- 1Key Laboratory of Zoonosis, Ministry of Education, College of Veterinary Medicine, Jilin University, Changchun, China
- 2Center of Infection and Immunity, The First Hospital, Jilin University, Changchun, China
Staphylococcus aureus (S.aureus) resistance, considered a dilemma for the clinical treatment of this bacterial infection, is becoming increasingly intractable. Novel anti-virulence strategies will undoubtedly provide a path forward in combating these resistant bacterial infections. Sortase A (SrtA), an enzyme responsible for anchoring virulence-related surface proteins, and alpha-hemolysin (Hla), a pore-forming cytotoxin, have aroused great scientific interest, as they have been regarded as targets for promising agents against S. aureus infection. In this study, we discovered that chalcone, a natural small compound with little anti-S. aureus activity, could significantly inhibit SrtA activity with an IC50 of 53.15 μM and Hla hemolysis activity with an IC50 of 17.63 μM using a fluorescence resonance energy transfer (FRET) assay and a hemolysis assay, respectively. In addition, chalcone was proven to reduce protein A (SpA) display in intact bacteria, binding to fibronectin, formation of biofilm and S. aureus invasion. Chalcone could down-regulate the transcriptional levels of the hla gene and the agrA gene, thus leading to a reduction in the expression of Hla and significant protection against Hla-mediated A549 cell injury; more importantly, chalcone could also reduce mortality in infected mice. Additionally, molecular dynamics simulations and mutagenesis assays were used to identify the mechanism of chalcone against SrtA, which implied that the inhibitory activity lies in the bond between chalcone and SrtA residues Val168, Ile182, and Arg197. Taken together, the in vivo and in vitro experiments suggest that chalcone is a potential novel therapeutic compound for S. aureus infection via targeting SrtA and Hla.
Introduction
Staphylococcus aureus (S. aureus) is the etiologic agent of a wide range of clinical infections, including bacteremia, infective endocarditis, and osteoarticular infections, as well as skin and soft tissue infections and metastatic abscess formation (Lowy, 1998; Coates et al., 2014). The continuous emergence of S. aureus strains developing a resistance to antibiotics, such as methicillin-resistant S. aureus (MRSA) and vancomycin-resistant S. aureus (VASA), has been largely responsible for the severe clinical complications and increase in the incidence rate of unfavorable prognoses (Ippolito et al., 2010; Gould, 2013). Simultaneously, there are few high-efficiency antibiotics in the drug discovery pipeline. Thus, treatment options are severely limited. The pressing challenge is the identification of new drug targets and the discovery of new agents against S. aureus infection.
A formidable array of secreted exotoxins and surface proteins anchored in the cell wall plays a crucial role in the pathogenic process of S. aureus (Dinges et al., 2000; Wardenburg et al., 2007). Among all of the virulence factors, sortase and alpha-hemolysin (Hla) generate the most interest. Interest in sortase as a target for the establishment of anti-virulence strategies primarily stems from studies in which loss of the gene encoding sortase led to the decreased virulence of S. aureus in a mouse model of S. aureus infection (Albus et al., 1991; Mazmanian et al., 2000). As the so-called “house-keeping” sortase, sortase A (SrtA) plays a critical role in the anchoring of surface proteins of S. aureus to the cell wall envelope. Surface proteins of Gram-positive bacteria, as one of the virulence factors, play a significant part in the process of invading the host. One of the shared features of these proteins that mediate bacterial adhesion and evade host immune defenses is that they all contain LPXTG (Leu-Pro-X-Thr-Gly) sorting signals, which SrtA can identify and use to catalyze the anchor further (Fischetti et al., 1990; Scott and Barnett, 2006). Soon after SrtA was discovered and cloned, many studies reported that the in vivo inhibition of SrtA could be measured. Subsequently, a number of SrtA inhibitors, including natural products and synthetic small molecules, among others, were identified (Clancy et al., 2010; Zhang et al., 2014, 2016; Lin et al., 2015).
Another important target, Hla, which is encoded by the hla gene and is generally secreted late in the exponential phase of growth, is a water-soluble pore-forming cytotoxin leading to the damage and death of cells, such as erythrocytes and epithelial cells, owing to its lytic property (Berube and Bubeck, 2013). Previous studies reported that Hla damaged the air-blood barrier of the lung in a rat model, and similar to sortase, S. aureus lacking Hla exhibited an obvious attenuated pathogenicity in a mouse model of S. aureus infection (Mcelroy et al., 1999; Wardenburg et al., 2007). A number of previous studies have shown that inhibitors targeting Hla could significantly prevent MASA infection, indicating a novel and effective strategy for combating S. aureus (Ragle et al., 2010).
Notably, an anti-virulence strategy targeting sortase or Hla was able to disrupt the pathogenesis of bacterial infections without a direct interference with bacterial growth (Levy et al., 1976) and, thus, may be less likely to induce selective pressures and may slow down the development of drug resistance. In addition, we reasoned that a strategy simultaneously targeting both sortase and Hla could cause a double blow to S. aureus infection and thereby represent a more effective anti-infection treatment. In this study, we first found that one class of dietary compound, called chalcone (Figure 1A), could be a promising inhibitor for targeting both S. aureus SrtA and Hla and then systematically evaluated the inhibitory activity of chalcone with a fluorescence resonance energy transfer (FRET) assay and a hemolysis assay, respectively, where chalcone was found to significantly neutralize SrtA activity and inhibit Hla production. The therapeutic effect in a S. aureus infection mouse model was found to be obvious as well. With these approaches, we provide powerful evidence that chalcone is a potential agent for the treatment of S. aureus infection via targeting SrtA and Hla.
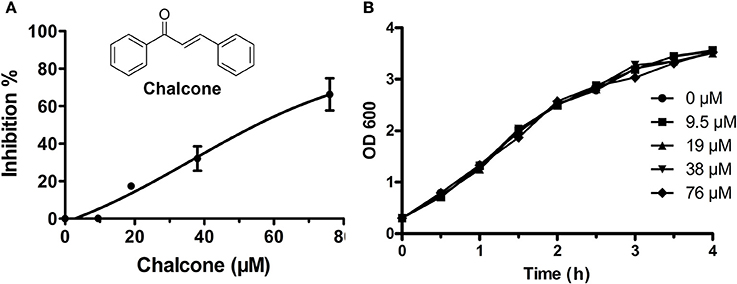
Figure 1. Chalcone inhibits S. aureus SrtA activity. (A) The inhibitory effect of chalcone on S. aureus SrtA activity. After pre-incubation with various concentrations of chalcone, followed by adding the model substrate peptide Dabcyl-QALPETGEE-Edans, a microplate reader was used to determine the catalytic activity of each sample. (B) The growth curve of S. aureus treated with the indicated concentrations of chalcone.
Methods
Bacterial Strains, Growth Conditions, and Reagents
Staphylococcus aureus strain USA 300 and S. aureus ΔSrtA strain USA 300 ΔSrtA, obtained from our lab, were used in the present study and cultured in brain-heart infusion (BHI) broth (Sigma) at 37°C. Chalcone was purchased from the Tianjin Yifang S&T Co., Ltd. (Tianjin, China). The fluorescent peptide Dabcyl-QALPETGEE-Edans was purchased from GL Biochem (Shanghai, China).
Construction of Plasmids Encoding Wild-Type (WT)-SrtA, V168A-SrtA, I182A-SrtA, and R197A-SrtA
The DNA sequence encoding the WT-SrtA protein was amplified from S. aureus USA 300 genomic DNA and used as a template along with the corresponding primers (Table 1) to amplify the S. aureus SrtA sequence through PCR. The PCR products were digested with Nde1 and BamH1 and then cloned into the pGEX-6P-1 expression vector. The pGEX-6P-1-srtA plasmid encoding WT-SrtA was generated after the sequence was confirmed by DNA sequencing. Site-directed mutagenesis for V168A-SrtA, I182A-SrtA, and R197A-SrtA was carried out using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). The mutagenic primer pairs employed to produce the three mutants are listed in Table 1.
Expression and Purification of WT-SrtA, V168A-SrtA, I182A-SrtA, and R197A-SrtA
WT-SrtA and all mutant constructs were transformed into Escherichia coli BL21 (DE3) cells. Subsequently, the transformants were grown and selected in Luria-Bertani (LB) media with ampicillin (100 mg/L) at 37°C. To induce protein expression, 1 mM IPTG (Sigma) was added into the bacteria suspension when the OD (600 nm) reached 0.6–0.8, followed by overnight growth at 16°C. The bacteria were harvested through centrifugation at 3,000 × g for 30 min at 4°C and resuspended in the reaction buffer (50 mM Tris-HCl, 5 mM CaCl2 and 150 mM NaCl, pH 7.5). The cell debris was removed through centrifugation at 10,000 × g for 1 h at 4°C after sonication. The supernatant was applied to a self-packaged GST-affinity column (2 ml glutathione Sepharose 4B) (GE Amersham Biosciences, Piscataway, NJ, USA). First, the reaction buffer was used to remove the unbound contaminating proteins, then Precision Protease was used to digest the GST-tagged protein at 4°C overnight. Finally, the reaction buffer was used again to wash off the target protein. The point mutations V168A-SrtA, I182A-SrtA, and R197A-SrtA were expressed and purified similarly to WT-SrtA.
WT and Mutant SrtA Activity Measurement
To measure the activity of WT and mutant SrtA, a FRET assay was used. As detailed earlier (Tonthat et al., 1999), 100 μl of a mixture consisting of the reaction buffer, purified proteins and different concentrations of chalcone were added in the appropriate order to 96-well plates and incubated for 30 min at 37°C. Subsequently, upon the addition of the fluorescent peptide substrate Dabcyl-QALPETGEE-Edans, the reaction was initiated, and after incubation for 1 h at 37°C, the fluorescence was read using emission and excitation wavelengths of 350 and 520 nm, respectively.
Anti-S. aureus Activity of Chalcone
The minimum inhibitory concentration (MIC) of chalcone against S. aureus was determined by broth microdilution according to the NCCLS guideline M31-A2. For growth curve plotting, 1 ml of the overnight bacterial cultures was transferred (1: 50) to BHI broth containing different concentrations of chalcone. The absorbance reading was taken at OD (600 nm).
Protein A (SpA)-Related Fluorescence Analysis
Overnight cultures of S. aureus wild-type strain (WT strain) were inoculated 1: 1000 into fresh BHI broth and grown to an OD (600 nm) of 1.0 with chalcone or dimethyl sulfoxide (DMSO, as the solvent control) at 37°C. Meanwhile, the USA300 ΔSrtA strain (WTΔSrtA strain) was used as the positive control. The bacteria were fixed with a 4% formaldehyde solution for 20 min after centrifugal collection (3,000 × g for 5 min) and washed twice with PBS. The bacteria were then resuspended in PBS containing a 1: 25 dilution of FITC-labeled goat anti-rabbit IgG (eBioscience) and incubated for 2.5 h at room temperature. Subsequently, the cells were washed three times with PBS and added to poly-L-lysine-coated glass slides. The SpA-related fluorescence was observed using a confocal laser-scanning microscope (Olympus, Shanghai, China).
Fibronectin-Binding Assay
The WT strain was grown in BHI broth to an OD (600 nm) of 0.5 with chalcone or DMSO in a shaking incubator with a shaker rate of 190 rpm at 37°C; the WTΔSrtA strain was used as a positive control. The bacteria were collected centrifugally (3,000 × g for 5 min), and after washing with PBS three times, the cells were resuspended using PBS and adjusted to an OD (600 nm) of 1.0. Fibronectin from bovine plasma with a concentration of 2 μg/ml was added into 96-well flat-bottom polystyrene microtiter plates, 100 μl in every well, and incubated overnight at 4°C. After washing three times with PBS, 100 μl of bovine serum albumin (BSA) at a concentration of 5% was added into the plate and incubated for 2 h at 37°C. The plates were washed three times afterwards, then 100 μl of the bacterial suspension was added and incubated for 2 h at 37°C. Subsequently, after removing the suspension and washing three times with PBS, 100 μl of crystal violet with a concentration of 0.4% was added and incubated for 30 min at 37°C. The absorbance of the plates was then read at 570 nm with a microplate reader (Tecan, Austria) after the plates were washed again and dried.
Biofilm Formation Assay
The WT strain was grown in BHI broth to an OD (600 nm) of 0.6 with chalcone or DMSO with a shaker rate of 190 rpm at 37°C (the WTΔSrtA strain was used as the positive control), and then, 10 μl of the bacterial solution was added into the 96-well flat-bottom polystyrene microtiter plates containing 290 ml BHI of broth and 3% (w/v) sucrose with or without chalcone. The mixture was incubated anaerobically under still culture conditions for 18 h at 37°C. After incubation, the liquid containing the bacteria and medium was removed, followed by the addition of 100 μl of 10% formaldehyde solution, which was then left overnight at room temperature to fix the biofilm. Subsequently, the formaldehyde was removed, and each well was stained with 100 μl of 0.1% v/v crystal violet for 30 min at room temperature. After rinsing with double distilled water and drying, 200 μl of 33% acetic acid was added to each well and all of the contents were mixed manually. The absorbance of the plates was subsequently read at 490 nm.
Cell Invasion Assays
J774 cells were suspended in DMEM / HIGH GLUCOSE supplemented with 10% heat-inactivated fetal bovine serum (HI-FBS; Invitrogen), and approximately 3 × 105 cells were seeded into each well of 24-well flat-bottom polystyrene microtiter plates containing 12 mm diameter coverslips overnight (37°C, 5% CO2). The WT strain was grown in BHI broth to an OD (600 nm) of 1.0 with chalcone or DMSO in a shaking incubator with a shaker rate of 190 rpm at 37°C; the WTΔSrtA strain was used as the positive control. The bacteria were then adjusted to an OD (600 nm) of 1.0, and 1 ml of the bacterial solution was added into each well and incubated for 60 min at 37°C. After each well was washed three times with PBS, 1 ml of DMEM / HIGH GLUCOSE supplemented with 300 μg / ml gentamicin was added and incubated for 30 min at 37°C. The J774 cells on the coverslips were then lysed in the sterile distilled water and plated onto BHI broth agar plates for CFU after washing three times with PBS. The plates were placed overnight at 37°C.
Molecular Modeling
In this work, the initial structure of SrtA was obtained from the X-ray crystallography 3D structure (PDB code: 3CI5). To obtain the starting structure of the ligand/SrtA complex for a molecular dynamics (MD) simulation, a standard docking procedure for a rigid protein and a flexible ligand was performed with AutoDock 4 (Morris et al., 2009; Hu et al., 2010). Subsequently, the molecular dynamics simulation of the complex's systems was performed, and the details of the processes of the computational biology method were described in a previous report (Dong et al., 2013; Lv et al., 2013; Niu et al., 2013).
Binding Affinity Determination of Ligands with Proteins
In our paper, the fluorescence-quenching method was used to measure the binding constants (KA) of ligands with proteins. A 280-nm excitation wavelength with a 5-nm bandpass and a 345-nm emission wavelength with a 10-nm bandpass were used for the measurements. Details of the measurements were described previously (Bandyopadhyay et al., 2002; Jurasekova et al., 2009).
Hemolysis Assay
Overnight cultures of the WT strain were inoculated 1: 100 into fresh BHI broth and grown to an OD (600 nm) of 0.3 at 37°C. DMSO or different concentrations of chalcone were then added for further culture. To determine the effect of chalcone on the hemolysis activity of S. aureus, 1 ml of supernatant was harvested (3,000 × g, 5 min), and 100 μl of the bacterial culture supernatants were incubated with rabbit erythrocytes whose final concentration was 2.5% in PBS at 37°C for 20 min, after which the samples were centrifuged (10,000 × g, 1 min). Finally, the release of hemoglobin was measured at OD (543 nm).
To determine the effect of chalcone on hemolysis induced by the bacterial culture supernatants, the bacterial supernatants were pre-cultured with different concentrations of chalcone as described above.
Western Blotting Assay
An equal volume of the bacterial culture supernatants that were harvested in the hemolysis assay was resolved using SDS-PAGE, and the proteins were transferred onto PVDF membranes. After blocking in 5% non-fat milk for 2 h, the membranes were incubated with a primary rabbit anti-Hla antibody (Sigma-Aldrich) diluted 1: 8,000 for 2 h and a horseradish peroxidase-conjugated secondary antibody (Proteintech) diluted 1: 4,000 for 2 h. The signals were visualized on a Tanon-4200 imager using Amersham ECL Western blotting detection reagents (GE Healthcare, Buckinghamshire, UK).
Real-Time RT-PCR Assay
The WT strain with DMSO or various concentrations of chalcone was cultured and grown to an OD (600 nm) of 2.5 at 37°C. As described previously (Qiu et al., 2011), the total RNA from the cultured bacteria was isolated and then reverse transcribed into cDNA using the Takara RNA PCR kit (AMV), ver. 3.0 (Takara, Kyoto, Japan). According to the manufacturer's instructions, the PCR reactions were performed in 25-μl volumes using SYBR Premix Ex Taq TM (Takara). The 7000 Sequence Detection System (Applied Biosystems, Courtaboeuf, France) was used to assess the PCR amplification. All primer pairs used for this assay conformed with a previous study (Qiu et al., 2011). It is worth noting that the housekeeping gene, gyrBRNA, was used as an endogenous control to normalize the expressional levels between the samples.
Live/Dead and Cytotoxicity Assays
Hla, as a vital factor, has been shown to participate in mediating A549 cell injury and death (Bubeck and Olaf, 2008). For this reason, A549 cells were cultured and transferred into 96-well flat-bottom polystyrene microtiter plates at a density of 2 × 104 cells per well in 200 μl of culture medium and incubated with the bacterial suspensions harvested above. After a 5-h incubation at 37°C, the Live/Dead (green/red) reagent (Roche) and the Cytotoxicity Detection kit (LDH, Roche) were used to assess the therapeutic effect of chalcone on A549 cells. The images of the Live/Dead cells were acquired using a confocal laser-scanning microscope, and the release of LDH was determined on a microplate reader (Tecan, Austria) at 490 nm.
Animal Experiments
The mice (6- to 8-week-old-female C57BL/6J) used for the animal experiments were obtained from the Experimental Animal Center of Jilin University. The animal experiments were approved by and conducted in accordance with the guidelines of the Animal Care and Use Committee of Jilin University. Overnight cultures of S. aureus were inoculated 1: 100 into fresh BHI broth and grown to an OD (600 nm) of 0.6 at 37°C. After centrifugal collection (3,000 × g for 5 min) and washing three times with PBS, the bacteria were resuspended in PBS to the required concentration for survival studies. Bacteria (4 × 108 CFUs) were dropped into the left nare of the mice. To investigate the therapeutic effect of chalcone, the mice were treated with a subcutaneous injection of chalcone of 150 mg/kg after infection with S. aureus and then at 12-h intervals thereafter. The control mice were treated with equal volumes of DMSO simultaneously. The mortality analysis was monitored after 96 h, with ten mice contained in each experimental group.
Statistical Analysis
The statistical significance of the treated and control group were assessed using the log-rank tests for the survival curves, and for other assays, a Student's t-test was used. The differences were analyzed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA) statistical software, and the differences were considered statistically significant when P < 0.05. The data are presented as the mean ± SD.
Results
Chalcone Inhibits the Activity of SrtA and the Expression of Hla
Chalcone (Figure 1A), one of the effective components of herbal plants, has been identified to be a remarkable inhibitor against bacteria virulence factors (Wallockrichards et al., 2015; Li et al., 2016). In this study, chalcone was shown to be a potential inhibitor against S. aureus SrtA and Hla. A FRET assay was used to determine the inhibitory activity of chalcone against SrtA, and the result indicated that the inhibition of SrtA activity was significant in the presence of various concentrations of chalcone (Figure 1A). We determined that the half maximal inhibitory concentration (IC50) of chalcone was 53.15 μM. It is worth noting that the minimum inhibitory concentration (MIC) of chalcone against the tested S. aureus strain was >4,864 μM. Meanwhile, the growth of S. aureus USA 300 was not visibly affected by chalcone at concentrations sufficient to inhibit SrtA (Figure 1B).
Furthermore, a hemolysis assay was used to determine the effect of chalcone on the hemolysis activity of the bacterial cultural supernatants. The results showed that chalcone at the concentrations tested in this study could significantly decrease the hemolysis activity of S. aureus in a dose-dependent manner when co-cultured with USA 300 strain (Figures 2A,B). Notably, minimal hemolysis was detected when the strain was cultured with 38 μM chalcone, and the IC50 value for chalcone-induced inhibition of Hla was 17.63 μM. In addition, pre-incubation with chalcone had almost no effect on the hemolytic activities of the bacterial culture supernatants (Figures 2A,B), implying that chalcone-induced inhibition of Hla might act by means of blocking its expression. To verify this, a Western blotting assay with the bacterial culture supernatants harvested above and a real-time RT-PCR assay were performed. In line with our conjecture, the Hla levels in the supernatants were reduced in a dose-independent manner (Figure 2C), and under our experimental conditions, the transcription levels of the hla gene and the agrA gene in USA 300 were both down-regulated by chalcone in a dose-independent manner (Figure 2D). Taken together, our results established that chalcone was able to effectively inhibit SrtA activity and the expression of Hla while exerting little antimicrobial activity, implying that chalcone could represent an effective anti-infective agent for S. aureus infection.
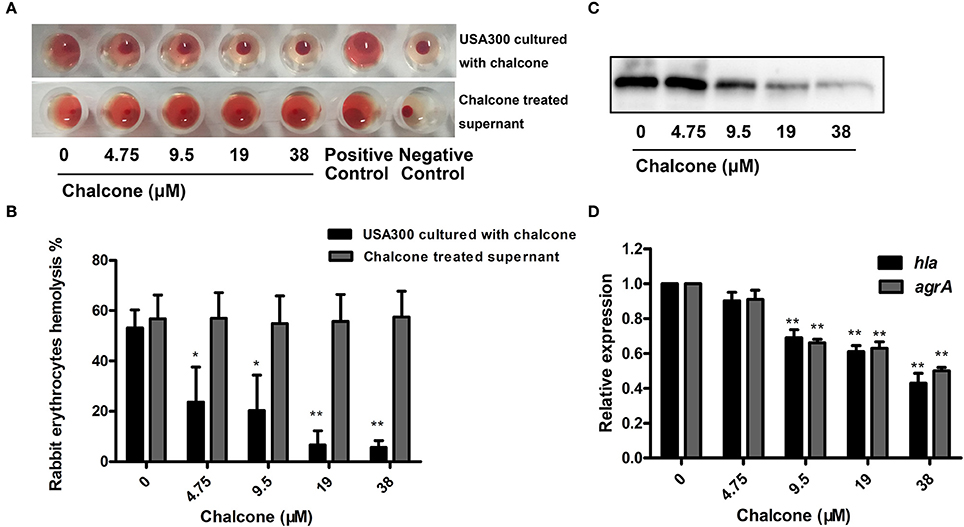
Figure 2. Chalcone inhibits S. aureus Hla expression. (A) The hemolysis activity of culture supernatants by S. aureus co-cultured with chalcone. (B) Bacterial supernatants pre-incubated with chalcone and then the hemolysis activity was determined. (C) Western blotting assay to detect the Hla expression in the bacterial culture supernatants by S. aureus co-cultured with or without chalcone. (D) The relative gene expression of the hla gene and the agrA gene in S. aureus exposed to chalcone at different concentrations. Three independent experiments were performed to obtain stable results. *P < 0.05 vs. the WT group and **P < 0.01 vs. the WT group.
Chalcone Influences the SpA Display in Intact Cells
Staphylococcus aureus SrtA anchors many different surface proteins in the cell wall, including SpA, a multifunctional molecule responsible for binding the Fcγ portion of host immunoglobulins (Falugi et al., 2013). Therefore, SpA is vital for immune evasion, and because of its function, a change in the amount of SpA in the cell wall after the addition of chalcone requires investigation. In this assay, S. aureus cultured with or without chalcone was stained with FITC-labeled goat anti-rabbit IgG, which made the cells display a green color whose strength was determined by the amount of SpA in the cell wall. With the aid of confocal laser-scanning microscope, the content variation of SpA in the cell wall was investigated. The result showed that the WTΔSrtA strain, used as a control, displayed much less SpA on its surface compared with the WT strain, and as expected, 76 μM chalcone present in the cell culture caused significant reductions in the SpA display (Figure 3). These consequences indicated that chalcone was capable of disturbing the assembly of sortase-mediated SpA in the cell wall.
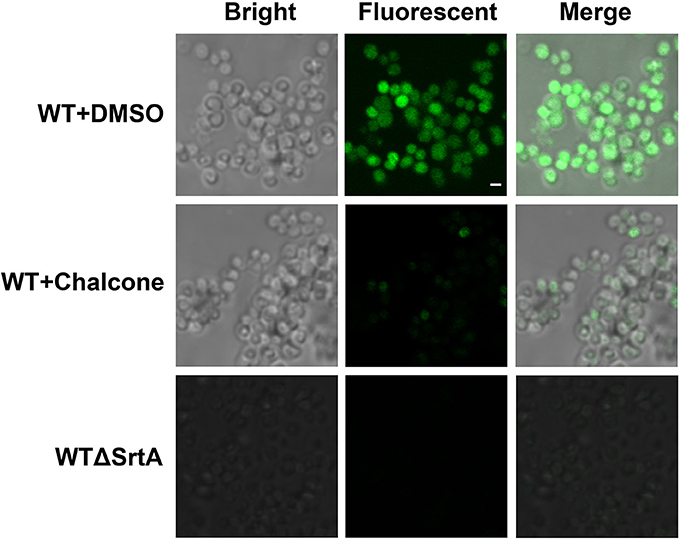
Figure 3. Effects of chalcone on SpA display in S. aureus. A confocal laser-scanning microscope was used to view the binding of FITC-labeled Ig to SpA. The strength of the green color represents the amount of the SpA anchored to the surface of bacteria. Scale bar, 1 μm.
Chalcone Reduces the Adherence of S. aureus to Fibronectin
In the previous study, we have drawn the conclusion that chalcone can visibly inhibit the SrtA-catalyzed transpeptidation at relatively low concentrations. The fibronectin-binding proteins FnBPA and FnBPB are LPXTG proteins expressed by S. aureus. Fibronectin (Fn) is regarded as a bridge between the bacterial adhesion FnBP and the mammalian cell integrin, accelerating the process of phagocytosis, thereby stimulating the internalization of bacteria; consequently, Fn and FnBPs play a vital role in the process of invading host cells (Foster and Höök, 1998; Roche et al., 2003). Because FnBPs anchored by S. aureus SrtA can make bacteria invasive, if their display in the cell wall is blocked by weakening SrtA activity, then bacterial invasion would be decreased. For this reason, we employed Fn-binding assays to determine if chalcone could reduce the adherence of S. aureus to Fn. As expected, the WTΔSrtA strain had a minimum adhesion rate to Fn owing to the damage to its Fn-binding function and no inhibition was observed in the samples treated with chalcone (Figure 4A). However, when the WT strain was treated with chalcone at different concentrations, the adhesion rates decreased in turn, respectively, indicating that the effect occurred in a dose-dependent manner. The variance between the 38 μM chalcone group and the WT group was a very significant difference, as was the 76 μM chalcone group (Figure 4A).
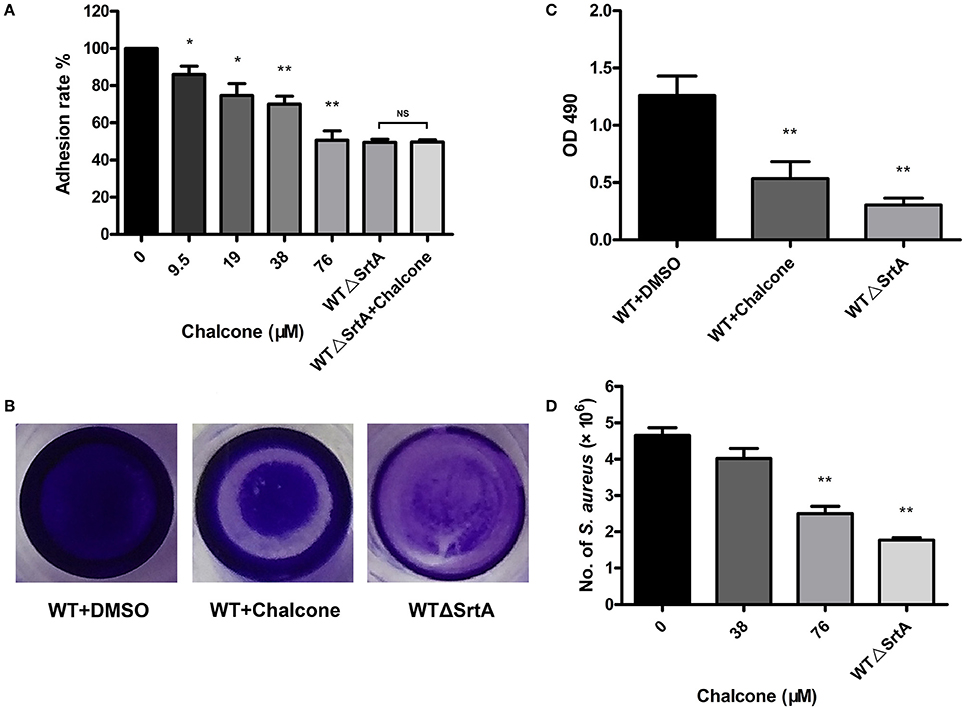
Figure 4. Chalcone reduces the adhesion of S. aureus to Fn, biofilm formation and S. aureus invasion. (A) Adhesion rate of S. aureus to Fn in the presence of different concentrations of chalcone. The WTΔSrtA strain was incubated with 76 μM chalcone. (B) Photographs of biofilms grown in 96-well flat-bottom polystyrene microtiter plates. (C) Quantification of the biofilm mass. (D) Chalcone weakened S. aureus invasion. Bacteria and J774 cells were cultured with chalcone at different concentrations at 37°C for 1 h, followed by gentamicin addition and incubation at 37°C for 30 min, after which number of intracellular bacteria was determined. Three independent experiments were performed to obtain stable results. *P < 0.05 vs. the WT group, **P < 0.01 vs. the WT group and NS represents no significance.
Chalcone Reduced Biofilm Formation
Bacteria enhance their ability to resist antibiotics and other antimicrobial agents by embedding in biofilms (Pugliese and Favero, 2002; Cerca et al., 2005; Høiby et al., 2010). Biofilm phenotypes are promoted by the FnBPs, the major cell wall autolysin or the icaADBC-encoded polysaccharide intercellular adhesin/poly-N-acetylglucosamine (PIA/PNAG) (O'Neill et al., 2007, 2008; Clarissa Pozzi et al., 2012). To investigate whether chalcone hindered biofilm formation, S. aureus biofilm formation was determined with or without chalcone. After dye treatment, the WTΔSrtA group was much more lightly stained than the WT group, and the visible difference between the biofilms formed under the conditions of untreated and treated with 76 μM chalcone was quite significant (Figure 4B). These results were in accordance with those of the quantitative analysis (Figure 4C). In summary, chalcone reduced biofilm formation, suggesting that the inhibitory effect of chalcone on S. aureus SrtA had occurred.
Chalcone Weakened the S. aureus Invasion
SrtA function is normally important for the display of sortase-mediated surface proteins, thus enabling bacteria to invade the host cells. To examine how chalcone affects S. aureus invasion, J774 cells were cultured with bacteria and chalcone at different concentrations. Not surprisingly, the numbers of bacteria entering the cells in the WTΔSrtA group showed a significant decrease compared with the WT group (Figure 4D). Consistent with the hypothesis, the addition of a dose of chalcone to the experimental system significantly decreased the quantity of bacteria, indicating that chalcone weakened the S. aureus invasion by inhibiting SrtA activity (Figure 4D).
Molecular Dynamics Simulation for SrtA-Chalcone
Through the computational biology method, the potential binding mode of chalcone with SrtA in the active site was explored in this study. The chalcone was bound to SrtA, and according to the binding mode given (Figures 5A,B), it was clear that chalcone could bind to SrtA via Van der Waals and electrostatic interactions. During the time course of the simulation, chalcone could localize to the catalytic pocket of SrtA (residue 160–200). In detail, the binding model of chalcone with SrtA revealed that chalcone could form strong interactions with Val166, Gly167, Val168, Ile182, Val193, and Arg197, respectively. The complex was found to reach equilibrium at 100 ns based on the analysis of the root-mean-square deviations (RMSD) of the backbone Cα atoms (Figure 5C), which indicated that the complex system has reached equilibrium.
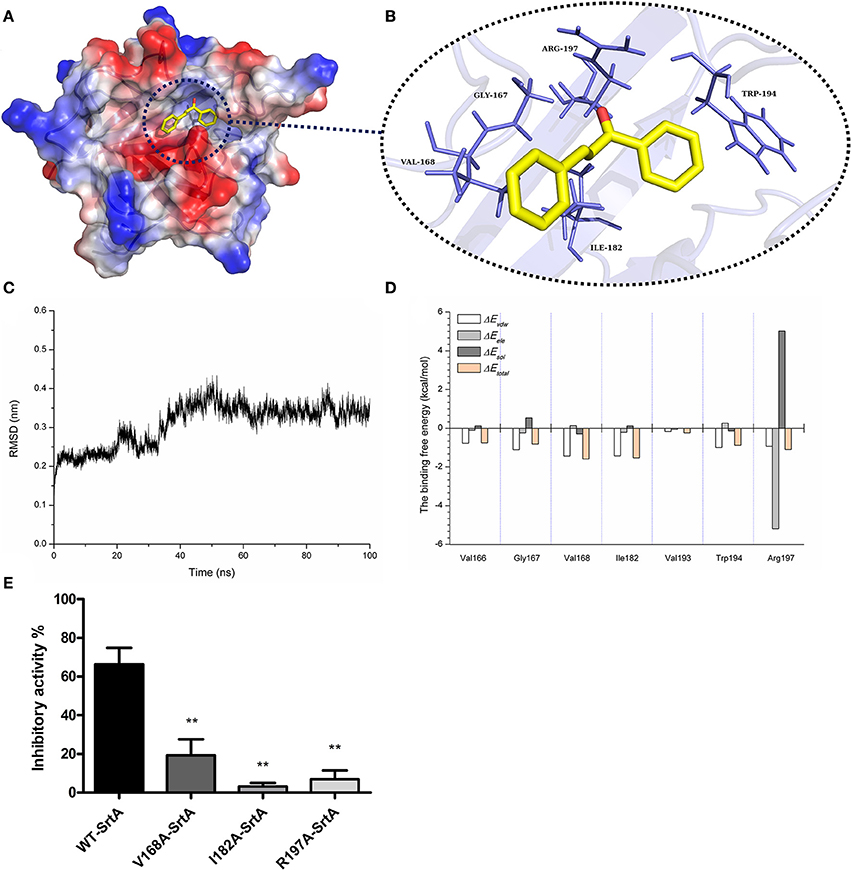
Figure 5. The 3D structural determination of a SrtA with chalcone complex by a molecular modeling method and the inhibitory effects of chalcone against WT-SrtA and SrtA mutants. (A) The structure of SrtA-chalcone. (B) The interaction of chalcone with the key residues of SrtA. (C) The RMSD displayed by the backbone atoms of the protein during MD simulations of SrtA-Chalcone is presented. (D) Decomposition of the binding energy on a per-residue basis at the binding sites of the SrtA-chalcone complex. (E) WT-SrtA and SrtA mutants (V168A-SrtA, I182A-SrtA and R197A-SrtA) were incubated with 76 μM chalcone, and the catalytic activity of the recombinant SrtA was determined as described in Figure 1A. **P < 0.01 vs. the WT group.
To explore the energy contributions from the residues of the binding sites in the SrtA- chalcone complex, the energy decomposition was calculated for the SrtA-chalcone complex system. Val168, Ile182 and Arg197 had a strong total binding energy contribution, with a ΔEtotal of < -1.0 kcal/mol (Figure 5D). In addition, residues Gly167 and Trp194 also had the appreciable total binding energy contribution, with a ΔEtotal of < -0.8 kcal/mol. These results suggested that these five residues were key residues for chalcone.
To confirm these theoretical results, the total binding free energy for the SrtA- chalcone complex and their detailed energy contributions calculated according to the MM-PBSA approach are summarized in Table 2. According to the calculation results, the binding free energy, ΔGbind, of the interaction between chalcone and the protein decreased in the following order: WT-SrtA > V168A-SrtA > R197A-SrtA > I182A-SrtA, which means that WT-SrtA had the strongest ability to bind to chalcone. Using fluorescence spectroscopy quenching, we measured the ΔGbind and the number of binding sites between chalcone and the three mutants, and these results were highly consistent with those obtained by computational methods (Table 2). To further validate the simulation results, three mutants, V168A-SrtA, I182A-SrtA, and R197A-SrtA, were constructed for the FRET assays. As expected, when compared with WT-SrtA, chalcone was significantly less sensitive for these three mutants (Figure 5E). These results indicated that the information generated by the MD simulation on the SrtA-Chalcone complex was reliable. Due to the binding of inhibitor, chalcone, with the activity region (residues of Gly167, Val168, Ile182, Cys184, Trp194, and Arg197), the biology activity of SrtA was inhibited.
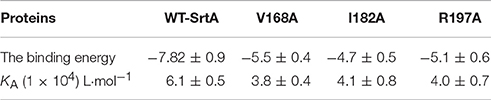
Table 2. The binding free energy (kcal/mol) of WT-Chalcone, V168A-Chalcone, I182A-Chalcone, and R197A-Chalcone systems based on computational method and the values of the binding constants (KA) based on the fluorescence spectroscopy quenching.
Chalcone Protects A549 Cells from Hla-Mediated Injury
In our co-culture system, the potential protective effect of chalcone on the Hla-induced injury of A549 cells was determined. In the Live/Dead assay, uninfected cells retained green fluorescence (Figure 6A), and inversely, injured cells retained red fluorescence. As expected, 38 μM chalcone showed an obvious reduction in cell injury, with almost no cytotoxic effect observed in A549 cells (Figures 6B–E). Simultaneously, the LDH assay showed that treatment with chalcone could significantly decrease the release of LDH into the supernatants in a dose-independent manner compared with the control group, indicating less cell death (Figure 6E). Above all, the results showed that chalcone had a protective effect on Hla–mediated A549 cell injury.
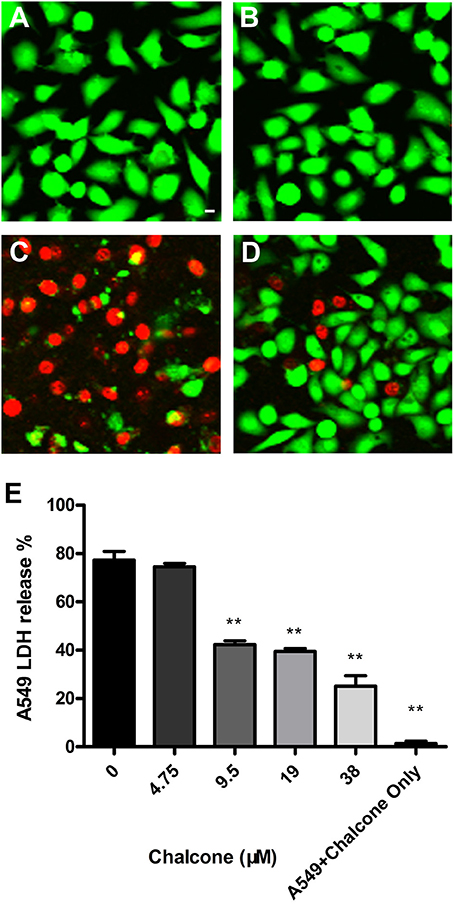
Figure 6. Chalcone protects A549 cells from injury mediated by S. aureus Hla. Live/Dead reagent-A549 cells were observed with fluorescent imaging. (A) The untreated A549 cells. (B) A549 cells treated with 38 μM chalcone only. (C) A549 cells infected with S. aureus culture supernatants harvested in the absence of chalcone. (D) A549 cells treated with S. aureus culture supernatants harvested in the presence of 38 μM chalcone. (E) The LDH release by A549 cells treated with S. aureus culture supernatants harvested previously and 38 μM chalcone only, respectively. Scale bar, 10 μm. **P < 0.01 vs. the WT group.
Chalcone Protected Mice from Fatal S. aureus Infection
To investigate the effect of chalcone on the survival rate of mice inoculated with S. aureus, we performed survival experiments. Ninety-six hours after infection with 4 × 108 CFUs of bacteria, only 20% of the WT-infected mice survived, in contrast to the WTΔSrtA group in which the survival rate was 100% (Figure 7). In the chalcone-treated group, the survival rate was significantly higher than in the group without treatment (Figure 7). The death of mice in the WT+chalcone group occurred only at 36, 48, and 72 h in the whole process of infection (Figure 7). These findings suggested that chalcone could prolong survival of the mice in S. aureus–induced infection.
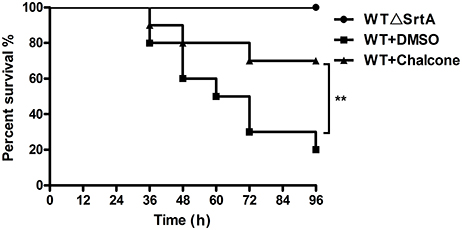
Figure 7. Effects of chalcone on survival rates after 96 hours in C57BL/6J mice. Mice were infected with 4 × 108 CFUs of S. aureus and the WTΔSrtA strain. Treatment with chalcone (150 mg/kg, twice a day) was initiated after infection. **P < 0.01 vs. the WT group.
Discussion
Staphylococcus aureus is a major human pathogen and is also a leading cause of sepsis and infective endocarditis. The continuous spread of antibiotic-resistant strains, such as MRSA and VRSA makes treatment very difficult (Lowy, 1998; Petti and Fowler, 2003; Menichetti, 2005), and new valid strategies are greatly needed to address this severe situation. The virulence factors of bacteria, such as adhesins, invasins and toxins, among others, facilitate infection by evading the immune response and leading to colonization, spread and tissue damage (Lowy, 1998; Foster, 2005; Rooijakkers et al., 2005). In addition, previous studies have demonstrated that the vast majority of these various virulence factors are nonessential for bacterial survival and are crucial in the process of targeting host cells and causing disease (Lowy, 1998; Berube and Bubeck, 2013). Some previous studies have also demonstrated that S. aureus lacking the genes encoding SrtA or Hla will show a weakened bacterial virulence (Albus et al., 1991; Mcelroy et al., 1999; Mazmanian et al., 2000; Wardenburg et al., 2007). For this reason, therapeutic agents targeting virulence factors, such as SrtA and Hla, which do not threaten survival, may not lead to the development of resistance as quickly as conventional antibiotics typically do, and this will have important implications for S. aureus infection. Some agents targeting S. aureus SrtA or Hla have been found previously (Liu et al., 2015; Zhou et al., 2015; Zhang et al., 2016). However, as these compounds are targeted only at one kind of virulence factor, the limitations for clinical treatment will certainly exist. A hypothesis was then proposed as to whether we could find an agent targeting SrtA and Hla simultaneously to produce a better therapeutic effect.
The scope of screening for SrtA and Hla inhibitors was wide, involving natural, synthetic and high-throughput screening methodologies. We sought to find inhibitors from natural small molecule compounds, from which chalcone, of the flavonoid class of natural products (Figure 1A), with relatively simple structures and various functions (Sahu et al., 2012), was discovered. First, a FRET assay and a hemolysis assay were used to identify the inhibition of chalcone on SrtA and Hla, respectively. After incubating S. aureus with chalcone at a certain concentration, the SrtA catalytic activity, hemolysis ratio and Hla expression in the bacterial culture supernatant were all decreased (Figures 1A, 2A–C). Additionally, chalcone indeed affected the transcriptional levels of the hly gene and the agrA gene (Figure 2D), indicating that the decreased expression of Hla might be caused by the decreased expression of the genes. In addition, the decreased expression of Hla conferred significant protection against Hla-mediated A549 cell injury (Figure 6). We also found that the inhibitory effect of chalcone against SrtA reduced the SpA display in the cell wall (Figure 3), decreased the adherence of S. aureus to fibronectin (Figure 4A) and resulted in the lower biofilm formation (Figures 4B,C). In addition, as far as we know, our study is the first to use a cell invasion assay to evaluate the inhibitory activity of a natural compound on SrtA, which showed that the quantity of bacterial entry into cells was significantly decreased by chalcone (Figure 4D). To explore the interaction mechanism between chalcone and SrtA, a molecular dynamics simulation for a SrtA-chalcone complex system was carried out. By means of molecular dynamics simulation, it was found that chalcone could localize to the catalytic pocket of SrtA (residues 160–200), which is very close to the binding site of substrate. Due to the binding of chalcone to SrtA, the binding of substrate to SrtA was blocked, leading to the loss of biological activity of SrtA (Figure 5). It is worth mentioning that just at a much lower concentration than the MIC, chalcone could significantly stop S. aureus SrtA-mediated transpeptidation and the hemolysis activity of Hla in vitro by inhibiting SrtA activity and the expression of Hla, respectively, instead of killing bacteria directly (Figure 1B), which means a less selective pressure for bacteria and a lower risk of drug resistance. More importantly, a C57BL/6J mouse model was established to determine the therapeutic effect of chalcone in vivo, and treatment with chalcone significantly attenuated the virulence of S. aureus and protected mice from infection caused by the bacteria (Figure 7). To the best of our knowledge, this is the first report of the discovery and proof of an inhibitor targeting S. aureus SrtA and Hla simultaneously, and thus, this kind of inhibitor can be considered as a novel promising candidate against S. aureus infection.
In conclusion, our study discovered that the natural small compound chalcone could effectively inhibit S. aureus SrtA activity and the expression of Hla by occupying the sites of the enzyme to prevent anchoring of surface proteins and decreasing transcription levels of the hla gene and the agrA gene, thereby attenuating S. aureus virulence both in vitro and in vivo. These findings could be the foundation for further design of novel anti-infection agents, and a SrtA-Hla-centered strategy has emerged accordingly, thus making a contribution for opening a new horizon for the treatment of S. aureus infection.
Author Contributions
JW and XN conceived and designed the experiments. BZ, ZT, XL, and GL performed the experiments. XD contributed reagents/materials/analysis tools. JW, BZ, and XD wrote the paper.
Funding
This work was supported by the National Key Technology R&D Program (No. 2016YFD05013), the National Basic Research Program of China (grant 2013CB127205) and the Project Funded by China Postdoctoral Science Foundation (Project No. 2016M591486).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Albus, A., Arbeit, R. D., and Lee, J. C. (1991). Virulence of Staphylococcus aureus mutants altered in type 5 capsule production. Infect. Immun. 59, 1008–1014.
Bandyopadhyay, S., Valder, C. R., Huynh, H. G., Ren, H., and Allison, W. S. (2002). The beta G156C substitution in the F1-ATPase from the thermophilic Bacillus PS3 affects catalytic site cooperativity by destabilizing the closed conformation of the catalytic site. Biochemistry 41, 14421–14429. doi: 10.1021/bi026243g
Berube, B. J., and Bubeck, W. J. (2013). Staphylococcus aureus α-Toxin: nearly a century of intrigue. Toxins (Basel) 5, 1140–1166. doi: 10.3390/toxins5061140
Bubeck, W. J., and Olaf, S. (2008). Vaccine protection against Staphylococcus aureus pneumonia. J. Exp. Med. 205, 287–294. doi: 10.1084/jem.20072208
Cerca, N., Martins, S., Cerca, F., Jefferson, K. K., Pier, G. B., Oliveira, R., et al. (2005). Cerca, N. Comparative assessment of antibiotic susceptibility of coagulase negative staphylococci in biofilm versus planktonic culture as assessed by bacterial enumeration or rapid XTT colorimetry. J. Antimicrob. Chemother. 56, 331–336. doi: 10.1093/jac/dki217
Clancy, K. W., Melvin, J. A., and Mccafferty, D. G. (2010). Sortase transpeptidases: insights into mechanism, substrate specificity, and inhibition. Biopolymers 94, 385–396. doi: 10.1002/bip.21472
Clarissa Pozzi, E. M. W., Justine Rudkin, K., Carolyn Schaeffer, R., Amanda Lohan, J., Pin, T., Brendan Loftus, J., et al. (2012). Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog. 8:e1002626. doi: 10.1371/journal.ppat.1002626
Coates, R., Moran, J., and Horsburgh, M. J. (2014). Staphylococci: colonizers and pathogens of human skin. Future Microbiol. 9, 75–91. doi: 10.2217/fmb.13.145
Dinges, M. M., Orwin, P. M., and Schlievert, P. M. (2000). Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13, 16–34, table of contents. doi: 10.1128/CMR.13.1.16-34.2000
Dong, J., Qiu, J., Zhang, Y., Lu, C., Dai, X., Wang, J., et al. (2013). Oroxylin A inhibits hemolysis via hindering the self-assembly of α-hemolysin heptameric transmembrane pore. PLoS Comput. Biol. 9:e1002869. doi: 10.1371/journal.pcbi.1002869
Falugi, F., Kim, H. K., Missiakas, D. M., and Schneewind, O. (2013). Role of protein a in the evasion of host adaptive immune responses by Staphylococcus aureus. MBio 4:e00575. doi: 10.1128/mBio.00575-13
Fischetti, V. A., Pancholi, V., and Schneewind, O. (1990). Conservation of a hexapeptide sequence in the anchor region of surface proteins from Gram-positive cocci. Mol. Microbiol. 4, 1603–1605. doi: 10.1111/j.1365-2958.1990.tb02072.x
Foster, T. J. (2005). Immune evasion by staphylococci. Nat. Rev. Microbiol. 3, 948–958. doi: 10.1038/nrmicro1289
Foster, T. J., and Höök, M. (1998). Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484. doi: 10.1016/S0966-842X(98)01400-0
Gould, I. M. (2013). Treatment of bacteraemia: meticillin-resistant Staphylococcus aureus (MRSA) to vancomycin-resistant S. aureus (VRSA). Int. J. Antimicrob. Agents 42, 23–30. doi: 10.1016/j.ijantimicag.2013.04.006
Høiby, N., Bjarnsholt, T., Givskov, M., Molin, S., and Ciofu, O. (2010). Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 35, 322–332. doi: 10.1016/j.ijantimicag.2009.12.011
Hu, R., Barbault, F., Maurel, F., Delamar, M., and Zhang, R. (2010). Molecular dynamics simulations of 2-Amino-6-arylsulphonylbenzonitriles analogues as HIV inhibitors: interaction modes and binding free energies. Chem. Biol. Drug Des. 76, 518–526. doi: 10.1111/j.1747-0285.2010.01028.x
Ippolito, G., Leone, S., Lauria, F. N., Nicastri, E., Wenzel, R. P., and Ippolito, G. (2010). Methicillin-resistant Staphylococcus aureus: the superbug. Int. J. Infect. Dis. 14(Suppl. 4), S7–S11. doi: 10.1016/j.ijid.2010.05.003
Jurasekova, Z., Marconi, G., Sanchez-Cortes, S., and Torreggiani, A. (2009). Spectroscopic and molecular modeling studies on the binding of the flavonoid luteolin and human serum albumin. Biopolymers 91, 917–927. doi: 10.1002/bip.21278
Levy, S. B., Fitzgerald, G. B., and Macone, A. B. (1976). Spread of antibiotic-resistant plasmids from chicken to chicken and from chicken to man. Nature 260, 40–42. doi: 10.1038/260040a0
Li, H., Chen, Y., Zhang, B., Niu, X., Song, M., Luo, Z., et al. (2016). Inhibition of sortase A by chalcone prevents Listeria monocytogenes infection. Biochem. Pharmacol. 106, 19–29. doi: 10.1016/j.bcp.2016.01.018
Lin, W., Bi, C., Cai, H., Liu, B., Zhong, X., Deng, X., et al. (2015). The therapeutic effect of chlorogenic acid against Staphylococcus aureus infection through sortase A inhibition. Front. Microbiol. 6:1031. doi: 10.3389/fmicb.2015.01031
Liu, S., Zhou, X., Li, W., Zhang, H., Zhang, B., Li, G., et al. (2015). Diosmetin inhibits the expression of alpha-hemolysin in Staphylococcus aureus. Antonie Van Leeuwenhoek 108:383. doi: 10.1007/s10482-015-0491-6
Lv, Z., Wang, H. S., and Niu, X. D. (2013). Molecular dynamics simulations reveal insight into key structural elements of aaptamines as sortase inhibitors with free energy calculations. Chem. Phys. Lett. 585, 171–177. doi: 10.1016/j.cplett.2013.08.097
Mazmanian, S. K., Liu, G., Jensen, E. R., Lenoy, E., and Schneewind, O. (2000). Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc. Natl. Acad. Sci. U.S.A. 97, 5510–5515. doi: 10.1073/pnas.080520697
Mcelroy, M. C., Harty, H. R., Hosford, G. E., Boylan, G. M., Pittet, J. F., and Foster, T. J. (1999). Alpha-toxin damages the air-blood barrier of the lung in a rat model of Staphylococcus aureus-induced pneumonia. Infect. Immun. 67:5541.
Menichetti, F. (2005). Current and emerging serious Gram-positive infections. Clin. Microbiol. Infect. 11(Suppl. S3), 22–28. doi: 10.1111/j.1469-0691.2005.01138.x
Morris, G. M., Huey, R., Lindstrom, W., Sanner, M. F., Belew, R. K., Goodsell, D. S., et al. (2009). AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791. doi: 10.1002/jcc.21256
Niu, X., Qiu, J., Wang, X., Gao, X., Dong, J., Wang, J., et al. (2013). Molecular insight into the inhibition mechanism of cyrtominetin to α-hemolysin by molecular dynamics simulation. Eur. J. Med. Chem. 62, 320–328. doi: 10.1016/j.ejmech.2013.01.008
O'Neill, E., Pozzi, C., Houston, P., Humphreys, H., Robinson, D. A., Loughman, A., et al. (2008). A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J. Bacteriol. 190, 3835–3850. doi: 10.1128/JB.00167-08
O'Neill, E., Pozzi, C., Houston, P., Smyth, D., Humphreys, H., Robinson, D. A., et al. (2007). Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolates from device-related infections. J. Clin. Microbiol. 45:1379. doi: 10.1128/JCM.02280-06
Petti, C. A., and Fowler, V. G. Jr. (2003). Staphylococcus aureus bacteremia and endocarditis. Cardiol. Clin. 21:219. doi: 10.1016/S0733-8651(03)00030-4
Pugliese, G., and Favero, M. S. (2002). biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167. doi: 10.1128/CMR.15.2.167-193.2002
Qiu, J., Li, H., Meng, H., Hu, C., Li, J., Luo, M., et al. (2011). Impact of luteolin on the production of alpha-toxin by Staphylococcus aureus. Lett. Appl. Microbiol. 53:238. doi: 10.1111/j.1472-765X.2011.03098.x
Ragle, B. E., Karginov, V. A., and Bubeck, W. J. (2010). Prevention and treatment of Staphylococcus aureus pneumonia with a -cyclodextrin derivative. Antimicrob. Agents Chemother. 54, 298–304. doi: 10.1128/AAC.00973-09
Roche, F. M., Massey, R., Peacock, S. J., Day, N. P., Visai, L., Speziale, P., et al. (2003). Characterization of novel LPXTG-containing proteins of Staphylococcus aureus identified from genome sequences. Microbiology 149(Pt 3), 643. doi: 10.1099/mic.0.25996-0
Rooijakkers, S. H. M., Kessel, K. P. M. V., and Strijp, J. A. G. V. (2005). Staphylococcal innate immune evasion. Trends Microbiol. 13:596. doi: 10.1016/j.tim.2005.10.002
Sahu, N. K., Balbhadra, S. S., Choudhary, J., and Kohli, D. V. (2012). Exploring pharmacological significance of chalcone scaffold: a review. Curr. Med. Chem. 19, 209–225. doi: 10.2174/092986712803414132
Scott, J. R., and Barnett, T. C. (2006). Surface proteins of gram-positive bacteria and how they get there. Annu. Rev. Microbiol. 60, 397–423. doi: 10.1146/annurev.micro.60.080805.142256
Tonthat, H., Liu, G., Mazmanian, S. K., Faull, K. F., and Schneewind, O. (1999). Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc. Natl. Acad. Sci. U.S.A. 96:12424. doi: 10.1073/pnas.96.22.12424
Wallockrichards, D. J., Marleswright, J., Clarke, D. J., Maitra, A., Dodds, M., Hanley, B., et al. (2015). Molecular basis of Streptococcus mutans sortase A inhibition by the flavonoid natural product trans-chalcone. Chem. Commun. 51:10483. doi: 10.1039/C5CC01816A
Wardenburg, J. B., Patel, R. J., and Schneewind, O. (2007). Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect. Immun. 75, 1040–1044. doi: 10.1128/IAI.01313-06
Zhang, B., Wang, X., Wang, L., Chen, S., Shi, D., and Wang, H. (2016). Molecular mechanism of the flavonoid natural product dryocrassin ABBA against Staphylococcus aureus sortase A. Molecules 21:1428. doi: 10.3390/molecules21111428
Zhang, J., Liu, H., Zhu, K., Gong, S., Dramsi, S., Wang, Y. T., et al. (2014). Antiinfective therapy with a small molecule inhibitor of Staphylococcus aureus sortase. Proc. Natl. Acad. Sci. U.S.A. 111, 13517–13522. doi: 10.1073/pnas.1408601111
Keywords: Staphylococcus aureus, sortase A, alpha-hemolysin, chalcone, inhibitor
Citation: Zhang B, Teng Z, Li X, Lu G, Deng X, Niu X and Wang J (2017) Chalcone Attenuates Staphylococcus aureus Virulence by Targeting Sortase A and Alpha-Hemolysin. Front. Microbiol. 8:1715. doi: 10.3389/fmicb.2017.01715
Received: 27 April 2017; Accepted: 24 August 2017;
Published: 06 September 2017.
Edited by:
Hui Wu, University of Alabama at Birmingham, United StatesReviewed by:
Shashank Gupta, Brown University, United StatesDevendra Hiraman Dusane, The Ohio State University, United States
Copyright © 2017 Zhang, Teng, Li, Lu, Deng, Niu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaodi Niu, bml1eGRAamx1LmVkdS5jbg==
Jianfeng Wang, d2pmOTI3QGpsdS5lZHUuY24=
†These authors have contributed equally to this work.
 Bing Zhang
Bing Zhang Zihao Teng1†
Zihao Teng1† Xianhe Li
Xianhe Li Gejin Lu
Gejin Lu Xuming Deng
Xuming Deng Jianfeng Wang
Jianfeng Wang