- 1Veterinary Public Health, College of Veterinary Medicine, Chonbuk National University, Iksan, South Korea
- 2College of Veterinary Medicine, Gyeongsang National University, Jinju, South Korea
Live, attenuated Salmonella Typhimurium vaccine candidate expressing BCSP31, Omp3b, and SOD proteins of Brucella abortus was constructed. Thirty BALB/c mice were divided equally into three groups, Group A, were intraperitoneally (IP) inoculated with 100 μl of approximately 1.2 × 106 colony-forming units (CFUs)/ml of the Salmonella containing vector only in 100 μl as a control. And groups B and C mice were orally and IP immunized with approximately 1.2 × 109 CFU/ml of the mixture of three delivery strains in 10 μl and IP immunized with approximately 1.2 × 106 CFU/ml of the mixture in 100 μl, respectively. The serum IgG, TNF-α and IFN-γ concentrations in groups B (except Omp3b) and C were significantly higher than those in group A. Following challenge with B. abortus strain 544; challenge strain was detected <103 CFU from the spleen of all mice of group C. These results suggest that IP immunization with the mixture of the vaccine candidate can induce immune responses, and can effectively protect mice against brucellosis.
Introduction
Brucellosis is a zoonotic disease cause infections approximately 500,000 people annually worldwide (Corbel, 1997; Pappas et al., 2005, 2006; Akhvlediani et al., 2010). The disease remains endemic in many regions of the world including Latin America, Middle East, Africa, Asia, and the Mediterranean basin (Pappas et al., 2006). Brucella is predominantly transmitted to humans via direct contact with fluid discharges from infected animals, but in endemic regions people usually get infected through the consumption of unpasteurized dairy products mainly goat’s milk and fresh soft cheese made out of unpasteurized milk (Avila-Calderón et al., 2013).
Brucellosis by Brucella species is facultative intracellular bacteria. Brucella infects domestic animals, causing abortion and infertility (Godfroid et al., 2005; Avila-Calderón et al., 2013), however; it can also infect humans producing undulant fever, endocarditis, arthritis and osteomyelitis (Pappas et al., 2005). Both humoral and cell mediated immunities are necessary to change the course of infection of Brucella, but cell mediated response is crucial for clearance of Brucella from host (Schurig et al., 2002). Th1 type of immune response mediated by IFN-γ helps in clearance of Brucella infection (Zhan et al., 1993). Therefore, live and attenuated strains such as Brucella abortus strain 19, B. abortus RB51, Brucella melitensis Rev. 1 and Brucella suis strain 2 have been used to control brucellosis in domestic animals (Cheville et al., 1993; Deqiu et al., 2002; Blaco, 2010; Avila-Calderón et al., 2013; Olsen, 2013). These live and attenuated strains may contain a risk because of its potential possibility to revert virulence bacteria (Goel and Bhatnagar, 2012; Avila-Calderón et al., 2013). In addition, these strains can confuse with the diagnosis of brucellosis in serum (Olsen et al., 2009; Avila-Calderón et al., 2013; Olsen, 2013). Because of these limitations, there is necessary for the development of better vaccines which are safer to use.
Brucella can cause host infections via mucosa and then the gastrointestinal tract has been known to be one of the major entrances of infection route of brucellosis (Gorvel et al., 2009). Therefore, mucosal immunity is needed to prevent this enteric infection. Oral vaccination can be successful in inducing levels of protective secretory IgA on the intestinal surface (Melkebeek et al., 2013), however, gastric digestion of vaccines before induction of the immune system is a big barrier in the development of oral vaccines (Melkebeek et al., 2013). Live, attenuated Salmonella strains have been proposed as an adequate vector to deliver orally heterologous proteins and its use can induces protective mucosal immune responses (Branger et al., 2009; Pathangey et al., 2009; Causey et al., 2010). The essential bacterial gene [such as aspartate β-semialdehyde dehydrogenase (asd)]-based balanced-lethal host-vector system has been used to continue plasmids co-expressing inserted antigens in Salmonella Δasd mutants and to escape the use of antimicrobials as marker (Cloeckaert et al., 2002; Hur and Lee, 2011b; Olsen, 2013).
Recombinant proteins are a valuable choice for vaccine and can induce antigen specific immune response (Zhao et al., 2009; Goel and Bhatnagar, 2012). An extensively purified protein from Strain 19, the cell surface 31-kilodalton (kDa) protein (or BCSP31), can serve as a protective subunit vaccine in rodents (Tabatabai and Deyoe, 1983; Tabatabai et al., 1989). It has reported that outer membrane proteins (Omps) can be protective antigens of the Brucella species (Verstreate et al., 1982). Omp3b, also known as Omp22, belongs to group 3 of the Brucella Omps (Vizcaino et al., 2004; Garcia-Yoldi et al., 2005), a highly conserved family that includes the most immunogenic Brucella proteins (Cloeckaert et al., 2002). The sodC gene encodes a Cu/Zn superoxide dismutase (SOD; Bricker et al., 1990; Sriranganathan et al., 1991). The SOD could function as virulence factor, since it clears harmful oxy-radicals following phagocytosis by macrophages (Tabatabai and Pugh, 1994).
The objective of this study was to evaluate immune responses against B. abortus BCSC31, Omp3b and SOD antigens expressing by live attenuated Salmonella in a murine model. After Salmonella delivery strains with BCSP31, Omp3b, and SOD antigens of B. abortus was constructed, mice were immunized by various inoculation routes with the delivery strains. Immune responses induced via intraperitoneal inoculation with the delivery strains were examined in mice. In addition, we evaluated the efficacy of the delivery strains for protection against experimental brucellosis in mice.
Materials and Methods
Bacterial Strains and Plasmids
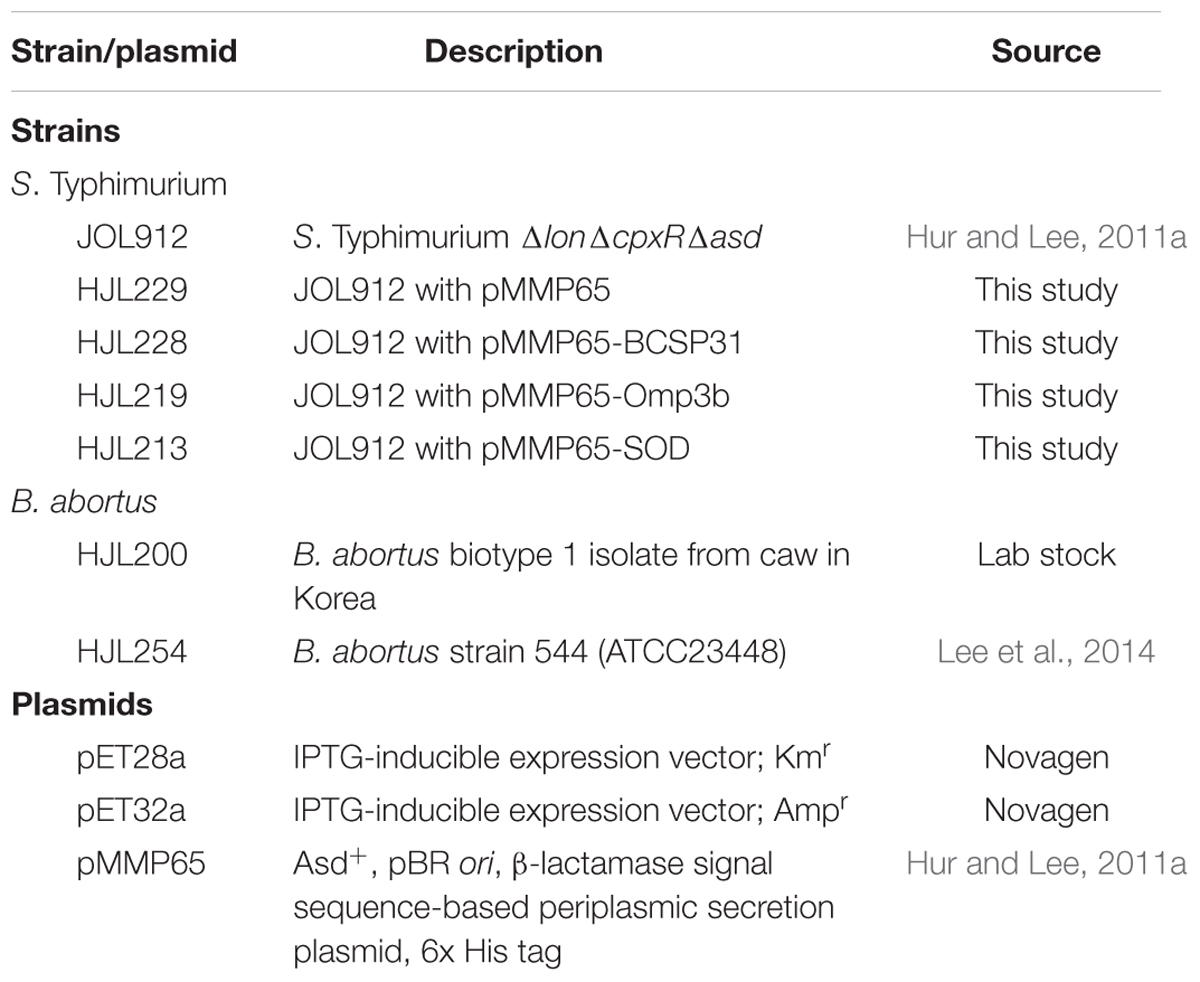
The bacterial strains and plasmids used in present study are listed in Table 1. Wild-type B. abortus biotype 1 isolated from cattle was used to amplify gene encoding BCSP31, Omp3b, and SOD antigens (Table 1). B. abortus strain 544 (strain 544) was used as the virulent challenge strain (Lee et al., 2014). The B. abortus biotype 1 isolate was kindly supplied by the National Veterinary Research and Quarantine Service, Korea. The attenuated Salmonella Typhimurium (Δlon ΔcpxR Δasd) mutant strain, JOL912 (Hur and Lee, 2011a) was used as a host for delivery of individual antigens. The pMMP65 plasmid was used as a vector for the expression/secretion of heterologous antigens in the delivery host (Hur and Lee, 2011a). B. abortus isolate and strain 544 were grown in Brucella agar (Becton, Dickinson and Company, Sparks, MD, USA). JOL912 was cultured according to the method in previous studies (Kang et al., 2002; Hur and Lee, 2011a,b).
Cloning for Recombinant BCSP31, Omp3b, and SOD Proteins
BCSP31, Omp3b, and SOD proteins were prepared from HJL206, HJL204, and HJL208, respectively (Table 1), and were used as coating antigens in enzyme-linked immunosorbent assay (ELISA). In addition, these antigens were used as splenocyte stimulating antigens for cytokines concentration measurement. Briefly, genes for BCSP31, Omp3b, and SOD proteins were amplified by polymerase chain reaction (PCR) using specific primer pairs described in Table 2. The amplified PCR fragments for each gene were digested with restriction enzymes. Subsequently, the digested fragments were inserted into commercial expression vectors such as pET28a or pET32a. And then these plasmids were transformed into Escherichia coli BL21 in order to create HJL206, HJL204, and HJL208 strains. The recombinant BCSP31, Omp3b, and SOD proteins were prepared using an affinity purification process with nickel-nitrilotriacetic acid-agarose (Qiagen, Valencia, CA, USA) from HJL206, HJL204, and HJL208 respectively. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used for the confirmation of the integrity of purified antigens. All purified antigens were stored at -70°C until use.
Preparation of Salmonella Delivery Strains
The delivery strains were prepared as previously described with a slight modification (Hur and Lee, 2011a). The genes for BCSP31, Omp3b, and SOD proteins were prepared by digestion with restriction enzymes from HJL206, HJL204, and HJL208 strains, respectively. And then each gene was inserted in pMMP65 for the construction of vaccine strains. Subsequently, these plasmids were electroporated into JOL912 to create HJL228 for BCSP31, HJL219 for Omp3b and HJL213 for SOD.
Western Blot Analysis
Western blot analysis was carried out to check the secretions of the individual BCSP31, Omp3b, and SOD proteins from HJL228, HJL219, and HJL213, respectively, using the modified method mentioned in previous study (Hur and Lee, 2011a). Briefly, the proteins in supernatant of culture were separated by 12% SDS-PAGE gel and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The individual proteins were reacted with anti-His antibodies (Invitrogen, Grand Island, NY, USA) and horseradish peroxidase-conjugated rabbit anti-mouse IgG antibodies (Southern Biotech., Birmingham, AL, USA). Immunoreactive bands were detected by addition of the AmershamTM ECL Prime Western Blotting Detection Reagent (GE Healthcare, Little Chalfont, Buckinghamshire, UK) and the CheBi illumination system (Neo science, Suwon, Gyeonggi, South Korea).
Immunization of Mice and Sample Collection
Thirty 5-weeks-old female BALB/c mice were equally distributed into three groups (n = 10 mice per group). All mice were immunized at 6-weeks-old. Group A mice were intraperitoneally (IP) inoculated with approximately 1.2 × 106 colony-forming units (CFUs)/ml of the Salmonella containing vector only (JHL229) in 100 μl as a control. Group B mice were orally immunized with approximately 1.2 × 109 CFU/ml of the mixture of the three delivery strains in 10 μl. And group C mice were IP immunized with approximately 1.2 × 106 CFU/ml of the mixture of the three delivery strains in 100 μl. Blood samples were collected before immunization [0 week post-immunization (WPI)] and again at the third WPI for the evaluation of serum IgG. All serum samples were stored at -20°C until use. The animal experiments mentioned in this study were performed under ethics approval (CBU 2015-052) from the Chonbuk National University Animal Ethics Committee in accordance with the guidelines of the Korean Council on Animal Care.
Immune Response Measurement by Enzyme-Linked Immunosorbent Assay (ELISA)
A standard ELISA was carried out to evaluate the immune response against BCSP31, Omp3b, and SOD antigens in serum samples of mice according to the modified method of the previous study (Hur and Lee, 2011b). Results of the ELISA are expressed as the mean optical density (OD) ± standard deviation.
Cytokine Quantitation of Splenocytes
At 3 WPI, five mice from each group were sacrificed and spleens were aseptically removed. Splenocytes were prepared according to the method described in previous study (Adone et al., 2012). Splenocytes were distributed in 24 well tissue culture plates with 2 × 106 cells per well (Velikovsky et al., 2003; Cloeckaert et al., 2004). Splenocytes were stimulated in vitro with each BCSP31, Omp3b, and SODC antigen (4 μg/well), Concavalin A (0.5 μg/well) as positive control or media as unstimulated control, and incubated at 37°C, 5% CO2 and 95% humidity (Adone et al., 2012). The supernatants of reaction were collected after 72 h of re-stimulation and used for cytokine measurement (Adone et al., 2012).
Cytokines Measurement by ELISA
The concentration of cytokines such as IFN-γ and TNF-α in the supernatants was evaluated by the mouse cytokine ELISA Ready-SET-GO reagent set according to the instructions of manufacturer (eBioscience, Inc., San Diego, CA, USA).
Challenge Experiments
For challenge experiments, the challenge strain, strain 544 was prepared. Briefly, the strain was cultured in Brucella broth at 37°C for 24 h. and resuspended to approximately 1 × 105 CFU/ml. All mice were IP challenged at 3 WPI with 100 μl of the challenge strain. The spleen weights from all mice were measured and were diluted as 1:100 using Brucella broth. A total of 100 μl of the diluted media was spread on blood agar to count the number of viable strain 544 from the spleens at 2 weeks after the challenge. If no colony detected on the blood agar, means the number of viable challenge strains from the spleen of mice is <103 CFU. The vaccine and challenge strains were confirmed by PCR using B. abortus-specific primer (GAC GAA CGG AAT TTT TCC AAT CCC), RB51/2308 primer (CCC CGG AAG ATA TGC TTC GAT CC) and IS711-specific primer (TGC CGA TCA CTT AAG GGC CTT CAT) in enhanced Brucella AMOS PCR primers (Bricker and Halling, 1994, 1995).
Statistical Analysis
The number of viable strain 544 from the spleens were normalized by log transformation and estimated by one-way analysis of variance, followed by Tukey’s Multiple Comparison Test using GraphPad program (InStat; GraphPad, La Jolla, CA, USA). The antibody and cellular immune responses were compared between the groups by Kruskal–Wallis test and one-way analysis of variance, respectively using SPSS version 16.0 software (SPSS, Chicago, IL, USA).
Results
Secretion of Recombinant BCSP31, Omp3b, and SOD Antigens from Vaccine Candidates
To express the recombinant BCSP31, Omp3b, and SOD proteins from Salmonella, the proteins encoding genes were individually cloned into pMMP65 and were transformed into a ΔlonΔcpxRΔasd S. Typhimurium strain. Western blot analysis was carried out to confirm the secretion of BCSP31, Omp3b and SOD proteins from the culture supernatants of the constructs. The expected sizes, 31 kDa for BCSP31, 22 kDa for Omp3b and 19 kDa for SOD, were observed from the precipitated supernatants of culture the individual constructs (Figure 1).
FIGURE 1. Identification of recombinant BCSP31, Omp3b, and SOD proteins secreted from attenuated Salmonella Typhimurium delivery system using Western blot analysis. Lanes: BCSP31, recombinant BCSP31 antigen secreted by JHL228; OMP3b, recombinant Omp3b antigen secreted by JHL219; and SOD, recombinant SOD antigen secreted by HJL213.
Humoral Immune Responses of the Vaccinated Mice
Antibody responses against each antigen in the serum samples are presented in (Figure 2). Serum IgG titers against all antigens in groups B (except Omp3b) and C were significantly increased compared to those of control group (P ≤ 0.05). In addition, serum IgG titers against all antigens in group C than group B were significantly higher (P ≤ 0.05).
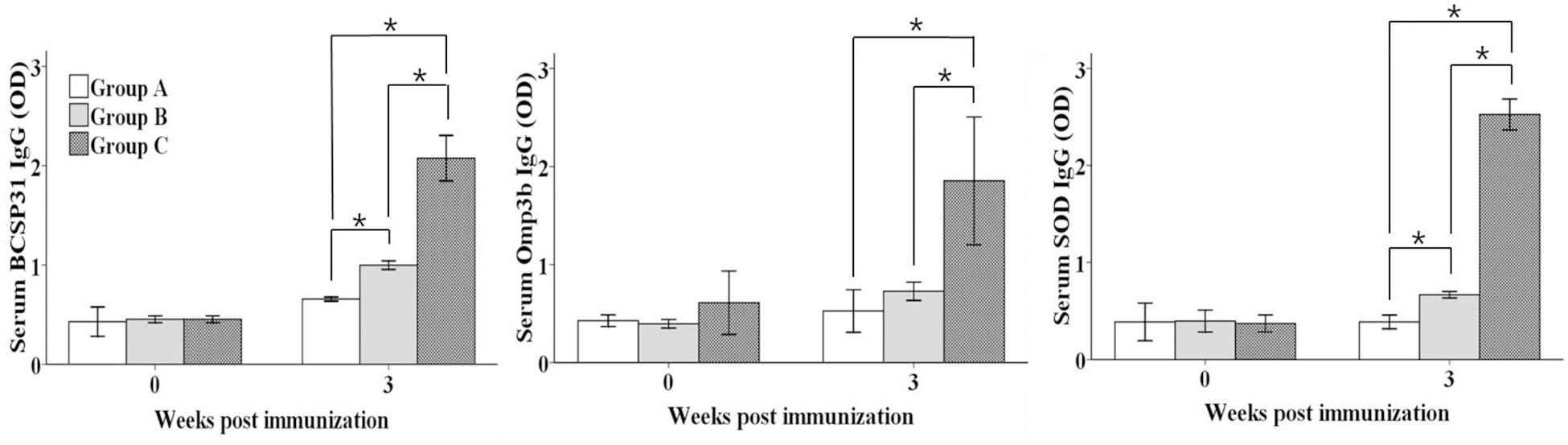
FIGURE 2. Serum IgG titers against BCSP31, Omp3b, and SOD antigens. Group A mice were intraperitoneally (IP) inoculated with approximately 1.2 × 106 CFU/ml of the Salmonella containing vector only in 100 μl as a control, group B mice were orally immunized with approximately 1.2 × 109 CFU/ml of the mixture of the three delivery strains in 10 μl and group C mice were IP inoculated approximately 1.2 × 106 CFU/ml of the mixture of the three strains in 100 μl. Data are the mean optical density (OD) of all mice in each group, and error bars show the standard deviations (SD). Asterisks indicate a significant difference between the titers of the immunized group and those of the control group (*P < 0.05).
Cytokine Analysis
The TNF-α and IFN-γ concentrations against BCSP31, Omp3b, and SOD antigens in splenocytes re-stimulated with each antigen of mice were measured using ELISA on 3 WPI. The levels of TNF-α and IFN-γ (except Omp3b in group B) were significantly elevated in the groups B and C than group A (P < 0.05; Figure 3).
FIGURE 3. Cytokine concentrations in the splenocytes at 3 weeks post-immunization. Groups A–C refer to Figure 2. Data are the mean of all mice in each group; error bars show SD. Asterisks indicate a significant difference between the values of each group mice (*P < 0.05).
Protection against Challenge
All mice were IP challenged with approximately 1 × 104 CFU of the challenge strain at 3 WPI. The level of protection was evaluated by the number of viable strain 544 from the spleens at 14 days after the challenge. As shown in (Table 3), groups B and C mice induced a significantly higher degree of protection than group A. In addition, a significantly higher degree of protection was observed in group C mice than group B mice. Furthermore, among five mice of groups A and B, the challenge strain was isolated from all mice. However, colony was not detected on blood agars spread with broth diluting the spleens of all mice of group C. It means that the number of viable strain 544 from the spleen of group C mice is <103 CFU.
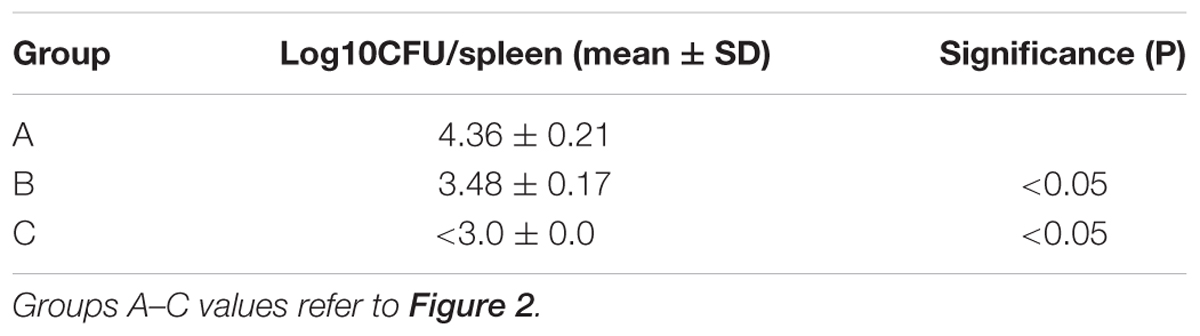
TABLE 3. Bacterial proliferations in spleen of mice challenged with a wild-type B. abortus strain 544.
Discussion
The small intestine is a major site of nutrient digestion and absorption (Moreto and Perez-Bosque, 2009). In addition, the intestinal epithelium plays a crucial role in the prevention of pathogenic microorganisms entering the interior space of animals (Moreto and Perez-Bosque, 2009). B. abortus can invade hosts through mucosa and is a facultative intracellular bacterium that survives inside phagocytes by escaping the endocytic pathway (Godfroid et al., 2005; Baldwin and Goenka, 2006; Avila-Calderón et al., 2013). The gastrointestinal tract has been known to be one of the major portals of B. abortus infection (Gorvel et al., 2009). Therefore, the host resistance to Brucella infections depends mainly on mucosal immunity and cell-mediated immunity (Baldwin and Parent, 2002; Paranavitana et al., 2005). Live attenuated Brucella vaccines that can stimulate strong mucosal and cell-mediated immune responses are usually very effective against brucellosis (Bricker and Halling, 1994; Murphy et al., 2001; Baldwin and Goenka, 2006). However, live vaccines induce antibodies that confuse the diagnosis of field infection in vaccinated animals (Olsen et al., 2009; Avila-Calderón et al., 2013; Olsen, 2013), which further hinder brucellosis eradication (Velikovsky et al., 2003; Cloeckaert et al., 2004; Izadjoo et al., 2004). In addition, vaccination of pregnant cows with some live vaccine may induce low level abortion or premature birth. Thus it is recommended to be used with caution in pregnant cattle (Olsen et al., 2009; Lin and He, 2012; Avila-Calderón et al., 2013).
In previous studies (Chen and Schifferli, 2003; Hur and Lee, 2011a; Hur et al., 2011, 2014) live attenuatted Salmonella, a facultative intracellular bacterium, has been known to induce a strong mucosal immune response as well as cell-mediated immune response. In addition, live attenuated Salmonella, as a vector to transfer heterologous proteins, has many benefits such as delivering the protein to the host, and easy preparation and handy inoculation (Chen and Schifferli, 2003; Hur et al., 2011). Especially, oral immunization with live attenuated Salmonella vaccine expressing the recombinant rBL protein induced significant protective effect, although this protection was lower than that by the inoculation with the pBL DNA vaccine (Zhao et al., 2009). Major Omps of Brucella species have been known to be immunogenic antigens for effective protection against brucellosis (Cloeckaert et al., 2002). In present study, we conducted a new Brucella vaccine candidate, attenuated Salmonella strain expressing Omps of Brucella. We analyzed the immunogenicity and protective efficacy of the vaccine strains against virulent Brucella infection in BALB/c mice immunized via various routes with the candidate. BALB/c mouse has been known to be a proven animal model to study the protection of Brucella vaccine candidates (Jain et al., 2014).
In the present study, the attenuated S. Typhimurium Δlon ΔcpxR Δasd strain and the pBP65 plasmid encoding the asd gene (Hur et al., 2011) was used as a delivery system to deliver BCSP31, Omp3b and SOD proteins of B. abortus. Secretion of each antigen from the vaccine candidate was demonstrated by Western blot analysis. This results indicated that the plasmids carrying the genes for BCSP31, Omp3b, and SOD proteins were stably maintained in the vaccine candidates, and that the recombinant BCSP31, Omp3b, and SOD proteins were effectively expressed and secreted. This combination of three S. Typhimurium strains expressing each other protein were determined from preliminary experiments to determine optimal union among various combinations using S. Typhimurium strains expressing each protein, such as BCSP31, Omp3b, Omp10, Omp25, SOD. Two different immunization routes of mice, such as oral (group B) and IP (group C), were used to evaluate the immunogenicity and limitation of colonization of B. abortus strain 544 in spleen. The mice of groups B (except Omp3b) and C induced significant higher amounts of serum IgG titers to BCSP31, Omp3b, and SOD antigens than mice of control group A. These results show that immunization with the vaccine candidate irrespective of inoculation routes can induce systemic immune response.
Brucella abortus is a facultative intracellular bacterium. Therefore, cell-mediated immune response is necessary for clearance of the bacteria (Baldwin and Parent, 2002; Paranavitana et al., 2005). Especially, Th1 type immune response is considered as necessary to protect completely brucellosis (Zhan et al., 1996; Murphy et al., 2001; Baldwin and Goenka, 2006). The role of TNF-α to limit colonization of B. abortus in spleen of mice has been known to be critical for activation of macrophages to kill B. abortus in the absence of IFN- γ (Zhan et al., 1996; Murphy et al., 2001). IFN- γ plays a critical role in the clearance of B. abortus by its ability to activate antibacterial functions of infected macrophages (Paranavitana et al., 2005; Baldwin and Goenka, 2006). We studied the cell mediated immune response generated by various immunization routes with the vaccine candidates expressing BSCP31, Omp3b, and SOD antigens of B. abortus. TNF-α and IFN-γ concentrations were evaluated from the supernatants of the re-stimulated splenocytes following restimulation of splenocytes with heat-inactivated B. abortus whole antigens of mice immunized with the vaccine candidates. TNF-α and IFN-γ (except Omp3b in group B) concentrations were significantly higher in groups B and C mice than in group A mice. These results indicate that immunization with the conducts irrespective of inoculation routes may induce strong Th1 type immune responses. Furthermore, significantly high levels of IFN-γ as well as TNF-α in splenocyte culture supernatants relative to group B could be observed in group C. It seems logical to conclude that TNF-α and IFN- γ produced by IP immunization with the candidate can have been associated with the increased protection.
The lack of well-established correlation of protection against Brucella is a critical difficulty of a novel vaccine candidate (Zhao et al., 2009). In previous study, oral immunization group with live attenuated Salmonella vaccine expressing the recombinant fusion protein rBL induced significant protection than control group after oral inoculation with B. abortus strain 544 (Zhao et al., 2009). However, the results only showed that the mice immunized with live attenuated Salmonella vaccine expressing the recombinant fusion protein could decreased the number of colonization of Brucella in spleen (Zhao et al., 2009). Similarly, groups B and C mice relative to group A mice significantly limited colonization of B. abortus strain 544 in spleen. These results show that immunization with the candidates irrespective of inoculation routes can protect mice from virulent B. abortus strain 544 infection. Furthermore, in this study, group C mice showed the best protection against virulent B. abortus strain 544 infection. The reason could be that IP immunization with the candidate coffered large amounts of antigens into antigen-presenting cells, which induced the strongest mucosal and cell-mediated immune responses.
Conclusion
The results of this study show that immunization with the Salmonella delivery system based Brucella vaccine induces robust mucosal and cell mediated immune response in mice. Immunization with the vaccine candidate irrespective of inoculation routes confers protection against B. abortus infection. Furthermore, in this study, mice IP immunized with the vaccine candidate showed the best protection against B. abortus strain 544 infections. On theses bases, we believe that IP immunization with the vaccine candidate can effectively protect brucellosis. In addition, these results imply that combination of live, attenuated Salmonella Typhi strains expressing each antigen such as BCSP31, Omp3b, and SOD, may attempt to study as vaccine candidate against human brucellosis, if the attenuated strains should prove safe use in human.
Author Contributions
WK: performed all the tests in this study and wrote this manuscript; JM: assisted the tests and drew the figures; SK: controlled mice; JH: managed this study and wrote this manuscript with WK.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MISP; No. 2013R1A4A1069486).
References
Adone, R., Francia, M., Pistoia, C., Petrucci, P., Pesciaroli, M., and Pasquali, P. (2012). Protective role of antibodies induced by Brucella melitensis B115 against B. melitensis and Brucella abortus infections in mice. Vaccine 30, 3992–3995. doi: 10.1016/j.vaccine.2012.04.009
Akhvlediani, T., Clark, D. V., Chubabria, G., Zenaishvili, O., and Hepburn, M. J. (2010). The changing pattern of human brucellosis: clinical manifestations, epidemiology, and treatment outcomes over three decades in Georgia. MBC Infect. Dis. 10:346. doi: 10.1186/1471-2334-10-346
Avila-Calderón, E. D., Lopez-Merino, A., Sriranganathan, N., Boyle, S. M., and Contreras- Rodriguez, A. (2013). A history of the development of Brucella vaccines. Biol. Med. Res. Int. 2013:743509. doi: 10.1155/2013/743509
Baldwin, C. L., and Goenka, R. (2006). Host immune responses to the intracellular bacteria Brucella: does the bacteria instruct the host to facilitate chronic infection? Crit. Rev. Immunol. 26, 407–442. doi: 10.1615/CritRevImmunol.v26.i5.30
Baldwin, C. L., and Parent, M. (2002). Fundamentals of host immune response against Brucella abortus: what the mouse model has revealed about control of infection. Vet. Microbiol. 90, 367–382. doi: 10.1016/S0378-1135(02)00222-5
Blaco, J. M. (2010). Control and eradication strategies for Brucella melitensis infection in sheep and goat. Prilozi 31, 145–165.
Branger, C. G., Torres-Escobar, A., Sun, W., Perry, R., Fetherston, J., Roland, K. L., et al. (2009). Oral vaccination with LcrV from Yersinia pestis KIM delivered by live attenuated Salmonella enterica serovar Typhimurium elicits a protective immune response against challenge with Yersinia pseudotuberculosis and Yersinia enterocolitica. Vaccine 27, 5363–5370. doi: 10.1016/j.vaccine.2009.06.078
Bricker, B., and Halling, S. M. (1994). Differentiation of Brucella abortus bv. 1, 2, and 4, Brucella melitensis, Brucella ovis, and Brucella suis bv. 1 by PCR. J. Clin. Microbiol. 32, 2660–2666.
Bricker, B., and Halling, S. M. (1995). Enhancement of the Brucella AMOS PCR assay for differentiation of Brucella aborus vaccine strains S19 and RB51. J. Clin. Microbiol. 33, 1640–1642.
Bricker, B. J., Tabatabai, L. B., Judge, B. A., Deyoe, B. L., and Mayfield, J. E. (1990). Cloning, expression, and occurrence of the Brucella Cu-Zn superoxide dismutase. Infect. Immun. 58, 2935–2939.
Causey, R. C., Artiushin, S. C., Crowley, I. F., Weber, J. A., Homola, A. D., Kelley, A., et al. (2010). Immunisation of the equine uterus against Streptococcus equi subspecies zooepidemicus using an intranasal attenuated Salmonella vector. Vet. J. 184, 156–161. doi: 10.1016/j.tvjl.2009.05.001
Chen, H., and Schifferli, D. M. (2003). Construction, characterization, and immunogenicity of an attenuated Salmonella enterica serovar typhimurium pgtE vaccine expressing fimbriae with integrated viral epitopes from the spiC promoter. Infect. Immun. 71, 4664–4673. doi: 10.1128/IAI.71.8.4664-4673.2003
Cheville, N. F., Stevens, M. G., Jensen, A. E., Tatum, F. M., and Halling, S. M. (1993). Immune responses and protection against infection and abortion in cattle experimentally vaccinated with mutant strains of B. abortus. Am. J. Vet. Res. 54, 1591–1597.
Cloeckaert, A., Jacques, I., Grillo, M. J., Marin, C. M., Grayon, M., Blasco, J. M., et al. (2004). Development and evaluation as vaccines in mice of Brucella melitensis Rev.1 single and double deletion mutants of the bp26 and omp31 genes coding for antigens of diagnostic significance in ovine brucellosis. Vaccine 22, 2827–2835. doi: 10.1016/j.vaccine.2004.01.001
Cloeckaert, A., Vizcaino, N., Paquet, J. Y., Bowden, R. A., and Elzer, P. H. (2002). Major outer membrane proteins of Brucella spp.: past, present and future. Vet. Microbiol. 90, 229–247. doi: 10.1016/S0378-1135(02)00211-0
Corbel, M. J. (1997). Brucellosis: an overview. Emerg. Infect. Dis. 3, 213–221. doi: 10.3201/eid0302.970219
Deqiu, S., Donglou, X., and Jiming, Y. (2002). Epidemiology and control of brucellosis in China. Vet. Microbiol. 90, 168–182. doi: 10.1016/S0378-1135(02)00252-3
Garcia-Yoldi, D., Marin, C. M., and Lopez-Goni, I. (2005). Restriction site polymorphisms in the genes encoding new members of group 3 outer membrane protein family of Brucella spp. FEMS. Microbiol. Lett. 245, 79–84. doi: 10.1016/j.femsle.2005.02.026
Godfroid, J., Cloeckaert, A., Liautard, J. P., Kohler, S., Fretin, D., Walravens, K., et al. (2005). From the discovery of the Malta fever’s agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet. Res. 36, 313–326. doi: 10.1051/vetres:2005003
Goel, D., and Bhatnagar, R. (2012). Intradermal immunization with outer membrane protein 25 protects Balb/c mice from virulent B. abortus 544. Mol. Immunol. 51, 159–168. doi: 10.1016/j.molimm.2012.02.126
Gorvel, J. P., Moreno, E., and Moriyon, I. (2009). Is Brucella an enteric pathogen? Nat. Rev. Microbiol. 7:250. doi: 10.1038/nrmicro2012-c2
Hur, J., Byeon, H., and Lee, J. H. (2014). Immunologic study and optimization of Salmonella delivery strains expressing adhesin and toxin antigens for protection against progressive atrophic rhinitis in a murine model. Can. J. Vet. Res. 78, 297–303.
Hur, J., Kim, M. Y., and Lee, J. H. (2011). Evaluation of efficacy of a new live Salmonella Typhimurium vaccine candidate in a murine model. Comp. Immun. Microbiol. Infect. Dis. 34, 171–177. doi: 10.1016/j.cimid.2010.11.001
Hur, J., and Lee, J. H. (2011a). Enhancement of immune responses by an attenuated Salmonella enterica serovar Typhimurium strain secreting an Escherichia coli heat-labile enterotoxin B subunit protein as an adjuvant for a live Salmonella vaccine candidate. Clin. Vaccine Immunol. 18, 203–209. doi: 10.1128/CVI.00407-10
Hur, J., and Lee, J. H. (2011b). Immune responses to new vaccine candidates constructed by a live attenuated Salmonella Typhimurium delivery system expressing Escherichia coli F4, F5, F6, F41 and intimin adhesin antigens in a murine model. J. Vet. Med. Sci. 73, 1265–1273. doi: 10.1292/jvms.11-0087
Izadjoo, M. J., Bhattacharjee, A. K., Paranavitana, C. M., Hadfield, T. L., and Hoover, D. L. (2004). Oral vaccination with Brucella melitensis WR201 protects mice against intranasal challenge with virulent Brucella melitensis 16M. Infect. Immun. 72, 4031–4039. doi: 10.1128/IAI.72.7.4031-4039.2004
Jain, S., Afley, P., Dohre, S. K., Saxena, N., and Kumar, S. (2014). Evaluation of immunogenicity and protective efficacy of a plasmid DNA vaccine encoding ribosomal protein L9 of Brucella abortus in BALB/c mice. Vaccine 32, 4537–4542. doi: 10.1016/j.vaccine.2014.06.012
Kang, H. Y., Srinivasan, J., and Curtiss, R. III (2002). Immune responses to recombinant pneumococcal PspA antigen delivered by live attenuated Salmonella enterica serovar Typhimurium vaccine. Infect. Immun. 70, 1739–1749. doi: 10.1128/IAI.70.4.1739-1749.2002
Lee, J. J., Lim, J. J., Kim, D. G., Simborio, H. L., Kim, D. H., Reyes, A. W., et al. (2014). Characterization of culture supernatant proteins from Brucella abortus and its protection effects against murine brucellosis. Comp. Immun. Microbiol. Infect. Dis. 37, 221–228. doi: 10.1016/j.cimid.2014.06.001
Lin, Y., and He, Y. (2012). Ontology representation and analysis of vaccine formulation and administration and their effects on vaccine immune responses. J. Biomed. Semantics. 3:17. doi: 10.1186/2041-1480-3-17
Melkebeek, V., Goddeeris, B. M., and Cox, E. (2013). ETEC vaccination in pigs. Vet. Immun. Immunopathol. 152, 37–42. doi: 10.1016/j.vetimm.2012.09.024
Moreto, M., and Perez-Bosque, A. (2009). Dietary plasma proteins, the intestinalimmune system, and the barrier functions of the intestinal mucosa. J. Anim. Sci. 87, E92–E100. doi: 10.2527/jas.2008-1381
Murphy, E. A., Sathiyaseelan, J., Parent, M. A., Zou, B., and Baldwin, C. L. (2001). Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103, 511–518. doi: 10.1046/j.1365-2567.2001.01258.x
Olsen, S. C. (2013). Recent developments in livestock and wildlife brucellosis vaccination. Rev. Sci. Technol. 32, 207–217.
Olsen, S. C., Boyle, S. M., Schurig, G. G., and Sriranganathan, N. N. (2009). Immune responses and protection against experimental challenge after vaccination of bison with Brucella abortus strain RB51 or RB51 overexpressing superoxide dismutase and glycosyltransferase genes. Clin. Vaccine. Immun. 16, 535–540. doi: 10.1128/CVI.00419-08
Pappas, G., Akritidis, N., Bosilkovski, M., and Tsianos, E. (2005). Brucellosis. New. Engl. J. Med. 352, 2325–2336. doi: 10.1056/NEJMra050570
Pappas, G., Papadimitriou, P., Akritidis, N., Christou, L., and Tsianos, E. V. (2006). The new global map of human brucellosis. Lancet. Infect. Dis. 6, 91–99. doi: 10.1016/S1473-3099(06)70382-6
Paranavitana, C., Zelazowska, E., Izadjoo, M., and Hoover, D. (2005). Interferon-gamma associated cytokines and chemokines produced by spleen cells from Brucella-immunemice. Cytokine 30, 86–92. doi: 10.1016/j.cyto.2004.12.009
Pathangey, L., Kohler, J. J., Isoda, R., and Brown, T. A. (2009). Effect of expression level on immune responses to recombinant oral Salmonella enterica serovar Typhimurium vaccines. Vaccine 27, 2707–2711. doi: 10.1016/j.vaccine.2009.02.072
Schurig, G. G., Sriranganathan, N., and Corbel, M. J. (2002). Brucellosis vaccines: past, present and future. Vet. Microbiol. 90, 479–496. doi: 10.1016/S0378-1135(02)00255-9
Sriranganathan, N., Boyle, S. M., Schurig, G., and Misra, H. (1991). Superoxide dismutases of virulent and avirulent strains of Brucella abortus. Vet. Microbiol. 26, 359–366. doi: 10.1016/0378-1135(91)90029-F
Tabatabai, L. B., and Deyoe, B. L. (1983). Isolation of two biologically active cell surface proteins from Brucella abortus by chromatofocusing. Fed. Proc. 42:2127.
Tabatabai, L. B., Deyoe, B. L., and Patterson, J. M. (1989). Immunogenicity of Brucella abortus salt- extractable proteins. Vet. Microbiol. 20, 49–58. doi: 10.1016/0378-1135(89)90006-0
Tabatabai, L. B., and Pugh, G. W. (1994). Modulation of immune responses in Balb/c mice vaccinated with Brucella abortus Cu-Zn superoxide dismutase synthetic peptide vaccine. Vaccine 12, 919–924. doi: 10.1016/0264-410X(94)90035-3
Velikovsky, C. A., Goldbaum, F. A., Cassataro, J., Estein, S., Bowden, R. A., Bruno, L., et al. (2003). Brucella lumazine synthase elicits a mixed Th1-Th2 immune response and reduces infection in mice challenged with Brucella abortus 544 independently of the adjuvant formulation used. Infect. Immun. 71, 5750–5755. doi: 10.1128/IAI.71.10.5750-5755.2003
Verstreate, D. R., Creasy, M. T., Caveney, N. T., Baldwin, C. L., Blab, M. W., and Winter, A. J. (1982). Outer membrane proteins of Brucella abortus: isolation and characterization. Infect. Immun. 35, 979–989.
Vizcaino, N., Caro-Hernandez, P., Cloeckaert, A., and Fernandez-Lago, L. (2004). DNA polymorphism in the omp25/omp31 family of Brucella spp.: identification of a 1.7-kb inversion in Brucella cetaceae and of a 15.1-kb genomic island, absent from Brucella ovis, related to the synthesis of smooth lipopolysaccharide. Microbes Infect. 6, 821–834. doi: 10.1016/j.micinf.2004.04.009
Zhan, Y., Kelso, A., and Cheers, C. (1993). Cytokine production in the murine response to Brucella infection or immunization with antigenic extracts. Immunology 80, 458–464.
Zhan, Y., Liu, Z., and Cheers, C. (1996). Tumor necrosis factor alpha and interleukin-12 Contribute to resistance to the intracellular bacterium Brucella abortus by different mechanisms. Infect. Immun. 64, 2782–2786.
Keywords: Brucella abortus, Salmonella Typhimurium delivery system, brucellosis, vaccine
Citation: Kim WK, Moon JY, Kim S and Hur J (2016) Comparison between Immunization Routes of Live Attenuated Salmonella Typhimurium Strains Expressing BCSP31, Omp3b, and SOD of Brucella abortus in Murine Model. Front. Microbiol. 7:550. doi: 10.3389/fmicb.2016.00550
Received: 08 January 2016; Accepted: 04 April 2016;
Published: 20 April 2016.
Edited by:
Johnan A. R. Kaleeba, Uniformed Services University of the Health Sciences, USAReviewed by:
Yongqun “Oliver” He, University of Michigan, USAHari Mohan Saxena, Guru Angad Dev Veterinary and Animal Sciences University, India
Thomas A. Ficht, Texas A&M University, USA
Copyright © 2016 Kim, Moon, Kim and Hur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Hur, aHVyamluQGpibnUuYWMua3I=
 Won K. Kim1
Won K. Kim1 Suk Kim
Suk Kim Jin Hur
Jin Hur