- 1Mycoplasmology-Bacteriology Unit, Ploufragan-Plouzané Laboratory, French Agency for Food, Environmental and Occupational Health and Safety (Anses), Ploufragan, France
- 2Bretagne-Loire University, Rennes, France
- 3Laboratory for Food Safety, French Agency for Food, Environmental and Occupational Health and Safety (Anses), Paris-Est University, Maisons-Alfort, France
- 4Laboratory for Hydrology, French Agency for Food, Environmental and Occupational Health and Safety (Anses), Nancy, France
- 5Epidemiology and Welfare in Poultry and Rabbit Farming, Ploufragan-Plouzané Laboratory, French Agency for Food, Environmental and Occupational Health and Safety (Anses), Ploufragan, France
- 6Epidemiology and Welfare in Pigs, Ploufragan-Plouzané Laboratory, French Agency for Food, Environmental and Occupational Health and Safety (Anses), Ploufragan, France
The importance of the role of environment in the dissemination of antimicrobial resistant bacteria is now well recognized. Thus, bacterial indicators to monitor the phenomena are required. The Aeromonas genus is autochthonous in the aquatic environment and easy to detect in any water type, such as freshwater, or wastewater. These microorganisms are also causing infections in humans and animals (including fish). Furthermore, as Aeromonas spp. is able to acquire antimicrobial resistance mechanisms, it is candidate for indicator bacteria to follow antimicrobial resistance dissemination in aquatic environments. Unfortunately, to date, interpretation criteria for Aeromonas spp. for antimicrobial susceptibility tests are scarce in the literature. No epidemiological cut-off values for Aeromonas are currently available at EUCAST to interpret Minimum Inhibitory Concentrations (MIC). The only interpretation criteria available are clinical breakpoints from CLSI that are adapted from Enterobacteriaceae. Based on the results of MIC distributions obtained for a collection of environmental isolates of Aeromonas, this study aimed at proposing tentative epidemiological cut-off values (COWT) for Aeromonas spp. assessing whether the genus is an acceptable level of definition. Thus, 233 isolates collected from 16 rivers were identified at species level using Maldi-Tof (Bruker). Eleven different species were identified, the most abundant were A. bestiarum (n = 54), A. salmonicida (n = 45), A. sobria (n = 41), and A. eucrenophila (n = 37). 96-well micro-plates containing different concentrations of 15 antimicrobials, namely cefotaxime, ceftazidime, chloramphenicol, colistin, enrofloxacin, erythromycin, florfenicol, flumequine, gentamicin, nalidixic acid, oxolinic acid, streptomycin, temocillin, tetracycline, and trimethoprim-sulfamethoxazole, were prepared. The broth micro-dilution method was used to determine the antimicrobial susceptibility of each isolate. The estimation of COWT values was satisfactory obtained at genus level for all antimicrobials except cefotaxime and erythromycin. This first step is an invitation for other research teams to increase the amount of antimicrobial resistance data collected. Then, robustness of our proposed provisional generic epidemiological cut-off values could be assessed by testing antimicrobial susceptibility of various Aeromonas collections.
Introduction
Antimicrobial agents have revolutionized medicine in many respects, but their use has been accompanied by a rapid emergence of resistant strains, resulting now in a global health issue. Shared use of antibiotics in both Humans and animals is a growing public health concern. Human and animal infectious diseases are so closely interlinked in a common environment that the One World - One Medicine - One Health concept fully applies to tackle the growing issue of antibiotic resistance. People and animals are connected to each other through the environment (including air, water, soil…). Aquatic environments may provide an ideal setting for acquisition and dissemination of antibiotic resistance: (i) they are frequently impacted by anthropogenic activities (wastewater, runoff, aquatic farms) (Marti et al., 2014), (ii) they contain an autochthonous bacterial microbiota which harbors antimicrobial resistance associated genes, (iii) they allow the mix of bacteria from different origins (human, livestock…) (Rizzo et al., 2013), and (iv) they may contain antimicrobials or biocides which may select resistant bacteria.
Aeromonas is an autochthonous bacteria of aquatic environment, which can be isolated from virtually any water source including freshwater (Goñi-Urriza et al., 2000), estuarine environments (Silva et al., 2014), drinking waters (Pablos et al., 2009), wastewaters and sewage (Imziln et al., 1996). This genus is a major causative agent of infections in fish (Austin, 2015), indeed an increasing range of Aeromonas, including A. allosaccharophila, A. bestiarum, A. caviae, A. hydrophila, A. jandaei, A. salmonicida, A. schubertii, A. sobria biovar sobria, and A. veronii biovar sobria, have become associated with disease of predominantly freshwater fish in most countries (Figueras and Baez-Higalgo, 2015). Among them, A. hydrophila, A. caviae, and A. veronii have been associated with human diarrheal diseases and wound infections (Janda and Abbott, 2010; Shin et al., 2015). Natural transformation is a general property of Aeromonas environmental isolates (Huddleston et al., 2013). Moreover, integrons, and other genetic elements are frequently detected in Aeromonas, in respect with these properties, Aeromonas spp. has been studied as an indicator of the dissemination of antimicrobial resistance in water (Usui et al., 2016; Varela et al., 2016) or in fish (Naviner et al., 2006, 2011) excepted for ampicillin, amoxicillin-clavulanate and cefazolin which is an intrinsic resistance for Aeromonas (CLSI, 2015). Monitoring Aeromonas susceptibilities would be much more relevant if standard interpretative criteria, internationally agreed, are applied to the generated data.
To study the antimicrobial susceptibility, clinicians and epidemiologists/ecologists/microbiologists have two totally different approaches, clinicians focus on the tryptic microorganism/antibiotic/host and others on the pair microorganism/antibiotic. Clinicians need to choose the right treatment in order to have the best chance to achieve the complete recovery of their patient and avoid development of antimicrobial resistance. In order to predict the outcome of the treatment, they need to use so called “Clinical breakpoints.” Clinical breakpoints allow them to interpret an in vitro measure or estimation of the minimum inhibitory concentration (MIC), to categorize their result as Susceptible/Intermediate/Resistant, meaning high likelihood of therapeutic success/uncertain therapeutic effect/ high likelihood of therapeutic failure.
Epidemiologists and microbiologists are mainly interested in evolution or emergence of bacterial populations displaying resistant traits, regardless of any therapeutic outcome. Epidemiological cut-off values allow them to interpret an in vitro measure or estimation of the MIC taking into account only the pair microorganism/antibiotic to categorize microorganisms as wild type or non-wild type, meaning for absence or presence of any acquired and mutational resistance mechanism to the drug in question. These interpretive criteria, called ECVs and ECOFFs by CLSI and EUCAST respectively, are based on data derived from diverse laboratories and represent the upper limit of the distribution of MIC data of fully susceptible (wild type) strains.
For Aeromonas, ECVs are available but only regarding the species A. salmonicida and for florfenicol, ormethoprim-sulfadimethoxine, oxytetracycline and oxolinic acid either MICs or for Inhibition Zone Diameter (IZD) obtained by disk diffusion and for gentamicin, erythromycin, and trimethroprim-sulfamethoxazole (only IZD) (VET03/VET04-S2) (CLSI, 2014b).
If antibiotic susceptibility of clinical isolates of Aeromonas has been extensively studied, less is known about environmental strains and particularly those from freshwater not directly impacted by wastewater input.
The aim of our study was to determine MICs of 15 antimicrobial agents for a collection of Aeromonas isolates from freshwater autochthonous flora. From these data, we propose a first set of presumptive interpretative criteria called COWT (Smith et al., 2016) for Aeromonas spp.
Materials and Methods
Bacterial Isolates
During 2014, 16 rivers located in the west part of France were sampled. Fourteen rivers were sampled once in winter (February/March) and once in summer (June/July) and two were sampled thrice in winter and thrice in summer. Each water sample was duplicated, and three volumes of each were analyzed. 10 and 1 mL were filtered onto 0.45 μm cellulose ester membranes (Millipore, Watford, UK), then filters were transferred onto glutamate starch phenol-red agar (GSP–Merck) and 0.1 mL was streaked onto GSP agar. The petri dishes were incubated at 22 ± 1°C for 48 h. Yellow colonies on GSP were considered as presumptive Aeromonas. Ten colonies per sample were purified on CHROMagar™ Orientation. The identification of isolates was confirmed at the genus level by PCR (Khan et al., 2009) and identification at the species level was done by Maldi-Tof (Microflex®Bruker V4.0.0.1_4613-5627). Up to three Aeromonas isolates per water sample were included in this study and stored at −20°C in peptone water with 20% glycerol.
Determination of MICs
The broth micro-dilution method (CLSI, 2006) (VET04-A) was used to determine the MICs of 15 antimicrobial agents for Aeromonas isolates. A stock solution of each antimicrobial agent at 200X concentration was prepared with the solvent recommended by CLSI (2016) (VET04-A) and aliquots were stored at −70°C. Solutions of antimicrobial were diluted 1:100 on the day of testing. 96-well microplates (tissue culture plate, 96 well flat bottom with low evaporation led, Corning) were used. One hundred microliters of the antimicrobial solution were added to the first column of the microplate, and 50 μL of sterile water were added into each well of the microplate (excepted those of the first column). Serial two-fold dilutions of antimicrobial solution were performed by transferring 50 μL from column 1 to column 2 and so on up to column 12, to obtain final concentrations of cefotaxime (0.031–64 mg/L), ceftazidime (0.031–64 mg/L), chloramphenicol (0.062–64 mg/L), colistin (0.025–51.2 mg/L), enrofloxacin (0.008–16 mg/L), erythromycin (0.031–64 mg/L), florfenicol (0.062–128 mg/L), flumequine (0.008–16 mg/L), gentamicin (0.062–64 mg/L), nalidixic acid (0.062–128 mg/L), oxolinic acid (0.008–16 mg/L), streptomycin (0.125–256 mg/L), temocillin (0.125–256 mg/L), tetracycline (0.062–128 mg/L) and trimethoprim-sulfamethoxazole (0.031/0.589–8/152 mg/L). In this study, the five antimicrobial agents labeled in French aquaculture (flumequine, oxolinic acid, trimethoprim-sulfamethoxazole, tetracycline, and florfenicol), and other main antimicrobial agents usually used to monitor the antimicrobial resistance of gram negative bacteria were tested. In addition, temocillin was used to evidence carbapenemase production (Woodford et al., 2014).
The day before the MIC determination assay, colonies of Aeromonas were inoculated onto Mueller Hinton (MH) agar and incubated at 22 ± 1°C for 24 h. 0.5 McFarland bacterial suspensions, prepared in physiological water, were diluted 1:100 in cation-adjusted MH broth, in order to reach the final concentration of 5 × 105 CFU/mL. Fifty microliters of the suspension were added to each well of the microplate. Two wells were used as positive controls (wells with only bacterial suspension) and two as negative controls (wells with only sterile cation-adjusted MH broth used to prepare the inoculum). The microplates were incubated at 22 ± 1 C for 24 h ± 2 h.
Escherichia coli ATCC 25922 and A. salmonicida subsp salmonicida ATCC 33658 were used as controls, and incubated respectively at 35 ± 1°C and 22 ± 1°C. For all the isolates of Aeromonas tested, the density of the inoculum was controlled by inoculation of MH agar with 10 μL of the suspension from the positive control well before incubation of the microplate.
MIC Analysis and Provisional Epidemiological Cut-Off Values (COWT) Determination
From the distribution of MICs values obtained, MIC50, MIC90, and COWT were calculated. The abbreviation COWT will be used to refer to these results as the values are proposals based on this isolate collection. The abbreviations ECV and ECOFF will not be used as they refer to consensus-based epidemiological cut-off values from CLSI and EUCAST, respectively.
Provisional COWT values were statistically determined according to two methods, one proposed by Turnidge et al. and second one by Kronvall (Turnidge et al., 2006; Kronvall, 2010) which will be referred to later on as “Turnidge method” or “Kronvall method.” Fully automated and freely available Excel spreadsheet calculators to apply the normalized resistance interpretation (NRI) method (Kronvall, 2010) [available at http://www.bioscand.se/nri/ used with permission from the patent holder, Bioscand AB, TÄBY, Sweden (European patent No 1383913, US Patent No. 7,465,559)] and ECOFFinder MS (available at http://clsi.org/standards/micro/ecoffinder/) were used. Following their author's recommendation, COWT were computed for 97.7 and 99% of the population level inclusion in the wild type population, respectively. Numbers and percentages of non-wild type isolates were calculated afterwards.
Calculations were performed for each antimicrobial, at genus level on the whole dataset and at species level, when at least 30 isolates from the same species were encountered in the collection (CLSI Report cited by Smith et al., 2013).
Results
Species Diversity
233 isolates of Aeromonas spp. were collected from the 16 rivers (Figure 1). Aeromonas spp. was detected in all the 16 sampled rivers, in winter and in summer. In four rivers, less than six isolates were included due to the non-confirmation of the presumptive identification Aeromonas (data not shown). Eleven species were detected and two isolates could not be identified at the species level. A. bestiarum (n = 54; 23.1%), A. salmonicida (n = 45; 19.3%), A. sobria, (n = 41; 17.6%), A. eucrenophila (n = 37; 15.9%), and A. veronii (n = 17; 7.3%) were the five most abundant species and they accounted for 83.3% of the 233 isolates of Aeromonas included in this study. The frequency of species isolation did not differ between summer and winter (Wilcoxon test p = 0.9). Pooling winter and summer samples from the same river together, at least four different species per river were detected.
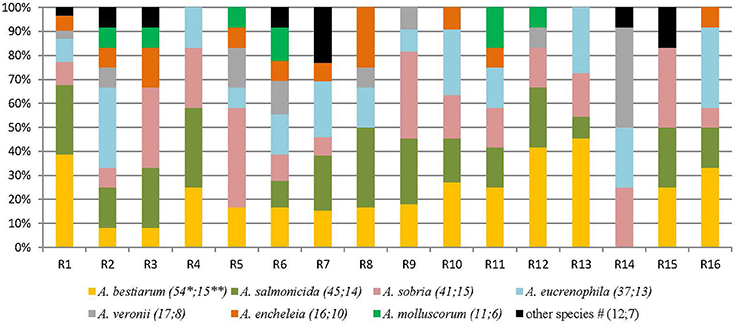
Figure 1. Diversity of Aeromonas species isolated from different freshwater sampling points (France, 16 rivers). R: river. *Number of isolates of this species. **Number of rivers where the species was detected at least one time. #Other species correspond to: 4 A. popoffii, 3 A. caviae, 2: A. media, 2 A. spp and 1 A. hydrophila; and were detected as follow: R1 1 A. popoffii; R2 1 A. spp; R3 1 A. media; R6 1 A. spp and 2 A. caviae; R7 1 A. popoffii, 1 A. hydrophila, and 1 A. caviae; R14: 1 A. media; R15: 2 A. popoffii.
Antimicrobial Susceptibility
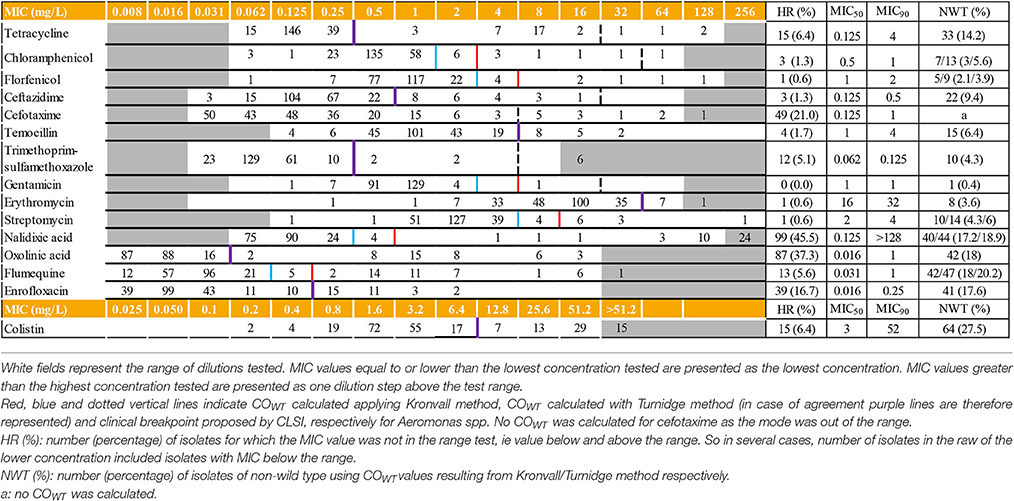
For each MIC determination assay, the results obtained for the reference strains and the density of the inoculum complied with CLSI recommendations (data not shown) (CLSI, 2006). Distribution of the MICs of 15 antimicrobials and the corresponding MIC50 and MIC90 are displayed in Table 1. MIC values below the tested ranges were observed for 12 out of the 15 antimicrobial agents. For six of them (florfenicol, trimethoprim-sulfamethoxazole, chloramphenicol, ceftazidime, streptomycin, and temocillin), less than 5% of the MIC values were concerned. The most important proportions of isolates displaying MIC below the tested range were observed for the quinolone class (oxolinic acid (n = 87; 37.3%), nalidixic acid (n = 75; 32.3%), enrofloxacin (n = 39; 16.7%) and flumequine (n = 12, 5.1%), followed by cefotaxime (n = 48; 20.6%) and tetracycline (n = 15; 6.4%).
On the opposite, MIC values above the tested ranges were observed for 44 isolates and six antimicrobial agents: cefotaxime, erythromycin, trimethoprim-sulfamethoxazole, nalidixic acid, flumequine, and colistin. Except for cefotaxime, the mode was always included in the range.
For gentamicin, the MIC50 and MIC90 values were the same: 1 mg/L. For seven out of the 15 tested antimicrobials (tetracycline, cefotaxime, nalidixic acid, oxolinic acid, flumequine, enrofloxacin and colistin), differences between MIC50 and MIC 90 were at least three dilutions.
MIC50 values calculated for Aeromonas spp. () (n = 233 isolates; 11 different species) were the same, within one dilution step, as those calculated for the four most abundant species (Table 2). Cefotaxime was the unique exception: MIC50 of the 41 isolates of A. sobria was smaller than the , 0.031 mg/L, and 0.125 mg/L, respectively.
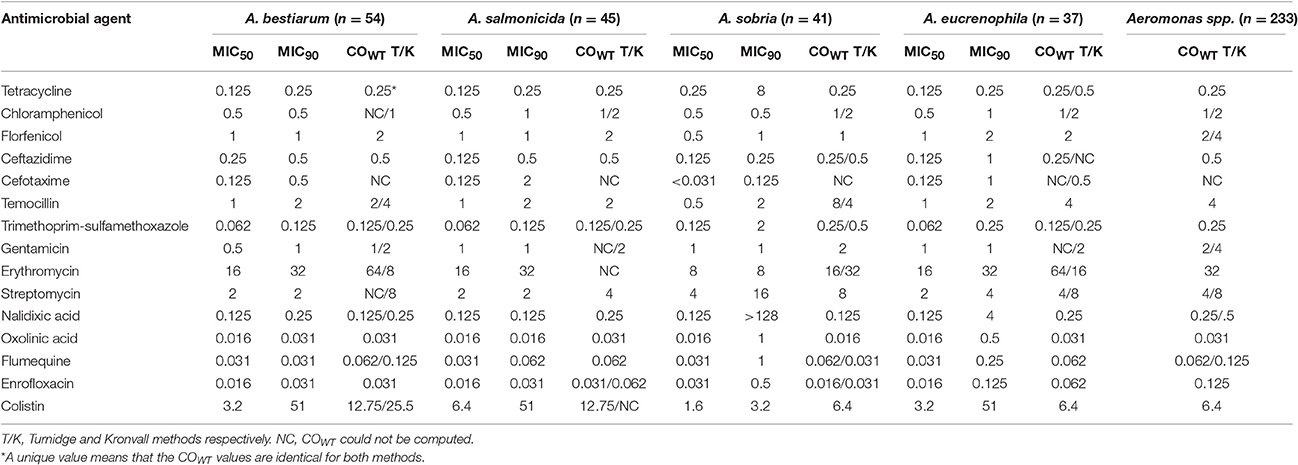
Table 2. COWT, MIC50, and MIC90 (mg/L) values specific for A. bestiarum, A. salmonicida, A. sobria, and A. eucrenophila and COWT for Aeromonas spp.
For the phenicols class (chloramphenicol and florfenicol), gentamicin and temocillin, the and MIC90 of the four most abundant species values were not significantly different, with a maximum of one dilution step variation.
COWT were calculated for 14 antimicrobial agents for the complete Aeromonas spp. dataset (Table 1). For all tested antimicrobials, MIC standard deviation never exceeded 1.2 log2 μg/ml, the limit value implemented in Kronvall method spreadsheet (Kronvall, 2010). No value was computed for cefotaxime due to the truncated distribution of isolates with a high number of strains out of the tested dilution range (Table 1). Similar COWT values were obtained by Kronvall and Turnidge methods applied for eight antimicrobials (61.5%). For the cases in which values were different, values obtained by the Turnidge method were consistently lower than the Kronvall method result by one dilution step. Percentages of non-wild type strains ranged from 0.4 to 27.5%, colistin, quinolone compounds, and tetracycline displaying the highest percentages. Excluding cefotaxime, 107 strains (45.9%) could be considered as wild type (WT) for all tested antimicrobials. The second most frequent phenotype, reduced susceptibility to colistin only, was represented by 47 strains (20.2%). Remaining isolates were distributed among 46 different combinations of non-wild type (NWT) and WT to the different antimicrobials (1 to 10 isolates per category).
Tentative species-specific COWT values (Table 2) could be computed by at least one statistical approach for all combinations excepted for cefotaxime. The values obtained by Turnidge or Kronvall methods were within one dilution step for a given pair antimicrobial-Aeromonas species, except for erythromycin. In the frame of one of the two methods, Aeromonas spp. and species-specific COWT were similar or within one dilution step for most of the species-antimicrobial combinations. Finally, combining all computed values obtained from both methods, for each antimicrobial tested except erythromycin, COWT ranges are at most three dilution step wide.
Discussion
The advantage of reporting MIC distributions is to allow comparison of studies over a long period of time even if interpretative criteria change over time (Schwarz et al., 2010), provided that the MIC determination methods are comparable. The ability to follow antimicrobial susceptibility trend over a long period of time is crucial to monitor antimicrobial resistance dissemination in the environment.
MIC distributions appeared to be bimodal for some agents: two clearly distinct populations were identified for oxolinic acid, tetracycline, and colistin. From these distributions, calculated MIC50 and MIC90 values were compared to previously published ones, even though laboratory methods were slightly different. Gentamicin MIC50 and MIC90, calculated in this study (1 mg/L), were equivalent to those obtained by Kämpfer et al. (1999) on a collection of 217 Aeromonas genomic species from various origins (1 and 2 mg/L) (Kämpfer et al., 1999), by Goñi-Urriza et al. (2000), on a collection of 138 Aeromonas spp. isolated from freshwater (1 and 2 mg/L) and by Lamy et al. (2012) on a collection of 146 isolates from clinical and environmental origins (0.5 and 1 mg/L) (Kämpfer et al., 1999; Goñi-Urriza et al., 2000; Lamy et al., 2012). For chloramphenicol, MIC50 and MIC90 were 0.5 mg/L and 1 mg/L, respectively; same as Kampfer et al. and very similar to Goñi et al. results, 1 mg/L and 2 mg/L, respectively. Here, MIC50 for trimethoprim-sulfamethoxazole (0.062/1.178 mg/L) were lower than those observed in the three latter studies (1/19 mg/L in Kämpfer et al., 0.25/4.75 mg/L for Lamy et al. and 8/152 mg/L for Goñi et al).
In their study, Goñi-Urriza et al. (2000), considered that Aeromonas spp. was poorly susceptible to streptomycin due to a MIC50 value of 16 mg/L; here was lower (2 mg/L). A MIC90 value of 16 mg/L was observed for the 41 studied isolates of A. sobria. Isolates here seemed to be more susceptible to streptomycin. Similarly, MIC50 for tetracycline was lower in the present study (0.125 mg/L vs. 0.5 mg/L). For cefotaxime, MIC50 was very similar (<0.1 mg/L vs. 0.125 mg/L). For colistin, MIC90 here was higher with a value of 52 mg/L vs. 2 mg/L which could be linked to the species composition of the collection. The MIC90 values for A. bestiarum, A. salmonicida, and A. eucrenophila were 52 mg/L although MIC90 for A. sobria was 3.2 mg/L.
Two types of thresholds are available: “Clinical breakpoints” to estimate the odds of therapeutic success to treat infections and “epidemiological cut-off values” to recognize any emerging resistance mechanism in the bacterial population studied. To delineate WT from NWT Aeromonas isolates, epidemiological cut-off values are needed.
In document M45-3rd Edition from CLSI, clinical breakpoints for 19 antimicrobial agents are proposed for Aeromonas spp. (CLSI, 2015). Aeromonas spp. includes members of Aeromonas caviae complex, Aeromonas hydrophila complex, and Aeromonas veronii complex. A footnote in the document mentions that most of the published data on susceptibility testing are limited to these three Aeromonas complexes. Moreover, the interpretative criteria are adapted from those for Enterobacteriaceae. To the best of our knowledge, all published studies on environmental sourced Aeromonas susceptibility tests, whatever their origin was freshwater (Rhodes and Kator, 1994; Imziln, 2001), wastewater (Imziln et al., 1996; Igbinosa and Okoh, 2012; Khor et al., 2015; Kim et al., 2015), aquaculture plants (Penders and Stobberingh, 2008), drinking water (Figueira et al., 2011), used these breakpoints. So, information gathered in those studies might document the hazard represented by these isolates in the context of human infections, but does not fully enquire about the issue of environmental antimicrobial resistance dissemination. In this specific environmental study, focusing on dissemination of antimicrobial resistance in an ecosystem, we found more relevant to consider epidemiological cut-off values as we only focus on the pair microorganism/antibiotic and no host or treatment option is involved.
Numerous methods were proposed to determine COWT, from “eye-ball” determination to statistically oriented ones (Turnidge et al., 2006; Turnidge and Paterson, 2007; Kronvall, 2010; Hombach et al., 2014; Jaspers et al., 2014). These methods were applied in the present study according to their authors' recommendation, computing COWT for 99 and 97.7% of the population level inclusion in the wild type population. Results from these methods were in accordance, with frequent full agreement or one dilution step difference. Values could also be computed using Jaspers method (Jaspers et al., 2014-data not shown) for three antimicrobials (colistin, erythromycin, and temocillin) and were in full agreement with those obtained with other methods. Erythromycin COWT here computed (32 mg/L) should be interpreted cautiously considering the fact that MICs of the supposed WT population are distributed over eight dilution steps instead of three to five usually.
Few epidemiological cut-off values for Aeromonas could be found in the literature. On The European Committee on Antimicrobial Susceptibility Testing website (www.eucast.org), MIC distributions are available for Aeromonas spp., but no ECOFFs have been proposed due to the low number of observations. In the frame of a simulation study to determine robustness of COWT, Smith and Kronvall published computed values for Aeromonas spp. and A. salmonicida, for oxytetracycline, oxolinic acid and florfenicol (Smith and Kronvall, 2015). The same value of 2 mg/L was proposed for florfenicol by both studies. For oxolinic acid, the value computed by Smith and Kronvall (2015) was 0.06 mg/L for Aeromonas spp. which is in accordance with our value (0.031 mg/L) considering the double dilution agreed variation for broth micro-dilution method (Smith and Kronvall, 2015). For A. salmonicida the value computed by Smith and Kronvall on a different dataset was 0.125 mg/L which is similar to the interpretive value proposed by CLSI (2014a). The CLSI ECVs for A. salmonicida were established based on visual inspection of MIC distributions for 217 isolates (Miller and Reimschuessel, 2006). In addition to oxolinic acid CLSI interpretive values were published for florfenicol (4 mg/L) oxytetracycline and ormetoprim-sulfadimethoxine (CLSI, 2015).
COWT were estimated for Aeromonas spp. and for the four main species encountered in the collection, in order to check for any species dependency upon the results. At species level, the available number of isolates was below the CLSI recommendation of 100 isolates (Smith and Kronvall, 2015) but close to the recommended minimal number of 30 WT isolates to form a Gaussian distribution as mentioned by Smith et al. (2013) and confirmed by Smith and Kronvall (2015). Nevertheless, some of the MICs distributions did not allow COWT calculation and values should be considered cautiously and unprecise (Smith et al., 2013) due to the low isolate number within a species. Species level values were mainly equal to or in the range of one dilution step around the COWT values determined for Aeromonas spp. (except 3/104 values: A. bestiarum and erythromycin COWT computed by Kronvall method, A. bestiarum and colistin COWT computed by Kronvall method, A. sobria, and enrofloxacin COWT computed by Turnidge method). These results do not preclude usage of COWT determined at the genus rather than the species level. The definition of COWT at the genus level has previously been considered (Miller and Reimschuessel, 2006; Smith et al., 2013) and applied (Miller and Reimschuessel, 2006; Smith et al., 2012; Smith and Kronvall, 2015), but contravenes to the generic principles established to set-up COWT values (Kronvall, 2010). Smith et al. (2012) addressed the question of the validity of Aeromonas genus defined COWT for antibiotic disk diffusion data, through the exploration of standard deviation of calculated normalized distribution (Smith et al., 2012). We addressed the same question comparing COWT values obtained at the genus and specific level, on a limited number of Aeromonas species. As concluded by Smith et al. (2012) for some agents, our results provide no reason why a single set of interpretative values could not be defined for application to all Aeromonas species included in our study. By projecting into daily business of a routine laboratory, identification of the genus Aeromonas is easy and reliable, even phenotypic methods could be used (Lamy et al., 2010) and several PCR were described, whereas identification at the species level requires either sequencing or Maldi-Tof methods. Moreover this genus is abundant and detectable easily through selective media. Thus, establishing COWT at genus level when possible seems more relevant to be widely used in future epidemiological studies. As emphasized by Smith et al. (2013) the workload would be greatly reduced defining interpretive criteria at the genus level.
Values obtained in this study are putative ones and should not be considered as official interpretative criteria. Our values are based on one laboratory only whereas multiple and diverse sources and a large number of isolates are recommended to offer more precise estimates (Smith et al., 2013) to reassess relevant generic COWTs.
Recently, Aeromonas spp. was proposed as a potential indicator of antimicrobial susceptibility for aquatic environment by several authors (Usui et al., 2016; Varela et al., 2016). Indeed, Aeromonas genus is ubiquitous and its abundance in aquatic environment allowed its detection all along the year. Identification methods of the Aeromonas genus are reliable and costless, which is not the case at species level (Lamy et al., 2010). Yet large environmental studies on such a complex matrix as water enforce the need of easy and cheap tools in order to analyze a large amount of samples. Indeed a large amount of samples is the only way to apprehend the complexity and the ecological condition variability of the water matrix. Harmonization of susceptibility tests at Aeromonas genus level would probably allow collecting multiple observations to follow antimicrobial resistance traits in the aquatic environment.
Conclusion
Thus, as a first step, it appears to be relevant to determine Aeromonas spp. COWT values. Further experiments might allow refining these values.
If Aeromonas spp. is used as an indicator of antimicrobial susceptibility for aquatic environment, it is absolutely essential to set epidemiological cut-off values; but this is far from being enough to be able to share and compare data. Indeed, if methods for assessing fecal contamination of water are standardized, it is absolutely not the case for Aeromonas spp. detection. Harmonization of the methods for detection, identification and characterization of Aeromonas is urgently needed.
Author Contributions
SB, IK, EL, EJ, CC, SG, and SL contributed to the design of the study. SB, EL, MC, BG, AW, and CC produced data. All authors contributed to the analysis of the data, to the redaction and/or the edition of the article.
Funding
This study was financed through “AQUARES,” research project supported by both French Ministries in charge of Environment and Agriculture. AQUARES is part of the national effort to reduce antimicrobial resistance in veterinary medicine called “EcoAntibio2017.”
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Authors would like to acknowledge the trainees implicated in AQUARES project: Camille Dorange, Philippe Fontaine, Anaïs Keraval, Louis Malard, Anthony Menard, Solène Spriet, Raphaël Veneziano, and Alexis Viel; our partners from ITAVI: Matthieu Gaumé, Aurélien Tocqueville, and Eumedica SA for providing Temocillin. Authors are especially grateful to Odile Balan for technical support and Stijn Jaspers, for his precious advice.
References
Austin, B. (2015). “Aeromonas fish pathogens,” in Aeromonas, ed J. Graf (Storrs, CT: Caister Academic Press), 45–65.
CLSI (2006). Methods for Broth Dilution Susceptibility Testing of Bacteria Isolated from Aquatic Animals; Approved Guidelinet. CLSI Document VET04-A. Wayne, PA: Clinical and Laboratory Standards Institute.
CLSI (2014a). Performance Standards for Antimicrobial Suceptibility Testing of Bacteria Isolated from Aquatic Animals; Second Informational Supplement. CLSI Document VET03/VET04-S2. Wayne, PA: Clinical and Laboratory Standards Institute.
CLSI (2014b). VET04-A2: Methods for Broth Dilution Susceptibility Testing of Bacteria Isolated from Aquatic Animals; Approved Guideline, 2nd Edn. Wayne, PA: Clinical and Laboratory Standards Institute.
CLSI (2015). Methods for Antimicrobial Dilution and Disk Susceptibility Testing of iInfrequently Isolated or Fastidious Bacteria, 3rd Edn CLSI Guideline M45. Wayne, PA: Clinical and Laboratory Standards Institute.
CLSI (2016). Performance Standards for Antimicrobial Suceptibility Testing, 26th Edn CLSI Supplement M100S. Wayne, PA: Clinical and Laboratory Standards Institute.
Figueira, V., Vaz-Moreira, I., Silva, M., and Manaia, C. M. (2011). Diversity and antibiotic resistance of Aeromonas spp. in drinking and waste water treatment plants. Water Res. 45, 5599–5611. doi: 10.1016/j.watres.2011.08.021
Figueras, M. J., and Baez-Higalgo, R. (2015). “Aeromonas infections in humans,” in Aeromonas, ed J. Graf (Storrs, CT: Caister Academic Press), 67–107.
Goñi-Urriza, M., Pineau, L., Capdepuy, M., Roques, C., Caumette, P., and Quentin, C. (2000). Antimicrobial resistance of mesophilic Aeromonas spp. isolated from two European rivers. J. Antimicrob. Chemother. 46, 297–301. doi: 10.1093/jac/46.2.297
Hombach, M., Courvalin, P., and Böttger, E. C. (2014). Validation of antibiotic susceptibility testing guidelines in a routine clinical microbiology laboratory exemplifies general key challenges in setting clinical breakpoints. Antimicrob. Agents Chemother. 58, 3921–3926. doi: 10.1128/AAC.02489-13
Huddleston, J. R., Brokaw, J. M., Zak, J. C., and Jeter, R. M. (2013). Natural transformation as a mechanism of horizontal gene transfer among environmental Aeromonas species. Syst. Appl. Microbiol. 36, 224–234. doi: 10.1016/j.syapm.2013.01.004
Igbinosa, I. H., and Okoh, A. I. (2012). Antibiotic susceptibility profile of Aeromonas species isolated from wastewater treatment plant. Sci. World J. 2012:764563. doi: 10.1100/2012/764563
Imziln, B. (2001). Occurrence and antibiotic resistance of mesophilic Aeromonas in three riverine freshwaters of Marrakech, Morocco. Sci. World J. 1, 796–807. doi: 10.1100/tsw.2001.284
Imziln, B., Lafdal, Y. M. O., and Jana, M. (1996). Effect of wastewater stabilization ponds on antimicrobial susceptibility and haemolysin occurrence among motile Aeromonas strains. World J. Microbiol. Biotechnol. 12, 385–390. doi: 10.1007/BF00340216
Janda, J. M., and Abbott, S. L. (2010). The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 23, 35–73. doi: 10.1128/CMR.00039-09
Jaspers, S., Aerts, M., Verbeke, G., and Beloeil, P. A. (2014). Estimation of the wild-type minimum inhibitory concentration value distribution. Stat. Med. 33, 289–303. doi: 10.1002/sim.5939
Kämpfer, P., Christmann, C., Swings, J., and Huys, G. (1999). In vitro susceptibilities of Aeromonas genomic species to 69 antimicrobial agents. Syst. Appl. Microbiol. 22, 662–669. doi: 10.1016/S0723-2020(99)80019-8
Khan, I. U. H., Loughborough, A., and Edge, T. A. (2009). DNA-based real-time detection and quantification of aeromonads from fresh water beaches on Lake Ontario. J. Water Health 7, 312–323. doi: 10.2166/wh.2009.041
Khor, W. C., Puah, S. M., Tan, J. A. M. A., Puthucheary, S. D., and Chua, K. H. (2015). Phenotypic and genetic diversity of Aeromonas species isolated from fresh water lakes in Malaysia. PLoS ONE 10:e0145933. doi: 10.1371/journal.pone.0145933
Kim, T. W., Joung, Y., Han, J. H., Jung, W., and Kim, S. B. (2015). Antibiotic resistance among aquatic bacteria in natural freshwater environments of Korea. J. Water Health 13, 1085–1097. doi: 10.2166/wh.2015.032
Kronvall, G. (2010). Normalized resistance interpretation as a tool for establishing epidemiological MIC susceptibility breakpoints. J. Clin. Microbiol. 48, 4445–4452. doi: 10.1128/JCM.01101-10
Lamy, B., Laurent, F., Kodjo, A., Roger, F., Jumas-Bilak, E., colBVH study group., et al. (2012). Which antibiotics and breakpoints should be used for Aeromonas susceptibility testing? Considerations from a comparison of agar dilution and disk diffusion methods using Enterobacteriaceae breakpoints. Eur. J. Clin. Microbiol. Infect. Dis. 31, 2369–2377. doi: 10.1007/s10096-012-1578-x
Lamy, B., Laurent, F., Verdier, I., Decousser, J. W., Lecaillon, E., Marchandin, H., et al. (2010). Accuracy of 6 commercial systems for identifying clinical Aeromonas isolates. Diagn. Microbiol. Infect. Dis. 67, 9–14. doi: 10.1016/j.diagmicrobio.2009.12.012
Marti, E., Variatza, E., and Balcazar, J. L. (2014). The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol. 22, 36–41. doi: 10.1016/j.tim.2013.11.001
Miller, R. A., and Reimschuessel, R. (2006). Epidemiologic cutoff values for antimicrobial agents against Aeromonas salmonicida isolates determined by frequency distributions of minimal inhibitory concentration and diameter of zone of inhibition data. Am. J. Vet. Res. 67, 1837–1843. doi: 10.2460/ajvr.67.11.1837
Naviner, M., Giraud, E., Le Bris, H., Armand, F., Mangion, C., and Ganière, J. P. (2006). Seasonal variability of intestinal microbiota in rainbow trout (Oncorhynchus mykiss), with a particular attention to Aeromonas spp. as candidate indicator of antimicrobial resistance. Rev. Med. Veterinaire 157, 597–602.
Naviner, M., Gordon, L., Giraud, E., Denis, M., Mangion, C., Le Bris, H., et al. (2011). Antimicrobial resistance of Aeromonas spp. isolated from the growth pond to the commercial product in a rainbow trout farm following a flumequine treatment. Aquaculture 315, 236–241. doi: 10.1016/j.aquaculture.2011.03.006
Pablos, M., Rodríguez-Calleja, J. M., Santos, J. A., Otero, A., and García-López, M. L. (2009). Occurrence of motile Aeromonas in municipal drinking water and distribution of genes encoding virulence factors. Int. J. Food Microbiol. 135, 158–164. doi: 10.1016/j.ijfoodmicro.2009.08.020
Penders, J., and Stobberingh, E. E. (2008). Antibiotic resistance of motile aeromonads in indoor catfish and eel farms in the southern part of The Netherlands. Int. J. Antimicrob. Agents 31, 261–265. doi: 10.1016/j.ijantimicag.2007.10.002
Rhodes, M. W., and Kator, H. (1994). Seasonal occurrence of mesophilic Aeromonas spp. as a function of biotype and water quality in temperate freshwater lakes. Water Res. 28, 2241–2251. doi: 10.1016/0043-1354(94)90039-6
Rizzo, L., Manaia, C., Merlin, C., Schwartz, T., Dagot, C., Ploy, M. C., et al. (2013). Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review. Sci. Total Environ. 447, 345–360. doi: 10.1016/j.scitotenv.2013.01.032
Schwarz, S., Silley, P., Simjee, S., Woodford, N., van duijkeren, E., Johnson, A. P., et al. (2010). Editorial: assessing the antimicrobial susceptibility of bacteria obtained from animals. J. Antimicrob. Chemother. 65, 601–604. doi: 10.1093/jac/dkq037
Shin, H. B., Yoon, J., Lee, Y., Kim, M. S., and Lee, K. (2015). Comparison of maldi-tof ms, housekeeping gene sequencing, and 16s rRNA gene sequencing for identification of Aeromonas clinical isolates. Yonsei Med. J. 56, 550–555. doi: 10.3349/ymj.2015.56.2.550
Silva, C. M., Evangelista-Barreto, N. S., Vieira, R. H. S. D. F., Mendonça, K. V., and Sousa, O. V. D. (2014). Population dynamics and antimicrobial susceptibility of Aeromonas spp. along a salinity gradient in an urban estuary in Northeastern Brazil. Mar. Poll. Bull. 89, 96–101. doi: 10.1016/j.marpolbul.2014.10.031
Smith, P., Alday-Sanz, V., Matysczak, J., Moulin, G., Lavilla-Pitogo, C. R., and Prater, D. (2013). Monitoring and surveillance of antimicrobial resistance in microorganisms associated with aquatic animals. OIE Rev. Sci. Tech. 32, 583–593. doi: 10.20506/rst.32.2.2237
Smith, P., Endris, R., Kronvall, G., Thomas, V., Verner-Jeffreys, D., Wilhelm, C., et al. (2016). Epidemiological cut-off values for Flavobacterium psychrophilum MIC data generated by a standard test protocol. J. Fish Dis. 39, 143–154. doi: 10.1111/jfd.12336
Smith, P., and Kronvall, G. (2015). How many strains are required to set an epidemiological cut-off value for MIC values determined for bacteria isolated from aquatic animals? Aquac. Int. 23, 465–470. doi: 10.1007/s10499-014-9827-x
Smith, P., Schwarz, T., and Verner-Jeffreys, D. W. (2012). Use of normalised resistance analyses to set interpretive criteria for antibiotic disc diffusion data produce by Aeromonas spp. Aquaculture 326–329, 27–35. doi: 10.1016/j.aquaculture.2011.11.011
Turnidge, J., Kahlmeter, G., and Kronvall, G. (2006). Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin. Microbiol. Infect. 12, 418–425. doi: 10.1111/j.1469-0691.2006.01377.x
Turnidge, J., and Paterson, D. L. (2007). Setting and revising antibacterial susceptibility breakpoints. Clin. Microbiol. Rev. 20, 391–408. doi: 10.1128/CMR.00047-06
Usui, M., Tagaki, C., Fukuda, A., Okubo, T., Boonla, C., Suzuki, S., et al. (2016). Use of Aeromonas spp. as General indicators of antimicrobial susceptibility among bacteria in aquatic environments in Thailand. Front. Microbiol. 7:710. doi: 10.3389/fmicb.2016.00710
Varela, A. R., Nunes, O. C., and Manaia, C. M. (2016). Quinolone resistant Aeromonas spp. as carriers and potential tracers of acquired antibiotic resistance in hospital and municipal wastewater. Sci. Total Environ. 542, 665–671. doi: 10.1016/j.scitotenv.2015.10.124
Woodford, N., Pike, R., Meunier, D., Loy, R., Hill, R., and Hopkins, K. L. (2014). In vitro activity of temocillin against multidrug-resistant clinical isolates of Escherichia coli, Klebsiella spp. and Enterobacter spp., and evaluation of high-level temocillin resistance as a diagnostic marker for OXA-48 carbapenemase. J. Antimicrob. Chemother. 69, 564–567. doi: 10.1093/jac/dkt383
Keywords: Aeromonas spp., epidemiological cut-off, freshwater, ECOFFinder, normalized resistance interpretation method, antimicrobial resistance, minimum inhibitory concentration
Citation: Baron S, Granier SA, Larvor E, Jouy E, Cineux M, Wilhelm A, Gassilloud B, Le Bouquin S, Kempf I and Chauvin C (2017) Aeromonas Diversity and Antimicrobial Susceptibility in Freshwater—An Attempt to Set Generic Epidemiological Cut-Off Values. Front. Microbiol. 8:503. doi: 10.3389/fmicb.2017.00503
Received: 21 October 2016; Accepted: 10 March 2017;
Published: 28 March 2017.
Edited by:
Magdalena Popowska, University of Warsaw, PolandReviewed by:
Jose Luis Balcazar, Catalan Institute for Water Research, SpainMaria Conceição Fontes, University of Trás-os-Montes and Alto Douro, Portugal
Etienne Giraud, Institut National de la Recherche Agronomique (INRA), France
Copyright © 2017 Baron, Granier, Larvor, Jouy, Cineux, Wilhelm, Gassilloud, Le Bouquin, Kempf and Chauvin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sandrine Baron, c2FuZHJpbmUuYmFyb25AYW5zZXMuZnI=
 Sandrine Baron
Sandrine Baron Sophie A. Granier
Sophie A. Granier Emeline Larvor1,2
Emeline Larvor1,2 Sophie Le Bouquin
Sophie Le Bouquin Isabelle Kempf
Isabelle Kempf Claire Chauvin
Claire Chauvin