin this synergistic activity was not observed as the reduction was comparable to nisin alone (Pol et al., 2001). While this combination is not synergistic against spores, food rarely contains spores alone but rather a mixed population of spores and vegetative cells. Therefore, this combination may still be an effective way of maintaining dormant spore numbers yet reducing the population of vegetative cells for increased food safety and shelf life.
Bacteriocins in Combination with Osmotic Activation
Stimulation of dormant bacterial spore germination followed by subsequent inactivation, as previously discussed, is a promising method used for spore inactivation. Small, non-polar, hydrophobic solutes that permeate the plasma membrane have been shown to stimulate B. cereus germination (Preston and Douthit, 1984). Inhibition of non-heat activated C. difficile spores was significantly increased when treated with nisin and single osmotic activators (ammonium, glycerol, and Tris) compared to heat activated spores treated with nisin and solutes in a germination medium. For example, nisin in combination with heating resulted in a 1–2.5 log10 spores/ml decrease in viable spores but when nisin was combined with osmotic activators this increased to >3.5 log10 spores/ml (Nerandzic and Donskey, 2013). Using flow cytometry, it was observed that the membrane permeability of spores was significantly increased when treated with osmotic activators. Spores treated with both nisin and solute transitioned to phase dark (as spores germinate they appear phase dark using phase contrast microscopy), whereas those incubated with nisin and osmotic activators separately did not transition to phase dark (Nerandzic and Donskey, 2013). The proposed synergistic ability of nisin and osmotic activators to inhibit outgrowth was attributed to the osmotically induced loss of membrane integrity. Although C. difficile is of clinical importance, this use of osmotic activation could be used to overcome limitations of the germination dependent activity of bacteriocins with other food related strains of clostridia.
Bacteriocins in Food Packaging
The preservation of sausage casings of preserved intestines of animals has been practiced for centuries. However, this preservation method has been modernized to suit modern consumer desires. Such a modernization is the binding of nisin to sausage casing in order to control Clostridium spore outgrowth. Wijnker et al. (2011) showed that nisin, at 100 μg/ml, when bound to casings and placed on agar plates seeded with Clostridium spores, produced zones whereas those casings with only 50 μg/ml did not. They also observed that addition of nisin at 50 μg/ml to the casings delayed C. sporogenes spore outgrowth between 1 and 8 days. Furthermore, at this concentration of 50 μg/ml, this sporostatic activity was observed for 30 days. In contrast, Meghrous et al. (1999) showed that a lower concentration of nisin, 23 μg/ml, delayed clostridial spore outgrowth by 10 days. It should also be noted that Wijnker et al. (2011) used 106 spores/ml whereas Meghrous et al. (1999) used 103 spores/ml. The reason that nisin at 50 μg/ml could inhibit outgrowth in vitro but not on the casings could be due to the irreversible binding of nisin to the collagen matrix of the casing wall. This would suggest that if outgrowth is to be prevented the casings need to contain a higher concentration of nisin in order to overcome the deleterious effect of irreversible binding to the casing.
Bacteriocins in Combination with Plant Extracts
Plants contain innumerable constituents and are valuable sources of new and biologically active molecules possessing antimicrobial properties. The plant family Piperaceae are found in tropical and subtropical regions and are commonly used as to generate medicinal herbs. Ruiz et al. (2013) showed that a combination of nisin and Piper aduncum exhibited a strong antibacterial activity against spores of A. acidoterrestris and also exhibited a synergism (FIC = 0.24) against A. acidoterrestris vegetative cells. Prenylated chromone was identified as the active compound in this plant extract. Piperaceae extract is a natural food preservation method that may be combined with nisin to lower (if any) heat treatment needed to reduce and inhibit spores outgrowth.
Nisin in Combination with Fatty Acid Esters
Sucrose fatty esters are approved internationally for use as emulsifiers and these non-toxic molecules have also been reported to inhibit Gram-positive bacteria. A combination of nisin and the fatty acid ester, sucrose palmitate (P-1570), displayed synergism against spores of B. cereus whereas sucrose fatty acid esters alone caused no decrease in growth (Thomas et al., 1998). Total inhibition of B. licheniformis spore outgrowth was achieved when nisin (0.75 μg/ml) was combined with the fatty acid ester monolaurin (100 μg/ml) whereas when these treatments were used separately at higher concentrations they only partially inhibited outgrowth (Mansour et al., 1999).
Nisin in Combination with Potassium Sorbate
Sorbates are extensively used in the food industry, as they are able to inhibit, or delay growth of, spores and vegetative populations of bacteria. Although their mechanism of action is not full defined for bacterial spores, it is has been shown that potassium sorbate inhibits the growth of spores of Bacillus species (Oloyede and Scholefield, 1994). A combination of nisin (1.25 μg/ml) and potassium sorbate (2% w/v) has been shown to cause a synergistic reduction in the number of heat activated B. sporothermodurans spores. After 8 h there was ~3 Log10 spores/ml reduction in spores. This reduction in spores continued albeit at a slower rate until 5 days where total inhibition of B. sporothermodurans spores occurred (Aouadhi et al., 2015). When tested separately at these levels, both nisin and potassium sorbate inhibited spore outgrowth. Nisin was not sporicidal but rather sporostatic, inhibiting spore outgrowth. While potassium sorbate was not sporicidal, it did significantly perturb germination of B. sporothermodurans and inhibited the outgrowth of spores (Aouadhi et al., 2015). This ability of potassium sorbate to inhibit spore germination has previously been reported for spores of B. cereus and C. botulinum (Smoot and Pierson, 1981).
Discussion
While spores are a widely recognized problem in the food industry the majority of bacteriocin-related studies have focused on the elimination of vegetative cells from food. The removal of spores and inhibition of their outgrowth in food is important for (i) increasing shelf life and (ii) protecting the consumer from harmful pathogenic spore-formers. Although, there are numerous bacteriocins which have been characterized as safe and effective molecules for use in food, to date, nisin is the only bacteriocin which is authorized for use as a food preservative. While this bacteriocin provides an effective and safe method to reduce spore outgrowth in food, it is important to recognize that this molecule has its limitations. Bacteriocins in food may be limited by: molecule specific solubility, the active pH range of the bacteriocin, inactivation by proteases in food, and the possible negative interactions that occur between certain bacteriocins and certain food components. One such limitation of nisin is its loss of activity as the pH of the food increases. There are a variety of bacteriocins which are more active than nisin at higher pH, such as gassericin A, pediocin AcM, and thermophilin T (Table 1), however they still need to be further characterized before their use in food may be authorized.
In the majority of cases nisin is only sporicidal against those spores in the outgrowth phase and therefore has no effect on those spores in the dormant phase. Although this model of nisin (and other bacteriocins) use in food suggests that germination is a prerequisite for its activity, it is important to note that there are relatively few studies which investigate bacteriocin/spore interactions. Furthermore, it should be recognized that the only detailed mechanism for bacteriocins/spore interaction is that of B. anthracis (Figure 2). Indeed, the limited number of existing studies highlights the need for further research in this area. Understanding these interactions and mechanisms will ultimately lead to a more precise and optimal use of bacteriocins in food. Undeniably, the mode of action for a great many bacteriocins has yet to be elucidated and a better understanding of the methods by which bacteriocins kill bacteria will facilitate a solid basis for engineering new and more potent derivatives with optimized potency and stability. Given that spores must germinate to exert their adverse effects, future research should focus on stimulating spore germination to enable spores to be more effectively targeted by bacteriocins in food settings. Indeed, recent research provides stimulating evidence for using a germination step prior to spore destruction for promoting inactivation of Bacillus and Clostridial spores (Gut et al., 2008). Furthermore, although numerous components of the spore germination machinery are conserved between spore forming members of bacilli and clostridia, significant differences between the germination of spores of Clostridium perfringens and that of spores of a number of Bacillus species, both in the proteins and in the signal transduction pathways involved have been revealed (Abhyankar et al., 2014; Setlow, 2014a; Olguín-Araneda et al., 2015). Indeed, as the number of microbial genome sequences has increased dramatically, bioinformatics data contained in the large number of spore-forming Bacillales and Clostridiales genomes that have been sequenced and the information gained from their analysis, can be used to guide researchers to develop novel strategies to achieve a complete and permanent loss of the spore's ability to germinate and grow in food products.
Regardless of the specific bacteriocin of choice, it is clear that there is considerable evidence of the potential value of bacteriocins with respect to controlling sporeforming bacteria in food. In the case of spores, while this activity more frequently tends to be sporostatic, there are also examples of sporicidal effects. As is the case for vegetative cells, the mechanisms via which bacteriocins inhibit spores may be heterogeneous but ultimately it is apparent that in general bacterial spores can be controlled using bacteriocins, and their application in combination with other novel non-thermal treatments makes their efficacy even greater. The use of the bacteriocins with other food processing hurdles, such as those previously described, thus has the potential to satisfy consumer demands for “clean label” products, enabling processors to produce foods of optimal quality and shelf life.
Author Contributions
KE drafted the manuscript. DF, MR, RR, CH, and PC revised and approved the final manuscript.
Funding
KE, DF, CH, PC, MR, RR are supported by the Irish Government under the National Development Plan, through the Food Institutional Research Measure, administered by the Department of Agriculture, Fisheries and Food, Ireland (DAFM 13/F/462) to PC and MR, a Science Foundation Ireland (SFI) Technology and Innovation Development Award (TIDA 14/TIDA/2286) to DF, SFI-PI funding (11/PI/1137) to PDC and the APC Microbiome Insitute under Grant Number SFI/12/RC/2273.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abhyankar, W., de Koning, L. J., Brul, S., and de Koster, C. G. (2014). Spore proteomics: the past, present and the future. FEMS Microbiol. Lett. 358, 137–144. doi: 10.1111/1574-6968.12568
Abriouel, H., Maqueda, M., Gálvez, A., Martínez-Bueno, M., and Valdivia, E. (2002). Inhibition of bacterial growth, enterotoxin production, and spore outgrowth in strains of Bacillus cereus by bacteriocin AS-48. Appl. Environ. Microbiol. 68, 1473–1477. doi: 10.1128/AEM.68.3.1473-1477.2002
Aktypis, A., Kalantzopoulos, G., Huis in't Veld, J. H. J., and Ten Brink, B. (1998). Purification and characterization of thermophilin T, a novel bacteriocin produced by Streptococcus thermophilus ACA-DC 0040. J. Appl. Microbiol. 84, 568–576. doi: 10.1046/j.1365-2672.1998.00383.x
Aouadhi, C., Mejri, S., and Maaroufi, A. (2015). Inhibitory effects of nisin and potassium sorbate alone or in combination on vegetative cells growth and spore germination of Bacillus sporothermodurans in milk. Food Microbiol. 46, 40–45. doi: 10.1016/j.fm.2014.07.004
Aouadhi, C., Rouissi, Z., Mejri, S., and Maaroufi, A. (2014). Inactivation of Bacillus sporothermodurans spores by nisin and temperature studied by design of experiments in water and milk. Food Microbiol. 38, 270–275. doi: 10.1016/j.fm.2013.10.005
Aouadhi, C., Simonin, H., Mejri, S., and Maaroufi, A. (2013). The combined effect of nisin, moderate heating and high hydrostatic pressure on the inactivation of Bacillus sporothermodurans spores. J. Appl. Microbiol. 115, 147–155. doi: 10.1111/jam.12220
Arnison, P. G., Bibb, M. J., Bierbaum, G., Bowers, A. A., Bugni, T. S., Bulaj, G., et al. (2013). Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 30, 108–160. doi: 10.1039/C2NP20085F
Atluri, S., Ragkousi, K., Cortezzo, D. E., and Setlow, P. (2006). Cooperativity between different nutrient receptors in germination of spores of Bacillus subtilis and reduction of this cooperativity by alterations in the GerB receptor. J. Bacteriol. 188, 28–36. doi: 10.1128/JB.188.1.28-36.2006
Ávila, M., Gómez-Torres, N., Hernández, M., and Garde, S. (2014). Inhibitory activity of reuterin, nisin, lysozyme and nitrite against vegetative cells and spores of dairy-related Clostridium species. Int. J. Food Microbiol. 172, 70–75. doi: 10.1016/j.ijfoodmicro.2013.12.002
Aymerich, T., Holo, H., Håvarstein, L. S., Hugas, M., Garriga, M., and Nes, I. F. (1996). Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl. Environ. Microbiol. 62, 1676–1682.
Basanta, A., Gómez-Sala, B., Sánchez, J., Diep, D. B., Herranz, C., Hernández, P. E., et al. (2010). Use of the yeast pichia pastoris as an expression host for secretion of enterocin L50, a leaderless two-peptide (L50A and L50B) bacteriocin from Enterococcus faecium L50. Appl. Environ. Microbiol. 76, 3314–3324. doi: 10.1128/AEM.02206-09
Bassi, D., Puglisi, E., and Cocconcelli, P. S. (2015). Understanding the bacterial communities of hard cheese with blowing defect. Food Microbiol. 52, 106–118. doi: 10.1016/j.fm.2015.07.004
Beard, B. M., Sheldon, B. W., and Foegeding, P. M. (1999). Thermal resistance of bacterial spores in milk-based beverages supplemented with nisin. J. Food Prot. 62, 484–491.
Beuchat, L. R., Clavero, M. R., and Jaquette, C. B. (1997). Effects of nisin and temperature on survival, growth, and enterotoxin production characteristics of psychrotrophic Bacillus cereus in beef gravy. Appl. Environ. Microbiol. 63, 1953–1958.
Birri, D. J., Brede, D. A., and Nes, I. F. (2012). Salivaricin D, a novel intrinsically trypsin-resistant lantibiotic from Streptococcus salivarius 5M6c isolated from a healthy infant. Appl. Environ. Microbiol. 78, 402–410. doi: 10.1128/AEM.06588-11
Black, E. P., Linton, M., McCall, R. D., Curran, W., Fitzgerald, G. F., Kelly, A. L., et al. (2008). The combined effects of high pressure and nisin on germination and inactivation of Bacillus spores in milk. J. Appl. Microbiol. 105, 78–87. doi: 10.1111/j.1365-2672.2007.03722.x
Blom, H., Katla, T., Holck, A., Sletten, K., Axelsson, L., and Holo, H. (1999). Characterization, production, and purification of leucocin H, a two-peptide bacteriocin from Leuconostoc MF215B. Curr. Microbiol. 39, 43–48. doi: 10.1007/PL00006825
Bogovic-Matijasić, B., Rogelj, I., Nes, I. F., and Holo, H. (1998). Isolation and characterization of two bacteriocins of Lactobacillus acidophilus LF221. Appl. Microbiol. Biotechnol. 49, 606–612. doi: 10.1007/s002530051221
Brötz, H., Bierbaum, G., Markus, A., Molitor, E., and Sahl, H.-G. (1995). Mode of action of the lantibiotic mersacidin: inhibition of peptidoglycan biosynthesis via a novel mechanism? Antimicrob. Agents Chemother. 39, 714–719. doi: 10.1128/AAC.39.3.714
Burgos, M. J. G., Pulido, R. P., Aguayo, M. C. L., Gálvez, A., and Lucas, R. (2014). The cyclic antibacterial peptide enterocin AS-48: isolation, mode of action, and possible food applications. Int. J. Mol. Sci. 15, 22706–22727. doi: 10.3390/ijms151222706
Cano, R. J., and Borucki, M. K. (1995). Revival and identification of bacterial-spores in 25-million-year-old to 40-million-year-old Dominican amber. Science 268, 1060–1064. doi: 10.1126/science.7538699
Carlin, F. (2011). Origin of bacterial spores contaminating foods. Food Microbiol. 28, 177–182. doi: 10.1016/j.fm.2010.07.008
Carmen Martínez-Cuesta, M., Bengoechea, J., Bustos, I., Rodríguez, B., Requena, T., and Peláez, C. (2010). Control of late blowing in cheese by adding lacticin 3147-producing Lactococcus lactis IFPL 3593 to the starter. Int. Dairy J. 20, 18–24. doi: 10.1016/j.idairyj.2009.07.005
Casaus, P., Nilsen, T., Cintas, L. M., Nes, I. F., Hernández, P. E., and Holo, H. (1997). Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin A. Microbiology 143(Pt 7), 2287–2294. doi: 10.1099/00221287-143-7-2287
Chen, Y., Ray, W. K., Helm, R. F., Melville, S. B., and Popham, D. L. (2014). Levels of germination proteins in Bacillus subtilis dormant, superdormant, and germinating spores. PLoS ONE 9:e95781. doi: 10.1371/journal.pone.0095781
Christiansson, A., Naidu, A. S., Nilsson, I., Wadström, T., and Pettersson, H. E. (1989). Toxin production by Bacillus cereus dairy isolates in milk at low temperatures. Appl. Environ. Microbiol. 55, 2595–2600.
Cintas, L. M., Casaus, P., Håvarstein, L. S., Hernández, P. E., and Nes, I. F. (1997). Biochemical and genetic characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl. Environ. Microbiol. 63, 4321–4330.
Cintas, L. M., Rodriguez, J. M., Fernandez, M. F., Sletten, K., Nes, I. F., Hernandez, P. E., et al. (1995). Isolation and characterization of pediocin L50, a new bacteriocin from Pediococcus acidilactici with a broad inhibitory spectrum. Appl. Environ. Microbiol. 61, 2643–2648.
Cortezzo, D. E., and Setlow, P. (2005). Analysis of factors that influence the sensitivity of spores of Bacillus subtilis to DNA damaging chemicals. J. Appl. Microbiol. 98, 606–617. doi: 10.1111/j.1365-2672.2004.02495.x
Cotter, P. D. (2014). An ‘Upp’-turn in bacteriocin receptor identification. Mol. Microbiol. 92, 1159–1163. doi: 10.1111/mmi.12645
Cotter, P. D., Hill, C., and Ross, R. P. (2005). Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3, 777–788. doi: 10.1038/nrmicro1273
Cotter, P. D., Ross, R. P., and Hill, C. (2013). Bacteriocins - a viable alternative to antibiotics? Nat. Rev. Microbiol. 11, 95–105. doi: 10.1038/nrmicro2937
Cui, Y., Zhang, C., Wang, Y., Shi, J., Zhang, L., Ding, Z., et al. (2012). Class IIa bacteriocins: diversity and new developments. Int. J. Mol. Sci. 13, 16668–16707. doi: 10.3390/ijms131216668
De Vuyst, L., Foulquié Moreno, M. R., and Revets, H. (2003). Screening for enterocins and detection of hemolysin and vancomycin resistance in enterococci of different origins. Int. J. Food Microbiol. 84, 299–318. doi: 10.1016/S0168-1605(02)00425-7
Diep, D. B., Skaugen, M., Salehian, Z., Holo, H., and Nes, I. F. (2007). Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc. Natl. Acad. Sci. U.S.A. 104, 2384–2389. doi: 10.1073/pnas.0608775104
Doyle, C. J., Gleeson, D., Jordan, K., Beresford, T. P., Ross, R. P., Fitzgerald, G. F., et al. (2015). Anaerobic sporeformers and their significance with respect to milk and dairy products. Int. J. Food Microbiol. 197, 77–87. doi: 10.1016/j.ijfoodmicro.2014.12.022
Draper, L. A., Cotter, P. D., Hill, C., and Ross, R. P. (2015). Lantibiotic resistance. Microbiol. Mol. Biol. Rev. 79, 171–191. doi: 10.1128/MMBR.00051-14
Durand, L., Planchon, S., Guinebretiere, M.-H., André, S., Carlin, F., and Remize, F. (2015). Contamination pathways of spore-forming bacteria in a vegetable cannery. Int. J. Food Microbiol. 202, 10–19. doi: 10.1016/j.ijfoodmicro.2015.02.019
Durban, E., Goodnow, R., and Grecz, N. (1970). Changes in resistance to radiation and heat during sporulation and germination of Clostridium botulinum 33A. J. Bacteriol. 102, 590–592.
Eguchi, T., Kaminaka, K., Shima, J., Kawamoto, S., Mori, K., Choi, S. H., et al. (2001). Isolation and characterization of enterocin SE-K4 produced by thermophilic enterococci, Enterococcus faecalis K-4. Biosci. Biotechnol. Biochem. 65, 247–253. doi: 10.1271/bbb.65.247
Elegado, F. B., Kim, W. J., and Kwon, D. Y. (1997). Rapid purification, partial characterization, and antimicrobial spectrum of the bacteriocin, Pediocin AcM, from Pediococcus acidilactici M. Int. J. Food Microbiol. 37, 1–11. doi: 10.1016/S0168-1605(97)00037-8
Enan, G., El-Essawy, A. A., Uyttendaele, M., and Debevere, J. (1996). Antibacterial activity of Lactobacillus plantarum UG1 isolated from dry sausage: characterization, production and bactericidal action of plantaricin UG1. Int. J. Food Microbiol. 30, 189–215. doi: 10.1016/0168-1605(96)00947-6
Field, D., Cotter, P. D., Ross, R. P., and Hill, C. (2015). Bioengineering of the model lantibiotic nisin. Bioengineered 6, 187–192. doi: 10.1080/21655979.2015.1049781
Franz, C. M., Holzapfel, W. H., and Stiles, M. E. (1999). Enterococci at the crossroads of food safety? Int. J. Food Microbiol. 47, 1–24. doi: 10.1016/S0168-1605(99)00007-0
Franz, C. M., Huch, M., Abriouel, H., Holzapfel, W., and Gálvez, A. (2011). Enterococci as probiotics and their implications in food safety. Int. J. Food Microbiol. 151, 125–140. doi: 10.1016/j.ijfoodmicro.2011.08.014
Fujita, K., Ichimasa, S., Zendo, T., Koga, S., Yoneyama, F., Nakayama, J., et al. (2007). Structural analysis and characterization of lacticin Q, a novel bacteriocin belonging to a new family of unmodified bacteriocins of gram-positive bacteria. Appl. Environ. Microbiol. 73, 2871–2877. doi: 10.1128/AEM.02286-06
Gálvez, A., Valdivia, E., Abriouel, H., Camafeita, E., Mendez, E., Martínez-Bueno, M., et al. (1998). Isolation and characterization of enterocin EJ97, a bacteriocin produced by Enterococcus faecalis EJ97. Arch. Microbiol. 171, 59–65. doi: 10.1007/s002030050678
Gao, Y., Qiu, W., Wu, D., and Fu, Q. (2011). Assessment of Clostridium perfringens spore response to high hydrostatic pressure and heat with nisin. Appl. Biochem. Biotechnol. 164, 1083–1095. doi: 10.1007/s12010-011-9196-0
García, M. T., Lucas, R., Abriouel, H., Omar, N. B., Pérez, R., Grande, M. J., et al. (2004). Antimicrobial activity of enterocin EJ97 against ‘Bacillus macroides/Bacillus maroccanus’ isolated from zucchini puree. J. Appl. Microbiol. 97, 731–737. doi: 10.1111/j.1365-2672.2004.02351.x
Georgalaki, M., Papadimitriou, K., Anastasiou, R., Pot, B., Van Driessche, G., Devreese, B., et al. (2013). Macedovicin, the second food-grade lantibiotic produced by Streptococcus macedonicus ACA-DC 198. Food Microbiol. 33, 124–130. doi: 10.1016/j.fm.2012.09.008
Georgalaki, M. D., Van Den Berghe, E., Kritikos, D., Devreese, B., Van Beeumen, J., Kalantzopoulos, G., et al. (2002). Macedocin, a food-grade lantibiotic produced by Streptococcus macedonicus ACA-DC 198. Appl. Environ. Microbiol. 68, 5891–5903. doi: 10.1128/AEM.68.12.5891-5903.2002
Ghosh, S., Zhang, P., Li, Y. Q., and Setlow, P. (2009). Superdormant spores of Bacillus species have elevated wet-heat resistance and temperature requirements for heat activation. J. Bacteriol. 191, 5584–5591. doi: 10.1128/JB.00736-09
Goh, H. F., and Philip, K. (2015). Isolation and mode of action of bacteriocin BacC1 produced by nonpathogenic Enterococcus faecium C1. J. Dairy Sci. 98, 5080–5090. doi: 10.3168/jds.2014-9240
González, B., Arca, P., Mayo, B., and Suárez, J. E. (1994). Detection, purification, and partial characterization of plantaricin C, a bacteriocin produced by a Lactobacillus plantarum strain of dairy origin. Appl. Environ. Microbiol. 60, 2158–2163.
González, L., and Zárate, V. (2015). Inhibitory activity of Lactobacillus plantarum TF711 against Clostridium sporogenes when used as adjunct culture in cheese manufacture. J. Dairy Res. 82, 236–241. doi: 10.1017/S0022029915000126
Götz, F., Perconti, S., Popella, P., Werner, R., and Schlag, M. (2014). Epidermin and gallidermin: staphylococcal lantibiotics. Int. J. Med. Microbiol. 304, 63–71. doi: 10.1016/j.ijmm.2013.08.012
Grande, M. J., Lucas, R., Abriouel, H., Omar, N. B., Maqueda, M., Martínez-Bueno, M., et al. (2005). Control of Alicyclobacillus acidoterrestris in fruit juices by enterocin AS-48. Int. J. Food Microbiol. 104, 289–297. doi: 10.1016/j.ijfoodmicro.2005.03.010
Grande, M. J., Lucas, R., Abriouel, H., Valdivia, E., Ben Omar, N., Maqueda, M., et al. (2006a). Inhibition of Bacillus licheniformis LMG 19409 from ropy cider by enterocin AS-48. J. Appl. Microbiol. 101, 422–428. doi: 10.1111/j.1365-2672.2006.02942.x
Grande, M. J., Lucas, R., Abriouel, H., Valdivia, E., Omar, N. B., Maqueda, M., et al. (2006b). Inhibition of toxicogenic Bacillus cereus in rice-based foods by enterocin AS-48. Int. J. Food Microbiol. 106, 185–194. doi: 10.1016/j.ijfoodmicro.2005.03.010
Gut, I. M., Blanke, S. R., and van der Donk, W. A. (2011). Mechanism of inhibition of Bacillus anthracis spore outgrowth by the lantibiotic nisin. ACS Chem. Biol. 6, 744–752. doi: 10.1021/cb1004178
Gut, I. M., Prouty, A. M., Ballard, J. D., van der Donk, W. A., and Blanke, S. R. (2008). Inhibition of Bacillus anthracis spore outgrowth by nisin. Antimicrob. Agents Chemother. 52, 4281–4288. doi: 10.1128/AAC.00625-08
Héchard, Y., and Sahl, H.-G. (2002). Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie 84, 545–557. doi: 10.1016/S0300-9084(02)01417-7
Hernández, D., Cardell, E., and Zárate, V. (2005). Antimicrobial activity of lactic acid bacteria isolated from Tenerife cheese: initial characterization of plantaricin TF711, a bacteriocin-like substance produced by Lactobacillus plantarum TF711. J. Appl. Microbiol. 99, 77–84. doi: 10.1111/j.1365-2672.2005.02576.x
Himeno, K., Rosengren, K. J., Inoue, T., Perez, R. H., Colgrave, M. L., Lee, H. S., et al. (2015). Identification, characterization, and three-dimensional structure of the novel circular bacteriocin, enterocin NKR-5-3B, from Enterococcus faecium. Biochemistry 54, 4863–4876. doi: 10.1021/acs.biochem.5b00196
Hofstetter, S., Gebhardt, D., Ho, L., Gänzle, M., and McMullen, L. M. (2013). Effects of nisin and reutericyclin on resistance of endospores of Clostridium spp. to heat and high pressure. Food Microbiol. 34, 46–51. doi: 10.1016/j.fm.2012.11.001
Holck, A., Axelsson, L., and Schillinger, U. (1996). Divergicin 750, a novel bacteriocin produced by Carnobacterium divergens 750. FEMS Microbiol. Lett. 136, 163–168. doi: 10.1111/j.1574-6968.1996.tb08043.x
Hu, C.-B., Malaphan, W., Zendo, T., Nakayama, J., and Sonomoto, K. (2010). Enterocin X, a novel two-peptide bacteriocin from Enterococcus faecium KU-B5, has an antibacterial spectrum entirely different from those of its component peptides. Appl. Environ. Microbiol. 76, 4542–4545. doi: 10.1128/AEM.02264-09
Hu, C. B., Zendo, T., Nakayama, J., and Sonomoto, K. (2008). Description of durancin TW-49M, a novel enterocin B-homologous bacteriocin in carrot-isolated Enterococcus durans QU 49. J. Appl. Microbiol. 105, 681–690. doi: 10.1111/j.1365-2672.2008.03798.x
Hu, M., Zhao, H., Zhang, C., Yu, J., and Lu, Z. (2013). Purification and characterization of plantaricin 163, a novel bacteriocin produced by Lactobacillus plantarum 163 isolated from traditional Chinese fermented vegetables. J. Agric. Food Chem. 61, 11676–11682. doi: 10.1021/jf403370y
Hu, X., Mao, R., Zhang, Y., Teng, D., Wang, X., Xi, D., et al. (2014). Biotechnical paving of recombinant enterocin A as the candidate of anti-Listeria agent. BMC Microbiol. 14:220. doi: 10.1186/s12866-014-0220-8
Huang, E., Zhang, L., Chung, Y. K., Zheng, Z., and Yousef, A. E. (2013). Characterization and application of enterocin RM6, a bacteriocin from Enterococcus faecalis. Biomed Res. Int. 2013, 206917. doi: 10.1155/2013/206917
Iancu, C., Grainger, A., Field, D., Cotter, P. D., Hill, C., and Ross, R. P. (2012). Comparison of the potency of the lipid II targeting antimicrobials nisin, Lacticin 3147 and vancomycin against gram-positive bacteria. Probiotics Antimicrob. Proteins 4, 108–115. doi: 10.1007/s12602-012-9095-x
Iwatani, S., Zendo, T., Yoneyama, F., Nakayama, J., and Sonomoto, K. (2007). Characterization and structure analysis of a novel bacteriocin, lacticin Z, produced by Lactococcus lactis QU 14. Biosci. Biotechnol. Biochem. 71, 1984–1992. doi: 10.1271/bbb.70169
Izquierdo, E., Bednarczyk, A., Schaeffer, C., Cai, Y., Marchioni, E., Van Dorsselaer, A., et al. (2008). Production of enterocins L50A, L50B, and IT, a new enterocin, by Enterococcus faecium IT62, a strain isolated from Italian ryegrass in Japan. Antimicrob. Agents Chemother. 52, 1917–1923. doi: 10.1128/AAC.01409-07
Jaouani, I., Abbassi, M. S., Ribeiro, S. C., Khemiri, M., Mansouri, R., Messadi, L., et al. (2015). Safety and technological properties of bacteriocinogenic enterococci isolates from Tunisia. J. Appl. Microbiol. 119, 1089–1100. doi: 10.1111/jam.12916
Jordan, K., Dalmasso, M., Zentek, J., Mader, A., Bruggeman, G., Wallace, J., et al. (2014). Microbes versus microbes: control of pathogens in the food chain. J. Sci. Food Agric. 94, 3079–3089. doi: 10.1002/jsfa.6735
Kabuki, T., Uenishi, H., Watanabe, M., Seto, Y., and Nakajima, H. (2007). Characterization of a bacteriocin, Thermophilin 1277, produced by Streptococcus thermophilus SBT1277. J. Appl. Microbiol. 102, 971–980. doi: 10.1111/j.1365-2672.2006.03159.x
Kayser, F. H. (2003). Safety aspects of enterococci from the medical point of view. Int. J. Food Microbiol. 88, 255–262. doi: 10.1016/S0168-1605(03)00188-0
Komitopoulou, E., Boziaris, I. S., Davies, E. A., Delves-Broughton, J., and Adams, M. R. (1999). Alicyclobacillus acidoterrestris in fruit juices and its control by nisin. Int. J. Food Sci. Technol. 34, 81–85. doi: 10.1046/j.1365-2621.1999.00243.x
Lasta, S., Ouzari, H., Andreotti, N., Fajloun, Z., Mansuelle, P., Boudabous, A., et al. (2012). Lacticin LC14, a new bacteriocin produced by Lactococcus lactis BMG6.14: isolation, purification and partial characterization. Infect. Disord. Drug Targets 12, 316–325. doi: 10.2174/187152612801319276
Lee, H., and Kim, H. Y. (2011). Lantibiotics, class I bacteriocins from the genus Bacillus. J. Microbiol. Biotechnol. 21, 229–235. doi: 10.4014/jmb.1010.10017
Liu, X., Vederas, J. C., Whittal, R. M., Zheng, J., Stiles, M. E., Carlson, D., et al. (2011). Identification of an N-terminal formylated, two-peptide bacteriocin from Enterococcus faecalis 710C. J. Agric. Food Chem. 59, 5602–5608. doi: 10.1021/jf104751v
Lozo, J., Vukasinovic, M., Strahinic, I., and Topisirovic, L. (2004). Characterization and antimicrobial activity of bacteriocin 217 produced by natural isolate Lactobacillus paracasei subsp. paracasei BGBUK2-16. J. Food Prot. 67, 2727–2734.
Lucas, R., Grande, M. J., Abriouel, H., Maqueda, M., Ben Omar, N., Valdivia, E., et al. (2006). Application of the broad-spectrum bacteriocin enterocin AS-48 to inhibit Bacillus coagulans in canned fruit and vegetable foods. Food Chem. Toxicol. 44, 1774–1781. doi: 10.1016/j.fct.2006.05.019
Luu, S., Cruz-Mora, J., Setlow, B., Feeherry, F. E., Doona, C. J., and Setlow, P. (2015). The effects of heat activation on Bacillus spore germination, with nutrients or under high pressure, with or without various germination proteins. Appl. Environ. Microbiol. 81, 2927–2938. doi: 10.1128/AEM.00193-15
Mansour, M., Amri, D., Bouttefroy, A., Linder, M., and Milliere, J. B. (1999). Inhibition of Bacillus licheniformis spore growth in milk by nisin, monolaurin, and pH combinations. J. Appl. Microbiol. 86, 311–324. doi: 10.1046/j.1365-2672.1999.00669.x
Marciset, O., Jeronimus-Stratingh, M. C., Mollet, B., and Poolman, B. (1997). Thermophilin 13, a nontypical antilisterial poration complex bacteriocin, that functions without a receptor. J. Biol. Chem. 272, 14277–14284. doi: 10.1074/jbc.272.22.14277
Martín-Platero, A. M., Valdivia, E., Ruíz-Rodríguez, M., Soler, J. J., Martín-Vivaldi, M., Maqueda, M., et al. (2006). Characterization of antimicrobial substances produced by Enterococcus faecalis MRR 10-3, isolated from the uropygial gland of the hoopoe (Upupa epops). Appl. Environ. Microbiol. 72, 4245–4249. doi: 10.1128/AEM.02940-05
Martínez, B., Böttiger, T., Schneider, T., Rodríguez, A., Sahl, H.-G., and Wiedemann, I. (2008). Specific interaction of the unmodified bacteriocin lactococcin 972 with the cell wall precursor lipid II. Appl. Environ. Microbiol. 74, 4666–4670. doi: 10.1128/AEM.00092-08
Martinez-Cuesta, M. C., Bengoechea, J., Bustos, I., Rodriguez, B., Requena, T., and Pelaez, C. (2010). Control of late blowing in cheese by adding lacticin 3147-producing Lactococcus lactis IFPL 3593 to the starter. Int. Dairy J. 20, 18–24. doi: 10.1016/j.idairyj.2009.07.005
Marugg, J. D., Gonzalez, C. F., Kunka, B. S., Ledeboer, A. M., Pucci, M. J., Toonen, M. Y., et al. (1992). Cloning, expression, and nucleotide sequence of genes involved in production of pediocin PA-1, and bacteriocin from Pediococcus acidilactici PAC1. 0. Appl. Environ. Microbiol. 58, 2360–2367.
Masuda, Y., Ono, H., Kitagawa, H., Ito, H., Mu, F., Sawa, N., et al. (2011). Identification and characterization of leucocyclicin Q, a novel cyclic bacteriocin produced by Leuconostoc mesenteroides TK41401. Appl. Environ. Microbiol. 77, 8164–8170. doi: 10.1128/AEM.06348-11
Masuda, Y., Zendo, T., Sawa, N., Perez, R. H., Nakayama, J., and Sonomoto, K. (2012). Characterization and identification of weissellicin Y and weissellicin M, novel bacteriocins produced by Weissella hellenica QU 13. J. Appl. Microbiol. 112, 99–108. doi: 10.1111/j.1365-2672.2011.05180.x
Mathot, A. G., Beliard, E., and Thuault, D. (2003). Streptococcus thermophilus 580 produces a bacteriocin potentially suitable for inhibition of Clostridium tyrobutyricum in hard cheese. J. Dairy Sci. 86, 3068–3074. doi: 10.3168/jds.S0022-0302(03)73906-X
Mazzotta, A. S., Crandall, A. D., and Montville, T. J. (1997). Nisin resistance in Clostridium botulinum spores and vegetative Cells. Appl. Environ. Microbiol. 63, 2654–2659.
Mazzotta, A. S., and Montville, T. J. (1999). Characterization of fatty acid composition, spore germination, and thermal resistance in a nisin-resistant mutant of Clostridium botulinum 169B and in the wild-type strain. Appl. Environ. Microbiol. 65, 659–664.
McAuliffe, O., Ryan, M. P., Ross, R. P., Hill, C., Breeuwer, P., and Abee, T. (1998). Lacticin 3147, a broad-spectrum bacteriocin which selectively dissipates the membrane potential. Appl. Environ. Microbiol. 64, 439–445.
Meghrous, J., Lacroix, C., and Simard, R. E. (1999). The effects on vegetative cells and spores of three bacteriocins from lactic acid bacteria. Food Microbiol. 16, 105–114. doi: 10.1006/fmic.1998.0221
Mills, S., Serrano, L. M., Griffin, C., O'Connor, P. M., Schaad, G., Bruining, C., et al. (2011). Inhibitory activity of Lactobacillus plantarum LMG P-26358 against Listeria innocua when used as an adjunct starter in the manufacture of cheese. Microb. Cell Fact. 10, S7. doi: 10.1186/1475-2859-10-S1-S7
Mkrtchyan, H., Gibbons, S., Heidelberger, S., Zloh, M., and Limaki, H. K. (2010). Purification, characterisation and identification of acidocin LCHV, an antimicrobial peptide produced by Lactobacillus acidophilus n.v. Er 317/402 strain Narine. Int. J. Antimicrob. Agents 35, 255–260. doi: 10.1016/j.ijantimicag.2009.11.017
Moir, A., Kemp, E. H., Robinson, C., and Corfe, B. M. (1994). The genetic analysis of bacterial spore germination. J. Appl. Bacteriol. 76, 9S–16S. doi: 10.1111/j.1365-2672.1994.tb04353.x
Munakata, N. (1974). Ultraviolet sensitivity of Bacillus subtilis spores upon germination and outgrowth. J. Bacteriol. 120, 59–65.
Nakamura, K., Arakawa, K., Kawai, Y., Yasuta, N., Chujo, T., Watanabe, M., et al. (2013). Food preservative potential of gassericin A-containing concentrate prepared from a cheese whey culture supernatant from Lactobacillus gasseri LA39. Anim. Sci. J. 84, 144–149. doi: 10.1111/j.1740-0929.2012.01048.x
Nerandzic, M. M., and Donskey, C. J. (2013). Activate to eradicate: inhibition of Clostridium difficile spore outgrowth by the synergistic effects of osmotic activation and nisin. PLoS ONE 8:e54740. doi: 10.1371/journal.pone.0054740
Nicholson, W. L., Munakata, N., Horneck, G., Melosh, H. J., and Setlow, P. (2000). Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64, 548–572. doi: 10.1128/MMBR.64.3.548-572.2000
Nieto-Lozano, J. C., Reguera-Useros, J. I., Pelaez-Martinez, M. D., Sacristan-Perez-Minayo, G., Gutierrez-Fernandez, A. J., and de la Torre, A. H. (2010). The effect of the pediocin PA-1 produced by Pediococcus acidilactici against Listeria monocytogenes and Clostridium perfringens in Spanish dry-fermented sausages and frankfurters. Food Control 21, 679–685. doi: 10.1016/j.foodcont.2009.10.007
Noonpakdee, W., Santivarangkna, C., Jumriangrit, P., Sonomoto, K., and Panyim, S. (2003). Isolation of nisin-producing Lactococcus lactis WNC 20 strain from nham, a traditional Thai fermented sausage. Int. J. Food Microbiol. 81, 137–145. doi: 10.1016/S0168-1605(02)00219-2
Okkers, D. J., Dicks, L. M., Silvester, M., Joubert, J. J., and Odendaal, H. J. (1999). Characterization of pentocin TV35b, a bacteriocin-like peptide isolated from Lactobacillus pentosus with a fungistatic effect on Candida albicans. J. Appl. Microbiol. 87, 726–734. doi: 10.1046/j.1365-2672.1999.00918.x
Olguín-Araneda, V., Banawas, S., Sarker, M. R., and Paredes-Sabja, D. (2015). Recent advances in germination of Clostridium spores. Res. Microbiol. 166, 236–243. doi: 10.1016/j.resmic.2014.07.017
Oloyede, O. B., and Scholefield, J. (1994). Inhibition of Bacillus spores by combinations of heat, potassium sorbate, NaCl and pH. World J. Microbiol. Biotechnol. 10, 579–582. doi: 10.1007/BF00367672
Oppedijk, S. F., Martin, N. I., and Breukink, E. (2015). Hit ‘em where it hurts: the growing and structurally diverse family of peptides that target lipid-II. Biochim. Biophys. Acta 1858, 947–957. doi: 10.1016/j.bbamem.2015.10.024
Oshima, S., Hirano, A., Kamikado, H., Nishimura, J., Kawai, Y., and Saito, T. (2014). Nisin A extends the shelf life of high-fat chilled dairy dessert, a milk-based pudding. J. Appl. Microbiol. 116, 1218–1228. doi: 10.1111/jam.12454
Park, S. H., Itoh, K., Kikuchi, E., Niwa, H., and Fujisawa, T. (2003). Identification and characteristics of nisin Z-producing Lactococcus lactis subsp. lactis isolated from Kimchi. Curr. Microbiol. 46, 385–388. doi: 10.1007/s00284-002-3898-z
Pei, J., Yuan, Y., and Yue, T. (2013). Characterization of bacteriocin bificin C6165: a novel bacteriocin. J. Appl. Microbiol. 114, 1273–1284. doi: 10.1111/jam.12145
Pei, J., Yue, T., and Yuan, Y. (2014). Control of Alicyclobacillus acidoterrestris in fruit juices by a newly discovered bacteriocin. World J. Microbiol. Biotechnol. 30, 855–863. doi: 10.1007/s11274-013-1491-1
Piard, J. C., Delorme, F., Giraffa, G., Commissaire, J., and Desmazeaud, M. (1990). Evidence for a bacteriocin produced by lactococcus-lactis Cnrz-481. Neth. Milk Dairy J. 44, 143–158.
Piard, J. C., Kuipers, O. P., Rollema, H. S., Desmazeaud, M. J., and de Vos, W. M. (1993). Structure, organization, and expression of the lct gene for lacticin 481, a novel lantibiotic produced by Lactococcus lactis. J. Biol. Chem. 268, 16361–16368.
Pirttijärvi, T. S., Wahlström, G., Rainey, F. A., Saris, P. E., and Salkinoja-Salonen, M. S. (2001). Inhibition of bacilli in industrial starches by nisin. J. Ind. Microbiol. Biotechnol. 26, 107–114. doi: 10.1038/sj.jim.7000078
Piva, A., and Headon, D. R. (1994). Pediocin A, a bacteriocin produced by Pediococcus pentosaceus FBB61. Microbiology 140(Pt 4), 697–702. doi: 10.1099/00221287-140-4-697
Pol, I. E., Mastwijk, H. C., Bartels, P. V., and Smid, E. J. (2000). Pulsed-electric field treatment enhances the bactericidal action of nisin against Bacillus cereus. Appl. Environ. Microbiol. 66, 428–430. doi: 10.1128/AEM.66.1.428-430.2000
Pol, I. E., van Arendonk, W. G., Mastwijk, H. C., Krommer, J., Smid, E. J., and Moezelaar, R. (2001). Sensitivities of germinating spores and carvacrol-adapted vegetative cells and spores of Bacillus cereus to nisin and pulsed-electric-field treatment. Appl. Environ. Microbiol. 67, 1693–1699. doi: 10.1128/AEM.67.4.1693-1699.2001
Preston, R. A., and Douthit, H. A. (1984). Stimulation of germination of unactivated Bacillus cereus spores by ammonia. J. Gen. Microbiol. 130, 1041–1050. doi: 10.1099/00221287-130-5-1041
Ramseier, H. (1960). The influence of nisin on Cl. butyricum Prazm. Arch. Mikrobiol. 37, 57–94. doi: 10.1007/BF00414627
Rea, M., Ross, R. P., Cotter, P., and Hill, C. (2011). “Classification of bacteriocins from gram-positive bacteria,” in Prokaryotic Antimicrobial Peptides, eds D. Drider and S. Rebuffat (New York, NY: Springer), 29–53.
Rea, M. C., Sit, C. S., Clayton, E., O'Connor, P. M., Whittal, R. M., Zheng, J., et al. (2010). Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc. Natl. Acad. Sci. U.S.A. 107, 9352–9357. doi: 10.1073/pnas.0913554107
Riley, M. A., and Wertz, J. E. (2002). Bacteriocins: evolution, ecology, and application. Annu. Rev. Microbiol. 56, 117–137. doi: 10.1146/annurev.micro.56.012302.161024
Rilla, N., Martínez, B., Delgado, T., and Rodríguez, A. (2003). Inhibition of Clostridium tyrobutyricum in Vidiago cheese by Lactococcus lactis ssp. lactis IPLA 729, a nisin Z producer. Int. J. Food Microbiol. 85, 23–33. doi: 10.1016/S0168-1605(02)00478-6
Rink, R., Wierenga, J., Kuipers, A., Kluskens, L. D., Driessen, A. J., Kuipers, O. P., et al. (2007). Dissection and modulation of the four distinct activities of nisin by mutagenesis of rings A and B and by C-terminal truncation. Appl. Environ. Microbiol. 73, 5809–5816. doi: 10.1128/AEM.01104-07
Rodríguez, J. M., Martínez, M. I., and Kok, J. (2002). Pediocin PA-1, a wide-spectrum bacteriocin from lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 42, 91–121. doi: 10.1080/10408690290825475
Rollema, H. S., Kuipers, O. P., Both, P., de Vos, W. M., and Siezen, R. J. (1995). Improvement of solubility and stability of the antimicrobial peptide nisin by protein engineering. Appl. Environ. Microbiol. 61, 2873–2878.
Ruiz, S. P., Anjos, M. M., Carrara, V. S., Delima, J. N., Cortez, D. A., Nakamura, T. U., et al. (2013). Evaluation of the antibacterial activity of Piperaceae extracts and nisin on Alicyclobacillus acidoterrestris. J. Food Sci. 78, M1772–M1777. doi: 10.1111/1750-3841.12283
Rumjuankiat, K., Perez, R. H., Pilasombut, K., Keawsompong, S., Zendo, T., Sonomoto, K., et al. (2015). Purification and characterization of a novel plantaricin, KL-1Y, from Lactobacillus plantarum KL-1. World J. Microbiol. Biotechnol. 31, 983–994. doi: 10.1007/s11274-015-1851-0
Russell, A. D. (1990). Bacterial spores and chemical sporicidal agents. Clin. Microbiol. Rev. 3, 99–119.
Sahingil, D., Isleroglu, H., Yildirim, Z., Akcelik, M., and Yildirim, M. (2011). Characterization of lactococcin BZ produced by Lactococcus lactis subsp lactis BZ isolated from boza. Turk. J. Biol. 35, 21–33. doi: 10.3906/biy-0906-48
Sandiford, S. K. (2015). Perspectives on lantibiotic discovery – where have we failed and what improvements are required? Expert Opin. Drug Discov. 10, 315–320. doi: 10.1517/17460441.2015.1016496
Sawa, N., Zendo, T., Kiyofuji, J., Fujita, K., Himeno, K., Nakayama, J., et al. (2009). Identification and characterization of lactocyclicin Q, a novel cyclic bacteriocin produced by Lactococcus sp. strain QU 12. Appl. Environ. Microbiol. 75, 1552–1558. doi: 10.1128/AEM.02299-08
Setlow, P. (2014a). Germination of spores of Bacillus species: what we know and do not know. J. Bacteriol. 196, 1297–1305. doi: 10.1128/JB.01455-13
Setlow, P. (2014b). Spore resistance properties. Microbiol. Spectr. 2. doi: 10.1128/microbiolspec.tbs-0003-2012
Smoot, L. A., and Pierson, M. D. (1981). Mechanisms of sorbate inhibition of Bacillus cereus T and Clostridium botulinum 62A spore germination. Appl. Environ. Microbiol. 42, 477–483.
Soliman, W., Wang, L., Bhattacharjee, S., and Kaur, K. (2011). Structure-activity relationships of an antimicrobial peptide plantaricin s from two-peptide class IIb bacteriocins. J. Med. Chem. 54, 2399–2408. doi: 10.1021/jm101540e
Song, D. F., Zhu, M. Y., and Gu, Q. (2014). Purification and characterization of Plantaricin ZJ5, a new bacteriocin produced by Lactobacillus plantarum ZJ5. PLoS ONE 9:e105549. doi: 10.1371/journal.pone.0105549
Soria, M. C., and Audisio, M. C. (2014). Inhibition of Bacillus cereus strains by antimicrobial metabolites from Lactobacillus johnsonii CRL1647 and Enterococcus faecium SM21. Probiotics Antimicrob. Proteins 6, 208–216. doi: 10.1007/s12602-014-9169-z
Steyn, C. E., Cameron, M., and Witthuhn, R. C. (2011). Occurrence of Alicyclobacillus in the fruit processing environment–a review. Int. J. Food Microbiol. 147, 1–11. doi: 10.1016/j.ijfoodmicro.2011.03.004
Stuy, J. H. (1956). Studies on the mechanism of radiation inactivation of micro-organisms III. Inactivation of germinating spores of Bacillus cereus. Biochim. Biophys. Acta 22, 241–246. doi: 10.1016/0006-3002(56)90146-9
Suda, S., Cotter, P. D., Hill, C., and Ross, R. P. (2012). Lacticin 3147–biosynthesis, molecular analysis, immunity, bioengineering and applications. Curr. Protein Pept. Sci. 13, 193–204. doi: 10.2174/138920312800785021
Suma, K., Misra, M. C., and Varadaraj, M. C. (1998). Plantaricin LP84, a broad spectrum heat-stable bacteriocin of Lactobacillus plantarum NCIM 2084 produced in a simple glucose broth medium. Int. J. Food Microbiol. 40, 17–25. doi: 10.1016/S0168-1605(98)00010-5
Svetoch, E. A., Eruslanov, B. V., Levchuk, V. P., Perelygin, V. V., Mitsevich, E. V., Mitsevich, I. P., et al. (2011). Isolation of Lactobacillus salivarius 1077 (NRRL B-50053) and characterization of its bacteriocin, including the antimicrobial activity spectrum. Appl. Environ. Microbiol. 77, 2749–2754. doi: 10.1128/AEM.02481-10
Tan, I. S., and Ramamurthi, K. S. (2014). Spore formation in Bacillus subtilis. Environ. Microbiol. Rep. 6, 212–225. doi: 10.1111/1758-2229.12130
Thomas, L. V., Davies, E. A., Delves-Broughton, J., and Wimpenny, J. W. (1998). Synergist effect of sucrose fatty acid esters on nisin inhibition of gram-positive bacteria. J. Appl. Microbiol. 85, 1013–1022. doi: 10.1111/j.1365-2672.1998.tb05266.x
Todorov, S., Onno, B., Sorokine, O., Chobert, J. M., Ivanova, I., and Dousset, X. (1999). Detection and characterization of a novel antibacterial substance produced by Lactobacillus plantarum ST 31 isolated from sourdough. Int. J. Food Microbiol. 48, 167–177. doi: 10.1016/S0168-1605(99)00048-3
Todorov, S. D., and Dicks, L. M. (2004). Characterization of mesentericin ST99, a bacteriocin produced by Leuconostoc mesenteroides subsp. dextranicum ST99 isolated from boza. J. Ind. Microbiol. Biotechnol. 31, 323–329. doi: 10.1007/s10295-004-0153-6
Tosukhowong, A., Zendo, T., Visessanguan, W., Roytrakul, S., Pumpuang, L., Jaresitthikunchai, J., et al. (2012). Garvieacin Q, a novel class II bacteriocin from Lactococcus garvieae BCC 43578. Appl. Environ. Microbiol. 78, 1619–1623. doi: 10.1128/AEM.06891-11
Udompijitkul, P., Paredes-Sabja, D., and Sarker, M. R. (2012). Inhibitory effects of nisin against Clostridium perfringens food poisoning and nonfood-borne isolates. J. Food Sci. 77, M51–M56. doi: 10.1111/j.1750-3841.2011.02475.x
van Heel, A. J., de Jong, A., Montalbán-López, M., Kok, J., and Kuipers, O. P. (2013). BAGEL3: automated identification of genes encoding bacteriocins and (non-)bactericidal posttranslationally modified peptides. Nucleic Acids Res. 41, W448–W453. doi: 10.1093/nar/gkt391
van Reenen, C. A., Dicks, L. M., and Chikindas, M. L. (1998). Isolation, purification and partial characterization of plantaricin 423, a bacteriocin produced by Lactobacillus plantarum. J. Appl. Microbiol. 84, 1131–1137. doi: 10.1046/j.1365-2672.1998.00451.x
Vessoni, P., and Moraes, D. A. (2002). The influence of nisin on the thermal resistance of Bacillus cereus. J. Food Prot. 65, 415–418.
Vidhyasagar, V., and Jeevaratnam, K. (2013). Bacteriocin activity against various pathogens produced by Pediococcus pentosaceus VJ13 isolated from Idly batter. Biomed. Chromatogr. 27, 1497–1502. doi: 10.1002/bmc.2948
Viedma, P. M., Abriouel, H., Ben Omar, N., López, R. L., Valdivia, E., and Gálvez, A. (2009). Inactivation of Geobacillus stearothermophilus in canned food and coconut milk samples by addition of enterocin AS-48. Food Microbiol. 26, 289–293. doi: 10.1016/j.fm.2008.12.007
Wada, T., Noda, M., Kashiwabara, F., Jeon, H. J., Shirakawa, A., Yabu, H., et al. (2009). Characterization of four plasmids harboured in a Lactobacillus brevis strain encoding a novel bacteriocin, brevicin 925A, and construction of a shuttle vector for lactic acid bacteria and Escherichia coli. Microbiology 155, 1726–1737. doi: 10.1099/mic.0.022871-0
Wandling, L. R., Sheldon, B. W., and Foegeding, P. M. (1999). Nisin in milk sensitizes Bacillus spores to heat and prevents recovery of survivors. J. Food Prot. 62, 492–498.
Wang, G., Manns, D. C., Guron, G. K., Churey, J. J., and Worobo, R. W. (2014). Large-scale purification, characterization, and spore outgrowth inhibitory effect of Thurincin H, a bacteriocin produced by Bacillus thuringiensis SF361. Probiotics Antimicrob. Proteins 6, 105–113. doi: 10.1007/s12602-014-9159-1
Weber, T., Blin, K., Duddela, S., Krug, D., Kim, H. U., Bruccoleri, R., et al. (2015). antiSMASH 3.0-a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 43, W237–W243. doi: 10.1093/nar/gkv437
Wei, J., Shah, I. M., Ghosh, S., Dworkin, J., Hoover, D. G., and Setlow, P. (2010). Superdormant spores of bacillus species germinate normally with high pressure, peptidoglycan fragments, and bryostatin. J. Bacteriol. 192, 1455–1458. doi: 10.1128/JB.01497-09
Wheeldon, L. J., Worthington, T., Lambert, P. A., Hilton, A. C., Lowden, C. J., and Elliott, T. S. (2008). Antimicrobial efficacy of copper surfaces against spores and vegetative cells of Clostridium difficile: the germination theory. J. Antimicrob. Chemother. 62, 522–525. doi: 10.1093/jac/dkn219
Wiedemann, I., Böttiger, T., Bonelli, R. R., Wiese, A., Hagge, S. O., Gutsmann, T., et al. (2006). The mode of action of the lantibiotic lacticin 3147–a complex mechanism involving specific interaction of two peptides and the cell wall precursor lipid II. Mol. Microbiol. 61, 285–296. doi: 10.1111/j.1365-2958.2006.05223.x
Wiedemann, I., Breukink, E., van Kraaij, C., Kuipers, O. P., Bierbaum, G., de Kruijff, B., et al. (2001). Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J. Biol. Chem. 276, 1772–1779. doi: 10.1074/jbc.M006770200
Wijnker, J. J., Weerts, E. A., Breukink, E. J., Houben, J. H., and Lipman, L. J. (2011). Reduction of Clostridium sporogenes spore outgrowth in natural sausage casings using nisin. Food Microbiol. 28, 974–979. doi: 10.1016/j.fm.2011.01.009
Yamazaki, K., Murakami, M., Kawai, Y., Inoue, N., and Matsuda, T. (2000). Use of nisin for inhibition of Alicyclobacillus acidoterrestris in acidic drinks. Food Microbiol. 17, 315–320. doi: 10.1006/fmic.1999.0309
Yang, S. C., Lin, C. H., Sung, C. T., and Fang, J. Y. (2014). Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front. Microbiol. 5:241. doi: 10.3389/fmicb.2014.00241
Yildirim, Z., and Johnson, M. G. (1998a). Detection and characterization of a bacteriocin produced by Lactococcus lactis subsp. cremoris R isolated from radish. Lett. Appl. Microbiol. 26, 297–304.
Yildirim, Z., and Johnson, M. G. (1998b). Characterization and antimicrobial spectrum of bifidocin B, a bacteriocin produced by Bifidobacterium bifidum NCFB 1454. J. Food Prot. 61, 47–51.
Yildirim, Z., Winters, D. K., and Johnson, M. G. (1999). Purification, amino acid sequence and mode of action of bifidocin B produced by Bifidobacterium bifidum NCFB 1454. J. Appl. Microbiol. 86, 45–54. doi: 10.1046/j.1365-2672.1999.00629.x
Yin, L. J., Wu, C. W., and Jiang, S. T. (2003). Bacteriocins from Pediococcus pentosaceus L and S from pork meat. J. Agric. Food Chem. 51, 1071–1076. doi: 10.1021/jf025838f
Zendo, T., Fukao, M., Ueda, K., Higuchi, T., Nakayama, J., and Sonomoto, K. (2003). Identification of the lantibiotic nisin Q, a new natural nisin variant produced by Lactococcus lactis 61-14 isolated from a river in Japan. Biosci. Biotechnol. Biochem. 67, 1616–1619. doi: 10.1271/bbb.67.1616
Zendo, T., Nakayama, J., Fujita, K., and Sonomoto, K. (2008). Bacteriocin detection by liquid chromatography/mass spectrometry for rapid identification. J. Appl. Microbiol. 104, 499–507. doi: 10.1111/j.1365-2672.2007.03575.x
Zhu, W. M., Liu, W., and Wu, D. Q. (2000). Isolation and characterization of a new bacteriocin from Lactobacillus gasseri KT7. J. Appl. Microbiol. 88, 877–886. doi: 10.1046/j.1365-2672.2000.01027.x
Keywords: antimicrobial peptide, bacteriocin, spore, Bacillus, Clostridium, food processing, LAB
Citation: Egan K, Field D, Rea MC, Ross RP, Hill C and Cotter PD (2016) Bacteriocins: Novel Solutions to Age Old Spore-Related Problems? Front. Microbiol. 7:461. doi: 10.3389/fmicb.2016.00461
Received: 28 January 2016; Accepted: 21 March 2016;
Published: 08 April 2016.
Edited by:
Amit Kumar Tyagi, The University of Texas MD Anderson Cancer Center, USAReviewed by:
Bradley D. Jones, The University of Iowa, USAStella Maris Reginensi Rivera, Universidad de la República, Uruguay
Riadh Hammami, Laval University, Canada
Beatriz Martínez, Consejo Superior de Investigaciones Científicas, Spain
Copyright © 2016 Egan, Field, Rea, Ross, Hill and Cotter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul D. Cotter, cGF1bC5jb3R0ZXJAdGVhZ2FzYy5pZQ==
 Kevin Egan
Kevin Egan Des Field
Des Field Mary C. Rea
Mary C. Rea R. Paul Ross3,4
R. Paul Ross3,4 Colin Hill
Colin Hill Paul D. Cotter
Paul D. Cotter