- 1W. Hirsch Regional Microbiology Laboratory, Clalit Health Services, Haifa, Israel
- 2Department of Evolutionary and Environmental Biology, Faculty of Natural Sciences, University of Haifa, Haifa, Israel
- 3National Institute of Infectious Diseases, Toyama, Japan
- 4Department of Biology and Environment, Faculty of Natural Sciences, University of Haifa at Oranim, Tivon, Israel
Infections due to Vibrio cholerae are rarely documented in Israel. Here we report a case of recurrent otitis media in a young male, caused by V. cholerae non-O1/O139. This extra-intestinal infection was caused by V. cholerae O100 and has been associated with freshwater exposure and travel. Symptoms of chronic periodic earaches along with purulent exudate began about one week after the patient suffered a water skiing accident on a river in Australia. The condition lasted for three years, until his ear exudate was examined in a clinical laboratory, diagnosed and treated. Five bacterial isolates were identified as V. cholerae O100. The isolates were screened for genetic characteristics and were found positive for the presence of hapA, hlyA, and ompU virulence genes. All isolates were negative for the presence of ctxA. Based on antibiogram susceptibility testing, ciprofloxacin ear drops were used until the patient’s symptoms disappeared. This case demonstrates that exposure to freshwater can cause otitis media by V. cholerae non-O1/O139 in young and otherwise healthy humans.
Introduction
Vibrio cholerae is a Gram-negative comma-shaped, facultative anaerobic, motile bacterium, belonging to the Vibrionaceae family. V. cholerae is both a human pathogen and a natural inhabitant of aquatic environments (Colwell et al., 1977; Cottingham et al., 2003). Infections are primarily associated with ingestion of contaminated foods and water, with subsequent diarrheal illness. This species is divided into more than 200 serogroups, of which only O1 and O139 cause cholera epidemics and pandemics (Harris et al., 2012; Clemens et al., 2017).
Vibrio cholerae is a waterborn bacterium and can be found in marine and freshwaters (Clemens et al., 2017). It has been associated with various reservoirs in the aquatic environment: chitinous organisms, i.e., crustaceans and especially copepods (Vezzulli et al., 2010); chironomids (Broza and Halpern, 2001; Halpern et al., 2004, 2006, 2007; Senderovich et al., 2008; Halpern and Senderovich, 2015); phytoplankton and aquatic plants (Islam et al., 2006; Seeligmann et al., 2008); protozoa (Barker and Brown, 1994); waterfowl, which may be the disseminators of V. cholerae between water bodies, both within and between continents (Halpern et al., 2008); fish, which consume chironomids and copepods, on one hand, and are consumed by waterfowl on the other, carry V. cholerae in their intestine and may play an important role in V. cholerae dissemination (Senderovich et al., 2010; Halpern and Izhaki, 2017). Furthermore, in addition to the particle-associated growth strategy of environmental V. cholerae, few reports have demonstrated that V. cholerae can also grow in water as a free-living organism in the planktonic phase (Worden et al., 2006).
Unlike the O1 and O139 serogroups, V. cholerae non-O1/O139 serogroups rarely produce the cholera toxin (CTX), and cause milder gastrointestinal infections, but are associated with extra-intestinal infections, such as septicemia, wound infection, peritonitis, skin infection, cellulitis, necrotizing fasciitis, endophthalmitis, cholecystitis, meningitis and ear infection (Hlady and Klontz, 1996; Centers for Disease Control and Prevention [CDC], 2014; Chen et al., 2015; Hao et al., 2015).
We report here a case of non-O1/O139 V. cholerae otitis media which developed in a young male after a water skiing accident.
Case Report
Ethics Statement
This study was carried out in accordance with the recommendations of the guidelines of Helsinki Committee and was approved by the Helsinki Committee of Clalit Health Services, Israel (approval no. 0192-15-COM1). The subject gave a written informed consent in accordance with the Declaration of Helsinki.
A 27-years old Caucasian male with acute otitis media presented to an otolaryngologist at a medical clinic in Haifa, Israel, after suffering for a period of three years from a periodic earache accompanied by a malodorous and purulent exudate from his right ear, without fever. As the patient recalled, the onset of the symptoms began approximately one week after his 2011 water skiing accident on the Murray River near Mildura, VIC, Australia, in which he fell and perforated his right eardrum.
Prior to his admission, and with the onset of the periodic ear exudates, the patient had undergone a series of ear cleansings and aspirations without any significant improvement. During his admission, an ear swab was used to sample his exudate, and later was sent to the microbiology laboratory of Clalit Health Services of Haifa and Western Galilee district for further analysis.
Twelve hours after initial incubation a prominent bacterial growth was noticed on Chocolate agar, Tryptic soy blood agar, and MacConkey agar plates (Novamed, Israel). The suspected bacterium was identified as V. cholerae by means of VITEK-2 bacterial identifier system (BioMerieux, France) and by using Vitek-MS MALDI-TOF technology (BioMerieux, France). Additionally, five single colonies were picked and streaked on Luria (LB) agar (HiMedia, Mumbai) in five successive days to verify the purity of the isolates; then their identities were verified by a PCR assay in accordance with Nandi et al. (2000). The results demonstrated that all the strains were positive for ompW, a gene of an outer membrane protein, specific to V. cholerae. The isolate was confirmed as V. cholerae non-O1/O139, cholera toxin-negative by the reference laboratory of the Israeli Ministry of Health. Somatic antigen serogrouping identification was employed on the five V. cholerae isolates according to Shimada et al. (1994), and all the isolates were identified as V. cholerae serogroup O100.
The isolates underwent complete antibiogram susceptibility testing by the disk diffusion technique (Oxoid, United Kingdom) with interpretation according to the V. cholerae CLSI guidelines (Clinical and Laboratory Standards Institute [CLSI], 2014). The strains proved resistant to polymyxin B sulfate and susceptible to co-amoxiclav, cefuroxime, co-trimoxazole, gentamicin, and ciprofloxacin. Based on the results of antibiotics activity against the isolates in vitro, ciprofloxacin ear drops were used until the patients symptoms disappeared completely. Three weeks later; an additional swab from his right ear was sent to our laboratory; it was found negative for V. cholerae.
Furthermore, the presence of virulence genes in the V. cholerae isolates was determined. All the isolates were positive for hapA (soluble hemagglutinin/protease), hlyA (haemolysin), ompU (outer membrane protein) and toxR (regulatory protein) genes, but negative for the virulence gene cassette such as the cholera toxin gene (ctxA), zonula occludens toxin (zot), accessory cholera enterotoxin (ace) toxin coregulated pilus (tcpA tcpI) and for the genes encoding the Type Three Secretion System (TTSS) (vcsC2, vcsN2, vspD, and vcsV2). The primers and the PCR procedures for the genes detection are described in Nandi et al. (2000), Dziejman et al. (2005), Halpern et al. (2006), Chatterjee et al. (2009).
Discussion
Vibrio cholerae belongs to the class of environmental pathogens defined as microorganisms that normally spend a substantial part of their life cycle outside the human host but, when introduced into humans, cause disease with measurable frequency (Clemens et al., 2017). While serogroups O1 and O139 cause cholera, the non-O1/O139 serogroups can cause a milder diarrhea, and are also associated with various human infections: blood, wound, ear, and other clinical sites (Dalsgaard et al., 2000).
The patient described in the current case is an immunocompetent young male with no history of chronic ear infections before the water skiing accident in Australia, during which his eardrum was perforated. V. cholerae O1 and non-O1/O139 had been isolated from river and marine water over a wide area in Australia since 1977 (Desmarchelier et al., 1988), which may link the ear injury in the Australian river to the subsequent V. cholerae otitis media. The water skiing accident happened in January, which is summer in Australia. Water temperatures and V. cholerae population size in the aquatic environment usually are higher during this season. Summer months are also the most common months of non-O1/O139 V. cholerae infections (Chen et al., 2015).
The isolates identified in this study didn’t harbor the virulence gene cassette such as the cholera toxin gene and the genes encoding Type Three Secretion System. Thus, these genes probably have no significant role in causing otitis media. On the other hand, the isolate harbored other virulence related genes, hlyA, hapA, toxR, and ompU. The pathogenic mechanisms of V. cholerae non-O1/139 in otitis media remain unclear, but these genes might play a role in the disease process. Using cytotoxicity and apoptosis assays, showed that hlyA-positive strains of V. cholerae non-O1/O139 had significantly higher cytotoxic activity and levels of apoptosis induction than hlyA-negative strains, and that a mitochondria-dependent apoptosis pathway is involved (Kanoktippornchai et al., 2014).
Furthermore, V. cholerae O100 isolate in the current study was positive to toxR and ompU genes. ToxR is the master regulator of V. cholerae pathogenicity (Faruque et al., 1998). Among other pathogenic genes, it regulates OmpU, one of the major outer membrane proteins of V. cholerae (Miller and Mekalanos, 1988). ompU, which is an outer membrane porin, has a role in the passage of certain substances through the outer membrane. Also, it has been shown that ToxR controls resistance to various cationic antibacterial proteins, for example, polymyxin B sulfate, through a porin-mediated mechanism (Mathur and Waldor, 2004). Indeed, in the current study, the isolates that were positive to toxR and ompU genes were resistant to polymyxin B sulfate antibiotics.
Moreover, OmpU is pro-inflammatory in nature. Interestingly, it can also down-regulate LPS (lipopolysaccharide)-mediated proinflammatory responses. OmpU causes suppression of LPS-mediated responses by attenuating the LPS-mediated toll-like receptor signaling pathway (Sakharwade and Mukhopadhaya, 2015). The presence of OmpU protein in the bacterial isolate may explain the phenomenon that was observed in the current case in which the patient suffered from symptoms of earache and purulent exudate for three years. He was cured only when the bacteria was identified and treated with ciprofloxacin ear drops. This antibiotic treatment was effective and all the symptoms completely disappeared.
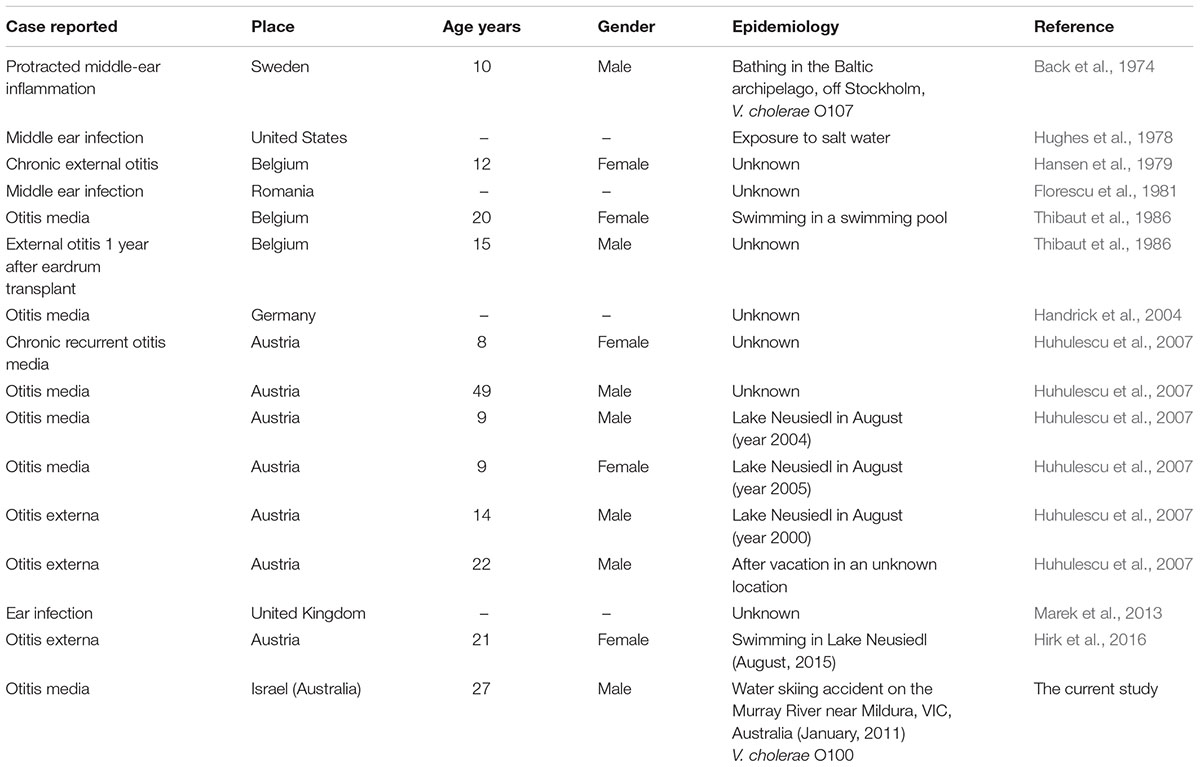
A few cases of otitis media or otitis externa caused by V. cholerae non-O1/O139 are reported in the literature. Most were children (n = 7, aged between 9 and 15 years) two young males and one female in their early twenties, and one 49 year- old-man (Table 1). The majority of the reported cases were from European countries and only one report was from the United States. In seven cases it was reported that the patients had been swimming in a swimming pool, a lake or in the Baltic Sea.
Untreated otitis media can cause complications such as loss of hearing, inner ear and facial nerve problems, including vertigo (Kitsko and Dohar, 2007), and extracranial and intracranial complications (Kangsanarak et al., 1993). Fortunately, the patient in the current case had not such complications. Nevertheless, physicians should be aware that non-O1/O139 V. cholerae can cause extra-intestinal infections that can be acquired from exposure to swimming pools and river, lake or marine water. Thus, V. cholerae should be added to the differential diagnosis of otitis infections.
Author Contributions
PK, YS, and SL-S identified the strains. EA, serotyped the strains. SL-S identified the virulence genes. PK and YS performed the antibiogram tests. PK and YS wrote the manuscript, SK-D and MH reviewed the manuscript. MH Edited the manuscript. MH, Contributed reagents/materials/publication fee.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
This study was supported by a grant from the German Research Foundation (Deutsche Forschungsgemeinschaft [DFG] grant GZ: HO 930/5-2).
References
Back, E., Ljunggren, A., and Smith, H. (1974). Non-cholera Vibrios in Sweden. Lancet 1, 723–724. doi: 10.1016/S0140-6736(74)92921-3
Barker, J., and Brown, M. R. W. (1994). Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology 140, 1253–1259. doi: 10.1099/00221287-140-6-1253
Broza, M., and Halpern, M. (2001). Chironomids egg masses and Vibrio cholerae. Nature 412, 40. doi: 10.1038/35083691
Centers for Disease Control and Prevention [CDC] (2014). “Non-O1, and Non-O139 Vibrio cholerae Infections”. Atlanta, GA: Centers for Disease Control and Prevention.
Chatterjee, S., Ghosh, K., Raychoudhuri, A., Chowdhury, G., Bhattacharya, M. K., Mukhopadhyay, A. K., et al. (2009). Incidence, virulence factors, and clonality among clinical strains of non-O1, non-O139 Vibrio cholerae isolates from hospitalized diarrheal patients in Kolkata, India. J. Clin. Microbiol. 47, 1087–1095. doi: 10.1128/JCM.02026-08
Chen, Y. T., Tang, H. J., Chao, C. M., and Lai, C. C. (2015). Clinical manifestations of non-O1 Vibrio cholerae infections. PLoS ONE 10:e0116904. doi: 10.1371/journal.pone.0116904
Clemens, J. D., Nair, G. B., Ahmed, T., Qadri, F., and Holmgren, J. (2017). Cholera. Lancet doi: 10.1016/S0140-6736(17)30559-7 [Epub ahead of print].
Clinical and Laboratory Standards Institute [CLSI] (2014). Performance Standards for Antimicrobial Susceptibility Testing. CLSI Document M45. Wayne, PA: Clinical and Laboratory Standards Institute.
Colwell, R. R., Kaper, J. B., and Joseph, S. W. (1977). Vibrio cholerae, Vibrio parahaemolyticus and other Vibrios: occurrence and distribution in Chesapeake Bay. Science 198, 394–396. doi: 10.1126/science.198.4315.394-a
Cottingham, K. L., Chiavelli, D. A., and Taylor, R. K. (2003). Environmental microbe and human pathogen: the ecology and microbiology of Vibrio cholerae. Front. Ecol. Environ. 1, 80–86. doi: 10.1890/1540-9295(2003)001[0080:EMAHPT]2.0.CO;2
Dalsgaard, A., Forslund, A., and Bruun, B. (2000). Clinical manifestations and characterization of extra-intestinal Vibrio cholerae non-O1, non-O139 infections in Denmark. Clin. Microbiol. Infect. 6, 625–627. doi: 10.1046/j.1469-0691.2000.00174.x
Desmarchelier, P. M., Momen, H., and Salles, C. A. (1988). A zymovar analysis of Vibrio cholerae isolated in Australia. Trans. R. Soc. Trop. Med. Hyg. 82, 914–917. doi: 10.1016/0035-9203(88)90041-7
Dziejman, M., Serruto, D., Tam, V. C., Sturtevant, D., Diraphat, P., Faruque, S. M., et al. (2005). Genomic characterization of non-O1, non-O139 Vibrio cholerae reveals genes for a type III secretion system. Proc. Natl. Acad. Sci. U.S.A. 102, 3465–3470. doi: 10.1073/pnas.0409918102
Faruque, S. M., Albert, M. J., and Mekalanos, J. J. (1998). Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 62, 1301–1314.
Florescu, D. P., Nacescu, N., and Ciufecu, C. (1981). Vibrio cholerae non group O1 associated with middle ear infection. Arch. Roum. Pathol. Exp. Microbiol. 40, 369–372.
Halpern, M., Broza, Y. B., Mittler, S., Arakawa, E., and Broza, M. (2004). Chironomid egg masses as a natural reservoir of Vibrio cholerae non-O1 and non-O139 in freshwater habitats. Microb. Ecol. 47, 341–349. doi: 10.1007/s00248-003-2007-6
Halpern, M., and Izhaki, I. (2017). Fish as possible reservoirs and vectors of Vibrio cholerae. Front. Microbiol. 8:282. doi: 10.3389/fmicb.2017.00282
Halpern, M., Landesberg, O., Raats, D., and Rosenberg, E. (2007). Culturable and VBNC Vibrio cholerae; Interactions with chironomid egg masses and their bacterial population. Microb. Ecol. 53, 285–293. doi: 10.1007/s00248-006-9094-0
Halpern, M., Raats, D., Lavion, R., and Mittler, S. (2006). Dependent population dynamics between chironomids (nonbiting midges) and Vibrio cholerae. FEMS Microb. Ecol. 55, 98–104. doi: 10.1111/j.1574-6941.2005.00020.x
Halpern, M., and Senderovich, Y. (2015). Chironomid microbiome. Microb. Ecol. 70, 1–8. doi: 10.1007/s00248-014-0536-9
Halpern, M., Senderovich, Y., and Izhaki, I. (2008). Waterfowl — the missing link in epidemic and pandemic cholera dissemination? PLoS Pathog. 4:e1000173. doi: 10.1371/journal.ppat.1000173
Handrick, W., Schwede, J., Schulz, K., Fitz, F. P., Pommerenke, G., and Reintanz, G. (2004). Otitis caused by Vibrio cholerae non-01/non-0139 strains acquired in Germany. MMW Fortschr. Med. 146, 38–39.
Hansen, W., Crokaert, F., and Yourassowsky, E. (1979). Two strains of Vibrio species with unusual biochemical features isolated from ear tracts. J. Clin. Microbiol. 9, 152–153.
Hao, Y., Wang, Y., Bi, Z., Sun, B., Jin, Y., Bai, Y., et al. (2015). A case of nonO1/non- O139 Vibrio cholerae septicemia and meningitis in a neonate. Int. J. Infect. Dis. 35, 117–119. doi: 10.1016/j.ijid.2015.05.004
Harris, J. B., LaRocque, R. C., Qadri, F., Ryan, E. T., and Calderwood, S. B. (2012). Cholera. Lancet 379, 2466–2476. doi: 10.1016/S0140-6736(12)60436-X
Hirk, S., Huhulescu, S., Allerberger, F., Lepuschitz, F., Rehak, S., Weil, S., et al. (2016). Necrotizing fasciitis due to Vibrio cholerae non-O1/non-O139 after exposure to Austrian bathing sites. Wieb Klin. Wochenschr. 128, 141–145. doi: 10.1007/s00508-015-0944-y
Hlady, W. G., and Klontz, K. C. (1996). The epidemiology of Vibrio infections in Florida, 1981–1993. J. Infect. Dis. 173, 1176–1183. doi: 10.1093/infdis/173.5.1176
Hughes, J. M., Hollis, D. G., Gangorosa, E. J., and Weaver, R. E. (1978). Non-cholera vibrio infections in the United States. Clinical, epidemiologic, and laboratory features. Ann. Intern. Med. 88, 602–606. doi: 10.7326/0003-4819-88-5-602
Huhulescu, S., Indra, A., Feierl, G., Stoeger, A., Ruppitsch, W., Sarkar, B., et al. (2007). Occurrence of Vibrio cholerae serogroups other than O1 and O139 in Austria. Wien Klin. Wochenschr. 119, 235–241. doi: 10.1007/s00508-006-0747-2
Islam, M. S., Goldar, M. M., Morshed, M. G., Bakht, H. B. M., Islam, M. S., and Sack, D. A. (2006). Chemotaxis between Vibrio cholerae O1 and a blue-green alga, Anabaena sp. Epidemiol. Infect. 134, 645–648. doi: 10.1017/S0950268805005297
Kangsanarak, J., Fooanant, S., Ruckhaount, K., Navacharoem, N., and Teotrakul, N. (1993). Extracranial and intracranial complications of suppurative otitis media, report of 102 cases. J. Laryngol. Otol. 107, 999–1004. doi: 10.1017/S0022215100125095
Kanoktippornchai, B., Chomvarin, C., Hahnvajanawong, C., and Nutrawong, T. (2014). Role of hlyA-positive Vibrio cholerae non-O1/non-O139 on apoptosis and cytotoxicity in a Chinese hamster ovary cell line. Southeast Asian J. Trop. Med. Public Health 45, 1365–1375.
Kitsko, D. J., and Dohar, J. E. (2007). Inner ear and facial nerve complications of acute otitis media, including vertigo. Curr. Allergy Asthma Rep. 7, 444–450. doi: 10.1007/s11882-007-0068-1
Marek, A., Inkster, T., Anderson, E., Jenkins, C., Boyed, J., Kerr, S., et al. (2013). Non-toxigenic Vibrio cholerae bacteraemia: case report and review of the literature. J. Med. Microbiol. 62, 1357–1359. doi: 10.1099/jmm.0.060400-0
Mathur, J., and Waldor, M. K. (2004). The Vibrio cholerae ToxR-regulated porin OmpU confers resistance to antimicrobial peptides. Infect. Immun. 72, 3577–3583. doi: 10.1128/IAI.72.6.3577-3583.2004
Miller, V. L., and Mekalanos, J. J. (1988). A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170, 2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988
Nandi, B., Nandy, R. K., Mukhopadhyay, S., Nair, G. B., Shimada, T., and Ghose, A. C. (2000). Rapid method for species-specific identification of Vibrio cholerae using primers targeted to the gene of outer membrane protein OmpW. J. Clin. Microbiol. 38, 4145–4151.
Sakharwade, S. C., and Mukhopadhaya, A. (2015). Vibrio cholerae porin OmpU induces LPS tolerance by attenuating TLR-mediated signaling. Mol. Immunol. 68, 312–324. doi: 10.1016/j.molimm.2015.09.021
Seeligmann, C. T., Mirande, V., Tracanna, B. C., Silva, C., Aulet, O., Cecilia, M., et al. (2008). Phytoplankton-linked viable non-culturable Vibrio cholerae O1 (VNC) from rivers in Tucuman, Argentina. J. Plankton Res. 30, 367–377. doi: 10.1093/plankt/fbn008
Senderovich, Y., Gershtein, Y., Halewa, E., and Halpern, M. (2008). Vibrio cholerae and Aeromonas; do they share a mutual host? ISME J. 2, 276–283. doi: 10.1038/ismej.2007.114
Senderovich, Y., Izhaki, I., and Halpern, M. (2010). Fish as reservoirs and vectors of Vibrio cholerae. PLoS ONE 5:e8607. doi: 10.1371/journal.pone.0008607
Shimada, T., Arakawa, E., Itoh, K., Okitsu, T., Matsushima, A., Asai, Y., et al. (1994). Extended serotyping scheme for Vibrio cholerae. Curr. Microbiol. 28, 175–178. doi: 10.1007/BF01571061
Thibaut, K., Van de Heyning, P., and Pattyn, S. R. (1986). Isolation of non-01 V. cholerae from ear tracts. Eur. J. Epidemiol. 2, 316–317. doi: 10.1007/BF00419497
Vezzulli, L., Pruzzo, C., Huq, A., and Colwell, R. R. (2010). Environmental reservoirs of Vibrio cholerae and their role in cholera. Environ. Microbiol. Rep. 2, 27–33. doi: 10.1111/j.1758-2229.2009.00128.x
Keywords: Vibrio cholerae, otitis media, case report, ear, serogroup identification, antibiogram, virulence genes
Citation: Kechker P, Senderovich Y, Ken-Dror S, Laviad-Shitrit S, Arakawa E and Halpern M (2017) Otitis Media Caused by V. cholerae O100: A Case Report and Review of the Literature. Front. Microbiol. 8:1619. doi: 10.3389/fmicb.2017.01619
Received: 28 April 2017; Accepted: 09 August 2017;
Published: 28 August 2017.
Edited by:
José Roberto Mineo, Federal University of Uberlandia, BrazilReviewed by:
Rudra Bhowmick, Oklahoma State University, United StatesAvi Peretz, Poria Medical Center, Israel
Copyright © 2017 Kechker, Senderovich, Ken-Dror, Laviad-Shitrit, Arakawa and Halpern. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Malka Halpern, bWhhbHBlcm5AcmVzZWFyY2guaGFpZmEuYWMuaWw=
 Peter Kechker1
Peter Kechker1 Shifra Ken-Dror
Shifra Ken-Dror Sivan Laviad-Shitrit
Sivan Laviad-Shitrit Eiji Arakawa
Eiji Arakawa Malka Halpern
Malka Halpern