- 1Division of Microbiology, Department of Pathology and Microbiology, Nihon University School of Medicine, Tokyo, Japan
- 2Division of Pediatric Dentistry, Meikai University School of Dentistry, Sakado, Japan
- 3Department of Pharmacy, College of Pharmacy, Hanyang University, Ansan, South Korea
- 4Institute of Pharmacological Research, Hanyang University, Ansan, South Korea
- 5Evelo Biosciences, Cambridge, MA, United States
- 6Institute for Life Science Entrepreneurship, Union, NJ, United States
- 7Department of Pharmacy Practice, Eugene Applebaum College of Pharmacy and Health Sciences, Wayne State University, Detroit, MI, United States
- 8Department of Laboratory Medicine, Ageo Central General Hospital, Ageo, Japan
Infections caused by multidrug-resistant Pseudomonas aeruginosa in hospitalized patients are often fatal, and nosocomial infections caused by Guiana extended-spectrum (GES) β-lactamase-producing strains are of growing concern. Several genotypes of the GES β-lactamase gene (blaGES) include a single missense mutation, a change from G to A at nucleotide position 493 (G493A) that changes glycine to serine; the mutant enzyme exhibits carbapenemase activity. Rapid and reliable identification of drug-resistance is important in clinical settings; however, culture methods remain the gold standard. Conventional and real-time PCR cannot identify carbapenemase-producing genotypes, and direct DNA sequencing is essential. We established a novel loop-mediated isothermal amplification (LAMP) method to detect various genotypes of blaGES and another LAMP method to discriminate carbapenemase genotypes of blaGES. We evaluated the two assays using clinical P. aeruginosa strains. Two primer sets targeting blaGES (GES-LAMP) and the point mutation (Carba-GES-LAMP) were designed and evaluated for specificity and sensitivity. The detection limit of the GES-LAMP method was assessed using purified DNA and DNA-spiked clinical samples (urine, sputum, and blood). To determine the clinical usefulness of the methods, we used different (genotypically and phenotypically) P. aeruginosa clinical isolates, collected from diverse geographical locations between 2003 and 2012. The novel LAMP assay targeting blaGES was highly specific. The detection limit was 10 DNA copies per reaction; the assay was 10-fold more sensitive than conventional PCR. The LAMP assay detected blaGES with high sensitivity in all DNA-spiked samples; PCR did not detect blaGES in blood samples. The GES-LAMP method correctly detected the 5 isolates containing blaGES among the 14 isolates tested. Using these isolates, we confirmed that our Carba-GES-LAMP method of detecting point mutations correctly identified the two blaGES positive organisms with carbapenemase activity. To the best of our knowledge, this is the first report of the GES β-lactamase gene detection assay using the LAMP method. Our new assays effectively detect blaGES and critical unique mutations.
Introduction
Multidrug-resistant bacteria have spread worldwide and become a major clinical concern and public health issue as well. Invasive infection by Pseudomonas aeruginosa is often fatal in the absence of appropriate antibiotic treatment (Miyoshi-Akiyama et al., 2017). As β-lactamase genes are plasmid-borne, a failure to treat drug-resistant bacteria may trigger nosocomial infections. Of the various β-lactamase genes, Guiana extended-spectrum (GES) β-lactamase has become of increasing concern (Poirel et al., 2012). GES was first described in 2000 in French Guiana (Poirel et al., 2000) and is an Amber class A extended-spectrum β-lactamase. However, certain variants (GES-4, -5, -6, -14, -15, -16, -18, -20, and -24) feature a single missense mutation at nucleotide position 493 (G493A) that changes glycine 165 to serine (Gly165Ser), which was previously reported as Gly170Ser (Bebrone et al., 2013). The resulting mutant enzyme exhibits carbapenemase activity (Bonnin et al., 2017). In Japan, GES-4 and GES-5 carbapenemase-producing P. aeruginosa have caused severe hospital-acquired infections (Kanayama et al., 2016; Yamasaki et al., 2017). Reports of GES-type enzymes remain rare but are steadily increasing (Naas et al., 2016).
Rapid and reliable identification of drug-resistance is essential to ensure that antibiotic use is appropriate. Conventional culture remains the gold standard for assessing antibiotic resistance despite being time-consuming, requiring sophisticated laboratory equipment and quality-control, and yielding ambiguous outcomes. Furthermore, the number of inoculated bacteria affect the drug minimum inhibitory concentration (MIC) (Bratu et al., 2005). Conventional PCR-based assays can detect β-lactamase genes but require well-equipped laboratories. Moreover, neither conventional PCR nor real-time PCR can identify carbapenemase-producing genotypes, and direct DNA sequencing is essential to do so (de Oliveira et al., 2017).
Loop-mediated isothermal amplification (LAMP) methods are becoming increasingly popular due to their relative simplicity and accuracy. The unique priming mechanism allows rapid and specific DNA amplification (Notomi et al., 2000), with no requirement for expensive equipment or a sophisticated laboratory. LAMP is a convenient and inexpensive alternative to PCR in terms of point-of-care testing (POCT). Here, we established two LAMP methods to detect the GES β-lactamase gene (blaGES, GES-LAMP) and carbapenemase genotypes (Carba-GES-LAMP) and applied the two assays to evaluate characterized (both genotypically and phenotypically) clinical P. aeruginosa strains from diverse geographical locations collected between 2003 and 2012 (Kos et al., 2015).
Materials and Methods
Bacterial Strains
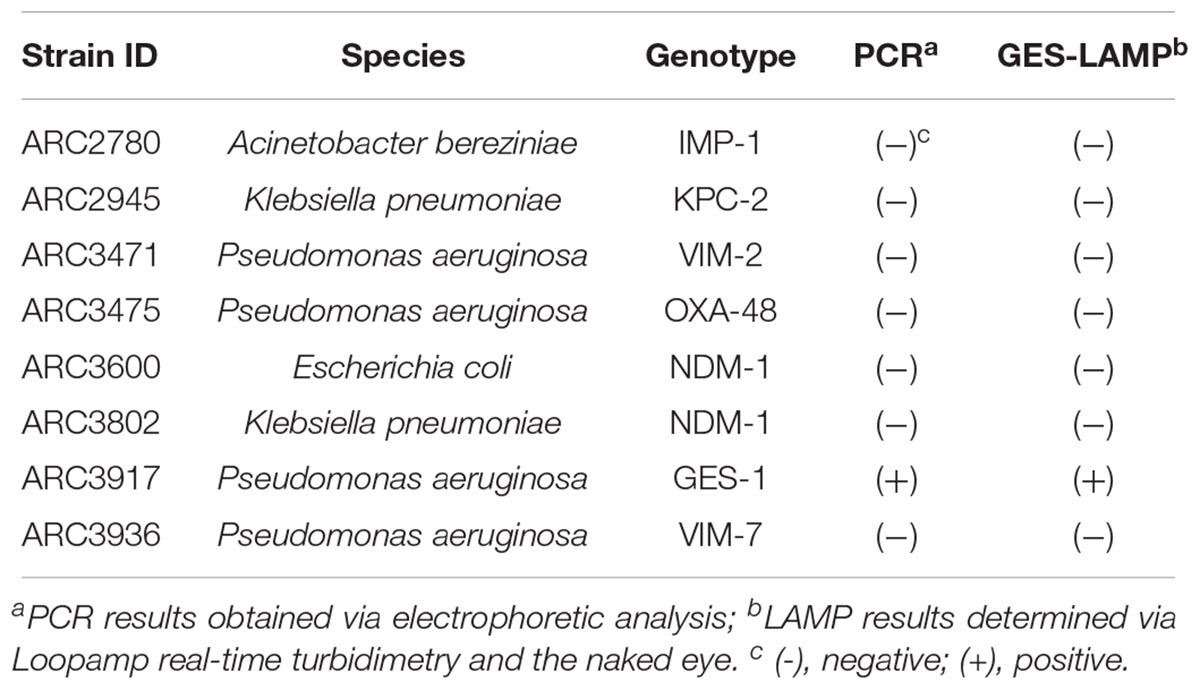
A total of 22 bacterial strains including 8 standard strains (Table 1) and 14 clinical P. aeruginosa strains (Table 2) were used to evaluate the LAMP methods. The eight standard strains included six kinds of genotypes of β-lactamase producers (KPC, NDM, VIM, IMP, OXA, and GES): two Klebsiella pneumoniae, one Escherichia coli, four P. aeruginosa, and one Acinetobacter bereziniae provided by AstraZeneca (Waltham, MA, United States). Genomic DNA was extracted using the Maxwell 16-cell DNA purification kit (Promega, Madison, WI, United States). DNA concentrations were measured using the NanoDrop 1000 (Thermo Fisher Scientific Inc., Waltham, MA, United States). Genome copy numbers were calculated based on genome sizes of 6.5 Mbp for P. aeruginosa (PB369; GenBank accession number, CP025049.1), 5.4 Mbp for K. pneumoniae (Kp52.145; GenBank accession number, FO834906.1), 5.2 Mbp for E. coli (CFT073; GenBank accession number, AE014075.1), and 4.5 Mbp for A. bereziniae (XH901; GenBank accession number, NZ_CP018259.1). Each DNA sample was normalized to the same concentration and used to evaluate assay specificity. To validate blaGES detection limits, we used genomic DNA from the P. aeruginosa strain ARC3917. Serial 10-fold-diluted DNA samples (105, 104, 103, 102, 10, and 1 genome copies) were amplified by LAMP and the results were compared with those from PCR assays. To confirm reproducibility, triplicate tests were performed over a 3-day period.
Clinical P. aeruginosa Strains
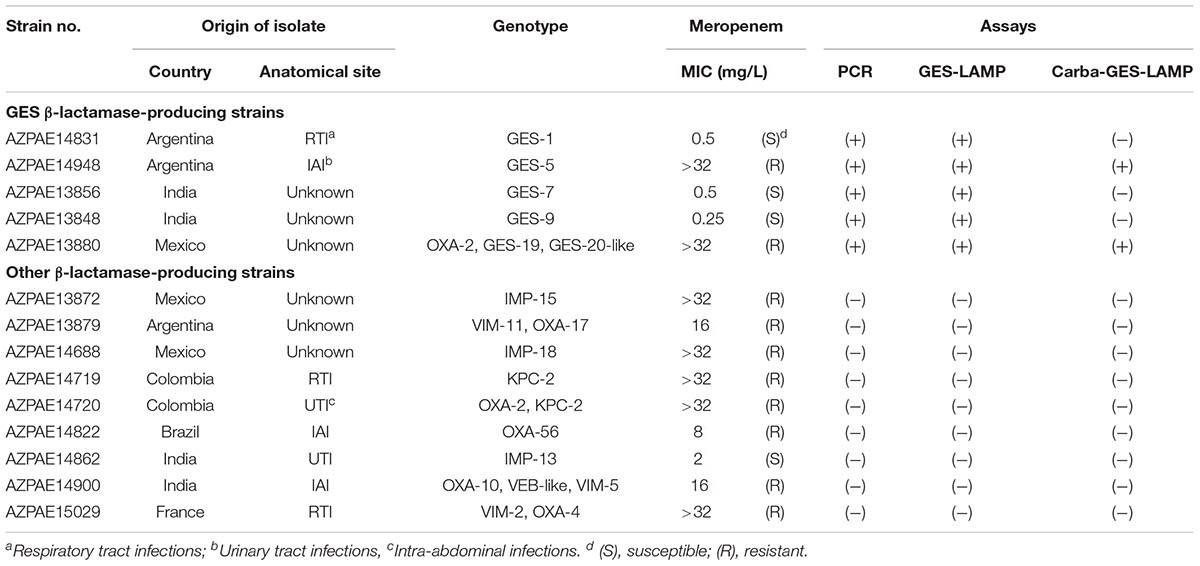
Fourteen clinical P. aeruginosa strains including five blaGES segments (GES-1, -5, -7, -9, and -19/20-like) were randomly selected from 388 strains with previously reported genotypes and phenotypes (Kos et al., 2015; Table 2) isolated from diverse geographical locations (Colombia, India, Spain, France, Greece, Germany, Argentina, Croatia, China, Brazil, Mexico, and the Philippines) between 2003 and 2012. Genomic DNA was extracted using the Maxwell 16-cell DNA purification kit (Promega). Whole-genome sequences were analyzed using the HiSeq 2000 or MiSeq platforms (Illumina, San Diego, CA, United States). Susceptibility to meropenem was explored using frozen Trek-Sensititre custom plates (Thermo Fisher Scientific Inc.) following the guidelines of the Clinical and Laboratory Standards Institute (2012). The results of meropenem MIC are listed in Table 2. We used genomic DNA from P. aeruginosa strain AZPAE4948 to evaluate the LAMP assay.
LAMP Primer Design
We targeted the blaGES gene (GES-1; GenBank accession number, AF355189.1; Supplementary Figure S1) using primers designed by Primer Explore V5 software (FUJITSU, 2016). The LAMP primers included two outer primers (F3 and B3), a forward inner primer (FIP), a backward inner primer (BIP), and loop primers (LF and LB) (Table 3). In addition, we developed primers targeting the G493A mutation of blaGES (GES-5; GenBank accession number, EF190326.1; Supplementary Figure S1) using an amplification-refractory mutation system (ARMS) (Newton et al., 1989; Ikeda et al., 2007). The BIP featured the addition of a single nucleotide mutation to the 5′-end, followed by the addition of two mutations in the second (G to C) and fifth (A to T) positions from the 5′ end. Other primers were designed using Primer Explorer V5 software (Table 3).
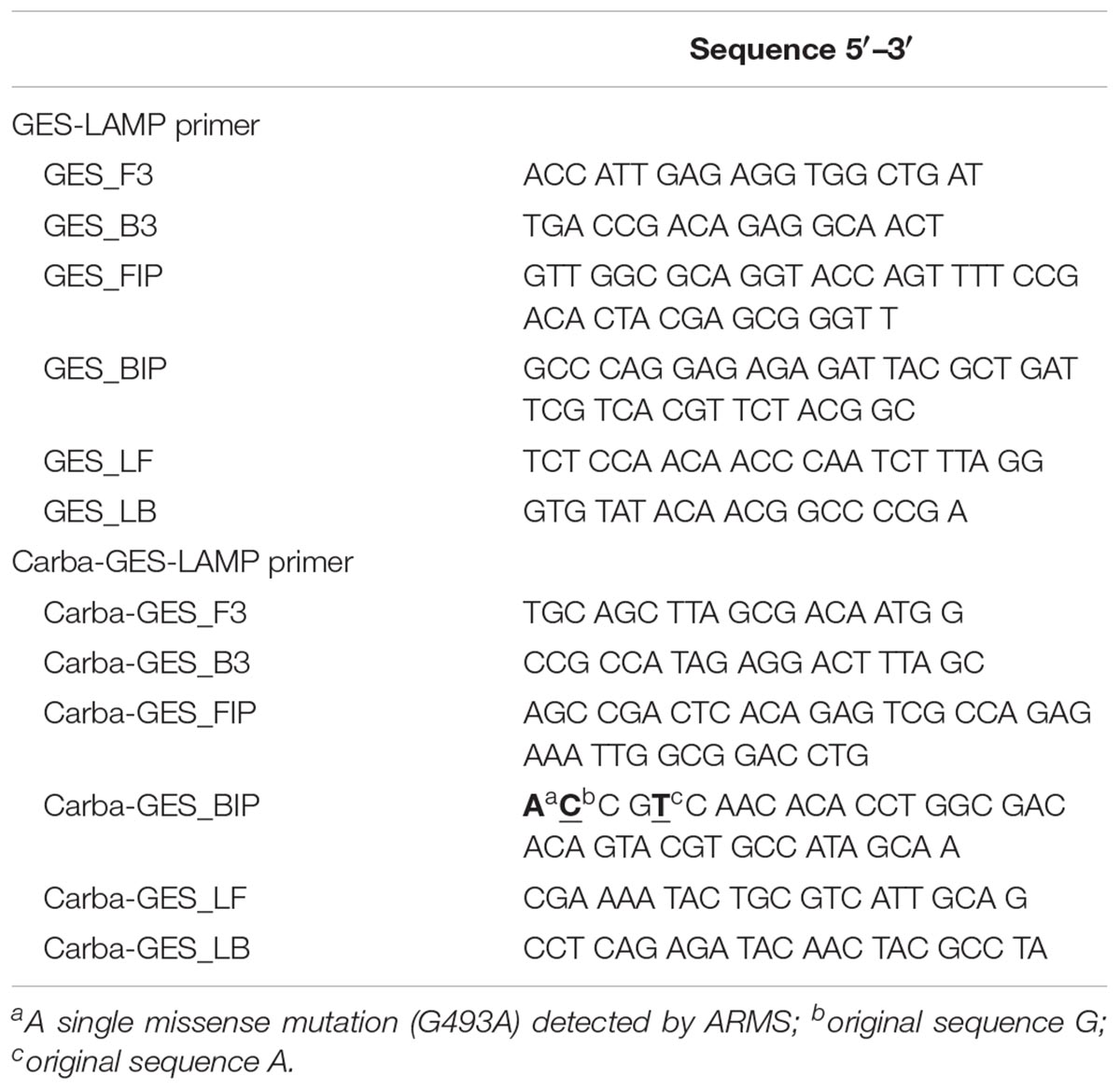
Table 3. LAMP primer sets used for genotypic identification of blaGES and the single missense mutation (G493A) that changes glycine 165 to a serine, endowing the enzyme with carbapenemase activity.
LAMP
The LAMP reaction mixture (25 μL) contained 1.6 μM FIP and BIP each, 0.2 μM F3 and B3 each, 0.4 μM LF, 8 U Bst DNA polymerase (large fragment) (New England Biolabs, Ipswich, MA, United States), 1.4 mM all four deoxynucleoside triphosphates, 0.8 M betaine (Sigma, St. Louis, MO, United States), 20 mM Tris–HCl (pH 8.8), 10 mM KCl, 10 mM (NH4)2SO4, 8 mM MgSO4, 0.1% (v/v) Tween 20, and template DNA (2 μL). Each mixture was incubated at 63°C for 60 min and then heated at 80°C for 2 min to terminate the reaction. For the Carba-GES-LAMP assay, the incubation time was 50 min. We monitored reaction tube turbidity in real time using a Loopamp turbidimeter (EXIA; Eiken Chemical Co., Tokyo, Japan) to read the optical density at 650 nm (OD650) at 6-s intervals. We recorded the time required to exceed a turbidity level of 0.1, in accordance with the manufacturer’s protocol. Amplified products could be seen with the naked eye.
Analysis of LAMP Products
Amplified LAMP products were sequenced at the Akita Prefectural University Biotechnology Center using the BigDye Terminator V3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA, United States) on the 3130xL genetic analyzer (Applied Biosystems). The following F2 primers for GES-LAMP and Carba-GES-LAMP were used to sequence the target regions; GES-F2, 5′-TTC TAG CAT CGG GAC ACA TG-3′ and Carba-GES-F2, 5′-AGA GAA ATT GGC GGA CCT G-3′, respectively.
PCR
blaGES genes were amplified by PCR using previously described primers: GES-F, 5′-CTA TTA CTG GCA GGG ATC G-3′; GES-R, 5′-CCT CTC AAT GGT GTG GGT-3′ (Monteiro et al., 2012). PCR assays were performed using the Ex Taq enzyme (Takara Bio, Tokyo, Japan) in a 25 μL reaction mixture containing 0.2 mM each deoxyribonucleoside triphosphate, 10 mM Tris–HCl buffer (pH 8.3), 50 mM KCl, 2 mM MgCl2, 0.5 μM each primer, 1 U Ex Taq DNA polymerase, and template DNA (2 μL). The PCR program sequence was 94°C for 1 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 60 s, and a final extension at 72°C for 15 min, followed by storage at 4°C. All reactions were performed in duplicate using the Veriti thermal cycler (Applied Biosystems). The resulting PCR products were subjected to agarose gel electrophoresis followed by staining with ethidium bromide. The expected size of the DNA fragment is 594 bp.
DNA-Spiked Specimens
To analyze the effects of biological substances on the established LAMP amplification, we studied the sensitivity of the LAMP assay using DNA-spiked clinical samples. We collected urine and blood specimens from five healthy volunteers in Nihon University School of Medicine. The blood specimens were heparinised and stored at -80°C. Sputum specimens were obtained from seven patients of the Ageo Central General Hospital and frozen at -80°C to inactivate bacteria. After approval was granted by the Biosafety Committee of Nihon University, the specimens were handled using the risk group 2 protocol of the laboratory biosafety manual of the World Health Organization, Geneva, 2004 (World Health Organization, 2004).
Urine specimens were boiled at 95°C for 5 min and then centrifuged at 1,500 rpm. Blood and sputum samples were subjected to a LoopampTM PURE DNA extraction kit (Eiken Chemical Co.) according to the manufacturer’s instructions. Purified blaGES DNA (from P. aeruginosa ARC3917) was spiked into the specimens and used to determine the detection limits of the GES-LAMP and PCR assays.
Ethics Statement
We utilized urine and blood specimens from five healthy volunteers in Nihon University School of Medicine. The study protocol was reviewed and approved by the Institutional Review Board of Nihon University School of Medicine (IRB # 28-9-0). Written informed consent was obtained from five healthy volunteers. Using the IRB approved protocol, seven patient sputum specimens (Ageo Central General Hospital) were collected in accordance with the recommendations of the Japan Society of Clinical Examination Medicine that supports “the use of specimens that have completed clinical tests for work, education, research.” This guidance provides access to specimens when it is difficult to obtain consent, the sample is anonymized, and where the scientific/ethical review committee (i.e., IRB) has approved the study protocol. Written consent was waived because specimens were anonymized discarded samples from the hospital clinical laboratory. The ethical approval for collection of those specimens were obtained from the ethical committee at the Ageo Central General Hospital (Approval # 434) and the Institutional Review Board of Nihon University School of Medicine (Approval # 28-9-0).
Results
Analytical Reactivity and Specificity of the GES-LAMP Assay
The LAMP assay successfully amplified the target sequence, as confirmed by visually evident turbidity in the reaction tube and by real-time turbidimetry (Figure 1). Of the various β-lactamase genes, the assay detected only blaGES (Table 1). The product was subjected to direct sequencing. The sequences were compared with those of the targeted region (bases 337–425) of the GES-1 gene (from F1 to B1c, Supplementary Figure S1A). The sequences obtained were identical to those expected (Supplementary Figure S2A).
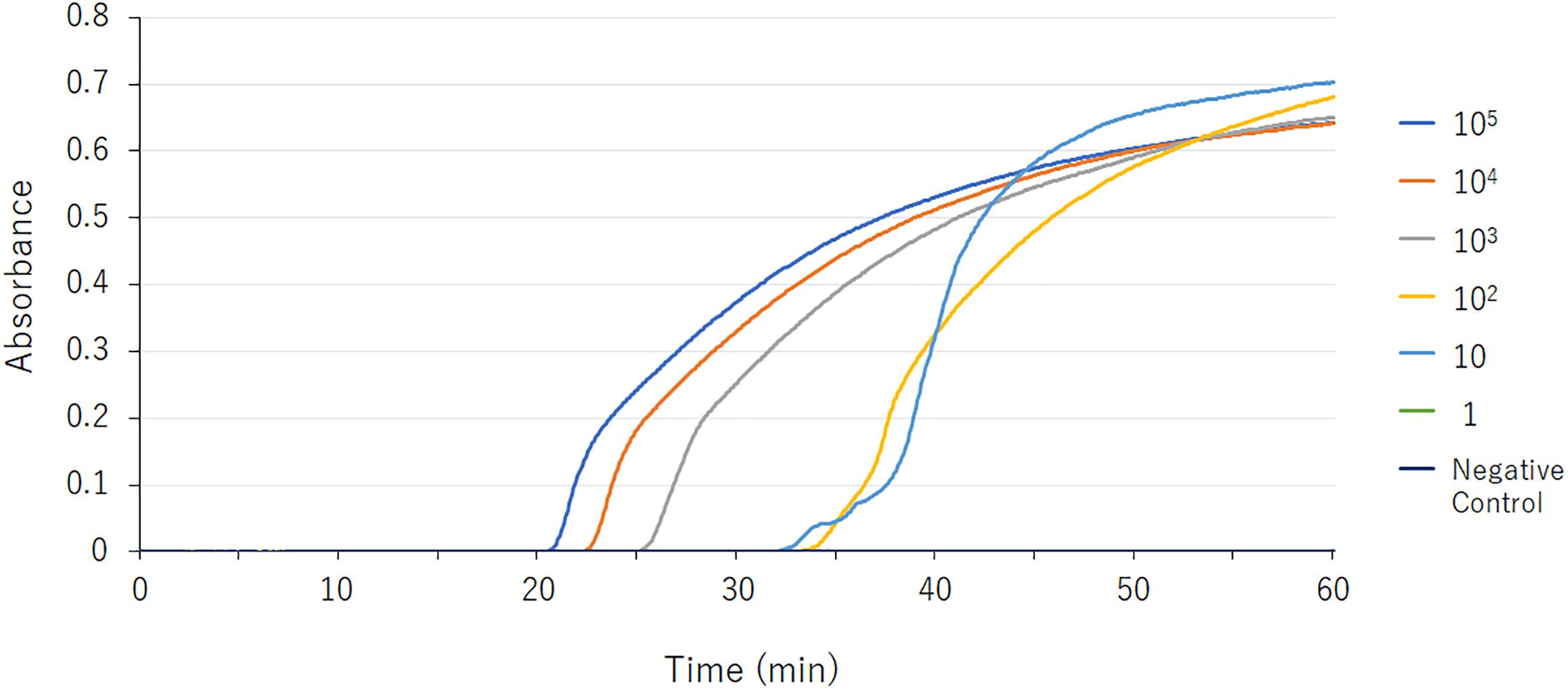
Figure 1. blaGES LAMP assay data derived via real-time turbidimetry. Serial 10-fold-diluted samples (105, 104, 103, 102, 10, and 1 DNA copies) were assayed by LAMP. The detection limit was 10 DNA copies.
Detection Limits
The detection limit of the GES-LAMP assay was 10 DNA copies per reaction, and that of the PCR assay was 100 copies. The LAMP assay was thus highly sensitive, 10-fold more so than PCR. LAMP products were measurable turbidimetrically in real-time and were evident with the naked eye. No false-positive reactions were observed.
DNA-Spiked Specimens
Assay detection limits were determined using DNA-spiked blood, urine, and sputum. The GES-LAMP detection limits were 10 DNA copies per reaction, thus identical to that reported above (Table 4). The PCR detection limits were 100 DNA copies for the DNA-spiked urine and sputum specimens, again identical to that reported above. However, the detection limit was >105 copies in DNA-spiked blood specimens (Table 4).
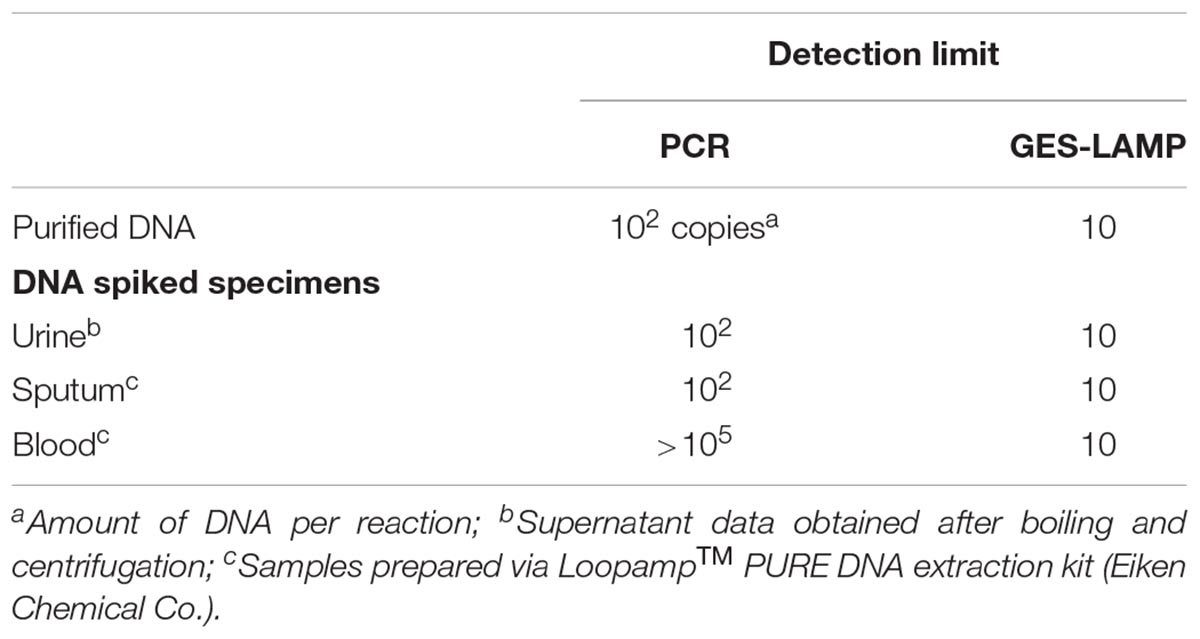
Table 4. Detection limits of the PCR and LAMP assays used to detect DNA from P. aeruginosa of genotype blaGES in DNA-spiked specimens.
Evaluation Using Clinical Strains
Using the 14 clinical P. aeruginosa strains, the GES-LAMP assay amplified the five blaGES segments (GES-1, -5, -7, -9, and- 19/20-like; Table 2), and no other genotype. The results were thus identical to those of PCR (Table 2 and Supplementary Figure S3).
Carba-GES-LAMP Assay
Of the five blaGES-positive samples, only GES-5 and -19/20-like β-lactamase-producing isolates exhibited carbapenem resistance (Table 2), consistent with their genotypes. Our Carba-GES-LAMP assay detected only GES-5 and -19/20-like alleles containing the blaGES Gly165Ser missense mutation. Using the ARMS, we successfully designed the Carba-GES-LAMP primers that distinguish the GES variants. The detection limit of the Carba-GES-LAMP assay was 104 DNA copies. No amplification of non-carbapenemase-producing blaGES genotypes (GES-1, -7, and -9) or any other genotype was evident within 50 min. The product was subjected to direct sequencing. The sequences were compared with those of the targeted region (bases 450–512) of the GES-5 gene (from F1 to B1c, Supplementary Figure S1B). The five base pairs of 3′ end of B1c region were matching to the Carba-GES-LAMP primer sequences (ACCGT; 493-497, Supplementary Figure S1B) including two mutations (Table 3) and obtained other sequences were identical to those expected (Supplementary Figure S2B).
Discussion
We established a novel LAMP assay detecting blaGES. Because β-lactamase genes spread widely via plasmids, rapid and accurate POCT drug-resistance assessment is imperative. Our blaGES LAMP assay was highly specific and more sensitive than PCR. blaGES was correctly identified in 14 clinical isolates expressing various β-lactamases. LAMP reactions are not inhibited by contaminants in DNA-spiked samples. On the other hand, PCR reactions are inhibited by such contaminants, especially heparin (Satsangi et al., 1994), and other blood components including heme, leukocyte DNA, and immunoglobulin G; such inhibitors must be removed prior to PCR (Al-Soud et al., 2000; Al-Soud and Radstrom, 2001). We used the LoopampTM PURE DNA extraction kit (Eiken Chemical Co.) to extract DNA over 30 min, and centrifugation was not required. LAMP can thus be performed at the bedside. We then sought to detect the G493A mutation. GES β-lactamases hydrolyse oxyimino-cephalosporins and those with the Gly165Ser mutation exhibit carbapenemase activity (Naas et al., 2016). P. aeruginosa blaGES strains may cause fatal nosocomial diseases. An outbreak of GES-5 β-lactamase-producing P. aeruginosa was reported in a long-term Japanese care facility (Kanayama et al., 2016). We designed GES-type carbapenemase-specific primers via ARMS, first demonstrated in 1989 (Newton et al., 1989) to detect any mutation via PCR. In 2007, Ikeda et al. used ARMS to design LAMP primers and detected a point mutation predicting the effects of the anti-lung cancer drug gefitinib (Ikeda et al., 2007). We previously used ARMS to establish a LAMP assay that distinguished Neisseria meningitidis serogroup Y from serogroup W (Lee et al., 2015). Here, we used ARMS to design the BIP, avoiding amplification of the wild-type gene. LAMP may be useful to detect GES-type carbapenemase genes.
Conclusion
We established a novel LAMP assay for blaGES with a comparable specificity and greater sensitivity to those of PCR. LAMP assays do not require substrate purification and are appropriate for POCT. LAMP can be used to detect GES-type carbapenemase genes with missense mutations. Further work involving more clinical specimens is required.
Author Contributions
CT, MS, DK, HG, RM, KK, and PK contributed the conception of this study. CT and MS designed the experiments. HG, RM, KK, and PK acquired the samples. CT and MS analyzed the data. CT, MS, DK, and SH interpreted the data, drafted the manuscript, and approved the manuscript.
Funding
This study was supported by JSPS Bilateral Open Partnership Joint Research Projects (MS). DK was supported by the grants NRF-2018R1A2A2A05018341 and NRF-2015M3C9A2054024 from National Research Foundation (NRF) of South Korea.
Conflict of Interest Statement
HG is an employee of Evelo Biosciences.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all staff of Division of Microbiology, Department of Pathology and Microbiology, Nihon University School of Medicine.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.00025/full#supplementary-material
References
Al-Soud, W. A., Jonsson, L. J., and Radstrom, P. (2000). Identification and characterization of immunoglobulin G in blood as a major inhibitor of diagnostic PCR. J. Clin. Microbiol. 38, 345–350.
Al-Soud, W. A., and Radstrom, P. (2001). Purification and characterization of PCR-inhibitory components in blood cells. J. Clin. Microbiol. 39, 485–493. doi: 10.1128/JCM.39.2.485-493.2001
Bebrone, C., Bogaerts, P., Delbruck, H., Bennink, S., Kupper, M. B., Rezende de Castro, R., et al. (2013). GES-18, a new carbapenem-hydrolyzing GES-Type beta-lactamase from Pseudomonas aeruginosa that contains Ile80 and Ser170 residues. Antimicrob. Agents Chemother. 57, 396–401. doi: 10.1128/AAC.01784-12
Bonnin, R. A., Jousset, A. B., Urvoy, N., Gauthier, L., Tlili, L., Creton, E., et al. (2017). Detection of GES-5 carbapenemase in Klebsiella pneumoniae, a newcomer in France. Antimicrob. Agents Chemother. 61, e2263–e2216. doi: 10.1128/AAC.02263-16
Bratu, S., Landman, D., Alam, M., Tolentino, E., and Quale, J. (2005). Detection of KPC carbapenem-hydrolyzing enzymes in Enterobacter spp. from Brooklyn, New York. Antimicrob. Agents Chemother. 49, 776–778. doi: 10.1128/AAC.49.2.776-778.2005
Clinical Laboratory Standards and Institute. (2012). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-two Informational Supplement. Vol.32.M100-S22. Wayne, PA: CLSI.
de Oliveira, D. V., Nunes, L. S., Barth, A. L., and Van Der Sand, S. T. (2017). Genetic background of beta-Lactamases in Enterobacteriaceae isolates from environmental samples. Microb. Ecol. 74, 599–607. doi: 10.1007/s00248-017-0970-6
FUJITSU, L. (2016). PrimerExplorer V5 [Online]. Available at: http://primerexplorer.jp/e/
Ikeda, S., Takabe, K., Inagaki, M., Funakoshi, N., and Suzuki, K. (2007). Detection of gene point mutation in paraffin sections using in situ loop-mediated isothermal amplification. Pathol. Int. 57, 594–599. doi: 10.1111/j.1440-1827.2007.02144.x
Kanayama, A., Kawahara, R., Yamagishi, T., Goto, K., Kobaru, Y., Takano, M., et al. (2016). Successful control of an outbreak of GES-5 extended-spectrum beta-lactamase-producing Pseudomonas aeruginosa in a long-term care facility in Japan. J. Hosp. Infect. 93, 35–41. doi: 10.1016/j.jhin.2015.12.017
Kos, V. N., Deraspe, M., McLaughlin, R. E., Whiteaker, J. D., Roy, P. H., Alm, R. A., et al. (2015). The resistome of Pseudomonas aeruginosa in relationship to phenotypic susceptibility. Antimicrob. Agents Chemother. 59, 427–436. doi: 10.1128/AAC.03954-14
Lee, D., Kim, E. J., Kilgore, P. E., Takahashi, H., Ohnishi, M., Tomono, J., et al. (2015). A novel loop-mediated isothermal amplification assay for serogroup identification of Neisseria meningitidis in cerebrospinal fluid. Front. Microbiol. 6:1548. doi: 10.3389/fmicb.2015.01548
Miyoshi-Akiyama, T., Tada, T., Ohmagari, N., Viet Hung, N., Tharavichitkul, P., Pokhrel, B. M., et al. (2017). Emergence and spread of epidemic multidrug-resistant Pseudomonas aeruginosa. Genome Biol. Evol. 9, 3238–3245. doi: 10.1093/gbe/evx243
Monteiro, J., Widen, R. H., Pignatari, A. C., Kubasek, C., and Silbert, S. (2012). Rapid detection of carbapenemase genes by multiplex real-time PCR. J. Antimicrob. Chemother. 67, 906–909. doi: 10.1093/jac/dkr563
Naas, T., Dortet, L., and Iorga, B. I. (2016). Structural and functional aspects of class a carbapenemases. Curr. Drug Targets 17, 1006–1028. doi: 10.2174/1389450117666160310144501
Newton, C. R., Graham, A., Heptinstall, L. E., Powell, S. J., Summers, C., Kalsheker, N., et al. (1989). Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res. 17, 2503–2516. doi: 10.1093/nar/17.7.2503
Notomi, T., Okayama, H., Masubuchi, H., Yonekawa, T., Watanabe, K., Amino, N., et al. (2000). Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 28:E63. doi: 10.1093/nar/28.12.e63
Poirel, L., Bonnin, R. A., and Nordmann, P. (2012). Genetic support and diversity of acquired extended-spectrum beta-lactamases in Gram-negative rods. Infect. Genet. Evol. 12, 883–893. doi: 10.1016/j.meegid.2012.02.008
Poirel, L., Le Thomas, I., Naas, T., Karim, A., and Nordmann, P. (2000). Biochemical sequence analyses of GES-1, a novel class A extended-spectrum beta-lactamase, and the class 1 integron In52 from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 44, 622–632. doi: 10.1128/AAC.44.3.622-632.2000
Satsangi, J., Jewell, D. P., Welsh, K., Bunce, M., and Bell, J. I. (1994). Effect of heparin on polymerase chain reaction. Lancet 343, 1509–1510. doi: 10.1016/S0140-6736(94)92622-0
World Health Organization (2004). Laboratory Biosafety Manual – 3rd Edn. Available at: http://www.who.int/csr/resources/publications/biosafety/WHO_CDS_CSR_LYO_2004_11/en/
Keywords: blaGES, β-lactamase, point mutation, carbapenemase, loop-mediated isothermal amplification, Pseudomonas aeruginosa
Citation: Takano C, Seki M, Kim DW, Gardner H, McLaughlin RE, Kilgore PE, Kumasaka K and Hayakawa S (2019) Development of a Novel Loop-Mediated Isothermal Amplification Method to Detect Guiana Extended-Spectrum (GES) β-Lactamase Genes in Pseudomonas aeruginosa. Front. Microbiol. 10:25. doi: 10.3389/fmicb.2019.00025
Received: 08 September 2018; Accepted: 09 January 2019;
Published: 04 February 2019.
Edited by:
Leonard Peruski, Centers for Disease Control and Prevention (CDC), United StatesReviewed by:
Amro Abd Al Fattah Amara, City of Scientific Research and Technological Applications, EgyptMariagrazia Perilli, University of L’Aquila, Italy
Copyright © 2019 Takano, Seki, Kim, Gardner, McLaughlin, Kilgore, Kumasaka and Hayakawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mitsuko Seki, c2VraS5taXRzdWtvQG5paG9uLXUuYWMuanA= Dong Wook Kim, ZG9uZ3dvb2tAaGFueWFuZy5hYy5rcg==
 Chika Takano
Chika Takano Mitsuko Seki
Mitsuko Seki Dong Wook Kim
Dong Wook Kim Humphrey Gardner
Humphrey Gardner Robert E. McLaughlin6
Robert E. McLaughlin6 Paul E. Kilgore
Paul E. Kilgore Satoshi Hayakawa
Satoshi Hayakawa