- 1State Key Laboratory of Agricultural Microbiology and Hubei Key Laboratory of Plant Pathology, Huazhong Agricultural University, Wuhan, China
- 2School of Environmental Sciences, University of Guelph, Guelph, ON, Canada
The bacterium, Streptomyces yanglinensis 3-10, shows promise in the control of many phytopathogenic fungi. In this study, S. yanglinensis and its antifungal substances, culture filtrate (CF3-10) and crude extracts (CE3-10), were evaluated for their activity in reducing growth and aflatoxin AFB1 production by Aspergillus flavus, both in vitro and in vivo on peanut kernels. The results showed that in dual culture conditions, S. yanglinensis reduced the mycelial growth of A. flavus about 41% as compared to control. The mycelial growth of A. flavus was completely inhibited on potato dextrose agar amended with CF3-10 at 3% (v/v) or CE3-10 at 2.5 μg/ml. In liquid culture experiments, growth inhibition ranged from 32.3 to 91.9% with reduction in AFB1 production ranging from 46.4 to 93.4% using different concentrations of CF3-10 or CE3-10. For in vivo assays, CF3-10 at 0.133 ml/g (v/w) or CE3-10 at 13.3 μg/g (w/w) reduced the postharvest decay of peanut kernels by inhibiting visible growth of A. flavus leading to an 89.4 or 88.1% reduction in AFB1 detected, respectively. Compared with the controls, CF3-10 and CE3-10 in A. flavus shake culture significantly reduced expression levels of two AFB1 biosynthesis genes, aflR and aflS. Furthermore, electron microscopy observation showed that CF3-10 (2%, v/v) caused hyphae growth to be abnormal and shriveled, cell organelles to degenerate and collapse, large vacuoles to appear. These results suggest that S. yanglinensis 3-10 has potential as an alternative to chemical fungicides in protecting peanut kernels and other agricultural commodities against postharvest decay from A. flavus.
Introduction
Aspergillus flavus is an important pathogenic fungus affecting peanuts during storage (Amaike and Keller, 2011). In infected peanuts it can cause seed rot and reduce seed viability and germination (Kumar et al., 2008). The pathogen can also produce aflatoxins, a group of fungal secondary metabolites, which are the most toxic carcinogens among known mycotoxins (Calvo et al., 2002; Klich, 2007; Krishnamurthy et al., 2008). These metabolites are considered potent hepatocarcinogens in animals and may also be involved in primary liver cancer and other cancers in kidney, lung, and colon tissues in humans (Bullerman, 1976; Amaike and Keller, 2011). The gene cluster for aflatoxin biosynthesis is 70 kb in length, containing 25 genes (Yu et al., 2004). In the aflatoxin biosynthesis gene cluster, aflR and aflS are the most important regulatory genes affecting the expression of aflatoxin biosynthesis genes (Yu et al., 2004; Amaike and Keller, 2011). The aflR gene encodes a zinc finger transcription factor, which binds to the consensus sequence 5′-TCGN5CGA-3′ in the promoter region of the aflatoxin biosynthetic genes, and positively regulates the transcription of these genes (Yu et al., 2004; Amaike and Keller, 2011). Another regulatory gene aflS (originally named aflJ) regulates aflatoxin production through direct interaction with aflR (Chang, 2004; Du et al., 2007).
To protect food and feedstuffs and to avoid aflatoxin contamination from A. flavus, there are several possible control strategies employed in peanut such as treating peanuts with fungicides, fumigants, or biocontrol agents (Kong et al., 2010). Although the use of fungicides for controlling A. flavus is an effective measure, there are several negative effects of this method including generation of fungicide-resistant strains of A. flavus, and fungicide residues affecting food safety and as a source of environmental pollution (Droby, 2006). Therefore, development of effective biological controls is a priority with presumed lower risk to humans and the environment.
Microorganisms have gained considerable attention in recent years because of their diversity and biological activities, mainly due to their ability to produce novel chemical compounds of high commercial value (Amador et al., 2003). As prokaryotes, Streptomyces spp. are Gram-positive bacteria that grow extensively in soil, on plants, and in air dust (Demain, 1999; Kettleson et al., 2013; Supong et al., 2016), and are known to produce over 7,500 bioactive compounds including anticancer agents, vitamins, and antibiotic compounds (Berdy, 2005; Gallagher et al., 2010; Manivasagan et al., 2014). Many Streptomyces species have been successfully evaluated as biocontrol agents against phytopathogens (Berg et al., 2001; Minuto et al., 2006; Law et al., 2017). Some antibiotic compounds (Al-Bari et al., 2007; Zucchi et al., 2008; Lyu et al., 2017) and other compounds such as chitinases (Gomes et al., 2001; Mander et al., 2016) produced by Streptomyces spp. have a strong antagonistic effect on growth and development of Aspergillus. Furthermore, some antibiotics, such as Blasticidin A (Sakuda et al., 2000a,b) and Dioctatin A (Yoshinari et al., 2007), produced by Streptomyces, can inhibit aflatoxin production in Aspergillus. Our previous study showed that crude extracts from liquid cultures of Streptomyces yanglinensis isolate 3-10 had high antifungal activity against several plant pathogenic fungi including A. flavus (Lyu et al., 2017). Therefore, the biocontrol potential of S. yanglinensis isolate 3-10 against A. flavus on peanut kernels was deserving of further study.
The objectives of this study were as follows: (i) to evaluate the efficacy of S. yanglinensis isolate 3-10 in inhibiting the growth of A. flavus in vitro and in vivo using peanuts as substrates; (ii) to determine the effect of S. yanglinensis on the biosynthesis of aflatoxin AFB1 and expression of aflR and aflS in A. flavus; and (iii) to investigate the mechanisms of the antifungal substance (AFS) of S. yanglinensis for suppression of A. flavus using electron microscopy.
Materials and Methods
Microorganisms and Media
Two microorganisms, S. yanglinensis 3-10 and A. flavus NRRL 3375, were used in this study. S. yanglinensis 3-10 was originally isolated from a healthy rice leaf grown in the field near Wuhan, China (Wan et al., 2008) and stored at -20°C. It was cultured on fermentation medium (soluble starch 3%, peptone 0.75%, yeast extract 0.025%, soybean meal 1%, K2HPO4⋅3H2O 0.5 g/l, KH2PO4 0.7 g/l, MgSO4⋅7H2O 0.4 g/l, MnSO4⋅H2O 0.02 g/l, ZnSO4⋅7H2O 0.01 g/l) at 28°C for 72 h for AFS production (Shakeel et al., 2016). A. flavus NRRL 3375 was kindly provided by Dr. Desheng Qi of Huazhong Agricultural University in China and cultured on potato dextrose agar (PDA). PDA and PDB (potato dextrose broth) were prepared with peeled potato tubers using the procedures described by Fang (1998). Both microorganisms were incubated at 28°C.
Preparation of the Culture Filtrates of S. yanglinensis 3-10 and Their Crude Extracts
The culture filtrate of S. yanglinensis 3-10 (CF3-10) was prepared by filtering a 3-day-old PDB shake culture through a 0.22-μm polycarbonate membrane filter, and the filtrate was extracted twice with ethyl acetate and dried in a vacuum to obtain total crude extract (CE3-10) (Shakeel et al., 2016). CE3-10 was dissolved in methanol at 12.5 mg/ml (w/v) and stored at 4°C for use as stock solution for subsequent tests.
Effects of Metabolites of S. yanglinensis 3-10 on Mycelial Growth of A. flavus on PDA
Dual culture was used to evaluate the potential antagonism of S. yanglinensis 3-10 against A. flavus. These were placed in a 9-cm-diameter Petri dish containing 20 ml PDA. An aliquot of 0.5 ml of spore suspension of S. yanglinensis (1 × 108 spores/ml) was streaked on one side of the plate, at least 3 cm from the center. After 24 h, a 6-mm-diameter agar plug from the leading edge of a 7-day-old culture of A. flavus was placed on the other side of the plate, 3 cm from the center. Plates without S. yanglinensis were used as control. The inoculated plates were incubated at 28°C. After 10 days, the colony diameter in each dish was measured.
PDA in Petri dishes amended with CF3-10 or CE3-10 was used to assess the effects of CF3-10 and CE3-10 on mycelia growth of A. flavus. In the CF3-10 treatment, the culture filtrate was amended into PDA to the final concentrations of 0, 0.5, 1.0, 1.5, 2.0, 2.5, or 3.0% (v/v), while fresh PDB was added to PDA as controls. In the CE3-10 treatment, the culture extract was added to PDA at final concentrations of 0, 0.5, 1.0, 1.5, 2.0, 2.5, or 3.0 μg/ml, while methanol was added to PDA at 0.2% (v/v) as the control. Mycelial plugs (6 mm diameter) of A. flavus cut from 7-day-old colonies were placed in the center of each dish. Plates were incubated at 28°C, with five replicate plates per treatment. Fungal growth was recorded after 7 days. Inhibition of growth (IG) of A. flavus by AFS of S. yanglinensis was calculated using the formula: IG (%) = (DCK - D3-10)/DCK × 100%, where DCK represents the colony diameter in the treatment of control, D3-10 represents the colony diameter after treatment with CF3-10 or CE3-10. The experiments were repeated three times.
Suppression of AFB1 Production in PDB by A. flavus Using S. yanglinensis 3-10 and Its AFS-Containing Products
To evaluate the effect of S. yanglinensis 3-10 on suppression of AFB1 production in PDB by A. flavus, spore suspensions of S. yanglinensis (S3-10) and AFS-containing products (CF3-10 and CE3-10) were tested. In the S3-10 treatment, S. yanglinensis was grown with A. flavus in 250 ml-Erlenmeyer flasks containing 50 ml PDB. The 50 ml PDB medium in 250 ml flasks was inoculated with 2.5 ml of spore suspension of A. flavus containing 1 × 108 spores/ml, and 2.5 ml of spore suspension of S. yanglinensis (S3-10) at 1 × 108 spores/ml. In AFS treatments, CF3-10 and CE3-10 were aseptically dispensed, before being inoculated with 2.5 ml of a 1 × 108 spores/ml spore suspension of A. flavus. The final concentrations of metabolites of S. yanglinensis were 1.25, 2.5, 3.75, 5% (v/v) for the CF3-10 treatment, and 1.25, 2.5, 3.75, 5 μg/ml for the CE3-10 treatment. PDB inoculated with 2.5 ml of spore suspension of A. flavus (1 × 108 spores/ml) was used as the control. All treatments (S3-10, CF3-10, CE3-10, and control) cultures were incubated at 28°C on a 150 rpm rotary shaker for 7 days, and then analyzed for fungal growth inhibition by weighted mycelial biomass of A. flavus, and AFB1 production with an aflatoxin plate kit (Agra Quant® Aflatoxin B1 assay, COKAQ8000/COKAQ8048, Romer Labs Singapore Pte Ltd.). There were five flasks (replications) for each treatment, and the experiment was repeated three times.
Antifungal Activity on Peanuts Under Storage Conditions
The inhibitory effect on A. flavus was determined following Zhang et al. (2013), with slight modifications. Peanut kernels with skins (cultivar Zhonghua No. 16) were surface sterilized with 5% NaOCl for 1 min and rinsed three times in sterilized water. In each Petri dish, 15 g peanut kernels were mixed with 2 ml of each treatment separately. The treatments were S3-10 (1 × 108 spores/ml giving final 1.33 × 107 spores/g peanut), CF3-10 (100%, v/v, giving final 0.133 ml/g peanut), and CE3-10 (100 μg/ml giving final 13.3 μg/g peanut). For the control treatment, 2 ml of sterile distilled water was added to 15 g of peanuts. There were five replicates dishes for each treatment. The plates were gently agitated by hand until the applied solutions were visibly absorbed by the kernel skins. Next, the kernels were inoculated with 1 ml of A. flavus spore suspension (1 × 108 spores/ml), and again agitated until absorption of all liquid. After incubation at 28°C for 7 days, the growth of A. flavus on kernels was evaluated visually. The amount of AFB1 associated with peanut kernels in each treatment was determined following the method stated above. The experiments were repeated three times.
Effect of S. yanglinensis 3-10 and Its AFS-Containing Products on aflR and aflS Expression
The effect of S. yanglinensis 3-10 metabolites on expression of AFB1 genes in A. flavus was studied with qRT-PCR. A spore suspension (2.5 ml) of A. flavus (1 × 108 spores/ml) was added to 50 ml PDB and incubated for 7 days with either S3-10 (5 × 106 spores/ml), CF3-10 (5%, v/v), or CE3-10 (5 μg/ml). As control A. flavus (2.5 ml, 1 × 108 spores/ml) was grown without any filtrates or extracts from S. yanglinensis. Mycelia were collected by centrifugation and total RNA was extracted using a Trizol method described by Lou et al. (2015). For quantitative and qualitative analysis of total RNA, the A260/A280 ratio was determined, and gel electrophoresis was performed. cDNA was synthesized by using cDNA Synthesis SuperMix of TransGen Biotech according to instructions of the manufacturer. qRT-PCR was performed by using UltraSYBR Mixture of CWBIO where the 18S sequence was used as internal control. Previously designed primers were used for amplification of 18S rRNA, aflR and aflS in qRT-PCR (Kong et al., 2010). Fungal mycelium grown in the absence of S. yanglinensis spores and products was used as a control, and relative quantification was accomplished by using the delta Ct method described by Lou et al. (2015). The experiment was repeated three times.
Scanning Electron Microscopy
Agar plugs of A. flavus were placed onto sterile cellophane films overlying PDA or PDA amended with CF3-10 2% final concentration in Petri dishes, with one plug per dish, and three replicate dishes per strain. The dishes were incubated at 28°C for 7 days. Then, small cellophane film pieces (2 mm × 2 mm) colonized by mycelia of A. flavus were sampled from the center of each colony and fixed at 4°C for 12 h in 2% (w/v) glutaraldehyde in 0.1 M phosphate buffer (PB; pH 7.0). The mycelial specimens were dehydrated in graded ethanol. After drying in a critical point dryer (Model 13200-AB, SPI SUPPLIES, PA, United States) and gold-coating in a sputter coater (Model JFC-1600, NTC, Japan), the mycelial specimens were examined under a scanning electron microscope (Model JSM-6390/LV, NTC, Japan).
Transmission Electron Microscopy
Morphology of the A. flavus samples was studied using transmission electron microscopy (TEM). Agar plugs, 6 mm in diameter, from 7-day-old culture were inoculated in the center of a piece of sterilized cellophane film (8 cm diameter) placed on PDA or PDA amended with CF3-10 at 2% in Petri dishes, with one plug per dish and three dishes per treatment. The dishes were incubated at 28°C for 7 days. The colonized films were cut into small pieces (3 mm × 3 mm) using a sharp razor. The cellophane film pieces were fixed in 2% (w/v) glutaraldehyde in 0.1 M PB (pH 7.0) at 4°C overnight. They were then washed in PB three times at room temperature (20–25°C), 10 min each time, and postfixed for 2 h in 1% osmium tetroxide, and stained for 1 h in 5% uranyl acetate (w/v) in 50% (v/v) of ethanol. Then, the fixed mycelial specimens were dehydrated in a graded series of ethanol, infiltrated with SPI-812 embedding medium and polymerized at 60°C for 12 h. Thin sections (50–60 nm) were cut with an ultra-microtome, mounted on copper grids, stained with 2% uranyl acetate and 5% aqueous lead citrate, and examined with a Hitachi transmission electron microscope (H-7650; Hitachi, Tokyo, Japan) at 80 kv. Images were recorded with a 4 KCCD camera (Model 832 ORIUS, Gatan, Pleasanton, CA, United States). At least 10 ultra-thin sections from each treatment were observed under TEM.
Data Analysis
All data were analyzed by one-way analysis of variance (ANOVA) with the statistical software SAS v. 9.1 (SAS Institute Inc., Cary, NC, United States). To meet the requirements of homogeneity of variance, the percent inhibition growth data of A. flavus by AFS of S. yanglinensis 3-10 was arcsin-transformed to angular data prior to ANOVA. Differences were analyzed by the least significant difference (LSD) post hoc test at α = 0.05. After each analysis, mean values were individually back-transformed to numerical values.
Results
Effects of S. yanglinensis 3-10 on Mycelial Growth of A. flavus in Vitro
The S. yanglinensis isolate 3-10 displayed significant inhibitory effects on the mycelial growth of A. flavus in dual culture in vitro assays. The average growth inhibition zone was 38.2 mm (Figure 1A). Compared to the control, the mycelial growth of A. flavus was reduced by 41%. In the tests on PDA plates, different concentrations of CF3-10 or CE3-10 incorporated in the PDA exhibited differential inhibitory effects on A. flavus. The efficacy (YCF) of inhibition of mycelial growth of A. flavus by the cultural filtrates of S. yanglinensis was positively related to the concentration of the cultural filtrates (XCF) incorporated in PDA. YCF = 47.006 Ln (XCF) + 19.305 (r = 0.9819, P < 0.01). With the increase of the concentration of the S. yanglinensis filtrate from 0.5 to 1.5%, the percentage inhibition of mycelial growth of A. flavus increased rapidly from 18.3 to 82.0% (Figure 1B). When the concentration of the filtrate was increased to 2 and 2.5%, the percentage inhibition slowly increased to 86.7 and 91.9%, respectively (Figure 1B). When the concentration of the filtrate was increased to 3%, the growth of A. flavus was completely inhibited (Figure 1B). The inhibition of mycelial growth of A. flavus by CE3-10 had a similar trend, yielding the equation YCE = 28.311 Ln (XCE) + 50.188 (r = 0.9838, P < 0.01) (Figure 1C). When the concentration of the CE3-10 was increased to 2.5%, the growth of A. flavus was completely inhibited (Figure 1C).
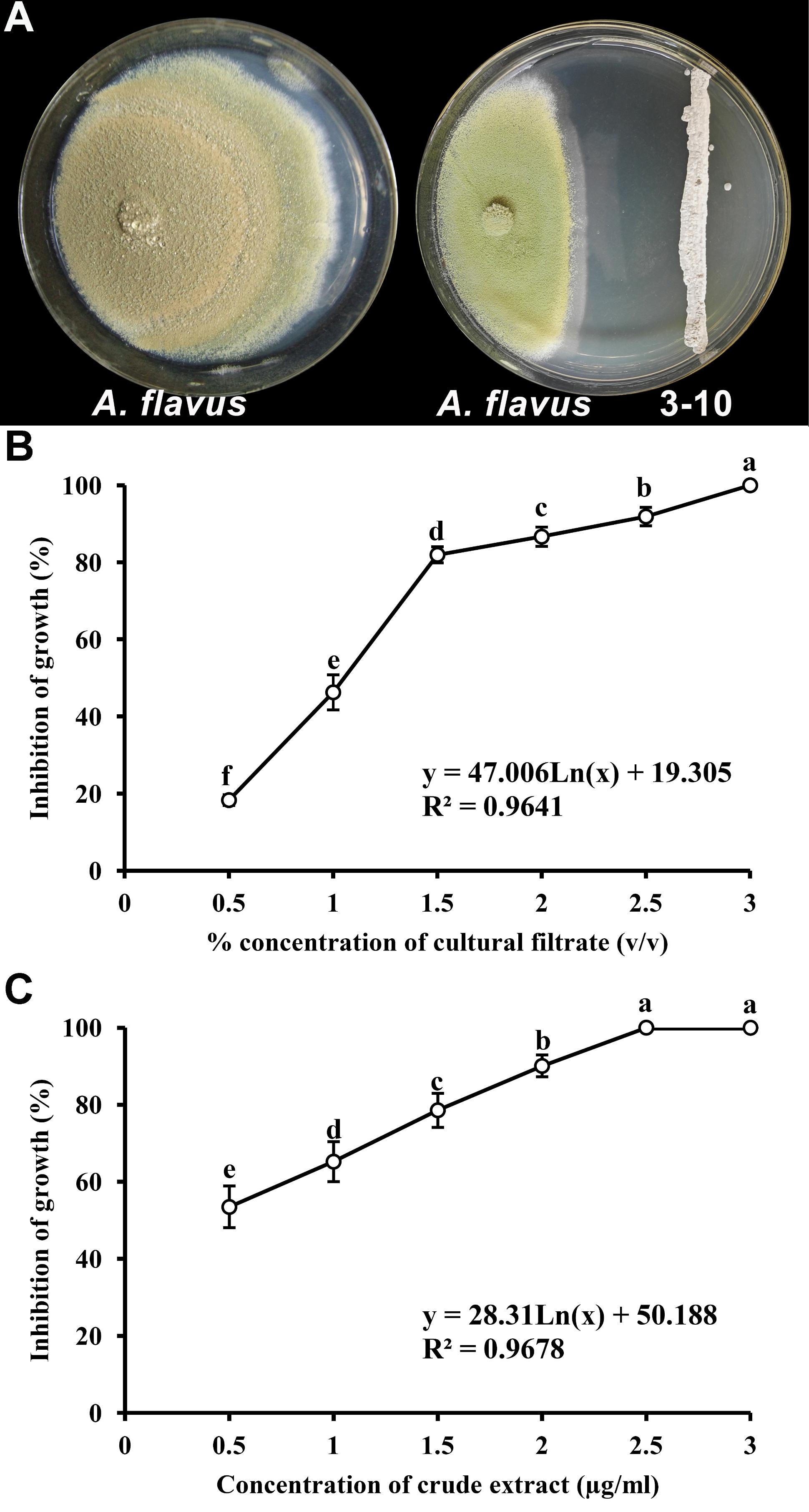
FIGURE 1. Inhibition of mycelial growth of Aspergillus flavus by Streptomyces yanglinensis 3-10. (A) Inhibitory effect of S. yanglinensis 3-10 against A. flavus in dual culture on PDA (28°C, 10 days). (B) Effect of culture filtrate of S. yanglinensis 3-10 on inhibition of growth of A. flavus. (C) Effect of crude extract of S. yanglinensis 3-10 on inhibition of growth of A. flavus. Means (circles) ± SD (whiskers) labeled with the same letter are not significantly different (P > 0.05) according to the least significant difference test.
Suppression of A. flavus Mycelial Growth and AFB1 Production by AFS of S. yanglinensis 3-10
The antifungal and anti-aflatoxigenic activities of S. yanglinensis and its AFS-containing products were analyzed using mycelial dry weights and AFB1 production by A. flavus. Inhibition of A. flavus mycelial growth and aflatoxin production in liquid culture were positively correlated with the concentration of different treatments. The average dry weight of A. flavus was 767.9 mg per flask in the negative control. With the increased concentration ranging from 1.25 to 5% for CF3-10, or from 1.25 to 5 μg/ml for CE3-10, the dry weight of A. flavus decreased significantly from 119.9 to 62.1 mg per flask (CF3-10) and 169 to 63.4 mg per flask (CE3-10), while in S3-10 it was 520 mg per flask (Table 1). The growth inhibition percentage of A. flavus in CF3-10 (5%, v/v) and CE3-10 (5 μg/ml) increased dramatically to 91.9 and 91.7%, respectively, but only 32.3% in S3-10 (Table 1). Similar trends were observed in suppression of AFB1. Reduction of AFB1 production ranged from 52.4 to 93.4% in CF3-10 treatment and from 48.5 to 79.4% in CE3-10 treatment, while it was only 46.41% in S3-10 (Table 1).
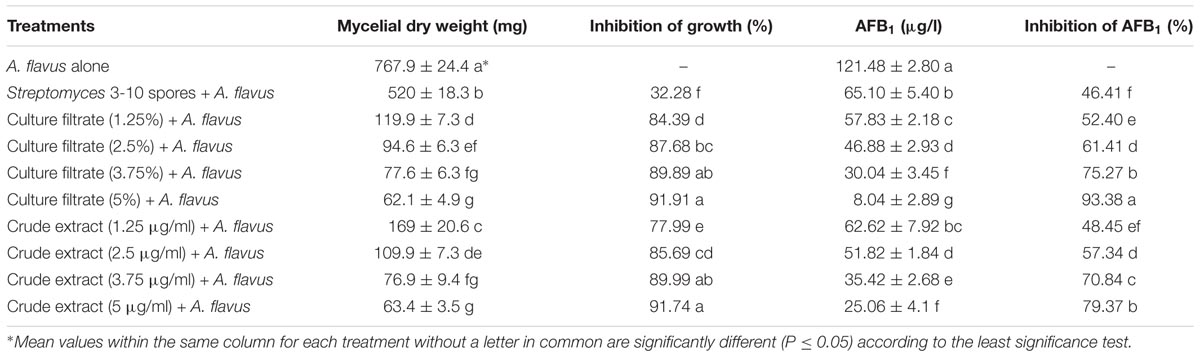
TABLE 1. Inhibition of mycelium and aflatoxin production from A. flavus by Streptomyces yanglinensis 3-10 and its metabolites in PDB.
Antifungal Activity on Peanuts Under Storage Conditions
The growth of A. flavus on fresh peanuts was examined after incubation for 7 days in the presence of S. yanglinensis and its AFS-containing products (Figure 2A). Compared with the control treatment (inoculated with A. flavus alone), growth of A. flavus was completely inhibited in peanuts treated with CF3-10 (100%, v/v) or CE3-10 (100 μg/ml). There was no visible growth of A. flavus on peanut kernels, while the S3-10 treatment had visible white mycelium on the kernels. Furthermore, in the CF3-10 and CE3-10 treatments, the amount of detected AFB1 was 14.4 and 16.1 μg/l, respectively, much lower than in the control treatment (135.6 μg/l). This result shows that both AFS products, CF3-10 and CE3-10, were very effective in suppressing not only growth but also AFB1 production by A. flavus. Surprisingly, S3-10 was not as effective as the other two treatments. The amount of detected AFB1 in the S3-10 treatment was 99.4 μg/l, which was still significantly lower than the in untreated control treatment (Figure 2).
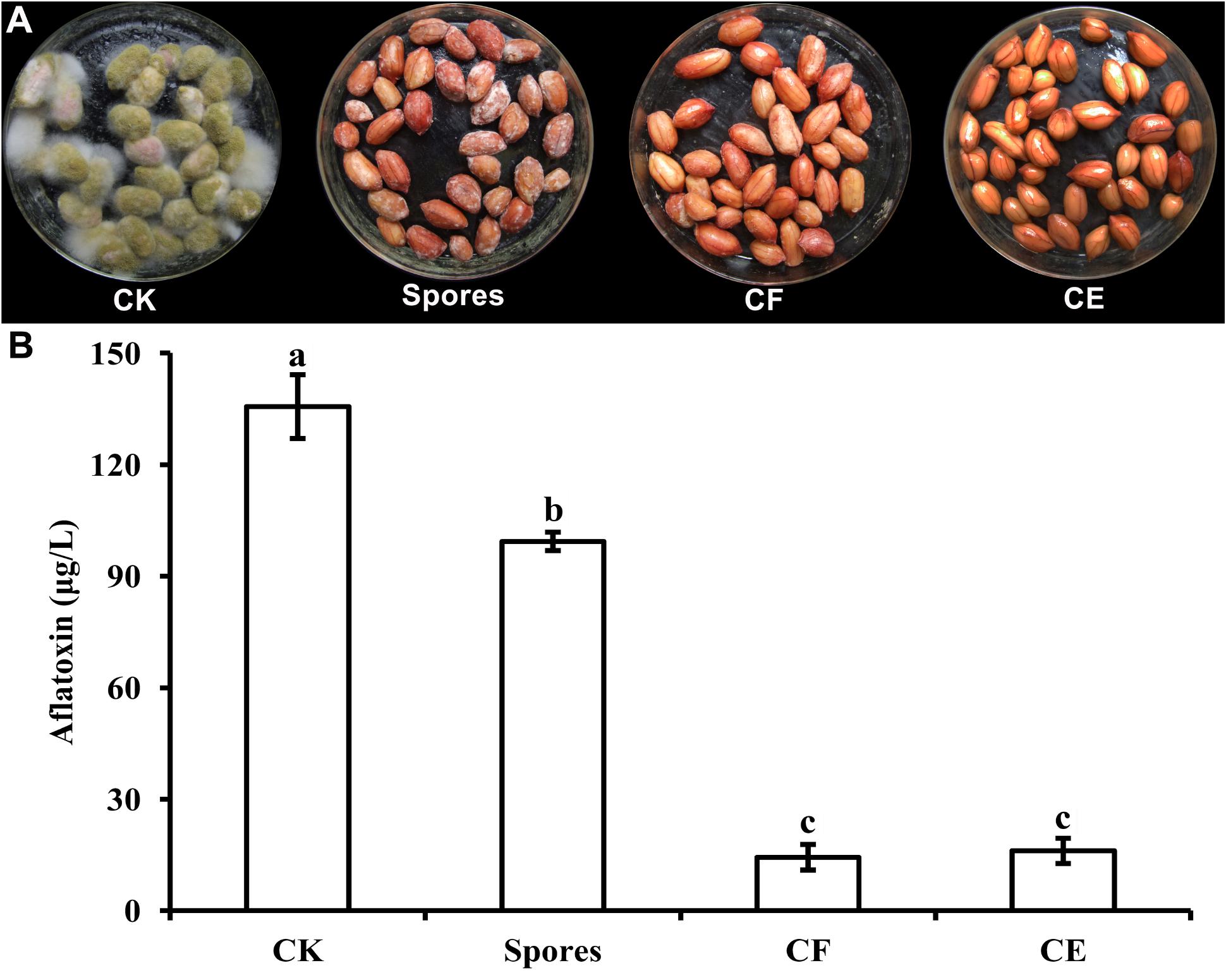
FIGURE 2. Streptomyces yanglinensis 3-10 and its AFS containing products inhibits A. flavus growth (A) and aflatoxin accumulation (B) in peanut kernels. CK, 1 × 108 spores/ml of A. flavus alone; Spores, washed cell mixture 1 × 108 spores/ml; CF, culture filtrate; CE, crude extract (100 μg/ml) (application volume 2 ml per 15 g peanut kernels). Treatment means (bars) ± SD (whiskers) labeled with the same letter are not significantly different (P > 0.05) according to the least significant difference test.
Effect of S. yanglinensis 3-10 and Its AFS Containing Products on aflR and aflS Genes Expression
In the control treatment (inoculated with A. flavus alone), the relative mRNA levels of aflR and aflS genes were 1.07 ± 0.12 and 1.06 ± 0.06, respectively. However, in the treatments of S3-10, CF3-10, and CE3-10, the expression levels of aflR were 0.98 ± 0.03, 0.09 ± 0.03, and 0.12 ± 0.02, respectively (Figure 3A). Similar trends were observed for the expression of aflS, at 0.58 ± 0.06, 0.34 ± 0.06, and 0.35 ± 0.05, respectively (Figure 3B). These results showed that S. yanglinensis could significantly suppress the expressions of these two genes (P < 0.05).
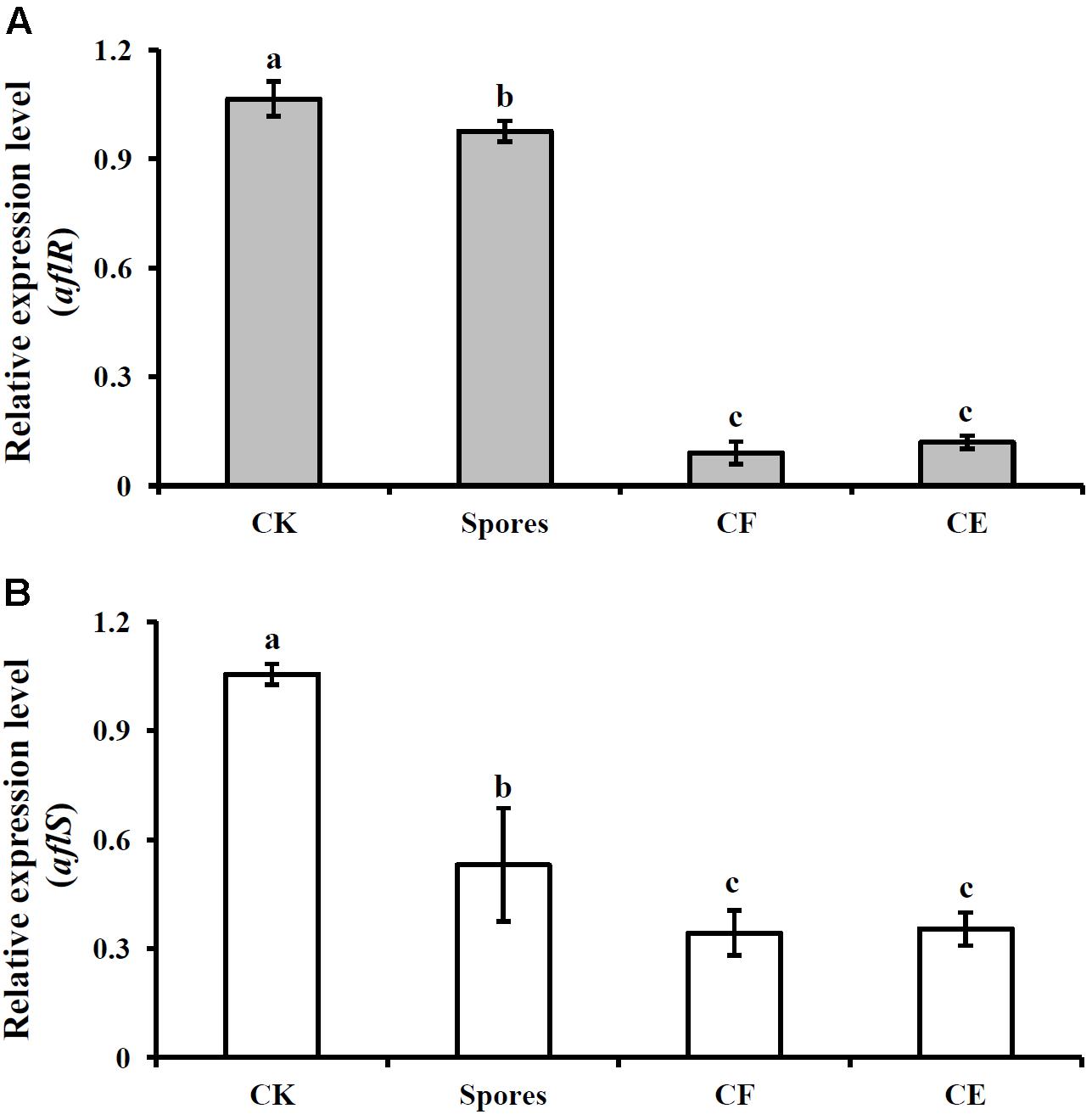
FIGURE 3. Expression of two aflatoxin biosynthesis genes aflR (A) and aflS (B) after treatment with S. yanglinensis 3-10 or its AFS containing products. The results are representative of the three independent experiments with similar results. CK, 5 × 106 spores/ml of A. flavus alone; Spores, washed cell 5 × 106 spores/ml; CF, culture filtrate (5%, v/v); CE, crude extract (5 μg/ml). Treatment means (bars) ± SD (whiskers) labeled with the same letter are not significantly different (P > 0.05) according to the least significant difference test.
Scanning Electron Microscopy
Aspergillus flavus was cultured on PDA amended with CF3-10 at 2% (v/v) and the effects on mycelial and conidiophore morphology of A. flavus was observed under SEM. In untreated controls (unamended PDA), development of mycelium and conidiophore was normal with abundant conidia (Figures 4A–D). While in CF3-10 (2%, v/v) treated culture, the development of A. flavus was suppressed. Under SEM, conidiophore development was obviously abnormal, where mycelia and conidiophores were shriveled compared to untreated controls (Figures 4E–H).
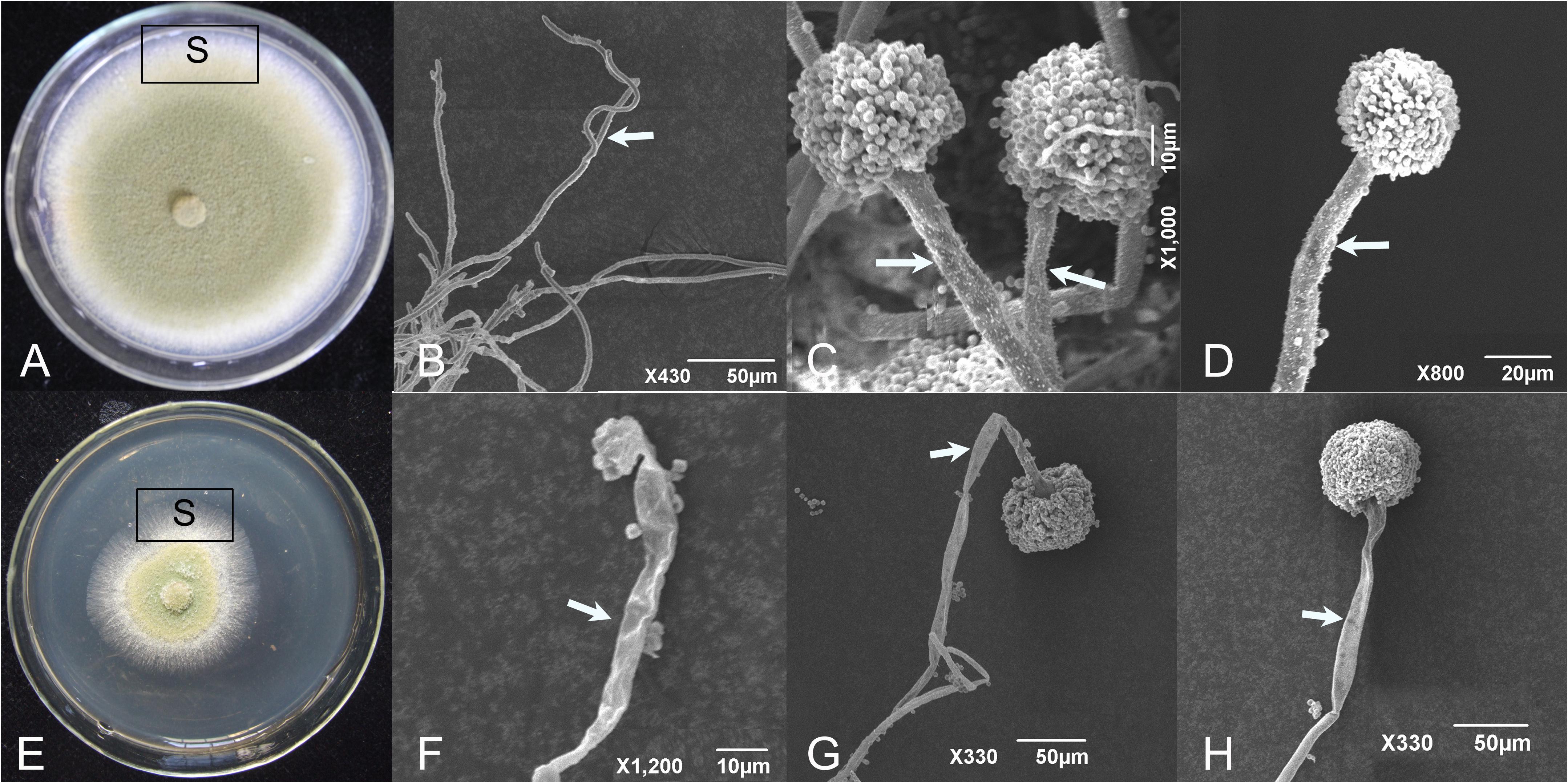
FIGURE 4. Scanning electron micrographs showing the healthy (A–D) and shriveled conidiophores (E–H) of A. flavus on PDA amended with culture filtrates of S. yanglinensis 3-10 at 2% (v/v). S, sampling site. The arrows mean hyphae or conidiophore.
Transmission Electron Microscopy
Thin sections examined by TEM showed that in control cultures, A. flavus possessed all the components of healthy eukaryotic cells (Figures 5A–D). But in cultures treated with CF3-10 (2%, v/v), only large vacuoles and disintegration of cytoplasm were observed (Figures 5E–H). In control treatments, there were fungal cells with maximum electron density and normal development of cell organelles (Figures 5B–D), while in cultures treated with CF3-10 (2%, v/v), the hyphae were degenerated and collapsed. The cell walls were well preserved and frequently visible (Figures 5F–H). Healthy cells contained obvious mitochondria and lipid bodies, while in treated cultures, development of these organelles was inhibited. In treated cultures, the lipid bodies were large and abundant and appeared to compress the cytoplasm content in the vicinity of plasma membranes making it difficult to recognize other cell organelles (Figures 5F–H). In a few healthy cells, single large nuclei were visible (Figures 5B,C), but because of the development of large vacuoles in treated cultures, the presence of nuclei was not obvious (Figures 5F–H).
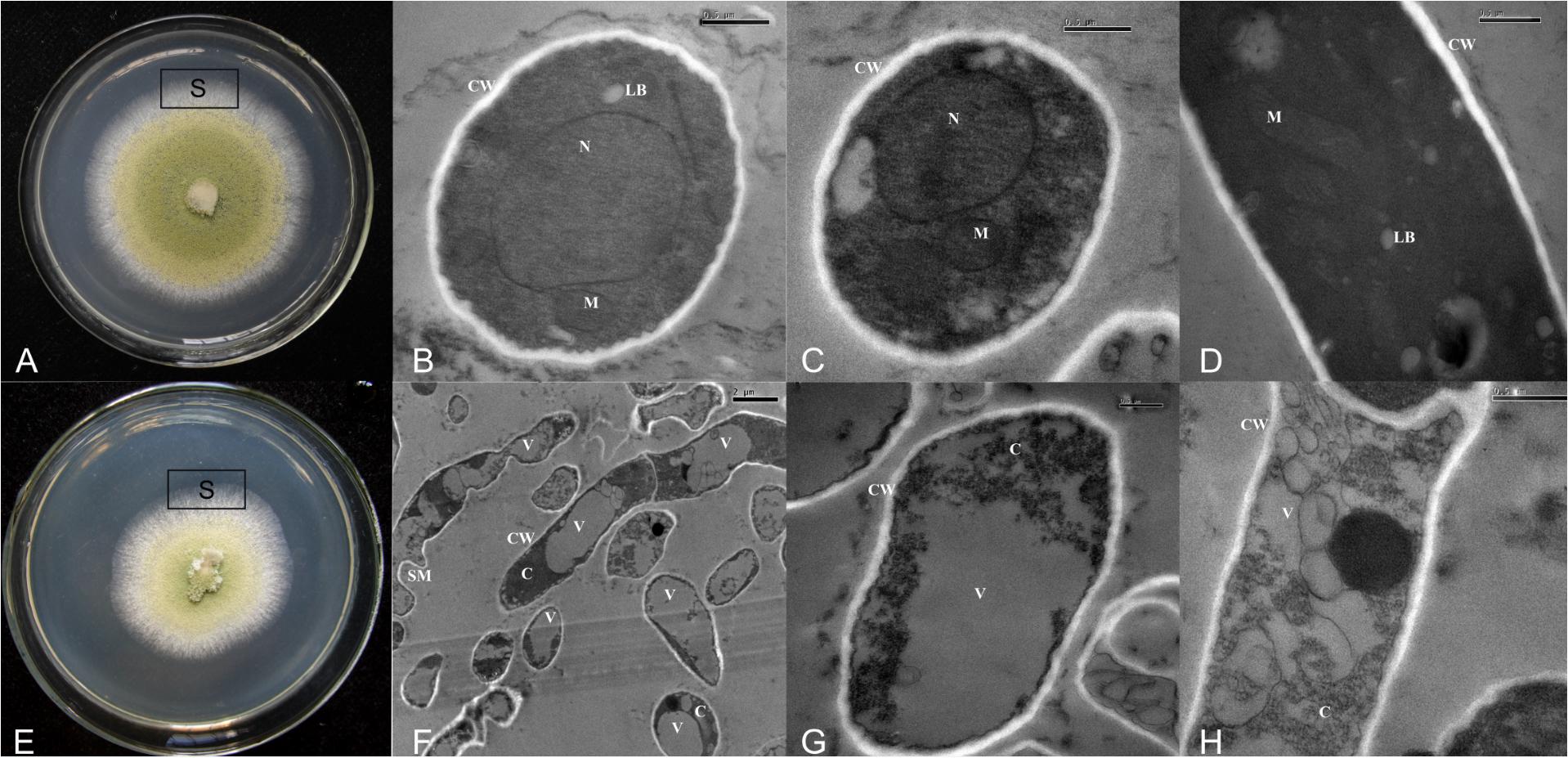
FIGURE 5. Transmission electron micrographs showing normal development of cell organelles (A–D) and disintegration of cytoplasm and cell organelles (E–H) when A. flavus was cultured on PDA amended with culture filtrates of S. yanglinensis 3-10 at 2% (v/v). VV, SM, N, M, LB, CW, C, vacuole; SM, shriveled mycelium; N, nucleus; M, mitochondria; LB, lipid bodies; CW, cell wall; C, cytoplasm; S, sampling site.
Discussion
Streptomyces yanglinensis isolate 3-10 was effective in suppressing postharvest disease caused by A. flavus both in vitro (liquid or solid media) and in vivo on peanut kernels. The cells of S. yanglinensis had inhibitory effects on A. flavus and the metabolites of S. yanglinensis were even more strongly inhibitory to A. flavus on PDA and in PDB. A comparison of our results with previous studies on A. flavus inhibition revealed that our compounds showed greater inhibitory effects than Bacillomycin D (Zhang et al., 2008) or extracts of Agave asperrima and Agave striata (Eduardo et al., 2005).
Our results are in agreement with those reported from other antagonists such as Streptomyces sp. ASBV-1 (Zucchi et al., 2008), Streptomyces hygroscopicus (Zhang et al., 2013), Streptomyces VITSVKS spp. (Kumar and Kannabiran, 2010), and Streptomyces VITSTK7 (Thenmozhi et al., 2013), which suggest the accumulation of bioactive metabolites in cultural medium or AFS production. Many Streptomyces isolates show antifungal activity against A. flavus, and can reduce aflatoxin B1 residual concentration, but the effectiveness depended on the particular isolate (Verheecke et al., 2014). Sultan and Magan (2011) reported that crude extracts of Streptomyces strain AS1 could achieve more than 85% inhibition of mycelial growth of A. flavus at 50 μg/ml, but in our results, the mycelial growth of A. flavus was completely inhibited by crude extracts of S. yanglinensis at 2.5 μg/ml. Results from the in vivo tests showed that the CF3-10 was as effective as the CE3-10, and even more effective in some cases. The concentration of the antagonist had significant effects on biocontrol effectiveness, and the higher the concentration of S. yanglinensis or its products were, the higher was the activity. The highest biocontrol activity was evident with CF3-10 rather than CE3-10 (100 μg/ml), indicating that some unknown bioactive metabolites could not be extracted with ethyl acetate.
Our findings also showed that S. yanglinensis was able to significantly inhibit the biosynthesis of aflatoxins in PDB and on peanut kernels (P < 0.05). We found that the biosynthesis of aflatoxins was related to the expression of aflatoxin pathway regulatory genes including aflR and aflS, as has been previously reported (Flaherty and Payne, 1997; Meyers et al., 1998). Quantitative PCR showed that in the aflS knockout mutants, the lack of aflS transcript led to a 5- to 20-fold reduction of expression of some aflatoxin pathway genes such as aflC (pksA), aflD (nor-1), aflM (ver-1), or aflP (omtA). The mutants lost the ability to synthesize aflatoxin intermediates and no aflatoxins were produced (Meyers et al., 1998). Deletion of aflR in Aspergillus parasiticus abolished the expression of other aflatoxin pathway genes (Cary et al., 2000). Overexpression of aflR in A. flavus up-regulated aflatoxin pathway gene transcription and aflatoxin accumulation (Flaherty and Payne, 1997), which was in accordance with a previous report in A. parasiticus (Chang et al., 1995). Our findings are in agreement with previous studies which demonstrated that aflS might be involved in the regulation of aflatoxin biosynthesis through the regulation of other genes, while aflR was more directly involved (Amare and Keller, 2014). However, to uncover the complete antagonistic physiological activities of S. yanglinensis and the underlying mechanisms, a more thorough investigation of all aflatoxin pathway genes should be investigated by the use of whole genome gene expression analyses such as RNA-Seq and differential gene expression, followed by specific quantification with targeted gene primers in real-time qPCR.
Lyu et al. (2017) identified active antifungal compounds purified from the crude extract of S. yanglinensis as reveromycins A and B. Reveromycins A is the main active AFS in the crude extract, which accounts for 37.7% of the crude extract. And the antifungal activity of reveromycins A is higher than that of reveromycins B (Lyu et al., 2017). Reveromycins A has been found to have a variety of effects, including inhibitory effects on mitogenic activity induced by epidermal growth factors (Osada et al., 1991), production of hormone-dependent tumors (Takahashi et al., 1997), induced proliferation of Candida species (Takahashi et al., 1992; Fremlin et al., 2011; Osada, 2016), and inhibition of mycelial growth of plant pathogenic fungi like Botrytis cinerea, Mucor hiemails, Rhizopus stolonifer, and Sclerotinia sclerotiorum (Lyu et al., 2017). Further research is needed on environmental effects and safety of reveromycins A, and on its antifungal activity against A. flavus and other food storage fungi.
Conclusion
In conclusion, this study demonstrated that S. yanglinensis isolate 3-10 has the potential for controlling peanut kernel postharvest disease caused by A. flavus and reducing the accumulation of aflatoxin. Still necessary are further studies to evaluate the potential risks of S. yanglinensis and its AFS for controlling A. flavus in peanut kernels and other agricultural commodities, as well as practical methods for delivery of optimal forms and concentrations of the antagonistic substances.
Author Contributions
QS, GL, and LY designed the research; QS and AL performed the research; QS, MW, JZ, and LY analyzed the data; QS, TH, and LY wrote the paper.
Funding
This research was funded by National Key Research and Development program of China (2017YFD0201100) and the Fundamental Research Funds for the Central Universities (Grant No. 2662015PY040).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Al-Bari, M. A. A., Sayeed, M. A., Khan, A., Islam, M. R., Khondokar, P., Rahman, M. M. S., et al. (2007). In vitro antimicrobial activities and cytotoxicity of ethyl acetate extract from Streptomyces maritimus. Biotechnology 6, 81–85. doi: 10.3923/biotech.2007.81.85
Amador, M. L., Jimeno, J., Paz-Ares, L., Cortes-Funes, H., and Hidalgo, M. (2003). Progress in the development and acquisition of anticancer agents from marine sources. Ann. Oncol. 14, 1607–1615. doi: 10.1093/annonc/mdg443
Amaike, S., and Keller, N. P. (2011). Aspergillus flavus. Annu. Rev. Phytopathol. 49, 107–133. doi: 10.1146/annurev-phyto-072910-095221
Amare, M. G., and Keller, N. P. (2014). Molecular mechanisms of Aspergillus flavus secondary metabolism and development. Fungal Genet. Biol. 66, 11–18. doi: 10.1016/j.fgb.2014.02.008
Berg, G., Marten, P., Minkwitz, A., and Brückner, S. (2001). Efficient biological control of plant fungal diseases by Streptomyces sp. DSMZ12424. J. Plant Dis. Protect. 108, 1–10.
Bullerman, L. B. (1976). Significance of mycotoxins to food safety and human health. J. Food Protect. 42, 65–86. doi: 10.4315/0362-028X-42.1.65
Calvo, A. M., Wilson, R. A., Bok, J. W., and Keller, N. P. (2002). Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 66, 447–459. doi: 10.1128/MMBR.66.3.447-459.2002
Cary, J. W., Ehrlich, K. C., Wright, M., Chang, P. K., and Bhatnagar, D. (2000). Generation of aflR disruption mutants of Aspergillus parasiticus. Appl. Microbiol. Biotechnol. 53, 680–684. doi: 10.1007/s002530000319
Chang, P. K. (2004). Lack of interaction between AFLR and AFLJ contributes to nonaflatoxigenicity of Aspergillus sojae. J. Biotechnol. 107, 245–253. doi: 10.1016/j.jbiotec.2003.10.012
Chang, P. K., Ehrlich, K. C., Yu, J., Bhatnagar, D., and Cleveland, T. E. (1995). Increased expression of Aspergillus parasiticus aflR, encoding a sequence-specific DNA binding protein, relieves nitrate inhibition of aflatoxin biosynthesis. Appl. Environ. Microbiol. 61, 2372–2377.
Demain, A. L. (1999). Pharmaceutically active secondary metabolites of microorganisms. Appl. Microbiol. Biotechnol. 52, 455–463. doi: 10.1007/s002530051546
Droby, S. (2006). Improving quality and safety of fresh fruits and vegetables after harvest by the use of biocontrol agents and natural materials. Acta Hortic. 709, 45–51. doi: 10.17660/ActaHortic.2006.709.5
Du, W., O’Brian, G. R., and Payne, G. A. (2007). Function and regulation of alfJ in the accumulation of aflatoxin early pathway intermediate in Aspergillus flavus. Food Addit. Contam. 24, 1043–1050. doi: 10.1080/02652030701513826
Eduardo, S., Norma, H., and Santos, G. (2005). Inhibition of growth and mycotoxin production of Aspergillus flavus and Aspergillus parasiticus by extracts of Agave species. Int. J. Food Microbiol. 98, 271–279. doi: 10.1016/j.ijfoodmicro.2004.07.009
Fang, Z. D. (1998). Research Methodology for Plant Diseases, 3rd Edn. Beijing, China: China Agriculture Press, 8–12.
Flaherty, J. E., and Payne, G. A. (1997). Overexpression of aflR leads to up regulation of pathway gene expression and increased aflatoxin production in Aspergillus flavus. Appl. Environ. Microbiol. 63, 3995–4000.
Fremlin, L., Farrugia, M., Piggott, A. M., Khalil, Z., Lacey, E., and Capon, R. J. (2011). Reveromycins revealed: new polyketide spiroketals from Australian marine-derived and terrestrial Streptomyces spp. A case of natural products vs. artifacts. Org. Biomol. Chem. 9, 1201–1211. doi: 10.1039/c0ob00654h
Gallagher, K. A., Fenical, W., and Jensen, P. R. (2010). Hybird isoprenoid secondry metabolite production in terrestrial and marine actinomycetes. Curr. Opin. Biotechnol. 21, 794–800. doi: 10.1016/j.copbio.2010.09.010
Gomes, R. C., Sêmedo, L. T., Soares, R. M., Linhares, L. F., Ulhoa, C. J., Alviano, C. S., et al. (2001). Purification of a thermostable endochitinase from Streptomyces RC1071 isolated from a cerrado soil and its antagonism against phytopathogenic fungi. J. Appl. Microbiol. 90, 653–661. doi: 10.1046/j.1365-2672.2001.01294.x
Kettleson, E., Kumar, S., Reponen, T., Vesper, S., Meheust, D., Grinshpun, S. A., et al. (2013). Stenotrophomonas, Mycobacterium, and Streptomyces in home dust and air: associations with moldiness and other home/family characteristics. Indoor Air 23, 387–396. doi: 10.1111/ina.12035
Klich, M. A. (2007). Aspergillus flavus: the major producer of aflatoxin. Mol. Plant Pathol. 8, 713–722. doi: 10.1111/J.1364-3703.2007.00436.X
Kong, Q., Shan, S., Liu, Q., Wang, X., and Yu, F. (2010). Biocontrol of Aspergillus flavus on peanut kernels by use of a strain of marine Bacillus megaterium. Int. J. Food Microbiol. 139, 31–35. doi: 10.1016/j.ijfoodmicro.2010.01.036
Krishnamurthy, Y. L., Shashikala, J., and Naik, B. S. (2008). Antifungal potential of some natural products against Aspergillus flavus in soybean seeds during storage. J. Stored Prod. Res. 44, 305–309. doi: 10.1016/j.jspr.2008.03.001
Kumar, S., and Kannabiran, K. (2010). Antifungal activity of Streptomyces VITSVK5 spp. against drug resistant Aspergillus clinical isolates from pulmonary tuberculosis patients. J. Med. Mycol. 20, 101–107. doi: 10.1016/j.mycmed.2010.04.005
Kumar, V., Basu, M. S., and Rajendran, T. P. (2008). Mycotoxin research and mycoflora in some commercially important agricultural commodities. Crop Prot. 27, 891–905. doi: 10.1016/j.cropro.2007.12.011
Law, J. W.-F., Ser, H.-L., Khan, T. M., Chuah, L.-H., Pusparajah, P., Chan, K.-G., et al. (2017). The potential of Streptomyces as biocontrol agents against the rice blast fungus, Magnaporthe oryzae (Pyricularia oryzae). Front. Microbiol. 8:3. doi: 10.3389/fmicb.2017.00003
Lou, Y., Han, Y. C., Yang, L., Wu, M. D., Zhang, J., Cheng, J. S., et al. (2015). CmpacC regulates mycoparasitism, oxalate degradation and antifungal activity in the mycoparasitic fungus Coniothyrium minitans. Environ. Microbiol. 17, 4711–4729. doi: 10.1111/1462-2920.13018
Lyu, A., Liu, H., Che, H. J., Yang, L., Zhang, J., Wu, M. D., et al. (2017). Reveromycins A and B from Streptomyces sp. 3-10: antifungal activity against plant pathogenic fungi in vitro and in a strawberry food model system. Front. Microbiol. 8:550. doi: 10.3389/fmicb.2017.00550
Mander, P., Cho, S. S., Choi, Y. H., Panthi, S., Choi, Y. S., Kim, H. M., et al. (2016). Purification and characterization of chitinase showing antifungal and biodegradation properties obtained from Streptomyces anulantus CS242. Arch. Pharm. Res. 39, 878–886. doi: 10.1007/s12272-016-0747-3
Manivasagan, P., Kang, K. H., Sivakumar, K., Li-Chan, E. C., Oh, H. M., and Kim, S. K. (2014). Marine actinobacteria: an important source of bioactive natural products. Environ. Toxicol. Pharmacol. 38, 172–188. doi: 10.1016/j.etap.2014.05.014
Meyers, D. M., Obrian, G., Du, W. L., Bhatnagar, D., and Payne, G. A. (1998). Characterization of aflJ, a gene required for conversion of pathway intermediates to aflatoxin. Appl. Environ. Microbiol. 64, 3713–3717.
Minuto, A., Spadaro, D., Garibaldi, A., and Gullino, M. L. (2006). Control of soilborne pathogens of tomato using a commercial formulation of Streptomyces griseoviridis and solarisation. Crop Protec. 25, 468–475. doi: 10.1016/j.cropro.2005.08.001
Osada, H. (2016). Chemical and biological studies of reveromycin A. J. Antibiot. 69, 723–730. doi: 10.1038/ja.2016.57
Osada, H., Koshino, H., Isono, K., Takahashi, H., and Kawanishi, G. (1991). Reveromycin A, a new antibiotic which inhibits the mitogenic activity of epidermal growth factor. J. Antibiot. 44, 259–261. doi: 10.7164/antibiotics.44.259
Sakuda, S., Ikeda, H., Nakamura, T., Kawachi, R., Kondo, T., Ono, M., et al. (2000a). Blasticidin A derivatives with highly specific inhibitory activity toward aflatoxin production in Aspergillus parasiticus. J. Antibiot. 53, 1378–1384.
Sakuda, S., Ono, M., Ikeda, H., Nakamura, T., Inagaki, Y., Kawachi, R., et al. (2000b). Blasticidin A as an inhibitor of aflatoxin production by Aspergillus parasiticus. J. Antibiot. 53, 1265–1271. doi: 10.7164/antibiotics.53.1265
Shakeel, Q., Lyu, A., Zhang, J., Wu, M. D., Chen, S. W., Chen, W. D., et al. (2016). Optimization of the cultural medium and conditions for production of antifungal substances by Streptomyces platensis 3-10 and evaluation of its efficacy in suppression of clubroot disease (Plasmodiophora brassicae) of oilseed rape. Biol. Control 101, 59–68. doi: 10.1016/j.biocontrol.2016.06.007
Sultan, Y., and Magan, N. (2011). Impact of a Streptomyces (AS1) strain and its metabolites on control of Aspergillus flavus and aflatoxin B1 contamination in vitro and in stored peanuts. Biocontrol Sci. Techn. 21, 1437–1455. doi: 10.1080/09583157.2011.632078
Supong, K., Thawai, C., Choowong, W., Kittiwongwattana, C. C., Thanaboripat, D., Laosinwattana, C., et al. (2016). Antimicrobial compounds from endophytic Streptomyces sp. BCC72023 isolated from rice (Oryza sativa L.). Res. Microbiol. 167, 290–298. doi: 10.1016/j.resmic.2016.01.004
Takahashi, H., Osada, H., Koshino, H., Sasaki, M., Onose, R., Nakakoshi, M., et al. (1992). Reveromycins, new inhibitors of eukaryotic cell growth II. Biological activities. J. Antibiot. 45, 1414–1419. doi: 10.7164/antibiotics.45.1414
Takahashi, H., Yamashita, Y., Takaoka, H., Nakamura, J., Yoshihama, M., and Osada, H. (1997). Inhibitory action of reveromycin A on TGF-alpha-dependent growth of ovarian carcinoma BG-1 in vitro and in vivo. Oncol. Res. 9, 7–11.
Thenmozhi, M., Kannabiran, K., Kumar, R., and Khanna, V. G. (2013). Antifungal activity of Streptomyces sp. VITSTK7 and its synthesized Ag2O/Ag nanoparticles against medically important Aspergillus pathogens. J. Med. Mycol. 23, 97–103. doi: 10.1016/j.mycmed.2013.04.005
Verheecke, C., Liboz, T., Darriet, M., Sabaou, N., and Mathieu, F. (2014). In vitro interaction of actinomycetes isolates with Aspergillus flavius: impact on aflatoxins B1 and B2 production. Lett. Appl. Microbiol. 58, 597–603. doi: 10.1111/lam.12233
Wan, M. G., Li, G. Q., Zhang, J. B., Jiang, D. H., and Huang, H. C. (2008). Effect of volatile substances of Streptomyces platensis F-1 on control of plant fungal diseases. Biol. Control 46, 552–559. doi: 10.1016/j.biocontrol.2008.05.015
Yoshinari, T., Akiyama, T., Nakamura, K., Kondo, T., Takahashi, Y., Muraoka, Y., et al. (2007). Dioctatin A is a strong inhibitor of aflatoxin production by Aspergillus parasiticus. Microbiology 153, 2774–2780. doi: 10.1099/mic.0.2006/005629-0
Yu, J. J., Chang, P. K., Ehrlich, K. C., Cary, J. W., Bhatnagar, D., Cleveland, T. E., et al. (2004). Clustered pathway genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 70, 1253–1262. doi: 10.1128/AEM.70.3.1253-1262.2004
Zhang, N., Song, Z., Xie, Y., Cui, P., Jiang, H., Yang, T., et al. (2013). Identification and characterization of antifungal active substances of Streptomyces hygroscopicus BS-112. World J. Microbiol. Biotechnol. 29, 1443–1452. doi: 10.1007/s11274-013-1307-3
Zhang, T., Shi, Z. Q., Hu, L. B., Cheng, L. G., and Wang, F. (2008). Antifungal compounds from Bacillus subtilis B-FS06 inhibiting the growth of Aspergillus flavus. World J. Microbiol. Biotechnol. 24, 783–788. doi: 10.1007/s11274-007-9533-1
Keywords: Streptomyces yanglinensis 3-10, peanut kernels, Aspergillus flavus, aflatoxin, biological control
Citation: Shakeel Q, Lyu A, Zhang J, Wu M, Li G, Hsiang T and Yang L (2018) Biocontrol of Aspergillus flavus on Peanut Kernels Using Streptomyces yanglinensis 3-10. Front. Microbiol. 9:1049. doi: 10.3389/fmicb.2018.01049
Received: 29 December 2017; Accepted: 02 May 2018;
Published: 23 May 2018.
Edited by:
Abd El-Latif Hesham, Assiut University, EgyptReviewed by:
Zuzana Hruska, Mississippi State University, United StatesLearn-Han Lee, Monash University Malaysia, Malaysia
Copyright © 2018 Shakeel, Lyu, Zhang, Wu, Li, Hsiang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Long Yang, eWFuZ2xvbmdAbWFpbC5oemF1LmVkdS5jbg==
†Present address: Qaiser Shakeel, Discipline of Plant Pathology, University College of Agriculture and Environmental Sciences, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
 Qaiser Shakeel
Qaiser Shakeel Ang Lyu
Ang Lyu Jing Zhang
Jing Zhang Mingde Wu1
Mingde Wu1 Guoqing Li
Guoqing Li Long Yang
Long Yang