- 1Laboratório de Glicobiologia de Eucariotos, Instituto de Microbiologia Professor Paulo de Góes, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil
- 2Department of Molecular Genetics and Microbiology, Stony Brook University, Stony Brook, NY, USA
- 3Veterans Administration Medical Center, Northport, NY, USA
- 4Department of Microbiology and Immunology and Medicine, Albert Einstein College of Medicine, Bronx, NY, USA
- 5Fundação Oswaldo Cruz, Centro de Desenvolvimento Tecnológico em Saúde, Rio de Janeiro, Brazil
Classic cell wall components of fungi comprise the polysaccharides glucans and chitin, in association with glycoproteins and pigments. During the last decade, however, system biology approaches clearly demonstrated that the composition of fungal cell walls include atypical molecules historically associated with intracellular or membrane locations. Elucidation of mechanisms by which many fungal molecules are exported to the extracellular space suggested that these atypical components are transitorily located to the cell wall. The presence of extracellular vesicles (EVs) at the fungal cell wall and in culture supernatants of distinct pathogenic species suggested a highly functional mechanism of molecular export in these organisms. Thus, the passage of EVs through fungal cell walls suggests remarkable molecular diversity and, consequently, a potentially variable influence on the host antifungal response. On the basis of information derived from the proteomic characterization of fungal EVs from the yeasts Cryptoccocus neoformans and Candida albicans and the dimorphic fungi Histoplasma capsulatum and Paracoccidioides brasiliensis, our manuscript is focused on the clear view that the fungal cell wall is much more complex than previously thought.
Introduction
Glucans, chitin, and glycoproteins are cross-linked to form the most essential structure of fungal cell walls (Free, 2013). This structure is responsible for cell shaping, as well as for osmotic and physical protection of the cell (Nimrichter et al., 2005). However, fungal morphogenesis and reproduction require elaborated cell wall remodeling. Therefore, the fungal cell wall must combine contrasting properties such as elasticity and rigidity, which demands a remarkable dynamism. In fact, different fungal species have distinct ways to assemble their cell wall (Erwig and Gow, 2016). A plethora of enzymes reach precise regions at the fungal cell surface to finely control remodeling, avoiding cellular damage (Fischer et al., 2008). The consequence of these rearrangements must impact directly on the recognition of fungal pathogens by the host, since they imply a high diversity in the molecular composition of the cell surface.
Several reviews discuss the interaction of fungi with host cells based on well-known surface components, such as α and β-glucans, mannoproteins, galactomannan, glucuronoxylomannan (GXM), and most recently chitin and its derivatives (Romani et al., 2002; Romani, 2011; Vautier et al., 2012; Paulovicova et al., 2014; Dambuza and Brown, 2015; Levitz et al., 2015; Underhill and Pearlman, 2015). However, atypical proteins originally characterized as cytoplasmic or plasma membrane constituents have been also found at cell wall (Alloush et al., 1997; Gil-Navarro et al., 1997; Gozalbo et al., 1998; Pitarch et al., 2002; Kneipp et al., 2003; Motshwene et al., 2003; Nimrichter et al., 2005; Barbosa et al., 2006; Batista et al., 2006; Castillo et al., 2008; da Silva Neto et al., 2009; Tomazett et al., 2010; Brito Wde et al., 2011; Karkowska-Kuleta et al., 2011; Puccia et al., 2011; Marcos et al., 2012; Gil-Bona et al., 2015b; Karkowska-Kuleta and Kozik, 2015). Most of these proteins share a common characteristic: they are released from the cell inside vesicular compartments that traverse the cell wall and reach the extracellular environment. These molecular carriers are called extracellular vesicles (EVs), which are part of a conserved secretion mechanism shared by all domains of life. EV composition, biogenesis, and immunobiological functions were discussed in recent reviews (Rodrigues et al., 2011, 2013, 2014, 2015; Oliveira et al., 2013; Brown et al., 2015) but there remains a significant need for additional information regarding the mechanisms through which EVs pass through the cell wall and how they influence host recognition. In fact, EVs from Cryptoccocus neoformans and Candida albicans are recognized and internalized by phagocytes culminating in host cell activation (Oliveira et al., 2010; Vargas et al., 2015). Considering the multiplicity in the composition of fungal EVs, a number of receptors are expected to participate in their recognition. For instance, EVs from Paracoccidioides brasiliensis carry membrane-bound mannose and N-acetylglucosamine and are recognized by DC-SIGN and DC-SIGNR, but not dectin-1 or -2 (Peres da Silva et al., 2015).
In this review we discuss both direct and indirect putative mechanisms of participation of EV-associated molecules during interaction of pathogenic fungi with host cells. In this context, host cell receptors and antibodies could target EV components. Moreover, enzymes carried by these compartments could modify the cell wall and its composition, which might impact cell wall architecture and the pathophysiology of distinct fungal diseases.
Enzymes From Metabolic Pathways
The mechanisms of EVs biogenesis remains obscure but apparently includes (i) multivesicular body formation followed by exosome release, (ii) vesicle shedding from the plasma membrane, and (iii) inverted macropinocytosis (Rodrigues et al., 2007, 2014, 2015; Vargas et al., 2015). All three mechanisms are in agreement with the compositional complexity of EVs, including membrane and cytoplasmic molecules (Albuquerque et al., 2008; Rodrigues et al., 2008; Vallejo et al., 2011; Wolf et al., 2014; Gil-Bona et al., 2015a; Vargas et al., 2015). Proteomic analyses of fungal EVs from diverse species clearly show a considerable number of enzymes that are associated with metabolic routes (Table 1) (Albuquerque et al., 2008; Rodrigues et al., 2008; Vallejo et al., 2011; Vargas et al., 2015). Some of these enzymes are actually conserved among EVs produced by distinct fungal species. Many of them are also considered as moonlighting proteins, implying primary and secondary biological functions (Jeffery, 2014). Major hits include enzymes required for glycolysis, fermentation, gluconeogenesis, pentose phosphate, tricarboxylic acid, and glyoxylate cycles (Table 1) (Albuquerque et al., 2008; Rodrigues et al., 2008; Vallejo et al., 2011; Vargas et al., 2015). In this group of molecules, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), enolase, and transaldolase were consistently detected in C. neoformans, C. albicans, P. brasiliensis, and Histoplasma capsulatum EVs by proteomic analysis (Rodrigues et al., 2008; Vallejo et al., 2011; Wolf et al., 2014; Gil-Bona et al., 2015a; Vargas et al., 2015). According to Gozalbo et al. (1998) GAPDH, a typical cytoplasmic protein, also decorates the outermost layer of the C. albicans cell wall where it mediates adhesion to laminin and fibronectin. The protein, however, is apparently a poor immunogen, since vaccination with GAPDH or exposure of mice to antibodies against GAPDH did not impact the outcome of disseminated candidiasis (Gil et al., 2006). GAPDH was also detected at the cell surface of P. brasiliensis yeast forms (Barbosa et al., 2006), where it also promoted binding to fibronectin, laminin, and type I collagen. Adherence and internalization of P. brasiliensis yeast forms by pneumocytes was reduced when fungal cells were pretreated with antibodies to P. brasiliensis GAPDH or in the presence of the purified enzyme (Barbosa et al., 2006).
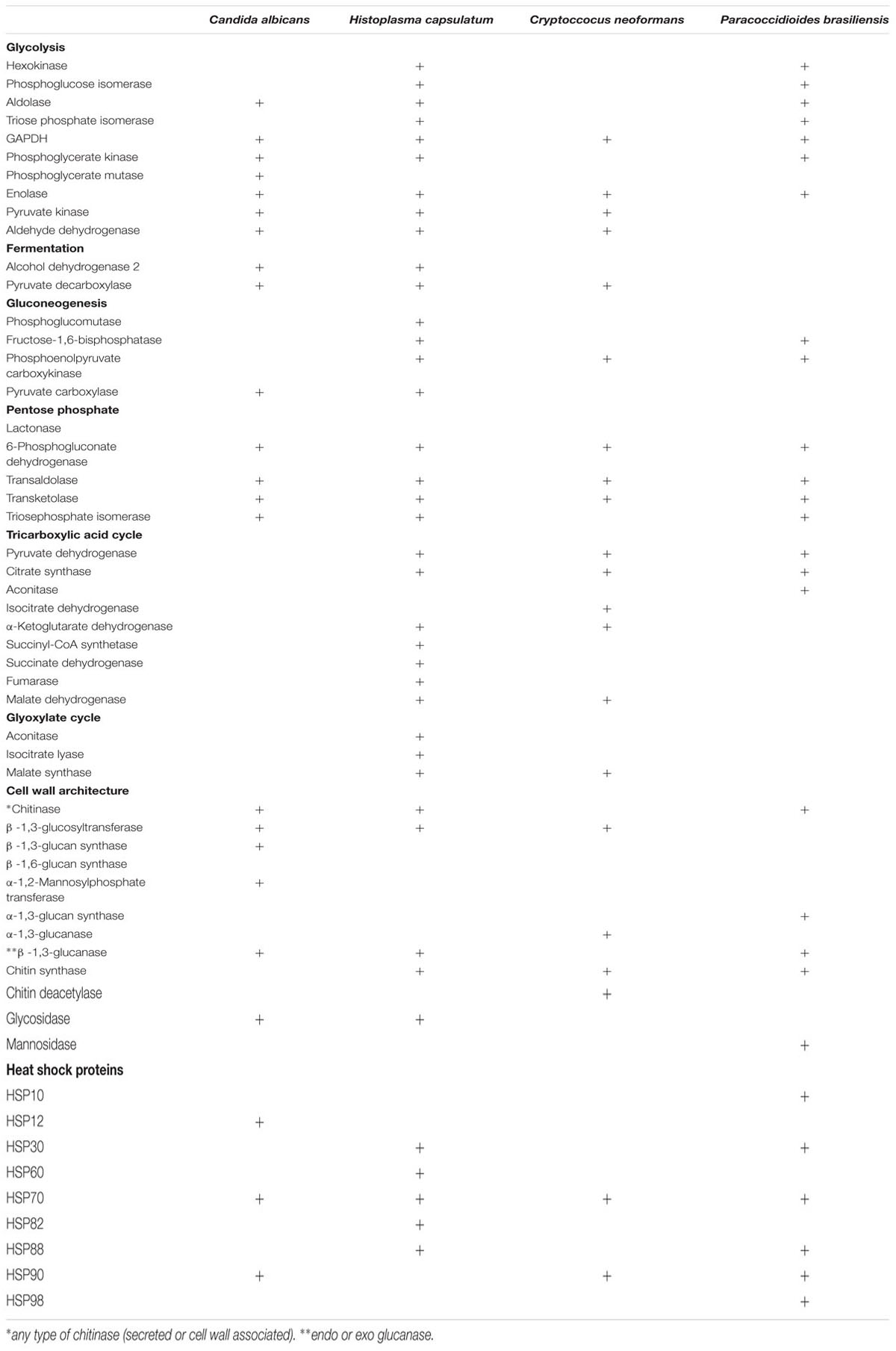
TABLE 1. Major proteins characterized in pathogenic fungi extracellular vesicles (EVs) and involved with metabolic routes, cell wall remodeling, and heat shock response.
Enolase is another example of an immunogenic, cytoplasmic protein that actively participates in the fungal-host cell interface. This antigen was detected at large amounts in C. albicans supernatants (Sundstrom and Aliaga, 1994). In addition, enolase is one of the main cell wall proteins of C. albicans (Angiolella et al., 1996). In fact, enolase is considered the humoral immunodominant antigen in germ free mice and in humans with disseminated candidiasis (Sundstrom et al., 1994; Pitarch et al., 2008). Li et al. (2013) suggested that IgG antibodies against Candida enolase and aldolase, in combination, could be markers to diagnose invasive candidiasis. In contrast to GAPDH, anti-enolase antibodies are at least partially protective in murine candidiasis (van Deventer et al., 1996; Montagnoli et al., 2004; Li et al., 2011). Besides its role in the glycolytic pathway and immunogenic properties, enolase participates in host cell adhesion. For instance, the C. albicans enzyme recognized plasmin and plasminogen (Jong et al., 2003). Furthermore, plasmin-bound yeast cells displayed an improved ability to induce fibrinolysis in a matrix-gel assay as well as to cross an in vitro blood brain barrier system. Likewise, enolase can participate during C. albicans intestinal colonization. Yeast adhesion to the intestinal epithelium was inhibited by enolase containing-disks or by pretreatment with antibodies to enolase (Silva et al., 2014). Similarly, adhesion of P. brasiliensis to host cells and fibronectin required enolase, which is also cell wall-bound in this fungus (Nogueira et al., 2010; Marcos et al., 2012). The relevance of enolase during infection was confirmed by the demonstration that its expression was upregulated in yeast cells of P. brasiliensis recovered from infected mice tissues (Nogueira et al., 2010). In addition, enolase and GADPH association with plasminogen resulted in plasmin formation through tissue plasminogen activator in a lysine dependent fashion. As a consequence fibronectin was degraded on the fungus surface. Recently, enolase was also detected at cell surface of A. fumigatus, A. flavus, A. terreus, A. nidulans, and C. glabrata (Funk et al., 2016). As observed for other fungal species, enolase from A. fumigatus binds to plasminogen remaining accessible to plasminogen activator uPA, which confirms its potential to participate during fungal dissemination (Funk et al., 2016).
Additional metabolism-related enzymes have been associated with host cell recognition. Phosphoglycerate mutase 1 (Pgmt1) from C. albicans binds to factor H, FHL-1 and plasminogen (Crowe et al., 2003; Poltermann et al., 2007). Lopez et al. (2014) also demonstrated that Pgmt1 interacted with fibronectin and vitronectin. Pgmt1 is recognized by human umbilical vein endothelial cells (HUVEC), keratinocytes (HaCaT cells), and U937 monocytic cells (Lopez et al., 2014). Consistent with these results, C. albicans mutants in which the enzyme was knocked out displayed a reduced capacity to bind HUVECS. Thus, Pgmt1 appears to be linked to fungal pathogenesis by activating the factor H, FHL-1, and plasminogen for immune evasion and degradation of extracellular matrices. The notion that metabolic enzymes in fact affect fungal pathogenesis was confirmed by results with triosephosphate isomerase (Tpi). This enzyme was also found at cell wall of P. brasiliensis and binds to laminin (Pereira et al., 2007). The use of polyclonal antibodies to Tpi inhibited the interaction of yeast with epithelial cells in vitro, suggesting that it also intermediates the association with host cells.
The enzymes mentioned above and others can operate through integrated mechanisms. Crowe et al. (2003) reported at least eight plasminogen-binding proteins at the cell wall of C. albicans (Crowe et al., 2003). Six of them were detected in EVs produced by C. albicans, including the enzymes Pgmt1, alcohol dehydrogenase, GAPDH, phosphoglycerate kinase, and aldolase (Gil-Bona et al., 2015a; Vargas et al., 2015). These proteins were associated with the capacity of C. albicans to activate plasminogen, resulting in more effective tissue invasion (Crowe et al., 2003).
The combination of proteins exported in EVs could influence recognition of other fungal species by host cells. Fibronectin, vitronectin and laminin recognize cytoplasmic proteins that are surface-exposed in C. parapsilosis and C. tropicalis pseudohyphae, including malate synthase, glucose-6-phosphate isomerase, 6-phosphogluconate dehydrogenase, enolase, fructose-1,6-bisphosphatase, transketolase, transaldolase, and elongation factor 2 (Kozik et al., 2015). In C. neoformans, phosphoglycerate kinase, transaldolase, aldolase, and glutamate dehydrogenase demonstrated the capacity to bind plasminogen (Stie et al., 2009). As shown in other species, surface-bound active plasmin in C. neoformans increased the ability of the fungus to penetrate the brain.
Association with extracellular matrix proteins and activation of plasmin are not the only potential activities of glycolytic enzymes in C. albicans. Karkowska-Kuleta et al. (2011) showed that enolase, Tgpm1, and Tpi are able to bind kininogen culminating with kinin activation (Karkowska-Kuleta et al., 2011). These studies support the hypothesis that fungal EVs correspond to antigen-rich compartments responsible for the delivery of metabolic enzymes interfering with host’s physiology.
Heat Shock Proteins
Similar to what is detailed for the above glycolytic enzymes, HSP70 is carried by fungal EVs through the cell wall (Albuquerque et al., 2008; Rodrigues et al., 2008; Vallejo et al., 2011; Wolf et al., 2014; Gil-Bona et al., 2015a; Vargas et al., 2015). Its participation during interaction with host cells has been investigated in C. neoformans and C. albicans. In the former, HSP70 is present at the fungal surface, within the capsular network (Silveira et al., 2013). Recombinant HSP70 (Cn-rHSP70) from C. neoformans is efficiently internalized by the macrophage like-cell line J774.1 and, to a minor extent, by A549 pneumocytes. Pre-treatment of J774.1 cells with Cn-rHSP70 does not impair phagocytosis, but increases fungal survival within macrophages accompanied by a decrease in nitric oxide (NO) production. In addition Cn-rHSP70 can upregulate TLR4 expression in macrophages (Silveira et al., 2013) and directly interfere with early macrophage polarization, limiting innate control of C. neoformans (Eastman et al., 2015). These results indicate that EVs carry proteins that facilitate C. neoformans survival within the host.
Candida albicans expresses two major HSP70 proteins, SSA1 and SSA2 (Lopez-Ribot et al., 1996). SSA2 has been immunolocalized at the plasma membrane and cell wall in both yeast and hyphal forms (Lopez-Ribot et al., 1996). The protein is recognized by histatin 5, a member of the family of small histidine-rich antifungal peptides secreted by salivary glands (Li et al., 2003), leading to its internalization and consequent fungal death (Li et al., 2006). On the other hand SSA1 is required for endocytosis by endothelial and epithelial cells in vitro through a cadherin-dependent recognition mechanism (Sun et al., 2010). Furthermore, SSA1 appears to act as an invasin contributing to C. albicans virulence in hematogenously disseminated and oropharyngeal candidiasis (Sun et al., 2010). In addition, SSA1 contributes at least partially to C. albicans penetration to microfold-like cells generated by the co-culture of enterocytes with B lymphocytes (Albac et al., 2016). Thus, proteins carried by C. albicans EVs can have opposite effects when in contact with host cells.
Vesicles from H. capsulatum carry distinct heat shock proteins (Albuquerque et al., 2008). HSP60 is one of the major hits in H. capsulatum EVs. A series of studies investigating binding and internalization of H. capsulatum yeasts by macrophages revealed a key function for this protein, which accumulates at the fungal cell wall (Long et al., 2003). HSP60 from H. capsulatum is recognized by the integrin CD18, a CR3 subunit at the macrophage cell surface (Long et al., 2003; Habich et al., 2006). Through this association yeasts of H. capsulatum are internalized and evade the macrophage defense (Strasser et al., 1999; Woods, 2003). In addition, HSP60 is considered an immunodominant antigen that orchestrates the adaptation to temperature stress (Deepe and Gibbons, 2002; Scheckelhoff and Deepe, 2002; Guimaraes et al., 2011b). Immunization of mice with recombinant HSP60 induces a protective response against H. capsulatum (Deepe and Gibbons, 2002; Scheckelhoff and Deepe, 2002). Furthermore, passive administration of IgG1 and IgG2a against HSP60 in a murine model of histoplasmosis promotes a protective effect associated with higher levels of IL-2, IL-12, and IFN-γ and decreased levels of IL-4 and IL-10 (Guimaraes et al., 2009). Different independent mechanisms could be linked to the antibody effect in vivo. First, antibodies to HSP60 alter the rates of phagocytosis and killing of H. capsulatum yeast cells by host effector cells (Guimaraes et al., 2009) as well as cause agglutination of H. capsulatum yeasts, which further alters interactions with macrophages and induces changes in macrophage antifungal functions (Guimaraes et al., 2011a). Recently, we demonstrated that H. capsulatum yeasts exposed to antibodies to HSP60 release EVs with different sizes and altered protein loads, including varying the quantity of virulence-associated products, when compared to untreated controls, which suggests that antibodies alter fungal susceptibility to host defenses (Baltazar et al., 2016). In this scenario, HSP60 emerges as an interesting target for the development of new therapies against H. capsulatum.
Polysaccharide Hydrolases
As mentioned previously, the complexity of the cell wall requires highly coordinated mechanisms to allow morphological rearrangements supporting fungal growth, budding and hyphal formation. In addition, cell wall composition can be robustly modified according to the species and growth conditions (Erwig and Gow, 2016). Figure 1 shows a simplified general picture of the fungal cell wall in which layers of chitin are displayed adjacent to the cell membrane, although oligomers of chitin have been observed in other regions of the fungal surface (Fonseca et al., 2009). The main chitin layers are connected to a glucan network that can include β1,3, β1,4, β1,6, and α1,3 linkages (Free, 2013; Erwig and Gow, 2016). Proteins are associated to the cell wall (cell wall proteins, CWP) through both covalent and non-covalent bonds (Chaffin, 2008; Heilmann et al., 2012; Orlean, 2012). At least three types of proteins can be covalently linked to fungal polysaccharides: (i) proteins with an alkali-sensitive linkage (ASL), which are linked to β1,3 glucans, (ii) proteins covalently bound to β-1,6-glucan via a remnant of a glycosylphosphatidylinositol (GPI) anchor, and (iii) proteins linked to wall polysaccharides through disulfide bonds. Non-covalently bound proteins encompass transitory polypeptides that are synthesized intracellularly and targeted for extracellular secretion (Chaffin, 2008).
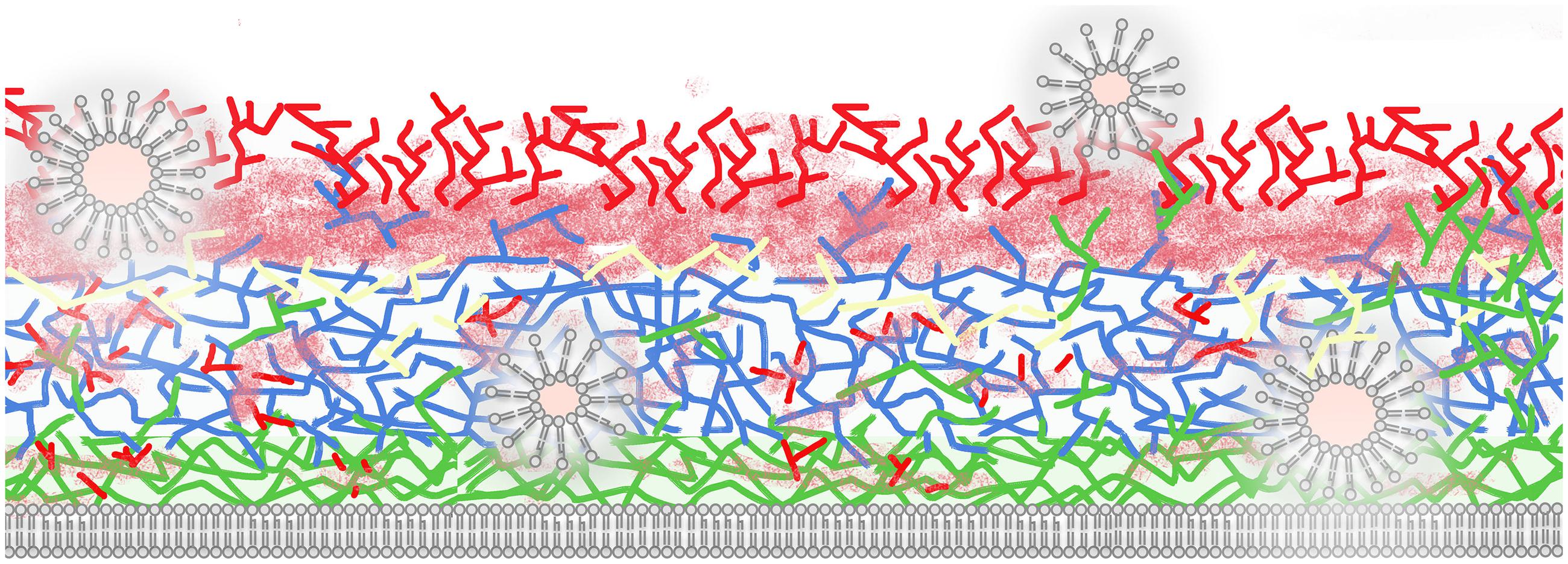
FIGURE 1. Schematic illustration of a fungal cell wall and its major polysaccharides and proteins (based on species with protein EV composition characterized). Extracellular vesicles (EVs) are shown as bilayered compartments. Structural polysaccharides include chitin (green, close to plasma membrane), β1,3 (blue), and β1,6 glucans (yellow). Chitin oligomers (green, distributed across the cell wall), mannans, and mannoproteins (solid and fuzzy red) are also illustrated. EVs traverse the cell wall potentially promoting remodeling through hydrolysis of polysaccharides and mannoproteins and exposing internal structural components to the extracellular environment. For didactic purposes, melanin, lipids, capsule, β 1,4, and α 1,4 glucans and other cell wall components are not illustrated in this model.
Environmental changes are associated with cell wall remodeling, including nutrient availability, pH and temperature (Sosinska et al., 2008; Heilmann et al., 2013; Ene et al., 2015). In a recent study, Ene et al. (2015) demonstrated substantial cell wall transformation after only thirty seconds in response to hyperosmotic stress. They showed changes in cell wall volume can be accompanied by ultrastructural adjustments, including (i) increase of the inner β-glucan and chitin layers and (ii) contraction of the mannoprotein layer. These drastic modifications require enzymatic activities of synthesis and degradation. To hydrolyze structural components, chitinases, mannosidases, and glucosidases (glucanases) are mandatory. In fungal EVs a number of hydrolases, including glycosidases, lipases, and proteases, were characterized (Rodrigues et al., 2007, 2008; Albuquerque et al., 2008; Vallejo et al., 2011; Gil-Bona et al., 2015a; Vargas et al., 2015). These compartments could be responsible for prompt changes at cell wall by releasing pre-formed enzymes during a stress response.
Several host cellular receptors for fungal cell wall polysaccharides are reported in the literature, including CR3 (CD18/CD11b), Toll like receptors (TLRs), Dectin 1 and 2, DC-SIGN, mannose receptors, CD14, lactosylceramide, and Mincle (for details, see Zimmerman et al., 1998; Kimberg and Brown, 2008; Barreto-Bergter and Figueiredo, 2014; Dalonso et al., 2015). Although functional studies of polysaccharide recognition have traditionally focused on typical cell wall components, it is important to mention the products of polysaccharide hydrolases have similar potential to be recognized by receptors and immunologically active. For instance, in the C. neoformans model, chitooligomers released through chitinase activity form soluble complexes with capsular GXM, resulting in hybrid glycans with unique immunological activity (Ramos et al., 2012). Enzymatically released oligosaccharides and (glyco)proteins could also modify the extracellular microenvironment and potentially impact the immune response, by scavenging antibodies and carbohydrate binding proteins (CBPs). Finally, cell wall components can also suffer modifications derived from the activity of host hydrolases. In C. neoformans, chitin cleavage via chitotriosidase promoted pathologic type-2 helper T cell responses (Wiesner et al., 2015).
The level of glucanase activities at the cell wall can potentially influence fungal recognition, finally interfering with receptor-ligand connections. For instance, along with CR3 (CD18/CD11b), dectin-1 is a major ligand responsible for β1,3 glucan cell wall detection culminating with phagocytosis, oxidative burst response and cytokine production (Brown and Gordon, 2001; Huang et al., 2015). Host cells that express dectin-1 include monocytes, macrophage, neutrophils, and dendritic cells (Taylor et al., 2002; Willment et al., 2005). Li et al. (2012) showed that treatment of C. albicans with β1,3 glucanase abolished fungal recognition through dectin-1 by neutrophils. Although some authors have shown that either soluble and particles of β1,3 glucans modulate the function of host cells (Drummond and Brown, 2011), studies by Goodridge et al. (2011) suggested that signaling is triggered only after dectin-1 binding to particulate β-glucans. The fact that particles of β1,3 glucans have a higher valence for dectin-1 recognition and consequent enhanced efficacy in the induction of cross-talks between other ligands, including CR3 and TLRs (O’Neill, 2008), suggests that the stimulatory mechanisms triggered by soluble and particle β1,3 glucans must be in fact distinct. The major β1,3 glucanases characterized in C. albicans EVs were Xog1p, Eng1, Sun41 and MP65 (Gil-Bona et al., 2015a; Vargas et al., 2015). Xog1p is the major β-1,3-exoglucanase in C. albicans (Gonzalez et al., 1997) that is a receptor for the antimicrobial peptide LL-37, produced by human neutrophils (Turner et al., 1998; Tsai et al., 2011a). LL-37 kills C. albicans and, in addition, reduces binding of C. albicans to plastic surfaces, oral epidermoid OECM-1 cells, and murine urinary bladders at sub-inhibitory concentrations (Tsai et al., 2011b). Additional ligands to LL-37 include mannans, glucans, and chitin (Tsai et al., 2011b). The mechanisms involved in inhibition of cell adhesion include direct competition and interference with glucanase activities leading to disturbance of cell wall remodeling (Tsai et al., 2011b; Chang et al., 2012). The putative glucosidase SUN41 and MP65 are also exported in C. albicans EVs (Gil-Bona et al., 2015a; Vargas et al., 2015). SUN41 is regularly involved with cytokinesis, cell wall biogenesis, adhesion to host tissue, and biofilm formation (Hiller et al., 2007).
MP65 is a major immunogenic mannoprotein secreted by C. albicans and other Candida species (Gomez et al., 1996; Karkowska-Kuleta et al., 2015). MP65 is found at the cell wall and its secretion occurs potentially due to the presence of a Kex2 site (Newport et al., 2003). A protective response generated after vaccination with a low-virulence Candida strain was associated with cell-mediated immunity disclosed by MP65 stimulation of splenocytes in vitro and a delayed-type hypersensitivity response in vivo (Mencacci et al., 1994; Cassone et al., 1998). Human DCs were stimulated by MP65 culminating with TNF-α and IL-6 release and the activation of IL-12 expression. Maturation of DCs was confirmed by increasing co-stimulatory molecules such as CD40, CD80, CD86, MHC class II, and decreasing CD16, CD32, and CD64 (Pietrella et al., 2006). Recombinant MP65 was also internalized by macrophages and DCs in a mechanism at least partially associated with a RGD peptide sequence (Pietrella et al., 2008). However, differently from the native mannoprotein, TNF-α and IL-6 were not induced by recombinant MP65 (Pietrella et al., 2008). These data suggested that cytokine production was potentially stimulated by the glycan moiety of MP65, probably through lectin and TLR-dependent mechanisms, as suggested by Netea et al. (2006). However, both cells were able to stimulate T-cell activation with IFNγ and IL-4 production, confirming the potential activity of the protein sequence of MP65 (Pietrella et al., 2008). In C. albicans, the activity of β1,3 glucanase also appears to be involved with yeast filamentation at 37°C, which also supports its relevance during infection progress (Xu et al., 2013). Influence of other glucanases exported in C. albicans, H. capsulatum, C. neoformans, and P. brasiliensis EVs during host-cell recognition has not been reported in the literature; however, as mentioned previously, their activities as cell wall remodeling enzymes could impact the distribution of native proteins, polysaccharides, and their products of hydrolysis, consequently modulating the immune response.
Concluding Remarks
The current literature shows that the fungal cell wall composition is highly complex and varies considerably according to the species investigated. The basic cell wall network is composed by structural components covalently connected to each other. Thus, molecular changes at this level require an intense participation of hydrolytic enzymes and transient molecules. Furthermore, such modifications interfere significantly with the way a fungal pathogen is coated and directly influence its engagement with a host cell. Based on the recent literature we believe that the complexity of the fungal cell wall could be significantly impacted by the presence and passage of EVs. The presence of enzymes and virulence regulators characterized in EVs produced by four distinct major pathogens suggests that these compartments could tailor the cell wall supporting significant changes in short periods of time. The ability of other medically relevant fungal species, including molds such as Aspergillus sp, to release EVs is still under investigation. In fact, typical cytoplasmic and membrane proteins from A. fumigatus were detected at the cell wall (Champer et al., 2016), supporting the hypothesis that they are trafficked in EVs. In this sense, hexokinase, aldolase, phosphoglycerate mutase, β1,3 glucosyltransferase, chitinase, mannosidase, β1,3 glucanase, among others, were characterized at cell wall extracts from A. fumigatus (Champer et al., 2016). In addition, enolase, transaldolase, β1,3 glucosyltransferase, β1,3 glucanase, α1,3 glucan synthase, α1,3 glucanase, chitinase, mannosidase were detected in the A. fumigatus secretome (Adav et al., 2015; Champer et al., 2016).
Through these heterogeneous compartments a number of proteins reach the fungal cell surface, consequently modifying cell wall composition and affecting fungal-host cell interactions. EVs can also release immunoactive compounds to the extracellular environment, impacting fungal pathogenesis. Consequently, biogenesis of EVs is a potential target for the development of novel antifungal drugs. In addition, the diversity of native immunogenic proteins carried by EVs suggests that these compartments are multi-antigen platforms that could be used in vaccine formulations.
Author Contributions
All authors listed, have made substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by grants from Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). MR acknowledges support from the Instituto Nacional de Ciência e Tecnologia de Inovação em Doenças Negligenciadas (INCT-IDN) and from the Wellcome Trust (Pathfinder award, grant number WT103212MF). JN is in part supported by AI52733, AI1033142, and AI124797-01. This work was supported by NIH grants AI56168, AI100631, AI116420, and by a Merit Review grant I01BX002624 from the Veterans Affairs Program in Biomedical Laboratory Research and Development to MP.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
The authors are very grateful to Jorge José-Jó Bastos Ferreira for valuable suggestions.
References
Adav, S. S., Ravindran, A., and Sze, S. K. (2015). Quantitative proteomic study of Aspergillus fumigatus secretome revealed deamidation of secretory enzymes. J. Proteomics 119, 154–168. doi: 10.1016/j.jprot.2015.02.007
Albac, S., Schmitz, A., Lopez-Alayon, C., d’Enfert, C., Sautour, M., Ducreux, A., et al. (2016). Candida albicans is able to use M cells as a portal of entry across the intestinal barrier in vitro. Cell. Microbiol. 18, 195–210. doi: 10.1111/cmi.12495
Albuquerque, P. C., Nakayasu, E. S., Rodrigues, M. L., Frases, S., Casadevall, A., Zancope-Oliveira, R. M., et al. (2008). Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell. Microbiol. 10, 1695–1710. doi: 10.1111/j.1462-5822.2008.01160.x
Alloush, H. M., Lopez-Ribot, J. L., Masten, B. J., and Chaffin, W. L. (1997). 3-phosphoglycerate kinase: a glycolytic enzyme protein present in the cell wall of Candida albicans. Microbiology 143(Pt 2), 321–330. doi: 10.1099/00221287-143-2-321
Angiolella, L., Facchin, M., Stringaro, A., Maras, B., Simonetti, N., and Cassone, A. (1996). Identification of a glucan-associated enolase as a main cell wall protein of Candida albicans and an indirect target of lipopeptide antimycotics. J. Infect. Dis. 173, 684–690. doi: 10.1093/infdis/173.3.684
Baltazar, L. M., Nakayasu, E. S., Tiago, J. P., Sobreira, T. J. P., Choi, H., Arturo Casadevall, A., et al. (2016). Antibody binding alters the characteristics and contents of extracellular vesicles released by Histoplasma capsulatum. Msphere 1, e00085-15. doi: 10.1128/mSphere.00085-15
Barbosa, M. S., Bao, S. N., Andreotti, P. F., de Faria, F. P., Felipe, M. S., dos Santos Feitosa, L., et al. (2006). Glyceraldehyde-3-phosphate dehydrogenase of Paracoccidioides brasiliensis is a cell surface protein involved in fungal adhesion to extracellular matrix proteins and interaction with cells. Infect. Immun. 74, 382–389. doi: 10.1128/IAI.74.1.382-389.2006
Barreto-Bergter, E., and Figueiredo, R. T. (2014). Fungal glycans and the innate immune recognition. Front. Cell. Infect. Microbiol. 4:145. doi: 10.3389/fcimb.2014.00145
Batista, W. L., Matsuo, A. L., Ganiko, L., Barros, T. F., Veiga, T. R., Freymuller, E., et al. (2006). The PbMDJ1 gene belongs to a conserved MDJ1/LON locus in thermodimorphic pathogenic fungi and encodes a heat shock protein that localizes to both the mitochondria and cell wall of Paracoccidioides brasiliensis. Eukaryot. Cell 5, 379–390. doi: 10.1128/EC.5.2.379-390.2006
Brito Wde, A., Rezende, T. C., Parente, A. F., Ricart, C. A., Sousa, M. V., Bao, S. N., et al. (2011). Identification, characterization and regulation studies of the aconitase of Paracoccidioides brasiliensis. Fungal Biol. 115, 697–707. doi: 10.1016/j.funbio.2011.02.011
Brown, G. D., and Gordon, S. (2001). Immune recognition. A new receptor for beta-glucans. Nature 413, 36–37. doi: 10.1038/35092620
Brown, L., Wolf, J. M., Prados-Rosales, R., and Casadevall, A. (2015). Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 13, 620–630. doi: 10.1038/nrmicro3480
Cassone, A., De Bernardis, F., Ausiello, C. M., Gomez, M. J., Boccanera, M., La Valle, R., et al. (1998). Immunogenic and protective Candida albicans constituents. Res. Immunol. 149, 289–299; discussion 504. doi: 10.1016/S0923-2494(98)80753-0
Castillo, L., Calvo, E., Martinez, A. I., Ruiz-Herrera, J., Valentin, E., Lopez, J. A., et al. (2008). A study of the Candida albicans cell wall proteome. Proteomics 8, 3871–3881. doi: 10.1002/pmic.200800110
Chaffin, W. L. (2008). Candida albicans cell wall proteins. Microbiol. Mol. Biol. Rev. 72, 495–544. doi: 10.1128/MMBR.00032-07
Champer, J., Ito, J. I., Clemons, K. V., Stevens, D. A., and Kalkum, M. (2016). Proteomic Analysis of Pathogenic Fungi Reveals Highly Expressed Conserved Cell Wall Proteins. J. Fungi (Basel) 2, 6. doi: 10.3390/jof2010006
Chang, H. T., Tsai, P. W., Huang, H. H., Liu, Y. S., Chien, T. S., and Lan, C. Y. (2012). LL37 and hBD-3 elevate the beta-1,3-exoglucanase activity of Candida albicans Xog1p, resulting in reduced fungal adhesion to plastic. Biochem. J. 441, 963–970. doi: 10.1042/BJ20111454
Crowe, J. D., Sievwright, I. K., Auld, G. C., Moore, N. R., Gow, N. A., and Booth, N. A. (2003). Candida albicans binds human plasminogen: identification of eight plasminogen-binding proteins. Mol. Microbiol. 47, 1637–1651. doi: 10.1046/j.1365-2958.2003.03390.x
da Silva Neto, B. R., de Fatima da Silva, J., Mendes-Giannini, M. J., Lenzi, H. L., de Almeida Soares, C. M., and Pereira, M. (2009). The malate synthase of Paracoccidioides brasiliensis is a linked surface protein that behaves as an anchorless adhesin. BMC Microbiol. 9:272. doi: 10.1186/1471-2180-9-272
Dalonso, N., Goldman, G. H., and Gern, R. M. (2015). beta-(1– > 3),(1– > 6)-Glucans: medicinal activities, characterization, biosynthesis and new horizons. Appl. Microbiol. Biotechnol. 99, 7893–7906. doi: 10.1007/s00253-015-6849-x
Dambuza, I. M., and Brown, G. D. (2015). C-type lectins in immunity: recent developments. Curr. Opin. Immunol. 32, 21–27. doi: 10.1016/j.coi.2014.12.002
Deepe, G. S. Jr., and Gibbons, R. S. (2002). Cellular and molecular regulation of vaccination with heat shock protein 60 from Histoplasma capsulatum. Infect. Immun. 70, 3759–3767. doi: 10.1128/IAI.70.7.3759-3767.2002
Drummond, R. A., and Brown, G. D. (2011). The role of Dectin-1 in the host defence against fungal infections. Curr. Opin. Microbiol. 14, 392–399. doi: 10.1016/j.mib.2011.07.001
Eastman, A. J., He, X., Qiu, Y., Davis, M. J., Vedula, P., Lyons, D. M., et al. (2015). Cryptococcal heat shock protein 70 homolog Ssa1 contributes to pulmonary expansion of Cryptococcus neoformans during the afferent phase of the immune response by promoting macrophage M2 polarization. J. Immunol. 194, 5999–6010. doi: 10.4049/jimmunol.1402719
Ene, I. V., Walker, L. A., Schiavone, M., Lee, K. K., Martin-Yken, H., Dague, E., et al. (2015). Cell wall remodeling enzymes modulate fungal cell wall elasticity and osmotic stress resistance. Mbio 6:e00986. doi: 10.1128/mBio.00986-15
Erwig, L. P., and Gow, N. A. (2016). Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 14, 163–176. doi: 10.1038/nrmicro.2015.21
Fischer, R., Zekert, N., and Takeshita, N. (2008). Polarized growth in fungi–interplay between the cytoskeleton, positional markers and membrane domains. Mol. Microbiol. 68, 813–826. doi: 10.1111/j.1365-2958.2008.06193.x
Fonseca, F. L., Nimrichter, L., Cordero, R. J., Frases, S., Rodrigues, J., Goldman, D. L., et al. (2009). Role for chitin and chitooligomers in the capsular architecture of Cryptococcus neoformans. Eukaryot. Cell 8, 1543–1553. doi: 10.1128/EC.00142-09
Free, S. J. (2013). Fungal cell wall organization and biosynthesis. Adv. Genet. 81, 33–82. doi: 10.1016/B978-0-12-407677-8.00002-6
Funk, J., Schaarschmidt, B., Slesiona, S., Hallstrom, T., Horn, U., and Brock, M. (2016). The glycolytic enzyme enolase represents a plasminogen-binding protein on the surface of a wide variety of medically important fungal species. Int. J. Med. Microbiol. 306, 59–68. doi: 10.1016/j.ijmm.2015.11.005
Gil, M. L., Dagan, S., Eren, R., and Gozalbo, D. (2006). Evaluation of the usefulness of anti-glyceraldehyde-3-phosphate dehydrogenase antibodies as a treatment for invasive candidiasis in a murine model. Antonie Van Leeuwenhoek 89, 345–350. doi: 10.1007/s10482-005-9037-7
Gil-Bona, A., Llama-Palacios, A., Parra, C. M., Vivanco, F., Nombela, C., Monteoliva, L., et al. (2015a). Proteomics unravels extracellular vesicles as carriers of classical cytoplasmic proteins in Candida albicans. J. Proteome Res. 14, 142–153. doi: 10.1021/pr5007944
Gil-Bona, A., Parra-Giraldo, C. M., Hernaez, M. L., Reales-Calderon, J. A., Solis, N. V., Filler, S. G., et al. (2015b). Candida albicans cell shaving uncovers new proteins involved in cell wall integrity, yeast to hypha transition, stress response and host-pathogen interaction. J. Proteomics 127(Pt B), 340–351. doi: 10.1016/j.jprot.2015.06.006
Gil-Navarro, I., Gil, M. L., Casanova, M., O’Connor, J. E., Martinez, J. P., and Gozalbo, D. (1997). The glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase of Candida albicans is a surface antigen. J. Bacteriol. 179, 4992–4999.
Gomez, M. J., Torosantucci, A., Arancia, S., Maras, B., Parisi, L., and Cassone, A. (1996). Purification and biochemical characterization of a 65-kilodalton mannoprotein (MP65), a main target of anti-Candida cell-mediated immune responses in humans. Infect. Immun. 64, 2577–2584.
Gonzalez, M. M., Diez-Orejas, R., Molero, G., Alvarez, A. M., Pla, J., Nombela, C., et al. (1997). Phenotypic characterization of a Candida albicans strain deficient in its major exoglucanase. Microbiology 143(Pt 9), 3023–3032. doi: 10.1099/00221287-143-9-3023
Goodridge, H. S., Reyes, C. N., Becker, C. A., Katsumoto, T. R., Ma, J., Wolf, A. J., et al. (2011). Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse.’ Nature 472, 471–475. doi: 10.1038/nature10071
Gozalbo, D., Gil-Navarro, I., Azorin, I., Renau-Piqueras, J., Martinez, J. P., and Gil, M. L. (1998). The cell wall-associated glyceraldehyde-3-phosphate dehydrogenase of Candida albicans is also a fibronectin and laminin binding protein. Infect. Immun. 66, 2052–2059.
Guimaraes, A. J., Frases, S., Gomez, F. J., Zancope-Oliveira, R. M., and Nosanchuk, J. D. (2009). Monoclonal antibodies to heat shock protein 60 alter the pathogenesis of Histoplasma capsulatum. Infect. Immun. 77, 1357–1367. doi: 10.1128/IAI.01443-08
Guimaraes, A. J., Frases, S., Pontes, B., de Cerqueira, M. D., Rodrigues, M. L., Viana, N. B., et al. (2011a). Agglutination of Histoplasma capsulatum by IgG monoclonal antibodies against Hsp60 impacts macrophage effector functions. Infect. Immun. 79, 918–927. doi: 10.1128/IAI.00673-10
Guimaraes, A. J., Nakayasu, E. S., Sobreira, T. J., Cordero, R. J., Nimrichter, L., Almeida, I. C., et al. (2011b). Histoplasma capsulatum heat-shock 60 orchestrates the adaptation of the fungus to temperature stress. PLoS ONE 6:e14660. doi: 10.1371/journal.pone.0014660
Habich, C., Kempe, K., Gomez, F. J., Lillicrap, M., Gaston, H., van der Zee, R., et al. (2006). Heat shock protein 60: identification of specific epitopes for binding to primary macrophages. FEBS Lett. 580, 115–120. doi: 10.1016/j.febslet.2005.11.060
Heilmann, C. J., Sorgo, A. G., and Klis, F. M. (2012). News from the fungal front: wall proteome dynamics and host-pathogen interplay. PLoS Pathog. 8:e1003050. doi: 10.1371/journal.ppat.1003050
Heilmann, C. J., Sorgo, A. G., Mohammadi, S., Sosinska, G. J., de Koster, C. G., Brul, S., et al. (2013). Surface stress induces a conserved cell wall stress response in the pathogenic fungus Candida albicans. Eukaryot. Cell 12, 254–264. doi: 10.1128/EC.00278-12
Hiller, E., Heine, S., Brunner, H., and Rupp, S. (2007). Candida albicans Sun41p, a putative glycosidase, is involved in morphogenesis, cell wall biogenesis, and biofilm formation. Eukaryot. Cell 6, 2056–2065. doi: 10.1128/EC.00285-07
Huang, J. H., Lin, C. Y., Wu, S. Y., Chen, W. Y., Chu, C. L., Brown, G. D., et al. (2015). CR3 and dectin-1 collaborate in macrophage cytokine response through association on lipid rafts and activation of Syk-JNK-AP-1 pathway. PLoS Pathog. 11:e1004985. doi: 10.1371/journal.ppat.1004985
Jeffery, C. J. (2014). An introduction to protein moonlighting. Biochem. Soc. Trans. 42, 1679–1683. doi: 10.1042/BST20140226
Jong, A. Y., Chen, S. H., Stins, M. F., Kim, K. S., Tuan, T. L., and Huang, S. H. (2003). Binding of Candida albicans enolase to plasmin(ogen) results in enhanced invasion of human brain microvascular endothelial cells. J. Med. Microbiol. 52(Pt 8), 615–622. doi: 10.1099/jmm.0.05060-0
Karkowska-Kuleta, J., Kedracka-Krok, S., Rapala-Kozik, M., Kamysz, W., Bielinska, S., Karafova, A., et al. (2011). Molecular determinants of the interaction between human high molecular weight kininogen and Candida albicans cell wall: identification of kininogen-binding proteins on fungal cell wall and mapping the cell wall-binding regions on kininogen molecule. Peptides 32, 2488–2496. doi: 10.1016/j.peptides.2011.10.021
Karkowska-Kuleta, J., and Kozik, A. (2015). Cell wall proteome of pathogenic fungi. Acta Biochim. Pol. 62, 339–351. doi: 10.18388/abp.2015_1032
Karkowska-Kuleta, J., Zajac, D., Bochenska, O., and Kozik, A. (2015). Surfaceome of pathogenic yeasts, Candida parapsilosis and Candida tropicalis, revealed with the use of cell surface shaving method and shotgun proteomic approach. Acta Biochim. Pol. 62, 807–819. doi: 10.18388/abp.2015_1140
Kimberg, M., and Brown, G. D. (2008). Dectin-1 and its role in antifungal immunity. Med. Mycol. 46, 631–636. doi: 10.1080/13693780802140907
Kneipp, L. F., Palmeira, V. F., Pinheiro, A. A., Alviano, C. S., Rozental, S., Travassos, L. R., et al. (2003). Phosphatase activity on the cell wall of Fonsecaea pedrosoi. Med. Mycol. 41, 469–477. doi: 10.1080/10683160310001615399
Kozik, A., Karkowska-Kuleta, J., Zajac, D., Bochenska, O., Kedracka-Krok, S., Jankowska, U., et al. (2015). Fibronectin-, vitronectin- and laminin-binding proteins at the cell walls of Candida parapsilosis and Candida tropicalis pathogenic yeasts. BMC Microbiol. 15:197. doi: 10.1186/s12866-015-0531-4
Levitz, S. M., Huang, H., Ostroff, G. R., and Specht, C. A. (2015). Exploiting fungal cell wall components in vaccines. Semin. Immunopathol. 37, 199–207. doi: 10.1007/s00281-014-0460-6
Li, D., Dong, B., Tong, Z., Wang, Q., Liu, W., Wang, Y., et al. (2012). MBL-mediated opsonophagocytosis of Candida albicans by human neutrophils is coupled with intracellular Dectin-1-triggered ROS production. PLoS ONE 7:e50589. doi: 10.1371/journal.pone.0050589
Li, F. Q., Ma, C. F., Shi, L. N., Lu, J. F., Wang, Y., Huang, M., et al. (2013). Diagnostic value of immunoglobulin G antibodies against Candida enolase and fructose-bisphosphate aldolase for candidemia. BMC Infect. Dis. 13:253. doi: 10.1186/1471-2334-13-253
Li, W., Hu, X., Zhang, X., Ge, Y., Zhao, S., Hu, Y., et al. (2011). Immunisation with the glycolytic enzyme enolase confers effective protection against Candida albicans infection in mice. Vaccine 29, 5526–5533. doi: 10.1016/j.vaccine.2011.05.030
Li, X. S., Reddy, M. S., Baev, D., and Edgerton, M. (2003). Candida albicans Ssa1/2p is the cell envelope binding protein for human salivary histatin 5. J. Biol. Chem. 278, 28553–28561. doi: 10.1074/jbc.M300680200
Li, X. S., Sun, J. N., Okamoto-Shibayama, K., and Edgerton, M. (2006). Candida albicans cell wall ssa proteins bind and facilitate import of salivary histatin 5 required for toxicity. J. Biol. Chem. 281, 22453–22463. doi: 10.1074/jbc.M604064200
Long, K. H., Gomez, F. J., Morris, R. E., and Newman, S. L. (2003). Identification of heat shock protein 60 as the ligand on Histoplasma capsulatum that mediates binding to CD18 receptors on human macrophages. J. Immunol. 170, 487–494. doi: 10.4049/jimmunol.170.1.487
Lopez, C. M., Wallich, R., Riesbeck, K., Skerka, C., and Zipfel, P. F. (2014). Candida albicans uses the surface protein Gpm1 to attach to human endothelial cells and to keratinocytes via the adhesive protein vitronectin. PLoS ONE 9:e90796. doi: 10.1371/journal.pone.0090796
Lopez-Ribot, J. L., Alloush, H. M., Masten, B. J., and Chaffin, W. L. (1996). Evidence for presence in the cell wall of Candida albicans of a protein related to the hsp70 family. Infect. Immun. 64, 3333–3340.
Marcos, C. M., de Fatima da Silva, J., de Oliveira, H. C., Moraes da Silva, R. A., Mendes-Giannini, M. J., and Fusco-Almeida, A. M. (2012). Surface-expressed enolase contributes to the adhesion of Paracoccidioides brasiliensis to host cells. FEMS Yeast Res. 12, 557–570. doi: 10.1111/j.1567-1364.2012.00806.x
Mencacci, A., Torosantucci, A., Spaccapelo, R., Romani, L., Bistoni, F., and Cassone, A. (1994). A mannoprotein constituent of Candida albicans that elicits different levels of delayed-type hypersensitivity, cytokine production, and anticandidal protection in mice. Infect. Immun. 62, 5353–5360.
Montagnoli, C., Sandini, S., Bacci, A., Romani, L., and La Valle, R. (2004). Immunogenicity and protective effect of recombinant enolase of Candida albicans in a murine model of systemic candidiasis. Med. Mycol. 42, 319–324. doi: 10.1080/13693780310001644653
Motshwene, P., Brandt, W., and Lindsey, G. (2003). Significant quantities of the glycolytic enzyme phosphoglycerate mutase are present in the cell wall of yeast Saccharomyces cerevisiae. Biochem. J. 369(Pt 2), 357–362. doi: 10.1042/BJ20021352
Netea, M. G., Gow, N. A., Munro, C. A., Bates, S., Collins, C., Ferwerda, G., et al. (2006). Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Invest. 116, 1642–1650. doi: 10.1172/JCI27114
Newport, G., Kuo, A., Flattery, A., Gill, C., Blake, J. J., Kurtz, M. B., et al. (2003). Inactivation of Kex2p diminishes the virulence of Candida albicans. J. Biol. Chem. 278, 1713–1720. doi: 10.1074/jbc.M209713200
Nimrichter, L., Rodrigues, M. L., Rodrigues, E. G., and Travassos, L. R. (2005). The multitude of targets for the immune system and drug therapy in the fungal cell wall. Microbes Infect. 7, 789–798. doi: 10.1016/j.micinf.2005.03.002
Nogueira, S. V., Fonseca, F. L., Rodrigues, M. L., Mundodi, V., Abi-Chacra, E. A., Winters, M. S., et al. (2010). Paracoccidioides brasiliensis enolase is a surface protein that binds plasminogen and mediates interaction of yeast forms with host cells. Infect. Immun. 78, 4040–4050. doi: 10.1128/IAI.00221-10
Oliveira, D. L., Freire-de-Lima, C. G., Nosanchuk, J. D., Casadevall, A., Rodrigues, M. L., and Nimrichter, L. (2010). Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect. Immun. 78, 1601–1609. doi: 10.1128/IAI.01171-09
Oliveira, D. L., Rizzo, J., Joffe, L. S., Godinho, R. M., and Rodrigues, M. L. (2013). Where do they come from and where do they go: candidates for regulating extracellular vesicle formation in fungi. Int. J. Mol. Sci. 14, 9581–9603. doi: 10.3390/ijms14059581
O’Neill, L. A. (2008). When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity 29, 12–20. doi: 10.1016/j.immuni.2008.06.004
Orlean, P. (2012). Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics 192, 775–818. doi: 10.1534/genetics.112.144485
Paulovicova, L., Paulovicova, E., and Bystricky, S. (2014). Immunological basis of anti-Candida vaccines focused on synthetically prepared cell wall mannan-derived manno-oligomers. Microbiol. Immunol. 58, 545–551. doi: 10.1111/1348-0421.12195
Pereira, L. A., Bao, S. N., Barbosa, M. S., da Silva, J. L., Felipe, M. S., de Santana, J. M., et al. (2007). Analysis of the Paracoccidioides brasiliensis triosephosphate isomerase suggests the potential for adhesin function. FEMS Yeast Res. 7, 1381–1388. doi: 10.1111/j.1567-1364.2007.00292.x
Peres da Silva, R., Heiss, C., Black, I., Azadi, P., Gerlach, J. Q., Travassos, L. R., et al. (2015). Extracellular vesicles from Paracoccidioides pathogenic species transport polysaccharide and expose ligands for DC-SIGN receptors. Sci. Rep. 5:14213. doi: 10.1038/srep14213
Pietrella, D., Bistoni, G., Corbucci, C., Perito, S., and Vecchiarelli, A. (2006). Candida albicans mannoprotein influences the biological function of dendritic cells. Cell. Microbiol. 8, 602–612. doi: 10.1111/j.1462-5822.2005.00651.x
Pietrella, D., Lupo, P., Rachini, A., Sandini, S., Ciervo, A., Perito, S., et al. (2008). A Candida albicans mannoprotein deprived of its mannan moiety is efficiently taken up and processed by human dendritic cells and induces T-cell activation without stimulating proinflammatory cytokine production. Infect. Immun. 76, 4359–4367. doi: 10.1128/IAI.00669-08
Pitarch, A., Jimenez, A., Nombela, C., and Gil, C. (2008). Serological proteome analysis to identify systemic candidiasis patients in the intensive care unit: analytical, diagnostic and prognostic validation of anti-Candida enolase antibodies on quantitative clinical platforms. Proteomics Clin. Appl. 2, 596–618. doi: 10.1002/prca.200780039
Pitarch, A., Sanchez, M., Nombela, C., and Gil, C. (2002). Sequential fractionation and two-dimensional gel analysis unravels the complexity of the dimorphic fungus Candida albicans cell wall proteome. Mol. Cell. Proteomics 1, 967–982. doi: 10.1074/mcp.M200062-MCP200
Poltermann, S., Kunert, A., von der Heide, M., Eck, R., Hartmann, A., and Zipfel, P. F. (2007). Gpm1p is a factor H-, FHL-1-, and plasminogen-binding surface protein of Candida albicans. J. Biol. Chem. 282, 37537–37544. doi: 10.1074/jbc.M707280200
Puccia, R., Vallejo, M. C., Matsuo, A. L., and Longo, L. V. (2011). The paracoccidioides cell wall: past and present layers toward understanding interaction with the host. Front. Microbiol. 2:257. doi: 10.3389/fmicb.2011.00257
Ramos, C. L., Fonseca, F. L., Rodrigues, J., Guimaraes, A. J., Cinelli, L. P., Miranda, K., et al. (2012). Chitin-like molecules associate with Cryptococcus neoformans glucuronoxylomannan to form a glycan complex with previously unknown properties. Eukaryot. Cell 11, 1086–1094. doi: 10.1128/EC.00001-12
Rodrigues, M. L., Franzen, A. J., Nimrichter, L., and Miranda, K. (2013). Vesicular mechanisms of traffic of fungal molecules to the extracellular space. Curr. Opin. Microbiol. 16, 414–420. doi: 10.1016/j.mib.2013.04.002
Rodrigues, M. L., Godinho, R. M., Zamith-Miranda, D., and Nimrichter, L. (2015). Traveling into outer space: unanswered questions about fungal extracellular vesicles. PLoS Pathog. 11:e1005240. doi: 10.1371/journal.ppat.1005240
Rodrigues, M. L., Nakayasu, E. S., Almeida, I. C., and Nimrichter, L. (2014). The impact of proteomics on the understanding of functions and biogenesis of fungal extracellular vesicles. J. Proteomics 97, 177–186. doi: 10.1016/j.jprot.2013.04.001
Rodrigues, M. L., Nakayasu, E. S., Oliveira, D. L., Nimrichter, L., Nosanchuk, J. D., Almeida, I. C., et al. (2008). Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot. Cell 7, 58–67. doi: 10.1128/EC.00370-07
Rodrigues, M. L., Nimrichter, L., Oliveira, D. L., Frases, S., Miranda, K., Zaragoza, O., et al. (2007). Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot. Cell 6, 48–59. doi: 10.1128/EC.00318-06
Rodrigues, M. L., Nosanchuk, J. D., Schrank, A., Vainstein, M. H., Casadevall, A., and Nimrichter, L. (2011). Vesicular transport systems in fungi. Future Microbiol. 6, 1371–1381. doi: 10.2217/fmb.11.112
Romani, L. (2011). Immunity to fungal infections. Nat. Rev. Immunol. 11, 275–288. doi: 10.1038/nri2939
Romani, L., Bistoni, F., and Puccetti, P. (2002). Fungi, dendritic cells and receptors: a host perspective of fungal virulence. Trends Microbiol. 10, 508–514. doi: 10.1016/S0966-842X(02)02460-5
Scheckelhoff, M., and Deepe, G. S. Jr (2002). The protective immune response to heat shock protein 60 of Histoplasma capsulatum is mediated by a subset of V beta 8.1/8.2+ T cells. J. Immunol. 169, 5818–5826. doi: 10.4049/jimmunol.169.10.5818
Silva, R. C., Padovan, A. C., Pimenta, D. C., Ferreira, R. C., da Silva, C. V., and Briones, M. R. (2014). Extracellular enolase of Candida albicans is involved in colonization of mammalian intestinal epithelium. Front. Cell. Infect. Microbiol. 4:66. doi: 10.3389/fcimb.2014.00066
Silveira, C. P., Piffer, A. C., Kmetzsch, L., Fonseca, F. L., Soares, D. A., Staats, C. C., et al. (2013). The heat shock protein (Hsp) 70 of Cryptococcus neoformans is associated with the fungal cell surface and influences the interaction between yeast and host cells. Fungal Genet. Biol. 60, 53–63. doi: 10.1016/j.fgb.2013.08.005
Sosinska, G. J., de Groot, P. W., Teixeira de Mattos, M. J., Dekker, H. L., de Koster, C. G., Hellingwerf, K. J., et al. (2008). Hypoxic conditions and iron restriction affect the cell-wall proteome of Candida albicans grown under vagina-simulative conditions. Microbiology 154(Pt 2), 510–520. doi: 10.1099/mic.0.2007/012617-0
Stie, J., Bruni, G., and Fox, D. (2009). Surface-associated plasminogen binding of Cryptococcus neoformans promotes extracellular matrix invasion. PLoS ONE 4:e5780. doi: 10.1371/journal.pone.0005780
Strasser, J. E., Newman, S. L., Ciraolo, G. M., Morris, R. E., Howell, M. L., and Dean, G. E. (1999). Regulation of the macrophage vacuolar ATPase and phagosome-lysosome fusion by Histoplasma capsulatum. J. Immunol. 162, 6148–6154.
Sun, J. N., Solis, N. V., Phan, Q. T., Bajwa, J. S., Kashleva, H., Thompson, A., et al. (2010). Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog. 6:e1001181. doi: 10.1371/journal.ppat.1001181
Sundstrom, P., and Aliaga, G. R. (1994). A subset of proteins found in culture supernatants of Candida albicans includes the abundant, immunodominant, glycolytic enzyme enolase. J. Infect. Dis. 169, 452–456. doi: 10.1093/infdis/169.2.452
Sundstrom, P., Jensen, J., and Balish, E. (1994). Humoral and cellular immune responses to enolase after alimentary tract colonization or intravenous immunization with Candida albicans. J. Infect. Dis. 170, 390–395. doi: 10.1093/infdis/170.2.390
Taylor, P. R., Brown, G. D., Reid, D. M., Willment, J. A., Martinez-Pomares, L., Gordon, S., et al. (2002). The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 169, 3876–3882. doi: 10.4049/jimmunol.169.7.3876
Tomazett, P. K., Felix, C. R., Lenzi, H. L., de Paula Faria, F., de Almeida Soares, C. M., and Pereira, M. (2010). 1,3-beta-d-Glucan synthase of Paracoccidioides brasiliensis: recombinant protein, expression and cytolocalization in the yeast and mycelium phases. Fungal Biol. 114, 809–816. doi: 10.1016/j.funbio.2010.07.007
Tsai, P. W., Yang, C. Y., Chang, H. T., and Lan, C. Y. (2011a). Characterizing the role of cell-wall beta-1,3-exoglucanase Xog1p in Candida albicans adhesion by the human antimicrobial peptide LL-37. PLoS ONE 6:e21394. doi: 10.1371/journal.pone.0021394
Tsai, P. W., Yang, C. Y., Chang, H. T., and Lan, C. Y. (2011b). Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PLoS ONE 6:e17755. doi: 10.1371/journal.pone.0017755
Turner, J., Cho, Y., Dinh, N. N., Waring, A. J., and Lehrer, R. I. (1998). Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 42, 2206–2214.
Underhill, D. M., and Pearlman, E. (2015). Immune interactions with pathogenic and commensal fungi: a two-way street. Immunity 43, 845–858. doi: 10.1016/j.immuni.2015.10.023
Vallejo, M. C., Matsuo, A. L., Ganiko, L., Medeiros, L. C., Miranda, K., Silva, L. S., et al. (2011). The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic alpha-Galactosyl epitopes. Eukaryot. Cell 10, 343–351. doi: 10.1128/EC.00227-10
van Deventer, H. J., Goessens, W. H., van Vliet, A. J., and Verbrugh, H. A. (1996). Anti-enolase antibodies partially protective against systemic candidiasis in mice. Clin. Microbiol. Infect. 2, 36–43. doi: 10.1111/j.1469-0691.1996.tb00198.x
Vargas, G., Rocha, J. D., Oliveira, D. L., Albuquerque, P. C., Frases, S., Santos, S. S., et al. (2015). Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans. Cell. Microbiol. 17, 389–407. doi: 10.1111/cmi.12374
Vautier, S., MacCallum, D. M., and Brown, G. D. (2012). C-type lectin receptors and cytokines in fungal immunity. Cytokine 58, 89–99. doi: 10.1016/j.cyto.2011.08.031
Wiesner, D. L., Specht, C. A., Lee, C. K., Smith, K. D., Mukaremera, L., Lee, S. T., et al. (2015). Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog. 11:e1004701. doi: 10.1371/journal.ppat.1004701
Willment, J. A., Marshall, A. S., Reid, D. M., Williams, D. L., Wong, S. Y., Gordon, S., et al. (2005). The human beta-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur. J. Immunol. 35, 1539–1547. doi: 10.1002/eji.200425725
Wolf, J. M., Espadas-Moreno, J., Luque-Garcia, J. L., and Casadevall, A. (2014). Interaction of Cryptococcus neoformans extracellular vesicles with the cell wall. Eukaryot. Cell 13, 1484–1493. doi: 10.1128/EC.00111-14
Woods, J. P. (2003). Knocking on the right door and making a comfortable home: Histoplasma capsulatum intracellular pathogenesis. Curr. Opin. Microbiol. 6, 327–331. doi: 10.1016/S1369-5274(03)00080-8
Xu, H., Nobile, C. J., and Dongari-Bagtzoglou, A. (2013). Glucanase induces filamentation of the fungal pathogen Candida albicans. PLoS ONE 8:e63736. doi: 10.1371/journal.pone.0063736
Zimmerman, J. W., Lindermuth, J., Fish, P. A., Palace, G. P., Stevenson, T. T., and DeMong, D. E. (1998). A novel carbohydrate-glycosphingolipid interaction between a beta-(1-3)-glucan immunomodulator, PGG-glucan, and lactosylceramide of human leukocytes. J. Biol. Chem. 273, 22014–22020. doi: 10.1074/jbc.273.34.22014
Keywords: fungal cell wall, extracellular vesicles, proteomics, host cell, cell wall remodeling
Citation: Nimrichter L, de Souza MM, Del Poeta M, Nosanchuk JD, Joffe L, Tavares PM and Rodrigues ML (2016) Extracellular Vesicle-Associated Transitory Cell Wall Components and Their Impact on the Interaction of Fungi with Host Cells. Front. Microbiol. 7:1034. doi: 10.3389/fmicb.2016.01034
Received: 20 April 2016; Accepted: 20 June 2016;
Published: 08 July 2016.
Edited by:
Agostinho Carvalho, University of Minho, PortugalReviewed by:
Olaf Kniemeyer, Hans-Knoell-Institute, GermanySophie Lev, University of Sydney at Westmead Hospital, Australia
Copyright © 2016 Nimrichter, de Souza, Del Poeta, Nosanchuk, Joffe, Tavares and Rodrigues. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leonardo Nimrichter, bmltcmljaHRlckBtaWNyby51ZnJqLmJy Marcio L. Rodrigues, bWFyY2lvbHJAY2R0cy5maW9jcnV6LmJy
 Leonardo Nimrichter
Leonardo Nimrichter Marcio M. de Souza1
Marcio M. de Souza1 Maurizio Del Poeta
Maurizio Del Poeta Joshua D. Nosanchuk
Joshua D. Nosanchuk Luna Joffe
Luna Joffe Patricia de M. Tavares
Patricia de M. Tavares Marcio L. Rodrigues
Marcio L. Rodrigues